Abstract
Background:
Although reductions in lingual strength are reported in individuals with amyotrophic lateral sclerosis (ALS) that are associated with dysphagia; determination of a functional lingual pressure threshold (FLPT) has not yet been established. The present study therefore sought to identify an FLPT for impaired swallowing safety and efficiency in individuals with ALS.
Methods:
Thirty individuals with ALS completed a standardized videofluoroscopic swallowing examination and maximum anterior isometric lingual pressure testing using the Iowa Oral Performance Instrument. Duplicate, blinded ratings of the validated Penetration-Aspiration Scale (PAS) and Analysis of Swallowing Physiology: Events, Kinematics and Timing (ASPEKT) were performed. Binary classifications of safety (unsafe: PAS: ≥ 3) and efficiency (inefficient: ≥ 3% worst total pharyngeal residue) were derived. Descriptives and receiver operating characteristic curve analyses (AUC, sensitivity, specificity) were performed.
Results:
Unsafe and inefficient swallowing were instrumentally confirmed in 57% and 70% of ALS patients respectively. Across the entire cohort, the mean maximum lingual physiologic capacity was 32.1 kilopascals (‘kPa’; SD: 18.1kPa). The identified FLPT for radiographically confirmed unsafe swallowing was 43kPa (sensitivity: 94%, specificity: 62%, AUC 0.82, p=0.003). FLPT for inefficient swallowing was 46kPa (sensitivity: 86%, specificity: 56%, AUC=0.77, p=0.02).
Conclusions:
These data provide preliminary FLPT data in a small cohort of individuals with ALS that need to be further investigated in larger cohorts to inform clinical screening practices.
Keywords: Lingual pressure, functional reserve, amyotrophic lateral sclerosis, deglutition, deglutition disorders
Introduction
Regulation of nutritional homeostasis relies on intact swallowing function for adequate nutrition and hydration via safe and efficient consumption of food and liquids [1,2]. Individuals with amyotrophic lateral sclerosis (ALS) demonstrate swallowing impairments (dysphagia) and dysregulated nutritional homeostasis. In ALS, dysphagia is characterized by progressive impairments in swallowing safety (airway invasion) and efficiency (pharyngeal residue) due to motor neuron degeneration impacting upper aerodigestive tract musculature. Among these, the tongue is observed to be preferentially affected [3,4] with relatively early morphological changes noted in this muscular hydrostat and reduced lingual force capacity noted in individuals with ALS that is associated with dysphagia (Robison, submitted).
Similar to other systems involved in the bodily homeostatic regulation of vital functions, the tongue has an inherent functional reserve representing its’ pressure generation capacity for speech and swallowing [5]. Functional reserves are found in cells, tissues, and organs and represent the difference between a structure’s maximum capacity and the amount needed for typical function [6]. Although functional reserves are not utilized during basal function, they are utilized when the body encounters unexpected stressors to maintain homeostasis of internal physiologic functions (e.g., blood glucose concentration and core body temperature) [7]. Homeostenosis refers to a diminishing or narrowing of functional reserves that occurs with aging and disease [8]. Reductions in functional reserve result from physiological changes that accompany aging and diseases such as sarcopenia, replacement of muscle with fatty tissue, and physical deconditioning [9,10]. Although homeostenosis is not necessarily pathologic within and of itself [11] as functional reserves are lost, the individual is brought closer to a ‘precipice’ (threshold) that once reached, disability and functional limitations become apparent [6,12].
These functional thresholds are documented across most physiologic systems and suggest once functional reserve is depleted by 30% functional limitations ensue, with complete function failure noted once a 70% depletion occurs [12,13]. Few studies have examined functional lingual pressure depletion thresholds for dysphagia. Steele [14] previously identified that a lingual pressure threshold of 40 kilopascals (kPa) was associated with an increased aspiration risk. Clark and colleagues [15] examined tongue strength in relation to oral phase swallowing impairments and suggested a cutoff of 20kPa might differentiate between those with and without oral phase swallowing impairments. Recently, Printza and colleagues [16] found in a cohort of 25 patients with ALS that a maximum anterior isometric lingual pressure of ≤22kPa discriminated aspirators versus non-aspirators. Importantly, the limited work that has been done in this area thus far has been primarily focused on identifying aspiration. Previously, Waito [17] highlighted that the ALS literature is overwhelmingly geared towards identifying safety versus efficiency impairments. This is concerning given our recent findings [18] that efficiency impairments were more prevalent than safety impairments in a large cohort of patients with ALS. Collectively, these data suggest in this patient population, lingual thresholds to identify impairments in both safety and efficiency may be clinically relevant. Such knowledge would aide our understanding of the emergence and progression of functional swallowing impairment profiles in ALS and inform potential clinical markers to indicate potential risk, guide assessment pathways, and inform subsequent clinical decision making. Motivated by these factors, we aimed to identify functional maximum lingual pressure thresholds (FLPT) associated with unsafe and inefficient swallowing in individuals with ALS. Given that generation of adequate lingual pressure behind the bolus is important to propel the bolus efficiently, we hypothesized that a higher FLPT (i.e. milder pressure reduction) would be identified for inefficient swallowing as compared to the FLPT for unsafe swallowing.
Methods
Participants
Thirty individuals were recruited from an academic outpatient neurology clinic as part of an ongoing natural history study. Inclusion criteria were: 1) confirmed diagnosis of probable or definite ALS (El-Escorial criteria revisited [19]) by a neuromuscular neurology specialist; 2) still consuming some form of oral intake (not feeding tube dependent); 3) no allergies to barium; 4) no history of stroke, head, and neck cancer, or other conditions impacting swallowing; and 5) not pregnant. As part of the longitudinal natural history study, participants attended serial research evaluations in our laboratory at regular three-month intervals. Thirty consecutive patients who attended their serial research evaluation during July 2019 to January 2020 were included in the current analysis. This study was approved by the University Institutional Review Board and conducted in accordance with the Declaration of Helsinki. Each participant provided written informed consent upon enrollment.
Procedures:
Lingual Physiologic Capacity
The lingual pressure experimental setup is fully described in the first author’s dissertation [20]. Briefly, lingual physiologic capacity (LPC; maximum tongue strength) for each participant was assessed using a handheld lingual manometer, the Iowa Oral Performance Instrument (IOPI version 2.3; Woodinville, WA). The IOPI device was connected to a 16/35 PowerLab (ADInstruments, Colorado Springs, CO) to enable real-time visualization and recording in LabChart 8 software (ADInstruments, Colorado Springs, CO). During data acquisition, the IOPI was placed into “peak” mode for all trials and a clean and individually wrapped IOPI tongue bulb was connected to the IOPI device via the “pressure in” connector located at the bottom of the device. The air-filled tongue bulb was then placed in the participant’s mouth on the alveolar ridge immediately behind the front teeth. The participants were instructed to press against the bulb as hard as possible using the front part of their tongue. As they pressed their tongue against the bulb, a corresponding lingual pressure pattern was generated within the LabChart software. The computer screen was placed out of view of the participants to prevent them from potentially modulating their pressure patterns. Three trials of LPC were performed by each participant with a one-minute rest period between each trial. The lingual waveforms were saved in the LabChart program for subsequent data extraction and analysis. To limit a potential ‘order effect’, in our protocol lingual testing was consistently performed prior to performing the videofluoroscopic swallowing examination.
Videofluoroscopic Swallowing Examination:
Procedures and analyses in the present study were conducted using previously detailed methodology from our laboratory [18]. A standardized videofluoroscopic swallowing evaluation (VF) assessed swallowing function of each participant. Participants were seated comfortably in the upright position in a TransMotion Medical TMM3 Videofluoroscopy Swallow Study Treatment Chair (Ocala, FL). A TIMS Dicom system (Version 3.2, TIMS Medical, TM, Chelmsford, MA) recorded high-resolution images continuously at a rate of 30 frames per second. Images were captured in the lateral plane using a properly collimated Phillips BV Endura System fluoroscopic C-arm unit (GE OEC 8800 Digital Mobile C-Arm System). The TIMS system automatically spliced each bolus trial into individual clips that were used in subsequent data analysis. A standardized bolus presentation was used consisting of Varibar® barium sulfate trials (Bracco Imaging, Monroe Township, NJ) and included: three 5-mL thin liquid trials from a 30-mL medicine cup (40% w/v ratio); a comfortable cup sip of 90-mL of thin liquid from a cup (Solo Clear Graduated Medicine Cups, Lake Forest, IL); consecutive cup sip challenge of remaining thin liquid; three 5-mL thin honey liquid from a tablespoon; two tablespoons of pudding; ¼ graham cracker (Honey Maid, Mondelez Global LLC, East Hanover, NJ) with pudding; and a 13mm E-Z-Disk™ barium tablet. Except the sequential swallow trial, participants were instructed to hold the bolus in their mouth and then cued to swallow. Participants self-administered each bolus trial, however, were assisted by the research clinician in the event they were unable to self-administer. A bailout criterion was used during the VF to maintain patient safety. Under the criterion, following the second instance of aspiration, the participant would move to the next thickest consistency. The VF was terminated if the third occurrence of aspiration occurred, or if pharyngeal residue totaling ≥75% accumulated in the valleculae or pyriform sinuses that could not be cleared.
Analyses and Outcomes of Interest:
Maximum Lingual Pressure:
The lingual pressure waveforms recorded in LabChart were subsequently extracted and analyzed using a custom MATrix LABoratory (MATLAB Version R2019a, Mathworks, Natick, Massachusetts, USA) program. Given that our interest was in identifying a maximum lingual pressure threshold for swallowing impairments; the highest LPC value recorded across the three trials for each participant was recorded and used in subsequent data analysis.
Videofluoroscopic Swallowing Examination Outcomes:
Two independent raters completed videofluoroscopic analyses for each participant post-hoc. Before completing analyses, raters completed a standardized training program that included reliability training of a benchmark set of previously-double-rated rated films of patients with ALS. Raters were required to obtain competencies of at least 90% accuracy to proceed with the analysis of the current dataset.
For the current videofluoroscopic analyses, bolus trials were blinded and presented in a randomized order consisting of one bolus per clip. One hundred percent agreement was required for duplicate ratings and a discrepancy meeting that included four expert raters was held to gain a final consensus.
Swallowing Safety:
The validated Penetration-Aspiration Scale (PAS) [21] was used to analyze swallowing safety. The PAS is an eight-point ordinal scale that objectively describes the depth of any airway invasion during a swallow and the patient’s response to any material entering above or below the airway. Scores on the PAS range from 1 indicating no airway invasion to a score of 8 which indicates the patient had no response to aspiration (‘silent aspiration’). Every swallow for each bolus trial was assigned PAS scores by two independent, blinded raters. The worst PAS score obtained across all trials and consistencies excluding the consecutive cup sips was used in subsequent statistical analyses. Based on the worst PAS score, binary swallowing safety classifications were also derived and were defined as Safe (PAS: ≤ 2) and Unsafe (PAS: ≥ 3)[18,22–24]. These binary safety classifications were chosen due to the accelerated course of ALS disease progression combined with previous findings of impaired laryngeal vestibule closure (LVC) timing and kinematics previously identified in this patient population. Specifically, ALS is known to be rapidly progressing with a median survival time of 40.2 months in younger adults (<65.2 years) and 20.5 months in older adults (>65.2 years)[25]. Therefore, it is important that any potentially meaningful changes in swallow function (such as penetration) be identified early so appropriate monitoring and management can be initiated. Further, previous research in patients with ALS has found reduced pharyngeal constriction [26], increased time-to-LVC [27], a high proportion of swallows with incomplete or partial LVC [27], and comprised airway protection due premature closure of the upper esophageal sphincter prior to the descent of the laryngeal muscles [28]. The nature of these impairments may have potential implications for entry of unwanted material into the vestibule during swallowing which may lead to penetration and/or aspiration.
Swallowing Efficiency:
Post-swallow residue in the valleculae, pyriform sinuses, and extra pharyngeal spaces were analyzed on the first and last swallow of the 5-mL thin (trials 1 and 2), cup sip, and 5-mL thin honey boluses (trials 1 and 2)for a total of ten swallows per participant analyzed. Swallows were analyzedusing the residue subcomponent of the Analysis of Swallowing Physiology: Events, Kinematics and Timing (ASPEKT [29]) method. Detailed methodology about residue ratings can be found in a previous investigation by our group [18] and also in the Appendix of the original publication by Steeleand colleagues which described the ASPEKT method in full detail and established reference values for thin to extremely thick liquids in healthy adults [29]. Worst residue in the valleculae, pyriform sinuses, and extra pharyngeal spaces was determined across boluses. Subsequently, a worst total percent pharyngeal residue was determined for each participant and represented the sum of worst residues across the three pharyngeal sites. Binary efficiency classifications were then determined. Efficient swallowing was defined as: worst total pharyngeal residue <3%. Inefficient swallowing was defined as: worst total pharyngeal residue ≥ 3%. This threshold was chosen based on recent findings that 3% represents the upper limit (2 standard deviations) of residue in normative healthy reference data [30].
Statistical Analysis
Descriptives were performed on participant demographics, lingual pressure, and videofluoroscopic evaluation data. FLPT thresholds for swallowing safety and efficiency impairments were determined using sensitivity, specificity, and receiver operating characteristic (ROC) curve analyses. Inter-rater reliability safety and efficiency ratings were determined using Cohen’s kappa and two-way mixed intraclass coefficients (ICCs), respectively. Levels of agreement for safety and efficiency reliability ratings were reported using published guidelines [31,32]. Statistical analysis was performed using SPSS statistical package for Windows (Version 26, IBM, Armonk, NY).
Results
Participant demographics are summarized in Table 1. Reliability outcomes indicated that there was substantial agreement between raters for PAS ratings (κ = 0.64; 70% agreement) and excellent inter-rater reliability for ASPEKT efficiency ratings (ICC: 0.89; 95% CI: 0.80–0.94).
Table 1.
Summary of study cohort demographic data (N=30).
| Demographic Variable: | Mean (SD) | Minimum | Maximum |
|---|---|---|---|
|
| |||
| Age (years) | 63.5 (9.4) | 36 | 81 |
| Disease Duration (months) | 43.6 (29.3) | 8 | 123 |
| ALSFRS-R Total Score | 32.5 (9.9) | 10 | 45 |
| ALSFRS-R Bulbar Score | 8.13 (3.1) | 3 | 12 |
| Sex | 50% Male 50% Female |
||
| Onset Type | 53.3% Spinal 46.7% Bulbar |
||
Note: ALSFRS-R – Amyotrophic Lateral Scale Functional Rating Scale-Revised [46].
The mean LPC for the entire cohort and between sex, swallowing safety, and swallowing efficiency groups is provided in Table 2. Swallowing safety and efficiency profiles are presented in Figure 1 and confirm inefficient swallowing in 70% (n=21) and unsafe swallowing in 56.7% (n = 17) of participants. ROC curves and FLPT data are presented in Table 3 and Figure 2, respectively. The FLPT that optimized sensitivity and specificity for detection of unsafe swallowing was ≤43kPa. This threshold demonstrated a sensitivity of 94%, a specificity of 61.5%, and an AUC of 0.824, p=0.003. The FLPT threshold that optimized sensitivity and specificity for detection of inefficient swallowing was ≤46kPa which demonstrated a sensitivity of 85.7%, a specificity of 55.6%, and an AUC of 0.77, p=0.02. Individual LPC values across safety and efficiency groups relative to the identified FLPTs are detailed in Figure 3.
Table 2.
Summary of mean (standard deviation; SD) lingual physiologic capacity (LPC) across the entire cohort and between sex, swallowing safety, and efficiency groups. LPC data are provided in kilopascals (kPa).
| Outcome | Mean LPC (kPa) |
SD |
|---|---|---|
|
| ||
| LPC and Cohort Demographics: | ||
| Entire Cohort (n=30) | 32.1 | 18.1 |
| Male (n=15) | 35.1 | 16.4 |
| Female (n=15) | 29.1 | 19.8 |
| LPC and Swallowing Safety: | ||
| Safe Swallowers (n=13) | 44.1 | 13.3 |
| Unsafe Swallowers (n=17) | 22.9 | 15.9 |
| LPC and Swallowing Efficiency: | ||
| Efficient Swallowers (n=9) | 44.2 | 13.1 |
| Inefficient Swallowers (n=21) | 26.9 | 17.7 |
Fig. 1.
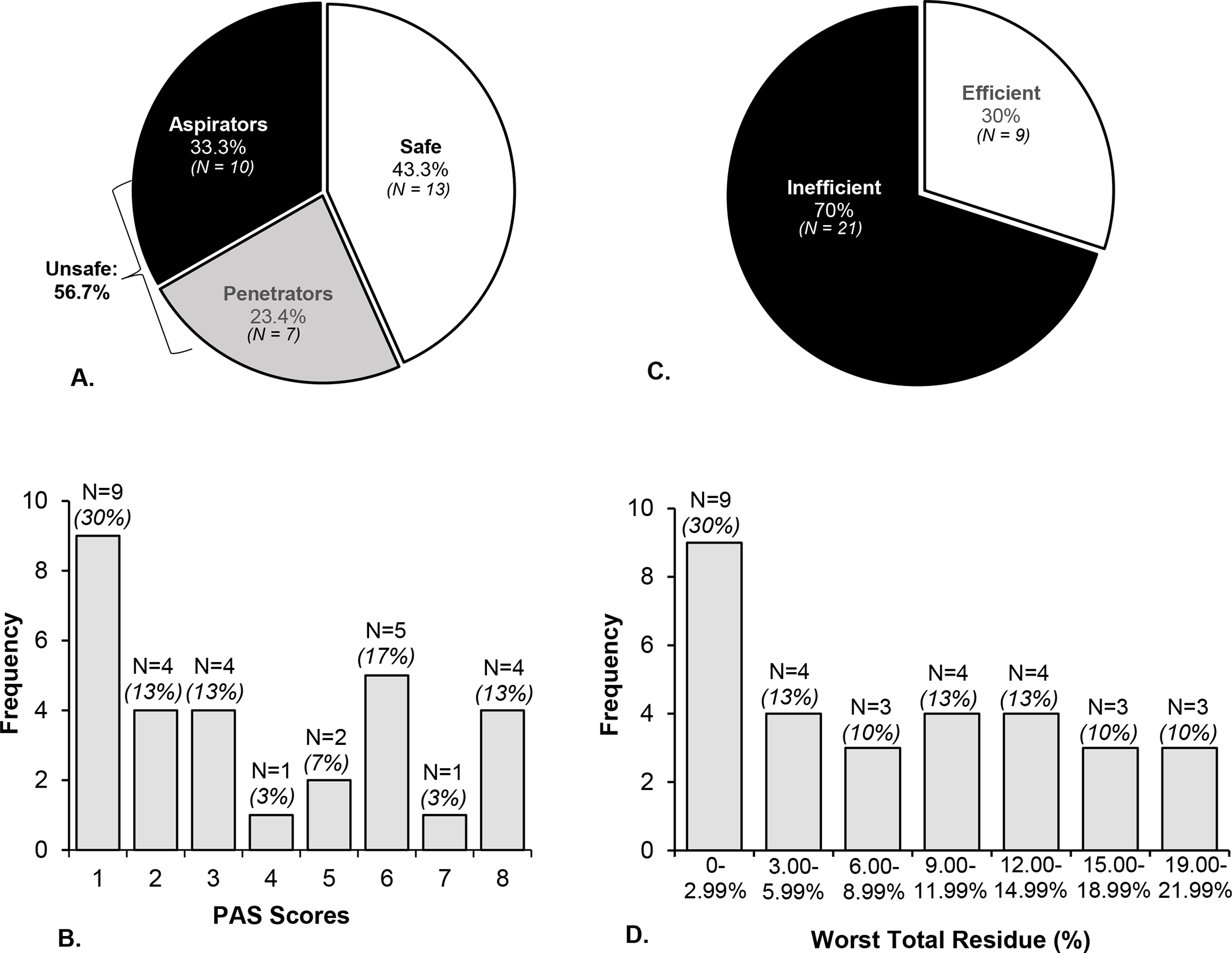
Profiles of swallowing safety (A) and efficiency (C) in this cohort of 30 individuals with amyotrophic lateral sclerosis (ALS). Swallowing safety classifications were derived using the validated Penetration-Aspiration Scale ‘PAS’ [21] (Safe: ≤2; Penetrators: 3–5; Aspirators: 6–8). Swallowing efficiency profiles were determined using the residue subcomponent of the Analysis of Swallowing Physiology: Events, Kinematics, Timing method [29] (Efficient: worst total residue <3%; Inefficient: worst total residue ≥3%). The frequency of PAS scores and worst residue percent ranges across participants are also depicted in Figure 3B and Figure 3D, respectively.
Table 3.
Summary of receiver operator characteristic curve results for lingual physiologic capacity and derived functional lingual pressure thresholds associated with unsafe and inefficient swallowing in this cohort of 30 individuals with amyotrophic lateral sclerosis.
| Swallowing Impairment | FLPT: | Area Under the Curve (95% C.I.) |
Sensitivity (%) |
Specificity (%) |
|---|---|---|---|---|
|
| ||||
| Unsafe Swallowing | ≤43kPa | 0.82 (0.70, 0.98) |
94% | 61.5% |
| Inefficient Swallowing | ≤46kPa | 0.77 (0.60, 0.94) |
85.7% | 55.6% |
Fig. 2.
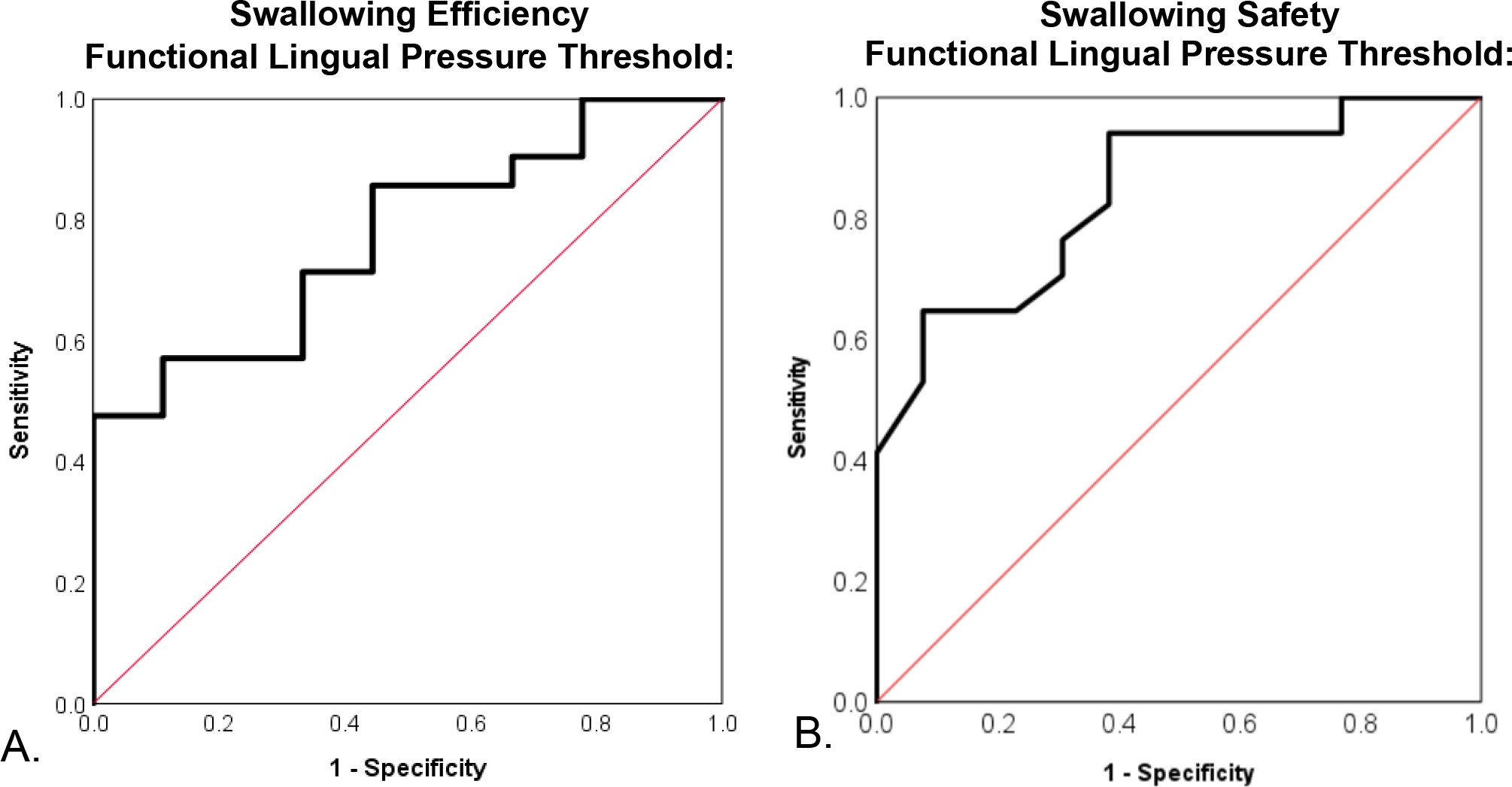
Receiver operating characteristic curve’s for functional lingual pressure thresholds (FLPT) to detect impairments in swallowing efficiency (A) and swallowing safety (B). A FLPT of ≤46kPa optimized sensitivity (86%) and specificity (56%) for detection of inefficient swallowing and correctly classified efficiency status in 77% of cases (AUC=0.77, p=0.02). A LPT of ≤43kPa optimized sensitivity (94%) and specificity (61%) for identification of unsafe swallowing and correctly classified swallowing safety status in 82% of cases (AUC 0.82, p=0.003).
Fig. 3.
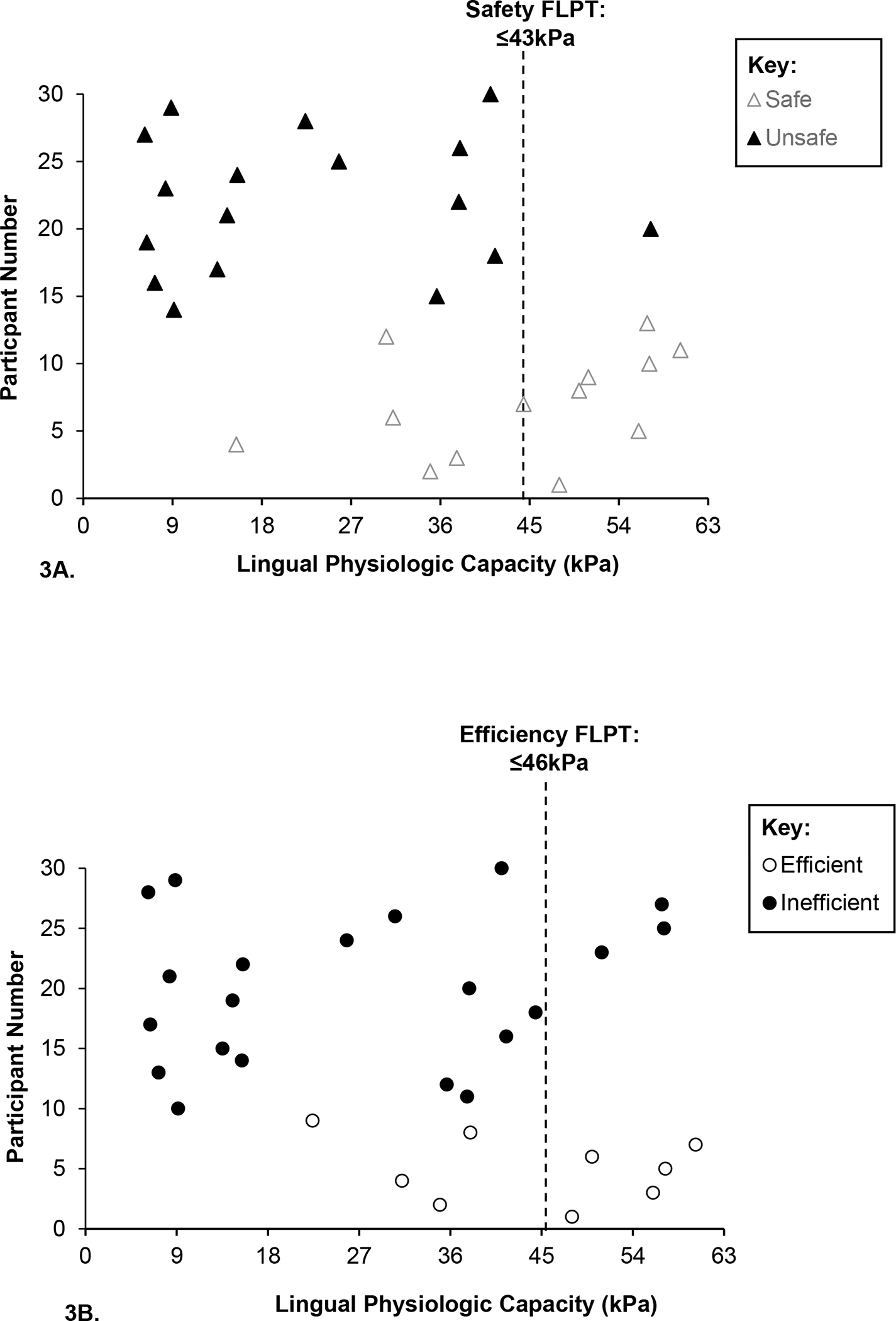
Individual lingual physiologic capacities (LPC; kilopascals (kPa)) across safety (3A) and efficiency groups (3B). Swallowing safety classifications were determined using the validated Penetration-Aspiration Scale ‘PAS’ [21] scale (Safe: ≤2; Unsafe: 3–8). Swallowing efficiency profiles were determined using the Analysis of Swallowing Physiology: Events, Kinematics, Timing methodology [29] (Efficient: worst total residue <3%; Inefficient: worst total residue ≥3%). Within the safety group, 38.5% (n=5) of safe swallowers demonstrated an LPC below the identified functional lingual pressure threshold (FLPT) of ≤43kPa. Within the efficiency group, 44.4% (n=4) of participants with efficient swallowing had an LPC below the FLPT of ≤46kPa.
Discussion
In the current investigation, the functional threshold identified to differentiate between safe and unsafe swallowers was 43kPa; with 94% of penetrators and aspirators demonstrating an LPC equal to or less than this value (sensitivity) and 61.5% of safe swallowers demonstrating an LPC above this threshold (specificity). The obtained AUC value indicated that there is an 82% chance this reference criterion can accurately distinguish between positive (those with safety impairments) and negative (those without safety impairments) classes.. The functional lingual pressure threshold for swallowing efficiency was 46kPa; with 85.7% of inefficient swallowers demonstrating an LPC equal to or less than this value (sensitivity) and 55.6% of efficient swallowers demonstrating an LPC above this threshold (specificity). The obtained AUC value indicated that there is a 77% chance that the identified reference criterion would correctly differentiate between positive (those with efficiency impairments) and negative (those without efficiency impairments) classes.
In typical aging and across most physiological systems, a 30% loss of physiologic capacity is noted to limit normal function and a 70% decline resulting in system failure [12]. In healthy older adults (> 60 years), the established expected or average LPC is ~57kPa [33]. Compared to this normative reference value, ALS participants in this study demonstrated an average LPC reduction in LPC of 25kPa or ~44%. These LPC data confirm previous reports of significantly reduced LPC in ALS [34–37].
Our hypothesis that swallowing efficiency impairments would be associated with a smaller reduction in lingual pressure generation capacity than safety impairments was somewhat confirmed by our data (i.e., higher FLPT), albeit by only 3kPa. These findings may relate to the physiological role the tongue typically plays in facilitating swallowing efficiency versus swallowing safety. Indeed, to ensure swallowing efficiency, the tongue is involved in bolus manipulation, transit, and propulsion during the oral preparatory and oral phases of the swallow [38]. Conversely, the tongue’s role in facilitating swallowing safety is less prominent and limited to the base of tongue retraction to help facilitate epiglottic inversion to seal off the laryngeal vestibule closure during swallowing [39]. In patients with ALS, it has been found that the tongue exhibits early and extensive physiologic impairment across both bulbar and spinal onset types [3,4,40] which likely contributes to the reduced tongue pressures, impaired bolus manipulation and transit, and early occurrence of oral residue noted in this patient population [36,41,42]. Thus, the current findings that milder lingual pressure reductions were associated with inefficient swallowing reflects the underlying bulbar pathology in ALS, wherein early and targeted changes to the tongue and its pressure generation capacity decrease the ability of ALS individuals to efficiently manipulate, transport, and propel ingested food and liquids.
Notably, the identified FLPT for swallowing safety is relatively similar to the functional threshold of 40kPa previously identified as a risk for aspiration in healthy older adults [14,43]. Printza and colleagues [16] recently examined maximum lingual pressure values in 25 individuals with ALS and reported that 22kPa discriminated instrumentally confirmed aspirators versus non-aspirators (sensitivity: 89.5%, specificity: 80.0%). Given that the focus of our investigation was to identify the FLPT for unsafe swallowing (including both penetrators and apirators), findings are not directly comparable. Factoring in these methodologic considerations, however, the higher safety FLPT derived in our study appears reasonable. An examination of mean LPC across unsafe swallowing categories within the current dataset indeed indicates that LPC is lower in aspirators (M: 17kPa; SD: 12.1kPa) versus penetrators (M: 30kPa; SD: 18.5kPa). These preliminary data suggest that as lingual pressure capacity progressively decreases in ALS, the degree of airway invasion during swallowing may increase.
While the FLPT for swallowing efficiency in the current cohort was higher than the FLPT for swallowing safety; their difference was minimal. This finding suggests lingual physiologic reserve homeostenosis (reduction) secondary to a decline in lingual pressure generation has important implications for both swallowing safety and efficiency. The extant literature examining associations between lingual pressure and both aspects of swallowing function in ALS is limited. A study by Pizzorni and colleagues found that decreased lingual pressure in individuals with ALS was associated with impairments in swallowing efficiency, however, not with impairments in swallowing safety [37]. Differences in methodologies used in the prior investigation (use of fiberoptic endoscopic evaluation of swallowing versus videofluoroscopy and differences in boluses administered and analyzed) and the current study may account for these contrasting findings. A recent investigation by our group (Robison, submitted) found in a larger cohort of 97 patients found that lower LPC was associated with significantly higher (worse) PAS scores and increased residue in the valleculae, pyriform sinuses, and extra pharyngeal spaces. Further, individuals classified as unsafe (aspirators and penetrators) and inefficient had significantly lower LPC than safe and efficient swallowers, respectively (Robison, submitted). Collectively, these findings contribute and expand upon the existing literature and suggest reductions to lingual function are an important consideration for maintenance of both aspects of swallowing in ALS.
Although findings from this study are preliminary, they hold potential clinical relevance to be further explored in larger scaled studies. Although instrumental swallowing evaluations (ISE) are considered the “gold standard” for assessing swallowing function [44], a recent national survey of multi-disciplinary ALS clinics revealed that only 55% perform routine clinical swallowing examinations and only 27% perform direct imaging of swallowing [45]. LPC is a quick, easy, and objective metric that can be obtained in real-time (under five minutes). Thus, lingual pressure testing could be considered by clinicians to incorporate into their visits when working in busy multidisciplinary clinics to objectively determine underlying lingual physiologic capacity to swallow safely and efficiently. In addition to other clinical signs, lingual pressure testing might also help with clinical decision-making to determine the timing for direct imaging of swallowing to optimize referral workflows and optimize resource utilization. For instance, if an individual has not yet reached the critical threshold identified for inefficient swallowing (46kPa), then a clinician might not yet recommend an ISE. On the other hand, if an individual has a reduced LPC near or below the safety FLPT (43kPa), a clinician might advocate for an ISE referral. Still, the optimal FLPTs identified in the current data set had relatively low AUCs highlighting the limitations of lingual pressure as a metric to identify safety and efficiency impairments. Namely that lingual pressure represents only one facet of swallowing function, therefore, it will be important for clinicians to consider LPC in the context of other bulbar sensorimotor assessment metrics and overall functioning (respiratory status, comorbid conditions, mobility etc) to inform their clinical decision-making.
There are limitations to the present study that must be acknowledged. First, while this study provided a preliminary foundation for understanding functional relationships with lingual pressure physiologic capacity in ALS, the number of participants was only 30. To improve the generalizability of these findings, validation in a larger cohort of ALS participants is warranted. Further, the cross-sectional nature of our data collection does not necessarily pinpoint the emergence or exact time point where an IMPAIRMENT appears over time. A longitudinal observational study is needed to answer these questions and is currently underway to evaluate FLPT at the early, middle, and late stages of the disease in a larger sample of patients with ALS.
In conclusion, lingual pressure generation capacity appears to contribute to both swallowing safety and efficiency, with relatively similar functional depletion thresholds observed in this cohort of ALS patients. Future longitudinal work in larger cohorts of patients is needed to elucidate the relative role and importance of FLPT on functional aspects of deglutition across the ALS disease course.
Acknowledgement:
We are grateful for the support of the Dr. Jon and Nancy McEwans Wilkins Research Fellowship and the individuals with ALS who participated. We would like to thank Amy Ashley, Kasey McElheny, and Kelly Leonard for their assistance with data analysis. This investigation was based on dissertation work submitted by the first author to the University of Florida in partial fulfillment of requirements for the degree of Doctor of Philosophy.
Funding:
This work was supported by National Institute of Neurological Disorders and Stroke (NINDS) Grant Numbers: 1R01 NS100859–01, 5R01NS10859–02S1, and 1F99 NS115339–01.
Footnotes
Disclosures: The authors have nothing to disclose.
References
- 1.Smith GP. The controls of eating: a shift from nutritional homeostasis to behavioral neuroscience. Nutrition. United States; 2000;16:814–20. [DOI] [PubMed] [Google Scholar]
- 2.Morton GJ, Meek TH, Schwartz MW. Neurobiology of food intake in health and disease. Nat Rev Neurosci [Internet]. 2014;15:367–78. Available from: https://www.ncbi.nlm.nih.gov/pubmed/24840801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.DePaul R, Brooks BR. Multiple orofacial indices in amyotrophic lateral sclerosis. J Speech Hear Res [Internet]. 1993/December/01. 1993;36:1158–67. Available from: http://www.ncbi.nlm.nih.gov/pubmed/8114482 [DOI] [PubMed] [Google Scholar]
- 4.DePaul R, Abbs JH, Caligiuri M, Gracco VL, Brooks BR. Hypoglossal, trigeminal, and facial motoneuron involvement in amyotrophic lateral sclerosis. Neurology [Internet]. 1988;38:281. Available from: http://n.neurology.org/content/38/2/281.abstract [DOI] [PubMed] [Google Scholar]
- 5.Steele CM. Optimal approaches for measuring tongue-pressure functional reserve. J Aging Res. Hindawi; 2013;2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Taffett GE. Physiology of Aging. Geriatr Med An Evidence-Based Approach [Internet]. New York, NY: Springer New York; 2003. p. 27–35. Available from: 10.1007/0-387-22621-4_3 [DOI] [Google Scholar]
- 7.Modell H, Cliff W, Michael J, McFarland J, Pat Wenderoth M, Wright A. A Personal View A physiologist’s view of homeostasis. Adv Physiol Educ [Internet]. 2015;39:259–66. Available from: www.physiology.org/journal/advances [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bettelli G Perioperative Care of the Elderly: Clinical and Organizational Aspects [Internet]. Cambridge University Press; 2017. Available from: https://books.google.com/books?id=rgRADwAAQBAJ [Google Scholar]
- 9.Strait JB, Lakatta EG. Aging-associated cardiovascular changes and their relationship to heart failure. Heart Fail Clin [Internet]. 2012;8:143–64. Available from: https://pubmed.ncbi.nlm.nih.gov/22108734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chun A Geriatric Practice: A Competency Based Approach to Caring for Older Adults [Internet]. Springer International Publishing; 2019. Available from: https://books.google.com/books?id=qCa7DwAAQBAJ [Google Scholar]
- 11.Silverstein J, Rooke A, Reves JG, McLeskey CH. Geriatric Anesthesiology [Internet]. Springer New York; 2008. Available from: https://books.google.com/books?id=wLDonTgNeGEC [Google Scholar]
- 12.Bortz 2nd WM. A conceptual framework of frailty: a review. J Gerontol A Biol Sci Med Sci [Internet]. 2002/May/02. 2002;57:M283–8. Available from: http://www.ncbi.nlm.nih.gov/pubmed/11983721 [DOI] [PubMed] [Google Scholar]
- 13.Marcell TJ. Sarcopenia : Causes, Consequences, and Preventions. 2018;58:911–6. [DOI] [PubMed] [Google Scholar]
- 14.Steele CM, Cichero JAY. Physiological Factors Related to Aspiration Risk: A Systematic Review. Dysphagia [Internet]. 2014;29:295–304. Available from: 10.1007/s00455-014-9516-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Clark HM, Henson PA, Barber WD, Stierwalt JAG, Sherrill M. Relationships among subjective and objective measures of tongue strength and oral phase swallowing impairments. Am J speech-language Pathol. United States; 2003;12:40–50. [DOI] [PubMed] [Google Scholar]
- 16.Printza A, Boziki M, Triaridis S, Kiousi V, Arnaoutoglou M, Constantinidis J, et al. Tongue strength, dysphagia questionnaire, pharyngeal secretions and FEES findings in dysphagia management in amyotrophic lateral sclerosis. Auris Nasus Larynx. Netherlands; 2020; [DOI] [PubMed] [Google Scholar]
- 17.Waito AA, Valenzano TJ, Peladeau-Pigeon M, Steele CM. Trends in Research Literature Describing Dysphagia in Motor Neuron Diseases (MND): A Scoping Review. Dysphagia. United States; 2017;32:734–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Robison R, DiBiase L, Ashley A, McElheny K, Anderson A, Wymer JP, et al. Swallowing Safety and Efficiency Impairment Profiles in Individuals with Amyotrophic Lateral Sclerosis. Dysphagia [Internet]. 2021; Available from: 10.1007/s00455-021-10315-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ludolph A, Drory V, Hardiman O, Nakano I, Ravits J, Robberecht W, et al. A revision of the El Escorial criteria - 2015. Amyotroph. Lateral Scler. Frontotemporal Degener. England; 2015. p. 291–2. [DOI] [PubMed] [Google Scholar]
- 20.Robison R Running on Reserve: Impact of Lingual Physiologic Reserve Homeostenosis on Deglutition in ALS [Internet]. University of Florida; 2020. Available from: https://ufdc.ufl.edu/UFE0056320/00001 [Google Scholar]
- 21.Rosenbek JC, Robbins JA, Roecker EB, Coyle JL, Wood JL. A penetration-aspiration scale. Dysphagia. 1996/January/01. 1996;11:93–8. [DOI] [PubMed] [Google Scholar]
- 22.Plowman EK, Tabor LC, Robison R, Gaziano J, Dion C, Watts SA, et al. Discriminant ability of the Eating Assessment Tool-10 to detect aspiration in individuals with amyotrophic lateral sclerosis. Neurogastroenterol Motil [Internet]. 2016;28:85–90. Available from: https://europepmc.org/articles/PMC4688134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chapin JL, Gray LT, Vasilopoulos T, Anderson A, DiBiase L, York JD, et al. Diagnostic utility of the amyotrophic lateral sclerosis Functional Rating Scale-Revised to detect pharyngeal dysphagia in individuals with amyotrophic lateral sclerosis. PLoS One [Internet]. Public Library of Science; 2020;15:e0236804–e0236804. Available from: https://pubmed.ncbi.nlm.nih.gov/32790801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Molfenter SM, Steele CM. Kinematic and temporal factors associated with penetration-aspiration in swallowing liquids. Dysphagia. 2014;29:269–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kjældgaard A-L, Pilely K, Olsen KS, Jessen AH, Lauritsen AØ, Pedersen SW, et al. Prediction of survival in amyotrophic lateral sclerosis: a nationwide, Danish cohort study. BMC Neurol [Internet]. 2021;21:164. Available from: 10.1186/s12883-021-02187-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Waito AA, Tabor-Gray LC, Steele CM, Plowman EK. Reduced pharyngeal constriction is associated with impaired swallowing efficiency in Amyotrophic Lateral Sclerosis (ALS). Neurogastroenterol Motil [Internet]. John Wiley & Sons, Ltd (10.1111); 2018;30:e13450. Available from: 10.1111/nmo.13450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Waito A, Plowman E, Barbon C, Peladeau-Pigeon M, Tabor-Gray L, Magennis K, et al. A Cross-Sectional, Quantitative Videofluoroscopic Analysis of Swallowing Physiology and Function in Individuals With Amyotrophic Lateral Sclerosis. J Speech, Lang Hear Res [Internet]. American Speech-Language-Hearing Association; 2020;63:948–62. Available from: 10.1044/2020_JSLHR-19-00051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ertekin C, Aydogdu I, Yüceyar N, Kiylioglu N, Tarlaci S, Uludag B. Pathophysiological mechanisms of oropharyngeal dysphagia in amyotrophic lateral sclerosis. Brain. 2000;123:125–40. [DOI] [PubMed] [Google Scholar]
- 29.Steele CM, Peladeau-Pigeon M, Barbon CAE, Guida BT, Namasivayam-MacDonald AM, Nascimento WV, et al. Reference Values for Healthy Swallowing Across the Range From Thin to Extremely Thick Liquids. J Speech Lang Hear Res. United States; 2019;62:1338–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Steele CM, Peladeau-Pigeon M, Barrett-Connor E, Wolkin TS. What is the Risk of Penetration-Aspiration Related to Residue in the Pharynx? Dysphagia Res Soc Annu Meet. San Juan, Puerto Rico; 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Koo TK, Li MY. A Guideline of Selecting and Reporting Intraclass Correlation Coefficients for Reliability Research. J Chiropr Med [Internet]. 2016;15:155–63. Available from: https://linkinghub.elsevier.com/retrieve/pii/S1556370716000158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McHugh ML. Interrater reliability: the kappa statistic. Biochem medica [Internet]. Croatian Society of Medical Biochemistry and Laboratory Medicine; 2012;22:276–82. Available from: https://pubmed.ncbi.nlm.nih.gov/23092060 [PMC free article] [PubMed] [Google Scholar]
- 33.Medical I IOPI Normal Values [Internet]. Tongue Press. Norm. Values. 2019. Available from: https://iopimedical.com/normal-values/ [Google Scholar]
- 34.Weikamp JG, Schelhaas HJ, Hendriks JC, de Swart BJ, Geurts AC. Prognostic value of decreased tongue strength on survival time in patients with amyotrophic lateral sclerosis. J Neurol. 2012/April/25. 2012;259:2360–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Easterling C, Antinoja J, Cashin S, Barkhaus PE. Changes in tongue pressure, pulmonary function, and salivary flow in patients with amyotrophic lateral sclerosis. Dysphagia. 2013;28:217–25. [DOI] [PubMed] [Google Scholar]
- 36.Hiraoka A, Yoshikawa M, Nakamori M, Hosomi N, Nagasaki T, Mori T, et al. Maximum Tongue Pressure is Associated with Swallowing Dysfunction in ALS Patients. Dysphagia. Springer US; 2017;32:542–7. [DOI] [PubMed] [Google Scholar]
- 37.Pizzorni N, Ginocchio D, Bianchi F, Feroldi S, Vedrodyova M, Mora G, et al. Association between maximum tongue pressure and swallowing safety and efficacy in amyotrophic lateral sclerosis. Neurogastroenterol Motil [Internet]. John Wiley & Sons, Ltd; 2020;n/a:e13859. Available from: 10.1111/nmo.13859 [DOI] [PubMed] [Google Scholar]
- 38.Matsuo K, Palmer JB. Anatomy and physiology of feeding and swallowing: normal and abnormal. Phys Med Rehabil Clin N Am [Internet]. 2008;19:691–vii. Available from: https://www.ncbi.nlm.nih.gov/pubmed/18940636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vose A, Humbert I. “Hidden in Plain Sight”: A Descriptive Review of Laryngeal Vestibule Closure. Dysphagia [Internet]. 2019;34:281–9. Available from: 10.1007/s00455-018-9928-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Langmore SE, Lehman ME. Physiologic deficits in the orofacial system underlying dysarthria in amyotrophic lateral sclerosis. J Speech Hear Res [Internet]. 1994/February/01. 1994;37:28–37. Available from: http://www.ncbi.nlm.nih.gov/pubmed/8170127 [DOI] [PubMed] [Google Scholar]
- 41.Watts CR, Vanryckeghem M. Laryngeal dysfunction in Amyotrophic Lateral Sclerosis: a review and case report. BMC Ear, Nose Throat Disord [Internet]. 2001;1:1. Available from: 10.1186/1472-6815-1-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hillel AD, Miller R. Bulbar amyotrophic lateral sclerosis: patterns of progression and clinical management. Head Neck. Wiley Online Library; 1989;11:51–9. [DOI] [PubMed] [Google Scholar]
- 43.Butler SG, Stuart A, Leng X, Wilhelm E, Rees C, Williamson J, et al. The relationship of aspiration status with tongue and handgrip strength in healthy older adults. J Gerontol A Biol Sci Med Sci. United States; 2011;66:452–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Costa MMB. Videofluoroscopy: the gold standard exam for studying swallowing and its dysfunction. Arq. Gastroenterol. Brazil; 2010. p. 327–8. [DOI] [PubMed] [Google Scholar]
- 45.Plowman EK, Tabor LC, Wymer J, Pattee G. The evaluation of bulbar dysfunction in amyotrophic lateral sclerosis: survey of clinical practice patterns in the United States. Amyotroph Lateral Scler Front Degener. 2017/April/21. 2017;18:351–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cedarbaum JM, Stambler N, Malta E, Fuller C, Hilt D, Thurmond B, et al. The ALSFRS-R: a revised ALS functional rating scale that incorporates assessments of respiratory function. J Neurol Sci. 1999;169:13–21. [DOI] [PubMed] [Google Scholar]


