Abstract
Aims
RECOVER AF evaluated the performance of whole-chamber non-contact charge-density mapping to guide the ablation of non-pulmonary vein (PV) targets in persistent atrial fibrillation (AF) patients following either a first or second failed procedure.
Methods and results
RECOVER AF was a prospective, non-randomized trial that enrolled patients scheduled for a first or second ablation retreatment for recurrent AF. The PVs were assessed and re-isolated if necessary. The AF maps were used to guide the ablation of non-PV targets through elimination of pathologic conduction patterns (PCPs). Primary endpoint was freedom from AF on or off antiarrhythmic drugs (AADs) at 12 months. Patients undergoing retreatment with the AcQMap System (n = 103) were 76% AF-free at 12 months [67% after single procedure (SP)] on or off AADs (80% free from AF on AADs). Patients who had only received a pulmonary vein isolation (PVI) prior to study treatment of non-PV targets with the AcQMap System were 91% AF-free at 12 months (83% SP). No major adverse events were reported.
Conclusion
Non-contact mapping can be used to target and guide the ablation of PCPs beyond the PVs in persistent AF patients returning for a first or second retreatment with 76% freedom from AF at 12 months. The AF freedom was particularly high, 91% (43/47), for patients enrolled having only a prior de novo PVI, and freedom from all atrial arrhythmias for this cohort was 74% (35/47). These early results are encouraging and suggest that guiding individualized targeted ablation of PCPs may therefore be advantageous to target at the earliest opportunity in patients with persistent AF.
Keywords: Atrial fibrillation, Charge density, Mapping, Ablation retreatment, Isolated veins, Reconnected veins, Non-pulmonary vein targets, Pathologic conduction patterns
Graphical Abstract
Graphical Abstract.
What’s new?
Visualization of the atrial activation during atrial fibrillation (AF), and the treatment of pathologic conduction patterns, leads to favourable clinical outcomes even in traditionally difficult-to-treat re-do patients.
76% of patients with persistent AF undergoing retreatment with the AcQMap System were AF-free at 12 months, 67% after a single procedure.
The most prominent pathologic conduction pattern observed during AF was ‘Localized Irregular Activity,’ (74% of patterns) indicative of pivoting propagating wavefronts as opposed to rotational or focal activity.
91% of patients who had only received a pulmonary vein isolation (PVI) ablation prior to treatment of non-PV targets with the AcQMap System were AF-free at 12 months, 83% after a single procedure. Eliminating pathologic conduction patterns during subsequent retreatment with only a previous PVI ablation is associated with high clinical success rates.
Acute termination guided by charge-density mapping predicts long-term freedom from AF in re-do patients.
Introduction
Long-term success with ablation in patients with persistent atrial fibrillation (AF) remains elusive with outcomes between 38% and 64% at 12 months, often necessitating second, third, and even fourth procedures to achieve sustained sinus rhythm (SR).1,2 The results of STAR AF II and CHASE AF, which showed no incremental value of additional ablation lines or complex fractionated atrial electrograms (CFAEs) over pulmonary vein isolation (PVI), suggest the first procedure treatment to be limited to PVI only. Studies investigating AF recurrence after PV isolation show that at retreatment, 63%–86% of patients will have 1–4 veins reconnected.3,4 However, the optimal ablation strategy to improve outcomes beyond PV isolation or re-isolation is unknown.3,4
Previously, the UNCOVER AF trial that utilized global chamber, charge density (CD) mapping to guide ablation of targets outside the PVs in de novo persistent AF patients demonstrated a 12-month, single-procedure freedom from AF of 73%. Within the UNCOVER AF trial, results showed a promising success rate (93%) for those patients that underwent a retreatment procedure during the study.5 The results of the UNCOVER AF trial motivated the RECOVER AF trial to investigate the clinical utility of targeted ablation of pathophysiologic propagation in patients receiving retreatment for AF.
Mapping the activation of the atria during AF allows for interrogation of spatiotemporal phenomena, such as patterns of activation propagation that is not achievable using conventional mapping systems. The capability to measure propagation during AF leads to the observation that there are reliable and recurring conduction patterns5 that are facilitated by and anchored to pathologic tissue that can be targeted and treated.6,7 Pathophysiologic conduction patterns are, in practice, wavefronts that exhibit highly refractive meandering, rotational, and ectopic behaviour. These behaviours arise from low safety-factor propagation and the interaction of multiple wavefronts due to a combination of the static syncytial state of the left-atrial (LA) myocardium and the dynamic spatiotemporal variance in restitution and repolarization.8–10 This pathophysiologic state is highly dynamic, particularly during AF, but is thought to be a result of a combination of the following factors: scar, fibrosis, fatty infiltration, poor inter-myocyte coupling, non-uniform refractoriness, and possibly many others.8,10,11
The RECOVER AF trial was designed to evaluate the performance and efficacy of global CD mapping to guide ablation of non-PV targets in persistent AF patients undergoing retreatment for recurrent AF following either a first or second failed ablation procedure. We show that treating targets of pathologic conduction patterns (PCPs) outside of the PVs, as identified by the AcQMap System, is a promising workflow for retreatment of AF patients beyond PVI. Utilizing the ability to map atrial activation during AF and treat pathophysiologic propagation patterns may reveal actionable detail about an individual's substrate and therefore may be a novel and efficacious tool in the treatment of AF.
Methods
Study design and participants
RECOVER AF (Utilizing Novel Charge Density Capabilities to Objectively Visualize the Etiology of Recurrent Atrial Fibrillation Following a Failed AF Ablation) was a prospective, single-arm, multi-centre, multi-national, non-randomized, pre-market study in Canada and post-market study in Europe designed to show the clinical performance of the AcQMap High Resolution Imaging and Mapping System in guiding ablation in patients undergoing retreatment for AF by targeting pathologic propagation patterns.
Patients aged 18 years or older, scheduled for repeat endocardial ablation for recurrent AF were recruited at 13 clinical sites throughout Europe and one clinical site in Canada (see Supplementary material online, Table S1). Patients were excluded if they had more than two previous LA ablation procedures for AF treatment, atrial arrhythmias (AAs) secondary to electrolyte imbalance or other reversible cause, structural heart disease or implanted devices including pacemakers, implantable cardioverter defibrillator, septal closure devices, LA appendage occlusion devices, history of blood clotting or bleeding, pregnant or lactating, or enrolled in any other study that may impact the results. The study was registered at www.clinicaltrials.gov (NCT03368781), complied with the Declaration of Helsinki, and was approved by local ethics committees, with all participants signing informed consent prior to study participation. Independent monitoring of the study was provided by a clinical research organization (ICON plc, Dublin, Ireland).
Comprehensive clinical history and procedural data were collected for each subject. Use of antiarrhythmic drugs (AADs) leading up to the procedure followed institutional standard of care. Discontinuation of amiodarone for 60 days prior to the ablation was recommended but not required. The date of the most recent direct current cardioversion (DCCV) and duration of resultant SR was recorded. Transesophageal echocardiography to rule out atrial thrombus was completed within 72 h prior to the procedure.
Clinical workflow
Catheter ablation retreatment procedures were conducted under either conscious sedation or general anaesthesia per institutional standard of care with central venous access gained and then fluoroscopic guidance used for transseptal access to the LA. Following LA access, intravenous heparin was administered to maintain ACT >350 s. The ultrasound imaging and CD mapping catheter (AcQMap 3D Imaging and Mapping Catheter, Acutus Medical, Carlsbad, CA) was positioned in the LA, via a steerable sheath, to generate 3D surface reconstructions and maps of atrial activation. The utilization of devices for 3D anatomic reconstruction, navigation, and electrical mapping was limited to the use of the AcQMap High Resolution Imaging and Mapping System. Functionality and features of the AcQMap System have been previously described.12–14 Investigators utilized the following stepwise approach for anatomy reconstruction, evaluation of PV reconnections, and CD mapping of non-PV targets (Figure 1).
Figure 1.
RECOVER AF procedural workflow. Pulmonary veins were initially evaluated and re-isolated as needed. Atrial rhythms present or induced after PVI were mapped. Identified non-PV targets were ablated, with re-mapping encouraged until no additional targets could be identified. The right atrium was mapped when left atrial ablation was not felt to be sufficient to treat the recurrent AF. Pulmonary vein isolation was confirmed following non-PV target ablation. AF, atrial fibrillation; PV, pulmonary vein; PVI, pulmonary vein isolation.
A patient-specific anatomical reconstruction was obtained using the AcQMap catheter-based ultrasound by roving the catheter throughout the LA without contacting the endocardial surface. Incidental contact by the catheter was permitted but was algorithmically excluded from the anatomic reconstruction. Limited post-processing was performed to sharpen the anatomic detail and remove non-conducting structures (e.g. mitral valve). For patients in AF, an initial 30-s unipolar recording of the electrical information throughout the LA chamber was obtained from a central and stationary location within the LA. Using the electrical information recorded from the central location, a high-resolution and fidelity reconstruction of the electrical propagation can be made through the CD mapping algorithm described previously.5,12,13,15 Using the propagation of the electrical wavefront during AF, clinicians could identify non-PV targets, defined here as pathological conduction patterns (PCPs), prior to (or at any point in the procedure) assessing isolation of the PVs. Optional induction of AF prior to assessment of PV reconnection was at the discretion of the investigator.
The PV isolation was documented via the investigator’s preferred strategy. Reconnected veins were documented and re-isolated using radiofrequency (RF) ablation catheters. A minimum power of 25–30 W was suggested with a flow rate of 15–30 cc/min as determined by the catheter manufacturer for irrigated RF. Balloon-based cryothermy was also allowed as an alternative to re-isolate reconnected veins. Prior to the conclusion of the procedure, PVI was documented by demonstrating absence of PV potentials with a circular mapping catheter (entrance block) and pacing from inside the vein for local capture with exit block towards the LA, all in accordance with international guidelines and consensus documents. If additional ablation was required, ablation parameters were recorded, and entrance and exit block was documented.
In patients who either converted to or remained in SR following PV re-isolation, programmed electrical stimulation with short-coupled extra stimuli and/or rapid pacing was used to induce clinically relevant AAs. The chamber-wide electrical propagation was reconstructed and visualized across the patient’s anatomy to identify non-PV PCPs.
Targeting pathologic conduction patterns
Three PCPs were characterized during the UNCOVER AF trial and systematically targeted as part of this study: (i) localized irregular activity (LIA), (ii) localized focal firing (FF), and (iii) localized partial rotational activity (LPRA). These targets, represented schematically in Figure 2A, are defined as follows:
Figure 2.
Pathologic conduction patterns and the suggested treatment strategy shown schematically. (A) Schematic illustrations showing the coarse algorithmic principles behind identification of pathologic conduction patterns. These pathologic conduction patterns are: (i) focal firing, (ii) localized partial rotational activity, and (iii) localized irregular activity. (B) Schematic illustration of the core-to-boundary ablation approach. (i) A target on the low posterior wall is identified, (ii) the core of the target is ablated, and (iii) an anti-arrhythmogenic ablation line is used to connect the targeted core to an inert boundary.
FF: Electrical activity that radially propagates outward from a given position is classified as FF. In practice, this can be due to ectopy or endocardial breakthrough in cases of intramural disassociation.
LPRA: Propagation that rotates greater than 270 degrees around a given position is classified as LPRA. In practice, these are sites of frequent but short-lived rotational activity (rotating for only one or a few cycles) before interruption, annihilation, splitting, or breakdown to non-rotational patterns or passive conduction.
LIA: Propagation that pivots and refracts (changes direction) greater than 90 degrees as it moves through a given position is classified as an LIA. In practice, sites of multidirectional entry/exit and ‘isthmus-like’ pivoting are typically classified as LIA.
Treatment of these PCPs included ablating the core and anchoring to the nearest anatomic boundary (core-to-boundary) or bisecting the LIA/LPRA target zone to prevent wavefront pivoting or rotation. An example of core-to-boundary being used to ablate a target and anchor those ablations to an inert non-propagating boundary can be seen in Figure 2B. Identification of the patterns was primarily done by the operator manually; however, an automated tool for detecting these PCPs, AcQTrack (Acutus Medical, Carlsbad, CA), was available for confirmation.
Re-mapping and ablation were repeated until all identified or subsequently uncovered targets were eliminated or sufficiently modified. Mapping of the RA was required when LA ablation was not successful in treating the recurrent AF. Ablation of additional RA targets or creation of a cavotricuspid isthmus (CTI) line of block was at the discretion of the investigator. Bi-directional block using pacing manoeuvers was documented following any linear ablation. The DCCV was used to convert any remaining arrhythmias to SR.
Follow-up
Patient information regarding follow-up was collected at hospital discharge, and at 3, 6, and 12 months. Safety was evaluated at each visit, and analysis of patient rhythm was completed at the 3-, 6-, and 12-month follow-up visits. Standard 12-lead ECG and 48-h continuous monitoring was performed at each visit to document recurrence of AAs. Recordings were independently analysed and reported by trained technicians/readers of the monitor manufacturer. All reports were confirmed by the site investigator, with any disagreements adjudicated between the site and the monitor manufacturer’s medical representative. Per protocol, recurrence of AF, atrial flutter (AFL), or atrial tachycardia (AT) outside of required visits was documented by ECG, 48-h continuous ECG monitor, or Holter and recorded as an unscheduled visit.
A 3-month blanking period was used during which recurrences of AF/AFL/AT were not counted as failures as per standard guidelines. Treatment to maintain SR in case of prolonged AF recurrence was allowed during the blanking period, including use of DCCV and/or AAD administration or dosage modification. The investigator could also elect to repeat an ablation; however, continuous monitoring for 48-h was required prior to the procedure to document the presenting rhythm. The AcQMap System was recommended to be used during repeat ablation procedures. Following repeat ablation, no adjustment was made to follow-up, and patients exited the study 12 months post the initial study retreatment procedure.
Outcomes
Long-term effectiveness was determined by evaluating all subjects who were free of AF lasting greater than 30 s in addition to all AAs in the follow-up period. Cardioversion outside of the blanking period was considered a study failure. A second ablation in the follow-up period was allowed as per study guidelines but considered a failure in the single-procedure results. Additional effectiveness measurements included subset analyses of patients who had only received PVI ablation prior to being treated with AcQMap for PCPs vs. those who were enrolled having had more extensive ablation. Further subsets included comparing those with vein reconnection or no vein reconnection, first or second retreatment procedure, and absence or presence of non-PV targets.
Safety outcomes were measured as freedom from device/procedural-related safety events within 24 h post-ablation. All adverse events were recorded and reported to the sponsor, with the investigator taking the responsibility of classifying any event as related to the System or the ablation procedure. Adverse events could be further categorized as serious (leading to death, deterioration of health resulting in life-threatening illness or injury, permanent impairment, prolonged hospitalization, or medical or surgical intervention to prevent life-threatening illness or injury) and adverse device effects caused by or related to use of the device. All procedure and device-related adverse events were adjudicated by the sponsor’s clinical representatives.
Procedural efficacy was measured using descriptive analysis of confirmation of electrical isolation of all PVs and elimination or modification of all non-PV targets as identified by the AcQMap System. Additional efficacy objectives included documentation of procedure data including total time, fluoroscopy time, PVI ablation time, and non-PV target ablation time.
Statistical analysis
Baseline and demographic characteristics were summarized for the procedural population, and effectiveness outcomes were summarized for all patients that completed the study. Continuous variables are presented as mean, SD, median and interquartile range as appropriate, and categorical variables are summarized via counts and percentages. A detailed description of the statistical analysis tests performed can be found in the Supplemental Material.
Results
Patient flow and characteristics
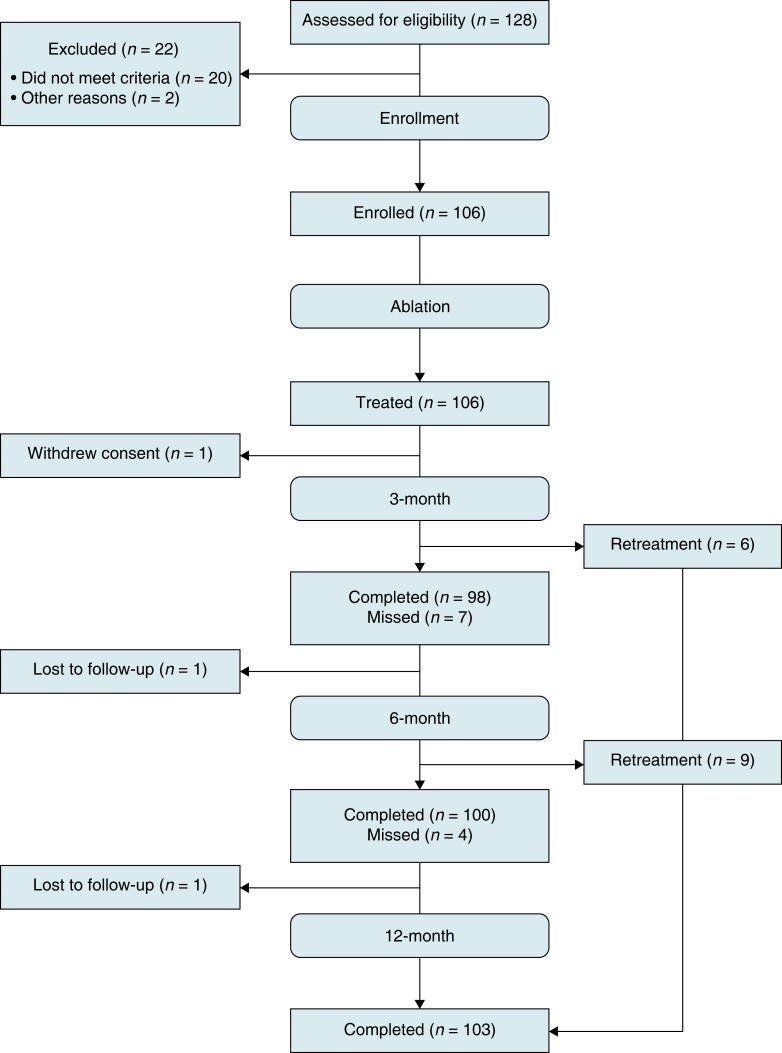
Between April 2018 and August 2019, 128 patients scheduled for a first or second retreatment ablation of recurrent AF were screened, and 106 patients were treated at 14 centres. Data for 106 patients were evaluated for safety and procedural efficacy, and 103 patients for effectiveness. Two patients were lost to follow-up, one patient prior to the 6-month follow-up, and one patient prior to the 12-month follow-up. The remaining patient withdrew consent prior to the 3-month follow-up. The study flow is outlined in Figure 3. Clinical follow-ups through the 12-month visit window were available for 103 (97.2%) of the treated patients. No deaths were reported during the 12-month follow-up period.
Figure 3.
Study consort diagram. Patient enrollment and flow through the study protocol.
Baseline characteristics for the 106 enrolled patients are shown in Table 1. Median time from first AF diagnosis to study enrollment was 6.1 years (IQR: 3.7–9.7 years). Of the 106 patients enrolled, 79 patients underwent a first retreatment procedure and 27 underwent a second retreatment procedure as part of this study. The median time from the de novo ablation procedure to the first retreatment was 1.8 years (IQR: 0.9–3.7 years), and median time from first retreatment to second retreatment was 1.5 years (IQR: 1.3–5.5 years).
Table 1.
Clinical characteristics
| Clinical characteristics | Patient cohort (n = 106) |
|---|---|
| Mean age ± SD, years | 63.0 ± 10.6 |
| Sex | |
| Men | 78 (73.6%) |
| Women | 28 (26.4%) |
| BMI, kg/m2 | 28.7 ± 4.9 |
| Time from first diagnosed AF, years, Median, (IQR) | 6.1, (3.7–9.7) |
| Average number of cardioversions since prior ablation | 1 |
| Retreatment | |
| First retreatment—de novo PVI only | 50 |
| First retreatment—de novo PVI + Additional Ablation | 29 |
| Second retreatment | 27 |
| Comorbidities | |
| Hypertension, n (%) | 55 (52) |
| Coronary artery disease, n (%) | 11 (10) |
| Diabetes mellitus, n (%) | 10 (9) |
| Valvular disease, n (%) | 4 (4) |
| Cardiomyopathy, n (%) | 8 (8) |
| Heart failure, n (%) | 1 (1) |
| Stroke or TIA, n (%) | 5 (5) |
| COPD, n (%) | 5 (5) |
| Obstructive sleep apnea, n (%) | 11 (10) |
| LA diameter, mm | 43.8 ± 6.8 |
| LVEF (%) | 57.1 ± 7.7 |
| Antiarrhythmic medications at time of procedure | |
| Class Ic, n, (%) | 23 (22) |
| Class III, n, (%) | 12 (11) |
| CHA2D2-VASc score, n (%) | |
| 0 | 22 (21) |
| 1 | 32 (30) |
| 2 | 27 (25) |
| >2 | 25 (24) |
COPD, chronic obstructive pulmonary disease; LA, left-atrial; LVEF, left ventricular ejection fraction; TIA, transient ischaemic attack.
Procedural characteristics
All patients enrolled were treated and mapped with AcQMap upon their index procedure (regular16 Pope and irregular arrhythmias5). Procedural data are shown in Table 2. Of the 106 patients who underwent treatment as part of this study (their first or second retreatment), 59% were in AF at the start of the procedure. The remaining patients were in SR (32%), AFL (4%), AT (4%), and unknown (1%). The PVI was confirmed in 29% of patients with the remaining 71% having between 1 and 4 veins reconnected. Following PV evaluation and re-isolation, despite aggressive pacing with or without isoproterenol, AF could not be induced in 22 patients. Six patients were non-inducible, and 16 patients could only be induced into AFL/AT/atrial ectopy. Ablation only in the LA was performed in 83 patients, 22 patients had both LA and right atrial ablation, and one (1) patient had only right atrial ablation for a CTI-dependent AFL. Overall, CTI linear ablation was performed in 12 patients, in 11 patients during their initial AcQMap procedure, and in one patient during a second study procedure. Mitral isthmus linear ablation was performed in three subjects during the initial procedure. The DCCV was performed at the end of the procedure in 64 patients, and 31 patients were ablated to SR during the procedure.
Table 2.
Procedural data
| Procedural data | ||
|---|---|---|
| Patient cohort (n = 106) | Percentage | |
| Rhythm at start of procedure, n (%) | ||
| SR | 34 | 32.1% |
| AF | 63 | 59.4% |
| AFL | 4 | 3.8% |
| AT | 4 | 3.8% |
| Unknown | 1 | 0.9% |
| Procedure time, h:min (defined as time of initial femoral venous access to end of procedure) | 3:18 ± 0:48 | |
| Total fluoroscopy time, min | 25.6 ± 12.5 | |
| Treatment received during initial procedure: | ||
| Re-isolation of the PVs | 19 | 18.0% |
| Re-isolation and ablation of PCPs | 56 | 52.8% |
| Ablation of PCPs alone | 28 | 26.4% |
| CTI/MIL/unknown | 3 | 2.8% |
| Number of veins reconnected: | ||
| 0 | 31 | 29.2% |
| 1 | 22 | 20.8% |
| 2 | 30 | 28.3% |
| 3 | 12 | 11.3% |
| 4 | 11 | 10.4% |
| Reconnected vein (number of points): | ||
| Left superior pulmonary vein | 33 | 31.1% |
| Left common pulmonary vein | 8 | 7.5% |
| Left inferior pulmonary vein | 36 | 34.0% |
| Right superior pulmonary vein | 42 | 39.6% |
| Right inferior pulmonary vein | 43 | 40.6% |
| Ablation time to re-isolate veins, min:sec | 8:30 ± 6:48 (n = 76) | |
| Ablation time non-PV targets, min:sec | 26:12 ± 19:18 (n = 84) | |
| Treatment to achieve terminal rhythm at initial procedure: | ||
| SR with cardioversion (DCCV or pharmacologic) | 64 | 60.4% |
| SR without cardioversion | 3111 | 29.2% |
| No ablation, DCCV or Pharmacologic conversion attempteda | 10.4% | |
| Rhythm at end of initial procedure: | ||
| SR | 99 | 93.4% |
| AFb | 6 | 5.7% |
| AFL | 1 | 0.9% |
AF, atrial fibrillation; AFL, atrial flutter; AT, atrial tachycardia; CTI, cavotricuspid isthmus; DCCV, direct current cardioversion; MIL, mitral isthmus line; PCP, pathologic conduction pattern; PV, pulmonary vein; SR, sinus rhythm.
One site, n = 11 patients, as standard of practice does not cardiovert patients in AF at the end of the procedure allowing the patient to heal. Patients either self-convert or are cardioverted between 1 and 3 months after the procedure. Four of the six patients remaining in AF were from this site.
Four of the six patients remaining in AF at the end of the initial procedure were from this site.
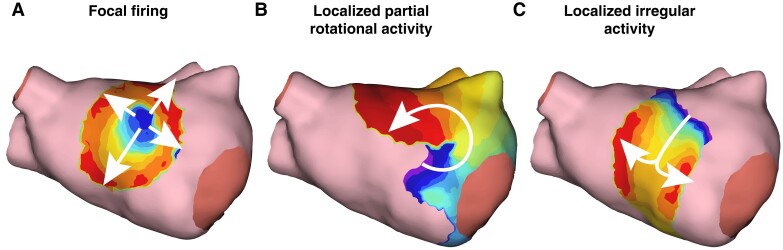
Patients with either baseline or induced AAs were mapped using the AcQMap system. For patients in AF, potential ablation targets were identified and characterized as FF, LPRA, and LIA. To identify areas of frequent occurrence, all targets were visually mapped to predefined zones on a generic anatomic shell of an LA or RA. An example of the three PCPs identified using the AcQMap System can be seen in Figure 4.
Figure 4.
Examples of pathologic conduction patterns within the AcQMap system. (A) Focal firing is defined as electrical activity that radially propagates outward from a given position. (B) Localized partial rotational activity is propagation that rotates greater than 270 degrees around a given position. (C) Localized irregular activity propagation that pivots and refracts (changes direction) greater than 90 degrees as it moves through a given position.
Left atrial targets
Zones 1–3 (1, 2, 3) were posterior and zones 4–5 (1, 2, 3) were anterior, zone 6 (1, 2) represents the anterior and posterior aspects of the roof, and zone 7 (1, 2) represents the surface below the appendage and atop the mitral valve. There were 352 targets identified in 83 patients. Of the 83 patients, 59 patients had targets in zone 2 and 72 patients had targets in zone 5. Target type was 262 (74.4%) LIA, 51 (14.5%) LPRA, and 39 (11.1%) focal. The median number of targets per patient was 4 (IQR: 3–5). Targets were evenly distributed across the anterior (51% of all targets) and posterior (49% of all targets) LA surfaces, similarly, occurring most frequently on the posterior wall between the antra of the right and left inferior PVs (Zone 2) and anteriorly in the region between the right superior PV antrum and 9 and 12 o’clock on the mitral valve (Zone 5). Number of patients with a target at that site, target type, and frequency of target by zone is detailed in Figure 5.
Figure 5.
Patient frequency, target type and target frequency by zone in the LA. (A) Shows the per cent of patients with targets in zone (black is per cent of patients and red is the zone location). (B) Shows the per cent of targets located in each zone (black is per cent of patients and red is the zone location). (C) Shows the number of patients with targets in each zone. (D) Shows the target type and frequency of occurrence in each zone.
A similar analysis for the RA targets and corresponding schematic figure (see Supplementary material online, Figure S2) can be found in the Supplemental Material.
Outcomes and safety
Outcomes data at 12 months was available for 103 patients. The primary effectiveness endpoint of freedom from AF on or off AADs at 12 months post the initial study retreatment procedure was achieved in 78 of 103 patients (75.7%). Sixty-nine of 78 patients that achieved 12-month freedom from AF had a single procedure resulting in a success rate of 67.0%. Freedom from any atrial arrhythmia at 12 months on or off AADs was 59.2%. Forty-seven patients who only received a de novo PVI ablation prior to treatment with the AcQMap System were free from AF on or off AADs at 12 months was 91.5% (74.5% freedom from all AAs). Single procedure success rates for the de novo PVI with AcQMap population was 83.0% free from AF and 65.9% free from all AAs. Additional outcome results were calculated based on retreatment (first or second), vein status (isolated or reconnected), and non-PV targets (absence or presence). Table 3 reports freedom from AF at 12 months post the initial study procedure and for all study procedures performed. Supplementary material online, Figure S1, reports AF freedom outcomes by site. Table 4 reports freedom from all AA at 12 months post the initial study procedure and for all study procedures performed.
Table 3.
Single-procedure effectiveness outcomes at 12 months (AF-free) or all in-study procedures on or off AADs
| Cohort (number of patients) | Single procedure success | Success | Failure | P-value | With retreatment procedure success | Success | failure | P-value |
|---|---|---|---|---|---|---|---|---|
| AF-free | AF-free | |||||||
| Full cohort (103) | 67.0% | 69 | 34 | n/a | 75.7% | 78 | 25 | n/a |
| De novo PVI w/AcQMap (47) | 83.0% | 39 | 8 | 0.0017 | 91.5% | 43 | 4 | 0.001 |
| PVI + w/AcQMap (56) | 53.6% | 30 | 26 | 62.5% | 35 | 21 | ||
| First retreatment (76) | 69.7% | 53 | 23 | 0.347 | 78.9% | 60 | 16 | 0.20 |
| Second retreatment (27) | 59.3% | 16 | 11 | 66.7% | 18 | 9 | ||
| No PV Reconnection (30) | 60.0% | 18 | 12 | 0.3626 | 70.0% | 21 | 9 | 0.45 |
| Reconnected Veins (73) | 69.9% | 51 | 22 | 78.1% | 57 | 16 | ||
| Absence of non-PV targets (21) | 71.4% | 15 | 6 | 0.796 | 81.0% | 17 | 4 | 0.78 |
| Presence of non-PV targets (82) | 65.9% | 54 | 28 | 74.4% | 61 | 21 |
AADs, antiarrhythmic drugs; AF, atrial fibrillation; PV, pulmonary vein; PVI, pulmonary vein isolation.
P-value calculated using Fishers exact test, significance level is P < 0.05.
Table 4.
Single-procedure effectiveness outcomes at 12 months (all atrial arrhythmia-free) or all in-study procedures on or off AADs
| Cohort (number of patients) | Single procedure success | Success | Failure | P-value | With retreatment procedure success | Success | Failure | P-value |
|---|---|---|---|---|---|---|---|---|
| AA-free | AA-free | |||||||
| Full cohort (103) | 52.4% | 54 | 49 | n/a | 59.2% | 61 | 42 | n/a |
| De novo PVI w/AcQMap (47) | 66.0% | 31 | 16 | 0.0055 | 74.5% | 35 | 12 | 0.0046 |
| PVI + w/AcQMap (56) | 37.5% | 21 | 35 | 46.4% | 26 | 30 | ||
| First retreatment (76) | 53.9% | 41 | 35 | 0.66 | 60.5% | 46 | 30 | 0.66 |
| Second retreatment (27) | 48.1% | 13 | 14 | 55.6% | 15 | 12 | ||
| No PV reconnection (30) | 36.7% | 11 | 19 | 0.051 | 46.7% | 14 | 16 | 0.12 |
| Reconnected Veins (73) | 58.9% | 43 | 30 | 64.4% | 47 | 26 | ||
| Absence of non-PV targets (21) | 57.1% | 12 | 9 | 0.81 | 57.1% | 12 | 9 | >0.99 |
| Presence of non-PV targets (82) | 51.2% | 42 | 40 | 59.8% | 49 | 33 |
AADs, antiarrhythmic drugs; AA, atrial arrhythmia; PV, pulmonary vein; PVI, pulmonary vein isolation.
P-value calculated using Fishers exact test, significance level is P < 0.05.
A second retreatment procedure (considered single procedure failures) was performed during the study in 15 (14.6%) patients, five patients with the AcQMap System and 10 patients using a conventional 3D anatomic mapping system. On the day of retreatment, 47% were in AF, 7% were in AFL, 13% were in AT, and 33% were in SR. The average time to a second retreatment procedure during the study was 6 months, six patients were retreated between 3 and 6 months, and nine patients were retreated between 6 and 12 months.
Demographic and procedural predictors of 12-month freedom from AF after a single procedure was evaluated and reported in Table 5. Predictors of freedom from AF at 12 months following a single procedure included ablation termination during the procedure and first retreatment following a de novo PVI-only procedure. Demographic and procedural predictors of 12-month freedom from all AAs after a single procedure was evaluated and reported in Table 6. Predictors of freedom from all AAs at 12 months following a single procedure included first retreatment following a de novo PVI-only procedure.
Table 5.
Multivariable analysisa for a single procedure outcome of freedom of AF at 12 months
| Variable or pairwise comparison, where applicable | Odds ratio (95% CI) | P-valueb |
|---|---|---|
| Treatment prior to enrollment: | — | 0.0005 |
| First retreatment following a de novo PVI-only procedure vs. first Retreatment following a de novo PVI + Procedure | 10.9 (3.14, 37.94) | 0.0002* |
| First retreatment following a de novo PVI-only procedure vs. second retreatment | 6.6 (1.91, 22.89) | 0.0028* |
| Ablation termination | 4.7 (1.36, 16.23) | 0.0144 |
AF, atrial fibrillation; PVI, pulmonary vein isolation.
Variables with univariate P ≤ 0.20 were considered for multivariable model. Stepwise selection was used to determine variables in final model.
Based on overall type 3 P-values or pairwise tests (denoted by *, no adjustments for multiple comparisons were made).
Table 6.
Multivariable analysisa for a single procedure outcome of freedom of all atrial arrhythmias at 12 months
| Variable or pairwise comparison, where applicable | Odds ratio (95% CI) | P-valueb |
|---|---|---|
| Treatment prior to enrollment: | — | 0.0005 |
| First retreatment following a de novo PVI-only procedure vs. first Retreatment following a de novo PVI + Procedure | 6.55 (2.18, 19.70) | 0.0008* |
| First retreatment following a de novo PVI-only procedure vs. second retreatment | 2.86 (1.03, 7.94) | 0.0434* |
| CHADS-VASC (1-unit increase) | 0.63 (0.43, 0.92) | 0.0163 |
PVI, pulmonary vein isolation.
Variables with univariate P ≤ 0.20 were considered for multivariable model. Stepwise selection was used to determine variables in final model.
Based on overall type 3 P-values or pairwise tests (denoted by *, no adjustments for multiple comparisons were made).
For the primary safety outcome, seven adverse events (6.6%) were reported in six patients. Five events were classified as definitely related to the procedure (urinary tract infection, arteriovenous fistula, arterial groin bleeding, prolonged fever, and groin pseudo-aneurysm), one event (pericardial effusion) was classified as probably related to the procedure, and one event (gallstone cholecystitis) was classified as not related to the procedure. One (1) event, an arteriovenous fistula, was also classified as possibly device related to usage of the AcQGuide Sheath. The arteriovenous fistula resolved without sequelae.
Discussion
Recently reported clinical studies on the persistent AF population demonstrate between 13% and 23% of persistent AF patients will opt for a second procedure within the first 12 months following their initial ablation.5,17,18 Outcomes in persistent AF patients retreated within the first year ranged from 43% to 50%.2,19 Most patients will be retreated due to AF recurrence, ∼4−50% will be retreated due to AFL or tachycardia.20 In the RECOVER AF study, the use of whole-chamber non-contact CD mapping to guide ablation demonstrated a 67% single procedure freedom from AF at 12-months follow-up in persistent AF patients requiring ablation after a first or second failed ablation, by targeting PCPs beyond the PVs. However, patients who only received a de novo PVI prior to being treated with the AcQMap system had a single procedure freedom from AF rate of 83% (91% with a follow-up treatment).
Success of atrial fibrillation retreatment with ablation beyond the pulmonary veins
Although a relatively small study, the RECOVER AF study provides some provocative insights into arrhythmia recurrence after two or three ablation procedures. While PVI is considered the foundation of AF ablation, AF does recur in patients with confirmed PVI suggesting that the arrhythmogenic nature of the patient's substrate was unaltered by isolation of the veins and may therefore be advantageous to target at the earliest opportunity in patients with persistent AF. Previous studies have suggested that ablation beyond the PVs add little clinical benefit, which leads to physicians struggling with the question of what additional therapy should be provided in cases where PVI is insufficient. These previous strategies included linear lesions,21 non-PV trigger ablation, empirical ablation of specific anatomic locations, CFAE,22 and/or rotor mapping,23,24 all showing limited to no success in reducing the recurrence of AF. In a case series reported by Baldinger et al., among AF patients (initially treated with PVI or PVI with lines and/or CFAE), with intact PVI returning for retreatment, those presenting with recurrent AF were less likely to be effectively retreated (20% 12-month freedom from AF) than those presenting with recurrent AT (67% 12-month freedom from AT).25 The study concluded that recurrent AF despite confirmed PV isolation may represent a population in which catheter ablation may be less successful. However, it should be noted that due to technical limitations in traditional mapping systems, the electrical activation of these patients in prior studies was not studied, and therefore not used to guide therapy. An explicit strength of the technology employed in our study allowed for the electrical activation to be directly visualized during episodes of AF and allow clinicians to observe and treat patient-specific PCPs, which may explain the high clinical success (76% AF-free at 12 months) observed in this study of traditionally difficult-to-treat patients. Other recent studies have also provided a critical appraisal of various technologies available for AF mapping to better ‘unravel the mechanisms and identify target sites for AF treatment’26 such as the meta-analysis from Junarta et al.,27 which demonstrated greater freedom from AF in patients with persistent AF who underwent more personalized ablations vs. empiric ablations.
Measurement of activation over an anatomy to deduce arrhythmogenic pathophysiology and plan treatment is one of the oldest practices in the field of patient-specific EP.28,29 However, the ability to measure fibrillatory waves, such as in AF, is traditionally difficult due to a lack of a stable temporal reference, which has led to several surrogate metrics (e.g. bipolar voltage, CFAE, and rotor mapping) for identifying substrate to be established. With the ability to measure the activation of fibrillatory waves, here with the AcQMap System, clinicians can observe direct measures of pathophysiologic propagation, or substrate, and plan treatment accordingly. The results presented here may highlight a misclassification or oversight of this type of AF mechanism in earlier studies where only CFAE, focal, and/or perpetual phase rotation were considered as non-PV targets.
Pathologic conduction pattern targets vs. posterior wall ablation
Further anatomic approaches beyond simply the PVs have been suggested and trialled previously, principally posterior wall isolation (PWI). In a mixed paroxysmal and persistent AF retreatment population, a recently published retrospective review after a single failed ablation procedure also demonstrated that empirical PWI did not confer benefit over just re-isolation of the PVs at 12 months.30 These results were supported by the CAPLA trial that similarly showed no benefit of empirical PWI over PVI.5,12,13,15,31 Conversely, in a direct comparison of AcQMap-guided ablation and empirical PWI, Shi et al. demonstrated a statistically significant difference in single procedure freedom from AAs off AADs at 24 months of 68% vs. 46%, respectively (P = 0.043).15 The mapping data from RECOVER AF demonstrated that while many patients had targets located in or around the posterior wall, an equivalent number of targets were equally distributed outside the atrial region a typical posterior box lesion set would encompass. Both studies suggest that identifying and ablating sites beyond the posterior wall may be important to achieving long-term success in de novo and retreatment procedures.
De novo PVI + PCP targeting
The population of persistent AF patients who only received a de novo PVI prior to enrollment is a particularly thought-provoking cohort with a freedom from all AAs of 74.5% at 12 months when retreated with the AcQMap System. These particularly high success rates for persistent AF retreatment patients could indicate that ablation beyond the PVs that are not informed by a patient’s activation during AF could be deleterious to future treatment. Indeed, misguided ablation beyond the PVs may disrupt the substrate in a manner non-characteristic to the ab initio arrhythmogenesis, which may further complicate diagnosis and treatment. Therefore, these results also suggest that, in the absence of direct visualization of AF propagation, the first treatment procedure for AF be limited to PVI alone, and in patients in whom AF recurs, the first retreatment procedure be limited to re-isolation of PVs as needed and targeted ablation of patient-specific pathological conduction patterns.
Predictors of success
Multivariate predictors of freedom from AF at 12 months included patients returning for retreatment after a failed PVI-only procedure and patients whose arrhythmia was terminated with ablation. Although not consistently shown to predict success, AF termination has been correlated with long-term success in de novo persistent populations.32,33 In aggregate, the addition of AcQMap-guided ablation achieved similar results in the first and second retreatment groups. However, when the first retreatment group was stratified by the de novo ablation strategy (first retreatment—PVI only or first retreatment—PVI plus additional ablation), those patients who underwent AcQMap-guided ablation after a de novo PVI were more likely to be AF-free at 12 months compared to patients returning for a second retreatment or following a failed initial procedure that included PVI plus additional ablation. It is possible that empirical ablation outside of PVI is proarrhythmic and creates a more complex and challenging substrate for the retreatment procedure. These predictors of success, based on the patient outcomes, suggest that minimizing ablations outside the PVs by ablating patient-specific pathological conduction patterns is associated with high clinical success rates. These findings warrant additional investigation to discern the possible electrophysiological or demographic characteristics and underscore the need for larger clinical trials to test the hypotheses.
Post-ablation atrial tachycardias
Recurrence due to AFL and AT is not unexpected after de novo and retreatment ablation procedures of persistent AF. It has been shown that the level of post-ablation flutters and tachycardias can be related to the duration of AF, amount of prior ablation, and/or incomplete ablation lines.34 In our study, 19 of 78 AF-free patients experienced AT recurrence at 12 months, 15 of whom had LPRA and/or LIA targets ablated during their retreatment procedure. The LPRA and LIA targets are ablated using a core-to-boundary strategy, which includes ablation at the centre of the activation pattern and linear ablation to anchor the site to an electrically inert boundary.15 Additional work in the literature suggests that anchoring lesions to inert boundary is likely anti-arrhythmic.35,36 While this ablation strategy was successfully applied in many of the study patients, incomplete linear ablation to anchor each ablated site may have contributed to the rate of post-ablation tachycardias. Several studies have reported better outcomes after repeat ablation for AT recurrence compared to recurrence of persistent AF. Ammar et al. proposed that recurrence of AT is reflective of significant substrate modification that no longer allows the tissue to sustain AF and provides a more definitive target for ablation.37
Study limitations
RECOVER AF was a non-randomized study design, the number of patients enrolled is relatively small, and heterogeneous in characteristics. Verification of rhythm status was 48 h in duration at 3, 6, and 12 months using a continuous ECG monitor or ECG if monitor data was not available, as is custom in most ablation studies. As these are retreatment patients, documentation was relatively complete as it relates to first ablation procedure, but less so for patients undergoing a second retreatment. These data represent outcomes obtained with a first-generation system design and specification that has since undergone upgrades enhancing stability of ablation catheter localization, broadening the range and type of ablation catheters operable with the system, and incorporation of newer software algorithms for diagnosing AT and AFL, verifying ablation lines and location of potential ablation targets. While operators were able to target PCPs at their discretion, adding a degree of ambiguity to assessing target stability and frequency, the results of this study are currently being used in an ongoing investigation to parameterize and refine the automated algorithm (AcQTrack). This tool is now more systematically being used in other clinical studies to automatically identify these PCPs within the AcQMap system.
Conclusion
The RECOVER AF study was designed to prospectively evaluate the safety and effectiveness of non-contact CD mapping to guide ablation in patients returning for a first or second retreatment following AF recurrence. Freedom from AF at 12 months following a single procedure was 67% (76% with a follow-up treatment). Ninety-one per cent of patients who had only received a PVI ablation prior to treatment of non-PV targets with the AcQMap System were AF-free at 12 months, 83% after a single procedure. The CD mapping identified potential targets throughout the LA substrate, highlighting the need for ablation beyond isolation of the PVs and posterior wall particularly when targeting PCPs. The results presented in this study, although preliminary, suggest the ability to identify PCPs, and guide therapy during AF is efficacious and provides clinical capability not achievable without direct observation of propagation during AF.
Supplementary Material
Acknowledgements
Janice Barstad and Nicole Marcovecchio (Acutus Medical, Carlsbad, CA) assisted with the preparation of tables, figures, and copyediting. Carlye Kraemer and Lindsay Lucas (NAMSA, Minneapolis, MN) assisted with statistical analysis.
Contributor Information
Timothy R Betts, Oxford Biomedical Research Centre, Oxford University Hospitals NHS Foundation Trust, Headley Way, Oxford, OX3 9DU, UK.
Wilson W Good, R&D Algorithms, Acutus Medical, Carlsbad, CA, USA.
Lea Melki, R&D Algorithms, Acutus Medical, Carlsbad, CA, USA.
Andreas Metzner, Cardiac Electrophysiology Department, Asklepios Klinik St. Georg, Hamburg, Germany.
Andrew Grace, Department of Cardiology, Royal Papworth Hospital NHS Foundation Trust, Cambridge, UK.
Atul Verma, Division of Cardiology, McGill University Health Centre, McGill University, Montreal, QC, Canada.
Stephen Murray, Cardiology Department, Freeman Hospital, Newcastle Upon Tyne, UK.
Simon James, Cardiology Department, The James Cook University Hospital, Middlesbrough, UK.
Tom Wong, Department of Cardiology, Royal Brompton Hospital, London, UK.
Lucas V A Boersma, Cardiology Department, Sint Antonius Hospital, Nieuwegein, The Netherlands.
Daniel Steven, Department of Electrophysiology, Heart Center, University of Cologne, Cologne, Germany.
Arian Sultan, Department of Electrophysiology, Heart Center, University of Cologne, Cologne, Germany.
Sonia Busch, Department Cardiology and Angiology, Klinikum Coburg, Coburg, Germany.
Petr Neužil, Department of Cardiology, Homolka Hospital (Na Homolce Hospital), Prague, Czech Republic.
Carlo de Asmundis, Heart Rhythm Management Centre, Cardiovascular Division, UZ Brussel—Vrije Universiteit Brussel, Brussels, Belgium.
Justin Lee, Cardiology and Cardiothoracic Surgery, Sheffield Teaching Hospitals NHS Foundation Trust, Sheffield, UK.
Tamás Szili-Török, Department of Cardiology, Erasmus Medical Center Rotterdam, Rotterdam, The Netherlands.
Supplementary material
Supplementary material is available at Europace online.
Funding
This study was sponsored and funded by Acutus Medical.
Data availability
The data underlying this article were provided by Acutus Medical under licence/by permission. Data will be shared on request to the corresponding author with permission of Acutus Medical.
References
- 1. Valderrabano M, Peterson LE, Swarup V, Schurmann PA, Makkar A, Doshi RNet al. . Effect of catheter ablation with vein of marshall ethanol infusion vs catheter ablation alone on persistent atrial fibrillation: the VENUS randomized clinical trial. JAMA 2020;324:1620–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Vogler J, Willems S, Sultan A, Schreiber D, Luker J, Servatius Het al. . Pulmonary vein isolation versus defragmentation: the CHASE-AF clinical trial. J Am Coll Cardiol 2015;66:2743–52. [DOI] [PubMed] [Google Scholar]
- 3. Das M, Wynn GJ, Saeed Y, Gomes S, Morgan M, Ronayne Cet al. . Pulmonary vein Re-isolation as a routine strategy regardless of symptoms: the PRESSURE randomized controlled trial. JACC Clin Electrophysiol 2017;3:602–11. [DOI] [PubMed] [Google Scholar]
- 4. Nery PB, Belliveau D, Nair GM, Bernick J, Redpath CJ, Szczotka Aet al. . Relationship between pulmonary vein reconnection and atrial fibrillation recurrence: a systematic review and meta-analysis. JACC Clin Electrophysiol 2016;2:474–83. [DOI] [PubMed] [Google Scholar]
- 5. Willems S, Verma A, Betts TR, Murray S, Neuzil P, Ince Het al. . Targeting nonpulmonary vein sources in persistent atrial fibrillation identified by noncontact charge density mapping: UNCOVER AF trial. Circ Arrhythm Electrophysiol 2019;12:e007233. [DOI] [PubMed] [Google Scholar]
- 6. Xie Y, Sato D, Garfinkel A, Qu Z, Weiss JN. So little source, so much sink: requirements for after depolarizations to propagate in tissue. Biophys J 2010;99:1408–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Qu Z, Liu MB, Olcese R, Karagueuzian H, Garfinkel A, Chen PSet al. . R-on-T and the initiation of reentry revisited: integrating old and new concepts. Heart Rhythm 2022;19:1369–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kleber AG, Rudy Y. Basic mechanisms of cardiac impulse propagation and associated arrhythmias. Physiol Rev 2004;84:431–88. [DOI] [PubMed] [Google Scholar]
- 9. Good WW, Zenger B, Bergquist JA, Rupp LC, Gillette KK, Gsell MAFet al. . Quantifying the spatiotemporal influence of acute myocardial ischemia on volumetric conduction velocity. J Electrocardiol 2021;66:86–94. [DOI] [PubMed] [Google Scholar]
- 10. Kalifa J, Tanaka K, Zaitsev AV, Warren M, Vaidyanathan R, Auerbach Det al. . Mechanisms of wave fractionation at boundaries of high-frequency excitation in the posterior left atrium of the isolated sheep heart during atrial fibrillation. Circulation 2006;113:626–33. [DOI] [PubMed] [Google Scholar]
- 11. Roney CH, Sim I, Yu J, Beach M, Mehta A, Alonso Solis-Lemus Jet al. . Predicting atrial fibrillation recurrence by combining population data and virtual cohorts of patient-specific left atrial models. Circ Arrhythm Electrophysiol 2022;15:e010253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Grace A, Willems S, Meyer C, Verma A, Heck P, Zhu Met al. . High-resolution noncontact charge-density mapping of endocardial activation. JCI Insight 2019;4:e126422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Shi R, Parikh P, Chen Z, Angel N, Norman M, Hussain Wet al. . Validation of dipole density mapping during atrial fibrillation and Sinus rhythm in human left atrium. JACC Clin Electrophysiol 2020;6:171–81. [DOI] [PubMed] [Google Scholar]
- 14. Bala G, De Asmundis C, Chierchia GB. A novel noncontact high-resolution charge density mapping system to guide ablation of complex atrial arrhythmias: overview of device technology and application. Expert Rev Med Devices 2021;18:343–50. [DOI] [PubMed] [Google Scholar]
- 15. Shi R, Chen Z, Pope MTB, Zaman JAB, Debney M, Marinelli Aet al. . Individualized ablation strategy to treat persistent atrial fibrillation: core-to-boundary approach guided by charge-density mapping. Heart Rhythm 2021;18:862–70. [DOI] [PubMed] [Google Scholar]
- 16. Pope MT, Leo M, Briosa E Gala A, Betts TR. Clinical utility of non-contact charge density ‘SuperMap’ algorithm for the mapping and ablation of organized atrial arrhythmias. EP Europace 2022;24:747–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mansour M, Calkins H, Osorio J, Pollak SJ, Melby D, Marchlinski FEet al. . Persistent atrial fibrillation ablation with contact force-sensing catheter: the prospective multicenter PRECEPT trial. JACC Clin Electrophysiol 2020;6:958–69. [DOI] [PubMed] [Google Scholar]
- 18. Su WW, Reddy VY, Bhasin K, Champagne J, Sangrigoli RM, Braegelmann KMet al. . Cryoballoon ablation of pulmonary veins for persistent atrial fibrillation: results from the multicenter STOP persistent AF trial. Heart Rhythm 2020;17:1841–7. [DOI] [PubMed] [Google Scholar]
- 19. Conti S, Weerasooriya R, Novak P, Champagne J, Lim HE, Macle Let al. . Contact force sensing for ablation of persistent atrial fibrillation: a randomized, multicenter trial. Heart Rhythm 2018;15:201–8. [DOI] [PubMed] [Google Scholar]
- 20. Mountantonakis S, Gerstenfeld EP. Atrial tachycardias occurring after atrial fibrillation ablation: strategies for mapping and ablation. JAFIB 2010;3:27–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Inoue K, Hikoso S, Masuda M, Furukawa Y, Hirata A, Egami Yet al. . OCVC Arrhythmia investigators. Pulmonary vein isolation alone vs. more extensive ablation with defragmentation and linear ablation of persistent atrial fibrillation: the EARNEST-PVI trial. EP Europace 2021;23:565–74. [DOI] [PubMed] [Google Scholar]
- 22. Scott PA, Silberbauer J, Murgatroyd FD. The impact of adjunctive complex fractionated atrial electrogram ablation and linear lesions on outcomes in persistent atrial fibrillation: a meta-analysis. Europace 2016;18:359–67. [DOI] [PubMed] [Google Scholar]
- 23. Tilz RR, Lenz C, Sommer P, Roza MS, Sarver AE, Williams CGet al. . Focal impulse and rotor modulation ablation vs. pulmonary vein isolation for the treatment of paroxysmal atrial fibrillation: results from the FIRMAP AF study. EP Europace 2021;23:722–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Spitzer SG, Miller JM, Sommer P, Szili-Torok T, Reddy VY, Nölker Get al. . Randomized evaluation of redo ablation procedures of atrial fibrillation with focal impulse and rotor modulation-guided procedures: the REDO-FIRM study. Europace 2023;25:74–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Baldinger SH, Chinitz JS, Kapur S, Kumar S, Barbhaiya CR, Fujii Aet al. . Recurrence of atrial arrhythmias despite persistent pulmonary vein isolation after catheter ablation for atrial fibrillation: a case series. JACC Clin Electrophysiol 2016;2:723–31. [DOI] [PubMed] [Google Scholar]
- 26. De Groot NM, Shah D, Boyle PM, Anter E, Clifford GD, Deisenhofer Iet al. . Critical appraisal of technologies to assess electrical activity during atrial fibrillation: a position paper from the European Heart Rhythm Association and European Society of Cardiology Working Group on eCardiology in collaboration with the Heart Rhythm Society, Asia Pacific Heart Rhythm Society, Latin American Heart Rhythm Society and Computing in Cardiology. EP Europace 2022;24:313–30. [DOI] [PubMed] [Google Scholar]
- 27. Junarta J, Siddiqui MU, Riley JM, Dikdan SJ, Patel A, Frisch DR. Low-voltage area substrate modification for atrial fibrillation ablation: a systematic review and meta-analysis of clinical trials. Europace 2022;24:1585–98. [DOI] [PubMed] [Google Scholar]
- 28. Durrer D, Van Der Tweel LH, Berreklouw S, Van Der Wey LP. Spread of activation in the left ventricular wall of the dog. IV. Two and three dimensional analysis. Am Heart J 1955;50:860–82. [DOI] [PubMed] [Google Scholar]
- 29. Durrer D, Van Dam RT, Freud GE, Janse MJ, Meijler FL, Arzbaecher RC. Total excitation of the isolated human heart. Circulation 1970;41:899–912. [DOI] [PubMed] [Google Scholar]
- 30. Pothineni NVK, Lin A, Frankel DS, Supple GE, Garcia FC, Lin Det al. . Impact of left atrial posterior wall isolation on arrhythmia outcomes in patients with atrial fibrillation undergoing repeat ablation. Heart Rhythm O2 2021;2:489–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kistler PM, Chieng D, Sugumar H, Ling LH, Segan L, Azzopardi Set al. . Effect of catheter ablation using pulmonary vein isolation with vs without posterior left atrial wall isolation on atrial arrhythmia recurrence in patients with persistent atrial fibrillation: the CAPLA randomized clinical trial. JAMA 2023;329:127–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kochhauser S, Jiang CY, Betts TR, Chen J, Deisenhofer I, Mantovan Ret al. . Impact of acute atrial fibrillation termination and prolongation of atrial fibrillation cycle length on the outcome of ablation of persistent atrial fibrillation: a substudy of the STAR AF II trial. Heart Rhythm 2017;14:476–83. [DOI] [PubMed] [Google Scholar]
- 33. Scherr D, Khairy P, Miyazaki S, Aurillac-Lavignolle V, Pascale P, Wilton SBet al. . Five-year outcome of catheter ablation of persistent atrial fibrillation using termination of atrial fibrillation as a procedural endpoint. Circ Arrhythm Electrophysiol 2015;8:18–24. [DOI] [PubMed] [Google Scholar]
- 34. Hung Y, Chang SL, Lin WS, Lin WY, Chen SA. Atrial tachycardias after atrial fibrillation ablation: how to manage? Arrhythm Electrophysiol Rev 2020;9:54–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Boyle PM, Zghaib T, Zahid S, Ali RL, Deng D, Franceschi WHet al. . Computationally guided personalized targeted ablation of persistent atrial fibrillation. Nat Biomed Eng 2019;3:870–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Azzolin L, Eichenlaub M, Nagel C, Nairn D, Sanchez J, Unger Let al. . Personalized ablation vs. conventional ablation strategies to terminate atrial fibrillation and prevent recurrence. Europace 2023;25:211–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ammar S, Hessling G, Reents T, Fichtner S, Wu J, Zhu Pet al. . Arrhythmia type after persistent atrial fibrillation ablation predicts success of the repeat procedure. Circ Arrhythm Electrophysiol 2011;4:609–14. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article were provided by Acutus Medical under licence/by permission. Data will be shared on request to the corresponding author with permission of Acutus Medical.