I. Introduction
The number of alkaloids identified from marine organisms continues to grow at an increasing rate, but few, if any, provide comparable sophistication in molecular architecture or as promising a biological significance as the manzamine class. The manzamines are a unique class of β-carboline-containing alkaloids with an unusual polycyclic system identified from marine sponges beginning in the late 1980s. The first representative of this class of alkaloids isolated by Higa’s group was identified as manzamine A (1) (Fig. 1) (1), and the relative, as well as absolute, configuration was considered unprecedented at the time. X-ray diffraction crystallographic analysis of manzamine A hydrochloride (1a), showed that apart from the β-carboline substituent, the molecule comprises a complicated array of 5-, 6-, 8-, and 13-membered rings. The piperidine and cyclohexene ring systems adopt chair and boat conformations, respectively, while the pyrrolidinium ring forms an envelope. The conformation of the 8-membered Z-olefinic ring is in an envelope-boat, with a mirror plane passing through C-32 and C-28. The two, six-membered rings of manzamine A are bridged by a chain of eight carbon atoms constituting a 13-membered macrocycle with a quadrangular conformation. The six bonds joining C-12 to C-19 of manzamine A form a “convex side” and a pseudo mirror plane transfixing the double bond and the C-36 atom (1).
Figure 1.
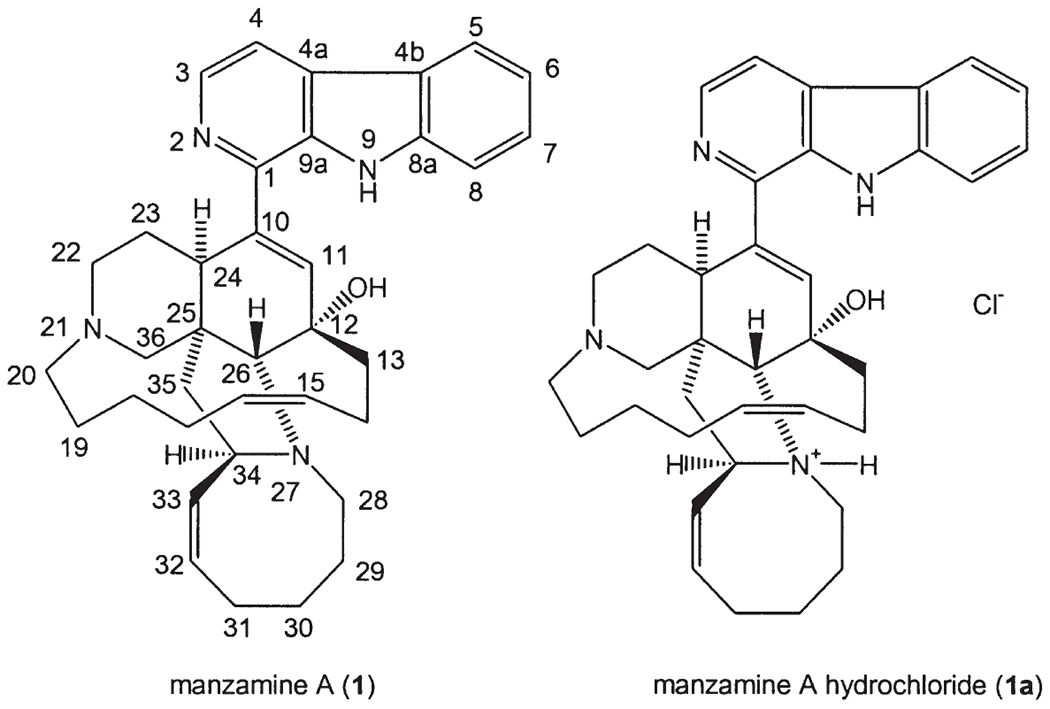
The structures of manzamine A (1) and its hydrochloride (1a).
In recent years, the manzamines have been regarded as an intriguing group of marine alkaloids with extraordinary biological activity, and as a result these compounds have been the subject of several reviews regarding their chemistry and pharmacology (2–4). In addition, the manzamines have also provoked a great deal of interest in their unprecedented biosynthetic pathway. In 1992 Baldwin et al. (5) first proposed a plausible biogenetic pathway involving an intramolecular Diels-Alder reaction for manzamines A (1) and B (2). This biogenetic scheme suggested that a macrocyclic bisdihydropyridine maybe derived from ammonia, a C3 unit, and a C10 unit. The bisdihydropyridine could then be converted through a Diels-Alder-type [4+2] intramolecular cycloaddition into a pentacyclic intermediate, which, in turn, would provide manzamines A and B via a tetracyclic intermediate. Manzamine C (3) could then easily be formed as a related product through a straightforward process involving four units including: tryptophan, ammonia, a C3 acrolein, and a C10 symmetrical dialdehyde (Scheme 1) (5).
Scheme 1.
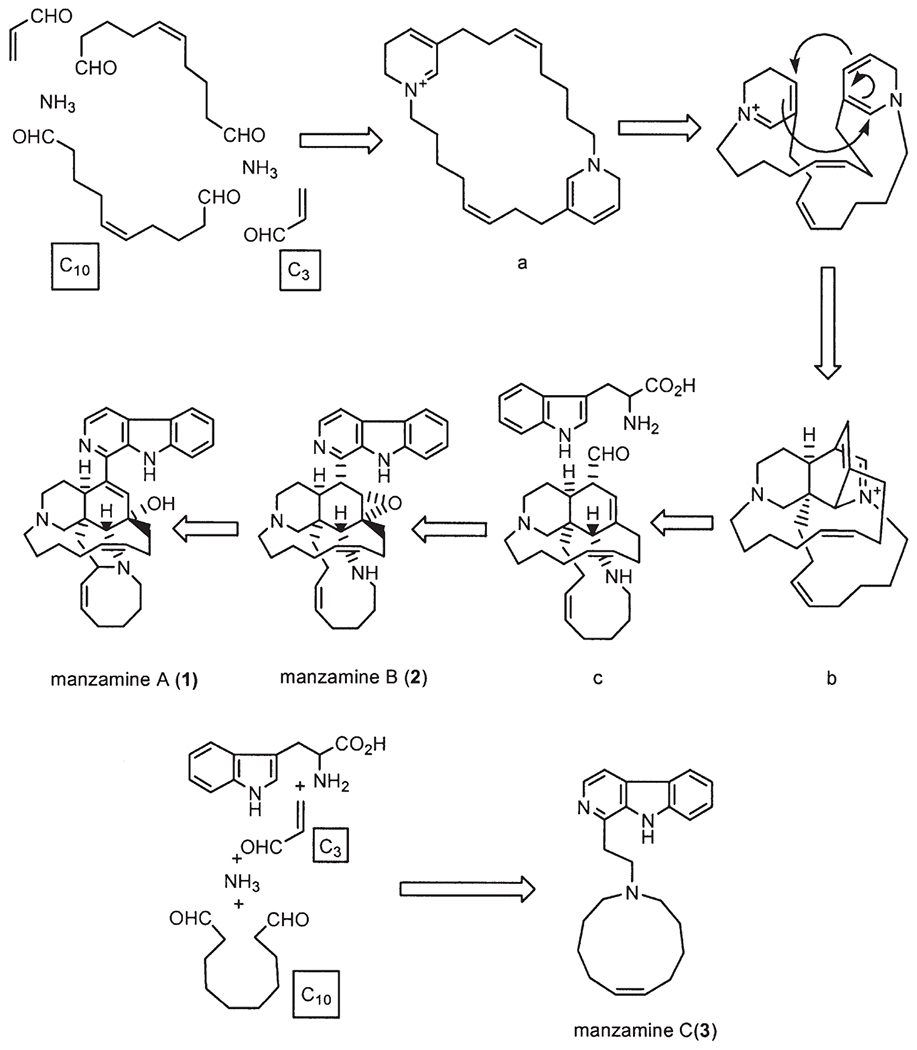
Biogenetic path of manzamines A (1), B (2), and C (3) proposed by Baldwin et al. (5).
Following manzamine A (1) (1), a series of β-carboline-containing manzamine alkaloids (2–29) (Fig. 2) (2,6–21) have been isolated from marine sponges over the past two decades, including the fascinating unsymmetrical manzamine dimer from the Scheuer group called kauluamine (25) (15) and a nearly symmetrical dimer called neo-kauluamine (26) (18). Based on Scheme 1, keramaphidin C (30) (22) may be regarded as the precursor of manzamine C (3). Keramaphidin C (30) and the closely related marine alkaloids 31 (23), 32–34 (24) (Fig. 3) are regarded as manzamine-related alkaloids due to their relationship to manzamine C presented in Scheme 1, despite the fact that they lack both the β-carboline and isoquinoline ring systems. From this same scheme it is also clear that ircinal A (35) (10) maybe a key precursor to manzamine A (1). Therefore, ircinal A, as well as the related marine alkaloids 36 (10), 37 (25), and 38 (25), are also regarded as part of the manzamine class of alkaloids (Fig. 3). Keramaphidin B (39) (26) is considered a key precursor to ircinals A (35) and B (36) (27), and, as a result, 39 and its related marine alkaloids 40 (28,29), 41 (28), 42 (30), 43 (30), 44 (31), 45–49 (32), 50 (33,34), 51–54 (35), and 55 (36) (Fig. 3) are also included in this review of manzamine-related alkaloids. In addition, there is a series of macrocyclic alkaloids isolated from marine sponges (37–52), which are similar in structure to compounds 39–55. However, these structures are not detailed in this review due to a diminished relationship to the manzamine alkaloids. Nakadomarin A (56) (53) is an example of a manzamine-related alkaloid that could be biogenetically derived from ircinal A (35) (3).
Figure 2.
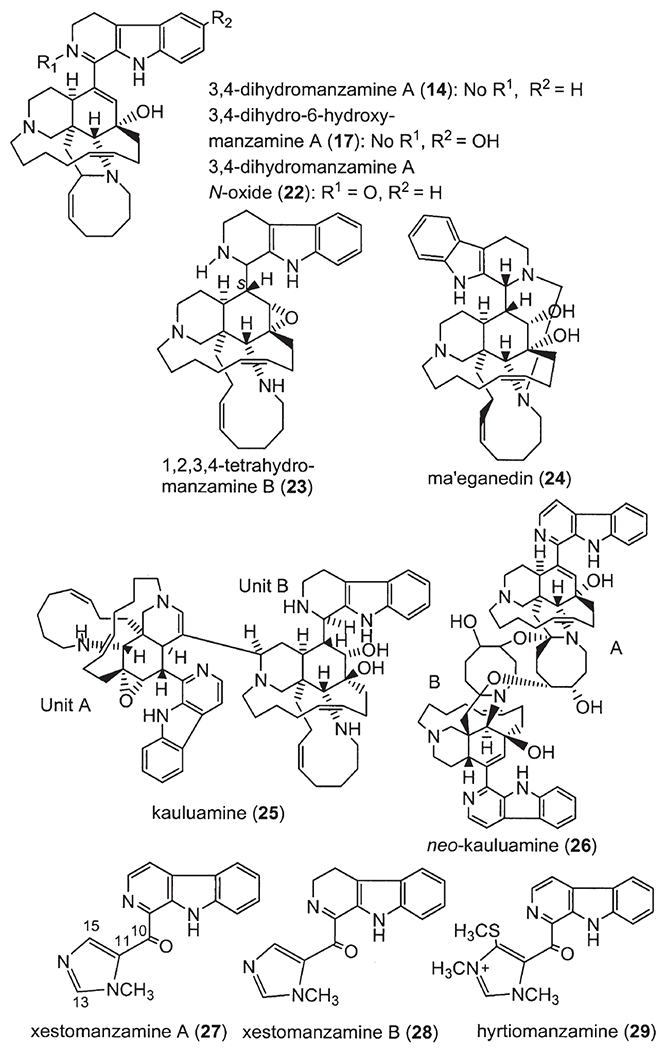
β-Carboline-containing manzamines.
Figure 3.
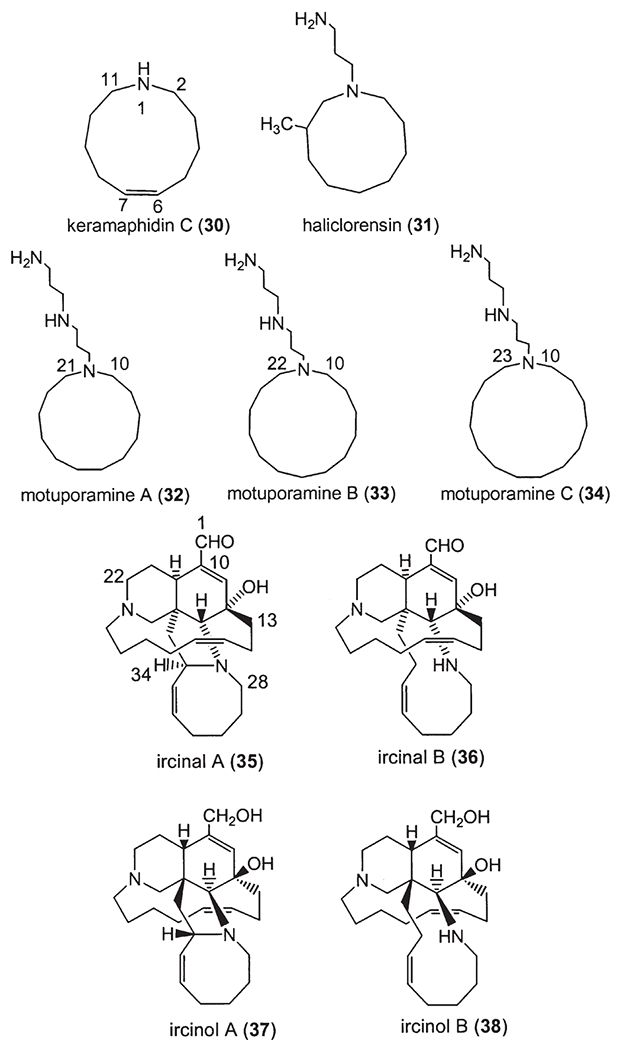
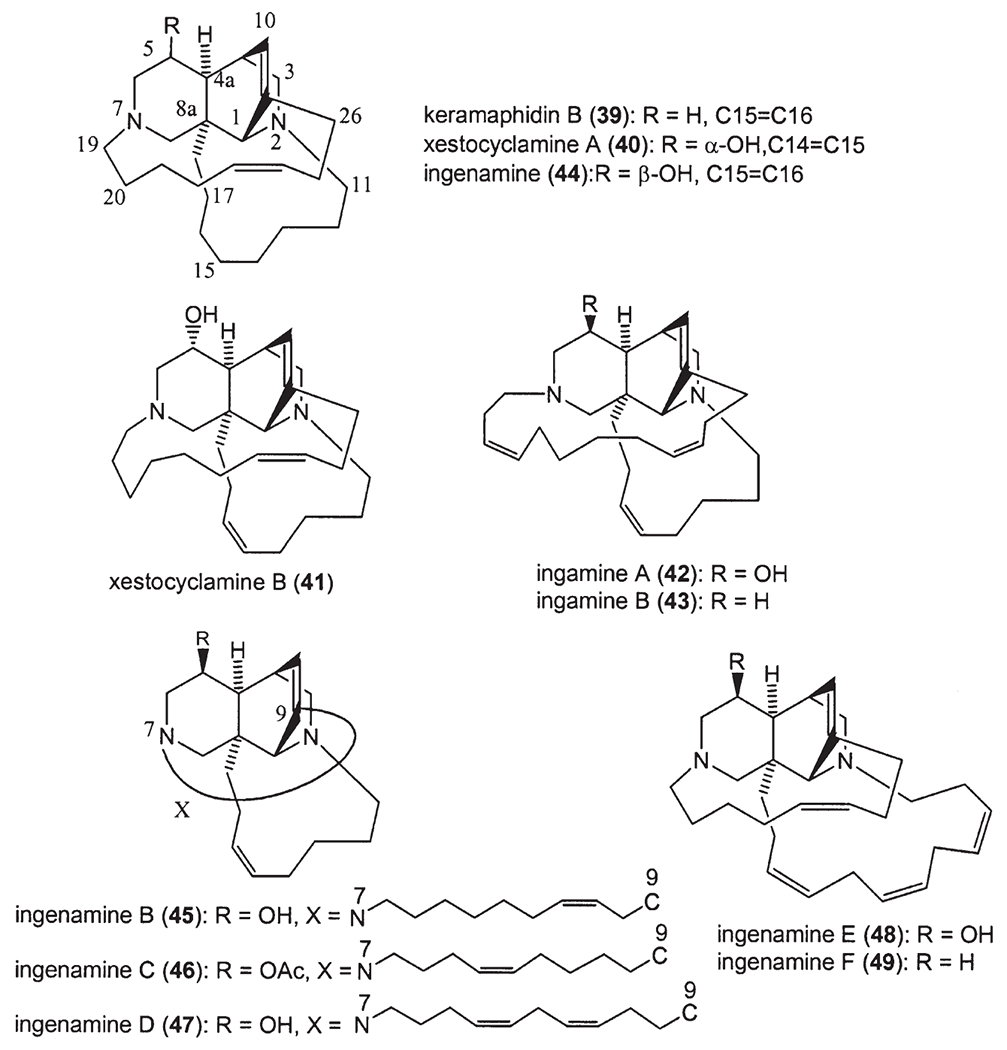
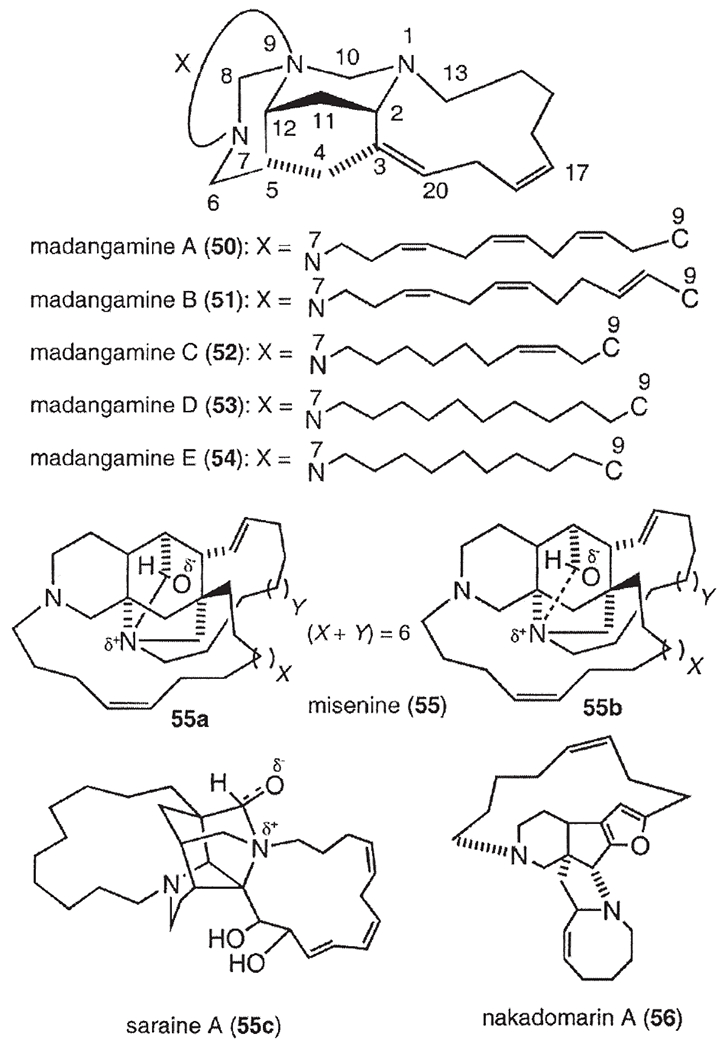
Manzamine-related marine alkaloids.
The manzamine alkaloids have shown a diverse range of bioactivities including: antitumor and cytotoxicity (1,7,9,10,12,15,16,54), anti-inflammatory (55), insecticidal (16,56), anti-infective and antiparasitic (17,27), with the greatest anti-infective activity against malaria and Mtb (18). The diversity of biological activity for this class of compounds provides additional evidence that they maybe of microbial origin and ultimately a novel class of lead, broad-spectrum, antiparasitic-antibiotics. To date, the greatest potential for the manzamine alkaloids appears to be against malaria with manzamine A (1), ent-8 hydroxymanzamine A (7a), as well as neo-kauluamine (26) showing improved activity over the clinically used drugs chloroquine and artemisinin in animal models (18). The isolation of the manzamine alkaloids from a growing number of sponge genera further implies the existence of a sponge-associated microorganism as the actual biosynthetic source for the manzamine alkaloids. A key tool for the study of the biosynthesis of these intriguing structures will clearly be the identification of such a microorganism.
II. Isolation and Structure Elucidation from Marine Sponges
To date, there are 17 or more species belonging to 5 families of marine sponges that have been reported to yield the β-carboline-containing manzamine and manzamine-related alkaloids (Table I). These sponges have been collected from Okinawa, Philippines, Indonesia, Red Sea, Italy, South Africa, and Papua New Guinea. Most species yielded a number of β-carboline-containing manzamine and manzamine-related alkaloids. The most productive species are those in the genera Amphimedon sp. (2,27), and Acanthostrongylophora ((58,59)); see Table I), which to date has yielded the greatest number of β-carboline-containing manzamine and manzamine-related alkaloids. Some species are particularly unusual due to their generation of enantiomers, such as 6 and 6b, as well as 7 and 7a (18).
TABLE I.
Marine Sponges Yielding Manzamines and Related Alkaloids.
| Taxonomy | Collection localities | Alkaloids | References |
|---|---|---|---|
| Order HAPLOSCLERIDA Topsent | |||
| Family CHALINIDAE Gray | |||
| Genus Haliclona Grant | |||
| Haliclona spp. | Manzamo and Amitori Bay (Iriomote Island), Okinawa | 1, 2, 3, 4, 13 | 1,7,8,14 |
| Haliclona tulearensis VV&La | Sodwana Bay, South Africa | 31 | 23 |
| Genus Reniera Nardo | |||
| Reniera sp. | Capo Miseno, Naples, Italy | 55 | 36 |
| Reniera sarai Pulitzeri-Finali | Bay of Naples | 55c | 53 |
| Family NIPHATIDAE van Soest | |||
| Genus Amphimedon D&Ma | |||
| Amphimedon spp. | Kerama Islands, Okinawa | 1, 2, 3, 3a, 4, 7, 8, 10, 11, 13, 14, 17, 18, 23, 24, 30, 35, 36, 37, 38, 39, 56 | 2,12,17,19,20,22,25–27,57 |
| Genus Cribrochalina Schmidt | |||
| Cribochalina sp. | Madang, Papua New Guinea | 16 | 13 |
| Family PETROSIIDAE van Soest | |||
| Genus Acanthostrongylophora Hooperb | |||
| Xestospongia spp.* | Miyako Island and Amitori Bay (Iriomote Island), Okinawa | 1, 2, 3, 4, 5, 6, 12, 27, 28, 40, 41 | 9,14,28,29 |
| [Prianos sp.] | Manado Bay, Sulawesi, Indonesia | 25 | 15 |
| [Xestospongia ashmorica Hooper] | Mindoro Island, Philippines | 1, 5, 6, 9, 19, 20, 21, 22 | 16 |
| [Xestospongia ingens Thiele] | Papua New Guinea | 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54 | 30–33,35 |
| [Petrosiidae n.g.] n. sp. | Manado Bay, Sulawesi, Indonesia | 6b, 7a, 26, 57, 58, 59, 62, 63, 64, 65, 66 | 18,58–60 |
| [Pachypellina sp.] | Manado Bay, Sulawesi, Indonesia | 1, 7 | 11 |
| Pellina sp.* | Kerama islands, Okinawa | 1, 6 | 6 |
| Genus Xestospongia de Laubenfels | |||
| Xestospongia exigua Kirkpatrick | Papua New Guinea | 32, 33, 34 | 24 |
| Genus Petrosia Vosmaer | |||
| Petrosia contignata Thiele | Milne Bay, Papua New Guinea | 15, 16 | 13 |
| Order DICTYOCERATIDA Minchin | |||
| Family THORECTIDAE Bergquist | |||
| Genus Hyrtios D&Ma | |||
| Hyrtios erecta Keller | Red Sea | 29 | 21 |
| Family IRCINIIDAE Gray | |||
| Genus Ircinia Nardo | |||
| Ircinia sp. | Kise, Okinawa | 1, 2, 4, 5, 8, 9, 35, 36 | 2,10 |
| Undetermined | Palau | 60, 61 | 61 |
Taxonomic authorities: VV&L = Vacelet, Vasseur & Lévi; D&M = Duchassaing & Michelotti.
The genus Acanthostrongylophora Hooper has been recently confirmed as the appropriate genus name for the group of sponges listed in square brackets above (63).
Those taxa followed by an asterisk have not been examined by MK but their descriptions conform to our understanding of the genus Acanthostrongylophora.
A. β-CARBOLINE-CONTAINING MANZAMINE ALKALOIDS
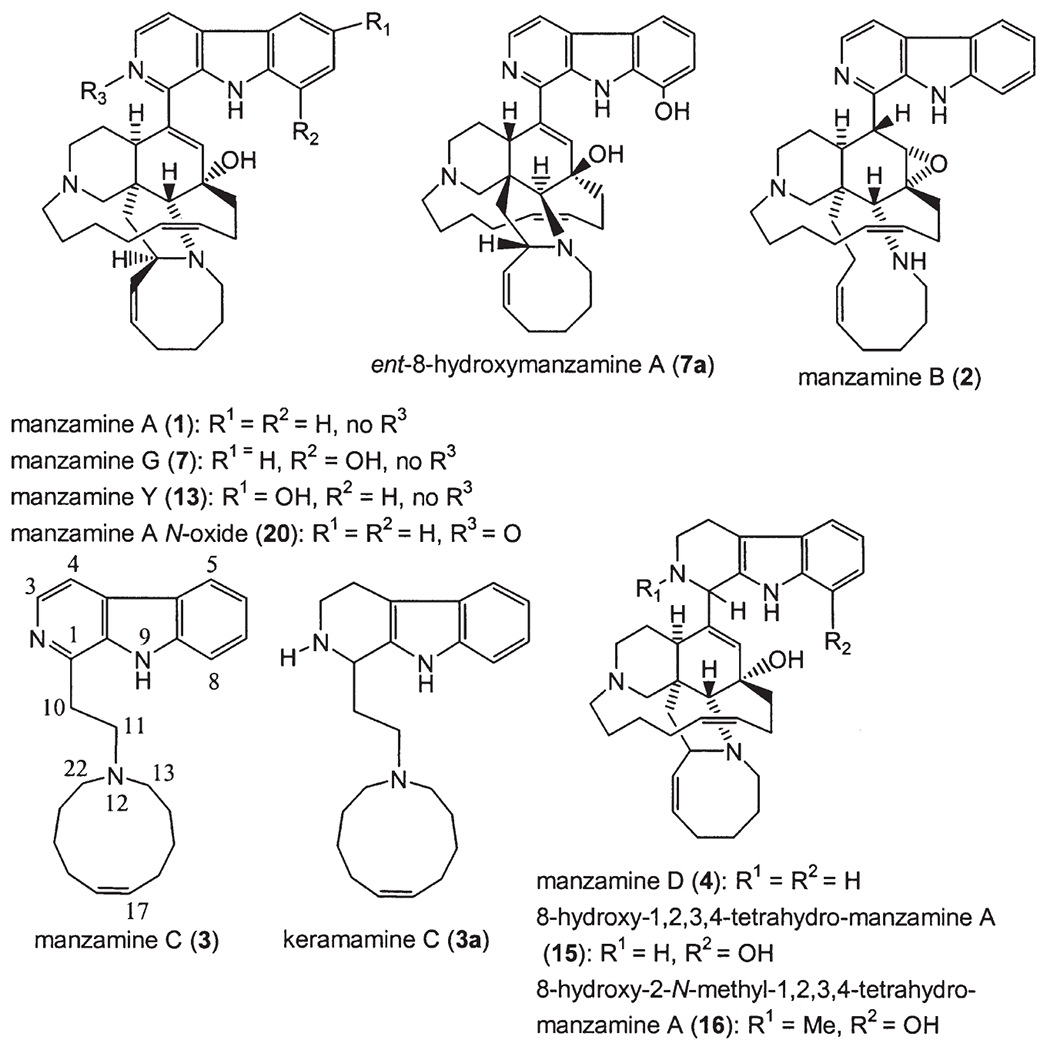
The β-carboline moiety is a distinct feature, which has been utilized in the classification of these alkaloids since the first report of manzamine A (1). In addition to manzamine A (1) (1), the following β-carboline-containing manzamines have since been reported and include: B (2) (7,8), C (3) (7,8), D (4) (7,8), E (5) (9), F (6) (9), G (7) (11,27), H (8) (10), J (9) (10), L (10) (27), M (11) (17), X (12) (14), Y (13) (12,27), 3,4-dihydromanzamine A (14) (12), 8-hydroxy-1,2,3,4-tetrahydromanzamine A (15) (13), 8-hydroxy-2-N-methyl-1,2,3,4-tetrahydromanzamine A (16) (13), 3,4-dihydro-6-hydroxymanzamine A (17) (17), 3,4-dihydromanzamine J (18) (17), and 6-deoxy-manzamine X (19) (19). Manzamines A (1) and F (6) were independently isolated almost at the same time and named as keramamine-A and B (6), respectively. The incorrect structural assignment of keramamine-B (6a) was revised quickly to be manzamine F (6) (9).
Manzamine G (7) (27) was first described using the name 8-hydroxymanzamine A (11), and it was also called manzamine K at a national meeting (34th Annual Meeting of The American Society of Pharmacognosy, July 18–22, 1993, San Diego, CA, Abstract No. P. 46) (11). 6-Hydroxymanzamine A (12) was named later as manzamine Y (13) (14,27). The Philippine sponge Xestospongia (=Acanthostrongylophora) ashmorica Hooper is an unusual species, which yielded the following manzamine N-oxides (16): manzamine A N-oxide (20), manzamine J N-oxide (21), and 3,4-dihydromanzamine A N-oxide (22) (Fig. 2).
Manzamine B (2) was the first epoxy alkaloid isolated in 1986 (7,8), and more than 10 years later the second epoxy 1,2,3,4-tetrahydromanzamine B (23) was isolated from a sponge identified as Amphimedon sp. (19). Ma’eganedin (24) is a tetrahydro-β-carboline alkaloid with a similar core structure to manzamine B (2), but possessing the unusual structural features of a methylene bridge between N-2 and N-27 and a C-11, C-12 vicinal cis-diol (20). The unsymmetrical manzamine dimer kauluamine (25) (15) and the nearly symmetrical manzamine neo-kauluamine (26) (18) were isolated from two species of Indonesian sponges independently. Manzamines H (8) and L (10) are C-1 isomers, (10,27) which were isolated from a single Okinawan species. Moreover, a striking feature of the manzamine series is that the two enantiomers of the β-carboline-containing manzamines, ent-8-hydroxymanzamine A (7a) (18) and ent-manzamine F (6b) (18) were obtained from the same sample collected in Manado, Indonesia. Compounds 7a and 6b possess opposite absolute configurations to those of 8-hydroxy manzamine A (manzamine G) and F. The β-carboline alkaloid keramamine C (3a) (22,27), regarded as the precursor of manzamine C (3), was isolated from the Okinawan sponge Amphimedon sp. Two β-carboline alkaloids xestomanzamines A (27) and B (28) (14) were identified from another Okinawan manzamine sponge Xestospongia sp. Hyrtiomanzamine (29) is structurally very similar to xestomanzamine A (27), and was isolated from the phylogenetically distant Red Sea sponge Hyrtios erecta (21).
Manzamine A (1) was first isolated as the major constituent from a sponge identified as belonging to the genus Haliclona (1). Subsequent studies with this same species have led to the isolation of the minor constituents manzamine B (2), C (3), and D (4) (7,8). The cytotoxic extract was purified over Si gel by successive elution with chloroform and acetone. The acetone eluate gave manzamine A hydrochloride (1a, 100 mg) as colorless crystals after recrystallization from methanol: mp>240°C dec, (c 0.28, CHCl3) (1). Almost at the same time, the brownish Okinawan marine sponge Pellina sp. was collected at the Kerama Islands. The chloroform soluble part of the 80% ethanol portion was chromatographed twice on Sephadex LH-20 columns (chloroform–methanol 1 : 1 and ethanol), followed by Si gel column chromatography (chloroform–methanol 98 : 2) to give pure manzamine A (called keramamine-A in the publication) hydrochloride (1a) (0.026% from wet sponge) (6).
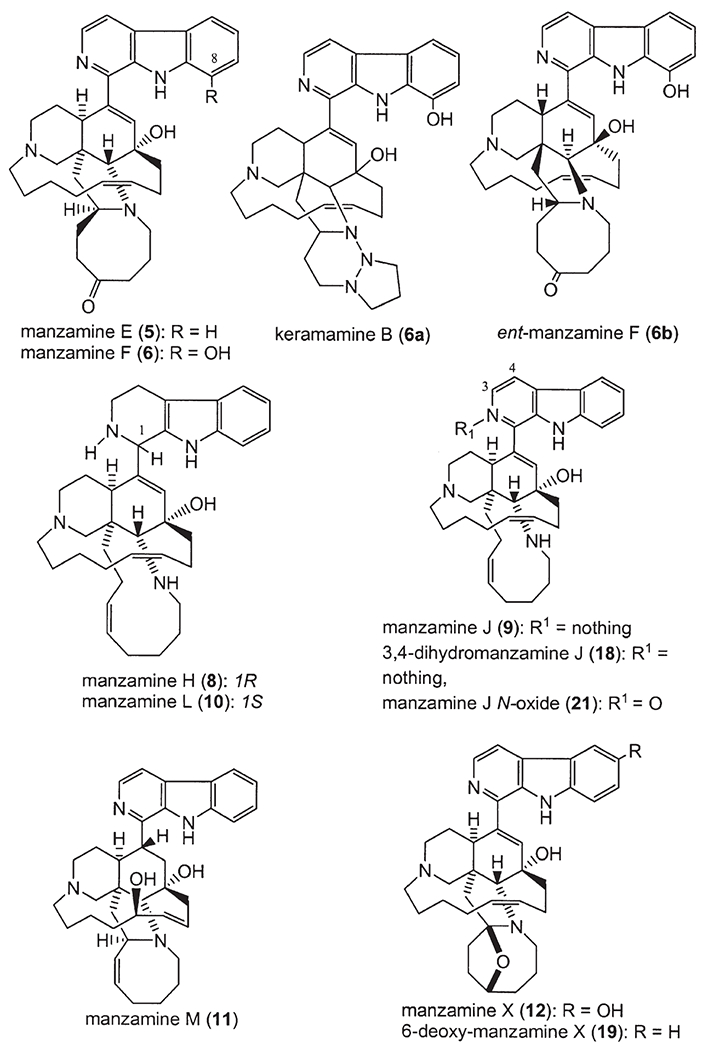
Manzamine E (5) and F (6) were isolated from an Okinawan Xestospongia sp. (9). Both 5 and 6 possess a ketonic carbonyl group in the eight-membered ring portion of the molecule. Manzamine F was found earlier from a sponge, Pellina sp., and named as keramamine B with an incorrectly assigned 1,2,3-triazacyclohexane moiety (6). Later, the unusual structure of keramamine B (6a) was revised as 6 (9). A sample (6 kg) of Xestospongia sp. was extracted by steeping in methanol. Purification of the fractions containing alkaloids by HPLC (LiChrosorb–NH2, CHCl3–MeOH 30 : 1) gave the free bases of manzamine E (5, 31 mg) and F (6, 111 mg) (9).
8-Hydroxymanzamine A (7, also called manzamine G or K) was isolated from an Indonesian sponge thought to be an undescribed species of Pachypellina by Ichiba et al. (11). The CH2Cl2-soluble fraction (320 mg) was separated by high-speed countercurrent chromatography with a solvent system of hexane–MeCN–CH2Cl2 (10 : 7 : 3, lower mobile phase) providing semi-pure 8-hydroxymanzamine A, which could be further purified by recrystallization from CH2Cl2/MeOH to furnish pure 8-hydroxymanzamine A (0.3%, based on dry weight) (11). This sponge was recently confirmed by MK to be in the genus Acanthostrongylophora Hooper.
Manzamines H (8) and J (9) were isolated from the Okinawan sponge Ircinia sp. (10). From this sponge, ircinals A (35) and B (36), two plausible biogenetic precursors of the manzamine alkaloids were also isolated. The sponge Ircinia sp. was collected off Kise Island, Okinawa, and kept frozen until processing. The methanol extract of the sponge was partitioned between ethyl acetate and water. The ethyl acetate soluble material was subjected to silica gel chromatography (hexane/acetone 4 : 1, CHCl3/MeOH 95 : 5, and hexane/acetone 9 : 1) to afford manzamines H (8, 0.0007% wet weight of the sponge) and J (9, 0.0022%) and ircinals A (35, 0.0057%) and B (36, 0.0020%) (10).
Manzamine M (11, 0.0015% wet weight), 3,4-dihydro-6-hydroxymanzamine A (17, 0.0015%), and 3,4-dihydromanzamine J (18, 0.0004%) were isolated from the sponge Amphimedon sp. collected off the Kerama Islands (17). Manzamine M (11) is the first manzamine congener with a hydroxyl group on the C-13–C-20 chain. 6-Hydroxymanzamine A (13) and 3,4-dihydromanzamine A (14) were obtained from another Okinawan sponge Amphimedon sp. (12). The sponge (1.5 kg) Amphimedon sp. was collected from Okinawa and kept frozen until extracted with MeOH and then evaporated under reduced pressure to give 68.4 g of extract. A fraction eluting from Si gel with CHCl3/MeOH (95 : 5) was further purified with a Si gel column (cyclohexane–Me2CO–Et2NH, 70 : 30 : 2) to give manzamine Y, also called 6-hydroxymanzamine A (13, 0.005%, wet weight). The fraction eluting with CHCl3/MeOH (98 : 2) was separated over a Si gel column (C6H6–Me2CO–Et2NH, 95 : 5 : 2) to afford 3,4-dihydromanzamine A (14, 0.002%) (12).
8-Hydroxy-1,2,3,4-tetrahydromanzamine A (15) and its N-methylated derivatives 8-hydroxy-2-N-methyl-1,2,3,4-tetrahydromanzamine A (16) were isolated from sponges of the genus Petrosia (13). The sponge P. contignata was preserved immediately after collection by immersion in an alcohol : H2O (1 : 1) solution. After approximately 24 h this solution was decanted and discarded. The damp organisms were placed in Nalgene™ bottles and shipped at ambient temperature. Final purification by HPLC (normal phase, hexane : EtOAC 1 : 1) provided 40 mg of 8-hydroxy-1,2,3,4-tetrahydromanzamine A (15) and 35 mg of 8-hydroxy-2-N-methyl-1,2,3,4-tetrahydromanzamine A (16). At the same time, compound 16 was also isolated from a Cribrochalina sp. (13). The manzamine-containing fractions were combined and further resolved with HPLC using a Si gel column and acetone : hexane (1 : 4). A less polar fraction was rechromatographed on Si gel HPLC (acetone : hexane 1 : 5) to give 50 mg of 8-hydroxy-2-N-methyl-1,2,3,4-tetrahydromanzamine A (16) (13).
Manzamine X (12) and the β-carboline alkaloids xestomanzamine A (27) and B (28) were isolated from an Okinawan marine sponge Xestospongia sp. (14). This sponge was collected in the shallow water (−2 m) off Amitori Bay, Okinawa. 6-Deoxymanzamine X (19) and the N-oxides of manzamine J (20–22) have been isolated from the Philippine sponge Xestospongia (=Acanthostrongylophora) ashmorica (Hooper), which was collected off the shores of Mindoro Island (16). The samples were freeze-dried prior to transport and extraction. The n-BuOH-soluble material was subjected to Si gel column chromatography, and seven major fractions were obtained. The first fraction yielded 6-deoxymanzamine X (19) together with manzamine J (9), and 6-deoxy-manzamine X (19) was obtained from the methanolic supernatant upon precipitation of manzamine J (9) at 5 °C for 24 h. The final three polar fractions yielded the manzamine N-oxides (20–22). The presence of manzamine N-oxides was evident in an HPLC chromatogram of the crude extract, indicating that these alkaloids are present as natural products and not as oxidation artifacts formed during isolation. The three N-oxides (20–22) were more polar and lack the characteristic fluorescence on Si 60 TLC plates when compared with their parent alkaloids (365 nm). In all cases, the mass spectral data of the N-oxides indicate that the molecular weight is 16 mass units higher than that expected after analysis of the NMR spectra. For each of the N-oxides, the 1D and 2D NMR spectra allowed signal assignments that readily confirm the chemical shift changes found in the aromatic system. These differences between the shifts of the N-oxides compared with those of their parent compounds appear to be characteristic, with large upfield shifts for aromatic carbons in the ortho and para positions to the substituent, caused by mesomeric redistribution of electron density and downfield shifts for directly bound sp3 carbon atoms. The decisive experiment for ascertaining the N-oxide character of the β-carboline moiety was its reduction with zinc dust and 1N HCl, which is a specific reducing agent for the conversion of an N-oxide to its corresponding tertiary base (16).
Manzamine L (10, 0.0056% wet weight, ) together with the known manzamines A (1), B (2), C (3), D (4), G (7), H (8), Y (13), and 3,4-dihydromanzamine A (14) were isolated from Amphimedon sp. collected off Kerama Islands, Okinawa (27). From this sponge, keramamine C (3a), ircinals A (35) and B (36), ircinols A (37) and B (38), keramaphidins B (40) and C (30) were also isolated.
Both enantiomers of keramaphidin B were separated by using chiral HPLC (27), of which one may be a plausible biogenetic precursor for both ircinals as well as manzamines A and B, while the other may be associated with the antipodes of the manzamine alkaloids, such as ircinols A and B. Ircinols A (37) and B (38), are the first reported antipodes of manzamine-related alkaloids and were isolated from an Okinawan sponge Amphimedon sp. collected off the Kerama Islands, Okinawa (25). The structures were determined to be enantiomers of the alcoholic forms at C-1 of ircinals A (35) and B (36) (10), respectively. Treatment of ircinal A (35), which was isolated from this sponge, with DIBALH afforded a reduced product, the spectral data of which were identical with those of ircinol A except for the optical rotation [reduction product of ircinal A, (c 0.2, MeOH); ircinol A, (c 0.5, MeOH)]. This result revealed that ircinol A was an enantiomer of the alcoholic derivative of ircinal A which has been shown to have the same absolute configuration as that of manzamine A. Manzamines A () and B () and ircinals A () and B () isolated from this sponge had the same absolute configurations as those reported previously (25,27).
Manzamines H (8) and L (10) were isolated from the same sponge, and both were shown to have the same 2D structure with a significant difference in the 13C NMR chemical shift of C-1 (8: 59.9 ppm, 10: 56.1 ppm, CDCl3). The absolute configuration of C-1 of manzamine L (10) was deduced to be 1S from a negative Cotton effect, while 8 showed the opposite sign implying the 1R-configuration. At the same time, manzamine D (4) was also isolated from this sponge, and showed a 1R-configuration as per a positive Cotton effect (27).
Dimeric manzamines: kauluamine (25) is the first report of a manzamine dimer, adding yet another level of complexity to the manzamine-type of alkaloids. Kauluamine was isolated by Scheuer’s group from an Indonesian sponge originally identified as Prianos sp. collected in Manado Bay, Indonesia (15). The fact that just a single bond holds two of these complex polycyclic systems together gives the molecule kauluamine the unusual appearance of being fragile. This sponge was also recently identified as a species of Acanthostrongylophora Hooper. The second unprecedented manzamine dimer isolated by Hamann’s group was named neo-kauluamine (26) and was isolated from what was originally identified as an undescribed petrosid genus, together with the new enantiomers of 8-hydroxymanzamine A (ent-8-hydroxymanzamine A, 7a) and manzamine F (ent-manzamine F, 6b) (18). neo-Kauluamine was also isolated from a sponge collected in Manado Bay, Indonesia as kauluamine. The relative stereochemistry of the nearly symmetric manzamine dimer neo-kauluamine (26) was established through a detailed analysis of the NOE-correlations combined with molecular modeling, while the enantiomers were elucidated through NOE measurements combined with optical rotation values (18). The undescribed petrosid genus is now known to conform to our understanding of the genus Acanthostrongylophora (63).
B. MANZAMINE-RELATED MARINE ALKALOIDS
Keramaphidin C (30) was isolated from Amphimedon sp. (22). Haliclorensin (31) was isolated from Haliclona sp. collected off Sodwana Bay, South Africa (23). Motuporamines A (32), B (33) and C (34) were isolated as an inseparable mixture from Xestospongia exigua collected in Papua New Guinea (24). Ircinals A (35) and B (36), two plausible biogenetic precursors of the manzamine alkaloids, were isolated from the Okinawan sponge Ircinia sp. (10). The antipodes of the manzamine-related alkaloids ircinols A (37) and B (38) were obtained from another Okinawan sponge Amphimedon sp., together with keramaphidins B (39) and C (30) (22,25–27). Ircinols A and B were determined to be enantiomers of the C-1 alcoholic forms of ircinals A and B, respectively. Xestocyclamine A (40) was first isolated from the Papua New Guinea marine sponge Xestospongia (=Acanthostrongylophora) ingens, and its structure was revised in the following year with the isolation of xestocyclamine B (41) (28,29). Ingamines A (42) and B (43) (30), ingenamine (44) (31), ingenamines B (45), C (46), D (47), E (48) and F (49) (32) were also isolated from the Papua New Guinea marine sponge Xestospongia (=A.) ingens (Fig. 3).
Madangamine A (50) was isolated from Xestospongia sp. collected off Madang, Papua New Guinea (33,34), and later madangamines B (51), C (52), D (53), and E (54) were also obtained from this same sponge (35). Misenine (55), a polycyclic ‘cage-like’ alkaloid, was isolated from an unidentified Mediterranean species Reniera sp. (36). The 1H-NMR spectrum of this unusual alkaloid showed significant variations with pH and it was concluded that the dominant species in neutral and basic solutions was 55a whereas under acidic conditions the structure 55b was preferred. A similar transannular N/C=O “proximity effect” had previously been observed in saraine A (55c) although, in this case, a lowering of pH enhanced the C–N linkage (53). Nakadomarin A (56) was isolated from Amphimedon sp., and its structure was reported to contain an unprecedented 8/5/5/5/15/6 ring system (57).
C. RECENTLY ISOLATED β-CARBOLINE-CONTAINING MANZAMINE ALKALOIDS
Recently, a number of Indo-Pacific sponges have yielded a novel class of manzamines, named 12,34-oxamanzamines (58). These alkaloids possess a novel ring system generated through a new ether bridge formed between carbons 12 and 34 of the typical manzamine structure. ent-12,34-Oxamanzamines E (57) and F (58), as well as 12,34-oxamanzamine A (59), were obtained from three Indo-Pacific sponges. The biocatalytic transformation of ent-8-hydroxymanzamine A (7a) to 58, using Nocardia sp. ATCC 21145 and Fusarium oxysporium ATCC 7601, has also been achieved, suggesting that these alkaloids maybe formed through biocatalysis by a sponge-associated microbe. In fact, the epi-isomers such as manzamines H (8, 1R configuration) and L (10, 1S configuration) were isolated from different sponges, but at the same time the sponge samples also yielded a series of manzamine-related compounds with 1R and/or 1S configuration. In 2000, Kingston’s group reported two new epi-manzamines (60, 61) (61). These two new β-carboline containing manzamines were isolated from a Palauan sponge, and both epi-manzamine D (60) and 2-N-methyl-epi-manzamine D (61) possess negative optical rotation values. Most recently, two unprecedented manzamine-related alkaloids called manadomanzamines A (62) and B (63) have been reported from an unidentified Indonesian sponge (59). Manadomanzamines A and B represent an unprecedented rearrangement of the manzamine skeleton. In addition three new β-carboline containing manzamines: 32,33-dihydro-31-hydroxymanzamine A (64), 32,33-dihydro-6,31-dihydroxymanzamine A (65), and 32,33-dihydro-6-hydroxymanzamine A-35-one (66) have recently been reported (60). Based on biogenetic considerations, compounds 64 and 65 are likely the reduced derivatives of manzamine E. Alkaloid 66 is unique in that it possesses a ketone moiety at C-35, instead of a typical C-31 ketone as seen in manzamine E and F (Fig. 4).
Figure 4.
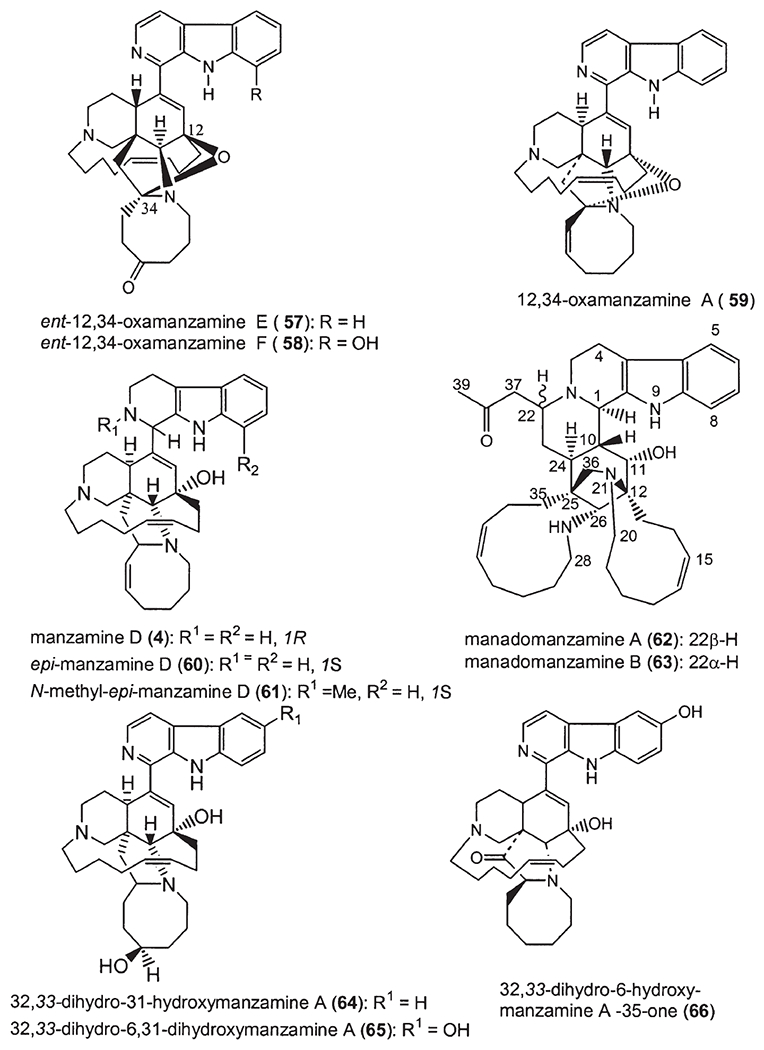
Recently isolated β-carboline manzamine alkaloids.
D. PHYSICAL AND SPECTRAL PROPERTIES
The physico-chemical properties of the manzamines are shown in Table II. Manzamines are solid powders or crystals, and most show strong UV absorption due to the β-carboline moiety. The majority of the manzamine alkaloids possess a positive optical rotation, except for ent-manzamine F (6b), ent-8-hydroxymanzamine A (7a), manzamine L (10), ircinols A (37) and B (38), epi-manzamine D (60), and 2-N-methyl-epi-manzamine D (61). The structures were completed by spectroscopic methods such as HR-MS, high-field 2D NMR, and X-ray diffraction analysis. The 13C- and 1H-NMR spectral data were recorded primarily in CDCl3, and their data are shown in Tables III and IV, respectively.
TABLE II.
Physico-chemical Properties of Manzamine Alkaloids.
| Alkaloids 1, 2, 3, 3a and 4 | |||||
|---|---|---|---|---|---|
| 1 (1,9)a | 2 (8) | 3 (8) | 3a (22) | 4 (7) | |
| Appearance | Colorless powder | Colorless crystals (EtOAc) | Colorless plates (CHCl3-CH3CN) | Colorless oil | Colorless powder |
| Molecular formula | C36H44N4O | C36H46N4O | C23H29N3 | C23H33N3 | C36H48N4O |
| HR-MS m/z | 548.3510a (Δ 0.5 mmu) (HREIMS) | 351.2687 (Δ 1.3 mmu) (HREIMS) | 552.3837 (Δ 0.9 mmu) (HREIMS) | ||
| MP (°C) | > 200 (dec.) | 198–203 | 77–82 | 165–168 | |
| [α]D | + 44.3° (c 1.09, CHCl3) | + 89° (c 1.8, CHCl3) | + 20° (c 0.92, MeOH) | + 60.6° (c 0.66, CHCl3) | |
| UV λmax (MeOH) nm (ε) | 219 (22900), 236 (18600), 280 (10800), 290 (sh, 9800), 346 (5300), 357 (5600) | 212 (18000), 235 (22000), 240 (sh, 20000), 250 (sh, 15000), 282 (sh, 6900), 288 (11000), 338 (3500), 351 (3500) | 212 (13500), 234 (22000), 239 (sh, 21000), 248 (sh, 14000), 282 (sh, 6100), 287 (9500), 335 (3000), 349 (3000) | 225 (sh), 271 (5600) 285 (sh), 290 (sh) | 223 (28800), 275 (sh, 6300), 281 (6700), 288 (5400) |
| IR vmax (KBr) cm−1 | 3280, 3150, 3050, 3000, 2920, 2800, 2760, 2630, 2560, 1617, 1555, 1488, 1448, 1418, 1385, 1370, 1315, 1270, 1230, 1180, 1142, 1110, 1095, 1065, 1025, 820, 740, 725, 700 | 3340, 3200, 3140, 3060, 3000, 2920, 2845, 1620, 1510, 1495, 1470, 1445, 1420, 1400, 1320, 1275, 1255, 1235, 1210, 1120, 870, 745, 710, 660, 620 | 3000, 2910, 2840, 2810, 1640, 1495, 1465, 1440, 1425, 1350, 1335, 1320, 1290, 1230, 1215, 1200, 1120, 740, 715, 660 | 3400, 2940 | 3460, 3000, 2920, 1450, 1440, 1365, 1340, 1290, 1240, 1210, 1145, 1105, 1070, 995, 970 |
| Alkaloids 5, 6, 6b, 7 and 7a | |||||
| 5 (9) | 6 (9) | 6b (18) | 7 (11) | 7a (18) | |
|
| |||||
| Appearance | Colorless crystals (CH3CN) | Colorless crystals (CH3CN) | Yellowish powder | Pale yellow crystals, (CH2Cl2/MeOH) | Yellowish powder |
| Molecular formula | C36H44N4O2 | C36H44N4O3 | C36H44N4O3 | C36H44N4O2 | C36H44N4O2 |
| HR-MS m/z | 565.3555 (M++H, Δ 1.2 mmu) (HRFABMS) | 581.3496 (M++H, Δ 0.4 mmu) (HRFABMS) | 581.3434 (Δ −5.8 mmu) [M+H]+ (HRESIMS) | 565.3507 (M++H, Δ 1.8 mmu) (HRFABMS) | 565.3433 (Δ −11.0 mmu) [M+H]+ (HRESIMS) |
| MP (°C) | 174–176 | > 200 (dec.) | 194 (dec) | > 230 (dec) | 196–198 (dec) |
| [α]D | + 63.7° (c 2.51, CHCl3) | + 59.9° (c 0.67, CHCl3) | − 44.6° (c 0.11, CHCl3) | + 118.5° (c 1.94,CHCl3) | − 112.0° (c 0.12,CHCl3) |
| UV λmax (MeOH) nm (ε) | 220 (35800), 237 (28100), 279 (18000), 290 (sh, 15300), 346 (8700), 359 (9300) | 220 (36000), 244 (31000), 265 (13900), 3559 (8100) | 266 (3.04), 300 (3.02), 380 (2.92) | 206 (sh,22700), 222 (32300), 245 (30600), 268 (14000), 360 (8100) | 266 (2.95), 282 (2.94), 390 (2.85) |
| IR vmax (KBr) cm−1 | 3400 (br), 3050, 3010, 2940, 2850, 2800, 1700, 1625, 1560, 1495, 1465, 1455, 1420, 1370, 1350, 1320, 1270, 1230, 1150, 1110, 1065, 1020, 820, 785, 740 | 3400, 3060, 3020, 2950, 2860, 2810, 1695, 1595, 1570, 1465, 1445, 1420, 1375, 1350, 1335, 1275, 1245, 1230, 1115, 1075, 790, 780, 740 | 3498–3260, 3026–2802, 1699, 1670, 1564, 1446, 1221 | 3280, 2900, 1570, 1540, 1420, 1410, 1330, 1260, 1230, 1220, 1200, 1055, 1035, 1010, 970, 940, 745, 720 | 3499–3267, 3017–2807, 1680, 1563, 1446, 1220 |
| Compounds 8–12 | |||||
| 8 (10,27) | 9 (10) | 10 (27) | 11 (17) | 12 (14) | |
|
| |||||
| Appearance | Colorless solid | Colorless amorphous solid | Colorless amorphous solid | Yellow prisms (n-hexane–acetone) | |
| Molecular formula | C36H50N4O | C36H46N4O | C36H50N4O | C36H44N4O2 | C36H44N4O3 |
| HR-MS m/z | 554.3980 (Δ −0.5 mmu) (HREIMS) | 550.3660 (Δ −1.2 mmu) (HREIMS) | 554.3975 (M+, Δ −0.9 mmu) (HREIMS) | 564.3459 (Δ −0.5 mmu) (HREIMS) | 581.3470 [M+H]+ (Δ −2.0 mmu) (HRFABMS) |
| MP (°C) | 145 | 140 | 143 | > 250 | |
| [α]D | +17° (c 1.1, CHCl3) | +47° (c 2.0, CHCl3) | −15° (c 0.42,CHCl3) | +16° (c 0.48, MeOH) | + 66.1° (c 1.93, CHCl3) |
| UV λmax (MeOH) nm (ε) | 225 (29000), 277 (6600), 282 (6800), 290 (5500) | 218 (26000), 236 (21000), 280 (11000), 290 (11000), 348 (5500), 356 (5600) | 223 (34000), 283 (6400) | 205 (14000), 233 (3000), 276 (1500), 359 (1000) | 215 (29500), 300 (17000), 378 (4800) |
| IR vmax (KBr) cm−1 | 3400, 3300, 2990, 2910, 2850, 2780, 1650, 1450, 1360, 1340, 1290, 1260, 1210, 1110, 1070, 1035, 1000, 910 | 3400, 3220, 2990, 2920, 2850, 2790, 1620, 1560, 1490, 1450, 1420, 1320, 1280, 1230, 1110, 1070, 1040 | 3400, 2900 | 3410 (br), 2925, 1630, 1405, 1070 | 3290, 2930, 1640, 1562, 1462 |
| CD (MeOH) λext (Δε) nm | 202 (+13.6), 222 (−10.8), 270 (+4.2) | ||||
| Compounds 13–16 | |||||
| 13 (12)b | 13’ (14)b | 14 (12) | 15 (13) | 16 (13) | |
|
| |||||
| Appearance | Yellowish amorphous solids | Yellow solid (n-hexane–acetone) | Colorless amorphous solids | White powder | |
| Molecular formula | C36H44N4O2 | C36H44N4O2 | C36H46N4O | C36H48N4O2 | C37H50N4O2 |
| HR-MS m/z | 564.3465 (M+, Δ −1.8 mmu) (HREIMS) | 565.3530 [M+H]+ (Δ −1.0 mmu) (HRFABMS) | 550.3653 (Δ −1.9 mmu) | 569.3832 [M+H]+ (Δ 2.3 mmu) (HRFABMS) | 583.4025 [M+H]+ (Δ −1.3 mmu) (HREIMS) |
| MP (°C) | 253 | > 250 | 237–241 | ||
| [α]D | +139° (c 1.10, MeOH) | +33° (c 2.50, CHCl3) | +86° (c 0.25, CHCl3) | +5° (c 0.03, CH2Cl2) | |
| UV λmax (MeOH) nm (ε) | 210 (32000), 293 (sh, 15000), 300 (16000), 370 (5100) | 215 (29500), 300 (11000), 378 (3000) | 230 (sh, 25000), 244 (21000), 323 (10000) | 240, 270, 326, 370 | |
| IR vmax (KBr) cm−1 | 3300, 2920, 1450 | 3228, 2930, 1670, 1562, 1462, 1200 | 3280, 2940, 1470, 1450 | 3005, 2935, 2847, 1648, 1627, 1578 | |
| Compounds 17–22 | ||||||
|---|---|---|---|---|---|---|
| 17 (17) | 18 (17) | 19 (16) | 20 (16) | 21 (16) | 22 (16) | |
| Appearance | Colorless amorphous solid | Colorless amorphous solid | Pale yellow amorphous powder | Yellow crystalline powder | Yellow crystalline powder | Yellow crystalline powder |
| Molecular formula | C36H46N4O2 | C36H48N4O | C36H44N4O2 | C36H44N4O2 | C36H46N4O2 | C36H46N4O2 |
| HR-MS m/z | 566.3604 (Δ −1.7 mmu) (HREIMS) | 552.3815 (Δ −1.4 mmu) (HREIMS) | 565 [M+H]+ (FABMS) | 565 [M+H]+ (FABMS) | 567 [M+H]+ (FABMS) | 567 [M+H]+ (FABMS) |
| MP (°C) | 140 | |||||
| [α]D | + 28.0° (c1.2, MeOH) | + 50.0° (c 0.10, MeOH) | + 30.1° (c 0.35, CHCl3) | + 18.6° (c 0.35, CHCl3) | + 15.0° (c 0.40, CHCl3) | + 34.1° (c 0.59, CHCl3) |
| UV λmax (MeOH) nm (ε) | 207 (9000), 225 (6500), 250 (3500), 337 (2500) | 209 (13000), 242 (9000), 322 (4500) | 210 (26000), 260 (11800), 312 (sh, 10000), 378 (3000) | 201 (26000), 241 (23000), 261 (23000), 310 (21000) | 261 (25000), 325 (19000) | 201 (25000), 355 (11000) |
| IR vmax (KBr) cm−1 | 3420 (br), 2920, 1630, 1400, 1070 | 3420 (br), 2920, 1630, 1090 |
| Compounds 25–28, 35 | |||||
|---|---|---|---|---|---|
| 25 (15) | 26 (18) | 27 (14) | 28 (14) | 35 (10) | |
| Appearance | Unstable pale yellow solid | Colorless needles | Yellow needles (CHCl3–MeOH) | Yellow oil | Colorless solid |
| Molecular formula | C72H94N8O3 | C72H88N8O6 | C16H12N4O | C16H14N4O | C26H38N2O2 |
| HR–MS m/z | 1101.7426 (Δ −0.4 mmu) for C72H93N8O2 [MH−H2O]+ (HRFABMS) | 1161.6905 (Δ −5.1 mmu) for C72H89N8O6 [M+H]+ (HRFABMS) | 277.1100 [M+H]+ (Δ −4.0 mmu) (HRFABMS) | 279.1250 [M+H]+ (Δ 0 mmu) (HREIMS) | 410.2924 [M]+ (Δ −0.9 mmu) (HREIMS) |
| MP (°C) | 184 | 185–186 | 70 | ||
| [α]D | + 0.7° (c 0.18, CHCl3) | + 94.6° (c 0.1, CHCl3) | + 48.0° (c 2.9, CHCl3) | ||
| UV λmax (MeOH) nm (ε) | 252 (4.20), 357 (3.85) | 221 (7400), 257 (1700), 300 (3900), 395 (1600) | 222 (27200), 270 (10300), 298 (16500), 388 (4500) | 231 (8500) | |
| IR λmax (KBr) cm−1 | 3380, 3150, 2990, 2900, 2840, 1645, 1620, 1450, 1440, 1320, 1235, 1150 | 3592, 3475–3250 (br), 3007–2802, 1626, 1560, 1454, 1215 | 3427, 3075, 1612, 1211, 1128 | 3451, 3110, 2926, 1641, 1190, 1130 | 3420, 2950, 2920, 2850, 2790, 1680, 1670, 1560, 1450, 1400, 1200, 1150, 1100, 1070, 730 |
| Compounds 30, 36–39 | |||||
| 30 (22) | 36 (10) | 37 (25) | 38 (25) | 39 (26,27) | |
|
| |||||
| Appearance | colorless amorphous solid | colorless solid | colorless amorphous solid | colorless amorphous solid | colorless needle |
| Molecular formula | C10H19N | C26H40N2O2 | C26H40N2O2 | C26H42N2O2 | C26H40N2 |
| HR–MS m/z | 153.1493 [M]+ (Δ −2.4 mmu) (HRFABMS) | 412.3118 [M]+ (Δ +2.9 mmu) (HREIMS) | 412.3107 [M]+ (Δ +1.7 mmu) (HREIMS) | 414.3248 [M]+ (Δ +0.2 mmu) (HREIMS) | 380.3199 [M]+ (Δ +0.8 mmu) (HREIMS) |
| MP (°C) | 106–109 | 95 | 83–85 | 78–79 | 131–132 |
| [α]D | 0 | +18.0° (c 1.1, CHCl3) | −19.0° (c 0.54, MeOH) | −2.8° (c 0.12, MeOH) | +22.2°c |
| UV λmax | 224 (12000) | ||||
| (MeOH) nm (ε) | |||||
| IR vmax (KBr) cm−1 | 3400, 2940 | 3400, 2920, 2850, 2800, 1680, 1450, 1190, 1170, 1120, 1040, 700 | 3400, 2940 | 3400, 2940 | 2940 |
| Compounds 57–61 | |||||
| 57 (58) | 58 (58) | 59 (58) | 60 (61) | 61(61) | |
|
| |||||
| Appearance | Brown amorphous solid | Yellowish powder | White powder | Amorphous powder | Orthorhombic crystals |
| Molecular formula | C36H42N4O2 | C36H42N4O3 | C36H42N4O | C36H48N4O | C37H50N4O |
| HR–MS m/z | 563.3386 [M+H]+ (Δ −3.0 mmu) (HRFABMS) | 579.3335 [M+H]+ (Δ −0.4 mmu) (HRFABMS) | 347.3408 [M+H]+ (Δ −5.0 mmu) (HRFABMS) | 552.3830 [M]+ (Δ +0.2 mmu) (HREIMS) | 566.3974 [M]+ (Δ −1.0 mmu) (HREIMS) |
| MP (°C) | 152 | 158 | 164 | 185–188 | |
| [α]D | −54.6° (c 0.3, CHCl3) | − 49.2° (c 0.10, CHCl3) | + 40.0° (c 0.6, CHCl3) | + 77.3° (c 0.165, CHCl3) | + 91.4° (c 0.27, CHCl3) |
| UV λmax | 252 (3.82) | 251 (3.83) | 252 (3.823) | 225 (4.23) | 223 (4.24) |
| (MeOH) nm (ε) | 275 (3.65) 354 (3.4l) | 273 (3.69) 356 (3.42) | 271 (3.71) 358 (3.41) | 281 (3.79) | 281 (3.74) |
| IR vmax (KBr) cm−1 | 3650, 3001–2818, 1714, 1620, 1592, 1533, 1452, 1267, 1144, 1052. | 3658, 3377, 3002–2822, 1714, 1620, 1592, 1533, 1452, 1267, 1144, 1052 | 3635, 3368, 3001–2815, 1715, 1625, 1590, 1535, 1451, 1265, 1145, 1050 | 3600–3200, 3004, 2931, 1650, 1620, 1454, 1071. | 3500–3200, 3004, 2937, 1651, 1616, 1454, 1070, 1060. |
| CD (MeOH) λext (Δε) nm | 204 (+22.1), 221.5 (−14.3), 226 (sh, −13.7), 269.5 (+11.7), 291.5 (+6.8). | 205 (+26.8), 223.5 (−13.7), 229 (sh, −12.2), 271 (+6.3), 295 (+3.4). | |||
| Compounds 62–66 | |||||
| 62 (59) | 63 (59) | 64 (60) | 65 (60) | 66 (60) | |
|
| |||||
| Appearance | White powder | White powder | Colorless crystals | Pale yellow powder | Pale yellow powder |
| Molecular formula | C39H54N4O2 | C39H54N4O2 | C36H46N4O2 | C36H46N4O3 | C36H44N4O3 |
| HR–MS m/z | 611.4348 [M+H]+ (Δ +2.9 mmu) (HRESIMS) | 611.4310 [M+H]+ (Δ −0.9 mmu) (HRESIMS) | 567.4052 [M+H]+ (Δ −3.5 mmu) (HRESIMS) | 583.3477 [M+H]+ (Δ −16.6 mmu) (HRESIMS) | 581.3467 [M+H]+ (Δ −2.5 mmu) (HRESIMS) |
| MP (°C) | > 200 (dec.) | ||||
| [α]D | −19° (c 0.11, MeOH) | −18° (c 0.11, MeOH) | + 34.44° (c 0.9, CHCl3) | + 25.9° (c 0.5, MeOH) | + 10.0° (c 1.0, MeOH) |
| UV λmax (MeOH) nm (ε) | 282 (7700) | 282 (7200) | 215, 248, 281, 291, 352, 359 | 218, 240, 280, 291, 356, 359 | 219, 248, 268, 356, 395 |
| IR vmax (KBr) cm−1 | 3372, 3002, 2919, 1707, 1468, 1354, 1164, 736 | 3387, 3001, 2917, 1711, 1460, 1355, 1162, 736 | 3280, 2954, 2927, 1560, 1493, 1453, 1370, 1276, 1150, 748, 665 | 3324, 2928, 1649, 1559, 1461, 1194, 675 | 3324, 2935, 1661, 1559, 1461, 1197, 664 |
| CD (MeOH) λext (Δε) nm | 226 (+ 13.4) 271 (−3.62) | 224 (+ 15.9) 269 (−3.68) | |||
Manzamine A hydrchloride (1a): Colorless crystals (MeOH); MP (°C) > 240°C (dec.); (c 0.28, CHCl3).
Compound 13 and compound 13′ should have the same structure as manzamine Y (6-hydroxymanzamine A), but the spectral data especially their optical rotations showed great difference between two different reports (12,14).
A small amount of crystals of keramaphidin B (39) obtained from CH3CN or CHCl3 was racemic (26).
TABLE III.
13C-NMR Data of Manzamine-type Alkaloids in CDCl3.
| Alkaloids 1-8 | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Atom | 1 (9) | 2 (8) | 3 (8) | 3a (22)a | 4 (7)b | 5 (9) | 6 (9) | 7 (11) | 8 (10,27) |
| 1 | 143.6s | 146.2s | 145.8s | 53.9d | 60.0d | 142.8s | 142.5s | 143.3s | 59.9d |
| 3 | 137.5d | 137.0d | 137.4d | 42.1t | 66.9t | 138.4d | 137.8d | 137.9d | 43.2t |
| 4 | 113.8d | 113.7d | 113.0d | 21.7t | 43.6t | 113.4d | 113.7d | 114.7d | 22.4t |
| 4a | 129.3s | 129.2s | 128.1s | 109.5s | 127.7s | 129.4s | 130.2s | 129.8s | 109.0s |
| 4b | 121.1s | 121.1s | 121.9s | 128.0s | 109.4s | 121.6s | 122.8s | 123.2s | 127.8s |
| 5 | 120.9d | 121.3d | 121.6d | 119.0d | 119.2d | 121.3d | 112.0d | 112.6d | 118.0d |
| 6 | 119.2d | 118.8d | 119.0d | 120.3d | 118.0d | 119.9d | 120.9d | 120.7d | 119.3d |
| 7 | 127.9d | 128.8d | 127.6d | 123.1d | 129.8d | 128.3d | 113.0d | 114.3d | 121.4d |
| 8 | 112.8d | 111.3d | 111.8d | 112.2d | 111.0d | 111.9d | 143.4s | 143.8s | 111.0d |
| 8a | 141.4s | 140.5s | 140.6s | 138.1s | 141.0s | 140.6s | 129.9s | 130.6s | 135.5s |
| 9a | 133.3s | 134.9s | 135.5s | 115.9s | 133.8s | 133.3s | 133.2s | 132.9s | 134.2s |
| 10 | 141.2s | 44.5d | 34.7t | 27.7t | 135.4s | 140.3s | 140.9s | 141.9s | 143.9s |
| 11 | 135.1d | 60.2d | 52.8t | 52.9t | 134.5d | 138.0d | 137.3d | 134.6d | 130.1d |
| 12 | 71.3s | 60.4s | 69.8s | 68.4s | 69.0s | 71.2s | 70.1s | ||
| 13 | 39.1t | 18.6t | 48.9t | 48.9t | 40.9t | 40.5t | 39.9t | 39.2t | 40.6t |
| 14 | 20.6t | 22.7t | 26.0t | 23.0t | 21.9t | 21.2t | 21.4t | 20.7t | 21.9t |
| 15 | 126.8d | 127.7d | 23.3t | 25.0 | 128.7d | 127.9d | 127.9d | 126.7d | 129.4d |
| 16 | 132.8d | 131.8d | 24.9t | 26.8t | 132.2d | 132.0d | 130.1d | 133.0d | 129.1d |
| 17 | 24.9t | 23.5t | 130.9d | 132.1d | 33.3t | 25.2t | 25.5t | 24.7t | 29.1t |
| 18 | 26.4t | 26.7t | 130.9d | 132.1d | 27.1t | 26.3t | 26.6t | 26.5t | 28.6t |
| 19 | 24.5t | 24.3t | 24.9t | 26.8t | 22.7t | 24.8t | 25.0t | 24.5t | 29.2t |
| 20 | 53.3t | 50.8t | 23.3t | 25.0t | 53.6t | 52.5t | 52.8t | 53.4t | 53.4t |
| 21 | 26.0t | 23.0t | 23.0t | ||||||
| 22 | 49.1t | 47.5t | 48.9t | 48.9t | 49.8t | 49.3t | 49.6t | 49.1t | 49.6t |
| 23 | 33.5t | 32.3t | 32.0t | 33.2t | 34.0t | 33.4t | 32.3t | ||
| 24 | 41.0d | 32.5d | 37.9d | 41.7d | 42.3d | 41.3d | 44.6d | ||
| 25 | 46.9s | 44.0s | 47.6s | 46.5s | 47.3s | 47.1s | 43.5s | ||
| 26 | 78.0d | 59.7d | 75.5d | 80.8d | 81.7d | 78.3d | 59.2d | ||
| 28 | 53.3t | 39.4t | 55.1t | 52.6t | 53.0t | 53.7t | 59.2t | ||
| 29 | 26.2t | 23.4t | 26.2t | 32.4t | 32.7t | 26.4t | 29.2t | ||
| 30 | 24.2t | 18.1t | 25.9t | 44.3t | 45.1t | 24.2t | 29.2t | ||
| 31 | 28.3t | 27.9t | 28.4t | 214.8s | 216.2s | 28.4t | 25.0t | ||
| 32 | 142.3d | 132.3d | 136.4d | 38.7t | 38.8t | 142.8d | 131.6d | ||
| 33 | 123.5d | 131.3d | 121.4d | 24.4t | 24.4t | 123.3d | 131.1d | ||
| 34 | 57.0d | 27.3t | 51.0t | 63.2d | 63.6d | 57.4d | 26.2t | ||
| 35 | 44.7t | 44.7t | 44.8t | 46.5t | 46.6t | 44.8t | 37.3t | ||
| 36 | 70.3t | 56.6t | 68.8t | 68.4t | 69.0t | 70.2t | 65.7t | ||
| Alkaloids 9–17 | |||||||||
| Atom | 9 (10) | 10 (27) | 11 (17) | 12 (14) | 13 (14) | 14 (12) | 15 (13) | 16 (13) | 17 (17) |
|
| |||||||||
| 1 | 144.1s | 56.1d | 145.6s | 142.9s | 143.3s | 158.9s | 60.8d | 69.5d | 158.8s |
| 3 | 138.6d | 41.3t | 139.6d | 137.9d | 137.0d | 48.8t | 43.9t | 53.0t | 48.8t |
| 4 | 113.4d | 21.7t | 115.3d | 113.4d | 113.9d | 19.1t | 22.3t | 21.9t | 19.2t |
| 4a | 129.4s | 109.7s | 123.5s | 129.0s | 129.1s | 117.1s | 110.3s | 110.3s | 116.2s |
| 4b | 121.9s | 126.6s | 132.1s | 122.3s | 121.7s | 125.5s | 129.5s | 129.0s | 126.0s |
| 5 | 121.6d | 117.1d | 123.1d | 106.7d | 106.0d | 119.7d | 109.5d | 109.3d | 103.4d |
| 6 | 120.0d | 118.6d | 121.7d | 150.3s | 149.8s | 120.2d | 119.9d | 119.9d | 149.8s |
| 7 | 129.4d | 120.6d | 130.3d | 118.5d | 118.4d | 124.3d | 106.8d | 106.9d | 114.5d |
| 8 | 111.7d | 110.0d | 114.1d | 112.3d | 113.3d | 121.1d | 143.0s | 143.1s | 112.9d |
| 8a | 140.2s | 134.8s | 135.7s | 134.7s | 136.2s | 136.1s | 125.1s | 125.3s | 135.2s |
| 9a | 133.8s | 132.6s | 141.3s | 134.3s | 134.0s | 127.6s | 144.8s | 144.9s | 128.3s |
| 10 | 142.5s | 143.3s | 143.5s | 139.8s | 141.3s | 139.0s | 132.8s | 131.6s | 138.9s |
| 11 | 131.7d | 128.6d | 138.4d | 136.6d | 134.9d | 140.8d | 132.7d | 132.1d | 140.2d |
| 12 | 70.4s | 68.9s | 70.7s | 69.4s | 71.2s | 69.9s | 70.6s | 71.1s | 69.9s |
| 13 | 40.9t | 39.6t | 50.5t | 41.7t | 39.2t | 40.5t | 39.6t | 39.8t | 40.3t |
| 14 | 22.2t | 21.0t | 128.3d | 21.8t | 20.8t | 21.6t | 20.6t | 20.6t | 21.4t |
| 15 | 128.2d | 128.4d | 140.6d | 128.5d | 126.9d | 128.4d | 127.6d | 126.8d | 128.9d |
| 16 | 131.2d | 127.9d | 74.4d | 132.3d | 132.8d | 132.3d | 131.7d | 132.9d | 132.4d |
| 17 | 32.5t | 28.1t | 37.2t | 26.1t | 25.0t | 25.9t | 24.9t | 24.8t | 25.5t |
| 18 | 28.7t | 27.5t | 27.4t | 26.3t | 26.4t | 25.8t | 26.4t | 26.3t | 26.2t |
| 19 | 29.2t | 28.2t | 22.5t | 22.9t | 24.6t | 25.6t | 24.4t | 24.4t | 25.1t |
| 20 | 53.5t | 52.8t | 55.9t | 53.2t | 53.4t | 53.4t | 53.3t | 53.2t | 53.4t |
| 22 | 49.7t | 48.6t | 45.3t | 49.8t | 49.2t | 49.5t | 49.1t | 49.1t | 49.4t |
| 23 | 29.2t | 31.7t | 33.3t | 32.5t | 33.4t | 32.5t | 33.7t | 33.4t | 32.6t |
| 24 | 46.7d | 46.2d | 42.2d | 39.3d | 40.8d | 40.8d | 37.5d | 37.4d | 38.2d |
| 25 | 43.5s | 42.9s | 49.1s | 45.4s | 47.0s | 46.9s | 46.7s | 44.6s | 46.8s |
| 26 | 59.2d | 58.1d | 74.7d | 75.2d | 78.0d | 75.1d | 78.9d | 78.9d | 75.2d |
| 28 | 37.5t | 58.1t | 53.1t | 55.3t | 53.4t | 50.9t | 53.5t | 53.3t | 51.2t |
| 29 | 26.2t | 28.2t | 34.5t | 28.0t | 26.4t | 31.3t | 26.3t | 26.3t | 29.6t |
| 30 | 25.1t | 28.2t | 27.2t | 26.8t | 24.3t | 25.6t | 24.0t | 24.0t | 28.2t |
| 31 | 29.2t | 24.1t | 30.1t | 79.6d | 28.3t | 28.1t | 28.6t | 28.7t | 25.8t |
| 32 | 131.0d | 130.6d | 136.9d | 40.7t | 142.4d | 134.9d | 142.5d | 142.1d | 135.2d |
| 33 | 129.4d | 130.0d | 131.4d | 36.9t | 123.6d | 129.6d | 123.6d | 123.6d | 128.2d |
| 34 | 59.2t | 25.1t | 57.3d | 103.9s | 57.1d | 55.0t | 57.5d | 57.5d | 55.2d |
| 35 | 29.2t | 37.0t | 45.3t | 51.4t | 44.7t | 44.5t | 43.2t | 42.5t | 44.6t |
| 36 | 65.6t | 64.9t | 65.6t | 66.5t | 70.3t | 68.6t | 70.9t | 70.9t | 68.9t |
| N-Me | 44.4q | ||||||||
| Alkaloids 18–22, and 25 | |||||||
|---|---|---|---|---|---|---|---|
| Atom | 18 (17) | 19 (16)c | 20 (16)c | 21 (16)c | 22 (16)c | 25 (15) | 25 (15) |
| Unit A | Unit B | ||||||
| 1 | 159.8s | 143.8s | 135.0s | 135.0s | 136.6s | 147.6s | 57.5d |
| 3 | 48.8t | 137.8d | 132.9d | 133.1d | 61.9t | 136.6d | 38.4t |
| 4 | 19.1t | 113.5d | 115.2d | 115.3d | 20.3t | 113.6d | 23.3t |
| 4a | 117.1s | 129.4s | 120.5s | 120.3s | 108.3s | 129.3s | 107.7s |
| 4b | 125.5s | 122.1s | 122.5s | 122.5s | 126.5s | 120.9s | 127.2s |
| 5 | 119.7d | 121.8d | 121.2d | 121.2d | 119.1d | 121.0d | 117.9d |
| 6 | 120.1d | 120.3d | 121.3d | 121.4d | 121.0d | 118.5d | 118.2d |
| 7 | 124.2d | 129.0d | 127.8d | 127.8d | 123.5d | 127.4d | 120.2d |
| 8 | 112.0d | 112.0d | 112.0d | 111.9d | 112.0d | 110.6d | 110.5d |
| 8a | 136.1s | 140.5s | 141.3s | 141.1s | 137.8s | 140.7s | 135.9s |
| 9a | 127.7s | 133.9s | 136.8s | 136.9s | 129.9s | 135.8s | 135.3s |
| 10 | 140.1s | 140.0s | 141.3s | 137.8s | 134.5s | 53.0d | 38.8d |
| 11 | 133.5d | 138.8d | 140.8d | 134.6d | 141.1d | 58.8d | 74.7d |
| 12 | 70.2s | 69.7s | 70.0s | 70.5s | 69.9s | 60.3s | 64.2s |
| 13 | 40.7t | 41.9t | 41.3t | 41.0t | 41.2t | 40.1t | 31.1t |
| 14 | 21.9t | 22.2t | 22.0t | 26.5t | 21.9t | 24.0t | 18.7t |
| 15 | 129.3d | 128.6d | 128.8d | 131.1d | 128.8d | 130.8d | 130.2d |
| 16 | 129.2d | 132.6d | 132.9d | 132.4d | 132.9d | 132.2d | 130.8d |
| 17 | 29.1t | NR | NR | NR | NR | 24.9t | 24.9t |
| 18 | 28.6t | NR | NR | NR | NR | 28.2t | 27.6t |
| 19 | 29.1t | NR | NR | NR | NR | 26.1t | 26.9t |
| 20 | 53.4t | NR | NR | NR | NR | 52.8t | 46.8t |
| 22 | 49.5t | NR | NR | NR | NR | 127.3d | 62.0d |
| 23 | 32.3t | NR | NR | NR | NR | 116.3s | 23.0t |
| 24 | 45.0d | NR | NR | NR | NR | 34.6d | 40.3d |
| 25 | 43.2s | NR | NR | NR | NR | 35.6s | 45.2s |
| 26 | 59.2d | 78.8d | 75.3d | 59.4d | 75.1d | 60.6d | 66.8d |
| 28 | 59.2t | 55.8t | 51.4t | 54.8t | 51.3t | 47.7t | 48.3t |
| 29 | 29.1t | 23.2t | 33.7t | 29.8t | 33.9t | 26.9t | 27.6t |
| 30 | 29.1t | 37.4t | 26.0t | 29.0t | 26.0t | 28.1t | 26.6t |
| 31 | 25.0t | 79.8d | 28.4t | 32.9t | 28.4t | 23.4t | 24.2t |
| 32 | 131.0d | 28.4t | 134.7d | 130.0d | 134.8d | 129.5d | 130.6d |
| 33 | 131.4d | 41.0t | 130.4d | 129.5d | 130.3d | 132.5d | 131.2d |
| 34 | 26.2t | 104.2s | 55.7d | 25.4t | 55.0d | 18.4t | 20.9t |
| 35 | 37.4t | 51.9t | 43.9t | 22.8t | 44.1t | 32.9t | 32.7t |
| 36 | 65.6t | 67.1t | 69.3t | 65.8t | 63.9t | 49.9t | 56.3t |
| 13C−, 15N-NMR Data of Manzamine-type Alkaloids 26, 57-59 in CDCl3d | |||||
|---|---|---|---|---|---|
| Atom | 26 (18) | 26 (18) | 57 (58) | 58 (58) | 59 (58) |
| Unit A | Unit B | ||||
| 1 | 142.8s | 143.0s | 143.9s | 142.6s | 143.8s |
| N2 | 273.7s | 273.7s | 298.0s | 299.0s | ND |
| 3 | 138.7d | 138.8d | 138.8d | 138.3d | 138.2d |
| 4 | 113.4d | 113.5d | 114.2d | 114.3d | 113.9d |
| 4a | 129.3s | 129.4s | 129.9s | 130.1s | 130.1s |
| 4b | 121.8s | 121.8s | 122.0s | 123.4s | 112.0s |
| 5 | 121.6d | 121.6d | 121.8d | 111.9d | 122.1d |
| 6 | 120.1d | 120.1d | 120.4d | 120.9d | 120.6d |
| 7 | 128.4d | 128.4d | 128.8d | 113.6d | 128.4d |
| 8 | 111.5d | 111.5d | 112.3d | 143.6s | 111.9d |
| 8a | 139.8s | 139.9s | 140.8s | 130.6s | 140.3s |
| N9 | 83.6p | 83.6p | 109.0p | 105.4p | ND |
| 9a | 133.5s | 133.6s | 133.8s | 133.2s | 133.2s |
| 10 | 139.8s | 140.8s | 142.8s | 140.1s | 142.9s |
| 11 | 137.2d | 137.2d | 132.7d | 132.2d | 135.5d |
| 12 | 70.8s | 69.3s | 80.5s | 80.3s | 80.4s |
| 13 | 40.4t | 41.6t | 40.3t | 39.8t | 41.4t |
| 14 | 21.7t | 21.8t | 23.1t | 22.5t | 23.6t |
| 15 | 128.0d | 128.3d | 129.9d | 129.3d | 127.8d |
| 16 | 132.5d | 132.8d | 129.8d | 129.4d | 133.2d |
| 17 | 25.9t | 25.8t | 25.4t | 25.0t | 24.6t |
| 18 | 26.7t | 26.7t | 30.0t | 29.7t | 29.7t |
| 19 | 25.6t | 26.1t | 30.1t | 29.6t | 30.1t |
| 20 | 44.6t | 53.1t | 59.3t | 58.9t | 58.8t |
| N21 | 13.5s | 13.0s | 36.1s | NO | ND |
| 22 | 49.6t | 49.7t | 50.1t | 49.7t | 49.3t |
| 23 | 32.4t | 32.2t | 32.1t | 32.8t | 33.8t |
| 24 | 39.8d | 39.2d | 46.3d | 45.9d | 43.2d |
| 25 | 45.3s | 45.3s | 38.6s | 38.0s | 39.9s |
| 26 | 75.5d | 75.9d | 67.2d | 66.8d | 68.8d |
| N27 | 57.4s | 37.4s | 73.5s | 73.2s | ND |
| 28 | 47.2t | 44.6t | 54.1t | 53.7t | 54.1t |
| 29 | 29.7t | 30.0t | 23.3t | 22.7t | 22.4t |
| 30 | 72.2d | 72.7d | 33.1t | 31.7t | 33.9t |
| 31 | 84.4d | 67.2d | 206.2s | 205.1s | 29.6t |
| 32 | 39.5t | 22.8t | 30.9t | 30.5t | 133.4d |
| 33 | 26.5t | 26.6t | 30.5t | 30.0t | 124.1d |
| 34 | 89.7s | 104.5s | 101.8s | 101.6s | 94.9s |
| 35 | 53.1t | 51.2t | 47.4t | 47.2t | 49.1t |
| 36 | 68.7t | 67.0t | 66.3t | 66.0t | 69.9t |
| 13C-NMR Data of Manzamine-related Natural Alkaloids 27, 28, 30, 35–39 in CDCl3. | ||||||||
|---|---|---|---|---|---|---|---|---|
| Atom | 27 (14) | 28 (14) | 30 (22)a | 35 (10) | 36 (10) | 37 (25) | 38 (25) | 39 (26) |
| 1 | 136.4s | 155.8s | 193.3d | 194.3d | 66.0t | 65.2t | 64.3d | |
| 2 | 42.8t | |||||||
| 3 | 137.9d | 49.1t | 23.8t | 53.6t | ||||
| 4 | 118.4d | 18.8t | 25.1t | 38.0d | ||||
| 4a | 131.5s | 118.0s | 43.3d | |||||
| 4b | 120.6s | 124.7s | ||||||
| 5 | 121.7d | 120.3d | 26.5t | 27.6t | ||||
| 6 | 120.5d | 119.9d | 132.2d | 47.4t | ||||
| 7 | 129.6d | 125.1d | 132.2d | |||||
| 8 | 111.8d | 112.2d | 26.5t | 50.8t | ||||
| 8a | 140.8s | 136.9s | 45.1s | |||||
| 9 | 25.1t | 141.8s | ||||||
| 9a | 136.5s | 125.1s | ||||||
| 10 | 184.2s | 182.9s | 23.8t | 142.6s | 144.7s | 144.3s | 143.8s | 122.6d |
| 11 | 129.7s | 126.3s | 42.8t | 157.6d | 151.7d | 130.4d | 126.8d | 54.1t |
| 12 | 70.2s | 69.9s | 71.6s | 70.6s | 26.1t | |||
| 13 | 143.6d | 144.2d | 38.9t | 40.4t | 42.1t | 41.2t | 25.6t | |
| 14 | 21.0t | 21.4t | 23.0t | 22.6t | 22.9t | |||
| 15 | 143.3d | 144.2d | 127.9d | 129.3d | 129.1d | 130.5d | 131.2d | |
| 16 | 132.5d | 131.4d | 134.6d | 128.9d | 130.9d | |||
| 17 | 25.6t | 29.2t | 27.1t | 30.0t | 21.0t | |||
| 18 | 26.7t | 29.2t | 28.6t | 29.6t | 41.6t | |||
| 19 | 25.3t | 26.2t | 26.9t | 25.5t | 56.2t | |||
| 20 | 53.5t | 37.1t | 55.0t | 59.4t | 21.1t | |||
| 21 | 27.2t | |||||||
| 22 | 49.4t | 49.5t | 51.2t | 49.9t | 25.0t | |||
| 23 | 31.6t | 31.4t | 33.6t | 30.2t | 131.5d | |||
| 24 | 34.0d | 40.2d | 39.9d | 44.1d | 132.0d | |||
| 25 | 46.4s | 42.6s | 48.7s | 43.9s | 25.6t | |||
| 26 | 76.3d | 59.8d | 78.8d | 60.3d | 37.0t | |||
| 28 | 51.4t | 53.5t | 54.5t | 52.4t | ||||
| 29 | 29.8t | 29.2t | 30.1t | 29.4t | ||||
| 30 | 25.3t | 25.0t | 26.5t | 29.7t | ||||
| 31 | 28.2t | 28.6t | 29.7t | 23.0t | ||||
| 32 | 137.1d | 131.2d | 141.5d | 130.9d | ||||
| 33 | 127.7d | 129.2d | 127.6d | 132.0d | ||||
| 34 | 55.4d | 59.3d | 55.8d | 29.7d | ||||
| 35 | 44.6t | 29.2t | 46.3t | 37.5t | ||||
| 36 | 69.2t | 65.6t | 71.3t | 65.3t | ||||
| N-Me | 35.2q | 35.2q | ||||||
| 13C−, 15N-NMR Data of Manzamine-type Alkaloids 60–66 in CDCl3d | |||||||
|---|---|---|---|---|---|---|---|
| Atom | 60 (61) | 61 (61) | 62 (59) | 63 (59) | 64 (60) | 65 (60) | 66 (60) |
| 1 | 61.3 | 69.2 | 53.5 | 60.1 | 143.1s | 143.5,s | 139.4,s |
| N2 | ND | ND | 46.3 | 40.4 | ND | ND | ND |
| 3 | 43.7 | 53.0 | 49.3 | 23.0 | 137.7d | 136.7d | 116.2d |
| 4 | 22.2 | 22.0 | 23.0 | 108.3 | 113.7d | 113.6d | 129.5d |
| 4a | 109.4 | 109.4 | 108.7 | 127.4 | 130.3s | 129.9s | 134.8s |
| 4b | 136.2 | 136.5 | 127.4 | 118.2 | 121.6s | 122.2s | 123.5s |
| 5 | 117.4 | 117.4 | 118.2 | 111.9 | 121.3d | 105.3d | 105.6d |
| 6 | 120.8 | 120.8 | 119.0 | 118.9 | 119.9d | 151.4s | 153.2s |
| 7 | 118.5 | 118.6 | 121.3 | 121.3 | 128.5d | 118.5d | 123.1d |
| 8 | 112.0 | 111.9 | 111.3 | 111.3 | 112.3d | 113.0d | 114.1d |
| 8a | 127.0 | 126.7 | 136.1 | 136.1 | 141.1s | 134.7s | 139.4s |
| N9 | ND | ND | 123.8 | ND | ND | ND | ND |
| 9a | 132.5 | 143.3 | 134.7 | 134.9 | 133.8s | 136.1s | 136.9s |
| 10 | 144.1 | 143.3 | 43.0 | 40.4 | 141.7s | 139.5s | 143.7s |
| 11 | 132.5 | 133.4 | 74.5 | 74.7 | 137.2d | 136.9d | 143.7d |
| 12 | 70.7 | 71.0 | 64.7 | 64.6 | 68.6s | 69.9s | 72.5s |
| 13 | 39.5 | 39.6 | 31.0 | 31.4 | 40.5t | 41.0t | 41.2t |
| 14 | 20.6 | 20.6 | 18.6 | 18.7 | 21.7t | 21.9t | 21.6t |
| 15 | 127.2 | 127.0 | 130.0 | 130.1 | 127.8d | 128.7d | 134.8d |
| 16 | 132.8 | 132.9 | 131.1 | 131.0 | 132.7d | 132.0d | 127.5d |
| 17 | 24.9 | 24.9 | 25.0 | 25.0 | 25.7t | 26.3t | 23.6t |
| 18 | 26.4 | 26.4 | 27.6 | 27.6 | 26.9t | 27.1t | 26.1t |
| 19 | 24.5 | 24.5 | 27.0 | 27.0 | 25.0t | 22.8t | 22.8t |
| 20 | 53.3 | 53.2 | 46.6 | 46.7 | 52.8t | 53.5t | 53.2t |
| N21 | ND | ND | 36.9 | ND | ND | ND | ND |
| 22 | 49.1 | 49.3 | 57.9 | 58.6 | 49.8t | 50.2t | 49.7t |
| 23 | 33.9 | 33.6 | 26.8 | 27.9 | 33.4t | 32.5t | 31.5t |
| 24 | 37.1 | 37.5 | 33.6 | 39.4 | 42.4d | 40.1d | 39.5d |
| 25 | 46.8 | 46.9 | 45.2 | 45.3 | 45.9s | 45.5s | 42.7s |
| 26 | 78.8 | 78.8 | 66.9 | 66.7 | 81.9d | 75.6d | 79.7d |
| N27 | ND | ND | 32.6 | ND | ND | ND | ND |
| 28 | 53.3 | 53.3 | 48.0 | 48.1 | 53.5t | 53.5t | 53.2t |
| 29 | 26.2 | 26.2 | 27.3 | 27.4 | 31.7t | 32.4t | 38.1t |
| 30 | 24.4 | 24.4 | 26.2 | 26.2 | 48.2t | 48.2t | 41.2t |
| 31 | 28.3 | 28.3 | 24.3 | 24.3 | 70.6d | 79.9d | 25.8t |
| 32 | 141.9 | 141.8 | 131.1 | 130.8 | 36.8t | 37.2t | 23.6t |
| 33 | 124.1 | 124.1 | 130.8 | 130.6 | 25.0t | 26.3t | 31.5t |
| 34 | 57.1 | 57.2 | 21.4 | 21.2 | 63.8d | 65.4d | 66.9d |
| 35 | 43.1 | 42.6 | 33.3 | 33.1 | 46.2t | 47.3t | 197.2s |
| 36 | 70.8 | 71.1 | 57.5 | 57.2 | 68.9t | 66.9t | 63.5t |
| 37 | 44.2 | 45.2 | 49.6 | ||||
| 38 | 208.6 | 207.4 | |||||
| 39 | 30.9 | 30.6 | |||||
Recorded in CD3OD.
Recorded in C6D6+CD3OD
19–22 were recorded in CD2Cl2. NR = not reported in the original literature.
Nitromethane was used as external standard for 15N-NMR, s = quaternary, p = protonated nitrogens. NO = not observed. ND = not determined.
TABLE IV.
1H-NMR Data of Manzamine-type Alkaloids in CDCl3.
| Alkaloids 1–4 | |||||
|---|---|---|---|---|---|
| Atom | 1 (9) | 2 (8) | 3 (8) | 3a (22)a | 4 (7)b |
| 1 | 4.51, s | ||||
| 3 | 8.34, d, 5.1–5.3 | 8.26, d, 5 | 8.26, d, 5 | 3.36, m 3.13, m |
2.35, 2H, m |
| 4 | 7.85, d, 5.1–5.3 | 7.84, d, 5 | 7.81, d, 5 | 2.83, m 2.88, m |
2.98, 2H, m |
| 5 | 8.08, d, 7.9 | 8.07, d, 7.8 | 8.11, d, 7.8 | 7.43, d, 7.8 | 7.49, d, 7.6 |
| 6 | 7.23, t, 7.9 | 7.46, dd, 7.8, 7.8 | 7.51, m | 7.02, t, 7.8 | 7.08, t, 7.3 |
| 7 | 7.52, t, 7.9 | 7.18, dd, 7.8, 7.8 | 7.34 m | 7.11, t, 7.8 | 7.14, t, 7.5 |
| 8 | 7.83, d, 7.9 | 7.40, d, 7.8 | 7.51 m | 7.32, d, 7.8 | 7.42, d, 7.9 |
| 10 | 3.80, dd, 9.5, 4.5 | 2.90, dd, 2H, 5.1, 5.1 | 2.22, m 2.31, m |
||
| 11 | 6.52, s | 3.52, d, 4.4 | 3.31 dd, 2H, 5.1, 5.1 | 3.04, m 2.92, m |
5.71, s |
| 13 | 2.15, m 1.75, m | 2.6–1.4, overlapped | 2.82 dd, 2H, 7.5, 7.5 | 2.99, m 2.95, m |
2.2–1.2, overlapped |
| 14 | 2.1–2.2, m | 2.6–1.4, overlapped | 2.32, 2H, m | 1.68, 2H, m | 2.2–1.2, overlapped |
| 15 | 5.57, m | 5.62, ddd, 10.8, 10.8, 5.4 | 1.75, 2H, m | 1.52, 2H, m | 5.85, m |
| 16 | 5.57, m | 5.47, ddd, 10.8, 10.8, 4.3 | 1.52, 2H, m | 2.27, 2H, m | 5.52, m |
| 17 | 2.50, m 1.60, m | 2.6–1.4, overlapped | 5.47, m | 5.43, m | 2.2–1.2, overlapped |
| 18 | 1.45, m 1.20, m | 2.6–1.4, overlapped | 5.47, m | 5.43, m | 2.2–1.2, overlapped |
| 19 | 1.81, m 1.45, m | 2.6–1.4, overlapped | 1.52, 2H, m | 2.27, 2H, m | 2.2–1.2, overlapped |
| 20 | 2.58, m 2.38, m | 2.6–1.4, overlapped | 1.75, 2H, m | 1.52, 2H, m | 2.62, 2H, m |
| 21 | 2.32, 2H, m | 1.68, 2H, m | 2.2–1.2, overlapped | ||
| 22 | 2.93, m; 1.88, m | 2.80, 2H, m | 2.82, dd, 2H, 7.5, 7.5 | 2.99, m; 2.95, m | 2.81, 2H, m |
| 23 | 2.95, m 1.78, m | 2.6–1.4, overlapped 1.30, dd, 13.2, 13.2 | 2.2–1.2, overlapped | ||
| 24 | 2.55, m | 2.6–1.4, overlapped | 2.2–1.2, overlapped | ||
| 26 | 3.72, s | 2.95, s | 3.40, s | ||
| 28 | 4.03, m 3.27, m |
3.47, m 3.09, br |
3.16, m 2.51, m |
||
| 29 | 2.60, m 2.00, m | 2.6–1.4, overlapped | 2.2–1.2, overlapped | ||
| 30 | 1.95, m 1.45, m | 2.6–1.4, overlapped | 2.2–1.2, overlapped | ||
| 31 | 2.30, m | 2.6–1.4, overlapped | 2.2–1.2, overlapped | ||
| 32 | 6.29, m | 5.30, brs | 5.64, m | ||
| 33 | 5.39, t, 7 | 5.30,brs | 5.20, dd, 9.3, 9.8 | ||
| 34 | 4.94, m | 2.6–1.4, overlapped | 4.18, t, 7.8 | ||
| 35 | 2.40, m 1.85, m | 1.14, d, 13.9 0.92, dd, 13.9, 7.9 |
2.2–1.2, overlapped | ||
| 36 | 2.88, m 2.32, m | 2.6–1.4, overlapped | 2.2–1.2, overlapped |
| Alkaloids 5–10 | ||||||
|---|---|---|---|---|---|---|
| Atom | 5 (7,9) | 6 (7,9) | 7 (11) | 8 (10) | 9 (10) | 10 (27) |
| 1 | 4.64,s | 4.65, m | ||||
| 3 | 8.42, d, 5.1 | 8.38, d, 5.3 | 8.33, d, 5.1 | 3.40–3.10 overlapped | 8.44, d5.4 | 3.6–1.0, m |
| 4 | 7.83, d, 5.1 | 7.80, d, 5.3 | 7.83, d, 5.1 | 3.40–3.10 overlapped | 7.83, d, 5.1 | 3.6–1.0, m |
| 5 | 8.10, d, 7.9 | 7.62, d, 7.7 | 7.62, brd, 7.2 | 7.48, d, 7.6 | 8.11, d, 7.8 | 7.48, d, 7.7 |
| 6 | 7.26, dt, 7.9, 1.1 | 7.14, m | 7.15, t, 7.2 | 7.14, m | 7.27, m | 7.14, t, 8.0 |
| 7 | 7.53, dt, 7.9, 1.1 | 7.14, m | 7.09, dd, 7.5, 0.9 | 7.09, m | 7.53, m | 7.09, t, 8.0 |
| 8 | 7.59, d, 7.9 | 7.33, d, 7.8 | 7.53, m | 7.31, d, 8.1 | ||
| 11 | 6.50, s | 6.65,s | 6.46,s | 5.61,s | 6.24,s | 5.64,s |
| 13 | 1.88, m 1.73, m |
2.05, m 1.87, m |
2.06, m 1.80, m |
3.40–3.10 overlapped | 3.6–1.0, m | |
| 14 | 2.2–2.1, m | 2.3–2.1, m | 2.23, m | 3.40–3.10 overlapped | 3.6–1.0, m | |
| 15 | 5.54, dt, 10.6, 7.8 | 5.63, dt, 10.3, 7.9 | 5.59, m | 5.62, m | 5.64, brt, 11.1 | 5.58, m |
| 16 | 5.45, td, 10.8, 4.6 | 5.52, ddd, 10.8, 10.8, 4.4 | 5.55, m | 5.45, m | 5.46, brtd, 11.1, 3.8 | 5.43, m |
| 17 | 2.50, m 1.64, m |
2.50, m 2.50, m 1.64, m |
2.47, m 2.47, m 1.64,m |
3.40–3.10 3.40–3.10 overlapped |
3.6–1.0,m 3.6–1.0, m |
|
| 18 | 1.42, m 1.30, m |
1.42, m 1.30, m |
1.54, m 1.23, m |
3.40–3.10 overlapped | 3.6–1.0, m | |
| 19 | 1.70, m 1.35, m |
1.75, m 1.40, m |
1.85, m 1.50, m |
3.40–3.10 overlapped | 3.6–1.0, m | |
| 20 | 2.60, m 2.38, m |
2.60, m 2.38, m |
2.62, m 2.43, m |
3.40–3.10 overlapped | 3.6–1.0, m | |
| 22 | 2.80, m 1.90, m |
2.80, m 1.90, m |
2.97, m 1.88, m |
3.40–3.10 overlapped | 3.6–1.0, m | |
| 23 | 2.03, m 1.45, m |
2.25, m 1.56, m |
2.97, m 1.86, m |
3.40–3.10 overlapped | 3.6–1.0, m | |
| 24 | 3.20, m | 3.20, m | 2.53, m | 3.40–3.10 overlapped | 3.33, brd, 11.0 | 3.6–1.0, m |
| 26 | 3.53, s | 3.70, s | 3.77, d, 6.9 | 3.71, brs | 3.85, brs | 3.74, brs |
| 28 | 3.23, m 2.65, m |
3.32, m 2.75, m |
4.03, dd, 12.06.9 3.32, q, 10.1 |
3.40–3.10 overlapped | 3.6–1.0, m | |
| 29 | 1.90, m 1.80, m |
2.05, m 1.95, m |
2.50, m 2.30, m |
3.40–3.10 overlapped | 3.6–1.0, m | |
| 30 | 2.48, m 2.23, m |
2.49, m 2.22, m |
2.03, m 1.54, m |
3.40–3.10 overlapped | 3.6–1.0, m | |
| 31 | 2.35, m | 3.40–3.10 overlapped | 3.6–1.0, m | |||
| 32 | 2.35, m 2.15, m |
2.63, m 2.33, m |
6.34, dt, 10.8, 6.8 | 5.31, m | 5.36, brt, 9.9 | 5.32, m |
| 33 | 1.95, m 1.60, m |
2.10, m 1.70, m |
5.42, t, 9.8 | 5.20, m | 5.25, brt, 10.7 | 5.19, m |
| 34 | 2.85, m | 2.98, m | 4.98, brq, 6.9 | 3.40–3.10 overlapped | 3.6–1.0, m | |
| 35 | 1.60, m 1.49, m |
1.65, m 1.57, m |
2.39, m 1.92, m |
3.40–3.10 overlapped | 3.6–1.0, m | |
| 36 | 2.50, m 2.35, m |
2.49, m 2.30, m |
2.92, m 2.40, m |
3.40–3.10 overlapped | 3.6–1.0, m |
| Alkaloids 11–15 | |||||
|---|---|---|---|---|---|
| Atom | 11 (17)a | 12 (14) | 13 (14) | 14 (12) | 15 (13) |
| 1 | 4.80,brs | ||||
| 3 | 8.35, d, 5.2 | 8.31, d, 5.1 | 8.13, d, 4.8 | 3.96, m 3.84, m |
3.27, brd, 11.0 2.94, dt, 11.0 |
| 4 | 8.00, d, 5.2 | 7.59, d, 5.1 | 7.48, d, 4.8 | 2.82, 2H, m | 2.81, m 2.62, brd, 13.8 |
| 5 | 8.19, d, 7.7 | 7.49, d, 2.5 | 7.39, d, 2.4 | 7.60, d, 7.9 | 6.96, d, 8.0 |
| 6 | 7.30, dd, 7.7, 7.1 | 7.13, t, 7.9 | 6.91, dt, 8.1, 4.0 | ||
| 7 | 7.59, dd, 7.1, 8.2 | 7.13, dd, 2.5, 8.6 | 7.08, dd, 2.4, 7.5 | 7.28, t, 7.9 | 6.63, d, 8.1 |
| 8 | 7.72, d, 8.2 | 7.26, d, 8.6 | 7.54, d, 7.5 | 7.41, d, 7.9 | |
| 11 | 6.54, s | 6.45, s | 6.51, s | 6.32, s | 5.84, s |
| 13 | 2.95, m 2.73, dd, 10.0, 14.4 |
2.13, m 1.68, m |
2.02, 2H, m | 1.93, m 1.67, m |
2.00, m 1.52, m |
| 14 | 5.73, ddd, 14.8, 10.0, 4.3 | 2.36, m 2.13, m |
2.26, 2H, m | 2.35, m 2.13, m |
2.13, 2H, m |
| 15 | 5.65, dd 14.8, 8.3 |
5.64, m | 5.51, m | 5.63, m | 5.55, m |
| 16 | 4.06, brt, 7.5 | 5.53, m | 5.51, m | 5.53, dt, 10.6, 4.7 | 5.53, m |
| 17 | 1.72, m 1.52, m |
2.58, m 1.70, m |
2.47, m 1.57, m |
2.53, m 1.70, m |
2.38, m 1.42, m |
| 18 | 1.68, m 1.45, m |
1.44, 2H, m | 1.42, m 1.24–1.17, m |
1.70, m 1.33, m |
1.38, m 1.13, m |
| 19 | 1.66, m 1.45, m |
1.69, m 1.43, m |
1.83, m 1.42, m |
1.73, m 1.42, m |
1.85, m 1.42, m |
| 20 | 2.60, tt, 4.4, 8.6 2.30, m | 2.67, m 2.45, m |
2.55, m 2.42, m |
2.60, dt, 13.2, 5.2 2.44, m | 2.27, m 2.45, dd, 12.0, 5.1 |
| 22 | 2.81, m 2.18, m |
2.71, m 1.95, m |
2.86, m 1.86, m |
2.76, brd, 10.8 1.95, m | 2.77, m 1.67, m |
| 23 | 1.98, m 1.72, m |
1.93, m 1.51, m |
2.86, m 1.74, m |
1.97, m 1.45, m |
2.16, m 1.33, m |
| 24 | 2.93, m | 3.00, dd, 12.0, 6.0 | 2.55, m | 2.00, m | 1.97, m |
| 26 | 4.20, s | 3.62, s | 3.67, s | 3.44, s | 3.55, s |
| 28 | 2.95, 2H, m | 3.34, m 2.88, dd, 11.1, 10.3 | 3.98, m 3.21, m |
3.17, 2H, m | 3.82, m 3.16, t,11.4 |
| 29 | 1.99, m 1.67, m |
1.70, m 1.58, m |
2.60, m 1.24–1.17, m |
1.96, m 1.62, m |
2.45, m 1.84, m |
| 30 | 1.95, m 1.45, m |
1.93, m 1.44, m |
1.95, m 1.42, m |
1.87, m 1.34, m |
1.90, m 1.42, m |
| 31 | 2.34, m 2.22, m | 4.55, m | 2.26, m | 2.35, m 2.13, m |
2.32, m (2H) |
| 32 | 5.98, dt, 10.9, 7.1 | 2.40, m 2.13, m |
6.20, m | 5.95, m | 6.14, m |
| 33 | 5.36, brt, 8.8 | 1.44, m | 5.30, m | 5.26, brd, 10.6 | 5.18, t, 9.9 |
| 34 | 4.32, brt, 8.3 | 4.89, m | 4.19, m | 4.70, t, 8.7 | |
| 35 | 2.41, dd, 8.6, 13.2 1.68, m | 2.40, d-like, 12.7 2.34, d-like, 12.7 | 2.45, m 1.83, m | 2.14, m 1.69, m | 1.71, m 1.42, m |
| 36 | 2.95, d, 11.6 1.95, d, 11.6 |
3.14, d, 12 2.27, d, 12 |
2.86, m 2.40, m |
2.78, d, 11.6 2.27, d, 11.6 |
2.78, m 2.10, m |
| Alkaloids 16–20 | |||||
| Atom | 16 (13) | 17 (17) | 18 (17) | 19 (16)c | 20 (16)c |
|
| |||||
| 1 | 3.92, s | ||||
| 3 | 3.00, dd, 11.0, 4.0 2.44, dd, 12.0, 3.0 | 3.93, m 3.89, m |
3.45, m 3.35, m |
8.42, d, 5.1 | 8.06, d, 6.7 |
| 4 | 2.85, t, 12.0 2.65, brd, 15.0 | 2.81, m 2.79, m |
2.88, m 2.86, m |
7.87, d, 5.2 | 7.77, d, 6.7 |
| 5 | 6.96, d, 8.0 | 6.97, brs | 7.59, d, 7.7 | 8.16, d, 7.8 | 8.04, d, 8.0 |
| 6 | 6.91, dt, 8.0, 4.0 | 7.14, dd, 7.7, 7.1 | 7.33, m | 7.32, t, 8.0 | |
| 7 | 6.64, d, 8.0 | 6.86, brd, 8.2 | 7.26, dd, 7.1, 8.2 | 7.57, m | 7.51, m |
| 8 | 7.29, d, 8.2 | 7.40, d, 8.2 | 7.57, m | 7.54, m | |
| 11 | 5.91, s | 6.36, s | 6.15, s | 6.41, s | 6.07, s |
| 13 | 1.92, m 1.75, m |
1.92, m 1.72, m |
2.05, m 1.48, m |
2.10, m 1.69, m |
2.15, m 1.85, m |
| 14 | 2.17, 2H, m | 1.73, m 1.43, m |
2.27, m 1.98, m |
2.40, m 2.14, m |
2.40, m 2.10, m |
| 15 | 5.56, dt, 8.0, 3.0 | 5.63, ddd, 7.3, 8.3, 10.8 | 5.32, ddd, 7.0, 8.3, 10.6 | 5.68, q, 10.0 | 5.72, m |
| 16 | 5.54, dt, 11.0, 4.0 | 5.54, dt, 10.8, 7.3 | 5.28, dt, 10.6, 7.6 | 5.58, dt, 10.8, 5.0 | 5.60, dt, 7.9, 4.7 |
| 17 | 2.36, m 1.55, m |
1.77, m 1.43, m |
2.93, m 2.90, m |
2.64, m 1.86–1.76, m |
2.55, m 1.75, m |
| 18 | 1.45, m 1.14, m |
1.91, m 1.43, m |
1.90, m 1.55, m |
1.51, 2H, m | 1.41, m 1.30, m |
| 19 | 1.72, m 1.40, m |
1.73, m 1.43, m |
1.70, m 1.20, m |
1.47, 2H, m | 1.81, m 1.41, m |
| 20 | 2.48, dd, 13.0, 5.0 2.26, m | 2.61, m 2.43, m |
2.91, m 2.82, m |
2.78, m 2.54, m |
2.80, m 2.38, m |
| 22 | 2.75, m 1.61, brd, 11.0 |
2.78, m 1.95, m |
2.95, m 1.94, m |
2.80, m 2.01, m |
2.98, m 2.50, m |
| 23 | 2.16, m 1.43, m |
2.01, m 1.48, m |
2.17, m 1.72, m |
1.58, 2H, m | 3.15, m 1.75, m |
| 24 | 1.86, m | 2.78, m | 2.45, m | 3.01, dt, 9.3, 6.4 | 3.00, dd, 11.5, 7.3 |
| 26 | 3.56, d, 7.5 | 3.47, s | 3.78, s | 3.62, s | 3.72, s |
| 28 | 3.88, m 3.16, q, 10.0 |
3.19, m 3.08, s |
2.62, m 2.35, m |
3.36, m 2.89, m |
3.96, m 3.20, m |
| 29 | 2.44, m 1.91, m |
1.93, m 1.27, m |
1.78, m 1.70, m |
1.86–1.76, 2H, m | 2.84, m 1.98, m |
| 30 | 1.97, m 1.42, m |
2.29, m 2.16, m |
1.78, m 1.28, m |
1.86–1.76, m 1.66, m |
1.91, m 1.38, m |
| 31 | 2.34, m 2.20, m |
1.74, m 1.48, m |
1.74, m 1.48, m |
4.54, brd, 7.9 | 2.30, 2H, m |
| 32 | 6.17, dt, 12.0, 6.0 | 5.97, t, 10.4, 7.6 | 5.61, dt, 10.9, 7.1 | 3.20, d, 11.9 2.05, m |
5.95, m |
| 33 | 5.18, t, 10.0 | 5.25, brt, 9.5 | 5.36, brt, 8.8 | 2.25–2.16, m 1.63, m |
5.35, m |
| 34 | 4.73, q, 8.1 | 4.30, m | 2.72, m 2.19, m |
4.29, m | |
| 35 | 1.69, dd, 14.0, 8.0 1.30, brd, 14.0 | 2.78, m 1.72, m | 2.18, m 1.45, m | 2.33, d, 1 3.9 1.94, d, 13.9 |
2.43, m 1.68, m |
| 36 | 2.74, brd, 12.1 2.09, brd, 12.0 |
2.80, m 2.37, m |
3.30, d, 12.1 2.08, d, 12.1 |
2.27, d, 11.9 | 2.80, d, 11.6 2.33, d, 11.3 |
| N-Me | 2.26, s | ||||
| Alkaloids 21, 22, 25 and 26 | ||||||
|---|---|---|---|---|---|---|
| Atom | 21 (16)c | 22 (16)c | 25 (15) | 25 (15) | 26 (18) | 26 (18) |
| unit A | unit B | unit A | unit B | |||
| 1 | 2.43, m | |||||
| 3 | 8.08, d, 6.6 | 4.36, m 4.21, m |
8.17, d, 5.0 | 2.57, m 2.34, m |
8.45, d, 5.1 | 8.46, d, 5.1 |
| 4 | 7.79, d, 6.2 | 3.22, 2H, m | 7.62, d, 5.0 | 2.13, m 1.89, m |
7.83, d, 5.3 | 7.83, d, 5.3 |
| 5 | 8.04, d, 7.5 | 7.51, d, 7.8 | 7.27, m | 6.84, brd, 8.0 | 8.10, d, 7.9 | 8.10, d, 7.9 |
| 6 | 7.32, m | 7.15, d, 8.0 | 6.51, brt, 8.0 | 6.78, brt, 8.0 | 7.29, dd, 8.0, 7.9 | 7.29, dd, 8.0, 7.9 |
| 7 | 7.51, m | 7.21, dt, 7.6, 1.1 | 7.11, m | 6.97, t, 8.0 | 7.55, dd, 8.3, 8.0 | 7.55, dd, 8.3, 8.0 |
| 8 | 7.51, m | 7.41, d, 8.0 | 7.17, brd, 8.0 | 7.14, d, 8.0 | 7.51, d, 8.4 | 7.51, d, 8.4 |
| 10 | 3.50, m | 1.22, m | ||||
| 11 | 6.06, s | 5.97, s | 3.44, d, 4.2 | 3.07, brs | 6.37, s | 6.41, s |
| 13 | 2.08, m 1.59, br |
2.02, m 1.75, m |
2.48, m 1.30, m |
2.33, m 1.11, dt, 14.5, 5.0 |
2.11, 2H, m | 2.13, m 1.70, m |
| 14 | 2.18, 2H, br | 2.41, m 2.14, m |
2.49, m 2.16, m |
2.51, m 1.68, m |
2.37, m 2.09, m |
2.37, m 2.11, m |
| 15 | 5.66, dt, 10.8, 4.4 | 5.69, m | 5.24, m | 5.44, dt, 11.0, 6.5 | 5.64, m | 5.64, m |
| 16 | 5.47, d, 10.9 | 5.59, dt, 10.8, 4.7 | 5.34, brt, 11.0 | 5.25, m | 5.58, m | 5.58, m |
| 17 | 2.52, m 1.85, m |
2.49, m 1.91, m |
2.64, m 1.78, m |
1.78, m 1.59, m |
1.77, m 1.55, m |
|
| 18 | 1.70, m 1.34, m |
1.57, m 1.51, m |
1.67, m 1.08, m |
1.44, 2H, m | 1.44, 2H, m | |
| 19 | 1.20, brd, 10.1 | 1.70, m 1.34, m |
1.73, m 1.53, m |
1.24, m 1.24, m |
1.76, m 1.39, m |
1.77, m 1.41, m |
| 20 | 2.59, brd, 11.6 2.38, br | 2.63, dt, 11.7, 4.8 2.42, m | 2.75, dt, 14.0, 4.5 3.47, m | 2.33, m 1.85, m |
2.64, m 2.49, m |
2.46, m 1.96, m |
| 22 | 2.88, br 1.81, br | 2.79, m 1.95, m |
5.47, brs | 2.88, m | 2.75, m 1.98, m |
2.76, m 1.99, m |
| 23 | 1.97, m 1.60, m |
1.29, m 0.96, m |
1.88, mm 1.54, m |
1.89, m 1.53, m |
||
| 24 | 2.90, brs | 2.98, m | 2.81, d, 8.5 | 1.36, m | 3.06, m | 3.05, m |
| 26 | 3.77, brs | 3.45, s | 3.13, s | 2.91, brs | 3.85, s | 3.66, s |
| 28 | 2.98, m 2.88, m |
3.19, 2H, m | 3.41, m 2.47, m |
3.15, m 2.62, m |
3.15, 2H, m | 3.57, m 3.18, m |
| 29 | 1.70, m 1.55, m |
1.95, m 1.62, m |
1.82, m 1.57, m |
2.05, m 1.56, m |
2.27, m 2.12, m |
|
| 30 | 1.50, m | 1.85, m 1.34, m |
1.82, m 1.67, m |
1.63, m 1.54, m |
3.76, brd, 6.1 | 4.14, d, 7.5 |
| 31 | 1.75, m 1.59, br |
2.35, m 2.15, m |
2.50, m 2.07, m |
2.26, m 1.95, m |
4.41, d, 8.7 | 3.69, brd, 6.1 |
| 32 | 5.35, m | 5.94, m | 5.53, td, 11.0, 3.5 | 5.63, m | 2.07, m 1.55, m |
2.12, m 1.52, m |
| 33 | 5.50, m | 5.30, t, 9.5 | 5.69, td, 11.0, 4.0 | 5.65, m | 1.75, m 1.45, m |
1.78, m 1.40, m |
| 34 | 1.48, m | 4.21, m | 2.40, m 1.94, m |
2.24, m 1.92, m |
||
| 35 | 2.47,br 2.19,br |
2.95, m 1.65, m |
2.34, m 0.91, m |
1.74, m 1.26, m |
2.65, m 2.45, m |
2.36, d, 13.1 2.05, d, 12.9 |
| 36 | 2.80, d, 11.6 2.30, d, 11.6 | 2.98, d, 10.5 2.69, d, 10.5 | 2.86, d, 9.5 2.07, d, 9.5 | 3.42, dd, 11.3, 2.0 2.30, m |
3.10, dd, 11.8, 2.1 2.28, d, 11.9 |
|
| Alkaloids 27, 28, 30 and 39 | ||||
|---|---|---|---|---|
| Atom | 27 (14) | 28 (14) | 30 (22)d | 39 (26) |
| 1 | 3.01,s | |||
| 2 | 3.08, 2H, t, 7.6 | |||
| 3 | 8.55, d, 5.0 | 4.16, dd, 9.0, 9.0 | 1.76, 2H, m | 2.86, dd, 8.5, 1.5 2.91, dd, 20.7, 9.7 1.64, dd, 9.0, 2.3 |
| 4 | 8.09, d, 5.0 | 2.97, dd, 9.0, 9.0 | 1.58, 2H, m | 2.22, m |
| 4a | 0.93, ddd, 11.6, 5.6, 1.9 | |||
| 5 | 8.12, d, 8.2 | 7.60, d, 7.9 | 2.30, 2H, m | 1.36, m 1.17, ddd, 13.0, 8.7, 4.4 |
| 6 | 7.30, dd, 8.2, 6.2 | 7.13, dd, 7.9, 7.0 | 5.46, m | 2.75, m 2.63, dt, 12.3, 3.6 |
| 7 | 7.55, dd, 7.3, 6.2 | 7.29, dd, 7.3, 7.0 | 5.46, m | |
| 8 | 7.57, d, 7.3 | 7.40, d, 7.3 | 2.30, 2H, m | 2.23, d, 12.3 2.08, d, 10.7 |
| 9 | 1.58, 2H, m | |||
| 10 | 1.76, 2H, m | 5.81, brd, 6.3 | ||
| 11 | 3.08, 2H, t, 7.6 | 2.23, m | ||
| 12 | 1.45, m 1.24, m | |||
| 13 | 8.93, s | 8.37, s | 1.58, m 1.46, m | |
| 14 | 2.35, m 1.57, m | |||
| 15 | 7.66, s | 7.63, s | 5.64, ddd, 13.6, 10.1, 5.2 | |
| 16 | 5.69, ddd, 13.6, 10.1, 6.3 | |||
| 17 | 2.27, m 1.78, m | |||
| 18 | 1.88, dt, 12.3, 7.6 1.61, m | |||
| 19 | 3.07, m 2.24, m | |||
| 20 | 1.55, m 1.34, m | |||
| 21 | 1.48, m 1.32, m | |||
| 22 | 2.14, m 1.96, brd, 15.2 | |||
| 23 | 5.24, brd, 10.8 | |||
| 24 | 5.36, brd, 10.8 | |||
| 25 | 2.29, m 2.12, m | |||
| 26 | 2.33, m 2.25, m | |||
| Alkaloids 35–38 | ||||
|---|---|---|---|---|
| Atom | 35 (10) | 36 (10) | 37 (25)a | 38 (25) |
| 1 | 9.45, s | 9.50, s | 4.00, s (2H) | 3.88, s (2H) |
| 11 | 6.75, s | 6.53, s | 5.68, s | 5.70, s |
| 13 | 1.77, m 1.61, m |
3.10–1.0 (overlapped) | 1.80, m 1.61, m | 2.18, brd, 10.8 1.64, m |
| 14 | 2.24, m 2.11, m |
3.10–1.0 (overlapped) | 2.26, m 1.96, m |
2.15, m 1.91, m |
| 15 | 5.56, m | 5.58, m | 5.49, m | 5.42, m |
| 16 | 5.50, dddd 10.7, 10.7, 10.7, 4.6 | 5.44, m | 5.52, m | 5.29, m |
| 17 | 2.43, m 1.63, m | 3.10–1.0 (overlapped) | 2.52, m 1.63, m |
2.98, brt, 12.7 1.74, m |
| 18 | 1.41, m 1.25, m |
3.10–1.0 (overlapped) | 1.42, m 1.22, dt, 9.2, 4.3 |
1.65, m 1.19, m |
| 19 | 1.71, m 1.38, m |
3.10–1.0 (overlapped) | 1.77, m 1.46, m |
1.36, m 1.34, m |
| 20 | 2.57, m 2.39, m |
3.10–1.0 (overlapped) | 2.67, dt, 12.0, 5.2 2.34, m | 2.49, dt, 12.1, 1.3 2.25, ddd, 16.9, 11.6, 4.6 |
| 22 | 2.77, m 1.86, m |
3.10–1.0 (overlapped) | 2.87, dd, 11.3, 5.5 1.83, m | 2.77, dd, 9.4, 4.7 1.74, m |
| 23 | 1.78, m 1.22, m |
3.10–1.0 (overlapped) | 1.90, m 1.41, m |
1.68, m 1.59, m |
| 24 | 2.58, dd, 12.2, 6.8 | 3.10–1.0 (overlapped) | 2.05, dd, 12.0, 6.8 | 1.71, m |
| 26 | 3.44, s | 3.71, s | 3.59, s | 3.66, s |
| 28 | 3.38, m 3.04, m |
3.25, m 3.10–1.0 (overlapped) |
3.56, dt, 12.7, 7.0 3.32, m |
3.00, m 2.90, m |
| 29 | 1.93, m 1.73, m |
3.10–1.0 (overlapped) | 1.98, m 1.58, m |
1.61, m 1.47, m |
| 30 | 1.89, m 1.35, m |
3.10–1.0 (overlapped) | 1.77, m 1.45, m |
1.65, m 1.34, m |
| 31 | 2.29, m 2.14, m |
3.10–1.0 (overlapped) | 2.36, m 2.24, m |
2.55, m 1.94, m |
| 32 | 6.03, dddd 11.0, 7.1, 7.1, 1.5 | 5.27, m | 6.18, dt, 10.7, 7.1 | 5.32, brt, 10.1 |
| 33 | 5.26, ddd 10.5, 10.3, 1.2 | 5.19, m | 5.40, dd, 10.7, 9.4 | 5.54, brt, 10.8 |
| 34 | 4.36, brt, 8.1 | 3.10–1.0 (overlapped) | 4.52, brt, 7.9 | 1.99, m 1.96, m |
| 35 | 1.86, m 1.67, m |
3.10–1.0 (overlapped) | 2.11, dd, 13.6, 8.0 1.71, m | 2.23, m 1.08, brt, 12.7 |
| 36 | 2.81, d, 11.2 2.29, d, 11.2 |
3.10–1.0 (overlapped) | 2.94, brd,1 1.5 2.29, d, 11.5 | 3.30, brd, 11.9 1.84, d, 11.9 |
| Alkaloids 57–59 and 61 | ||||
| Atom | 57 (58) | 58 (58) | 59 (58) | 61 (61) |
|
| ||||
| 1 | 3.97, s | |||
| 3 | 8.41, d, 5.2 | 8.39, d, 5.6 | 8.46, d, 5.0 | 2.49, m 3.03, ddd, 12.9, 4.7, 1.4 |
| 4 | 7.84, d, 5.2 | 7.82, d, 5.6 | 7.84, d, 5.0 | 2.73, ddd, 14.9, 12.9, 1.5 2.89, m |
| 5 | 8.08, d, 7.8 | 7.63, d, 7.7 | 8.12, d, 7.7 | 7.43, d, 7.6 |
| 6 | 7.26, t, 8.0 | 7.13, dd, 7.8, 7.7 | 7.29, t, 7.5 | 7.09, d, 7.6 |
| 7 | 7.51, t, 7.4 | 7.02, d, 7.8 | 7.53, t, 7.6 | 7.02, dt, 7.6, 1.1 |
| 8 | 7.55, d, 8.0 | – | 7.49,d, 7.6 | 7.59,d, 7.6 |
| N9 | 8.85, s | 9.12, s | ||
| 11 | 6.24, s | 6.33, s | 6.58,s | 5.94,s |
| 13 | 2.35, m 1.66, m |
2.27, m 2.09, m |
2.25, m 2.12, m |
1.75, m, 1.92, m |
| 14 | 2.85, m, 2.45, m | 2.24, m, 1.83, m | 2.31, m, 2.01, m | 2.17, m |
| 15 | 5.34, brs | 5.33, brs | 5.65, m | 5.59, dt, 3.1, 7.4 |
| 16 | 5.29, brs | 5.30, brs | 5.57, m | 5.54, dt, 10.6, 4.0 |
| 17 | 1.86, m, 1.73, m | 1.61, m, 1.49, m | 1.65, m, 1.53, m | 1.59, m, 2.41, m |
| 18 | 1.52, m, 1.24, m | 1.81, m, 1.63, m | 1.64, m, 1.73, m | 1.15, m, 1.45, m |
| 19 | 1.46, m 1.38, m | 1.79, m 160, m | 1.81, m 1.67, m | 1.40, m, 1.72, m |
| 20 | 2.71, m 2.28, m | 2.67, m 2.36, m | 2.65, m 2.34, m | 2.26, m 2.53, dd, 12.2, 5.7 |
| 22 | 3.02, m 2.04, m | 3.03, brd, 9.3 2.07, m | 3.05, m 2.15, m | 2.76, m |
| 23 | 2.59, m 2.67, m | 2.59, m 2.20, m | 2.46, m 2.31, m | 1.43, m, 2.16, m |
| 24 | 2.52, dd, 11.8, 5.5 | 2.57, dd, 12.0, 5.6 | 2.47, dd, 12.0, 5.4 | 1.84, dd, 7.0, 11.9 |
| 26 | 4.36, s | 4.39, s | 4.38, s | 3.53, brs |
| 28 | 3.38, dd, 12.5, 11.3 2.84, dd, 12.5, 4.4 | 3.37, dd, 12.8, 11.9 2.84, dd, 12.8, 4.7 | 3.35, dd,1 2.7, 11.6, 2.83, dd, 12.8, 4.6 | 3.12, dd, 9.6, 11.4 3.94, m |
| 29 | 1.72, m 1.76, m | 1.57, m 1.48, m | 1.63, m 1.46, m | 1.91, m, 2.48, m |
| 30 | 1.64, m 1.78, m | 1.83, 2H, m | 1.58, m 1.82, m | 1.42, m, 1.97, m |
| 31 | – | – | 2.32, m 1.86, m | 2.34, m |
| 32 | 3.20, m 2.75, m | 1.79, m 1.51, m | 5.37, brs | 6.18, dt, 9.9, 7.1 |
| 33 | 2.25, m, 2.15, m | 2.53, m, 1.57, m | 5.38, brs | 5.21, dd, 9.9, 9.7 |
| 34 | 4.68, m | |||
| 35 | 2.27, d, 12.5 2.34, d, 12.5 | 2.34, d, 12.4 2.24, d, 12.4 | 2.35, d, 12.5 2.21, d, 12.3 | 1.26, dd, 12.5, 1.3 1.69, m |
| 36 | 3.15, d, 11.0 2.24, d, 11.0 | 3.16, d, 11.1 2.30, d, 11.1 | 3.14, d, 11.2 2.26, d, 11.1 | 2.09, d, 11.3 2.76, d, 12.3 |
| 37 | 2.30, s | |||
| Alkaloids 62–66 | |||||
|---|---|---|---|---|---|
| Atom | 62 (59) | 63 (59) | 64 (60) | 65 (60) | 66 (60) |
| 1 | 4.16, d, 9.6 | 4.13, d, 9.3 | |||
| 3 | 3.25, m, 2.84, m | 2.96, m 2.90, m |
8.30, d, 5.2 | 8.22, d, 5.3 | 8.44, d, 5.3 |
| 4 | 2.80, 2H, m | 2.77, 2h, m | 7.93, d, 5.2 | 7.84, d, 5.3 | 8.35, d, 5.3 |
| 5 | 7.46, d, 7.6 | 7.45, d, 7.6 | 8.14, d, 8.14 | 7.49, d, 2.3 | 7.63, d, 2.3 |
| 6 | 7.04, t, 7.6 | 7.03, t, 7.6 | 7.24, t, 8.0 | ||
| 7 | 7.11, t, 7.6 | 7.10, t, 7.6 | 7.53, t, 8.0 | 7.09, dd, 2.3, 8.8 | 7.33, dd, 2.3, 8.8 |
| 8 | 7.31, d, 8.0 | 7.30, d, 7.6 | 7.66, d, 8.5 | 7.52, d, 8.8 | 7.68, d, 8.8 |
| N9 | 9.17, s | 9.04, s | |||
| 10 | 1.58, m | 1.63, m | |||
| 11 | 3.52, m | 3.39, m | 6.44, s | 6.29, s | 6.65, s |
| 13 | 2.48, m, 1.37, m | 2.45, m 1.20, m |
1.83, m, 1.88, m | 1.81, m, 1.89, m | 1.85, m, 1.99, m |
| 14 | 2.63, m, 1.80, m | 2.54, m 1.73, m |
2.15, m, 2.18, m | 2.11, m, 2.20, m | 2.11–2.20, m |
| 15 | 5.48, m | 5.46, m | 5.63, q, 12.08 | 5.65, dt, 4.7, 11.38 | 5.62, q, 9.8 |
| 16 | 5.29, m | 5.30, m | 5.52, dt, 4.4, 10.8 | 5.52, q, 9.09 | 5.66, q, 10.1 |
| 17 | 2.70, m, 1.81, m | 2.68, m, 1.82, m | 1.64, m, 2.49, m | 1.66, m, 2.48 m | 1.65, m, 2.51, m |
| 18 | 1.15, m, 1.77, m | 1.16, m 1.76, m |
1.35, m, 1.42, m | 1.35, m, 1.41, m | 1.32, m, 1.40, m |
| 19 | 1.73, m, 1 .41, m | 1.37, m 1.74, m |
1.36, m, 1.69, m | 1.34, m, 1.71, m | 1.42, m, 1.73, m |
| 20 | 2.54, m, 2.12, m | 2.52, m 2.08, m |
2.39, m, 2.60, m | 2.37, m, 2.62, m | 2.36, m, 2.59, m |
| 22 | 3.75, m | 3.54, m | 1.82, m, 2.70, m | 1.83, m, 2.71, m | 1.93, m, 2.81, m |
| 23 | 1.43, m 1.78, m |
1.49, m 1.74, m |
1.48, m, 1.95, m | 1.46, m, 1.85, m | 1.55, m, 2.75, m |
| 24 | 2.11, m | 1.98, m | 3.15, dt, 6.4, 9.3 | 3.15, dd, 7.3, 11.2 | 3.05, dd, 7.1, 10.8 |
| 26 | 2.93, s | 2.95, s | 3.79, s | 3.64, s | 5.01, s |
| 28 | 3.07, m 2.70, m |
3.10, m 2.69, m |
2.66, m, 3.56, m | 2.66, m, 3.56, m | |
| 29 | 1.74, 2H, m | 1.65, 2H, m | 1.83, m, 1.90, m | 1.83, m, 1.90, m | 1.83, m, 2.10, m |
| 30 | 1.47, m 1.86, m |
1.46, m 1.72, m |
2.65, m, 2.98, m | 2.65, m, 2.98, m | 2.45, m, 2.87, m |
| 31 | 2.22, m 2.03, m |
2.25, m 1.98, m |
4.05, m | 4.05, m | 2.05, m |
| 32 | 5.53, m | 5.55, m | 1.80, m, 2.21, m | 1.80, m, 2.21, m | 1.98, m, 2.31, m |
| 33 | 5.53, m | 5.55, m | 1.60, m, 1.79, m | 1.60, m, 1.79, m | 1.70, m, 2.10, m |
| 34 | 2.05, m 2.24, m |
2.26, m 2.00, m |
3.05, m | 3.2, m | 4.13, m |
| 35 | 1.50, m 1.60, m |
1.63, m 1.44, m |
1.35, m, 1.42, m | 1.34, m, 1.42, m | |
| 36 | 2.89, d, 10.0 2.39, d, 10.0 | 2.93, d, 9.6 2.28, d, 9.6 | 2.30, m, 2.65, m | 2.31, m, 2.65, m | 2.33, m, 2.55, m |
| 37 | 3.06, m 2.63, m |
2.54, dd, 15.8, 7.6 2.89, m | |||
| 39 | 2.17, 3H, s | 2.27, s | |||
Recorded in CD3OD
Recorded in C6D6+CD3OD
Recorded in CD2Cl2
Recorded in CD3OH
III. Biogenesis and Biosynthesis
A. BIOGENETIC PATHWAYS
Since the first representative, manzamine A (1) with a fused and bridged pentacyclic ring system joined to a β-carboline moiety, was isolated in 1986, the manzamines have been regarded as an intriguing group of marine alkaloids (2–4), which have provoked a great interest in their unprecedented biosynthetic pathway. In 1992 Baldwin et al. (5) proposed a biogenetic pathway with an intramolecular Diels-Alder reaction for manzamines A (1) and B (2). The proposal by Tsuda et al. suggested that the macrocyclic bisdihydropyridine maybe derived from ammonia, a C3 unit, and a C10 unit. The bisdihydropyridine could then be converted through a Diels-Alder-type [4+2] intramolecular cycloaddition into a pentacyclic intermediate, which in turn could provide manzamines A and B via a tetracyclic intermediate (Scheme 1) (5). 8-hydroxy-1,2,3,4-tetrahydromanzamine A (15) and its N-methylated derivative 8-hydroxy-2-N-methyl-1,2,3,4-tetrahydromanzamine A (16) are of further interest as it provides yet another intermediate in the biosynthetic path from acyclic precursors to the fully aromatized manzamines (13).
The isolation of keramaphidin C and keramamine C, together with manzamine C (3) and tryptamine, appears to substantiate, in part, the biogenetic path of manzamine C (3), which may be derived from the coupling of keramaphidin C with tryptamine and a C3 unit via keramamine C. On the other hand, keramaphidin C is probably generated from a C10 unit and ammonia (Scheme 2) (2). The biogenesis has not yet been investigated experimentally.
Scheme 2.
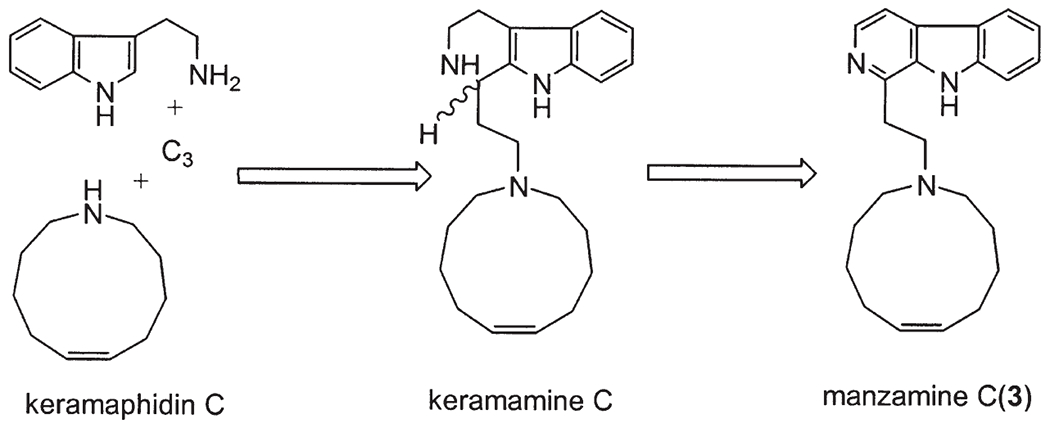
Biogenetic Pathway for Manzamine C (3) Proposed by Tsuda et al. (2).
The following proposed schemes have been published for the rational biogenesis of a number of the manzamine and manzamine-related alkaloids and are shown in Schemes 3–6.
Scheme 3.
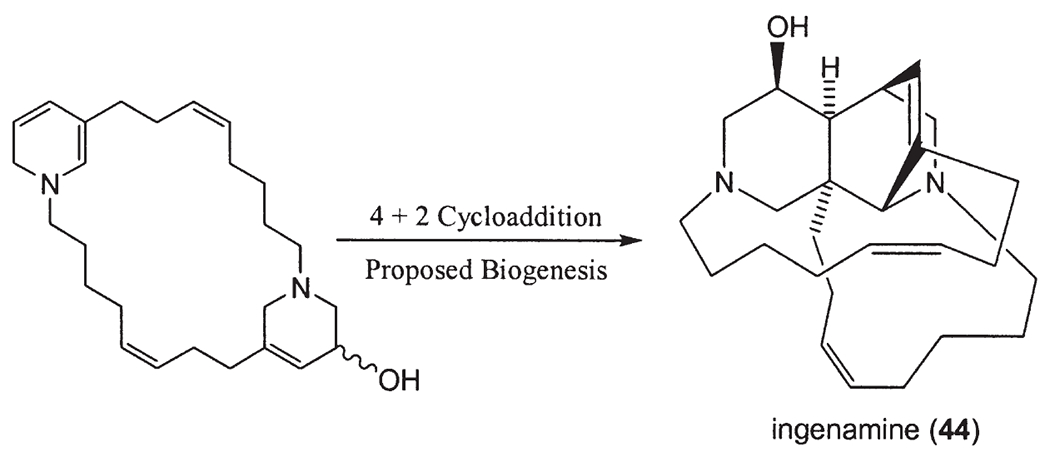
Biogenesis of ingenamine (44), proposed by Kong et al. (31).
Scheme 6.
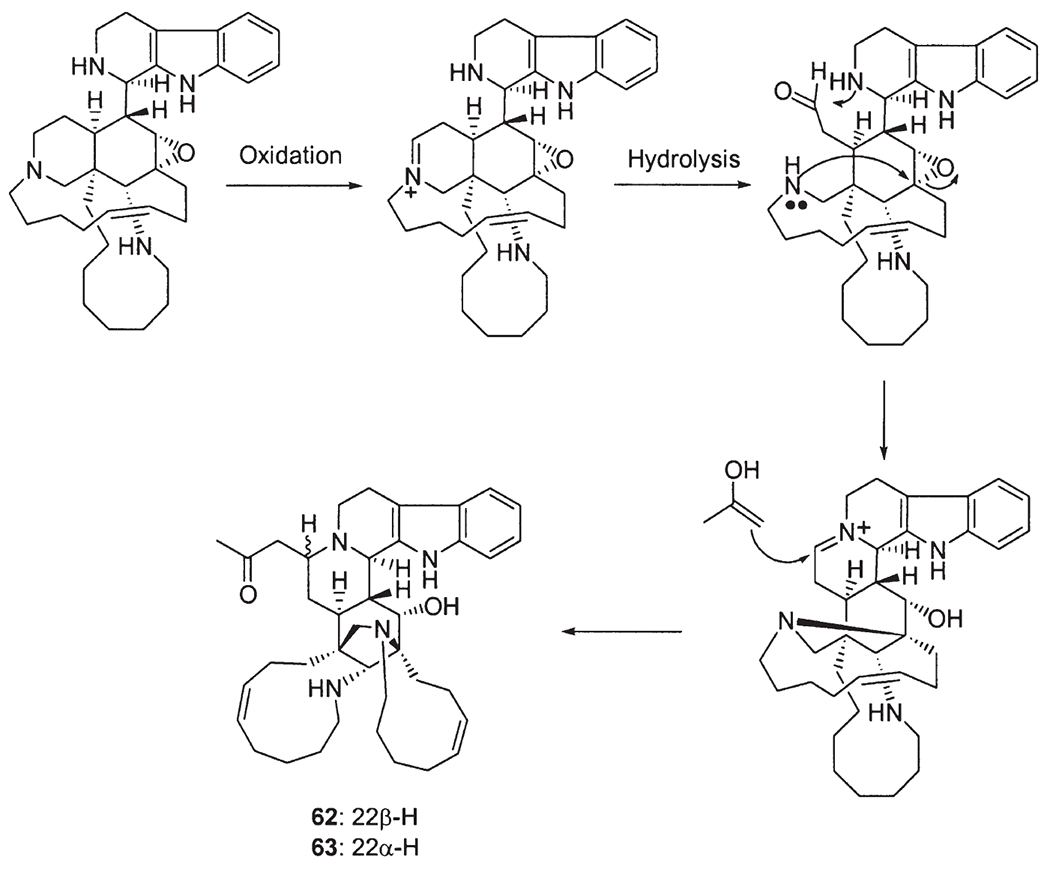
Biogenetic pathway of manadomanzamine A (62) and B (63) (59).
B. INTERMEDIATES
As shown in the Scheme 1, Baldwin et al. proposed a biogenetic pathway for manzamines A–C, where the manzamines were presumed to be biosynthesized from an intermediate composed of two dihydropyridine rings with an alkyl residue and a tryptophan unit (5). The proposal was based on the isolation of several reasonable intermediates in the biosynthetic pathway, which include structures similar to the bis-3-alkyldihydropyridine macrocyclic intermediate (a) and the pentacyclic intermediate (b). While the isolation of the plausible biosynthetic intermediates ircinals A (35) and B (36) (10), ircinol A (37) (25), ingenamine (31), keramaphidin B (2,26), and xestocyclamine B (28) have facilitated piecing together a reasonable biogenesis, it is noteworthy to mention that these alkaloids have been isolated from sponges belonging to a number of different genera. As a result, the manzamines are certain to be the key to a better understanding of the bioorganic evolution of the sponges that produce these alkaloids, as well as the evolutionary pressures that have allowed for the accumulation of metabolites that fit so neatly as intermediates into a sophisticated biosynthetic scheme. In addition, it would seem clear that the microbial associations with the manzamine-producing sponges will play a critical role in understanding the biosynthesis of many of these manzamine or manzamine-related alkaloids.
C. A TAXONOMIC SURVEY OF SPONGES THAT PRODUCE MANZAMINES AND RELATED ALKALOIDS
A large variety of manzamines and related alkaloids thought to be their biogenetic precursors have been reported primarily from the Order Haplosclerida (Porifera: Demospongiae); 15 or more species in 7 genera and 3 families have been listed as producers (Table I). Two species in 2 genera and 2 families within the phylogenetically distant Order Dictyoceratida have also been found to produce manzamines and related alkaloids.
Haliclona, Reniera, and Prianos (Order Haplosclerida: Family Chalinidae)-
The first manzamine identified (A (1)) was extracted from a sponge identified as a Haliclona sp. collected off Manzamo Island, Okinawa, in April 1985 by Higa’s group (1). Manzamines B, C, and D (2,3,4) were subsequently extracted from possibly the same species after further collections (8). In 1992, Kobayashi et al. (14) collected another unidentified Haliclona sp. from Iriomote Island, Okinawa, reporting the new alkaloid manzamine Y (13) (12,27). No taxonomic description of the sponge was given in these early papers, other than the sponge was “brownish”, and no taxonomic authority was acknowledged for identification.
A further species of Haliclona collected from Sodwana Bay, Durban in September 1992 (23), containing haliclorensin (31), was identified as Haliclona tulearensis Vacelet, Vasseur & Lévi (62) by an unknown taxonomic authority. The sponge was described as a “fine muddy orange laminate sponge with large oscules on ridges on the surface”, but no details of the skeleton were given. Although this sponge appears to be somewhat similar to the description of this type of species (62), spiculation and arrangement of the skeleton will need to be checked to confirm the generic identification. A species of the manzamine-containing genus Acanthostrongylophora (Table I) is known from Pemba Island, off Zanzibar. The sponge is a bright orange hemisphere with flush oscules, and has a Haliclona-like skeleton of small strongyles. To the inexperienced eye, this sponge could easily be mis-identified as an “unusual species of Haliclona” or a thick species of Prianos.
Misenine (55) and saraine A (55c), the only two manzamines reported from Mediterranean waters, were isolated from an unidentified species of Reniera (36) and Reniera sarai Pulitzer-Finali (37), both sponges were collected from the vicinity of Naples, Italy. Neither report contains descriptive details of morphology, skeletal architecture, or spiculation, nor is there a taxonomic authority named. Confirmation of these identifications can only come from examination of the vouchers that are available (36,37).
The genus Haliclona contains species that are characterized by a very simple spiculation of small oxea spicules joined together at their ends to form a regular network. In some species, the spicules form tracts, but the sponges are always soft, compressible and often feel soggy and slightly velvety to the touch. Reniera is considered by some taxonomists to be a subgenus of Haliclona. These sponges are typically brightly colored and quite small; it is doubtful whether the identifications for the material are correct, as “brownish” is not a common chalinid color, and rather a large amount of material was harvested (8 kg) (8) suggesting a more dense sponge of a different, perhaps petrosid genus. However, without recourse to examination of the specimens that the compounds were isolated from (no sample numbers were given or retained for reference), it is impossible to confirm the taxonomic identity of these sponges.
It is now known that the sponge described as Prianos sp., from Manado, Indonesia (15) is closely related to the sponges identified as Pachypellina (11), Xestospongia (16,28–33,35), and to those reported as an undescribed new genus and species from the Family Petrosiidae (18,58,59) (Table I). These taxa have all been confirmed as species of Acanthostrongylophora Hooper, which was previously unrecognizable (63).
Amphimedon, Cribrochalina (Order Haplosclerida: Family Niphatidae):
Some 22 alkaloids have been isolated from at least 8 specimens of a sponge identified as an undescribed species of Amphimedon (see Table I). Examination of the specimen SS-264 (17,20,57) from Kerama Island, Okinawa, kindly supplied by the original taxonomic authority, Dr J. Fromont, Museum of Western Australia, confirms that the generic identification is correct. Several specimens, SS-326 (12), and SS-932 (19) from Okinawa Island, Okinawa, also appear to be correctly identified by the descriptions given of spicule complement and skeletal arrangements in the literature. However, the remaining reports contain no taxonomic or morphological information, other than that the specimens were collected from Kerama Island, Okinawa. Although we cannot assume that these latter specimens are the same undescribed species of Amphimedon, the likelihood that they are is quite high since the material had been collected many times by the same groups. Although no description of the sponge material, spiculation or skeletal arrangements is reported for Cribrochalina sp. (13), the taxonomic authority was reputable and thus the identification is most probably correct.
Amphimedon and Cribrochalina are sister taxa within the Family Niphatidae and are characterized by the possession of small oxeas usually embedded in well-developed spongin. Differences in fiber size and architecture, especially at the sponge surface, and spicule dimensions, differentiate the genera.
Acanthostrongylophora, Xestospongia, Petrosia (Order Haplosclerida: Family Petrosiidae):
All of the species listed in brackets under Acanthostrongylophora in Table I are now considered to be species of that genus (MK, R. van Soest, University of Amsterdam, unpublished data), recently re-described by Desqueyroux-Faundez (2002) in (63). Along with the specimens in publications (18,58,59) (Table I), these specimens clearly comprise several species, possibly up to five (58), and are the subject of ongoing taxonomic evaluation. The genus is characterized by the possession of small curved to slightly sinuous strongyles arranged in loose ladder-like tracts to large loose irregular rounded meshes. The sponges are usually quite crumbly, soft and compressible, and have a rough surface due to projecting tracts of spicules. They form irregular thick encrustations to massive lumps with raised oscular chimneys, or can form columns with apical oscular vents. The color is usually brown externally with a deep yellow interior, and the surface is frequently tinged with the maroon of a symbiotic cyanobacteria.
One of the authors (11) considered the blackish-brown sponge that they collected in Sulawesi, Indonesia, to be reminiscent of Xestospongia collected off the Coast of Miyako Island, Okinawa in June, 1986 (9). The former sponge was identified as Pachypellina (11), but has since been confirmed as a species of Acanthostrongylophora (58). Thus, it is highly likely that the material extracted in publication (9) is also a species of Acanthostrongylophora. However, confirmation can only be given by examination of voucher material. It seems quite possible that the “brownish” sponge from Kerama Island, Okinawa, identified as Pellina sp. (6) by Hoshino, may also be a species of Acanthostrongylophora, as this genus typically possesses oxeas or strongyles in tracts.
In contrast to the above specimens, it is likely that the identification of Xestospongia exigua (Kirkpatrick), given to the material extracted by Williams et al. (24) is correct, as the taxonomic authority is reputable and Acanthostrongylophora is exceedingly rare in southern Papua New Guinea, having been found in only two locations: Eastern Fields and the Louisiade Archipelago (MK, unpublished data). The great majority of species are found in Indonesia, with some species known from the Philippines, the northern coast of Papua New Guinea, Micronesia, Fiji, and Tanzania (MK, unpublished data). The geographic distribution is very similar to that of the genus Diacarnus (Poecilosclerida: Podospongiidae) (64), but, unlike Diacarnus, Acanthostrongylophora is less common in the southern Indo-Pacific locations such as the Great Barrier Reef and southern Papua New Guinea, and absent, as far as is known, from New Caledonia.
The identification of Petrosia contignata Thiele (13) is probably correct, as the sponge was described as being gray to tan, barely compressible, with oxeas in two size categories, arranged in a dense isotropic multispicular reticulate skeleton.
Hyrtios (Order Dictyoceratida: Family Thorectidae) and Ircinia (Order Dictyoceratida: Family Irciniidae):
A manzamine alkaloid has been obtained from Red Sea specimens of Hyrtios erecta (Keller) (21) and Okinawan specimens of Ircinia sp. (10), both of which would be very difficult to mistake taxonomically, as dictyoceratid sponges do not contain spicules as haplosclerid sponges do. These sponges are fleshy and rubbery and contain only sand-grains embedded in large spongin (collagen) fibres. These sponges are very distantly related to the haplosclerid sponges considered above, and are in completely different taxonomic orders. It is interesting to note that a similar situation exists with the distribution of cytotoxic latrunculins found typically in the Red Sea sponge genus Negombata (65a), latrunculins are also found in the dictyoceratid sponge Petrosaspongia mycofijiensis (Bakus).
Following this survey (Table I), it now appears that manzamines and related alkaloids are restricted to eight genera (Haliclona, Reniera, Amphimedon, Cribrochalina, Acanthostrongylophora, Petrosia, Hyrtios, Ircinia) within five families (Chalinidae, Niphatidae, Petrosiidae, Thorectidae, Irciniidae) in two orders (Haplosclerida, Dictyoceratida). The isolation of these alkaloids from such a range of genera has led to the speculation that production of these alkaloids may be due to the biosynthetic participation of microorganisms (6,9,14). While there is an increasing level of data to support a microbial origin, the vast majority of alkaloids are produced by petrosid species, particularly those in Acanthostrongylophora (Table I). It may thus be interesting to investigate the potential congruence between the producer microorganisms and sponge phylogenies once the sponge species are confirmed and the microorganism populations are fully characterized. Future taxonomic studies based on morphological examination and comparison of all relevant voucher specimens should reveal the taxonomic and chemotaxonomic relationships within Acanthostrongylophora in particular, and shed further light on the distribution of the manzamines and related alkaloids across the Orders Haplosclerida and Dictyoceratida.
D. MANZAMINE SPONGE-ASSOCIATED MICROBES
Sixteen different species of sponges from five families have been shown to produce manzamine-related alkaloids to date and the discovery of additional manzamine producing sponges seems highly likely (Table I). One interpretation for the fact that these compounds can be isolated from a diverse array of sponges is that the manzamines are produced by common or closely related microorganism(s) present as symbionts in all of these sponges. This possibility was proposed by Kobayashi et al. in 1995 (14) after six species of sponges were known to contain manzamines and appears even more likely now that an even greater diversity of sponges are known to yield manzamines. This possibility warrants careful investigation as the pharmaceutical potential of manzamine alkaloids continues to grow. If these compounds are actually produced by symbiotic bacteria within the sponge, isolation and culture of the producing bacteria may provide an efficient method for production of the compound in fermentation systems. This could ensure a ready supply of a particular manzamine in the high likelihood that one of these drug-leads will advance into clinical trials and applications. Certainly, if it could be shown that manzamines are microbial products, the pharmaceutical and biotechnology community would express a greater interest in this group of compounds and sponge metabolites in general. The marine natural products community in particular is highly sensitive to the difficulty associated with the supply of sufficient quantities of invertebrate metabolites to allow the preclinical and clinical development. Sustainable sourcing for development has been a strong justification for thorough microbiological evaluation of manzamine-producing sponges. In addition, evidence that sponge-associated microbes may play a significant role in the bioconversion of manzamines to the growing number of alkaloids found in manzamine producing sponges is provided by the biotransformation of 8-hydroxymanzamine A (7) and the entantiomer 7a to manzamine A (1) and ent-12,34-oxamazamine F (58), respectively (58).
The culturable microbial communities associated with two undescribed Acanthostrongylophra species also known as 01IND35 (58) and 01IND52 (59), were investigated to obtain isolates that could be examined for manzamine production.
A full molecular community analysis of the entire bacterial community (both culturable and unculturable) was completed for sponge 01IND35 (Fig. 5). This molecular approach is essential to explore the full diversity of microbes associated with sponges since typically less than 1% of the bacteria present are culturable by conventional approaches.
Fig. 5.
Neighbor-joining phylogenetic tree from analysis of ca. 500 bp of 16S rRNA gene sequence from clones obtained from Acanthostrongylophora sp. (01IND035) (58). The scale bar represents 0.1 substitutions per nucleotide position. Culturable isolates from the sponge are boxed. Sequences shown in bold are those whose nearest relatives, based on BLAST searches, are also from sponges.
Culturable isolates of heterotrophic bacteria were obtained from sponges 01IND 35 and 01IND52 and unequivocally identified by 16S ribosomal RNA gene sequence analysis. Ten isolates were obtained and the nearest relative of each isolate was found by BLAST analysis (Table V). Phylogenetic trees can then be inferred for selected isolates, exemplified in Fig. 5. Homologous nucleotides were compared using the neighbor-joining, Fitch–Margoliash and maximum parsimony algorithms in the PHYLIP package. Tree topologies were evaluated after 1000 bootstrap re-samplings of the neighbor-joining data. Isolates of this undescribed Petrosiidae genus included several closely-related strains of α-proteobacteria (Fig. 5 and Table V), a group previously found to be important in culturable sponge-associated bacteria (65b). Interestingly, two actinomycetes (designated M41 and M42) were also present in the culturable assemblage (Table V). Actinomycetes are recognized as a significant component of sponge-associated microbiota (65c) and are of particular interest considering the excellent track-record of these microbes in production of bioactive compounds. The great diversity of bacteria present in the total bacterial community associated with sponge 011ND35 (Fig. 5) is striking. Clearly, sponges can be a valuable source of novel microbial isolates for biological screening if methods can be developed to culture a greater proportion of these microbes. Microbiological analysis of manzamine-containing sponges provides valuable insights into the potential of the bacterial communities to produce bioactive metabolites, including manzamines, considering the diversity of these communities and the presence of bacteria known to be producers of important compounds. The role that these microbes play in the biosynthesis or metabolism of the manzamine alkaloids remains to be determined.
TABLE V.
Nearest Relatives of Isolates from Two Undescribed Sponges from Acanthostrongylophora sp. (01IND035) (58) and (01IND052) (59) Based on BLAST.
| Strain designation | Source sponge | Closest relative from BLAST analysis | Accession no. of closest relative |
|---|---|---|---|
| M28 | 01IND35 | Bacillus sp. strain VAN35 | AF286486 |
| M29 | 01IND35 | Staphylococcus arlettae | AB009933 |
| M30 | 01IND35 | Brevibacillus borstelensis | AF378230 |
| M31 | 01IND35 | Alpha proteobacterium MBIC3368 | AB012864 |
| M34 | 01IND35 | Unidentified firmicute strain HTE831 | AB010863 |
| M36 | 01IND35 | Alpha proteobacterium strain NW001 | AF295099 |
| M37 | 01IND35 | Pseudomonas sp. strain PB1 | AF482708 |
| M39 | 01IND35 | Unidentified eubacterium clone BSV04 | AJ229178 |
| M40 | 01IND35 | Bacillus sp.strain VAN35 | AF286486 |
| M41 | 01IND35 | Microbacterium barkeri | X77446 |
| M42 | 01IND35 | Micromonospora sp. strain IM-7416 | AF131427 |
| M44 | 01IND52 | Shewanella alga | U91547 |
| M45 | 01IND52 | Alpha proteobacterium strain NW001 | AF295099 |
| M46 | 01IND52 | Alpha proteobacterium strain NW001 | AF295099 |
| M47 | 01IND52 | Alpha proteobacterium MBIC3368 | AB012864 |
| M52 | 01IND52 | Shewanella woodyi | AF003549 |
| M53 | 01IND52 | Alpha proteobacterium NW001 | AF295099 |
| M56 | 01IND52 | Pseudomonas sp. strain PB1 | AF482708 |
IV. Synthesis
The unusual ring system of the manzamines has attracted great interest as one of the most challenging natural product targets for total synthesis. Synthetic studies of the manzamines have been reported by a number of groups and this part of the review is limited to the most recently completed total syntheses, as well as semisynthetic studies that have been the key for the structural and stereochemical determination of these alkaloids.
A. TOTAL SYNTHESIS
Among the more reasonable manzamine targets is manzamine C (3), which has been synthesized by Torisawa et al. (Scheme 7) (66,67). A number of research groups have contributed syntheses of manzamine C or its precursors (68–73).
Scheme 7.
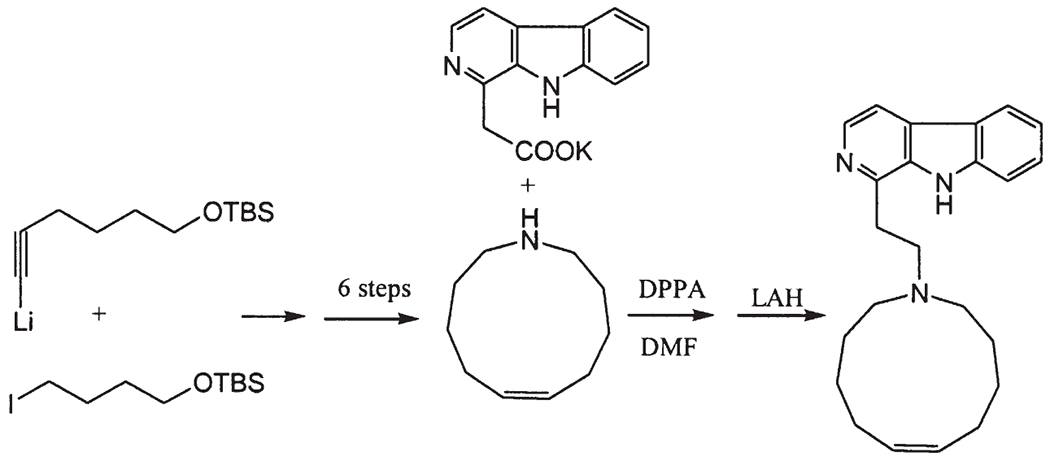
A total synthesis of the sophisticated manzamine A (1) skeleton was not achieved until Pandit’s group published their work in 1996 (Scheme 8) (74). They first reported a total synthesis of the pentacyclic nuclei of manzamine A (1) (74). Before Pandit’s report, Nakagawa et al. reported the synthesis of a tetracyclic core of manzamine A (1) (Scheme 9) (75).
Scheme 8.
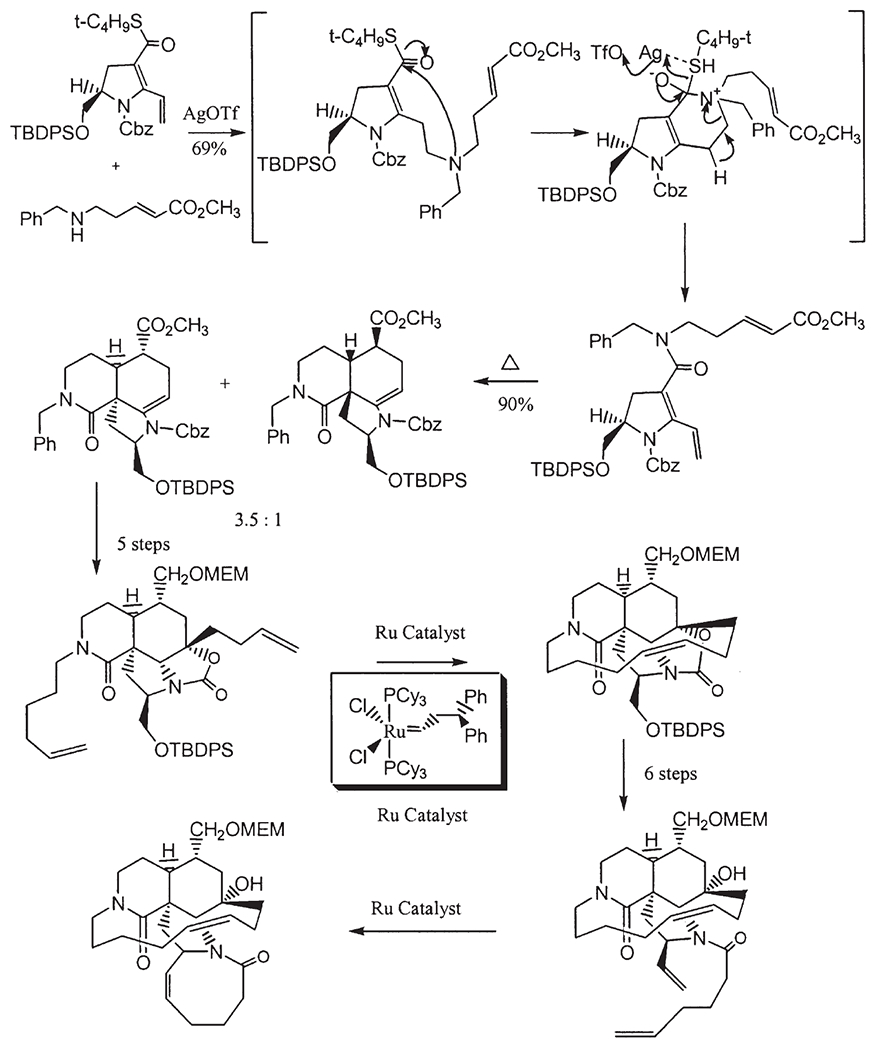
Synthesis of the pentacyclic core of manzamine A (1) by Pandit et al. (74).
Scheme 9.
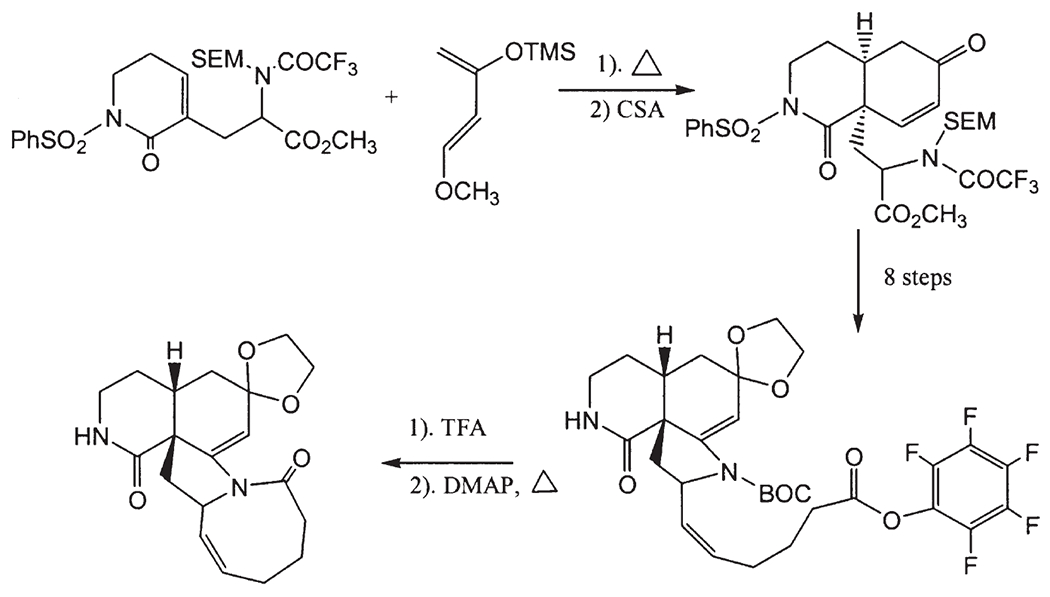
Synthesis of the tetracyclic core of manzamine A (1) by Nakagawa et al. (75).
In 1998, Winkler’s group reported the first total syntheses of ircinol A, ircinal A, and manzamines A & D (Scheme 10) (76). There are over 77 publications reporting the total synthesis of manzamine A, related alkaloids, potential key intermediates and substructures, attesting to the level of interest and challenge involved in the synthesis of the manzamine alkaloids (77–153).
Scheme 10.
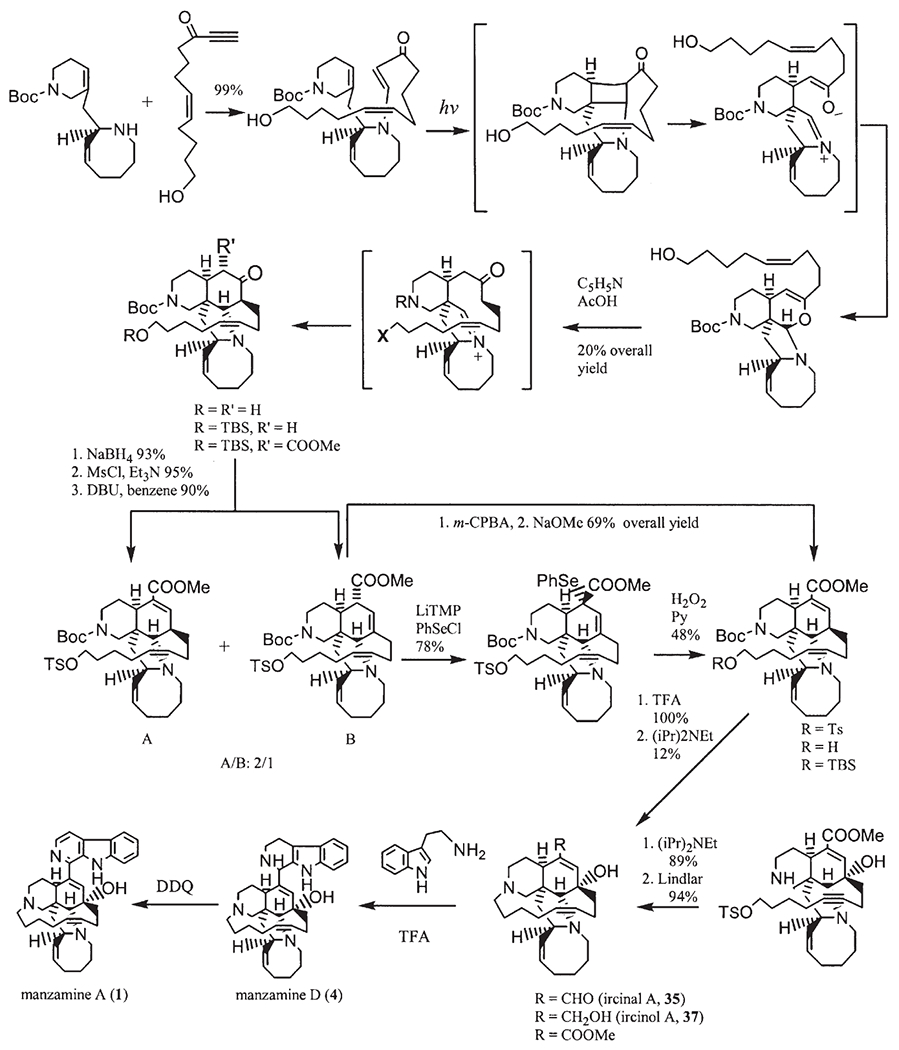
The Total Synthesis of Ircinal A (35), Ircinol A (37), Manzamines A (1) and D (4) by Winkler et al. (76).
B. SEMISYNTHESIS
Ircinals A (35) and B (36), the biogenetic precursors of the manzamine alkaloids, were isolated from an Okinawan sponge Ircinia sp. (10). Aldehydes 35 and 36 were successfully converted into manzamines A (1) and J (9), respectively, through a Pictet-Spengler cyclization (154) with tryptamine (step I, yield: 37%) followed by 2,3-dichloro-5,6-dicyano-p-benzoquinone (DDQ) oxidation (step II, yield: 54%) (Scheme 11) (10).
Scheme 11.
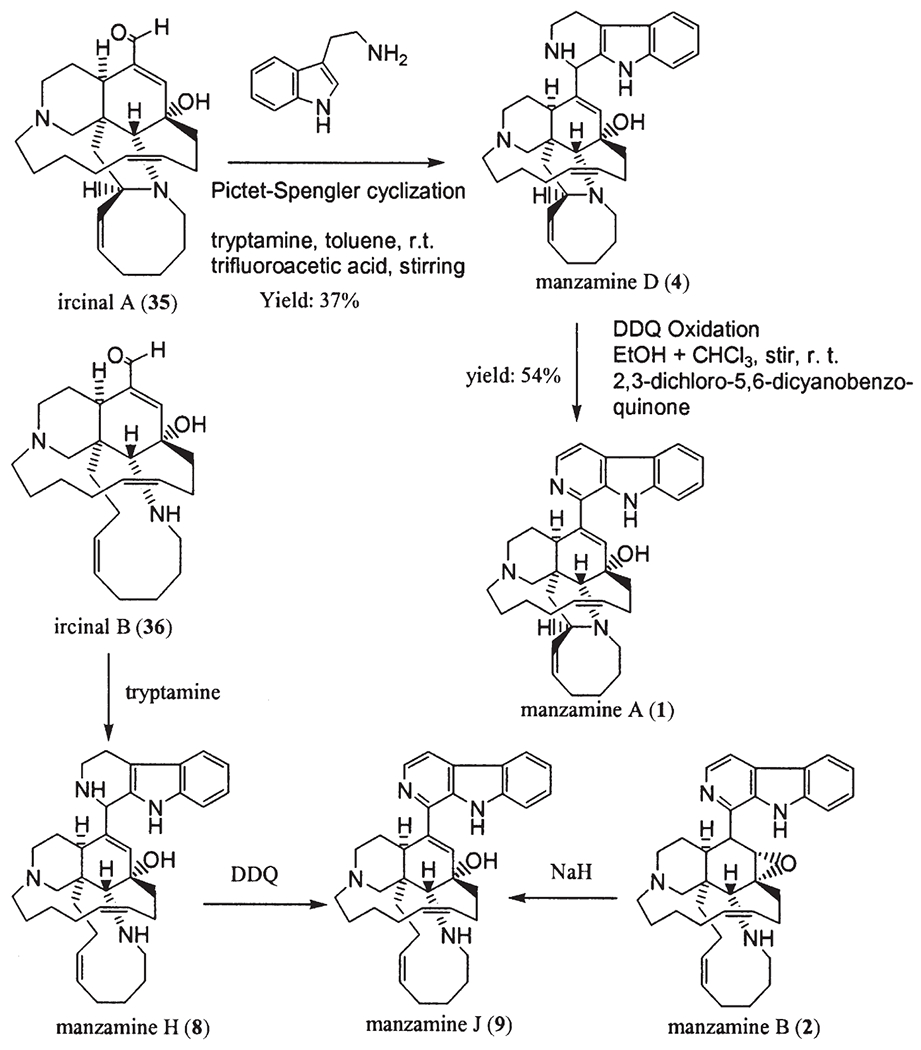
Semisynthesis of Manzamines A (1), D (4), H (8) and J (9) via Pictet–Spengler Cyclization (2,10).
C. BIOMIMETIC METHODS
8-Methoxymanzamine A was generated from 8-hydroxymanzamine A (manzamine G, 7) by using TMSCHN2 (11). Treatment of manzamine L (10) with DDQ yielded the corresponding manzamine J (9) (27). DDQ oxidation of 3,4-dihydro-6-hydroxymanzamine A (17) and 3,4-dihydromanzamine J (18) yielded 6-hydroxymanzamine A (manzamine Y, 13) and manzamine J (9), respectively (17). Manzamine A (1) was shown to be generated from 8-hydroxy manzamine A (7) in good yields using Fusarium solani and Streptomyces seokies providing further evidence for the biogenesis of some of these alkaloids from a sponge-associated microbe and the potential biocatalysis and biotransformations have for the production of analogs of these complex alkaloids (58b). The 3,4-dihydro analogs of the manzamine alkaloids, e.g., 3,4-dihydromanzamine A (14), are likely the direct precursors which generate manzamine A through dehydrogenation (12). In fact, 14 can be easily converted into 1 by daylight (2). Manzamines X (12) and Y (13) are examples of 6-hydroxymanzamine-type alkaloids (12,14), and 13 is presumed to follow manzamine A (1) via oxidation at the C-6 position. The tetrahydrofuran ring moiety in 12 is then presumed to be biosynthesized from 13 via initial allylic oxidation at C-31 in 13, subsequent migration of the double bond (Δ32 → Δ33), and cyclization between the hydroxyl at C-31 and C-34 as depicted in Scheme 12 (14). On the other hand, a biogenetic pathway of the xestomanzamines A (27) and B (28) is presumed to occur as depicted in Scheme 13 (14). That is, these alkaloids could potentially be biosynthesized from an N-methyl histidine and a tryptamine unit.
Scheme 12.
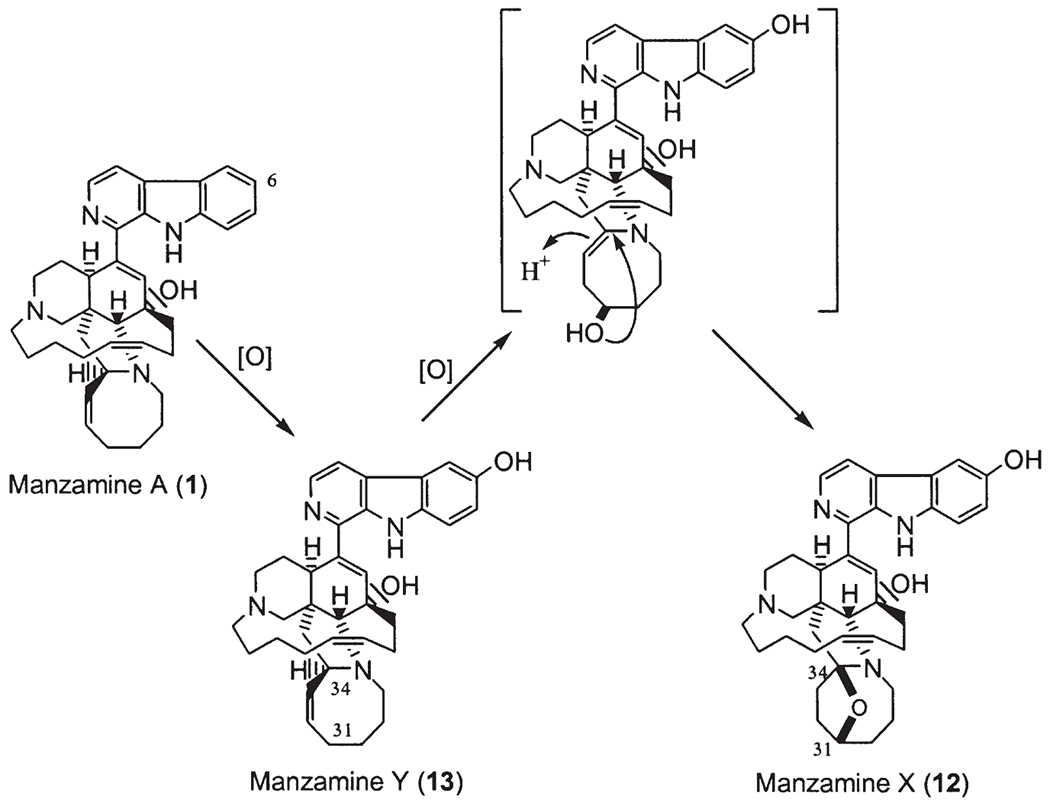
Biomimetic Transformations among Manzamines A (1), X (12), and Y (13) (14).
Scheme 13.
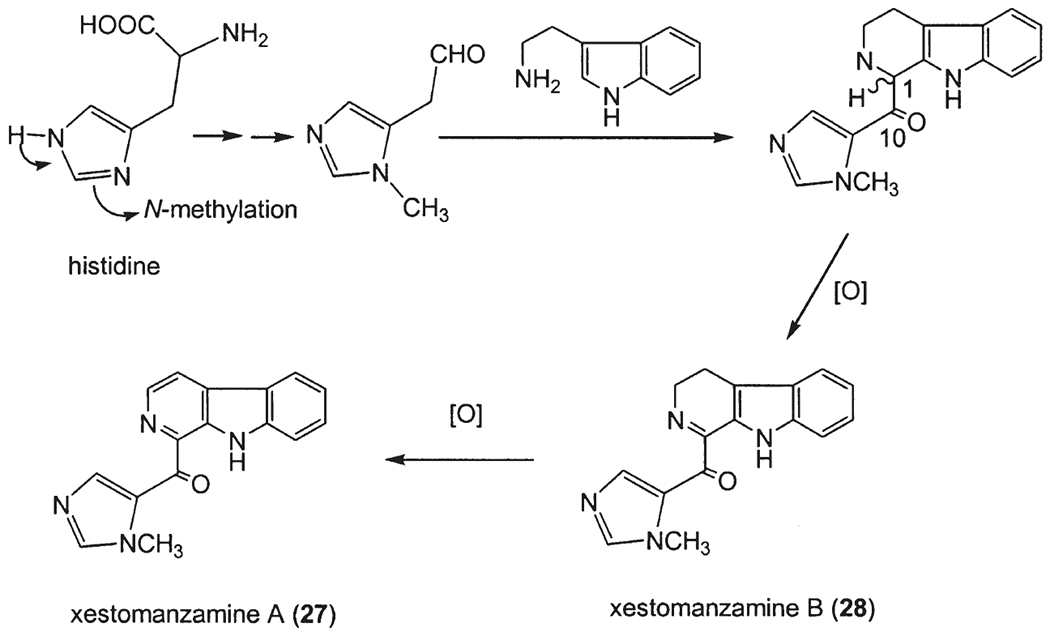
Biogenetic Pathway of Xestomanzamines A (27) and B (28) (14)
As discussed earlier, a species of Amphimedon sp. yielded ircinals A (35) and B (36), as well as ircinols A (37) and B (38) (27). Ircinals A (35) and B (36) were then converted into manzamines A (1) and J (9), respectively, through a Pictet-Spengler cyclization with tryptamine, followed by DDQ oxidation. Treatment of ircinal A (35) with DIBALH afforded a reductive product (35a), whose spectral data were identical with those of ircinol A (37). However, the sign of the optical rotation was opposite {35a, (c 0.2, MeOH); 37, (c 0.5, MeOH)} (Scheme 14) (2,25). This result revealed that compound 37 is clearly an enantiomer of the alcoholic form at C-1 of ircinal A (35) (2,25). In the same way, 38 was shown to be an enantiomer of the alcoholic form at C-1 of 36 (2,25). Alkaloids 37 and 38 were the first examples possessing the opposite absolute configurations to those of the previously reported manzamine alkaloids, followed by the recently reported new enantiomers (6b and 7a) of manzamine F (6) and 8-hydroxymanzamine A (manzamine G, 7).
Scheme 14.
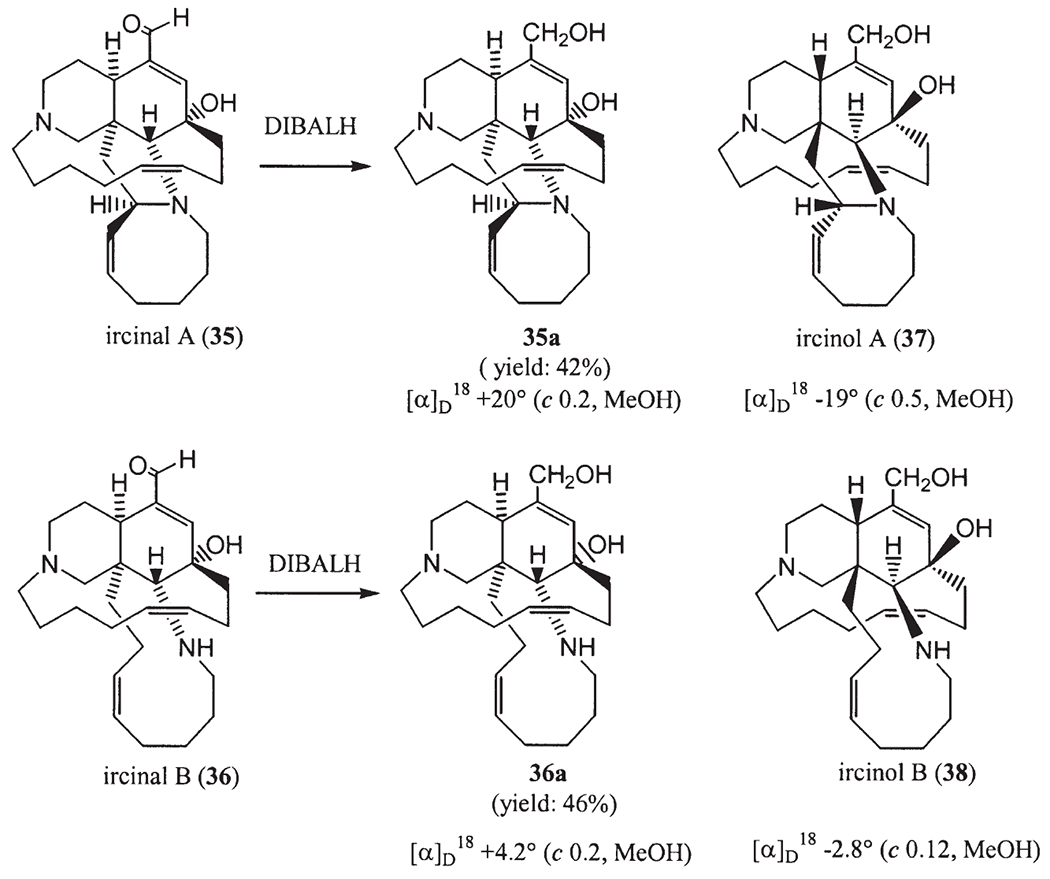
Conversion of Ircinals A (35) and B (36) into their Alcohol Forms (35a and 36a) (2,25).
Keramaphidin B (39) is a unique pentacyclic alkaloid with an unprecedented skeleton isolated from Amphimedon sp., and in addition, several alkaloids with similar skeletons to that of 39 such as ingenamine, ingenamines B-F, ingamines A and B, and xestocyclamines A and B from Xestospongia have also been reported. These structures are very close to those of the corresponding biogenetic intermediate of manzamines A (1) and B (2) proposed by Baldwin et al., in which a bis-3-alkyldihydropyridine macrocycle may be converted through a Diels-Alder-type [4+2] intramolecular cycloaddition into a pentacyclic intermediate like 39, which in turn provides manzamines A (1) and B (2) via a tetracyclic intermediate such as ircinals A (35) and B (36). This may explain the fact that manzamines A (1) and B (2) and keramaphidin B (39) possess the same absolute configurations, and indeed, ircinals A (35) and B (36) were found to have the same absolute configurations as those of manzamines A (1) and B (2). Keramaphidin B (39) was optically active as mentioned previously (26,27). However, the crystal of 39 employed for X-ray analysis was revealed to be racemic. On the other hand, ingenamine, ingamine A, and ingenamine E were reported to be antipodal to the manzamines and ircinals. Both enantiomers of keramaphidin B (39) were separated by chiral HPLC [the ratio of (+)- and (−)-forms of the crystals was ca. 1 : 1], of which one maybe a biogenetic precursor of ircinals A (35) and B (36) and manzamines A (1) and B (2), while the other may be associated with the antipodes of the manzamine alkaloids, such as ircinols A (37) and B (38) (27). Synthetic strategies that utilized the likely biogenesis to varying degrees for the preparation of the manzamines are reported by a number of research groups (155–169).
V. Pharmacology
In addition to being the first reported manzamine alkaloid, manzamine A (1,1a) has also been the subject of the greatest number of pharmacology studies revealing a portion of the biological activity for this complex alkaloid. Manzamine A hydrochloride (1a) showed an IC50 of 0.07 μg/mL inhibiting the growth of P388 mouse leukemia cells (1). Keramamine-A (manzamine A) showed antimicrobial activity against Staphylococcus aureus with a minimum inhibitory concentration (MIC) of 6.3 μg/mL (6). Manzamine A (1) showed significant activity against KB (IC50 0.05 μg/mL), LoVo (IC50 0.15 μg/mL), and HSV-II (MIC 0.05 μg/mL) cells in vitro (11). Manzamine A can elicit an 80% growth inhibition of the insect Spodoptera littoralis larvae at 132 ppm (16). In addition, manzamine A exhibited insecticidal activity toward neonate larvae of the polyphagous pest insect Spodoptera littoralis with an ED50 of 35 ppm when incorporated into an artificial diet and offered to larvae in a chronic feeding bioassay.
Manzamine A was also active (antibacterial) against the Gram-positive bacteria Bacillus subtilis and Staphylococcus aureus. Manzamine A exhibited cytotoxicity against L1578y mouse lymphoma cells with an ED50 1.8 μg/mL. Among the most promising activities of manzamine A is the fact that it inhibits the growth of the rodent malaria parasite Plasmodium berghei in vivo. More than 90% of the asexual erythrocytic stages of P. berghei were inhibited after a single intraperitoneal injection of manzamine A into infected mice. A remarkable aspect of manzamine A treatment is its ability to prolong the survival of highly parasitemic mice, with 40% recovery 60 days after a single injection. Oral administration of an oil suspension of manzamine A or (−)-8-hydroxymanzamine A (2 × 100 μM/kg) produced a significant reduction (90%) in parasitemia. The plasma manzamine A concentration peaked 4 h after injection and remained high even at 48 h. Morphological changes of P. berghei were observed 1 h after treatment of infected mice. Manzamine A also induced 98–99% inhibition of Mycobacterium tuberculosis (H37Rv) with an MIC < 12.5 μg/mL, and it exhibits an MIC endpoint of 1.56 μg/mL (18,170).
Initial in vivo studies of manzamine A against P. berghei provided a number of intriguing characteristics for this drug-lead as compared with either chloroquine or artemisinin. At dosages of 50 and 100 μmol/kg (i.p.) manzamine A (and 8 hydroxymanzamine A) showed significant improvements in survival times over mice treated with either chloroquine or artemisinin (170). In addition, it was observed that manzamine A possessed a rapid onset of action (1–2 hours) against malaria in mice and provided a continuous and sustained level of the drug in plasma when measured as long as 48 hours after administration. Manzamine A and chloroquine were both shown to be toxic to mice at an intraperitoneal (i.p.) dose of 500 μmol/kg. However, the toxicity of manzamine A is slower acting than chloroquine suggesting that the in vivo toxicity of manzamine A may be associated with its cytotoxicity. The fact that mice treated with a single 100 μmol/kg dose of manzamine A could survive longer carrying fulminating recurrent parasitemia and in some cases clear the parasite lead to speculation that manzamine A induced an immunostimulatory effect.
In order to further evaluate the effect that manzamine A may have on the immune system of P. berghei infected mice the serum concentrations of immunoglobulin G (IgG), interferon-γ (IFN-γ), interleukin-10 (IL-10), and tumor necrosis factor-α (TNF-α) were evaluated (18). Th1-mediated immunity was found to be suppressed in mice infected with P. berghei and treated with manzamine while Th2-mediated immunity was found to be up-regulated. IL-10 and IgG concentrations did not increase with manzamine A alone suggesting that the immune-mediated clearance of malaria in mice maybe a product of the long half-life of manzamine A resulting in a delayed rise of the parasitemia. This delayed rise in parasitemia may provide the infected animal the time needed to up-regulate a Th2-mediated response. In addition, the possibility that manzamine A and the other active manzamines form a conjugate with a malaria protein may also help explain the Th2-mediated immune response.
Manzamine A was also selected for in vivo testing against Toxoplasma gondii because it was the most effective in vitro of the manzamines assayed. A daily i.p. dose of 8 mg/kg of manzamine A, for 8 consecutive days, beginning on day 1 following the infection prolonged the survival of Swiss Webster (SW) mice to 20 days, as compared with 16 days for the untreated control. These data indicate that the manzamines are valuable candidates for further investigations and development as leads against several serious infectious diseases, and in particular manzamine A is quite clearly a new and promising antimalarial agent (18,170). The effectiveness of manzamine A against malaria in laboratory mice, as well as tuberculosis in vitro suggests that they could have an extraordinary impact on infectious diseases in developing countries. The fact that manzamine A is found in a diversity of organisms and in reasonable high yields would facilitate the potential development of cost-effective antimalarial drugs in regions of the world that are saddled with the disease burden. In addition, the diversity of biological activity associated with this molecule further supports the growing possibility that these alkaloids are broad-spectrum antiparasitic-antibiotics generated ultimately by the sponge-associated microbial communities.
Manzamine B (2) with the C-11,12 epoxide group exhibited an IC50 of 6 μg/mL against P388 leukemia cells in vitro (7). In the above assay, manzamines C (3) and D (4) exhibited IC50 values of 3 and 0.5 mg/mL (7).
Manzamine E (5) showed an IC50 of 5 μg/mL against P388 murine leukemia cells (9). Manzamine F (6, keramamine-B) showed antimicrobial activity against Staphylococcus aureus with a minimum inhibitory concentration (MIC) of 25 μg/mL (6). Manzamine F (6) also showed an IC50 of 5 μg/mL against P388 murine leukemia cells (9), 50.6% growth inhibition of the insect S. littoralis larvae (dose = 132 ppm), and cytotoxicity against L5178 mouse lymphoma cells of an ED50 of 2.3 μg/mL. Manzamine F did not exhibit antimalarial activity. However, ent-manzamine F (6b) induced 98–99% inhibition of Mycobacterium tuberculosis (H37Rv) with a MIC of < 12.5 μg/mL (18).
(+)-8-Hydroxymanzamine A (7, also known as manzamine G or manzamine K) was relatively active in the KB (IC50 0.30 μg/mL), LoVo (IC50 0.26 μg/mL), and HSV-II (MIC 0.1 μg/mL) assays (11). (−)-8-Hydroxymanzamine A exhibits improved activity against P388 with an IC50 of 0.25 μg/mL. This enantiomer displayed antimalarial activity in vivo, which was assayed against P. berghei with a single intraperitoneal (i.p.) dose of 100 μM/kg and no apparent toxicity. It efficiently reduced parasitemia with an increase in the average survival days of P. berghei-infected mice (9–12 days), as compared with untreated controls (2–3 days). Three 50 μmoles/kg i.p. doses were found to be curative and totally cleared the parasite and two oral doses (100 μmoles/kg) provided a notable reduction of parasitemia. (−)-8-Hydroxymanzamine A (7a) induced 98–99% inhibition of Mycobacterium tuberculosis (H37Rv) with MIC < 12.5 μg/mL, and it exhibits a MIC endpoint of 3.13 μg/mL (18).
Manzamines H (8) and J (9) exhibited cytotoxicity against L1210 murine leukemia cells with IC50 values of 1.3 and 2.6 μg/mL, and KB human epidermoid carcinoma cells with IC50 values of 4.6 and > 10 μg/mL in vitro, respectively (10). Manzamine L (10) exhibited cytotoxicity against murine lymphoma L1210 cells and human epidermoid carcinoma KB cells (IC50 3.7 and 11.8 μg/mL, respectively) and antibacterial activity against: Sarcina lutea, Staphylococcus aureus, Bacillus subtilis, and Mycobacterium 607 (MIC 10, 10, 10, and 5 μg/mL, respectively) (27).
Manzamine M (11) showed cytotoxicity against murine leukemia L1210 cells (IC50 1.4 μg/mL) (17). Manzamine X (12) exhibited weak cytotoxicity against KB cells with an IC50 of 7.9 μg/mL (14). Manzamine Y (6-hydroxymanzamine A, 13) and 3,4-dihydromanzamine A (14) showed antibacterial activity against the Gram-positive bacteria Sarcina lutea (MIC value, 1.25 and 4 μg/mL, respectively). Alkaloids 13 and 14 were cytotoxic against L-1210 (IC50 values, 1.5 and 0.48 μg/mL, respectively) and KB cells (IC50 2.5 and 0.61 μg/mL, respectively) in vitro (12). 8-Hydroxy-2-N-methyl-1,2,3,4-tetrahydromanzamine A (16) was cytotoxic to P388 leukemia cells, and exhibited an ED50 of 0.8 μg/mL (13). 3,4-Dihydro-6-hydroxymanzamine A (17) and 3,4-dihydromanzamine J (18) showed cytotoxicity against murine leukemia L1210 cells (IC50 3.1 and 12.5 μg/mL, respectively) (17). The N-oxides (19, 21) showed cytotoxicity in vitro against L1578y mouse lymphoma cells with an ED50 value of 1.6 μg/mL (16).
Kauluamine (25) was inactive against tumor cell lines, but showed moderate immunosuppressive activity (MLR IC50 1.57 μg/mL, LcV IC50 L > 25 μg/mL, LcV/MLR > 16) in the mixed lymphoma reaction (15). neo-Kauluamine (26) possesses cytotoxicity with an IC50 of 1.0 g/mL, against human lung and colon carcinoma cells (18). It also displayed significant antimalarial activity in vivo, which was assayed against Plasmodium berghei with a single intraperitoneal (i.p.) dose of 100 μM/kg and no apparent toxicity. It efficiently reduced parasitemia with an increase in the average survival time of P. berghei-infected mice (9–12 days), as compared with untreated controls (2–3 days).
Xestomanzamine B (28) exhibited weak cytotoxicity against KB cells with an IC50 14.0 μg/mL (14). Ircinals A (35) and B (36) exhibited cytotoxicity against L1210 murine leukemia cells with IC50 values of 1.4 and 1.9 μg/mL and KB human epidermoid carcinoma cells with IC50 values of 4.8 and 3.5 μg/mL in vitro, respectively (10). Ircinols A (37) and B (38) were cytotoxic against L1210 cells (IC50 values: 2.4 and 7.7 μg/mL, respectively) and KB cells (IC50 values: 6.1 and 9.4 μg/mL, respectively). Ircinol A (37) showed inhibitory activity against endothelin converting enzyme (IC50: 55 μg/mL) (25).
Keramaphidin B (39) was cytotoxic against P388 murine leukemia and KB human epidermoid carcinoma cells with an IC50 of 0.28 and 0.3 μg/mL, respectively (26). Xestocyclamine A (40) was moderately potent against PKC (IC50 4 μg/mL) and also exhibited activity in a whole cell IL-1 release assay with an IC50 of 1 μM. This action appeared to be selective, as compound 40 was inactive against other cancer-relevant targets, including PTK and IMPDH. Finally, compound 40, at doses as high as 100 μM, did not show in vitro growth inhibition effects against cancer cells in the NCI’s disease oriented screening program (29). Ingamines A (42) and B (43) both showed in vitro cytotoxicity against murine leukemia P388 with an ED50 of 1.5 μg/mL (30). Ingenamine (44) showed cytotoxicity against murine leukemia P388 (ED50 1 μg/mL) (31). Madangamine A (50) showed significant cytotoxic activity toward a number of tumor cell lines, including murine leukemia P388 (ED50 0.93 μg/mL) and human lung A549 (ED50 14 μg/mL), brain U373 (ED50 5.1 μg/mL), and breast MCF-7 (ED50 5.7 μg/mL) cancer cell lines (33). The saraines, such as saraine A (55c), generally display significant biological properties including, vasodilative, antineoplastic, and cytotoxic activities (53). Nakadomarin A (56) showed cytotoxicity against murine lymphoma L1210 cells (IC50 1.3 μg/mL) and inhibitory activity against cyclin dependent kinase 4 (IC50 9.9 μg/mL). Compound 56 exhibited antimicrobial activity against a fungus (Trichophyton mentagrophytes, MIC 23 μg/mL) and a Gram-positive bacterium (Corynebacterium xerosis, MIC 11 mg/mL) (57).
ent-12,34-Oxamanzamines E (57) and F (58), as well as 12,34-oxamanzamine A (59) were isolated from three Indo-Pacific sponges (58). The biocatalytic transformation of ent-8-hydroxymanzamine A (7a) to 58, using Nocardia sp. and Fusarium oxysporium ATCC 7601, has also been achieved (58). Eleven heterotrophic bacterial isolates, including actinomycetes and α-proteobacteria, were isolated from one of these sponges in a preliminary investigation to identify a possible microbial origin for these alkaloids. The potent in vitro activity of the manzamines against malaria and the AIDS opportunistic infection (OI) pathogen, Mycobacterium tuberculosis, is also presented. The in vitro activity of manzamines against Mycobacterium tuberculosis (H37Rv) and the malaria parasite Plasmodium falciparum is reported with most manzamines showing activity against M. tuberculosis with MICs < 12.5 g/mL. (+)-8-Hydroxymanzamine A had an MIC 0.91 μg/mL, indicating improved activity for the (+) over the (−) enantiomer. The significant activity of ircinol A (1.93 μg/mL) indicates that the β-carboline moiety is not essential for activity against Mtb in vitro. This result suggests the candidacy of ircinol A as a possible antituberculosis lead for further development since it showed minimal toxicity and reduced structural complexity. The decreased activity of 57, 58, and 59 against M. tuberculosis and P. falciparum is clearly associated with the changes in the molecule that result during the formation of the new C-12, C-34 oxygen bridge. This data provides significant improvements in the understanding of the SAR against malaria and Mtb (58).
A crude extract of an unidentified Palauan marine sponge showed initial inhibitory bioactivities in a yeast assay for inhibitors of methionine aminopeptidase-2 (Met AP-2). Bioassay-directed fractionation indicated that the activity was concentrated in the CH2Cl2-soluble fraction, and chromatography on silica gel led to the isolation of two new bioactive alkaloids epi-manzamine D (60) and N-methyl-epi-manzamine D (61) (61). The author’s initial interest in the extract was due to its potential antiangiogenic activity, the differential activity observed between the Δmap1 and Δmap2 yeast strains was not significant for either compound 60 or 61, and the authors thus conclude that neither compound has anti-angiogenic activity. Both 60 and 61 did, however, show cytotoxic activity against HeLa and B16F10 cell lines. The greatest potency (IC50 0.1 μg/mL) was observed for 61 against the B16F10 cell line (61).
The two novel manzamine-related alkaloids, manadomanzamines A (62) and B (63), were obtained from an Indonesian species of Acanthostrongylophora (59). The manadomanzamines 62 and 63 represent an unprecedented rearrangement of the manzamine skeleton and exhibit significant activities against Mtb and human immunodeficiency virus (HIV-1), with moderate cytotoxicity (59).
Both 62 and 63 exhibited strong activity against Mtb with MIC values of 1.86 and 1.53 μg/mL, indicating that the manadomanzamines are a new class of antituberculosis leads. Manadomanzamines A and B exhibited modest cytotoxic activity against human tumor cells. Manadomanzamine A is active against human lung carcinoma A-549 and human colon carcinoma H-116 with IC50 values of 2.5 and 5.0 μg/mL while manadomanzamine B is only active against H-116 with an IC50 value of 5.0 μg/mL. Manadomanzamines A (62), B (63), and xestomanzamine A (27) are active against human immunodeficiency virus (HIV-1) with EC50 values of 11.4, 62.7, and 42.7 μM, respectively. Manadomanzamine B and xestomanzamine A are active against the fungus Cryptococcus neoformans with IC50 values of 3.5 and 6.0 μg/mL. Manadomanzamine A was active against the fungus Candida albicans with an IC50 of 20 μg/mL. It is worthy to note that manadomanzamines A, B, and xestomanzamine A, unlike manzamine A, 8-hydroxymanzamine A, and neo-kualuamine which are extraordinarily active antimalarial agents (18,170), only exhibited marginal activity against the malaria parasite indicating that the polycyclic ring system of the manzamine structure is important for antimalarial activity (59).
β-Carboline containing manzamines 32,33-dihydro-31-hydroxymanzamine A (64), 32,33-dihydro-6,31-dihydroxymanzamine A (65), and 32,33-dihydro-6-hydroxymanzamine A-35-one (66) were isolated from an Indonesian sponge (60). Additional data regarding the in vitro activity of the manzamines against Mtb (H37Rv), P. falciparum, and Leishmania donovani, the causative agent for visceral leishmaniasis, is reported. Most manzamines were active against M. tuberculosis with MICs < 12.5 μg/mL. Although alkaloids 64 and 66 were inactive against malaria and leishmania, these results provide valuable information on the structural moieties required for activity against malaria and leishmania. This observation further supports the previous report (18), which indicates that reduction of the C32–C33 olefin and oxidation of C-31 also significantly reduces the antimalarial activity for the manzamine alkaloids in vivo. These combined data strongly suggest that the ability of the C-34 allylic carbon to form a stabilized carbocation after oxidation both in cell culture and in animals, followed by the inherent nucleophilic attack, may play a critical role in the biological activity of the manzamine alkaloids against the malaria parasite. The significant difference in biological activities of manzamine A, manzamine E, and their corresponding 12,34-oxa-derivatives indicate that the C-12 hydroxy, the C-34 methine, or the conformation of the lower aliphatic rings play a key role in the antimalarial and leishmanicidal activity and provides valuable insight into the structural moieties required for activity against malaria and leishmania parasites. The significant leishmanicidal activity of ircinol A (IC50 0.9 μg/mL and IC90 1.7 μg/mL) indicates that the β-carboline moiety is not essential for activity against the leishmania parasite in vitro. The cytotoxic values of 6-deoxymanzamine X and manzamine X against A-548, HT-29, H-116, and MS-1 cell lines with IC50 (μg/mL), respectively, are as follows.1, 5.1; 0.5, 0.5; 0.5, 5.1; 1, 5.1. The anti HIV (EC50) activity of manzamine A, 8-hydroxymanzamine A, and 6-deoxymanzamine X against human PBM cells acutely infected with HIV-1/LA1 is 0.59, 4.2, and 1.6 μM, respectively (60).
VI. Conclusions
The manzamine alkaloids are unique and viable, leads to the treatment of malaria, as well as other infectious or tropical parasitic diseases, based on their significant activity in animal models. In addition, the relatively wide range of biological activity for the manzamines in vitro raises the question that perhaps these molecules maybe broad-spectrum antiparasitic antibiotics generated by a sponge-associated microbe. The ecological relationship between the microbial communities and the sponge in the case of the manzamines is particularly intriguing due to the structural complexity of these alkaloids.
In spite of the necessity of the β-carboline moiety for in vitro antimalarial activity, it has little effect on the antituberculosis activity in vitro, suggesting that several different possible mechanisms of action are likely to exist. Although further investigations are required to completely understand the SAR for this class of compounds, the absence of activity associated with the C-12, C-34 oxygen bridge system provides valuable insight into the structural moieties required for activity against Mtb and malaria. In addition, the formation of this new oxygen bridge and its reduced biological activity suggests that this alkaloid maybe a potential intermediate in the development of resistance to this class of bioactive alkaloids. The significant reduction in biological activity observed against P. falciparum for the C-12, C-34 oxygen bridge system indicates that the C-12 hydroxy, C-34 methine, or the conformation of the lower aliphatic rings, plays a key role in the antimalarial activity. The reduction of the C32𠄶C33 olefin and oxidation of C-31 also significantly reduces the antimalarial activity for the manzamine alkaloids in vivo. These data combined suggest that the ability of the C-34 allylic carbon to form a stabilized carbocation after oxidation both in cell culture and in animals, followed by the inherent nucleophilic attack, may play a critical role in the biological activity of the manzamine alkaloids.
Few classes of alkaloids are as unique and intriguing as the manzamine class. The biological activity of the manzamines against infectious diseases, cancer, and inflammatory diseases, combined with their unusual structure, strongly suggests that these alkaloids will ultimately yield useful clinical candidates. In addition, the manzamine alkaloids are clearly a key to understanding the sophisticated, but poorly understood, ecological and phylogenetic relationships between a diverse group of sponges and their associated microbial communities. Understanding the biosynthesis of this group of alkaloids, and the role that invertebrates and their microbial association play, will certainly provide a better understanding of the bioorganic evolution of these complex secondary metabolites and the evolutionary pressures required to ultimately produce them.
Scheme 4.
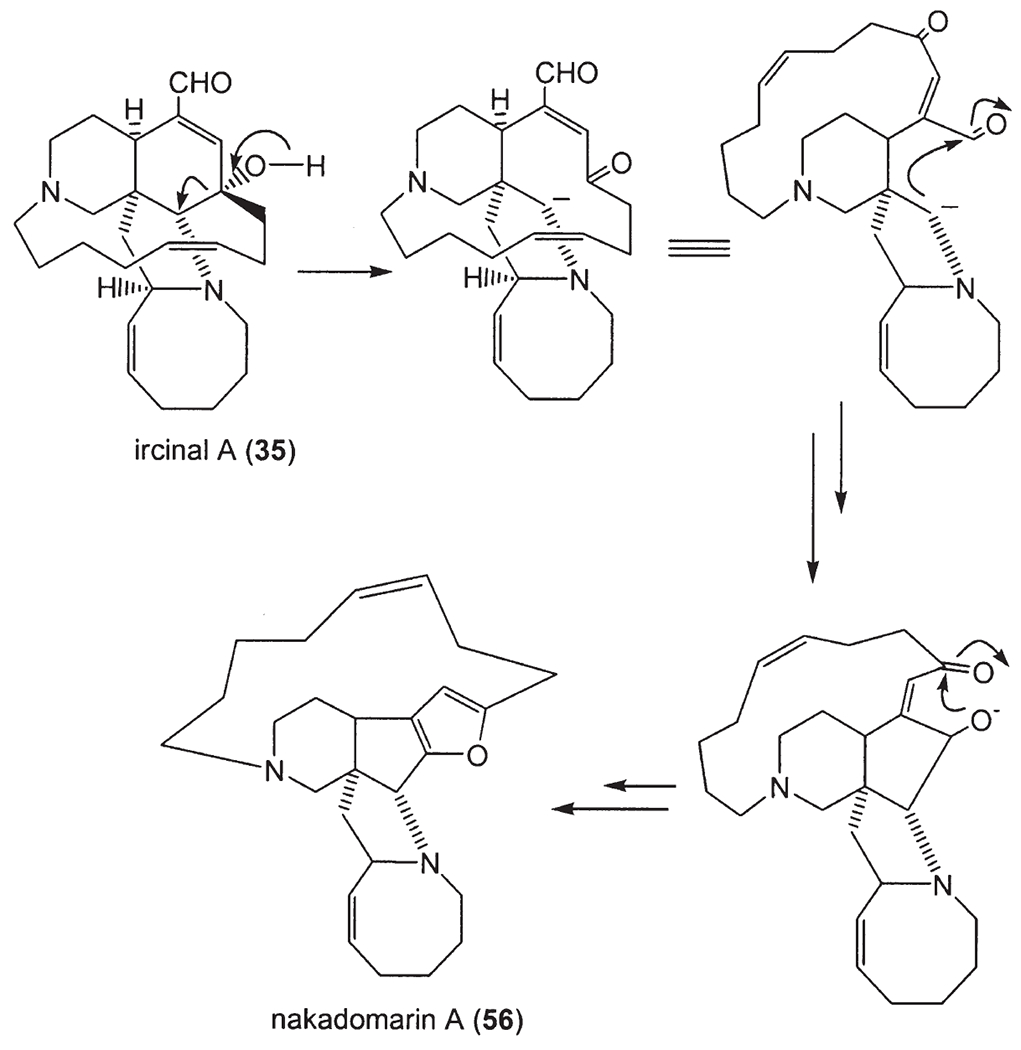
Biogenesis of nakadomarin A (56) from ircinal A (35) (3,57).
Scheme 5.
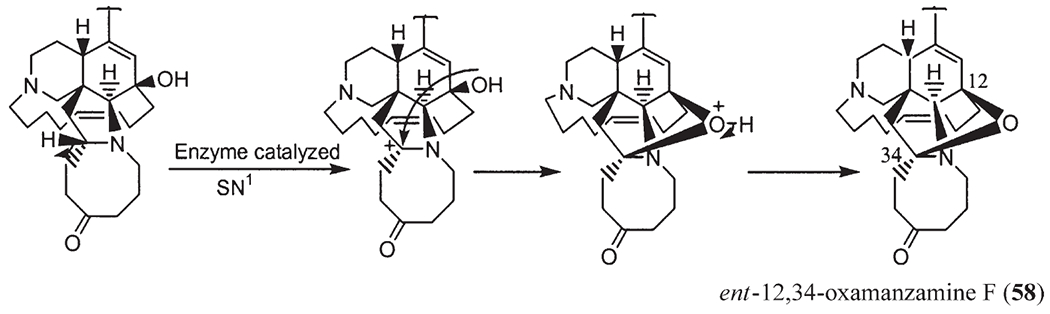
Biogenesis of the 12,34-oxaether bridge in alkaloid 58 (58).
Acknowledgements
The preparation of this chapter was supported, in part, by NIH grants R01AI 36596 and KO2AI01502 from the National Institute of Allergy and Infectious Diseases. Microbiological studies were supported in part by NSF Microbial Observatories MCB-0238515 to RTH. MH thanks his research group for their assistance in the preparation of this chapter.
References
- 1.Sakai R, Higa T, Jefford CW, and Bernardinelli G, J. Am. Chem. Soc 108, 6404–6405 (1986). [Google Scholar]
- 2.(a) Tsuda M and Kobayashi J, Heterocycles 46, 765–794 (1997). [Google Scholar]; (b) Baker BJ, Beta-carboline and isoquinoline alkaloids from marine organisms, “Alkaloids: Chemical and Biological Perspectives” (Pelletier W, ed.), vol. 10, pp. 357–407. Pergamon, Oxford, 1996. [Google Scholar]; (c) Ihara M and Fukumoto K, Nat. Prod. Rep 12, 277–301 (1997). [Google Scholar]
- 3.Whitehead R, Annu. Rep. Prog. Chem. Sect. B 95, 183–205 (1999). [Google Scholar]
- 4.(a) Urban S, Hickford SJH, Blunt JW, Munro MHG, Curr. Org. Chem 4, 765–807 (2000). [Google Scholar]; (b) Higa T, Ohtani II, and Tanaka J, ACS Symposium Series, Vol. 745 (Natural and Selected Synthetic Toxins; ), 12–21 (2000). [Google Scholar]; (c) Rodríguez J, Studies in Natural Products Chemistry, Vol. 24 [Bioactive Natural Products (Part E)], 573–681 (2000). [Google Scholar]; (d) Higa T, Tanaka J, Ohtani II, Musman M, Roy MC, and Kuroda I, Pure Appl. Chem 73, 589–593 (2000). [Google Scholar]; (e) Higa T, Tanaka J, and Tan LT, Cytotoxic Macrocycles from Marine Sponges, New Trends Nat. Prod. Chem (Int. Symp. Nat. Prod. Chem., 6th, Meeting Data 1996) (Atta-ur-Rahman and Choudhary MI, eds.), pp. 109–120. Harwood, Amsterdam, 1998. CA129: 185141. [Google Scholar]; (f) Barriault L, and Paquette LA, Chemtracts 12, 76–281 (1999). CA131: 73818. [Google Scholar]
- 5.Baldwin JE and Whitehead RC, Tetrahedron Lett. 33, 2059–2062 (1992). [Google Scholar]
- 6.Nakamura H, Deng S, Kobayashi J, Ohizumi Y, Tomotake Y, Matsuzaki T, and Hirata Y, Tetrahedron Lett. 28, 621–624 (1987). [Google Scholar]
- 7.Higa T, Sakai R, Kohmoto S, and Lui MS, “Isolation of Antitumor Manzamines B, C, and D from Haliclona” Eur. Pat. Appl. EP 272,056 (Cl. C07D471/04), 22 Jun 1988, US Appl. 943,609, 18 Dec 1986; 14 pp. [Google Scholar]
- 8.(a) Sakai R, Kohmoto S, Higa T, Jefford CW, and Bernardinelli G, Tetrahedron Lett. 28, 5493–5496 (1987). [Google Scholar]; (b) Seki H, Nakagawa M, Hashimoto A, and Hino T, Chem. Pharm. Bull 41, 1173–1176 (1987). [Google Scholar]; (c) Seki H, Hashimoto A, and Hino T, Chem. Pharm. Bull 41, 1169–1172 (1987). [Google Scholar]
- 9.(a) Ichiba T, Sakai R, Kohmoto S, Saucy G, and Higa T, Tetrahedron Lett. 29, 3083–3086 (1988). [Google Scholar]; (b) Higa T, Sakai R, and Ichiba T, “Manzamine E and F from Xestospongia as Neoplasm Inhibitor” Patent CODEN: USXXAM US 4895852 A 19900123 (1990). [Google Scholar]; (c) Kitagawa I, and Kobayashi M, Yuki Gosei Kagaku Kyokaishi 49, 1053–1061 (1991). CA116: 50671. [Google Scholar]
- 10.(a) Kondo K, Shigemori H, Kikuchi Y, Ishibashi M, Sasaki T, and Kobayashi J, J. Org. Chem 57, 2480–2483 (1992) [Google Scholar]; (b) Kondo K, Shigemori H, Kikuchi Y, Ishibashi M, Kobayashi J, and Sasaki T, Tennen Yuki Kagobutsu Toronkai 34, 463–469 (1992). CA120: 86212. [Google Scholar]
- 11.Ichiba T, Corgiat JM, Scheuer PJ, and Kelly-Borges M, J. Nat. Prod 57, 168–170 (1994). [DOI] [PubMed] [Google Scholar]
- 12.Kobayashi J, Tsuda M, Kawasaki N, Sasaki T, and Mikami Y, J. Nat. Prod 57, 1737–1740 (1994). [DOI] [PubMed] [Google Scholar]
- 13.Crews P, Cheng XC, Adamczeski M, Rodriguez J, Jaspars M, Schmitz FJ, Traeger SC, and Pordesimo EO, Tetrahedron 50, 13567–13574 (1994). [Google Scholar]
- 14.Kobayashi M, Chen YJ, Aoki S, In Y, Ishida T, and Kitagawa I, Tetrahedron 51, 3727–3736 (1995). [Google Scholar]
- 15.(a) Ohtani II, Ichiba T, Isobe M, Kelly-Borges M, and Scheuer PJ, J. Am. Chem. Soc 117, 10743–10744 (1995), and Ohtani Supplemental Pages 1–16. [Google Scholar]; (b) Ohtani II, Ichiba T, Isobe M, Kelly-Borges M, and Scheuer PJ, Tennen Yuki Kagobutsu Toronkai Koen Yoshishu 37, 236–241 (1995). CA124: 202710. [Google Scholar]
- 16.Edrada RA, Proksch P, Wray V, Witte L, Müeller WEG, and Van Soest RWM, J. Nat. Prod 59, 1056–1060 (1996). [DOI] [PubMed] [Google Scholar]
- 17.Watanabe D, Tsuda M, and Kobayashi J, J. Nat. Prod 61, 689–692 (1998). [DOI] [PubMed] [Google Scholar]
- 18.(a) El Sayed KA, Kelly M, Kara UAK, Ang KKH, Katsuyama I, Dunbar DC, Khan AA, and Hamann MT, J. Am. Chem Soc 123, 1804–1808 (2001). [DOI] [PubMed] [Google Scholar]; (b) Hamann MT, and El-Sayed KA, “Methods of Treating Drug-Resistant Infections through Administration of Pharmaceutical Compositions Containing Manzamine Alkaloids” PCT Int. Appl CODEN: PIXXD2 WO 0217917 A1 200020307 (2002). [Google Scholar]; (c) Ang KKH, Holmes MJ, and Kara UA, Parasitology Research 87, 715–721 (2001). [DOI] [PubMed] [Google Scholar]; (d) Kara AU, Higa T, Holmes M, and Ang KH, “Antimalarial Activity of Beta-Carboline Alkaloids” PCT Int. Appl CODEN: PIXXD2 WO 9959592 A1 19991125 (1999 US Patent 6,143,756. CA131: 346498. [Google Scholar]
- 19.Tsuda M, Watanabe D, and Kobayashi J, Heterocycles 50, 485–488 (1999). [Google Scholar]
- 20.(a) Tsuda M, Watanabe D, and Kobayashi J, Tetrahedron Lett. 39, 1207–1210 (1998). [Google Scholar]; (b) Kobayashi J, Tsuda M, and Ishibashi M, Pure and Applied Chem. 71, 1123–1126 (1998). [Google Scholar]
- 21.Bourguet-Kondracki ML, Martin MT, and Guyot M, Tetrahedron Lett. 37, 3457–3460 (1996). [Google Scholar]
- 22.(a) Tsuda M, Kawasaki N, and Kobayashi J, Tetrahedron Lett. 35, 4387–4388 (1994). [Google Scholar]; (b) Tsuda M, Kawasaki N, and Kobayashi J, Tennen Yuki Kagobutsu Toronkai Koen Yoshishu 36, 509–516 (1994). CA123: 193590. [Google Scholar]
- 23.Koren-Goldshlager G, Kashman Y, and Schleyer M, J. Nat. Prod 61, 282–284 (1998). [DOI] [PubMed] [Google Scholar]
- 24.Williams DE, Lassota P, and Andersen RJ, J. Org. Chem 63, 4838–4841 (1998). [Google Scholar]
- 25.Tsuda M, Kawasaki N, and Kobayashi J, Tetrahedron 50, 7957–7960 (1994). [Google Scholar]
- 26.(a) Kobayashi J, Tsuda M, Kawasaki N, Matsumoto K, and Adachi T, Tetrahedron Lett. 35, 4383–4386 (1994). [Google Scholar]; (b) Kobayashi J, Kawasaki N, and Tsuda M, Tetrahedron Lett. 37, 8203–8204 (1994). [Google Scholar]
- 27.(a) Tsuda M, Inaba K, Kawasaki N, Honma K, and Kobayashi J, Tetrahedron 52, 2319–2324 (1996). [Google Scholar]; (b) Shen Y-C, Tai H-R, and Duh C-Y, Chin. Pharm. J. (Taipei) 48, 1–10 (1996). [Google Scholar]
- 28.Rodriguez J and Crews P, Tetrahedron Lett. 35, 4719–4722 (1994). [Google Scholar]
- 29.Rodriguez J, Peters BM, Kurz L, Schatzman RC, McCarley D, Lou L, and Crews P, J. Am. Chem. Soc 115, 10436–10437 (1993). [Google Scholar]
- 30.Kong F, Andersen RJ, and Allen TM, Tetrahedron 50, 6137–6144 (1994). [Google Scholar]
- 31.Kong F, Andersen RJ, and Allen TM, Tetrahedron Lett. 35, 1643–1646 (1994). [Google Scholar]
- 32.Kong F and Andersen RJ, Tetrahedron 51, 2895–2906 (1995). [Google Scholar]
- 33.Kong F, Andersen RJ, and Allen TM, J. Am. Chem. Soc 116, 6007–6008 (1994). [Google Scholar]
- 34.Matzanke N, Gregg RJ, Weinreb SM, and Parvez M, J. Org. Chem 62, 1920–1921 (1997). [DOI] [PubMed] [Google Scholar]
- 35.Kong F, Graziani EI, and Andersen RJ, J. Nat. Prod 61, 267–271 (1998). [DOI] [PubMed] [Google Scholar]
- 36.Guo Y, Trivellone E, Scognamiglio G, and Cimino G, Tetrahedron 54, 541–550 (1998). [Google Scholar]
- 37.Kaiser A, Billot X, Gateau-Olesker A, Marazano C, and Das BC, J. Am. Chem. Soc 120, 8026–8034 (1998). [Google Scholar]
- 38.Fusetani N, Asai N, Matsunaga S, Honda K, and Yasumuro K, Tetrahedron Lett. 35, 3967–3970 (1994). [Google Scholar]
- 39.Kobayashi M, Kawazoe K, and Kitagawa I, Chem. Pharm. Bull 37(6), 1676–1678 (1989). [DOI] [PubMed] [Google Scholar]
- 40.Cimino G, Mattia CA, Mazzarella L, Puliti R, Scognamiglio G, Spinella A, and Trivellone E, Tetrahedron 45, 3863–3872 (1989). [Google Scholar]
- 41.Braekman JC, Daloze D, Macedo de Abreu P, Piccinni-Leopardi C, Germain G, and Van Meerssche M, Tetrahedron Lett. 23, 4277–4280 (1982). [Google Scholar]
- 42.Nakagawa M, Endo M, Tanaka N, and Gen-Pei L, Tetrahedron Lett. 25, 3227–3230 (1984). [Google Scholar]
- 43.Fusetani N, Yasumuro K, Matsunaga S, and Hirota H, Tetrahedron Lett. 30, 6891–6894 (1989). [Google Scholar]
- 44.Kobayashi M, Kawazoe K, and Kitagawa I, Tetrahedron Lett. 30, 4149–4152 (1989). [Google Scholar]
- 45.Cimino G, Spinella A, and Trivellone E, Tetrahedron Lett. 30, 133–136 (1989). [Google Scholar]
- 46.Guo YW, Madaio A, Trivellone E, Scognamiglio G, and Cimino G, Tetrahedron 52, 8341–8348 (1996). [Google Scholar]
- 47.Guo YW, Madaio A, Trivellone E, Scognamiglio G, and Cimino G, Tetrahedron 52, 14961–14974 (1996). [Google Scholar]
- 48.Charan RD, Garson MJ, Brereton IM, Willis AC, and Hooper JNA, Tetrahedron 52, 9111–9120 (1996). [Google Scholar]
- 49.Quirion J-C, Sevenet T, Husson H-P, Weniger B, and Debitus C, J. Nat. Prod 55, 1505–1508 (1992). [DOI] [PubMed] [Google Scholar]
- 50.Baker BJ, Scheuer PJ, and Shoolery JN, J. Am. Chem. Soc 110, 965–966 (1988). [Google Scholar]
- 51.Jaspars M, Pasupathy V, and Crews P, J. Org. Chem 59, 3253–3255 (1994). [Google Scholar]
- 52.Harrison B, Talapatra S, Lobkovsky E, Clardy J, and Crews P, Tetrahedron Lett. 37, 9151–9154 (1996). [Google Scholar]
- 53.Cimino G, Scognamiglio G, Spinella A, and Trivellone E, J. Nat. Prod 53, 1519–1525 (1990). [Google Scholar]
- 54.Toshima K, Ohta K, Ohashi A, Nakamura T, Nakata M, Tatsuta K, and Matsumura S, J. Am. Chem. Soc 117, 4822–4831 (1995). [Google Scholar]
- 55.Mayer AMS, Gunasekera SP, Pomponi SA, and Sennett SH, “Anti-inflammatory Uses of Manzamines” PCT Int. Appl, CODEN: PIXXD2 WO 0056304 A2 20000928 (2000). [Google Scholar]
- 56.(a) El Sayed KA, Dunbar DC, Perry TL, Wilkins SP, Hamann MT, Greenplate JT, and Wideman MA, Agricultural and Food Chemistry 45, 2735–2739 (1997). [Google Scholar]; (b) Nugroho BW, Edrada RA, Bohnenstengel F, Supriyono A, Eder C, Handayani D, and Proksch P, “Spodoptera littoralis as a Test Model for Insecticidal Bioassays” Natural Product Analysis: Chromatography, Spectroscopy, Biological Testing (Symposium), pp. 373–375. Wuerzburg, Germany, 1997. [Google Scholar]
- 57.(a) Kobayashi J, Watanabe D, Kawasaki N, and Tsuda M, J. Org. Chem 62, 9236–9239 (1997). [Google Scholar]; (b) Tsuda M, Watanabe D, and Kobayashi J, Tennen Yuki Kagobutsu Toronkai Koen Yoshishu 40, 467–472 (1998). CA131: 142191. [Google Scholar]
- 58.(a) Yousaf M, El Sayed KA, Rao KV, Lim CW, Hu J-F, Kelly M, Franzblau SG, Zhang F, Peraud O, Hill RT, and Hamann MT, Tetrahedron 58, 7397–7402 (2002). [Google Scholar]; (b) Kasanah N, Rao KV, Yousaf M, Wedge DE, and Hamann MT, Tetrahedron Lett. 44, 1291–1293 (2002). [Google Scholar]
- 59.Peng J, Hu J-F, Kazi AB, Franzblau SG, Zhang F, Schinazi RF, Wirtz SS, Tharnish P, Kelly M, and Hamann MT, J. Am. Chem. Soc In press (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rao KV, Santarsiero BD, Mesecar AD, Schinazi RF, Tewani BL, and Hamann MT, J. Nat. Prod 66, 823–828 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhou B-N, Slebodnick C, Johnson RK, Mattern MR, and Kingston DGI, Tetrahedron 56, 5781–5784 (2000). [Google Scholar]
- 62.Vacelet J, Vasseur P, and Lévi C, Mémoires du Muséum National D’Histoire Naturelle Serie A Zoologie XLIX, 1–116 (1976). [Google Scholar]
- 63.Desqueyroux-Faúndez R and Valentine C, Family Petrosiidae Van Soest, 1980, in “Systema Porifera: A Guide to the Classification of Sponges” (Hooper JNA and van Soest RWM, eds.), pp. 906–917. Kluwer Academic/Plenum Publishers, New York, 2002. [Google Scholar]
- 64.Kelly-Borges M and Vacelet J, Memoirs of the Queensland Museum 38, 477–503 (1995). [Google Scholar]
- 65.(a) Gillor O, Carmeli S, Rahamim Y, Fishelson Z, and Ilan M, Marine Biotechnology 2, 213–223 (2000). [DOI] [PubMed] [Google Scholar]; (b) Webster NS, and Hill RT, Marine Biol. 138, 843–851 (2000). [Google Scholar]; (c) Webster NS, Wilson KJ, Blackall LL, and Hill RT, Appl. Environ. Microbiol 67, 434–444 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Torisawa Y, Hashimoto A, Nakagawa M, and Hino T, Tetrahedron Lett. 30, 6549–6550 (1989). [Google Scholar]
- 67.Torisawa Y, Hashimoto A, Nakagawa M, Seki H, Hara R, and Hino T, Tetrahedron 47, 8067–8078 (1991). [Google Scholar]
- 68.MaGee DI and Beck EJ, Can. J. Chem 78, 1060–1066 (2000). [Google Scholar]
- 69.Arisawa M, Kato C, Kaneko H, Nishida A, and Nakagawa M, Perkin 1, 1873–1876 (2000). CA133: 237976. [Google Scholar]
- 70.Vidal T, Magnier E, and Langlois Y, Tetrahedron 54, 5959–5966 (1998). [Google Scholar]
- 71.Torisawa Y, Hashimoto A, Okouchi M, Iimori T, Nagasawa M, Hino T, and Nakagawa M, Bioorg. & Med. Chem. Lett 6, 2565–2570 (1996). [Google Scholar]
- 72.Nowak W and Gerlach H, Liebigs Ann. Chem, 153–159 (1993). [Google Scholar]
- 73.Nakagawa M, Torisawa Y, Hosaka T, Tanabe K, Tavet F, and Hino T, Tennen Yuki Kagobutsu Toronkai Toen Yoshishu 34, 408–415 (1992). [Google Scholar]
- 74.Pandit UK, Borer BC, and Bieraugel H, Pure & Appl. Chem 68, 659–662 (1996). [Google Scholar]
- 75.Nakagawa M, Torisawa Y, Hosaka T, Tanabe K, Da-te T, Okamura K, and Hino T, Tetrahedron Lett. 34, 4543–4546 (1993). [Google Scholar]
- 76.Winkler JD and Axten JM, J. Am. Chem. Soc 120, 6425–6426 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Turet L, Markó IE, Tinant B, Declercq J-P, and Touillaux R, Tetrahedron Lett. 43, 6591–6595 (2002). [Google Scholar]
- 78.Solberghe GF, Marko IE, and Batiment L, Tetrahedron Lett. 43, 5061–5065 (2002). [Google Scholar]
- 79.Humphrey JM, Liao YS, Ali A, Rein T, Wong Y-L, Chen H-J, Courtney AK, and Martin SF, J. Am. Chem. Soc 124, 8584–8592 (2002). [DOI] [PubMed] [Google Scholar]
- 80.Magnus P, Fielding MR, Wells C, and Lynch V, Tetrahedron Lett. 43, 947–950 (2002). [Google Scholar]
- 81.Karle M, and Koert U, Org. Syn. Highlights IV, 91–96 (2000). CA136: 134290. [Google Scholar]
- 82.Gomez J-M, Gil L, Ferroud C, Gateau-Olesker A, Martin M-T, and Marazano C, J. Org. Chem 66, 4898–4903 (2001). [DOI] [PubMed] [Google Scholar]
- 83.Lindsay HA, Salisbury CL, Cordes W, and McIntosh MC, Org. Lett 3, 4007–4010 (2001). [DOI] [PubMed] [Google Scholar]
- 84.Urban D, Duval E, and Langlois Y, Tetrahedron Lett. 41, 9251–9256 (2000). [Google Scholar]
- 85.Arisawa M, Takahashi M, Takezawa E, Yamaguchi T, Torisawa Y, Nishida A, and Nakagawa M, Chem. Pharm. Bull 48, 1593–1596 (2000). [DOI] [PubMed] [Google Scholar]
- 86.Nakagawa M, J. Heterocyclic Chem 37, 567–581 (2000). [Google Scholar]
- 87.Kotsukibi K and Gamou K, Chokoatsu Yuki Gosei, 299–314 (1999). [Google Scholar]
- 88.Nakagawa M, Torisawa Y, Uchida H, and Nishida A, Yuki Gosei Kagaku Kyokaishi (J. Synth. Org. Chem. Japan), 57, 1004–1015 (1999). CA131: 351494. [Google Scholar]
- 89.Coldham I, Crapnell KM, Fernández J-C, Moseley JD, and Rabot R, J. Org. Chem 67, 6181–6187 (2002). [DOI] [PubMed] [Google Scholar]
- 90.Coldham I, Fernandez J-C, Rabot R, and Crapnell KM, Abstr. Pap.-Am. Chem. Soc 221st ORGN-027 (2001). [Google Scholar]
- 91.Coldham I, Crapnell KM, Fernandez J-C, Haxell TFN, Treacy AB, Coles SJ, Hursthouse MB, and Moseley JD, Chem. Commun 1757–1758 (1999). [Google Scholar]
- 92.Bland D, Chambournier G, Dragan V, and Hart DJ, Tetrahedron 55, 8953–8966 (1999). [Google Scholar]
- 93.Uchida H, Kimura Y, Yamabe M, Nishida A, and Nakagawa M, Tennen Yuki Kagobutsu Toronkai Koen Yoshishu 41, 67–72 (1999). CA132: 279387. [Google Scholar]
- 94.Uchida H, Nishida A, and Nakagawa M, Tetrahedron Lett. 40, 113–116 (1999). [Google Scholar]
- 95.Uchida H, Takezawa E, Kawate T, Nishida A, and Nakagawa M, Tennen Yuki Kagobutsu Toronkai Koen Yoshishu 40, 601–606 (1998). CA131: 228862. [Google Scholar]
- 96.Morimoto Y, Yokoe C, Kurihara H, and Kinoshita T, Tetrahedron 54, 12197–12214 (1998). [Google Scholar]
- 97.Lee ML, and MaGee DI, 216th ACS National Meeting (Boston, August 23–27), ORGN-553 (1998). [Google Scholar]
- 98.Goldring WPD, Hodder AS, and Weiler L, Tetrahedron Lett. 39, 4955–4958 (1998). [Google Scholar]
- 99.Morimoto Y and Yokoe C, Tetrahedron Lett. 38, 8981–8984 (1997). [Google Scholar]
- 100.Bland D, Hart DJ, and Lacoutiere S, Tetrahedron 53, 8871–8880 (1997). [Google Scholar]
- 101.Sageot O, Monteux D, Langlois Y, Riche C, and Chiaroni A, Tetrahedron Lett. 37, 7019–7022 (1996). [Google Scholar]
- 102.Torisawa Y, Soe T, Katoh C, Motohashi Y, Nishida A, Hino T, and Nakagawa N, Heterocycles 47, 655–659 (1998). [Google Scholar]
- 103.Torisawa Y, Hosaka T, Tanabe K, Suzuki N, Motohashi Y, Hino T, and Nakagawa M, Tetrahedron 52, 10597–10608 (1996). [Google Scholar]
- 104.Torisawa Y, Ali MA, Tavet F, Kageyama A, Aikawa M, Fukui N, Hino T, and Nakagawa M, Heterocycles 42, 677–689 (1996). [Google Scholar]
- 105.Torisawa Y, Motohashi Y, and Nakagawa M, Tennen Yuki Kagobutsu Toronkai Koen Yoshishu 37, 451–456 (1995). CA124: 202699. [Google Scholar]
- 106.Magnier E and Langlois Y, Tetrahedron 54, 6201–6258 (1998). [Google Scholar]
- 107.Magnier E and Langlois Y, Tetrahedron Lett. 39, 837–840 (1998). [Google Scholar]
- 108.Magnier E, Langlois Y, and Mérienne C, Tetrahedron Lett. 36, 9475–9478 (1995). [Google Scholar]
- 109.Bieraugel H, Borer BC, and Pandit UK, Heterocyclic Commun. 1, 115–118. CA123: 56358 (1995). [Google Scholar]
- 110.Clark JS, Hodgson PB, Goldsmith MD, Blake AJ, Cooke PA, and Street LJ, J. Chem. Soc., Perkin Trans 1, 3325–3337 (2001). [Google Scholar]
- 111.(a) Clark JS, Townsend RJ, Blake AJ, Teat SJ, and Johns A, Tetrahedron Lett. 42, 3235–3238 (2001). [Google Scholar]; (b) Townsend RJ, and Clark JS, 216th ACS National Meeting (Boston, August 23–27), ORGN-551 (1998). [Google Scholar]
- 112.Clark JS and Hodgson PB, Tetrahedron Lett. 36, 2519–2522 (1995). [Google Scholar]
- 113.Li SM and Yamamura S, Tetrahedron 54, 869–8710 (1998). [Google Scholar]
- 114.Li SM, Kosemura S, and Yamamura S, Tetrahedron 54, 6661–6676 (1998). [Google Scholar]
- 115.Li SM, Yamamura S, Hosomi H, and Ohba S, Tetrahedron Lett. 39, 2601–2604 (1998). [Google Scholar]
- 116.Li SM and Yamamura S, Tetrahedron Lett. 39, 2597–2600 (1998). [Google Scholar]
- 117.Li SM, Ohba S, Kosemura S, and Yamamura S, Tetrahedron Lett. 37, 7365–7368 (1996). [Google Scholar]
- 118.Li SM, Kosemura S, and Yamamura S, Tetrahedron Lett. 35, 8217–8220 (1994). [Google Scholar]
- 119.Leonard J, Fearnley SP, Finlay MR, Knight JA, and Wong G, J. Chem. Soc, Perkin Trans. 1: Organic and Bio-Organic Chemistry 2359–2361 (1994). [Google Scholar]
- 120.Ma J, Nakagawa M, Torisawa Y, and Hino T, Heterocycles 38, 1609–1618 (1994). [Google Scholar]
- 121.Kamenecka TM and Overman LE, Tetrahedron Lett. 35, 4279–4282 (1994). [Google Scholar]
- 122.Borer BC, Deerenberg S, Bieräugel H, and Pandit UK, Tetrahedron Lett. 35, 3191–3194 (1994). [Google Scholar]
- 123.Pandit UK, Overkleeft HS, Borer BC, and Bieräugel H, Eur. J. Org. Chem, 959–968 (1999). [Google Scholar]
- 124.Pandit UK, Synthetic Studies on Anti-tumor Alkaloids. New Trends in Natural Product Chemistry, [International Symposium on Natural Product Chemistry], 15–22 (1998). CA129: 216790. [Google Scholar]
- 125.Pandit UK, Farmaco 50, 749–754 (1995) CA124: 87434. [PubMed] [Google Scholar]
- 126.Pandit UK, Borer BC, Bieraugel H, and Deerenberg S, Pure & Appl. Chem 66, 2131–2134 (1994). [Google Scholar]
- 127.Pandit UK, J. Heterocycl. Chem 31, 615–624 (1994). CA121: 109360. [Google Scholar]
- 128.Hino T and Nakagawa M, J. Heterocyclic. Chem 31, 625–630 (1994). [Google Scholar]
- 129.Martin SF, Humphrey JM, Ali A, and Hillier MC, J. Am. Chem. Soc 121, 866–867 (1999). [Google Scholar]
- 130.Martin SF, Chen H-J, Courtney AK, Liao YS, Pätzel M, Ramser MN, and Wagman AS, Tetrahedron 52, 7251–7264 (1996). [Google Scholar]
- 131.Martin SF, Liao Y, Wong Y, and Rein T, Tetrahedron Lett. 35, 691–694 (1994). [Google Scholar]
- 132.Winkler JD, Stelmach JE, Siegel MG, Haddad N, Axten J, and Dailey WP III., Isr. J. Chem 37, 47–67 (1997). [Google Scholar]
- 133.(a) Winkler JD, Axten J, Hammach AH, Kwak Y-S, Lengweiler U, Lucero MJ, and Houk KN, Tetrahedron 54, 7045–7056 (1998). [Google Scholar]; (b) Axten JM, and Winkler JD, 216th ACS National Meeting (Boston, August 23–27), ORGN-525 (1998). [Google Scholar]
- 134.Winkler JD, Siegel MG, and Stelmach JE, Tetrahedron Lett. 34, 6509–6512 (1993). [Google Scholar]
- 135.Nakagawa M, Torisawa Y, Hosaka T, Tanabe K, Da-te T, Okamura K, and Hino T, Tetrahedron Lett. 34, 4543–4546 (1993). [Google Scholar]
- 136.Longley RE, McConnell OJ, Essich E, and Harmody D, J. Nat. Prod 56, 915–920 (1993). [DOI] [PubMed] [Google Scholar]
- 137.Campbell JA and Hart DJ, J. Org. Chem 58, 2900–2903 (1993). [Google Scholar]
- 138.Campbell JA and Hart DJ, Tetrahedron Lett. 33, 6247–6250 (1992). [Google Scholar]
- 139.Leonard J, Fearnley SP, and Hickey DMB, Synlett, 272–274 (1992). [Google Scholar]
- 140.Lynch VM, Liao YS, Martin SF, and Davis BE, Acta Cryst. Sect. C: Cryst. Struct. Commun 48, 1703–1705 (1992). [DOI] [PubMed] [Google Scholar]
- 141.Torisawa Y, Nakagawa M, Hosaka T, Tanabe K, Lai Z, Ogata K, Nakata T, Oishi T, and Hino T, J. Org. Chem 57, 5741–5747 (1992). [Google Scholar]
- 142.Marko IE, Southern JM, and Adams H, Tetrahedron Lett. 33, 4657–4660 (1992). [Google Scholar]
- 143.Marko IE and Chesney A, Synlett, 275–278 (1992). [Google Scholar]
- 144.Brands KMJ and DiMichele LM, Tetrahedron Lett. 39, 1677–1680 (1998). [Google Scholar]
- 145.Brands KMJ, Meekel AAP, and Pandit UK, Tetrahedron 47, 2005–2026 (1991). [Google Scholar]
- 146.Martin SF, Rein T, and Liao YS, Tetrahedron Lett. 32, 6481–6484 (1991). [Google Scholar]
- 147.Imbroisi DDO and Simpkins NS, J. Chem. Soc. Perkin Trans 1, 1815–1823 (1991). [Google Scholar]
- 148.Brands KMJ and Pandit UK, Heterocycles 30, 257–261 (1990). [Google Scholar]
- 149.Nakagawa M, Lai ZP, Torisawa Y, and Hino T, Heterocycles 31, 999–1002 (1990). [Google Scholar]
- 150.Chesney A and Marko IE, Synth. Commun 20, 3167–3180 (1990). [Google Scholar]
- 151.Torisawa Y, Nakagawa M, Arai H, Lai ZP, Hino T, Nakata T, and Oishi T, Tetrahedron Lett. 31, 3195–3198 (1990). [Google Scholar]
- 152.Brands KMJ and Pandit UK, Tetrahedron Lett. 30, 1423–1426 (1989). [Google Scholar]
- 153.Hart DJ and McKinney JA, Tetrahedron Lett. 30, 2611–2614 (1989). [Google Scholar]
- 154.Mohan R, Chou YL, and Morrissey MM, Tetrahedron Lett. 37, 3963–3966 (1996). [Google Scholar]
- 155.Herdemann M, Gateau-Olesker A, Al Mourabit A, and Marazano C, Biomimetic Model of Manzamines: Cycloaddition of Aminopentadienes and Dihydropyridiniums. 2nd Euroconference on Marine Natural Products (Spain, Sept.) 168 (1999). [Google Scholar]
- 156.Matzanke N, Gregg RJ, and Weinreb SM, Org. Prep. Proced. Int 30, 1–51 (1998). [Google Scholar]
- 157.Baldwin JE, Claridge TDW, Culshaw AJ, Heupel FA, Lee V, Spring DR, and Whitehead RC, Chem. Eur. J 5, 3154–3161 (1999). [Google Scholar]
- 158.Baldwin JE, Claridge TDW, Culshaw AJ, Heupel FA, Lee V, Spring DR, Whitehead RC, Boughtflower RJ, Mutton IM, Upton RJ, Angew. Chem. Int. Ed 37, 2661–2663 (1998). [DOI] [PubMed] [Google Scholar]
- 159.Baldwin JE, Bischoff L, Claridge TDW, Heupel FA, Spring DR, and Whitehead RC, Tetrahedron 53, 2271–2290 (1997). [Google Scholar]
- 160.Baldwin JE, Claridge TDW, Culshaw AJ, Heupel FA, Smrcková S, and Whitehead RC, Tetrahedron Lett. 37, 6919–6922 (1996). [Google Scholar]
- 161.Baldwin JE, Claridge TDW, Heupel FA, and Whitehead RC, Tetrahedron Lett. 35, 7829–7832 (1994). [Google Scholar]
- 162.Herdemann M, Al-Mourabit A, Martin M-T, and Marazano C, J. Org. Chem 67, 1890–1897 (2002). [DOI] [PubMed] [Google Scholar]
- 163.Jakubowicz K, Ben Abdeljelil K, Herdemann M, Martin M-T, Gateau-Olesker A, Al Mourabit A, Marazano C, and Das BC, J. Org. Chem 64, 7381–7387 (1999). [Google Scholar]
- 164.Kobayashi J and Tsuda M, Yuki Gosei Kagaku Kyokaishi (J. Synth. Org. Chem. Japan) 55, 1114–1123 (1997). CA128: 61660. [Google Scholar]
- 165.Kobayashi J, Farumashia 31, 869–873 (1995). CA123: 139226. [Google Scholar]
- 166.Kobayashi J, Gendai Kagaku 29, 47–53 (1995). CA123: 79881. [Google Scholar]
- 167.Gil L, Baucherel X, Martin M-T, Marazano C, and Das BC, Tetrahedron Lett. 36, 6231–6234 (1995). [Google Scholar]
- 168.Gil L, Gateau-Olesker A, Marazano C, and Das BC, Tetrahedron Lett. 36, 707–710 (1995). [Google Scholar]
- 169.Shen GQ and Baker BJ, Tetrahedron Lett. 35, 1141–1144 (1994). [Google Scholar]
- 170.Ang KKH, Holmes MJ, Higa T, Hamann MT, and Kara UAK, Antimicrobial Agents and Chemotherapy 44, 1645–1649 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]


