Abstract
Everyday experience suggests that sleep and affect are closely linked, with daytime affect influencing how we sleep, and sleep influencing subsequent affect. Yet empirical evidence for this bidirectional relationship between sleep and affect in non-clinical adult samples remains mixed, which may be due to heterogeneity in both construct definitions and measurement. This conceptual review proposes a granular framework that deconstructs sleep and affect findings according to three subordinate dimensions, namely domains (which are distinct for sleep and affect), methods (i.e., self-report vs. behavioral/physiological measures), and timescale (i.e., shorter vs. longer). We illustrate the value of our granular framework through a systematic review of empirical studies published in PubMed (N = 80 articles). We found that in some cases, particularly for sleep disturbances and sleep duration, our framework identified robust evidence for associations with affect that are separable by domain, method, and timescale. However, in most other cases, evidence was either inconclusive or too sparse, resulting in no clear patterns. Our review did not find support for granular bidirectionality between sleep and affect. We suggest a roadmap for future studies based on gaps identified by our review and discuss advantages and disadvantages of our granular dimensional framework.
Keywords: Affect, Sleep, Conceptual review, Framework, Granularity, Methods, Timescales
1. Introduction
Everyday experience tells us that certain affective states can make it difficult to fall asleep, sleep well, or sleep enough. It also suggests that poor sleep might impair affective functioning. These common experiences are congruent with the widespread view that sleep and affect have bidirectional links (see Fig. 1) [1,2]. Despite the intuitive appeal of this idea, the empirical evidence for links between sleep and affect remains mixed [1–6]. Among individuals with mental health disorders such as insomnia, anxiety, depressive, post-traumatic stress, and bipolar disorders, affective states do seem to be related to sleep [7,8]. However, while it is commonly assumed that the link between sleep and affect is also evident in non-clinical populations, the empirical evidence is not as strong [1,5] as might be expected from widespread lay beliefs.
Fig. 1.
Bidirectional spiral depicting relationships between sleep and affect unfolding dynamically over time.
In this article, we describe a framework for organizing the complex and heterogeneous literature on associations between sleep and affect, combining distinctions from sleep science and affective science to illuminate patterns of empirical results at the intersection of the two fields. We then demonstrate how this framework can be used to clarify where empirical research currently stands on the following three questions, related to three broad study designs: 1) What are the cross-sectional associations between sleep and affect? 2) What can we learn from studies in which affective experience temporally precedes sleep experience? and 3) What can we learn from studies in which sleep experience temporally precedes affective experience? We organize our results in three sections corresponding to each of these three questions. We conclude with suggestions for researchers moving forward.
2. Our granular dimensional framework
Sleep scientists and affective scientists each make distinctions within their respective fields in terms of three dimensions: domains, methods, and timescales. We believe that attending to these three dimensions at once (see schematic of our framework in Fig. 2A) could deepen our understanding of points of contact between sleep and affect, help explain current inconsistencies, and identify gaps in knowledge. We discuss each of these three dimensions below.
Fig. 2.
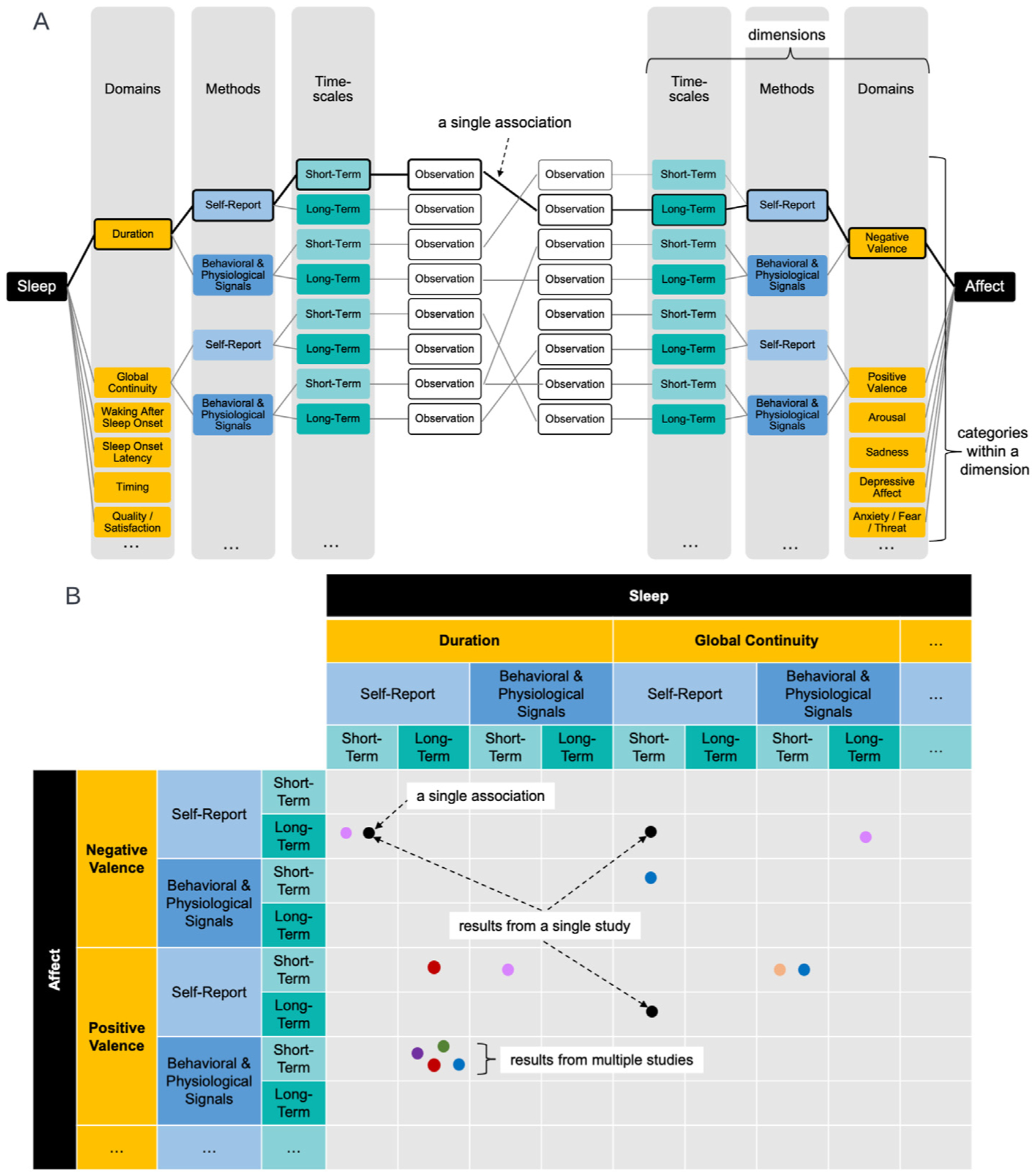
Organizing framework concept and execution.
Panel A: Conceptual representation of our framework describing sleep and affect constructs (in black) as three dimensions. Domains are represented in gold and populated with six example categories. Methods are represented in blue and populated with two example categories. Timescales are represented in teal and populated with two categories. These specific categories are flexible, as represented by the ellipses, so the number and identity of categories within each dimension can change, as well as the number and identity of dimensions. In the present review, we used the following categories: 10 sleep and 14 affect domains (see Tables S2–S4 for the full set of domains used in the present review), two methods, self-report (in light blue) and behavioral/physiological signals (in bold blue); and two timescales, short-term (in light teal) and long-term (in bold teal).
The diagonal gray lines represent a hypothetical study in which sleep and affect are each assessed within two categories of domain, and for each of those, two methods at two timescales, for a total of eight observations. In this example, there are seven unique associations. As an example of how to interpret each association, we draw attention to one single association (represented by a thick black line); the association is between short-term self-reported sleep duration and long-term self-reported negative valence.
Panel B: Representation of the practical execution of organizing multiple studies into matrices (see Tables S2–S4) using the conceptual structure depicted in Panel A. The single association from Panel A is entered in one cell (represented as a black dot). Multiple associations from one study table (multiple black dots) as well as from different studies (different colored dots) can be entered into the table. In order to derive conclusions about associations, we analyze results from multiple studies in a given cell. Different study designs, population samples, or other categorical moderators can be expressed as different versions of this matrix.
2.1. Domains
Sleep and affect are each multi-faceted constructs that encompass a suite of experiences, behaviors, and physiological responses with separable and overlapping aspects [9,10]. They each contain distinct domains (depicted in gold in Fig. 2A); therefore we describe them separately.
For sleep, we start with Buysse’s [11] five sleep health domains, namely: 1) sleep duration, usually assessed by total sleep time; 2) sleep continuity, which can be captured by sleep efficiency, defined as the percent of time spent asleep, or as sleep fragmentation, using fragmentation indices, minutes awake after sleep onset, or percentage of time awake during specific sleep stages; 3) sleep timing, indexed by bedtime, wake time, dim light melatonin onset, circadian nadir, or sleep timing relative to circadian preference; 4) sleep quality or satisfaction, a subjective perception of sleep typically assessed by self-report; and 5) sleepiness and alertness, which can be measured either as a subjective perception or by performance on tasks that require cognitive and/or attentional vigilance. For this review, we broke the domain of sleep continuity into global and specific measures of continuity. For global sleep continuity, we included, for example, sleep efficiency and total wake time (time to sleep onset plus time awake after sleep onset). For specific sleep continuity, we included two domains: sleep onset latency (SOL) and wakefulness after sleep onset (WASO; e.g., minutes awake after sleep onset and number of awakenings). We also expanded the satisfaction/quality domain by adding a sleep disturbance domain, as measured by global sleep disturbance questionnaires such as the Pittsburgh Sleep Quality Index [12] and the Insomnia Severity Index [13]. Sleep research also examines aspects of sleep that have not been fully mapped to these five domains. Therefore, we added the following domains: a) sleep architecture, in order to capture macro- and micro-level neural activity such as sleep stages and spectral frequency during sleep, and b) autonomic activity, encompassing neuroendocrine, cardiovascular, and neuromodulatory systems, in order to capture activation in the body during sleep.
For affect, we included four domains: negative valence, positive valence, arousal, and specific emotions. We draw these categories both from the range of published studies on bidirectional sleep-affect links and from well-accepted categorization of affect along two core axes, valence and arousal, that correspond to a continuum of pleasantness to unpleasantness and a continuum from low to high activation, respectively. Specific emotions such as sadness, joy, anger, and fear are thought to occupy different positions on a cir-cumplex of affect defined by the axes of valence and arousal [14]. As with sleep, some affective constructs show empirical separability, while others represent a mixture of related domains. We included domains for both positive and negative valence because empirical work indicates that they are separable and can co-occur in the same affective experience [15,16]. The specific emotion categories we focus on represent the constructs described in the literature reviewed for this article, but do not represent all specific emotions.
2.2. Methods
We distinguish two broad methods of assessment (depicted in blue in Fig. 2A), namely self-report and behavioral and physiological signals. We favor this terminology over the common “subjective vs. objective” distinction because, for both sleep and affect, we face limitations in our ability to ground-truth the measurements typically deemed “objective” without relating them to “subjective” measures.
Self-report measures of sleep include questionnaires and daily diaries. Behavioral/physiological measures of sleep include measures of movement, such as actigraphy, brain and muscle activity, usually captured with polysomnography (PSG), and other indices of sleep health such as electrocardiography (ECG) [17,18]. In general, sleep researchers tend to place the greatest confidence in the precision of polysomnography, considered the “gold standard,” moderate confidence in measures like actigraphy and daily diary, and least confidence in questionnaires about habitual sleep. At the same time, PSG is not a feasible measure of habitual sleep over extended periods of time, nor is it able to assess aspects of sleep related to one’s perception. There is general acknowledgement that actigraphy and daily diary assessed across multiple weeks have greater ecological validity than a few nights of laboratory polysomnography [18,19]. Self-reported sleep quality has only moderate agreement with behavioral/physiological measures of sleep [19,20], suggesting that the subjective experience of sleep is not fully captured by behavioral/physiological measures. The agreement between self-report and behavioral/physiological measures of sleep vary by population and sleep variable of interest. One can argue that there is valuable information in the level of disagreement between self-report and behavioral/physiological sleep measures (e.g., “sleep state misperception”) and that it might be best to view the two as complementary. We also note that, unlike behavioral/physiological measures of sleep, self-reported measures of sleep are inherently retrospective as they cannot be obtained during the experience of sleeping.
In affective science, affect is usually assessed via a combination of self-report measures, such as questionnaires or ratings of momentary states, and a host of behavioral/physiological measures of affect that assess responses to affective stimuli. The latter include reaction time, measures of facial expressions using electromyography or facial coding, autonomic measures, such as ECG and skin conductance response to affective stimuli, and neural measures, such as electroencephalography (EEG) and functional magnetic resonance imaging (fMRI) [21]. Affective scientists argue that use of the terms “subjective”/“objective” can prevent researchers from taking seriously internal qualia that subjects report as well as behavioral/physiological indices of internal processes. Instead, affect researchers tend to consider a suite of “loosely coupled” [22] measures as providing a holistic picture of the construct under study [23]. Measures of affect tend to demonstrate moderate coherence at best, but, as is the case for sleep scientists, affective scientists argue that the degree of coherence between channels may itself carry important information.
2.3. Timescales
We distinguish measures based on the time window they capture (i.e., timescale of interest; depicted in teal in Fig. 2A). For simplicity, we use two broad categories, short-term and long-term. We define short-term as measures examining one or a few instances of sleep or affect, collected over 48 h or less, and long-term as measures examining either multiple instances or summarizing across multiple instances, collected over more than 48 h. The choice of 48 h as a cutoff is a compromise between timescales used in sleep and affective literatures and is somewhat arbitrary. The translation of the terminology of short- and long-term timescales from the terminology of “state vs. trait” is complex. For example, one individual’s single instance measurement may reflect their person-level average, as well as the variance away from their average at that particular instance. Likewise, measuring multiple instances over the long-term allows for calculation of person-level averages as well as estimates of within-person variation.
Temporal dynamics are extremely important to consider when studying sleep, since sleep patterns on one night can influence subsequent sleep in the next hours or days. This is because homeostatic sleep drive builds up over the course of a day and, if unfulfilled, continues to increase until full recovery sleep is attained [24]. The timescale of interest, the resolution of measurement, and the proximity of measurement to the sleep period are all relevant in sleep research. The timescale of interest may be a single or multiple sleep opportunities, depending on the research question. The resolution, captured by the sampling rate and summary strategy, may be milliseconds, minutes, hours, or longer. Examples of coarse resolution measurements include asking individuals to summarize their sleep across periods of weeks, months, or years. For instance, sleep disturbances are usually measured with questionnaires that assess habitual sleep and hence most measures are inherently long-term. In most cases, sampling at a higher frequency over a longer period is considered more ecologically valid, even when observations are summarized, than sampling fewer nights or shorter periods. The timing of sleep assessments is also relevant. Sleep can be measured as it occurs, or later. In the case of later reporting, the time since sleep occurred may vary widely from a few hours to weeks or longer. Characterizing the timing of sleep and its measurements is also important because sleep’s internal structure and duration are impacted by the circadian regulation of sleep [24].
Affective states, even when measured at a single moment in time, represent a process of generation and regulation that ranges from seconds to minutes, hours or days [10]. Initially, we attend to some internal or external state of the world, make an appraisal or valuation of its meaning, and generate a response, whether an external behavior or an internal thought. Each action then begets a new state of the world, setting off the cycle again. Essentially, affect can be considered as a time series consisting of fluctuating states set off by perceptions, valuations, and responses to states of the world. One affective state may continue to influence future affective states. The timescales for these affective states are variable. For example: valuations and appraisals that form within hundreds of milliseconds, thoughts within seconds, autonomic system responses within seconds, cortisol and hypothalamic-pituitary-adrenal axis activation over the course of minutes, moods that last minutes or hours, and personality traits such as neuroticism that remain stable across contexts for weeks, months, and years [10,25]. As in the case of timescales for assessing sleep, assessing affect may vary across experimental designs in terms of the time-scale of interest, the resolution of measurement (i.e., number, timing of assessments, and summary strategies), and the proximity of the measure to the affective experience. For example, some studies of affect measure it at a single time and others at repeated time points; some use measures reflecting affect over several days and others measure affect as it is experienced at multiple time points (e.g., ecological momentary assessments).
3. Review of empirical studies from PubMed
We applied our framework to a systematic review of the PubMed database [26]. The review was pre-registered on Open Science Framework, available at https://osf.io/7rksp/?view_only=bee231f528424e0e90e5c557e7da63be. This registration in cludes the search terms and inclusion/exclusion criteria. The PubMed database was searched from inception through September 1, 2020 with terms combining “affect,” “mood,” or “emotion” (and appropriate derivations) with “sleep.” Search limitations were placed on age (adults/young adults/middle aged) and subjects (human). The search string is provided in the supplemental materials. Rayyan web-based software [27] was used to organize and screen studies.
The following inclusion criteria were used to select studies: 1) original, published peer-reviewed articles that included healthy human participants between the ages of 18–65; 2) assessed affective experience with either self-report or behavioral/physiological data; (3) assessed sleep parameters with either self-report or behavioral/physiological data; and (4) had the full text available in English. Studies were excluded if they 1) only assessed affective memory, learning, specific cognitions relating to sleep or affect, stress, or affect regulation (and did not otherwise include a measure of affective experience)1; 2) used a mixed-age sample which included participants outside of the 18–65 range; 3) did not focus on a healthy (i.e., non-clinical) sample or did not include a sub-sample/control group/subgroup analysis of healthy participants, 4) included participants likely to have a shift work or non-traditional sleep schedule (e.g., nighttime caregivers); 5) included a sleep or affect intervention without reporting relationships between sleep and affect at baseline; 6) were systematic reviews, meta-analyses, dissertations, editorials, or conference proceedings; or 7) had an N < 25.
The search process is reported according to PRISMA guidelines, when applicable (see PRISMA checklist in Supplementary Materials). See Fig. 3 for the description of the flow of the review process. Publications were independently reviewed at the title/abstract level by two authors (JRD & JT) against the inclusion/exclusion criteria. Studies that progressed to the next phase of review were then reviewed, further screened, and categorized by both authors, as described below, and discrepancies were resolved by consensus involving a third author (MtB, RM) as needed.
Fig. 3.
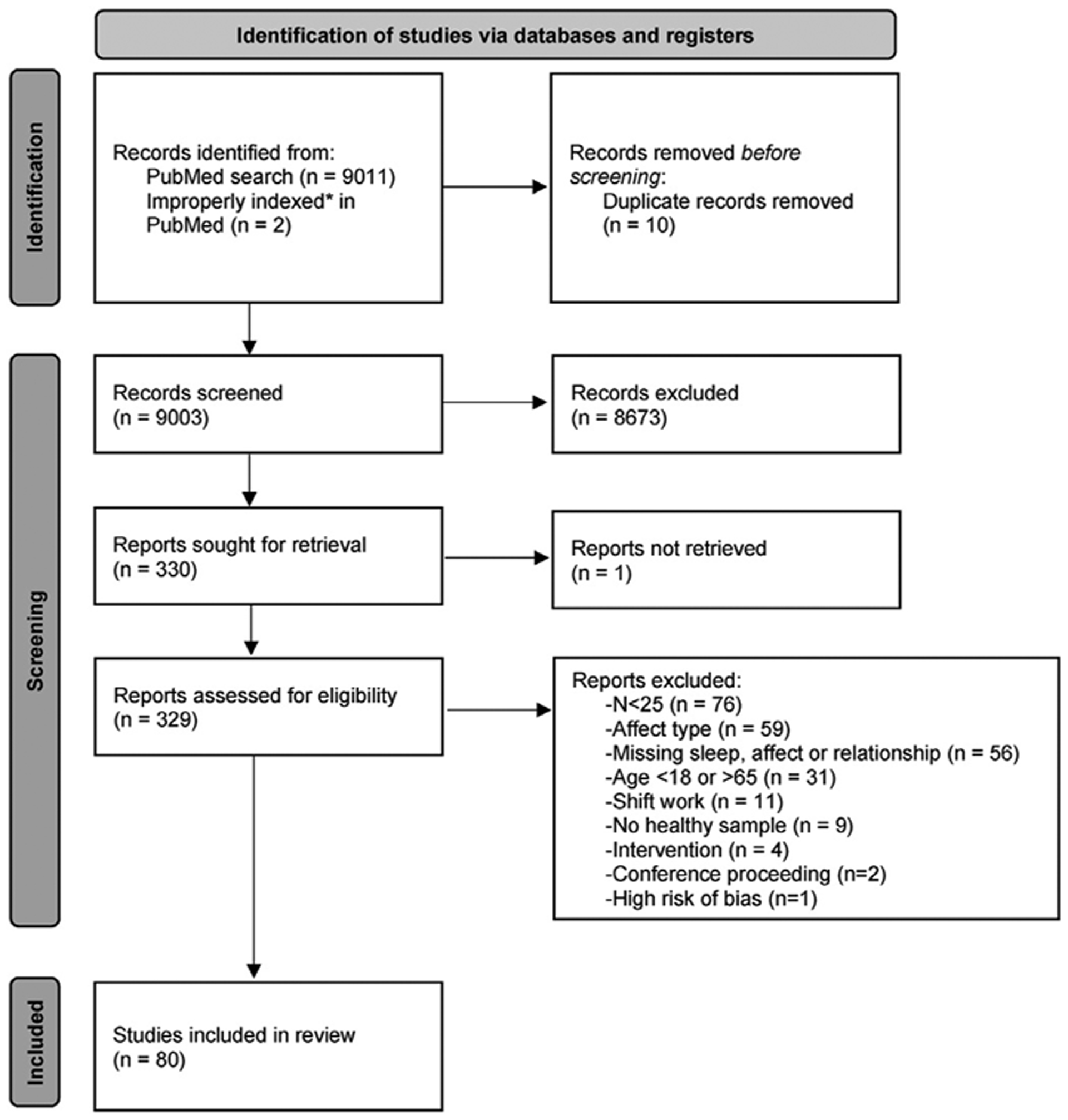
PRISMA 2020 flow diagram for new systematic reviews that included searches of databases and registers only.
Note: Two studies (indicated by an asterisk, *) that were known to the authors were not picked up by the search due to a PubMed classification error.
We also conducted a systematic risk of bias assessment using the National Heart Lung and Blood Institute Quality Assessment Tool for Observational Cohort and Cross-Sectional Studies [30]. Two independent raters assessed the risk of bias of each manuscript (see Table S1). There was a high inter-rater agreement, with only 7 manuscripts requiring discussion to reach agreement. A final exclusion criterion was having high risk of bias (poor quality).
We entered the citation for each coded study in Table S1. As shown in the schematic matrix in Fig. 2B, we entered the findings from each study into three tables. We classified studies as “cross-sectional” when participants’ sleep and affect experiences occurred in overlapping time windows, or if the temporal ordering of the experiences could not be determined (Table S2). We classified studies as “affect to sleep” when the affective experience temporally preceded the sleep experience (Table S3), and as “sleep to affect” when the sleep experience temporally preceded the affective experience (Table S4). Studies were categorized as temporally sequential based on the period of time the sleep/affect was experienced, ignoring the timing of the measurement. We note that, although we included in Tables S3 and S4 mostly studies in which both sleep and affect were measured repeatedly or at different times, we did also include studies in which sleep and affect were each measured at a single time point, even though the affective experience and the sleep experience referred to in the measurement were temporally sequenced and non-overlapping. We therefore caution against interpreting temporal sequencing as causal effects.
Each column and row is split by dimensions (see Fig. 2B): domain, method (either self-report or behavioral/physiological signals), and timescale (either short- or long-term). Each column represents one particular domain of affect measured using one methodology (either self-report or behavioral/physiological signals) at a particular timescale (either short- or long-term). Each cell represents the association between that particular sleep and affect sub-construct.
For each study, we extracted and coded the direction of associations between sleep and affect variables based on whether there was a significant positive or negative linear association or no evidence for a linear association. In addition, we coded the consistency of associations when different measures were reported within a single study that we categorized as belonging in the same domain, method, and timescale; namely, we coded whether there were a combination of multiple significant and non-significant associations, or a combination of significant associations in opposing directions. Studies sometimes appeared in multiple cells (see black dots in Fig. 2B) and occasionally appeared in multiple tables, as a given study frequently included multiple methods and findings.
We then summarized broad patterns in Figs. 4–6, with each figure corresponding to Tables S2, S3, and S4, respectively. In each of the three figures, we included only sleep and affect variables (columns and rows) in which at least one of the cells was populated by at least three results (see schematic in Fig. 2B) coded as low risk of bias (i.e., “good” in Table S1). We used the following color coding to indicate our conclusions for directions of association: orange for no evidence of an association, red for negative, and green for positive; and bold or light hues to indicate the robustness of a given association. In each cell of each figure (see Figs. 4–6), the number indicates how many results were available in that cell and the bubble size indicates our confidence in the result.
Fig. 4.
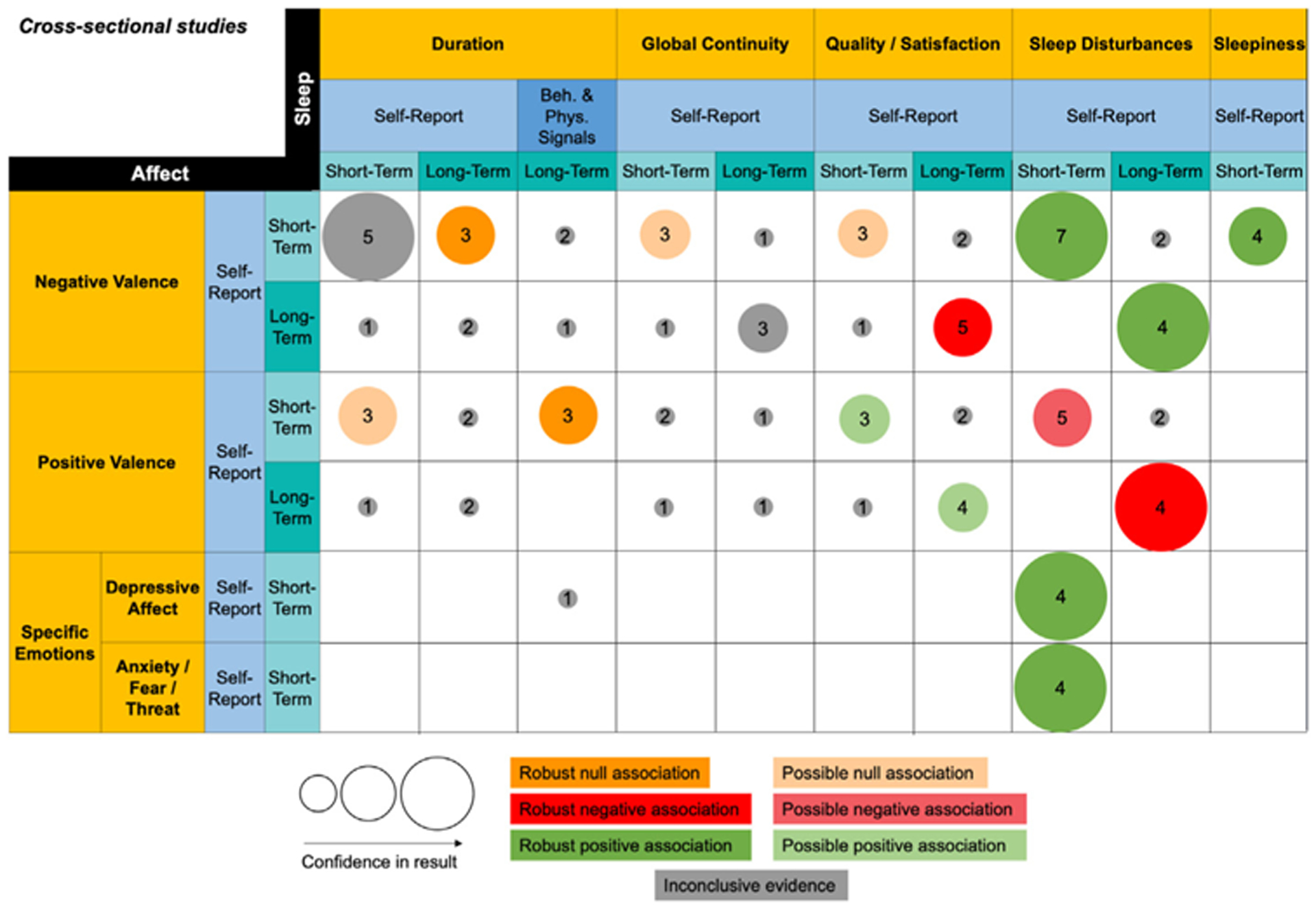
Summary of findings for cross-sectional associations between sleep and affect.
This figure summarizes Table S2. Only columns and rows from Table S2 that contain at least three studies in one cell are included. The column and row headers represent domains (gold), methods (blue), and timescales (teal). The size of the bubble represents our confidence in the strength of the result. Low, moderate, and high confidence correspond to small, medium, and large bubbles respectively. The number in the center of the bubble represents the number of studies in a given cell. The color of the bubble represents the direction of evidence for the association. Light hues represent possible evidence for association and bold hues represent robust evidence for association. All associations are in the direction represented by their domain labels. For a positive association between duration and negative valence, for example, that means that higher negative valence is associated with longer duration.
Fig. 6.
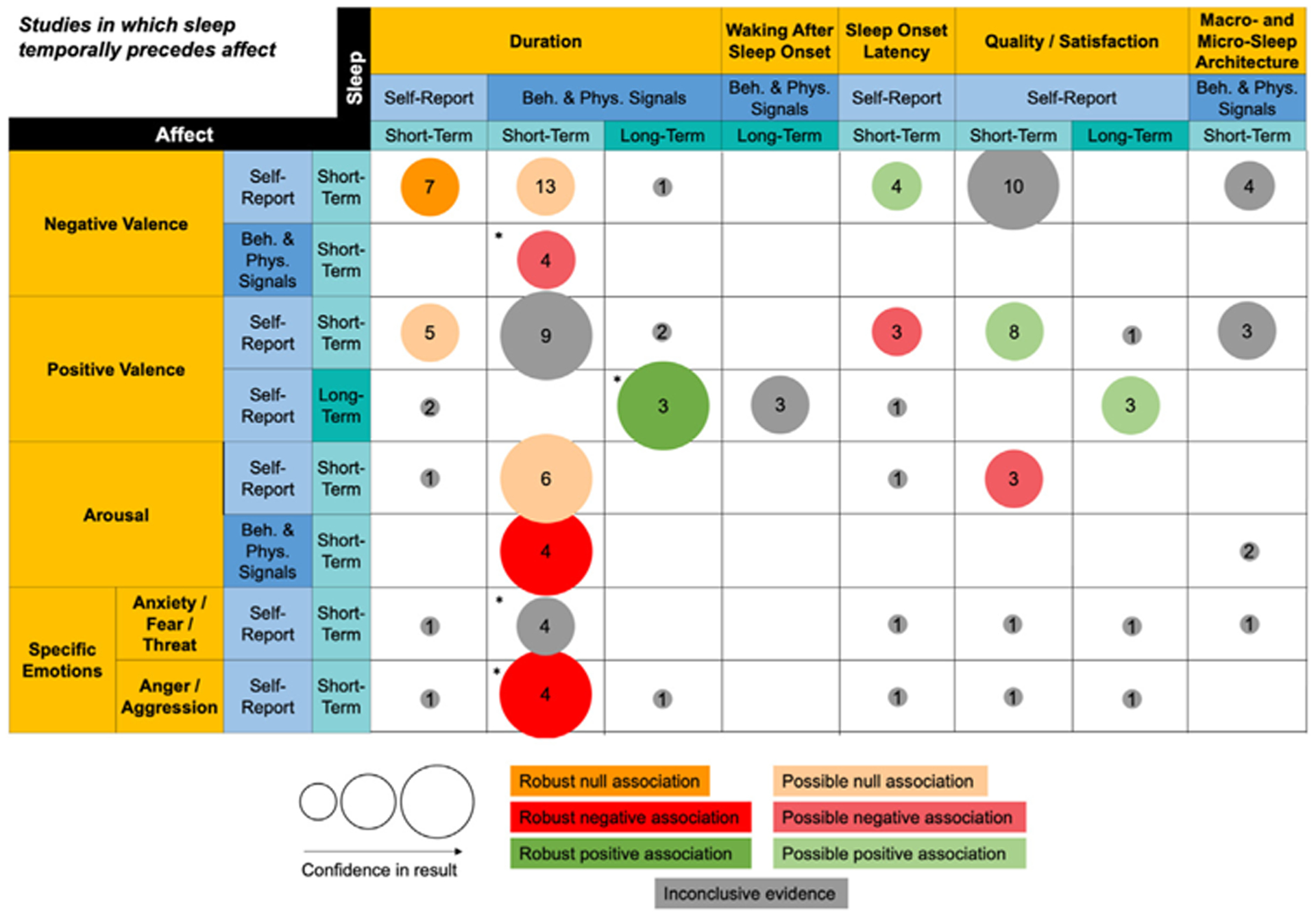
Summary of findings for sequential studies in which sleep temporally precedes affect.
This figure summarizes Table S4. Only columns and rows from Table S4 that contain at least three studies in one cell are included. The column and row headers represent domains (gold), methods (blue), and timescales (teal). The size of the bubble represents our confidence in the strength of the result. Low, moderate, and high confidence correspond to small, medium, and large bubbles respectively. The number in the center of the bubble represents the number of studies in a given cell. The color of the bubble represents the direction of evidence for the association. Light hues represent possible evidence for association and bold hues represent robust evidence for association. All associations are in the direction represented by their domain labels. For a positive association between duration and negative valence, for example, that means that higher negative valence is associated with longer duration. Asterisks (*) indicate that the conclusions are impacted if sleep manipulation studies are excluded.
Where studies’ conclusions diverged, we designated confidence in the associations (possible or robust) based on our assessment of study quality, sample size, and proportion of studies with similar findings. Cells that contained fewer than three results were considered to reflect “inconclusive evidence” (gray hue). Otherwise, we applied the following rules: 1) Cells where at least 60% of results within a cell converged in a given direction were summarized as “possible associations” (light hue) in that direction; 2) Cells where there was convergence across at least 85% of results in a given direction were summarized as “robust associations” (bold hue) in that direction; 3) Cells where convergence was below 60% were summarized as “inconclusive evidence” (gray hue). When computing the above proportions, we weighted studies taking the risk of bias assessment into account, so that “good” study quality translated to a weight of 1 and “fair” translated to a weight of 0.5. We also adjusted for multiple convergent results that were based on the same sample by considering them as a single sample both in the numerator and denominator. Some cells include both naturalistic and sleep manipulation studies (i.e., partial or total sleep deprivation); this pertains mostly to the sleep duration domain in studies where sleep temporally precedes affective experience (Table S4 and Fig. 6). Consequently, we conducted sensitivity analysis to document the impact of excluding the sleep manipulation studies on the conclusions.
Notably, we organized measures according to the actual operationalization of the construct, not the label provided by the authors. For example, a rating of sleepiness was categorized as belonging to the “sleepiness” domain even if authors referred to it as “sleep quality,” and purported “anger” measures that only asked about unpleasantness rather than the specific experience of anger were classified as “negative valence.” We also note that we organized results according to main findings, ignoring moderators, but denoted in the supplementary tables wherever effects were moderated.
4. Results
Our PubMed search returned 9,011 studies. We deleted 10 duplicates and added 2 studies that were known to the authors to be indexed in PubMed but did not appear in the search results due to errors in the MeSH terms for a total of 9,003 papers. The most common reason for exclusion was studies that had N < 25 (see Fig. 3). The final sample included 80 papers. Studies were published between 1994 and 2020. Across all papers, there were 295,730 participants (68% female). We present the results below in three sections corresponding to the three questions we presented in the introduction. In each section, results are presented grouped by sleep domain (corresponding to columns in Figs. 4–6).
4.1. Cross-sectional associations
Fig. 4 summarizes patterns of cross-sectional associations between both self-report and behavioral/physiological measures of sleep and self-reported affect, drawing from Table S2. Regarding sleep domains, we found that by far, the largest number of studies was in the domain of sleep disturbances, followed by quality/satisfaction, and then duration. We note the paucity of cross-sectional studies reporting on the sleep domains of sleep onset latency (SOL), waking after sleep onset (WASO), timing, sleep architecture, and autonomic activity. We also note the dearth of studies in the affect domains of arousal and numerous specific emotions, particularly specific positive emotions. Regarding methods, the dominant methodology used in cross-sectional studies was self-report. Regarding timescales, the number of cross-sectional studies examining both short- and long-term timescales of interest were relatively balanced, compared to studies in which affective experience temporally preceded sleep experience (Fig. 5) or vice versa (Fig. 4).
Fig. 5.
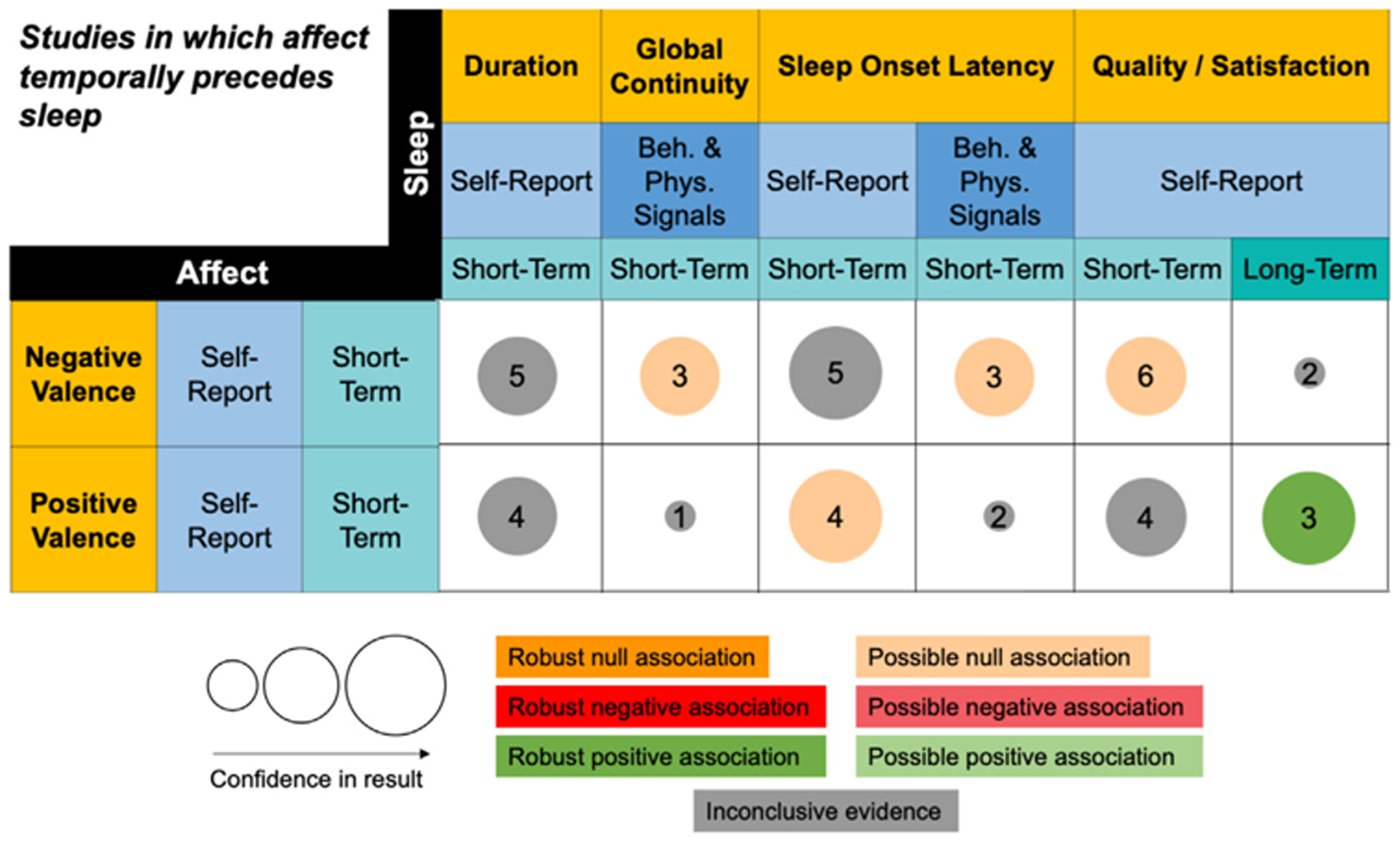
Summary of findings for sequential studies in which affect temporally precedes sleep.
This figure summarizes Table S3. Only columns and rows from Table S3 that contain at least three studies in one cell are included. The column and row headers represent domains (gold), methods (blue), and timescales (teal). The size of the bubble represents our confidence in the strength of the result. Low, moderate, and high confidence correspond to small, medium, and large bubbles respectively. The number in the center of the bubble represents the number of studies in a given cell. The color of the bubble represents the direction of evidence for the association. Light hues represent possible evidence for association and bold hues represent robust evidence for association. All associations are in the direction represented by their domain labels. For a positive association between duration and negative valence, for example, that means that higher negative valence is associated with longer duration.
Cross-sectional associations between sleep disturbances and affect.
The largest number of studies and the most robust associations were identified between self-reported sleep disturbances and self-reported affect. Results for the domains of sleep disturbances, duration, timing, continuity, and quality were convergent. This is not surprising given that many measures of sleep disturbances were composites of the other domains. We identified robust positive associations between matching timescales for self-reports, such that greater short-term sleep disturbances were associated with greater short-term negative valence in general [31–37], as well as with specific negative emotions of depressive affect (which captured the combination of sadness and anhedonia) [38–41] and anxiety, fear, or threat specific emotions [38–40,42]. Greater long-term sleep disturbances were also associated with greater long-term negative valence [43–46]. The pattern of matched time-scales also emerged for positive valence, reflecting possible short-term [31,33,34,38,47] and robust long-term [43,44,46,48] negative associations. There was a dearth of studies examining short-term sleep disturbance associations with long-term affect and vice versa.
Cross-sectional associations between sleep quality/satisfaction and affect.
The pattern of associations in the quality domain was consistent with results for the sleep disturbances domain: better sleep quality is associated with lower negative valence (long-term: [49–53]) and higher positive valence (short-term [49,54,55]: and long-term: [50–53]). However, when quality and negative valence were both measured at short-term timescales, we identified a possible null association [50,54,55]. This divergence from the results pertaining to the domain of sleep disturbance supports the value of considering sleep qualityand sleep disturbances as separate domains.
Cross-sectional associations between sleep duration and affect.
Cross-sectional associations between self-reported sleep duration and affect seemed to be non-existent or inconclusive, with possible null associations with short-term positive valence [47,50,54], and a mix of null, positive, and negative associations [37,50,54,56] with short-term negative valence. There did not seem to be evidence for associations when timescales of sleep duration and affect were mismatched. There was evidence for robust null associations between short-term self-reported measures of negative and positive valence and long-term self-reported [40,57,58] and behavioral/physiological measures [34,58,59] of duration. The pattern of findings in the sleep duration domain highlights the insights that can be gained by considering distinctions between short- and long-term timescales and between self-reported and behavioral/physiological measurement methods.
Cross-sectional associations between sleep continuity and affect.
There were very few cross-sectional associations in the domain of sleep continuity. There were not enough studies reporting SOL or WASO separately to derive any conclusions about specific sleep continuity. In the global sleep continuity domain, two of three studies reviewed [50,54,60] did not find evidence for an association between short-term self-reported continuity and negative valence, while the evidence for an association between long-term self-reported continuity and negative valence was inconclusive [49,50,61]. There were also too few studies with mismatching time-scales to draw conclusions.
Cross-sectional associations between sleepiness and affect.
There was a robust cross-sectional association between short-term self-reported sleepiness and negative valence [32,35,37,62]. There were too few studies in other affect domains to draw any conclusions.
4.2. Affect to sleep associations
Fig. 5 summarizes results from studies coded in Table S3. These studies report associations between short-term self-reported affect and subsequently experienced behavioral/physiological and self-reported sleep. Only five domains of sleep (duration, global sleep continuity, SOL, and sleep quality/satisfaction) had a sufficient number of studies to be included in the figure and interpreted. Nearly all sleep domains contained studies measuring at short-term timescales. Only two affect domains, negative and positive valence, were well represented, and they were dominated by self-report measures at short-term timescales. Across all studies of affect temporally preceding sleep, there was no support for associations between affect and subsequent sleep, with the exception of sleep quality.
Affect temporally preceding sleep quality/satisfaction.
We identified a robust association between self-reports of short-term positive valence and long-term sleep quality/satisfaction [52,63,64]. However, the evidence for association with short-term quality was inconclusive [63,65–67]. This divergence of results for long- and short-term sleep quality highlights the value of considering timescale in our framework. Evidence suggested a possible null association between short-term self-reported quality and negative valence [63,65–69], supporting negative and positive valence as separate domains in the framework.
Affect temporally preceding sleep continuity.
Within both the global sleep continuity domain [70–72] and the specific sleep continuity domain of SOL [70,71,73] measured at short-term timescales with behavioral/physiological signals, we found a possible null association with short-term self-reported negative valence. We identified a divergence of results when short-term SOL was measured using self-report versus behavioral/physiological signals. In the former case, there was no conclusive evidence for any associations with short-term self-reported negative valence [63,65,67,68,73]; in the latter case, we identified a possible null association with short-term self-reported negative valence [70,71,73]. With short-term self-reported SOL, we saw the opposite pattern to the one in the sleep quality domain described above. Specifically, we identified a possible null association with positive valence [63,65,67,73], while the evidence was inconclusive for an association with negative valence.
Affect temporally preceding sleep duration.
Despite a fairly large number of studies in the sleep duration domain, there was no clearly conclusive association between self-reports of short-term sleep duration and short-term negative [63,65–68] or positive valence [63,65–67].
4.3. Sleep to affect associations
Fig. 6 summarizes patterns from studies coded in Table S4. Just over half of all results in this figure were in the sleep duration domain. However, only three sleep duration results remained conclusive after excluding sleep manipulation studies (either partial or total deprivation, denoted in the text with †) [74–86]. In contrast, most other domains were sparsely populated. Fig. 6 is the only one of the three figures where the domain of micro- and macro-sleep architecture had at least three studies in a cell. There were very few studies of arousal and specific emotions.
Sleep duration temporally preceding affect.
For short-term self-reported duration, we found no evidence for an association with negative valence [54,65,66,68,69,87,88] and a possible null association with positive valence [54,65–67,88]. For short-term behavioral/physiological measures of duration, we found a possible null association with self-reported negative valence [49,70,76†,77†,79–82†,86†,89–92] (46% of studies involved sleep deprivation) but a negative association with behavioral/physiological measures of negative valence [49,76†,77†,90] (50% of studies involved sleep deprivation). Likewise, different findings depending on methods also emerged for arousal, where we found a possible null association with self-reported arousal [49,76†,80†,83,90,93] (33% of studies involved sleep deprivation) but a negative association with behavioral/physiological measures of arousal [76†,90,92,93] (25% of studies involved sleep deprivation). Sensitivity analyses for results pertaining to behavioral/physiological signals of sleep duration revealed that excluding studies involving partial or total sleep deprivation only changed the conclusions for the association between short-term duration and short-term behavioral/physiological signals of negative valence; specifically, after excluding sleep studies involving manipulation of sleep duration, there were not enough remaining studies to draw conclusions.
We identified a negative association between short-term behavioral/physiological signals of duration and short-term self-report of the specific emotions of anger and aggression [74†,79 ,81†,82] that replicated the behavioral/physiological negative valence and arousal findings, but interestingly, not the self-report findings. Because 100% of studies examining this association involved partial or total sleep deprivation, it is more accurate to say that we identified a negative association between short-term behavioral/physiological measures of manipulated sleep deprivation and short-term self-reported anger and aggression.
Examining associations at short-term timescales, we could not conclusively identify an association between behavioral/physiological measures of duration and self-reported positive valence [70,76†,77†,79 ,82†,86† ,89,91,92] (56% of studies involved sleep deprivation). also could not conclusively identify an association between behavioral/physiological measures of duration and self-reported specific emotions of anxiety, fear, and threat [74†,81†,82†,92] (75% of studies involved sleep deprivation); half of studies found a null association similar to the self-reported results for negative valence and arousal, while the other half found a negative association similar to the behavioral/physiological signal results for negative valence and arousal. At long-term timescales, we found a robust positive association between behavioral/physiological signals measuring duration and self-reported positive valence [84†,85†,94] (67% of studies involved sleep deprivation). It bears noting that, wherever we did so, excluding studies involving partial or total sleep deprivation did not yield a clearer pattern of associations. Even examining sleep deprivation studies alone, when the cells included more than three studies, a conclusive pattern of association could not be identified.
Sleep quality/satisfaction temporally preceding affect.
We identified possible positive associations between matching timescales and methodologies for self-reports. Better short-term sleep quality was associated with greater short-term positive valence [54,59,65–67,88,95,96] and possibly also with lower short-term arousal [59,68,95]. Better long-term sleep quality was associated with greater long-term positive valence [48,94,97]. Finally, despite the large number of studies [54,59,65–69,88,95,96], there was no clear pattern of association between short-term sleep quality and short-term negative valence. There were no studies of long-term self-reported sleep quality temporally preceding short-term self-report or behavioral/physiological measures of negative valence.
Sleep continuity temporally preceding affect.
We identified possible associations for the specific continuity domain of SOL with affect along matching methods and timescales. Longer short-term self-reported SOL was possibly associated with higher short-term self-reported negative valence [65,67,68,88] and lower positive valence [65,67,88]. Behavioral/physiological measures of the specific continuity domain of WASO, however, showed inconclusive associations with self-reported positive valence measured at matching long-term timescales [94,98,99]. There were very few studies populating the WASO domain.
Sleep architecture temporally preceding affect.
The few relevant existing studies did not conclusively reveal patterns of association for short-term macro- and micro-sleep architecture with either short-term self-reported negative [72,75†,89,91] or positive [75†,89,91] valence. Sensitivity analyses revealed that excluding the single study involving partial or total sleep deprivation did not change the inconclusive pattern of evidence (for negative valence) or did not leave enough studies to draw conclusions (for positive valence).
5. Discussion
Our granular dimensional framework allowed us to visualize patterns of converging results as well as discrepancies and gaps in the literature. Below, we contextualize specific findings from Figs. 4–6 relative to prior published reviews. Earlier reviews often struggled to make sense of seemingly contradictory associations and draw conclusions based on the limited pool of studies. Some recent reviews [1,3,4,100] have begun to make headway by taking a more granular approach to patterns of association, similar to the current paper. Konjarski’s [1] review of studies with a sequential design is notable in that it separated the domains of positive and negative affect, and, like our review, excluded stress as an affective experience. We begin with a summary of our findings for each of the three questions we posed, corresponding to each of the three study designs. We note that findings are not always consistent across study designs. For example, we found cross-sectional evidence that more long-term self-reported disturbed sleep and, separately, worse sleep quality/satisfaction are each associated with more negative valence and less positive valence. However, this association was not evident in studies that examined how sleep temporally preceded affect. Despite repeated claims of broad construct bidirectionality in the sleep and affect literature, we did not identify any clear bidirectional patterns with our granular dimensional framework, such that there was no cell where there was a temporal association from both affect to sleep and sleep to affect.
5.1. What are the cross-sectional associations between sleep and affect?
Overall, results were more robust and consistent across matching timescales for cross-sectional study designs compared to temporally sequential designs. We conclude that long-term self-reported sleep disturbances and long-term self-reported sleep quality show convergent cross-sectional associations with long-term self-reported negative as well as positive valence at matching timescales (see Fig. 4). At short-term timescales, these associations are less robust or null, except for the association with self-reported negative valence, which remains robust. These conclusions are consistent with past reviews, which identified cross-sectional associations between trait-like measures of negative affect and long-term self-reported sleep quality [3,5]. There do not appear to be any reviews of non-clinical adult samples to corroborate our findings of positive associations between short-term self-reported sleep disturbances and short-term self-reported depressive affect and anxiety/fear/threat, but these patterns are consistent with the clinical literature [101,102], adolescent literature [103], and with the related perseverative cognitions of rumination and worry [104].
In addition, we conclude that sleep duration and global continuity show a null association or inconclusive evidence for associations with short- and long-term self-reported positive and negative valence (see Fig. 4). This finding is contrary to the broad conclusions of some reviews [2,7,105]. However, other reviews acknowledge mixed evidence for both duration and continuity [4,5,106] and suggest that sleep duration’s association with negative valence may operate via affect regulation processes [5,105] or memory processes [107]. In addition, there is a literature correlating excessive sleep with greater negative affect [2], suggesting that the association may be non-linear.
Finally, our finding that greater short-term self-reported sleepiness is associated with greater short-term self-reported negative valence (see Fig. 4) fits with a pattern more broadly reported in the literature for sleep disturbances and deprivation in clinical and non-clinical populations [7]. However, we note that a number of commonly used affect measures such as the Profile of Mood States questionnaire include items relating to fatigue and alertness, potentially inflating apparent associations [108]. We therefore carefully examined the wording of specific items when reviewing and organizing studies within our framework in order to avoid conflation.
5.2. What can we learn from studies in which affective experience temporally precedes sleep experience?
For positive affect, we find that increased short-term self-reported positive valence is associated with better long-term self-reported sleep quality, but we could not draw a conclusion about short-term sleep quality (see Fig. 5). In addition, the direction of influence of positive valence was inconclusive for short-term self-reported duration, and there was a possible null association with short-term self-reported SOL (see Fig. 5). Although some reviews suggest links between sleep and positive affect [1,66], Ong [3] concluded that more than 65% of the studies they reviewed suffered from bias or design weaknesses and noted that between-person variation in positive affect seemed more predictive of sleep outcomes than within-person variation. We also note that we were not able to find past reviews that focused exclusively on non-clinical samples. Most combined results from clinical and non-clinical samples.
For negative affect, we find that there is either inconclusive evidence of an association or possible null association between short-term self-reported negative valence that temporally precedes short-term self-reported as well as behavioral/physiological measures of sleep (see Fig. 5). A review by Konjarski and colleagues [1] came to similar conclusions in naturalistic sequential studies that excluded stress as an affective experience. However, earlier reviews [2,6], which summarized both naturalistic and experimental studies and did include stress as an affective experience concluded that there is a strong influence of negative affect on sleep. This discrepancy highlights the value of the granular framework we proposed and used in our review.
We did not find enough studies meeting inclusion criteria to draw conclusions about affective arousal preceding sleep (see Fig. 5). However, work in clinical and adolescent populations suggests that we would expect to see robust effects of arousal on a variety of sleep domains, particularly SOL [5,7,109]. More systematic exploration of the arousal domain in a healthy adult sample is clearly needed.
5.3. What can we learn from studies in which sleep experience temporally precedes affective experience?
Several important patterns are evident. First, we found a larger number of studies with behavioral/physiological signals measuring short-term sleep duration relative to any of the other sleep domains (see Fig. 6), including a large number of experimental sleep deprivation and restriction studies. Second, both self-report and behavioral/physiological measures of sleep at short- and long-term timescales seem not to predict subsequent short-term self-reported negative affect, even after excluding sleep deprivation and restriction studies (see Fig. 6). Examining only sleep deprivation and restriction studies also did not show any conclusive pattern of evidence. An exception is our finding about short-term self-reported SOL, which corroborates Konjarski’s conclusion [1]. This is surprising given the number of qualitative review papers that report increased negative affect following sleep loss and deprivation [2,7,110–112]. Our findings for duration are similar to Konjarksi et al.’s [1] and Tempesta et al.’s [4] conclusions of mixed results. Our findings for sleep quality run counter to Konjarski and colleagues’ [1] report of a robust inverse relationship between sleep quality and next-day negative affect. However, their review included heterogeneous samples including children, adolescents, older adults, and clinical populations. Fairholme and Manber [5] conclude that sleep’s influence on negative affect tends to appear in experimental contexts, not in daily naturalistic studies. This distinction in contexts may be helpful in future iterations of our framework.
Third, our findings indicate mixed associations across a variety of sleep domains with positive valence, which largely matches other reviews [3,6], as well as arousal (see Fig. 6). Our null and inconclusive findings for the association of short-term self-reported and behaviorally/physiologically measured sleep duration with affect is consistent with two past reviews [1,3], and our findings of a possible positive association between sleep quality and subsequent positive valence are similar to Konjarski and colleages’ review [1]. However, our conclusions diverge from theirs in two ways. First, whereas they reported a null association between short-term behavioral/physiological measures of global sleep continuity and self-reported positive affect, we did not find evidence for a null association, though we found that studies reported different directions of association. Second, they reported [1] mixed results for the association between short-term self-reported SOL and short-term self-reported positive affect, whereas we identified a possible positive association. The fact that surveying a reasonably broad array of studies finds contradictory results may suggest that our categories of short-term behavioral/physiological signals of duration may not be capturing the most relevant methodological distinctions for self-reported positive valence. For instance, inconclusive links may be attributable to differences in the degree of deviation in observed sleep duration from habitual sleep duration, ranging from total deprivation to different levels of partial or restricted sleep, as well as to the effect of cumulative sleep insufficiency [112]. The inconclusive results may also stem from the presence of non-linear associations. Indeed, Konjarski and colleagues [1] identified a U-shaped relationship between sleep duration and affect, such that either shortened or extended self-reported duration decreased positive and increased negative affect the next day. Further research may need to reassess patterns in terms of non-linear associations. Kahn and colleagues [2] also suggested that examining the ratio of positive to negative affect as an index of healthy affective functioning may be more informative than either positive or negative affect alone.
Finally, we conclude that greater short-term behavioral/physiological sleep duration is associated with higher negative valence and arousal when both are measured with short-term behavioral/physiological signals, but not self-report (see Fig. 6). This discrepancy based on methodology has been noted by Fairholme & Manber [5]. Interestingly, the specific emotions of anxiety/fear/threat and anger/aggression, which are characterized by high arousal and high negative valence [14], show discrepant patterns of association as well (see Fig. 6). We are unable to conclude whether there is any association of short-term behavioral/physiological measures of duration and self-reported anxiety, both excluding and including sleep manipulation studies (see Fig. 6), which partially aligns with Pires and colleagues’ [100] review concluding that sleep deprivation led to increased state anxiety, but sleep restriction did not. Our finding of a robust negative association with anger (see Fig. 6) driven by sleep deprivation and restriction is consistent with other reviews [6,7]. This indicates that conceptualizing self-reported affect using discrete as well as continuous categories may help reveal patterns of association with sleep.
5.4. Gaps identified by the framework
We organized selected empirical findings using a framework that emphasized categorical distinctions across three dimensions: 1) subordinate domains within sleep and affect; 2) methodological distinctions between self-report and behavioral/physiological signals; and 3) timescale distinctions between short-term and long-term measures of phenomena. Most findings in the systematic review focused on sleep experience preceding affective experience, while the fewest findings focused on affective experience preceding sleep experience.
Overall, we noticed that studies tended to be concentrated in just a few domains: the sleep domains of sleep disturbances, quality/satisfaction, and duration, and the affect domains of negative and positive valence. We also identified areas that were particularly understudied. Within sleep, these included the domains of WASO, sleep timing, sleepiness, macro- and micro-sleep architecture, and autonomic activity during sleep. Within affect, these included the domains of affective arousal and specific emotions beyond anxiety/fear/threat, anger/aggression, and depressive affect. There was a paucity of studies measuring both constructs, but particularly affect, with behavioral/physiological signals. In studies where sleep temporally preceded affect or vice versa, there were very few studies at long-term timescales.
5.5. The value of granularity
Granularity in domains, methods, and timescales revealed interesting patterns of association, supporting the main premise of this conceptual review. The granular approach was helpful both for clarifying prior inconsistencies and for clarifying where associations exist. Our framework revealed a number of cells that were populated by studies that indicated null results, thus helping distinguish between associations that are truly null and inconsistencies in findings. The power of our framework to identify null patterns is particularly notable given a general publication bias whereby null results tend not to be published.
Domain granularity was also important, as was apparent in Fig. 6, where our review clarified what was previously a set of inconsistent results relating to sleep in relation to subsequent negative affect. In this case, the added granularity revealed that short-term behavioral/physiological signals measuring sleep duration were associated with specific emotion domains, such as anger and anxiety, but not with the more general domains of affective valence and arousal. Domain granularity helps to clarify what might have previously been interpreted as inconsistent results. In addition, our review revealed different patterns of cross-sectional association with negative and positive valence for the composite domain of sleep disturbances than for granular domains of global continuity, quality/satisfaction, and sleepiness. This may be because composite sleep disturbance reflects an individual’s integration not only across other specific sleep domains but also their personal impact. This suggests that sleep disturbance is an important domain to consider and is partially separable from other domains.
We also found that adding methodological granularity to our framework mattered. Whereas collapsing across methods may provide more power to detect associations between domains, it might also lead to interpreting inconsistent results as evidence of no association. Separating results based on methodology can reveal potentially meaningful patterns, even across domains. For example, this was apparent for self-reported sleep quality in Fig. 5 (affect preceding sleep) as well as for sleep duration in Fig. 6 (sleep preceding affect), where behavioral/physiological signals for both sleep and affect show different associations compared to self-report. At the same time, we find that in many cases, even a single result in a single cell could encompass multiple, sometimes contradictory findings. For instance, studies that measured associations between behaviorally/physiologically measured WASO and subsequent positive valence over a long timescale had contradictory findings. We also note that in order to avoid shared methods bias, it is important to consider both self-reported and behavioral/physiological measures, and ideally consider cross-methods associations when examining links between sleep and affect.
Finally, timescale granularity in Fig. 5 (affect preceding sleep) was valuable, as it revealed discrepant patterns of association for matched compared to mismatched timescales. In general, where there was sufficient information to interpret the results, patterns of association tended to match on timescale (i.e., short-term to short-term and long-term to long-term).
5.6. Interpreting inconsistent findings
Despite being populated by three or more studies, there was no clear direction of association for some cells. These include the following: 1) the cross-sectional association between short-term self-reported sleep duration and negative valence; 2) the cross-sectional association between long-term global sleep continuity and negative valence; 3) the association between short-term self-reported negative valence and subsequent sleep duration and 4) sleep onset latency; 5) the association between short-term self-reported positive valence and subsequent sleep duration and 6) sleep quality; 7) the association between short-term behavioral/physiological signals measuring sleep duration and subsequent self-reported positive valence and 8) anxiety/fear/threat; 9) the association between short-term behavioral/physiological signals of WASO and subsequent self-reported positive valence; 10) the association between short-term self-reported sleep quality and subsequent short-term self-reported negative valence; and 11) the association between short-term behavioral/physiological signals measuring sleep architecture and subsequent short-term self-reported negative and 12) positive valence.
The absence of clear associations in these cells suggests that our framework may have overlooked some important distinctions or, alternatively, identified distinctions that are not the relevant ones to explain associations. Thus, future research may need to identify which features of these studies might explain contradictory findings, as this could guide the conduct of future, more definitive research.
Refining our framework could provide a roadmap for such explorations. For example, we found that some single columns (such as macro- and micro- sleep architecture) encompassed an extremely broad set of outcomes, suggesting that finer-grained categorization might provide additional insights. Similarly, our framework also includes overlapping domains that might need to be separated. For instance, for affect, specific emotions combine different aspects of valence and arousal, and, for sleep, neural and autonomic activity, such as slow wave sleep, may overlap with other sleep domains, such as a physiological measure of sleep quality. It is also possible that greater clarity can be gained by greater granularity in methods; for example, by further separating naturalistic from experimental methods, as Konjarski and colleagues [1] did in focusing their review exclusively on naturalistic studies. In addition, it is possible that different timescale categories than the ones we used (shorter or longer than 48 h) might be even more relevant.2 Categorizing studies based on information about the sampling resolution or proximity of measurement to the experience may reveal more clear patterns [112]. We expect that our proposed framework will keep evolving as more findings emerge and new assessment techniques develop.
We note that there are advantages and disadvantages to different approaches to placing boundaries between categories within each dimension. Indeed, it may not be possible to achieve fully orthogonal categories within dimensions and trying to do so might not be important. In addition, methodology and timescale are not fully separable. For instance, sleep disturbances are typically measured with self-report questionnaires that refer to the past week or month and hence are long-term. Another important distinction that could prove useful relates to between- or within-person analyses. Such distinctions may reveal bidirectional associations for one level of analysis but not the other. The process of identifying relevant dimensions and categories should be guided by past theoretical work as well as empirical efforts.
Beyond refining our framework, other possible explanations for the inconsistent findings are that the assumption of linear association between sleep and affect may be mistaken and that there are moderators not characterized in this review that may explain variations in association. These moderators may reveal different patterns of association, depending on the methods and timescales used to measure sleep and affect variables.
5.7. Exploring moderators
The current review did not systematically characterize moderators.3 There are four categories of moderators that are likely to be particularly important to explore: demographic factors (such as sex, age, race, and socioeconomic status), sleep moderators, affect moderators, and individual differences that moderate both sleep and affect.
Regarding sleep moderators, we highlight the following three: 1) different levels of “severity” for each sleep construct, 2) typical sleep habits and deviations from them, and 3) individual differences in resilience to sleep perturbations. Essentially, there may be different effects of receiving a lower or higher “dosage” of restful sleep, and these effects may be non-linear, vary individually, and depend on prior sleep. “Healthy” and “unhealthy” sleep likely represent a continuum, rather than a dichotomy [11]. Therefore, it will be important to collect repeated data over time in order to determine the effects of sleep changes relative to a person’s mean or baseline. It is also important to understand how individuals react to atypical sleep conditions, since their ability to function cognitively, attentionally, and physically can strongly interact with their affective function [2].
Regarding affect moderators, we propose the following three potential moderators: 1) affective stimuli, 2) individual differences in affective reactivity, and 3) affect regulation. One way to conceptualize the importance of affective stimuli or antecedents involves thinking about the severity or “dosage” of an affective experience when comparing across studies. It is also important to consider that, even with identical antecedents, individuals experience different affective reactions. Finally, affect regulation processes can arise almost immediately and nearly inseparably from an initial affective reaction [10]. Using certain affect regulation strategies [5,105,113] has been shown to impact sleep outcomes, and, conversely, poor sleep seems to impair some affect regulation capabilities [105].
Regarding moderators that can simultaneously impact both sleep and affect, we propose to focus on individual differences in beliefs about sleep [114], affect [115], and the relationship between them. These beliefs could alter particular domains within sleep and affect, alter patterns of associations differently at different time-scales, and impact perceptions (self-report measures) differently from behaviors and physiology.
5.8. Other future directions
We limited the scope of this review by excluding two specific domains of affect: pain [116] and stress [117]. These complex affective states, whose definitions combine negative valence, arousal, and appraisals of goals, self-efficacy, and coping resources, are each the subject of extensive literatures and have been previously reviewed (for example, pain: [118,119]; stress: [120,121]). The utility of our framework for organizing these two bodies of literature remains unknown. For feasibility, we also constrained our review to a single search index. Future work should draw from even broader pools of indexed articles.
6. Conclusion
Scientific understanding requires not only amassing empirical evidence but also developing appropriate frameworks to interpret findings. Currently, researchers interested in bidirectional links between sleep and affect have gathered data with a variety of samples and measures but have not been able to fully synthesize the many disparate findings. In this article, we proposed that studies of sleep, affect, and their relationship can be more clearly understood by appreciating how they differ in domains, methods, and timescale. We presented a new organizing granular dimensional framework that incorporates insights from both sleep science and affective science and opens the door for fruitful collaboration at the intersection of the two fields.
Based on our review, we identified several patterns. First, in cross-sectional studies measured with self-report, more disturbed sleep was robustly associated with greater self-reported negative valence, depressive affect, and anxiety/fear/threat as well as lower positive valence. The pattern appeared similar, though less robust, for worse sleep quality. Second, there were relatively fewer studies in which affect was measured preceding sleep, and we found either a lack of evidence or inconclusive evidence for the association between negative and positive valence and sleep domains. Third, most studies in which sleep experience preceded affect examined how short-term behavioral/physiological measures sleep duration related to subsequent affect. We identified null or inconclusive associations with short-term self-reported negative valence, positive valence, arousal, and anxiety/fear/threat, but negative associations for behavioral/physiological measures of negative valence, arousal, and anger/aggression (though this last association was entirely driven by sleep deprivation or restriction studies). Fourth, we found no support for granular bidirectionality from affect to sleep and sleep to affect.
As the supplementary tables make clear, cells are unevenly populated by empirical studies. We view the sparseness as gaps that call for future exploration. We believe that such exploration can benefit from the conceptual framework we have laid out here and from collaboration between sleep and affective scientists. We recognize that our granular dimensional framework is likely to evolve over time and hope this review serves as a jumping-off point.
Supplementary Material
Practice points.
The empirical evidence for a bidirectional link between sleep and affect in non-clinical adult samples is not as strong as might be expected from widespread lay beliefs. In fact, contradictory findings suggest a more nuanced relationship.
Bringing insights from both sleep science and affective science to bear, we offer an organizing framework that distinguishes findings according to important differences in both sleep and affect domains, methods, timescales.
The results of conducting a review using this framework reveal a concentration of studies examining 1) cross-sectional associations between sleep disturbances and positive and negative affect valence, and 2) temporally sequential associations between behavioral/physiological measures of sleep duration and subsequent affect. They also reveal large gaps in scientific knowledge, particularly for studies where affect temporally precedes sleep.
We found inconclusive or no evidence for several associations, particularly between sleep duration and negative valence, that had been expected based on past reviews.
We did not find evidence for bidirectionality between sleep and affect when the two constructs were examined at a more granular resolution.
A granular framework, such as the one we propose could help identify important factors that do or do not impact the links between sleep and affect.
Research agenda.
Efforts should be made to organize existing and future empirical work according to distinctions within the domains, methods, and timescales measured.
Future research should systematically test other categories and boundaries within each of the three dimensions (domains, methods, timescales) to address inconclusive patterns of association and continue refining our framework. In particular, we believe three timescale distinctions will be important to characterize more thoroughly: timescale of interest, sampling resolution, and proximity of measurement to experience.
Future reviews could apply our framework to additional affective domains, such as pain and stress, that were beyond the scope of this review.
We encourage more exploration of individual and sample demographic moderators, sleep moderators (in particular, different levels of severity/dosage for each sleep domain, typical sleep habits, and individual differences in resilience to sleep disturbance), affect moderators (in particular, affective stimuli, individual differences in affective reactivity, and use of affect regulation), and individual differences that moderate both sleep and affect (in particular, clinical status and beliefs).
Acknowledgements
MtB is funded by a National Science Foundation Graduate Research Fellowship. The authors have no conflicts of interest to report. Enormous thanks to Annabelle T. Wang for help with the initial stages of literature review. Thank you to Taqwa Ramadan for help organizing citations and Pilleriin Sikka for comments on drafts.
Footnotes
We made one exception, however, and included studies of affect recognition because a large portion of the articles operationalized affect with a task where participants had to recognize and label affect from faces or pictures. Affect recognition relies on a distinct set of psychological processes of perception, memory, and theory of mind to evaluate how people generally rate the affect of a picture [28]. Affective experience, however, involves a more automatic assessment of one’s own state, involving interception of internal sensations and introspective processes. These processes also appear to be dissociated in the brain [29]. Given how often studies blurred this distinction, we decided to include but explicitly separate affective experience from affect recognition results in the empirical review in order to illustrate how those measures perform differently from measures of affective experience (Table S4).
We believe that the three temporal aspects we described above, namely timescale of interest, sampling resolution, and proximity of reporting relative to the period reported on, provide greater granularity and clarity than the traditional labels of “retrospective” (i.e., measuring experiences from the past) versus “prospective” (i.e., measuring real time experiences over an extended period in order to observe subsequent developments).
Although we summarized main effects, numerous studies in our empirical review reported moderation by affect regulation, which we denoted in the supplementary tables by asterisks.
Appendix A. Supplementary data
Supplementary data related to this article can be found at https://doi.org/10.1016/j.smrv.2022.101670.
References
* key citations
- [1]*.Konjarski M, Murray G, Lee VV, Jackson ML Reciprocal relationships between daily sleep and mood: a systematic review of naturalistic prospective studies. Sleep Med Rev 2018;42:47–58. 10.1016/j.smrv.2018.05.005. [DOI] [PubMed] [Google Scholar]
- [2]*.Kahn M, Sheppes G, Sadeh A. Sleep and emotions: bidirectional links and underlying mechanisms. Int J Psychophysiol 2013;89:218–28. 10.1016/j.ijpsycho.2013.05.010. [DOI] [PubMed] [Google Scholar]
- [3].Ong AD, Kim S, Young S, Steptoe A. Positive affect and sleep: a systematic review. Sleep Med Rev 2016. [DOI] [PubMed] [Google Scholar]
- [4]*.Tempesta D, Socci V, De Gennaro L, Ferrara M Sleep and emotional processing. Sleep Med Rev 2018;40:183–95. 10.1016/j.smrv.2017.12.005. [DOI] [PubMed] [Google Scholar]
- [5]*.Fairholme CP, Manber R. Sleep, emotions, and emotion regulation. Sleep affect. Elsevier; 2015. p. 45–61. [Google Scholar]
- [6].Deliens G, Gilson M, Peigneux P. Sleep and the processing of emotions. Exp Brain Res 2014;232:1403–14. 10.1007/s00221-014-3832-1. [DOI] [PubMed] [Google Scholar]
- [7].Baglioni C, Spiegelhalder K, Lombardo C, Riemann D. Sleep and emotions: a focus on insomnia. Sleep Med Rev 2010;14:227–38. 10.1016/j.smrv.2009.10.007. [DOI] [PubMed] [Google Scholar]
- [8].Ong AD, Bastarache ED, Steptoe A. Positive affect as resilience and vulnerability in sleep. Elsevier; 2015. p. 275–91. 10.1016/B978-0-12-417188-6.00013-X. Sleep Affect. [DOI] [Google Scholar]
- [9]*.Katulka EK, Berube FR, D’Agata MN Dreaming of better health: quantifying the many dimensions of sleep . Sleep 2020;43:zsz275. 10.1093/sleep/zsz275. [DOI] [PubMed] [Google Scholar]
- [10]*.Gross JJ Emotion regulation: current status and future prospects. Psychol Inq 2015;26:1–26. 10.1080/1047840X.2014.940781. [DOI] [Google Scholar]
- [11]*.Buysse DJ Sleep health: can we define it? Does it matter?. Sleep 2014;37: 9–17. 10.5665/sleep.3298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Buysse DJ, Reynolds III CF, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh sleep quality index: a new instrument for psychiatric practice and research. Psychiatr Res 1989;28:193–213. 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- [13].Bastien C Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep Med 2001;2:297–307. 10.1016/S1389-9457(00)00065-4. [DOI] [PubMed] [Google Scholar]
- [14]*.Russell JA Core affect and the psychological construction of emotion. Psychol Rev 2003;110:145–72. 10.1037/0033-295X.110.1.145. [DOI] [PubMed] [Google Scholar]
- [15].Kron A, Goldstein A, Lee DH-J, Gardhouse K, Anderson AK. How are you feeling? Revisiting the quantification of emotional qualia. Psychol Sci 2013;24: 1503–11. 10.1177/0956797613475456. [DOI] [PubMed] [Google Scholar]
- [16].Kreibig SD, Gross JJ. Understanding mixed emotions: paradigms and measures. Curr Opin Behav Sci 2017;15:62–71. 10.1016/j.cobeha.2017.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Keenan SA, Hirshkowitz M. Monitoring and staging human sleep. In: Kryger MH, Roth T, Dement WC, editors. Princ. Pract. Sleep med fifth ed. St. Louis: Elsevier Saunders; 2011. p. 1602–9. [Google Scholar]
- [18].Ancoli-Israel S, Martin JL, Blackwell T, Buenaver L, Liu L, Meltzer LJ, et al. The SBSM guide to actigraphy monitoring: clinical and research applications. Behav Sleep Med 2015;13:S4–38. 10.1080/15402002.2015.1046356. [DOI] [PubMed] [Google Scholar]
- [19].Kaplan KA, Hirshman J, Hernandez B, Stefanick ML, Hoffman AR, Redline S, et al. When a gold standard isn’t so golden: lack of prediction of subjective sleep quality from sleep polysomnography. Biol Psychol 2017;123:37–46. 10.1016/j.biopsycho.2016.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Harvey AG, Stinson K, Whitaker KL, Moskovitz D, Virk H. The subjective meaning of sleep quality: a comparison of individuals with and without insomnia. Sleep 2008;31:383–93. 10.1093/sleep/31.3.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Mauss IB, Robinson MD. Measures of emotion: a review. Cognit Emot 2009;23: 209–37. 10.1080/02699930802204677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Gross JJ, Jazaieri H. Emotion, emotion regulation, and psychopathology: an affective science perspective. Clin Psychol Sci 2014;2:387–401. 10.1177/2167702614536164. [DOI] [Google Scholar]
- [23].Mauss IB, Levenson RW, McCarter L, Wilhelm FH, Gross JJ. The tie that binds? Coherence among emotion experience, behavior, and physiology. Emotion 2005;5:175–90. 10.1037/1528-3542.5.2.175. [DOI] [PubMed] [Google Scholar]
- [24].Borbely AA. A two process model of sleep regulation. Hum Neurobiol 1982;1: 195–204. [PubMed] [Google Scholar]
- [25].Hollenstein T, Lichtwarck-Aschoff A, Potworowski G. A model of socioemotional flexibility at three time scales. Emot Rev 2013;5:397–405. 10.1177/1754073913484181. [DOI] [Google Scholar]
- [26].Canese K, Jentsch J, Myers C. PubMed: the bibliographic database. NCBI Handb 2003;2:1–12. [Google Scholar]
- [27].Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan—a web and mobile app for systematic reviews. Syst Rev 2016;5:210. 10.1186/s13643-016-0384-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Robinson MD, Clore GL. Belief and feeling: evidence for an accessibility model of emotional self-report. Psychol Bull 2002;128:934. 10.1037/0033-2909.128.6.934. [DOI] [PubMed] [Google Scholar]
- [29].Lindquist KA, Gendron M, Barrett LF, Dickerson BC. Emotion perception, but not affect perception, is impaired with semantic memory loss. Emotion 2014;14:375–87. 10.1037/a0035293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Bortoletto TG, Silva TV, Borovac-Pinheiro A, Pereira CM, Silva AD, França MS, et al. Cervical length varies considering different populations and gestational outcomes: results from a systematic review and meta-analysis. PLoS One 2021;16:e0245746. 10.1371/journal.pone.0245746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Rehman A, Gumley A, Biello S. Sleep quality and paranoia: the role of alex-ithymia, negative emotions and perceptual anomalies. Psychiatr Res 2018;259: 216–22. 10.1016/j.psychres.2017.09.066. [DOI] [PubMed] [Google Scholar]
- [32].Benham G, Charak R. Stress and sleep remain significant predictors of health after controlling for negative affect. Stress Health 2019;35:59–68. 10.1002/smi.2840. [DOI] [PubMed] [Google Scholar]
- [33].Machado L, Souza CTN de, Nunes R de O, de Santana CN, Araujo CF de, Cantilino A Subjective well-being, religiosity and anxiety: a cross-sectional study applied to a sample of Brazilian medical students. Trends Psychiatry Psychother 2018;40:185–92. 10.1590/2237-6089-2017-0070. [DOI] [PubMed] [Google Scholar]
- [34].Lemola S, Ledermann T, Friedman EM. Variability of sleep duration is related to subjective sleep quality and subjective well-being: an actigraphy study. PLoS One 2013;8:e71292. 10.1371/journal.pone.0071292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Kurina LM, Knutson KL, Hawkley LC, Cacioppo JT, Lauderdale DS, Ober C. Loneliness is associated with sleep fragmentation in a communal society. Sleep 2011;34:1519–26. 10.5665/sleep.1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Wassing R, Benjamins JS, Dekker K, Moens S, Spiegelhalder K, Feige B, et al. Slow dissolving of emotional distress contributes to hyperarousal. Proc Natl Acad Sci USA 2016;113:2538–43. 10.1073/pnas.1522520113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Simor P, Zavecz Z, Pálosi V, Török C, Köteles F. The influence of sleep complaints on the association between chronotype and negative emotionality in young adults. Chronobiol Int 2015;32:1–10. 10.3109/07420528.2014.935786. [DOI] [PubMed] [Google Scholar]
- [38].Pilcher JJ, Ginter DR, Sadowsky B. Sleep quality versus sleep quantity: relationships between sleep and measures of health, well-being and sleepiness in college students. J Psychosom Res 1997;42:583–96. 10.1016/S0022-3999(97)00004-4. [DOI] [PubMed] [Google Scholar]
- [39].Mathur A, Graham-Engeland JE, Slavish DC, Smyth JM, Lipton RB, Katz MJ, et al. Recalled early life adversity and pain: the role of mood, sleep, optimism, and control. J Behav Med 2018;41:504–15. 10.1007/s10865-018-9917-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Prather AA, Bogdan R, Hariri AR. Impact of sleep quality on amygdala reactivity, negative affect, and perceived stress. Psychosom Med 2013;75:350–8. 10.1097/PSY.0b013e31828ef15b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Smith SS, Kozak N, Sullivan KA. An investigation of the relationship between subjective sleep quality, loneliness and mood in an Australian sample: can daily routine explain the links? Int J Soc Psychiatr 2012;58:166–71. 10.1177/0020764010387551. [DOI] [PubMed] [Google Scholar]
- [42].Wang Z, Chen B, Li W, Xie F, Loke AY, Shu Q. Sleep quality and its impacts on quality of life among military personnel in remote frontier areas and extreme cold environments. Health Qual Life Outcome 2020;18:227. 10.1186/s12955-020-01460-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Carciofo R, Du F, Song N, Zhang K. Mind wandering, sleep quality, affect and chronotype: an exploratory study. PLoS One 2014;9:e91285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Cellini N, Duggan KA, Sarlo M. Perceived sleep quality: the interplay of neuroticism, affect, and hyperarousal. Sleep Health 2017;3:184–9. 10.1016/j.sleh.2017.03.001. [DOI] [PubMed] [Google Scholar]
- [45].Lund HG, Reider BD, Whiting AB, Prichard JR. Sleep patterns and predictors of disturbed sleep in a large population of college students. J Adolesc Health 2010;46:124–32. 10.1016/j.jadohealth.2009.06.016. [DOI] [PubMed] [Google Scholar]
- [46].Howell AJ, Digdon NL, Buro K, Sheptycki AR. Relations among mindfulness, well-being, and sleep. Pers Indiv Differ 2008;45:773–7. 10.1016/j.paid.2008.08.005. [DOI] [Google Scholar]
- [47].Allen MS, Magee CA, Vella SA. Personality, hedonic balance and the quality and quantity of sleep in adulthood. Psychol Health 2016;31:1091–107. 10.1080/08870446.2016.1178745. [DOI] [PubMed] [Google Scholar]
- [48].Pilcher JJ, Ott ES. The relationships between sleep and measures of health and weil-being in college students: a repeated measures approach. Behav Med 1998;23:170–8. 10.1080/08964289809596373. [DOI] [PubMed] [Google Scholar]
- [49].Morin CM, Rodrigue S, Ivers H. Role of stress, arousal, and coping skills in primary insomnia. Psychosom Med 2003;65:259–67. 10.1097/01.PSY.0000030391.09558.A3. [DOI] [PubMed] [Google Scholar]
- [50].Pressman SD, Jenkins BN, Kraft-Feil TL, Rasmussen H, Scheier MF. The whole is not the sum of its parts: specific types of positive affect influence sleep differentially. Emotion 2017;17:778. 10.1037/emo0000256.0213. [DOI] [PubMed] [Google Scholar]
- [51].Brand S, Beck J, Hatzinger M, Harbaugh A, Ruch W, Holsboer-Trachsler E. Associations between satisfaction with life, burnout-related emotional and physical exhaustion, and sleep complaints. World J Biol Psychiatr 2010;11: 744–54. 10.3109/15622971003624205. [DOI] [PubMed] [Google Scholar]
- [52].Sonnentag S, Binnewies C, Mojza EJ. Did you have a nice evening?” A day-level study on recovery experiences, sleep, and affect. J Appl Psychol 2008;93: 674–84. 10.1037/0021-9010.93.3.674. [DOI] [PubMed] [Google Scholar]
- [53].Flueckiger L, Lieb R, Meyer AH, Mata J. How health behaviors relate to academic performance via affect: an intensive longitudinal study. PLoS One 2014;9:e111080. 10.1371/journal.pone.0111080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Leger KA, Charles ST. Affective recovery from stress and its associations with sleep. Stress Health 2020;36:693–9. 10.1002/smi.2966. [DOI] [PubMed] [Google Scholar]
- [55].Hartmann JA, Wichers M, van Bemmel AL, Derom C, Thiery E, Jacobs N, et al. The serotonin transporter 5-HTTLPR polymorphism in the association between sleep quality and affect. Eur Neuropsychopharmacol 2014;24:1086–90. 10.1016/j.euroneuro.2014.01.015. [DOI] [PubMed] [Google Scholar]
- [56].Seixas AA, Auguste E, Butler M, James C, Newsome V, Auguste E, et al. Differences in short and long sleep durations between blacks and whites attributed to emotional distress: analysis of the National Health Interview Survey in the United States. Sleep Health 2017;3:28–34. 10.1016/j.sleh.2016.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Ong JC, Carde NB, Gross JJ, Manber R. A two-dimensional approach to assessing affective states in good and poor sleepers: two-dimensional approach to measuring affect. J Sleep Res 2011;20:606–10. 10.1111/j.1365-2869.2011.00907.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Jackowska M, Ronaldson A, Brown J, Steptoe A. Biological and psychological correlates of self-reported and objective sleep measures. J Psychosom Res 2016;84:52–5. 10.1016/j.jpsychores.2016.03.017. [DOI] [PubMed] [Google Scholar]
- [59].Tinajero R, Williams PG, Cribbet MR, Rau HK, Bride DL, Suchy Y. Nonrestorative sleep in healthy, young adults without insomnia: associations with executive functioning, fatigue, and pre-sleep arousal. Sleep Health 2018;4:284–91. 10.1016/j.sleh.2018.02.006. [DOI] [PubMed] [Google Scholar]
- [60].Jackowska M, Dockray S, Hendrickx H, Steptoe A. Psychosocial factors and sleep efficiency: discrepancies between subjective and objective evaluations of sleep. Psychosom Med 2011;73:810–6. 10.1097/PSY.0b013e3182359e77. [DOI] [PubMed] [Google Scholar]
- [61].Amaral AP, Soares MJ, Pinto AM, Pereira AT, Madeira N, Bos SC, et al. Sleep difficulties in college students: the role of stress, affect and cognitive processes. Psychiatr Res 2018;260:331–7. 10.1016/j.psychres.2017.11.072. [DOI] [PubMed] [Google Scholar]
- [62].Jean-Louis G, Von Gizycki H, Zizi F, Nunes J. Mood states and sleepiness in college students: influences of age, sex, habitual sleep, and substance use. Percept Mot Skills 1998;87:507–12. 10.2466/pms.1998.87.2.507. [DOI] [PubMed] [Google Scholar]
- [63].Kane HS, Slatcher RB, Reynolds BM, Repetti RL, Robles TF. Daily self-disclosure and sleep in couples. Health Psychol 2014;33:813. 10.1037/hea0000077. 0728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Ong AD, Exner-Cortens D, Riffin C, Steptoe A, Zautra A, Almeida DM. Linking stable and dynamic features of positive affect to sleep. Ann Behav Med 2013;46:52–61. 10.1007/s12160-013-9484-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Kalmbach DA, Pillai V, Roth T, Drake CL. The interplay between daily affect and sleep: a 2-week study of young women. J Sleep Res 2014;23:636–45. 10.1111/jsr.12190. [DOI] [PubMed] [Google Scholar]
- [66].Sin NL, Almeida DM, Crain TL, Kossek EE, Berkman LF, Buxton OM. Bidirectional, temporal associations of sleep with positive events, affect, and stressors in daily life across a week. Ann Behav Med 2017;51:402–15. 10.1007/s12160-016-9864-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].de Wild-Hartmann JA, Wichers M, van Bemmel AL, Derom C, Thiery E, Jacobs N, et al. Day-to-day associations between subjective sleep and affect in regard to future depression in a female population-based sample. Br J Psychiatry 2013;202:407–12. 10.1192/bjp.bp.112.123794. [DOI] [PubMed] [Google Scholar]
- [68].Kalmbach DA, Arnedt JT, Swanson LM, Rapier JL, Ciesla JA. Reciprocal dynamics between self-rated sleep and symptoms of depression and anxiety in young adult women: a 14-day diary study. Sleep Med 2017;33:6–12. 10.1016/j.sleep.2016.03.014. [DOI] [PubMed] [Google Scholar]
- [69].Park Y, Kim S. Customer mistreatment harms nightly sleep and next-morning recovery: job control and recovery self-efficacy as cross-level moderators. J Occup Health Psychol 2018;24:256. 10.1037/ocp0000128. 0628. [DOI] [PubMed] [Google Scholar]
- [70].Takano K, Sakamoto S, Tanno Y. Repetitive thought impairs sleep quality: an experience sampling study. Behav Ther 2014;45:67–82. 10.1016/j.beth.2013.09.004. [DOI] [PubMed] [Google Scholar]
- [71].Vandekerckhove M, Kestemont J, Weiss R, Schotte C, Exadaktylos V, Haex B, et al. Experiential versus analytical emotion regulation and sleep: breaking the link between negative events and sleep disturbance. Emotion 2012;12: 1415–21. 10.1037/a0028501. [DOI] [PubMed] [Google Scholar]
- [72].Talamini LM, Bringmann LF, de Boer M, Hofman WF. Sleeping worries away or worrying away sleep? Physiological evidence on sleep-emotion interactions. PLoS One 2013;8:e62480. 10.1371/journal.pone.0062480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Talbot LS, Hairston IS, Eidelman P, Gruber J, Harvey AG. The effect of mood on sleep onset latency and REM sleep in interepisode bipolar disorder. J Abnorm Psychol 2009;118:448–58. 10.1037/a0016605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Minkel JD, Banks S, Htaik O, Moreta MC, Jones CW, McGlinchey EL, et al. Sleep deprivation and stressors: evidence for elevated negative affect in response to mild stressors when sleep deprived. Emotion 2012;12:1015–20. 10.1037/a0026871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Finan PH, Quartana PJ, Smith MT. The effects of sleep continuity disruption on positive mood and sleep architecture in healthy adults. Sleep 2015;38: 1735–42. 10.5665/sleep.5154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Franzen PL, Buysse DJ, Dahl RE, Thompson W, Siegle GJ. Sleep deprivation alters pupillary reactivity to emotional stimuli in healthy young adults. Biol Psychol 2009;80:300–5. 10.1016/j.biopsycho.2008.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Franzen PL, Siegle GJ, Buysse DJ. Relationships between affect, vigilance, and sleepiness following sleep deprivation. J Sleep Res 2008;17:34–41. 10.1111/j.1365-2869.2008.00635.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Holding BC, Laukka P, Fischer H, Bänziger T, Axelsson J, Sundelin T. Multimodal emotion recognition is resilient to insufficient sleep: results from cross-sectional and experimental studies. Sleep 2017;40. 10.1093/sleep/zsx145. [DOI] [PubMed] [Google Scholar]
- [79].Krizan Z, Hisler G. Sleepy anger: restricted sleep amplifies angry feelings. J Exp Psychol Gen 2018. 10.1037/xge0000522. [DOI] [PubMed] [Google Scholar]
- [80].Tempesta D, Alessandro C, Curcio G, Moroni F, Cristina M, De Gennaro L, et al. Lack of sleep affects the evaluation of emotional stimuli. Brain Res Bull 2010;82:104–8. 10.1016/j.brainresbull.2010.01.014. [DOI] [PubMed] [Google Scholar]
- [81].Selvi Y, Gulec M, Agargun MY, Besiroglu L. Mood changes after sleep deprivation in morningness–eveningness chronotypes in healthy individuals. J Sleep Res 2007;16:241–4. [DOI] [PubMed] [Google Scholar]
- [82].Schwarz J, Axelsson J, Gerhardsson A, Tamm S, Fischer H, Kecklund G, et al. Mood impairment is stronger in young than in older adults after sleep deprivation. J Sleep Res 2019;28:e12801. 10.1111/jsr.12801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Schwarz JFA, Popp R, Haas J, Zulley J, Geisler P, Alpers GW, et al. Shortened night sleep impairs facial responsiveness to emotional stimuli. Biol Psychol 2013;93:41–4. 10.1016/j.biopsycho.2013.01.008. [DOI] [PubMed] [Google Scholar]
- [84].Haack M, Mullington JM. Sustained sleep restriction reduces emotional and physical well-being. Pain 2005;119:56–64. 10.1016/j.pain.2005.09.011. [DOI] [PubMed] [Google Scholar]
- [85].Dickinson DL, Drummond SPA, McElroy T. The viability of an ecologically valid chronic sleep restriction and circadian timing protocol: an examination of sample attrition, compliance, and effectiveness at impacting sleepiness and mood. PLoS One 2017;12:e0174367. 10.1371/journal.pone.0174367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Cote KA, Milner CE, Smith BA, Aubin AJ, Greason TA, Cuthbert BP, et al. CNS arousal and neurobehavioral performance in a short-term sleep restriction paradigm. J Sleep Res 2009;18:291–303. 10.1111/j.1365-2869.2008.00733.x. [DOI] [PubMed] [Google Scholar]
- [87].de Wild-Hartmann JA, Wichers M, van Bemmel AL, Derom C, Thiery E, Jacobs N, et al. Day-to-day associations between subjective sleep and affect in regard to future depressionin a female population-based sample. Br J Psychiatry 2013;202:407–12. 10.1192/bjp.bp.112.123794. [DOI] [PubMed] [Google Scholar]
- [88].Totterdell P, Reynolds S, Parkinson B, Briner RB. Associations of sleep with everyday mood, minor symptoms and social interaction experience. Sleep 1994;17:466–75. 10.1093/sleep/17.5.466. [DOI] [PubMed] [Google Scholar]
- [89].Jones BJ, Schultz KS, Adams S, Baran B, Spencer RMC. Emotional bias of sleep-dependent processing shifts from negative to positive with aging. Neurobiol Aging 2016;45:178–89. 10.1016/j.neurobiolaging.2016.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Cunningham TJ, Crowell CR, Alger SE, Kensinger EA, Villano MA, Mattingly SM, et al. Psychophysiological arousal at encoding leads to reduced reactivity but enhanced emotional memory following sleep. Neurobiol Learn Mem 2014;114:155–64. 10.1016/j.nlm.2014.06.002. [DOI] [PubMed] [Google Scholar]
- [91].Gujar N, McDonald SA, Nishida M, Walker MP. A role for REM sleep in recalibrating the sensitivity of the human brain to specific emotions. Cerebr Cortex 2011;21:115–23. 10.1093/cercor/bhq064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Bolinger E, Cunningham TJ, Payne JD, Bowman MA, Bulca E, Born J, et al. Sleep’s benefits to emotional processing emerge in the long term. Cortex 2019;120:457–70. 10.1016/j.cortex.2019.07.008. [DOI] [PubMed] [Google Scholar]
- [93].van der Helm E, Yao J, Dutt S, Rao V, Saletin JM, Walker MP. REM sleep depotentiates amygdala activity to previous emotional experiences. Curr Biol 2011;21:2029–32. 10.1016/j.cub.2011.10.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Studer RK, Nielsen C, Klumb PL, Hildebrandt H, Nater UM, Wild P, et al. The mediating role of mood in the relationship between perseverative cognition, sleep and subjective health complaints in music students. Psychol Health 2019;34:754–70. 10.1080/08870446.2019.1574014. [DOI] [PubMed] [Google Scholar]
- [95].Strahler J, Nater UM, Skoluda N. Associations between health behaviors and factors on markers of healthy psychological and physiological functioning: a daily diary study. Ann Behav Med 2020;54:22–35. 10.1093/abm/kaz018. [DOI] [PubMed] [Google Scholar]
- [96].Flueckiger L, Lieb R, Meyer AH, Witthauer C, Mata J. Day-to-day variations in health behaviors and daily functioning: two intensive longitudinal studies. J Behav Med 2017;40:307–19. 10.1007/s10865-016-9787-x. [DOI] [PubMed] [Google Scholar]
- [97].Bouwmans MEJ, Bos EH, Hoenders HJR, Oldehinkel AJ, de Jonge P. Sleep quality predicts positive and negative affect but not vice versa. An electronic diary study in depressed and healthy individuals. J Affect Disord 2017;207:260–7. 10.1016/j.jad.2016.09.046. [DOI] [PubMed] [Google Scholar]
- [98].Finan PH, Whitton AE, Letzen JE, Remeniuk B, Robinson ML, Irwin MR, et al. Experimental sleep disruption and reward learning: moderating role of positive affect responses. Sleep 2019;42:zsz026. 10.1093/sleep/zsz026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Finan PH, Quartana PJ, Remeniuk B, Garland EL, Rhudy JL, Hand M, et al. Partial sleep deprivation attenuates the positive affective system: effects across multiple measurement modalities. Sleep 2017;40. 10.1093/sleep/zsw017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100]*.Pires GN, Bezerra AG, Tufik S, Andersen ML. Effects of acute sleep deprivation on state anxiety levels: a systematic review and meta-analysis. Sleep Med 2016;24:109–18. 10.1016/j.sleep.2016.07.019. [DOI] [PubMed] [Google Scholar]
- [101].Babson KA. The interrelations between sleep and fear/anxiety. Sleep affect. Elsevier; 2015. p. 143–62. 10.1016/B978-0-12-417188-6.00007-4. [DOI] [Google Scholar]
- [102].Baglioni C, Battagliese G, Feige B, Spiegelhalder K, Nissen C, Voderholzer U, et al. Insomnia as a predictor of depression: a meta-analytic evaluation of longitudinal epidemiological studies. J Affect Disord 2011;135:10–9. 10.1016/j.jad.2011.01.011. [DOI] [PubMed] [Google Scholar]
- [103].O’Callaghan VS, Couvy-Duchesne B, Strike LT, McMahon KL, Byrne EM, Wright MJ. A meta-analysis of the relationship between subjective sleep and depressive symptoms in adolescence. Sleep Med 2021;79:134–44. 10.1016/j.sleep.2021.01.011. [DOI] [PubMed] [Google Scholar]
- [104].Clancy F, Prestwich A, Caperon L, Tsipa A, O’Connor DB. The association between worry and rumination with sleep in non-clinical populations: a systematic review and meta-analysis. Health Psychol Rev 2020;14:427–48. 10.1080/17437199.2019.1700819. [DOI] [PubMed] [Google Scholar]
- [105].Palmer CA, Alfano CA. Sleep and emotion regulation: an organizing, integrative review. Sleep Med Rev 2017;31:6–16. 10.1016/j.smrv.2015.12.006. [DOI] [PubMed] [Google Scholar]
- [106].Reynolds AC, Banks S. Total sleep deprivation, chronic sleep restriction and sleep disruption. Prog Brain Res 2010;185:91–103. 10.1016/B978-0-444-53702-7.00006-3.Elsevier. [DOI] [PubMed] [Google Scholar]
- [107].Goldstein AN, Walker MP. The role of sleep in emotional brain function. Annu Rev Clin Psychol 2014;10:679–708. 10.1146/annurev-clinpsy-032813-153716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].O’Connor PJ. Evaluation of four highly cited energy and fatigue mood measures. J Psychosom Res 2004;57:435–41. 10.1016/j.jpsychores.2003.12.006. [DOI] [PubMed] [Google Scholar]
- [109].Buckley TM, Schatzberg AF. On the interactions of the hypothalamic-pituitary-adrenal (HPA) axis and sleep: normal HPA axis activity and circa-dian rhythm, exemplary sleep disorders. J Clin Endocrinol Metab 2005;90: 3106–14. 10.1210/jc.2004-1056. [DOI] [PubMed] [Google Scholar]
- [110].Altena E, Micoulaud-Franchi J-A, Geoffroy P-A, Sanz-Arigita E, Bioulac S, Philip P. The bidirectional relation between emotional reactivity and sleep: from disruption to recovery. Behav Neurosci 2016;130:336–50. 10.1037/bne0000128. [DOI] [PubMed] [Google Scholar]
- [111].Vandekerckhove M, Cluydts R. The emotional brain and sleep: an intimate relationship. Sleep Med Rev 2010;14:219–26. 10.1016/j.smrv.2010.01.002. [DOI] [PubMed] [Google Scholar]
- [112].Watling J, Pawlik B, Scott K, Booth S, Short MA. Sleep loss and affective functioning: more than just mood. Behav Sleep Med 2017;15:394–409. 10.1080/15402002.2016.1141770. [DOI] [PubMed] [Google Scholar]
- [113].Sadeh A, Keinan G, Daon K. Effects of stress on sleep: the moderating role of coping style. Health Psychol 2004;23:542–5. 10.1037/0278-6133.23.5.542. [DOI] [PubMed] [Google Scholar]
- [114].Adan ANA, Fabbri M, Natale V, Prat G. Sleep beliefs scale (SBS) and circadian typology. J Sleep Res 2006;15:125–32. [DOI] [PubMed] [Google Scholar]
- [115].Tamir M, John OP, Srivastava S, Gross JJ. Implicit theories of emotion: affective and social outcomes across a major life transition. J Pers Soc Psychol 2007;92: 731–44. 10.1037/0022-3514.92.4.731. [DOI] [PubMed] [Google Scholar]
- [116].Gilam G, Gross James J, Wager Tor D, Keefe Francis J, Mackey SC. What is the relationship between pain and emotion? Bridging constructs and communities. Neuron 2020;107:17–21. 10.1016/j.neuron.2020.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Epel ES, Crosswell AD, Mayer SE, Prather AA, Slavich GM, Puterman E, et al. More than a feeling: a unified view of stress measurement for population science. Front Neuroendocrinol 2018;49:146–69. 10.1016/j.yfrne.2018.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Kelly GA, Blake C, Power CK, O’Keeffe D, Fullen BM. The association between chronic low back pain and sleep: a systematic review. Clin J Pain 2011;27: 169–81. 10.1097/AJP.0b013e3181f3bdd5. [DOI] [PubMed] [Google Scholar]
- [119].Stroemel-Scheder C, Kundermann B, Lautenbacher S. The effects of recovery sleep on pain perception: a systematic review. Neurosci Biobehav Rev 2020;113:408–25. 10.1016/j.neubiorev.2020.03.028. [DOI] [PubMed] [Google Scholar]
- [120].Kim E-J, Dimsdale JE. The effect of psychosocial stress on sleep: a review of polysomnographic evidence. Behav Sleep Med 2007;5:256–78. 10.1080/15402000701557383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Van Reeth O, Weibel L, Spiegel K, Leproult R, Dugovic C, Maccari S. Interactions between stress and sleep: from basic research to clinical situations. Sleep Med Rev 2000;4:201–19. 10.1053/smrv.1999.0097. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


