Keywords: fasting, free cortisol, overfeeding, stress
Abstract
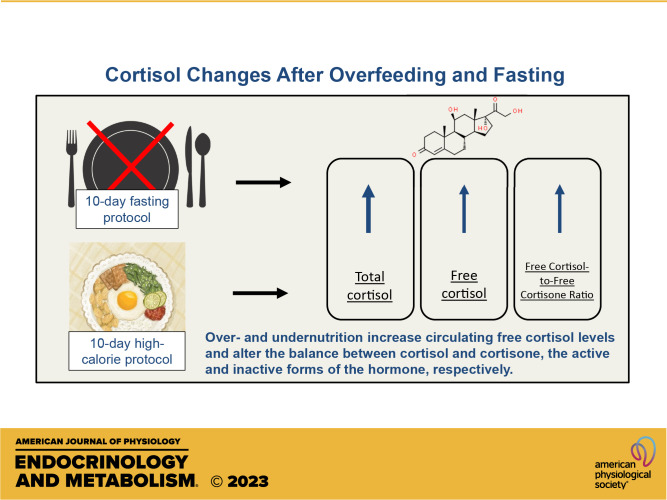
Chronic caloric deprivation and obesity are complicated by hypercortisolemia. The effects of acute overfeeding and fasting on circulating free cortisol levels and conversion of cortisone to free cortisol are unknown. We hypothesized that serum-free cortisol and free cortisol-to-cortisone ratio would increase after both overfeeding and fasting. This is a prospective study of 22 healthy volunteers who completed a 10-day high-calorie protocol followed by a 10-day fast, separated by a 2-wk washout. Morning free and total cortisol and free cortisone levels (LC/MS) were measured at baseline and after 10 days of each intervention. Both high-calorie feeding and fasting increased total and free cortisol and the free cortisol-to-free cortisone ratio (P = 0.001 to P = 0.046). There were sex interactions, with significant effects in men (P < 0.001), but not in women (P = 0.898 and 1.000, respectively) in subset analyses examining the effects of fasting on free cortisol and the free-to-total cortisol ratio. Overfeeding and fasting both increase circulating free cortisol levels and appear to alter the balance between cortisol and its inactive metabolite, cortisone. Further study is warranted to determine whether elevated cortisol levels contribute to complications of starvation and obesity, such as bone fragility.
NEW & NOTEWORTHY Overfeeding and fasting both increase circulating free cortisol levels and appear to alter the balance between cortisol and its inactive metabolite, cortisone. The effect of fasting on free cortisol levels is modified by sex. Further study is needed to determine the mechanisms driving the increases in cortisol.
INTRODUCTION
Cortisol is a critical element of the human stress response and contributes to immune system modulation, cardiovascular function, and energy availability (1–3). In studies of acute fasting (up to 10 days), elevated plasma total cortisol levels have been reported, with variable changes in total cortisol levels reported with less severe calorie restriction (fewer than 800 kcal/day; 4, 5). About a third of individuals with anorexia nervosa, a state of chronic calorie deprivation, have elevated serum and urine cortisol levels (6–9). Studies examining the effect of short-term overfeeding on cortisol levels have inconsistent findings, reporting no change or elevation in cortisol levels (10–12). Cortisol, as measured by 24-h urine-free cortisol (UFC) and mean overnight serum cortisol, demonstrates a U-shaped relationship with body mass index (BMI), with highest levels at the extremes of weight (13). High cortisol states, such as Cushing’s disease, anorexia nervosa, and prolonged exogenous glucocorticoid exposure, are associated with complications including low bone mineral density, fractures, hypercoagulability, and immunosuppression (14–17). Examining the effects of fasting and overfeeding on free cortisol and mechanisms for increasing free cortisol relative to total cortisol may further our understanding of complications of overnutrition and undernutrition, including increased bone fragility.
The cortisol stress response to critical illness has been well studied and includes suppression of 11β-hydroxysteroid dehydrogenase (HSD) 2 activity, which results in higher cortisol levels (18). In addition, inflammatory cytokines, including plasma TNF-α and IL-6 levels, are associated with increased cortisol production (18). Reduced breakdown of cortisol, increased cortisol production and decreased cortisol binding globulin binding have also been implicated (19). However, little is known about the mechanisms of the cortisol stress response in states of over- and underfeeding.
We previously published the effects of 10 days of overfeeding and 10 days of fasting on a number of endpoints, including bone marrow adipose tissue (BMAT) and inflammatory markers in 23 healthy subjects (20). We now leverage this model to examine the cortisol stress response of acute nutrient changes (overfeeding and fasting), including effects on serum-free cortisol and serum-free cortisol-to-cortisone ratio.
MATERIALS AND METHODS
Study Participants
The study included 23 normal or overweight individuals (10 women and 13 men) without a history of diabetes mellitus or an eating disorder. Seventeen of 22 subjects were overweight (BMI ≥ 25 kg/m2). All women were premenopausal and had a history of regular menstrual cycles, and none had used exogenous estrogen within 3 mo of her baseline visit. An additional female participant was enrolled in the study but was not able to comply with study procedures; therefore, her data were not included in any of the analyses. For 1 of the 23 individuals who completed the study, there was inadequate sample to run serum cortisol levels; therefore, this individual was excluded from this analysis.
Study Protocol
The study protocol was previously described in detail (20). In brief, subjects were admitted to the Translational and Clinical Research Center at the Massachusetts General Hospital for two inpatient study visits totaling 20 days. Participants were initially admitted for a 10-day high-calorie visit with the aim of achieving a 7% weight gain based on body weight. Subjects met with a bionutritionist, who calculated their caloric needs for 7% weight gain based on body weight using the Mifflin St Jeor equation with an activity factor of 1.3. Participants were permitted to select menu items with a macronutrient content consisting of 45%–55% carbohydrates, 30%–40% fat, and no more than 25% protein. The mean kilocalories consumed by participants per day ranged from 4,761 ± 614 to 7,388 ± 174 (Table 1). After completion of the high-calorie protocol, subjects were discharged home to resume their normal diet for 13–18 days. Subjects were subsequently readmitted for a second inpatient visit (fasting visit), during which subjects did not consume any calories for 10 days but were permitted to drink water ad libitum and received 20 mEq of potassium chloride and a multivitamin containing 400 IU of cholecalciferol daily. On the first (baseline) and final inpatient day of the high-calorie and fasting interventions, blood was drawn (fasting) in the morning for laboratory studies. One subject ended the fast and had post-fasting laboratory reports drawn after 7 days.
Table 1.
Kilocalories and macronutrient content consumed by participant during the high-calorie protocol
| Kilocalories per Day | Fat | Carbohydrate | Protein | |
|---|---|---|---|---|
| 1 | 5,895.5 ± 134.3 | 32.1 ± 1.1% | 47.3 ± 0.7% | 20.5 ± 1.1% |
| 2 | 5,817.0 ± 477.6 | 31.5 ± 1.3% | 48.3 ± 1.9% | 17.3 ± 2.2% |
| 3 | 5,562.0 ± 1189.8 | 32.4 ± 1.3% | 48.7 ± 1.4% | 19.8 ± 1.2% |
| 4 | 6,258.7 ± 1163.9 | 32.5 ± 1.3% | 50.4 ± 1.8% | 22.5 ± 11.0% |
| 5 | 6,444.2 ± 987.1 | 32.7 ± 0.9% | 49.5 ± 2.0% | 17.9 ± 1.5% |
| 6 | 5,600.5 ± 661.3 | 33.4 ± 1.6% | 50.5 ± 1.6% | 18.2 ± 1.7% |
| 7 | 6,361.0 ± 1660.4 | 33.2 ± 1.1% | 48.9 ± 1.6% | 18.7 ± 1.9% |
| 8 | 5,029.2 ± 539.5 | 33.1 ± 1.2% | 51.1 ± 1.2% | 17.1 ± 1.1% |
| 9 | 4,760.9 ± 614.3 | 33.3 ± 1.6% | 50.0 ± 1.6% | 17.8 ± 1.2% |
| 10 | 5,276.5 ± 1039.6 | 33.6 ± 1.3% | 48.1 ± 1.3% | 18.0 ± 1.1% |
| 11 | 7,387.5 ± 173.9 | 34.1 ± 1.3% | 49.7 ± 1.4% | 16.9 ± 1.7% |
| 12 | 6,626.0 ± 1640.8 | 34.4 ± 1.7% | 48.9 ± 1.8% | 17.1 ± 1.6% |
| 13 | 7,242.8 ± 278.1 | 33.0 ± 1.5% | 49.3 ± 1.6% | 18.3 ± 2.1% |
| 14 | 5,613.9 ± 1482.4 | 33.4 ± 2.1% | 50.1 ± 1.9% | 17.1 ± 1.4% |
| 15 | 7,065.5 ± 232.0 | 33.1 ± 0.9% | 49.2 ± 1.5% | 18.2 ± 1.2% |
| 16 | 6,202.2 ± 610.9 | 33.8 ± 1.7% | 49.9 ± 2.1% | 16.7 ± 1.4% |
| 17 | 6,839.6 ± 604.5 | 33.9 ± 2.3% | 50.8 ± 1.7% | 16.3 ± 1.6% |
| 18 | 4,769.4 ± 1185.0 | 32.9 ± 1.5% | 50.6 ± 1.8% | 17.4 ± 1.4% |
| 19 | 6,018.0 ± 650.8 | 34.3 ± 2.0% | 49.1 ± 2.2% | 17.7 ± 1.3% |
| 20 | 5,796.7 ± 683.0 | 34.9 ± 1.3% | 49.7 ± 1.9% | 17.0 ± 2.0% |
| 21 | 5,365.7 ± 267.4 | 34.7 ± 1.6% | 50.1 ± 2.0% | 16.6 ± 1.5% |
| 22 | 5,949.3 ± 471.3 | 34.0 ± 1.8% | 49.9 ± 2.0% | 16.9 ± 1.0% |
Laboratory Methods
Serum was stored at −80°C until analysis. Total cortisol was measured by high-performance liquid chromatography with triple quad mass spectrometer (LC-MS/MS; Mayo Labs, Rochester, MN). Free cortisol and cortisone were measured by equilibrium dialysis-LC-MS (Mayo Labs, Rochester, MN). For total cortisol, deuterated stable isotopes (d4-cortisol) are added to a 0.1 mL of serum sample as internal standards. Cortisol and the internal standards are extracted from specimens online using a Cohesive TX system with a C18 4 × 3 Security Guard Cartridge. The analytes are transferred online to a Kinetex 2.6-μm C8 100 Å, 50 × 3-mm analytical column and are analyzed by LC-MS/MS. The analytical measuring range (AMR) for total cortisol is 0.2–40 μg/dL. The reportable range was 0.2–400 μg/dL. The coefficient of variation for total cortisol was 10.8%, 11.6%, and 11.4% at total cortisol concentrations of 2, 9.7, and 19.2 μg/dL, respectively. To measure free cortisol in serum, the unbound and conjugated forms were separated without disrupting the equilibrium of the sample. The rapid equilibrium dialysis (RED, ThermoFisher) plate is used to perform a simple means of equilibrium dialysis in a 96-well format. Sample is placed into one of the two chambers separated by a vertical cylinder of equilibrium membrane (molecular weight cut-off 8,000). The second chamber is filled with dialysis buffer. Both chambers reside in a polypropylene plate with standard 96-well format. After gently shaking overnight at 37°C, the free cortisol is at equilibrium within the dialysis chamber. The dialysate is removed and d3-cortisol is added as an internal standard. The dialysate mixture is then analyzed using turbo flow liquid chromatography combined with a heated nebulizer ion source (atmospheric pressure chemical ionization) and tandem mass spectrometry. The AMR of free cortisol was 0.03–10 μg/dL. The coefficient of variation for free cortisol was 12.1%, 10.5%, and 12.4% at total cortisol concentrations of 0.046, 0.405, and 3.44 μg/dL, respectively. The method and performance of free cortisone were similar to that for free cortisol. IL-6, TNF-α, and C-reactive protein (CRP) were previously reported. IL-6 and TNF-α were measured by human DuoSet ELISA (R&D Systems, Bio-Techne). CRP was measured by a clinical laboratory (Quest Diagnostics).
Statistical Analysis
Statistical analysis was performed using R version 4.1.0. Continuous data were reported as median [interquartile range]. Normality was assessed with the Shapiro–Wilk test. Normally distributed data were compared using the paired two-tailed t test and nonnormally distributed data were compared using the paired Wilcoxon rank-sum test. For normally distributed data, Pearson’s correlation coefficients (represented by r) were calculated to assess univariate relationships. For nonnormally distributed data, Spearman’s coefficients (represented by ρ) were calculated to assess univariate relationships. A two-tailed P value of less than 0.05 was considered significant.
Study Approval
The study was approved by the Mass General Brigham Institutional Review Board (Boston, MA, protocol 2015P000624) and complied with the Health Insurance Portability and Accountability Act Guidelines. Written informed consent was obtained from all subjects. The study is registered at ClinicalTrials.gov (NCT02482519).
RESULTS
Characteristics of Study Participants
For subjects included in this study, at baseline, median age was 32.5 yr (interquartile range (IQR) 28.0, 39.8) and median BMI was 26.4 kg/m2 (IQR 25.1, 27.2; 20). During the 10-day high-calorie protocol, subjects gained a median 4.6% of body weight (IQR 3.9, 5.0). Subjects lost a median 8.9% (IQR −9.7, −8.1) of body weight during the 10-day fast (Table 2).
Table 2.
Biochemical and anthropometric characteristics of participants throughout the study
| High Calorie |
Fasting |
|||
|---|---|---|---|---|
| Pre | Post | Pre | Post | |
| BMI, kg/m2 | 26.4 [25.1, 27.2] | 27.8 [26.9, 28.7] | 27.3 [26.0, 27.9] | 24.9 [23.8, 25.7] |
| Weight, kg | 73.4 [66.9, 80.9] | 78.2 [72.2, 87.0] | 75.5 [70.2, 83.6] | 69.0 [63.3, 76.4] |
| Systolic blood pressure, mmHg | 117.0 [110.0, 132.0] | 119.5 [113.0, 135.0] | 119.0 [110.8, 136.0] | 115.0 [107.0, 128.0] |
| Diastolic blood pressure, mmHg | 71.0 [67.0, 86.5] | 70.0 [65.8, 76.8] | 71.5 [62.8, 81.0] | 76.0 [73.0, 85.0] |
| Glucose, mg/dL | 89.0 [83.0, 94.5] | 94.0 [89.0, 98.8] | 91.0 [83.0, 96.0] | 68.0 [65.0, 72.0] |
| Sodium, mmol/L | 140.0 [140.0, 141.0] | 140.0 [139.0, 140.8] | 140.0 [138.0, 141.0] | 136.0 [136.0, 137.0] |
| Potassium, mmol/L | 4.1 [4.0, 4.2] | 4.1 [4.0, 4.3] | 4.0 [3.9, 4.1] | 4.1 [3.8, 4.4] |
| Calcium, mg/dL | 9.2 [9.1, 9.5] | 9.4 [9.2, 9.6] | 9.2 [9.1, 9.5] | 9.8 [9.5, 10.0] |
| Uric acid, mg/dL | 4.9 [4.0, 5.8] | 5.0 [4.4, 5.6] | 5.1 [4.0, 6.3] | 11.3 [10.7, 13.3] |
| Creatinine, mg/dL | 0.9 [0.8, 1.1] | 0.8 [0.7, 1.0] | 0.9 [0.8, 1.0] | 1.1 [0.9, 1.2] |
| BUN, mg/dL | 12.5 [10.3, 14.8] | 16.0 [14.0, 18.0] | 12.0 [10.0, 15.0] | 9.0 [8.0, 11.0] |
| Albumin, g/dL | 4.5 [4.2, 4.6] | 4.4 [4.1, 4.6] | 4.3 [4.2, 4.5] | 4.7 [4.6, 4.9] |
| Visceral adipose tissue by MRI, cm2 | 37.4 [19.0, 81.6] | 48.6 [17.8, 82.9] | 44.8 [22.1, 79.2] | 40.0 [22.1, 81.6] |
| Subcutaneous adipose tissue by MRI, cm2 | 194.4 [150.2, 230.8] | 217.9 [171.5, 258.0] | 225.3 [168.1, 245.4] | 182.2 [150.8, 233.5] |
| Trunk fat, kg, by DXA | 9.2 [7.3, 11.4] | 9.9 [8.3, 11.6] | 10.2 [8.1, 11.8] | 9.4 [7.0, 10.9] |
| Total fat mass, kg, by DXA | 54.8 [43.4, 60.5] | 57.7 [47.2, 60.4] | 55.7 [43.7, 59.9] | 51.0 [39.9, 54.1] |
| Lean mass, kg, by DXA | 22.3 [17.9, 24.5] | 24.1 [19.3, 26.2] | 24.7 [19.3, 25.7] | 22.9 [17.5, 25.0] |
Values shown as median with interquartile range. BMI, body mass index; BUN, blood urea nitrogen; DXA, dual-energy X-ray absorptiometry.
Serum Cortisol Level Changes during High-Calorie Protocol
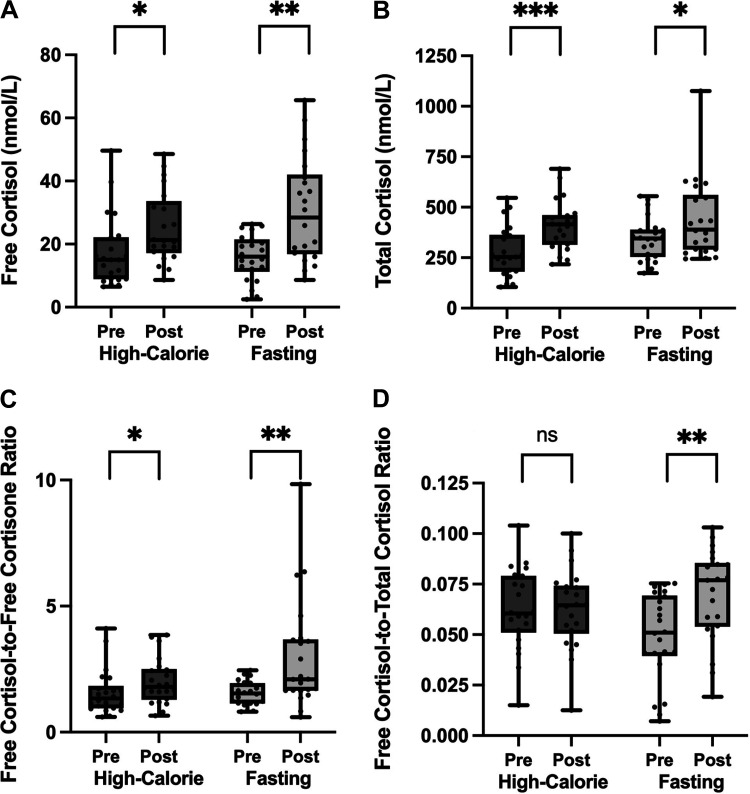
Median free cortisol increased after a 10-day high-calorie protocol from 14.0 to 21.2 nmol/L (P = 0.021). Median total cortisol increased from 253.8 to 415.2 nmol/L (P < 0.001). Median free cortisol-to-free cortisone ratio increased from 1.3 to 1.8 (P = 0.046). After the high-calorie protocol, there was no change in median free-to-total cortisol ratio (0.06–0.06, P = 0.989; Fig. 1).
Figure 1.
There was an increase in free cortisol (A), total cortisol (B), and free cortisol-to-free cortisone ratio (C) after a 10-day high-calorie and fasting protocol. There was no change in the free cortisol-to-total cortisol ratio after high-calorie visit, but there was an increase after fasting visit (D). “Pre” indicates baseline levels at start of each 10-day visit. “Post” indicates levels at the end of each 10-day visit. Data presented as box and whisker plots with boxes representing median and interquartile range and whiskers representing minimum and maximum values. ns (not significant) indicates P > 0.05, *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001.
Serum Cortisol Level Changes during 10 Days of Fasting
Median free cortisol increased from 16.0 to 28.4 nmol/L (P = 0.001). Median serum total cortisol increased after a 10-day fast from 344.9 to 389.0 nmol/L (P = 0.021). The median free cortisol-to-free cortisone ratio increased 1.4 times from 1.5 to 2.1 (P = 0.002) and the free cortisol-to-total cortisol ratio increased 1.5 times from 0.05 to 0.08 (P = 0.003; Fig. 1).
Sex Differences in Cortisol Level Changes during High-Calorie and Fasting Protocols
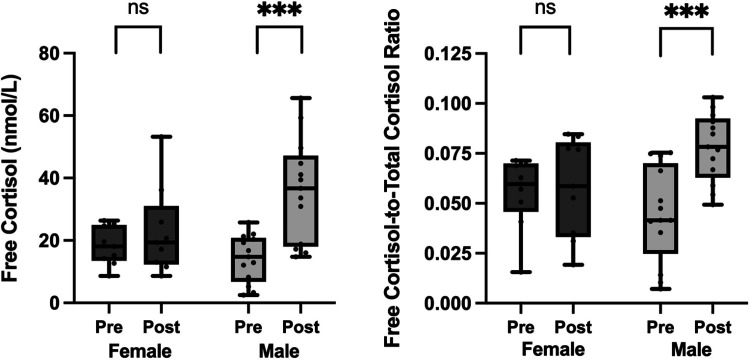
During the high-calorie protocol, there was no significant interaction between sex and time for any of the cortisol parameters (Table 3). During the fasting visit, the changes in free cortisol and free-to-total cortisol ratio varied by sex (P = 0.014 and 0.004, respectively, for interaction term). There was a trend toward a significant interaction between sex and time in the change in free cortisol-to-free cortisone ratio (P = 0.054). In men, mean free cortisol levels increased from 14.2 to 36.0 nmol/L (P < 0.001) and mean free cortisol-to-total cortisol ratio increased 1.8 times from 0.04 to 0.08 (P < 0.001) after fasting. In contrast, there was no change in median free cortisol or free cortisol-to-total cortisol ratio in women (P = 0.898 and 1.000, respectively; Fig. 2).
Table 3.
Repeated-measure ANOVA for change in cortisol parameters after high-calorie and fasting protocol by sex
| High Calorie | ||||
|---|---|---|---|---|
| Pre | Post | P Value for Sex Interaction | P Value for Change | |
| Free cortisol, nmol/L | 14.90 [8.83, 21.24] | 21.24 [17.93, 32.83] | 0.096 | 0.005 |
| Total cortisol, nmol/L | 253.83 [185.40, 354.53] | 415.23 [325.01, 455.24] | 0.189 | <0.001 |
| Free cortisol-to-free cortisone ratio | 1.33 [0.97, 1.71] | 1.79 [1.39, 2.46] | 0.204 | 0.038 |
| Free cortisol-to-total cortisol ratio | 0.06 [0.05, 0.08] | 0.06 [0.05, 0.07] | 0.989 | 0.273 |
| Fasting | ||||
|---|---|---|---|---|
| Pre | Post | P Value for Sex Interaction | P Value for Change | |
| Free cortisol, nmol/L | 16.00 [12.14, 16.00] | 28.42 [9.93, 40.83] | 0.014 | <0.001 |
| Total cortisol, nmol/L | 344.88 [260.45, 385.71] | 389.02 [292.45, 543.52] | 0.340 | 0.018 |
| Free cortisol-to-free cortisone ratio | 1.54 [1.15, 1.93] | 2.10 [1.65, 3.64] | 0.054 | <0.001 |
| Free cortisol-to-total cortisol ratio | 0.05 [0.04, 0.07] | 0.07 [0.06, 0.08] | 0.004 | <0.001 |
Free cortisol and free cortisol-to-total cortisol ratio change varied by sex after fasting. Cortisol variables shown as median with interquartile range. Bold indicates P < 0.05.
Figure 2.
Free cortisol and free cortisol-to-total cortisol ratio increased after 10-day fast in men but not in women. “Pre” indicates baseline levels at start of each 10-day visit. “Post” indicates levels at the end of each 10-day visit. Data presented as box and whisker plots with boxes representing median and interquartile range and whiskers representing minimum and maximum values. ns (not significant) indicates P > 0.05, ***P ≤ 0.001.
Predictors of Change in Cortisol Levels during High-Calorie Protocol
Change in weight, baseline percent body fat by dual-energy X-ray absorptiometry (DXA), and inflammatory markers (TNF-α, IL-6, and CRP) did not correlate with change in free cortisol, total cortisol, free cortisol-to-total cortisol ratio, or free cortisol-to-free cortisone ratio (data not shown). In addition, percent of kilocalories from carbohydrate, fat, or protein did not correlate with change in free cortisol, total cortisol, free cortisol-to-total cortisol ratio, or free cortisol-to-free cortisone ratio. Baseline BMI correlated with the change in free cortisol-to-total cortisol ratio (ρ = −0.45, P = 0.036) but not other cortisol parameters.
Predictors of Change in Cortisol Levels during Fasting Protocol
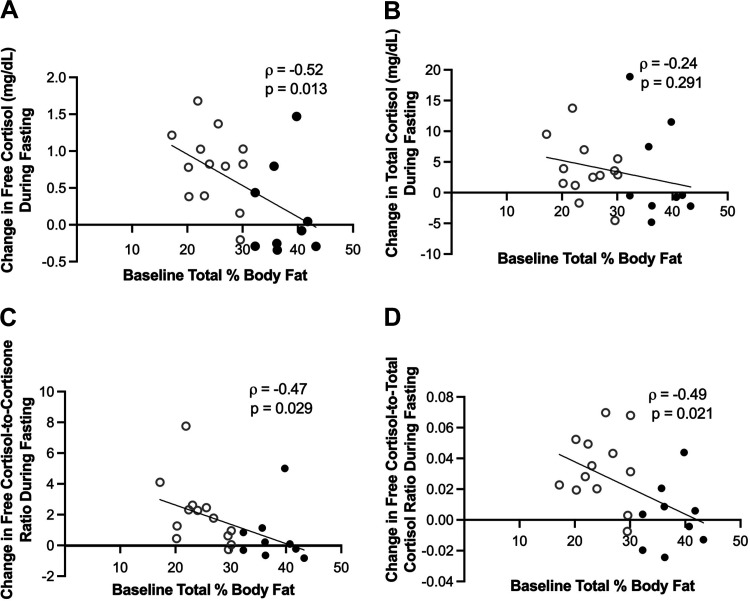
Change in weight and inflammatory markers (TNF-α, IL-6, and CRP) did not correlate with change in free cortisol, total cortisol, free cortisol-to-total cortisol ratio, or free cortisol-to-free cortisone ratio (data not shown). Percent body fat at the start of the fasting visit as measured by DXA was inversely associated with change in free cortisol (ρ = −0.52, P = 0.013), change in free cortisol-to-total cortisol ratio (ρ = −0.49, P = 0.021), and change in free cortisol-to-cortisone ratio (ρ = −0.47, P = 0.029) over the 10-day fasting period. Baseline percent body fat did not correlate with change in total cortisol (ρ = −0.24, P = 0.291; Fig. 3). When an interaction term for sex was added, the associations between percent body fat by DXA and change in free cortisol, change in free cortisol-to-total cortisol ratio, and change in free cortisol-to-cortisone ratio were no longer significant. Baseline BMI was associated with the change in free cortisol-to-cortisone ratio (ρ = −0.50, P = 0.0.19), but there was no association with the change in free cortisol (P = 0.103), total cortisol (P = 0.610), and free cortisol-to-total cortisol (P = 0. 168) ratios.
Figure 3.
Baseline (prefasting) percent body fat as measured by DXA was inversely associated with change in free cortisol (A), free cortisol-to-free cortisone ratio (C), and free cortisol-to-total cortisol ratio (D) during fasting. Change in total cortisol (B) was not associated with baseline percent body fat. Black ● represents females. Gray ○ represents males. DXA, dual-energy X-ray absorptiometry.
DISCUSSION
We demonstrated that fasting and high-calorie feeding result in increases in free and total cortisol and free cortisol-to-free cortisone ratio. The effect of fasting, but not overfeeding, on free cortisol levels is modified by sex with free cortisol and free cortisol-to-total cortisol ratio increasing in men, but not in women. The changes in circulating free cortisol, free cortisol-to-free cortisone ratio, and free cortisol-to-total cortisol ratio during fasting are inversely associated with baseline percent body fat, which raises the question whether higher percent body fat in women is relatively protective against fasting-induced hypercortisolemia.
It is known that circulating cortisol levels (free and total) are elevated in states of stress (2, 21). In critical illness, reduced breakdown is the primary driver of elevated serum-free cortisol levels with smaller contributions from increased production and decreased binding (18). In humans, critical illness suppresses 11β-hydroxysteroid dehydrogenase 2 activity; 5-α reductase and 5-β reductase activity and plasma TNF-α and IL-6 levels are positively correlated with cortisol production (18). In addition, there is accumulating evidence for the importance of bile acids in the regulation of cortisol metabolism during critical illness (18, 22, 23). In a cross-sectional study of hospitalized patients, CRP concentrations correlated with increasing cortisol-to-cortisone ratio (24). In our previous report, we observed increased CRP levels after fasting and overfeeding, increased TNF-α after overfeeding, and no change in IL-6 during overfeeding or fasting (20). In this analysis, we found no association between TNF-α, CRP, and IL-6 and changes in cortisol during overfeeding or fasting, suggesting a different mechanism drives hypercortisolemia in states of acute nutrient changes.
In prolonged fasting, another high-stress state, plasma total cortisol levels are elevated (4, 5). In two prior studies of healthy women undergoing a 2.5- or 4-day fasts, mean 24-h serum total cortisol levels, measured by frequent sampling, increased significantly (25, 26). A 5-day fast in eight healthy men induced a 1.8-fold increase in mean 24-h cortisol production without a change in cortisol half-life (27). Mean total cortisol levels increased on days 2 through 9 of a 10-day fast in nine subjects; however, mean total cortisol levels were not higher than baseline at the completion of the fast (5). To our knowledge, effects of fasting and overfeeding on free cortisol, the free cortisol-to-free cortisone ratio, or sex-specific effects in the same study have not been previously investigated.
In this study, the free cortisol and free-to-total cortisol ratio response to fasting varied by sex, with a trend for a sex interaction in the change in free cortisol-to-free cortisone ratio. Sexually dimorphic expression and activity of 11β-HSD1 in the liver have previously been demonstrated in rats and sex differences in cortisol secretion and metabolism in humans have been described (28, 29). Our findings suggest that women experience less of a stress response to prolonged fasting than men, which is driven by unclear factors. Based on the inverse associations between changes in cortisol measures and baseline body fat percentage, the difference in the stress response between sexes may at least partially be related to the greater body fat stores of women. There was not a clear association between BMI and change in cortisol. It is also possible that menstrual cycle phase affects cortisol levels in women, as a meta-analysis found circulating cortisol levels are higher in the follicular than the luteal phase (30). However, the effect size found in this meta-analysis was small (Hedges’ g of 0.13), and this finding has not been consistent among studies. Therefore, although the menstrual cycle phases in which the female subjects were studied are not known, it is unlikely that menstrual cycle phase variability in circulating cortisol levels is an explanation for our findings.
The sex differences observed in the percent change in L4 BMAT previously reported (20), with male subjects experiencing significantly greater increases in L4 BMAT than female subjects, mirrors the sexually dimorphic change in free cortisol and suggests increased circulating free cortisol as a possible mechanism for depot-specific fat accumulation. In contrast, as previously reported, although there was no significant change in BMAT at the femoral metaphysis in the group as a whole, female subjects gained significantly more BMAT at this site than male subjects during fasting (20). The mechanisms driving different responses of various BMAT locations to acute nutrient changes are unknown and require further study. Rodent models have also demonstrated sex and skeletal site differences in regulation of BMAT (31) and a role for circulating glucocorticoid levels has been hypothesized as a driver for increased BMAT in mice undergoing calorie restriction (32).
The macronutrient composition of the kilocalories consumed during the overfeeding protocol did not correlate with change in total or free cortisol levels. Prior studies have had inconsistent findings regarding the impact of meal carbohydrate content on serum cortisol levels with increased levels (33) and unchanged levels found (34). Body fat distribution is a possible explanation for the variable findings (33). Participants in this study had a narrow range of percent kilocalories from carbohydrate (47%–51%) and no correlations were found between macronutrient content during the overfeeding protocol and change in total or free cortisol levels. This may be due to the fairly consistent macronutrient content consumed across all participants. It is also possible that the impact of macronutrients on cortisol levels differs when subjects are undergoing high-calorie feeding versus eucaloric feeding as performed in prior studies.
The increase in the free cortisol-to-free cortisone ratio during overfeeding and fasting may reflect a change in 11β-HSD activity with a shift toward cortisol generation. 11β-HSD1 regulation is complex and incompletely understood with endocrine, inflammatory, and metabolic factors contributing to tissue-specific regulation (35). 11β-HSD1 modulates cortisol activity at the cellular level (intracrine effects; 35), but the circulating cortisol-to-cortisone ratio also reflects the overall balance between 11β-HSD1 and 11β-HSD2 activity (endocrine effects). The mechanism by which acute nutrient changes modify 11β-HSD activity requires further study but appears independent of inflammation.
Limitations
Our findings should be interpreted in the context of several limitations. Cortisol levels were measured from fasting morning blood samples collected at baseline and on day 10 of the overfeeding and fasting intervention; thus, diurnal variation was not captured by the once-daily measures available in this study. In addition, all subjects had completed 10-day high-calorie intervention 14 days before fasting, and the implications of this for cortisol regulation are unknown. Without measures of cortisol metabolism and measures of cortisol production, the mechanisms driving the increases in free and total cortisol cannot be determined. Although the macronutrient content of meals during the high-calorie protocol was documented, the fructose and sucrose contents were not collected and therefore the impact of these components could not be analyzed.
Conclusions
In conclusion, this study demonstrated increases in free cortisol and free cortisol-to-free cortisone ratio after 10 days of overfeeding and fasting. We observed a sexually dimorphic response of free cortisol and free-to-total cortisol ratio to fasting. Further study is warranted to determine the mechanisms driving the increases in free and total cortisol and whether elevated circulating cortisol levels contribute to the expansion of BMAT observed in starvation and obesity.
DATA AVAILABILITY
The study is registered at https://clinicaltrials.gov/ (NCT02482519).
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
GRANTS
This study is supported by NIH grant R24 DK084970, Harvard Catalyst/The Harvard Clinical and Translational Science Center (National Center for Advancing Translational Sciences, NIH, award UL 1TR002541), and NIH grants P30 DK040561, U19 AG060917S1, T32 DK007028, K24 HL092902, K24 DK109940.
DISCLOSURES
K.K.M. has received study medication from Pfizer and investigator-initiated research grants from Amgen, and has had equity in Bristol-Myers Squibb, General Electric, Boston Scientific, Amgen, and Becton Dickinson. P.K.F. is a consultant for Regeneron and Strongbridge Biopharma. None of the other authors has any conflicts of interest, financial or otherwise, to disclose.
AUTHOR CONTRIBUTIONS
M.A.B., P.K.F., C.J.R., and K.K.M. conceived and designed research; M.A.B., P.K.F., G.P-P., R.J.S., and C.J.R. performed experiments; C.C. analyzed data; C.C., M.A.B., R.J.S., C.J.R., and K.K.M. interpreted results of experiments; C.C. prepared figures; C.C. drafted manuscript; M.A.B., P.K.F., G.P-P., R.J.S., C.J.R., and K.K.M. edited and revised manuscript; C.C., M.A.B., P.K.F., G.P-P., R.J.S., C.J.R., and K.K.M. approved final version of manuscript.
ACKNOWLEDGMENTS
Present address of P. K. Fazeli: Neuroendocrinology Unit, University of Pittsburgh, Pittsburgh, Pennsylvania, United States.
REFERENCES
- 1. Chrousos GP. The hypothalamic-pituitary-adrenal axis and immune-mediated inflammation. N Engl J Med 332: 1351–1362, 1995. doi: 10.1056/NEJM199505183322008. [DOI] [PubMed] [Google Scholar]
- 2. Widmer IE, Puder JJ, König C, Pargger H, Zerkowski HR, Girard J, Müller B. Cortisol response in relation to the severity of stress and illness. J Clin Endocrinol Metab 90: 4579–4586, 2005. doi: 10.1210/jc.2005-0354. [DOI] [PubMed] [Google Scholar]
- 3. Finlay WE, McKee JI. Serum cortisol levels in severely stressed patients. Lancet 1: 1414–1415, 1982. doi: 10.1016/s0140-6736(82)92531-4. [DOI] [PubMed] [Google Scholar]
- 4. Nakamura Y, Walker BR, Ikuta T. Systematic review and meta-analysis reveals acutely elevated plasma cortisol following fasting but not less severe calorie restriction. Stress 19: 151–157, 2016. doi: 10.3109/10253890.2015.1121984. [DOI] [PubMed] [Google Scholar]
- 5. Steinhauser ML, Olenchock BA, O’Keefe J, Lun M, Pierce KA, Lee H, Pantano L, Klibanski A, Shulman GI, Clish CB, Fazeli PK. The circulating metabolome of human starvation. JCI Insight 3: e121434, 2018. doi: 10.1172/jci.insight.121434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Biller BM, Saxe V, Herzog DB, Rosenthal DI, Holzman S, Klibanski A. Mechanisms of osteoporosis in adult and adolescent women with anorexia nervosa. J Clin Endocrinol Metab 68: 548–554, 1989. doi: 10.1210/jcem-68-3-548. [DOI] [PubMed] [Google Scholar]
- 7. Putignano P, Dubini A, Toja P, Invitti C, Bonfanti S, Redaelli G, Zappulli D, Cavagnini F. Salivary cortisol measurement in normal-weight, obese and anorexic women: comparison with plasma cortisol. Eur J Endocrinol 145: 165–171, 2001. doi: 10.1530/eje.0.1450165. [DOI] [PubMed] [Google Scholar]
- 8. Doerr P, Fichter M, Pirke KM, Lund R. Relationship between weight gain and hypothalamic pituitary adrenal function in patients with anorexia nervosa. J Steroid Biochem 13: 529–537, 1980. doi: 10.1016/0022-4731(80)90209-5. [DOI] [PubMed] [Google Scholar]
- 9. Boyar RM, Hellman LD, Roffwarg H, Katz J, Zumoff B, O'Connor J, Bradlow HL, Fukushima DK. Cortisol secretion and metabolism in anorexia nervosa. N Engl J Med 296: 190–193, 1977. doi: 10.1056/NEJM197701272960403. [DOI] [PubMed] [Google Scholar]
- 10. Dirlewanger M, di Vetta V, Guenat E, Battilana P, Seematter G, Schneiter P, Jéquier E, Tappy L. Effects of short-term carbohydrate or fat overfeeding on energy expenditure and plasma leptin concentrations in healthy female subjects. Int J Obes Relat Metab Disord 24: 1413–1418, 2000. doi: 10.1038/sj.ijo.0801395. [DOI] [PubMed] [Google Scholar]
- 11. Vinales KL, Schlögl M, Piaggi P, Hohenadel M, Graham A, Bonfiglio S, Krakoff J, Thearle MS. The consistency in macronutrient oxidation and the role for epinephrine in the response to fasting and overfeeding. J Clin Endocrinol Metab 102: 279–289, 2017. doi: 10.1210/jc.2016-3006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ibrahim M, Bonfiglio S, Schlögl M, Vinales KL, Piaggi P, Venti C, Walter M, Krakoff J, Thearle MS. Energy expenditure and hormone responses in humans after overeating high-fructose corn syrup versus whole-wheat foods. Obesity (Silver Spring) 26: 141–149, 2018. doi: 10.1002/oby.22068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Schorr M, Lawson EA, Dichtel LE, Klibanski A, Miller KK. Cortisol measures across the weight spectrum. J Clin Endocrinol Metab 100: 3313–3321, 2015. doi: 10.1210/JC.2015-2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Maurice F, Dutour A, Vincentelli C, Abdesselam I, Bernard M, Dufour H, Lefur Y, Graillon T, Kober F, Cristofari P, Jouve E, Pini L, Fernandez R, Chagnaud C, Brue T, Castinetti F, Gaborit B. Active cushing syndrome patients have increased ectopic fat deposition and bone marrow fat content compared to cured patients and healthy subjects: a pilot 1H-MRS study. Eur J Endocrinol 179: 307–317, 2018. doi: 10.1530/EJE-18-0318. [DOI] [PubMed] [Google Scholar]
- 15. Vande Berg BC, Malghem J, Lecouvet FE, Devogelaer JP, Maldague B, Houssiau FA. Fat conversion of femoral marrow in glucocorticoid-treated patients: a cross-sectional and longitudinal study with magnetic resonance imaging. Arthritis Rheum 42: 1405–1411, 1999. doi:. [DOI] [PubMed] [Google Scholar]
- 16. Ferraù F, Giovinazzo S, Messina E, Tessitore A, Vinci S, Mazziotti G, Lania A, Granata F, Cannavò S. High bone marrow fat in patients with Cushing's syndrome and vertebral fractures. Endocrine 67: 172–179, 2020. doi: 10.1007/s12020-019-02034-4. [DOI] [PubMed] [Google Scholar]
- 17. Pivonello R, De Martino MC, De Leo M, Simeoli C, Colao A. Cushing's disease: the burden of illness. Endocrine 56: 10–18, 2017. doi: 10.1007/s12020-016-0984-8. [DOI] [PubMed] [Google Scholar]
- 18. Boonen E, Vervenne H, Meersseman P, Andrew R, Mortier L, Declercq PE, Vanwijngaerden YM, Spriet I, Wouters PJ, Vander Perre S, Langouche L, Vanhorebeek I, Walker BR, Van den Berghe G. Reduced cortisol metabolism during critical illness. N Engl J Med 368: 1477–1488, 2013. doi: 10.1056/NEJMoa1214969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Beishuizen A, Thijs LG, Vermes I. Patterns of corticosteroid-binding globulin and the free cortisol index during septic shock and multitrauma. Intensive Care Med 27: 1584–1591, 2001. doi: 10.1007/s001340101073. [DOI] [PubMed] [Google Scholar]
- 20. Fazeli PK, Bredella MA, Pachon-Peña G, Zhao W, Zhang X, Faje AT, Resulaj M, Polineni SP, Holmes TM, Lee H, O’Donnell EK, MacDougald OA, Horowitz MC, Rosen CJ, Klibanski A. The dynamics of human bone marrow adipose tissue in response to feeding and fasting. JCI Insight 6: e138636, 2021. doi: 10.1172/jci.insight.138636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mesotten D, Vanhorebeek I, Van den Berghe G. The altered adrenal axis and treatment with glucocorticoids during critical illness. Nat Clin Pract Endocrinol Metab 4: 496–505, 2008. doi: 10.1038/ncpendmet0921. [DOI] [PubMed] [Google Scholar]
- 22. McNeilly AD, Macfarlane DP, O'Flaherty E, Livingstone DE, Mitić T, McConnell KM, McKenzie SM, Davies E, Reynolds RM, Thiesson HC, Skøtt O, Walker BR, Andrew R. Bile acids modulate glucocorticoid metabolism and the hypothalamic-pituitary-adrenal axis in obstructive jaundice. J Hepatol 52: 705–711, 2010. doi: 10.1016/j.jhep.2009.10.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rose AJ, Berriel Díaz M, Reimann A, Klement J, Walcher T, Krones-Herzig A, Strobel O, Werner J, Peters A, Kleyman A, Tuckermann JP, Vegiopoulos A, Herzig S. Molecular control of systemic bile acid homeostasis by the liver glucocorticoid receptor. Cell Metab 14: 123–130, 2011. doi: 10.1016/j.cmet.2011.04.010. [DOI] [PubMed] [Google Scholar]
- 24. Vogeser M, Zachoval R, Felbinger TW, Jacob K. Increased ratio of serum cortisol to cortisone in acute-phase response. Horm Res 58: 172–175, 2002. doi: 10.1159/000065486. [DOI] [PubMed] [Google Scholar]
- 25. Bergendahl M, Evans WS, Pastor C, Patel A, Iranmanesh A, Veldhuis JD. Short-term fasting suppresses leptin and (conversely) activates disorderly growth hormone secretion in midluteal phase women–a clinical research center study. J Clin Endocrinol Metab 84: 883–894, 1999. doi: 10.1210/jcem.84.3.5536. [DOI] [PubMed] [Google Scholar]
- 26. Schurgin S, Canavan B, Koutkia P, Depaoli AM, Grinspoon S. Endocrine and metabolic effects of physiologic r-metHuLeptin administration during acute caloric deprivation in normal-weight women. J Clin Endocrinol Metab 89: 5402–5409, 2004. doi: 10.1210/jc.2004-1102. [DOI] [PubMed] [Google Scholar]
- 27. Bergendahl M, Vance ML, Iranmanesh A, Thorner MO, Veldhuis JD. Fasting as a metabolic stress paradigm selectively amplifies cortisol secretory burst mass and delays the time of maximal nyctohemeral cortisol concentrations in healthy men. J Clin Endocrinol Metab 81: 692–699, 1996. doi: 10.1210/jcem.81.2.8636290. [DOI] [PubMed] [Google Scholar]
- 28. Low SC, Chapman KE, Edwards CR, Wells T, Robinson IC, Seckl JR. Sexual dimorphism of hepatic 11 beta-hydroxysteroid dehydrogenase in the rat: the role of growth hormone patterns. J Endocrinol 143: 541–548, 1994. doi: 10.1677/joe.0.1430541. [DOI] [PubMed] [Google Scholar]
- 29. Andrew R, Phillips DI, Walker BR. Obesity and gender influence cortisol secretion and metabolism in man. J Clin Endocrinol Metab 83: 1806–1809, 1998. doi: 10.1210/jcem.83.5.4951. [DOI] [PubMed] [Google Scholar]
- 30. Hamidovic A, Karapetyan K, Serdarevic F, Choi SH, Eisenlohr-Moul T, Pinna G. Higher circulating cortisol in the follicular vs. luteal phase of the menstrual cycle: a meta-analysis. Front Endocrinol (Lausanne) 11: 311, 2020. doi: 10.3389/fendo.2020.00311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lecka-Czernik B, Stechschulte LA, Czernik PJ, Sherman SB, Huang S, Krings A. Marrow adipose tissue: skeletal location, sexual dimorphism, and response to sex steroid deficiency. Front Endocrinol (Lausanne) 8: 188, 2017. doi: 10.3389/fendo.2017.00188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Cawthorn WP, Scheller EL, Parlee SD, Pham HA, Learman BS, Redshaw CM, Sulston RJ, Burr AA, Das AK, Simon BR, Mori H, Bree AJ, Schell B, Krishnan V, MacDougald OA. Expansion of bone marrow adipose tissue during caloric restriction is associated with increased circulating glucocorticoids and not with hypoleptinemia. Endocrinology 157: 508–521, 2016. doi: 10.1210/en.2015-1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Vicennati V, Ceroni L, Gagliardi L, Gambineri A, Pasquali R. Comment: response of the hypothalamic-pituitary-adrenocortical axis to high-protein/fat and high-carbohydrate meals in women with different obesity phenotypes. J Clin Endocrinol Metab 87: 3984–3988, 2002. doi: 10.1210/jcem.87.8.8718. [DOI] [PubMed] [Google Scholar]
- 34. Martens MJ, Rutters F, Lemmens SG, Born JM, Westerterp-Plantenga MS. Effects of single macronutrients on serum cortisol concentrations in normal weight men. Physiol Behav 101: 563–567, 2010. doi: 10.1016/j.physbeh.2010.09.007. [DOI] [PubMed] [Google Scholar]
- 35. ChapmanHolmes KM, Seckl J. 11β-hydroxysteroid dehydrogenases: intracellular gate-keepers of tissue glucocorticoid action. Physiol Rev 93: 1139–1206, 2013. doi: 10.1152/physrev.00020.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The study is registered at https://clinicaltrials.gov/ (NCT02482519).
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.