Keywords: bone remodeling, energy availability, female athlete triad, stress fracture
Abstract
This study investigated sex differences in, and the effect of protein supplementation on, bone metabolism during a 36-h military field exercise. Forty-four British Army Officer cadets (14 women) completed a 36-h field exercise. Participants consumed either their habitual diet [n = 14 women (Women) and n = 15 men (Men Controls)] or the habitual diet with an additional 46.6 g·day−1 of protein for men [n = 15 men (Men Protein)]. Women and Men Protein were compared with Men Controls to examine the effect of sex and protein supplementation. Circulating markers of bone metabolism were measured before, 24 h after (postexercise), and 96 h after (recovery) the field exercise. Beta C-telopeptide cross links of type 1 collagen and cortisol were not different between time points or Women and Men Controls (P ≥ 0.094). Procollagen type I N-terminal propeptide decreased from baseline to postexercise (P < 0.001) and recovery (P < 0.001) in Women and Men Controls. Parathyroid hormone (PTH) increased from baseline to post-exercise (P = 0.006) and decreased from postexercise to recovery (P = 0.047) in Women and Men Controls. Total 25(OH)D increased from baseline to postexercise (P = 0.038) and recovery (P < 0.001) in Women and Men Controls. Testosterone decreased from baseline to post-exercise (P < 0.001) and recovery (P = 0.007) in Men Controls, but did not change for Women (all P = 1.000). Protein supplementation in men had no effect on any marker. Men and women experience similar changes to bone metabolism—decreased bone formation and increased PTH—following a short-field exercise. Protein had no protective effect likely because of the energy deficit.
NEW & NOTEWORTHY Energy deficits are common in arduous military training and can cause disturbances to bone metabolism. This study provides first evidence that short periods of severe energy deficit and arduous exercise—in the form of a 36-h military field exercise—can suppress bone formation for at least 96 h, and the suppression in bone formation was not different between men and women. Protein feeding does not offset decreases in bone formation during severe energy deficits.
INTRODUCTION
Military personnel are exposed to high exercise volumes and severe energy deficits (energy intake lower than total energy expenditure) during training courses and field exercises (1). Short periods of military training (from several days to 8 wk) in energy deficit result in endocrine changes in male soldiers—increased cortisol and decreased insulin-like growth factor-I (IGF-I), testosterone, estradiol, and thyroid hormones (2–9). There is some evidence that these endocrine disturbances lead to decreased markers of bone formation in men after 8 wk of training (8, 10), but evidence for the effects of acute periods (several days) of military field exercises on bone metabolism is limited, with even fewer data in women (1).
Acute periods (several days) of low energy availability (energy intake minus exercise energy expenditure) in women increase circulating markers of bone resorption and decrease circulating markers of bone formation (11, 12). Chronic low energy availability is associated with decreased areal bone mineral density and increased stress fracture risk, classically observed in female athletes (13). There is emerging evidence that male athletes experience similar endocrine and bone metabolic responses to low energy availability, although men may be more resistant to these metabolic effects than women (14); to our knowledge, only one study has compared the bone metabolic response to energy deficits in men and women (12). Women have recently been allowed to enter combat roles alongside men in the UK Armed Forces and other nations, but there is a lack of data on women examining the bone metabolic responses to the physiological stressors—high levels of physical activity, energy deficiency, and sleep deprivation—associated with combat training (1, 15). Alongside energy deficiency, sleep deprivation can also increase circulating markers of bone resorption and decrease circulating markers of bone formation (16), whereas exercise can increase markers of bone resorption and formation (17). The primary aim of this study was to investigate sex differences in markers of bone metabolism following a short arduous military field exercise. A better understanding of the effects of short periods of military field exercise, and subsequent recovery, on bone metabolism, will help develop strategies to protect skeletal health in operationally relevant settings and military training. We hypothesized that the field exercise would increase bone resorption and decrease bone formation—primarily due to the effects of energy deficiency—more in women than in men.
Evidence for the effect of additional dietary energy during military training in energy deficits on metabolic and endocrine markers is mixed (1). However, supplementary energy increased bone formation (8) and attenuated changes to the thyroid hormones (3) and IGF-I axis (8) but did not influence the changes in reproductive hormones (2, 3, 8). Although providing supplemental energy is one strategy to overcome energy deficits in military training, complete mitigation of energy deficits in this environment is difficult and impractical due to high total energy expenditures, limited time to eat or other logistical barriers, and suppressed appetite (1). Targeted specific macronutrient or micronutrient supplementation during energy deficits may help protect bone metabolism. Protein plays a structural role in the bone matrix, and protein feeding increases intestinal calcium absorption and may attenuate changes in concentrations of anabolic and metabolic hormones (18). Increasing protein intake during 8 to 10 days of military field exercise in energy deficit did not prevent changes in testosterone, thyroid hormones, or the IGF-I axis (19, 20), and a ∼40 g·day−1 protein supplement had no effect on markers of bone metabolism compared with a carbohydrate supplement during 9 wk of basic military training (21). There are limited data examining the effect of protein supplementation on bone metabolism in military training and no study has examined a short-term military field exercise in energy deficit. The secondary aim of this trial was to examine the effect of protein supplementation during a short and arduous field exercise on markers of bone metabolism in men. We hypothesized the field exercise would increase bone resorption and decrease bone formation, and supplementary protein would protect against these disturbances.
METHODS
Participants
Forty-five British Army Officer Cadets (15 women, 30 men) volunteered to take part in this mixed methods trial. All participants were recruited in July 2019 during week 7 of their 44-wk British Army Officer Commissioning Course at the Royal Military Academy, Sandhurst, United Kingdom. The Officer Commissioning Course is a basic military training course comprising three 14-wk terms, each separated by 2 or 3 wk of leave, with 2 wk of adventure training following term two. The Officer Commissioning Course teaches soldiering skills and military leadership and is physically arduous. Officer Cadets complete aerobic and resistance training, military-specific fitness training, military drill, progressive loaded marching, learn basic military skills, and complete several arduous field exercises. The study was advertised to all women and men on the Officer Commissioning Course and the first 15 women and 30 men to volunteer were accepted into the study. The 15 women consumed the habitual diet (Women), whereas the 30 men were randomized (1:1) using block randomization to either the habitual diet (Men Controls) or the habitual diet with additional protein (Men Protein). The first part of this trial compared Women with Men Controls in an observational cohort study to examine sex differences in our outcomes. The second part of this trial was an unblinded randomized controlled trial with a parallel group design, whereby Men Controls with Men Protein were compared to examine the effect of protein supplementation on our outcomes. The low numbers of women in British Army Officer training (∼25 women and ∼200 men in each course) meant it was not possible to randomize a group of women to be supplemented with protein. All participants passed an initial military medical assessment and were confirmed injury free and medically fit before starting training. Exclusion criteria for entry to the military were pregnancy; adrenal, ovarian, or gonadotropin-releasing hormone insufficiency; pituitary disease; thyroid disease in the past year; diabetes; hyperparathyroidism; osteopenia; glucocorticoid use; or musculoskeletal injury. Each participant had the study procedures and risks fully explained verbally and in writing before providing written informed consent. This study was approved by the Ministry of Defense Research Ethics Committee (Ref: 931/MoDREC/18).
Experimental Design
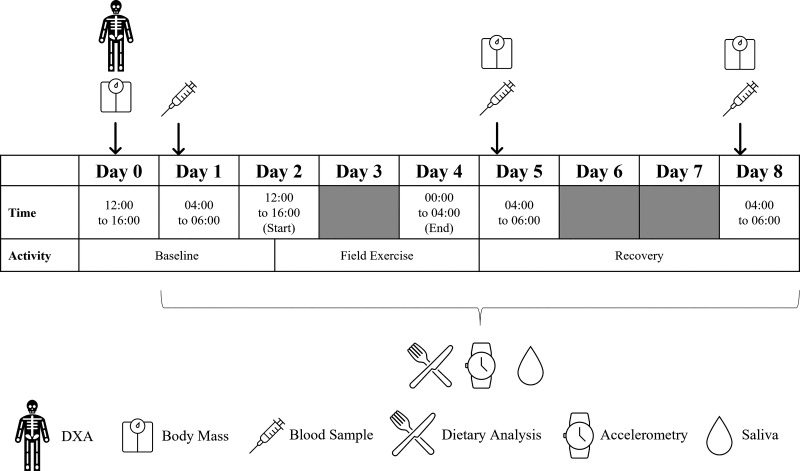
All participants completed a 36-h field exercise in the Brecon Beacons, Wales, UK, during week 8 of their training course. The first 7 wk of military training involves a progressive increase in physical training intensity volume and intensity in the camp where sleep and food intake are protected. The field exercise consisted of completing ∼70 km of load carriage carrying 25 kg in a rucksack, helmet, and rifle across undulating and hilly terrain in teams of six. The 70-km course required each team of six to pass through 12 checkpoints within 36 h with ≤ 4-h sleep. Each team had a staggered start and finish to the field exercise resulting in all participants completing the field exercise over a ∼40-h period. Each team could pass the checkpoints in any order. Participants were enforced to take a 4-h break where they had the opportunity to sleep after 24 h. Each checkpoint required a team of six to complete a leadership or problem-solving task, and the checkpoints could be completed in any order as decided by each team. Total distance and elevation were recorded by GPS worn by one member of each team of six. One woman, one man in the control group, and one man supplemented with protein were part of each group of six to control for differences in the self-selected route. Following the field exercise, participants returned to normal training in camp where they were permitted to sleep between 2200 and 0600 h. Venous blood samples were drawn ∼18 h before (baseline), ∼24 h (postexercise), and 96 h (recovery) after the field exercise and analyzed for biochemical markers of bone formation, bone resorption, calcium metabolism, and reproductive and adrenal hormones (Fig. 1). A follow-up time of 96 h of recovery was chosen because participants had a break from military training following the end of the field exercise with a resumption of training after 96 h. Body mass was measured by calibrated scales at all time points. Whole body lean and fat mass were measured by dual-energy X-ray absorptiometry (DXA) at baseline. Energy expenditure was measured by accelerometry and using the doubly labeled water method. Energy intake was measured from food diaries when eating in camp and food wrappers and discards from the ration pack when on field exercise.
Figure 1.
Overview of study design. DXA, dual-energy X-ray absorptiometry.
Dietary Intervention and Dietary Assessment
Participants ate ad libitum from the military canteen when not on field exercise and ate from an operational ration pack during the field exercise. Participants could also supplement their diet with their own food at any time. The operational ration pack provides 4,000 kcal·day−1 in the form of ready-to-eat meals and snacks. The men supplemented with protein were provided an additional two protein-rich bars (217 kcal, 23.3 g protein, 13.6 g carbohydrate, and 8.2 g fat per bar) to consume per day throughout the trial. Dietary intake was measured by food diaries and the collection of all wrappers (including the protein-rich bars) for the 7 days of the trial. During the field exercise, participants carried the food diaries as part of their kit and recorded consumed items whenever they stopped to eat. Investigators were placed at 4 of the 12 checkpoints to assist with the collection of discards from the ration packs and any food wrappers. Nutritional intake was calculated for the 24 h before the field exercise (baseline), for the 48 h that included the field exercise (field exercise), and for the 96 h after the field exercise (recovery). Absolute energy, carbohydrate, protein, and fat intake were determined using Nutritics software (Nutritics, Ireland) and calculated as the mean per day for each of the three monitoring periods. Relative values were also calculated by dividing the absolute values by the body weight measured at that time point.
Energy Expenditure
Total energy expenditure was estimated using a wrist-worn triaxial accelerometer (GENEActiv, Activinsights, UK). Participants were instructed to wear the accelerometers at all times. The accelerometers were set at a sampling frequency of 50 Hz and calibrated to each participant’s sex, age, height, and body mass. Raw acceleration data were analyzed to estimate metabolic equivalents (METs) using proprietary software (Activinsights, UK) and summed to calculate MET minutes (MET·min−1). Minutes with a zero value were replaced with 0.9 METs to reflect resting metabolism. Daily data were excluded if the device was worn <65% of the day. Total daily energy expenditure was calculated as MET.min × 3.5 × body mass (kg)/200 with an adjustment applied using a previously developed equation validated against doubly labeled water in a military training population: 563.116 + (0.886 × total daily energy expenditure) (22). Total energy expenditure was calculated for the 24 h before the field exercise (baseline), for the 48 h that included the field exercise (field exercise), and for the 96 h after the field exercise (recovery).
Total energy expenditure was measured using the doubly labeled water method (23). Following a baseline saliva sample, participants consumed a single-weighed oral dose of deuterium (2H) and oxygen-18 (18O) before a 7-day measurement period. Daily saliva samples were then collected at ∼0700 h for the following 7 days and stored at 4°C until analysis. Saliva samples were analyzed by isotope ratio mass spectrometry for the determination of rCO2. A food quotient was calculated for each participant from the dietary assessment data and used to estimate energy expenditure from rCO2 (23). Total energy expenditure was calculated for the total 7-day period. Absolute total energy expenditure values—measured from both accelerometry and doubly labeled water—were also converted to relative values by dividing by the body weight measured at the same time-point.
Biochemical Markers of Bone Formation, Bone Resorption, and Calcium Metabolism
Venous blood was drawn from a vein in the antecubital fossa between 0400 and 0600 after an overnight fast from 2200 h. Serum separator vacutainers and EDTA vacutainers were stood at room temperature for 30 min before being centrifuged (Becton Dickinson) at 2,000 g at 4°C for 10 min. Serum and plasma were fractioned and stored at −80°C until analysis. Plasma samples were analyzed for procollagen type I N-terminal propeptide (PINP), c-terminal cross links telopeptide of type 1 collagen (βCTX), and intact parathyroid hormone (PTH) by electrochemiluminescence immunoassay on Cobas e601 platform (Roche Diagnostics, Germany) with interassay CVs of < 5.0% across their respective analytical ranges. Plasma testosterone and cortisol were analyzed by liquid chromatography-tandem mass spectrometry (LC-MS/MS) calibrated using commercial standards (Chromsystems, München, Germany) traceable to standard reference material SRM971 from the National Institute of Science and Technology (NIST). Plasma testosterone and cortisol had an interassay CV < 6.0% across the working range of 0.1– 39.9 nmol·L−1 and 0.1–806.0 nmol·L−1, respectively. Serum samples were analyzed for 25-hydroxyvitamin D (25(OH)D3 and 25(OH)D2) and 24,25-dihydroxyvitamin D (24,25(OH)2D3 and 24,25(OH)2D2) by LC-MS/MS and calibrated using standard reference material SRM972a from NIST. Total 25(OH)D and total 24,25(OH)2D were calculated from the sum of the measurements of D3 and D2 forms with an interassay CV < 10.0% across the working range of 0.1–200.0 nmol·L−1 and 0.1–30.0 nmol·L−1, respectively. Total 1,25-dihydroxyvitamin D (1,25(OH)2D) was analyzed by the DiaSorin LIAISON XL 1,25(OH)2D chemiluminescent immunoassay (Stillwater, MN) with an interassay CV ≤ 9.2% across the working range of 12–480 pmol·L−1. Serum total calcium, albumin, and phosphate were measured by spectrophotometric methods on the Cobas c501 platform (Roche Diagnostics, Germany) according to the manufacturer’s instructions with interassay CVs ≤ 2.1% across the working ranges of 0.20–5.00 mmol·L−1, 2–60 g·L−1, and 0.81–1.45 mmol·L−1, respectively. Albumin-adjusted calcium was calculated as = −0.8 × [albumin] − 4) + [total calcium]. All biochemical analysis was undertaken by the Good Clinical Laboratory Practice-certified Bioanalytical Facility at the University of East Anglia. All analytical processes meet the requirements specified by external national quality assurance schemes.
Body Composition
Whole body lean mass and fat mass were assessed by DXA (Lunar iDXA, GE Healthcare, UK) at baseline (2 days before the field exercise) with participants wearing shorts and a T-shirt. Body mass was measured with calibrated scales (SECA, UK).
Statistical Analyses
All data were analyzed using the R programming language (v.4.2.0). A minimum of 13 women and 13 men were necessary to detect a sex × time interaction for βCTX (ηp2 = 0.04) (24) with an ɑ of 0.05, 1 - β of 0.80, and correlation among repeated measures of 0.7 (G*Power, v.3.1.9.2). Distribution of the data was checked using Shapiro–Wilk tests and frequency distribution histograms. Participant demographics were compared between Women and Men Controls with independent samples t tests or a Welch’s t test for groups with unequal variances; Men Controls and Men Protein were randomized to group and so were not compared. Field trial characteristics, total energy expenditure (doubly labeled water), and energy balance (doubly labeled water) were compared between Women and Men Controls and Men Controls and Men Protein with independent samples t tests or a Welch’s t test for groups with unequal variances. Linear mixed-effect models with restricted maximum likelihood estimation were used to examine changes in energy intake, carbohydrate intake, fat intake, protein intake, energy expenditure (accelerometry), energy balance (accelerometry), βCTX, PINP, PTH, albumin-adjusted calcium, phosphate, total 25(OH)D, total 1,25(OH)D2 total 24,24(OH)D2, cortisol, and testosterone (lme4 package v1.1.29). Separate linear mixed-effects models were run to examine the effect of sex and the effect of protein supplementation. Sex (Women vs. Men Controls), time (baseline vs. postexercise vs. recovery), and their interaction were included as fixed effects to examine sex differences. Group (Men Controls vs. Men Protein), time (baseline vs. postexercise vs. recovery), and their interaction were included as fixed effects to examine the effect of protein supplementation. The comparison of Men Protein with Men Controls was made with an intention-to-treat analysis. Random intercepts were assigned to each participant to account for within-participant correlation for repeated measures. Significance of the fixed effects from each model was determined with Satterthwaite degrees of freedom (lmerTest package v.3.1.3). Normality of the residuals for each model was checked visually by plotting the residuals against the fitted values and from Q–Q plots. In the event of a significant main effect of time or significant interaction, pairwise comparisons with Bonferroni corrections and Kerward-Roger degrees of freedom were used on the linear mixed-effects model to identify differences between time points or group (emmeans package v.1.7.3). Pooled data were used for main effects when there was no significant interaction, and each group was analyzed independently when there was a significant interaction. Effect sizes are presented as partial eta-squared (ηp2) for main and interaction effects, Hedges’ g for between-group comparisons, and paired Hedges’ g for within-group paired comparisons (effectsize package v.0.6.0.1). Figures were drawn in the ggplot2 package (v.3.3.5). Significance was accepted as P ≤ 0.05.
RESULTS
Participants
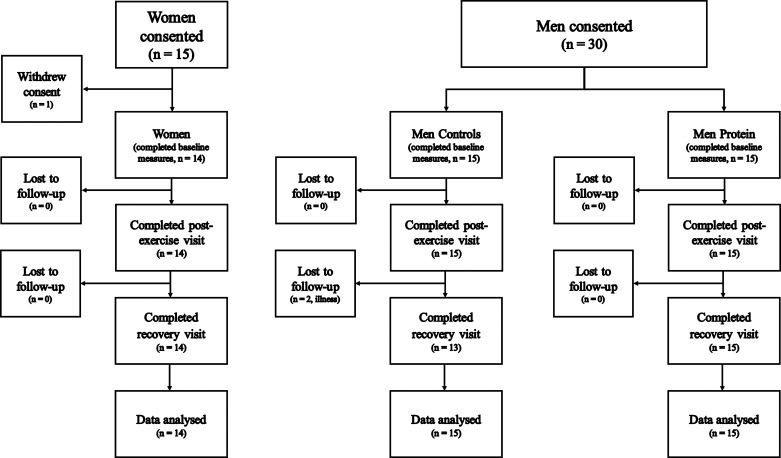
Participant flow through the study is shown in Fig. 2. One woman withdrew consent before baseline measures, and two men from Men Controls were unavailable for blood samples at the recovery time point due to illness. Nutritional intake data were missing for five observations across four participants due to incomplete food diaries. Energy expenditure data estimated from accelerometers were missing for 20 observations across seven participants due to insufficient wear time. Total energy expenditure data measured by doubly labeled water data were missing for four Women, five Men, and three Men Protein due to missing saliva samples. There were no differences between Women and Men Controls for age (P = 0.670, g = 0.16), total 25(OH)D (P = 0.691, g = 0.14), or fat mass (P = 0.711, g = 0.14) but Women were shorter, lighter, and had less lean mass than Men Controls (all P < 0.001, g ≥ 2.15) (Table 1). There was no difference between Women and Men Controls (P ≥ 0.878, g ≤ 0.06) or Men Protein and Men Controls (P ≥ 0.645, g ≤ 0.17) for distance covered, elevation gain, or completion time during the field exercise (Table 1).
Figure 2.
Participant flow through the study. Women were compared with Men Controls to examine sex differences. Men Controls were compared with men supplemented with protein (Men Protein) to examine the effects of protein supplementation.
Table 1.
Participant demographics and field exercise characteristics
| n | Women 14 | Men Controls 15 | Men Protein 15 |
|---|---|---|---|
| Age, yr | 23 ± 1 | 23 ± 2 | 25 ± 3 |
| Height, m | 1.66 ± 0.07a | 1.81 ± 0.07 | 1.84 ± 0.08 |
| Body mass, kg | 61.6 ± 6.6a | 81.4 ± 7.9 | 84.4 ± 12.5 |
| Lean mass, kg | 45.3 ± 5.4a | 63.5 ± 5.8 | 66.8 ± 8.5 |
| Fat mass, kg | 14.2 ± 2.4 | 14.6 ± 3.3 | 14.0 ± 4.8 |
| Total 25(OH)D, nmol·L−1 | 73.2 ± 8.1 | 71.1 ± 17.5 | 80.5 ± 11.6 |
| Distance, km | 67.5 ± 12.4 | 66.8 ± 12.2 | 67.2 ± 11.9 |
| Elevation gain, m | 4,486 ± 1,158 | 4,424 ± 1,141 | 4,350 ± 1,100 |
| Completion time, h:min | 33:53 ± 3:00 | 33:52 ± 2:53 | 34:20 ± 2:39 |
Data are means (SD). aP < 0.05 vs. men controls.
Sex Differences in Nutritional Intake
Nutritional intake for Women and Men Controls is displayed in Table 2. Absolute and relative energy intake, absolute protein intake, and relative fat intake were not different between time points (main effects of time, P ≥ 0.173, ηp2 ≤ 0.07) or Women and Men Controls (main effects of sex, P ≥ 0.093, ηp2 ≤ 0.10; sex × time interaction, P ≥ 0.105, ηp2 ≤ 0.09). There was a main effect of time for absolute carbohydrate intake (P = 0.004, ηp2 = 0.19), but no difference between Women and Men Controls (main effect of sex, P = 0.314, ηp2 = 0.04; sex × time interaction, P = 0.455, ηp2 = 0.03). Absolute carbohydrate intake was lower in recovery than in baseline (P = 0.005, g = 1.12) and field exercise (P = 0.043, g = 0.39), with no difference between baseline and field exercise (P = 1.000, g = 0.24). There was a main effect of time for relative carbohydrate intake (P = 0.016, ηp2 = 0.15), but Women and Men Controls changed similarly (sex × time interaction, P = 0.795, ηp2 < 0.01). Relative carbohydrate intake was lower in recovery than in baseline (P = 0.021, g = 0.63), with no difference between baseline (P = 1.000, g = 0.12) or recovery (P = 0.071, g = 0.36) with field exercise. Relative carbohydrate intake was higher in Women than in Men Controls (main effect of group, P = 0.047, ηp2 = 0.14). Absolute fat intake was not different between time points (main effect of time, P = 0.193, ηp2 = 0.06; sex × time interaction, P = 0.658, ηp2 = 0.02), but was lower in Women than in Men Controls (main effect of sex, P = 0.038, ηp2 = 0.15). Relative protein intake was not different between time points (main effect of time, P = 0.759, ηp2 < 0.01; sex × time interaction, P = 0.062, ηp2 = 0.07), but was higher in Women than in Men Controls (main effect of sex, P = 0.033, ηp2 = 0.06).
Table 2.
Body mass, energy balance, and macronutrient intake
| Women (n = 14) |
Men Controls (n = 15) |
Men Protein (n = 15) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | Exercise* | Recovery | Total | Baseline | Exercise* | Recovery | Total | Baseline | Exercise* | Recovery | Total | |
| Body mass, kg | 61.6 ± 6.6g | 60.8 ± 7.2g | 61.5 ± 7.2g | 81.4 ± 7.9 | 79.8 ± 8.3 | 81.4 ± 6.5 | 84.4 ± 12.5 | 84.2 ± 12.4 | 83.0 ± 14.6 | |||
| Energy intake | ||||||||||||
| Absolute, kcal·day−1 | 3,202 ± 1,013 | 3,007 ± 1,040 | 2,891 ± 725 | 2,924 ± 649 | 3,737 ± 770 | 3,612 ± 1,543 | 3,145 ± 804 | 3,296 ± 714 | 4,363 ± 866 | 5,006 ± 2,153 | 3,916 ± 1,219 | 4,189 ± 848g |
| Relative, kcal·kg·day−1 | 52 ± 15 | 49 ± 13 | 48 ± 11 | 48 ± 9 | 46 ± 10 | 45 ± 20 | 41 ± 9 | 42 ± 10 | 52 ± 9 | 61 ± 27 | 48 ± 13 | 50 ± 9g |
| Carbohydrate intake | ||||||||||||
| Absolute, g·day−1 | 376 ± 133 | 374 ± 130 | 321 ± 103a,b | 335 ± 89 | 439 ± 96 | 415 ± 148 | 319 ± 80a,b,c,d | 357± 77 | 493 ± 138 | 546 ± 235 | 382 ± 163c,d | 431 ± 115g |
| Relative, g·kg·day−1 | 6.1 ± 2.1 | 6.2 ± 1.8 | 5.3 ± 1.6a | 5.5 ± 1.3g | 5.5 ± 1.4 | 5.2 ± 2.0 | 4.1 ± 1.0a,c,d | 4.5 ± 1.1 | 5.9 ± 1.4 | 6.6 ± 2.9 | 4.6 ± 1.7c,d | 5.2 ± 1.2 |
| Fat intake | ||||||||||||
| Absolute, g·day−1 | 125 ± 51 | 115 ± 49 | 111 ± 38 | 112 ± 34g | 156 ± 30 | 152 ± 84 | 126 ± 36d | 135 ± 31 | 176 ± 47 | 210 ± 98 | 152 ± 61d | 167 ± 39g |
| Relative, g·kg·day−1 | 2.0 ± 0.8 | 1.9 ± 0.6 | 1.9 ± 0.5 | 1.9 ± 0.4 | 1.9 ± 0.4 | 1.9 ± 1.1 | 1.7 ± 0.4 | 1.8 ± 0.4 | 2.1 ± 0.5 | 2.6 ± 1.3 | 1.8 ± 0.7 | 2.1 ± 0.4 |
| Protein intake | ||||||||||||
| Absolute, g·day−1 | 129 ± 34 | 109 ± 40 | 98 ± 29 | 103 ± 26 | 112 ± 41 | 132 ± 73 | 126 ± 44 | 122 ± 35 | 172 ± 48 | 211 ± 91 | 160 ± 35 | 172 ± 21g |
| Relative, g·kg·day−1 | 2.1 ± 0.5 | 1.8 ± 0.6 | 1.6 ± 0.4 | 1.7 ± 0.3g | 1.4 ± 0.5 | 1.7 ± 0.9 | 1.6 ± 0.6 | 1.6 ± 0.5 | 2.1 ± 0.6 | 2.6 ± 1.2 | 2.0 ± 0.5 | 2.1 ± 0.3g |
| Accelerometry | ||||||||||||
| Absolute EE, kcal·day−1 | 2,473 ± 722h | 5,087 ± 915e,h | 2,496 ± 692f,h | 3,244 ± 729 | 3,460 ± 265 | 6,697 ± 542c,e | 3,514 ± 296d,f | 4,373 ± 459 | 3,818 ± 596 | 7,193 ± 951c | 3,933 ± 574d | 4,895 ± 677 |
| Relative EE, kcal·kg·day−1 | 40 ± 11 | 82 ± 11a | 41 ± 10b | 53 ± 10 | 44 ± 4 | 87 ± 7a,c | 44 ± 4b,d | 55 ± 7 | 45 ± 4 | 86 ± 4c | 48 ± 5d | 59 ± 4 |
| Absolute EB, kcal·day−1 | 764 ± 1,261 | −1,998 ± 1,359a | 439 ± 949b | −281 ± 952g | 220 ± 587 | −2,870 ± 1,699a,c | −433 ± 713b,d | −1,121 ± 562 | 545 ± 894 | −2,187 ± 2,508c | −17 ± 989d | −706 ± 741 |
| Relative EB, kcal·kg·day−1 | 12 ± 20 | −33 ± 22a | 7 ± 16b | −5 ± 15 | 3 ± 7 | −36 ± 21a,c | −4 ± 7b | −13 ± 7 | 7 ± 11 | −25 ± 28c | 0 ± 12d | −8 ± 9 |
| Doubly labeled water | ||||||||||||
| Absolute EE, kcal·day−1 | 3,557 ± 1,299 | 3,998 ± 1,242 | 5,159 ± 1,395 | |||||||||
| Relative EE, kcal·kg·day−1 | 60 ± 25 | 50 ± 15 | 64 ± 20 | |||||||||
| Absolute EB, kcal·day−1 | −762 ± 1,304 | −415 ± 1,068 | −1,033 ± 1,407 | |||||||||
| Relative EB, kcal·kg·day−1 | −13 ± 23 | −5 ± 13 | −13 ± 19 | |||||||||
Data are means (SD). aP < 0.05 vs. baseline (main effects, women and men controls pooled); bP < 0.05 vs. exercise (main effects, women and men controls pooled); cP < 0.05 vs. baseline (main effects, men controls and men protein pooled); dP < 0.05 vs. exercise (main effects, men controls and men protein pooled); eP < 0.05 vs. baseline (within group); fP < 0.05 vs. exercise (within group); gP < 0.05 vs. men controls (main effect of group); hP < 0.05 vs. men controls (post hoc).
*Post exercise for body mass only.
EB, energy balance; EE, energy expenditure; total, the average of the total 7-day period.
Sex Differences in Energy Balance
Energy expenditure and energy balance data for Women and Men Controls are displayed in Table 2. Body mass was not different between time points (main effect of time, P = 0.106, ηp2 = 0.08; sex × time interaction, P = 0.623, ηp2 = 0.02), but was higher in Men than in Women (main effect of sex, P < 0.001, ηp2 = 0.67). There was a sex × time interaction for absolute accelerometry-estimated energy expenditure (P < 0.001, ηp2 = 0.27). Absolute accelerometry-estimated energy expenditure increased from baseline to field exercise (P < 0.001, g ≥ 4.48) and decreased from field exercise to recovery (P < 0.001, g ≥ 5.55), with baseline and recovery not different (P = 1.000, g ≤ 0.13) in Women and Men Controls; the increase from baseline to field exercise was lower in Women than in Men Controls. Absolute accelerometry-estimated energy expenditure was lower in Women than in Men Controls at all time points (P < 0.001, g ≥ 1.75). There was a main effect of time for relative accelerometry-estimated energy expenditure and relative accelerometry-estimated energy balance (P < 0.001, ηp2 ≥ 0.98), but no difference between Women and Men Controls (main effect of sex, P ≥ 0.134, ηp2 ≤ 0.09; sex × time interaction, P ≥ 0.583, ηp2 ≤ 0.03). Relative accelerometry-estimated energy expenditure increased from baseline to field exercise (P < 0.001, g = 6.41) and decreased from field exercise to recovery (P < 0.001, g = 7.75), with baseline and recovery not different (P = 1.000, g < 0.01). Relative accelerometry-estimated energy balance decreased from baseline to field exercise (P < 0.001, g = 1.89) and increased from field exercise to recovery (P < 0.001, g = 1.82), with baseline and recovery not different (P = 0.670, g = 0.36). There was a main effect of time for absolute accelerometry-estimated energy balance (P < 0.001, ηp2 = 0.75), but Women and Men Controls changed similarly (sex × time interaction, P = 0.890, ηp2 = 0.01). Absolute accelerometry-estimated energy balance decreased from baseline to field exercise (P < 0.001, g = 1.79) and increased from field exercise to recovery (P < 0.001, g = 1.71), with baseline and recovery not different (P = 0.398, g = 0.64). Absolute accelerometry-estimated energy balance was higher in Women than in Men Controls (main effect of sex, P = 0.038, ηp2 = 0.17). Absolute and relative total energy expenditure and energy balance measured by doubly labeled water were not different between Women and Men Controls (P ≥ 0.296, g ≤ 0.49).
The Effect of Protein Supplementation on Nutritional Intake
Nutritional intake for Men Controls and Men Protein can be seen in Table 2. Absolute and relative energy intake and protein intake were not different between time points (main effect of time, P ≥ 0.076, ηp2 ≤ 0.09; group × time interaction, P ≥ 0.352, ηp2 ≤ 0.04), but were higher in Men Protein than in Men Controls (main effect of group, P ≤ 0.018, ηp2 ≥ 0.18). There was a main effect of time (P ≤ 0.037, ηp2 ≥ 0.20) and group (P ≤ 0.025, ηp2 ≥ 0.16) for absolute carbohydrate and absolute fat intake, but no group × time interactions (P ≥ 0.449, ηp2 ≤ 0.03). Absolute carbohydrate intake was lower in recovery than in baseline (P = 0.011, g = 0.80) and field exercise (P = 0.006, g = 0.52), with no difference between baseline and field exercise (P = 1.000, g = 0.03). Absolute fat intake decreased from field exercise to recovery (P = 0.037, g = 0.39), but baseline and field exercise (P = 1.000, g = 0.18) and baseline and recovery (P = 0.287, g = 0.40) were not different. Absolute carbohydrate and absolute fat intake were higher in Men Protein than in Men Controls. There was a main effect of time for relative carbohydrate intake (P = 0.003, ηp2 = 0.11), but no difference between Men Protein and Men Controls (main effect of group, P = 0.077, ηp2 = 0.11; group × time interaction, P = 0.513, ηp2 = 0.02). Relative carbohydrate intake was lower in recovery than in baseline (P = 0.018, g = 0.77) and field exercise (P = 0.006, g = 0.53), but baseline and field exercise were not different (P = 1.000, g = 0.05). Relative fat intake was not different between time points (main effect of time, P = 0.064, ηp2 = 0.10) or Men Controls and Men Protein (main effect of group, P = 0.075, ηp2 = 0.11; group × time interaction, P = 0.406, ηp2 = 0.03).
The Effect of Protein Supplementation on Energy Balance
Energy expenditure and energy balance data for Men Controls and Men Protein are displayed in Table 2. Body mass was not different between time points (main effect of time, P = 0.393, ηp2 = 0.03) or Men Controls and Men Protein (main effect of group, P = 0.438, ηp2 = 0.02; group × time interaction, P = 0.175, ηp2 = 0.06). There was a main effect of time for absolute and relative accelerometry-estimated energy expenditure and energy balance (P < 0.001, ηp2 ≥ 0.43), but no difference between Men Protein and Men Controls (main effect of group, P ≥ 0.066, ηp2 ≤ 0.13; group × time interaction, P ≥ 0.058, ηp2 ≤ 0.12). Absolute and relative accelerometry-estimated energy expenditure increased from baseline to field exercise (P < 0.001, g ≥ 6.01) and decreased from field exercise to recovery (P < 0.001, g ≥ 5.76), but baseline and recovery were not different (P = 1.000, g ≤ 0.30). Absolute and relative accelerometry-estimated energy balance decreased from baseline to field exercise (P < 0.001, g ≥ 1.12) and increased from field exercise to recovery (P < 0.001, g ≥ 0.91), but baseline and recovery were not different (P ≥ 0.449, g ≤ 0.43). Absolute and relative total energy expenditure and energy balance measured by doubly labeled water were not different between Men Protein and Men Controls (P ≥ 0.052, g ≤ 0.84).
Sex Differences in Biochemical Markers of Bone Resorption, Bone Formation, and Calcium Metabolism
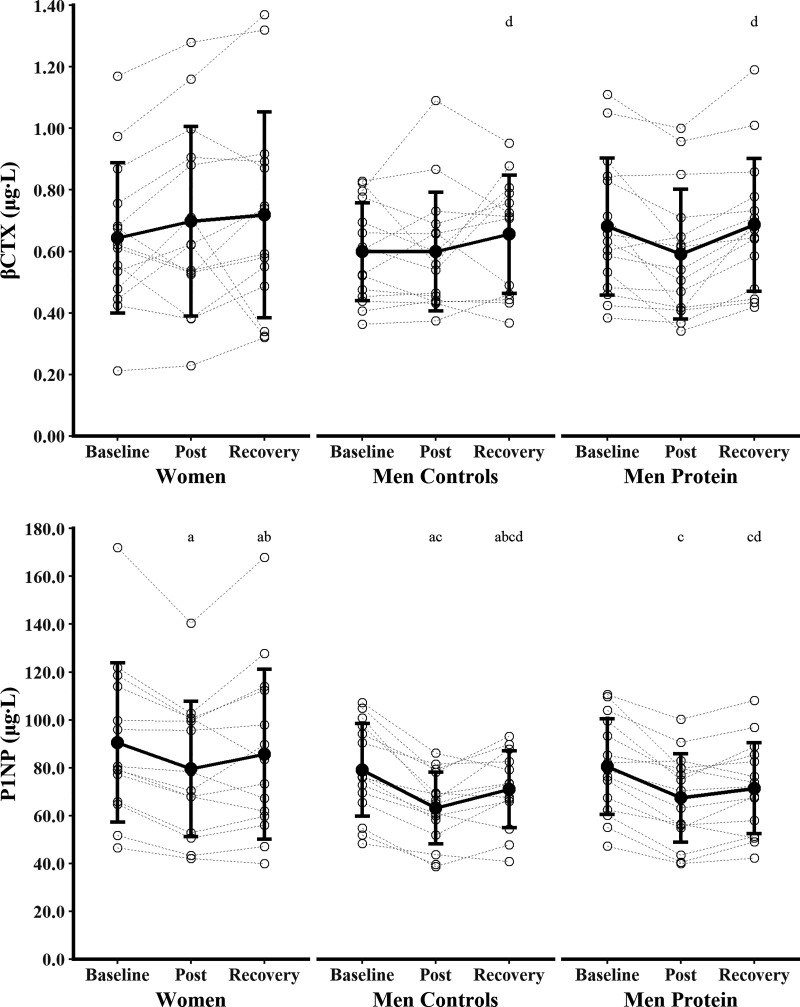
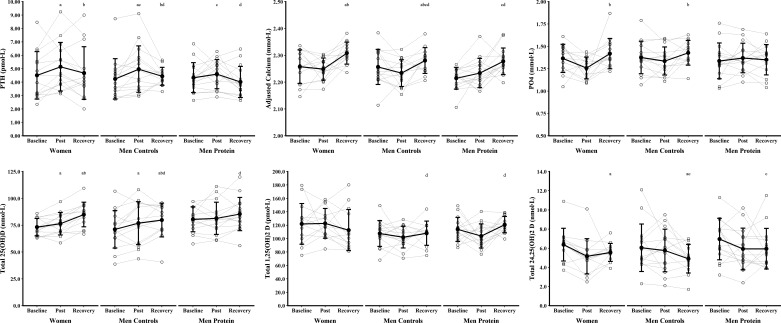
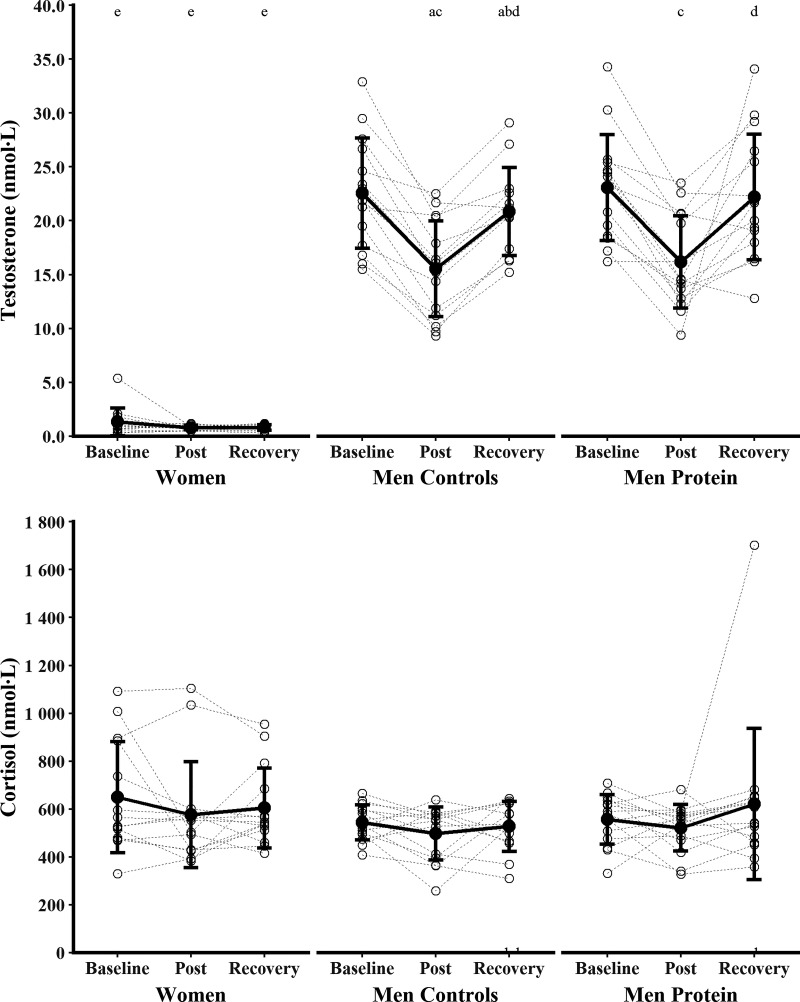
Biochemical markers of bone metabolism and calcium metabolism are presented in Figs. 3, 4, and 5 with mean absolute differences presented in Table 3. βCTX, total 1,25(OH)2D, and cortisol were not different between time points (main effects of time, P ≥ 0.094, ηp2 ≤ 0.09) or Women and Men Controls (main effect of sex, P ≥ 0.069, ηp2 ≤ 0.12; sex × time interaction, P ≥ 0.245, ηp2 ≤ 0.05). There were main effects of time for PINP, PTH, albumin-adjusted calcium, phosphate, total 25(OH)D, and total 24,25(OH)2D (P < 0.005, ηp2 ≥ 0.18), but no difference between Women and Men Controls (main effects of sex, P ≥ 0.122, ηp2 ≤ 0.09; sex × time interactions, P ≥ 0.125, ηp2 ≤ 0.08). PINP decreased from baseline to postexercise (P < 0.001, g = 1.52) and recovery (P < 0.001, g = 0.68), with postexercise lower than recovery (P = 0.010, g = 0.52). PTH increased from baseline to postexercise (P = 0.006, g = 0.63) and decreased from postexercise to recovery (P = 0.047, g = 0.44), with no difference between baseline and recovery (P = 1.000, g = 0.12). Albumin-adjusted calcium increased from baseline to recovery (P = 0.006, g = 0.54) and from postexercise to recovery (P < 0.001, g = 0.98), but baseline and postexercise were not different (P = 0.434, g = 0.27). Phosphate increased from postexercise to recovery (P = 0.001, g = 0.67), but baseline and postexercise (P = 0.082, g = 0.46) and baseline and recovery were not different (P = 0.369, g = 0.27). Total 25(OH)D increased from baseline to postexercise (P = 0.038, g = 0.45) and recovery (P < 0.001, g = 0.96), and from postexercise to recovery (P = 0.016, g = 0.59). Total 24,25(OH)2D decreased from baseline to recovery (P = 0.011 g = 0.51), but baseline and postexercise (P = 0.100, g = 0.43) and postexercise and recovery (P = 1.000, g = 0.16) were not different. There was a sex × group interaction for testosterone (P < 0.001, ηp2 = 0.52). Testosterone decreased from baseline to postexercise (P < 0.001, g = 1.97) and recovery (P = 0.007, g = 0.50), and increased from postexercise to recovery (P < 0.001, g = 2.05), in Men Controls. Testosterone did not change for Women at any time point (all P = 1.000, g ≤ 0.41). Testosterone was lower in Women than in Men Controls at all time points (all P < 0.001, g ≥ 4.48).
Figure 3.
Biochemical markers of bone resorption (top) and bone formation (bottom) before (baseline), 24 h after (post), and 96 h after (recovery) the field exercise. Women (n = 14) and men supplemented with protein (Men Protein, n = 15) were independently compared with non-supplemented men (Men Controls, n = 15) to examine the effect of sex and protein supplementation. Data were analyzed with linear mixed-effects models. aP < 0.05 vs. baseline (main effects, Women and Men Controls pooled); bP < 0.05 vs. post (main effects, Women and Men Controls pooled); cP < 0.05 vs. baseline (main effects, Men Controls and Men Protein pooled); dP < 0.05 vs. post (main effects, Men Controls and Men Protein pooled). βCTX, beta C-telopeptide cross-links of type 1 collagen; PINP, procollagen I N-terminal propeptide.
Figure 4.
Biochemical markers of calcium metabolism before (baseline), 24 h after (post), and 96 h after (recovery) the field exercise. Women (n = 14) and men supplemented with protein (Men Protein, n = 15) were independently compared with nonsupplemented men (Men Controls, n = 15) to examine the effect of sex and protein supplementation. Data were analyzed with linear mixed-effects models. aP < 0.05 vs. baseline (main effects, Women and Men Controls pooled); bP < 0.05 vs. post (main effects, Women and Men Controls pooled); cP < 0.05 vs. baseline (main effects, Men Controls and Men Protein pooled); dP < 0.05 vs. post (main effects, Men Controls and Men Protein pooled). PO4, phosphate; PTH, parathyroid hormone; total 25(OH)D, total 25-hydroxyvitamin D; total 24,25(OH)2D, total 24,25-dihydroxyvitamin D; total 1,25(OH)2D, total 1,25-dihydroxyvitamin D.
Figure 5.
Testosterone and cortisol before (baseline), 24 h after (post), and 96 h after (recovery) the field exercise. Women (n = 14) and men supplemented with protein (Men Protein, n = 15) were independently compared with nonsupplemented men (Men Controls, n = 15) to examine the effect of sex and protein supplementation. Data were analyzed with linear mixed-effects models. aP < 0.05 vs. baseline (within group); bP < 0.05 vs. post (within group); cP < 0.05 vs. baseline (main effects, Men Controls and Men Protein pooled); dP < 0.05 vs. post (main effects, Men Controls and Men Protein pooled); eP < 0.05 vs. Men Controls (main effect of sex).
Table 3.
Mean absolute changes [95% confidence intervals] of biochemical markers of bone formation, bone resorption, and calcium metabolism
| Baseline vs. Post Exercise | Baseline vs. Recovery | Post Exercise vs. Recovery | |
|---|---|---|---|
| Women | |||
| βCTX, μg·L−1 | 0.05 [−0.02, 0.13] | 0.07 [−0.01, 0.16] | 0.02 [−0.08, 0.12] |
| PINP, μg·L−1 | −11.0 [−15.9, −6.1] | −4.9 [−11.5, 1.6] | 6.1 [−1.4, 13.6] |
| PTH, pmol·L−1 | 0.6 [0.0, 1.3] | 0.2 [−0.5, 0.8] | −0.5 [−1.1, 0.2] |
| Adjusted calcium, mmol·L−1 | −0.01 [−0.04, 0.02] | 0.05 [0.01, 0.09] | 0.06 [0.03, 0.09] |
| Phosphate, mmol·L−1 | −0.11 [−0.21, 0.00] | 0.05 [−0.07, 0.18] | 0.16 [0.04, 0.28] |
| Total 25(OH)D, nmol·L−1 | 3.2 [−1.0, 7.4] | 11.7 [7.3, 16.1] | 8.5 [4.9, 12.1] |
| Total 1,25(OH)2D, nmol·L−1 | 0.7 [−16.2, 17.6] | −9.3 [−25.0, 6.4] | −10.0 [−24.5, 4.4] |
| Total 24,25(OH)2D, nmol·L−1 | −1.2 [−1.7, −0.7] | −0.8 [−1.9, 0.2] | 0.4 [−0.7, 1.5] |
| Testosterone, nmol·L−1 | −0.5 [−1.2, 0.2] | −0.5 [−1.2, 0.2] | 0.0 [−0.1, 0.1] |
| Cortisol, nmol·L−1 | −73 [−182, 35] | −46 [−144, 52] | 28 [−56, 111] |
| Men Controls | |||
| βCTX, μg·L−1 | 0.00 [−0.07, 0.07] | 0.04 [−0.02, 0.09] | 0.03 [−0.06, 0.13] |
| PINP, μg·L−1 | −15.9 [−20.6, −11.2] | −10.4 [−16.3, 4.6] | 5.6 [−1.4, 13.6] |
| PTH, pmol·L−1 | 0.7 [0.2, 1.3] | 0.1 [−0.6, 0.8] | −0.6 [−1.4, 0.2] |
| Adjusted calcium, mmol·L−1 | −0.02 [−0.06, 0.01] | 0.02 [−0.02, 0.05] | 0.04 [0.01, 0.07] |
| Phosphate, mmol·L−1 | −0.04 [−0.10, 0.02] | 0.05 [−0.05, 0.15] | 0.09 [0.00, 0.18] |
| Total 25(OH)D, nmol·L−1 | 5.7 [−0.8, 12.4] | 7.2 [0.4, 14.0] | 2.2 [−4.1, 8.6] |
| Total 1,25(OH)2D, nmol·L−1 | −5.5 [−17.2, 6.2] | −0.3 [−12.1, 11.4] | 5.0 [−7.0, 17.0] |
| Total 24,25(OH)2D, nmol·L−1 | −0.3 [−1.5, 0.9] | −1.4 [−2.9, 0.1] | −1.1 [−2.1, 0.1] |
| Testosterone, nmol·L−1 | −7.0 [−8.9, −5.2] | −2.2 [−4.7, 0.3] | 4.9 [3.5, 6.2] |
| Cortisol, nmol·L−1 | −46 [−106, 13] | −14 [−81, 53] | 34 [−47, 115] |
| Men Protein | |||
| βCTX, μg·L−1 | −0.09 [−0.15, −0.03] | 0.01 [−0.05, 0.06] | 0.10 [0.05, 0.14] |
| PINP, μg·L−1 | −13.1 [−17.4, −8.8] | −9.1 [−12.7, 5.5] | 4.1 [0.5, 7.6] |
| PTH, pmol·L−1 | 0.3 [−0.3, 0.8] | −0.3 [−0.9, 0.2] | −0.6 [−1.2, 0.0] |
| Adjusted calcium, mmol·L−1 | 0.02 [−0.01, 0.05] | 0.06 [0.02, 0.10] | 0.04 [0.01, 0.08] |
| Phosphate, mmol·L−1 | 0.03 [−0.05, 0.12] | 0.01 [−0.08, 0.11] | −0.02 [−0.10, 0.06] |
| Total 25(OH)D, nmol·L−1 | 1.0 [−4.7, 6.7] | 4.9 [−1.7, 11.5] | 3.9 [−1.4, 9.2] |
| Total 1,25(OH)2D, nmol·L−1 | −9.8 [−25.4, 5.8] | 7.1 [−4.1, 18.3] | 16.9 [5.3, 28.5] |
| Total 24,25(OH)2D, nmol·L−1 | −1.0 [−2.1, 0.0] | −1.0 [−2.3, 0.3] | 0.0 [−1.3, 1.3] |
| Testosterone, nmol·L−1 | −6.9 [−8.9, −4.9] | −0.9 [−4.8, 3.0] | 6.0 [2.4, 9.6] |
| Cortisol, nmol·L−1 | −36 [−105, 34] | 64 [−122, 249] | 99 [−64, 263] |
βCTX, beta C-telopeptide cross-links of type 1 collagen; PINP, procollagen I N-terminal propeptide; PTH, parathyroid hormone; total 25(OH)D, total 25-hydroxyvitamin D; total 1,25(OH)2D, total 1,25-dihydroxyvitamin D; total 24,25(OH)2D, total 24,25-dihydroxyvitamin D.
The Effect of Protein Supplementation on Biochemical Markers of Bone Resorption, Bone Formation, and Calcium Metabolism
Biochemical markers of bone metabolism and calcium metabolism are presented in Figs. 3, 4, and 5 with mean absolute differences presented in Table 3. There were main effects of time for βCTX, PINP, PTH, albumin-adjusted calcium, total 25(OH)D, total 1,25(OH)2D, total 24,25(OH)2D, and testosterone (P ≤ 0.023, ηp2 ≥ 0.13), but no effect of protein supplementation (main effects of group, P ≥ 0.111, ηp2 ≤ 0.09; group × time interactions, P ≥ 0.084, ηp2 ≤ 0.09). βCTX did not change from baseline to postexercise (P = 0.089, g = 0.36) or recovery (P = 0.899, g = 0.20), but increased between postexercise and recovery (P = 0.007, g = 0.55). PINP decreased from baseline to postexercise (P < 0.001, g = 1.75) and recovery (P < 0.001, g = 1.18) and increased between postexercise and recovery (P = 0.006, g = 0.62). PTH increased from baseline to postexercise (P = 0.048, g = 0.47) and decreased from postexercise to recovery (P = 0.015, g = 0.50), with baseline and recovery not different (P = 1.000, g = 0.12). Albumin-adjusted calcium increased from baseline to recovery (P = 0.002, g = 0.57) and from postexercise to recovery (P = 0.001, g = 0.71), but baseline and postexercise were not different (P = 1.000, g = 0.03). Total 25(OH)D increased from baseline to recovery (P = 0.010, g = 0.51), but baseline and postexercise (P = 0.289, g = 0.29) and postexercise and recovery (P = 0.469, g = 0.31) were not different. Total 1,25(OH)2D increased from postexercise to recovery (P = 0.024, g = 0.53), but baseline and postexercise (P = 0.181, g = 0.30) and baseline and recovery (P = 1.000, g = 0.18) were not different. Total 24,25(OH)2D decreased from baseline to recovery (P = 0.018, g = 0.49), but baseline and postexercise (P = 0.301, g = 0.32) and postexercise and recovery (P = 0.666, g = 0.23) were not different. Testosterone decreased from baseline to postexercise (P < 0.001, g = 1.96) and increased from postexercise to recovery (P < 0.001, g = 1.08), but baseline and recovery were not different (P = 0.351, g = 0.25). Phosphate and cortisol were not different between time points (main effects of time, P ≥ 0.244, ηp2 ≤ 0.05) or groups (main effect of group, P ≥ 0.259, ηp2 ≤ 0.04; group × time interaction, P ≥ 0.144, ηp2 ≤ 0.07).
DISCUSSION
A 36-h field exercise involving ∼70 km of load carriage carrying 25 kg, ≤4 h of total sleep, and a severe energy deficit (∼2,000–3,000 kcal·day−1) decreased PINP and increased PTH in women and men, decreased testosterone in men, and had no effect on βCTX. Men supplemented with protein consumed ∼50 g·day−1 more protein and ∼900 kcal·day−1 more energy than men consuming the habitual diet, but protein supplementation had no effect on any metabolic marker. Although there are data examining the bone metabolic response to several months of basic military training in female and male recruits (24–30) and 8-wk specialist combat training courses in trained male soldiers (8, 10), there are no data examining acute responses to short periods of military operational stress. Women have recently been allowed to enter UK Armed Forces combat roles alongside men, but there is a lack of data on women in response to the physiological stressors associated with combat—high levels of physical of activity, energy deficiency, and sleep deprivation (1, 15). The data in this study provide new insight into the suppression of a metabolic marker of bone formation in both men and women in response to an acute period of extreme exercise and nutritional stress.
Biochemical Markers of Bone Resorption and Bone Formation
We observed no change in βCTX—a measure of type I collagen degradation—in the comparison of women and men. There was an increase in βCTX between postexercise and recovery in men (pooled analysis of Men Controls and Men Protein). It is not clear if this increased βCTX between postexercise and recovery is because of suppressed βCTX immediately after the field exercise or increased βCTX following recovery. Prolonged moderate-intensity running has been shown to decrease βCTX (31) and could explain suppressed βCTX immediately after the field exercise, but high-intensity or exhaustive running had no effect (31) or increased βCTX (32, 33). Exercise mode appears to influence the βCTX response, with low-impact prolonged aerobic activities generally causing the biggest increases (17). Short periods of low energy availability (5 days) increased βCTX in women (11, 12). A 61-day Antarctic traverse with severe energy deficit (∼13% body mass loss) had no effect on βCTX in Servicewomen; however, the sample size was small, measures were taken after 4 days of recovery feeding, and there were large effect sizes for increased βCTX (34). Our sample size was determined to detect an effect size (sex × interaction) of ηp2 = 0.04 (small effect). Sensitivity power analysis revealed that our study was actually able to detect any effect size (sex × interaction) of ηp2 ≥ 0.05 with 80% power, but our observed effect size for βCTX was ηp2 = 0.02. Our βCTX findings could therefore be type II error; however, any effect is likely to be small. Our data do not provide sufficient evidence for increased bone resorption in response to a short military field exercise in energy deficit, or a difference between women and men. The duration of the field exercise was short, and 24 h of energy deficit did not have any effect on βCTX in men or women in a laboratory trial (35). The βCTX response to longer periods of military training is complex with decreased (8, 29, 36), increased (25, 26, 28), and unchanged (10, 30) βCTX reported in military training studies of 8–16 wk in men and/or women. Some of these studies also report adaptive bone formation at the tibia, demonstrating a complex relationship between βCTX and skeletal adaptation (27–30, 36). One study reported similar increases in βCTX between sexes during 16 wk of basic military training (25), and another study reported no effect of protein supplementation on βCTX during 9 wk basic military training (21), supporting our findings that the bone resorption response to military activities does not differ between women and men and is not influenced by an additional protein intake of ∼50 g·day−1. The lack of effect of protein supplementation must be interpreted with caution as the control group still consumed a high amount of protein (122 ± 35 g·day−1 or 1.6 ± 0.5 g·kg·day−1).
Procollagen type I N-terminal propeptide—a measure of type I collagen synthesis—decreased from baseline to postexercise and recovery. The PINP data suggest that a short period of military field exercise suppressed bone formation, which remained lower than baseline following 96 h of ad libitum food intake and recovery. The PINP response was not different between men and women and was not protected by an additional intake of ∼50 g·day−1 protein supplementation for men. The observed sex × interaction effect size for PINP was small (ηp2 = 0.05) and any effect was not detectable with our statistical power. Laboratory studies show that 5-day low energy availability decreased PINP production in women and men, with no difference between sexes (12), but ≥ 60 min treadmill running had no effect on (32) or increased (31, 33) PINP production. Acute exercise typically increases markers of bone formation (17), and therefore the decrease in PINP production was likely due to energy deficiency, although 24 h of energy restriction had no effect on PINP in men or women in another laboratory trial (35), and acute periods of sleep deprivation can also decrease bone formation (16). Women were in a smaller absolute energy deficit compared with men (∼2,000 kcal vs. 2,900 kcal·day−1), and so women may experience disturbances in bone formation at lower severities of energy deficits. The PINP response to military training in energy deficit is inconsistent; PINP was unchanged in women following severe energy deficit during a 61-day Antarctic crossing (34) and increased in men during 8-wk combat training in moderate energy deficit (∼500 kcal·day−1) (8). Other studies have reported decreased bone-specific alkaline phosphatase (bone ALP) following 8-wk military combat courses in energy deficits (∼500–1,000 kcal·day−1) (8, 10), but PINP and bone ALP represent different bone formation processes with different responses to training and nutrition (8) and so comparisons between markers should be made with caution. Basic military training studies report increased (25, 26) or unchanged (27–30, 36) PINP production in men and women over 8–16 wk, alongside adaptive bone formation at the tibia (27–30, 36). The increase in PINP during 16 wk of basic military training was similar between men and women (25), and protein supplementation had no effect on PINP during 9 wk of basic military training (21). We similarly observed no evidence of a sex difference when PINP production was decreased by a military field exercise and no protective effect of protein supplementation. The implications for acute decreases in type I collagen formation for stress fracture risk and adaptive bone formation are unclear, but a high incidence of stress fractures (1.9% for men, 11.4% for women) has been reported during this training course (37).
Biochemical Markers of Calcium Metabolism
Parathyroid hormone increased 24 h after the field exercise compared with baseline and decreased between postexercise and recovery. The observed sex × interaction effect size for PTH was very small (ηp2 < 0.01). There was no sex difference and no effect of protein supplementation on the PTH response. Increases in PTH have previously been reported after several months of basic military training (26, 27), although decreased (25) and unchanged (10, 29, 36) PTH have also been shown in men and women. Parathyroid hormone secretion is regulated by serum ionized calcium (38) and phosphate (39), and PTH mobilizes skeletal calcium by stimulating bone resorption (38). The increase in PTH was not accompanied by an increase in βCTX, but the anabolic and catabolic actions of PTH are complex (38). Our study design makes it challenging to identify the mechanisms for increases in PTH as PTH increases within minutes following a decrease in serum-ionized calcium and changes in serum-ionized calcium and phosphate are both causes and consequences of changes in PTH. Albumin-adjusted calcium—an estimate of ionized calcium—and phosphate were not different from baseline after the field exercise and so the direct mechanism for the increase in PTH is unclear. Exercise acutely decreases ionized calcium and increases phosphate resulting in increased PTH production (40, 41), although an increase in PTH only occurs when the exercise intensity is high (31) or the exercise is prolonged (38). The demands of British Army military training are typically higher for women than for men (42), which might explain our previous finding that PTH increased in women but not in men (24). The field exercise in this study was high-intensity and prolonged for both men and women as evidenced by the high total energy expenditures, which may have masked any sex differences in the PTH response. Parathyroid hormone secretion follows a circadian rhythm, which is also disturbed by sleep disturbances and fasting (43), and so our PTH changes may represent a shift in this circadian rhythm. The implications of an increase in PTH for stress fracture risk and adaptive bone formation are not clear; intermittent increases in PTH are osteogenic (38) yet higher PTH has been associated with increased stress fracture risk (44). Previous studies showed a higher protein diet (2.1 g·day−1 vs. 1.0 g·day−1) increased intestinal calcium absorption (45) and a lower protein diet (0.7 g·day−1 vs. 1.0 g·day−1) decreased PTH (46), although increasing dietary protein intake during energy deficit (from 0.8 g·day−1 to 1.6 g·day−1 or 2.4 g·day−1) had no effect on calcium absorption or PTH (47). The protein supplement in our study did not influence markers of calcium metabolism likely because of the greater contribution of high-intensity and prolonged exercise on disruptions to PTH, but also potentially because of the high volume of protein consumed in the control group.
Total 25(OH)D increased from baseline to postexercise and from postexercise to recovery, with no difference between women and men, and no effect of protein supplementation. The increase in total 25(OH)D was high (5–12 nmol·L−1 depending on group) in the short time frame in this study. The mechanism is likely an increase in fat oxidation with prolonged exercise and energy deficit (48). An increase in total 25(OH)D could have contributed to the decreased PTH from postexercise to recovery and increased calcium and phosphate in recovery. The active 25(OH)D metabolite 1,25(OH)2D contributes to calcium and phosphate homeostasis by providing negative feedback of PTH secretion (38) and increasing calcium and phosphate absorption from the gastrointestinal tract (39). Total 1,25(OH)2D was unchanged, which is unsurprising considering the tight regulation of 1,25(OH)2D independently of total 25(OH)D concentrations (49). An increase in total 25(OH)D coincided with a decrease in total 24,25(OH)2D from baseline to recovery, which is in contrast to the positive linear relationship between 25(OH)D and 24,25(OH)2D and could be due to disturbances to the hydroxylase enzymes (49). Unchanged total 1,25(OH)2D and decreased total 24,25(OH)2D increase the ratio between these two metabolites (vitamin D metabolite ratio) (49). The implications of changes in 24,25(OH)2D are not clear, but higher vitamin D metabolite ratios are associated with poorer physical performance (50) and higher PTH (49). These data present a novel analysis of changes in vitamin D metabolites following acute physiological stress.
Reproductive and Adrenal Hormones
Testosterone decreased from baseline to postexercise and recovery and increased from postexercise to recovery in men. Military training in energy deficit has consistently been shown to decrease testosterone in men over training courses ranging from several days to 8 wk (2–6, 9, 51), but to our knowledge, this study provides the first evidence that a military field exercise as short as 36 h can decrease testosterone. The sex steroids testosterone and estradiol are important regulators of bone metabolism (52). Testosterone can have a direct effect on bone through the androgen receptor, but estradiol is the main regulator of bone metabolism in men through peripheral aromatization of testosterone (52). Estradiol suppresses osteoclast activity (53) and low concentrations of estradiol with energy deficiency increase bone resorption in physically active women (11). The effect of energy restriction on sex steroid concentrations and bone in men is less well understood, but here we observed low testosterone and decreased PINP in men. We observed no change in bone resorption despite decreased testosterone, although we did not measure free testosterone or estradiol and increases in sex hormone binding globulin are observed after arduous military training courses in energy deficits decreasing free testosterone and estradiol (3, 6, 8). The decrease in bone formation may also be due to a decrease in IGF-I and/or other alterations to the IGF axis caused by energy deficiency (8). We did not measure IGF-I or the IGF-binding proteins in this study, but IGF-I is an important regulator of bone formation (54), and military training has consistently shown to decrease IGF-I and alter concentrations of the binding proteins, even after just several days (3–8). Cortisol was not different across time points in either men or women (sex × interaction, ηp2 < 0.01) and so was unlikely to contribute to decreased bone formation.
The few military training studies that have provided supplementary energy found no protective effect on sex steroid concentrations (2, 3, 8, 51), consistent with our data. Increasing protein intake to 2 g·kg−1·day−1 during a 10-day military field exercise in energy deficit did not protect the disturbances to testosterone, thyroid hormones, or IGF-I compared with the habitual ration packs (1 g·kg−1·day−1) (19). Although 0.9 g·kg−1·day−1 of protein intake attenuated a decrease in IGF-I compared with 0.5 g·kg−1·day−1 of protein intake, there were no effects of increased protein intake on other parts of the IGF-I axis or testosterone (20). A randomized controlled trial showed that increasing protein intake to two or three times the recommended daily allowance during a 40% energy deficit had no effect on endocrine markers, calcium absorption or metabolism, or bone metabolism (20, 47). Supplementary protein had no protective effect on testosterone in our study, and these previous studies, likely because the additional energy was insufficient to eliminate the energy deficit, or mechanisms other than energy deficiency such as sleep restriction or high levels of physical activity, were responsible for the reduction in testosterone.
Limitations
The findings in this study are limited by the small sample size, the limited number of time points captured, and the short study duration, which likely meant some of our outcomes were underpowered or some effects were undetectable with our study design. Sensitivity power analysis revealed that our study was able to detect any sex × interaction effect size of ηp2 ≥ 0.05 (small effects) with 80% power, and so our study would have only been underpowered for detecting small effects and the impact of any type II error on our conclusions would have been minimal. Our postexercise measures were taken 24 h after the field exercise and so acute changes in our markers may have been missed. The low numbers of women going through British Army Officer training meant we were unable to include a group of women supplemented with protein. We were also unable to blind the control group, but do not believe the unblinded nature of the trial impacted the results. We did not measure estradiol, sex hormone binding globulin, or IGF-I, which may have helped in the interpretation of the bone metabolism data. However, the measurement and interpretation of estradiol over the time frame in this study were unfeasible and lacked external validity as some of the women took a range of hormonal contraceptives and others were at different stages of the menstrual cycle. We did not adjust our circulating measures of bone metabolism for potential changes in plasma volume. Finally, we did not have a measure of calcium or phosphate intake; calcium may interact with protein to increase calcium intestinal absorption, and phosphate intake is important in the circadian rhythm of PTH.
Conclusions
A 36-h field exercise suppressed a marker of bone formation for 4 days in men and women, with no difference between sexes. Protein supplementation had no protective effect on the decrease in bone formation or testosterone. The mechanism for this decrease in bone formation is unclear but could be due to the acute effects of low energy availability on metabolic regulators of bone metabolism. The implications of acute decreased bone formation for skeletal adaptations and stress fracture risk warrant further investigation.
DATA AVAILABILITY
Data will be made available upon reasonable request.
GRANTS
This study was funded by the UK Ministry of Defense (Army).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
T.J.O., V.C.E., S.L.W., and J.P.G. conceived and designed research; T.J.O., C.V.C., V.C.E., R.L.K., F.N.K., J.C.Y.T., W.D.F., and S.L.W. performed experiments; T.J.O. analyzed data; T.J.O., C.V.C., S.L.W., and J.P.G. interpreted results of experiments; T.J.O. prepared figures; T.J.O. drafted manuscript; T.J.O., C.V.C., V.C.E., S.D.B., R.L.K., J.C.Y.T., W.D.F., S.L.W., and J.P.G. edited and revised manuscript; T.J.O., C.V.C., V.C.E., S.D.B., R.L.K., F.N.K., J.C.Y.T., W.D.F., S.L.W., and J.P.G. approved final version of manuscript.
ACKNOWLEDGMENTS
We acknowledge Dr. Katrina Hinde, Dr. Andy Siddall, Dr. Sarah Needham-Beck, Dr. Steve Powell, Dr. Emma Bostock, and Sgt Romeo Torriro for help with data collection, and Prof. Kenny Smith, Dr. Jessica Cegielski, and Dr. Joe Bass for the doubly labeled water analysis.
REFERENCES
- 1. O'Leary TJ, Wardle SL, Greeves JP. Energy deficiency in soldiers: the risk of the athlete triad and relative energy deficiency in sport syndromes in the military. Front Nutr 7: 142, 2020. doi: 10.3389/fnut.2020.00142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Opstad PK, Aakvaag A. Decreased serum levels of oestradiol, testosterone and prolactin during prolonged physical strain and sleep deprivation, and the influence of a high calorie diet. Eur J Appl Physiol Occup Physiol 49: 343–348, 1982. doi: 10.1007/BF00441295. [DOI] [PubMed] [Google Scholar]
- 3. Friedl KE, Moore RJ, Hoyt RW, Marchitelli LJ, Martinez-Lopez LE, Askew EW. Endocrine markers of semistarvation in healthy lean men in a multistressor environment. J Appl Physiol (1985) 88: 1820–1830, 2000. doi: 10.1152/jappl.2000.88.5.1820. [DOI] [PubMed] [Google Scholar]
- 4. Nindl BC, Barnes BR, Alemany JA, Frykman PN, Shippee RL, Friedl KE. Physiological consequences of U.S. Army Ranger training. Med Sci Sports Exerc 39: 1380–1387, 2007. doi: 10.1249/MSS.0b013e318067e2f7. [DOI] [PubMed] [Google Scholar]
- 5. Nindl BC, Friedl KE, Frykman PN, Marchitelli LJ, Shippee RL, Patton JF. Physical performance and metabolic recovery among lean, healthy men following a prolonged energy deficit. Int J Sports Med 18: 317–324, 1997. doi: 10.1055/s-2007-972640. [DOI] [PubMed] [Google Scholar]
- 6. Henning PC, Scofield DE, Spiering BA, Staab JS, Matheny RW Jr, Smith MA, Bhasin S, Nindl BC. Recovery of endocrine and inflammatory mediators following an extended energy deficit. J Clin Endocrinol Metab 99: 956–964, 2014. doi: 10.1210/jc.2013-3046. [DOI] [PubMed] [Google Scholar]
- 7. Nindl BC, Castellani JW, Young AJ, Patton JF, Khosravi MJ, Diamandi A, Montain SJ. Differential responses of IGF-I molecular complexes to military operational field training. J Appl Physiol (1985) 95: 1083–1089, 2003. doi: 10.1152/japplphysiol.01148.2002. [DOI] [PubMed] [Google Scholar]
- 8. O'Leary TJ, Walsh NP, Casey A, Izard RM, Tang JCY, Fraser WD, Greeves JP. Supplementary energy increases bone formation during arduous military training. Med Sci Sports Exerc 53: 394–403, 2021. doi: 10.1249/MSS.0000000000002473. [DOI] [PubMed] [Google Scholar]
- 9. Nindl BC, Rarick KR, Castellani JW, Tuckow AP, Patton JF, Young AJ, Montain SJ. Altered secretion of growth hormone and luteinizing hormone after 84 h of sustained physical exertion superimposed on caloric and sleep restriction. J Appl Physiol (1985) 100: 120–128, 2006. doi: 10.1152/japplphysiol.01415.2004. [DOI] [PubMed] [Google Scholar]
- 10. Hughes JM, Smith MA, Henning PC, Scofield DE, Spiering BA, Staab JS, Hydren JR, Nindl BC, Matheny RW Jr. Bone formation is suppressed with multi-stressor military training. Eur J Appl Physiol 114: 2251–2259, 2014. [Erratum in Eur J Appl Physiol 114: 2261, 2015]. doi: 10.1007/s00421-014-2950-6. [DOI] [PubMed] [Google Scholar]
- 11. Ihle R, Loucks AB. Dose-response relationships between energy availability and bone turnover in young exercising women. J Bone Miner Res 19: 1231–1240, 2004. doi: 10.1359/JBMR.040410. [DOI] [PubMed] [Google Scholar]
- 12. Papageorgiou M, Elliott-Sale KJ, Parsons A, Tang JCY, Greeves JP, Fraser WD, Sale C. Effects of reduced energy availability on bone metabolism in women and men. Bone 105: 191–199, 2017. doi: 10.1016/j.bone.2017.08.019. [DOI] [PubMed] [Google Scholar]
- 13. Nattiv A, Loucks AB, Manore MM, Sanborn CF, Sundgot-Borgen J, Warren MP; American College of Sports M. American College of Sports Medicine position stand. The female athlete triad. Med Sci Sports Exerc 39: 1867–1882, 2007. doi: 10.1249/mss.0b013e318149f111. [DOI] [PubMed] [Google Scholar]
- 14. De Souza MJ, Koltun KJ, Williams NI. The role of energy availability in reproductive function in the female athlete triad and extension of its effects to men: an initial working model of a similar syndrome in male athletes. Sports Med 49: 125–137, 2019. doi: 10.1007/s40279-019-01217-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Conkright WR, O'Leary TJ, Wardle SL, Greeves JP, Beckner ME, Nindl BC. Sex differences in the physical performance, physiological, and psycho-cognitive responses to military operational stress. Eur J Sport Sci 22: 99–111, 2022. doi: 10.1080/17461391.2021.1916082. [DOI] [PubMed] [Google Scholar]
- 16. Staab JS, Smith TJ, Wilson M, Montain SJ, Gaffney-Stomberg E. Bone turnover is altered during 72 h of sleep restriction: a controlled laboratory study. Endocrine 65: 192–199, 2019. doi: 10.1007/s12020-019-01937-6. [DOI] [PubMed] [Google Scholar]
- 17. Dolan E, Dumas A, Keane KM, Bestetti G, Freitas LHM, Gualano B, Kohrt WM, Kelley GA, Pereira RMR, Sale C, Swinton PA. The bone biomarker response to an acute bout of exercise: a systematic review with meta-analysis. Sports Med 52: 2889–2908, 2022. doi: 10.1007/s40279-022-01718-8. [DOI] [PubMed] [Google Scholar]
- 18. Dolan E, Sale C. Protein and bone health across the lifespan. Proc Nutr Soc 78: 45–55, 2019. doi: 10.1017/S0029665118001180. [DOI] [PubMed] [Google Scholar]
- 19. Øfsteng SJ, Garthe I, Jøsok O, Knox S, Helkala K, Knox B, Ellefsen S, Rønnestad BR. No effect of increasing protein intake during military exercise with severe energy deficit on body composition and performance. Scand J Med Sci Sports 30: 865–877, 2020. doi: 10.1111/sms.13634. [DOI] [PubMed] [Google Scholar]
- 20. Henning PC, Margolis LM, McClung JP, Young AJ, Pasiakos SM. High protein diets do not attenuate decrements in testosterone and IGF-I during energy deficit. Metabolism 63: 628–632, 2014. doi: 10.1016/j.metabol.2014.02.007. [DOI] [PubMed] [Google Scholar]
- 21. Sefton JM, Lyons KD, Beck DT, Haun CT, Romero MA, Mumford PW, Roberson PA, Young KC, Roberts MD, McAdam JS. Markers of bone health and impact of whey protein supplementation in army initial entry training soldiers: a double-blind placebo-controlled study. Nutrients 12: 2225, 2020. doi: 10.3390/nu12082225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Blacker SD, Siddall AG, Needham-Beck S, Powell SD, Edwards VC, Kefyalew SS, Singh PA, Orford ER, Venables M, Jackson S, Greeves JP, Myers SD. Equation to estimate total energy expenditure in military populations using a wrist-worn physical activity monitor. Med Sci Sports Exerc 51: 275–276, 2019. doi: 10.1249/01.mss.0000561329.12994.43. [DOI] [Google Scholar]
- 23. Coward WA. Stable isotopic methods for measuring energy expenditure. The doubly-labelled-water (2H2(18)O) method: principles and practice. Proc Nutr Soc 47: 209–218, 1988. doi: 10.1079/pns19880037. [DOI] [PubMed] [Google Scholar]
- 24. O'Leary TJ, Izard RM, Tang JCY, Fraser WD, Greeves JP. Sex differences in tibial adaptations to arduous training: an observational cohort study. Bone 160: 116426, 2022. doi: 10.1016/j.bone.2022.116426. [DOI] [PubMed] [Google Scholar]
- 25. Evans RK, Antczak AJ, Lester M, Yanovich R, Israeli E, Moran DS. Effects of a 4-month recruit training program on markers of bone metabolism. Med Sci Sports Exerc 40: S660–S670, 2008. doi: 10.1249/MSS.0b013e318189422b. [DOI] [PubMed] [Google Scholar]
- 26. Lutz LJ, Karl JP, Rood JC, Cable SJ, Williams KW, Young AJ, McClung JP. Vitamin D status, dietary intake, and bone turnover in female soldiers during military training: a longitudinal study. J Int Soc Sports Nutr 9: 38, 2012. doi: 10.1186/1550-2783-9-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gaffney-Stomberg E, Lutz LJ, Rood JC, Cable SJ, Pasiakos SM, Young AJ, McClung JP. Calcium and vitamin D supplementation maintains parathyroid hormone and improves bone density during initial military training: a randomized, double-blind, placebo controlled trial. Bone 68: 46–56, 2014. doi: 10.1016/j.bone.2014.08.002. [DOI] [PubMed] [Google Scholar]
- 28. Hughes JM, Gaffney-Stomberg E, Guerriere KI, Taylor KM, Popp KL, Xu C, Unnikrishnan G, Staab JS, Matheny RW Jr, McClung JP, Reifman J, Bouxsein ML. Changes in tibial bone microarchitecture in female recruits in response to 8 weeks of U.S. Army Basic Combat Training. Bone 113: 9–16, 2018. doi: 10.1016/j.bone.2018.04.021. [DOI] [PubMed] [Google Scholar]
- 29. O'Leary TJ, Izard RM, Walsh NP, Tang JCY, Fraser WD, Greeves JP. Skeletal macro- and microstructure adaptations in men undergoing arduous military training. Bone 125: 54–60, 2019. doi: 10.1016/j.bone.2019.05.009. [DOI] [PubMed] [Google Scholar]
- 30. O'Leary TJ, Wardle SL, Gifford RM, Double RL, Reynolds RM, Woods DR, Greeves JP. Tibial macrostructure and microarchitecture adaptations in women during 44 weeks of arduous military training. J Bone Miner Res 36: 1300–1315, 2021. doi: 10.1002/jbmr.4290. [DOI] [PubMed] [Google Scholar]
- 31. Scott JP, Sale C, Greeves JP, Casey A, Dutton J, Fraser WD. The role of exercise intensity in the bone metabolic response to an acute bout of weight-bearing exercise. J Appl Physiol (1985) 110: 423–432, 2011. doi: 10.1152/japplphysiol.00764.2010. [DOI] [PubMed] [Google Scholar]
- 32. Scott JP, Sale C, Greeves JP, Casey A, Dutton J, Fraser WD. The effect of training status on the metabolic response of bone to an acute bout of exhaustive treadmill running. J Clin Endocrinol Metab 95: 3918–3925, 2010. doi: 10.1210/jc.2009-2516. [DOI] [PubMed] [Google Scholar]
- 33. Scott JP, Sale C, Greeves JP, Casey A, Dutton J, Fraser WD. Effect of fasting versus feeding on the bone metabolic response to running. Bone 51: 990–999, 2012. doi: 10.1016/j.bone.2012.08.128. [DOI] [PubMed] [Google Scholar]
- 34. O'Leary TJ, Gifford RM, Double RL, Reynolds RM, Woods DR, Wardle SL, Greeves JP. Skeletal responses to an all-female unassisted Antarctic traverse. Bone 121: 267–276, 2019. doi: 10.1016/j.bone.2019.02.002. [DOI] [PubMed] [Google Scholar]
- 35. Clayton DJ, James LJ, Sale C, Templeman I, Betts JA, Varley I. Severely restricting energy intake for 24 h does not affect markers of bone metabolism at rest or in response to re-feeding. Eur J Nutr 59: 3527–3535, 2020. doi: 10.1007/s00394-020-02186-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Gaffney-Stomberg E, Nakayama AT, Guerriere KI, Lutz LJ, Walker LA, Staab JS, Scott JM, Gasier HG, McClung JP. Calcium and vitamin D supplementation and bone health in Marine recruits: effect of season. Bone 123: 224–233, 2019. doi: 10.1016/j.bone.2019.03.021. [DOI] [PubMed] [Google Scholar]
- 37. O'Leary TJ, Wardle SL, Rawcliffe AJ, Chapman S, Mole J, Greeves JP. Understanding the musculoskeletal injury risk of women in combat: the effect of infantry training and sex on musculoskeletal injury incidence during British Army basic training. BMJ Mil Health 169: 57–61, 2023. doi: 10.1136/jramc-2019-001347. [DOI] [PubMed] [Google Scholar]
- 38. Lombardi G, Ziemann E, Banfi G, Corbetta S. Physical activity-dependent regulation of parathyroid hormone and calcium-phosphorous metabolism. Int J Mol Sci 21: 5388, 2020. doi: 10.3390/ijms21155388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Peacock M. Phosphate metabolism in health and disease. Calcif Tissue Int 108: 3–15, 2021. doi: 10.1007/s00223-020-00686-3. [DOI] [PubMed] [Google Scholar]
- 40. Townsend R, Elliott-Sale KJ, Pinto AJ, Thomas C, Scott JP, Currell K, Fraser WD, Sale C. Parathyroid hormone secretion is controlled by both ionized calcium and phosphate during exercise and recovery in men. J Clin Endocrinol Metab 101: 3231–3239, 2016. doi: 10.1210/jc.2016-1848. [DOI] [PubMed] [Google Scholar]
- 41. Kohrt WM, Wherry SJ, Wolfe P, Sherk VD, Wellington T, Swanson CM, Weaver CM, Boxer RS. Maintenance of serum ionized calcium during exercise attenuates parathyroid hormone and bone resorption responses. J Bone Miner Res 33: 1326–1334, 2018. doi: 10.1002/jbmr.3428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. O'Leary TJ, Saunders SC, McGuire SJ, Venables MC, Izard RM. Sex differences in training loads during british army basic training. Med Sci Sports Exerc 50: 2565–2574, 2018. doi: 10.1249/MSS.0000000000001716. [DOI] [PubMed] [Google Scholar]
- 43. Fraser WD, Ahmad AM, Vora JP. The physiology of the circadian rhythm of parathyroid hormone and its potential as a treatment for osteoporosis. Curr Opin Nephrol Hypertens 13: 437–444, 2004. doi: 10.1097/01.mnh.0000133985.29880.34. [DOI] [PubMed] [Google Scholar]
- 44. Välimäki VV, Alfthan H, Lehmuskallio E, Loyttyniemi E, Sahi T, Suominen H, Välimäki MJ. Risk factors for clinical stress fractures in male military recruits: a prospective cohort study. Bone 37: 267–273, 2005. doi: 10.1016/j.bone.2005.04.016. [DOI] [PubMed] [Google Scholar]
- 45. Kerstetter JE, O'Brien KO, Caseria DM, Wall DE, Insogna KL. The impact of dietary protein on calcium absorption and kinetic measures of bone turnover in women. J Clin Endocrinol Metab 90: 26–31, 2005. doi: 10.1210/jc.2004-0179. [DOI] [PubMed] [Google Scholar]
- 46. Kerstetter JE, Caseria DM, Mitnick ME, Ellison AF, Gay LF, Liskov TA, Carpenter TO, Insogna KL. Increased circulating concentrations of parathyroid hormone in healthy, young women consuming a protein-restricted diet. Am J Clin Nutr 66: 1188–1196, 1997. doi: 10.1093/ajcn/66.5.1188. [DOI] [PubMed] [Google Scholar]
- 47. Cao JJ, Pasiakos SM, Margolis LM, Sauter ER, Whigham LD, McClung JP, Young AJ, Combs GF Jr. Calcium homeostasis and bone metabolic responses to high-protein diets during energy deficit in healthy young adults: a randomized controlled trial. Am J Clin Nutr 99: 400–407, 2014. doi: 10.3945/ajcn.113.073809. [DOI] [PubMed] [Google Scholar]
- 48. Sun X, Cao ZB, Taniguchi H, Tanisawa K, Higuchi M. Effect of an acute bout of endurance exercise on serum 25(OH)D concentrations in young adults. J Clin Endocrinol Metab 102: 3937–3944, 2017. doi: 10.1210/jc.2017-00146. [DOI] [PubMed] [Google Scholar]
- 49. Tang JCY, Jackson S, Walsh NP, Greeves J, Fraser WD; Bioanalytical Facility team. The dynamic relationships between the active and catabolic vitamin D metabolites, their ratios, and associations with PTH. Sci Rep 9: 6974, 2019. doi: 10.1038/s41598-019-43462-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Carswell AT, Jackson S, Swinton P, O'Leary TJ, Tang JCY, Oliver SJ, Sale C, Izard RM, Walsh NP, Fraser WD, Greeves JP. Vitamin D metabolites are associated with physical performance in young healthy adults. Med Sci Sports Exerc 54: 1982–1989, 2022. doi: 10.1249/MSS.0000000000002987. [DOI] [PubMed] [Google Scholar]
- 51. Guezennec CY, Satabin P, Legrand H, Bigard AX. Physical performance and metabolic changes induced by combined prolonged exercise and different energy intakes in humans. Eur J Appl Physiol Occup Physiol 68: 525–530, 1994. doi: 10.1007/BF00599524. [DOI] [PubMed] [Google Scholar]
- 52. Khosla S, Monroe DG. Regulation of bone metabolism by sex steroids. Cold Spring Harb Perspect Med 8: a031211, 2018. doi: 10.1101/cshperspect.a031211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Almeida M, Laurent MR, Dubois V, Claessens F, O'Brien CA, Bouillon R, Vanderschueren D, Manolagas SC. Estrogens and androgens in skeletal physiology and pathophysiology. Physiol Rev 97: 135–187, 2017. doi: 10.1152/physrev.00033.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Govoni KE, Baylink DJ, Mohan S. The multi-functional role of insulin-like growth factor binding proteins in bone. Pediatr Nephrol 20: 261–268, 2005. doi: 10.1007/s00467-004-1658-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available upon reasonable request.