Abstract
Background:
Surgical outcomes for non-small cell lung cancer after neoadjuvant immune checkpoint inhibitors continue to be debated. We assessed perioperative outcomes of patients treated with Nivolumab or Nivolumab plus Ipilimumab (NEOSTAR) and compared them with patients treated with chemotherapy or previously untreated patients with stage I-IIIA non-small cell lung cancer.
Methods:
Forty-four patients with stage I to IIIA non-small cell lung cancer (American Joint Committee on Cancer Staging Manual, seventh edition) were randomized to nivolumab (N; 3 mg/kg intravenously on days 1, 15, and 29; n = 23) or nivolumab with ipilimumab (NI; I, 1 mg/kg intravenously on day 1; n = 21). Curative-intent operations were planned between 3 and 6 weeks after the last dose of neoadjuvant N. Patients who completed resection upfront or after chemotherapy from the same time period were used as comparison.
Results:
In the N arm, 21 (91%) were resected on-trial, 1 underwent surgery off-trial, and one was not resected (toxicity-related). In the NI arm, 16 (76%) resections were performed on-trial, one off-trial, and 4 were not resected (none toxicity-related). Median time to operation was 31 days, and consisted of 2 (5%) pneumonectomies, 33 (89%) lobectomies, and 1 (3%) each of segmentectomy and wedge resection. The approach was 27 (73%) thoracotomy, 7 (19%) thoracoscopy, and 3 (8%) robotic-assisted. Conversion occurred in 17% (n = 2/12) of minimally invasive cases. All 37 achieved Ro resection. Pulmonary, cardiac, enteric, neurologic, and wound complications occurred in 9 (24%), 4 (11%), 2 (5%), 1 (3%), and 1 (3%) patient, respectively. The 30- and 90-day mortality rate was 0% and 2.7% (n = 1), respectively. Postoperative complication rates were comparable with lung resection upfront or after chemotherapy.
Conclusions:
Operating after neoadjuvant N or NI is overall safe and effective and yields perioperative outcomes similar to those achieved after chemotherapy or upfront resection.
Keywords: neoadjuvant, immunotherapy, nivolumab, ipilimumab, lung surgery, lung resection, lobectomy
Complete surgical extirpation of non–small cell lung cancer (NSCLC) remains the best option for locoregional disease control and potential cure. Although the risk of recurrence and metastatic disease spread exists even in very early stages,1 chemotherapy either before2 or after3 surgery has added modest additional survival benefit to the treatment regimen of stage IB-IIIA NSCLC, and remains the standard of care at this time. Within the past 5 years, the successes of immune checkpoint inhibitor (ICI) therapy options for NSCLC in the metastatic setting4 ushered in clinical trials in earlier stages.5 ICIs in the neoadjuvant setting offers the unique opportunity to measure therapeutic effectiveness pathologically and radiographically, and allow for translational and exploratory end points.6 However, in the setting in which curative-intent surgical therapy for lung cancer is possible, the balance between therapeutic drug effects and potential toxicities needs to be carefully weighed, because medications administered before surgery might potentially influence patient eligibility for lung cancer resection and/or potentially affect perioperative outcomes.7
The recently presented results of the Checkmate-816 trial (nivolumab with chemotherapy vs chemotherapy) met its co-primary end point of pathological complete response after combination chemoimmunotherapy,8 lending more evidence that immunotherapy might evolve as a component of the standard of care neoadjuvant regimens in the near future. Thus, from a surgical perspective, it is important to characterize the effects of various neoadjuvant immunotherapy regimens. The early reports from initial neoadjuvant ICI trials have been promising,9 however, early closure of the neoadjuvant nivolumab and ipilimumab feasibility trial10 with only 66% (6/9) resection rate, and high 9% postoperative mortality in the Immune Neoajuvant Therapy in Early-Stage Non-Small Cell Lung Cancer (IoNESCO) trial11 highlight the need for careful analysis of perioperative outcomes in these novel trials.
Herein, we report the surgical outcomes of the Nivolumab With or Without Ipilimumab in Treating Patients With Previously Untreated Stage I-IIIA Non–Small Cell Lung Cancer (NEOSTAR) phase II randomized trial of neoadjuvant nivolumab or nivolumab with ipilimumab followed by curative-intent surgical resection. NEOSTAR was the only randomized phase II trial of neoadjuvant single and dual immunotherapy, which allows for exploratory comparisons between the 2 regimens, and is currently the largest patient cohort completing neoadjuvant dual immunotherapy. We focus our report on surgical resection, timing, completeness of resection, and operative complexity, as well as perioperative morbidity and mortality. To set our report in the context of outcomes after primary lung cancer resections or after chemotherapy, we compared NEOSTAR perioperative results with perioperative outcomes of the 2 mentioned cohorts from the same era.
METHODS
Patients
NEOSTAR (NCT03158129) was a randomized parallel clinical trial of neoadjuvant immunotherapy followed by curative-intent surgical resection for NSCLC. All patients were enrolled in the trial from June 16, 2017, to November 15, 2018, and were provided with and signed written informed consent. The Data Safety Monitoring Board provided oversight of the trial until patients completed treatment in the randomized study. This study was approved by the institutional review board at The University of Texas MD Anderson Cancer Center (2016-0982) on February 15, 2017.
Of the 53 screened patients, 44 patients with stage IA to IIIA (N2 single station) NSCLC according to the seventh edition of the American Joint Committee on Cancer Staging Manual were randomized to receive either nivolumab (3 mg/kg intravenously on days 1, 15, and 29) with or without ipilimumab (1 mg/kg intravenously on day 1 only). Surgical resection was recommended between 21 and 42 days after the last dose of nivolumab. The approach and extent of resection were at the surgeons’ discretion. The administration of adjuvant systemic or radiation therapies were also at clinicians’ discretion. Details of the study protocol, and clinical and select correlative results of the study were previously published.12
The primary outcome of the trial was the major pathologic response (MPR; defined as ≤10% viable tumor)13,14 rate in resected tumors compared with the MPR rate to neoadjuvant chemotherapy in historical controls, with prespecified boundary for a treatment arm to be considered promising for further testing at ≥6 MPRs in 21 evaluable patients. Select secondary outcomes included radiographic response rate according to Response Evaluation Criteria in Solid Tumors version 1.1 criteria, toxicity, recurrence-free and overall survival, and commonly collected thoracic surgical outcomes captured by The Society of Thoracic Surgeons database which included the type of surgery, approach, operative time, blood loss, hospital length of stay, perioperative morbidity and mortality.15 We performed additional exploratory subjective data collection and analyses of surgeons’ opinions on surgical complexity and correlated it with MPR.
The Immunogenomic Profiling of NSCLC Cohort
The Immunogenomic Profiling of NSCLC (ICON) project enrolled, between April 2016 and August 2018, 150 patients with surgically resected NSCLC.16,17 All patients were provided written informed consent, and the project was approved by the Institutional Review Board at The University of Texas MD Anderson Cancer Center. All clinical, pathological, and perioperative data were collected prospectively in accordance with The Society of Thoracic Surgeons database. For our comparison of perioperative outcomes in the NEOSTAR cohort, we identified 141 NSCLC patients from the ICON cohort, 34 who underwent neoadjuvant chemotherapy, and 107 who underwent primary resection.
Outcomes
In both NEOSTAR study arms, we evaluated the resection rate, timing to surgery, operative approach, operative time, and estimated blood loss (EBL), completeness of resection, 30-day postoperative morbidities including cardiac, pulmonary, gastrointestinal, neurologic, and other, and 30- and 90-day postoperative mortality. Postoperative morbidities at 30 days were reported individually and also according to the Clavien–Dindo classification system.18 Pulmonary function tests (PFTs) before and after immunotherapy were also evaluated as an exploratory analysis of operative complexity.
To determine whether operations after immunotherapy are potentially more complex than other “routine” lung cancer operations, the operating surgeon used the following scores to assess operative complexity in terms of tissue dissection compared with a “normal” anatomical lobectomy with mediastinal lymph node dissection for stage I lung cancer:
Easier than normal tissue dissection (tissues easily separate mostly with blunt dissection);
Normal tissue dissection (similar to routine anatomical lobectomy, blunt and sharp dissection used);
Difficult tissue dissection because of inflammation (tissue planes somewhat obliterated requiring mostly sharp dissection);
Very complex tissue dissection (tissue and anatomical planes completely obliterated similar to fibrosis or long-term post radiation effect).
Statistical Analyses
We used descriptive statistics to summarize data. Categorical variables were compared between groups using a χ2 or Fisher exact test as appropriate, continuous variables were compared using a Wilcoxon rank sum test for 2 group comparisons or a Kruskal–Wallis test for 3 or more groups. The exact Wilcoxon signed rank test was used to compare matched-pair PFTs conducted before and after neoadjuvant treatment. The Jonckheere Terpstra test was used to test if there was an ordered difference in median operative times and EBL among the ordered complexity scores. All statistical tests were performed using SPSS version 24.0 (IBM Corp) and R version 4.0.4.
RESULTS
The main results of the NEOSTAR trial were reported previously.12 Briefly, of the 53 patients screened for eligibility, 44 patients were randomized to receive nivolumab (n = 23) or nivolumab with ipilimumab (n = 21; Figure 1). Of these patients, 37 (84%) underwent resection on-trial and were included in the final analysis of perioperative outcomes.
FIGURE 1.
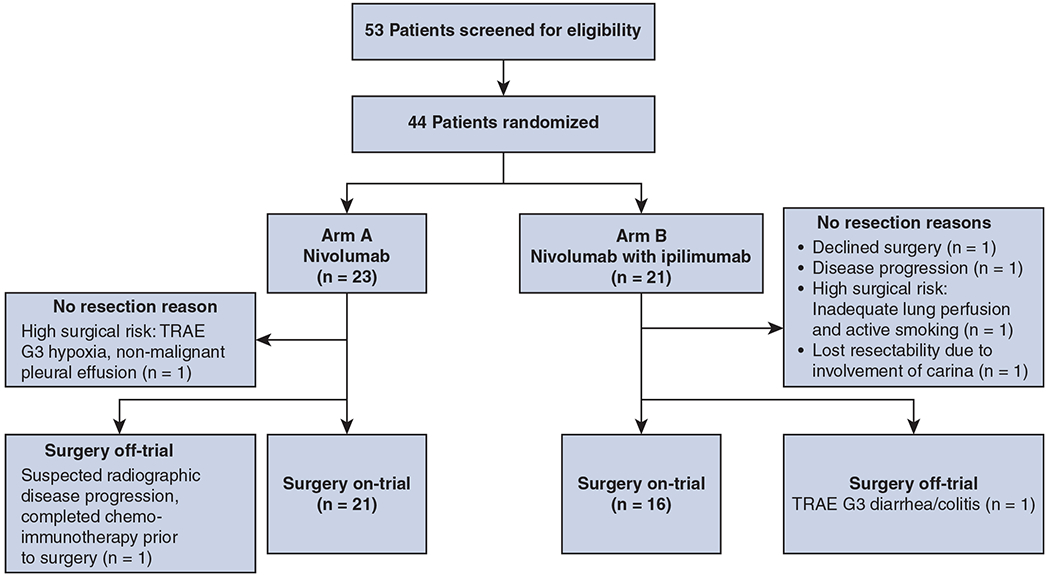
Consolidated Standards of Reporting Trials diagram for patients in Nivolumab With or Without Ipilimumab in Treating Patients With Previously Untreated Stage I-IIIA Non–Small Cell Lung Cancer (NEOSTAR). TRAE, Treatment-related adverse events.
Patients
The baseline characteristics for the 37 patients who underwent resection on-trial are summarized in Table 1. The median age was 66 (range, 43–83) years, 65% were male, and 89% were white. There were 22 (59%) adenocarcinomas. Twenty-two (59%), 9 (24%), and 6 (16%) patients had clinical stage I, II, and III disease, respectively.
TABLE 1.
Characteristics of NEOSTAR and ICON Patients
| Variables | NEOSTAR |
ICON |
P value | ||
|---|---|---|---|---|---|
| Arm A nivolumab (n = 21) | Arm B nivolumab with ipilimumab (n = 16) | Chemotherapy (n = 34) | Upfront resection (n = 107) | ||
| Age, y | 66 (58-83) | 66 (43-76) | 63 (42-81) | 69 (38-85) | .09 |
|
| |||||
| Male sex | 14 (67) | 10 (63) | 16 (47) | 48 (45) | .22 |
|
| |||||
| Smoking status | .01 | ||||
| Current smoker | 4 (19) | 5 (31) | 0 | 7 (7) | |
| Former smoker | 12 (57) | 9 (56) | 29 (85) | 81 (76) | |
| Never smoker | 5 (24) | 2 (13) | 5 (15) | 19 (18) | |
|
| |||||
| Resection approach | .20 | ||||
| Open | 15 (71) | 12 (75) | 28 (82) | 85 (79) | |
| VATS | 5 (24) | 2 (13) | 2 (6) | 18 (17) | |
| RATS | 1 (5) | 2 (13) | 4 (12) | 4 (4) | |
|
| |||||
| Conversion from VATS or RATS to open | 1 (17) | 1 (25) | 2 (33) | 0 | .03 |
|
| |||||
| Resection type | .02 | ||||
| Sublobar | 2 (10) | 0 | 0 | 5 (5) | |
| Lobectomy | 15 (71) | 15 (94) | 20 (59) | 88 (82) | |
| Bilobectomy | 0 | 1 (6) | 5 (15) | 3 (3) | |
| Sleeve lobectomy | 2 (10) | 0 | 2 (6) | 1 (1) | |
| Pneumonectomy | 2 (10) | 0 | 7 (21) | 10 (9) | |
|
| |||||
| Histology | .01 | ||||
| Adenocarcinoma | 11 (52) | 11 (69) | 20 (59) | 78 (73) | |
| SCC/adenosquamous | 10 (48) | 5 (31) | 10 (29) | 29 (27) | |
| Large cell | 0 | 0 | 4 (12) | 0 | |
|
| |||||
| Clinical stage | <.01 | ||||
| I | 11 (52) | 11 (69) | 1 (3) | 64 (60) | |
| II | 6 (29) | 3 (19) | 13 (38) | 38 (36) | |
| III | 4 (19) | 2 (13) | 20 (59) | 5 (5) | |
Data are n(%) or median (range). NEOSTAR, Nivolumab With or Without Ipilimumab in Treating Patients With Previously Untreated Stage I-IIIA Non-Small Cell Lung Cancer; ICON, The Immunogenomic Profiling of NSCLC; VATS, video-assisted thoracoscopic surgery; RATS, robot-assisted thoracic surgery; SCC, squamous cell carcinoma.
Resection Rates
The overall resection rate was 89% (39/44), with 84% (37/44) resected on-trial (Figure 1). In the nivolumab arm (n = 23), 22 (96%) patients completed surgical resection with 21 (91%) resected on-trial. One patient developed a grade 3 treatment-related adverse event (TRAE) hypoxia due to nonmalignant pleural effusion and was no longer medically eligible for surgery. One patient developed suspected radiographic disease progression (nodal immune flare, subsequentially not biopsy-proven) and was switched to a chemo-immunotherapy regimen to which the disease responded and underwent subsequent resection off-trial.
Of the 21 patients in the nivolumab with ipilimumab arm, 17 (81%) completed surgical resection, with 16 (76%) resected on-trial. Of the 4 patients (19%) who did not undergo resection, 1 declined surgery despite radiographic response, 1 developed disease progression from single station to multistation N2 and N3 disease, 1 was judged to be high surgical risk after neoadjuvant therapy because of continued smoking and decreasing pulmonary function. The final patient had a right upper lobe cancer extending to the right main stem bronchus and it was initially thought that sleeve lobectomy would be possible. Although radiographically the patient had stable disease post therapy, preoperative bronchoscopy showed tumor encroaching into the carina and the resection was not performed; the patient subsequently underwent definitive chemoradiation therapy. One patient who underwent resection out of the trial developed a grade 3 TRAE (diarrhea/colitis) after 1 dose of combined ICIs, and was switched to platinum-based chemotherapy before resection. Overall, only 2 patients (5%), 1 in each study arm, did not undergo resection on-trial because of toxicity from neoadjuvant ICI, and, among those, 1 patient received curative-intent surgery off-trial.
Resection Timing
For 37 patients resected on-trial, the median time to operation was 31 days from the last dose of nivolumab (range, 21-87 days). For 8 patients (22%; 3 of 21 [14%] after nivolumab and 5 of 16 [31%] after nivolumab with ipilimumab treatment), resection was delayed beyond 42 days. In the nivolumab arm, the reasons for delay were accidental fall (resection on day 48), pulmonary embolism (day 73), and pneumonia (day 77). In the nivolumab with ipilimumab arm, the reasons for delay were scheduling issues (day 46), accidental fall (day 49), hyperthyroidism and hypoglycemia (day 52), new onset chest pain (day 87), and pneumonitis requiring steroids (day 71). Only the patient with pneumonitis had surgery delay because of TRAE.
Resection Type and Approach
Of the 37 patients who underwent resection on-trial, 2 (5%) underwent pneumonectomy, 30 (81%) underwent lobectomy, 2 (5%) underwent sleeve lobectomy, 1 (3%) each underwent bilobectomy, segmentectomy, and wedge resection (Figure 2, A). Surgical approaches included thoracotomy in 27 patients (73%), video-assisted thoracoscopic surgery (VATS) in 7 (19%), and robot-assisted thoracic surgery (RATS) in 3 (8%) patients (Figure 2, B). Of 12 patients for whom minimally invasive surgery with VATS or RATS was planned, 2 (17%) electively converted to thoracotomy, and both were VATS cases. One conversion was because of significant intrathoracic adhesions visible upon camera placement into the pleural cavity, and the other was because of significant hilar fibrosis that impeded adequate nodal dissection. Complete R0 resections were achieved in all 37 patients resected on-trial (R0 rate: 100%).
FIGURE 2.
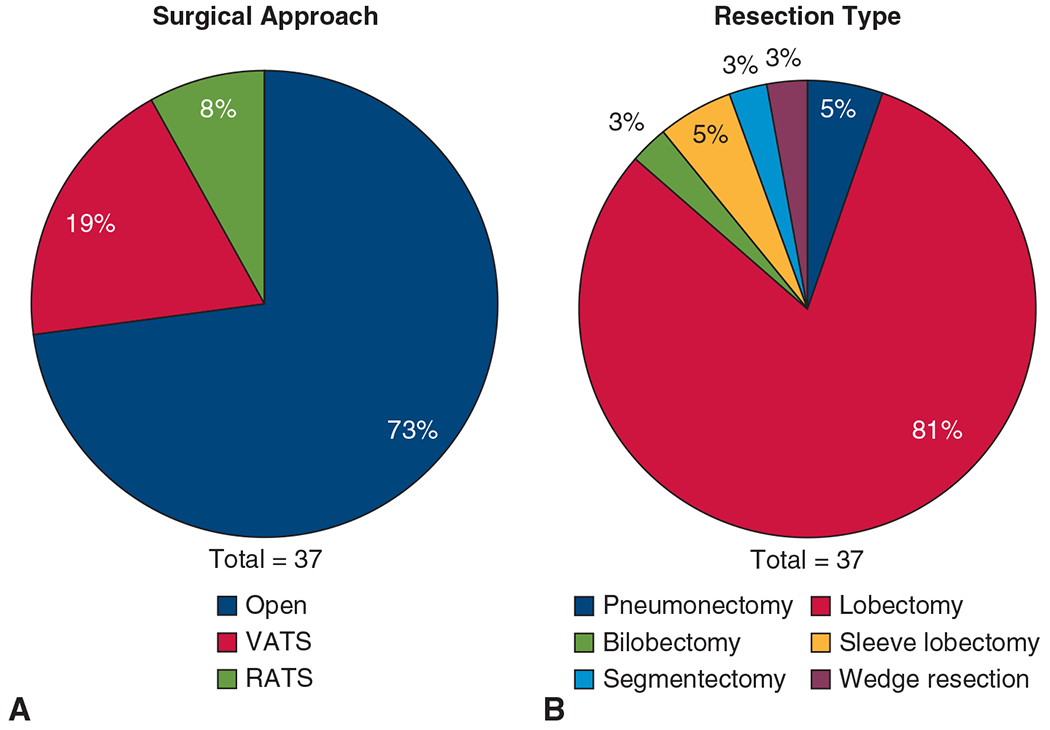
Surgical approach and resection type. A, Proportion of surgical approach: open (n = 27; 73%), VATS (n = 7; 19%), and RATS (n = 3; 8%). B, Proportion of resection type: pneumonectomy (n = 2; 5%), lobectomy (n = 30; 81%), bilobectomy (n = 1; 3%), sleeve lobectomy (n = 2; 5%), segmentectomy (n = 1; 3%), and wedge resection (n = 1; 3%). VATS, Video-assisted thorascopic surgery; RATS, robot-assisted thoracoscopic surgery.
Perioperative Outcomes
The perioperative outcomes of the 37 patients who underwent resection on-trial are summarized in Table 2. The overall median operative time was 147 (range, 71-315) minutes, with no significant difference between the nivolumab and nivolumab with ipilimumab arms (158 vs 142 minutes; P = .25). The overall median EBL was 100 (range, 50-1000) mL, with no significant differences between the nivolumab and nivolumab with ipilimumab arms (150 vs 100; P = .24).
TABLE 2.
Perioperative outcomes of NEOSTAR and ICON patients
| Variable | NEOSTAR |
ICON |
P value | ||
|---|---|---|---|---|---|
| Neoadjuvant nivolumab (n = 21) | Neoadjuvant Nivolumab with ipilimumab (n = 16) | Neoadjuvant chemotherapy (n = 34) | Upfront resection (n = 107) | ||
| R0 resection | 21 (100) | 16 (100) | 29 (85) | 100 (94) | .16 |
|
| |||||
| 30-Day mortality | 0 | 0 | 1 (3) | 1 (1) | .64 |
|
| |||||
| 90-Day mortality | 1 (5) | 0 | 1 (3) | 2 (2) | .60 |
|
| |||||
| EBL, mL | 150 (50-1000) | 100 (50-400) | 250 (10-2750) | 150 (20-2100) | .02 |
|
| |||||
| Operative time, minutes | 158 (71-315) | 142 (95-278) | 174 (87-508) | 156 (74-506) | .16 |
|
| |||||
| Length of stay, d | 4 (1-18) | 5 (1-15) | 6 (3-11) | 5 (2-37) | .04 |
|
| |||||
| Complications | 8 (38) | 5 (31) | 16 (47) | 58 (54) | .24 |
| Pulmonary | 4 (19) | 5 (31) | 11 (32) | 31 (29) | .75 |
| Cardiac | 4 (19) | 0 | 4 (12) | 20 (19) | .22 |
| Gastrointestinal | 3 (14) | 0 | 0 | 3 (3) | .07 |
| Genitourinary | 0 | 0 | 0 | 9 (8) | .15 |
| Neurological | 0 | 1 (6) | 0 | 2 (2) | .45 |
| Wound | 1 (5) | 0 (0) | 1 (3) | 6 (6) | 1.00 |
|
| |||||
| pRBC transfusion | 0 | 0 | 3 (9) | 7 (7) | .53 |
Data are n(%) or median (range). NEOSTAR, Nivolumab With or Without Ipilimumab in Treating Patients With Previously Untreated Stage I-IIIA Non–Small Cell Lung Cancer; ICON, The Immunogenomic Profiling of NSCLC; EBL, estimated blood loss; pRBC, packed red blood cells.
The 30-day postoperative complication rates for the nivolumab arm and nivolumab with ipilimumab arm were 38% (8/21) and 31% (5/16), respectively. The overall complication rate was 35% (13/37). Pulmonary complications occurred in 9 patients (24% ) and included prolonged air leak (air leak lasting >5 days) in 8 patients (22%) and perioperative pneumonitis and pneumonia in 2 patients (5 %); in addition, 1 of the patients with prolonged air leak developed empyema that resolved with antibiotics. Four patients (11%) had atrial fibrillation. Two patients (5%) had gastrointestinal complications (mild diarrhea and ileus in 1 patient each). One patient developed symptoms that were consistent with a transient ischemic attack and resolved within 24 hours. One patient had a wound seroma that was managed with a percutaneous drain.
There was no postoperative mortality at 30 days; however, 1 patient in the nivolumab arm had a pulmonary complication that resulted in death within 90 days. This 83-year-old patient developed pneumonitis-like symptoms after right upper lobectomy. The patient was treated with steroids and discharged from the hospital in stable condition but was later readmitted because of pneumonia and bronchopleural fistula caused by the failed healing of the right upper bronchial stump. The patient required intubation and ventilator management. Reoperative options were contemplated but rejected because of the overall picture, and the patient subsequently died on day 65 postoperatively. Incidentally, this patient’s primary tumor showed a pathological complete response after nivolumab treatment.
PFT Analysis
Among the 37 patients who underwent resection in the trial, the median forced expiratory volume in 1 second decreased after immunotherapy (86% vs 79%; P < .01), as did the median diffusion of lung capacity for carbon monoxide (93% vs 85%; P = .01).
Comparison With ICON Cohorts
The baseline characteristics of patients who received neoadjuvant chemotherapy or underwent upfront resection in the ICON project are summarized alongside those of the patients in the NEOSTAR trial in Table 1. The characteristics of the NEOSTAR patients and those of the ICON patients who underwent upfront resection were similar, whereas the ICON patients who received neoadjuvant chemotherapy had higher rates of stage III disease and pneumonectomy.
The perioperative outcomes of the ICON patients are shown alongside those of the NEOSTAR patients in Table 2. The 4 groups had similar 30- and 90-day mortality rates and operative times. Whereas the NEOSTAR patients had an R0 resection rate of 100%, the ICON patients had R0 resection rates of 85% in the neoadjuvant chemotherapy arm and 94% in the upfront resection arm. Neoadjuvant chemotherapy patients had greater EBL and a longer length of stay. The groups’ overall complication rates and rates of pulmonary, cardiac, genitourinary, gastrointestinal, neurological, wound/infection, or postoperative packed red blood cell transfusions were comparable, as were the rates of 30-day postoperative morbidity according to Clavien-Dindo classification (P = .31; Figure 3).
FIGURE 3.
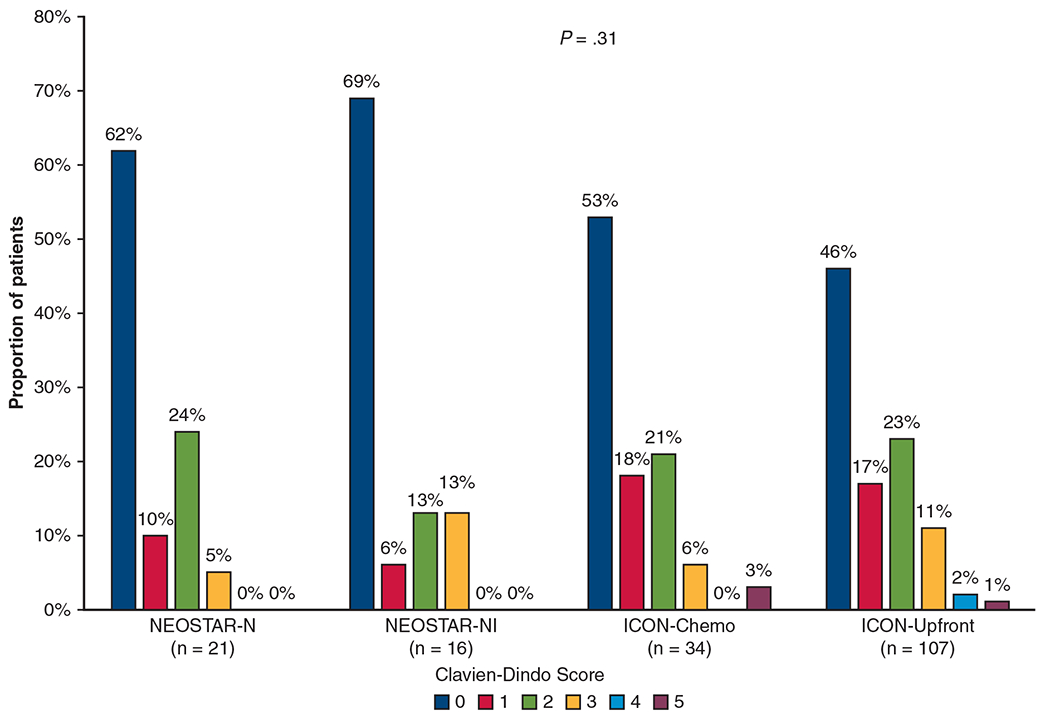
Distribution of 30-day Clavien–Dindo postoperative morbidity scores for Nivolumab With or Without Ipilimumab in Treating Patients With Previously Untreated Stage I-IIIA Non–Small Cell Lung Cancer (NEOSTAR) and The Immunogenomic Profiling of Non–Small Cell Lung Cancer (ICON) patients. Clavien–Dindo classification 1 = deviation from normal postoperative course without the need for intervention; 2 = complication requiring pharmacological intervention including blood transfusion; 3 = complication requiring invasive intervention; 4 = complication requiring intensive care unit care; and 5 = death. NEOSTAR-N, NEOSTAR-neoadjuvant nivolumab; NEOSTAR-NI, NEOSTAR-neoadjuvant nivolumab with ipilimumab; ICON-Chemo, ICON-neoadjuvant chemotherapy; ICON-Upfront, ICON-upfront surgical resection.
Resection Complexity
Of the 37 resections performed on-trial, 3 (8%) were rated as 1, 19 (51%) as 2, 12 (32%) as 3, and 3 (8%) as 4. The distribution of the surgical complexity scores according to treatment arm are shown in Figure 4, A. The median scores for the nivolumab arm and nivolumab with ipilimumab arm were 3 (range, 1-4) and 2 (range, 1-3), respectively (P = .07). Overall, surgeons rated 15 operations (40%) as a 3 or 4 and therefore more complex than a typical lobectomy for stage I disease. Notably 7/37 (19%) operations lasted more than 4 hours; 6 in the nivolumab arm and 1 in the nivolumab with ipilimumab arm. Five were thoracotomies and 2 were RATS approaches.
FIGURE 4.
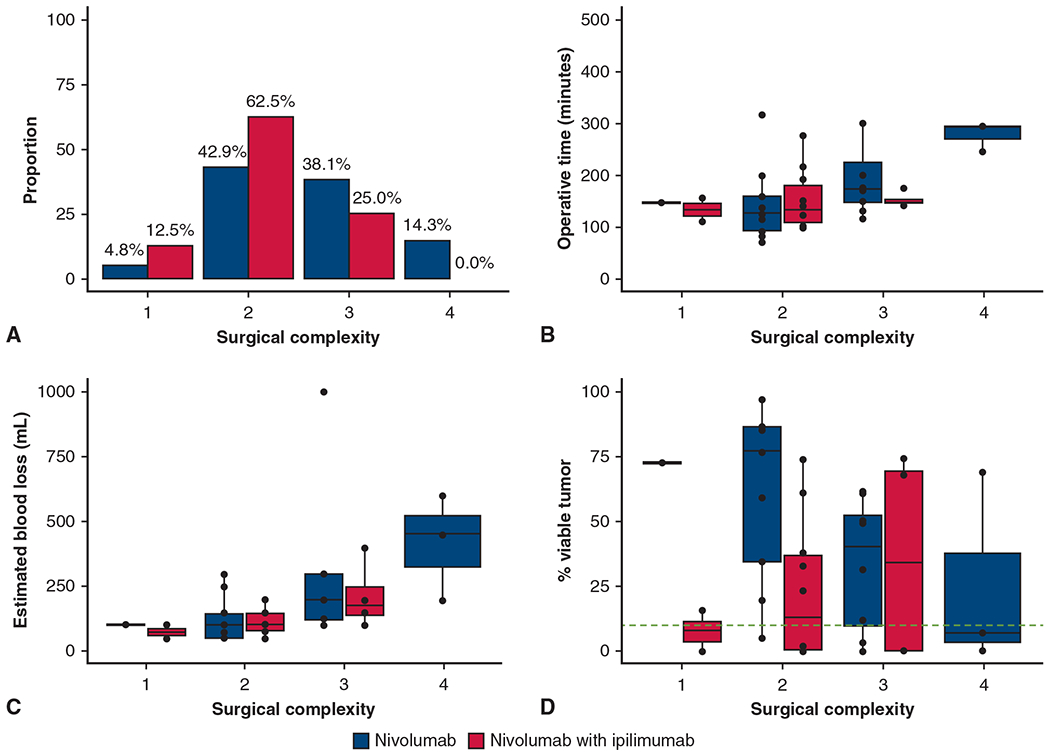
Surgical complexity rating. A, Distribution of surgical complexity after neoadjuvant treatment with nivolumab versus nivolumab with ipilimumab. B, Operative time (minutes) for each surgical complexity score after neoadjuvant treatment with nivolumab versus nivolumab with ipilimumab. C, Estimated blood loss (mL) for each surgical complexity score after neoadjuvant treatment with nivolumab versus nivolumab with ipilimumab. D, Percent viable tumor for surgical complexity score after neoadjuvant treatment with nivolumab versus nivolumab with ipilimumab. The green dashed line indicates 10% viable tumor threshold for major pathologic response. The box is drawn from the first quartile to the third quartile with inside line indicating the median value. The whiskers extend from the ends of the box to the minimum and maximum nonoutliers. Outliers are 1.5 times outside the interquartile range above the upper quartile and below the lower quartile. Surgical complexity scale: 1 = easier than normal tissue dissection; 2 = normal tissue dissection; 3 = difficult tissue dissection because of inflammation; and 4 = very complex tissue dissection.
For the resections with complexity scores of 1, 2, 3, and 4, the median operative times were 137, 147, 179, and 278 minutes, respectively (P = .01; Figure 4, B), and the median EBLs were 83, 123, 265, and 417 mL, respectively (P < .01; Figure 4, C). Surgical complexity was not associated with the percent of viable tumor on pathology (P = .78). Of clinical interest, resection complexity scores of 4 were recorded for 2 patients who had MPR and for 1 patient who did not have MPR (Figure 4, D). We reiterate that the complexity scores reflect different surgeons’ subjective impressions of the operations.
DISCUSSION
Our perioperative results from the randomized NEOSTAR trial of nivolumab or nivolumab with ipilimumab show that perioperative 30- and 90-day mortality rate, and morbidity outcomes on the basis of Clavien-Dindo classification are overall comparable with upfront lung cancer resections or after neoadjuvant chemotherapy in historical controls from the same era. This is specifically with regard to the routinely measured postoperative complications such as cardiac, pulmonary, genitourinary, gastrointestinal, neurological, wound, or blood transfusions. Therefore, neoadjuvant single or dual immunotherapy does not appear to negatively influence objectively measured postoperative outcomes used in thoracic surgery (Figure 5). There are a number of nuances regarding neoadjuvant immunotherapy and surgical patient care, which deserve further discussion.
FIGURE 5.
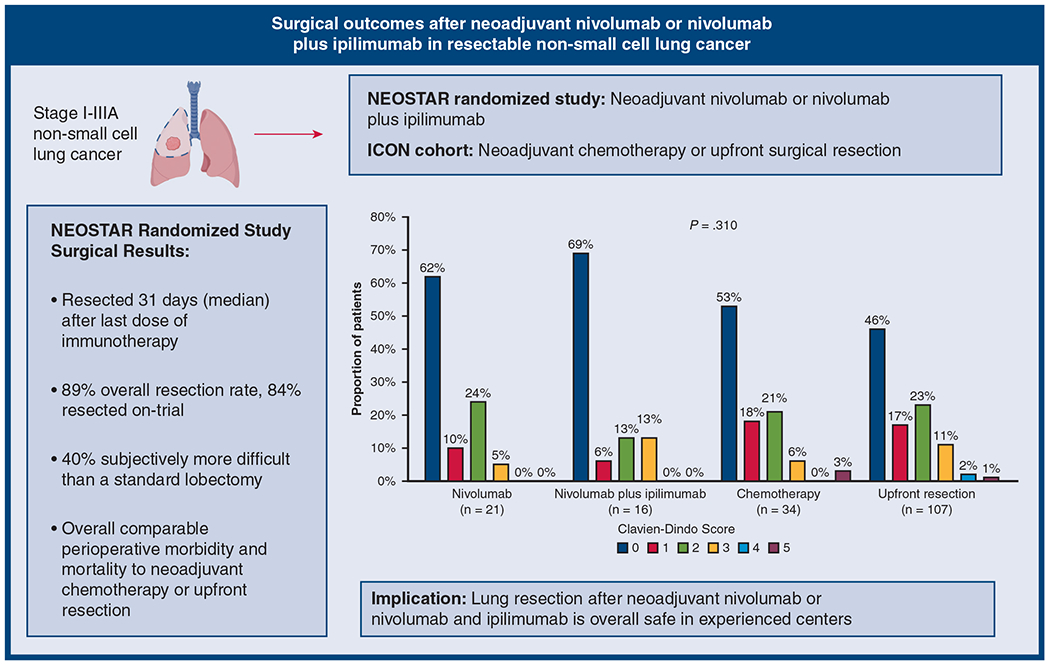
Summarization of the main purpose and outcome of the study. Clavien–Dindo classification 1 = deviation from normal postoperative course without the need for intervention; 2 = complication requiring pharmacological intervention including blood transfusion; 3 = complication requiring invasive intervention; 4 = complication requiring intensive care unit care; and 5-death. NEOSTAR, Nivolumab With or Without Ipilimumab in Treating Patients With Previously Untreated Stage I-IIIA Non–Small Cell Lung Cancer; ICON, The Immunogenomic Profiling of NSCLC.
Perhaps the most important topic is the surgical resection rate. Currently, all neoadjuvant trials require surgeon’s judgement on resectable disease, thus, theoretically 100% of patients should complete an operation as part of their treatment plan. However, there is a consistent 10% to 17% “fall off” rate in neoadjuvant NSCLC trials. Post neoadjuvant resection rates after chemotherapy were 89% in the S9900 trial19 and only 75% (135/179) in the Checkmate-816 trial.8 Resection rate was 95% (20/21) in the nivolumab pilot trial by Forde and colleagues20 with an additional unresectable case at exploration, 88% (159/181) in the Lung Cancer Mutation Consortium 3 (LCMC3) trial21 with atezolizumab, 83% (149/176) in the Checkmate-816 trial,8 and 89% (41/46) in the Neoadjuvant Chemotherapy and Nivolumab in Resectable Non-Small Cell Lung Cancer (NADIM) trial22 after nivolumab with chemotherapy, and 97% (29/30) in a trial with atezolizumab with chemotherapy.23 Essentially all of these trials showed the resection rate of approximately 83% to 95% to be acceptable. In NEOSTAR, our resection rate was overall 89% including 2 patients who underwent operation off-trial after additional therapy. Only 2 (4.5%) patients overall did not proceed to resection on-trial because of TRAEs, and of those, 1 in the nivolumab with ipilimumab group, underwent curative-intent surgery off-trial after platinum-based chemotherapy, reducing the overall TRAE-related inability to resect rate to 2.3% (1/44, in the nivolumab arm). To improve resection rates after neoadjuvant immunotherapy, some variables such as patient selection could be modified. Factors such as patients’ social issues, change of mind, or other accidental events that occur to patients are not predicable. The 10% “fall off” rate seems to be generally acceptable for neoadjuvant trials at this point.
Delay in primary disease control by delayed surgical resection is another important factor to consider. Although 8 (22%) NEOSTAR patients were resected past the recommended 42 days, only 1 patient was delayed because of TRAEs. In the Checkmate-816 trial, 21% (31/179) of patients were delayed, although only 4% were because of an adverse event. For NEOSTAR, we followed the dictum of operating no sooner than 21 days after the last therapy dose (nivolumab) on the basis of historical chemotherapy regimens, which influenced blood counts and potential wound healing. We have since learned that operations after immunotherapy could be performed sooner, as shown by Forde and colleagues.20
Another important question relates to surgical approaches after neoadjuvant immunotherapy and conversion rates from minimally invasive to open approach. VATS, RATS, and open thoracotomy approach are all appropriate surgical approaches for lung cancer. The approach is dependent on a surgeon’s judgement on the basis of tumor, stage, patient characteristics, surgeon’s training, experience, and preference to achieve the task safely. Our conversion rate of 17% is less than the 65% conversion reported by Bott and colleagues9; however, we initiated most operations via thoracotomy. In the era of enhanced recovery pathways, we have previously shown the same perioperative outcomes for VATS and open approaches.24
Completeness of resection is a major variable in prognosticating lung cancer recurrence and overall survival.25 In the NEOSTAR trial, all 100% of patients underwent complete microscopic cancer negative R0 resection compared with 83% in the Checkmate-816 trial, 87% in the chemotherapy and atezolizumab trial,23 100% in the NADIM trial,22 90% in the IONESCO trial,11 or 97% in the Atezolizumab as Induction Therapy in Non-Small Cell Lung Cancer (PRINCEPS) study.26
Perioperative 30-day mortality for lung cancer at our institution has been <1% for more than a decade.24 The 1 death within 90 days in NEOSTAR certainly requires attention as does the 9% 90-day mortality rate in the IONESCO trial11 of neoadjuvant durvalumab, which included deaths from surgical complications at 45 days post surgery and tracheal fistula 8 days post surgery. Whether these mortalities should be attributed to neoadjuvant therapy or surgical misadventures could be debated. However, it is important to state that perioperative mortality of surgical therapy in a multimodality treatment setting must be low, otherwise the long-term oncological benefit of surgical therapy will be offset by the early postoperative mortality as in some former trials.27
Immunotherapy is known to induce pulmonary toxicity in a form of pneumonitis.28,29 Therefore, we measured patients’ PFTs before and after neoadjuvant immunotherapy. The effect of short-course immunotherapy on PFTs has not yet been reported. Although we observed clinically minor (<10%) median decline in forced expiratory volume in 1 second and diffusion of lung capacity for carbon monoxide before and after immunotherapy, these results did not influence surgical decision-making. Further studies are needed to elucidate whether immunotherapy causes subclinical asymptomatic decline in lung function, or whether this was related to patients’ effort during the PFT or inherent variability of PFT interpretation.
Last, the question of operative complexity has arisen since the early experiences of operating after immunotherapy. This has mainly been through anecdotal reports and personal communication among surgeons about tissue quality and anatomical planes encountered during these operations. Thus, we developed an “empirical” scale to grade surgical complexity scored by the 8 surgeons who performed all operations for NEOSTAR. We acknowledge that the scale is not validated, and these data are purely subjective but overall might help quantify surgeons’ impression. It appears that in 40% of cases, surgeons believed that the operation was more difficult than an anatomical lobectomy for a stage I lung cancer without previous treatment. This impression correlated with the operative time and EBL, but not with pathologic response to therapy. However, it is difficult to conclude whether operating after immunotherapy is more difficult than other lung cancer surgery, because factors such as larger tumors, presence of gross nodal disease, and cancer desmoplastic reaction can all make operations inherently more complex. Thoracic surgeons operating after immunotherapy should be well trained in all surgical approaches and exercise sound judgment to complete these operations safely.
CONCLUSIONS
We conclude that perioperative outcomes for lung cancer operations after either nivolumab or nivolumab with ipilimumab are comparable with outcomes of upfront lung cancer resections or resections after neoadjuvant chemotherapy. All surgical approaches are feasible after immunotherapy, although individual judgement is required to complete operations safely. Pulmonary function is not adversely affected in otherwise asymptomatic patients after neoadjuvant immunotherapy. As the experience with operating after immunotherapy grows, additional nuances of perioperative thoracic surgery patient care will need to be studied including patient-reported outcomes.
CENTRAL MESSAGE.
Surgical outcomes after neoadjuvant nivolumab or nivolumab with ipilimumab were comparable with upfront resection or resection after neoadjuvant chemotherapy.
PERSPECTIVE.
Neoadjuvant immunotherapy in NSCLC is rapidly gaining favor. Although 40% of resections in NEOSTAR were subjectively more difficult than a standard lobectomy, postoperative morbidity and mortality were comparable with outcomes after primary resection or after neoadjuvant chemotherapy. The operating surgeon should be experienced in all surgical approaches and exercise sound judgement for safe operations.
Conflict of Interest Statement
B.S. has received speakers’ fees from Astra Zeneca and PeerView; Consultation fees from BMS and Medscape. T.C. has received speakers’ fees from the Society for Immunotherapy of Cancer, Bristol Myers Squibb, Roche, Peer-View, and Medscape Oncology; reports consulting/advisory role fees from MedImmune, AstraZeneca, Bristol Myers Squibb, Merck & Co, Genentech, Arrowhead Pharmaceuticals, and EMD Serono; reports institutional clinical research funding from Boehringer Ingelheim, MedImmune, AstraZeneca, EMD Serono, and Bristol Myers Squibb. D.L.G. reported honoraria for scientific advisory boards from AstraZeneca, Sanofi, Alethia Biotherapeutics, and Eli Lily, research support from Janssen, Takeda, Ribon Therapeutics, Astellas, NGM Biopharmaceuticals, and AstraZeneca. All other authors reported no conflicts of interest.
The Journal art policy requires editors and reviewers to disclose conflicts of interest and to decline handling or reviewing manuscripts for which they may have a conflict of interest. The editors and reviewers of this article have no conflicts of interest.
Funding support for the Nivolumab With or Without Ipilimumab or Chemotherapy in Treating Patients With Previously Untreated Stage I-IIIA Non–Small Cell Lung Cancer (NEOSTAR) clinical trial was provided by Bristol Myers Squibb. Support for this study was partially provided by the National Institutes of Health/National Cancer Institute through The University of Texas Lung Specialized Program of Research Excellence (SPORE) grant 5P50CA070907 (to T.C., D.L.G., J.V.H.), the National Institutes of Health/National Cancer Institute P30 CA016672 Cancer Center Support Grant, the Conquer Cancer Foundation of the American Society of Clinical Oncology Career Development Award 2018 Project ID 12895 (T.C.). This work was also partially supported by the generous philanthropic contributions to The University of Texas MD Anderson Cancer Center Lung Cancer Moon Shots Program, the Gil and Dody Weaver Foundation and Bill and Katie Weaver Charitable Trust, the Bob Mayberry Foundation, the T.J. Martell Foundation, the University of Texas MD Anderson Cancer Center Physician Scientist Program, the Khalifa Bin Zayed Al Nahyan Foundation, the Rexanna’s Foundation for Fighting Lung Cancer, and The Mason Family Research Fund.
Abbreviations and Acronyms
- EBL
estimated blood loss
- ICI
immune checkpoint inhibitor
- ICON
Immunogenomic Profiling of NSCLC
- MPR
major pathologic response
- NEOSTAR
Nivolumab With or Without Ipilimumab in Treating Patients With Previously Untreated Stage I-IIIA Non–Small Cell Lung Cancer
- NSCLC
non–small cell lung cancer
- PFT
pulmonary function test
- RATS
robot-assisted thoracic surgery
- TRAE
treatment-related adverse event
- VATS
video-assisted thoracoscopic surgery
References
- 1.Amin MB, Edge S, Greene F, Compton CC, Gershenwald JE, Brookland RK, et al. AJCC Cancer Staging Manual. 8th ed. Springer International Publishing; 2017. [Google Scholar]
- 2.NSCLC Meta-analysis Collaborative Group. Preoperative chemotherapy for non-small-cell lung cancer: a systematic review and meta-analysis of individual participant data. Lancet. 2014;383:1561–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pignon JP, Tribodet H, Scagliotti GV, Douillard JY, Shepard FA, Stephens RJ, et al. Lung adjuvant cisplatin evaluation: a pooled analysis by the LACE Collaborative Group. J Clin Oncol. 2008;26:3552–9. [DOI] [PubMed] [Google Scholar]
- 4.Brahmer JR, Govindan R, Anders RA, Antonio SJ, Sargorsky S, Davies MJ, et al. The Society for Immunotherapy of Cancer consensus statement on immunotherapy for the treatment of non-small cell lung cancer (NSCLC). J Immunother Cancer. 2018;6:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huynh C, Walsh LA, Spicer JD. Surgery after neoadjuvant immunotherapy in patients with resectable non-small cell lung cancer. Transl Lung Cancer Res. 2021;10:563–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pradhan M, Chocry M, Gibbons DL, Sepesi B, Cascone T. Emerging biomarkers for neoadjuvant immune checkpoint inhibitors in operable non-small cell lung cancer. Transl Lung Cancer Res. 2020;10:590–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yendamuri S, Groman A, Miller A, Demmy T, Hennon M, Dexter E, et al. Risk and benefit of neoadjuvant therapy among patients undergoing resection for non-small-cell lung cancer. Eur J Cardiothorac Surg. 2018;53:656–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Forde PM, Spicer J, Lu S, Provencio M, Mitsudomi T, Awad MM, et al. Nivolumab (NIVO) + platinum-doublet chemotherapy (chemo) vs chemo as neoadjuvant treatment (tx) for resectable (IB-IIIA) non-small cell lung cancer (NSCLC) in the phase 3 CheckMate 816 trial. Cancer Res. 2021;81(suppl 13):CT003. [Google Scholar]
- 9.Bott MJ, Yang SC, Park BJ, Adusumilli PS, Rusch VW, Isbell JM, et al. Initial results of pulmonary resection after neoadjuvant nivolumab in patients with resectable non–small cell lung cancer. J Thorac Cardiovasc Surg. 2019;158:269–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reuss JE, Anagnostou V, Cottrell TR, Smith KN, Verde F, Zahurak M, et al. Neoadjuvant nivolumab plus ipilimumab in resectable non-small cell lung cancer. J Immunother Cancer. 2020;8:e001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wislez M, Mazieres J, Lavole A, Zalcman O, Carre T, Egenod R, et al. 1214O Neoadjuvant durvalumab in resectable non-small cell lung cancer (NSCLC): preliminary results from a multicenter study (IFCT-1601 IONESCO). Ann Oncol. 2020;31:S794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cascone T, William WN, Weissferdt A, Leung CH, Lin HY, Pataer A, et al. Neoadjuvant nivolumab or nivolumab plus ipilimumab in operable non-small cell lung cancer: the phase 2 randomized NEOSTAR trial. Nat Med. 2021;27:504–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hellmann MD, Chaft JE, William WN Jr, Rusch V, Pisters KM, Kalhor N, et al. Pathological response after neoadjuvant chemotherapy in resectable non-small--cell lung cancers: proposal for the use of major pathological response as a surrogate endpoint. Lancet Oncol. 2014;15:e42–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weissferdt A, Pataer A, Vaporciyan AA, Correa AM, Sepesi B, Moran CA, et al. Agreement on major pathological response in NSCLC patients receiving neoadjuvant chemotherapy. Clin Lung Cancer. 2020;21:341–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.The Society of Thoracic Surgeons. STS National database. Accessed June 1, 2021. https://www.sts.org/registries/sts-national-database
- 16.Negrao M, Reuben A, Chen R, Sepesi B, Karpinets E, Parra L, et al. MA06.02 Prospective immunogenomic profiling of non-small cell lung cancer: genomic and immune profiling updates from project ICON. J Thorac Oncol. 2018;13:S375. [Google Scholar]
- 17.Sepesi B, Team I, Heymach J, Sharma P, Allison J, Fang B, et al. OA20.06 Prospective ImmunogenomiC PrOfiling of Non-small cell lung cancer - the ICON project. J Thorac Oncol. 2017;12:S324–5. [Google Scholar]
- 18.Clavien PA, Barkun J, De Oliveira ML, Vauthey JN, Dindo D, Schulick RD, et al. The Clavien–Dindo classification of surgical complications: five-year experience. Ann Surg. 2009;250:187–96. [DOI] [PubMed] [Google Scholar]
- 19.Pisters KMW, Vallières E, Crowley JJ, Franklin WA, Bunn PA Jr, Ginsberg RJ, et al. Surgery with or without preoperative paclitaxel and carboplatin in early-stage non-small-cell lung cancer: Southwest Oncology Group Trial S9900, an intergroup, randomized, phase III trial. J Clin Oncol. 2010;28:1843–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Forde PM, Chaft JE, Smith KN, Anagnostou V, Cottrell TR, Hellmann MD, et al. Neoadjuvant PD-1 blockade in resectable lung cancer. N Engl J Med. 2018;378:1976–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee J, Chaft J, Nicholas A, Patterson A, Waqar S, Toloza E, et al. PS01.05 Surgical and clinical outcomes with neoadjuvant atezolizumab in resectable stage IB–IIIB NSCLC: LCMC3 trial primary analysis. J Thorac Oncol. 2021;16:S59–61. [Google Scholar]
- 22.Provencio M, Nadal E, Insa A, García-Campelo MR, Casal-Rubio J, Domine M, et al. Neoadjuvant chemotherapy and nivolumab in resectable non-small-cell lung cancer (NADIM): an open-label, multicentre, single-arm, phase 2 trial. Lancet Oncol. 2020;21:1413–22. [DOI] [PubMed] [Google Scholar]
- 23.Shu CA, Gainor JF, Awad MM, Chiuzan C, Grigg CM, Pabani A, et al. Neoadjuvant atezolizumab and chemotherapy in patients with resectable non-small-cell lung cancer: an open-label, multicentre, single-arm, phase 2 trial. Lancet Oncol. 2020;21:786–95. [DOI] [PubMed] [Google Scholar]
- 24.Van Haren RM, Mehran RJ, Mena GE, Correa AM, Antonoff MB, Baker CM, et al. Enhanced recovery decreases pulmonary and cardiac complications after thoracotomy for lung cancer. Ann Thorac Surg. 2018;106:272–9. [DOI] [PubMed] [Google Scholar]
- 25.Osarogiagbon RU, Lin CC, Smeltzer MP, Jemal A. Prevalence, prognostic implications, and survival modulators of incompletely resected non-small cell lung cancer in the U.S. National Cancer Data Base. J Thorac Oncol. 2016;11:e5–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Besse B, Adam J, Cozic N, Chaput-Gras N, Planchard D, Mezquita L, et al. 1215O - SC Neoadjuvant atezolizumab (A) for resectable non-small cell lung cancer (NSCLC): results from the phase II PRINCEPS trial. Ann Oncol. 2020;31:S794–5. [Google Scholar]
- 27.Albain KS, Swann RS, Rusch VW, Turrisi AT III, Shepard FA, Smith C, et al. Radiotherapy plus chemotherapy with or without surgical resection for stage III non-small-cell lung cancer: a phase III randomised controlled trial. Lancet. 2009;374:379–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Naidoo J, Page DB, Li BT, Connell LC, Schindler K, Lacouture ME, et al. Toxicities of the anti-PD-1 and anti-PD-L1 immune checkpoint antibodies. Ann Oncol. 2015;26:2375–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Naidoo J, Wang X, Woo KM, Lyriboz T, Halpenny D, Cunningham J, et al. Pneumonitis in patients treated with anti–programmed death-1/programmed death ligand 1 therapy. J Clin Oncol. 2017;35:709–17. [DOI] [PMC free article] [PubMed] [Google Scholar]


