Abstract
Transgenic alfalfa (Medicago sativa L. cv Regen) roots carrying genes encoding soybean lectin or pea (Pisum sativum) seed lectin (PSL) were inoculated with Bradyrhizobium japonicum or Rhizobium leguminosarum bv viciae, respectively, and their responses were compared with those of comparably inoculated control plants. We found that nodule-like structures formed on alfalfa roots only when the rhizobial strains produced Nod factor from the alfalfa-nodulating strain, Sinorhizobium meliloti. Uninfected nodule-like structures developed on the soybean lectin-transgenic plant roots at very low inoculum concentrations, but bona fide infection threads were not detected even when B. japonicum produced the appropriate S. meliloti Nod factor. In contrast, the PSL-transgenic plants were not only well nodulated but also exhibited infection thread formation in response to R. leguminosarum bv viciae, but only when the bacteria expressed the complete set of S. meliloti nod genes. A few nodules from the PSL-transgenic plant roots were even found to be colonized by R. leguminosarum bv viciae expressing S. meliloti nod genes, but the plants were yellow and senescent, indicating that nitrogen fixation did not take place. Exopolysaccharide appears to be absolutely required for both nodule development and infection thread formation because neither occurred in PSL-transgenic plant roots following inoculation with an Exo− R. leguminosarum bv viciae strain that produced S. meliloti Nod factor.
Bacteria belonging to the family Rhizobiaceae (Rhizobium, Bradyrhizobium, Azorhizobium, Mesorhizobium, and Sinorhizobium) induce the formation of nitrogen-fixing nodules on their leguminous hosts. This symbiotic interaction, which is governed by sequential signal exchange between rhizobia and their symbiotic partners, exhibits a high degree of specificity, and a number of signal molecules involved in the initial stages of this specificity have been extensively studied. Nod factors are synthesized by the products of rhizobial nod genes, which are induced by plant-secreted molecules such as flavonoids (Hirsch, 1992; Long, 1996). The Sinorhizobium meliloti Nod factor consists of a variable-length N-acetylglucosamine oligomer with a C-16 acyl tail at the non-reducing end and a sulfate at the reducing end, whereas the Rhizobium leguminosarum bv viciae Nod factor has a C-18 fatty acyl residue and no sulfate (for review, see Schultze and Kondorosi, 1998). Nod factors are considered the main rhizobial inducËer molecules for nodulation because the purified molecules elicit, in a host-specific way, many of the plant responses observed in the early stages of nodule formation. These responses include changes in free calcium levels and ion balance, alterations in cytoskeletal organization and morphology of root hairs, the initiation of cortical cell divisions (Ccd), and the triggering of nodule development (Spaink et al., 1991; Truchet et al., 1991; Ehrhardt et al., 1992, 1996; Relic et al., 1993; Felle et al., 1995; Gehring et al., 1997; Cárdenas et al., 1998). However, species that produce well-developed nodule primordia in response to Nod factor are limited; many plant roots undergo just a few Ccd, whereas others show no response at all. The legumes that exhibit an obvious response to purified Nod factor are alfalfa (Medicago sativa L. cv Regen), red clover, Glycine soja, Phaseolus, Acacia, and Lotus corniculatus (Truchet et al., 1991; Stokkermans and Peters, 1994; López-Lara et al., 1995b; Stokkermans et al., 1995; van Rhijn et al., 1998; Díaz et al., 2000). Nevertheless, many legumes have not been tested.
Nod factors are not the only molecules that are involved in host recognition, however. The specific binding of a legume lectin to a saccharide moiety, as yet unidentified, on the cell surface of a compatible Rhizobium allows the two symbionts to recognize each other (Bohlool and Schmidt, 1974; Dazzo and Hubbell, 1975). Halverson and Stacey (1985, 1986) reported that a nodulation-defective mutant of Bradyrhizobium japonicum, capable of initiating root hair attachment and curling, was restored to normal nodulation capability by pretreatment with soybean lectin (SBL). Díaz et al. (1989) demonstrated that transgenic white clover plants carrying a cloned pea (Pisum sativum) seed lectin (PSL) gene were nodulated (although at low efficiency and somewhat later than control plants) by the pea symbiont, R. leguminosarum bv viciae. Subsequent site-directed mutagenesis demonstrated that the carbohydrate-binding domain of the lectin was responsible for the change in host-specificity (Kijne et al., 1994; van Eijsden et al., 1995). After introducing the PSL gene into red clover, Díaz et al. (2000) found that the transgenic hairy roots formed nodule primordia-like structures after inoculation with heterologous rhizobia. Here also, the sugar-binding activity of the pea lectin was reported to be essential for the plant responses.
To test the universality of the lectin recognition hypothesis as well as to apply it to legumes that are distantly related, we introduced the SBL gene into L. corniculatus, which is nodulated by Mesorhizobium loti and not by B. japonicum (van Rhijn et al., 1998). From these experiments, we learned (a) that SBL was properly targeted to L. corniculatus root hairs, (b) that infection threads were formed within the root hair cells, but they rarely penetrated into the next cell layer, (c) that the transgenic Lotus plants were nodulated by B. japonicum, which normally nodulates soybean and not Lotus, and (d) that mutating the SBL sugar-binding site eliminated both infection thread formation and nodule development.
In this report, we introduced either the SBL gene or the PSL gene expressed from the 35S cauliflower mosaic virus promoter into alfalfa (Bingham, 1991) as a further test of the universality of the lectin recognition hypothesis. For these experiments, we used Agrobacterium tumefaciens-mediated transformation because concerns have been expressed about hairy root transformation and whether or not the resultant hairy roots exhibit phytohormone alterations that obscure the plant's response. All other published experiments (Díaz et al., 1989, 2000; van Rhijn et al., 1998) have been performed on plants transformed with A. rhizogenes. In addition, we wanted to examine the effects of heterologous rhizobial inoculation on subsequent generations of lectin-expressing plants. However, this report is concerned only with the responses of the primary transgenic alfalfa plants.
RESULTS
Transgenic Plants
During the generation of the primary transgenic plants, no significant differences in growth and development could be detected in vector control plants versus those harboring the SBL (Hirsch et al., 1995) or the PSL genes. Total protein was isolated from the transgenic plants and subjected to SDS-gel electrophoresis. Western-blot analysis demonstrated that the transgenic alfalfa lines with an introduced lectin gene produced either SBL (Hirsch et al., 1995) or PSL (Fig. 1). All wild-type R. leguminosarum bv viciae (Rlv) strains were found to elicit normal nodule formation on vetch, the compatible host, whereas the Nod− and Exo− strains elicited no nodules on vetch (data not shown) just as previously described (Downie et al., 1985; Borthakur et al., 1988).
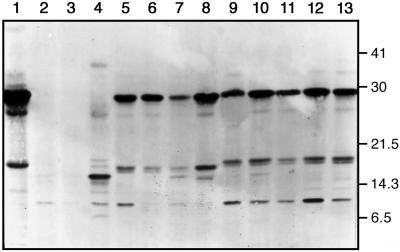
Figure 1.
Western-blot analysis. Protein and western-blot analyses were performed as described earlier (Hirsch et al., 1995). Lanes left to right: 1, PSL, positive control; 2, 121-6, control plant; 3, 121-4, control plant; 4, 2813-E, mutated pea lectin plant (2813-E does not express the right form of lectin and was used as an additional control); 5, 2813-C, mutated pea lectin plant; 6, 2813-5, mutated pea lectin plant; 7, 2813-B, mutated pea lectin plant; 8, 2813-A, mutated pea lectin plant; 9, 2809-T11, pea lectin plant; 10, 2809-T10, pea lectin plant; 11, 2809-T9, pea lectin plant; 12, 2809-T7, pea lectin plant; and 13, 2809-T2, pea lectin plant.
Nodules Form on SBL-Transgenic Alfalfa Only When B. japonicum Produces S. meliloti Nod Factor
The different primary transgenic plant lines were inoculated and found to be nodulated normally by S. meliloti strain Rm1021 (Fig. 2). We previously reported that primary transgenic lines of alfalfa carrying the SBL gene were completely unresponsive to inoculation with B. japonicum strain USDA110 except for showing some minor root hair deformation (Hirsch et al., 1995). The same result is reported here. Moreover, nodules were not formed in response to B. japonicum even when the bacteria were pre-incubated with genistein, one of several inducers of B. japonicum nod genes (Kosslak et al., 1987) (Fig. 2).
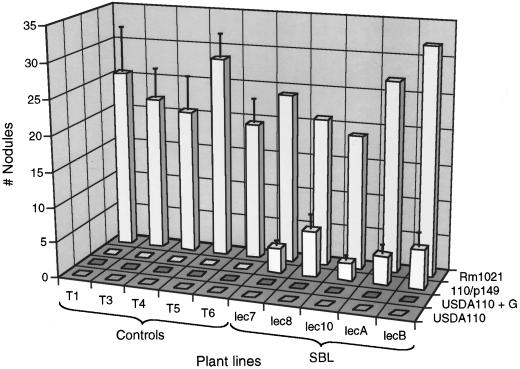
Figure 2.
Responses of different primary transgenic lines of alfalfa to inoculation with wild-type Sinorhizobium meliloti (Rm1021) and with different bradyrhizobial strains. All lines develop nodules in response to Rm1021, but only the lines containing SBL exhibit any response to USDA110 (p149). The data are an average of two independent experiments.
We then introduced the plasmid p149 (Truchet et al., 1985) into B. japonicum USDA110. Plasmid p149 contains the full complement of essential S. meliloti genes for synthesizing S. meliloti Nod factor. Whereas the vector control and SBL lines developed an average of 25 nodules per plant after inoculation with S. meliloti, no nodules were detected on the vector control roots, and fewer than five nodules per plant were found on the roots of different transgenic alfalfa lines expressing SBL (Fig. 2). The structures elicited on the SBL roots were small, underdeveloped, and completely bacteria-free (Fig. 3A).
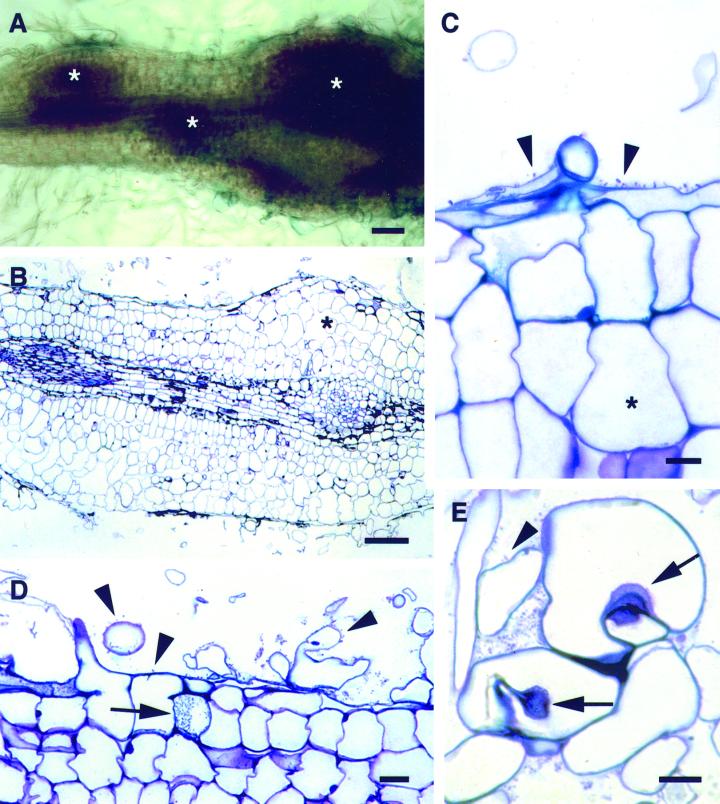
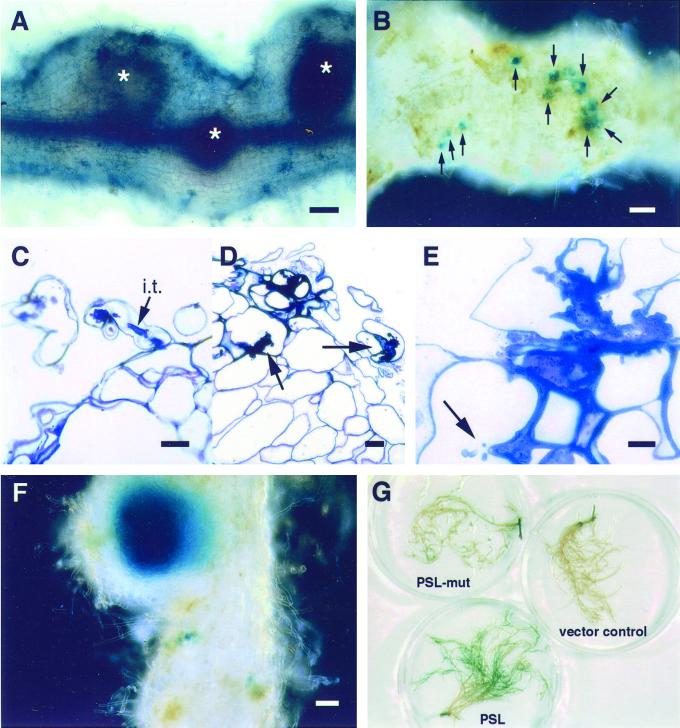
Figure 3.
Responses of transgenic alfalfa lines carrying SBL to B. japonicum (p149). A, Methylene blue-stained root inoculated with USDA110 (p149); three regions indicating Ccds (*) are evident. Scale bar = 100 μm. B, Longitudinal section through a root with a region showing Ccd (*) developed in response to NodZ− (p149). Scale bar = 100 μm. C, Section through the edge of a root showing cortical cell expansion (*) and polar attachment of bradyrhizobia (arrowheads) to the epidermal and root hair cells. Scale bar = 10 μm. D, USDA110 (p149) cells are polarly attached to the root hairs and epidermal cells (arrowheads). Bradyrhizobia are also evident within cells (arrow) and intercellular spaces. Scale bar = 20 μm. E, Polar attachment of USDA110 (p149) cells to root hairs (arrowhead). Hyaline spots indicating the beginnings of bacterial penetration into the root hairs (arrows). Scale bar = 10 μm.
We next examined the possibility that the endogenous Nod factor from B. japonicum interfered with nodule formation on alfalfa. To do this, we inoculated the transgenic alfalfa roots with the B. japonicum NodZ− strain carrying p149. We found that Ccd took place and that nodule primordia were induced on the SBL plants (Fig. 3B). Detailed examination of sectioned material indicated that both cell expansion and Ccd occurred in response to inoculation with NodZ− (p149) (Fig. 3, B and C). The bradyrhizobia were in direct contact with the root epidermal cells of the alfalfa SBL plant lines and were frequently attached in a polar fashion (arrowheads, Fig. 3, C–E). In some instances, the bradyrhizobia appeared to colonize epidermal or hypodermal root cells (arrow, Fig. 3D). However, we did not observe infection threads in any of the 20-plus SBL-roots inoculated with either USDA110 (p149) or NodZ− (p149) bacteria although in a few cases, hyaline spots, which represent the start of infection thread formation, were observed (Fig. 3E).
Taken together, these results indicate that nodule development on the SBL-alfalfa roots in response to B. japonicum occurred only when the compatible Nod factor is produced by bacteria attached to the root hairs. However, no infection threads were observed in the root hairs of the transgenic alfalfa lines, which contrasts with our previous results with SBL-transgenic L. corniculatus. Accordingly we hypothesized that there might be a difference in response of an indeterminate nodule-forming legume such as alfalfa or pea to inoculation with bradyrhizobia in comparison with a determinate nodule-forming host such as Lotus or soybean. To test this hypothesis, we introduced cDNA clones for the PSL gene and a PSL gene with a mutation in the sugar-binding site (PSL-mut) into alfalfa as described in “Materials and Methods.”
Nodules Form on PSL-Transgenic Alfalfa in Response to R. leguminosarum bv viciae, But Only When S. meliloti Nod Factor Is Produced
From the kanamycin-resistant plants, 10 independent primary transgenic lines were selected. These showed: (a) normal shoot and root formation; (b) normal nitrogen-fixing root nodule development within 4 weeks of inoculation with the wild-type S. meliloti strain Rm1021; and (c) a positive hybridization signal using the nptII gene as a probe for the vector control plants or a detectable amount of cross-reacting anti-PSL bands for the PSL transgenic plants (data not shown). The Mr of the lectin produced by the transgenic plants did not differ from that in pea seeds (Fig. 1).
When we inoculated roots with 2 × 104 cells/mL of wild-type S. meliloti, all the alfalfa plant lines, vector control, as well as PSL and PSL-mut plant lines nodulated (Fig. 4, lavender bars). In contrast, there was no root hair curling, infection thread formation, or Ccds detected after either spot (data not shown) or flood inoculations (Fig. 4, blue-gray squares) in response to the same inoculum level of wild-type Rlv strains.
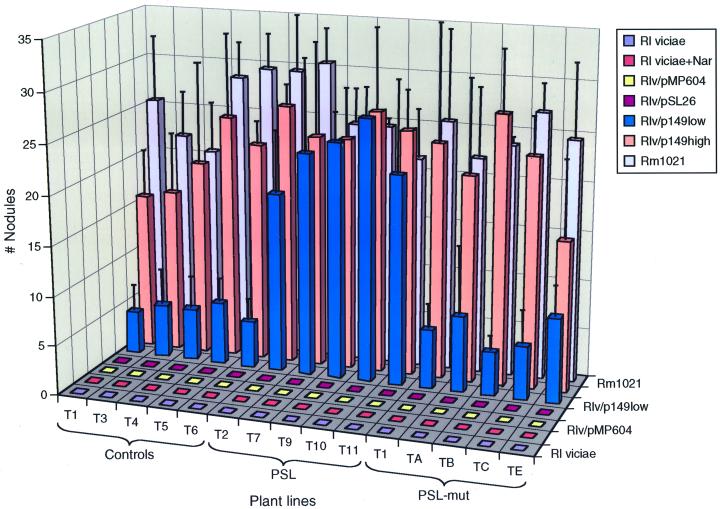
Figure 4.
Responses of different primary transgenic of lines of alfalfa to inoculation with wild-type Sinorhizobium meliloti (Rm1021; lavendar bars) and different rhizobial strains. There is no response to R. leguminosarum bv viciae without induction (blue-gray bars) or with induction (orange bars) by naringenin or in response to R. leguminosarum bv viciae carrying a mutant nodD (yellow bars) or pRmSL26 (magenta bars). The data are an average of two independent experiments. Introducing the S. meliloti nod genes on p149 into R. leguminosarum bv viciae results in nodules formed on all plant lines at high inoculum concentrations (2 × 104 to 2 × 105 cells/mL; coral bars). At low inoculum concentrations (approximately 200 cells/mL) only the PSL-containing lines develop nodules above background levels (blue bars). The data are an average of three independent experiments.
We also inoculated the control, PSL, and PSL-mut alfalfa lines with wild-type Rlv induced with the flavonoid naringenin, which should activate endogenous nod gene expression. The different plant lines were also inoculated with R. leguminosarum bv trifolii carrying the Rlv pSym (Priem and Wijffelman, 1984), a strain that should behave essentially the same as wild-type Rlv. No nodules were induced in either case (Fig. 4, orange squares; data not shown). Inoculation with Rlv, either a wild-type or a NodC− strain, carrying the S. meliloti nodulation genes on plasmid pSL26, which contains the common nodulation genes, nodDABC (Long et al., 1982) also did not elicit nodule formation (Fig. 4, magenta squares; data not shown). Strains carrying pSL26 produce Nod factor lacking side chain modifications that are critical for host-specific alfalfa nodulation. There were also no nodules induced after inoculation with R. leguminosarum bv viciae strains containing pMP604, which results in the constitutive expression of the nodulation genes by a hybrid nodD (Spaink et al., 1989) (Fig. 4, yellow squares). Together, these results demonstrate that alfalfa is unresponsive to R. leguminosarum bv viciae, even when Rlv Nod factor production is enhanced. We also inoculated the transgenic and control alfalfa with an exopolysaccharide-minus (Exo−) strain. Four weeks after inoculation, we could not detect Ccd or infection threads in either sectioned or in whole-mount material in roots inoculated with the Exo− strain or with Exo− (pSL26) (data not shown).
We next introduced the plasmid p149 into both wild-type and Exo− Rlv. In response to the wild-type Rlv strain carrying the entire set of S. meliloti nod genes, nodule primordia or uninfected nodules (Fig. 4, coral bars) developed on the roots of all the alfalfa plant lines when 2,000 to 20,000 cells/mL were used as inoculum. However, there was a significant difference in the number of nodules formed on the vector control, PSL, and PSL-mut plant lines when only 200 cells/mL were inoculated. There was an approximately 3.5- to 4-fold increase in the number of nodules, comparable with the number of S. meliloti-induced nodules (20–25 per plant), formed on the PSL transgenic alfalfa lines in contrast to the vector control and the PSL-mut lines, which developed fewer than five nodules per plant (Fig. 4, blue bars). In addition, the nodules formed on the PSL plants were generally larger and showed more apical growth than those developed on the vector control and PSL-mutant roots (Fig. 5A). From these results, we conclude that PSL facilitates nodule development in response to Rlv, but only if compatible Nod factor is provided.
Figure 5.
Responses of transgenic alfalfa lines carrying PSL to Rlv (p149). A, Well-developed nodule-like structures (*) on a root of alfalfa carrying the PSL gene. Scale bar = 100 μm. B, Dark-field microscopy of PSL-transgenic alfalfa root inoculated with a Gus-marked Rlv (p149). The arrows point to the numerous infection threads. Scale bar = 100 μm. C, Section through the edge of a nodule illustrating an infection thread (i.t.) in the root hair. Scale bar = 20 μm. D, Multiple threads are evident in the root hair and hypodermal cells (arrows). Scale bar = 20 μm. E, Convoluted infection thread with narrow branches protruding into the host cell (arrow). Scale bar = 10 μm. F, An infected nodule formed on a PSL-transgenic alfalfa root inoculated with Gus-marked Rlv (p149). Scale bar = 100 μm. G, Attachment assays with Gus marked strains. Attachment is stronger to the PSL transgenic roots as evidenced by the dark-blue color of the roots.
Transgenic Alfalfa Plants Producing Pea Lectin Exhibit Increased Infection Thread Formation
A detailed examination of the nodules formed by different transgenic lines in response to the low inoculum concentration of Rlv (p149) was made. The strain was marked with Gus so it was possible to monitor infection thread development.
Table I shows that there is a significant difference in the number of Ccd per centimeter of root among the three groups of transgenic plants; the PSL lines exhibit an almost 2-fold increase over the vector control and PSL-mut lines. The number of infection threads per centimeter of root was also found to be significantly different (almost 10 times higher) in the PSL transgenic plant lines. The data were recalculated to illustrate the number of infection threads formed per Ccd. Again, the PSL plants exhibited the greatest number of infection threads/Ccd, approximately 4-fold, over the vector control and PSL-mut lines (Table I; Fig. 5B).
Table I.
Detailed study of responses of transgenic alfalfa plants after inoculation with R. leguminosarum bv viciae (pGMI149)
| Responses to R. leguminosarum bv viciae-Gus (pGMI149) Low Inoculum | Vector control-T1 | Vector control-T3 | Vector Control-T4 | Vector Control-T6 | PSL-T2 | PSL-T7 | PSL-T9 | PSL-T10 | PSL-T11 | PSL-mut-T1 | PSL-mut-TA | PSL-mut-TB | PSL-mut-TC | PSL-mut-TE |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ccd/cm | 2.00 ± 0.96 | 1.30 ± 0.54 | 0.96 ± 0.41 | 0.94 ± 0.58 | 2.97 ± 1.02 | 3.38 ± 1.80 | 2.74 ± 1.54 | 3.76 ± 1.45 | 3.60 ± 1.20 | 0.77 ± 0.38 | 1.01 ± 0.59 | 0.98 ± 0.52 | 1.39 ± 0.68 | 1.42 ± 0.56 |
| Inf/cm | 1.32 ± 0.80 | 1.34 ± 0.92 | 1.14 ± 0.63 | 1.29 ± 0.85 | 12.49 ± 6.72 | 13.32 ± 6.63 | 9.77 ± 4.42 | 13.79 ± 6.18 | 17.63 ± 4.95 | 0.98 ± 0.43 | 1.09 ± 0.48 | 1.15 ± 0.80 | 1.06 ± 0.92 | 2.03 ± 0.77 |
| Inf/Ccd | 0.75 ± 0.31 | 0.94 ± 0.64 | 1.05 ± 0.71 | 1.27 ± 0.81 | 4.01 ± 1.42 | 4.56 ± 1.81 | 3.61 ± 0.46 | 3.61 ± 0.46 | 5.04 ± 0.95 | 1.21 ± 0.63 | 1.19 ± 0.78 | 1.19 ± 0.90 | 0.85 ± 0.73 | 1.45 ± 0.22 |
Sections and whole-mounts were examined to visualize the sites of infection thread formation and to verify that bona fide infection threads had developed. Figure 5, C to E, shows infection threads penetrating into root hairs of PSL lines inoculated with Rlv (p149). The threads often extended beyond the root hair and into the interior of the nodule (Fig. 5D). In some experiments, nodules stained blue indicating that the Rlv (p149) bacteria had invaded the central zone of the nodule (Fig. 5F). However, the plants were yellow suggesting that the nodules were ineffective. Bacteria were recovered from these nodules and exhibited the appropriate antibiotic resistance markers and DNA restriction pattern, demonstrating that they were Rlv (p149).
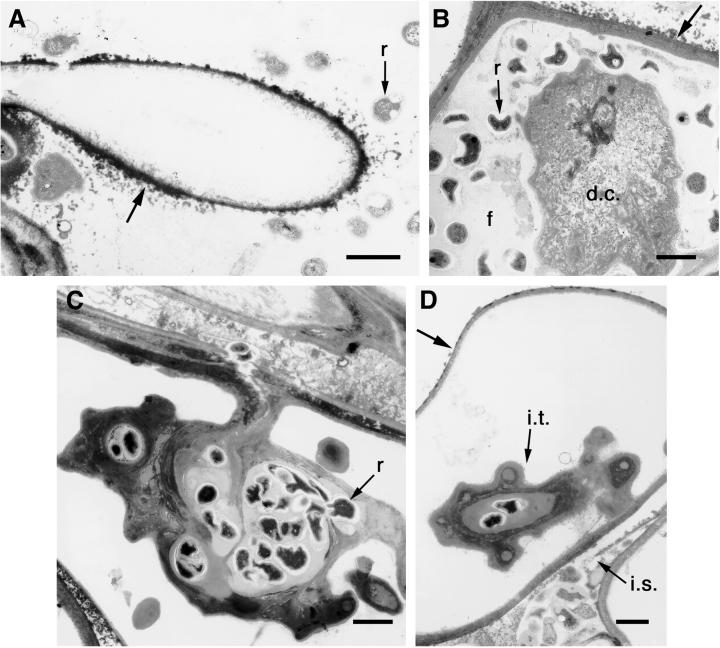
Transmission electron microscopy verified that large numbers of rhizobia were either attached or closely associated with root hairs, which were frequently covered with an irregular, electron-dense matrix (Fig. 6A). Non-membrane bound rhizobia frequently are observed between the layers of the epidermal cell walls (data not shown) and also within intercellular spaces (Fig. 6D). Many root hairs and epidermal cells contained infection threads, but the threads were convoluted and highly branched or barbed (Fig. 6, C and D). It was difficult to ascertain whether rhizobia were released from such threads; no membrane-bound rhizobia were detected. However, we observed numerous rhizobia surrounded by a fibrillar matrix in host cells where the cytoplasm and nucleus appeared degenerated (Fig. 6B). This fibrillar matrix is probably derived from the infection thread.
Figure 6.
Transmission electron micrographs of PSL-transgenic alfalfa root inoculated with Rlv (p149). A, Part of a root hair with attached and associated rhizobia (r). An electron-dense, particulate matrix surrounds the root hair (arrow). Scale bar = 1 μm. B, An epidermal cell filled with rhizobia (r) that are surrounded by a fibrillar matrix (f). The cytoplasm and nucleus of the host cell have degenerated (d.c.). The outer edge of the cell is covered with an electron-dense material (arrow). Scale bar = 1 μm. C, Highly convoluted, extensively branched infection thread within a hypodermal cell. Rhizobia (r) are encapsulated within the thread. Scale bar = 1 μm. D, Another infection thread (i.t.). Rhizobia are also present in an adjacent intercellular space (i.s.). The arrow points to the outer edge of the cell. Scale bar = 1 μm.
Exopolysaccharide Is Necessary for Root Hair Penetration
The Rlv Exo− (p149) strain was used to test whether infection thread development would take place in the absence of rhizobial exopolysaccharide (EPS). Based on our previous results with exoB bradyrhizobia on SBL-transgenic L. corniculatus (van Rhijn et al., 1998), we predicted that it would not. In roots inoculated with Rlv Exo− (p149), rhizobia were infrequently observed along the deformed root hairs and at those sites, swellings and even more rarely, very small, nodule-like structures were observed. We believe that small, bacteria-free nodules developed because the Exo− (p149) strain produces compatible Nod factor, and alfalfa undergoes Ccd in response to Nod factor alone (Truchet et al., 1991). Thus, the plant response to Exo− (p149) was essentially Nod−, strongly suggesting that some component of EPS interacts with lectin or is the ligand for lectin.
The Introduced Pea Lectin Mediates Rhizobial Attachment
We monitored attachment by examining the amount of Gus staining on the PSL-transgenic roots as well as the vector control and PSL-mut plant roots after inoculation with Rlv-Gus and Rlv-Gus carrying a constitutive nodD (data not shown). The intense blue staining of the PSL roots indicates that there is greater rhizobial attachment to these roots compared with the PSL-mut or vector control plant roots (Fig. 5G).
DISCUSSION
We have shown that the PSL gene (with an intact sugar-binding site), when introduced into alfalfa, augments both attachment to and infection thread formation in root hairs by a non-host bacterial strain, R. leguminosarum bv viciae. This result is consistent with our previous observations showing that introduction of the SBL gene into L. corniculatus enables the non-host B. japonicum strain USDA110 to attach, elicit nodule formation, and form infection threads within root hairs (van Rhijn et al., 1998). Moreover, attachment is a prerequisite for nodulation and infection thread formation in the root hair because an Exo− mutant of R. leguminosarum bv viciae, even if it carries S. meliloti nod genes, is essentially Nod− and does not promote infection thread formation in root hairs of PSL-transgenic plants. However, in contrast to our previous results where no infection threads were detected on SBL-mutant and vector control L. corniculatus lines, both the vector control and PSL-mut alfalfa lines developed a basal level of infection threads when inoculated with wild-type Rlv (p149) (Table I). To our knowledge, this is the first report of a heterologous rhizobial strain, albeit one that makes the compatible Nod factor, inducing infection thread development on alfalfa.
In contrast, SBL-containing alfalfa plants did not form infection threads in the root hairs of the SBL-transgenic alfalfa plants in response to Bj (p149). This result differs with what we described earlier for transgenic L. corniculatus (van Rhijn et al., 1998). One explanation may be that alfalfa develops indeterminate nodules, whereas soybean and Lotus establish determinate nodules. EPS-deficient mutants are known to have much more severe effects on indeterminate-nodule versus determinate-nodule forming hosts, perhaps in part because the length and thickness of the infection thread in each nodule type differ significantly (Becker and Pühler, 1998; Kijne, 1992). Nevertheless, even when infection threads developed in the SBL-transgenic L. corniculatus plants (van Rhijn et al., 1998), they aborted indicating that a subsequent stage in the developmental process was not activated. In the PSL-transgenic alfalfa plants (this report), we observed an occasional “blue” nodule indicating that the heterologous rhizobia colonized the central part of the nodule, triggering some yet unknown process that enables host cell infection. However, the nodules were Fix−; the plants were yellow and senescent. We have subsequently determined that the rhizobia isolated from these nodules are more mucoidy than Rlv (p149), suggesting that some type of cell surface mutation has occurred. These results suggest that an unknown element(s) may be required for full entry and release of bacteria into the nodule. We are analyzing these altered rhizobia further.
Although infection thread formation did not take place in the nodules formed on the SBL alfalfa plants, the introduced lectin was required for cortical cell expansion and division as well as primordium formation in response to Bj (p149) because there was no response in the control plants. Moreover, we found that numerous rhizobia were attached to the root surface of the SBL-alfalfa lines, whereas negligible numbers were attached to the vector control plants. Taken together, these data suggest that attachment brings together on the root surface a collection of rhizobia that produces an effective concentration of Nod factor. They further suggest that a threshold of Nod factor must be reached for triggering the intensity of the plant's response. If too little Nod factor is produced or if the Nod factor is not completely compatible, then only root hair deformation and cell expansion take place. If sufficient Nod factor is produced or recognized, then Ccd and nodule primordium formation are elicited.
Earlier, we found that the SBL-transgenic L. corniculatus plants responded to B. japonicum Nod factor and synthetic lipochitin oligosaccharides when present at relatively high concentrations (van Rhijn et al., 1998); soybean and Lotus Nod factors show some overlap (Sanjuan et al., 1992; Carlson et al., 1993; López-Lara et al., 1995a). Rlv Nod factor differs from S. meliloti Nod factor in several significant ways: at the reducing end of the molecule and in the length and saturation of the acyl tail. The ability of Rlv to elicit nodule formation on alfalfa has an absolute requirement for the compatible Nod factor from S. meliloti. By itself, Rlv induces only insignificant root hair deformation on alfalfa. We did not observe any cortical cell expansion, Ccd, or nodule primordia formation on the PSL-transgenic alfalfa lines in response to heterologous rhizobia. This contrasts with the results of Díaz et al. (2000), who found that exogenous lipooligosaccharide molecules having substitutions that are characteristic of a number of heterologous strains induced swellings or inner Ccds of transgenic red clover roots. In our experiments, the Rlv (p149) strain (wild-type R. leguminosarum bv viciae that produces S. meliloti Nod factor) was the only strain to elicit extensive root hair deformation, shepherd's crook formation, infection thread development, and nodulation, strongly suggesting that these responses in alfalfa are dependent on the presence of compatible Nod factor. Because Rlv strains carrying pSL26 did not elicit any response on the transgenic alfalfa lines, we conclude that the substitutions on glucosamine portion of the Nod factor, most likely the sulfate, are absolutely necessary for alfalfa to react to the “wrong” rhizobia. The requirement for compatible Nod factor suggests that Nod factor is critical not only for root hair deformation and nodule development (Long, 1996), but also for the formation and penetration of the infection threads into the root hair (Hirsch, 1999).
In summary, in addition to what has been previously established regarding the essentiality of rhizobial EPS for infection thread entry into the root hair, we conclude the following: (a) Lectin mediates nodule development and infection thread formation on the “wrong” host by facilitating rhizobial attachment, and (b) compatible Nod factor is absolutely required for infection thread development on alfalfa by the “wrong” rhizobial strain. Nevertheless, these threads abort strongly, suggesting that some component(s) is (are) missing in either the transgenic alfalfa or in the genetically modified rhizobia. What these may be is the focus of our future work.
MATERIALS AND METHODS
Bacterial Strains and Plasmids
The Bradyrhizobium strains used in this study were USDA110, a wild-type Bradyrhizobium japonicum, and NAD138, a nodZ::Tn5 mutant that lacks the 2-O-methyl-Fuc on the reducing end of the Nod factor molecule (Nieuwkoop et al., 1987; Stacey et al., 1994). The Rhizobium leguminosarum bv viciae (Rlv) wild-type strain used as a host for the various plasmids in this study was constructed by moving the transposon TP003 containing Gus into strain 128C53 (this work). In addition to the wild-type strain, the following Rlv mutants were used: NodC− (nodC::Tn5; Downie et al., 1985) and Exo− (A168; Scheu et al., 1992). Strain A168 is mutated in pssA (Borthakur et al., 1986, 1988; van Workum et al., 1997), an Rlv gene homologous to Sinorhizobium meliloti exoY, which encodes the first glycosyl transferase in EPS biosynthesis. The strains Rlv (pSL26), Rlv (p149), Rlv (pMP604), NodC− (pSL26), NodC− (p149), Exo− (pSL26), and Exo− (p149) were constructed by triparental matings. Plasmid pSL26 (pRmSL26) is an IncP plasmid containing the S. meliloti nodDABC genes (Long et al., 1982), whereas plasmid p149 (pGMI149) is an IncP plasmid containing the entire S. meliloti nod gene region (Truchet et al., 1985). Plasmid pMP604 is an IncP plasmid containing a hybrid nodD gene allowing flavonoid-independent gene expression (Spaink et al., 1989). The plasmids were mated into either USDA110 or Rlv by a triparental mating using pRK2013 (Figurski and Helinski, 1979) as a helper plasmid.
Bradyrhizobium and Rhizobium strains were grown at 28°C to 30°C in peptone-salt-yeast extract medium for bradyrhizobia (Regensburger and Hennecke, 1983) or for rhizobia, in yeast mannitol broth medium (Vincent, 1970) or tryptone-yeast extract medium (Beringer, 1974) supplemented with the appropriate antibiotics: 50 μg/mL streptomycin; 25 μg/mL kanamycin; and tetracycline, 10 μg/mL for Rlv and 100 μg/mL for Bj strains.
Construction of Transgenic Plants
Leaves of alfalfa (Medicago sativa L. cv Regen) were transformed with Agrobacterium tumefaciens LBA4404 (Hoekema et al., 1983) carrying the SBL gene as described previously (Hirsch et al., 1995). Six independent transgenic lines, originating from different alfalfa leaves, were chosen for analysis (Hirsch et al., 1995). For the pea (Pisum sativum)-lectin transgenic plant lines, A. tumefaciens carried the control vector pBI121 or either the wild-type PSL cDNA or the mutated PSL cDNA, where the conserved Asn-125 of the sugar-binding site was mutated to Asp (PSLN125D), on the binary vector pAGS HB35S (van Eijsden et al., 1995). The different plasmids were electroporated into A. tumefaciens LBA4404, and these cells were then used to infect alfalfa leaves (Hirsch et al., 1995). The following nomenclature was adopted for the pea-lectin transgenic lines: vector control plants; PSL, plants expressing the PSL gene; and PSL-mut, plants carrying the mutated pea lectin gene. Ten PSL-expressing and eight PSL-mut-expressing independent lines were constructed. All produced a protein that cross-reacted with an antibody to PSL on western blots (Fig. 1) except for one line, which was used as an additional control. The transgenic plants were started in a Conviron growth cabinet with a 16-h-light/8-h-dark photoperiod and a 23°C-day/20°C-night thermoperiod. Mature plants were maintained in the UCLA greenhouses, and rooted cuttings were used for analysis.
Western-Blot Analysis
Protein and western-blot analyses were performed as described earlier (Hirsch et al., 1995). The blots containing extracts from the control, and alfalfa-SBL lines were probed with a commercial soybean seed lectin antibody as described in Hirsch et al. (1995). Blots containing extracts from control and alfalfa-PSL plants were incubated with the appropriate dilution of the polyclonal anti-PSL antibody RAL439, which had been raised against SDS-denatured seed PSL (Díaz et al., 1986).
Plant Inoculations
Rooted cuttings were transferred to Magenta jars (Magenta Corporation, Chicago) filled with a mixture of 2:1 vermiculite/perlite saturated with Jensen's medium minus nitrogen (Vincent, 1970) after they had been rooted in one-half-strength Murashige and Skoog medium (Szabados et al., 1990). The roots were flood-inoculated with rhizobia, which had grown in YMB medium and were then rinsed in sterile water prior to inoculation at two different concentrations: either 106 to 107 cells per Magenta jar (approximately 2 × 104 to 2 × 105 cells/mL) or 102 to 103 cells per jar (approximately 2 × 101 to 2 × 102 cells/mL). The bacterial medium was supplemented with naringenin (10 μm) for the induction of nod genes in Rlv and with genistein (10 μm) for USDA110. The plants were incubated for 4 to 6 weeks in a growth cabinet with a 16-h-light/8-h-dark photoperiod and a 23°C-day/20°C-night temperature regime.
Seeds of Vicia sativa subsp. nigra (vetch) were surface sterilized for 5 min in 95% (v/v) ethanol and 60 min in full-strength commercial bleach. After copious rinsing with sterile distilled water, the seeds were germinated on water agar and then transferred to Magenta jars containing sterilized vermiculite/perlite watered with one-quarter- strength Hoagland medium minus N. The seedlings were inoculated with the various Rlv strains 4 to 6 d post-germination, and the plants were harvested 28 days post-inoculation.
Microscopic Analysis
Roots inoculated with the different rhizobial strains were examined under a Axiophot microscope (Zeiss, Jena, Germany) for root hair deformation, root hair curling, Ccd, and infection thread formation. The plants were harvested 5 to 6 weeks after inoculation. Excess vermiculite/perlite was carefully removed, and some root segments were cleared following the procedure of Stokkermans et al. (1995). Other segments were fixed and stained overnight for β-glucuronidase activity (Jefferson et al., 1987). Some roots were embedded in Spurr's plastic for determining the specific loci of Ccds. These roots were fixed in phosphate-buffered glutaraldehyde:paraformaldehyde and embedded as described previously (Yang et al., 1992). Sections cut at 1- to 2-μm thickness were stained with toluidine blue (Yang et al., 1992). Photographs were taken with Ektachrome 160 film (Eastman-Kodak, Rochester, NY), the slides were scanned into the computer, and composites were made using Photoshop (Adobe Systems, Mountain View, CA). Thin sections were stained with lead citrate and uranyl acetate as described in Yang et al. (1992) and examined under a JEOL JEM-100CX electron microscope (JEOL, Tokyo).
Bacterial Recovery and Attachment Assays
Bacteria were recovered from nodules as previously described (van Rhijn et al., 1998) and plated onto the appropriate medium containing antibiotics.
The degree of bacterial attachment was measured by collecting the roots aseptically in 50 mL of phosphate-buffered saline and washing them five times in phosphate-buffered saline to eliminate loosely attached cells (van Rhijn et al., 1998). The roots were stained for Gus histochemical activity (Jefferson et al., 1987).
ACKNOWLEDGMENTS
We gratefully acknowledge R.B. Goldberg for the SBL clone and J. Kijne and C. Díaz for the PSL and PSL-PSLN125D cDNA clones and the PSL antibody. Our thanks are also extended to J. Dénarié, J.A. Downie, S.R. Long, and H.P. Spaink for rhizobial strains and plasmids and to G. Stacey for the bradyrhizobial strains. We also thank an anonymous reviewer for suggestions on improving the manuscript. We are grateful to B. Sjostrand for her help with sectioning the plastic-embedded material, M. Kowalczyk for the final photographs, and W. Yang for growing and maintaining the transgenic plants in the UCLA greenhouses. We also thank members of our laboratory group for comments on the manuscript, and our special thanks go to M. Lum for help with the Gus staining.
Footnotes
This work was supported by the National Research Competitive Grant Program (grant nos. 93–37305–9144 and 96–35305–3583 to A.M.H.) and by the D. Collen Research Foundation, K.U. Leuven, Belgium (to P.v.R.).
LITERATURE CITED
- Becker A, Pühler A. Production of exopolysaccharides. In: Spaink HP, Kondorosi A, Hooykaas PJJ, editors. The Rhizobiaceae: Molecular Biology of Model Plant-Associated Bacteria. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1998. pp. 97–118. [Google Scholar]
- Beringer JE. R-factor transfer in Rhizobium leguminosarum. J Gen Microbiol. 1974;120:421–429. doi: 10.1099/00221287-84-1-188. [DOI] [PubMed] [Google Scholar]
- Bingham ET. Registration of alfalfa hybrid Regen-SY germplasm for tissue culture and transformation research. Crop Sci. 1991;31:1098. [Google Scholar]
- Bohlool BB, Schmidt EL. Lectins: a possible basis for specificity in the Rhizobium-legume root nodule symbiosis. Science. 1974;185:269–271. doi: 10.1126/science.185.4147.269. [DOI] [PubMed] [Google Scholar]
- Borthakur D, Barber CE, Lamb JW, Daniels MJ, Downie JA, Johnston AWB. A mutation that blocks exopolysaccharide synthesis prevents nodulation of peas by Rhizobium leguminosarum but not of beans by R. phaseoli and is corrected by cloned DNA from Rhizobium or the phytopathogen Xanthomonas. Mol Gen Genet. 1986;203:320–323. [Google Scholar]
- Borthakur D, Barker RF, Latchford JW, Rossen L, Johnston AWB. Analysis of pss genes in Rhizobium leguminosarum required for exopolysaccharide synthesis and nodulation of peas: their primary structure and their interaction with psi and other nodulation genes. Mol Gen Genet. 1988;213:155–162. doi: 10.1007/BF00333413. [DOI] [PubMed] [Google Scholar]
- Cárdenas L, Vidali L, Dominguez J, Perez H, Sánchez F, Hepler PK, Quinto C. Rearrangement of actin microfilaments in plant root hairs responding to Rhizobium etli nodulation signals. Plant Physiol. 1998;116:871–877. doi: 10.1104/pp.116.3.871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson RW, Sanjuan J, Ramadas Bhat U, Glushka J, Spaink JP, Wijfjes AHM, van Brussel AAN, Stokkermans TJW, Peters NK, Stacey G. The structures and biological activities of the lipo-oligosaccharide nodulation signals produced by type I and II strains of Bradyrhizobium japonicum. J Biol Chem. 1993;268:18372–18381. [PubMed] [Google Scholar]
- Dazzo FB, Hubbell HD. Cross-reactive antigens and lectins as determinants of symbiotic specificity in the Rhizobium-clover association. Appl Microbiol. 1975;30:1017–1033. doi: 10.1128/am.30.6.1017-1033.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Díaz CL, Melchers LS, Hooykaas PJJ, Lugtenberg EJJ, Kijne JW. Root lectin as a determinant of host-plant specificity in the Rhizobium-legume symbiosis. Nature. 1989;338:579–581. [Google Scholar]
- Díaz CL, Spaink HP, Kijne JW. Heterologous rhizobial lipochitin oligosaccharides and chitin oligomers induce cortical cell divisions in red clover roots, transformed with the pea lectin gene. Mol Plant Microbe Interact. 2000;13:268–276. doi: 10.1094/MPMI.2000.13.3.268. [DOI] [PubMed] [Google Scholar]
- Díaz CL, van Spronsen PC, Bakhuizen R, Logman GJJ, Lugtenberg EJJ, Kijne JW. Correlation between infection by Rhizobium leguminosarum and lectin on the surface of Pisum sativum L. roots. Planta. 1986;168:350–359. doi: 10.1007/BF00392360. [DOI] [PubMed] [Google Scholar]
- Downie JA, Knight CD, Johnston AWB, Rossen L. Identification of genes and gene products involved in the nodulation of peas by Rhizobium leguminosarum. Mol Gen Genet. 1985;198:255–262. [Google Scholar]
- Ehrhardt DW, Atkinson EM, Long SR. Depolarization of alfalfa root hair membrane potential by Rhizobium meliloti Nod factors. Science. 1992;256:998–1000. doi: 10.1126/science.10744524. [DOI] [PubMed] [Google Scholar]
- Ehrhardt DW, Wais R, Long SR. Calcium spiking in plant root hairs responding to Rhizobium nodulation signals. Cell. 1996;85:673–681. doi: 10.1016/s0092-8674(00)81234-9. [DOI] [PubMed] [Google Scholar]
- Felle HH, Kondorosi E, Kondorosi A, Schultze M. Nod signal-induced plasma membrane potential changes in alfalfa root hairs are differentially sensitive to structural modifications of the lipochitooligosaccharide. Plant J. 1995;7:939–947. [Google Scholar]
- Figurski DH, Helinski DR. Replication of an origin-containing derivative of plasmid RK2 dependent on a plasmid function provided in trans. Proc Natl Acad Sci USA. 1979;73:1648–1652. doi: 10.1073/pnas.76.4.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehring CA, Irving HR, Kabbara AA, Parish RW, Boukli NM, Broughton WJ. Rapid, plateau-like increases in intracellular free calcium are associated with Nod factor-induced root-hair deformation. Mol Plant Microbe Interact. 1997;10:791–802. [Google Scholar]
- Halverson LJ, Stacey G. Host recognition in the Rhizobium-soybean symbiosis: evidence for the involvement of lectin in nodulation. Plant Physiol. 1985;77:621–625. doi: 10.1104/pp.77.3.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halverson LJ, Stacey G. Effect of lectin on nodulation of wild type Bradyrhizobium japonicum and a nodulation defective mutant. Appl Environ Microbiol. 1986;51:753–760. doi: 10.1128/aem.51.4.753-760.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch AM. Tansley review no. 40: developmental biology of legume nodulation. New Phytol. 1992;122:211–237. doi: 10.1111/j.1469-8137.1992.tb04227.x. [DOI] [PubMed] [Google Scholar]
- Hirsch AM. Role of lectins (and rhizobial exopolysaccharides) in legume nodulation. Curr Opin Plant Biol. 1999;2:320–326. doi: 10.1016/S1369-5266(99)80056-9. [DOI] [PubMed] [Google Scholar]
- Hirsch AM, Brill LM, Lim PO, Scambray J, van Rhijn P. Steps toward defining the role of lectins in nodule development in legumes. Symbiosis. 1995;19:155–173. [Google Scholar]
- Hoekema A, Hirsch PR, Hooykaas PJJ, Schilperoort RA. A binary plant vector strategy based on separation of the vir- and T-region of Agrobacterium tumefaciens Ti plasmid. Nature. 1983;303:179–180. [Google Scholar]
- Jefferson RA, Kavanagh TA, Bevan MW. GUS fusions: β-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J. 1987;6:3901–3907. doi: 10.1002/j.1460-2075.1987.tb02730.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kijne JW. The Rhizobium infection process. In: Stacey G, Burris R, Evans H, editors. Biological Nitrogen Fixation. New York: Chapman & Hall; 1992. pp. 349–398. [Google Scholar]
- Kijne JW, Díaz CL, van Eijsden R, Booij P, Demel R, van Workum W, Wijffelman C, Spaink H, Lugtenberg B, de Pater S. Lectin and Nod factors in Rhizobium-legume symbiosis. In: Kiss GB, Endre G, editors. Proceedings of the First European Nitrogen Fixation Conference. Officina Press, Szeged. 1994. pp. 106–110. [Google Scholar]
- Kosslak R, Bookland R, Barkei J, Paaren H, Appelbaum E. Induction of Bradyrhizobium japonicum common nod genes by isoflavones isolated from Glycine max. Proc Natl Acad Sci USA. 1987;84:7428–7432. doi: 10.1073/pnas.84.21.7428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long SR. Rhizobium symbiosis: Nod factors in perspective. Plant Cell. 1996;8:1885–1898. doi: 10.1105/tpc.8.10.1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long SR, Buikema WJ, Ausubel FM. Cloning of Rhizobium meliloti nodulation genes by direct complementation of Nod− mutants. Nature. 1982;298:485–488. [Google Scholar]
- López-Lara IM, van den Berg JDJ, Thomas-Oates JE, Glukshka J, Lugtenberg BJJ, Spaink HP. Structural identification of the lipo-chitin oligosaccharide nodulation signals of Rhizobium loti. Mol Microbiol. 1995a;15:627–638. doi: 10.1111/j.1365-2958.1995.tb02372.x. [DOI] [PubMed] [Google Scholar]
- López-Lara IM, van Der Drift KMGM, van Brussel AAN, Haverkamp J, Lugtenberg BJJ, Thomas-Oates JE, Spaink HP. Induction of nodule primordia on Phaseolus and Acacia by lipo-chitin oligosaccharide nodulation signals from broad-host-range Rhizobium strain GRH2. Plant Mol Biol. 1995b;29:465–477. doi: 10.1007/BF00020978. [DOI] [PubMed] [Google Scholar]
- Nieuwkoop AJ, Banfalvi Z, Deshmane N, Gerhold D, Schell MG, Sirotkin KM, Stacey G. A locus encoding host range is linked to the common nodulation genes of Bradyrhizobium japonicum. J Bacteriol. 1987;169:2631–2638. doi: 10.1128/jb.169.6.2631-2638.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priem WJE, Wijffelman CA. Selection of strains cured of the Rhizobium leguminosarum Sym-plasmid pRJ1J1 by using small bacteriocin. FEMS Microbiol Lett. 1984;25:245–251. [Google Scholar]
- Regensburger B, Hennecke H. RNA polymerase from Rhizobium japonicum. Arch Microbiol. 1983;135:103–109. doi: 10.1007/BF00408017. [DOI] [PubMed] [Google Scholar]
- Relic B, Talmont F, Kopcinska J, Golinowski W, Prome J-C, Broughton WJ. Biological activity of Rhizobium sp. NGR234 Nod-factors on Macroptilium atropurpureum. Mol Plant Microbe Interact. 1993;6:764–774. doi: 10.1094/mpmi-6-764. [DOI] [PubMed] [Google Scholar]
- Sanjuan J, Carlson RW, Spaink HP, Bhat UR, Barbour WM, Glushka J, Stacey G. A 2-O-methylfucose moiety in the lipooligosaccharide nodulation signal of Bradyrhizobium japonicum. Proc Natl Acad Sci USA. 1992;89:8789–8793. doi: 10.1073/pnas.89.18.8789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheu AK, Economou A, Hong GF, Ghelani S, Johnston AWB, Downie JA. Secretion of the Rhizobium leguminosarum nodulation protein NodO by haemolysin-type systems. Mol Microbiol. 1992;6:231–238. doi: 10.1111/j.1365-2958.1992.tb02004.x. [DOI] [PubMed] [Google Scholar]
- Schultze M, Kondorosi A. Regulation of symbiotic root nodule development. Annu Rev Genet. 1998;32:33–57. doi: 10.1146/annurev.genet.32.1.33. [DOI] [PubMed] [Google Scholar]
- Spaink HP, Sheeley DM, van Brussel ANN, Glushka JN, York WS, Tak T, Geiger O, Kennedy EP, Reinhold VN, Lugtenberg GJ. A novel highly unsaturated fatty acid moiety of lipo-oligosaccharide signals determines host specificity of Rhizobium. Nature. 1991;354:125–129. doi: 10.1038/354125a0. [DOI] [PubMed] [Google Scholar]
- Spaink HP, Wijffelman CA, Okker RJH, Lugtenberg EJJ. Localization of functional regions of the Rhizobium nodD product using hybrid nodD genes. Plant Mol Biol. 1989;12:59–73. doi: 10.1007/BF00017448. [DOI] [PubMed] [Google Scholar]
- Stacey G, Luka S, Sanjuan J, Banfalvi Z, Nieuwkoop AJ, Chun JY, Forsberg LS, Carlson R. nodZ, a unique host-specific nodulation gene, is involved in the fucosylation of the lipooligosaccharide nodulation factor of Bradyrhizobium japonicum. J Bacteriol. 1994;176:620–633. doi: 10.1128/jb.176.3.620-633.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokkermans TJW, Peters NK. Bradyrhizobium elkanii lipo-oligosaccharide signals induce complete nodule structures on Glycine soja Siebold et Zucc. Planta. 1994;193:413–420. doi: 10.1007/BF00201821. [DOI] [PubMed] [Google Scholar]
- Stokkermans TJW, Ikeshita S, Cohn J, Carlson RW, Stacey G, Ogawa T, Peters NK. Structural requirements of synthetic and natural product lipo-chitin oligosaccharides for induction of nodule primordia on Glycine soja. Plant Physiol. 1995;108:1587–1595. doi: 10.1104/pp.108.4.1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabados L, Ratet P, Grunenberg B, deBruijn F. Functional analysis of the Sesbania rostrata leghemoglobin glb3 gene 5′-upstream region in transgenic Lotus corniculatus and Nicotiana tabacum plants. Plant Cell. 1990;2:973–973. doi: 10.1105/tpc.2.10.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truchet G, Debellé F, Vasse J, Terzaghi B, Garnerone A-M, Rosenberg C, Batut J, Maillet F, Dénarié J. Identification of a Rhizobium meliloti pSym2011 region controlling the host specificity of root hair curling and nodulation. J Bacteriol. 1985;164:1200–1210. doi: 10.1128/jb.164.3.1200-1210.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truchet G, Roche P, Lerouge P, Vasse J, Camut S, de Billy F, Promé JC, Dénarié J. Sulphated lipooligosaccharide signals from Rhizobium meliloti elicit root nodule organogenesis in alfalfa. Nature. 1991;351:670–673. [Google Scholar]
- van Eijsden RR, Díaz CL, de Pater BS, Kijne JW. Sugar-binding activity of pea (Pisum sativum) lectin is essential for heterologous infection of transgenic white clover hairy roots by Rhizobium leguminosarum biovar viciae. Plant Mol Biol. 1995;29:431–439. doi: 10.1007/BF00020975. [DOI] [PubMed] [Google Scholar]
- van Rhijn P, Goldberg RB, Hirsch AM. Lotus corniculatus nodulation specificity is changed by the presence of a soybean lectin gene. Plant Cell. 1998;10:1233–1250. doi: 10.1105/tpc.10.8.1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Workum WAT, Canter Cremers HCJ, Wijfjes AHM, van der Kolk C, Wijffelman CA, Kijne JW. Cloning and characterization of four genes of Rhizobium leguminosarum bv. trifolii involved in exopolysaccharide production and nodulation. Mol Plant Microbe Interact. 1997;10:290–301. doi: 10.1094/MPMI.1997.10.2.290. [DOI] [PubMed] [Google Scholar]
- Vincent JM. A Manual for the Practical Study of Root Nodule Bacteria: IBP Handbook No 15. Oxford: Blackwell Scientific Publications; 1970. [Google Scholar]
- Yang C, Signer ER, Hirsch AM. Nodules initiated by Rhizobium meliloti exopolysaccharide mutants lack a discrete, persistent nodule meristem. Plant Physiol. 1992;98:143–151. doi: 10.1104/pp.98.1.143. [DOI] [PMC free article] [PubMed] [Google Scholar]