Abstract
Inhibition of specific lignin biosynthetic steps by antisense strategy has previously been shown to alter lignin content and/or structure. In this work, homozygous tobacco (Nicotiana tabacum) lines transformed with cinnamoyl-coenzyme A reductase (CCR) or caffeic acid/5-hydroxy ferulic acid-O-methyltransferase I (COMT I) antisense sequences have been crossed and enzyme activities, lignin synthesis, and cell wall structure of the progeny have been analyzed. In single transformed parents, CCR inhibition did not affect COMT I expression, whereas marked increases in CCR activity were observed in COMT I antisense plants, suggesting potential cross talk between some genes of the pathway. In the progeny, both CCR and COMT I activities were shown to be markedly decreased due to the simultaneous repression of the two genes. In these double transformants, the lignin profiles were dependent on the relative extent of down-regulation of each individual enzyme. For the siblings issued from a strongly repressed antisense CCR parent, the lignin patterns mimicked the patterns obtained in single transformants with a reduced CCR activity. In contrast, the specific lignin profile of COMT I repression could not be detected in double transformed siblings. By transmission electron microscopy some cell wall loosening was detected in the antisense CCR parent but not in the antisense COMT I parent. In double transformants, immunolabeling of non-condensed guaiacyl-syringyl units was weaker and revealed changes in epitope distribution that specifically affected vessels. Our results more widely highlight the impact of culture conditions on phenotypes and gene expression of transformed plants.
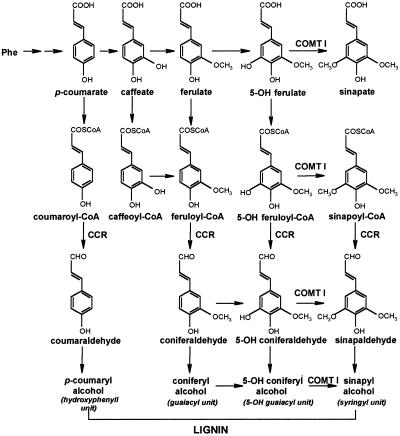
Lignin is a complex phenolic heteropolymer that provides strength and water hydrophobicity to the vessels and fibers of vascular plants. In angiosperms this polymer is mainly composed of three units: p-hydroxyphenyl, guaiacyl (G), and syringyl (S) units, derived from the phenylpropanoid metabolic grid (Fig. 1) and differing by their degree of methoxylation. Lignin composition changes during plant development and is affected by environmental cues (Boudet et al., 1995; Campbell and Sederoff, 1996; Whetten et al., 1998).
Figure 1.
Lignin biosynthetic pathway. CCR, Cinnamoyl-coenzyme A (CoA) reductase. Caffeic acid/5-OH ferulic acid O-methyltransferase (COMT I) catalyzes the second step of methylation as deduced by the analysis of transgenic tobacco inhibited for this enzyme (Atanassova et al., 1995), but in vitro studies indicate that COMT I may use 5-OH ferulic acid as well as its CoA, aldehyde, and alcohol derivatives (Humphreys et al., 1999; Maury et al., 1999; Osakabe et al., 1999).
From the economical point of view, lignin contributes to the calorific value of the wood but also limits the industrial utilization of the biomass because delignification during kraft pulping is an expensive and polluting process. Moreover, lignin has a negative impact on forage crop digestibility (Jung and Vogel, 1986). Therefore, much current effort is being directed to the reduction of lignin content or the modification of lignin composition by genetic engineering (Baucher et al., 1998; Chapple and Carpita, 1998; Grima-Pettenati and Goffner, 1999). The analysis of transgenic plants affected in distinct biosynthetic steps recently has revealed unexpected results that have led to a profound reappraisal of our view of the phenylpropanoid metabolic grid (Atanassova et al., 1995; Van Doorsselaere et al., 1995) and point to the occurrence of alternative pathways (Kajita et al., 1997; Sewalt et al., 1997; Zhong et al., 1998; Hu et al., 1999). In particular, a significant degree of plasticity was established for lignin biosynthesis because transgenic plants were shown to incorporate unusual components into their lignins (Boudet, 1998; Chapple and Carpita, 1998; Ralph et al., 1998; Whetten et al., 1998; Sederoff et al., 1999). For instance, plants down-regulated in cinnamyl alcohol dehydrogenase (CAD) activity were shown to incorporate coniferaldehyde, benzaldehyde, and sinapaldehyde into their lignins (Halpin et al., 1994; Ralph et al., 1998; Yahiaoui et al., 1998). Lignin of transgenic tobacco (Nicotiana tabacum) similarly inhibited CCR activity and contained unusual units such as tyramine ferulate (Ralph et al., 1998). In COMT I down-regulated plants, total lignin content was not affected but transgenic lignin was shown to include abnormally high amounts of 5-hydroxyguaiacyl (5-OH G) units, significantly higher amounts of G units, and a strongly decreased content in S units (Atanassova et al., 1995; Van Doorsselaere et al., 1995). Tobacco plants whose phenotype was visually undistinguishable from the controls displayed lower lignin degradability during kraft pulping (M. Petit-Conil, personal communication) but better cell wall digestibility (Bernard-Vailhé et al., 1996). As far as quantitative aspects are concerned, a dramatic decrease in the lignin content of transgenic tobacco down-regulated for CCR activity was demonstrated and was associated with improved pulping characteristics (J. Piquemal, J. Grima-Pettenati, M. Petit-Conil, and A.M. Boudet, unpublished data). In these plants, lignin thioacidolysis showed a dramatic decrease in the recovery of S and G monomers together with a relative increase in the corresponding S:G ratio. Taken together, these characteristics suggest that lignins in CCR down-regulated tobacco are enriched in condensed bonds, namely carbon-carbon and diphenyl ether interunit bonds, more particularly at the level of G units that can participate in condensed linkages at the C-5 position. Unusual amounts of cell wall-linked phenolics, such as ferulic and sinapic acids, were found that could contribute to the abnormally high cross-linking degree of the lignins (Piquemal et al., 1998; C. Lapierre, unpublished data).
Analysis of single transformants inhibited in COMT I or CCR expression has shown that the repression of each individual gene has differential effects on lignin synthesis and structure. In addition, despite a strong reduction in lignin content that could be useful for the pulp industry, CCR down-regulated plants cannot have a practical utilization in this area due to their reduced size and vigor. In relation to the potential plasticity of the lignin polymer and with the aim of identifying new lignin profiles better adapted to agro-industrial purposes, we have tested the effects of the concomitant inhibition of COMT I and CCR inhibition in double-transformed tobacco plants. Homozygous lines obtained from transformants highly repressed for each activity (Atanassova et al., 1995; Piquemal et al., 1998) were cross-pollinated and progeny that had integrated one copy of each gene was analyzed for enzyme activity and effects on lignin synthesis. Depending on growth conditions and developmental stage, some variability was observed in plant growth and lignin content and composition. In-depth lignin analysis on single transformants and on double transformed siblings by histological, immunocytological, and chemical methods revealed a predominant effect of the CCR transgene in most of the hybrid lines.
RESULTS
Phenotypes of Single and Double Transformants in Different Culture Conditions
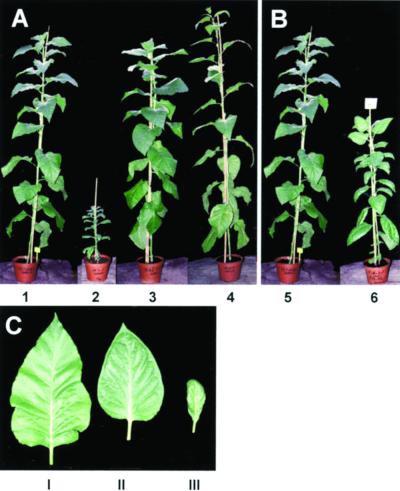
Two individual tobacco lines (B31 and B68) homozygous for the CCR transgene but exhibiting a different extent of CCR down-regulation have been crossed with a tobacco line homozygous for the COMT I transgene (B10). These two antisense CCR (ASCCR) parents have been selected because they represent two distinct situations. The B31 line is characterized by a severe alteration of CCR activity (6% residual activity relative to the control; Table I) accompanied by an important decrease in lignin content. In addition, this line displays a reduction of the size of the whole plant and of the different organs, with a spoon-like shape of the leaves (Piquemal et al., 1998; Fig. 2). These phenotypes were observed under culture room (with a 2-fold reduction of the size, not shown) as well as greenhouse growth (with a 4-fold reduction of the size, Fig. 2A, plant no. 2) conditions. In contrast to and despite a subtantial reduction of CCR activity (9% residual activity relative to the control; Table I), the B68 line has a normal development (Fig. 2A, plant no. 4). The differences observed between the two lines are likely related to the occurrence of a threshold level for residual CCR activity below which the effects on development and lignification can be observed. The antisense COMT (ASCOMT) B10 parent never exhibited any abnormal phenotype under the different growth conditions tested (Fig. 2A, plant no. 3).
Table I.
Lignin analysis of antisense parent lines and their progeny
| Culture Room Conditions
|
Greenhouse Conditions
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Line | CCRa | COMT Ia | LKb | S:Gc | CCRa | COMT Ia | LKb | β-O-4 bonds | S"Gc |
| % | % | ||||||||
| Control | 100 ± 7 | 100 ± 13 | 20.9 ± 0.1 | 0.91 ± 0.02 | 100 ± 23 | 100 ± 8 | 15.3 ± 0.3 | 44 ± 5 | 1.2 ± 0.1 |
| B68 | 30 ± 2 | 87 ± 4 | 20.7 ± 1.5 | 1.35 ± 0.13 | 9 ± 3 | 73 ± 6 | 9.7 ± 1.4 | 33 ± 1 | 2.4 ± 0.1 |
| B31 | 9 ± 3 | 137 ± 13 | 10.7 ± 0.2 | 3.16 ± 0.06 | 6 ± 1 | 83 ± 12 | 8.8 ± 0.9 | 8 ± 0.6 | 2.5 ± 0.1 |
| B10 | 150 ± 4 | 27 ± 8 | 21.2 ± 0.6 | 0.52 ± 0.01 | 230 ± 70 | 7 ± 5 | 13.8 ± 2.7 | 28 ± 3 | 0.2 ± 0.1 |
| B31 × B10 progeny | 12 ± 0.2 | 45 ± 4 | 10.7 ± 0.3 | 3.29 ± 0.17 | 10 ± 4 | 41 ± 2 | 7.6 ± 0.6 | 17 ± 2 | 2.3 ± 0.4 |
| B68 × B10 progeny | 71 ± 2 | 42 | 21.9 ± 1.0 | 0.90 ± 0.06 | nd | 34 | 14.8 ± 0.1 | 41 ± 2 | 1.1 ± 0.1 |
Eleven-week-old plants grown under culture room or greenhouse conditions were analyzed. Klason lignin (LK) values reflect the total lignin content. For plants grown in the culture room, a micromethod was used. The analysis of lignin monomers issuing from the thioacidolysis of extractive free samples allowed us to estimate the molar percentage of S and G units only involved in β-O-4 bonds and the corresponding S:G ratio according to Lapierre et al. (1995). Three to five plants of each line were analyzed and all samples were duplicated. nd, Not determined.
Enzyme activities are expressed relative to the mean value of the control population taken as 100%.
Klason lignin values are expressed as percentage of extractive-free walls.
Lignin monomeric composition is expressed as S:G ratio determined by thioacidolysis analysis.
Figure 2.
Phenotypic analysis of single and double transformants grown in the greenhouse. A, Eleven-week-old control and single transformants: 1, control plant; 2, B31 antisense (AS) CCR parent; 3, B10 AS COMT parent; and 4, B68 AS CCR parent. B, Eleven-week-old control and double transformants: control plant (5) and double transformant (6) issued from B31 × B10 cross showing an intermediate phenotype. C, Leaves of control plant (I), double transformant from B31 × B10 cross (II), and B31 AS CCR parent (III).
In the progeny from the different crosses, we found no particular phenotype for the double transgenics obtained from the B68 line, but a modified phenotype appeared at various stages of development when the parent was the B31 line. In culture room conditions, the progeny of the B31 parent exhibited a very slight reduction in size when compared with the controls and the same result was observed whatever the direction of the cross, i.e. with either the ASCCR plant or the ASCOMT I plant as a female (data not shown). Under greenhouse conditions, 11-week-old plants were much smaller when compared with untransformed tobacco (Fig. 2B, plant no. 6 to be compared with control no. 5) and the leaf shape was intermediate between the two parents (Fig. 2C, leaf type II). In field conditions, the observed effects were even more pronounced with a greater reduction of the size and leaf morphology similar to that of the B31 ASCCR parent (Fig. 2C, leaf phenotype III). Taken together, these observations show that growth conditions may strongly influence the phenotype of transgenic plants. In addition, we also frequently observed that the effects of the transformation were the most pronounced in the late stages of development.
Inhibition of COMT I and CCR Activities in Parents and Progeny
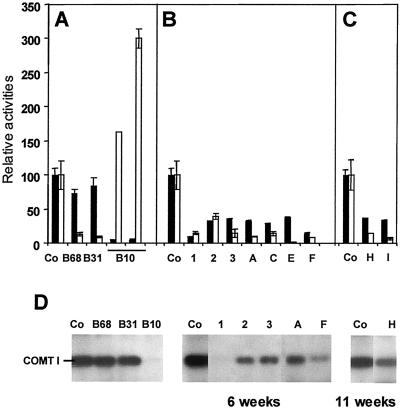
The occurrence of both COMT I and CCR AS transgenes in the hybrid plants was first confirmed by PCR (data not shown). COMT I and CCR activities were assayed on a series of individual 6- and 11-week-old plants. In AS CCR parents, CCR inhibition had no effect on COMT I activity, which was close to the control level (Fig. 3A, B68 and B31 plants). In contrast and surprisingly enough, COMT I inhibition in B10 plants induced a strong increase in CCR activity, ranging from 160% to 300% of the control level (Fig. 3A, B10 plants). In double-transformed progenies, residual enzyme activities ranged from 10% to 38% for COMT I and from 3% to 39% for CCR (Fig. 3, B and C). These values demonstrate that the inhibition of both enzyme activities was effective during the development and that the increase in CCR activity in the low COMT I activity background was no longer observed when the AS CCR transgene was present. Concerning COMT I, the inhibition level was not maintained in the progeny to the same extent as in the ASCOMT I parent: Only three plants (exemplified by plants 1 and F, Fig. 3B) out of 15 plants analyzed from the sexual crossing between ASCOMT I and ASCCR parents exhibited a high level of COMT I inhibition as observed for the B10 parent (i.e. 80% inhibition). In contrast, the CCR inhibition level of the progenies resembled that of their respective ASCCR parents. This result further confirmed that COMT I down-regulation did not interfere with CCR inhibition in the progeny as might have been anticipated from the data for ASCOMT parent. Immunoblotting assays also showed that COMT I levels in transgenic samples were lower than in controls (Fig. 3D). These data confirm that COMT I inhibition occurs in the double transgenics, but to a lower extent than in the B10 parent where the COMT I protein was undetectable (Fig. 3D).
Figure 3.
CCR and COMT I expression in single and double transformants. COMT I and CCR activities were measured on stems of single transformants (A), 6-week-old double transformants (B), and 11-week-old double transformants (C) grown in the greenhouse. Activities are expressed relative to the mean value of five (B) or three (A and C) untransformed controls. Black bars represent the relative COMT I activities and white bars show the relative CCR activities. For each individual transformant, the mean value of duplicates and error bars are presented. In several cases the error bar was too short to be drawn. D, Western-blot analysis of plants tested for enzyme activities in A, B, and C using anti-COMT I antibodies. Co, Control plants. B10, B68, and B31 are ASCOMT I and ASCCR parents, respectively. The progeny of B68 × B10 is indicated by numbers, and the progeny of B31 × B10 is indicated by letters.
Variation in the Lignification of Double Transformants Revealed by Histochemistry
The consequences of COMT I and CCR inhibition on the lignification of the double transformants grown in the greenhouse were first evaluated by Wiesner's and Maüle's histochemical methods. Previous studies based on thioacidolysis or NMR analyses of tobacco and poplar samples (Atanassova et al., 1995; Robert et al., 1999) revealed that COMT I inhibition induced a global decrease in the proportion of S units in lignin without significantly affecting the total lignin amount. In contrast, the lignin content was dramatically reduced by CCR down-regulation, which also caused pronounced structural alterations of the lignin (Piquemal et al., 1998; Ralph et al., 1998). Wiesner staining roughly reflects the total lignin content due to the reactivity of hydroxycinnamaldehyde groups always incorporated into native lignins (Adler, 1977; Nakano and Meshitsuka, 1992). Consistent with a lower lignin content and previous histological studies (Piquemal et al., 1998), Wiesner staining of the stem sections from the parents carrying the CCR transgene in AS orientation was pale red (Fig. 4A, c and d) compared with the bright-red staining of the untransformed control (Fig. 4A, a). In contrast and as previously described (Atanassova et al., 1995), equivalent staining intensity was observed for COMT I-repressed and control samples (Fig. 4A, sections b and a, respectively), which suggests similar lignin levels. Concerning the double transformants, the plants issued from the B31 × B10 cross displayed the typical pale red coloration (Fig. 4A, e) similar to that of the B31 parent with reduced lignin content. However, double transformants issued from the B68 × B10 cross behaved differently without any visible change in staining intensity (Fig. 4A, f).
Figure 4.
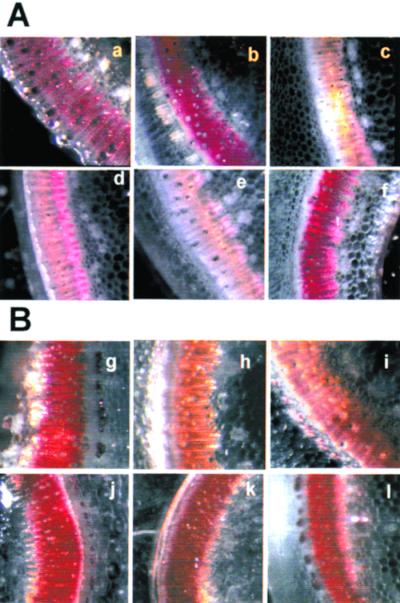
Histochemistry of stem transversal sections from controls and single or double transformants. Staining with Wiesner (A) or Mäule (B) reagent was carried out on sections of control plants (a and g), B10 (ASCOMT I, b and h), B31 (ASCCR, c and i), and B68 (ASCCR, d and j) single transformants, or double transformants issued from B31 × B10 cross (e and k) or B68 × B10 cross (f and l).
As expected from previous work (Atanassova et al., 1995), the ASCOMT parent (Fig. 4B, h) displayed typical yellow-brown staining that reflects a decrease in S-unit content due to COMT activity inhibition. The ASCCR parents showed two distinct phenotypes: The B31 line stained pale red to red (Fig. 4B, i) in contrast to the B68 parent (Fig. 4B, j), which was stained as red as the control (Fig. 4B, g). In the progeny of the two crosses (Fig. 4B, k and l), Maüle coloration similar to control (Fig. 4B, g) was obtained, indicating no important difference in S-unit content of the lignin.
Impact of Genetic Transformation on the Ultrastructure of Cell Walls
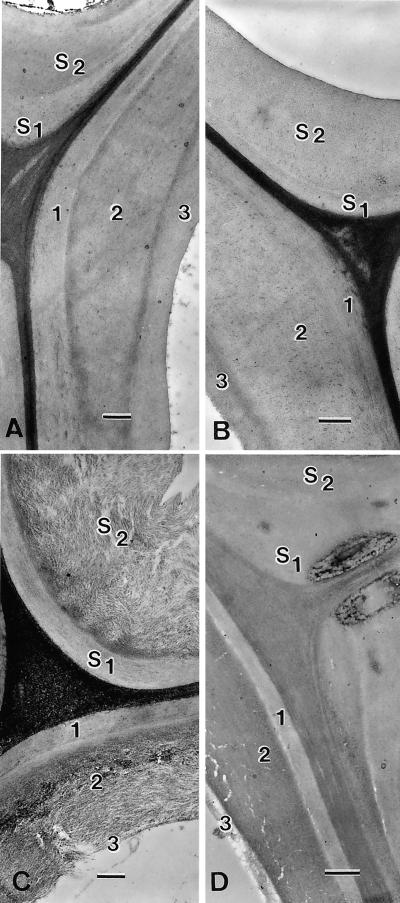
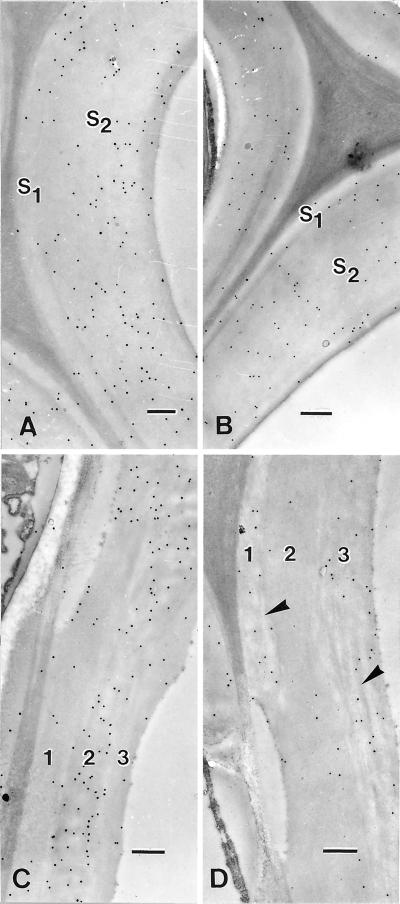
Investigation by transmission electron microscopy (TEM) revealed the micromorphology of B10 and B31 parent lines and their progeny (Fig. 5). The B31 line, whose CCR activity was highly inhibited, showed important alterations of the fiber cell wall (Fig. 5C), consisting of a loosening in the arrangement of cellulose microfibrils not observed in control (Fig. 5A). As shown in Figure 5C, the micromorphological alterations were clearly localized in the S2 sublayer, whereas the S1 layer did not exhibit detectable ultrastructural modification. In the B10 parent, the cell walls of fibers and vessels showed good cohesion with no visible sign of loosening, indicating that the repression of COMT I did not alter the interactions between wall polymers (Fig. 5B). In the B31 × B10 progeny, despite the significant loss measured in total lignin content (see Table II), no clear indication of loosening of vessel or fiber cell walls was observed generally (Fig. 5D). In fact, only a few instances of loosening have been observed in the progeny (see also Fig. 6), which may be due to a slight reduction of CCR inhibition compared with that of the B31 parent (Fig. 3 and Table I).
Figure 5.
Ultrastructural morphology of fibers and vessels observed in TEM after transverse section of the stem. A through C, periodic acid-thiocarbohydrazide-Ag proteinate staining. D, Uranyl acetate staining. A, Wild-type plant: the fiber secondary wall and its sublayers S1 and S2 appear very compact. Note the typical subdivision of the vessel wall in the three sub-layers noted 1, 2, and 3. B, B10 (ASCOMT I) single transformant. No visible ultrastructural alteration. C, B31 (ASCCR) single transformant exhibits a pronounced loosening of its cellulosic framework in S2 of the fiber walls. Sub-layers 2 and 3 of the vessel wall also are affected. D, Double transformant from B31 × B10 cross: Only a slight alteration of ultrastucture is detected in sub-layer 3 of vessel; no particular loosening in sub-layer 2 of the vessel wall is visible (the white cracks in internal S2 are artifacts due to embedding). Bars in A and C represent 0. 5 μm; in B and D, they represent 0.7 μm.
Table II.
Analysis of individual plants issued from the B31 × B10 cross at two stages of development
| Age | Plant | CCR Relative Activity | COMT I Relative Activity | LK | Units Only Involved in β-O-4 Bonds
|
|
|---|---|---|---|---|---|---|
| % | S:G | |||||
| % | ||||||
| 6 Weeks | Controls | 100 ± 21 | 100 ± 8 | 11.8 ± 1.0 | 38.5 ± 3 | 0.9 ± 0.1 |
| A | 10 | 32 | 10.7 | 7.2 | 1.7 | |
| C | 14 | 28 | nd | nd | 1.2 | |
| E | 2 | 38 | 11.0 | 35.2 | 0.8 | |
| F | 7 | 14 | 10.6 | 34.0 | 0.9 | |
| G | nd | 21 | 8.2 | 22.9 | 0.8 | |
| 11 Weeks | Controls | 100 ± 23 | 100 ± 8 | 15.3 ± 0.8 | 44.0 ± 5 | 1.2 ± 5 |
| H | 14 | 36 | 8.4 | 18.2 | 2.3 | |
| I | 7 | 34 | 7.6 | 20.2 | 2.3 | |
| J | nd | nd | 7.0 | 19.2 | 2.1 | |
| K | nd | 32 | 8.3 | 15.2 | 2.7 | |
Analyses were conducted on untransformed controls or double transformants that were 6 or 11 weeks old. COMT I and CCR activities are expressed relative to the controls. Klason lignin (LK) and thioacidolysis evaluation of lignin units involved only in β-O-4 bonds were determined as described in Table I. Nd, Not determined. Values of controls were calculated from the data of four or three plants for 6- and 11-week-old tobaccos, respectively. All measurements were duplicated.
Figure 6.
Immunocytochemical labeling in TEM of stem transverse sections from control and double transformant. Labeling with antibodies directed against non-condensed GS substructures. A and B, Fiber cell walls. C and D, Vessel cell walls. A, Fiber of control plant: mixed non-condensed GS lignin subunits are more concentrated in S2. B, B31 × B10 progeny, the cell wall appears thinner and the labeling reduced in S2. C, Vessel of control plant. Non-condensed lignin epitopes are unevenly distributed in the sub-layers 1, 2, and 3, with the highest concentration in sub-layer 2. D, B31 × B10 progeny. Non-condensed epitopes appear concentrated in sub-layers 1 and 3 of the vessel wall, which also shows signs of loosening (arrowheads). Bar in A represents 0.6 μm, and in B through D, the bar represents 0.7 μm.
Immunocytochemical Topochemistry of Lignin Epitope Distribution
The predominant lignin epitopes, consisting of G and mixed G-S rings involved in condensed or non-condensed interunit linkages, were localized in situ by using antibodies raised against these structures (Ruel et al., 1994; Joseleau and Ruel, 1997). Figure 6 shows the results of labeling of B31 × B10 progeny in which the mixed S-G substructures of the non-condensed type were selectively identified. In general, the labeling was weaker in double transformants issued from the B31 × B10 cross (Fig. 6, B and D) as compared with the untransformed control (Fig. 6, A and C), indicating a diminution of the frequency of non-condensed bonds. The observed diminution of GS non-condensed lignin subunits in fiber and vessel walls of the double transformant is in accordance with the results of chemical analysis that showed a decrease in total lignin content, a slight reduction in the relative amount of S rings, but much lower yields of thioacidolysis as indicated by the decreased content in β-O-4 bonds (see Table II). Because thioacidolysis and immunological labeling account for the non-condensed interunit linkages, the results given by the two methods can be directly compared. Although the overall labeling was lower, the distribution of non-condensed GS epitopes was almost unaffected in fibers of the transformant (Fig. 6B) compared with the control (Fig. 6A). In contrast, the results showed that a modification in the labeling distribution occurred in vessels. Non-condensed GS subunits that were mostly concentrated in the medial sublayer 2 of the control vessel wall (Fig. 6C) appeared principally localized in sublayers 1 and 3 in the double transformant (Fig. 6D), also in low amounts. These observations demonstrate that the genetic transformation has differentially affected the lignification process in different cell types.
Chemical Analysis of Lignin in the Various Tobacco Genotypes
Changes in lignin content and structure detected by histochemical and immunocytochemical methods were more comprehensively studied by chemical analysis of the walls. Total lignin was estimated by the gravimetric method of Klason (Monties, 1989) and lignin structure was evaluated using the thioacidolysis degradative method. In the latter method, the gas chromatography determination of the G, S, and 5-OH G monomers recovered from the thioacidolysis of in situ lignins provides information about the percentage and ring type (G, S, or 5-OH G) of lignin units involved only in β-O-4 bonds (Lapierre et al., 1995). Low thioacidolysis yields conversely are indicative of a high content in condensed carbon-carbon and diphenyl ether bonds (Lapierre et al., 1995). To more accurately evaluate the effects of the transformation, we studied lignin content and structure of transgenic plants at different developmental stages and under various growth conditions, together with the CCR and COMT I residual activity (Table I).
Based on the data gathered in Table I, the B68 parent line appears extremely responsive to growth conditions. Its lignin content equaled that of controls in the culture chamber but was markedly decreased in the greenhouse compared with the corresponding control plants. This discrepancy may be related to different CCR activity levels in the culture chamber (30% of the control value) and in the greenhouse (9% of the control value). In contrast, for the B31 parent a similarly strong CCR inhibition was measured whatever the growth conditions, leading to a marked decrease in lignin content. For all plants grown in the greenhouse and relative to the control, the percentages of lignin units involved only in β-O-4 bonds (estimated from the recovery of thioacidolysis monomers when expressed on the basis of lignin content; Lapierre et al., 1995) were substantially lower in B68, B31, and B10 lines (Table I and data not shown). This trait is particularly pronounced in B31 lignin, which comprises only 8% of the lignin units involved in β-O-4 bonds and thus, conversely, 92% of lignin units involved in condensed bonds. The higher thioacidolysis S:G ratio in B68 and B31 lignins may suggest that the condensed bonds more particularly involve G units and/or that the overall content in G units is decreased. However, the relative importance of G signals in NMR (Ralph et al., 1998) and infrared (C. Lapierre, unpublished data) spectra of isolated lignin fractions from control and B31 samples does not argue for a pronounced decrease in G units.
In agreement with previous results (Atanassova et al., 1995) and histochemical observations (Fig. 4), the ASCOMT B10 parent did not display any change in lignin content. In contrast, the lignin structure was markedly altered as revealed by the aforementioned higher content in condensed bonds, by the severe decrease in thioacidolysis S:G ratio (a diagnostic for a shortage in the precursors of S units; Table I), and by the appearance of 5-OH G units in substantial amounts (about 5% of β-O-4 linked lignin units, data not shown). The S:G decrease was maximum under greenhouse conditions. It is noteworthy that the strong increase in CCR activity detected in COMT I down-regulated plants grown in the greenhouse (Fig. 3) also occurred in the culture chamber (Table I).
In both the B31 × B10 progeny and the B31 parent, CCR inhibition clearly affected lignin biosynthesis because a 2-fold reduction in lignin content was measured whatever the growth conditions (Table I). This finding is in accordance with the histological and immunocytochemical observations (Figs. 4, 5, and 6). The thioacidolysis profile of B31 × B10 progeny was similar to that of the B31 parent, as shown by the substantial enrichment in condensed bonds (involving 82% of lignin units from the data of Table I) and the concomitant increase in thioacidolysis S:G ratio. In contrast, the most specific trait of COMT I inhibition detected in the B10 parent, namely 5-OH G units in substantial amounts, could not be evidenced and the 5-OH G thioacidolysis monomers were only detected as trace components as in the control samples (data not shown).
In B68 × B10 double transformants, no significant change in lignin content or structure could be observed whatever the growth conditions (Table I). The percentage of lignin units involved only in β-O-4 bonds and the thioacidolysis S:G ratio were restored to the control levels (Table I).
Because some variability in enzyme inhibition was detected in double transformed progeny (Fig. 3, B and C), individual enzymatic and chemical analyses of B31 × B10 hybrids grown in the greenhouse were performed at 6- and 11-week developmental stages (Table II). Consistent with our current knowledge on lignification (Terashima et al., 1993), the Klason lignin content in the control line increased together with the proportion of β-O-4 bonds and S units in lignin as the plants aged (Table II). In most 6-week-old double transformants, inhibition of both CCR and COMT I activities did not substantially alter lignin content and structure relative to the control (except for plant A and to a lesser extent plant G; Table II). In contrast and relative to the corresponding controls, 11-week-old double transformants displayed a substantial alteration in lignification, similar to that measured in the B31 line (2-fold decrease in lignin content and β-O-4 content and 2-fold increase in thioacidolysis S:G ratio; Table II). These changes in lignin quantity and quality were most likely responsible for the typical phenotypes illustrated in Figures 2 and 4.
DISCUSSION
Outcomes of Simultaneous Repression of COMT I and CCR Activity in Double Transformants
When tobacco plants homozygous for COMT I or CCR transgene were cross fertilized, the progenies that harbored each transgene in the hemizygous state displayed a substantial inhibition of both CCR and COMT I activities (Fig. 3). Two down-regulated CCR parent lines (B31 and B68) differing in their CCR inhibition level were used to more easily identify potential additive, synergic, or antagonist effects between COMT I and CCR AS transgenes. Although both enzyme activities were systematically reduced in the hybrid plants, the B68 × B10 cross produced hybrids phenotypically undistinguishable from the wild type and no alteration of lignin content and structure was detected (Table I). In contrast, the B31 × B10 cross produced plants strongly modified when compared with the control plants; an intermediate reduction of plant size was observed for the hybrids and the modifications of lignin pattern were reminiscent of the B31 parent line. However, no signature of COMT I down-regulation could be detected, probably due to insufficient down-regulation of COMT activity, although an effect of lower CCR levels cannot be excluded. Previous studies (Piquemal et al., 1998) have shown that the hemizygous CCR down-regulated line obtained by outcrossing B31 tobacco displayed a lower reduction in lignin content than the B31 homozygous line, pointing to a potential gene dosage effect. These observations have been made under both culture chamber and greenhouse conditions. In the hybrid plants hemizygous for both transgenes and grown under the same greenhouse conditions, this effect was no longer observed, suggesting that simultaneous interference at two different points of the lignin biosynthetic pathway can more efficiently reduce the lignin level. However, the lignin thioacidolysis profiles revealed a large predominance of CCR transgene effects in the hybrids. This predominance could reflect different down-regulation levels, with a COMT I activity too moderately inhibited to have any detectable impact. It could also be related to the relative position of COMT I and CCR in the lignin pathway (Fig. 1). Because COMT I has 5-OH coniferaldehyde and 5-OH coniferylalcohol as main substrates (Humphreys et al., 1999; Maury et al., 1999; Osakabe et al., 1999), the upstream CCR inhibition may prevent any COMT deficiency-related accumulation of 5-OH G derivatives. Finally, the phenotypes of the plants grown in greenhouse conditions do not lend themselves in a straightforward manner to diagnosis of reduction in lignin content because some plants displayed a similarly reduced lignin content but distinct morphology (e.g. plants nos. 2 and 6 in Fig. 2). However, the hybrid plants were always taller than the AS CCR homozygous parent.
Manifestation of COMT I and CCR Repression on Cell Wall Ultrastructural Organization
The study of the impact of modifications in lignin synthesis on cell wall organization at the ultrastructural level necessitates TEM. At this scale of observation, and with the use of immunoprobes for the identification of lignin substructures, it became possible to visualize both the micromorphological alterations and the variations in lignin topochemical distribution resulting from the genetic transformation. The specific traits observed in the ASCCR parent line, particularly the wall loosening that characterized CCR inhibition, were detected in the progeny but to a lesser extent (Fig. 6D). It is noteworthy that signs of loosening were not always detected in the progeny (Fig. 5D), indicating that cell wall alterations were less pronounced (and perhaps less frequent) than in the ASCCR parent. This is in agreement with the lower levels of CCR inhibition measured in the progeny compared to those of parent lines (Fig. 3). Immunological topochemistry suggested that not only the amount but also the type of lignin deposited in the wall is important for cohesion between cellulose microfibrils. From chemical analysis data (Table II, G plant), it can be calculated that the net amount of non-condensed units (β-O-4 bonds) in the cell walls was reduced by a factor of two in comparison with the controls. This explains the significant diminution of labeling by the antibody directed against non-condensed GS epitopes despite an almost unchanged S:G ratio (Table II). Immunolocalization of lignin substructures also demonstrated the differential impact of the genetic transformation on the lignification process of fibers and vessels of the double transformants (Fig. 6). It is interesting to note that an Arabidopsis gene specifically implicated in fiber differentiation has been characterized recently (Zhong and Ye, 1999), in accordance with the regulation of lignification at the cellular level. The study of a variety of AS plants affected in different lignin biosynthesis steps should quickly improve our understanding of the mechanisms of wood and fiber cell wall edification.
Impact of the Culture Conditions and Developmental Stages on the Characteristics of AS Plants
In the present study we examined the effects of AS transformation by CCR and COMT I genes in different tobacco genotypes, namely homozygous mono-transformants and their double-transformed progenies obtained by cross fertilization. In addition, this investigation was carried out at different developmental stages and under various growth conditions. It is interesting enough that the phenotypic and biochemical alterations appeared to be more pronounced at the latest developmental stages and for the plants cultured in the less favorable environment, i.e. under greenhouse and field conditions in comparison with culture chamber conditions. Phenotypic variations with growth conditions have been previously reported for transgenic plants (Brandle et al., 1995), but such investigations are infrequent and have never been applied to lignin-engineered plants. The fact that the impact of the transformation is maximum under field conditions should be considered in relation to the future exploitation of transgenic plants and particularly with respect to forest biotechnology.
Metabolic Regulation May Interfere with Expected Transgene Effects
When parent lines (ASCCR or ASCOMT I) were examined for COMT I and CCR expression, CCR inhibition was shown to be highly effective and to have no detectable effect upon COMT I expression. In contrast, the repression of COMT I activity triggered a strong increase in CCR activity, which may be due to the accumulation of metabolic intermediates. In-depth analysis of the phenolic profiles of COMT I-repressed tobacco lines should shed some light on the mechanisms underlying such metabolic regulation. When CCR transgene was present in double transgenic lines depressed in COMT I activity, the increase in CCR activity was no longer observed and CCR inhibition levels similar to those of ASCCR parents were measured. Thus, in the double-transformed plants, the CCR AS transgene not only repressed the basal expression of endogenous CCR gene but also prevented CCR induction in a low COMT I activity context.
In parallel studies, we observed that the down-regulation of COMT I, but not that of CCR, has a negative effect on the expression of caffeoyl-CoAOMT (Martz et al., 1998); data not shown). These results further emphasize that potential cross regulation may occur between genes as a consequence of altered metabolic profiles and accumulation of intermediates in the transgenic plants. Future experiments using DNA arrays will be useful to probe the extent of cross talk between the genes involved in phenylpropanoid metabolism.
MATERIALS AND METHODS
Plant Material and Culture Conditions
Tobacco (Nicotiana tabacum) plants used for the crosses carried, in AS orientation, COMT I or CCR sequences as described (Atanassova et al., 1995; Piquemal et al., 1998). Double transformants were obtained by fertilizing AS CCR lines (B31 and B68) with the pollen of ASCOMT I line (B10). Five parents of each genotype and 15 double transformants from each cross were directly grown in soil in the greenhouse under 3,000 lux lighting and a light/dark period of 16/8 h. Under culture room conditions, 12 to 15 progenies were grown along with control and parent genotypes under 14,000 lux lighting with a light/dark period of 14/10 h.
Enzyme Assays
CCR and COMT I assays were performed on tobacco stem extracts. Greenhouse-grown tobacco plants were individually analyzed and 1 cm of the fifth internode tissues (from the plant bottom) was extracted and assayed. For plants grown under culture room conditions, activity measurements were carried out on 5 cm of the stem bottom of three pooled plants. COMT I assays were conducted as described (Atanassova et al., 1995). CCR activities measurements were performed using a radiochemical test: 7 − 15 × 103 dpm of 14C-radiolabeled feruloyl-CoA was added to the reaction mixture (final volume of 350 μL). The mixture was incubated at 30°C for 10 min and the reaction was stopped by the addition of 10 μL of 200 mm coniferaldehyde. One milliliter of toluene:ethyl acetate (1:1, v/v) mixture was added immediately, vortexed, and centrifuged for 30 s at maximum speed. A 500-μL aliquot of organic phase that contained reaction product was counted by scintillation after addition of 10 mL of Ready Safe solution (Beckman Coulter, Fullerton, CA). Protein quantities were determined according to Bradford (1976).
Histology
Stem sections were hand cut with a razor blade from the second internode of 6- and 11-week-old tobacco plants grown in the greenhouse. Transversal sections were stained with Wiesner reagents according to Atanassova et al. (1995). Photographs were taken at 50-fold magnification with a binocular microscope (Stemi SV, Carl Zeiss, Jena, Germany).
Chemical Analysis of Lignin
Thioacidolysis and Klason lignin analysis were performed as previously described (Lapierre et al., 1995). For plants grown in the culture room, a micromethod for Klason lignin analysis was used (Piquemal et al., 1998).
Electron Microscopy
The plant material was the same as for histology .One-millimeter-thick stem sections were cut just after harvesting and immediately fixed for 4 h in a freshly prepared mixture of 0.3% (w/v) glutaraldehyde and 2% (w/v) paraformaldehyde in 50 mm phosphate buffer (pH 7.2). There were usually 20 to 30 ultrathin sections per grid. Several tens of photographs were taken on each grid. Immunocytochemical labeling and observations were conducted as described by Joseleau and Ruel (1997).Each immunolabeling experiment was done in triplicate.
ACKNOWLEDGMENTS
We are grateful to Frédéric Legée for the Klason lignin determinations. We thank Dr. Kenneth Richards (Institut de Biologie Moléculaire des Plantes) for critical reading.
Footnotes
This work was supported by the Commission of European Communities (project nos. Optimization of Lignin in Crop and Industrial Plants through Genetic Engineering AGRE0021 and Tree Improvement Based on Lignin Engineering CT95–0424) and by the Ministère de l'Education Nationale, de la Recherche, et de la Technologie (grant no. ACC–SV14).
LITERATURE CITED
- Adler A. Lignin chemistry: past, present and future. Wood Sci Technol. 1977;11:169–218. [Google Scholar]
- Atanassova R, Favet N, Martz F, Chabbert B, Tollier MT, Monties B, Fritig B, Legrand M. Altered lignin composition in transgenic tobacco expressing O-methyltransferase sequences in sense and antisense orientation. Plant J. 1995;8:465–477. [Google Scholar]
- Baucher M, Monties B, Van Montagu M, Boerjan W. Biosynthesis and genetic engineering of lignin. Crit Rev Plant Sci. 1998;17:125–197. [Google Scholar]
- Bernard-Vailhé MA, Migné C, Cornu A, Maillot MP, Grenet E, Besled JM, Atanassova R, Martz F, Legrand M. Effect of modification of the O-methyltransferase activity on the cell wall composition, ultrastructure and degradability of transgenic tobacco. J Sci Food Agric. 1996;72:385–391. [Google Scholar]
- Boudet A-M. A new view of lignification. Trends Plant Sci. 1998;3:67–71. [Google Scholar]
- Boudet AM, Lapierre C, Grima-Pettenati J. Biochemistry and molecular biology of lignification. New Phytol. 1995;129:203–236. doi: 10.1111/j.1469-8137.1995.tb04292.x. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brandle JE, McHugh L, James H, Miki BL. Instability of transgene expression in field grown tobacco carrying the csr1-1 gene for sulfonylurea herbicide resistance. Bio-Technology. 1995;13:994–998. [Google Scholar]
- Campbell MM, Sederoff RR. Variation in lignin content and composition: mechanisms of control and implications for the genetic improvement of plants. Plant Physiol. 1996;110:3–13. doi: 10.1104/pp.110.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapple C, Carpita N. Plant cell walls as targets for biotechnology. Curr Opin Plant Biol. 1998;1:179–185. doi: 10.1016/s1369-5266(98)80022-8. [DOI] [PubMed] [Google Scholar]
- Grima-Pettenati J, Goffner D. Lignin genetic engineering revisited. Plant Sci. 1999;145:51–65. [Google Scholar]
- Halpin C, Knight ME, Foxon GA, Campbell MM, Boudet AM, Boon JJ, Chabbert B, Tollier M-T, Schuch W. Manipulation of lignin quality by down-regulation of cinnamyl alcohol dehydrogenase. Plant J. 1994;6:339–350. [Google Scholar]
- Hu WJ, Hardings SA, Lung J, Popko JL, Ralph J, Stokke DD, Tsai CJ, Chiang VL. Repression of lignin biosynthesis promotes cellulose accumulation and growth in transgenic trees. Nat Biotechnol. 1999;17:808–812. doi: 10.1038/11758. [DOI] [PubMed] [Google Scholar]
- Humphreys JM, Hemm MR, Chapple C. New routes for lignin biosynthesis defined by biochemical characterization of recombinant ferulate 5-hydroxylase, a multifunctional cytochrome P450-dependent monooxygenase. Proc Natl Acad Sci USA. 1999;96:10045–10050. doi: 10.1073/pnas.96.18.10045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseleau J-P, Ruel K. Study of lignification by noninvasive techniques in growing maize internodes. Plant Physiol. 1997;114:1123–1133. doi: 10.1104/pp.114.3.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung HG, Vogel KP. Influence of lignin on digestibility of forage cell wall material. J Anim Sci. 1986;62:1703–1712. doi: 10.2527/jas1986.6261703x. [DOI] [PubMed] [Google Scholar]
- Kajita S, Hishiyama S, Tomimura Y, Katayama Y, Omuri S. Structural characterization of modified lignin in transgenic tobacco plants in which the activity of 4-coumarate:coenzyme A ligase is depressed. Plant Physiol. 1997;114:871–879. doi: 10.1104/pp.114.3.871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapierre C, Pollet B, Rolando C. New insights into the molecular architecture of hardwood lignins by chemical degradative methods. Res Chem Intermed. 1995;21:397–412. [Google Scholar]
- Martz F, Maury S, Pinçon G, Legrand M. cDNA cloning, substrate specificity and expression study of tobacco caffeoyl-CoA 3-O-methyltransferase, a lignin biosynthetic enzyme. Plant Mol Biol. 1998;36:427–437. doi: 10.1023/a:1005969825070. [DOI] [PubMed] [Google Scholar]
- Maury S, Geoffroy P, Legrand M. Tobacco O-methyltransferases involved in phenylpropanoid metabolism: the different CCoAOMT and COMT classes have distinct substrate specificities and expression patterns. Plant Physiol. 1999;121:215–224. doi: 10.1104/pp.121.1.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monties B. Lignins. In: Dey PM, Harborne JB, editors. Methods in Plant Biochemistry. London: Academic Press; 1989. pp. 113–157. [Google Scholar]
- Nakano J, Meshitsuka G. The detection of lignin. In: Dence C, Lin S, editors. Methods in Lignin Chemistry. Berlin: Springer-Verlag; 1992. pp. 23–32. [Google Scholar]
- Osakabe K, Tsao CC, Li L, Popko JL, Umezawa T, Carraway DT, Smeltzer RH, Joshi CP, Chiang VL. Coniferyl aldehyde 5-hydroxylation and methylation direct syringyl lignin biosynthesis in angiosperms. Proc Natl Acad Sci USA. 1999;96:8955–8960. doi: 10.1073/pnas.96.16.8955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piquemal J, Lapierre C, Myton K, O'Connell A, Schuch W, Grima-Pettenati J, Boudet AM. Down-regulation of cinnamoyl-CoA reductase induces significant changes of lignin profiles in transgenic tobacco plants. Plant J. 1998;13:71–83. [Google Scholar]
- Ralph J, Hatfield RD, Piquemal J, Yahiaoui N, Pean M, Lapierre C, Boudet AM. NMR characterization of altered lignins extracted from tobacco plants down-regulated for lignification enzymes cinnamyl-alcohol dehydrogenase and cinnamoyl-CoA reductase. Proc Natl Acad Sci USA. 1998;95:12803–12808. doi: 10.1073/pnas.95.22.12803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert D, Piersantelli D, Jouanin L, Ferret V, Pollet B, Lapierre C. 13C NMR spectroscopy of lignins from transgenic poplars deficient in O-methyltransferase and cinnamyl alcohol dehydrogenase. Tenth International Symposium on Wood Pulping Chemistry. Yokohama, Japan: Tappi; 1999. pp. 18–21. [Google Scholar]
- Ruel K, Faix O, Joseleau JP. New immunogold probes for studying the distribution of the different lignin types during plant cell wall biogenesis. J Trace Microprobe Technol. 1994;12:267–276. [Google Scholar]
- Sederoff RR, MacKay JJ, Ralph J, Hatfield RD. Unexpected variation in lignin. Curr Opin Plant Biol. 1999;2:145–152. doi: 10.1016/S1369-5266(99)80029-6. [DOI] [PubMed] [Google Scholar]
- Sewalt VJH, Ni W, Blount JW, Jung HG, Masoud SA, Howles PA, Lamb C, Dixon RA. Reduced lignin content and altered lignin composition in transgenic tobacco down-regulated in expression of L-phenylalanine ammonia-lyase or cinnamate 4-hydroxylase. Plant Physiol. 1997;115:41–50. doi: 10.1104/pp.115.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terashima N, Fukushima K, He LF, Takabe K. Comprehensive model of the lignified plant cell wall. In: Jung HG, Buxton DR, Hatfield RD, Ralph J, editors. Forage Cell Wall Structure and Digestibility. Madison, WI: American Society of Agronomy; 1993. pp. 133–166. [Google Scholar]
- Van Doorsselaere J, Baucher M, Chognot E, Chabbert B, Tollier MT, Petit-Conil M, Leplé JC, Pilate G, Cornu D, Monties B. A novel lignin in poplar trees with a reduced caffeic acid/5-hydroxyferulic acid O-methyltransferase activity. Plant J. 1995;8:855–864. [Google Scholar]
- Whetten RW, MacKay JJ, Sederoff RR. Recent advances in understanding lignin biosynthesis. Annu Rev Plant Physiol Plant Mol Biol. 1998;49:585–609. doi: 10.1146/annurev.arplant.49.1.585. [DOI] [PubMed] [Google Scholar]
- Yahiaoui N, Marque C, Myton KE, Negrel J, Boudet AM. Impact of different level of cinnamyl alcohol dehydrogenase down-regulation on lignins of transgenic tobacco plants. Planta. 1998;204:8–15. [Google Scholar]
- Zhong R, Morrison WH, III, Negrel J, Ye Z-H. Dual methylation pathways in lignin biosynthesis. Plant Cell. 1998;10:2033–2045. doi: 10.1105/tpc.10.12.2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong R, Ye ZH. IFL1, a gene regulating interfascicular fiber differentiation in Arabidopsis, encodes a homeodomain-leucine zipper protein. Plant Cell. 1999;11:2139–2152. doi: 10.1105/tpc.11.11.2139. [DOI] [PMC free article] [PubMed] [Google Scholar]