Abstract
Purpose:
To correlate microvascular changes and assess the relationship between microvascular changes and cardiovascular disease (CVD) risk in patients with retinal vein occlusion (RVO).
Methods:
Patients over 40 years of age with unilateral RVO were included in this prospective study. Those known to have cardiovascular disease were excluded. A detailed medical history was taken and physical exam was done to measure the height, weight, body mass index (BMI), and systolic blood pressure (SBP). A comprehensive eye check-up was followed by optical coherence tomography angiography (OCTA). Microvascular indices such as vessel density (VD) and perfusion density (PD) were noted. A statistical model was developed for prediction of CVD risk and was integrated with the World Health Organization (WHO)’s risk prediction charts.
Results:
This study included 42 patients with RVO and 22 controls with an age range of 42–82 years. There were 40 males (62.5%) and 24 females (37.5%). Along with age, SBP, and gender, perfusion density was found to have significant impact on CVD risk (P = 0.030). Reduction in PD was associated with increase in CVD risk. PD had a greater influence on CVD in <50 years age than in >70 years group. Using linear regression, a model with accuracy of 72.1% was developed for CVD risk prediction and was converted into color coded charts similar to WHO risk prediction charts.
Conclusion:
These findings suggest a significant correlation between microvascular parameters and CVD risk in RVO patients. Based on these parameters, an easy-to-use and color-coded risk prediction chart was developed.
Keywords: Cardiovascular risk, microvascular changes in RVO, perfusion density, retinal vein occlusion, WHO risk prediction charts
Retinal vein occlusion (RVO) is a common cause of vision loss in the middle aged and elderly population.[1] Its incidence is seen to vary from 0.8% to 3.64% in various studies.[2–4] It can either present as central retinal vein occlusion (CRVO) or branch retinal vein occlusion (BRVO). The patient experiences sudden decrease in vision due to occlusion of the retinal vein that results in retinal hemorrhages, venous dilation and tortuosity, capillary loss, and macular edema.[5] If left untreated, it can eventually progress to neovascularization, vitreous hemorrhage, tractional retinal detachment, and neovascular glaucoma.[6,7] All of these can cause severe vision loss or irreversible blindness.[6]
RVO is associated with various local as well as systemic risk factors such as glaucoma,[8] short axial length, diabetes mellitus,[9–11] hypertension,[10–12] hyperhomocystinemia,[13] and dyslipidemia[14]. Several studies have shown an association with CVD with increased mortality as compared to normal population.[13,15–21] How well is the systemic risk being assessed and managed by the ophthalmic fraternity? Since the ophthalmologist might be the first point of contact for patients with RVO, the responsibility to assess this risk and manage it lies with them. This study attempts to provide a simple risk calculation scale. This can possibly help the ophthalmologist assess the cardiovascular risk, prioritize the referral to a cardiologist, and educate the patient about lifestyle changes and other preventive measures, if necessary.
Methods
This prospective study was conducted at a tertiary care ophthalmic center in south India. The research was approved by the Institutional Ethics Committee and the National Board of Education, New Delhi. Patients above 40 years of age reporting to the clinic with unilateral retinal vein occlusion (RVO) and with no history of heart disease were included in the study. Patients with previous history of any ocular surgery or other pathology and significant media haze where fundus could not be visualized were excluded. Age- and sex-matched controls with no history of ocular pathologies were included in the study. Only one eye from controls was included and it was chosen randomly. In the case of patients, both the fellow and affected eye data were captured. But only the affected eye data were used in the analysis. Systemic parameters like age, gender, history of smoking, past medical history including history of diabetes, hypertension, and ischemic heart disease were noted. Body weight and height were documented for all the participants. The body mass index (BMI) of each participant was calculated from the values for weight and height. Blood pressure measurement was done using the mercury sphygmomanometer. All present smokers and those who quit smoking less than one year before the assessment were considered as smokers. Subjects with systolic blood pressure (SBP) ≥140 mmHg or those already on treatment for high SBP were considered as hypertensive. SBP was categorized into four groups, that is, 120–139 mmHg, 140–159 mmHg, 160–179 mmHg, and ≥180 mmHg.
All subjects underwent optical coherence tomography angiography (OCTA) after pupillary dilation with 1% tropicamide instilled three times at an interval of 10 minutes. The Cirrus HD-OCT AngioPlex 5000 machine (Carl Zeiss Meditech, Inc, Dublin, CA, USA; Ver 11.0.0.29946) was used in this study. Parameters like vessel density (VD), perfusion density (PD), and foveal avascular zone (FAZ) were assessed. With the AngioPlex 5000, 6 × 6–mm scans centered on the fovea were taken. Vessel density (VD) is defined as the total length of perfused vasculature per unit area in a region of measurement, and perfusion density (PD) is defined as the total area of perfused vasculature per unit area in a region of measurement. The superficial plexus is between the internal limiting membrane (ILM) and the inner plexiform layer (IPL). The segmentation as well as the calculation of VD, PD, and FAZ were done automatically by a built-in software.
The regions of the tissue were subdivided according to the Early Treatment of Diabetic Retinopathy Study (ETDRS) subfields with diameters of 1, 3, and 6 mm, and each ring was divided into quadrants. All RVO subjects were analyzed collectively. The VD and PD from the affected quadrant of the RVO patients were taken for analysis.
The systemic data collected was used to calculate the 10-year risk for major cardiovascular event using the updated World Health Organization (WHO) 2019 risk prediction charts for the South Asia region.[22] The prediction charts provide the 10-year risk of a fatal or non-fatal major cardiovascular event, such as myocardial infarction or stroke based on age, sex, blood pressure, BMI, and smoking status. For each region, two sets of charts were developed based on the availability of laboratory-based tests. In this study, the WHO cardiovascular disease (CVD) risk non-laboratory-based chart developed for South Asia (including Bangladesh, Bhutan, India, Nepal, and Pakistan) was used. The WHO CVD risk was categorized into ≤5%, 6%–10%, 11%–15%, 16%–25%, and >25%.
Statistical analysis
Statistical analysis was performed using Statistical Package for the Social Sciences (SPSS) version 20 software. One-way analysis of variance (ANOVA) was used to find the differences in age of controls and cases, post hoc Bonferroni test for the comparison of ocular parameters such as VD, PD between affected eye, fellow eye, and control eye. Chi-squared test was used to find the difference between the numbers of cases in all the categories of SBP. Mean and standard deviation were presented for age, SBP, PD, and VD. Frequencies were presented for gender, smoking status, and history of diabetes. The Pearson correlation coefficient was done for SBP versus VD and PD, and age versus VD and PD. Multiple linear regression was used to predict the risk of CVD. Models were developed based on forward method where each variable was added to the model, starting from PD followed by age, SBP, and gender. The model with PD, SBP, age, and gender was found to be more accurate. Risk was calculated based on the new model, with PD as one of the independent variables for different age and SBP groups, separately for each gender. The risk predicted from the WHO chart was used as the dependent variable. Multicollinearity among the independent variables was ruled out using the Pearson correlation coefficient.
Results
This study included a total of 64 participants, of which 36 were diagnosed with BRVO, 6 were diagnosed with CRVO, and 22 were controls. The overall age range of participants in our study was 42–82 years. There was no significant difference in the mean (SD) age of RVO patients (59.5 [11.5] years) and controls (51.5 [6.6] years; P = 0.353) [Table 1]. Table 2 describes the OCTA parameters. All the parameters were significantly different between the affected eyes and control eyes. But there was no significant difference between the fellow eyes of patients and control eyes. The risk of developing CVD for each patient was calculated from their systemic data, which included SBP, age, BMI, gender, and smoking status. For this calculation, we used the models and validated charts provided by the WHO for South Asia region which includes India, Bangladesh, Bhutan, Nepal, and Pakistan. The non-laboratory-based charts were used in this study. With WHO CVD risk value being the outcome variable, PD, age, and SBP were taken as independent variables for a simple regression analysis to predict the CVD risk in this study. Gender was coded as “0” for male and “1” for female in this model calculation. All the three variables had a significant influence on CVD risk, but when compared with age both PD and SBP had a lesser influence on CVD risk [Table 3]. Using the forward method and with addition of variables such as PD to age, SBP, and gender, the model achieved an accuracy of 72.1%. Addition of more variables such as smoking and BMI increased the accuracy, but these also increased the complexity of the model. The Pearson correlation matrix was used for assessing and ruling out multicollinearity between the independent variables [Table 4].
Table 1.
Demographic data of subjects with retinal vein occlusion and controls
| RVO (n=42) | Controls (n=22) | |
|---|---|---|
| Age – mean (SD) years | 59.5 (11.5) | 51.5 (6.6) |
| Systolic blood pressure – mean (SD) mmHg | 138.3 (17.2) | 122.9 (14.2) |
| Gender – n (%) | ||
| Male | 28 (66.7) | 12 (54.5) |
| Female | 14 (33.3) | 10 (44.5) |
| Smoker – n (%) | 20 (47.6) | 1 (4.5) |
| Diabetes mellitus – n (%) | 29 (69) | 5 (22.7) |
RVO=Retinal vein occlusion
Table 2.
Difference in optical coherence tomography angiography parameters in subjects with RVO and controls
| RVO (n=42) Mean (SD) | Control (n=22) Mean (SD) | P | |
|---|---|---|---|
| FAZ area | 0.4 (0.1) | 0.3 (0.1) | 0.002 |
| FAZ circularity | 0.5 (0.1) | 0.6 (0.07) | 0.001 |
| FAZ perimeter | 3 (0.4) | 2.3 (0.4) | 0.007 |
| Perfusion density 6×6 mm scan | 35.7 (9.4) | 41.9 (4.0) | 0.046 |
RVO=Retinal vein occlusion, FAZ=Foveal avascular zone, SD=Standard deviation
Table 3.
Linear regression model for cardiovascular disease risk prediction from WHO risk prediction model
| Model | Unstandardized Coefficients | Sig. | |
|---|---|---|---|
|
| |||
| B | Std. Error | ||
| 1 | |||
| (Constant) | −25.378 | 4.57 | 0.000 |
| Systolic blood pressure | 0.144 | 0.024 | 0.000 |
| Age | 0.376 | 0.043 | 0.000 |
| Perfusion density | −0.127 | 0.058 | 0.030 |
| Gender | −3.021 | 0.83 | 0.000 |
Table 4.
Pearson’s correlations matrix for assessing multicollinearity between the independent variables
| RVO | Controls | |||
|---|---|---|---|---|
|
|
|
|||
| VD r2 (P) | PD r2 (P) | VD r2 (P) | PD r2 (P) | |
| SBP (mmHg) | 0.179 (0.298) | −0.002 (0.992) | 0.241 (0.279) | −0.162 (0.470) |
| Age (years) | −0.181 (0.264) | −0.325 (0.041) | 0.319 (0.148) | −0.280 (0.206) |
RVO=Retinal vein occlusion, VD=Vessel density, PD=Perfusion density, SBP=Systolic blood pressure
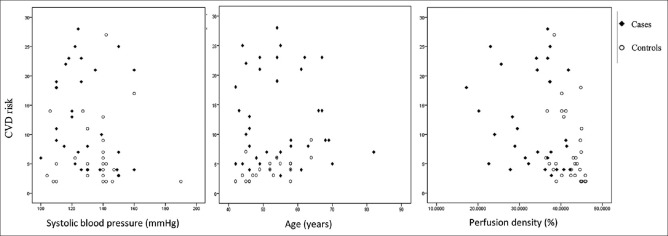
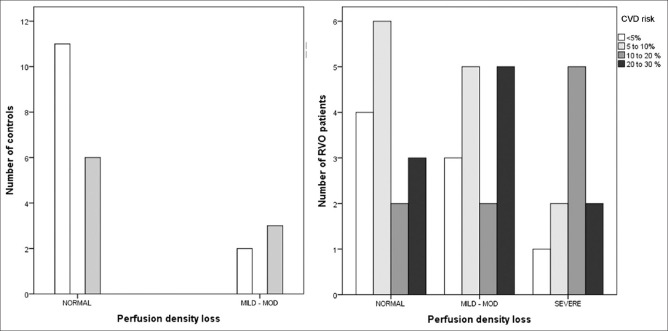
PD showed a significant but weak negative linear relationship with CVD risk, SBP a moderate positive correlation, and age a strong positive relationship with CVD risk [Fig. 1]. The risk of developing CVD increased with age. Similarly, reduction in PD was associated with increased risk of CVD. Fig. 2 shows increased CVD risk with mild to moderate and severe reduction in the PD. Based on the values of controls, a PD of 50% or higher was considered to be normal. PD of 40%–50% was categorized as mild loss, 30%–40% as moderate loss, and less than 30% as severe loss. The amount of increase in risk with decrease in PD showed a greater impact for younger age, but in the elderly group the change in PD even for a drastic amount influenced the risk to a lesser extent. For the age group less than 50 years, a PD of less than two standard deviations increased the risk by 16%–18%; for 50 to 70 years, it was by 10–13% and for above 70 years, it was 8%.
Figure 1.
Correlation of systolic blood pressure, age, and perfusion density with cardiovascular risk
Figure 2.
Cardiovascular disease risk distribution for perfusion density loss
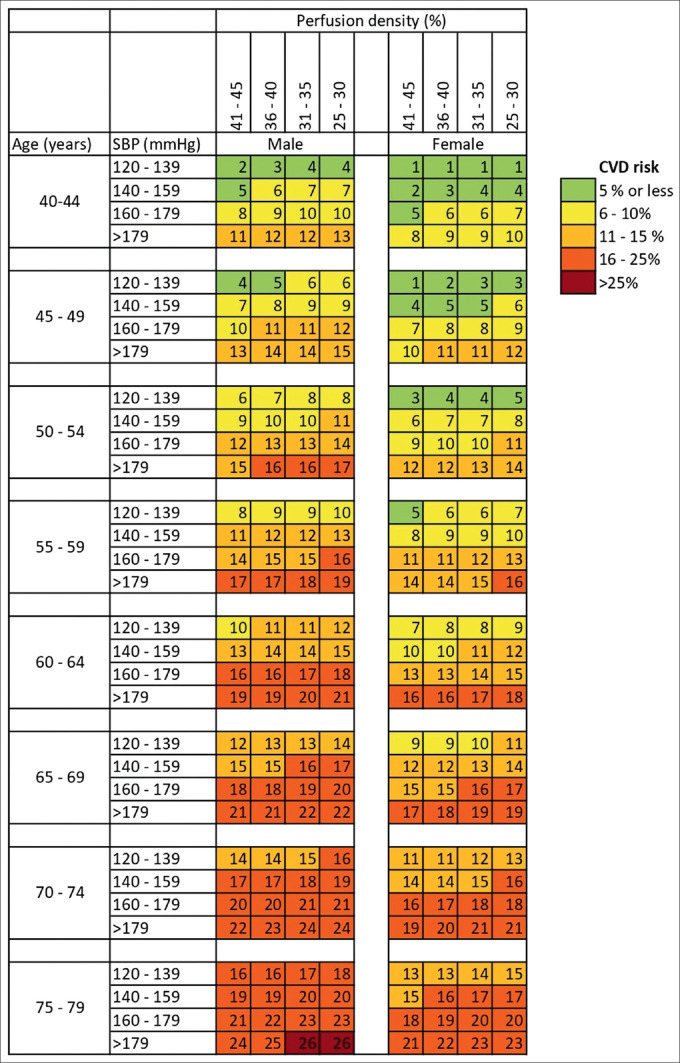
To calculate the CVD risk for a patient with RVO for a given age, gender, SBP, and PD, we created color-coded charts similar to the WHO risk calculation charts [Fig. 3]. By using the method described above, our model for prediction achieved an accuracy value of 72.1%. These charts based on our model are easy to use and interpret. It divides the age into eight groups, from 40 to 79 years, and for each age group the SBP and PD are divided into four groups. The risk is given separately for males and females. The risk is divided in five categories similar to the WHO charts, that is, ≤5%, 6%–10%, 11%–15%, 16%–25%, and >25%. The color coding makes it easier to understand.
Figure 3.
Chart for calculating the cardiovascular disease risk in patients with retinal vein occlusion
Discussion
We feel that this cardiovascular risk calculation scale will be beneficial for two reasons. First, since the RVO patients are likely to present to the ophthalmologist first this chart can help the ophthalmologist assess their patients’ CVD risk. Thus, it would make it easy for them to integrate the CVD risk assessment as a part of management protocol and help them streamline the investigations and prioritize the referral to a cardiologist. In a study by Christoffersen et al.,[23] reduced mortality was noted among patients with BRVO following the diagnosis of RVO which the authors attributed to the measures taken to reduce the risk.
Secondly, this scale also incorporates the severity of RVO which can be quantified easily with the non-invasive OCTA. The risk of cardiovascular event is different for CRVO and BRVO. The risk was seen to be significantly higher for CRVO than for BRVO.[16,24] On the other hand, another study reported higher coronary disease risk in BRVO due to presence of systolic hypertension.[18] Increased risk profile was noted particularly in ischemic CRVO than in non-ischemic CRVO.[25] This suggests that the risk is related to the severity of the vascular occlusion. OCTA is an easy and non-invasive way to quantify the severity of the vascular occlusion. Our study showed the association of increased cardiovascular risk with reduced PD, possibly indicating that the risk can be correlated to the severity of the vascular occlusion.
Previous studies correlating retinal vascular changes such as vascular caliber, arteriolar-to-venule ratio and arteriovenous nicking have shown distinct association with CVD and mortality.[26,27] They have suggested that when reliably quantified, the retinal microvascular abnormalities may be a useful indicator of cardiovascular risk. The present study attempted to correlate the cardiovascular risk to the quantifiable microvascular parameters measured with OCTA, such as VD and PD. Recently, Chua et al.[28] demonstrated significant correlation between superficial retinal capillary density and adverse cardiac remodeling markers in hypertensive patients, indicating that OCTA could be used as a simple, non-invasive index of microcirculation alteration for stratification of vascular risk in people with hypertension. In a multivariable linear regression analysis, after adjusting for age, sex, SBP, diabetes, and OCTA signal strength, the authors found that patients with reduced superficial retinal capillary density had significantly higher left ventricular mass, higher interstitial volume, and worse global longitudinal strain. These adverse markers put the patients in a higher risk group as these myocardial changes can eventually lead to ventricular dysfunction and heart failure.
This relationship between the microvascular changes and cardiovascular risk was seen to be age-dependent. In the younger age group, the risk of CVD was higher while in older age groups, for the same percentage change in PD, the risk was lower. Several other researchers have also noted a higher risk in younger patients.[13,20] In a pooled study from two large population studies, namely, the Beaver Dam Eye study and the Blue Mountains Eye study, the cardiovascular risk doubled for patients aged between 43 and 69 years.[17] Asian patients might be at higher risk as RVO is seen to occur at a younger age with higher association of diabetes mellitus and hypertension.[9] Retinal microvascular abnormalities might be better indicators of cardiovascular risk in younger patients.[27]
It can be argued that RVO mainly occurs due to local factors such as endothelial damage and turbulent flow, but a close relationship exists between endothelial dysfunction and arterial stiffness.[29] Adding microvascular parameters such as PD to risk factors help us assess the risk better. VD and PD represent a dynamic state of the vasculature with cumulative damage from aging, hypertension, and other factors. They are more consistently related to cardiovascular risk than other factors.[28] Moreover, it is easy to measure the microvascular parameters in a non-invasive, reliable, and accurate manner with OCTA. Using the output from the WHO model, we used PD as one of the independent variables to predict the risk of CVD. We found a definite correlation between PD and the cardiovascular risk. The risk increased with worsening PD in RVO. In the study, it was observed that PD had a statistically significant impact on CVD risk. We used the validated charts by the WHO and recalibrated it by adding a new independent variable. Among all the variables, the addition of PD to age, gender, and SBP gave the strongest predictive value to the model. It would be useful to employ this risk prediction model in assessing the cardiovascular risk in patients with RVO in clinical practice.
This study has limitation on account of limited sample size. Furthermore, the risk prediction model needs to be checked with a larger population and requires longitudinal data. It can nevertheless be an important addition to the ophthalmologist’s armamentarium of diagnostic tests.
Conclusion
This study developed a risk prediction model for calculation of CVD risk in patients with RVO. The risk was seen to be dependent on the severity of vascular occlusion. PD showed a significant impact on cardiovascular risk. Incorporating PD in the WHO risk prediction model resulted in a statistically robust model with strongest predictive capacity. The chart based on this model can help the ophthalmologist assess the CVD risk in a patient with RVO and prioritize referral to a cardiologist.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Hayreh SS, Zimmerman MB, Podhajsky P. Incidence of various types of retinal vein occlusion and their recurrence and demographic characteristics. Am J Ophthalmol. 1994;117:429–41. doi: 10.1016/s0002-9394(14)70001-7. [DOI] [PubMed] [Google Scholar]
- 2.Bhattacharjee H, Barman M, Misra D, Multani PK, Dhar S, Behera UC, et al. Spectrum of Eye disease in diabetes (SPEED) in India:A prospective facility-based study. Report #3. Retinal vascular occlusion in patients with type 2 diabetes mellitus. Indian J Ophthalmol. 2020;68((Suppl 1)):S27–31. doi: 10.4103/ijo.IJO_1934_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rosenblatt TR, Vail D, Saroj N, Boucher N, Moshfeghi DM, Moshfeghi AA. Increasing incidence and prevalence of common retinal diseases in retina practices across the United States. Ophthalmic Surg Lasers Imaging Retina. 2021;52:29–36. doi: 10.3928/23258160-20201223-06. [DOI] [PubMed] [Google Scholar]
- 4.Jonas JB, Nangia V, Khare A, Sinha A, Lambat S. Prevalence and associations of retinal vein occlusions:The Central India Eye and Medical Study. Retina. 2013;33:152–9. doi: 10.1097/IAE.0b013e318260246f. [DOI] [PubMed] [Google Scholar]
- 5.Hayreh SS. Prevalent misconceptions about acute retinal vascular occlusive disorders. Prog Retin Eye Res. 2005;24:493–519. doi: 10.1016/j.preteyeres.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 6.McIntosh RL, Rogers SL, Lim L, Cheung N, Wang JJ, Mitchell P, et al. Natural history of central retinal vein occlusion:An evidence-based systematic review. Ophthalmology. 2010;117:1113–23.e15. doi: 10.1016/j.ophtha.2010.01.060. [DOI] [PubMed] [Google Scholar]
- 7.Natural history and clinical management of central retinal vein occlusion. The Central Vein Occlusion Study Group. Arch Ophthalmol. 1997;115:486–91. doi: 10.1001/archopht.1997.01100150488006. [DOI] [PubMed] [Google Scholar]
- 8.Klein R, Moss SE, Meuer SM, Klein BE. The 15-year cumulative incidence of retinal vein occlusion:The Beaver Dam Eye Study. Arch Ophthalmol. 2008;126:513–8. doi: 10.1001/archopht.126.4.513. [DOI] [PubMed] [Google Scholar]
- 9.Dodson PM, Kritzinger EE, Clough CG. Diabetes mellitus and retinal vein occlusion in patients of Asian, west Indian and white European origin. Eye (Lond) 1992;6:66–8. doi: 10.1038/eye.1992.13. [DOI] [PubMed] [Google Scholar]
- 10.Elman MJ, Bhatt AK, Quinlan PM, Enger C. The risk for systemic vascular diseases and mortality in patients with central retinal vein occlusion. Ophthalmology. 1990;97:1543–8. doi: 10.1016/s0161-6420(90)32379-5. [DOI] [PubMed] [Google Scholar]
- 11.Lee JY, Yoon YH, Kim HK, Yoon HS, Kang SW, Kim JG, et al. Baseline characteristics and risk factors of retinal vein occlusion:A study by the Korean RVO Study Group. J Korean Med Sci. 2013;28:136–44. doi: 10.3346/jkms.2013.28.1.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pacella F, Bongiovanni G, Malvasi M, Trovato Battagliola E, Pistone A, Scalinci SZ, et al. Impact of cardiovascular risk factors on incidence and severity of Retinal Vein Occlusion. Clin Ter. 2020;171:e534–81. doi: 10.7417/CT.2020.2269. doi: 10.7417/ct. 2020.2269. [DOI] [PubMed] [Google Scholar]
- 13.Kuo JZ, Lai CC, Ong FS, Shih CP, Yeung L, Chen TL, et al. Central retinal vein occlusion in a young Chinese population:Risk factors and associated morbidity and mortality. Retina. 2010;30:479–84. doi: 10.1097/IAE.0b013e3181b9b3a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shih CH, Ou SY, Shih CJ, Chen YT, Ou SM, Lee YJ. Bidirectional association between the risk of comorbidities and the diagnosis of retinal vein occlusion in an elderly population:A nationwide population-based study. Int J Cardiol. 2015;178:256–61. doi: 10.1016/j.ijcard.2014.10.110. [DOI] [PubMed] [Google Scholar]
- 15.Khan Z, Almeida DR, Rahim K, Belliveau MJ, Bona M, Gale J. 10-Year Framingham risk in patients with retinal vein occlusion:A systematic review and meta-analysis. Can J Ophthalmol. 2013;48:40–5.e1. doi: 10.1016/j.jcjo.2012.08.017. [DOI] [PubMed] [Google Scholar]
- 16.Bertelsen M, Linneberg A, Christoffersen N, Vorum H, Gade E, Larsen M. Mortality in patients with central retinal vein occlusion. Ophthalmology. 2014;121:637–42. doi: 10.1016/j.ophtha.2013.07.025. [DOI] [PubMed] [Google Scholar]
- 17.Cugati S, Wang JJ, Knudtson MD, Rochtchina E, Klein R, Klein BE, et al. Retinal vein occlusion and vascular mortality:Pooled data analysis of 2 population-based cohorts. Ophthalmology. 2007;114:520–4. doi: 10.1016/j.ophtha.2006.06.061. [DOI] [PubMed] [Google Scholar]
- 18.Martin SC, Butcher A, Martin N, Farmer J, Dobson PM, Bartlett WA, et al. Cardiovascular risk assessment in patients with retinal vein occlusion. Br J Ophthalmol. 2002;86:774–6. doi: 10.1136/bjo.86.7.774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rim TH, Han JS, Oh J, Kim DW, Kang SM, Chung EJ. Retinal vein occlusion and the risk of acute myocardial infarction development:A 12-year nationwide cohort study. Sci Rep. 2016;6:22351. doi: 10.1038/srep22351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xu L, Liu WW, Wang YX, Yang H, Jonas JB. Retinal vein occlusions and mortality:The Beijing Eye Study. Am J Ophthalmol. 2007;144:972–3. doi: 10.1016/j.ajo.2007.07.015. [DOI] [PubMed] [Google Scholar]
- 21.Zhou Y, Zhu W, Wang C. Relationship between retinal vascular occlusions and incident cerebrovascular diseases:A systematic review and meta-analysis. Medicine (Baltimore) 2016;95:e4075. doi: 10.1097/MD.0000000000004075. doi: 10.1097/md. 0000000000004075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.World Health Organization cardiovascular disease risk charts: Revised models to estimate risk in 21 global regions. Lancet Glob Health. 2019;7:e1332–45. doi: 10.1016/S2214-109X(19)30318-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Christoffersen N, Gade E, Knudsen L, Juel K, Larsen M. Mortality in patients with branch retinal vein occlusion. Ophthalmology. 2007;114:1186–9. doi: 10.1016/j.ophtha.2007.01.031. [DOI] [PubMed] [Google Scholar]
- 24.Chen YY, Sheu SJ, Hu HY, Chu D, Chou P. Association between retinal vein occlusion and an increased risk of acute myocardial infarction:A nationwide population-based follow-up study. PLoS One. 2017;12:e0184016. doi: 10.1371/journal.pone.0184016. doi: 10.1371/journal.pone. 0184016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Risk factors for central retinal vein occlusion. The Eye Disease Case-Control Study Group. Arch Ophthalmol. 1996;114:545–54. [PubMed] [Google Scholar]
- 26.Wang JJ, Liew G, Wong TY, Smith W, Klein R, Leeder SR, et al. Retinal vascular calibre and the risk of coronary heart disease-related death. Heart. 2006;92:1583–7. doi: 10.1136/hrt.2006.090522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wong TY, Klein R, Klein BE, Tielsch JM, Hubbard L, Nieto FJ. Retinal microvascular abnormalities and their relationship with hypertension, cardiovascular disease, and mortality. Surv Ophthalmol. 2001;46:59–80. doi: 10.1016/s0039-6257(01)00234-x. [DOI] [PubMed] [Google Scholar]
- 28.Chua J, Le TT, Sim YC, Chye HY, Tan B, Yao X, et al. Relationship of quantitative retinal capillary network and myocardial remodeling in systemic hypertension. J Am Heart Assoc. 2022;11:e024226. doi: 10.1161/JAHA.121.024226. doi: doi: 10.1161/JAHA.121.024226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gouliopoulos N, Siasos G, Moschos MM, Oikonomou E, Rouvas A, Bletsa E, et al. Endothelial dysfunction and impaired arterial wall properties in patients with retinal vein occlusion. Vasc Med. 2020;25:302–8. doi: 10.1177/1358863X20913609. [DOI] [PubMed] [Google Scholar]