Abstract
OBJECTIVES:
To evaluate whether a nurse navigator-led, multicomponent Sepsis Transition And Recovery program improves 30-day mortality and readmission outcomes after sepsis hospitalization.
DESIGN:
Multisite pragmatic randomized clinical trial.
SETTING:
Three hospitals in North Carolina from January 2019 to March 2020.
PATIENTS:
Eligible patients hospitalized for suspected sepsis and deemed high-risk for mortality or readmission by validated internal risk models.
INTERVENTIONS:
Patients were randomized to receive usual care alone (i.e., routine transition support, outpatient care; n = 342) or additional Sepsis Transition And Recovery support (n = 349). The 30-day intervention involved a multicomponent transition service led by a nurse navigator through telephone and electronic health record communication to facilitate best practice postsepsis care strategies during and after hospitalization including: postdischarge medication review, evaluation for new impairments or symptoms, monitoring comorbidities, and palliative care approach when appropriate. Clinical oversight was provided by a Hospital Medicine Transition Services team.
MEASUREMENTS AND MAIN RESULTS:
The primary outcome was a composite of mortality or hospital readmission at 30 days. Logistic regression models were constructed to evaluate marginal and conditional odds ratios (adjusted for prognostic covariates: age, comorbidity, and organ dysfunction at enrollment). Among 691 randomized patients (mean age = 63.7 ± 15.1 yr; 52% female), a lower percentage of patients in the Sepsis Transition And Recovery group experienced the primary outcome compared with the usual care group (28.7% vs 33.3%; risk difference, 4.7%; odds ratio, 0.80; 95% Cl, 0.58–1.11; adjusted odds ratio, 0.80; 95% Cl, 0.64–0.98). There were 74 deaths (Sepsis Transition And Recovery: 33 [9.5%] vs usual care: 41 [12.0%]) and 155 rehospitalizations (Sepsis Transition And Recovery: 71 [20.3%] vs usual care: 84 [24.6%]).
CONCLUSIONS:
In a multisite randomized clinical trial of patients hospitalized with sepsis, patients provided with a 30-day program using a nurse navigator to provide best practices for postsepsis care experienced a lower proportion of either mortality or rehospitalization within 30 days after discharge. Further research is needed to understand the contextual factors associated with successful implementation.
Keywords: critical illness recovery, mortality, readmission, sepsis, survivorship
Sepsis is a common, life-threatening condition defined by organ dysfunction due to a dysregulated response to infection (1). Aggressive early sepsis identification and treatment initiatives have decreased hospital mortality for patients with sepsis over the last few decades (2). However, sepsis survivors face challenges after the acute illness episode, experiencing new functional, cognitive, and psychologic deficits, new or recurrent infections, exacerbation of chronic diseases, and high rates of hospital readmission and postdischarge mortality (3–5).
To address persistent morbidity and mortality for sepsis survivors, we developed the Sepsis Transition and Recovery (STAR) program, based on the widely adopted Chronic Care Model (6). The STAR program built on our evaluation of transition interventions in complex patients, which suggested benefit in the subgroup of patients with sepsis (7), with the following modifications: 1) the program elements specifically addressed best practices for postsepsis care (3) and 2) the program was delivered through a remote navigator to alleviate the challenges of low adherence to in-person follow-up. The navigator provides disease education, helps patients overcome medical system barriers to recommended care, and bridges gaps in service that can serve as points of failure for sepsis patients. The STAR program specifically targets delivery of best practice postsepsis care including: 1) medication optimization, 2) screening for new impairments, 3) anticipation and mitigation of risk of health deterioration, and 4) palliative care when appropriate (3). Receipt of these care elements is associated with reduced postdischarge mortality and rehospitalization (8).
The Improving Morbidity during Post-Acute Care Transitions for Sepsis (IMPACTS) trial tests the hypothesis that implementation of the STAR program reduces 30-day readmission and mortality rates for high-risk patients with suspected sepsis compared with usual care (UC) alone.
METHODS
The Atrium Health Institutional Review Board (IRB) approved this study (IRB No. 01–19-24E) with waiver of informed consent as the intervention is considered within the range of standard practice and study data are limited to those routinely collected in usual clinical practice. Detailed study methods are described elsewhere (9). The study protocol is available in Appendix 1 (http://links.lww.com/CCM/G815).
Study Design and Setting
IMPACTS was a two-arm parallel-group pragmatic randomized clinical trial comparing UC alone versus UC plus transition support from the STAR program. The study occurred at three hospitals in North Carolina. Enrollment began January 2019 and ended in March 2020, with follow-up completed in May 2020.
The target population was patients hospitalized for suspected sepsis at high risk for 30-day mortality or readmission. Patients were eligible if they were admitted to a participating hospital from the emergency department (ED) and met the following inclusion criteria: 1) greater than or equal to 18 years old; 2) antibiotic or bacterial culture order within 24 hours of ED arrival and either a) culture drawn first and antibiotics ordered within 48 hours or b) antibiotics ordered first and culture ordered within 48 hours; 3) remaining hospitalized at the time a daily list of eligible patients was generated each weekday morning; and 4) deemed high risk for either 30-day readmission or mortality, defined as a readmission risk probability greater than or equal to 20% or mortality risk probability greater than or equal to 10%.
Patients were excluded if they transferred from other acute care hospitals; had code status documented as “do not resuscitate” or “do not intubate” within 24 hours after admission (due to possible limitations on aggressive treatment); resided more than 2.5 hours drive time from the treating hospital (to be eligible for Transition Services if care escalation required); or were previously randomized to either treatment arm.
Detailed information about readmission and mortality risk models is provided in Table S1 (http://links.lww.com/CCM/G816). Broadly, risk models included clinical and administrative data selected based on their association with postsepsis outcomes and conceptual models of postsepsis pathophysiology. Model covariates were sourced from routine clinical data captured during hospitalization and billing history at the time of hospital admission to produce a near-real-time score that identifies risk for 30-day hospital readmission or mortality. Risk models were locally developed and tested on data from the three participating hospitals. In validation studies conducted prior to trial enrollment, the 30-day mortality risk model demonstrated an area under the receiver operating characteristic curve (AUC) of 0.85 and negative predictive value (NPV) of 0.97. The 30-day hospital readmission model demonstrated AUC of 0.70 and NPV of 0.89.
Figure S1 (http://links.lww.com/CCM/G816) depicts the identification and enrollment of eligible trial patients. The electronic health record (EHR)-based algorithm ran each morning and automatically generated lists of eligible, high-risk patients admitted over the prior 72 hours. Eligible patients were then randomly allocated 1:1 to receive UC or the STAR program using a computer-based randomization tool and maximally tolerated imbalance procedure with up to 10% allowable deviation to maintain similar comparison group sizes while limiting predictability of future treatment assignments. The total daily number of patients randomized was constrained to maintain a feasible caseload over the study duration, approximately 5–10 new patients per week per navigator. We randomized up to six consecutively identified patients each weekday, enumerated by time of ED presentation. The randomization constraint was reevaluated biweekly and adjusted as needed to match the STAR navigators capacity (approximately 50 concurrent patients).
Study Group Descriptions
Intervention Group.
The STAR program was developed by a multidisciplinary stakeholder group including clinicians, researchers, and operational leaders at Atrium Health. The intervention was designed to facilitate the delivery of best practice care for sepsis survivors including: 1) identification and treatment of new physical, mental, and cognitive deficits; 2) review and adjustment of medications; 3) surveillance of treatable conditions that commonly lead to poor outcomes, including chronic conditions that may destabilize during sepsis and recovery; and 4) focus on care alignment, including palliative care when appropriate (3).
The intervention delivery was led by a centrally located sepsis nurse navigator (i.e., a registered nurse with special focus on supporting sepsis survivors; described previously) (10). The primary roles of the STAR navigator were to promote care planning and self-management, proactive follow-up, and patient, provider, and community engagement during care transitions after sepsis. The STAR navigator began providing support during the hospital stay and continued after discharge, at close, regular intervals through 30 days after discharge. Protocols guided care escalation under the direction of the Hospital Medicine Transition Services team physician, who reviewed cases with the navigator weekly and was available on an as-needed basis. Detailed descriptions of STAR intervention activities are shown in Table S2 (http://links.lww.com/CCM/G816).
Comparison Group.
Participants in the comparison group received usual transitional and outpatient care. This was not prescribed but could include patient education and follow-up instructions at discharge, routine recommendations for follow-up visits with primary care providers, arrangements for home health services, transitional care, or care management. Intervention characteristics are reported according to the template for intervention description and replication checklist in Table S3 (http://links.lww.com/CCM/G816) (11).
Data Collection
All clinical and outcomes data (Table S4, http://links.lww.com/CCM/G816) were collected directly from the EHR and Enterprise Data Warehouse. Baseline variables included physiologic measurements (e.g., mean arterial pressure), laboratory values (complete blood count, basic/comprehensive metabolic panel, lactate), basic sociodemographic characteristics (e.g., age, gender, race), past medical history (e.g., comorbidities, prior healthcare use, medication history), and care delivered during the index hospitalization (e.g., intensive care, mechanical ventilation). Charlson Comorbidity Index (CCI) scores at enrollment were calculated from diagnoses during the prior 12 months. The navigator documented completion of STAR workflow processes in the patients EHR through the ambulatory care management electronic documentation form, which also captured information on symptoms, background, assessment, and STAR recommendations. EHR data were exported into a secure, research database (Research Electronic Data Capture) (12).
Outcomes
The study’s prespecified primary outcome was a composite, dichotomous endpoint of all-cause mortality or hospital readmission assessed 30 days postindex hospital discharge. Death dates were determined using EHR data along with linked national death records integrated monthly into the Enterprise Data Warehouse. Hospital réadmissions included any inpatient or observation encounter to any of 47 Atrium Health hospitals.
Prespecified secondary outcomes assessed 30 days after hospital discharge included the proportion of patients who experienced: all-cause mortality; all-cause hospital readmission; and cause-specific hospital readmissions related to infection, chronic lung disease, heart failure, or acute renal failure. The count of ED encounters and the number of acute care-free days alive were also analyzed during the interval from hospital discharge to day 30. Intervention implementation was tracked as the proportion of patients engaging with the navigator at least once after discharge. Process measures were tracked in both groups, including: inpatient physical therapy consult; mental health assessment by Patient Health Questionnaire (PHQ)-2 or PHQ-9 (13,14); out-patient referrals to physical therapy, speech therapy, and behavioral health; outpatient follow-up visit within 10 days; and documented medication reconciliation in the EHR during the 30 days postdischarge. We also measured place of death (i.e., hospital or other location), the proportion of patients who received palliative care consult, had documentation of end-of-life care preferences, or were discharged to hospice.
Statistical Analyses
Baseline variables were described using mean values and SDS for continuous measures and proportions for categorical measures. Primary analyses were conducted under an intention-to-treat approach. To test the STAR treatment effect on the proportion of patients who experienced the composite mortality or hospital readmission event, logistic regression models were constructed with an indicator for treatment assignment. Conditional odds ratios (ORs) with 95% CIs were calculated to measure patient-level effects adjusting for prespecified prerandomization covariates known to be associated with the primary outcome: age, comorbidity burden (CCI score), and organ dysfunction at enrollment (15). Analyses of secondary outcome measures involved a similar approach. Given variation in known risk factors across the eligible study population, we evaluated heterogeneity of treatment effect by risk-based (16) (i.e., predicted 30-d mortality probability quartile) and traditional subgroups (i.e., by age, comorbidity burden, acute illness severity).
Sensitivity analyses were conducted in a modified intention-to-treat population who survived the index hospitalization. Per-protocol analyses were performed among patients who were discharged alive and not under hospice care, only including patients in the STAR group if they received transition follow-up through the program and only including patients in UC if they were not enrolled into an alternative care transitions program at discharge. Analyses were completed using SAS Enterprise Guide v7.1 (SAS Institute, Cary, NC). All statistical tests were two-sided with p value of less than 0.05 considered for statistical significance.
Statistical Power and Sample Size
Sample size was calculated to detect a 25% relative reduction in the composite rate of 30-day readmission and mortality, based on prior literature suggesting that approximately 2% of deaths and between 22% and 42% of hospital readmissions after sepsis are preventable. Based on historical data from the study sites, the control group was estimated to have a 40% combined readmission and mortality rate. To achieve at least 80% power (α = 0.05, two-sided), the estimated sample size included 354 patients per group.
RESULTS
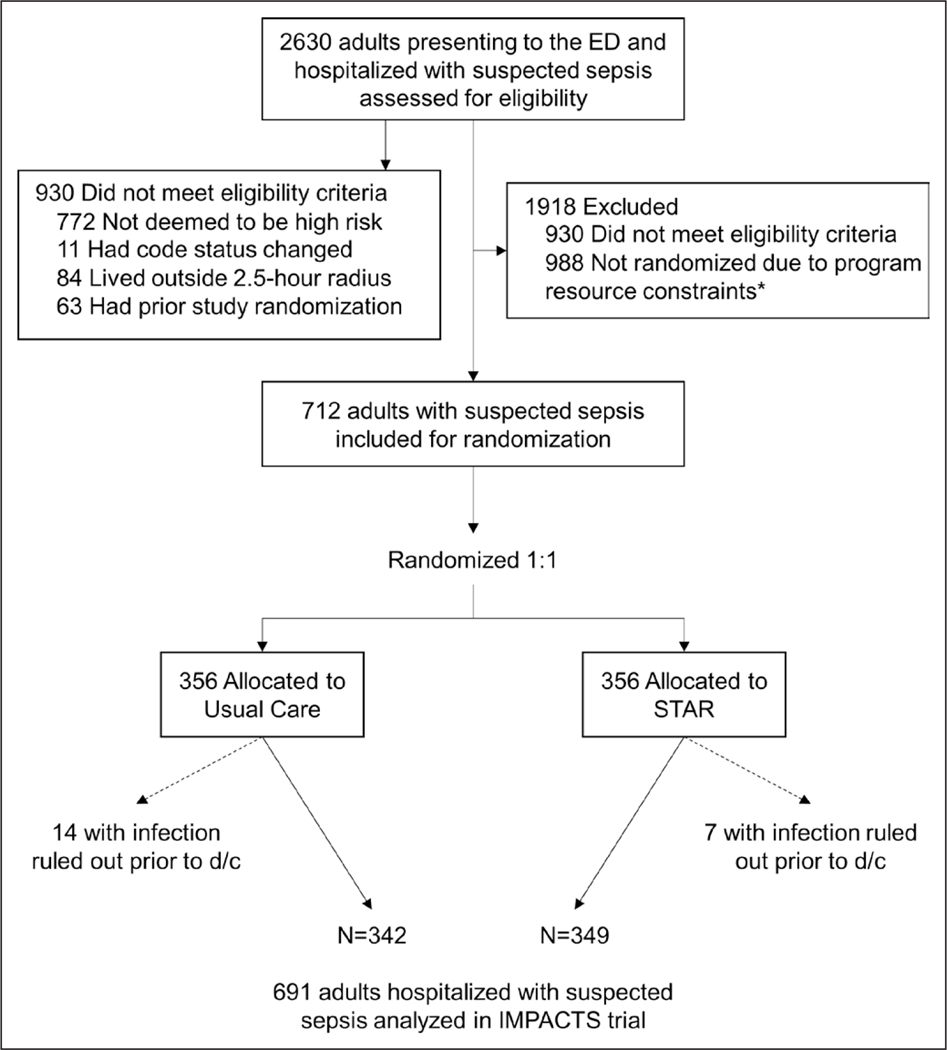
Of 2,630 patients screened for participation, 1,700 met eligibility criteria and 712 were randomized within program resource constraints (Fig. 1; and Table S6, http://links.lww.com/CCM/G816). Of the randomized patients, 14 patients in UC and seven patients in STAR were excluded because a diagnosis of infection was ruled out before discharge. Among the remaining 691 patients (349 in STAR, 342 in UC), the mean age was 63.7 years (SD = 15.1 yr), 52% were female, and 65% were White. At baseline, 92% met the Sepsis-3 definition of suspected infection plus at least one organ failure (17), and patients in the intervention and UC groups had similar characteristics (Table 1) and predicted readmission and mortality probabilities (Figs. S2 and S3, http://links.lww.com/CCM/G816).
Figure 1.
Consolidated Standards of Reporting Trials flow diagram of patient enrollment into Improving Morbidity during Post-Acute Care Transitions for Sepsis (IMPACTS) trial. ED = emergency department, STAR = Sepsis Transition And Recovery.
TABLE 1.
Baseline Demographics and Clinical Characteristics of Patients With Suspected Sepsis Enrolled Into Improving Morbidity During Post-Acute Care Transitions for Sepsis Trial
| Variable | Usual Care (n = 342) | Sepsis Transition and Recovery Program (n = 349) |
|---|---|---|
|
| ||
| Mean age at admission, yr | 62.7 ± 15.5 | 64.7 ± 14.9 |
| Female sex | 174 (50.9) | 185 (53.0) |
| Race | ||
| Black | 111 (32.5) | 97 (27.8) |
| White | 209 (61.1) | 239 (68.5) |
| Other | 22 (6.4) | 13 (3.7) |
| Mean Charlson Comorbidity Index | 4.5 ± 3.1 | 4.6 ± 3.2 |
| Comorbid conditions | ||
| Chronic lung disease | 128 (37.4) | 126 (36.1) |
| Chronic renal disease | 129 (37.7) | 132 (37.8) |
| Diabetes | 164 (48.0) | 172 (49.3) |
| Heart failure | 107 (31.3) | 128 (36.7) |
| Malignancy | 72 (21.1) | 63 (18.1) |
| Prior hospital admission < 6 mo | 193 (56.4) | 191 (54.7) |
| Clinical characteristics | ||
| Mean number of organ dysfunction criteria at presentation | 2.0 ± 1.2 | 2.0 ±1.2 |
| Mean arterial pressure, mm Hg | 62.6 ± 15.2 | 62.1 ± 13.7 |
| Mean serum creatinine, mg/dL | 2.2 ± 2.4 | 2.0 ± 2.2 |
| Mean bilirubin, mg/dL | 1.1 ± 1.3 | 1.2 ± 1.5 |
| Mean platelet count, cells/μL | 203.8 ± 120.6 | 219.0 ±125.3 |
| Mean serum lactate, mmol/L | 3.0 ± 2.2 | 3.4 ± 2.9 |
| Required ICU admission | 147 (42.9) | 142 (40.7) |
| Required vasopressor medication | 122 (35.7) | 133 (38.1) |
| Mean hospital length of stay, d | 8.3 ± 8.0 | 9.0 ± 11.2 |
| Discharge disposition | ||
| Expired | 15 (4.3) | 16 (4.6) |
| Home | 224 (65.5) | 230 (65.9) |
| Hospice | 11 (3.2) | 10 (2.9) |
| Skilled nursing facility | 72 (21.1) | 71 (20.3) |
| Long-term care facility or inpatient rehabilitation | 15 (4.4) | 17 (4.9) |
| Other acute care hospital | 5(1.5) | 5(1.4) |
Mean values are presented with corresponding sds (denoted as ±).
Hospital Readmission and Mortality
Fewer patients in the STAR group experienced the composite primary outcome within 30 days postdischarge compared with UC (28.7% vs 33.3%; risk difference, −4.7%; unadjusted OR, 0.80; 95% Cl, 0.58–1.11; adjusted OR, 0.80; 95% Cl, 0.64–0.98; Table 2). There were 74 deaths (STAR 33 [9.5%] vs UC: 41 [12.0%]) and 155 rehospitaüzations (STAR 71 [20.3%] vs UC: 84 [24.6%]) (Fig. S4, http://links.lww.com/CCM/G816)
TABLE 2.
Improving Morbidity During Post-Acute Care Transitions for Sepsis Trial Primary Outcome Measure
| Outcome Measure | Usual Care | Sepsis Transition and Recovery | Risk Difference (95% Cl) | OR (95% Cl) | P |
|---|---|---|---|---|---|
|
| |||||
| 30-d all-cause mortality and readmission | 114 (33.3) | 100 (28.7) | −4.7 (−11.6 to 2.2) | ||
| Unadjusted effect estimate | 0.80 (0.58–1.11) | 0.18 | |||
| Conditional effect estimate | 0.80 (0.64–0.98) | 0.03 | |||
| Among hospital survivors | 99/327 (30.3) | 84/333 (25.2) | −5.1 (−11.9 to 1.8) | 0.78 (0.64–0.95) | 0.02 |
| Components of primary outcome | |||||
| 30-d all-cause mortality | 41 (12.0) | 33 (9.5) | −2.5 (−7.2 to 2.1) | ||
| Unadjusted effect estimate | 0.77 (0.47–1.24) | 0.28 | |||
| Conditional effect estimate | 0.68 (0.51–0.92) | 0.01 | |||
| Among hospital survivors | 26/327 (8.0) | 17/333 (5.1) | −2.9 (−6.6 to 0.9) | 0.55 (0.47–0.66) | <0.01 |
| 30-d all-cause readmission | 84 (24.6) | 71 (20.3) | −4.3 (−10.4 to 2.0) | ||
| Unadjusted effect estimate | 0.78 (0.55–1.12) | 0.18 | |||
| Conditional effect estimate | 0.79 (0.65–0.97) | 0.03 | |||
| Among hospital survivors | 84/327 (25.7) | 71/333 (21.3) | −4.4 (−10.8 to 2.1) | 0.80 (0.65–0.98) | 0.03 |
Conditional estimates are adjusted for covariates known a priori to be associated with the outcome: age, comorbidity burden, and organ dysfunction at time of enrollment.
Risk-based variation in treatment effect is shown in Table 3. Combining patients in 30-day mortality risk quartiles 1–3 (predicted probability < 39%), 30-day mortality or readmission occurred in 62 patients (23.7%) in the STAR group versus 84 (32.8%) in UC (risk difference, −9.2%; 95% Cl, −16.9 to −1.4). In the top risk quartile (predicted probability ≥ 39%), 30-day mortality or readmission occurred in 38 patients (43.4%) in the STAR group versus 30 (34.9%) in UC (risk difference, 8.8; 95% Cl, −5.7 to 23.3). Additional risk-based variation in outcome components is shown in Table S5 (http://links.lww.com/CCM/G816).
TABLE 3.
Comparison of Primary Outcome Events Within 30 Days in Strata Defined by Baseline Predicted Risk Probability
| Usual Care |
Sepsis Transition and Recovery |
Group Differences in Composite Outcome |
||||
|---|---|---|---|---|---|---|
| Predicted Probability Range* | n | Composite (%) | n | Composite (%) | Risk Differences (95% Cl) | OR (95% Cl) |
|
| ||||||
| Q1, P | mortality: 1–12% | 86 | 18 (20.9) | 88 | 25 (28.4) | 7.5 (−5.3 to 20.2) | 1.50 (0.75–3.01) |
| Q2, P | mortality: 13–21% | 85 | 33 (38.8) | 87 | 14 (16.1) | −22.7 (−35.7 to −9.8) | 0.30 (0.15–0.62) |
| Q3, P | mortality: 22–38% | 85 | 33 (38.8) | 87 | 23 (26.4) | −12.4 (−26.3 to 1.5) | 0.57 (0.30–1.08) |
| Q4, P | mortality: 39–99% | 86 | 30 (34.9) | 87 | 38 (43.4) | 8.8 (−5.7 to 23.3) | 1.45 (0.78–2.67) |
| Predicted Probability Range, Mortality “AND” Readmission | n | Composite (%) | n | Composite (%) | RD (95% Cl) | OR (95% Cl) |
| Q1-Q3, P | mortality: 1–38% | 256 | 84 (32.8) | 262 | 62 (23.7) | −9.2 (−16.9 to−1.4) | 0.63 (0.43–0.93) |
| P | readmission: ≤ 20% | 119 | 24 (20.2) | 134 | 25 (18.7) | −1.5 (−11.3 to 8.3) | 0.91 (0.49–1.69) |
| P | readmission: > 20% | 137 | 60 (43.8) | 128 | 37 (28.9) | −14.9 (−26.3 to −3.5) | 0.52 (0.31–0.87) |
| Q4, P | mortality: 39–99% | 86 | 30 (34.9) | 87 | 38 (43.4) | 8.8 (−5.7 to 23.3) | 1.45 (0.78–2.67) |
| P | readmission: ≤ 20% | 34 | 9 (26.5) | 35 | 9 (25.7) | −0.8 (−21.5 to 20.0) | 0.96 (0.33–2.82) |
| P | readmission: > 20% | 52 | 21 (40.3) | 52 | 29 (55.8) | 15.4 (−3.6 to 34.4) | 1.86 (0.85–4.05) |
OR = odds ratio, P = probability, Q = quartile.
Predicted probability of outcome calculated at baseline enrollment using internal risk stratification models (described in Table S1, http://links.Iww.com/CCM/G816).
There were no significant differences in intervention effects on the primary outcome between groups defined by age, comorbidity burden, or sepsis severity (Fig. S5, http://links.lww.com/CCM/G816). In sensitivity intention-to-treat population who survived hospitalization and per-protocol analysis population who complied with treatment assignment (Tables S6–S9, http://links.lww.com/CCM/G816).
Secondary Outcomes
The number of days alive and outside the hospital (Figs. S6–S8, http://links.lww.com/CCM/G816), proportion with cause-specific rehospitalization, and count of ED visits (Fig. S9, http://links.lww.com/CCM/G816) did not significantly differ between STAR and UC groups (Table S10, http://links.lww.com/CCM/G816).
In an exploratory evaluation among patients who survived to index discharge but died during the intervention period (n = 43), fewer patients in the STAR group had an inhospital death compared with patients in UC (12% vs 31%; Table 4).
TABLE 4.
Description of Processes of Care Received by Patients Enrolled in the Improving Morbidity During Post-Acute Care Transitions for Sepsis Trial
| Process Measure | Usual Care | Sepsis Transition and Recovery | P |
|---|---|---|---|
|
| |||
| Functional assessment | |||
| Physical or occupational therapy consult during hospitalization | 209 (61) | 226 (65) | 0.98 |
| Outpatient rehabilitation or physical or occupational therapy during follow-up | 55 (16) | 60 (17) | 0.99 |
| Speech therapy consult during hospitalization | 79 (23) | 81 (23) | 0.99 |
| Outpatient speech therapy during follow-up | 17(5) | 27 (8) | 0.14 |
| Mental health assessment | |||
| Behavioral health consult or goals documented during hospitalization | 68 (20) | 68 (20) | 0.99 |
| Behavioral health screening (Patient Health Questionnaire) during follow-up | 33 (10) | 192 (55) | <0.01 |
| Outpatient follow-up after index hospitalization | |||
| Physician office visit within 10 d | 107 (31) | 136 (39) | 0.03 |
| Medication review documented in the electronic health record during follow-up | 172 (50) | 265 (76) | <0.01 |
| Goal-concordant care | |||
| Care alignment tool completed during follow-up | 80 (23) | 148 (42) | <0.01 |
| Advance directive completed during follow-up | 124 (36) | 134 (38) | 0.56 |
| Palliative care consult during follow-up | 59 (17) | 69 (20) | 0.39 |
| Code status changed to do not resuscitate or do not intubate during follow-up | 34 (10) | 39 (11) | 0.60 |
| In hospital death after index hospital discharge | |||
| Among all hospital survivors | 8/327 (2) | 2/333 (1) | 0.05 |
| Among posthospital decedents only | 8/26 (31) | 2/17 (12) | 0.15 |
Postsepsis Care Delivery
Navigators engaged patients a median of 15 times over the study period for a total of 170 minutes (Table S7, http://links.lww.com/CCM/G816). Table 4 compares process measures between STAR and UC groups. More patients in the STAR group had outpatient follow-up within 10 days of the index sepsis discharge compared with the UC group (39% vs 31%).
Additionally, during 30 days following index hospital discharge, more patients in the STAR group had documented medication reconciliation (76% vs 50%), depression screening (55% vs 10%), and outpatient speech therapy (8% vs 4%) compared with UC. For processes of care related to goal-concordant care, more patients in the STAR group than in the UC group had a care alignment tool documented in the EHR (42% vs 23%) and a palliative care consult during follow-up (20% vs 17%).
DISCUSSION
In a multisite randomized pragmatic trial of high-risk patients hospitalized with sepsis, a STAR program reduced the proportion of patients who experienced either mortality or rehospitalization within 30 days after discharge. Consistent with Consolidated Standards of Reporting Trials (CONSORT) guidelines (18), we report both unadjusted and adjusted analyses, and interpreting the statistical significance of the results requires consideration of whether unadjusted or adjusted effects are most relevant in this study Trial methodology experts recommend using multivariable models to adjust for prognostic baseline covariates in a randomized trial, even if groups are generally balanced, due to benefits including improved precision of the effect estimate and transportability of findings to populations with different covariate distributions (19–21). Within this context and considering the observed risk-based heterogeneity, we believe the ORs adjusted for prognostic covariates provide the most meaningful estimates of the intervention effect in this study. The effect direction and magnitude of both unadjusted and adjusted findings in the current trial have encouraging implications for the advancement of postsepsis care, a key priority in addressing the worldwide burden of sepsis (22), for which little is currently known.
The STAR program was developed with a multidisciplinary stakeholder group of clinicians and healthcare leaders with a focus on delivering evidence-based postsepsis practices through a high-value platform integrated with existing care resources (i.e., patients’ primary care providers, Hospital Medicine Transition Services). The STAR program is an example of a complex intervention that addresses contextual issues and supports patients’ capacity for self-care—a strategy shown to be effective in reducing adverse outcomes during care transitions (23). Additionally, the STAR program extends evidence demonstrating the effectiveness of nurse navigators to facilitate delivery of recommended care for complex patients (24–26) and incorporates lessons learned from prior evaluations of postdischarge care after serious illness to increase intervention reach and limit attrition (7, 27, 28).
Our analysis of risk-based variation in treatment effect suggests that patients in the highest quartile of mortality and readmission risk derive no mortality or readmission benefit from the STAR intervention. This is consistent with current thinking that endpoints like mortality or rehospitalization may not be avoidable or even undesirable among patients with serious illness and deteriorating health status (29–31). Instead, comfort- or function-oriented outcomes may be more relevant (32). Given the high rates of morbidity and mortality after sepsis, early integration of goals of care discussions into clinical care is important, particularly for patients with deteriorating health prior to sepsis (3). Timely discussion about care preferences enables delivery of goal-concordant care, improves patient and caregiver satisfaction, and may reduce undesired, high-intensity healthcare interventions (33–36). Our findings related to goal-concordant care indicate that STAR may have benefits in aligning care with patients’ goals, including increased EHR documentation of care goals, palliative care consultations, and death outside of the hospital among those who died following index discharge. These results suggest a nurse navigator may be able to help patients receive goal-concordant care and warrants further study.
The strengths of our study include randomized treatment assignment, multicenter enrollment of a diverse sepsis population within a pragmatic, real-world context, low attrition, and evaluation of an endpoint that is clinically meaningful to patients and prioritized by healthcare systems. Our findings are timely given the urgency to identify strategies to facilitate recovery for coronavirus disease 2019 (COVID-19) survivors. Although this study did not specifically evaluate patients with COVID-19, it is reasonable to extend STAR program effects to these patients, who share many illness and outcome features with survivors of sepsis due to other causes (37).
Findings should be considered within the context of several limitations. First, because we used risk modeling to enrich our sample for patients with high risk of 30-day mortality or rehospitalization, findings are relevant to adults at high risk for these outcomes. We identified patients using locally derived risk stratification tools, but similar approaches could apply other readily accessible risk models. Second, excluding some patients, prerandomization (due to navigator capacity) and postrandomization (due to having infection ruled out) may have biased results, although included and excluded patients had similar characteristics. Third, the observed event rate was lower than expected, although predicted probabilities were balanced across trial arms at baseline. Fourth, our study was not powered to detect differences in secondary outcome measures. Most findings were directionally aligned but not statistically significant. Fifth, postsepsis health effects have been shown to persist for months or years; thus, outcome evaluations beyond 30 days and including patient-reported measures of common sequelae (e.g., functional, cognitive, psychologic deficits) may be valuable. Finally, this study could not disaggregate effects of individual intervention components to determine which were influential in effecting overall program benefit, and further implementation and outcomes evaluations are needed to assess whether these effects can be replicated and scaled in rural or other lower-resource settings (38).
CONCLUSIONS
In this multisite randomized clinical trial of patients hospitalized with sepsis, a multicomponent transition and recovery intervention reduced 30-day mortality and rehospitalizations compared with UC. Further research is needed to understand the generalizability of the findings to other healthcare settings and identify key implementation factors for intervention success.
Supplementary Material
Acknowledgments
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (http://journals.Iww.com/ccmjournal).
Supported, in part, internally by Atrium Health Acute Care Outcomes Research Network.
Dr. Taylor reports grant support from National Institutes of Health (NIH) outside of the submitted work. Dr. Kowalkowski reports grant support from NIH and Patient-Centered Outcomes Research Institute outside of the submitted work Drs. Taylor and Kowalkowski received funding from the National Institute of Nursing Research and the National Library of Medicine. Drs. Rios and Hetherington received support for article research from the NIH. Drs. Rios and Kowalkowski disclosed work for hire. Dr. McWilliams’ institution received funding from The Heineman Foundation; he disclosed he is the co-founder and administrative member of ¡Enroll, LLC. Drs. McWilliams’s and Hetherington’s institutions received funding from the NIH. Dr. Russo received funding from Moderna.
Footnotes
The remaining authors have disclosed that they do not have any potential conflicts of interest.
Trial Registration: ClinicalTrials.gov NCT03865602.
REFERENCES
- 1.Singer M, Deutschman CS, Seymour CW, et al. : The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA 2016; 315:801–810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fleischmann C, Scherag A Adhikari NK, et al. ; International Forum of Acute Care Trialists: Assessment of global incidence and mortality of hospital-treated sepsis. Current estimates and limitations. Am J Respir Crit Care Med 2016; 193:259–272 [DOI] [PubMed] [Google Scholar]
- 3.Prescott HC, Angus DC: Enhancing recovery from sepsis: A review. JAMA 2018; 319:62–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Iwashyna TJ, Ely EW, Smith DM, et al. : Long-term cognitive impairment and functional disability among survivors of severe sepsis. JAMA 2010; 304:1787–1794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Prescott HC, Langa KM, Iwashyna TJ: Readmission diagnoses after hospitalization for severe sepsis and other acute medical conditions. JAMA 2015; 313:1055–1057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wagner EH, Austin BT, Davis C, et al. : Improving chronic illness care: Translating evidence into action. Health Aff (Millwood) 2001;20:64–78 [DOI] [PubMed] [Google Scholar]
- 7.McWilliams A Roberge J, Anderson WE, et al. : Aiming to improve réadmissions through integrated hospital transitions (AIRTIGHT): A pragmatic randomized controlled trial. J Gen Intern Med 2019; 34:58–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Taylor SP, Chou SH, Sierra MF, et al. : Association between adherence to recommended care and outcomes for adult survivors of sepsis. Ann Am Thorac Soc 2020; 17:89–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kowalkowski M, Chou SH, McWilliams A, et al. ; Atrium Health ACORN Investigators: Structured, proactive care coordination versus usual care for improving morbidity during post-acute care transitions for sepsis (IMPACTS): A pragmatic, randomized controlled trial. Trials 2019; 20:660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Taylor SP, Rios A Kowalkowski MA: Translating postsepsis care to post-COVID-19 care. The case for a virtual recovery program. Ann Am Thorac Soc 2021; 18:938–941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hoffmann TC, Glasziou PP, Boutron I, et al. : Better reporting of interventions: Template for intervention description and replication (TIDieR) checklist and guide. BMJ 2014; 348:g1687. [DOI] [PubMed] [Google Scholar]
- 12.Harris PA, Taylor R, Minor BL, et al. ; REDCap Consortium: The REDCap consortium: Building an international community of software platform partners. J Biomed Inform 2019; 95:103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kroenke K, Spitzer RL, Williams JB: The PHQ-9: Validity of a brief depression severity measure. J Gen Intern Med 2001; 16:606–613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kroenke K, Spitzer RL, Williams JB: The patient health questionnaire-2: Validity of a two-item depression screener. Med Care 2003; 41:1284–1292 [DOI] [PubMed] [Google Scholar]
- 15.Rhee C, Zhang Z, Kadri SS, et al. ; CDC Prevention Epicenters Program: Sepsis surveillance using adult sepsis events simplified eSOFA criteria versus Sepsis-3 Sequential Organ Failure Assessment criteria. Crit Care Med 2019; 47:307–314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kent DM, Paulus JK, van Klaveren D, et al. : The Predictive Approaches to Treatment effect Heterogeneity (PATH) statement. Ann Intern Med 2020; 172:35–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shankar-Hari M, Phillips G, Levy M: Assessment of definition and clinical criteria for septic shock: For the third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA 2016;315:801–810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schulz KF, Altman DG, Moher D; CONSORT Group: CONSORT 2010 statement: Updated guidelines for reporting parallel group randomised trials. BMJ 2010; 340:c332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Steyerberg EW, Bossuyt PM, Lee KL: Clinical trials in acute myocardial infarction: Should we adjust for baseline characteristics? Am Heart J 2000; 139:745–751 [DOI] [PubMed] [Google Scholar]
- 20.Hauck WW, Anderson S, Marcus SM: Should we adjust for covariates in nonlinear regression analyses of randomized trials? Control Clin Trials 1998; 19:249–256 [DOI] [PubMed] [Google Scholar]
- 21.Pocock SJ, Assmann SE, Enos LE, et al. : Subgroup analysis, covariate adjustment and baseline comparisons in clinical trial reporting: Current practice and problems. Stat Med 2002; 21:2917–2930 [DOI] [PubMed] [Google Scholar]
- 22.Prescott HC, Iwashyna TJ, Blackwood B, et al. : Understanding and enhancing sepsis survivorship. Priorities for research and practice. Am J Respir Crit Care Med 2019; 200:972–981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leppin AL, Gionfriddo MR, Kessler M, et al. : Preventing 30-day hospital réadmissions: A systematic review and meta-analysis of randomized trials. JAMA Intern Med 2014; 174:1095–1107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Parker VA, Lemak CH: Navigating patient navigation: Crossing health services research and clinical boundaries. Adv Health Care Manag 2011; 11:149–183 [DOI] [PubMed] [Google Scholar]
- 25.Reuland DS, Brenner AT, Hoffman R, et al. : Effect of combined patient decision aid and patient navigation vs usual care for colorectal cancer screening in a vulnerable patient population: A randomized clinical trial. JAMA Intern Med 2017; 177:967–974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shlay JC, Barber B, Mickiewicz T, et al. : Peer reviewed: Reducing cardiovascular disease risk using patient navigators, Denver, Colorado, 2007–2009. Prev Chronic Dis 2011; 8:A143. [PMC free article] [PubMed] [Google Scholar]
- 27.Bloom SL, Stollings JL, Kirkpatrick 0, et al. : Randomized clinical trial of an ICU recovery pilot program for survivors of critical illness. Crit Care Med 2019; 47:1337–1345 [DOI] [PubMed] [Google Scholar]
- 28.Haines KJ, McPeake J, Hibbert E, et al. : Enablers and barriers to implementing ICU follow-up clinics and peer support groups following critical illness: The thrive collaboratives. Crit Care Med 2019; 47:1194–1200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rubin EB, Buehler AE, Halpern SD: States worse than death among hospitalized patients with serious illnesses. JAMA Intern Med 2016; 176:1557–1559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fried TR, Bradley EH, Towle VR, et al. : Understanding the treatment preferences of seriously ill patients. N Engl J Med 2002; 346:1061–1066 [DOI] [PubMed] [Google Scholar]
- 31.Rhee C, Jones TM, Hamad Y, et al. ; Centers for Disease Control and Prevention (CDC) Prevention Epicenters Program: Prevalence, underlying causes, and preventability of sepsis-associated mortality in US acute care hospitals. JAMA Netw Open 2019; 2:e187571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Auriemma CL, Harhay MO, Haines KJ, et al. : What matters to patients and their families during and after critical illness: A qualitative study. Am J Crit Care 2021; 30:11–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brinkman-Stoppelenburg A Rietjens JA van der Heide A: The effects of advance care planning on end-of-life care: A systematic review. Palliat Med 2014; 28:1000–1025 [DOI] [PubMed] [Google Scholar]
- 34.Klingler C, in der Schmitten J, Marckmann G: Does facilitated advance care planning reduce the costs of care near the end of life? Systematic review and ethical considerations. Palliat Med 2016; 30:423–433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Heyland DK, Barwich D, Pichora D, et al. ; ACCEPT (Advance Care Planning Evaluation in Elderly Patients) Study Team; Canadian Researchers at the End of Life Network (CARENET): Failure to engage hospitalized elderly patients and their families in advance care planning. JAMA Intern Med 2013; 173:778–787 [DOI] [PubMed] [Google Scholar]
- 36.Khandelwal N, Curtis JR, Freedman VA, et al. : How often is end-of-life care in the United States inconsistent with patients’ goals of care? J Palliat Med 2017; 20:1400–1404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Prescott HC, Girard TD: Recovery from severe COVID-19: Leveraging the lessons of survival from sepsis. JAMA 2020; 324:739–740 [DOI] [PubMed] [Google Scholar]
- 38.ClinicalTrials.gov: Implementation and Effectiveness of Engagement and Collaborative Management to Proactively Advance Sepsis Survivorship. 2020. Available at: https://ClinicalTrials.qov/show/NCT04495946. Accessed Auqust 31,2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.