Abstract
The global population of people over the age of 65 is increasing and expected to reach 1.5 billion by 2050. While aging is associated with a number of chronic illnesses including dementia, the underlying contribution of alcohol misuse in the elderly is understudied. Long-term chronic alcohol misuse can lead to alcohol-associated liver disease, consisting of a spectrum of pathologies including steatosis and cirrhosis; liver disease can be rapidly accelerated by non-resolving inflammation. Despite this knowledge, the mechanistic underpinnings of dysregulated host immunity and accelerated liver disease progression in the aged by alcohol is unknown. Alcohol misuse in the elderly is on the rise and aging is associated with progressive increases in pro-inflammatory cytokine production. The goals of current study are to characterize bioactive lipid mediators of inflammation by making use of a murine model of ethanol-induced liver disease in 3-month old and 20-month old mice by quantitatively profiling selected oxylipins in liver, brain and plasma. Following chronic ethanol exposure, liver injury, steatosis, and senescence markers were robustly increased in aged mice compared to young adult mice. Expression of proinflammatory cytokines and lipid metabolizing enzymes were increased in liver by both age and ethanol feeding. Lipoxygenase-derived lipid metabolites 9- and 13-hydroxy-octadecadienoic acid and 15-hydroxyeicosatetraenoic acid were increased in liver and plasma in ethanol-fed aged mice and positively correlated with liver injury. In plasma, 9,10-dihydroxy-octadecenoic acid/epoxy-octadecenoic acid plasma ratios correlated with liver injury in ethanol-fed aged mice. Finally, 15-hydroxyeicosatetraenoic acid and 9,10-dihydroxy-octadecenoic acid positively correlated between liver and plasma. Importantly, leukotriene E4, 9,10-dihydroxy-octadecenoic acid and 15-hydroxyeicosatetraenoic acid increased lipid accumulation and ER stress in cultured AML12 hepatocytes. These data highlight the complexity of lipid metabolite networks but identify key mediators that may be used for diagnostic and prognostic markers in early stages of alcohol-related liver disease in patients of all ages.
Keywords: Alcohol-associated liver disease, aging, inflamm-aging, oxylipins, LC-MS/MS
Graphical Abstract

Introduction
A growing population at risk to the damaging effects of alcohol misuse and organ damage are the aged. The average lifespan of United States residents is ever increasing and reports indicate that ~45% of adults age 60 years or older drink alcohol1. The sensitivity of the elderly population (>65 years) to alcohol is compounded by multiple risk factors contributing to impaired elimination of alcohol and prolonged ethanol effects2. While individuals with advanced age have increased risk for chronic diseases including liver disease3, there remain large gaps in knowledge related to the mechanisms and consequences of alcohol misuse in the aged. Importantly, because progressive liver disease is associated with hepatic encephalopathy and cognitive decline4,5, it is paramount to identify mechanisms leading to alcohol-induced end organ damage, including identifying potential factors contributing to organ-organ crosstalk in early stages of disease pathogenesis.
A shared theme among alcohol-related pathologies and aging include persistent, non-resolving inflammation. Despite clinical and experimental evidence demonstrating that “healthy” individuals with advanced age have a basal inflammatory state referred to as “inflamm-aging”6,7, these age-related perturbations in host immunity have been linked to both 1) “hypoinflammatory” (e.g. delayed or reduced) responses including a slower response to infection8 or 2) “hyperinflammatory” (e.g. excessive) responses leading to tissue injury9. While coordinated and controlled inflammatory processes are associated with normal immune function, this rheostat becomes imbalanced with age and the mechanisms driving immune dysregulation are not well understood. Heavy alcohol use of 7 or more drinks/week in older adults is linked to elevated serum interleukin-6 (IL-6) levels10 and recent reports using murine models of ethanol exposure have described elevated inflammatory responses to ethanol, including increased hepatic expression of select proinflammatory cytokines (e.g. interleukin-1beta [IL-1β]) and chemokines (e.g. C–C motif chemokine ligand 2 [CCL2]) compared to young counterparts9. Despite our appreciation that “inflamm-aging” is associated with biologic aging and frailty11, we have limited understanding regarding the impact of alcohol misuse on “inflamm-aging” and liver disease pathogenesis in the aged.
Chronic inflammation can have profound effects on cellular metabolism12. Under conditions of oxidative stress or inflammation, n3- and n6-polyunsaturated fatty acids (PUFA) containing phospholipids and cholesterol esters in cellular membranes and lipoproteins can readily be oxidized to a number of oxidation products termed oxylipins13; oxidation can occur non-enzymatically via free-radical induced lipid peroxidation or enzymatically via lipoxygenases (LOXs), cyclooxygenases (COXs), and cytochrome P450 (CYP450) epoxygenases. Oxylipins are formed as a consequence of inflammation (increased free radical formation and upregulation of LOX/COX/CYP genes) and, depending on which mediators are produced, can further amply14, or accelerate the resolution15–18, of inflammation. For example, CYP450–formed epoxy fatty acid metabolites of Linoleic Acid (LA) (EpOMEs), which are hydrolyzed by the soluble epoxide hydrolase enzyme (sEH) to dihydroxyoctadecenoic acids (DiHOMEs), are associated with acute respiratory distress syndrome in severe COVID-19 patients and in murine models of burn injury19. 15-hydroxyeicosatetraenoic acid (15-HETE), an eicosanoid derived from LOX, CYP and non-enzymatic oxidation of arachidonic acid (AA), is well characterized to modulate vasoconstriction and drive hypoxic conditions20. Variations in PUFA-derived metabolite production have been described with liver disease13,21–27. Lipid mediators derived from n6-PUFA AA and n3-PUFA eicosapentaenoic acid (EPA) and docosahexanenoic acid (DHA) are particularly increased in individuals with alcohol use disorder and alcohol-associated hepatitis (AH), positively correlating with clinical outcomes of steatosis and inflammation and negatively correlating with 90-day survival22. Moreover, linoleic acid (LA) metabolites, including 13-hydroxy-octadecadienoic acid (13-HODE) and 9,10-dihydroxy-octadecenoic acid (9,10-DiHOME [also known as leukotoxin diol]) positively correlate with liver damage while anti-inflammatory cytochrome P450-derived LA epoxides 9,10-epoxy-octadecenoic acid (9,10-EpOME) and 12,13-EpOME decreased in early stages of AH13. Proinflammatory oxylipins, including LOX-derived 5-, 12-, and 15-hydroxyeicosatetraenoic acid (5-, 12-, 15-HETE) and 9- and 13- hydroxy-octadecadienoic acids (9- and 13-HODE) are increased in patients with alcohol-related cirrhosis24. Taken together, oxylipin production is skewed with pro-inflammatory oxylipins surpassing pro-resolving oxylipins in alcohol-associated liver disease (ALD).
Despite these key findings, there is a gap in research defining the specific changes in oxylipins associated with alcohol misuse, liver disease and advanced age. There is evidence of impaired lipid metabolism with advanced age28, and the increase in certain oxylipin metabolites could reflect “inflamm-aging” and dysregulated host immunity and increased tissue damage associated with alcohol abuse in the elderly. Identifying key lipid mediators specifically impacted by age, and ethanol, could have important implications to normalize inflammation in elderly individuals who consume alcohol. Therefore, to begin to elucidate these specific metabolic changes, we made use of a chronic, ad libitum model of ethanol feeding superimposed with advanced age and quantitative metabolomic methods to profile the plasma and tissue oxylipidome. We find that aged mice, compared to young, have increased liver injury and steatosis, and age- but not ethanol-related increases in pro-inflammatory cytokines and LOX expression in the liver after chronic ethanol feeding. Importantly, we find LOX-derived 9- and 13-HODE from liver tissue, and 15-Hydroxyeicosatetraenoic acid (15-HETE) and leukotriene E4 (LTE4) plasma levels are significantly increased in aged, ethanol-fed mice compared to young. Moreover, challenge with LTE4, 9,10-DiHOME and 15-HETE, alone and in combination with ethanol, increased lipid accumulation and measures of endoplasmic reticulum (ER) stress in cultured hepatocytes. Taken together, these data demonstrate for the first time that specific oxylipins are impacted by ethanol and advanced age, which may provide insight to dysregulated host immunity in aged individuals who consume alcohol. Importantly, we find for the first time, that specific oxylipins impacted by age can negatively influence hepatocyte health during ethanol exposure. As the number of individuals aged 65 or older is expected to exceed 1.5 billion by 205029, gaining a better understanding of the mechanisms leading to dysregulated host immunity occurring with advanced age will aid in better patient healthcare and outcomes.
Materials and biochemical methods
Reagents:
Oxylipin Standards were purchased from Cayman Chemical (Ann Arbor, Michigan) or Biomol (Plymouth Meeting, PA). Oasis HLB (Hydrophilic-Lipophilic Balanced) solid phase extraction cartridges were obtained from Waters (Milford Massachusetts). Acetic acid, water (HPLC grade), methanol (LC/MS grade), acetonitrile (LC/MS grade) and ammonium hydroxide were obtained from Fisher Scientific (Fair Lawn, New Jersey). cOmplete Protease Inhibitor tablets were from Roche. Irradiated control and ethanol Lieber-DeCarli Diets were from Bio-Serv (Frenchtown, NJ).
Ethanol feeding trials:
All animal procedures were approved by the Institutional Animal Care and Use Committee at the University of Colorado Anschutz Medical Campus. Women are at greater risk ALD30; accordingly, female mice were used. Two independent experiments were performed (Cohort 1 and 2). Lieber-DeCarli ‘82 Shake and Pour ethanol (catalog: F1258SP) and control (catalog: F1259SP) diets were purchased from Bio-Serv (Flemington, NJ). The caloric profile of the ethanol diet consisted of 318, 135, 359, and 151 kcal/L of ethanol, carbohydrates, fat, and protein while the control diet consisted of 490, 359, and 151 kcal/L of carbohydrates, fat, and protein, respectively. The fat profile of the diet consisted of C18:2 Linoleic Acid (9.3 g/L), C18:3 Linolenic Acid (0,3 g/L), saturated fat (5.2 g/L), monounsaturated fat (23.5 g/L) and polyunsaturated fats (9.7 g/L); fat sources were from casein, olive oil, corn oil and safflower oil. 50 mL glass feeding tubes (catalog: 9019) were employed to facilitate ethanol and control diet feeding within rodent cages and were also purchased from Bio-Serv (Flemington, NJ). Female mice were purchase from Jackson Laboratories (Bar Harbor, ME). C57BL/6J wild-type [WT], aged 3 months (young) and 18 months (aged) were randomized (2 mice/cage) into pair- and ethanol-fed groups, adapted to control liquid diet for 2 days and fed increasing concentrations of ethanol for 25 days as previously described31–33; briefly, ethanol-fed mice were acclimated to ethanol as follows: 1% v/v for 2 days, 2% v/v for 2 days, 4% v/v (22% kcal) for 1 week, 5% v/v (27% kcal) for 1 week, and lastly 6% v/v (32% kcal) for 1 week and is denoted as 32%,d25. Ethanol-fed mice were allowed ad libitum access to liquid diet. Control mice were pair-fed a diet that received isocalorically-substituted maltose dextrins for ethanol. Diets were changed daily in the afternoon at 2pm. Ethanol and pair-fed mice increased their body mass over the course of the 25d protocol. At the conclusion of these experiments, mice were anesthetized, blood was taken from the inferior vena cava, and livers were perfused with 0.9% saline via the hepatic portal vein. Livers were excised and portions were stored in RNAlater (Ambion, Austin, TX), fixed in formalin or frozen in optimal cutting temperature (OCT) compound (Sakura Finetek USA, Inc, Torrance, CA) for histological analysis, or snap frozen in liquid nitrogen and stored at −80°C for later analysis. Blood was transferred into EDTA-containing microtainer tubes (BD Biosciences) and plasma was isolated following centrifugation and stored at −80°C until further analysis. For histological analysis, formalin-fixed tissues were paraffin-embedded, sectioned and stained with hematoxylin and eosin. Images were captured using an Olympus Microscope.
Plasma Ethanol Measurements:
Blood samples were collected from mice on the chronic ethanol diet (Cohort 1) during the active drinking phase, between 10:30pm and 12:00am; samples were collected on days 4, 11, 18, and 24 to measure blood ethanol levels when mice were consuming 2%, 4%, 5%, and 6% ethanol-containing diets. Blood was collected via tail nick into heparinized microhematocrit capillary tubes (Fisher Scientific, Pittsburgh, PA) and centrifuged in a microcentrifuge with a microhematocrit rotor for 3 minutes. Fresh plasma samples were assayed for ethanol using a commercially available enzymatic assay kit (Ethanol L3K kit, Sekisui Diagnostics, Burlington, MA).
Biochemical Assays:
Plasma samples were assayed for alanine and aspartate aminotransferase activity using an enzymatic assay kit according to the manufacturers protocol (Sekisui Diagnostics, Burlington, MA). Flash-frozen liver samples and plasma were used to quantify hepatic and plasma triglyceride levels using a commercially available kit from Pointe Scientific (Lincoln Park, MI) per the manufacturer’s protocol.
Liver Histology and Immunohistochemistry:
For histologic analysis, formalin-fixed tissues were paraffin embedded, sectioned, and stained with hematoxylin and eosin. Paraffin-embedded liver sections were deparaffinized and stained with antibodies against NIMP-R14 (Novus Biologicals, cat NB600–1387), PCNA (Millipore, cat# MAB424R) and p53 (Novus Biologicals, cat# NB200–103). Frozen liver sections were mounted on glass slides and stained for Oil Red O as previously described34 or β-Galactosidase activity according to the manufacturer’s protocol (Cell Signaling cat#9860). Images were acquired using an upright Olympus BX43 Microscope (Waltham, MA). Formalin and OCT images are coded at the time of collection for a blinded analysis; at least three images were acquired per tissue section, and positive staining was quantified using Image J software. No specific immunostaining was seen in sections incubated with PBS/blocking buffer rather than the primary antibody (data not shown). Pathologic changes in the liver were quantified using a procedure adapted for mice35,36 from the validated histologic scoring system established by Kleiner and Brunt37. Briefly, the extent of steatosis, the presence of inflammatory cells and foci, features of hepatocyte injury (e.g. ballooning, acidophilic bodies and necrotic cells), reactive tissue changes (e.g. ductal reaction, thickened peri-ductal tissue and hepatocyte polyploidization) were determined by microscopic evaluation of H&E-stained tissue sections by a trained histopathologist. The entire liver section from each mouse was scored by the histopathologist who was blinded to the treatment of the mice.
Liver Homogenates and Western Blotting:
Protein lysates were made from frozen liver in lysis buffer containing: 0.5% Triton X-100, 20 mM HEPES (pH 7.4), 150 mM MgCl2, 2 mM EGTA and protease and phosphate inhibitor cocktail (Roche Diagnostics, Indianapolis, IN). Protein concentrations were determined by the DC Protein Assay (Biorad, Hercules, CA) and samples were denatured at 95°C in laemmli buffer. Samples were separated on SDS-PAGE gels, transferred to Immobilon-Psq PVDF membranes using a semi-dry transfer apparatus (Biorad, Hercules, CA), and blocked in 3% bovine serum albumin/Tris-buffered saline containing 0.01% Tween-20. Membranes were probed with antibodies specific for ADH1 (Cell Signaling cat#52955) C3 (ICL labs, cat#GC3–90A-Z), CYP2E1 (Abcam, ab28146), GRP78 (Stressgen cat#SPA-826), XBP-1 (Abcam, cat#37152), HSC70 (Santa Cruz Biotechnologies, cat# sc-7298), Actin (Sigma, A5441), and GAPDH (Millipore, MAB374). Horseradish peroxidase- and AF488-conjugated secondary antibodies (Jackson ImmunoResearch, West Grove, PA; Invitrogen, Waltham, MA) were applied, and membranes were developed using Immobilon Western Developing reagents (Millipore, Temecula, CA). Chemiluminescence or fluorescence was visualized using a ChemiDoc Imaging System (Bio-Rad, Hercules, CA).
Alcohol Dehydrogenase 1 (ADH1) Activity in Liver Homogenates:
ADH activity was assessed according to Pikkarainen PH et al38. Briefly, livers (50 mg) were homogenized in 400μL of 0.25M ice-cold sucrose buffer containing 1% Triton X-100 and supernatants were assayed for protein (DC Lowry, Biorad) and ADH1 activity after centrifugation at 5,000 x g for 10 minutes at 4°C. The total reaction volume was 200μl consisting of the lysate, pyrophosphate-glycine buffer, 1.0mM NAD; 20μM 4-methylpyrazole to inhibit ADH activity was used as a negative control. The plate reader was heated to 37 °C prior to the addition of ethanol as substrate. Conversion of NAD+ to NADH was measured at 340nm. One unit of ADH1 activity was defined as the amount of enzyme needed to catalyze the formation of 1 μmol NADH over ten minutes and are expressed as μunits of enzyme activity/mg of protein sample.
RNA Isolation and Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR):
RNA was isolated from liver (<30mg) stored in RNAlater using RNeasy Mini kits per the manufacturer’s instructions (Qiagen, Germantown, MD). 4 μg of liver RNA was reverse transcribed using SuperScript IV VILO master mix and analyzed with PowerSYBR qRT-PCR kits (ThermoFisher, Grand Island, NY) on a QuantStudio Real-Time PCR analyzer (Applied Biosystems, La Jolla, CA). Relative messenger RNA (mRNA) expression was determined using gene-specific primers (supplementary table 1). Statistical analyses were performed on the ΔCt values (average Ct of gene of interest – average Ct of 18S).
Standard and Sample Preparation for Oxylipin Measurement:
All standards and internal standards used for LC/MS/MS analysis of oxylipins were purchased from Cayman Chemical (Ann Arbor, MI). All HPLC solvents and extraction solvents were HPLC grade or better. An internal standard solution was made by combining lipid mediators of interest at a concentration of 1ug/mL in ethanol. This solution was then diluted into 10 calibration standards over a concentration range of 0.05ng/mL to 50ng/ml. Concentrations were adjusted for liver samples (1ng/mL to 1000ng/mL). The internal standard solution containing 10 representative, isotopically labeled lipid mediators (8-iso-PGF2a-d4, PGE2-d4, PGF2a-D9, Resolvin D2-d5, Resolvin D1-d5, LTD4-d5, LTE4-d5, 9(S)-HODE-d4, 5(S)-HETE-d8) were used for both calibration and sample extraction. All stock solutions were stored at −80 until use.
Tissue (100mg) and plasma (100ul) from Cohort 2 were used for oxylipin quantitation. Immediately after sample collection at the time of tissue harvest, samples were snap frozen in liquid nitrogen and stored for less than one week prior to processing. Plasma samples were prepared using solid-phase extraction (SPE) according to published reports39,40. Briefly, proteins were precipitated from 100 uL of plasma by addition 400 uL ice-cold methanol and 10uL of an internal standard solution in 1.5mL microfuge tube that was vortexed and incubated on ice for 15 minutes. Precipitates were removed via centrifugation for 10 minutes (14,000 RPM) and supernatant was transferred to a new tube. The pellet was subjected to a second extraction round (100uL ice-cold methanol and pellets separated via centrifugation) and supernatants were combined with the first supernatant. Samples were dried to completion in a vacuum centrifuge at 55°C then immediately reconstituted in 1 mL of 90:10 water:methanol prior to purification by SPE. Liver tissue was processed as previously described41. Briefly, homogenization tubes that were prewashed with methanol were chilled in dry ice for 15 minutes prior to adding 100mg of pulverized liver tissue. After adding 500uL of pre-chilled methanol (−20°C), lipids were extracted from liver tissue for 10 minutes and pellets were separated via centrifugation at 18,000xg at 4°C for 10 minutes. Supernatants were transferred to clean 1.5 mL tubes and 10uL of internal standard was added to each sample prior to sample evaporation in a vacuum centrifuge. Samples were reconstituted in 1mL 90:10 methanol:water before SPE.
Oxylipins were enriched using SPE. Briefly, Strata-X 33 um 30 mg/1 mL polymeric Reverse Phase SPE cartridges (Phenomenex, Torrance, CA) were prewashed with 2 × 1 mL of LC/MS grade methanol and preconditioned with 2 × 1 mL of LC/MS grade water. Plasma and Liver samples were loaded into the cartridges, washed with 1mL 10% methanol and analytes were eluted into MRQ vials with 1mL methyl formate and dried under nitrogen. Residues were reconstituted with 20uL ethanol (200-proof) and vortexed.
Liquid Chromatography-Tandem Mass Spectrometry (LC/MS/MS):
Lipid mediators were separated using on-line trapping and enrichment followed by reverse phase chromatography as previously described41 using an Agilent 1260 autosampler (Agilent Technologies, Santa Clara, CA, USA), an Agilent 1260 binary loading pump (pump 1), an Agilent 1260 binary analytical pump (pump 2), and a 6-port switching valve. Pump 1 buffers consisted of 0.1% formic acid in water (solvent A) and 9:1 v:v acetonitrile:water with 0.1% formic acid (solvent B). Pump 2 buffers consisted of 0.01% formic acid in water (solvent C) and 1:1 v:v acetonitrile:isopropanol (solvent D). 10 μL of the extracted sample or calibration standard was injected onto an Agilent SB-C18 2.1X5mm 1.8um trapping column using pump 1 at 2 mL/min for 0.5 min with a solvent composition of 97% solvent A: 3% solvent B. At 0.51 min the switching valve changed the flow to the trapping column from pump 1 to pump 2. The flow was reversed and the trapped oxylipins were eluted onto an Agilent Eclipse Plus C-18 2.1X150 mm 1.8 um analytical column using the following gradient at a flow rate of 0.3 mL/min: hold at 75% solvent A:25% solvent D from 0–0.5 min, a linear gradient from 25–75% D over 20 min followed by an increase from 75–100% D from 20–21 min, then holding at 100% D for 2 min. During the analytical gradient, pump 1 washed the injection loop with 100% B for 22.5 min at 0.2 mL/min. Both the trapping column and the analytical column were re-equilibrated at starting conditions for 5 min before the next injection.
Tandom mass spectrometric analysis was performed on an Agilent 6490 triple quadrupole mass spectrometer in negative ionization mode. The HPLC column eluent was diverted to waste until 4 minutes, then diverted to the electrospray source from 4 – 23 minutes. Droplet desolvation was achieved using heated (250°C) drying gas flowing at 15 L/minute and a nebulizer pressure of 35 PSI. Spray was induced with a capillary voltage of 3500 V. The optimal fragmentor voltage and collision energy for each analyte of interest was determined using flow injection analysis41. Quantitation of lipid mediators was performed using a routine internal standard method. All target analytes and internal standards were monitored for quantitation by extracting ion chromatograms for each respective transition ion as previously described41 using Agilent Masshunter Quantitative Analysis software. Calibration curves for each analyte were calculated using linear regression. Plasma samples were quantitated using the calibration curves to obtain the column concentration, followed by multiplication of the results by the appropriate dilution factor to obtain the concentration in pg/mL. To ensure consistent quantitation throughout the study and standard solution stability, reference standard calibration curves were analyzed for consistency of slope.
Culture and Treatment of AML12 Cells:
AML12 mouse hepatocytes were obtained from ATCC and cultured in a 1:1 mixture of Dulbecco’s modified Eagle’s medium and Ham’s F12 medium (Sigma Aldrich) with 1X insulin, transferrin and selenium (Gibco), 1X dexamethasone (Cayman Chemical), 10% fetal bovine serum and 1% penicillin streptomycin. AML12 cells were used because they replicate and differentiate while remaining non-tumorigenic42 and efficiently metabolize ethanol43. Cells were sub-cultured at a density of 0.044 × 106 cells/cm2 in a 24-well plate; 24hrs later cells were then treated with 50mM ethanol33. Following 24hr culture with ethanol, cells were challenged with 100 ng/mL, 500ng/mL or 1ug/mL of LTE4 (Cayman Chemical Item No. 20410), 9,10-DiHOME (Cayman Chemical Item No. 53400), or 15-HETE (Cayman Chemical Item No. 34700) with 50mM ethanol, or not, in serum- and dexamethasone-free media for 14–24 hours. Cells were stained with Bodipy493/503 (Invitrogen) according to the manufacturer’s instructions; after two consecutive 1xPBS washes, live cell imaging solution (Molecular Probes, cat A14291DJ) was added to the cells and live cell imaging was performed using the Incucyte Live Cell Imaging System (Sartorius, Arvada CO). Images were exported and fluorescence intensity/area was calculated using Image J software. Cell lysates were prepared using RIPA buffer containing protease and phosphatase inhibitor cocktail (Roche); proteins were quantified using the DC Lowry Kit (BioRad) and samples were denatured at 95°C in laemmli buffer.
Statistical Analysis:
Values reported are means ± SEM. Data were collected from two independent different feeding trials. The data were analyzed using GraphPad Prism Version 9.3.1 (San Diego, CA) using unpaired student t-test for parametric data or two-way analysis of variance (ANOVA) to compare differences between groups and Sidak’s post-hoc analysis was used to adjust for multiple comparisons. Oxylipin molecules with >20% missing observations were excluded from analyses. Concentrations are reported as picogram/mL (pg/mL) and pg/mg tissue. Spearman correlation was conducted to correlate liver oxylipins with liver injury parameters (ALT/TG) and between liver and plasma compartments.
Results
Aged mice have elevated chronic ethanol-induced liver injury
Aging impacts a multitude of cellular processes, including the metabolism of ethanol2. In an effort to better understand the impact of chronic ethanol feeding in aged mice, young adult (3 mo) and aged adult (18mo) mice were fed ethanol-containing diets for 25 days (32%, d25). To first determine baseline differences in ethanol metabolism, plasma ethanol was quantified throughout the feeding paradigm, the night prior to increasing the percentage of ethanol in the diet on days 4, 11, 18, and 24 to capture measurements when mice consumed 2%, 4%, 5%, and 6% ethanol-containing diets, respectively (Figure 1A). Plasma ethanol levels increased throughout the feeding trial in both young and aged adult mice; no differences in plasma ethanol levels were detected between the age groups at d4, d11, d18. Plasma ethanol levels were decreased in aged adult mice compared to young adult mice at d24. Mice receiving isocaloric diets (pair-fed) had no detectable levels of level (data not shown). Because plasma ethanol levels can be largely influenced by when, and how much diet is consumed two hours prior to blood draws, we made use of an acute ethanol exposure paradigm in young and aged mice to control a bolus ethanol dose and to eliminate variability associated with ad libitum ethanol feeding (Supplemental Figure 1). Young and aged adult mice were gavaged with 3g/kg ethanol and blood was drawn 30-, 60-, and 90-min after the ethanol gavage. Plasma ethanol levels dynamically and acutely increased after ethanol gavage, with levels peaking at 60 minutes in aged mice and trending to still increase 90 minutes after ethanol gavage in young mice (FigS1). Plasma ethanol levels trended to be higher in aged compared to young mice (p = 0.07) at 30 minutes but were not significantly different from each other.
Figure 1. Aged adult mice have more pronounced liver injury after chronic ethanol feeding compared to young adult mice.
Young and aged adult WT mice were allowed free access to ethanol (32%,d25) or pair-fed control diets. (A) Plasma ethanol concentrations were measured 3h into the feeding/dark cycle from WT mice throughout the chronic ethanol feeding protocol. Values represent means ± SEM from Cohort 1, n=4 for pair-fed (data not shown) and n=6 for ethanol-fed mice. Liver Lysates were prepared and proteins separated by SDS-PAGE. ADH1 and HSC70 (loading control) were measured by Western blot. Values with different superscripts are significantly different from each other (P < 0.05). Liver lysates were assayed for ADH1 activity. (B) Body weights were measured throughout the chronic ethanol feeding period and reported as initial and final weights. (C) Diet consumption was measured daily and weekly averages were calculated/cage. N= 6 cages/group (n=12 mice/group). Hepatomegaly was determined by taking liver weights at the time of tissue harvest/final body weight. (D) ALT and AST activity was measured in plasma. (E) Liver lysates were prepared and proteins separated by SDS-PAGE. C3, CYP2E1 and GAPDH (loading control) were measured by Western blot. Relative expression is denoted as arbitrary units of density. Data, unless denoted, are combined from two independent feeding trials; values represent means ± SEM, n=8 for pair-fed and n=12 for ethanol-fed mice. *Values are significantly different from each other (P<0.05).
Since we found that aged mice had lower BAC compared to young counterparts on d24, and because the elimination kinetics of ethanol after a bolus gavage versus ad libitum in the diet involve different alcohol dehydrogenase (ADH) isoforms44, we next measured hepatic ADH1 expression and activity in both age groups that did, and did not receive chronic ethanol in the diet (Figure 1A). ADH1 expression was lower in aged pair-fed animals compared to young pair-fed mice. Despite no differences between pair- and ethanol-fed young mice, ADH1 expression was normalized by ethanol feeding in aged mice. Hepatic ADH1 activity was increased by ethanol feeding in young mice; ADH1 activity trended higher at baseline in aged mice which was not further changed by ethanol feeding (Figure 1A).
Body weights and diet consumption was monitored throughout the study (Figure 1B/C). Aged mice were heavier at the start and finish of the feeding paradigm compared to young adult mice, but there were no differences in weight gain between pair- and ethanol-fed groups of both ages (Figure 1B). Aged mice consumed more diet/day compared to young adult mice, but when adjusted for body weight (data not shown) there were no differences in diet consumption between the age groups (Figure 1C). Liver weights were normalized to final body weight to determine hepatomegaly; both age- and ethanol-feeding increased liver:body weight ratios (Figure 1C). After 25d, plasma alanine transaminase (ALT) and aspartate transaminase (AST) activities were increased in young adult mice compared to pair-fed controls, which were further increased in ethanol-fed, aged mice (Fig1D).
In murine ALD (mALD) and ALD, the deposition of complement activation fragments and expression of complement components increases in the plasma and liver31,33,34,45,46. The influence on aging on complement activation and deposition in liver is unclear, therefore, we evaluated a common markers of complement activation in the liver as a measure of tissue damage in our 25d ethanol exposure model. Complement component 3 (C3) is readily cleaved into activation fragments including C3b, C3c and C3d which deposit on damaged cells34; ethanol-feeding increased hepatic expression C3c in young adult mice, while C3b and C3d fragments were greatly increased in both pair-fed and ethanol-fed aged mice (Fig1E). Because the activity of the ethanol metabolizing enzyme, cytochrome P450 2E1 (CYP2E1) is reported to decline in aged male, but not female rats47, we next determined the hepatic expression of CYP2E1. Ethanol feeding increased the expression of CYP2E1; the magnitude of CYP2E1 upregulation was higher in young mice compared to aged mice (Fig1E).
Chronic ethanol feeding accelerates steatosis, reactive changes and cell injury in aged adult mice
The progression of ALD is characterized by marked histopathological changes including steatosis, hepatocyte ballooning, inflammation, ductular reaction and the presence of Mallory-Denk bodies48. After ethanol feeding, both young and aged adult mice developed steatosis characterized by microvesicular and macrovesicular structures observed on H&E-stained liver sections; aged mice exhibited more pronounced macrovesicular steatosis compared to ethanol-fed, young adult mice (Figure 2A). Importantly, aged mice on isocaloric diets had baseline elevations in steatosis compared to young counterparts. Histopathologic scoring of H&E stained liver sections revealed both age- and ethanol-dependent changes to the liver architecture. Inflammatory cell infiltrates and foci were increased in aged mice, but not further increased by ethanol feeding. While not observed in young mice, ethanol feeding increased the number of vesiculated macrophage clusters in aged mice (yellow arrows). Reactive changes, characterized by mild ductular reaction, polyploidization of hepatocytes, and thickening of peri-ductular tissue, was observed in ethanol-fed aged mice. Cell injury, characterized by acidophilic bodies and necrotic cells, was increased in aged mice after chronic ethanol feeding. Hepatic triglycerides were increased in ethanol-fed young mice compared to pair-fed controls; hepatic triglycerides were increased in aged ethanol-fed mice compared with young counterparts (Figure 2B). Plasma triglycerides were increased by ethanol feeding in young, but not aged mice (Figure 2B). Oil Red O staining of neutral lipid was consistent with the biochemical assessment of triglyceride and histopathologic scoring of liver sections (Figure 2 C/D). Immunohistochemical staining with the neutrophil detection antibody NIMPR-14 revealed a trending (but not significant) increase in hepatic neutrophils in ethanol-fed young mice; NIMPR-14 positive cells were slightly higher in aged mice, but were not different between ethanol-fed and pair-fed mice (Figure 2 C/D).
Figure 2. Inflammation, steatosis and reactive changes are increased by advanced age and chronic ethanol feeding.
Young and aged adult WT mice were allowed free access to ethanol (32%,d25) or pair-fed control diets. (A) Paraffin-embedded liver sections were deparaffinized followed by staining with hematoxylin and eosin and images were acquired using a 10x objective. Periportal (PV) and centrilobular (CV) regions are indicated. Vesiculated macrophages are highlighted (yellow arrows). Histopathologic scoring of 1) inflammation consisting of the average number of inflammatory foci/200x field (0 = no foci, 1 = 1–2 foci, 3 = 3–4 foci) and portal inflammation (0 = none-minimal; 2 = >30% of PVs); 2) reactive changes consisting of ductal reaction (0 = none, 1 = minor, 3 = robust) and hepatocyte polyploidization (no = <10% hepatocytes with >8N DNA/c, yes = >10% hepatocytes with >8N DNA/c); 3) cell injury consisting of acidophilic bodies (0 = none, 2 = few, 4 = many), necrotic cells (0 = none, 2 = present (1≥ per 100x field); 4) steatosis consisting of macrovesicular steatosis (0= <10%, 1= 10–33%, 2= 33–66%, 3= > 66%) and microvesicular steatosis (0 = not present (<5% of hepatocytes), 1 = present (5–25% of hepatocytes), 2 = present (>25% of hepatocytes). B) Hepatic triglyceride content was measured in whole liver tissue. Triglyceride content was measured in plasma. C/D) Frozen liver sections were stained with Oil Red O. Paraffin-embedded liver sections were deparaffinized followed by immunodetection of neutrophil marker NIMPR-14 and nuclei were counterstained with hematoxylin. Images were acquired using a ×10 objective. Oil Red O stained areas and NIMPR-14 positive cells were quantified using Image-J. E) Liver RNA was isolated and expression of pro-inflammatory mediators tnfα, il-6, ccl2, c5ar1 mRNA was detected in mouse livers using qRT-PCR. Data are from two independent trials; individual values are reported and represented as means ± SEM, n=8 pair-fed and 12 EtOH-fed mice/group. *Values were significantly different from each other (P<0.05).
Chronic ethanol feeding and advanced age increase hepatic pro-inflammatory mediator expression
Ethanol activates cellular components of host immunity, including tissue resident macrophages (e.g. Kupffer cells [KC]), to secrete cytokines/chemokines; tumor necrosis factor alpha (TNF-α) and interleukin-6 (IL-6) are key pro-inflammatory cytokines that contribute to liver injury during ethanol exposure and C-C motif chemokine ligand 2 (CCL2) drives monocyte and neutrophil recruitment into the liver to additionally control inflammation, infection and tissue injury. Chronic ethanol feeding increased tnfα, il-6 and ccl2 mRNA expression in young adult mice (Figure 2E). Hepatic tnfα, il-6 and ccl2 expression was increased in ethanol-fed aged mice compared to young, pair-fed mice; however, there were no differences between pair-fed and ethanol-fed aged mice. Pro-inflammatory cytokine mRNA expression was increased in aged pair-fed mice compared to young pair-fed mice, consistent with other studies9,49. Ethanol-induced complement activation can generate the potent anaphylatoxin C5a which can bind to its cognate g-protein coupled receptor (GPCR), Complement component C5a Receptor 1 (C5aR1) to increase the activation of innate immune cells. Ethanol feeding increased hepatic c5ar1 mRNA expression in young adult and aged adult mice compared to young pair-fed controls (Figure 2E).
Chronic ethanol feeding induces premature senescence in young mice
Cellular senescence is implicated to play an important role for aging and inflammation50. Because chronic alcohol abuse can increase cellular stress and reactive oxygen species (ROS) production51, and cellular senescence leads to the secretion of chemokines and cytokines (i.e. senescence-associated secretory phenotype [SASP])50, we next measured the growth arrest markers, p16 and p21(Figure 3A). Expression of p16 mRNA was increased by age, but not ethanol feeding. Ethanol feeding increased p21 mRNA expression in liver from young adult mice compared to pair-fed controls (Figure 3A). Age-associated increases in p21 were observed between young and aged mice; ethanol feeding further increased hepatic p21 mRNA expression in aged, compared to young mice. Senescence was further confirmed by reduced expression of the proliferation-associated marker, proliferating cell nuclear antigen (PCNA) in ethanol-fed young mice and aged mice (pair- and ethanol-fed) compared to young pair-fed controls (Figure 3B/C). p53 plays a pivotal role in the cellular response to stress and can lead to cellular senescence via p21-dependent cell-cycle arrest52. Aged pair-fed mice had increased p53 expression compared to young pair-fed mice. Ethanol feeding increased p53 expression and nuclear localization in both young and aged mice compared to pair-fed controls (Figure 3B/C). Furthermore, the number of β-galactosidase (β-gal) positive cells reflected trends of other measures of senescence, whereby ethanol and aging increased the number of β-gal cells with the morphological appearance of hepatocytes in the hepatic parenchyma (Figure 3B/C). Taken together, these data highlight that even in young mice, chronic ethanol feeding can elicit stress-induced premature senescence and that in the aged liver, selected measures of senescence are further increased by ethanol.
Figure 3. Senescence is accelerated by chronic ethanol feeding in young mice.
Young and aged adult WT mice were allowed free access to ethanol (32%,d25) or pair-fed control diets. A) Liver RNA was isolated and expression of senescence markers, p16 and p21 mRNA was detected in mouse livers using qRT-PCR. B/C) Paraffin-embedded liver sections were deparaffinized followed by immunodetection of PCNA and p53 and nuclei were counterstained with hematoxylin. Frozen liver sections were stained for β-galactosidase activity. Images were acquired using a ×10 objective and positive staining was quantified using Image-J. Data are from two independent trials; individual values are reported and represented as means ± SEM, n=8 pair-fed and 12 EtOH-fed mice/group. *Values were significantly different from each other (P<0.05).
COX/LOX genes are induced by ethanol and age
Several metabolic genes, including CYP450s, COX (COX-1/2) and LOX (5/12/15-LOX) enzymes, participate in the metabolism of lipid species. Inflammation and oxidative stress are key activators of COX-2 and 5/12/15-LOX53–55. While the aged are known to have suppressed ethanol metabolism, including the expression of cytochrome P450 genes2, the consequence of ethanol feeding on COX and LOX gene expression is not known. Hepatic prostaglandin synthetase 2 (ptges2/cox-2) mRNA expression was upregulated by ethanol in young and aged mice (Figure 4A). The response of our young animals is consistent with published reports of ptges2 upregulation in models of ALD using both rats and mice54,56. Ethanol feeding increased alox12 (12-LOX) mRNA expression in liver from young adult mice; alox5 (5-LOX), alox12 and alox15 (15-LOX) was increased in aged pair-fed and ethanol fed mice (Figure 4B).
Figure 4. Expression of cyclooxygenase and lipoxygenase enzymes are differentially impacted by ethanol and age.
Young and aged adult WT mice were allowed free access to ethanol (32%,d25) or pair-fed control diets. Liver RNA was isolated and expression of lipid metabolizing enzymes (A) ptgs2 and (B) alox5, alox12, alox15 mRNA was detected in mouse livers using qRT-PCR. Data are from two independent trials; individual values are reported and represented as means ± SEM, n=8 pair-fed and 12 EtOH-fed mice/group. *Values were significantly different from each other (P<0.05).
The Hepatic Oxylipidome is impacted by both ethanol and age
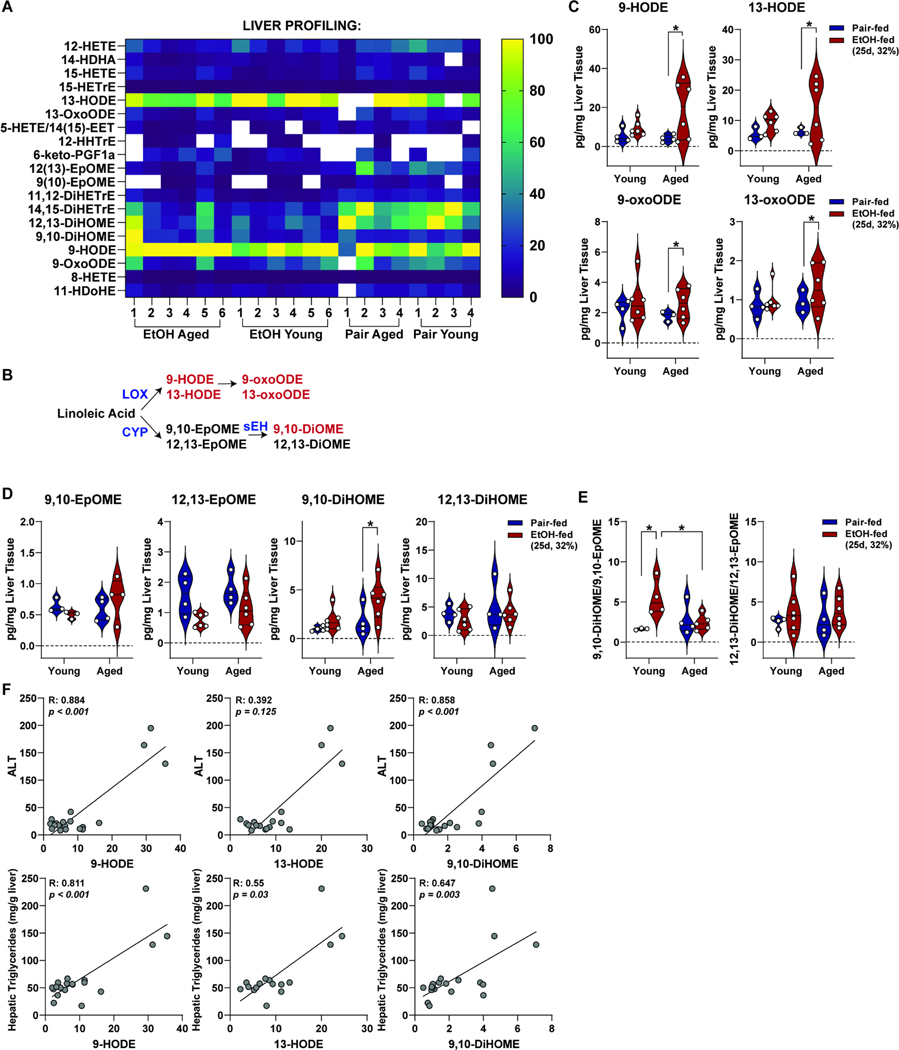
ALD is often referred to as a disease consisting of non-resolving inflammation. Proinflammatory mediators coordinating the dynamics of inflammation consist of protein mediators (e.g. cytokines) and bioactive lipids (e.g. oxylipins). To better understand the effects of age and ethanol on the oxipidome, we profiled bioactive lipid mediators in liver tissue from young and aged mice; of 55 selected metabolites for the panel, 19 metabolites were detected and are shown in a normalized heatmap to illustrate the least (dark blue) and most (yellow) abundant lipid species in liver (Figure 5A). A schematic of PUFA oxidation by LOX and P450/soluble epoxide hydrolase (s-EH) (Figure 5B) and absolute values of metabolites are shown (Figure 5C/D). Ethanol feeding to aged, but not young, mice increased LOX-derived 9-HODE, 13-HODE, and 9- and 13-oxooctadecadienoic acid (9-/13-OxoODE) metabolites in liver. No significant differences were observed in 9,10-EpOME, 12,13-EpOME and 12,13-DiHOME between pair-fed and ethanol-fed, young and aged mice; ethanol feeding increased 9,10-DiHOME in aged, but not young, mice.
Figure 5. Ethanol feeding to aged mice increased lipoxygenase-derived oxylipins in liver tissue.
Young and aged adult WT mice were allowed free access to ethanol (32%,d25) or pair-fed control diets. Lipids were extracted from liver tissue, enriched using solid-phase extraction and subjected to LS-MS/MS. (A) Heatmap of detected oxylipins from liver. Data are normalized by smallest to largest value in the dataset, with the largest value set as 100% (yellow) and the smallest values set as 0% (dark blue). Undetected values (lower limit was detection) are denoted as white. (B) n-6 Polyunsaturated fatty acid metabolism. Lipoxygenase (LOX)-derived metabolites 9- and 13-hydroxyoctadecadienoic acid (9-HODE, 13-HODE) and 9- and 13-Hydroxyoctadecadienoic acid (9-oxoODE and 13-oxoODE) and cyclooxygenase (CYP)-derived metabolites 9,10- and 12,13-Epoxy-9-octadecenoic acid (9,10-EpOME and 12,13-EpOME) and 9,10- and 12,13-Dihydroxyocadecenoic acid (9.10-DiHOME and 12,13-DiHOME). (C) Absolute values of LOX-derived metabolites 9-HODE, 13-HODE, 9-oxoODE, and 13-oxoODE are shown. (D) Absolute values of CYP-derived metabolites 9,10-EpOME, 12,13-EpOME, 9,10-DiHOME and 12,13-DiHOME are shown. E) Diol:epoxide ratios of 9,10-DiHOME/9,10-EpOME and 12,13-DiHOME/12,13-EpOME were calculated. Individual data points are reported and represented using violin plots. F) Spearman correlation of 9-HODE, 13-HODE, and 9,10-DiHOME with plasma ALT and hepatic TG content was performed. Individual data points are reported; n=4 pair-fed and 6 EtOH-fed mice from Cohort 2. *Values were significantly different from each other (P<0.05).
The ratio of diol:epoxide pairs is an indirect measure of s-EH activity and plasma ratios are associated with liver disease and inflammation13. We find that the 9,10-DiHOME/9,10-EpOME ratio is significantly increased by ethanol feeding in young, but not aged mice (Figure 5E). The 12,13-DiHOME/12,13-EpOME ratio was not impacted by ethanol or age. To next determine whether selected oxylipins correlate with liver injury, we performed Spearman correlation between plasma ALT activity and hepatic triglycerides and 9-HODE, 13-HODE, 9,10-DiHOME. Significant positive correlations between ALT was observed between 9-HODE and 9,10-DiHOME while hepatic TGs positively correlated with 9-HODE, 13-HODE, and 9,10-DiHOME (Figure 5F).
Profiling of the plasma oxylipidome revealed specific age- and ethanol-induced changes that were not reflective of the liver oxylipin profile
Many of the foundational studies investigating oxylipins and ALD have utilized plasma and serum patient samples due, in part, to lack of availability of liver tissue but also for the prognostic utility for plasma biomarkers. Here, in an effort to characterize both liver and plasma compartments, we performed quantitative metabolomics on plasma samples from our chronic model of ethanol feeding. Detected, and normalized metabolites are shown in a heatmap to highlight low and high abundant metabolites (Figure 6A). PUFA metabolism by LOX and CYP/s-EH enzymes (Figure 6B/C) and absolute values of selected metabolites are shown (Figure 6D/E/G). Ethanol feeding decreased LOX-derived 9-HODE, 13-HODE, and 9- and 13-oxooctadecadienoic acid (9-/13-oxoODE) metabolites in young mice (Figure 6D). 9-HODE, 13-HODE, 9-oxoODE and 13-oxoODE were similar between pair-fed young and aged mice and ethanol feeding negatively impacted 13-oxoODE (Figure 6D). Ethanol feeding decreased CYP-derived 9,10-EpOME and 12,13-EpOME in aged, but not young mice. Plasma 9,10-DiHOME was increased in ethanol-fed mice, independent of age and positively correlated with the liver injury marker, ALT (Figure 6E). 9,10-DiHOME/9,10-EpOME, but not 12,13-DiHOME/12,13-EpOME, ratios were significantly increased by ethanol and age (Figure 6F). 9,10-DiHOME/9,10-EpOME positively correlated with liver injury. LOX-derived 15-HETE was increased in plasma by ethanol feeding in young mice which was further increased in aged mice; plasma LTE4 was increased in ethanol-fed aged mice (Figure 6G).
Figure 6. Plasma oxylipins are differentially impacted by age and ethanol.
Young and aged adult WT mice were allowed free access to ethanol (32%,d25) or pair-fed control diets. Lipids were extracted from plasma, enriched using solid-phase extraction and subjected to LS-MS/MS. (A) Heatmap of detected oxylipins from plasma. Data are normalized by smallest to largest value in the dataset, with the largest value set as 100% (yellow) and the smallest values set as 0% (dark blue). Undetected values (lower limit was detection) are denoted as white. (B) n-6 Polyunsaturated fatty acid metabolism. Lipoxygenase (LOX)-derived metabolites 9- and 13-hydroxyoctadecadienoic acid (9-HODE, 13-HODE) and 9- and 13-Hydroxyoctadecadienoic acid (9-oxoODE and 13-oxoODE) and cyclooxygenase (CYP)-derived metabolites 9,10- and 12,13-Epoxy-9-octadecenoic acid (9,10-EpOME and 12,13-EpOME) and 9,10- and 12,13-Dihydroxyocadecenoic acid (9.10-DiHOME and 12,13-DiHOME). (C) Arachidonic Acid metabolism by LOX produces 5-Hydroxyeicosatetraenoic acid (5-HETE), leukotriene A4 (LTA4), and Leukotriene(s) B4, C4, D4, and E4. (D) Absolute values of LOX-derived metabolites 9-HODE, 13-HODE, LTE4 and CYP-derived 9,10-DiHOMEDE are shown. E) Absolute values of CYP- and sEH-derived metabolites, 9,10-EpOME, 12,13-EpOME, 9,10-DiHOME, and 12,13-DiHOME are shown. Spearman correlation of 9,10-DiHOME with plasma ALT was performed. F) Diol:epoxide ratios of 9,10-DiHOME/9,10-EpOME and 12,13-DiHOME/12,13-EpOME were calculated. Spearman correlation of 9,10-DiHOME/9,10-EpOME with plasma ALT was performed. G) Absolute values of 15-HETE and LTE4 are shown. Individual data points are reported and represented using violin plots; n=4 pair-fed and 6 EtOH-fed mice for each age group from Cohort 2. *Values were significantly different from each other (P<0.05).
The brain oxylipidome was not differentially impacted by Lieber-DeCarli ethanol feeding in young or aged mice
Progressive liver disease is associated with hepatic encephalopathy and cognitive decline4,5. Because aging and alcohol abuse are independent risk factors for dementia, we next asked if during early stages of ALD, there are changes to the bioactive lipid mediator signature in brain tissue that may correlate with liver damage or plasma oxylipins. Only 17 of 55 metabolites were detected in half hemisphere whole brain tissue; the most abundant oxylipins were prostaglandin D2 (PGD2) and PGF2a Isomers but the levels of all detected metabolites were similar between age groups that did, and did not receive ethanol in the liquid diet (Figure S2).
15-HETE and 9,10-DiHOME positively correlate between liver and plasma compartments
To establish if plasma oxylipin levels are reflective of liver-derived mediators in early stages of mALD, we next performed correlation analysis between selected metabolites that were the most impacted by ethanol and/or age in liver and plasma. LOX-derived 15-HETE and CYP-derived 9,10-DiHOME were positively and significantly correlated while 9- and 13-HODE showed no correlation between the compartments (Figure 7).
Figure 7. 15-HETE and 9,10-DiHOME metabolites positively correlate between plasma and liver compartments.
Young and aged adult WT mice were allowed free access to ethanol (32%,d25) or pair-fed control diets. Lipids were extracted from plasma and liver tissue, enriched using solid-phase extraction and subjected to LS-MS/MS. Spearman correlation of 15-HETE, 9-HODE, 13-HODE, and 9,10-DiHOME between liver and plasma was performed. Values of young and aged, pair-fed and ethanol-fed were included and denoted at grey circles. Spearman correlation coefficients are reported and P-values are shown. n=4 pair-fed and 6 EtOH-fed mice for each age group from Cohort 2.
LTE4, 9,10-DiHOME and 15-HETE increase hepatocyte lipid accumulation and ER stress in cultured AML12 cells
Despite the finding that selected oxylipins positively associate with liver injury (Figures 5/6/7), these data do not provide causative evidence for hepatocyte damage. Because 13-HODE can directly induce hepatocyte damage in vitro57, we next asked if LTE4, 9,10-DiHOME and/or 15-HETE, which were significantly increased in the plasma or liver (or both) of ethanol-fed aged mice, could directly impact hepatocyte health (Figure 8). Ethanol challenge increased Bodipy493/503 fluorescence of neutral lipids, which was further increased in combination with increasing concentrations of LTE4, 9,10-DiHOME or 15-HETE (Figure 8A/B). Notably, LTE4, 9,10-DiHOME or 15-HETE were sufficient to increase Bodipy493/503 staining of AML12 cells at 500ng/mL and 1ug/mL, but not at the lower 100 ng/mL concentration, in the absence of ethanol challenge. Excessive accumulation of lipids in hepatocytes is associated with ER stress and the unfolded protein response (UPR)58. Challenge with 1 ug/mL 9,10-DiHOME and 15-HETE, but not LTE4, increased expression of the ER stress sensor glucose-regulated protein 78 (GRP78) and splicing of X-box binding protein 1 (XBP-1) (Figure 8C/D). Exposure to ethanol (50mM) increased GRP78 and sXBP-1 expression that was maintained when AML12 cells were co-challenged with LTE4, 9,10-DiHOME or 15-HETE (Figure 8C/D). No differences in cytotoxicity as measured by lactate dehydrogenase activity from cell supernatants was observed after ethanol and/or oxylipin challenge for 24hr (data not shown).
Figure 8. LTE4, 9,10-DiHOME and 15-HETE increase lipid content and ER stress in ethanol-sensitized AML12 cells.
AML12 cells were cultured with or with 50mM ethanol for 24 hours. After 24hrs, AML12 cells were treated with leukotriene E4, 9,10-dihydroxy-octadecenoic acid or 15-hydroxyeicosatetraenoic acid (100ng/mL, 500ng/mL or 1ug/mL) alone, or in combination with 50mM EtOH for an additional 14 or 24 hours. A) Neutral lipid content was visualized using Bodipy493/503 using the Incucyte live cell imaging system. Fluorescence intensity was determined after normalizing to total cell area using Image J software. Data are representative of 4 independent, biologic replicates/group. Data are expressed as means ± SEM; *values are significantly different due to ethanol within the treatment group (P<0.05); #values are significantly different from untreated control (P<0.01); $values are significantly different from cells treated only with ethanol (P<0.01). B) Representative fluorescent images from Bodipy493/503 stained AML12 cells treated with, or without ethanol and/or 1ng/mL leukotriene E4, 9,10-dihydroxy-octadecenoic acid or 15-hydroxyeicosatetraenoic acid. C/D) Expression of GRP78 and splicing of XBP-1 was determined by Western Blot in AML12 cells after culture in 50mM ethanol for 24hrs and then challenged with leukotriene E4, 9,10-dihydroxy-octadecenoic acid or 15-hydroxyeicosatetraenoic acid (1ug/mL) with and without 50mM ethanol for and additional 14 hours. GRP78 and sXBP-1 were normalized to GAPDH and β-actin as a loading control. Data are representative of 6 independent, biologic replicates/group. Data are expressed as means ± SEM; *values are significantly different due to ethanol within the treatment group (P<0.05); #values are significantly different from untreated control (P<0.05).
Discussion
In addition to cytokines, endogenous bioactive lipids consist of a vast network of soluble mediators that govern immunity by triggering, coordinating, and confining inflammatory processes59. Importantly, oxylipins can play important roles in the transition from acute to chronic inflammation via activating proinflammatory processes and also repressing the activity of anti-inflammatory, pro-resolving mediators. Collectively, a comprehensive mapping of the oxylipidome is required to determine if the balance of mediators being produced lead to, and are a consequence of, disease progression. Investigating these processes in alcohol-related pathologies is of particular importance as ALD is associated with many cell stressors dynamically affecting oxylipins, including ROS and non-resolving inflammation. Several seminal publications in the field have profiled plasma oxylipins in individuals with alcohol use disorder, alcohol-associated hepatitis, and alcohol-associated cirrhosis and found a skewing of the pro-inflammatory:anti-inflammatory mediator ratios that correlate with liver damage, hepatic function, and intestinal permeability/endotoxin13,22,24. Profiling of other compartments, including fecal oxylipins, has also highlighted a shift in specific metabolites in alcohol use disorder and alcohol-associated hepatitis, including elevations in 14-hydroxydocosahexaenoic acid (14-HDoHE), 11,12-epoxyeicosatrienoic acid (11,12-EpETrE) and 9-, 8S- and 11-hydroxyeicosatetraenoic acid (9/8S/11-HETE) and decreases in thromboxane B222. Correlations between fecal oxylipins and clinical parameters were found; however, these correlations were not as strong as the correlations between serum metabolites22. It is important to note that these studies reported oxylipin profiles in young and middle-aged adults (defined by adults ages 19–48 and 49–6460) and there remain gaps in knowledge of specific changes associated in the oxylipidome in ALD patients with advanced age.
Oxylipin profiling of mALD models has also identified key metabolites affected by alcohol in young adult mice57,61,62. Hepatic 13-HODE, produced via upregulated 15-LOX, was identified after ethanol feeding57. Proinflammatory 13-HODE, 5-, 15-, and 20-HETE, derived from AA and LA-derived omega-6 PUFAs, are increased in liver when diets are enriched with unsaturated fats and combined with ethanol61. Moreover, positive correlations between 8,9-DiHETrE and ccl2 mRNA, and 11,12-DiHETrE and the stress marker and danger-associated molecular pattern molecule, high mobility group box protein 1 mRNA were observed61. Our study is the first to directly compare oxylipin profiles in liver and plasma, between young and aged adult mice challenged with ethanol. Importantly, our data support previous observations that chronic ethanol feeding can increase pro-inflammatory 9- and 13-HODE in liver and show new findings that in aged mice, these increases are amplified compared to young counterparts (Figure 5). While these changes were not observed in plasma, these data specify the liver as an important driver of heightened inflammation in mALD in both young and aged mice. We also observed specific reductions in anti-inflammatory CYP-derived 9,10-EpHOME and 12,13-EpOME in liver, which trended to decrease by ethanol feeding while in plasma, ethanol-fed aged mice had significant reductions in these metabolites. Furthermore, because the ratio of diol:epoxide pairs is an indirect measure of s-EH activity, our data suggest that s-EH activity is significantly impacted in aged liver as evidenced by reduced diol:epoxide ratios of 9,10-DiHOME/9,10-EpOME. These measures in plasma were more reflective of the injury profile of aged, ethanol-fed mice, as demonstrated by significant reductions in epoxides and significant increases in pro-inflammatory diol species (Figure 6). Because sEH inhibition strategies are currently under consideration to reduce DiHOME production during inflammatory challenges19, it will particularly useful to determine if advanced age will differentially impact efficacy compared to young and middle-aged adults. Overall, CYP-derived metabolites in plasma more strongly correlated with liver injury compared to liver, including the diol:epoxide ratios of 9,10-DiHOME/9,10-EpOME, suggesting these measures in plasma are better prognostic biomarkers of liver damage throughout life.
Two notable metabolites were identified from the plasma that were increased by advanced age and/or chronic ethanol feeding: 9,10-DiHOME and 15-HETE. As previously discussed, both of these metabolites are increased in models of trauma in chronic inflammatory diseases and have been identified in patients that have moderate AH and in mALD13,61,62. Dihydroxy fatty acids, or diols, have significant biologic activity and are derived from neutrophils and macrophages62–64. Importantly, EpOMEs and DiHOMEs exert G-protein-mediated chemotaxis of neutrophils65. Despite no statistical differences between neutrophils in our data, histopathologic scoring of livers from aged mice demonstrated higher levels of inflammatory cells infiltrate and foci, suggesting diol production may contribute to inflammatory cell recruitment and/or activation during “inflamm-aging” and chronic ethanol exposure. In addition to their proinflammatory function, HETEs possess pro-apoptotic activity as 5-HETE can increase the pro-apoptotic action of TNFα in hepatocytes66 Conversely, 15-HETE specifically is a PPARγ agonist and has been linked to adipocyte differentiation67 and macrophage suppression68. Our data support the agonistic nature of 15-HETE to PPARα69, as evidenced from our findings in AML12 cells (Figure 8), but more details studies are needed to determine the specific role of 15-HETE in models of advanced aging and ethanol exposure.
A black sheep among these data includes the profiling of brain oxylipins, and the unexpected lack of change associated with both age and ethanol. Because alcohol misuse and ALD are strongly associated with oxidative stress, cellular damage, and inflammation and progressive liver disease can lead to early onset dementia, our prediction was that early changes in the brain oxylipidome would also reflect alcohol-mediated damage in other organs, including liver. Our observations may likely be explained by the fact that Lieber-DeCarli liquid diet ethanol feeding is a better model of early, rather than late ALD, with pronounced hepatic steatosis and the initiation of systemic inflammation that lacks hepatic fibrosis70. Moreover, clinical observations linking liver disease to dementia occur during cirrhosis due to pronounced portal hypertension, a stage of the disease that is beyond the manifestations occurring in mALD.
The complex interaction of age and ethanol on complement activation has not previously been reported. Controlled activation and resolution of complement is implicated as an important contributor to tissue injury in mALD31,34,71,72 and selected components and activation fragments are newly identified diagnostic and prognostic indicators for AH73. While our steatosis and liver injury profiles are supported by previous reports9,49,74, we find that ethanol feeding induces activation and deposition of complement fragments of C3 in liver of young mice and age dramatically increases the deposition of C3b (independent of alcohol exposure), C3c (dependent on alcohol) and C3d (dependent on age and alcohol). Tissue-bound C3 fragments are durable biomarkers of tissue inflammation75 and our data support that hepatic inflammation is heighted both by age and ethanol feeding. Terminal activation of complement can generate the potent anaphylatoxins C3a and C5a, which bind to cognate receptors C3aR1 and C5aR1. We find that alcohol feeding, independent of age, readily upregulates C5aR1 expression in liver and we have previously found that C5aR1 upregulation on myeloid cells lead to hepatic inflammation while on hepatocytes, C5aR1 signaling is hepatoprotective71. Taken together, these findings indicate that compared to young, aged mice have elevated tissue bound C3 and upregulate C5aR1 to modulate both inflammation and hepatocyte regenerative signals after ethanol feeding.
Alcohol is a well-defined cell stressor, capable of inducing DNA damage, hypoxia, and reactive oxygen species generation. Similarly, these pathways lead to stress-induced senescence via tumor suppressor p53-dependent apoptosis and/or transient growth arrest via p2150. We find here that ethanol feeding elevates senescence-associated markers, including p21, p53- and β-gal-positive cells and SASP factors (e.g. il-6, ccl2, tnfα) in liver from young mice. Senescence was further confirmed with reduced PCNA-positive cells and are consistent with previous reports76. Despite age-related increases in hepatic p16 and p21 expression coupled with p53- and β-gal positive cells, ethanol feeding further increased selected senescence markers, including clusters of p53 positive cells in the hepatic parenchyma of ethanol-fed aged mice. Importantly, pro-inflammatory mediator expression and SASP factors were increased by age, suggesting that ethanol does not further impact classic markers of “inflamm-aging” in the liver. Our data are consistent with recent reports making use of alternative ethanol exposure paradigms and strains of mice, including differential expression of selected cytokines impacted by only age (tnfα) and ethanol feeding to aged mice (il-1β, ccl2) 9,49. Indeed, inductions in proinflammatory mediators (nlrp3, il-6, ccl3/4) have been reported in primary neutrophils from aged, chronic-plus-binge challenged mice49. Despite age- and not ethanol-related increases in hepatic il-6 and tnfα mRNA expression, our oxylipin profiling from liver suggest other inflammatory oxylipins, including omega-6 PUFAs (e.g. 9- and 13-HODE) are likely contributing to persistent inflammation and liver injury in aged, ethanol-fed mice. While nuclear factor kappa beta (NF-kβ) is a critical factor leading to pro-inflammatory mediator production, constitutive activation can also lead to inhibitory feedforward regulation affecting multiple facets of its vast network of critical functions77. It is possible that because ethanol feeding did not further increase inflammatory markers in aged mice, especially with respect to cytokines and lipid metabolizing enzyme (COX/LOX) gene expression, there may be either be a compensatory stress response to mitigate systemic damage or it is reflective of constitutive activation of NF-kβ in aged mice due to “inflamm-aging”. While beyond the scope of the current project, it will be important in future studies to dissect these regulatory mechanisms associated with age to better understand mechanisms of “inflamm-aging” and ethanol exposure on disease progression.
It is well established that ethanol can directly lead to hepatocyte stress, including the production of lipids and ER Stress-mediated activation of the UPR. The direct effects of ethanol on mitochondria, coupled with oxidative metabolism, generation of ROS and depletion of cellular NAD+, lead to impairments in fatty acid oxidation and accumulation of neutral lipids, including triglycerides78–80. In addition to increasing uptake of extrahepatic lipids, hepatocytes increase de novo lipogenesis which all augment hepatic triglyceride accumulation and progression to steatosis. Despite this knowledge, there are limited studies employing a direct role for lipid metabolites and the pathogenesis of steatosis. Indeed, 13-HODE challenge to cultured Hepa-1c1c7 hepatocytes increases oxidative stress, ER stress and apoptosis but these studies were not performed in combination with ethanol57. Here, we find alone or in combination with ethanol, that selected metabolites found to be uniquely increased in liver and plasma from aged mice after ethanol feeding can increase the neutral lipid content of AML12 cells (Figure 8). In addition to the aforementioned mechanism of steatosis listed above, the UPR has been also implicated to regulate lipid metabolism via activation and expression of key lipid rheostats, including but not limited to, sterol regulatory element-binding proteins, carbohydrate-responsive element-binding protein, peroxisome proliferator-activated receptor (PPAR), and CCAAT/enhancer-binding protein81. Our findings demonstrate that both ethanol and oxylipins increase selected measures of ER Stress. The cascade of the ER stress response is controlled by three sensors, including inositol-requiring enzyme 1 (IRE1), activating transcription factor 6 and PKR-like ER kinase81. Upon activation, IRE1 splices XBP-1 to its active form (sXBP-1), allowing for transcription of PPARα81 which may be a likely mechanism driving lipid production by 9,10-DiHOME and 15-HETE. LTE4-mediated activation of the LTE4 receptor, GPR99, is not well understood but implicated generally in inflammatory diseases82. Because LTE4 increased lipid load, but not GRP78 or sXBP-1 expression, the mechanisms of hepatocyte injury are likely distinct and separate from 9,10-DiHOME and 15-HETE. Despite these findings, it remains to be determined if cells from aged animals differentially produce lipids or activate ER stress pathways in response to ethanol and/or oxylipin challenge and should be a focus of future studies.
It should be noted that our study was limited by high variability in the response to ethanol in our aged mice with respect to oxylipin profiling (Cohort 2). It has been reported that phenotypic variability amongst age groups increases after 25 weeks of age (the biologic equivalent of 25–30 years for humans) and our aged mice are approximately 20 months of age at the termination of the study83–85. In the future, it may be appropriate to assess frailty prior to the start of feeding trials in an effort to establish if frailty stratifies endpoint data more clearly86. Moreover, our findings support some, but not all of the reported increases in oxylipins from young adult mice after chronic ethanol feeding which could be explained by differences in sex, housing facilities, extraction methodologies and instrumentation between research institutes. We must also acknowledge that compartmental metabolomic profiling does not specifically identify metabolites formed by a particular tissue or cell, which precludes our ability to determine specific cellular sources driving phenotypes in our study. Additional limitations to this study include sex as a biologic variable, which would allow us to determine if certain oxylipins could be used for prognostic/diagnostic purposes amongst everyone, of all ages.
Taken together, our data confirm that ethanol can skew the oxylipidome in both plasma and liver, and that these perturbations are further impacted with advanced age. Importantly, we identify specific mediators that positively correlate with liver injury and can drive early hepatocyte stress, suggesting that they may be good diagnostic biomarkers in individuals that misuse alcohol across the lifespan.
Supplementary Material
Highlights:
Ethanol-induced liver injury is amplified in aged mice compared to young adult mice
Chronic alcohol increases hepatic markers of senescence
Hepatic 9- and 13-HODE are increased in ethanol-fed aged mice and correlate with liver injury
9,10-DiHOME and 15-HETE are strongly associated between plasma and liver
LTE4, 9,10-DiHOME and 15-HETE increase hepatocyte ER Stress and lipid accumulation
Acknowledgements:
This study was supported, in part, by pilot funds to support Metabolomics Core usage from the ADR (Skaggs School of Pharmacy). The authors would also like to thank the members of the Metabolomics Core (Skaggs School of Pharmacy, CU Anschutz) and especially Mike Armstrong and Jon Manke for technical assistance with plasma and tissue processing and quantitation of lipid mediators for this study. We would also like to thank Kristofer Fritz, PhD and Peter Harris for generously providing cofactors and 4-MP for the ADH activity assay.
Financial support:
this work was supported in part by NIH grants R00AA025386 (RLM), R00AA025386-S1 (RLM), pilot grant funds from the Associate Dean for Research, Skaggs School of Pharmacy (RLM), and startup funds from the department of Pharmaceutical Sciences in the School of Pharmacy and the GI and Liver Innate Immune Program at CU.
Abbreviations:
- ALD
alcohol-associated liver disease
- ALT
Alanine transaminase
- AH
Alcohol-associated hepatitis
- ALD
Alcohol-associated liver disease
- AA
Arachidonic acid
- AST
Aspartate transaminase
- CCL2
Chemokine ligand 2
- C3
Complement component 3
- C5aR1
Component C5a Receptor 1
- COXs
Cyclooxygenases
- CYP450
Cytochrome P450
- DiHOME
Dihydroxy-octadecenoic acid
- DHA
Docosahexanenoic acid
- EPA
Eicosapentaenoic acid
- ER
Endoplasmic Reticulum
- EpOME
Epoxy-octadecenoic acid
- GRP78
Glucose-regulated protein 78
- GPCR
G-protein coupled receptor
- HODE
Hydroxy-octadecadienoic acid
- HETE
Hydroxyeicosatetraenoic acid
- IRE1
Inositol-requiring enzyme 1
- IL-6
Interleukin-6
- IL1-β
Interleukin-1 beta
- LA
Linoleic acid
- LOXs
Lipoxygenases
- LTE4
Leukotriene E4
- mALD
Murine ALD
- NF-kβ
Nuclear Factor Kappa Beta
- oxoODE
Oxooctadecadienoic acid
- PPAR
Peroxisome proliferator-activated receptor
- PUFA
Polyunsaturated fatty acids
- PGD2
Prostaglandin D2
- ptges2/cox-2
Prostaglandin synthetase 2
- ROS
Reactive oxygen species
- SASP
Senescence-associated secretory phenotype
- s-EH
Soluble epoxide hydrolase
- TNFα
Tumor Necrosis Factor alpha
- XBP-1
X-box binding protein 1
Footnotes
Conflicts of Interest: The authors declare no conflicts of interest
Data Availability Statement: The data presented in this study are available on request from the corresponding author.
CRediT author statement
Paige Anton: Conceptualization, Validation, Formal Analysis, Investigation, Writing – Review & Editing; Lauren Rutt: Investigation, Writing – Review & Editing; Courtney Capper: Conceptualization, Investigation; David J Orlicky: Formal Analysis, Review & Editing; Rebecca McCullough, PhD: Conceptualization, Validation, Formal analysis, Investigation, Writing – Original Draft, Writing – Review & Editing, Visualization, Supervision, Project administration, Funding acquisition
Publisher's Disclaimer: This is a PDF file of an article that has undergone enhancements after acceptance, such as the addition of a cover page and metadata, and formatting for readability, but it is not yet the definitive version of record. This version will undergo additional copyediting, typesetting and review before it is published in its final form, but we are providing this version to give early visibility of the article. Please note that, during the production process, errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Moos RH, Schutte KK, Brennan PL & Moos BS Older adults’ alcohol consumption and late-life drinking problems: a 20-year perspective. Addiction 104, 1293–1302, doi: 10.1111/j.1360-0443.2009.02604.x (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Meier P. & Seitz HK Age, alcohol metabolism and liver disease. Curr Opin Clin Nutr Metab Care 11, 21–26, doi: 10.1097/MCO.0b013e3282f30564 (2008). [DOI] [PubMed] [Google Scholar]
- 3.Kim IH, Kisseleva T. & Brenner DA Aging and liver disease. Curr Opin Gastroenterol 31, 184–191, doi: 10.1097/MOG.0000000000000176 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Koch M. et al. Alcohol Consumption and Risk of Dementia and Cognitive Decline Among Older Adults With or Without Mild Cognitive Impairment. JAMA Netw Open 2, e1910319, doi: 10.1001/jamanetworkopen.2019.10319 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xu G. et al. Alcohol consumption and transition of mild cognitive impairment to dementia. Psychiatry Clin Neurosci 63, 43–49, doi: 10.1111/j.1440-1819.2008.01904.x (2009). [DOI] [PubMed] [Google Scholar]
- 6.Franceschi C., Garagnani P., Parini P., Giuliani C. & Santoro A. Inflammaging: a new immune–metabolic viewpoint for age-related diseases. Nature Reviews Endocrinology 14, 576–590, doi: 10.1038/s41574-018-0059-4 (2018). [DOI] [PubMed] [Google Scholar]
- 7.Minciullo PL et al. Inflammaging and Anti-Inflammaging: The Role of Cytokines in Extreme Longevity. Arch Immunol Ther Exp (Warsz) 64, 111–126, doi: 10.1007/s00005-015-0377-3 (2016). [DOI] [PubMed] [Google Scholar]
- 8.Puzianowska-Kuźnicka M. et al. Interleukin-6 and C-reactive protein, successful aging, and mortality: the PolSenior study. Immunity & Ageing 13, 21, doi: 10.1186/s12979-016-0076-x (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McMahan RH et al. A novel murine model of multi-day moderate ethanol exposure reveals increased intestinal dysfunction and liver inflammation with age. Immunity & Ageing 18, 37, doi: 10.1186/s12979-021-00247-8 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maraldi C. et al. Impact of Inflammation on the Relationship Among Alcohol Consumption, Mortality, and Cardiac Events: The Health, Aging, and Body Composition Study. Archives of Internal Medicine 166, 1490–1497, doi: 10.1001/archinte.166.14.1490 (2006). [DOI] [PubMed] [Google Scholar]
- 11.Pansarasa O. et al. Altered immune system in frailty: Genetics and diet may influence inflammation. Ageing Res Rev 54, 100935, doi: 10.1016/j.arr.2019.100935 (2019). [DOI] [PubMed] [Google Scholar]
- 12.Kominsky DJ, Campbell EL & Colgan SP Metabolic shifts in immunity and inflammation. J Immunol 184, 4062–4068, doi: 10.4049/jimmunol.0903002 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Warner D. et al. Linoleic Acid-Derived Oxylipins Differentiate Early Stage Alcoholic Hepatitis From Mild Alcohol-Associated Liver Injury. Hepatol Commun 5, 947–960, doi: 10.1002/hep4.1686 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pecorelli A. et al. 13-HODE, 9-HODE and ALOX15 as potential players in Rett syndrome OxInflammation. Free Radic Biol Med 134, 598–603, doi: 10.1016/j.freeradbiomed.2019.02.007 (2019). [DOI] [PubMed] [Google Scholar]
- 15.Node K. et al. Anti-inflammatory properties of cytochrome P450 epoxygenase-derived eicosanoids. Science 285, 1276–1279, doi: 10.1126/science.285.5431.1276 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lopez-Vicario C. et al. Inhibition of soluble epoxide hydrolase modulates inflammation and autophagy in obese adipose tissue and liver: role for omega-3 epoxides. Proc Natl Acad Sci U S A 112, 536–541, doi: 10.1073/pnas.1422590112 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morin C., Sirois M., Echave V., Albadine R. & Rousseau E. 17,18-epoxyeicosatetraenoic acid targets PPARgamma and p38 mitogen-activated protein kinase to mediate its anti-inflammatory effects in the lung: role of soluble epoxide hydrolase. Am J Respir Cell Mol Biol 43, 564–575, doi: 10.1165/rcmb.2009-0155OC (2010). [DOI] [PubMed] [Google Scholar]
- 18.Gilroy DW et al. CYP450-derived oxylipins mediate inflammatory resolution. Proc Natl Acad Sci U S A 113, E3240–3249, doi: 10.1073/pnas.1521453113 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bergmann CB et al. sEH-derived metabolites of linoleic acid drive pathologic inflammation while impairing key innate immune cell function in burn injury. Proc Natl Acad Sci U S A 119, e2120691119, doi: 10.1073/pnas.2120691119 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhu D. & Ran Y. Role of 15-lipoxygenase/15-hydroxyeicosatetraenoic acid in hypoxia-induced pulmonary hypertension. The Journal of Physiological Sciences 62, 163–172, doi: 10.1007/s12576-012-0196-9 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zein CO et al. Pentoxifylline decreases oxidized lipid products in nonalcoholic steatohepatitis: new evidence on the potential therapeutic mechanism. Hepatology 56, 1291–1299, doi: 10.1002/hep.25778 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gao B. et al. Serum and Fecal Oxylipins in Patients with Alcohol-Related Liver Disease. Dig Dis Sci 64, 1878–1892, doi: 10.1007/s10620-019-05638-y (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Loomba R, Quehenberger O, Armando A. & Dennis EA Polyunsaturated fatty acid metabolites as novel lipidomic biomarkers for noninvasive diagnosis of nonalcoholic steatohepatitis. J Lipid Res 56, 185–192, doi: 10.1194/jlr.P055640 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Raszeja-Wyszomirska J. et al. Lipidic last breath of life in patients with alcoholic liver disease. Prostaglandins Other Lipid Mediat 99, 51–56, doi: 10.1016/j.prostaglandins.2012.06.001 (2012). [DOI] [PubMed] [Google Scholar]
- 25.Feldstein AE et al. Mass spectrometric profiling of oxidized lipid products in human nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. J Lipid Res 51, 3046–3054, doi: 10.1194/jlr.M007096 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Puri P. et al. The plasma lipidomic signature of nonalcoholic steatohepatitis. Hepatology 50, 1827–1838, doi: 10.1002/hep.23229 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Azbukina NV et al. Oxylipin Profiles in Plasma of Patients with Wilson’s Disease. Metabolites 10, doi: 10.3390/metabo10060222 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Das UN “Cell Membrane Theory of Senescence” and the Role of Bioactive Lipids in Aging, and Aging Associated Diseases and Their Therapeutic Implications. Biomolecules 11, doi: 10.3390/biom11020241 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stahl EC., Haschak MJ., Popovic B. & Brown BN. Macrophages in the Aging Liver and Age-Related Liver Disease. Front Immunol 9, 2795, doi: 10.3389/fimmu.2018.02795 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gao B. & Bataller R. Alcoholic liver disease: pathogenesis and new therapeutic targets. Gastroenterology 141, 1572–1585, doi: 10.1053/j.gastro.2011.09.002 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cohen JI, Roychowdhury S, McMullen MR, Stavitsky AB & Nagy LE Complement and alcoholic liver disease: role of C1q in the pathogenesis of ethanol-induced liver injury in mice. Gastroenterology 139, 664–674, 674 e661, doi: 10.1053/j.gastro.2010.04.041 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McCullough RL et al. Complement Factor D protects mice from ethanol-induced inflammation and liver injury. American Journal of Physiology-Gastrointestinal and Liver Physiology 315, G66–G79 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McCullough RL et al. Differential contribution of complement receptor C5aR in myeloid and non-myeloid cells in chronic ethanol-induced liver injury in mice. Mol Immunol 75, 122–132, doi: 10.1016/j.molimm.2016.05.006 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McCullough RL et al. Complement Factor D protects mice from ethanol-induced inflammation and liver injury. Am J Physiol Gastrointest Liver Physiol 315, G66–G79, doi: 10.1152/ajpgi.00334.2017 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lanaspa MA et al. Ketohexokinase C blockade ameliorates fructose-induced metabolic dysfunction in fructose-sensitive mice. J Clin Invest 128, 2226–2238, doi: 10.1172/JCI94427 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Monks J. et al. Maternal obesity during lactation may protect offspring from high fat diet-induced metabolic dysfunction. Nutr Diabetes 8, 18, doi: 10.1038/s41387-018-0027-z (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kleiner DE et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 41, 1313–1321, doi: 10.1002/hep.20701 (2005). [DOI] [PubMed] [Google Scholar]
- 38.Pikkarainen PH & Räihä NCR Development of Alcohol Dehydrogenase Activity in the Human Liver. Pediatric Research 1, 165–168, doi: 10.1203/00006450-196705000-00001 (1967). [DOI] [PubMed] [Google Scholar]
- 39.Armstrong M. et al. Leukotriene-E4 in human urine: Comparison of on-line purification and liquid chromatography-tandem mass spectrometry to affinity purification followed by enzyme immunoassay. J Chromatogr B Analyt Technol Biomed Life Sci 877, 3169–3174, doi: 10.1016/j.jchromb.2009.08.011 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vander Ploeg M. et al. SPM pathway marker analysis of the brains of obese mice in the absence and presence of eicosapentaenoic acid ethyl esters. Prostaglandins Leukot Essent Fatty Acids 175, 102360, doi: 10.1016/j.plefa.2021.102360 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Armstrong M, Manke J, Nkrumah-Elie Y, Shaikh SR & Reisdorph N. Improved quantification of lipid mediators in plasma and tissues by liquid chromatography tandem mass spectrometry demonstrates mouse strain specific differences. Prostaglandins Other Lipid Mediat 151, 106483, doi: 10.1016/j.prostaglandins.2020.106483 (2020). [DOI] [PubMed] [Google Scholar]
- 42.Wu JC, Merlino G. & Fausto N. Establishment and characterization of differentiated, nontransformed hepatocyte cell lines derived from mice transgenic for transforming growth factor alpha. Proc Natl Acad Sci U S A 91, 674–678, doi: 10.1073/pnas.91.2.674 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hu M. et al. Regulation of hepatic lipin-1 by ethanol: role of AMP-activated protein kinase/sterol regulatory element-binding protein 1 signaling in mice. Hepatology 55, 437–446, doi: 10.1002/hep.24708 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cederbaum AI Alcohol metabolism. Clin Liver Dis 16, 667–685, doi: 10.1016/j.cld.2012.08.002 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bykov I, Junnikkala S, Pekna M, Lindros KO & Meri S. Complement C3 contributes to ethanol-induced liver steatosis in mice. Annals of medicine 38, 280–286, doi: 10.1080/07853890600664608 (2006). [DOI] [PubMed] [Google Scholar]
- 46.Shen H, French BA, Liu H, Tillman BC & French SW Increased activity of the complement system in the liver of patients with alcoholic hepatitis. Experimental and molecular pathology 97, 338–344, doi: 10.1016/j.yexmp.2014.09.004 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Seitz HK et al. Effect of aging on in vivo and in vitro ethanol metabolism and its toxicity in F344 rats. Gastroenterology 97, 446–456, doi: 10.1016/0016-5085(89)90082-6 (1989). [DOI] [PubMed] [Google Scholar]
- 48.Ventura-Cots M. et al. Clinical, histological and molecular profiling of different stages of alcohol-related liver disease. Gut, doi: 10.1136/gutjnl-2021-324295 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ren R. et al. Aging exaggerates acute-on-chronic alcohol-induced liver injury in mice and humans by inhibiting neutrophilic sirtuin 1-C/EBPalpha-miRNA-223 axis. Hepatology 75, 646–660, doi: 10.1002/hep.32152 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lasry A. & Ben-Neriah Y. Senescence-associated inflammatory responses: aging and cancer perspectives. Trends Immunol 36, 217–228, doi: 10.1016/j.it.2015.02.009 (2015). [DOI] [PubMed] [Google Scholar]
- 51.Cederbaum AI, Lu Y. & Wu D. Role of oxidative stress in alcohol-induced liver injury. Arch Toxicol 83, 519–548, doi: 10.1007/s00204-009-0432-0 (2009). [DOI] [PubMed] [Google Scholar]
- 52.Mijit M, Caracciolo V, Melillo A, Amicarelli F. & Giordano A. Role of p53 in the Regulation of Cellular Senescence. Biomolecules 10, doi: 10.3390/biom10030420 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Martinez FO, Gordon S, Locati M. & Mantovani A. Transcriptional profiling of the human monocyte-to-macrophage differentiation and polarization: new molecules and patterns of gene expression. J Immunol 177, 7303–7311, doi: 10.4049/jimmunol.177.10.7303 (2006). [DOI] [PubMed] [Google Scholar]
- 54.Han JH et al. Astaxanthin alleviated ethanol-induced liver injury by inhibition of oxidative stress and inflammatory responses via blocking of STAT3 activity. Sci Rep 8, 14090, doi: 10.1038/s41598-018-32497-w (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mashima R. & Okuyama T. The role of lipoxygenases in pathophysiology; new insights and future perspectives. Redox Biol 6, 297–310, doi: 10.1016/j.redox.2015.08.006 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nanji AA et al. Enhanced cyclooxygenase-2 gene expression in alcoholic liver disease in the rat. Gastroenterology 112, 943–951, doi: 10.1053/gast.1997.v112.pm9041257 (1997). [DOI] [PubMed] [Google Scholar]
- 57.Zhang W, Zhong W, Sun Q, Sun X. & Zhou Z. Hepatic overproduction of 13-HODE due to ALOX15 upregulation contributes to alcohol-induced liver injury in mice. Sci Rep 7, 8976, doi: 10.1038/s41598-017-02759-0 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Song MJ & Malhi H. The unfolded protein response and hepatic lipid metabolism in non alcoholic fatty liver disease. Pharmacol Ther 203, 107401, doi: 10.1016/j.pharmthera.2019.107401 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chiurchiù V, Leuti A. & Maccarrone M. Bioactive Lipids and Chronic Inflammation: Managing the Fire Within. Frontiers in Immunology 9, doi: 10.3389/fimmu.2018.00038 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Geifman N, Cohen R. & Rubin E. Redefining meaningful age groups in the context of disease. Age (Dordr) 35, 2357–2366, doi: 10.1007/s11357-013-9510-6 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Warner DR et al. Ethanol and unsaturated dietary fat induce unique patterns of hepatic omega-6 and omega-3 PUFA oxylipins in a mouse model of alcoholic liver disease. PLoS One 13, e0204119, doi: 10.1371/journal.pone.0204119 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Warner DR et al. Dietary Linoleic Acid and Its Oxidized Metabolites Exacerbate Liver Injury Caused by Ethanol via Induction of Hepatic Proinflammatory Response in Mice. Am J Pathol 187, 2232–2245, doi: 10.1016/j.ajpath.2017.06.008 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bettaieb A. et al. Soluble epoxide hydrolase deficiency or inhibition attenuates diet-induced endoplasmic reticulum stress in liver and adipose tissue. J Biol Chem 288, 14189–14199, doi: 10.1074/jbc.M113.458414 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hayakawa M. et al. Neutrophils biosynthesize leukotoxin, 9, 10-epoxy-12-octadecenoate. Biochem Biophys Res Commun 137, 424–430, doi: 10.1016/0006-291x(86)91227-1 (1986). [DOI] [PubMed] [Google Scholar]
- 65.Totani Y. et al. Leukotoxin and its diol induce neutrophil chemotaxis through signal transduction different from that of fMLP. Eur Respir J 15, 75–79, doi: 10.1183/09031936.00.15107500 (2000). [DOI] [PubMed] [Google Scholar]
- 66.Martinez-Clemente M. et al. 5-lipoxygenase deficiency reduces hepatic inflammation and tumor necrosis factor alpha-induced hepatocyte damage in hyperlipidemia-prone ApoE-null mice. Hepatology 51, 817–827, doi: 10.1002/hep.23463 (2010). [DOI] [PubMed] [Google Scholar]
- 67.Lecka-Czernik B. et al. Divergent effects of selective peroxisome proliferator-activated receptor-gamma 2 ligands on adipocyte versus osteoblast differentiation. Endocrinology 143, 2376–2384, doi: 10.1210/endo.143.6.8834 (2002). [DOI] [PubMed] [Google Scholar]
- 68.Lefevre L. et al. LRH-1 mediates anti-inflammatory and antifungal phenotype of IL-13-activated macrophages through the PPARgamma ligand synthesis. Nat Commun 6, 6801, doi: 10.1038/ncomms7801 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Naruhn S. et al. 15-hydroxyeicosatetraenoic acid is a preferential peroxisome proliferator-activated receptor beta/delta agonist. Mol Pharmacol 77, 171–184, doi: 10.1124/mol.109.060541 (2010). [DOI] [PubMed] [Google Scholar]
- 70.Gao B. et al. Animal Models of Alcoholic Liver Disease: Pathogenesis and Clinical Relevance. Gene Expr 17, 173–186, doi: 10.3727/105221617X695519 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.McCullough RL et al. Anaphylatoxin Receptors C3aR and C5aR1 Are Important Factors That Influence the Impact of Ethanol on the Adipose Secretome. Frontiers in Immunology 9, doi: 10.3389/fimmu.2018.02133 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Smathers RL et al. Soluble IgM links apoptosis to complement activation in early alcoholic liver disease in mice. Mol Immunol 72, 9–18, doi: 10.1016/j.molimm.2016.02.008 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Fan X. et al. Diagnostic and Prognostic Significance of Complement in Patients With Alcohol-Associated Hepatitis. Hepatology 73, 983–997, doi: 10.1002/hep.31419 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ramirez T. et al. Aging aggravates alcoholic liver injury and fibrosis in mice by downregulating sirtuin 1 expression. J Hepatol 66, 601–609, doi: 10.1016/j.jhep.2016.11.004 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Thurman JM et al. Detection of complement activation using monoclonal antibodies against C3d. J Clin Invest 123, 2218–2230, doi: 10.1172/JCI65861 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chavez PRG et al. Long-Term Ethanol Consumption Promotes Hepatic Tumorigenesis but Impairs Normal Hepatocyte Proliferation in Rats. The Journal of Nutrition 141, 1049–1055, doi: 10.3945/jn.110.136531 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Prescott JA, Mitchell JP & Cook SJ Inhibitory feedback control of NF-κB signalling in health and disease. Biochemical Journal 478, 2619–2664, doi: 10.1042/bcj20210139 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Jeon S. & Carr R. Alcohol effects on hepatic lipid metabolism. J Lipid Res 61, 470–479, doi: 10.1194/jlr.R119000547 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Blomstrand R, Kager L. & Lantto O. Studies on the ethanol-induced decrease of fatty acid oxidation in rat and human liver slices. Life Sci 13, 1131–1141, doi: 10.1016/0024-3205(73)90380-9 (1973). [DOI] [PubMed] [Google Scholar]
- 80.Cederbaum AI, Lieber CS, Beattie DS & Rubin E. Effect of chronic ethanol ingestion on fatty acid oxidation by hepatic mitochondria. J Biol Chem 250, 5122–5129 (1975). [PubMed] [Google Scholar]
- 81.Moncan M. et al. Regulation of lipid metabolism by the unfolded protein response. J Cell Mol Med 25, 1359–1370, doi: 10.1111/jcmm.16255 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sasaki F. & Yokomizo T. The leukotriene receptors as therapeutic targets of inflammatory diseases. International Immunology 31, 607–615, doi: 10.1093/intimm/dxz044 (2019). [DOI] [PubMed] [Google Scholar]
- 83.Brayton CF, Treuting PM & Ward JM Pathobiology of aging mice and GEM: background strains and experimental design. Vet Pathol 49, 85–105, doi: 10.1177/0300985811430696 (2012). [DOI] [PubMed] [Google Scholar]
- 84.Laboratory TJ Aged C56BL/6J Mice for Reserach Studies: Considerations, Applications, and Best Practices, <https://resources.jax.org/white-papers/whitepaper-aged-b6> (2020). [Google Scholar]
- 85.Ackert-Bicknell CL et al. Aging Research Using Mouse Models. Curr Protoc Mouse Biol 5, 95–133, doi: 10.1002/9780470942390.mo140195 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Whitehead JC. et al. A clinical frailty index in aging mice: comparisons with frailty index data in humans. J Gerontol A Biol Sci Med Sci 69, 621–632, doi: 10.1093/gerona/glt136 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.