Abstract
Objectives
Adiponectin and resistin are adipokines involved in insulin resistance, glucometabolic control and adiposity. There is evidence that hypoadiponectinemia and hyperresistinemia are associated with cardiovascular disease. Whether the ratio of Adiponectin-Resistin (AR) and Insulin Resistance Adiponectin-Resistin (IRAR) indices can be used as non-invasive biomarker of cardiovascular disease needs more attention. Therefore, the aim of this study was to assess the relationships of AR and IRAR indices with adiposity, glucometabolic control and cardiovascular risk incurred by high-sensitivity C-reactive protein (hsCRP) in healthy subjects and patients with Type 2 Diabetes Mellitus.
Methods
This observational case control study was conducted in the Department of Physiology and Medicine, King Saud University, Riyadh. A total of 191 (control = 84 and diabetic = 107) subjects were recruited. Body composition was assessed by bioelectrical impendence analyzer (BIA). Fasting blood samples were analyzed for glucose, glycosylated hemoglobin (HbA1c), high-sensitivity C-reactive protein (hsCRP), lipid profile, adiponectin, and resistin levels. The AR and IRAR indices were determined by formulas.
Results
Serum adiponectin levels were significantly lower in diabetics compared to control (95.45 ± 39.27 ng/ml vs 146.64 ± 56.36 ng/ml, p < .001) while serum resistin was significantly higher in diabetic when compared to control (2.94 ± 1.30 ng/ml vs 2.40 ± 1.09 ng/ml, p = .003). Furthermore, AR and IRAR indices were significantly increased in diabetic subjects when compared to control (.82 ± .29 vs .48 ± .35, p < .001) and (.30 ± .10 vs .17 ± .12, p < .001) respectively. ROC analysis revealed that these indices predicted increased cardiovascular risk with area under the curve (AUC) for adiponectin = .717 ( p = .001), resistin = .635 ( p = .002), AR index = .740 ( p < .001), and IRAR index = .737 ( p < .001) respectively. AR index correlated positively with Triglycerides (r = .354, p < .01), hsCRP (r = .264, p < .01), HbA1c (r = .425, p < .01), fat mass (r = .164, p < .05), Waist/Hip Ratio (WHR) (r = .248, p < .01), and negatively with high density lipoprotein (r=−.327, p < .01). Furthermore, IRAR index more strongly correlated with Triglycerides (r = .409, p < .01), hsCRP (r = .268, p < .01), HbA1c (r = .508, p < .01), fat mass (r = .152, p < .05), WHR (r = .256, p < .01), and negatively with high density lipoprotein (r = −.340, p < .01).
Conclusions
AR and IRAR indices correlate significantly with adiposity, glucometabolic control and cardiovascular risk in type 2 diabetic patients and non-diabetic individuals. They may prove to be useful integrated biomarkers to predict metabolic dysregulation and cardiovascular risk.
Keywords: Adiponectin-resistin index, Insulin resistance adiponectin-resistin index, Cardiovascular risk, Diabetes mellitus, High-sensitivity C-Reactive protein
1. Introduction
Cardiovascular diseases (CVD) remain the biggest cause of death worldwide. As the global population becomes increasingly sedentary, CVD and related diseases such as diabetes (T2DM) and obesity will increase. In 2019, according to World Health Organization (WHO), cardiovascular diseases accounted for 32% of mortality globally [1]. By 2020, cardiovascular disease was the cause of 11 million deaths worldwide and it is estimated that cardiovascular diseases would be the leading cause of death by 2030 [2,3].
One of the leading risk factors of cardiovascular diseases is diabetes mellitus which needs special concern and attention in addition to other risk factors such as increased body mass index (BMI), high level of Triglycerides and Cholesterol, and sedentary lifestyle (poor nutrition and lack of activities) [4–9]. In addition, it has been observed that Saudi Arabia has high obesity prevalence with low physical fitness score [10].
The role of adipokines, adiponectin and resistin secreted by adipocytes, is crucial in regulation of insulin sensitivity [11–13]. In addition, adiponectin plays an essential role as cardioprotective guard against inflammatory processes and hence decreases the risk of atherosclerosis [11–13]. Number of studies have reported that adiponectin concentrations are reduced in obese and cardiovascular diseases while plasma resistin concentrations are increased in metabolic syndromes and cardiovascular risk [11–14]. Not only metabolic syndrome disorders but also other diseases such as asthma [15] and polycystic ovary syndrome [16] have been mentioned in the literature to have correlation with adiponectin, resistin, and Adiponectin-Resistin (AR) index. On the other hand, resistin, secreted from white adipocytes and is involved in inflammation in humans, has been observed as an important link between obesity and T2DM and contributes harmfully to the inflammation process [17].
The chronic inflammatory process in atherosclerosis usually can be well predicted by high-sensitivity C-reactive protein (hsCRP) [18,19]. Many large prospective trials have shown that the inflammatory biomarker hsCRP is an independent predictor of future cardiovascular events [20].
An interesting study conducted by Lau et al., they proposed novel AR and Insulin Resistance Adiponectin-Resistin (IRAR) indices that can be used as valid and reliable predictors of T2DM and metabolic syndrome [21]. The scientific society aims to find trustful non-invasive biomarkers to predict cardiovascular risk and its complications. Whether AR and IRAR ratios can be used as indices for cardiovascular risk factors in healthy as well as diabetic subjects needs to be assessed. The aim of this study was to assess the relationships of AR and IRAR indices with adiposity, glucometabolic control and cardiovascular risk incurred by high-sensitivity C-reactive protein (hsCRP) in healthy subjects and patients with T2DM.
2. Methods
This case control study was conducted from January 2021 to December 2021 in the Department of Physiology and Medicine, College of Medicine & King Khalid University Hospital, King Saud University, Riyadh, Saudi Arabia. The study was approved by the Institutional Review Board (IRB) of College of Medicine, King Saud University (10/2664/IRB). We had a total of 191 individuals: 107 patients with T2DM (67 males and 40 females) and control group including 84 healthy subjects (45 males and 39 females) matched for age, gender and weight recruited from patients’ companions. Personal and demographic information from all subjects were obtained on a predesigned form including age, gender, Waist/Hip Ratio (WHR), weight, height, and BMI measurements. American Diabetes Association (ADA) criteria of blood glucose level were used for diagnosis and patients were in stable metabolic condition with at least one year of duration of T2DM [22]. Exclusion criteria included acute or chronic renal disorders, thyroid diseases, acute & chronic infections, stroke, acute diabetic states, and recent surgery in the last month. All subjects signed the consent form for participation in the study.
Bioelectrical impendence analysis was used to measure body composition with an InBody3.0 (Bio-Space, Korea) body analyzer according to the manufacturer’s instructions. All assessments were made in the early morning fasting state, wearing light clothing, and after emptying of the urinary bladder. We collected fasting venous blood samples after 10–12 h of overnight fasting and stored at −80 °C for the evaluation of total Cholesterol (TC), Triglycerides (TG), Low density Lipoprotein (LDL), High density lipoprotein (HDL), fasting blood glucose (FBG), glycosylated hemoglobin (HbA1c). Basal insulin, adiponectin and resistin levels immunoassays were carried out by a quantitative standard sandwich ELISA technique using monoclonal antibody specific for resistin with kits supplied by R&D Systems, (Abingdon, United Kingdom) were used to assess basal insulin, adiponectin and resistin levels. To maintain the overall reliability and precision of ELISA assays all tests were run in duplicate keeping inter-assay % CV <15% while intra-assay %CV <10%. To measure hsCRP, we used a turbidimetric assay (Quantex CRP ultra-sensitive kits, BIOKIT, S.A., Barcelona, Spain) on auto-analyzer Hitachi 911, (ROCHE diagnostics, Indianapolis, Indiana, USA). The US-CRP kits measured ranges from .10 to 20.0 mg/L. The outcome parameter for increased cardiovascular risk in our study was hsCRP. We followed American Heart Association criteria and guidelines for measurement, evaluation and expression of low and high-risk levels of hsCRP [23].
2.1. Formulation of the adiponectin-resistin (AR) index
2.2. Formulation of the Insulin Resistance Adiponectin-Resistin (IRAR) index
2.3. IRAR index = AR index/QUICKI
{R0 = serum total resistin (ng/mL); A0 = serum total adiponectin (μg/mL),
I0 = fasting serum insulin (μU/mL), G0 = fasting plasma glucose (mg/dL)} [21].
2.4. Statistical analysis
Statistical Package for Social Sciences (SPSS Version 19, Chicago, IL, USA) was used for data analysis. Descriptive characteristics were expressed as Mean ± standard deviation (SD). Group comparisons were performed using t-tests. Comparative correlation coefficients were determined using Spearman’s correlations between adiponectin, resistin, AR and IRAR indices with glycemic indices, insulin resistance indices, hsCRP, and body composition. A p value ≥ .05 was considered significant. hsCRP was dichotomized into low and high-risk categories based on AHA guidelines cut off points (low risk ≥3.0 mg/L and high risk >3 mg/L) to use it as state variable for Receiver Operating Curve (ROC) analysis which was carried out to assess the performance of hsCRP in relation to adiponectin, resistin, AR and IR AR indices for increased cardiovascular risk incurred by hsCRP as standard. Since adiponectin is cardio protective at high levels, therefore, we used inverse adiponectin (invadiponectin) values in ROC predictors to keep all variables above midline.
3. Results
Comparison of demographic data and body composition analysis between control and T2DM patients is shown in Table 1. There were no significant differences in age ( p = .066) and height ( p = .535) between control and T2DM patients, while the diabetic patients had higher weight ( p = .010), BMI ( p = .017), body fat mass ( p = .047), and WHR ( p < .001) compared to controls. Table 2 compares HOMA-IR and hsCRP between control and patients with T2DM. HOMA-IR, and hsCRP were significantly higher in T2DM patients than control (9.52 ± 4.98 vs 5.16 ±1.82, p< .001) and (4.53 ± 2.75 mg/L vs 3.55 ± 2.23 mg/L, p = .008) respectively.
Table 1.
Comparison of descriptive characteristics and body composition between control and diabetic subjects.
| Variables | Total N = 191 |
Control n = 84 | T2DM n = 107 | p-value |
|---|---|---|---|---|
| Male/Female | 98/93 | 45/39 | 58/49 | – |
| Age (years) | 51.15 ± 11.15 | 49.51 ± 11.31 | 52.51 ± 10.88 | .066 |
| Height (cm) | 166.31 ± 11.59 | 166.88 ± 8.50 | 165.82 ± 13.68 | .535 |
| Weight (Kg) | 81.41 ± 18.40 | 77.65 ± 14.69 | 84.58 ± 20.56 | .010* |
| BMI (kg/m2) | 28.94 ± 5.16 | 27.96 ± 4.93 | 29.77 ± 5.23 | .017* |
| Intracellular fluid (ICF) (Liters) | 27.43 ± 4.32 | 26.87 ± 4.73 | 27.91 ± 3.91 | .100 |
| Extracellular fluid (ECF) (Liters) | 13.92 ± 7.26 | 13.93 ± 10.45 | 13.90 ± 2.38 | .983 |
| Total body water (TBW) (Liters) | 42.73 ± 27.39 | 43.94 ± 40.11 | 41.70 ± 5.83 | .581 |
| Protein Mass (Kg) | 10.97 ± 1.73 | 10.75 ± 1.89 | 11.16 ± 1.56 | .109 |
| Bone mass (Kg) | 3.53 ± .47 | 3.46 ± .49 | 3.60 ± .43 | .053* |
| Body Fat Mass (Kg) | 25.74 ± 10.13 | 24.13 ± 9.09 | 27.09 ± 10.80 | .047* |
| Fat mass/Muscle mass ratio | 2.36 ± .89 | 2.28 ± .90 | 2.42 ± .87 | .276 |
| Soft Lean Mass (Kg) | 51.80 ± 8.09 | 50.53 ± 8.74 | 52.86 ± 7.37 | .050* |
| Lean body mass (Kg) | 55.25 ± 8.60 | 53.87 ± 9.26 | 56.41 ± 7.86 | .044* |
| Body Fat % (BF%) | 31.40 ± 9.26 | 30.30 ± 8.13 | 32.34 ± 10.06 | .135 |
| Waist/Hip-Ratio (WHR) | .97 ± .11 | .94 ± .12 | 1.00 ± .09 | <.001* |
Data are represented as mean and standard deviation.
Significant p-values of t-test,
p ≤ .05.
Table 2.
Comparison of insulin resistance indices and hsCRP between control and patients with T2DM.
| Variable | All N = 191 |
Control n = 84 | T2DM n = 107 | p-value |
|---|---|---|---|---|
| FBG mmol/dl | 7.08 ± 3.09 | 5.13 ± 1.45 | 8.68 ± 3.16 | <.001* |
| Insulin (μI/ml) | 23.76 ± 8.09 | 22.64 ± 6.11 | 24.69 ± 9.35 | .084 |
| FBG Log10 | 2.07 ± .16 | 1.96 ± .08 | 2.17 ± .14 | <.001* |
| Insulin Log10 | 1.36 ± .13 | 1.34 ± .11 | 1.37 ± .14 | .133 |
| HOMA-IR | 7.55 ± 4.44 | 5.16 ± 1.82 | 9.52 ± 4.98 | <.001* |
| HbA1c (%) | 6.82 ± 2.45 | 5.18 ± 1.46 | 8.15 ± 2.29 | <.001* |
| hsCRP mg/L | 4.07 ± 2.56 | 3.55 ± 2.23 | 4.53 ± 2.75 | .008* |
Data are represented as Mean and standard deviation.
Significant p-values of t-test,
p ≤ .05,
FBG: Fasting blood glucose, HOMA-IR: Homeostatic Model Assessment for Insulin Resistance, HbA1c: glycosylated hemoglobin, hs-CRP: high-sensitivity C-reactive protein.
Adiponectin, resistin, AR, IR, and IRAR indices comparisons between control and patients with T2DM are expressed in Table 3. Serum adiponectin levels were significantly lower in diabetics compared to control (95.45 ± 39.27 ng/ml vs 146.64 ± 56.36 ng/ml, p < .001) while serum resistin was significantly higher in diabetic when compared to control (2.94 ± 1.30 ng/ml vs 2.40 ± 1.09 ng/ml, p = .003). Furthermore, AR and IRAR indices were significantly increased in diabetic subjects when compared to control (.82 ± .29 vs .48 ± .35, p < .001) and (.30 ± .10 vs .17 ± .12, p < .001) respectively.
Table 3.
Comparison of adiponectin, resistin, AR index and IR AR indices between control and patients with T2DM.
| Variable | All N = 191 |
Control n = 84 | T2DM n = 107 | p-value |
|---|---|---|---|---|
| QUICKI | 2.82 ± .17 | 2.71 ± .10 | 2.91 ± .15 | <.001* |
| Adiponectin ng/ml | 118.84 ± 54.13 | 146.64 ± 56.36 | 95.45 ± 39.27 | <.001* |
| Resistin ng/ml | 2.70 ± 1.24 | 2.40 ± 1.09 | 2.94 ± 1.30 | .003* |
| Adiponectin Log10 | 2.03 ± .19 | 2.14 ± .15 | 1.95 ± .17 | <.001* |
| Resistin Log10 | .38 ± .22 | .32 ± .24 | .42 ± .20 | .002* |
| AR index | .63 ± .36 | .48 ± .35 | .82 ± .29 | <.001* |
| IR AR index | .23 ± .13 | .17 ± .12 | .30 ± .10 | <.001* |
Data are represented as mean and standard deviation.
Significant p-values of t-test,
p ≤ .05,
AR index: Adiponectin-Resistin index, IRAR index: Insulin Resistance Adiponectin-Resistin index, QUICKI: Quantitative insulin-sensitivity check index.
Comparison of correlation coefficients of adiponectin, resistin, AR, and IRAR indices with demographic data, body composition and insulin resistance indices is revealed in Table 4. AR index correlated positively with Triglycerides (r = .354, p < .01), hsCRP (r = .264, p < .01), HbA1c (r = .425, p < .01), fat mass (r = .164, p < .05), WHR (r = .248, p < .01), and negatively with high density lipoprotein (r = −.327, p < .01). Furthermore, IRAR index was more strongly correlated with Triglycerides (r = .409, p < .01), hsCRP (r = .268, p < .01), HbA1c (r = .508, p < .01), fat mass (r = .152, p < .05), WHR (r = .256, p < .01), and negatively with high density lipoprotein (r = −.340, p < .01).
Table 4.
Correlation coefficients of adiponectin, resistin, AR index and IRAR index with glycemic indices, insulin resistance indices, hsCRP and body composition in all subjects, N = 191.
| Variable | Adiponectin | Resistin | AR index | IRAR index |
|---|---|---|---|---|
| FBG mmol/dl | −.422** | .101 | .409** | .502** |
| Insulin (μI/ml) | −.180* | .157* | .209** | .170* |
| QUICKI | .325** | .020 | .283** | .387** |
| HOMA-IR | −.398** | .166* | .423** | .475** |
| HbA1c (%) | −.490** | .079 | .425** | .508** |
| TG mmol/L | −.358** | −.088 | .354** | .409** |
| TC mmol/L | −.071 | .009 | .065 | .092 |
| HDL mmol/L | .316** | .146 | −.327** | −.340** |
| LDL mmol/L | −.044 | −.053 | .026 | .050 |
| hsCRP mg/L | −.246** | .097 | .264** | .268** |
| Body Fat Mass (Kg) | −.093 | .196** | .164* | .152* |
| Body Fat % | .025 | .166* | .090 | .080 |
| Protein Mass (Kg) | −.321** | .039 | .166* | .165* |
| Fat mass/Muscle mass | .035 | .172* | .092 | .082 |
| BMI (kg/m2) | −.158* | .141 | .174* | .165* |
| Waist/Hip-Ratio (WHR) | −.209** | .159* | .248** | .256** |
p ≤ .05,
p < .01.
FBG: Fasting blood glucose, TG: Triglycerides, TC: Total cholesterol, HDL: high density lipoprotein, LDL: low density lipoprotein, HbA1c: glycosylated hemoglobin, hs-CRP: high-sensitivity C-reactive protein, QUICKI: Quantitative insulin-sensitivity check index.
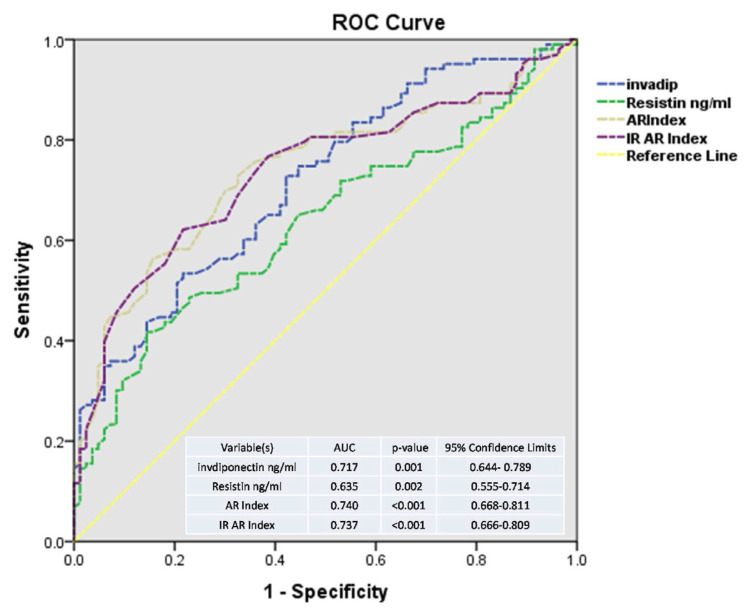
Fig. 1 shows ROC analysis comparing predictive value of adiponectin, resistin, AR, and IRAR indices for increased cardiovascular risk revealed by hsCRP high risk levels. It revealed that area under the curve (AUC) was significant for all these indices with maximum value for IRAR index. AUC values were adiponectin = .717 ( p = .001), resistin = .635 ( p = .002), AR index .740 ( p < .001), and IRAR index = .737 ( p < .001).
Fig. 1.
ROC curve analysis comparing predictive value of adiponectin, resistin, AR and IRAR indices for increased cardiovascular risk incurred by hsCRP. AUC: area under the curve. invadip: inverse adiponectin.
4. Discussion
This study compared the relationships of adiponectin, resistin, AR, and IRAR indices with increased levels of cardiovascular risk markers and adiposity indices in healthy subjects and in patients with T2DM. We found that these composite indices are significantly related with cardiovascular risk markers and body adiposity and have better prediction of cardiovascular risk in patients with T2DM compared to adiponectin, resistin and insulin resistance alone. AR and specifically IRAR indices are significantly increased in diabetic subjects when compared to control. Furthermore, AR and IRAR indices correlated positively with Triglycerides, hsCRP, HbA1c, fat mass, WHR, and negatively with high density lipoprotein.
Our study is concomitant with the findings of Hameed et al. who reported that the AR index correlated significantly with HOMA-IR in type 2 diabetic subjects [24]. Furthermore, we found IRAR index was more positively correlated with cardiovascular biomarkers.
A study in hypertensive type 2 diabetic patients reported that AR index strongly correlated with atherosclerosis, and hence, it may be a good marker of cardiovascular risk in these patients [25]. Singh P et al. reported that AR index was the best predictor of acute coronary syndrome [26]. These studies supported our findings regarding the utility of AR and IRAR indices as cardiovascular risk biomarkers. On the other hand, our findings are not in agreement with Toczylowski et al. who found that patients with coronary artery disease who were obese, or diabetic have a limited impact on adipokines level [27]. In addition to the well-known role of adipokines, several signaling pathways still need to be studied thoroughly in order to understand the full role of adipokines. Sawaguchi et al. suggested that adiponectin could be used as a predictor of progression of heart failure in cardiovascular surgery due its link to sarcopenia, inflammation, and malnutrition [28]. Also, adiponectin and resistin can be involved in cardiac remodeling mechanism [29]. Furthermore, the relationship of adiponectin to chemerin, adiponectin/chemerin ratio, could play an important role in affecting lipids and metabolism especially in reproductive system dysfunctions such as Polycystic ovary syndrome [30]. Mooldijk et al. suggested that adipokines might participate in the pathophysiology of dementia [31]. Whether AR and IRAR indices can be used as biomarkers in these situations or not is the area of further investigations. Moreover, other adipokines like leptin, chemerin and visfatin can be targets of further research in future.
5. Conclusions and recommendations
AR and IRAR indices are more strongly associated with adiposity, glycemia, dyslipidemia and increased risk of cardiovascular diseases. They may prove to be useful integrated biomarkers to predict metabolic dysregulation and cardiovascular risk. We recommend that further, prospective study at a large scale is required to explore the true homeostatic roles of adiponectin and resistin and their correlation to each other in patients with T2DM. Since they are related to glucose and lipid metabolism, it would be worth studying them as an integrated approach with various pharmacological therapies and exercise approaches. They may prove to be useful predictive biomarkers to predict increased cardiovascular risk & metabolic dysregulation in patients with T2DM.
Acknowledgments
The authors extend their appreciation to the Deanship of Scientific Research, King Saud University for funding this project (Grant No. RGP-1438-048).
Abbreviations
- ADA
American Diabetes Association
- AR
Adiponectin-Resistin index
- BIA
Bioelectrical Impendence Analyzer
- BMI
Body Mass Index
- CVD
Cardiovascular diseases
- FBG
Fasting Blood Glucose
- HbA1c
Glycosylated Hemoglobin
- HDL
High density lipoprotein
- HOMA-IR
Homeostatic Model Assessment for Insulin Resistance
- hsCRP
High-sensitive C-reactive Protein
- IRB
Institutional Review Board
- IRAR
Insulin Resistance Adiponectin-Resistin index
- QUICKI
Quantitative insulin-sensitivity check index
- TC
Total cholesterol
- TG
Triglycerides
- T2DM
Type 2 diabetes mellitus
- WHO
World Health Organization
- WHR
Waist hip ratio
Funding Statement
The authors extend their appreciation to the Deanship of Scientific Research, King Saud University for funding this project (Grant No. RGP-1438-048).
Footnotes
Conflicts of interest
None declared.
Author contribution
Conception and design of Study: SSH, TAK, KAR. Literature review: SSH, TAK, MAB, SMH. Acquisition of data: SSH, TAK, MAB, SMH, HAK, KAR. Analysis and interpretation of data: SSH, TAK, SMH. Research investigation and analysis: SSH, TAK, SMH. Data collection: SSH, TAK, MAB, SMH, HAK, KAR. Drafting of manuscript: SSH, TAK, MAB, SMH, HAK, KAR. Revising and editing the manuscript critically for important intellectual contents: SSH, SMH, HAK, KAR. Data preparation and presentation: SSH, TAK, MAB, SMH, HAK, KAR. Supervision of the research: SSH, TAK, KAR. Research coordination and management: SSH, TAK, KAR. Funding for the research: SSH, TAK, KAR.
References
- 1.WHO. cardiovascular diseases (CVDs) World Health organization; Available at: http://www.who.int/mediacentre/factsheets/fs317/en/ [Google Scholar]
- 2. Habib SS, Al-Regaiey KA, Al-Khlaiwi T, Habib SM, Bashir S, Al-Hussain F, et al. Serum inducible and endothelial nitric oxide synthase in coronary artery disease patients with Type 2 Diabetes mellitus. Eur Rev Med Pharmacol Sci. 2022 May;26(10):3695–702. doi: 10.26355/eurrev_202205_28865. [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization. A vital investment. World Health; 2005. p. 202. [Google Scholar]
- 4. Alzamil H, Aldokhi L, Habib SS. Physical fitness and its relationship to plasma leptin, leptin soluble receptor, and free leptin index in a Saudi population: a comparison between diabetic and non-diabetic individuals. Med Sci Monit Basic Res. 2018 Aug 9;24:113–9. doi: 10.12659/MSMBR.910573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.International Diabetic Federation. Diabetes atlas. 9th edition. [Accessed 12 February 2020]. Available online, http://www.diabetesatlas.org/keymessages.html. accessed on.
- 6. Al-Khlaiwi T, Alsabih AO, Khan A, Habib SH, Sultan M, Habib SS. Reduced pulmonary functions and respiratory muscle strength in Type 2 diabetes mellitus and its association with glycemic control. Eur Rev Med Pharmacol Sci. 2021 Dec;25(23):7363–8. doi: 10.26355/eurrev_202112_27430. [DOI] [PubMed] [Google Scholar]
- 7. Zeitouni M, Clare RM, Chiswell K, Abdulrahim J, Shah N, Pagidipati NP, et al. Risk factor burden and long-term prognosis of patients with premature coronary artery disease. J Am Heart Assoc. 2020 Dec 15;9(24):e017712. doi: 10.1161/JAHA.120.017712. . Epub 2020 Dec 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Alshaikh MK, Filippidis FT, Baldove JP, Majeed A, Rawaf S. Women in Saudi Arabia and the prevalence of cardiovascular risk factors: a systematic Review. J Environ Public Health. 2016;2016:7479357. doi: 10.1155/2016/7479357. . Epub 2016 Sep 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lavie CJ, Ozemek C, Carbone S, Katzmarzyk PT, Blair SN. Sedentary behavior, exercise, and cardiovascular Health. Circ Res. 2019 Mar;124(5):799–815. doi: 10.1161/CIRCRESAHA.118.312669. [DOI] [PubMed] [Google Scholar]
- 10. Habib SS. Body composition analysis and estimation of physical fitness by scoring grades in Saudi adults. J Pakistan Med Assoc. 2013 Oct;63(10):1285–9. [PubMed] [Google Scholar]
- 11. Liu W, Zhou X, Li Y, Zhang S, Cai X, Zhang R, et al. Serum leptin, resistin, and adiponectin levels in obese and nonobese patients with newly diagnosed type 2 diabetes mellitus: a population-based study. Medicine (Baltimore) 2020 Feb;99(6):e19052. doi: 10.1097/MD.0000000000019052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Achari AE, Jain SK. Adiponectin, a therapeutic target for obesity, diabetes, and endothelial dysfunction. Int J Mol Sci. 2017 Jun 21;18(6):1321. doi: 10.3390/ijms18061321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hjort R, Ahlqvist E, Andersson T, Alfredsson L, Carlsson PO, Grill V, et al. Physical activity, genetic susceptibility, and the risk of latent autoimmune diabetes in adults and type 2 diabetes. J Clin Endocrinol Metab. 2020 Nov 1;105(11):e4112–23. doi: 10.1210/clinem/dgaa549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Habib SS, Sultan M, Khan A, Al-Khlaiwi T, Bashir S. Circulating adiponectin and resistin levels are associated with adiposity indices and physical fitness in healthy adult males. Med Sci Monit Basic Res. 2021 Jun 23;27:e930322. [PubMed] [Google Scholar]
- 15. Ballantyne D, Scott H, MacDonald-Wicks L, Gibson PG, Wood LG. Resistin is a predictor of asthma risk and resistin: adiponectin ratio is a negative predictor of lung function in asthma. Clin Exp Allergy. 2016 Aug;46(8):1056–65. doi: 10.1111/cea.12742. . Epub 2016 May 27. [DOI] [PubMed] [Google Scholar]
- 16. De Medeiros SF, Rodgers RJ, Norman RJ. Adipocyte and steroidogenic cell crosstalk in polycystic ovary syndrome. Hum Reprod Update. 2021 Jun 22;27(4):771–96. doi: 10.1093/humupd/dmab004. [DOI] [PubMed] [Google Scholar]
- 17. Hjort R, Ahlqvist E, Andersson T, Alfredsson L, Carlsson PO, Grill V, et al. Physical activity, genetic susceptibility, and the risk of latent autoimmune diabetes in adults and type 2 diabetes. J Clin Endocrinol Metab. 2020;105(11):dgaa549. doi: 10.1210/clinem/dgaa549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Habib SS, Al Masri AA. Relationship of high sensitivity C-reactive protein with presence and severity of coronary artery disease Pakistan J Med Sci 2013. 29 6 1425 9 10.12669/pjms.296.3302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Aseri ZA, Habib SS, Alhomida AS, Khan HA. Relationship of high sensitivity C-reactive protein with cardiac biomarkers in patients presenting with acute coronary syndrome. J Coll Physicians Surg Pak. 2014;24(6):387–91. [PubMed] [Google Scholar]
- 20. Ridker PM. C-reactive protein and the prediction of cardiovascular events among those at intermediate risk: moving an inflammatory hypothesis toward consensus. J Am Coll Cardiol. 2007;49(21):2129–38. doi: 10.1016/j.jacc.2007.02.052. [DOI] [PubMed] [Google Scholar]
- 21. Lau CH, Muniandy S. Novel adiponectin-resistin (AR) and insulin resistance (IRAR) indexes are useful integrated diagnostic biomarkers for insulin resistance, type 2 diabetes and metabolic syndrome: a case control study. Cardiovasc Diabetol. 2011 Jan 21;10:8. doi: 10.1186/1475-2840-10-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. American Diabetes Association Classification and diagnosis of diabetes: standards of medical care in diabetes Diabetes Care 2018. 1 Suppl 1 S13 27 10.2337/dc18-S002. [DOI] [PubMed] [Google Scholar]
- 23. Carrero JJ, Andersson Franko M, Obergfell A, Gabrielsen A, Jernberg T. hsCRP level and the risk of death or recurrent cardiovascular events in patients with myocardial infarction: a healthcare-based study J Am Heart Assoc 2019. 8 11 e012638 10.1161/JAHA.119.012638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hameed IK, Rashid NF, Abed BA. Homeostasis Model Assessment-Adiponectin ratio and Adiponectin-Resistin index as markers of insulin resistance in type 2 diabetes mellitus. J Fac Med Baghdad. 2013;55:175–8. [Google Scholar]
- 25. Rubio-Guerra AF, Cabrera-Miranda LJ, Vargas-Robles H, Maceda-Serrano A, Lozano-Nuevo JJ, Escalante-Acosta BA. Correlation between levels of circulating adipokines and adiponectin/resistin index with carotid intima-media thickness in hypertensive type 2 diabetic patients. Cardiology. 2013;125(3):150–3. doi: 10.1159/000348651. . Epub 2013 May 30. [DOI] [PubMed] [Google Scholar]
- 26. Singh P, Sridhar MG, Rajappa M, Balachander J, Kadhiravan T. Adiponectin-resistin index and its strong association with acute coronary syndrome in South Indian men. Inflamm Res. 2014 Nov;63(11):961–8. doi: 10.1007/s00011-014-0771-z. . Epub 2014 Sep 14. [DOI] [PubMed] [Google Scholar]
- 27. Toczylowski K, Hirnle T, Harasiuk D, Zabielski P, Lewczuk A, Dmitruk I, et al. Plasma concentration and expression of adipokines in epicardial and subcutaneous adipose tissue are associated with impaired left ventricular filling pattern. J Transl Med. 2019 Sep 18;17(1):310. doi: 10.1186/s12967-019-2060-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sawaguchi T, Nakajima T, Haruyama A, Hasegawa T, Shibasaki I, Nakajima T, et al. Association of serum leptin and adiponectin concentrations with echocardiographic parameters and pathophysiological states in patients with cardiovascular disease receiving cardiovascular surgery. PLoS One. 2019 Nov 8;14(11):e0225008. doi: 10.1371/journal.pone.0225008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Puchałowicz K, Kłoda K, Dziedziejko V, Rać M, Wojtarowicz A, Chlubek D, et al. Association of adiponectin, leptin and resistin plasma concentrations with echocardiographic parameters in patients with coronary artery disease. Diagnostics. 2021 Sep 26;11(10):1774. doi: 10.3390/diagnostics11101774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Singh A, Choubey M, Bora P, Krishna A. Adiponectin and chemerin: contrary adipokines in regulating reproduction and metabolic disorders. Reprod Sci. 2018 Oct;25(10):1462–73. doi: 10.1177/1933719118770547. . Epub 2018 Apr 18. [DOI] [PubMed] [Google Scholar]
- 31. Mooldijk SS, Ikram MK, Ikram MA. Adiponectin, leptin, and resistin and the risk of dementia. J Gerontol A Biol Sci Med Sci. 2022 Jun 1;77(6):1245–9. doi: 10.1093/gerona/glab267. [DOI] [PMC free article] [PubMed] [Google Scholar]