Abstract
Bats are presumed reservoirs of diverse α- and β- coronaviruses (CoVs) and understanding the diversity of bat-CoVs and the role bats play in CoV transmission is highly relevant in the context of the current COVID pandemic. We sampled bats in Côte d'Ivoire (2016–2018) living at ecotones between anthropogenic and wild habitats in the Marahoué National Park, a recently encroached protected area, to detect and characterize the CoVs circulating in bats and humans. A total of 314 bats were captured, mostly during the rainy season (78%), and CoV RNA was detected in three of the bats (0.96%). A CoV RNA sequence similar to Chaerephon bat coronavirus/Kenya/KY22/2006 (BtKY22) was found in a Chaerephon cf. pumilus and a Mops sp. fecal swab, while a CoV RNA sequence similar to the two almost identical Kenya bat coronaviruses BtKY55 and BtKY56 (BtKY55/56) was detected in an Epomops buettikoferi oral swab. Phylogenetic analyses indicated differences in the degree of evolutionary host-virus co-speciation for BtKY22 and BtKY55/56. To assess potential for human exposure to these viruses, we conducted human syndromic and community-based surveillance in clinics and high-risk communities. We collected data on participant characteristics, livelihoods, animal contact, and high-risk behaviors that may be associated with exposure to zoonotic diseases. We then collected biological samples for viral testing from 401 people. PCR testing of these biological samples revealed no evidence of CoV infection among the enrolled individuals. We identified higher levels of exposure to bats in people working in crop production and in hunting, trapping and fishing. Finally, we used the ‘Spillover’ risk-ranking tool to assess the potential for viral spillover and concluded that, while there is no evidence to suggest imminent risk of spillover for these CoVs, their host range and other traits suggest caution and vigilance are warranted in people with high exposure risk.
Keywords: Coronavirus, Côte d'Ivoire, wildlife, behavior, Chaerephon, Kenya bat
Highlights
-
•
Coronavirus RNA was detected in three bats caught in Côte d'Ivoire in 2018.
-
•
Coronavirus RNA detected are very similar to BtKY55 and BtKY56.
-
•
Crop farming, hunting, trapping, and fishing have a high risk of bat exposure.
-
•
There is no evidence to suggest imminent risk of spillover of those strains.
-
•
Host range, genetics of CoVs and habitat encroachment call for continuous vigilance.
1. Introduction
In response to the ongoing SARS-CoV-2 pandemic, past deadly coronavirus (CoV) epidemics (SARS-CoV-1 and MERS-CoV), high CoV diversity in wildlife reservoirs, and their zoonotic potential [[1], [2], [3]], surveillance of CoVs in wildlife and humans should be a public health priority. One of the human β-CoVs that cause the common cold, (HCoV-OC43) in Côte d'Ivoire (Lau et al., 2011; Ekaza et al., 2014) and globally, has also been found infecting chimpanzees, threatening the apes' habituated group in the Tai National Park [4]. However, despite our knowledge about CoV origins and spillover potential between humans and animals, no research has specifically been dedicated to bat-CoVs in Côte d'Ivoire.
Bats are presumed reservoirs of diverse α- and β- CoVs [2,5,6] and understanding the role bats play in CoV transmission is highly relevant from both a public health and conservation perspective. However, due in part to the realities that not all injuries from wildlife are reported, the mechanisms of bat-to-human CoV transmission [7] and the evolution and diversification of CoVs remain poorly understood [8]. Between 2006 and 2022, bat-CoVs have been detected in at least 17 Afrotropical bat species from various countries including the Democratic Republic of the Congo, the Republic of Congo [9], Rwanda [10], Kenya [11], Gabon [12], Cameroon [13] and islands from the West Indian Ocean [14].
Given the role of bats as potential sources of zoonotic spillover, it is critically important to understand the circumstances and behaviors that bring human populations into close contact with bats [15]. Behavioral risk investigations across several countries recently identified how some at-risk groups, such as bushmeat hunters and wildlife/guano farmers underestimate risks linked to their work activities due to accepted generational practice or lack of information regarding potential risks [16]. Fruit bats are widely hunted and sold as bushmeat in Africa, including Côte d'Ivoire, while the smaller insectivorous bats are largely ignored by hunters (PREDICT CIV unpublished data). In 2011 in a rural area of south-eastern Côte d'Ivoire, RNA of a novel Hantavirus, the Mouyassue Hantavirus (MOYV), (GenBank JQ 287717) was detected in liver biopsies of two banana serotines (Afronycteris nanus) [17]. Moreover, in rural areas of Sierra Leone where the new Ebola Bombali virus has been identified [18], and in Côte d'Ivoire, insectivorous bats are found inside the roofs of houses and in hollow trees in crop fields and are regularly caught by children [19,20]. The majority of fruit bat species are in decline, primarily as a result of habitat loss and hunting [21], while insectivorous bats are indirectly threatened by loss of roost sites [22] and disease [23]. This may result in altered foraging and behavioral patterns, supplemented by virus niche expansion or alteration, which may move within closer proximity to humans and livestock [21]. Vigilance is thus required to monitor the potential zoonotic microbes carried by these bats living in close proximity to people, and to assess risk of spillover in behaviors undertaken by people in West Africa. Consequently, we sampled and tested bats from a recently encroached protected area, the Marahoué National Park, Côte d'Ivoire (MNP), and conducted surveys (semi-structured interviews and focus groups) to assess human behavior.
2. Methods and study site
We carried out bat surveys, human syndromic and community-based surveillance between March 2017 and August 2018 in a rural setting at the edge of the MNP, district of Bouaflé, Côte d'Ivoire. The park in the center of Côte d'Ivoire was established in 1968 and occupies an area of approximately 100,000 ha. The MNP consists of a forest and savanna mosaic and is relatively flat with a mean elevation of ±250 m. It is located in the mesophilic Guinean sector, within a climatic transition zone between the wetter southern half (1200 to 1800 mm rainfall/year) and the drier north (1100 to 1600 mm rainfall/year), and with average annual temperatures between 25 °C and 28 °C [24]. The MNP is one of the most degraded protected areas in Côte d'Ivoire due to conversion of forests into agricultural land (rubber, cocoa) and intense poaching and fishing pressure. Study sites with a high chance for human-wildlife interactions were selected, four rural localities were selected for bat collection and community-based surveillance, and two health centers for ambulatory patients' syndromic surveillance.
3. Sample collection and species identification
3.1. Bat sampling
During the 2017 and 2018 rainy (May to June and August to September) and dry seasons (October to April) [25], bats were caught with mist nets at the interface between village and crop fields, in clearings, around houses, fruit trees, and colonized hollowed tree trunks and permanently monitored. Nets were deployed between 6:00 pm and 11:00 pm. Captured bats were placed separately in cloth bags prior to anesthesia (isofluorane) and sample collection. Morphometric measurements were recorded and samples (oral, rectal and nasal swabs, and veinous blood and serum) collected and preserved in VTM and Trizol media following the PREDICT guidelines (https://ohi.vetmed.ucdavis.edu/programs-projects/predict-project/publications#Guides). Samples were stored in liquid nitrogen in the field prior to transfer to either the Pasteur Institute Côte d'Ivoire (IPCI), Adiopodoumé site or the National Laboratory for Agricultural Development and Support (LANADA), Bingerville site in Abidjan. All animals were released at the capture site. Bats were identified in the field following regional identification keys [26,27], a picture atlas of West African Biodiversity [24], and the iNaturalist citizen science platform (https://www.inaturalist.org/guides/74).
3.2. Human syndromic surveillance and behavioral risk factors of exposure
Participants in this study were consenting individuals who were living in, working in, or visiting the selected communities, or admitted to nearby clinics or hospitals with symptoms (fever, cough, encephalitis, diarrhea, hemorrhagic syndrome). In the community, participants were enrolled through cluster sampling at the same sites where animal surveillance was conducted. In hospital settings, patients were enrolled according to national procedures and standards in a 50 km radius of the zoonotic surveillance site. Samples were taken from ambulatory sick patients exhibiting fever, cough, diarrhea, hemorrhagic syndrome, or skin rash. An urban health center was initially selected as the catchment site for patients originating in the rural communities, and a regional hospital center was added in 2018 after interviews with villagers suggested this was also used frequently. Oral, nasal, rectal and venous blood samples were collected from consenting individuals, preserved in VTM and Trizol media following the PREDICT SOPs (see ethical statement), then stored in liquid nitrogen in the field prior to transfer to the IPCI Adiopodoumé biobank. A standardized questionnaire was administered to enrolled individuals in the high-risk communities and clinic settings to collect data on health, livelihoods, animal contact, and behaviors that may be associated with high risk of exposure to zoonotic diseases. In-depth, one-on-one interviews and focus groups were also conducted to better understand the contexts within which people interacted with bushmeat and animals, including a specific focus on interactions with bats. All analysis were performed in the open source R environment for statistical computing [38].
3.3. Viral screening
Testing was performed at LANADA, Bingerville and IPCI, Adiopodoumé. RNA was extracted using the Qiagen Viral RNA Mini Kit according to the manufacturer's instructions and converted into cDNA using a FIREScriptRT cDNA Synthesis KIT (Solis Biodyne). The cDNA was stored at −20 °C until analysis. Two conventional nested broad range PCR assays, both targeting conserved regions within the RNA-Dependent RNA Polymerase gene (RdRp), were used to test the samples for CoV RNA [28]; [29]. In total 686 tests were performed for CoV RNA detection (53 feces, 312 oral swabs, and 142 rectal swabs). A total of 179 specimens were screened with both the Quan and Watanabe essays, 253 only with Watanabe, and 75 only with Quan [28]; [29].
To confirm field-identification of bats, samples from all CoV RNA-positive animals and from representatives of different field-identified species were tested with a Cytochrome b (CytB) PCR assay [30].
For visualization, PCR products ran on an agarose gel, and products corresponding to the expected size were excised. DNA was extracted using the Qiagen QIAquick Gel Extraction Kit and were sent for cloning and Sanger sequencing at GENEWIZ (Germany). Sequencing results were assessed and processed using Geneious 11.1 and compared to the GenBank database (BLAST N).
For phylogenetic evaluation, sequences with >90% identities to the detected ones were downloaded from GenBank. Sequences with >99% with others from the same country were excluded from the analysis. For host species identification, full or close to full (>1029 nt) CytB sequences were used for phylogenetic analysis. Multiple sequence alignments were made in Geneious (version 11.1.3, MUSCLE Alignment). Bayesian phylogenies were inferred using MrBayes 3.2 [31]; Datatype = DNA, Nucmodel = 4by4, Nst = 1, Coavion = No, # States = 4, Rates = Equal, two runs, four chains of 10,000,000 generations for virus PCR target regions, and 1000,000 generations for host gene sequences. Two CoV sequences with <90% identities to the Côte d'Ivoire sequences served as outgroups to root each of the PCR trees; Gallus gallus and Anas platyrhynchos were used as outgroup for CytB trees. Trees were sampled after every 1000 steps during the process to monitor phylogenetic convergence. The average standard deviation of split frequencies was below 0.005 for the BtKY22-like CoV analysis, below 0.003 for the BtKY56/BtKY55-like CoV analysis, and below 0.001 for the CytB analyses. The first 10% of the trees were discarded and the remaining ones combined using TreeAnnotator (versions 1.10.4 and 2.5.1; http://beast.bio.ed.ac.uk) and displayed with FIGTREE (1.4.4; http://tree.bio.ed.ac.uk/). Sequences obtained from the CytB PCR were blasted against the GenBank database and were considered to match a species if identities were at 98% or higher.
For cospeciation analysis, the Jane software tool (version 4) with default settings for generations and population size was used with the host and virus trees (Fig. 1, Fig. 2) [32]. Default costs for events were used (Cospeciation 0, Duplications 1, Duplications and host switches 2, Losses 1, Failure to diverge 1) and more extreme costs (10) for each category explored. CoV sequences not shown in the phylogenetic trees (Fig. 1, Fig. 2) due to >99% identities with others from the same country were included for this analysis if found in different hosts. Correlations between the genetic differences of CoV sequences (0–10%) and geographical location/distances and year/time difference of sample collection were evaluated using a Pearson correlation. In cases where exact sampling locations were not published, county, province, or country centroids were used.
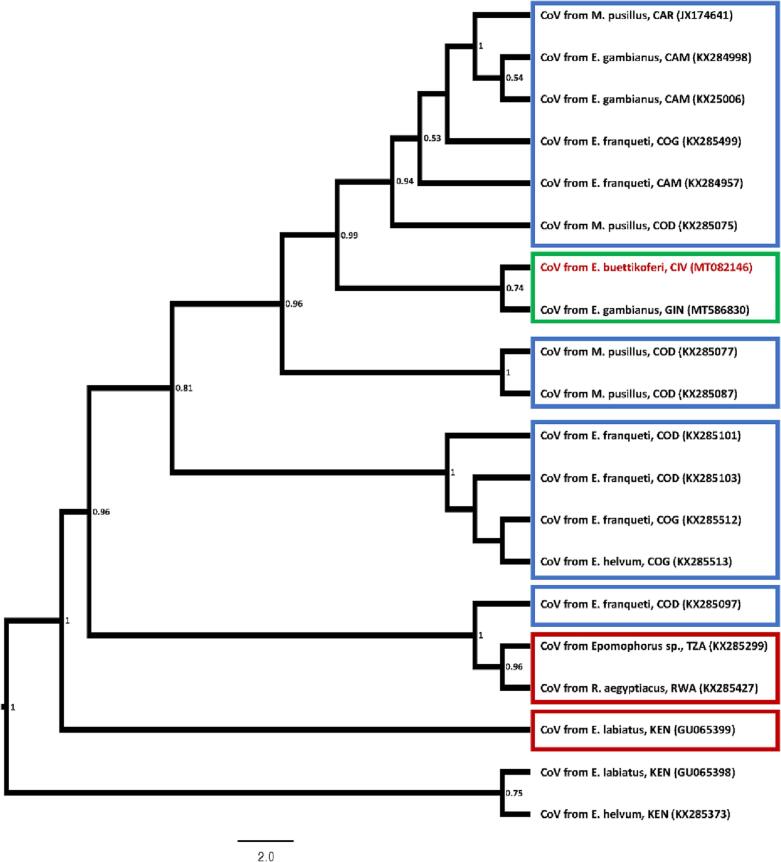
Fig. 1.
Maximum likelihood phylogenetic tree of BtKY56/BtKY55-like CoVs based on the amplified PCR fragment of the RdRp gene, including sequences closest (>90% identities) to Cote d'Ivoire CoV isolate from Epomops buettikoferi. Isolates are identified by GenBank ID and host (bat species) from which the CoV sequence was obtained as well as country three letter code (ISO 3166-1 alpha-3) were sampling took place are indicated. Geographic regions are indicated with boxes for sequences >90% identical with Cote d'Ivoire isolates, with green indicating West Africa, blue Central Africa, and red West Africa (Compare Supplement 4). Numbers at nodes indicate bootstrap support. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
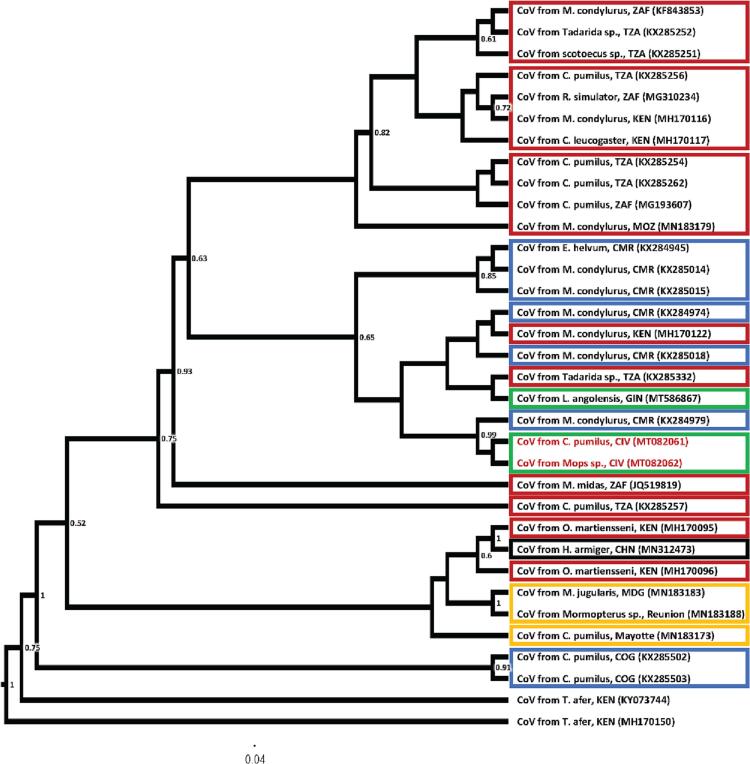
Fig. 2.
Maximum likelihood phylogenetic tree of BtKY22-like CoVs based on the amplified PCR fragment of the RdRp gene, including sequences closest (>90% identities) to Cote d'Ivoire CoV isolate from Chaerephon cf. pumilus and Mops sp.. Isolates are identified by GenBank ID and host (bat species) from which the CoV sequence was obtained as well as country three letter code (ISO 3166-1 alpha-3) or place were sampling took place are indicated. Geographic regions are indicated with boxes for sequences >90% identical with Cote d'Ivoire isolates, with green indicating West Africa, blue Central Africa, red West & South Africa, orange Islands in the Indian Ocean, and black “other continent” (Compare Supplement 5). Numbers at nodes indicate bootstrap support. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
4. Results
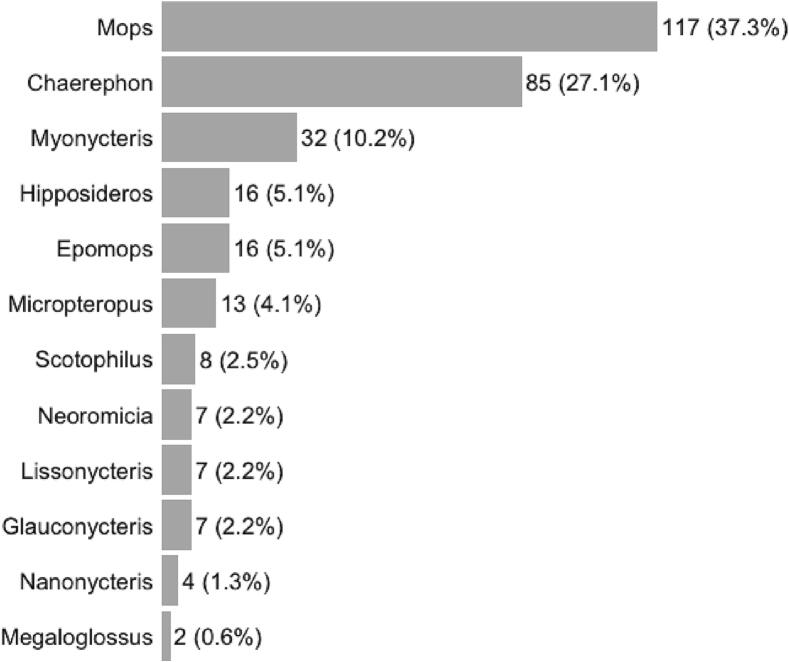
A total of 314 bats were caught between 2017 and 2018, with the majority (n = 245; 78.0%) collected during the rainy season. Bats belonged to 14 genera and at least 25 species, including Mops sp. (n = 117; 37.3%), Chaerephon sp. (n = 85; 27.1%), and Myonycteris sp. (n = 32; 10.2%) (Fig. 3; Supplement 1). Bats were primarily reproductive adults (n = 288; 91.7%), with 20 individual subadults or juveniles (6.4%) and 6 not characterized (1.9%). 183 (58.3%) of the bats were female, 125 (39.8%) were male, and 6 not characterized (1.9%). CoV RNA was detected in three of the 314 bats (0.96%), with two of the three samples collected in the wet season. [29]. Two of the three samples were collected in the wet season. Three species of bats were positive for CoV RNA: Chaerephon cf. pumilus, Epomops buettikoferi, and an unidentified Mops sp. CoV RNA sequence analysis indicates that the Chaerephon cf. pumilus and the Mops sp. (fecal swabs) were both harboring a CoV similar to Chaerephon bat coronavirus/Kenya/KY22/2006 (BtKY22) while the Epomops buettikoferi (oral swab) was harboring a CoV similar to the two almost identical Kenya bat coronaviruses BtKY55 and BtKY56 (BtKY55/56). Both BtKY22 and BtKY55/56 have previously been detected in bats from multiple countries across Africa (Supplements 3 and 4). The three sequences from Côte d'Ivoire were deposited in GenBank under accession numbers MT082046 (Epomops buettikoferi), MT082061 (Chaerephon cf. pumilus), and MT082062 (Mops sp.).
Fig. 3.
Chiroptera by genera captured and sampled in rural villages of the Marahoué National Park region, Côte d'Ivoire, between 2017 and 2018.
Phylogenetic analysis of the BtKY55/56 variants showed that the Côte d'Ivoire CoV clusters were related to a geographically and temporally close variant from neighboring Guinea, while in the BtKY22 tree, Côte d'Ivoire CoVs cluster closely with a CoV from Cameroon rather than with a Guinean CoV (Fig. 1, Fig. 2). Cospeciation analysis with Jane indicates differences in the driving forces for the calculated phylogenies of BtKY22 and BtKY55/56 (Table 1).
Table 1.
Cospeciation analysis results.
| Species | Cospeciation | Duplications | Duplications and Host Switching | Losses | Failures to Diverge | Cost |
|---|---|---|---|---|---|---|
| BtKY55/56 | 3 | 4 | 9 | 0 | 1 | 24 |
| BtKY22 | 0 | 12 | 19 | 7 | 7 | 64 |
Correlation coefficients between genetic distances and geographic distances were 0.58 for MT082146 and 0.44 for MT082061, while coefficients between genetic distances and temporal distances were 0.05 for MT082146 and − 0.27 for MT082061 (Supplement 5).
4.1. Human syndromic surveillance and behavioral risk factors of exposure
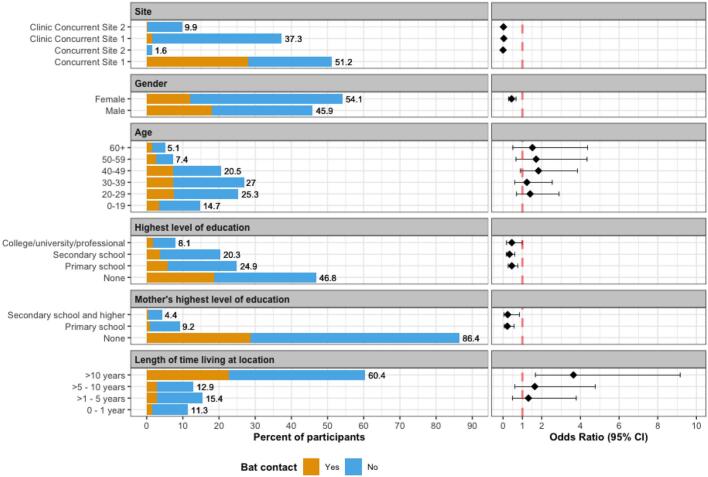
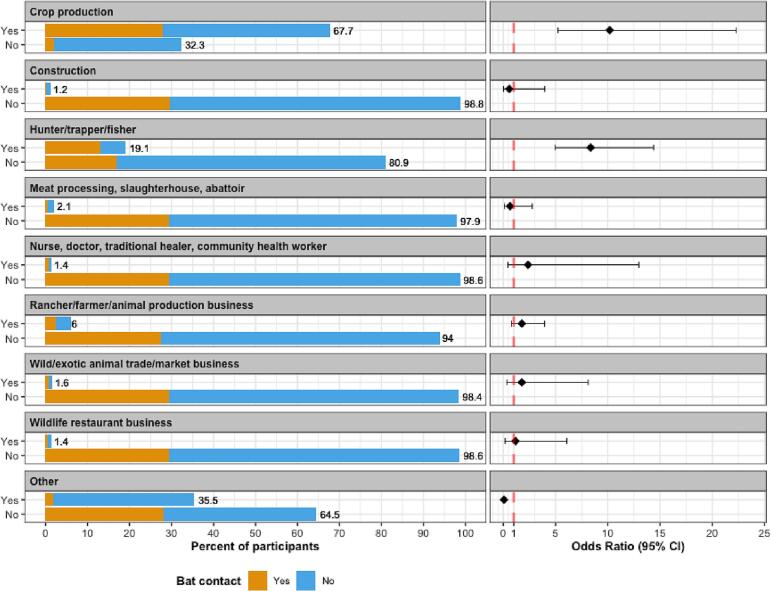
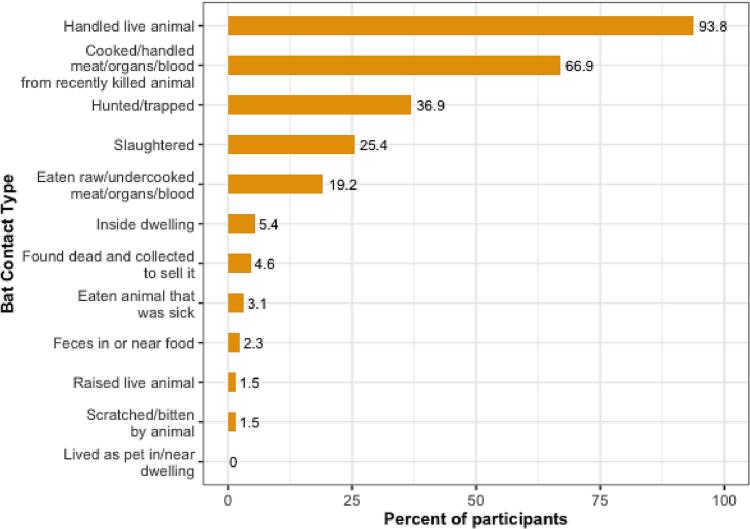
A total of 434 people (n = 235 female; n = 199 male) were interviewed in clinic (n = 205) and rural sites (n = 229), of which 401 consented to provide at least one specimen sample (n = 230 female; n = 171 male; Fig. 4). All 281 oral and 281 nasal swabs, and the 94 rectal swabs tested were negative for CoV RNA using either of the PCR assays [28,29]. Of the 130 participants who reported bat contact (30%) in the last 12 months, the three most common types of contact were handling a live bat (94%), cooking or handling the meat, organs, or blood of a recently killed bat (67%), and hunting or trapping a bat (37%). Using unadjusted odds ratios, females were less likely to report bat contact within the last 12 months than males (OR 0.44, CI 0.29–0.67) (Table 2 and Fig. 4). Higher levels of education were generally associated with less bat contact; compared to those reporting no formal education, those whose highest level of education was primary school, secondary school, or college/university/professional reported lower levels of bat contact (OR 0.45, CI 0.26–0.76, OR 0.33, CI 0.18–0.60, and OR 0.45, CI 0.18–0.99, respectively). The two livelihood groups associated with higher levels of bat contact were crop production (OR 10.2, CI 5.25–22.3) and hunter/trapper/fisher (OR 8.35, CI 4.96–14.4), compared to those not in those livelihood groups (Table 2, Table 3 and Fig. 5). Those who reported their livelihood as “other”, which included extraction industry, homemaker, child, non-animal business, student, and unemployed, were less likely to report bat contact when compared to those who did not (OR 0.07, CI 0.03–0.14).
Fig. 4.
Questionnaire participant characteristics: prevalence of and unadjusted odd ratios for bat contact by demographic group within the 12 months prior to questionnaire (n = 434).
Table 2.
Questionnaire participant characteristics: prevalence of and unadjusted odd ratios for bat contact within the 12 months prior to questionnaire (n = 434).
| Bat Contact1 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| Total |
No |
Yes |
|||||||
| n | % | n | % | n | % | OR | 95% CI | ||
| Total | 434 | 100.0 | 304 | 70.0 | 130 | 30.0 | – | – | |
| Site | |||||||||
| Concurrent Site 1 | 222 | 51.2 | 100 | 23.0 | 122 | 28.1 | – | – | |
| Concurrent Site 2 | 7 | 1.6 | 7 | 1.6 | 0 | 0.0 | 0.00 | – | |
| Clinic Concurrent Site 1 | 162 | 37.3 | 155 | 35.7 | 7 | 1.6 | 0.04 | 0.02, 0.08 | |
| Clinic Concurrent Site 2 | 43 | 9.9 | 42 | 9.7 | 1 | 0.2 | 0.02 | 0.00, 0.09 | |
| Sex | |||||||||
| Male | 199 | 45.9 | 121 | 27.9 | 78 | 18.0 | -- | -- | |
| Female | 235 | 54.1 | 183 | 42.2 | 52 | 12.0 | 0.44 | 0.29, 0.67 | |
| Age | |||||||||
| 0–19 | 52 | 12.0 | 41 | 9.4 | 11 | 2.5 | -- | -- | |
| 20–29 | 110 | 25.3 | 77 | 17.7 | 33 | 7.6 | 1.60 | 0.75, 3.61 | |
| 30–39 | 117 | 27.0 | 85 | 19.6 | 32 | 7.4 | 1.40 | 0.66, 3.17 | |
| 40–49 | 89 | 20.5 | 57 | 13.1 | 32 | 7.4 | 2.09 | 0.97, 4.79 | |
| 50–59 | 32 | 7.4 | 21 | 4.8 | 11 | 2.5 | 1.95 | 0.72, 5.30 | |
| 60+ | 22 | 5.1 | 15 | 3.5 | 7 | 1.6 | 1.74 | 0.55, 5.29 | |
| Highest Education | |||||||||
| None | 203 | 46.8 | 122 | 28.1 | 81 | 18.7 | -- | -- | |
| Primary School | 108 | 24.9 | 83 | 19.1 | 25 | 5.8 | 0.45 | 0.26, 0.76 | |
| Secondary School | 88 | 20.3 | 72 | 16.6 | 16 | 3.7 | 0.33 | 0.18, 0.60 | |
| College/University/Professional | 35 | 8.1 | 27 | 6.2 | 8 | 1.8 | 0.45 | 0.18, 0.99 | |
| Mother's Highest Education | |||||||||
| None | 375 | 86.4 | 251 | 57.8 | 124 | 28.6 | – | – | |
| Primary School | 40 | 9.2 | 36 | 8.3 | 4 | 0.9 | 0.22 | 0.07, 0.58 | |
| Secondary School | 14 | 3.2 | 12 | 2.8 | 2 | 0.5 | 0.34 | 0.05, 1.26 | |
| College/University/Professional | 5 | 1.2 | 5 | 1.2 | 0 | 0.0 | 0.00 | – | |
| Length of Time Living at Location | |||||||||
| 0–1 year | 49 | 11.3 | 42 | 9.7 | 7 | 1.6 | – | – | |
| >1–5 years | 67 | 15.4 | 55 | 12.7 | 12 | 2.8 | 1.31 | 0.48, 3.79 | |
| >5–10 years | 56 | 12.9 | 44 | 10.1 | 12 | 2.8 | 1.64 | 0.60, 4.77 | |
| >10 years | 262 | 60.4 | 163 | 37.6 | 99 | 22.8 | 3.64 | 1.67, 9.15 | |
| Crop Production | |||||||||
| No | 140 | 32.3 | 131 | 30.2 | 9 | 2.1 | – | – | |
| Yes | 294 | 67.7 | 173 | 39.9 | 121 | 27.9 | 10.2 | 5.25, 22.3 | |
| Wildlife Restaurant Business | |||||||||
| No | 428 | 98.6 | 300 | 69.1 | 128 | 29.5 | – | – | |
| Yes | 6 | 1.4 | 4 | 0.9 | 2 | 0.5 | 1.17 | 0.16, 6.08 | |
| Wild/exotic animal trade/market business | |||||||||
| No | 427 | 98.4 | 300 | 69.1 | 127 | 29.3 | – | – | |
| Yes | 7 | 1.6 | 4 | 0.9 | 3 | 0.7 | 1.77 | 0.34, 8.15 | |
| Rancher/farmer/animal production business | |||||||||
| No | 408 | 94.0 | 289 | 66.6 | 119 | 27.4 | – | – | |
| Yes | 26 | 6.0 | 15 | 3.5 | 11 | 2.5 | 1.78 | 0.78, 3.97 | |
| Meat processing, slaughterhouse, abattoir | |||||||||
| No | 425 | 97.9 | 297 | 68.4 | 128 | 29.5 | – | – | |
| Yes | 9 | 2.1 | 7 | 1.6 | 2 | 0.5 | 0.66 | 0.10, 2.79 | |
| Hunter/trapper/fisher | |||||||||
| No | 351 | 80.9 | 278 | 64.1 | 73 | 16.8 | -- | -- | |
| Yes | 83 | 19.1 | 26 | 6.0 | 57 | 13.1 | 8.35 | 4.96, 14.4 | |
| Nurse, doctor, traditional healer, community health worker | |||||||||
| No | 428 | 98.6 | 301 | 69.4 | 127 | 29.3 | – | – | |
| Yes | 6 | 1.4 | 3 | 0.7 | 3 | 0.7 | 2.37 | 0.43, 13.0 | |
| Construction | |||||||||
| No | 429 | 98.8 | 300 | 69.1 | 129 | 29.7 | -- | -- | |
| Yes | 5 | 1.2 | 4 | 0.9 | 1 | 0.2 | 0.58 | 0.03, 3.98 | |
| Other2 | |||||||||
| No | 280 | 64.5 | 158 | 36.4 | 122 | 28.1 | – | – | |
| Yes | 154 | 35.5 | 146 | 33.6 | 8 | 1.8 | 0.07 | 0.03, 0.14 | |
Defined as participant-reported bat contact within 12 months prior to interview.
Includes extraction, homemaker, child, non-animal business, student, and unemployed. Note: livelihood is select all that apply.
Table 3.
Human-bat interaction types (n = 130).
| Bat Contact Type | n | % | |
|---|---|---|---|
| Lived as pet in/near dwelling | 0 | 0.0 | |
| Handled live animal | 122 | 93.8 | |
| Raised live animal | 2 | 1.5 | |
| Feces in or near food | 3 | 2.3 | |
| Inside dwelling | 7 | 5.4 | |
| Cooked/handled meat/organs/blood from recently killed animal | 87 | 66.9 | |
| Eaten raw/undercooked meat/organs/blood | 25 | 19.2 | |
| Eaten animal that was sick | 4 | 3.1 | |
| Found dead and collected to sell it | 6 | 4.6 | |
| Scratched/bitten by animal | 2 | 1.5 | |
| Hunted/trapped | 48 | 36.9 | |
| Slaughtered | 33 | 25.4 |
Fig. 5.
Questionnaire participant characteristics: prevalence of and unadjusted odd ratios for bat contact by livelihood within the 12 months prior to questionnaire (n = 434).
In interviews and focus groups, there was wide variation between how much direct contact participants had with bats (Fig. 6). While some described recent contact (within the last 12 months) or observation of local contact through activities such as hunting, sale, or consumption, others shared that they had only done so in the past, that there were no bats around, or that they did not like to consume bats. In some descriptions of local consumption observations, respondents shared that bat species found in the home (e.g. Mops condylurus) were not the ones that were typically consumed (e.g. Megaloglossus woermanii, Epomops buettikoferi). Some respondents shared that killed bats were sometimes handed off to children and women for preparation, while other reports described bat butchering and meat preparation happening while outside the home. In descriptions of handling dead bats, some respondents described bare-handed contact during the process. Among participants who described hunting and slaughtering practices, the use of a slingshot, a catapult, or a gun/rifle were among the most common ways to hunt bats. Some respondents described leaving bats found dead alone, and others linked recent messaging about Ebola with bats.
Fig. 6.
Human-bat interaction types (n = 130).
5. Discussion
Bat-CoV-RNA has previously been detected in African bats. The apparent prevalence of bat CoV RNAs measured in our study is relatively low (<1%) compared to other studies that reported 5–10% of bats carrying CoV RNA [13]. However, this lower apparent prevalence should be considered with caution, as many ecological factors may influence the spatial and temporal patterns of virus circulation. There can be a wide variation in apparent prevalence among bat species, based on season, sex, and age class [9,12,13,33]. The species composition of sampled bat populations may be a major factor contributing to differences in prevalence among studies.
The two bat CoVs detected in the current study appear to belong to close relatives (genetic variants) of BtKY22 and BtKY55/56. Both viruses were first detected in Kenya and have subsequently been found to circulate in different bat species across Africa, and with a close relative found in China (BtKY22-like CoV) (Supplements 4 and 5) [8]. Phylogenetic analysis and the geographic location of the bats sampled suggests that virus-host coevolution played an important role in the evolution of BtKY55/56 and related variants, but this was not as significant for BtKY22. It is unknown if this is due to differences in host behavior, ecological factors, or virus-specific traits.
A weak correlation between genetic distance and geographic distance appears to exist for both BtKY22 and BtKY55/56, however as the sequences are very short and the geographical data points limited, the significance of this is uncertain. No correlation was observed between genetic distance and temporal distance, which might be attributed to the relatively small window in which the samples (published in the literature) were collected over 11 or 14 years respectively, with most samples being collected within 6 years [[9], [10], [11], [12], [13], [14]].
Human α- and β-CoVs are associated with seasonal common cold, and β-CoVs also include viruses with pandemic potential such as SARS-CoV-1, SARs-CoV-2, and MERS-CoV. In this study, we found an α-CoV, the Chaerephon bat coronavirus/Kenya/KY22/2006 (HQ728486), with a spillover position at 20/889 and a risk score of 78/155 in the “Spillover” open-source risk virus ranking tool (https://spillover.global/) [accessed on 05/16/2022] [34]. The β-CoV we detected and the Kenya bat coronavirus BtKY56/BtKY55 (GU065400) has a spillover position at 39/889 and a risk score of 72/155 in the “Spillover” open-source tool [accessed on 05/16/2022] [34]. While there is currently no evidence to suggest that BtKY22 and BtKY56/BtKY55 pose an imminent threat to human health, their host range and properties as reflected in the risk ranking should be considered as reasons for caution and vigilance, particularly where specific risk behaviors are engaged in. While none of samples from people we tested were positive for CoVs, hunting and handling bats is common among the surveyed population, and colonies of Mops sp. and Chaerephon pumilus have been recorded in the MNP region. The risk for bat-borne zoonotic disease transmission therefore remains a public health concern [35]. While the overall CoV prevalence in bats was low, the circulation of bat-CoVs with both fecal and oral tropism in bats in an area with substantial anthropogenic activity suggests that human exposure to these viruses is likely. Both Mops sp. and Epomops sp. bats found positive in this study have been found to harbor Bombali Ebola virus in the region [18,36], and Zaire Ebola virus antibodies have been detected in Epomops buettikoferi in West Africa [37]. These past detections have motivated recent awareness campaigns on ways to live safely with bats (https://p2.predict.global/living-safely-with-bats-book) [15]. The viral screening and behavioral risk data in the current paper suggest that further targeted sampling to identify host range for the bat-CoVs identified, as well as the diversity of other viruses with potential for zoonotic spillover, are warranted.
5.1. Limitations
While producing new and valuable results on the interactions between humans and bats in rural Côte d'Ivoire and potential spillover risks, there are limits to the degree to which the biological data can be interpreted. The sample size of both the human and of the bat population sampled was small compared to some other studies, which might explain the relatively low rate of CoV detections, since “random” effects will have a higher impact. The relatively short amplicons produced by the PCRs also do not allow for a definite classification and risk assessment of the viruses, even though the virus species allocation appears solid.
6. Conclusions
The results of the study confirm a high potential for the spillover of viruses from bats to humans in Côte d'Ivoire, even though we did not detect an imminent viral threat. Community awareness campaigns about the value of bats to conservation and human well-being (e.g. in pollinating trees and reducing pest insects), and the potential for contact with bats to lead to exposure to their viruses may assist in reducing risk of spillover to these or other potential zoonoses. Surveillance efforts directed at a better characterization of viruses and other pathogens in wildlife through for example whole genome sequencing would be beneficial, since most existing tools and algorithms rely in part or solely on such data to predict the risk for humans.
Ethical statement
This study was made possible by the generous support of the American people through the United States Agency for International Development (USAID) Emerging Pandemic Threats PREDICT project (cooperative agreement number GHN-A-OO-09-00010-00). The results from the study do not indicate the opinion of the United States of America government. Sampling was conducted under a University of California, Davis Animal Care and Use Committee approved protocol (UC Davis IACUC Protocol No. 16048). There is no animal ethics committee in Côte d'Ivoire (IACUC), therefore the study protocol was submitted to the National Ethics Committee for Health Research (CNERS) which approved the fieldwork (permit number 035–18/MHS/CNER/kp). The Directorate of fauna and hunting resources (DFRC) and the Ivorians office for Parcs and Reserves (OIPR) also provided authorization to sample animals in the protected area and its periphery (permit number 768/MINSEDD/OIPR/DG).
Author contributions
Formal analysis, Visualization, Writing - original draft: CEL.
Writing - review & editing: CEL, AL, KS, JLF, DOJ, ESM, SB, CL, JKC, ABAA, SNV, VK, STK, CY, MD, FJL.
Conceptualization & Methodology: CEL, AL, JLF, DOJ, KS, JKC.
Statistical analysis: SB, ESM.
Biological field sampling (animals): JKC, AL, EKK.
Laboratory work and sequence analysis: CL, JKC, ABAA, SNV, VK, STK, CY.
Human syndromic surveillance (sampling): JKC.
Ethnographic questionnaires and focus groups: KS, AL, LF, SM, JM, DB.
Supervision & Project administration: KS, JLF, AL, DOJ, PD, JKC, ABAA, MD.
Funding acquisition: DOJ, PD.
Declaration of Competing Interest
The authors have no competing interests to declare.
Acknowledgements
The authors would like to thank the government of Côte d'Ivoire for the permission to conduct this study, Commander Seka and Ivorian Office of Parks and Reserves (OIPR) for making its forest guards available to us for the security of the teams, Dr. Saraka, Dr. Tape and his team at the Bonon Urban Health Center, Dr. Kone, Director of the Bouaflé Regional Hospital, Dr. Yao, Head of the Bouaflé Regional Hospital Laboratory and their respective teams for their valuable help in collecting samples, the populations of the villages bordering the Marahoué National Park and their respective chiefs for their assistance and collaboration, the staff of Metabiota and Ecohealth Alliance who assisted in sample collection and other members of the PREDICT-2 consortium (https://ohi.sf.ucdavis.edu/programs-projects/predict-project/authorship). The study was undertaken as part of the global USAID-funded Emerging Pandemic Threats (EPT) PREDICT project that focuses on enhancing the global capacity for the detection and discovery of potentially zoonotic viruses at the human-animal interface. It was made possible by the generous support of the American people through the United States Agency for International Development (USAID) Emerging Pandemic Threats PREDICT program Project Award AID-OAA-A-14-00102. The contents are the responsibility of the authors and do not necessarily reflect the views of USAID or the United States Government.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.onehlt.2023.100569.
Appendix A. Supplementary data
Supplement 1 (Table): Number of bats captured per species (2017-2018).
Supplement 2 (Figure): Map of Africa showing the countries from which the sequences included in Figure 2 (KY55/56) derived. In green are Guinea and Côte d’Ivoire, in blue Cameroon, the Republic of the Congo, the Democratic Republic of the Congo, and the Central African Republic, and in red Ruwanda, Tanzania, and Kenya.
Supplement 3 (Figure): Map of Africa showing the countries from which the sequences included in Figure 3 (KY22) derived. In green are Guinea and Côte d’Ivoire, in blue Cameroon, the Republic of the Congo, and the Democratic Republic of the Congo, in red Rwanda, Tanzania, Kenya, Mozambique, and South Africa, and in yellow Madagascar, Mayotte, and Reunion.
Supplement 4 (Figure): Scatter plots showing the correlation between genetic difference in percent and the distance between collection sites in kilometers (A and B) and the correlation between genetic difference in percent and the temporal distance between collection events in years (C and D).
Data availability
Bat data collected for this project is available through USAID (https://data.usaid.gov/Global-Health-Security-in-Development-GHSD-/PREDICT-Emerging-Pandemic-Threats-Project/tqea-hwmr). Human quantitative data is available upon request from study authors. Human qualitive interview data is not available to protect the privacy of study participants. Data analysis was performed in R version 4.0.4 [38]. Code used in this project are available for download at https://github.com/ecohealthalliance/predict-civ-bat-coronaviruses.
References
- 1.Gryseels S., De Bruyn L., Gyselings R., Calvignac-Spencer S., Leendertz F.H., Leirs H. Risk of human-to-wildlife transmission of SARS-CoV-2. Mammal Rev. 2021;51:272–292. doi: 10.1111/mam.12225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anthony S.J., Johnson C.K., Greig D.J., Kramer S., Che X., Wells H., Hicks A.L., Joly D.O., Wolfe N.D., Daszak P., Karesh W., Lipkin W.I., Morse S.S., PREDICT Consortium, Mazet J.A.K., Goldstein T. Global patterns in coronavirus diversity. Virus Evol. 2017;3(1) doi: 10.1093/ve/vex012. vex012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Parrish C.R., Holmes E.C., Morens D.M., Park E.-C., Burke D.S., Calisher C.H., Laughlin C.A., Saif L.J., Daszak P. Cross-species virus transmission and the emergence of new epidemic diseases. Microbiol. Mol. Biol. Rev. 2008;72(3):457–470. doi: 10.1128/MMBR.00004-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Patrono L.V., Samuni L., Corman V.M., Nourifar L., Röthemeier C., Wittig R.M., Drosten C., Calvignac-Spencer S., Leendertz F.H. Human coronavirus OC43 outbreak in wild chimpanzees, Côte d’Ivoire, 2016. Emerg. Microbes Infect. 2018;7(1):118. doi: 10.1038/s41426-018-0121-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Drexler J.F., Corman V.M., Drosten C. Ecology, evolution and classification of bat coronaviruses in the aftermath of SARS. Antivir. Res. 2014;101:45–56. doi: 10.1016/j.antiviral.2013.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Letko M., Seifert S.N., Olival K.J., Plowright R.K., Munster V.J. Bat-borne virus diversity, spillover and emergence. Nat. Rev. Microbiol. 2020;18(8):461–471. doi: 10.1038/s41579-020-0394-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Frutos R., Serra-Cobo J., Pinault L., Lopez Roig M., Devaux C.A. Emergence of bat-related betacoronaviruses: hazard and risks. Front. Microbiol. 2021;12(437) doi: 10.3389/fmicb.2021.591535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Latinne A., Hu B., Olival K.J., Zhu G., Zhang L., Li H., Chmura A.A., Field H.E., Zambrana-Torrelio C., Epstein J.H., et al. Origin and cross-species transmission of bat coronaviruses in China. Nat. Commun. 2020;11(1):4235. doi: 10.1038/s41467-020-17687-3. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 9.Kumakamba C., Niama F.R., Muyembe F., Mombouli J.V., Kingebeni P.M., Nina R.A., Lukusa I.N., Bounga G., N'Kawa F., Nkoua C.G., et al. Coronavirus surveillance in wildlife from two Congo basin countries detects RNA of multiple species circulating in bats and rodents. PLoS One. 2021;16(6) doi: 10.1371/journal.pone.0236971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nziza J., Goldstein T., Cranfield M., Webala P., Nsengimana O., Nyatanyi T., Mudakikwa A., Tremeau-Bravard A., Byarugaba D., Tumushime J.C., et al. Coronaviruses detected in bats in close contact with humans in Rwanda. Ecohealth. 2020;17(1):152–159. doi: 10.1007/s10393-019-01458-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tao Y., Tang K., Shi M., Conrardy C., Li K.S.M., Lau S.K.P., Anderson L.J., Tong S. Genomic characterization of seven distinct bat coronaviruses in Kenya. Virus Res. 2012;167(1):67–73. doi: 10.1016/j.virusres.2012.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maganga G.D., Pinto A., Mombo I.M., Madjitobaye M., Mbeang Beyeme A.M., Boundenga L., Ar Gouilh M., N’Dilimabaka N., Drexler J.F., Drosten C., et al. Genetic diversity and ecology of coronaviruses hosted by cave-dwelling bats in Gabon. Sci. Rep. 2020;10(1):7314. doi: 10.1038/s41598-020-64159-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ntumvi N.F., Ndze V.N., Gillis A., Le Doux Diffo J., Tamoufe U., Takuo J.-M., Mouiche M.M.M., Nwobegahay J., LeBreton M., Rimoin A.W., et al. Wildlife in Cameroon harbor diverse coronaviruses, including many closely related to human coronavirus 229E. Virus Evol. 2022;8(1) doi: 10.1093/ve/veab110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Joffrin L., Goodman S.M., Wilkinson D.A., Ramasindrazana B., Lagadec E., Gomard Y., Le Minter G., Santos A. Dos, Schoeman M. Corrie, Sookhareea R., et al. Bat coronavirus phylogeography in the Western Indian Ocean. Sci. Rep. 2020;10(1):6873. doi: 10.1038/s41598-020-63799-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Euren J., Bangura J., Gbakima A., Sinah M., Yonda S., Lange C.E., McIver D.J., LeBreton M., Wolking D., Grigorescu Monagin C., et al. Human interactions with bat populations in Bombali, Sierra Leone. Ecohealth. 2020;17(3):292–301. doi: 10.1007/s10393-020-01502-y. [DOI] [PubMed] [Google Scholar]
- 16.Saylors K., Wolking D.J., Hagan E., Martinez S., Francisco L., Euren J., Olson S.H., Miller M., Fine A.E., Thanh N.N.T., et al. Socializing one health: an innovative strategy to investigate social and behavioral risks of emerging viral threats. One Health Outlook. 2021;3(1):11. doi: 10.1186/s42522-021-00036-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sumibcay L., Kadjo B., Gu S.H., Kang H.J., Lim B.K., Cook J.A., Song J.W., Yanagihara R. Divergent lineage of a novel hantavirus in the banana pipistrelle (Neoromicia nanus) in Côte d'Ivoire. Virol. J. 2012;9:34. doi: 10.1186/1743-422X-9-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goldstein T., Anthony S.J., Gbakima A., Bird B.H., Bangura J., Tremeau-Bravard A., Belaganahalli M.N., Wells H.L., Dhanota J.K., Liang E., et al. The discovery of Bombali virus adds further support for bats as hosts of ebolaviruses. Nat. Microbiol. 2018;3(10):1084–1089. doi: 10.1038/s41564-018-0227-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.PREDICT Consortium . Edited by One Health Institute UoC; Davis: 2020. Advancing Global Health Security at the Frontiers of Disease Emergence. [Google Scholar]
- 20.Kupferschmidtt K. 2019. This Bat Species may be the Source of the Ebola Epidemic that Killed more than 11,000 People in West Africa.https://www.science.org/content/article/bat-species-may-be-source-ebola-epidemic-killed-more-11000-people-west-africa (accessed 13 March 2023) [DOI] [Google Scholar]
- 21.Calisher C.H., Childs J.E., Field H.E., Holmes K.V., Schountz T. Bats: important reservoir hosts of emerging viruses. Clin. Microbiol. Rev. 2006;19(3):531–545. doi: 10.1128/CMR.00017-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weier S.M., Linden V.M.G., Grass I., Tscharntke T., Taylor P.J. The use of bat houses as day roosts in macadamia orchards, South Africa. PeerJ. 2019;7 doi: 10.7717/peerj.6954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Olival K.J., Cryan P.M., Amman B.R., Baric R.S., Blehert D.S., Brook C.E., Calisher C.H., Castle K.T., Coleman J.T.H., Daszak P., et al. Possibility for reverse zoonotic transmission of SARS-CoV-2 to free-ranging wildlife: a case study of bats. PLoS Pathog. 2020;16(9) doi: 10.1371/journal.ppat.1008758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Konaté S., Kampmann D. Abidjan & Frankfurt; Côte d’Ivoire: 2010. Biodiversity Atlas of West Africa, Volume III. [Google Scholar]
- 25.Climate Knowledge Portal. Côte d'Ivoire. 2021. https://climateknowledgeportal.worldbank.org/country/cote-divoire/climate-data-historical (accessed 13 March 2023)
- 26.Bergmans W., Bellier L., Vissault J. A taxonomical report on a collection of Megachiroptera (Mammalia) from the Ivory Coast. Rev. Zool. Afr. 1974;88:18–48. [Google Scholar]
- 27.Koopman K.F.K., Kofron C.P., Chapman A. The bats of Liberia: systematics, ecology and distribution. Am. Mus. Novit. 1995;3148:1–24. http://hdl.handle.net/2246/3673 [Google Scholar]
- 28.Quan P.L., Firth C., Street C., Henriquez J.A., Petrosov A., Tashmukhamedova A., Hutchison S.K., Egholm M., Osinubi M.O., Niezgoda M., et al. Identification of a severe acute respiratory syndrome coronavirus-like virus in a leaf-nosed bat in Nigeria. mBio. 2010;1(4) doi: 10.1128/mBio.00208-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Watanabe S., Masangkay J.S., Nagata N., Morikawa S., Mizutani T., Fukushi S., Alviola P., Omatsu T., Ueda N., Iha K., et al. Bat coronaviruses and experimental infection of bats, the Philippines. Emerg. Infect. Dis. 2010;16(8):1217–1223. doi: 10.3201/eid1608.100208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Townzen J.S., Brower A.V., Judd D.D. Identification of mosquito bloodmeals using mitochondrial cytochrome oxidase subunit I and cytochrome b gene sequences. Med. Vet. Entomol. 2008;22(4):386–393. doi: 10.1111/j.1365-2915.2008.00760.x. [DOI] [PubMed] [Google Scholar]
- 31.Ronquist F., Teslenko M., van der Mark P., Ayres D.L., Darling A., Höhna S., Larget B., Liu L., Suchard M.A., Huelsenbeck J.P. MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 2012;61(3):539–542. doi: 10.1093/sysbio/sys029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Conow C., Fielder D., Ovadia Y., Libeskind-Hadas R. Jane: a new tool for the cophylogeny reconstruction problem. Algorithms Mol. Biol. 2010;5:16. doi: 10.1186/1748-7188-5-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sánchez C.A., Li H., Phelps K.L., et al. A strategy to assess spillover risk of bat SARS-related coronaviruses in Southeast Asia. Nat. Commun. 2022;13:4380. doi: 10.1038/s41467-022-31860-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Grange Z.L., Goldstein T., Johnson C.K., Anthony S., Gilardi K., Daszak P., Olival K.J., O’Rourke T., Murray S., Olson S.H., et al. Ranking the risk of animal-to-human spillover for newly discovered viruses. Proc. Natl. Acad. Sci. 2021;118(15) doi: 10.1073/pnas.2002324118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shapiro J.T., Mollerup S., Jensen R.H., Olofsson J.K., N-pD Nguyen T.A., Hansen L., Vinner A., Monadjem R.A., McCleery A.J. Hansen. Metagenomic analysis reveals previously undescribed bat coronavirus strains in Eswatini. Ecohealth. 2021;18(4):421–428. doi: 10.1007/s10393-021-01567-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Karan L.S., Makenov M.T., Korneev M.G., Sacko N., Boumbaly S., Yakovlev S.A., Kourouma K., Bayandin R.B., Gladysheva A.V., Shipovalov A.V., et al. Bombali virus in Mops condylurus bats, Guinea. Emerg. Infect. Dis. 2019;25(9):1774–1775. doi: 10.3201/eid2509.190581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Marí Saéz A., Weiss S., Nowak K., Lapeyre V., Zimmermann F., Düx A., Kühl H.S., Kaba M., Regnaut S., Merkel K., et al. Investigating the zoonotic origin of the West African Ebola epidemic. EMBO Mol. Med. 2015;7(1):17–23. doi: 10.15252/emmm.201404792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.R Core Team R: A Language and Environment for Statistical Computing. 2022. https://www.R-project.org/ (accessed 22 December 2022)
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplement 1 (Table): Number of bats captured per species (2017-2018).
Supplement 2 (Figure): Map of Africa showing the countries from which the sequences included in Figure 2 (KY55/56) derived. In green are Guinea and Côte d’Ivoire, in blue Cameroon, the Republic of the Congo, the Democratic Republic of the Congo, and the Central African Republic, and in red Ruwanda, Tanzania, and Kenya.
Supplement 3 (Figure): Map of Africa showing the countries from which the sequences included in Figure 3 (KY22) derived. In green are Guinea and Côte d’Ivoire, in blue Cameroon, the Republic of the Congo, and the Democratic Republic of the Congo, in red Rwanda, Tanzania, Kenya, Mozambique, and South Africa, and in yellow Madagascar, Mayotte, and Reunion.
Supplement 4 (Figure): Scatter plots showing the correlation between genetic difference in percent and the distance between collection sites in kilometers (A and B) and the correlation between genetic difference in percent and the temporal distance between collection events in years (C and D).
Data Availability Statement
Bat data collected for this project is available through USAID (https://data.usaid.gov/Global-Health-Security-in-Development-GHSD-/PREDICT-Emerging-Pandemic-Threats-Project/tqea-hwmr). Human quantitative data is available upon request from study authors. Human qualitive interview data is not available to protect the privacy of study participants. Data analysis was performed in R version 4.0.4 [38]. Code used in this project are available for download at https://github.com/ecohealthalliance/predict-civ-bat-coronaviruses.