Abstract
Objectives:
In the U.S., vaccination coverage is lower in rural versus urban areas. Spatial accessibility to immunization services has been a suspected risk factor for undervaccination in rural children. Our objective was to identify whether geographic factors, including driving distance to immunization providers, were associated with completion of recommended childhood vaccinations.
Methods:
We analyzed records from Montana’s immunization information system for children born 2015–2017. Using geolocated address data, we calculated distance in road miles from children’s residences to the nearest immunization provider. A multivariable log-linked binomial mixed model was used to identify factors associated with completion of the combined 7-vaccine series by age 24 months.
Results:
Among 26,085 children, 16,503 (63.3%) completed the combined 7-vaccine series by age 24 months. Distance to the nearest immunization provider ranged from 0 to 81.0 miles (median=1.7; IQR=3.2), with the majority (92.1%) of children living within 10 miles of a provider. Long distances (>10 miles) to providers had modest associations with not completing the combined 7-vaccine series (adjusted prevalence ratio [aPR]: 0.97, 95% confidence interval [CI]: 0.96–0.99). After adjustment for other factors, children living in rural areas (measured by rural-urban commuting area) were significantly less likely to have completed the combined 7-vaccine series than children in metropolitan areas (aPR: 0.88, 95% CI: 0.85–0.92).
Conclusions:
Long travel distances do not appear to be a major barrier to childhood vaccination in Montana. Other challenges, including limited resources for clinic-based strategies to promote timely vaccination and parental vaccine hesitancy, may have greater influence on rural childhood vaccination.
Keywords: rural health, childhood immunisations, vaccination barriers, distance to care, immunisation services
INTRODUCTION
Early childhood immunization, a key public health achievement of the past century, is responsible for preventing millions of illnesses, hospitalizations, and deaths in the U.S.1–3 Currently, the Centers for Disease Control and Prevention’s (CDC) Advisory Committee on Immunization Practices (ACIP) recommends that children complete 11 vaccine series within the first two years of life to protect against 15 diseases, including the COVID-19 vaccine series recently recommended for children 6 months and older.3, 4
Childhood vaccination rates lag in rural areas of the U.S. compared to urban and suburban areas.5–9 In rural areas, barriers related to accessing preventive health care may play a prominent role in lower childhood vaccination coverage.10–13 Moreover, disparities in childhood vaccination coverage by other sociodemographic characteristics such as living below the poverty level or being insured by Medicaid, are also more prevalent in rural areas, suggesting barriers to health care access due to financial constraints might be heightened in certain rural communities.5–8, 14–17 Vaccines for Children (VFC), a federal program that provides free vaccines to children who are uninsured or face financial barriers to vaccination, has helped to close much of the historical gap in vaccination coverage among children with lower socioeconomic status.2 In rural areas, VFC providers and public health clinics play a large role in childhood and adolescent immunization services.8, 11, 18 Despite VFC and public health clinics, rural-urban vaccination disparities still persist.5–9
One suspected vaccination barrier for rural families is lack of geographically accessible immunization providers, as families in rural areas may have to travel farther for primary care and vaccination services.7 Geographic proximity to providers has been previously investigated as an important indicator of health care access and health outcomes.19–23 Greater travel burden to access primary and specialist care is associated with later-stage diagnosis and time-to-treatment after diagnosis for certain cancers.21, 24 Increased travel burden and distance for perinatal and obstetric care have been negatively associated with adequate care access and positive health outcomes in urban and rural areas, with distinct racial and ethnic disparities.25, 26 However, few studies have investigated proximity to providers as a predictor of childhood immunization, with mixed results across both urban27–29 and rural11, 30 settings. Of these studies, physical distance was not associated with childhood immunization rates in an urban setting29 or delayed infant vaccinations in a metropolitan area.27 However, a 2002 study in rural Colorado did find that living within 10 miles of a clinic was positively associated with vaccination coverage among low-income, Latino children.30
To date, focused investigation of barriers to and facilitators of early childhood immunization in rural areas has been limited, largely due to a lack of data. Recent advances in state immunization information systems (IIS) are allowing rural populations to be more robustly included in immunization services delivery research.31 IIS are confidential, centralized systems that consolidate vaccination records across providers in a geographic area. These systems are used at point of care to identify which vaccinations children are due for; however, IIS are also valuable tools for investigating gaps in vaccination uptake and identifying strategies to address immunization inequities.32
Montana is the 4th largest U.S. state by physical size and the 8th least populous state.33 The majority of the population lives in micropolitan, small town, or rural areas, based on grouped delineations of rural-urban commuting area (RUCA) codes.34, 35 Our primary objective was to examine the association between proximity to immunization providers and completion of recommended childhood vaccine series by age 24 months in Montana. Our main analysis focused on distance in road miles to the nearest immunization provider; however, we also conducted secondary analyses examining distance to nearest VFC provider and distance to nearest public health clinic.
METHODS
Data source
Our study relied on vaccination records from Montana’s immunization information system (IIS), imMTrax, which is operated by the Montana Department of Public Health and Human Services (MT DPHHS). State birth records are imported from vital statistics into imMTrax and linked with immunization data. This IIS does not routinely capture children who receive no vaccines, as it only tracks vaccinations reported to imMTrax by providers who have obtained parental consent to do so.10 Many clinics have bidirectional interoperability between their electronic health record systems and imMTrax.10 Over 90% of clinics providing early childhood vaccinations in Montana (including private facilities, public health departments, Indian Health Service sites, tribal clinics, and other types of facilities providing immunizations) routinely report all vaccination records to imMTrax.10 When compared to National Immunization Survey-Child (NIS-Child) vaccination coverage estimates, per CDC and National Vaccine Advisory Committee (NVAC) recommendations for evaluating IIS data quality, imMTrax vaccination coverage metrics among children born in Montana between 2015–2017 are very similar to corresponding NIS-Child survey year reports.10, 36
Study population
The study cohort included children born in Montana in 2015–2017 who had at least one vaccination recorded in imMTrax after their first birthday. As imMTrax does not routinely track children who move out of state, this inclusion criterion was applied to ensure all children in the study cohort had the opportunity to receive MMR and varicella vaccines, which are recommended at ages 12–15 months. Children with only influenza vaccines were excluded due to potential issues with data quality. Children with other data anomalies, such as records of vaccines not approved for children, were also excluded. Children with out-of-state addresses or missing residential address data were excluded. Children with only P.O. box addresses recorded were also excluded, as these addresses may not accurately reflect the actual distance children must travel to access providers. Children with residential addresses within Malmstrom Air Force Base in Great Falls, Montana were considered a dynamic population with a higher likelihood of moving out-of-state between their first and second birthdays. Due to concerns regarding incomplete imMTrax vaccination records in this population, children living on Malmstrom Air Force Base were excluded.
Proximity to immunization providers
In 2020, we acquired and merged three lists of Montana primary care facilities to quantify accessibility to immunization services. The lists included facilities providing childhood immunizations identified through imMTrax, primary care providers identified by WIM (Wyoming Idaho Montana) Health Workforce Information Services, and facilities participating in VFC (obtained via MT DPHHS). The imMTrax and VFC lists identified the majority of primary care facilities and WIM facilitated the creation of a more complete list. By linking with the WIM list, we were able to identify whether clinics had primary care providers who specialized in family medicine, pediatrics, or both. From descriptions in imMTrax, we were able to identify which facilities were public health clinics. For a subset of facilities from our combined list (n=50), we could not confirm they provided early childhood vaccinations, participated in the VFC program, or utilized imMTrax. To collect this missing information, we consulted with MT DPHHS, reviewed facility websites, and called 44 individual facilities. Our final list included 246 primary care facilities providing early childhood vaccinations; of these, 223 clinics participated in the VFC program and 58 were public health clinics.
To protect individuals’ anonymity, MT DPHHS provided children’s addresses from imMTrax via a dataset that was not linked to vaccination records or any other identifying information, with randomly generated study IDs. We used address data to create proximity to provider variables and then MT DPHHS deleted address data and linked new variables back to vaccination data. To assess proximity to immunization providers, we used the Google Distance Matrix application programming interface in conjunction with R statistical computing language to calculate driving distances from child’s residence to nearest primary care provider. Upon a review of previous research using distance variables and operationalization in the literature, we categorized distance to nearest immunization provider into three categories: 1) short (≤2 miles), 2) medium (>2–10 miles), and 3) long (>10 miles) distances.30 Using these methods, we also calculated distance to nearest VFC provider and distance to nearest public health clinic.
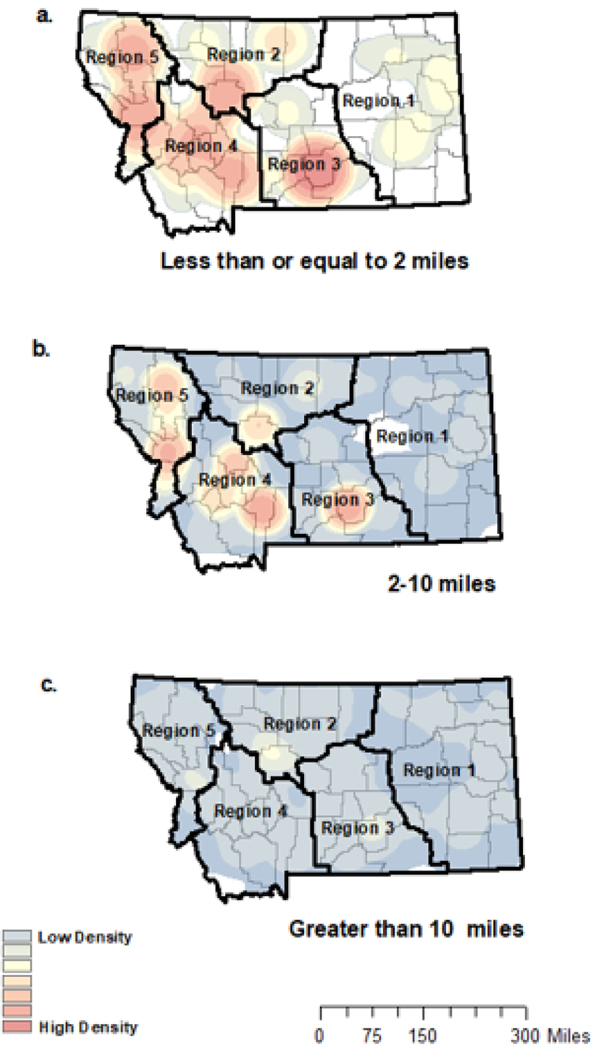
To display proximity to immunization providers visually, we created three maps of Montana (ArcGIS, version 10.8.1, ESRI)37 depicting density of children in each distance category (short [Figure 1a], medium [Figure 1b], and long [Figure 1c]). Addresses were geocoded as (latitude, longitude) and geomasked using the random perturbation method (and 2010 Census Bureau census block-level population density estimates) making each address indistinguishable from the nearest 100 individuals.38 Geomasked addresses were then linked with vaccination data for analyses, as described above. To ensure anonymity, we created heat maps using kernel density estimation in ArcMap with an output cell size of 1 kilometer and a search radius of 100 kilometers for each map. We used equivalent density scales to produce maps with comparable density delineations. Additionally, each map shows Montana county delineations39 and Montana health planning regions.40
Figure 1.
Density of geomasked locations of children born between 2015–2017 in Montana by distance to immunization provider
Each heat map represents the density of geomasked location of children born 2015–2017 in Montana is presented by driving distance to immunization provider. Maps were created using kernel density estimation (100km search radius). Figure 1a depicts density of children who live short distance (<2 road miles) from the nearest immunization provider. Figure 1b depicts density of children who live medium distances (>2–10 road miles) from the nearest provider. Figure 1c depicts density of children who live long distances (>10 road miles) from the nearest provider. Colors represent equal parameters across maps. County borders are outlined and “region” indicates the different Montana Health Planning Regions.
Vaccine series completion by age 24 months
Using imMTrax records, we identified series completion as receipt of all vaccines in the combined 7-vaccine series by age 24 months. This metric is routinely tracked by the CDC to measure progress toward vaccination goals and includes seven vaccine series: diphtheria-tetanus-acellular pertussis (DTaP; four doses), poliovirus (three doses), measles-mumps-rubella (MMR; one dose), hepatitis B (three doses), Haemophilus influenzae type b (Hib; three or four doses depending on brand), varicella (one dose), and pneumococcal conjugate vaccines (four doses).3–6 Previous work using Montana IIS data has found lower completion and on-time receipt of the combined 7-vaccine series by age 24 months in rural areas.10
Demographic, geographic and health care access characteristics
Other variables obtained via imMTrax included child’s year of birth, sex, race, ethnicity, and facility where each vaccination was provided. Due to missing race and ethnicity data and small numbers among certain racial and ethnic groups, the two variables were combined and collapsed into four groups: 1) American Indian/Alaska Native, all ethnicities, 2) non-Hispanic White, 3) other known race/ethnicity (which includes children who identified as Black, Asian, Native Hawaiian or Pacific Islander, or Hispanic ethnicity), and 4) missing race/ethnicity. Using immunization facility data from imMTrax and relevant WIM Tracking data, we identified whether children 1) only visited clinics with providers certified in pediatrics, 2) sometimes visited clinics with pediatric providers, or 3) never visited clinics with pediatric providers. Montana is divided into five regions by MT DPHHS for health planning and public health purposes.10, 41 We used address data to identify a child’s location by Montana health planning region and primary RUCA codes, since disparities in vaccination coverage have been previously observed across health region and rurality in Montana.10 RUCA codes are a measure of rurality and were calculated using Census commuting data at the tract level.34, 35 For the purposes of our study, RUCA codes were used to classify children into four groups: 1) metropolitan areas with primary commuting flow to an urbanized area (UA) (>50,000 people), 2) micropolitan areas with primary commuting flow to a large urban cluster (UC) (10,000–49,999 people), 3) small towns that primarily commute to small UCs (2,500–9,999 people), and 4) rural areas with a primary flow to tracts outside of UAs or UCs (<2,499 people).35
To capture other socioeconomic factors, we used a neighborhood deprivation index (NDI) at Census tract level. An NDI is a measure of socioeconomic status extracted using the Social Explorer Tool from 2014–2018 American Community Surveys, with higher values indicating more socioeconomically deprived areas.42 Through an existing principal components analysis starting with 21 variables, the NDI was constructed from a weighted linear combination of the most informative seven variables: percent with Bachelor degree; percent in managerial occupations; median home value; percent with at least a high school education; percent interest, dividend, or rental income; median household income; and percent with annual household income greater than $50,000. A provider density variable, the number of pediatric and family medicine providers per 1,000 children aged 0–5 years for each county, was created to capture spatial accessibility to care as a covariate using WIM provider data and 2010 Census Bureau population estimates.
Statistical analyses
We described population demographics and immunization provider characteristics as well as distance to provider measures and vaccine series completion by age 24 months. We conducted bivariate and multivariable log-linked binomial mixed models to identify factors associated with combined 7-vaccine series completion by age 24 months, with year of birth included as a random effect. The primary independent variable of interest was distance to nearest immunization provider. Other fixed effect covariates included in the final multivariable model were identified a priori based on literature review and included child sex, race/ethnicity, type of immunization providers available at clinics accessed, health region, RUCA, NDI, and provider density.8, 10, 11, 27, 28 Tests for interaction between distance to immunization provider and health region and distance to immunization provider and RUCA were performed in multivariable analyses. We conducted tests for multicollinearity in the final multivariable model.43 Secondary analyses were conducted using two alternative distance (in road miles) measures: distance to nearest VFC provider and distance to nearest public health clinic. Unadjusted and adjusted prevalence ratios (PRs) and 95% confidence intervals (CIs) were reported. All study analyses were conducted using SAS 9.4 (Cary, NC). This study was approved by the University of Montana Institutional Review Board under the exempt category of review.
RESULTS
Sample size
Vaccination records were available for 36,505 children, of which 32,656 (89.5%) children had one or more vaccinations after their first birthday. We excluded children with only influenza vaccination records (n=326), vaccine data errors (n=908), addresses that were in error, or unable to be geolocated, or were for PO boxes (n=5,260), and children with addresses on Malmstrom Air Force base (n=77). Our final analytic cohort consisted of 26,085 children, which represents 71.2% of 36,654 children born in Montana from 2015 to 2017.10 Compared to the final analytic cohort (Table 1), children excluded due to addresses that were in error, or unable to be geolocated, or were for PO boxes (n=5,260) were disproportionately American Indian/Alaska Native (35.0% versus 7.0%) and more likely to live in rural areas (49.2% versus 12.0%) with lower provider density (Supplemental Table 1).
Table 1.
Demographics and provider characteristics among study-eligible children born 2015–2017 in Montana
| Total analytic cohort n=26,085 | Children who completed the combined 7-vaccine series by age 24 months n= 16,503 | Children who did not complete the combined 7-vaccine series by age 24 months n= 9,582 | |
|---|---|---|---|
| Characteristics | n (%) | ||
| Year of Birth | |||
| 2015 | 9,388 (36.0) | 5,466 (33.1) | 3,922 (40.9) |
| 2016 | 9,090 (34.9) | 5,825 (35.3) | 3,265 (34.1) |
| 2017 | 7,607 (29.2) | 5,212 (31.6) | 2,395 (25.0) |
| Sex* | |||
| Female | 12,610 (48.8) | 7,899 (48.3) | 4,711 (49.5) |
| Male | 13,241 (51.2) | 8,444 (51.7) | 4,797 (50.5) |
| Race and ethnicity | |||
| American Indian/Alaska Native, all ethnicities | 1,790 (6.9) | 1,047 (6.3) | 743 (7.8) |
| Other known race/ethnicity* | 1,033 (4.0) | 615 (3.7) | 418 (4.4) |
| Missing race/ethnicity data | 7,676 (29.4) | 4,737 (28.7) | 2,939 (30.7) |
| Non-Hispanic White | 15,586 (59.8) | 10,104 (61.2) | 5,482 (57.2) |
| Categories based on rural-urban commuting area (RUCA) codes | |||
| Rural areas | 3,137 (12.0) | 1,808 (11.0) | 1,329 (13.9) |
| Small towns | 4,618 (17.7) | 2,833 (17.2) | 1,785 (18.6) |
| Micropolitan areas | 7,587 (29.1) | 4,856 (29.4) | 2,731 (28.5) |
| Metropolitan areas | 10,743 (41.2) | 7,006 (42.5) | 3,737 (39.0) |
| Availability of pediatric providers at clinics accessed | |||
| Only accessed clinics with pediatric providers | 5,128 (19.7) | 3,127 (19.0) | 2,001 (20.9) |
| Sometimes accessed clinics with pediatric providers | 13,001 (49.8) | 8,889 (53.9) | 4,112 (42.9) |
| Never accessed clinics with pediatric providers | 7,956 (30.5) | 4,487 (27.2) | 3,469 (36.2) |
| Health Region | |||
| 1 | 1,781 (6.8) | 1,003 (6.1) | 778 (8.1) |
| 2 | 3,724 (14.3) | 2,594 (15.7) | 1,130 (11.8) |
| 3 | 5,984 (22.9) | 4,046 (24.5) | 1,938 (20.2) |
| 4 | 7,325 (28.1) | 4,721 (28.6) | 2,604 (27.2) |
| 5 | 7,271 (27.9) | 4,139 (25.1) | 3,132 (32.7) |
| Tract-level neighborhood deprivation index (NDI), mean (SD) | −0.1 (1.9) | −0.1 (1.9) | 0.0 (1.9) |
| County-level family medicine and pediatric provider density per 1,000 children age 0–5 | |||
| <10 | 1,365 (5.2) | 922 (5.6) | 443 (4.6) |
| 10–14.99 | 15,666 (60.1) | 10,022 (60.7) | 5,644 (58.9) |
| 15–19.99 | 6,227 (23.9) | 3,856 (23.4) | 2,371 (24.7) |
| ≥20 | 2,827 (10.8) | 1,703 (10.3) | 1,124 (11.7) |
| Distance (miles) to nearest immunization provider, median (IQR) | 1.7 (3.2) | 1.7 (3.2) | 1.6 (3.2) |
| Distance (miles) to nearest immunization provider | |||
| Short (≤2 miles) | 14,577 (55.9) | 9,147 (55.4) | 5,430 (56.7) |
| Medium (2.01–10 miles) | 9,453 (36.2) | 6,049 (36.7) | 3,404 (35.5) |
| Long (>10 miles) | 2,055 (7.9) | 1,307 (7.9) | 748 (7.8) |
Summary statistics
More than half (59.8%) of children were non-Hispanic White, followed by 29.4% of children who had missing race/ethnicity data and 1,790 (7.0%) who were American Indian/Alaska Native (Table 1). While 19.7% of children only visited clinics with pediatric providers, about 30.5% of children only visited clinics without pediatric providers and 49.8% of children visited multiple clinics with and without pediatric providers. The majority (60.1%) of children lived in a county with 10–14.99 primary care providers per 1,000 children aged 0–5 years, followed by 23.9% of children with 15–19.99 providers per 1,000 children aged 0–5 years. Most children lived in non-metropolitan areas, with 29.1% living in micropolitan areas, 17.7% living in small towns, and 12.0% living in rural areas. About 63.3% of children completed the combined 7-vaccine series by 24 months of age.
Proximity to immunization providers
Distance from a child’s residence to the nearest immunization provider ranged from 0 to 81.0 road miles. On average, children were located 3.7 road miles (standard deviation [SD]=5.7, median=1.7, interquartile range [IQR]=3.2) from the nearest immunization provider. Among children in rural areas, the mean distance was 9.0 road miles (SD=10.2, median=5.8, IQR=10.7). The mean driving distance to immunization provider was highest in health regions 1 (mean=5.5, SD=9.9, median=1.2, IQR=3.9) and 2 (mean=4.5, SD=7.5, median=1.4, IQR=2.7). When mapping distance categories, 14,577 (55.9%) children fell into the short (≤2 miles) group (Figure 1a), 9,453 (36.2%) fell into the medium (>2–10 miles) group (Figure 1b), and 2,055 (7.9%) fell into the long (>10 miles) group (Figure 1c). As depicted in Figure 1, children with short distances to providers largely live close to one another and along city and town centers; as distance to providers increases, children become more and more dispersed across the state (Figure 1a-c).
Distance in road miles to VFC clinics was very similar to the above results, with children located, on average, 3.9 road miles (SD=6.1, median=1.7, IQR=3.3) from the nearest VFC provider. Over 91% of children lived ≤10 miles from a VFC clinic. Children were located a bit farther from public health clinics at an average of 8.6 road miles (SD=10.6, median=4.4, IQR=9.2) to the nearest site, with about 72% of children located ≤10 miles from a clinic.
Factors associated with vaccine series completion by age 24 months
In adjusted analyses, there was a modest association between long distances to nearest immunization provider and decreased likelihood of vaccine series completion by age 24 months (aPR: 0.97, 95% CI: 0.96–0.99), compared to children with short distances to nearest immunization provider (Table 2). In secondary analyses, we saw similar results, with an attenuated association between medium/long distances to both VFC providers and public health clinics and series completion by age 24 months (Supplemental Table 2).
Table 2.
Factors associated with completion of the combined 7-vaccine series by age 24 months among children born in Montana 2015–2017, n=26,085*
| Completion of the combined 7-vaccine series | ||
|---|---|---|
| Distance (miles) to nearest immunization provider | Unadjusted PR (95% CI) | Adjusted PR (95% CI) |
| Short (≤2 mi) | Reference | Reference |
| Medium (2.01–10 mi) | 0.99 (0.96–1.03) | 0.99 (0.95–1.02) |
| Long (>10 mi) | 0.98 (0.96–1.00) | 0.97 (0.96–0.99) |
| Sex* | ||
| Female | 0.98 (0.96–1.00) | 0.99 (0.97–1.00) |
| Male | Reference | Reference |
| Race and ethnicity | ||
| American Indian/Alaska Native, all ethnicities | 0.90 (0.87–0.94) | 0.92 (0.88–0.95) |
| Other known race/ethnicity | 0.92 (0.87–0.97) | 0.93 (0.88–0.97) |
| Missing race/ethnicity data | 0.95 (0.93–0.97) | 0.99 (0.97–1.01) |
| Non-Hispanic White | Reference | Reference |
| Categories based on rural-urban commuting area (RUCA) codes | ||
| Rural areas | 0.88 (0.86–0.91) | 0.88 (0.85–0.92) |
| Small towns | 0.94 (0.92–0.97) | 0.96 (0.92–1.00) |
| Micropolitan areas | 0.98 (0.96–1.00) | 0.98 (0.94–1.02) |
| Metropolitan areas | Reference | Reference |
| Availability of pediatric providers at clinics accessed | ||
| Only accessed clinics with pediatric providers | 1.08 (1.05–1.11) | 1.04 (1.01–1.07) |
| Sometimes accessed clinics with pediatric providers | 1.21 (1.19–1.24) | 1.19 (1.16–1.22) |
| Never accessed clinics with pediatric providers | Reference | Reference |
| Health Region | ||
| 1 | 0.99 (0.95–1.04) | 0.89 (0.84–0.94) |
| 2 | 1.22 (1.19–1.26) | 1.10 (1.06–1.15) |
| 3 | Reference | Reference |
| 4 | 1.13 (1.10–1.16) | 0.89 (0.86–0.93) |
| 5 | 0.88 (0.86–0.91) | 1.05 (1.01–1.09) |
| Tract-level neighborhood deprivation index (NDI) | 0.99 (0.98–0.99) | 0.99 (0.98–0.99) |
| County-level family medicine and pediatric provider density per 1,000 children ages 0–5 years | ||
| <10 providers | 1.12 (1.07–1.18) | 1.06 (1.01–1.11) |
| 10–14.99 providers | 1.06 (1.03–1.10) | 0.91 (0.88–0.95) |
| 15–19.99 providers | 1.03 (0.99–1.07) | 0.97 (0.93–1.01) |
| ≥20 providers | Reference | Reference |
Adjusting for year of birth as a random effect, with 234 observations excluded due to missing sex data
In our main multivariable model (Table 2), living in a rural area (compared to metropolitan) was associated with lower series completion by age 24 months (aPR: 0.88, 95% CI: 0.85–0.92). There was no evidence of interaction between distance to provider and RUCA (interaction p-value=0.15) or distance to provider and health region (interaction p-value=0.51). Compared to non-Hispanic White children, American Indian/Alaska Native children (aPR: 0.92, 95% CI: 0.88–0.95) were less likely to complete the vaccine series by age 24 months. Children who only accessed clinics with pediatric providers (aPR: 1.04, 95% CI: 1.01–1.07) or sometimes accessed clinics with pediatric providers (aPR: 1.19, 95% CI: 1.16–1.22) had increased likelihood of series completion by age 24 months, compared to children who did not visit clinics with pediatric providers. Compared to children in counties with ≥20 family medicine and pediatric providers per 1,000 children ages 0–5 years, children in counties with 10–14.99 providers per 1,000 children had a lower likelihood of series completion by age 24 months (aPR: 0.91, 95% CI: 0.88–0.95), whereas lower provider density (<10 providers per 1,000 children) was slightly associated with increased likelihood of series completion by age 24 months (aPR: 1.06, 95% CI: 1.01–1.11).
DISCUSSION
The current study investigated physical distance to immunization providers as a potential barrier to completing recommended childhood vaccine series in Montana by age 24 months. While distance to immunization services was higher in rural areas and certain health regions (1 and 2), most (>90%) children in our study lived within 10 miles to immunization providers. In multivariable analyses, long distances to providers (>10 miles) were only modestly associated with not completing the combined 7-vaccine series by 24 months. Other important factors associated with not completing the series by age 24 months included being American Indian/Alaska Native or any other known race and ethnicity other than non-Hispanic White, as well as living in rural and other non-metropolitan areas. Factors associated with increased likelihood of completing recommended childhood vaccinations included a history of visiting clinics with pediatric providers.
The high proportion of Montana children living near immunization providers and VFC-participating clinics reflects initiatives by state health officials to ensure widespread access to immunization services across this large state. Priorities related to such access have been recently elevated nationwide due to the COVID-19 pandemic, bringing more investigations into local availability of immunization providers and COVID-19 vaccines.44–46 However, there are few studies on spatial accessibility and little to no recent studies on physical distance to immunization providers as a potential factor associated with completing routine childhood vaccinations. Among low-income, Latino children in rural Colorado in 2002, researchers found living near a clinic (0–10 miles) versus far from a clinic (>10 miles) was associated with being up-to-date on early childhood vaccinations.30 A study using IIS data from 2002–2004 found direct measurement of distance to immunization providers was not a factor associated with late initiation of childhood vaccinations (>3 months of age) in metropolitan Philadelphia.27 In our current study, long distances to providers (>10 road miles) were modestly associated with combined 7-vaccine series completion by age 24 months.
Our study also found an association between children who lived in counties with medium provider density levels (10–14.99 providers per 1,000 children) and not completing the combined 7-vaccine series by age 24 months, compared to higher provider density (≥20 providers). Additionally, we found modest associations between children in counties with low provider density (<10 providers) and series completion by age 24 months. The latter finding was unexpected given existing literature suggesting higher pediatric and family medicine provider density is related to greater vaccination coverage.28, 47 A potential limitation of our county-level density measure is it combines pediatric and family medicine providers. While a majority of children in Montana see family medicine providers, it is possible some family medicine providers do not routinely see very young children as part of their practice.10 Another study, using IIS data, found individual-level provider-to-population density was positively associated with pediatric vaccination coverage among low-income children in Washington, D.C.28 However, this measure weighted providers to adjust for training type, level, and provider type (e.g., pediatrics vs. family medicine; attendings vs. residents; physician vs. nurse practitioner). Further work is needed to identify more appropriate measures of provider density and spatial accessibility for rural children’s health outcomes.
Although research on this topic is limited, there is evidence to suggest multiple concurrent barriers to early childhood vaccination access and timely vaccination in rural areas.8, 10, 31 While results from the current study suggest distance to care is perhaps not a primary explanation of why children do not complete early childhood vaccinations, transportation barriers not reflected in distance to care measures may still play a role. Lack of access to a vehicle or public transportation may hinder access to care, regardless of distance.48, 49 Alternatively, it is unclear how frequently evidence-based, health systems-level approaches for promoting vaccination uptake are used in rural areas.8, 30, 50 These approaches include electronic provider prompts that populate within EHR systems indicating which vaccinations are due and reminder-recall initiatives that involve reaching out to parents with reminders of recommended vaccinations and upcoming well-child visits.8, 30, 50, 51 Many rural areas of the U.S., especially in Montana, rely heavily on small, private clinics and public health departments for immunization services.8, 10, 18 Such facilities may have limited funds and capabilities for implementing these strategies as compared to larger health systems providing immunization services in more urban areas.8, 52, 53
It is also possible that refusal of vaccines due to parental hesitancy occurs more often in rural areas. A recent analysis of 2018 and 2019 National Immunization Survey-Child data found parents in non-metropolitan statistical areas reported more concern about long-term serious side effects from vaccines and were more likely to report personally knowing someone with serious long-term side effects from a vaccine.54 As the COVID-19 pandemic has progressed, research and surveillance efforts have indicated increased COVID-19 vaccine hesitancy in rural versus urban areas.55 However, more work is needed to further understand the role vaccine hesitancy plays in urban-rural childhood vaccination disparities. State-level IIS provide a unique opportunity to monitor vaccination coverage and undervaccination patterns consistent with vaccine hesitancy and to identify other potential barriers to childhood vaccination.10, 36, 56
Limitations
Our study has several limitations to consider. First, this study may not be representative of rural populations outside of Montana. Rural areas across the nation are diverse and vary widely; thus, vaccination barriers in early childhood may be different for rural areas in Montana than other places. Second, completely unvaccinated children are not consistently captured in imMTrax. According to NIS-Child data for children born 2017–2018, approximately 1.0% (95% CI: 0.8%−1.1%) of U.S. children had received zero vaccinations by their 2nd birthday.7 Since completely unvaccinated children and children without vaccinations after their first birthday were not included in this study, vaccine series completion by age 24 months may be slightly overestimated in our study. Third, as is common with some IIS, race and ethnicity data was limited in imMTrax with 29.4% of our analytic cohort missing race and ethnicity data. Rather than exclude this large group from analyses, we chose to include these children in their own “missing race and ethnicity” category. Previous analyses using these data found children with missing race and ethnicity data had immunization characteristics similar to non-Hispanic White children in Montana.31 Fourth, in this study, we focused on the outcome of combined 7-vaccine series completion by age 24 months, since this is a standard metric for monitoring vaccination coverage across multiple vaccine series.5–7 However, other outcomes, such as timeliness of vaccine receipt, may also be important with regard to geographic barriers and should be considered in future work. Finally, children who were excluded due to having PO Box addresses (or addresses that could not be geolocated) in imMTrax instead of residential addresses were disproportionately rural and American Indian/Alaska Native. This may lead to a bias towards the null and an underestimate of the true association between distance to providers and vaccine series completion by age 24 months, as children in these groups had lower series completion rates and may live further from town, thus requiring PO Boxes. However, since PO Box locations do not reflect the actual distance children must travel to access providers, it was not appropriate to include them in this analysis.
CONCLUSION
Research on barriers to and facilitators of childhood immunization in rural areas is limited.8 We used state IIS data to investigate proximity to immunization providers as a potential barrier to combined 7-vaccine series completion by age 24 months and found that the majority of children live close to an immunization provider and long distances (>10 miles) were only modestly associated with higher likelihood of not completing the combined 7-vaccine series by age 24 months. In rural areas, proximity to immunization providers must be considered in context with other known barriers to vaccination, such as transportation challenges and vaccine hesitancy, as well as facilitators of vaccination uptake, such as reminder/recall programs.
Supplementary Material
Article Highlights.
Over 90% of children in Montana live within 10 miles of an immunization provider
Long distances had modest associations with not completing childhood vaccinations
Immunization information systems provide opportunities to identify vaccination barriers
Acknowledgments:
We thank Erin L. Landguth for her assistance with figure development.
Funding:
This research was supported by a Center for Biomedical Research Excellence award (1P20GM130418) from the National Institute of General Medical Sciences of the National Institutes of Health. The study sponsor did not have any role in the study design; collection, analysis, and interpretation of data; writing the report; or the decision to submit the report for publication.
Footnotes
Disclosures: Preliminary data from this study were presented at the 2022 Society for Epidemiologic Research Annual Meeting in Chicago, IL.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement:
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Orenstein WA, Ahmed R. Simply put: Vaccination saves lives. Vol 114: National Acad Sciences; 2017:4031–4033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Whitney CG, Zhou F, Singleton J, Schuchat A. Benefits from immunization during the vaccines for children program era—United States, 1994–2013. MMWR. Morbidity and mortality weekly report. 2014;63(16):352. [PMC free article] [PubMed] [Google Scholar]
- 3.Institute of Medicine. The Childhood Immunization Schedule and Safety: Stakeholder Concerns, Scientific Evidence, and Future Studies. The National Academies Press, 2013. [PubMed] [Google Scholar]
- 4.Fleming-Dutra KE, Wallace M, Moulia DL, et al. Interim recommendations of the Advisory Committee on Immunization Practices for use of Moderna and Pfizer-BioNTech COVID-19 vaccines in children aged 6 months–5 years—United States, June 2022. [DOI] [PubMed] [Google Scholar]
- 5.Hill HA, Singleton JA, Yankey D, Elam-Evans LD, Pingali SC, Kang Y. Vaccination coverage by age 24 months among children born in 2015 and 2016—National Immunization Survey-Child, United States, 2016–2018. Morbidity and Mortality Weekly Report. 2019;68(41):913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hill HA, Yankey D, Elam-Evans LD, Singleton JA, Pingali SC, Santibanez TA. Vaccination coverage by age 24 months among children born in 2016 and 2017— National Immunization Survey-Child, United States, 2017–2019. Morbidity and Mortality Weekly Report. 2020;69(42):1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hill HA, Yankey D, Elam-Evans LD, Singleton JA, Sterrett N. Vaccination Coverage by Age 24 Months Among Children Born in 2017 and 2018—National Immunization Survey-Child, United States, 2018–2020. Morbidity and Mortality Weekly Report. 2021;70(41):1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Albers AN, Thaker J, Newcomer SR. Barriers to and facilitators of early childhood immunization in rural areas of the United States: a systematic review of the literature. Preventive Medicine Reports. 2022:101804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhai Y, Santibanez TA, Kahn KE, Srivastav A, Walker TY, Singleton JA. Rural, urban, and suburban differences in influenza vaccination coverage among children. Vaccine. 2020;38(48):7596–7602. [DOI] [PubMed] [Google Scholar]
- 10.Newcomer SR, Freeman RE, Wehner BK, Anderson SL, Daley MF. Timeliness of Early Childhood Vaccinations and Undervaccination Patterns in Montana. American Journal of Preventive Medicine. 2021;61(1):e21–e29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fagnan LJ, Shipman SA, Gaudino JA, Mahler J, Sussman AL, Holub J. To give or not to give: approaches to early childhood immunization delivery in Oregon rural primary care practices. The Journal of Rural Health. 2011;27(4):385–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shipman SA, Lan J, Chang C-h, Goodman DC. Geographic maldistribution of primary care for children. Pediatrics. 2011;127(1):19–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weigel PA, Ullrich F, Shane DM, Mueller KJ. Variation in primary care service patterns by rural‐urban location. The Journal of Rural Health. 2016;32(2):196–203. [DOI] [PubMed] [Google Scholar]
- 14.Freeman RE, Thaker J, Daley MF, Glanz JM, Newcomer SR. Vaccine timeliness and prevalence of undervaccination patterns in children ages 0–19 months, US, National Immunization Survey-Child 2017. Vaccine. 2022;40(5):765–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gong G, Phillips SG, Hudson C, Curti D, Philips BU. Higher US rural mortality rates linked to socioeconomic status, physician shortages, and lack of health insurance. Health Affairs. 2019;38(12):2003–2010. [DOI] [PubMed] [Google Scholar]
- 16.Probst JC, Barker JC, Enders A, Gardiner P. Current state of child health in rural America: how context shapes children’s health. The Journal of Rural Health. 2018;34:s3–s12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Douthit N, Kiv S, Dwolatzky T, Biswas S. Exposing some important barriers to health care access in the rural USA. Public health. 2015;129(6):611–620. [DOI] [PubMed] [Google Scholar]
- 18.Newcomer SR, Freeman RE, Albers AN, et al. Missed opportunities for human papillomavirus vaccine series initiation in a large, rural US state. Human Vaccines & Immunotherapeutics. 2022;18(1):2016304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ludwick A, Fu R, Warden C, Lowe RA. Distances to emergency department and to primary care provider’s office affect emergency department use in children. Academic Emergency Medicine. 2009;16(5):411–417. [DOI] [PubMed] [Google Scholar]
- 20.Luo W, Wang F. Measures of spatial accessibility to health care in a GIS environment: synthesis and a case study in the Chicago region. Environment and planning B: planning and design. 2003;30(6):865–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Scoggins JF, Fedorenko CR, Donahue SM, Buchwald D, Blough DK, Ramsey SD. Is distance to provider a barrier to care for medicaid patients with breast, colorectal, or lung cancer? The Journal of Rural Health. 2012;28(1):54–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McGrail MR. Spatial accessibility of primary health care utilising the two step floating catchment area method: an assessment of recent improvements. International journal of health geographics. 2012;11(1):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gips J, Psoter KJ, Sufrin C. Does distance decrease healthcare options for pregnant, incarcerated people? Mapping the distance between abortion providers and prisons. Contraception. 2020;101(4):266–272. [DOI] [PubMed] [Google Scholar]
- 24.Massarweh NN, Chiang Y-J, Xing Y, et al. Association between travel distance and metastatic disease at diagnosis among patients with colon cancer. Journal of Clinical Oncology. 2014;32(9):942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hung P, Henning-Smith CE, Casey MM, Kozhimannil KB. Access to obstetric services in rural counties still declining, with 9 percent losing services, 2004–14. Health Affairs. 2017;36(9):1663–1671. [DOI] [PubMed] [Google Scholar]
- 26.Thorsen ML, Harris S, McGarvey R, Palacios J, Thorsen A. Evaluating disparities in access to obstetric services for American Indian women across Montana. The Journal of Rural Health. 2022;38(1):151–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Feemster KA, Spain CV, Eberhart M, Pati S, Watson B. Identifying infants at increased risk for late initiation of immunizations: maternal and provider characteristics. Public Health Reports. 2009;124(1):42–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fu LY, Cowan N, McLaren R, Engstrom R, Teach SJ. Spatial accessibility to providers and vaccination compliance among children with medicaid. Pediatrics. 2009;124(6):1579–1586. [DOI] [PubMed] [Google Scholar]
- 29.Baumgardner DJ, Halsmer SE, Steber DL, Shah DS, Mundt MP. Does proximity to clinic affect immunization rates and blood pressure? The International Journal of Psychiatry in Medicine. 2006;36(2):199–209. [DOI] [PubMed] [Google Scholar]
- 30.Hicks P, Tarr GA, Hicks XP. Reminder cards and immunization rates among Latinos and the rural poor in Northeast Colorado. The Journal of the American Board of Family Medicine. 2007;20(6):581–586. [DOI] [PubMed] [Google Scholar]
- 31.Michels SY, Freeman RE, Williams E, et al. Evaluating vaccination coverage and timeliness in American Indian/Alaska Native and non-Hispanic White children using state immunization information system data, 2015–2017. Preventive Medicine Reports. 2022:101817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Scharf LG, Coyle R, Adeniyi K, et al. Current challenges and future possibilities for immunization information systems. Academic pediatrics. 2021;21(4):S57–S64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.U.S. Census Bureau. 2020 Census State Profiles: Montana. 2021. Accessed March 1, 2023. https://www.census.gov/library/stories/state-by-state/montana-population-change-between-census-decade.html
- 34.Hart LG, Larson EH, Lishner DM. Rural definitions for health policy and research. American journal of public health. 2005;95(7):1149–1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cromartie J. Rural-urban commuting area codes. 2005. [Google Scholar]
- 36.NVAC. Assessing the state of vaccine confidence in the United States: recommendations from the National Vaccine Advisory Committee: approved by the National Vaccine Advisory Committee on June 10, 2015. Public Health Reports. 2015. Nov;130(6):573–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Environmental Systems Research Institute (ESRI). ArcGIS version 10.8.1. 2020. Redlands, CA. [Google Scholar]
- 38.Montana 2010 Census Blocks with Population Data, US Dept. of Commerce, US Census Bureau, Geography Division, 2011.
- 39.Montana State Library. Geographic Information Clearinghouse. Montana county. Available at: https://geoinfo.msl.mt.gov/home/msdi/administrative_boundaries. Published July 2021. Accessed July 11, 2022.
- 40.Montana Department of Public Health and Human Services. Montana health planning regions. https://dphhs.mt.gov/qad/licensure/healthcarefacilitylicensure/certificateofneed/healthplanningregions
- 41.Duthie M. Burden report: an overview of asthma in Montana. Montana Department of Public Health and Human Services. 2021. [Google Scholar]
- 42.Christine PJ, Auchincloss AH, Bertoni AG, et al. Longitudinal associations between neighborhood physical and social environments and incident type 2 diabetes mellitus: the Multi-Ethnic Study of Atherosclerosis (MESA). JAMA internal medicine. 2015;175(8):1311–1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Craney TA, Surles JG. Model-dependent variance inflation factor cutoff values. Quality engineering. 2002;14(3):391–403. [Google Scholar]
- 44.Murthy BP, Sterrett N, Weller D, et al. Disparities in COVID-19 vaccination coverage between urban and rural counties—United States, December 14, 2020–April 10, 2021. Morbidity and Mortality Weekly Report. 2021;70(20):759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.DiRago NV, Li M, Tom T, et al. COVID-19 vaccine rollouts and the reproduction of urban spatial inequality: disparities within large US cities in March and April 2021 by racial/ethnic and socioeconomic composition. Journal of Urban Health. 2022;99(2):191–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rader B, Astley CM, Sewalk K, et al. Spatial Accessibility Modeling of Vaccine Deserts as Barriers to Controlling SARS-CoV-2. medRxiv. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.LeBaron CW, Massoudi M, Stevenson J, Lyons B. Vaccination coverage and physician distribution in the United States, 1997. Pediatrics. 2001;107(3):e31–e31. [DOI] [PubMed] [Google Scholar]
- 48.Thomas M, Kohli V, King D. Barriers to childhood immunization: findings from a needs assessment study. Home health care services quarterly. 2004;23(2):19–39. [DOI] [PubMed] [Google Scholar]
- 49.Syed ST, Gerber BS, Sharp LK. Traveling towards disease: transportation barriers to health care access. Journal of community health. 2013;38(5):976–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Szilagyi PG, Bordley C, Vann JC, et al. Effect of patient reminder/recall interventions on immunization rates: a review. Jama. 2000;284(14):1820–1827. [DOI] [PubMed] [Google Scholar]
- 51.Harvey H, Reissland N, Mason J. Parental reminder, recall and educational interventions to improve early childhood immunisation uptake: a systematic review and meta-analysis. Vaccine. 2015;33(25):2862–2880. [DOI] [PubMed] [Google Scholar]
- 52.Albright K, Saville A, Lockhart S, Racich KW, Beaty B, Kempe A. Provider attitudes toward public-private collaboration to improve immunization reminder/recall: a mixed-methods study. Academic pediatrics. 2014;14(1):62–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Deutchman M, Brayden R, Siegel CD, Beaty B, Crane L. Childhood immunization in rural family and general practices: current practices, perceived barriers and strategies for improvement. Ambulatory Child Health. 2000;6(3):181–189. [Google Scholar]
- 54.Nguyen KH, Srivastav A, Lindley MC, et al. Parental Vaccine Hesitancy and Association with Childhood Diphtheria, Tetanus Toxoid, and Acellular Pertussis; Measles, Mumps, and Rubella; Rotavirus; and Combined 7-Series Vaccination. American journal of preventive medicine. 2022;62(3):367–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kricorian K, Civen R, Equils O. COVID-19 vaccine hesitancy: Misinformation and perceptions of vaccine safety. Human Vaccines & Immunotherapeutics. 2022;18(1):1950504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Robison SG, Groom H, Young C. Frequency of alternative immunization schedule use in a metropolitan area. Pediatrics. 2012;130(1):32–38. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.