Abstract
Vitamins D and K are essential fat-soluble nutrients that intervene in bone development processes among other biological functions. The present study is aimed at investigating the potential combined effect of dietary supplementation with vitamin D3 (cholecalciferol) and vitamin K3 (menadione) in gilthead seabream (Sparus aurata) larvae. For that purpose, seabream diets were supplemented with different combinations of vitamin D3/vitamin K3 (mg/kg diet) as follows: 0.00/0, 0.06/70, 0.06/170, 0.13/70, 0.13/170, 0.40/70, and 0.40/170. Feeding gilthead seabream larvae (22 days post hatch) for 21 days with the diets supplemented with 0.06-0.13 mg/kg vitamin D3 and 70 mg/kg vitamin K3 (diets 0.06/70 and 0.13/70) led to the highest larval growth and survival and the highest expression of important biomarkers of both bone development and health, such as bmp2, osx, and mgp, and calcium homeostasis, such as pthrp and casr. However, the increased supplementation with both vitamins at 0.40 mg/kg vitamin D3 and 170 mg/kg vitamin K3 (diet 0.40/170) reduced larval growth and survival, downregulated bmp2 and pthrp expressions, and upregulated osx and mgp, causing an unbalance in the relative expression of these genes. The results of the present study have shown the interaction between vitamin D3 supplementation and vitamin K3 supplementation in larval performance and gene expression related to bone development and calcium homeostasis, denoting the significance of a correct balance between both vitamins in larval diets.
1. Introduction
Culturing marine fish larvae at commercial hatcheries is a demanding task, given their high sensitivity to biotic and abiotic factors [1]. Skeletal anomalies in larvae are more likely to develop during embryonic and postembryonic development, making it crucial to ensure proper nutrition for the larvae [2–5]. Micronutrients play a critical role in ensuring proper nutrition, but imbalances can lead to skeletal anomalies and affect larval performance during rearing [5–8]. Therefore, feed plays a significant role in larval performance, and the nutritional requirements for marine fish larvae differ from those of juveniles and broodstock [9, 10]. The larval stage is marked by numerous morphological and physiological changes that may affect nutrient requirements [11], which also vary across species [10, 12, 13]. Hatcheries typically feed larvae live prey, such as rotifers and Artemia, which have varying nutritional compositions and may lack some essential vitamins and minerals [11, 12, 14–16].
Among the different nutrients studied, fat-soluble vitamins D and K play crucial roles in the skeletal development of aquatic animals [17]. Vitamin D is vital for regulating plasma calcium and maintaining bone health, as well as for receptor-based biological functions in various tissues such as the gills, kidney, and intestine [18]. Vitamin K is essential for blood coagulation and the posttranslational modification of vitamin K-dependent proteins, which play critical roles in bone metabolism and growth control [19]. In juvenile fish studies, replacing fishmeal and fish oil with terrestrial ingredients in feed may decrease the dietary content of these vitamins, underscoring the need to establish their optimal dietary levels [20]. Several marine fish species have proposed individual dietary requirements for both vitamins, including Atlantic salmon (Salmo salar) postsmolt, Wuchang bream (Megalobrama amblycephala) fingerling, orange-spotted grouper (Epinephelus coioides) juveniles, and gilthead seabream (Sparus aurata) juveniles [18, 21–30]. However, quantitative requirements for larvae are still scarce, and only a few species, such as European seabass (Dicentrarchus labrax) and gilthead seabream for vitamin D and Senegalese sole (Solea senegalensis) for vitamin K, have had their optimal levels determined [31–33]. Additionally, live prey used in commercial hatcheries exhibit wide variations in their vitamin D and vitamin K contents [15], which could contribute to the high incidence of skeletal anomalies. Moreover, deficiencies or imbalances in either vitamin can lead to skeletal anomalies and affect the overall performance of the larvae during rearing.
Studies on humans or terrestrial animals demonstrate that vitamin D and vitamin K interact with each other to improve bone health and other biological functions [34–44]. However, this type of study is very scarce in fish [17], and none of them has been conducted during larval stages. The bone-forming cells (osteocytes) and bone-resorbing cells (osteoclasts) play significant roles in bone formation and remodeling in fish [19, 45, 46]. Proliferation and differentiation of these bone-forming cells are regulated by bone biomarkers [46–50]. The bone biomarkers analyzed in the present study and their role in regulating bone and calcium metabolism are listed in Table 1.
Table 1.
List of bone biomarkers and their function on bone development and calcium metabolism.
| Bone biomarkers | Function | Reference |
|---|---|---|
| Bone morphogenic protein 2 (bmp2) | Bone cell differentiation | [20, 30, 31, 47, 50–52, 82–85] |
| Development of cartilage and bone | ||
| Transforming growth factor beta (TGF-β) signaling pathway | ||
| RUNX family transcription factor 2 (runx2) | Osteoblast differentiation | |
| Development of cartilage and bone | ||
| Osterix (osx) | Bone formation and remodeling | |
| Activates gene cascade during differentiation of preosteoblast to mature osteoblast | ||
| Alkaline phosphatase (alp) | Bone mineralization | |
| Reduces the extracellular pyrophosphate concentration | ||
| Osteocalcin (oc) | Noncollagenous bone protein synthesized in the extracellular matrix of osteoblast | |
| Contributes to bone mineralization by promoting calcium deposition | ||
| Matrix Gla protein (mgp) | Inhibits calcification | |
| Regulates osteoclastogenesis | ||
| Calcium-sensing receptor (casr) | Regulates calcium homeostasis | |
| Can act directly on bone cells to induce bone modeling or remodeling | ||
| Parathyroid hormone 1 receptor (pthr1) | Modifies the gene involved in mineralization | |
| Parathyroid hormone-related protein (pthrp) | Hypercalcemic hormone | |
| Calcium homeostasis |
The bone-forming molecules work as a cascade during the bone development process and help in regulating other physiological pathways in fish [51]. The up- and downregulation of these biomarkers can be modulated by dietary nutrients [19, 20]. An abnormal expression of bone biomarkers causes severe problems in skeletal development and might lead to skeletal anomalies in cultured fish [52]. In marine fish species, the effect of dietary vitamin D and vitamin K on skeletal development has been studied individually by very few authors [20, 22, 31–33, 53–55]. Although the vitamin D and K interaction studies in fish species are scarce, a series of trials on Atlantic salmon studied the effects of dietary vitamin D and vitamin K on growth, bone minerals, and health performance [17, 56]. However, the combined effect of dietary vitamins D and K has not yet been studied in gilthead seabream larvae, despite its importance to understand the versatile functions of these vitamins in growth and skeletal development. Thus, considering that our previous studies determining the dietary vitamin D3 [32] and vitamin K3 [57] requirements in gilthead seabream larvae pave the way to study the interaction between both vitamins, the present study is aimed at understanding the potential interaction between vitamin D3 and vitamin K3 in gilthead seabream larvae concerning larval performance and expression of selected genes related to bone development and calcium regulation.
2. Materials and Methods
2.1. Larval Rearing
Natural spawns of gilthead seabream larvae were obtained from selected broodstock (PROGENSA (Spanish National Breeding Program) project [2] from GIA (Grupo de Investigación en Acuicultura, ECOAQUA Institute, Las Palmas de Gran Canaria University (ULPGC), Spain). Larvae (initial total length 6.27 ± 0.46 mm, dry body weight 0.22 ± 0.03 mg, mean ± SD) previously fed rotifers (Brachionus plicatilis) enriched with ORIGREEN (Skretting, Norway) until 22 days post hatch (dph) [58] were randomly distributed in 12 experimental tanks at a density of 1200 larvae in each tank. After the distribution, larvae were fed with experimental diets for 21 days (43 dph). All tanks (200 L, light grey color cylinder fiberglass tanks) were supplied with filtered seawater (36 g L−1 salinity) at an increasing rate of 0.3–1.0 L min−1 as a flow-through system along the feeding trials. Water entered the tank through a bottom mesh and was let out from the top to ensure water quality. Water was continuously aerated (125 mL min−1), attaining 5-8 g L−1 dissolved oxygen and saturation ranging between 60 and 80%. Water temperature (20 ± 2°C), photoperiod (12 h light : 12 h dark), and light intensity (1700 lux) were maintained constant throughout the experimental period (21 days).
2.2. Experimental Diets
Seven experimental microdiets of isoproteic (64.8 ± 0.56%) and isolipidic (20.48 ± 0.52%) contents were prepared containing four different levels of vitamin D3 (VD—cholecalciferol) (Sigma-Aldrich, CAS-67970) combined with two different levels of vitamin K3 (VK—menadione) (Sigma-Aldrich, CAS-58275) and one deficient diet (nonsupplemented) (Table 2). The chosen dietary vitamin levels were based on our previous study in gilthead seabream larvae fed different level of dietary vitamin D3 [32] and vitamin K3 [57]. The microdiets were prepared by grinding and sieving the ingredients below 125 microns. The ingredients were mixed in the following order: squid powder, water-soluble components, lipids, and fat-soluble vitamins, and finally, gelatin was mixed to obtain a homogenized mix. Then, the mix was pressed through a compress pelletizer (Severin, Suderm, Germany) and dried in the oven at 38°C for 24 h (Ako, Barcelona, Spain). After drying, pellets were grounded using a grinder (Braun, Kronberg, Germany) and sieved (Filtra, Barcelona, Spain) to achieve a pellet size of 250 and 500 μm. Then, the microdiets were stored at refrigerated condition (2-8°C) throughout the experiment to avoid vitamin deterioration. Proximate analysis of the feed was conducted at GIA laboratories (Table 3). Each diet was tested in triplicates, and the diets were fed manually from 8.00 to 20.00 with 45-minute intervals for 21 days. Daily feed supply was increased from 3 g to 5 g along with the pellet size of 250–500 μm, gradually during the experimental period. Larvae were periodically observed under stereoscope (Leica, M125, using Leica Application Suite software, Wetzlar, Germany) to determine the feed acceptance.
Table 2.
Supplemented levels of VD3 and VK3 in the experimental diets (mg/kg).
| Diet | 0 | 0.06/70 | 0.06/170 | 0.13/70 | 0.13/170 | 0.40/70 | 0.40/170 |
|---|---|---|---|---|---|---|---|
| VD3 (mg/kg) | 0 | 0.06 | 0.06 | 0.13 | 0.13 | 0.40 | 0.40 |
| VK3 (mg/kg) | 0 | 70 | 170 | 70 | 170 | 70 | 170 |
Table 3.
Ingredients (g/kg diet) and analyzed proximate composition (%) of the experimental diets supplemented with different levels of vitamin D3 and vitamin K3.
| Experimental diet | |||||||
|---|---|---|---|---|---|---|---|
| Diet | 0 | 0.06/70 | 0.06/170 | 0.13/70 | 0.13/170 | 0.40/70 | 0.40/170 |
| Ingredients (%) | |||||||
| Squid powder∗ | 70.2 | 70.2 | 70.2 | 70.2 | 70.2 | 70.2 | 70.2 |
| Gelatin | 3 | 3 | 3 | 3 | 3 | 3 | 3 |
| Krill oil† | 13 | 13 | 13 | 13 | 13 | 13 | 13 |
| Mineral premix˟ | 4.5 | 4.5 | 4.5 | 4.5 | 4.5 | 4.5 | 4.5 |
| Sel-Plex | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 |
| Vitamin premix˟ | 6 | 6 | 6 | 6 | 6 | 6 | 6 |
| Attractantsᵜ | 3 | 3 | 3 | 3 | 3 | 3 | 3 |
| Proximate analysis (% wet weight) | |||||||
| Moisture | 8.2 ± 0.16 | 6.79 ± 0.01 | 7.47 ± 0.04 | 7.47 ± 0.16 | 7.99 ± 0.09 | 8.9 ± 0.04 | 7.86 ± 0.03 |
| Ash | 7.44 ± 0.03 | 7.59 ± 0.02 | 7.51 ± 0.02 | 7.49 ± 0.04 | 7.48 ± 0.02 | 7.39 ± 0.01 | 7.07 ± 0.49 |
| Lipid | 22.03 ± 0.96 | 20.36 ± 0.33 | 20.09 ± 0.38 | 20.25 ± 0.01 | 20.63 ± 0.58 | 19.46 ± 0.54 | 19.83 ± 0.83 |
| Protein | 63.72 ± 0.76 | 65.33 ± 0.28 | 63.9 ± 1.85 | 65.46 ± 0.52 | 64.71 ± 0.4 | 64.88 ± 0.02 | 65.34 ± 0.06 |
∗Bacarel Express, code: 70400, United Kingdom. †Krill, high phospholipids, Aker BioMarine, Fjordalléen, Norway. ˟Vitamin and mineral premix used according to [86] with modifications: vitamin premix (mg/100 g): water-soluble vitamins (cyanocobalamin: 0.03, astaxanthin: 5, folic acid: 5.44, pyridoxine-HCl: 17.28, thiamine-HCl: 21.77, riboflavin: 72.53, calcium pantothenate: 101.59, P-aminobenzoic acid: 145, nicotinic acid: 290.16, and inositol: 1450.9) and fat-soluble vitamins (retinol acetate: 0.24, alpha-tocopherol acetate: 150, and vitamin C: 180). Mineral premix (mg/100 g): sodium chloride (NaCl): 215.133, magnesium sulfate heptahydrate (MgSO4.7H2O): 677.545, sodium dihydrogen phosphate monohydrate (NaH2PO4.H2O): 381.453, dipotassium hydrogen phosphate (K2HPO4): 758.949, calcium dihydrogen phosphate dihydrate (Ca(H2PO4).2H2O): 671.61, ferric citrate (FeC6H5O7): 146.884, calcium lactate (C3H5O3.1/2Ca): 1617.21, aluminum sulfate hexahydrate (Al2(SO4)3.6H2O): 0.693, zinc sulfate heptahydrate (ZnSO4.7H2O): 14.837, copper sulfate pentahydrate (CuSO4.5H2O): 1.247, manganese sulfate monohydrate (MnSO4.H2O): 2.998, potassium iodide (KI): 0.742, and cobalt sulfate heptahydrate (CoSO4.7H2O): 10.706. ᵜAttractants were used based on [87].
2.3. Larval Performance
Growth performance was determined at each sampling points at 7th, 14th, and 21st days of feeding, by measuring total length and dry body weight. Total length of 20 larvae (anesthetized with clove oil) from each tank was measured by microscope (Leica microsystem, Leica Application Suite software, Germany). Whole-body weight was determined by triplicates of 10 starved larvae washed with distilled water and dried in a glass slide at an oven at 110°C for 24 h, followed by 1 h periods until constant weight was achieved. To evaluate the survival rate of larvae, the total live larvae from each treatment were collected and counted at the end of the experimental period.
2.4. Gene Expression Analysis
Larvae were collected for molecular studies at days 7 and 21 of the trial and preserved in 500 μL RNALater (SIGMA, Madrid, Spain) and stored at -80°C. Total RNA was extracted from seabream larvae (approximately 100-150 mg per treatment) using the RNeasy Mini Kit (Qiagen, Hilden, Germany). Larvae were homogenized using TissueLyser-II (Qiagen, Germany) with QIAzol lysis reagent (Qiagen, Germany). Homogenized samples were centrifuged with chloroform for phase separation (12000 g, 15 min, 4°C). The upper aqueous phase was carefully pipetted into a tube containing 75% ethanol and mixed. This mix was transferred into an RNeasy spin column, where total RNA bonded to a membrane. RW1 and RPE buffers (Qiagen, Germany) were used to wash away contaminants. Finally, purified RNA was eluted with 30 μL of RNase-free water. NanoDrop 1000 spectrophotometer (Thermo Scientific, Wilmington, DE, USA) was used to determine the quality and quantity of the eluted RNA. Synthesis of cDNA was conducted using the iScript cDNA Synthesis Kit (Bio-Rad, Hercules, CA, USA) according to manufacturer's instructions in an iCycler thermal cycler (Bio-Rad, USA). Primer efficiency was tested with serial dilutions of a cDNA pool (1, 1 : 10, 1 : 100, 1 : 200, and 1 : 1000). Real-time quantitative PCR was performed in an iQ5 Multicolor Real-Time PCR detection system (Bio-Rad, USA) using beta-actin (β-actin), ribosomal protein L27 (rpl27), and elongation factor 1 alpha 1 (ef1a) as the housekeeping genes in a final volume of 20 μL per reaction well, and 100 ng of total RNA was reverse transcribed to complementary cDNA. The PCR conditions were the following: denaturation 95°C for 3 min and 30 s, followed by annealing at 40 cycles of 95°C for 15 s, 58.1-61°C for 30 s (Table 4), 72°C for 30 s, and 95°C for 1 min, and a final denaturation step from 58 to 95°C for 10 s. The nucleotides of the housekeeping and target gene primers are listed in Table 4.
Table 4.
Sequences of primers used in gene expression studies.
| Gene | Nucleotide sequence (5′-3′) | Annealing temperature | Accession number |
|---|---|---|---|
| Beta-actin (β-actin) | F: TCTGTCTGGATCGGAGGCTC | 58.1 | X89920 |
| R: AAGCATTTGCGGTGGACG | |||
| Elongation factor 1-alpha (ef1α) | F: CTTCAACGCTCAGGTCATCAT | 60 | AF184170 |
| R: GCACAGCGAAACGACCAAGGGGA | |||
| Ribosomal protein L27 (rpl27) | F: AAGAGGAACACAACTCACTGCCCCAC | 68 | AY188520 |
| R: GCTTGCCTTTGCCCAGAACTTTGTAG | |||
| Bone morphogenic protein 2 (bmp2) | F: GTGGCTTCCATCGTATCAACATTTT | 60 | JF261172.1 |
| R: GCTCCCCGCCATGAGT | |||
| RUNX family transcription factor 2 (runx2) | F: GCCTGTCGCCTTTAAGGTGGTTGC | 61 | AJ619023 |
| R: TCGTCGTTGCCCGCCATAGCTG | |||
| Osterix (osx) | F: CAGTCAGGGATTCAGCAACA | 60 | ERR22591_isotig06993 |
| R: GGTGAAGGAGCCAGTGTAGG | |||
| Alkaline phosphatase (alp) | F: AGAACGCCCTGACGCTGCAA | 61 | AY266359 |
| R: TTCAGTATACGAGCAGCCGTCAC | |||
| Osteocalcin (oc) | F: GGCAGCCATCTGTCTGACTT | 58.1 | AF048703 |
| R: GGTCCGTAGTAGGCCGTGTA | |||
| Matrix Gla protein (mgp) | F: CGCCCGAAATACACCTCAGA | 60 | AY065652 |
| R: GACGGACGGATACTAGGAGTCTA | |||
| Calcium-sensing receptor (casr) | F: GCTTCTCCAGCTCGCTCATC | 60 | AJ289717 |
| R: AGGCGGGCTGGCGTAA | |||
| Parathyroid hormone 1 receptor (pthr1) | F: GAACCTGCCCGGCTACGTGAAG | 60 | AJ619024 |
| R: GCTCCTGTCCCG ACGAGGGTAT | |||
| Parathyroid hormone-related protein (pthrp) | F: GAGGCAAATGAATGGAACAG | 60 | AF197904 |
| R: TGGCCAGCTCAAAACTTGT |
2.5. Skeletal Anomalies
To determine the occurrence of skeletal anomalies, 150 larvae per treatment (i.e., 50 larvae per tank) were sampled at 43 dph and fixed in 4% formalin in phosphate buffer, pH 7.2, 0.1 M. Fixed larvae were stained for whole mount staining and examined under stereomicroscope (Leica M125, Wetzlar, Germany), and larval image (photographed using Leica DFC295 digital camera, Leica, Wetzlar, Germany) was processed using the Leica application suite (LAS 32167, Leica, Wetzlar, Germany). The stained larvae were examined for severe skeletal anomalies [5].
2.6. Data Analysis
All data were statistically analyzed using IBM SPSS Statistics v26.0. (IBM Corp., Chicago, IL, USA) and are expressed as means ± S.D. Data were treated for normality and homogeneity of variances using Levene's statistic, and means were compared to understand the statistical difference among the groups using Tukey's post hoc test (p < 0.05). To determine the effect of individual diet, one-way analysis of variance (ANOVA) was used, and for the interaction, two-way ANOVA was performed. SigmaPlot 12.3 (Systat Software, Inc., California, USA) was used to plot data in a three-dimensional mesh surface response plot to illustrate the interaction effect.
2.7. Ethical Statement
The study was conducted according to the European Union Directive (2010/63/EU) on the protection of animals for scientific purposes at Aquaculture Research Group (GIA) of ECOAQUA Institute, University of Las Palmas de Gran Canaria (ULPGC), Canary Islands, Spain. All experimentation performed at the ULPGC was approved by the Bioethical Committee of the University of Las Palmas de Gran Canaria (REF: 05/2021 CEBA ULPGC).
3. Results
3.1. Larval Performance
Microscopic observation showed that all the experimental diets were well accepted by gilthead seabream larvae. After a period of 21-day feeding, the best survival was found in larvae fed 0.06/70 VD/VK diet and the lowest in those fed the 0.40/170 VD/VK diet (p = 0.08) (Table 5). The two-way ANOVA denoted a significant negative effect of increased dietary vitamin K3 levels on survival rates. Regarding the growth of larvae, there was no significant difference in total length on day 7 (29 dph) and day 14 (36 dph) (Figure 1). At the end of the trial, due to high mortality, no samples were recorded for length and weight in the group of larvae fed with high 0.40/170 VD/VK diet; hence, the highest growth in terms of total length was found in fish fed the 0.13/70 diet and the lowest in those fed the 0.40/70 diet (Table 5). Thus, increase in dietary vitamin D3 supplementation from 0.06 to 0.13 mg/kg significantly improved total length at the lower vitamin K3 supplementation level (70 mg/kg), but it did not at the higher supplementation level of vitamin K3 (170 mg/kg), denoting the interaction between both vitamins. Besides, further increase in dietary vitamin D3 from 0.13 to 0.40 mg/kg significantly reduced larval total length. Accordingly, the two-way ANOVA denoted that there was no significant effect on day 7 and day 14. But on day 21, there was a significant effect of dietary VD on total length, with an interaction between both vitamins. No significant effect was found on dry body weight, which is a less sensitive parameter at larval stages.
Table 5.
Growth performance of gilthead seabream larvae fed diets supplemented with several levels of vitamin D3 and vitamin K3 for 21 days.
| 0 | 0.06/70 | 0.06/170 | 0.13/70 | 0.13/170 | 0.40/70 | 0.40/170 | One-way ANOVA(p value) | Two-way ANOVA(p value) | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| VD | VK | VD∗VK | |||||||||
| Survival (%) | 19.03 ± 5.04ab | 28.39 ± 4.38b | 19.81 ± 5.96ab | 22.92 ± 11.95ab | 19.53 ± 9.83ab | 24.56 ± 5.14ab | 7.92 ± 0.00a | 0.08 | 0.183 | 0.012 | 0.291 |
| Total length (mm/larvae) | 8.58 ± 0.25ab | 8.20 ± 0.16a | 8.42 ± 0.34ab | 9.06 ± 0.05b | 8.74 ± 0.22ab | 8.24 ± 0.40a | _ | 0.03 | 0.001 | 0.536 | 0.023 |
| Body weight (mg/larvae) | 0.54 ± 0.02 | 0.52 ± 0.02 | 0.52 ± 0.07 | 0.55 ± 0.05 | 0.49 ± 0.13 | 0.44 ± 0.12 | _ | 0.64 | 0.316 | 0.604 | 0.581 |
∗Different letters in a row denote significant differences between groups fed different diets (mean ± SD, n = 3, p < 0.05).
Figure 1.
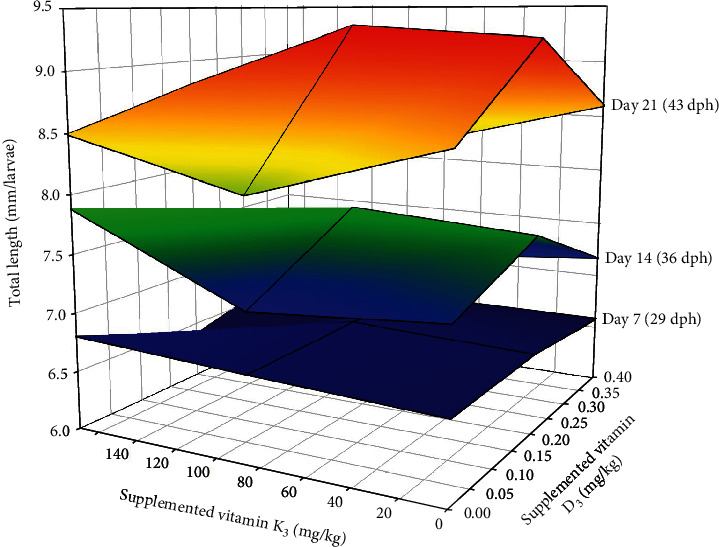
Effect of dietary vitamin D3 and vitamin K3 interaction on total length of gilthead seabream larvae at different sampling points (day 7: one-way ANOVA—p = 0.552 and two-way ANOVA—VD = 0.454, VK = 0.485, and VD∗VK = 0.826; day 14: one-way ANOVA—p = 0.061 and two-way ANOVA—VD = 0.925, VK = 0.551, VD∗VK = 0.315; day 21: one-way ANOVA—p = 0.03 and two-way ANOVA—VD = 0.001, VK = 0.536, and VD∗VK = 0.023).
3.2. Bone Biomarker Gene Expression
Among the genes related to bone metabolism, the relative gene expression of the bone morphogenic protein 2 (bmp2) was not affected by the diet after 7 days of feeding the experimental diets (Table 6) (p > 0.05), whereas at day 21, it was significantly upregulated in fish fed 0.06/70 diet in comparison to those fed the 0.40/170 diet. The runx2 expression was not affected by the different dietary vitamin D3 and vitamin K3 levels tested neither at day 7 nor at day 21 (Table 6). The relative expression of osterix (osx) showed a similar expression pattern as bmp2, with no differences at day 7 (p < 0.05), but an upregulation at day 21 in fish fed 0.06/70 diet (Table 6). Moreover, in osx gene expression, a significant (p < 0.05) interaction between both vitamins was found at day 21 by the two-way ANOVA, denoting that although at the lowest vitamin D3 levels the increase in vitamin K3 downregulated osx (0.06/70 vs. 0.06/170), this effect was reversed at the highest vitamin D3 levels (0.40/70 vs. 0.40/170). Indeed, there was a very strong lineal regression (R2 = 0.99, p < 0.05; y = 0.9988x–0.0024) between bmp2 and osx relative expressions at day 21 (Figure 2), except for the value at highest level of both vitamins (0.40/170 diet) that showed a higher expression in osx in relation to the bmp2 expression. The expression of alkaline phosphatase (alp) was not significantly affected on day 7, although at day 21 there was a tendency for an upregulation at the highest vitamin D3 levels (0.40/70 and 0.40/170) (p = 0.054, Table 6). The osteocalcin (oc) expression was significantly downregulated in fish fed diet 0.13/170 at day 7 (p < 0.05), the two-way ANOVA suggesting an effect of vitamin D3 levels (p = 0.055, Table 6). At day 21, besides the mild vitamin D3 effect (p = 0.06), the two-way ANOVA also denoted the significant (p < 0.05) downregulatory effect of vitamin K3 increase (Table 6 and Figures 3(a) and 4(a)). After 7 days of feeding, the matrix-GLA protein (mgp) was significantly (p < 0.05) lowest in fish fed diet 0.13/170 and highest in fish fed 0.40/170 followed by those fed 0.40/70 (Table 6), and the two-way ANOVA analysis suggested the downregulatory effect of vitamin D3 increase (p = 0.056) and the interaction of both vitamins leading to an upregulation at the highest dietary levels of both vitamins (p = 0.067). At day 21, mgp expression followed a similar pattern at day 7, with the downregulation of this gene in fish fed the 0.40/70 diet followed by those fed intermediate levels of these vitamins (p < 0.05) and the highest in fish fed 0.06/70 followed by those fed 0.40/170 diet. The two-way ANOVA suggested the downregulatory effect of vitamin D3 (p = 0.054) and vitamin K3 (p = 0.059) and showed a significant interaction between both vitamins (p < 0.05, Figures 3(b) and 4(b)). Moreover, there was a strong lineal regression (R2 = 0.92, p < 0.05; y = 1.1285x − 0.2077) between mgp and oc relative expressions at day 7 (Figure 5). At day 21, a lineal regression (R2 = 0.5242, y = 1.1635x + 0.0946) was also observed between mgp and oc relative expressions (Figure 6), except for the value at highest level of both vitamins (0.40/170 diet) that showed a lower expression in oc in relation to the mgp expression.
Table 6.
Relative gene expression of bone biomarkers and calcium regulators in gilthead seabream larvae fed diets supplemented with several levels of vitamin D3 and vitamin K3 for 21 days.
| Diet | One-way ANOVA (p value) | Two-way ANOVA (p value) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gene | 0 | 0.06/70 | 0.06/170 | 0.13/70 | 0.13/170 | 0.40/70 | 0.40/170 | VD | VK | VD∗VK | ||
| Day 7 (29 dph) | bmp2 | 1.08 ± 0.40 | 1.13 ± 1.11 | 1.09 ± 0.62 | 0.96 ± 0.64 | 0.42 ± 0.15 | 1.11 ± 0.79 | 0.79 ± 0.33 | 0.659 | 0.751 | 0.267 | 0.48 |
| runx2 | 1.04 ± 0.31 | 0.77 ± 0.44 | 1.27 ± 0.53 | 1.16 ± 0.72 | 1.04 ± 0.55 | 1.14 ± 0.56 | 1.12 ± 0.56 | 0.892 | 0.473 | 0.592 | 0.185 | |
| osx | 1.06 ± 0.41 | 0.83 ± 0.14 | 1.24 ± 0.71 | 0.78 ± 0.21 | 0.80 ± 0.45 | 0.67 ± 0.08 | 1.53 ± 1.19 | 0.201 | 0.189 | 0.859 | 0.164 | |
| alp | 1.00 ± 0.10 | 1.15 ± 0.07 | 1.74 ± 0.71 | 1.34 ± 0.36 | 1.09 ± 0.22 | 1.16 ± 0.51 | 1.31 ± 0.44 | 0.102 | 0.674 | 0.459 | 0.371 | |
| oc | 1.05 ± 0.32b | 0.92 ± 0.57b | 1.15 ± 0.47b | 0.70 ± 0.48ab | 0.29 ± 0.07a | 0.52 ± 0.15ab | 1.14 ± 0.72b | 0.03 | 0.055 | 0.547 | 0.344 | |
| mgp | 1.03 ± 0.24ab | 1.01 ± 0.28ab | 1.07 ± 0.32ab | 0.87 ± 0.31ab | 0.44 ± 0.12a | 0.68 ± 0.27ab | 1.31 ± 0.57b | 0.009 | 0.056 | 0.297 | 0.067 | |
| casr | 1.16 ± 0.66ab | 0.88 ± 0.32ab | 0.92 ± 0.60ab | 1.03 ± 0.65ab | 0.35 ± 0.06a | 0.76 ± 0.14ab | 1.90 ± 1.30b | 0.054 | 0.104 | 0.501 | 0.015 | |
| pthr1 | 1.04 ± 0.29ab | 0.88 ± 0.45ab | 0.82 ± 0.49ab | 0.76 ± 0.38ab | 0.34 ± 0.02a | 0.55 ± 0.16ab | 1.15 ± 0.53b | 0.035 | 0.488 | 0.457 | 0.113 | |
| pthrp | 1.09 ± 0.45ab | 1.04 ± 0.69ab | 0.99 ± 0.51ab | 1.10 ± 0.84ab | 0.35 ± 0.05a | 0.80 ± 0.23ab | 1.97 ± 0.95b | 0.035 | 0.068 | 0.595 | 0.005 | |
|
| ||||||||||||
| Day 21 (43 dph) | bmp2 | 1.03 ± 0.29ab | 1.19 ± 0.47b | 0.70 ± 0.30ab | 0.67 ± 0.31ab | 0.75 ± 0.33ab | 0.64 ± 0.26ab | 0.55 ± 0.26a | 0.026 | 0.178 | 0.352 | 0.51 |
| runx2 | 1.02 ± 0.25 | 0.93 ± 0.36 | 0.80 ± 0.43 | 1.02 ± 0.31 | 1.02 ± 0.70 | 1.35 ± 0.90 | 0.95 ± 0.29 | 0.705 | 0.746 | 0.28 | 0.519 | |
| osx | 1.01 ± 0.17ab | 1.20 ± 0.23b | 0.67 ± 0.19a | 0.67 ± 0.22a | 0.75 ± 0.19ab | 0.66 ± 0.29a | 1.12 ± 0.46ab | 0.002 | 0.122 | 0.185 | 0.043 | |
| alp | 1.03 ± 0.26 | 1.12 ± 0.33 | 1.05 ± 0.38 | 1.37 ± 0.47 | 1.20 ± 0.24 | 1.77 ± 0.81 | 1.68 ± 0.55 | 0.054 | 0.141 | 0.555 | 0.529 | |
| oc | 1.06 ± 0.43 | 1.71 ± 0.46 | 0.98 ± 0.61 | 1.50 ± 0.82 | 1.04 ± 0.60 | 0.78 ± 0.50 | 0.79 ± 0.51 | 0.149 | 0.058 | 0.002 | 0.284 | |
| mgp | 1.03 ± 0.29abc | 1.33 ± 0.50c | 0.81 ± 0.24ab | 0.83 ± 0.27ab | 0.85 ± 0.19ab | 0.74 ± 0.18a | 1.22 ± 0.54bc | 0.037 | 0.054 | 0.059 | 0.039 | |
| casr | 1.03 ± 0.27 | 1.21 ± 0.75 | 0.70 ± 0.29 | 1.20 ± 0.53 | 0.98 ± 0.21 | 0.99 ± 0.23 | 1.40 ± 0.48 | 0.215 | 0.667 | 0.662 | 0.175 | |
| pthr1 | 1.00 ± 0.12b | 0.84 ± 0.25ab | 0.49 ± 0.26a | 0.63 ± 0.21ab | 0.48 ± 0.12a | 0.64 ± 0.38ab | 0.51 ± 0.25a | 0.014 | 0.694 | 0.103 | 0.691 | |
| pthrp | 1.02 ± 0.19ab | 1.64 ± 0.50b | 0.78 ± 0.20a | 1.61 ± 0.31b | 1.00 ± 0.27ab | 1.13 ± 0.58ab | 1.18 ± 0.23ab | 0.002 | 0.531 | 0.002 | 0.053 | |
∗Different letters in a row denote significant differences between groups fed different diets (mean ± SD, n = 3, p < 0.05).
Figure 2.
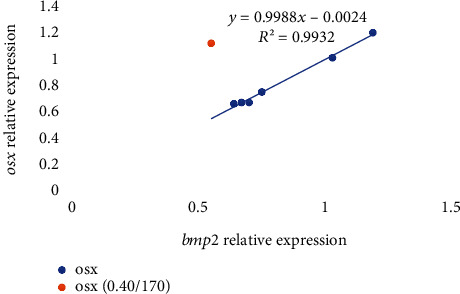
Relative expression of osx in relation to bmp2 expression in gilthead seabream larvae fed diets supplemented with several levels of vitamin D3 and vitamin K3 for 21 days (n = 3, p < 0.05; blue dots for values of diets 0.00/0, 0.06/70, 0.06/170, 0.13/70, 0.13/170, and 0.40/70 and orange dot for 0.40/170).
Figure 3.
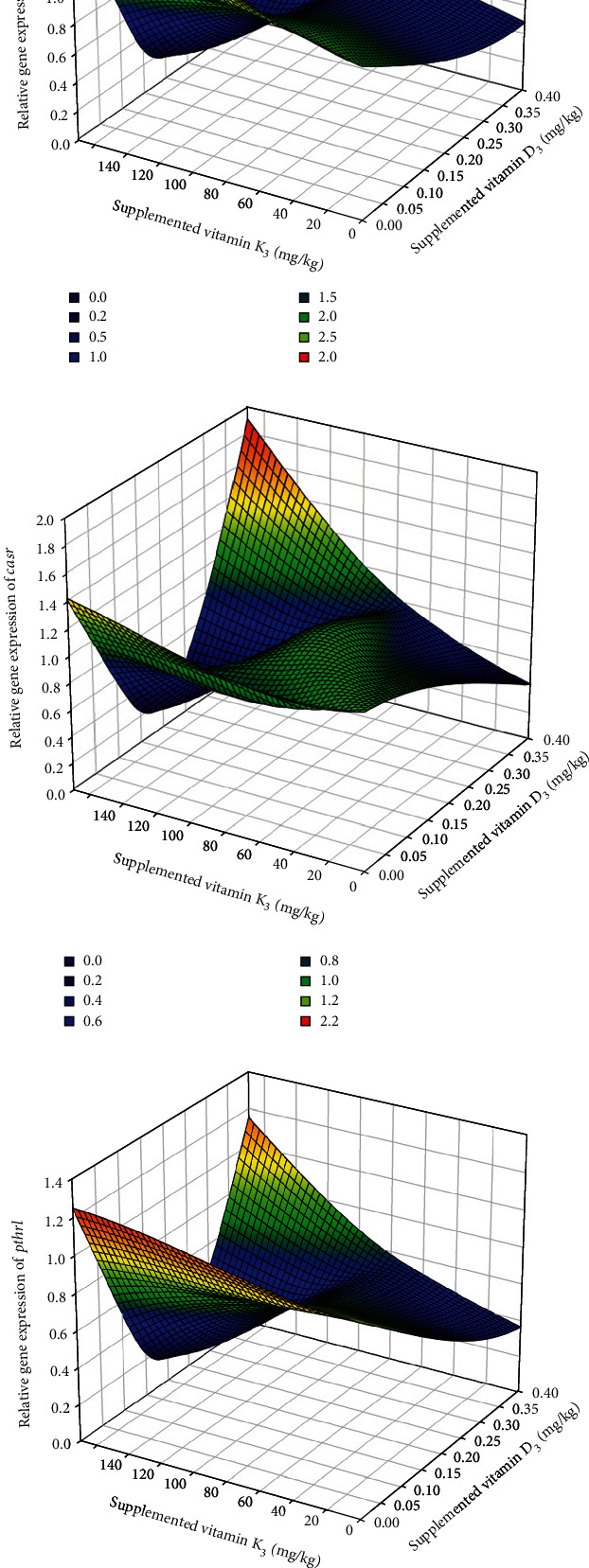
Effect of dietary vitamin D3 and vitamin K3 interaction on gene expression of vitamin K-dependent proteins (a) oc and (b) mgp and calcium regulators (c) casr, (d) pthr1, and (e) pthrp in gilthead seabream larvae at 29 dph.
Figure 4.
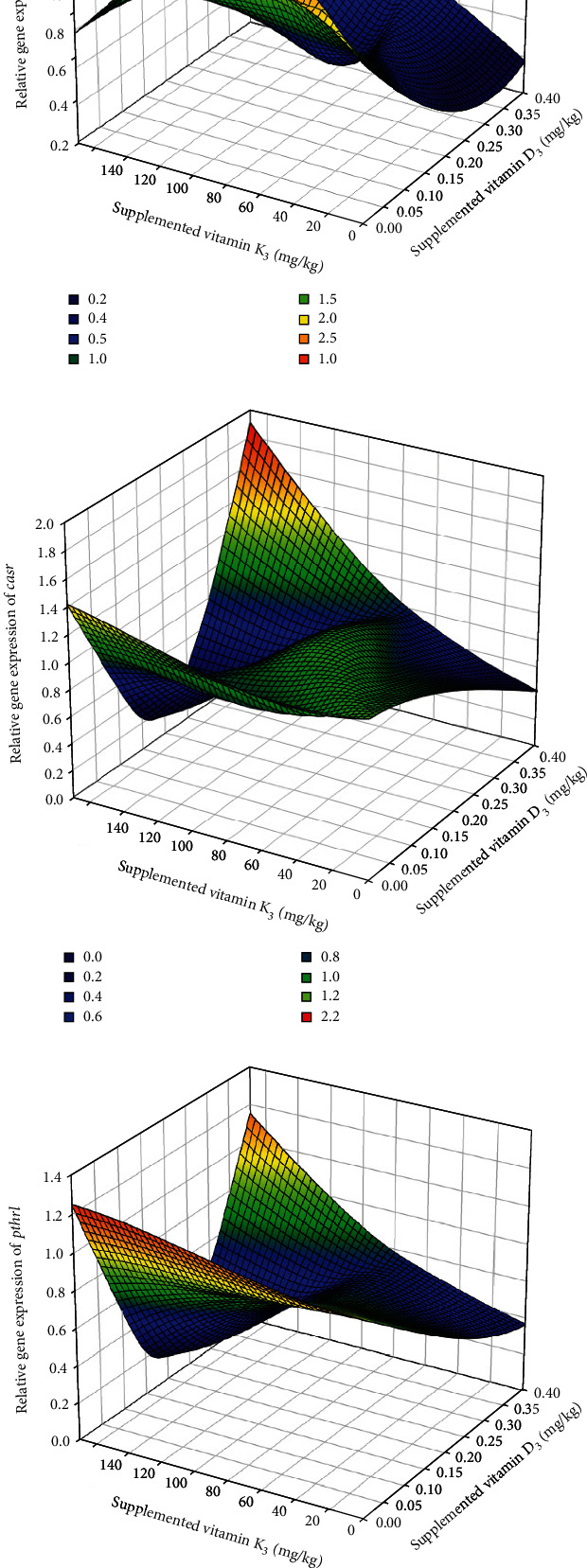
Effect of dietary vitamin D3 and vitamin K3 interaction on gene expression of vitamin K-dependent proteins (a) oc and (b) mgp and calcium regulators (c) casr, (d) pthr1, and (e) pthrp in gilthead seabream larvae at 43 dph.
Figure 5.
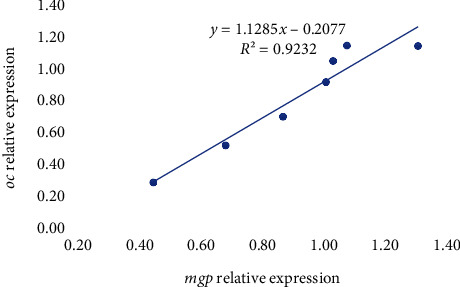
Relative expression of oc in relation to mgp expression in gilthead seabream larvae fed diets supplemented with several levels of vitamin D3 and vitamin K3 for 7 days (n = 3, p < 0.05; blue dots for values of diets 0.00/0, 0.06/70, 0.06/170, 0.13/70, 0.13/170 and 0.40/70 and orange dot for 0.40/170).
Figure 6.
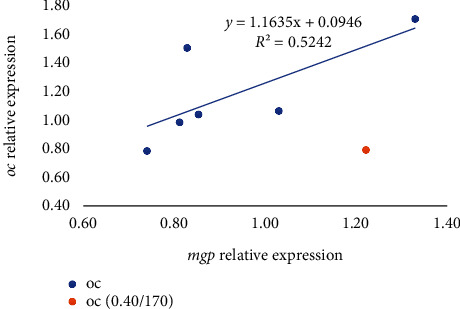
Relative expression of oc in relation to mgp expression in gilthead seabream larvae fed diets supplemented with several levels of vitamin D3 and vitamin K3 for 21 days (n = 3, p < 0.05; blue dots for values of diets 0.00/0, 0.06/70, 0.06/170, 0.13/70, 0.13/170 and 0.40/70 and orange dot for 0.40/170).
Regarding the expression of other genes related to vitamin D and K metabolism, the calcium-sensing receptor (casr) expression at day 7 was significantly (p < 0.05) highest in fish fed 0.40/170 diet in comparison to those fed 0.13/170, whereas at day 21, no significant differences were found among the different dietary groups (Table 6 and Figures 3(c) and 4(c)). The expression of the parathyroid hormone receptor 1 (pthr1) and the parathyroid hormone-related protein (pthrp) at day 7 and increase in dietary vitamin K3 tend to downregulate these genes leading to the significantly (p < 0.05) lowest expression in fish fed the 0.13/170 diet, except in those fish fed the highest vitamin D3 levels, which showed the highest expression (Table 6 and Figures 3(d) and 3(e) and 4(d) and 4(e)). Moreover, there were strong logarithmic regressions between casr expression and pthr1 (R2 = 0.8325, y = 0.4795ln(x) + 0.8173) or pthrp (R2 = 0.8975, y = 0.6917ln(x) + 1.0387) relative expressions at day 7 (Figure 7). After 21 days of feeding, increase in vitamin K3 downregulated both parathyroid hormone-related genes, which showed the lowest expression in fish fed 0.06/170 and 0.13/170 diets. In the case of pthrp, the two-way ANOVA also suggested (p = 0.053) the interaction between both vitamins, and, hence the increase in vitamin K3 in fish fed the highest levels of dietary vitamin D3 did not upregulate pthrp expression. There was also an exponential regression (R2 = 0.9162, y = 0.2552e1.4825x) between casr and pthrp expressions except for the value at highest level of both vitamins (0.40/170 diet) that showed a lower expression in pthrp in relation to the casr expression (Figure 8). However, the comparison between expression of bone biomarker and calcium regulators did not show any significant changes with respect to different sampling points between different dietary groups (Table 7).
Figure 7.
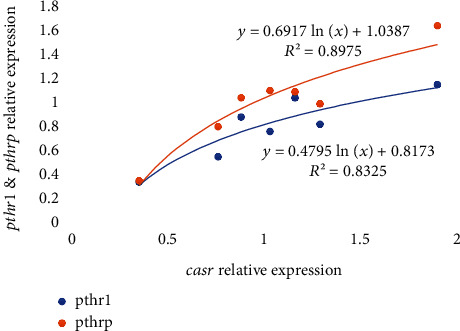
Relative expression of pthr1 and pthrp in relation to casr expression in gilthead seabream larvae fed diets supplemented with several levels of vitamin D3 and vitamin K3 for 7 days (n = 3, p < 0.05; blue dots for pthr1 values and orange dots for pthrp).
Figure 8.
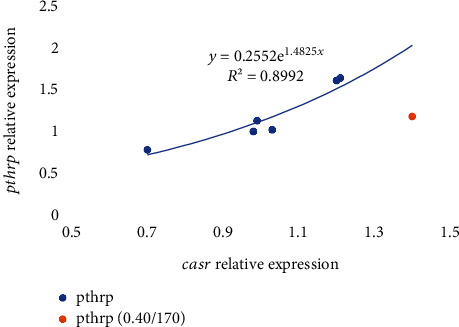
Relative expression of pthrp in relation to casr expression in gilthead seabream larvae fed diets supplemented with several levels of vitamin D3 and vitamin K3 for 21 days (n = 3, p < 0.05; blue dots for values of diets 0.00/0, 0.06/70, 0.06/170, 0.13/70, 0.13/170 and 0.40/70 and orange dot for 0.40/170).
Table 7.
Comparison of bone biomarkers and calcium regulator gene expression in gilthead seabream larvae between two sampling points day 7 (29 dph) and day 21 (43 dph) fed diets supplemented with several levels of vitamin D3 and vitamin K3.
| Gene | Two-way ANOVA (p value) | ||
|---|---|---|---|
| Group | Day | Group ∗ day | |
| bmp2 | 0.819 | 0.114 | 0.811 |
| runx2 | 0.995 | 0.404 | 0.956 |
| osx | 0.043 | 0.368 | 0.918 |
| alp | 0.812 | 0.694 | 0.193 |
| oc | 0.401 | 0.912 | 0.985 |
| mgp | 0.429 | 0.461 | 0.947 |
| casr | 0.707 | 0.293 | 0.836 |
| pthr1 | 0.839 | 0.095 | 0.749 |
| pthrp | 0.77 | 0.399 | 0.880 |
3.3. Skeletal Studies
Supplementation with different combinations of dietary VD and VK showed no significant difference in frequency of skeletal anomalies (Figure 9). The occurrence of abdominal kyphosis and haemal lordosis (Table 8) did not differ among the different dietary treatments. Similarly, two-way ANOVA also showed no effect on skeletal anomalies.
Figure 9.
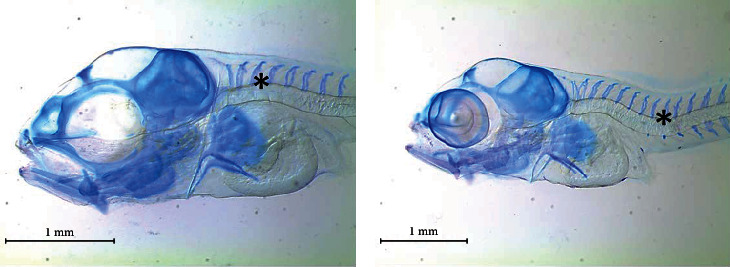
Skeletal anomalies found in gilthead seabream larvae fed diets supplemented with several levels of vitamin D3 and vitamin K3 for 21 days: (a) abdominal kyphosis and (b) haemal lordosis.
Table 8.
Frequency of skeletal anomalies in gilthead seabream larvae fed diets supplemented with several levels of vitamin D3 and vitamin K3 for 21 days.
| Skeletal anomalies (%) | 0 | 0.06/70 | 0.06/170 | 0.13/70 | 0.13/170 | 0.40/70 | 0.40/170 | One-way ANOVA (p value) | Two-way ANOVA (p value) | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| VD | VK | VD∗VK | |||||||||
| Abdominal kyphosis | 21.03 ± 20.08 | 24.94 ± 2.84 | 23.78 ± 6.66 | 15.22 ± 8.63 | 16.41 ± 12.45 | 19.57 ± 10.66 | 13.03 ± 7.94 | 0.810 | 0.353 | 0.684 | 0.828 |
| Haemal lordosis | 0.83 ± 1.44 | 5.13 ± 5.13 | 0.85 ± 1.48 | 2.24 ± 2.07 | 2.30 ± 2.19 | 0.83 ± 1.44 | 0.65 ± 1.13 | 0.332 | 0.307 | 0.231 | 0.267 |
| Total anomalies | 21.86 ± 21.28 | 30.07 ± 7.64 | 24.64 ± 6.69 | 17.46 ± 8.59 | 18.71 ± 12.77 | 20.40 ± 10.80 | 13.68 ± 9.00 | 0.731 | 0.287 | 0.527 | 0.826 |
4. Discussion
Optimum dietary levels of either vitamin D or vitamin K are necessary for larval performance and skeletal development in marine fish larvae [31, 32]. Despite the clear interaction between these vitamins in mammals [42], studies in fish are very scarce and none were conducted during larval stages [56, 59]. The present study showed the significant interaction between both vitamins in larval performance and gene expression related to bone development, denoting the importance of a correct balance between both vitamins in larval diets.
Regarding larval performance, neither growth nor survival was significantly reduced in fish fed diets without supplementation with vitamin D3 or vitamin K3. However, an increase in both vitamins led to the best larval performance, suggesting that the basal dietary levels were only marginally deficient. Thus, the increase in dietary vitamin D3 supplementation from 0.06 to 0.13 mg/kg improved the total length of larvae fed the lower dietary vitamin K3 supplementation level (70 mg/kg). This agrees well with the growth-promoting effect of vitamin D observed in mammals [60] and fish [25, 27, 61], particularly during early developmental stages [31]. In contrast with the present results, in gilthead seabream juveniles, increased supplementation of vitamin D3 had only a mild effect on growth improvement [20], confirming the stronger growth-promoting effect and importance of this vitamin in early stages. However, increase in vitamin D3 from 0.06 to 0.13 mg/kg did not improve growth at the higher vitamin K3 supplementation level (170 mg/kg), denoting a clear interaction between both vitamins confirmed by the 2-way ANOVA statistical analysis. The present study is the first one that shows the interaction effect on the larval growth. On the contrary, in the present study, the increase in vitamin K3 supplementation level from 70 or 170 mg/kg did not affect seabream growth, in agreement with the lack of effect of vitamin K supplementation on growth of salmon fry [62].
In the present study, the further increase in the dietary vitamin D3 up to 0.4 mg/kg significantly reduced the larval growth, denoting the negative effects of excessive dietary vitamin D3, and this conclusion is in agreement with the previous studies on seabream [32] and other species [18]. In turbot (Psetta maxima), the overdose of vitamin D3 produced an intestinal inflammation and reduced the gut microbiota diversity [63] that could be related with an impairment of growth. Despite the increase in vitamin K3 supplementation from 70 to 170 mg/kg did not affect seabream growth, at the highest vitamin D3 and K3 supplementation levels, the survival rate was significantly reduced. Poor survival rates may be attributed to the toxic effect of vitamin overdose, where the larvae fed 0.4 mg/kg vitamin D3 with 70 mg/kg showed higher survival than larvae fed 170 mg/kg vitamin K3. This denotes that the highest dietary vitamin D3 content accompanied with the lower vitamin K3 level had better effect on survival than in larvae fed on the higher vitamin K3 content. This in agreement with the authors' previous study, where the larvae were fed on diets supplemented with vitamin D3 at 0.50 mg/kg and vitamin K3 at 173 mg/kg supplementation levels that reduced the larval survival rate [32]. Further studies in mammals and other species showed that the excess menadione supplementation would impair the growth and survival rate [23] and increase the binding capacity of vitamin D transporter to vitamin D, which results in increased vitamin D receptor activity. The vitamin D receptor participates in the pathophysiological process by enhancing the metabolite effect in larvae [64, 65].
The highest larval survival rate was recorded in larvae fed the diet supplemented with 0.06 and 70 mg/kg vitamin D3 and vitamin K3, respectively. These larvae also showed, at the end of the trial, the highest expression of bmp2, osx, and mgp, which are important biomarkers of bone development and health. The bmp2 is an osteochondrogenic factor which initiates bone formation and bone healing, inducing the expression of other bmps, whereas osx regulates late osteogenesis and bone matrix mineralization [66, 67]. In the present study, osx expression followed a linear relation with bmp2 expression, in larvae fed the diet supplemented with 0.06 and 70 mg/kg vitamin D3 and vitamin K3, respectively, showing the highest values for both genes. The mgp promotes osteoblast proliferation and bone formation through the Wnt/β-Catenin signaling pathway [68], affecting mineralization and osteoclast differentiation [69]. The expression of mgp occurs during early development of gilthead seabream [70]. In the present study, mgp expression was associated with increased oc expression, with the highest values for both genes found in the larvae fed the diet supplemented with 0.06 and 70 mg/kg vitamin D3 and vitamin K3, respectively. Osteocalcin is produced by osteoblasts and incorporated into the bone matrix, but it is also involved in energy metabolism and other metabolic functions [71]. Vitamin K plays an important role in the carboxylation of osteocalcin and its posterior binding to hydroxyapatite [17]. Besides, both vitamins regulate oc expression [72, 73]. The highest expression of mgp and its correlation with oc in larvae fed the diet supplemented with 0.06 mg/kg vitamin D3 and 70 mg/kg vitamin K3 agree well with the combined effect of vitamin D and vitamin K on osteoblast activity and osteoclast formation, upregulation of osteoblastic genes, and bone health improvement found in other species [43, 74]. Besides, vitamin D enhances the vitamin K-dependent proteins to induce bone formation along with vitamin K [38]. Thus, in mammals, the combination of both vitamins is more effective in the prevention of bone loss and fracture risk [42, 75].
However, further elevation of vitamin K3 supplementation from 70 to 170 mg/kg at 0.06 mg/kg vitamin D3 supplementation downregulated bmp2, osx, and mgp, denoting the negative effect of increased vitamin K3. These results agree well with the indirect effect of vitamin K on bmp2 production translating into MK-7 [76]. Besides, vitamin K3 intervenes in the activation by carboxylation of the matrix Gla protein (mgp), which is a main inhibitor of mgp expression. Moreover, the combined higher concentrations of both dietary vitamin D3 and K3 (0.40 and 170 mg/kg, respectively) further downregulated bmp2 expression and upregulated osx and mgp, causing an imbalance in the expression of osx in relation to bmp2 and in the expression of oc in relation to mgp. These results reflected the interaction of both vitamins denoted by the two-way ANOVA and agree well with the downregulation of bmp2 found in fish larvae fed high doses of vitamin D3 [31, 32]. Indeed, vitamin D is actively involved in the regulation of bmp2 and osx expression [77]. Whereas bmp2 is an osteochondrogenic factor, osx inhibits chondrogenesis, and the imbalance in the expression of both genes could be responsible for an impairment in bone formation, particularly in endochondral bones. This agrees well with the high incidence of cranial anomalies found in gilthead seabream larvae fed excessive levels of vitamin D3 [32].
There is much evidence that, particularly during early developmental stages, both vitamin D and K are necessary for the formation of strong bones and the prevention of skeletal anomalies [38, 53, 78]. Besides, feeding gilthead seabream with high levels of vitamin D3 leads to cranial malformations [32], whereas excessive levels of vitamin K3 cause a high incidence of kyphosis [57]. In the present study, despite the imbalance found in the relative expression of bone health related genes and their significant differences among larvae fed different vitamin supplementation levels, no significant differences were found in the incidence of skeletal anomalies. This lack of evidence on the skeletal anomalies could be attributed to the sufficient contents of vitamins D3 and K3 in the diet without supplementation of these vitamins, preventing the marked deficiency symptoms such as poor growth or high incidence of bone anomalies. On the other hand, the highest supplementation levels of vitamins D3 and K3 (0.40/170 mg/kg) caused an imbalance in the expression of some bone biomarkers, significantly reduced larval survival rate (7.92% vs. 28.39%), and induced the weakest and deformed larvae. Moreover, the supplementation levels of vitamins D3 and K3 tested in the present study were lower than those inducing skeletal anomalies in the previous studies [57]. Nevertheless, further studies should be conducted to find out the potential interaction between these two vitamins in relation to the occurrence of skeletal anomalies.
After 21 days of feeding the experimental diets, the expression of pthrp followed an exponential regression with casr expression, with the highest values found in larvae fed the diets 0.06/70 and 0.13/70. These results agree well with the highest survival and growth found in these larvae and with the upregulation of pthrp found in juveniles of seabream when dietary vitamin D is increased over the deficient levels [79]. Besides, the results also agree with the strong correlation between pthrp and casr found in mammals [80]. The parathyroid hormone-related protein (pthrp) is related to the function of PTH and among other roles regulates endochondral bone development. The calcium-sensing receptor (CaSR) is a class C G-protein coupled receptor, sensitive to extracellular levels of calcium. It is expressed in the parathyroid gland, among other organs, and calcium inhibits parathyroid hormone (PTH) release. Both factors are the main regulators of calcium homeostasis [81], and their ratios are determinant for the correct balance of intra- and extracellular calcium.
However, the increase in the dietary vitamin K3 supplementation level from 70 to 170 mg/kg at the lowest dietary vitamin D3 supplementary level downregulated pthrp. Moreover, the highest supplementation level of both vitamins up to 0.40 vitamin D3 mg/kg and 170 mg/kg vitamin K3 markedly reduced the expression of pthrp in relation to cars expression. This unbalance in the expression of these two factors, which are important for calcium homeostasis, could be responsible for an excessive elevation of plasma calcium concentrations that could in turn inhibit pthrp release [80].
5. Conclusions
The present study showed the interaction between vitamin D3 supplementation and vitamin K3 supplementation in larval performance and gene expression related to bone development and calcium homeostasis, denoting the significance of a correct balance between both vitamins in larval diets. Supplementation with 0.06-0.13 mg/kg vitamin D3 and 70 mg/kg vitamin K3 led to the highest larval growth and survival and the highest expression of important biomarkers of both bone development and health, such as bmp2, osx, and mgp, and calcium homeostasis, such as pthrp and casr. However, the combined increase in the supplementation of both vitamins up to 0.40 mg/kg vitamin D3 and 170 mg/kg vitamin K3 reduced the larval growth and survival, downregulated bmp2 and pthrp expression, and upregulated osx and mgp, causing an unbalance in the relative expression of these genes.
Acknowledgments
This research was funded by the European Union's Horizon 2020 research and innovation programme under the Marie Skłodowska-Curie (Grant Agreement No. 766347). Open access funding is enabled and organized by CRUE-UNIRIS Gold.
Data Availability
The data used to support the findings of this study are included in the article. However, more data could be available from the corresponding author upon request.
Disclosure
This output reflects the views of the authors only, and the European Union cannot be held responsible for any use which may be made of the information contained therein.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- 1.Sorgeloos P., Dehasque M., Dhert P., Lavens P. Review of some aspects of marine fish larviculture. ICES Marine Science Symposia . 1995;201:138–142. [Google Scholar]
- 2.Afonso J. M., Montero D., Robaina L., Astorga N., Izquierdo M. S., Ginés R. Association of a lordosis-scoliosis-kyphosis deformity in gilthead seabream (Sparus aurata) with family structure. Fish Physiology and Biochemistry . 2000;22(2):159–163. doi: 10.1023/A:1007811702624. [DOI] [Google Scholar]
- 3.Andrades J. A., Becerra J., Fernández-Llebrez P. Skeletal deformities in larval, juvenile and adult stages of cultured gilthead sea bream (Sparus aurata L.) Aquaculture . 1996;141(1–2):1–11. doi: 10.1016/0044-8486(95)01226-5. [DOI] [Google Scholar]
- 4.Berillis P. Skeletal deformities in seabreams. Understanding the genetic origin can improve production? Journal of FisheriesSciences.com . 2017;11(2):57–59. [Google Scholar]
- 5.Boglione C., Gagliardi F., Scardi M., Cataudella S. Skeletal descriptors and quality assessment in larvae and post-larvae of wild-caught and hatchery-reared gilthead sea bream (Sparus aurata L. 1758) Aquaculture . 2001;192(1):1–22. doi: 10.1016/S0044-8486(00)00446-4. [DOI] [Google Scholar]
- 6.Divanach P. B. C. M. B., Boglione C., Menu B., Koumoundouros G., Kentouri M., Cataudella S. Handbook of contributions and short communications presented at the International Workshop on 'Seabass and Seabream Culture: Problems and Prospects . Verona, Italy: Oostende : European Aquaculture Society; 1996. Abnormalities in finfish mariculture: an overview of the problem, causes and solutions; pp. 45–66. [Google Scholar]
- 7.Izquierdo M. S., Socorro J., Roo J. Studies on the appearance of skeletal anomalies in red porgy: effect of culture intensiveness, feeding habits and nutritional quality of live preys. Journal of Applied Ichthyology . 2010;26(2):320–326. doi: 10.1111/j.1439-0426.2010.01429.x. [DOI] [Google Scholar]
- 8.Izquierdo M., Domínguez D., Jiménez J. I., et al. Interaction between taurine, vitamin E and vitamin C in microdiets for gilthead seabream (Sparus aurata) larvae. Aquaculture . 2019;498:246–253. doi: 10.1016/j.aquaculture.2018.07.010. [DOI] [Google Scholar]
- 9.Izquierdo M., Fernandez-Palacios H. Nutritional requirements of marine fish larvae and broodstock. Cahiers Options Méditerranéennes . 1997;22:243–264. [Google Scholar]
- 10.National Research Council. National Academies Press; 2011. Nutrient requirements of fish and shrimp. [Google Scholar]
- 11.Izquierdo M. S., Socorro J., Arantzamendi L., Hernández-Cruz C. M. Recent advances in lipid nutrition in fish larvae. Fish Physiology and Biochemistry . 2000;22(2):97–107. doi: 10.1023/A:1007810506259. [DOI] [Google Scholar]
- 12.Izquierdo M. S., Koven W. Lipids in larval fish nutrition. In: Holt J., editor. Chichester: Wiley Blackwell, John Wiley and Sons Publishers; 2011. [Google Scholar]
- 13.Hamre K., Yufera M., Rønnestad I., Boglione C., Conceição L. E., Izquierdo M. Fish larval nutrition and feed formulation: knowledge gaps and bottlenecks for advances in larval rearing. Reviews in Aquaculture . 2013;5:S26–S58. doi: 10.1111/j.1753-5131.2012.01086.x. [DOI] [Google Scholar]
- 14.Curnow J., King J., Partridge G., Kolkovski S. Effects of two commercial microdiets on growth and survival of barramundi (Lates calcarifer Bloch) larvae within various early weaning protocols. Aquaculture Nutrition . 2006;12(4):247–255. doi: 10.1111/j.1365-2095.2006.00399.x. [DOI] [Google Scholar]
- 15.Hamre K. Nutrient profiles of rotifers (Brachionus sp.) and rotifer diets from four different marine fish hatcheries. Aquaculture . 2016;450:136–142. doi: 10.1016/j.aquaculture.2015.07.016. [DOI] [Google Scholar]
- 16.Atalah E. IU de Sanidad Animal y Seguridad Alimentaria; 2008. Importance of the proportions of dietary polyunsaturated fatty acids and antioxoidants in larval development of marine fish, [Ph.D. thesis] [Google Scholar]
- 17.Graff I. E., Øyen J., Kjellevold M., et al. Reduced bone resorption by intake of dietary vitamin D and K from tailor-made Atlantic salmon: a randomized intervention trial. Oncotarget . 2016;7(43):69200–69215. doi: 10.18632/oncotarget.10171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lock E. J., WaagbØ R., Wendelaar Bonga S., Flik G. The significance of vitamin D for fish: a review. Aquaculture Nutrition . 2010;16(1):100–116. doi: 10.1111/j.1365-2095.2009.00722.x. [DOI] [Google Scholar]
- 19.Lall S. P., Lewis-McCrea L. M. Role of nutrients in skeletal metabolism and pathology in fish -- an overview. Aquaculture . 2007;267(1–4):3–19. doi: 10.1016/j.aquaculture.2007.02.053. [DOI] [Google Scholar]
- 20.Dominguez D., Montero D., Zamorano M. J., et al. Effects of vitamin D3 supplementation in gilthead seabream (Sparus aurata) juveniles fed diets high in plant based feedstuffs. Aquaculture . 2021;543, article 736991 doi: 10.1016/j.aquaculture.2021.736991. [DOI] [Google Scholar]
- 21.Krossøy C., Waagbø R., Ørnsrud R. Vitamin K in fish nutrition. Aquaculture Nutrition . 2011;17(6):585–594. doi: 10.1111/j.1365-2095.2011.00904.x. [DOI] [Google Scholar]
- 22.Udagawa M. The effect of dietary vitamin K (phylloquinone and menadione) levels on the vertebral formation in mummichog Fundulus heteroclitus. Fisheries Science . 2001;67(1):104–109. doi: 10.1046/j.1444-2906.2001.00205.x. [DOI] [Google Scholar]
- 23.Prabhu P. A. J., Lock E.-J., Hemre G.-I., et al. Recommendations for dietary level of micro-minerals and vitamin D3 to Atlantic salmon (Salmo salar) parr and post-smolt when fed low fish meal diets. PeerJ . 2019;(7, article e6996) doi: 10.7717/peerj.6996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ling-hong M., Xian-ping G., Jun X., et al. Dietary vitamin D3 requirement of Wuchang bream (Megalobrama amblycephala) Aquaculture . 2015;436:104–109. doi: 10.1016/j.aquaculture.2014.10.049. [DOI] [Google Scholar]
- 25.Xie S., Tian L., Liu Y., et al. Vitamin D3 requirement of grouper (Epinephelus coioides) in practical diet. South China Fisheries Science . 2019;15(4):61–67. [Google Scholar]
- 26.He S., Ding M., Watson Ray G., et al. Effect of dietary vitamin D levels on growth, serum biochemical parameters, lipid metabolism enzyme activities, fatty acid synthase and hepatic lipase mRNA expression for orange-spotted grouper (Epinephelus coioides) in growth mid-stage. Aquaculture Nutrition . 2021;27(3):655–665. doi: 10.1111/anu.13212. [DOI] [Google Scholar]
- 27.Cheng L., Zhang W., Lin S., Xu W., Mai K. Effects of dietary vitamin K on growth performances, blood coagulation time and menaquinone-4 (MK-4) concentration in tissues of juvenile large yellow croaker Pseudosciaena crocea. Aquaculture Research . 2015;46(5):1269–1275. doi: 10.1111/are.12278. [DOI] [Google Scholar]
- 28.Yuan J., Feng L., Jiang W. D., et al. Effects of dietary vitamin K levels on growth performance, enzyme activities and antioxidant status in the hepatopancreas and intestine of juvenile Jian carp (Cyprinus carpiovar. Jian) Aquaculture Nutrition . 2016;22(2):352–366. doi: 10.1111/anu.12264. [DOI] [Google Scholar]
- 29.Wei X., Hang Y., Li X., et al. Effects of dietary vitamin K3 levels on growth, coagulation, calcium content, and antioxidant capacity in largemouth bass, Micropterus salmoides. Aquaculture and Fisheries . 2023;8(2):159–165. [Google Scholar]
- 30.Dominguez D., Castro P., Lall S., et al. Effects of menadione sodium bisulphite (vitamin K3) supplementation of the diets based on plant feed ingredients on growth and bone health of gilthead seabream (Sparus aurata) fingerlings. Aquaculture Nutrition . 2022;2022, article 1613030:1–8. doi: 10.1155/2022/1613030. [DOI] [Google Scholar]
- 31.Darias M., Mazurais D., Koumoundouros G., et al. Dietary vitamin D3 affects digestive system ontogenesis and ossification in European sea bass (Dicentrachus labrax, Linnaeus, 1758) Aquaculture . 2010;298(3–4):300–307. doi: 10.1016/j.aquaculture.2009.11.002. [DOI] [Google Scholar]
- 32.Sivagurunathan U., Dominguez D., Tseng Y., et al. Effects of dietary vitamin D3 levels on survival, mineralization, and skeletal development of gilthead seabream (Sparus aurata) larvae. Aquaculture . 2022;560, article 738505 doi: 10.1016/j.aquaculture.2022.738505. [DOI] [Google Scholar]
- 33.Richard N., Fernández I., Wulff T., et al. Dietary supplementation with vitamin K affects transcriptome and proteome of Senegalese sole, improving larval performance and quality. Marine Biotechnology . 2014;16(5):522–537. doi: 10.1007/s10126-014-9571-2. [DOI] [PubMed] [Google Scholar]
- 34.Abawi F. G., Sullivan T. W. Interactions of vitamins A, D3, E, and K in the diet of broiler chicks. Poultry Science . 1989;68(11):1490–1498. doi: 10.3382/ps.0681490. [DOI] [PubMed] [Google Scholar]
- 35.Koshihara Y., Hoshi K., Ishibashi H., Shiraki M. Vitamin K2 promotes 1,25(OH)2 vitamin D3-induced mineralization in human periosteal osteoblasts. Calcified Tissue International . 1996;59(6):466–473. doi: 10.1007/BF00369212. [DOI] [PubMed] [Google Scholar]
- 36.Matsunaga S., Ito H., Sakou T. The effect of vitamin K and D supplementation on ovariectomy-induced bone loss. Calcified Tissue International . 1999;65(4):285–289. doi: 10.1007/s002239900700. [DOI] [PubMed] [Google Scholar]
- 37.Meunier P. J. International osteoporosis day. Nutrition & Food Science . 2011;41(1):83–88. [Google Scholar]
- 38.Ushiroyama T., Ikeda A., Ueki M. Effect of continuous combined therapy with vitamin K2 and vitamin D3 on bone mineral density and coagulofibrinolysis function in postmenopausal women. Maturitas . 2002;41(3):211–221. doi: 10.1016/S0378-5122(01)00275-4. [DOI] [PubMed] [Google Scholar]
- 39.Iwamoto J., Yeh J. K., Takeda T., Ichimura S., Sato Y. Comparative effects of vitamin K and vitamin D supplementation on prevention of osteopenia in calcium-deficient young rats. Bone . 2003;33(4):557–566. doi: 10.1016/S8756-3282(03)00249-7. [DOI] [PubMed] [Google Scholar]
- 40.Masterjohn C. Vitamin D toxicity redefined: vitamin K and the molecular mechanism. Medical Hypotheses . 2007;68(5):1026–1034. doi: 10.1016/j.mehy.2006.09.051. [DOI] [PubMed] [Google Scholar]
- 41.Kidd P. M. Vitamins D and K as pleiotropic nutrients: clinical importance to the skeletal and cardiovascular systems and preliminary evidence for synergy. Alternative Medicine Review . 2010;15(3):199–222. [PubMed] [Google Scholar]
- 42.Van Ballegooijen A. J., Pilz S., Tomaschitz A., Grübler M. R., Verheyen N. The synergistic interplay between vitamins D and K for bone and cardiovascular health: a narrative review. International Journal of Endocrinology . 2017;2017:12. doi: 10.1155/2017/7454376.7454376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kuang X., Liu C., Guo X., Li K., Deng Q., Li D. The combination effect of vitamin K and vitamin D on human bone quality: a meta-analysis of randomized controlled trials. Food & Function . 2020;11(4):3280–3297. doi: 10.1039/C9FO03063H. [DOI] [PubMed] [Google Scholar]
- 44.Ziemińska M., Sieklucka B., Pawlak K. Vitamin K and D supplementation and bone health in chronic kidney disease—apart or together? Nutrients . 2021;13(3):809–834. doi: 10.3390/nu13030809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cohen L., Dean M., Shipov A., Atkins A., Monsonego-Ornan E., Shahar R. Comparison of structural, architectural, and mechanical aspects of cellular and acellular bone in two teleost fish. The Journal of Experimental Biology . 2012;215(11):1983–1993. doi: 10.1242/jeb.064790. [DOI] [PubMed] [Google Scholar]
- 46.Lavajoo F., Perelló-Amorós M., Vélez E. J., et al. Regulatory mechanisms involved in muscle and bone remodeling during refeeding in gilthead sea bream. Scientific Reports . 2020;10(1):184–214. doi: 10.1038/s41598-019-57013-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rafael M. S., Laizé V., Cancela M. L. Identification of Sparus aurata bone morphogenetic protein 2: molecular cloning, gene expression and in silico analysis of protein conserved features in vertebrates. Bone . 2006;39(6):1373–1381. doi: 10.1016/j.bone.2006.06.021. [DOI] [PubMed] [Google Scholar]
- 48.Cavaco S., Williamson M. K., Rosa J., et al. Teleost fish osteocalcin 1 and 2 share the ability to bind the calcium mineral phase. Fish Physiology and Biochemistry . 2014;40(3):731–738. doi: 10.1007/s10695-013-9880-9. [DOI] [PubMed] [Google Scholar]
- 49.Vieira F. A., Thorne M. A. S., Stueber K., et al. Comparative analysis of a teleost skeleton transcriptome provides insight into its regulation. General and Comparative Endocrinology . 2013;191:45–58. doi: 10.1016/j.ygcen.2013.05.025. [DOI] [PubMed] [Google Scholar]
- 50.Saleh R., Betancor M. B., Roo J., Benítez-Santana T., Zamorano M. J., Izquierdo M. Biomarkers of bone development and oxidative stress in gilthead sea bream larvae fed microdiets with several levels of polar lipids and α-tocopherol. Aquaculture Nutrition . 2015;21(3):341–354. doi: 10.1111/anu.12166. [DOI] [Google Scholar]
- 51.Fernández A. L., El-Diasty M. M., Martínez A., Alvarez J., García-Bengochea J. B. A simple technique to rule out occlusion of right coronary artery after aortic valve surgery. The Annals of Thoracic Surgery . 2011;92(6):2281–2282. doi: 10.1016/j.athoracsur.2011.06.016. [DOI] [PubMed] [Google Scholar]
- 52.Riera-Heredia N., Vélez E. J., Gutiérrez J., Navarro I., Capilla E. Gene expression analyses in malformed skeletal structures of gilthead sea bream (Sparus aurata) Journal of Fish Diseases . 2019;42(8):1169–1180. doi: 10.1111/jfd.13019. [DOI] [PubMed] [Google Scholar]
- 53.Roy P. K., Lall S. P. Vitamin K deficiency inhibits mineralization and enhances deformity in vertebrae of haddock (Melanogrammus aeglefinus L.) Comparative Biochemistry and Physiology. Part B, Biochemistry & Molecular Biology . 2007;148(2):174–183. doi: 10.1016/j.cbpb.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 54.Haga Y., Takeuchi T., Murayama Y., Ohta K., Fukunaga T. Vitamin D3 compounds induce hypermelanosis on the blind side and vertebral deformity in juvenile Japanese flounder Paralichthys olivaceus. Fisheries Science . 2004;70(1):59–67. doi: 10.1111/j.1444-2906.2003.00771.x. [DOI] [Google Scholar]
- 55.Domínguez D., Robaina L., Zamorano M. J., Karalazos V., Izquierdo M. Effects of zinc and manganese sources on gilthead seabream (Sparus aurata) fingerlings. Aquaculture . 2019;505:386–392. doi: 10.1016/j.aquaculture.2019.03.004. [DOI] [Google Scholar]
- 56.Graff I. E., Høie S., Totland G. K., Lie Ø. Three different levels of dietary vitamin D3 fed to first-feeding fry of Atlantic salmon (Salmo solar L.): effect on growth, mortality, calcium content and bone formation. Aquaculture Nutrition . 2002;8(2):103–111. doi: 10.1046/j.1365-2095.2002.00197.x. [DOI] [Google Scholar]
- 57.Sivagurunathan U., Dominguez D., Tseng Y., Zamorano M. J., Prabhu A. J., Izquierdo M. Deficiency and excess in dietary vitamin K3 negatively affect gilthead seabream (Sparus aurata) larvae performance and bone health. Aquaculture . 2023;574, article 739646 doi: 10.1016/j.aquaculture.2023.739646. [DOI] [Google Scholar]
- 58.Tseng Y., Eryalçın K. M., Sivagurunathan U., et al. Effects of the dietary supplementation of copper on growth, oxidative stress, fatty acid profile and skeletal development in gilthead seabream (Sparus aurata) larvae. Aquaculture . 2023;568, article 739319 doi: 10.1016/j.aquaculture.2023.739319. [DOI] [Google Scholar]
- 59.Graff I. E., Waagbo R., Fivelstad S., Vermeer C., Lie O., Lundebye A. K. A multivariate study on the effects of dietary vitamin K, vitamin D3 and calcium, and dissolved carbon dioxide on growth, bone minerals, vitamin status and health performance in smolting Atlantic salmon Salmo salar L. Journal of Fish Diseases . 2002;25(10):599–614. doi: 10.1046/j.1365-2761.2002.00403.x. [DOI] [Google Scholar]
- 60.Zhang L., Li J., Mai K., et al. Effects of different dietary vitamin D contents on growth performance, calcium and phosphorus metabolism of juvenile Japanese seabass (Lateolabrax japonicas) Chinese Journal of Animal Nutrition . 2016;28(5):1402–1411. [Google Scholar]
- 61.Luqman S., Fatima M., Shah S. Z. H., Afzal M., Bilal M. Concurrent supplementation effects of dietary formic acid and vitamin D3 on the growth, digestive enzyme activities and bone mineralization of pond cultured Cirrhinus mrigala fingerlings. Aquaculture Nutrition . 2021;27(6):2629–2636. doi: 10.1111/anu.13390. [DOI] [Google Scholar]
- 62.Krossøy C., WaagbØ R., Fjelldal P. G., et al. Dietary menadione nicotinamide bisulphite (vitamin K3) does not affect growth or bone health in first-feeding fry of Atlantic salmon (Salmo salar L.) Aquaculture Nutrition . 2009;15(6):638–649. doi: 10.1111/j.1365-2095.2008.00633.x. [DOI] [Google Scholar]
- 63.Shao R., Liu J., Lan Y., et al. Vitamin D impacts on the intestinal health, immune status and metabolism in turbot (Scophthalmus maximus L.) British Journal of Nutrition . 2022;128(11):2083–2096. doi: 10.1017/S0007114522000125. [DOI] [PubMed] [Google Scholar]
- 64.Calvo M. S., Whiting S. J., Barton C. N. Overview of the Proceedings from Experimental Biology 2004 Symposium: Vitamin D Insufficiency: A Significant Risk Factor in Chronic Diseases and Potential Disease-Specific Biomarkers of Vitamin D Sufficiency. The Journal of Nutrition . 2005;135(2):301–303. doi: 10.1093/jn/135.2.301. [DOI] [PubMed] [Google Scholar]
- 65.Cusano N. E., Thys-Jacobs S., Bilezikian J. P. Hypercalcemia due to vitamin D toxicity. Vitamin D . 2018:507–526. doi: 10.1016/B978-0-12-809963-6.00082-1. [DOI] [Google Scholar]
- 66.Chen H., Li J., Wang Q. Associations between bone-alkaline phosphatase and bone mineral density in adults with and without diabetes. Medicine . 2018;97(17, article e0432) doi: 10.1097/MD.0000000000010432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sinha K. M., Zhou X. Genetic and molecular control of osterix in skeletal formation. Journal of Cellular Biochemistry . 2013;114(5):975–984. doi: 10.1002/jcb.24439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fraser J. D., Price P. A. Induction of matrix Gla protein synthesis during prolonged 1, 25-dihydroxyvitamin D3 treatment of osteosarcoma cells. Calcified Tissue International . 1990;46(4):270–279. doi: 10.1007/BF02555007. [DOI] [PubMed] [Google Scholar]
- 69.Zhang J., Ma Z., Yan K., Wang Y., Yang Y., Wu X. Matrix gla protein promotes the bone formation by up-regulating Wnt/β-catenin signaling pathway. Frontiers in Endocrinology . 2019;10:p. 891. doi: 10.3389/fendo.2019.00891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pinto J. P., Conceição N., Gavaia P. J., Cancela M. L. Matrix Gla protein gene expression and protein accumulation colocalize with cartilage distribution during development of the teleost fish Sparus aurata. Bone . 2003;32(3):201–210. doi: 10.1016/S8756-3282(02)00981-X. [DOI] [PubMed] [Google Scholar]
- 71.Diegel C. R., Hann S., Ayturk U. M., et al. An osteocalcin-deficient mouse strain without endocrine abnormalities. PLoS Genetics . 2020;16(5, article e1008361) doi: 10.1371/journal.pgen.1008361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wolf G. Function of the bone protein osteocalcin: definitive evidence. Nutrition Reviews . 1996;54(10):332–333. doi: 10.1111/j.1753-4887.1996.tb03798.x. [DOI] [PubMed] [Google Scholar]
- 73.Aguayo-Ruiz J. I., García-Cobián T. A., Pascoe-González S., et al. Effect of supplementation with vitamins D3 and K2 on undercarboxylated osteocalcin and insulin serum levels in patients with type 2 diabetes mellitus: a randomized, double-blind, clinical trial. Diabetology and Metabolic Syndrome . 2020;12(1):p. 73. doi: 10.1186/s13098-020-00580-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Anderson P. H., Atkins G. J. The skeleton as an intracrine organ for vitamin D metabolism. Molecular Aspects of Medicine . 2008;29(6):397–406. doi: 10.1016/j.mam.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 75.Finnes T. E., Lofthus C. M., Meyer H. E., et al. A combination of low serum concentrations of vitamins K1 and D is associated with increased risk of hip fractures in elderly Norwegians: a NOREPOS study. Osteoporosis International . 2016;27(4):1645–1652. doi: 10.1007/s00198-015-3435-0. [DOI] [PubMed] [Google Scholar]
- 76.Katsuyama H., Saijoh K., Otsuki T., Tomita M., Fukunaga M., Sunami S. Menaquinone-7 regulates gene expression in osteoblastic MC3T3E1 cells. International Journal of Molecular Medicine . 2007;19(2):279–284. doi: 10.3892/ijmm.19.2.279. [DOI] [PubMed] [Google Scholar]
- 77.Tong Z., Qi-you X., Hong X., Chang-an W., Lian-yu S. Effects of dietary vitamin D3 supplementation on body composition and activity of alkaline phosphatase in the serum of juvenile mirror carp (C. carpio Songpu mirror carp) Acta Agriculturae Boreali-Sinica . 2011;26(Supplement 1):258–263. [Google Scholar]
- 78.Udagawa M. The effect of parental vitamin K deficiency on bone structure in mummichog Fundulus heteroclitus. Journal of the World Aquaculture Society . 2004;35(3):366–371. doi: 10.1111/j.1749-7345.2004.tb00100.x. [DOI] [Google Scholar]
- 79.Abbink W., Flik G. Parathyroid hormone-related protein in teleost fish. General and Comparative Endocrinology . 2007;152(2-3):243–251. doi: 10.1016/j.ygcen.2006.11.010. [DOI] [PubMed] [Google Scholar]
- 80.Boisen I. M., Nielsen J. E., Verlinden L., et al. Calcium transport in male reproduction is possibly influenced by vitamin D and CaSR. The Journal of Endocrinology . 2021;251(3):207–222. doi: 10.1530/JOE-20-0321. [DOI] [PubMed] [Google Scholar]
- 81.Hang X. M., Power D., Flik G., Balment R. J. Measurement of PTHrP, PTHR1, and CaSR expression levels in tissues of sea bream (Sparus aurata) using quantitative PCR. Annals of the New York Academy of Sciences . 2005;1040(1):340–344. doi: 10.1196/annals.1327.056. [DOI] [PubMed] [Google Scholar]
- 82.Abbink W., Hang X. M., Guerreiro P. M., et al. Parathyroid hormone-related protein and calcium regulation in vitamin D-deficient sea bream (Sparus auratus) The Journal of Endocrinology . 2007;193(3):473–480. doi: 10.1677/JOE-06-0164. [DOI] [PubMed] [Google Scholar]
- 83.Cheng D., Hassan M. M., Ma Z., Yang Q., Qin J. G. Skeletal ontogeny and anomalies in larval and juvenile crimson snapper, Lutjanus erythropterus bloch, 1790. Pakistan Journal of Zoology . 2018;50(3):799–807. doi: 10.17582/journal.pjz/2018.50.3.799.807. [DOI] [Google Scholar]
- 84.Sivagurunathan U., Srivastava P. P., Gupta S., Krishna G. Responses of Corpuscles of Stannius to intra-peritoneal vitamin- D3 administration in teleost Labeo rohita (Hamilton, 1822) reared in water with two different levels of calcium concentration. Saudi Journal of Biological Sciences . 2020;27(12):3593–3600. doi: 10.1016/j.sjbs.2020.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yang C., Du X., Hao R., Wang Q., Deng Y., Sun R. Effect of vitamin D3 on immunity and antioxidant capacity of pearl oyster Pinctada fucata martensii after transplantation: insights from LC–MS-based metabolomics analysis. Fish & Shellfish Immunology . 2019;94:271–279. doi: 10.1016/j.fsi.2019.09.017. [DOI] [PubMed] [Google Scholar]
- 86.Teshima S. Microparticulate diets for the larvae of aquatic animals. Min. Rev. Data File Fish. Res. . 1982;2:67–86. [Google Scholar]
- 87.Kanazawa A., Koshio S., Teshima S. I. Growth and survival of larval red sea bream Pagrus major and Japanese flounder Paralichthys olivaceus fed microbound diets. Journal of the World Aquaculture Society . 1989;20(2):31–37. doi: 10.1111/j.1749-7345.1989.tb00521.x. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to support the findings of this study are included in the article. However, more data could be available from the corresponding author upon request.


