ABSTRACT
Background
Various glomerular pathologies have been reported in patients who have undergone haematopoietic stem cell transplantation (HSCT), but the data on clinico-pathological correlations and clinical outcome remain limited.
Methods
We analysed the clinical and histopathological data of patients who had biopsy-proven de novo glomerular diseases after HSCT since 1999.
Results
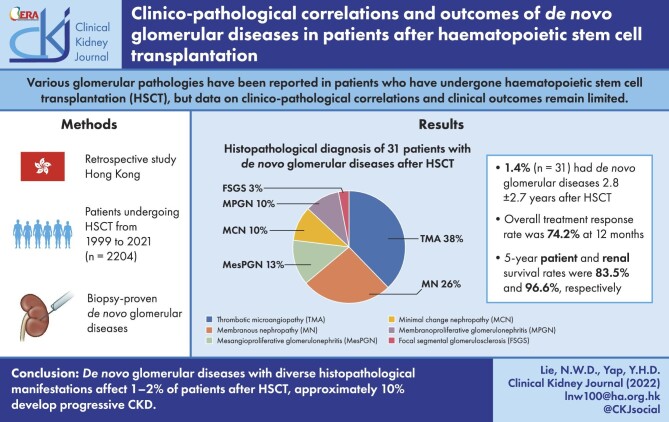
A total of 2204 patients underwent HSCT during the period 1999–2021, and 31 patients (1.4%) developed de novo glomerular diseases after a mean duration of 2.8 ± 2.7 years after HSCT. Fifteen of these patients (48.4%) had graft-versus-host-disease prior to or concomitant with renal abnormalities. Proteinuria and eGFR at the time of kidney biopsy were 4.1 ± 5.3 g/day and 50.8 ± 25.4 mL/min/1.73 m2, respectively. Kidney histopathologic diagnoses included thrombotic microangiopathy (TMA) (38.7%), membranous nephropathy (MN) (25.8%), mesangial proliferative glomerulonephritis (12.9%), minimal change disease (9.7%), focal segmental glomerulosclerosis (9.7%) and membranoproliferative glomerulonephritis (3.2%). Immunosuppressive treatment was given to patients who presented with nephrotic-range proteinuria and/or acute kidney injury, while renin–angiotensin–aldosterone blockade was given to all patients with proteinuria ≥1 g/day, with complete and partial response rates of 54.8% and 19.4%, respectively. One patient with TMA progressed to end-stage kidney disease after 24 weeks, and two patients, one with TMA and one with MN, (6.4%) progressed to chronic kidney disease (CKD) Stage ≥3. Kidney and patient survival rates were 96.6% and 83.5%, respectively, at 5 years.
Conclusion
De novo glomerular diseases with diverse histopathologic manifestations affect 1.4% of patients after HSCT, and approximately 10% develop progressive CKD.
Keywords: glomerulonephritis, haematopoietic stem cell transplantation, outcomes
Graphical Abstract
Graphical Abstract.
INTRODUCTION
Haematopoietic stem cell transplantation (HSCT) is an increasingly important treatment for malignant or non-malignant haematological conditions, severe congenital immunodeficiency syndromes and refractory autoimmune disorders [1]. HSCT is a major medical procedure that is associated with many short- and long-term complications [2]. With growing patient numbers and improved survival after HSCT, various long-term sequelae including uncommon complications continue to emerge. Kidney dysfunction after HSCT can result from toxicities of chemotherapeutic or antimicrobial agents, irradiation, excessive calcineurin inhibitor exposure or graft-versus-host disease (GVHD) [3]. De novo glomerular diseases are uncommon, and various renal histopathologic features have been reported including renal thrombotic microangiopathy (TMA), membranous nephropathy (MN), mesangial proliferative glomerulonephritis (MesPGN), minimal change nephropathy (MCN), focal segmental glomerulosclerosis (FSGS) and membranoproliferative glomerulonephritis (MPGN) [4–7]. The literature to date comprises mostly anecdotal reports or autopsy findings [7, 8]. The overall incidence, relative preponderance of histopathologic varieties and clinical outcome of this uncommon complication remain uncertain [4, 5, 7].
MATERIALS AND METHODS
Patients
We retrospectively reviewed the records of all patients who underwent HSCT at the Bone Marrow Transplantation Unit of Queen Mary Hospital, Hong Kong, from 1999 to 2021. Patients with de novo glomerular diseases confirmed with kidney biopsy were included for analysis. Data were retrieved from electronic patient records. This study was approved by the HKU/HKWC Institutional Review Board (Reference number: HKU/HKWC IRB UW-18-656).
Conditioning and maintenance treatment after HSCT
Since the 1990s, we adopted standardized conditioning immunosuppressive protocols based on the underlying haematological disease, type of HSCT and the patient's general state. For autologous HSCT, conditioning therapy for patients with multiple myeloma was single-agent melphalan, and for patients with non-Hodgkin's lymphoma were Big cyclophosphamide/carmustine/etoposide (Big-CBV) or carmustine/etoposide/cytarabine/melphalan (BEAM). For allogeneic HSCT, standardized conditioning regimens are used ranging from myeloablative to reduced intensity for different donor types.
Anti-GVHD immunosuppression was used in patients who underwent allogeneic HSCT. Human leucocyte antigen (HLA)-matched sibling HSCT patients were maintained on single-agent cyclosporin A (CYA) at 1–1.5 mg/kg/day with twice daily dosing and tapered off after 3 months. For unrelated donor (URD) HSCT, maintenance therapy comprised CYA (target 12-h trough level ∼250–300 µg/L), mycophenolate mofetil (MMF, 500 mg twice daily) and methotrexate (15 mg on day 1, 10 mg on Days 3, 6 and 11 post-transplant). Corticosteroids were not routinely used unless the patient developed GVHD, in whom methylprednisolone (MP) was commenced at 2 mg/kg/day followed by prednisolone (PRED, 1 mg/kg/day) tapered at 5-10 mg per week depending on clinical progress.
Follow-up and monitoring schedule
Patients were seen weekly after discharge for 4–6 weeks; after that the follow-up intervals were gradually increased according to clinical status. At each clinic visit, complete blood counts, liver and renal biochemistry, urine protein-to-creatinine ratio and 24-h urine protein levels were monitored. Clinically significant events were documented.
Renal assessment and management
Patients presenting with renal abnormalities were referred to nephrologists for assessment. All patients referred underwent ultrasound of the kidneys performed by radiologists. The indications for kidney biopsy included proteinuria ≥1 g/day or acute kidney injury (AKI) without identifiable causes. Kidney biopsy was performed within 2 weeks from the onset of renal abnormalities. Renin–angiotensin–aldosterone system (RAAS) blockade was used in patients with persistent proteinuria ≥1 g/day despite satisfactory blood pressure control. Patients with biopsy-proven TMA were treated with PRED, starting dose 1 mg/kg/day then tapered to reach 5–7.5 mg/day after 6 months with or without MMF (500–1000 mg/day) for steroid-sparing. Patients with crescentic MesPGN were given PRED in combination with intravenous cyclophosphamide 1 g monthly for six doses or oral cyclophosphamide 50 mg daily for 6 months. Patients with MN were treated with PRED and CYA or tacrolimus. Patients with MCN were treated with PRED, starting dose 0.8–1 mg/kg/day then tapered to reach 5–7.5 mg/day at approximately 4 months. Immunosuppressive treatment was given in tapering dose for a minimum of 26 weeks. Patients with systemic thrombotic thrombocytopenic purpura associated with low ADAMTS-13 activity were treated with plasmapheresis with or without anti-CD20. Patients with FSGS were put on maximally tolerated RAAS blockade.
Outcome measures and statistical analysis
De novo glomerular diseases were defined as the occurrence of biopsy proven glomerulonephritis after HSCT. Patients with known history of glomerular diseases were excluded from analysis. AKI was defined according to the KDIGO guideline, namely an increase in serum creatinine by ≥26 µmol/L within 48 h or >1.5 times from the baseline within the last 7 days. Complete renal remission (CR) was defined as 24-h urine protein <0.5 g/day and serum creatinine not higher than 10% above baseline. Partial renal remission (PR) was defined as 24-h urine protein reduced by ≥50% and within the range of 0.5–1.0 g/day and stable serum creatinine as defined. Other outcome parameters included new-onset chronic kidney disease (CKD) Stage 3 or above, and end-stage kidney disease (ESKD) defined as Stage 5 CKD or continued requirement for renal replacement therapy, mortality and cause of death. Continuous variables were expressed as mean (standard deviation) or median (range), and analysed with Student's t-test or Mann–Whitney test where appropriate. Categorical variables were expressed as frequency (percentages) and analysed by Chi square test or Fisher's exact test where appropriate. Patient and renal survival was estimated by Kaplan–Meier method. All statistical analyses were performed by SPSS (Version 24) and P-values <.05 were considered statistically significant.
RESULTS
Patient characteristics
A total of 2204 patients underwent HSCT during the period 1 January 1999 to 31 December 2021. A total 112 patients were indicated for kidney biopsy and 35 actually underwent the procedure. The reasons for not performing kidney biopsy in the remaining 77 patients were largely related to improvement in renal function, technical difficulties of procedure, high bleeding risk or the presence of multiple renal cysts. Thirty-one patients (1.4%) showed de novo glomerular diseases, accounting for 88.6% of all biopsy samples (Table 1). These patients were followed for a mean duration of 91.0 ± 76.7 months (2820 patient-months) after the presentation of renal abnormality. The mean durations until glomerular disease onset were 2.16 ± 2.36 years for TMA, 3.84 ± 3.21 years for MN, 4.08 ± 3.51 years for MesPGN/MPGN, 2.30 ± 2.23 years for MCD and 2.70 ± 3.5 years for FSGS. The underlying haematological conditions for these patients included leukaemia (61.3%), lymphoma (16.1%), myeloma (6.4%) and other miscellaneous diseases. HSCT donor type was URD in 12 patients (38.7%), matched siblings in 10 patients (32.3%), autologous in 6 patients (19.4%) and haploidentical related donor in 3 (9.6%). The number of mismatched HLA was 0.68 ± 1.25. Sixteen patients (51.6%) received total body irradiation (TBI) in conditioning regimen. Fifteen of the 31 patients with kidney abnormalities (48.4%) had history of GVHD, of which 12 were prior to while three were concomitant with the occurrence of renal abnormalities.
Table 1:
Clinical characteristics of 31 patients who underwent HSCT and then developed de novo glomerulonephritis.
| Age (years) | 49.0 ± 14.3 |
| Sex (M/F) | 19/12 |
| Underlying haematological disorders, n (%) | |
| ALL | 8 (25.8) |
| AML | 9 (29.0) |
| Lymphoma | 5 (16.1) |
| MDS/SAA | 2 (6.4) |
| Multiple myeloma | 2 (6.4) |
| CML | 2 (6.4) |
| Others | 3 (9.7) |
| Type of HSCT | |
| URD | 12 (38.7) |
| HLA-matched sibling | 10 (32.3) |
| Haploidentical related | 3 (9.6) |
| Autologous | 6 (19.4) |
| Number of HLA mismatches | 0.68 ± 1.25 |
| Conditioning regimen, n (%) | |
| Bu-CYC | 7 (22.6) |
| CYC-TBI | 11 (35.5) |
| Mini-TBI | 2 (6.4) |
| Flu-CYC | 3 (9.7) |
| Big CBV | 2 (6.4) |
| Others | 6 (19.4) |
| Maintenance regimen, n (%) | |
| PRED + CYA ± MMF | 7 (22.6) |
| CYA + MMF | 8 (25.8) |
| PRED alone | 3 (9.7) |
| Others | 13 (41.9) |
| Presence of GVHD, n (%) | 15 (48.4) |
| Time to GN (years) | 2.8 ± 2.7 |
| Renal histology, n (%) | |
| TMA | 12 (38.7) |
| MN | 8 (25.8) |
| MesPGN | 4(12.9) |
| MCN | 3 (9.7) |
| FSGS | 3 (9.7) |
| MPGN | 1 (3.2) |
| Patient demographics and medical comorbidities | |
| Body weight (kg) | 63.1 ± 14.1 |
| Diabetes mellitus, n (%) | 6 (19.4) |
| Hypertension, n (%) | 11 (35.5) |
| Presence of monoclonal gammopathy, n (%) | 1 (3.2) |
| Urinary protein prior to development of renal disease (g/day) | 0.24 ± 0.42 |
| SCr level prior to development of renal disease (µmol/L) | 99 ± 37 |
| Use of anti-hypertensives (including RAAS blockade), n (%) | |
| None | 20 (64.5) |
| One anti-hypertensive | 8 (25.8) |
| Two anti-hypertensives | 3 (9.7) |
| Use of RAAS blockade | 2 (6.5) |
| Renal parameters at presentation | |
| eGFR (mL/min/1.73 m2) | 50.8 ± 25.4 |
| Urinary protein excretion (g/day) | 4.1 ± 5.3 |
| Haemoglobin level (g/dL) | 11.3 ± 2.1 |
| Platelet count (×109/L) | 169.4 ± 94.8 |
Data are presented as mean ± SD or n (%).
ALL, acute lymphocytic leukemia; AML, acute myeloid leukemia; BEAM, carmustine/etoposide/cytarabine/melphalan; Bu, busulphan; CBV, cyclophosphamide/carmustine/etoposide; CML, chronic myeloid leukemia; CYC, cyclophosphamide; MDS, myelodysplastic syndrome; RAAS, renin–angiotensin–aldosterone system; SAA, severe aplastic anemia; SCr, serum creatinine.
Clinico-pathological correlations
De novo glomerular diseases occurred at a mean duration of 2.8 ± 2.7 years after HSCT. Five cases (16%) of de novo glomerular diseases occurred within 6 months after HSCT (i.e. early onset) and the histological diagnosis were renal TMA (n = 2), MesPGN (n = 1), MCN (n = 1) and FSGS (n = 1). The remaining 26 cases (84%) occurred over 6 months after HSCT (renal TMA, n = 10; MesPGN, n = 3, MPGN, n = 1, MCN, n = 2; and FSGS, n = 2). Twenty-seven patients (87.1%) presented with proteinuria ≥1 g/day, and 12 (38.7%) had nephrotic-range proteinuria. Four patients (12.9%) presented with proteinuria <1 g/day. Eighteen patients (58.1%) presented with AKI, but none required dialysis. Nine patients (29%) had microscopic hematuria and one patient (3%) had leukocyturia on presentation. All patients had ultrasound assessment before kidney biopsies, and none had evidence of urinary tract obstruction in our cohort. Proteinuria, serum creatinine level and eGFR at the time of kidney biopsy were 4.1 ± 5.3 g/day, 151.1 ± 69.3 µmol/L and 50.8 ± 25.4 mL/min/1.73 m2 respectively. The histological diagnoses included renal TMA in 12 patients (38.7%), MN in 8 patients (25.8%), MesPGN in 4 patients (12.9), MCN in 3 patients (9.7%), FSGS in 3 patients (9.7%) and MPGN in 1 patient (3.2%) (Table 1). The MesPGN and MPGN cases in the cohort are of immune-complex type. De novo glomerulonephritis of various histological diagnosis showed no relationship with the type of HSCT (P > .05, for all). Proteinuria at kidney biopsy amounted to 2.5 ± 1.8 g/day, 5.2 ± 3.2 g/day, 1.5 ± 0.6 g/day, 14.2 ± 12.8 g/day and 2.0 ± 2.1 g/day in the TMA, MN, MesPGN/MPGN, MCN and FSGS groups, respectively; eGFR was 39.3 ± 19.6 mL/min/1.73 m2, 72.5 ± 23.0 mL/min/1.73 m2, 47.4 ± 19.5 mL/min/1.73 m2, 43.7 ± 19.4 mL/min/1.73 m2 and 52.3 ± 42.1 mL/min/1.73 m2, respectively (Table 1). Two patients (16.7%) with renal TMA also showed systemic TMA. The renal TMA changes were primarily glomerular and without significant vascular involvement.
Among 12 patients with TMA, 10 patients showed negative direct immunofluorescence (IF) staining for IgG, IgA, IgM, C3 and C1q, while the other 2 patients with TMA had trace C3 granular capillary loop deposits on IF staining. On electron microscopy, 10 patients had mild to moderate effacement of foot processes. For histological diagnosis with MN, the majority (seven out of eight) had IgG deposits along the capillary loops. One had negative IgG staining on IF but was compatible with MN due to presence of subepithelial electron dense deposition. All the four MesPGN had an increase in mesangial matrix and cellularity with either scanty IgM, C3 or IgA staining on IF.
Short- and long-term clinical outcomes
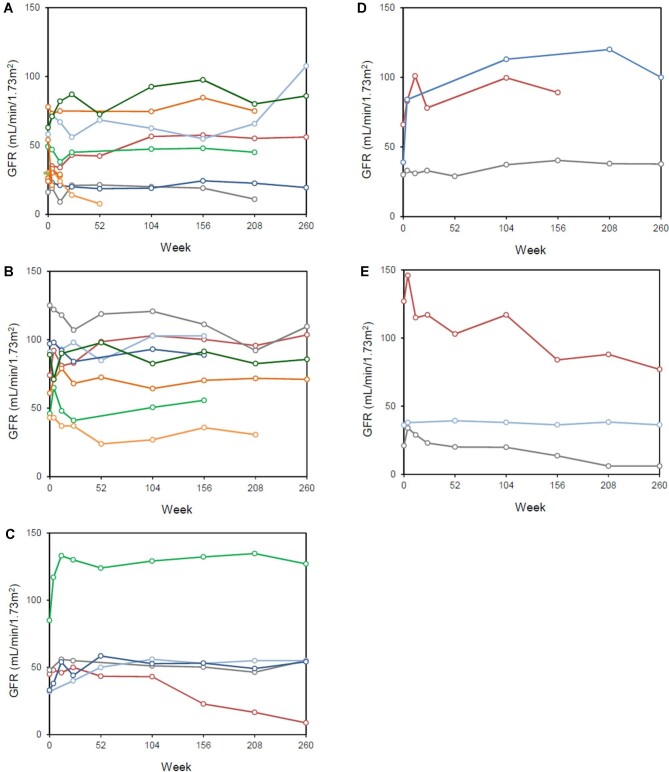
Immunosuppressive treatment was added for the glomerular diseases in 16 patients (51.6%). The mean duration of immunosuppressive treatments was 13.4 ± 7.1 months. The rate of CR and PR after 12 months was 54.8% and 19.4%, respectively, for the entire cohort; the time to achieve PR was 2.6 ± 0.9 months while that for CR was 5.1 ± 3.7 months. The 12-month CR rate was 41.6%, 75.0%, 60.0%, 66.7% and 33.3% in patients with renal TMA, MN, MesPGN/MPGN, MCN and FSGS, respectively, with no significant difference between groups (P > .05, for all) (Table 2). The 12-month PR rate was 16.6%, 25.0%, 0%, 33.3% and 33.3% in the corresponding groups, respectively, also with no between-group difference (P > .05, for all) (Table 2). The overall response rate (CR + PR) for the entire cohort was 74.2%. Serial changes in eGFR and proteinuria according to different glomerular pathologies were shown in Fig. 1 and Fig. 2. In responding patients, proteinuria reduction occurred within the first 6 months and was sustained during follow-up of approximately 5 years. None of the patients showed a significant increase of proteinuria during follow-up.
Table 2:
Clinical outcomes of patients who developed de novo glomerular diseases after HSCT.
| Renal parameters at presentation | Clinical outcomes | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient | Renal histology | Age at renal presentation (years) | Haematological disease | HSCT | Donor type | Onset after HSCT (years) | SCr (µmol/L) | eGFR (mL/min/1.73 m2) | 24-h UP (g/day) | Management | CR | PR | NR | Stage ≥3 CKD/ESKD | Death |
| 1 | Renal TMA | 29 | Leukaemia | Allo | URD | 1.2 | 274 | 25 | 0.3 | RAAS blockade | Y | N | N | ||
| 2 | Renal TMA | 43 | Leukaemia | Allo | URD | 1.0 | 102 | 57 | 1.89 | RAAS blockade | Y | N | N | ||
| 3 | Renal TMA | 38 | Lymphoma | Allo | URD | 2.2 | 218 | 24 | 0.23 | Continue MMF; ↓CsA | Y | N | N | ||
| 4 | Renal TMA | 69 | Myeloma | Auto | 1.9 | 101 | 49 | 6.6 | Stop pomalidomide and observe | Y | N | N | |||
| 5 | Renal TMA | 50 | SAA | Allo | URD | 7.0 | 97 | 78 | 2.75 | MMF | Y | N | N | ||
| 6 | Renal TMA | 49 | Leukaemia | Allo | Sibling | 1.5 | 216 | 28 | 4.63 | RAAS blockade | Y | Y | N | ||
| 7 | Renal TMA | 54 | Leukaemia | Allo | Sibling | 1.7 | 344 | 16 | 2 | MMF + anti-CD20; stopped CYA | Y | N | N | ||
| 8 | Renal TMA | 32 | Leukaemia | Allo | URD | 7.1 | 129 | 61 | 1.68 | PLEX + anti-CD20 | Y | N | Y | ||
| 9 | Renal TMA | 66 | Leukaemia | Allo | Haploidentical | 0.7 | 157 | 30 | 2.97 | PRED | Y | N | N | ||
| 10 | Renal TMA | 56 | Lymphoma | Allo | Haploidentical | 0.7 | 240 | 26 | 2.0 | RAAS blockade | Y | N | N | ||
| 11 | Renal TMA | 65 | Leukaemia | Allo | Haploidentical | 0.5 | 195 | 23 | 2.8 | PRED + MMF | Y | N | N | ||
| 12 | Renal TMA | 48 | Leukaemia | Allo | Sibling | 0.4 | 131 | 54 | 1.62 | PRED+↓CYA | Y | Y | Y | ||
| 13 | MN | 50 | Leukaemia | Allo | Sibling | 1.6 | 101 | 71 | 3.13 | PRED + CYA | Y | N | N | ||
| 14 | MN | 27 | Lymphoma | Allo | URD | 2.0 | 67 | 100 | 5.45 | PRED + CYA | Y | N | N | ||
| 15 | MN | 49 | Leukaemia | Allo | URD | 4.5 | 68 | 89 | 2.26 | PRED + CYA + MMF | Y | N | Y | ||
| 16 | MN | 59 | Leukaemia | Allo | URD | 2.7 | 112 | 46 | 4.42 | PRED + MMF | Y | N | N | ||
| 17 | MN | 32 | Leukaemia | Allo | URD | 5.0 | 133 | 55 | 7.3 | ↑MMF and continue CYA | Y | N | N | ||
| 18 | MN | 39 | Leukaemia | Allo | Sibling | 11.0 | 93 | 81 | 1.05 | RAAS blockade | Y | N | N | ||
| 19 | MN | 63 | Leukaemia | Allo | URD | 3.0 | 67 | 97 | 10.55 | RAAS blockade | Y | N | N | ||
| 20 | MN | 54 | MDS | Allo | Sibling | 0.9 | 157 | 41 | 7.62 | PRED + CsA + MMF | Y | Y | Y | ||
| 21 | MesPGN | 39 | Leukaemia | Allo | Sibling | 4.2 | 155 | 42 | 1.4 | RAAS blockade | Y | N | N | ||
| 22 | MesPGN | 19 | Systemic sclerosis | Auto | 7.3 | 86 | 81 | 2.12 | PRED, CYC, FK, RAAS blockade | Y | N | N | |||
| 23 | MesPGN | 52 | Lymphoma | Auto | 0.3 | 195 | 30 | 0.61 | PRED + CYC | Y | N | Y | |||
| 24 | MesPGN | 12 | JIA | Auto | 0.8 | 226 | 40 | 1.5 | RAAS blockade | Y | N | N | |||
| 25 | MPGN | 53 | Lymphoma | Auto | 7.8 | 151 | 44 | 2 | PRED | Y | N | N | |||
| 26 | MCN | 45 | Leukaemia | Allo | URD | 1.9 | 116 | 65 | 8.5 | PRED | Y | N | N | ||
| 27 | MCN | 40 | Leukaemia | Allo | Sibling | 4.7 | 230 | 27 | 5.25 | PRED + RAAS blockade | Y | N | N | ||
| 28 | MCN | 6 | Beta Thalassemia Major | Allo | Sibling | 0.3 | 107 | 39 | 28.85 | PRED + MMF + anti-CD20 | Y | N | N | ||
| 29 | FSGS | 13 | Leukaemia | Allo | URD | 6.7 | 47 | 100 | 1.37 | RAAS blockade | Y | N | N | ||
| 30 | FSGS | 62 | Myeloma | Auto | 1.2 | 212 | 20 | 4.27 | RAAS blockade | Y | N | N | |||
| 31 | FSGS | 35 | Leukaemia | Allo | Sibling | 0.2 | 158 | 37 | 0.22 | Observe | Y | N | N | ||
Auto, autologous; Allo, allogeneic; CYA, cyclosporine A; CYC, cyclophosphamide; FK, tacrolimus; JIA, juvenile idiopathic arthritis; MMF, mycophenolate mofetil; NR, no response; PLEX, plasmapheresis; PRED, prednisolone; RAAS, renin–angiotensin–aldosterone system; SAA, severe aplastic anemia; UP, urine protein; SCr, serum creatinine; URD, unrelated donor.
Figure 1:
Serial changes in eGFR in patients with (A) TMA, (B) MN, (C) MesPGN/MPGN, (D) MCN and (E) FSGS after HSCT.
Figure 2:
Serial changes in proteinuria in patients with (A) TMA, (B) MN, (C) MesPGN/MPGN, (D) MCN and (E) FSGS after HSCT.
Ten patients (32.3%) showed improvement in eGFR by 20%. Four patients (12.9%) showed progressive CKD. Two patients (one with renal TMA and one with MN) (6.4%) developed CKD Stage ≥3 and one patient with TMA progressed to ESKD after 6 months. The 5-year renal survival rate was 96.6% for the entire cohort. Five patients died (16.1%), three due to infections and two because of relapsed haematological malignancies. The 5-year patient survival rate was 83.5%.
DISCUSSION
While de novo glomerular diseases are a recognized complication after HSCT, the true incidence remains uncertain [4, 6, 9–11]. Results from the present series that included over 2000 patients showed that this complication was uncommon, occurring in 1.4% of patients, and the kidney histopathological changes were diverse, with variable renal outcomes. Nevertheless, the incidence rate was still substantially higher than that in the general population, which was in the range of ∼0.001%–0.0017% [4, 10, 12]. We found that renal TMA (38.7%) and MN (25.8%) were the most common renal histological diagnoses, similar to previous reports [4–8, 13–16]. Notably, de novo MPGN was rare after HSCT and literature on this entity was scarce. Our series included 3.2% (1 out of 31 cases) that showed MPGN features after HSCT. In another two series of both with 15 patients, one patient had MPGN after HSCT [17] and the other had none with MPGN changes [11], highlighting the rarity of this entity. With regard to clinical presentation, proteinuria was the most common abnormality, occurring in over 80% of patients, and almost half were in the nephrotic range. Proteinuria appeared more severe in our series compared with previous report [4, 6, 7, 9–12, 18, 19]. The histological diagnosis of high range proteinuria belonged to membranous nephropathy and minimal change disease. The specific findings for those individuals demonstrated marked effacement of foot processes on electron microscopy. AKI was present in 60% of our patients, similar to previous reports [20]. The clinical presentation of the different renal pathologies was similar to that in the non-HSCT or ‘idiopathic’ setting [21]. It is noteworthy that this is not necessarily an early presentation, as the mean time of onset of renal abnormalities was around 3 years after HSCT [6, 20]. Whether renal impairment is indeed more common in MCN following HSCT requires confirmation with further studies [21].
The pathogenesis of de novo glomerular diseases after HSCT remains unclear, although dysregulated immune responsiveness and autoimmunity are likely involved. The risks factors for developing de novo glomerular diseases in HSCT patients, and factors that determine which renal histological changes would manifest, are also poorly understood. Factors that have been proposed to affect renal histological changes include conditioning regimes, TBI, immunosuppressive agents and concurrent viral infections. Renal pathologies can be a manifestation of chronic GVHD [6, 22–25], as highlighted by the high percentage in our cohort (∼50%) and others (47%–72%) [5, 6, 22]. GVHD largely occurred prior to renal manifestation in 12 of the 15 patients, the earliest was 11 years prior to the occurrence of de novo GN. Three had GVHD concomitant with de novo GN. The findings concurred with other series [17, 26]. Immune complex–mediated injury during the course of GVHD may have a key role in the pathogenesis of membranous nephropathy [27, 28]. In this context, recent reduction of immunosuppression was associated with the development of nephrotic syndrome [22]. In patients with renal TMA, vascular endothelial injury due to chemotherapy, calcineurin inhibitors, TBI and sepsis could also be a contributory pathogenic factor [24, 29]. TMA represented the major cause of glomerulonephritis in our cohort for which 75% received TBI. TBI was suggested to induce vascular endothelial injury causing the development of TMA and this association between the two were demonstrated in our cohort [30].
Owing to the lack of high-quality evidence, the optimal management of de novo glomerular diseases after HSCT remains undefined. The treatment strategies in our cohort were largely based on experience in idiopathic cases in the non-HSCT setting [21]. Based on the severity of clinical and histopathological abnormalities, approximately half of our patients received immunosuppressive treatments, in addition to non-specific RAAS blockade for renoprotection where appropriate. It is encouraging to note CR and PR rates of 54.8% and 19.4%, respectively, with renal responses usually achieved within 6 months of treatment initiation and sustained during long-term follow-up. Relatively favourable outcomes were also reported by other investigators [6, 7, 22]. It is important to note that renal TMA or FSGS appeared to be associated with less favourable outcomes despite immunosuppressive treatment, in contrast to MN and MCN, for which all patients achieved CR or PR within 12 months [6, 7, 21, 22]. The overall renal survival in our series, with only one ESKD in a patient who presented with TMA and AKI, was relatively favourable compared with previous studies [4, 7]. Another TMA patient, who also presented with AKI, progressed to CKD Stage 3 after 1 year. Patient survival rates reported in previous studies were quite variable (30%–87%) [4, 6, 7, 31–33]. In the present study patient survival rate at 5 years was 85%, and the mortalities were unrelated to renal causes.
The dominant antigen in post-transplant membranous nephropathy was found to be associated with Protocadherin FAT1 [34]. The data on FAT-1 was lacking as the test was not available in our unit. Data for serum anti-PLA2R assay and PLA2R immunohistochemical staining was only recently available in our unit, which was not possible to do on histological blocks that have been discarded. One limitation of this study was that the quantification of proteinuria in earlier patients was by 24-h urine collection, which could be inaccurate, and that urine protein-to-creatinine ratio measurement has only become the standard in our cohort in recent years. Furthermore, the Modification of Diet in Renal Disease equation was used to assess GFR until Jun 2017, after which the CKD Epidemiology Collaboration formula was used. As the study spanned a period of 20 years, the bias from the change in formula affected most of the patients in this retrospective study. There is also possibility of loss of information during the long-term follow-up but the retrieval of data from electronic records has minimized the amount of missing data. The merit of this study was that our centre is the only centre for adult allogeneic HSCT in Hong Kong and was the only centre for autologous HSCT up to 2011. Hence, our data was complete and covered the entire territory with a population of 7.5 million over a long observation period especially with allogeneic HSCT. Also, all patients received standard conditioning protocols and anti-GVHD immunosuppression and clinical assessments. However, one should also appreciate that the induction regimens could have evolved over time as our study spans a long period of time (1999–2021). Notwithstanding, our results are a good representation of real-world data of de novo glomerular pathologies after HSCT and the data from over 2000 HSCT patients makes it one of the largest series of this uncommon disease entity to date.
CONCLUSION
De novo glomerular diseases affect 1%–2% of patients after HSCT, and show diverse histopathological changes with variable renal outcomes.
ACKNOWLEDGEMENTS
D.Y.H.Y. received research donations from the Wai Im Charitable Foundation and the Chan Sui Kau Family Benefits and Charitable Foundation. D.Y.H.Y. and T.M.C. received research funding support from the Mr and Mrs Tam Wing Fun Edmund Renal Research Fund. T.M.C. received research funding support from the Wai Hung Charitable Foundation and Mr S Ho.
Contributor Information
Desmond Y H Yap, Division of Nephrology, Department of Medicine, Queen Mary Hospital, The University of Hong Kong, Hong Kong.
Davina Lie, Division of Nephrology, Department of Medicine, Queen Mary Hospital, The University of Hong Kong, Hong Kong.
Tiffany Lau, Department of Pathology, Queen Mary Hospital, The University of Hong Kong, Hong Kong.
Alex Tang, Department of Pathology, Queen Mary Hospital, The University of Hong Kong, Hong Kong.
Gavin Chan, Department of Pathology, Queen Mary Hospital, The University of Hong Kong, Hong Kong.
Thomas S Y Chan, Division of Haematology, Medical Oncology and Haematopoietic Stem Cell Transplantation, Queen Mary Hospital, The University of Hong Kong, Hong Kong.
Joycelyn Sim, Division of Haematology, Medical Oncology and Haematopoietic Stem Cell Transplantation, Queen Mary Hospital, The University of Hong Kong, Hong Kong.
Albert K W Lie, Division of Haematology, Medical Oncology and Haematopoietic Stem Cell Transplantation, Queen Mary Hospital, The University of Hong Kong, Hong Kong.
Tak Mao Chan, Division of Nephrology, Department of Medicine, Queen Mary Hospital, The University of Hong Kong, Hong Kong.
DATA AVAILABILITY STATEMENT
The datasets used and analysed during the current study are available from the corresponding author on reasonable request.
CONFLICT OF INTEREST STATEMENT
The authors have no conflict of interest to declare.
REFERENCES
- 1. Duarte RF, Labopin M, Bader Pet al. Indications for haematopoietic stem cell transplantation for haematological diseases, solid tumours and immune disorders: current practice in Europe, 2019. Bone Marrow Transplant 2019;54:1525–52. [DOI] [PubMed] [Google Scholar]
- 2. Tabbara IA, Zimmerman K, Morgan Cet al. Allogeneic hematopoietic stem cell transplantation: complications and results. Arch Intern Med 2002;162:1558–66. [DOI] [PubMed] [Google Scholar]
- 3. Hingorani S. Chronic kidney disease in long-term survivors of hematopoietic cell transplantation: epidemiology, pathogenesis, and treatment. J Am Soc Nephrol 2006;17:1995–2005. [DOI] [PubMed] [Google Scholar]
- 4. Srinivasan R, Balow JE, Sabnis Set al. Nephrotic syndrome: an under-recognised immune-mediated complication of non-myeloablative allogeneic haematopoietic cell transplantation. Br J Haematol 2005;131:74–9. [DOI] [PubMed] [Google Scholar]
- 5. Chang A, Hingorani S, Kowalewska Jet al. Spectrum of renal pathology in hematopoietic cell transplantation: a series of 20 patients and review of the literature. Clin J Am Soc Nephrol 2007;2:1014–23. [DOI] [PubMed] [Google Scholar]
- 6. Hu SL. The role of graft-versus-host disease in haematopoietic cell transplantation-associated glomerular disease. Nephrol Dial Transplant 2011;26:2025–31. [DOI] [PubMed] [Google Scholar]
- 7. Brinkerhoff BT, Houghton DC, Troxell ML. Renal pathology in hematopoietic cell transplant recipients: a contemporary biopsy, nephrectomy, and autopsy series. Mod Pathol 2016;29:637–52. [DOI] [PubMed] [Google Scholar]
- 8. El-Seisi S, Gupta R, Clase CMet al. Renal pathology at autopsy in patients who died after hematopoietic stem cell transplantation. Biol Blood Marrow Transplant 2003;9:683–8. [DOI] [PubMed] [Google Scholar]
- 9. Colombo AA, Rusconi C, Esposito Cet al. Nephrotic syndrome after allogeneic hematopoietic stem cell transplantation as a late complication of chronic graft-versus-host disease. Transplantation 2006;81:1087–92. [DOI] [PubMed] [Google Scholar]
- 10. Reddy P, Johnson K, Uberti JPet al. Nephrotic syndrome associated with chronic graft-versus-host disease after allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant 2006;38:351–7. [DOI] [PubMed] [Google Scholar]
- 11. Troxell ML, Pilapil M, Miklos DBet al. Renal pathology in hematopoietic cell transplantation recipients. Mod Pathol 2008;21:396–406. [DOI] [PubMed] [Google Scholar]
- 12. Imai H, Oyama Y, Miura ABet al. Hematopoietic cell transplantation-related nephropathy in Japan. Am J Kidney Dis 2000;36:474–80. [DOI] [PubMed] [Google Scholar]
- 13. Garcia-Martin P, Alarcon-Payer C, Lopez-Fernandez Eet al. Transplantation-associated thrombotic microangiopathy in patients treated with sirolimus and cyclosporine as salvage therapy for graft-versus-host disease. Ann Pharmacother 2015;49:986–94. [DOI] [PubMed] [Google Scholar]
- 14. Jodele S, Davies SM, Lane Aet al. Diagnostic and risk criteria for HSCT-associated thrombotic microangiopathy: a study in children and young adults. Blood 2014;124:645–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ye Y, Zheng W, Wang Jet al. Risk and prognostic factors of transplantation-associated thrombotic microangiopathy in allogeneic haematopoietic stem cell transplantation: a nested case control study. Hematol Oncol 2017;35:821–7. [DOI] [PubMed] [Google Scholar]
- 16. Hingorani S, Pao E, Stevenson Pet al. Changes in glomerular filtration rate and impact on long-term survival among adults after hematopoietic cell transplantation: a prospective cohort study. Clin J Am Soc Nephrol 2018;13:866–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cho YH, Kang SH, Kim Yet al. De novo glomerulitis associated with graft-versus-host disease after allogeneic hematopoietic stem cell transplantation: a single-center experience. Kidney Res Clin Pract 2013;32:121–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hingorani SR, Seidel K, Lindner Aet al. Albuminuria in hematopoietic cell transplantation patients: prevalence, clinical associations, and impact on survival. Biol Blood Marrow Transplant 2008;14:1365–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Saddadi F, Hakemi M, Najafi Iet al. Chronic kidney disease after hematopoietic cell transplantation: frequency, risk factors, and outcomes. Transplant Proc 2009;41:2895–7. [DOI] [PubMed] [Google Scholar]
- 20. Kanduri SR, Cheungpasitporn W, Thongprayoon Cet al. Incidence and mortality of acute kidney injury in patients undergoing hematopoietic stem cell transplantation: a systematic review and meta-analysis. QJM 2020;113:621–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kidney Disease: Improving Global Outcomes Glomerular Diseases Work Group. KDIGO 2021 clinical practice guideline for the management of glomerular diseases. Kidney Int 2021;100:S1–276. [DOI] [PubMed] [Google Scholar]
- 22. Brukamp K, Doyle AM, Bloom RDet al. Nephrotic syndrome after hematopoietic cell transplantation: do glomerular lesions represent renal graft-versus-host disease? Clin J Am Soc Nephrol 2006;1:685–94. [DOI] [PubMed] [Google Scholar]
- 23. Tichelli A, Gratwohl A. Vascular endothelium as ‘novel’ target of graft-versus-host disease. Best Pract Res Clin Haematol 2008;21:139–48. [DOI] [PubMed] [Google Scholar]
- 24. Changsirikulchai S, Myerson D, Guthrie KAet al. Renal thrombotic microangiopathy after hematopoietic cell transplant: role of GVHD in pathogenesis. Clin J Am Soc Nephrol 2009;4:345–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mii A, Shimizu A, Kaneko Tet al. Renal thrombotic microangiopathy after hematopoietic stem cell transplantation: involvement of chronic graft-Versus-Host disease. Kidney Int Rep 2018;3:743–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hiramatsu R, Ubara Y, Sawa Net al. Clinicopathological analysis of allogeneic hematopoietic stem cell transplantation-related membranous glomerulonephritis. Hum Pathol 2016;50:187–94. [DOI] [PubMed] [Google Scholar]
- 27. Bruijn JA, van Elven EH, Hogendoorn PCet al. Murine chronic graft-versus-host disease as a model for lupus nephritis. Am J Pathol 1988;130:639–41. [PMC free article] [PubMed] [Google Scholar]
- 28. Nergizoglu G, Keven K, Ateş Ket al. Chronic graft-versus-host disease complicated by membranous glomerulonephritis. Nephrol Dial Transplant 1999;14:2461–3. [DOI] [PubMed] [Google Scholar]
- 29. Parikh CR, Coca SG. Acute renal failure in hematopoietic cell transplantation. Kidney Int 2006;69:430–5. [DOI] [PubMed] [Google Scholar]
- 30. Takatsuka H, Wakae T, Mori Aet al. Effects of total body irradiation on the vascular endothelium. Clin Transplant 2002;16:374–7. [DOI] [PubMed] [Google Scholar]
- 31. Cho BS, Yahng SA, Lee SEet al. Validation of recently proposed consensus criteria for thrombotic microangiopathy after allogeneic hematopoietic stem-cell transplantation. Transplantation 2010;90:918–26. [DOI] [PubMed] [Google Scholar]
- 32. Martinez MT, Bucher C, Stussi Get al. Transplant-associated microangiopathy (TAM) in recipients of allogeneic hematopoietic stem cell transplants. Bone Marrow Transplant 2005;36:993–1000. [DOI] [PubMed] [Google Scholar]
- 33. Heybeli C, Sridharan M, Alkhateeb HBet al. Characteristics of late transplant-associated thrombotic microangiopathy in patients who underwent allogeneic hematopoietic stem cell transplantation. Am J Hematol 2020;95:1170–9. [DOI] [PubMed] [Google Scholar]
- 34. Sethi S, Madden B, Casal Moura Met al. Hematopoietic stem cell transplant-membranous nephropathy is associated with protocadherin FAT1. J Am Soc Nephrol 2022;33:1033–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and analysed during the current study are available from the corresponding author on reasonable request.