Abstract
Aims
Left ventricular assist devices (LVADs) improve quality of life and survival in patients with advanced heart failure, but device-related infections (DRIs) remain cumbersome. We evaluated the diagnostic capability of [18F]FDG PET/CT, factors affecting its accuracy, and the additive value of semi-quantitative analysis for the diagnosis of DRI.
Methods and results
LVAD recipients undergoing [18F]FDG PET/CT between 2012 and 2020 for suspected DRI were retrospectively included. [18F]FDG PET/CT was performed and evaluated in accordance with EANM guidelines. The final diagnosis of DRI, based on multidisciplinary consensus and findings during surgery, whenever performed, was used as the reference for diagnosis. 41 patients were evaluated for 59 episodes of suspected DRI. The clinical evaluation established driveline infection in 32 (55%) episodes, central device infection in 6 (11%), and combined infection in 2 (4%). Visual analysis of [18F]FDG PET/CT achieved a sensitivity and specificity for driveline infections of 0.79 and 0.71, respectively, whereas semi-quantitative analysis achieved a sensitivity and specificity of 0.94 and 0.83, respectively. For central device component infection, visual analysis of [18F]FDG PET/CT achieved a sensitivity and specificity of 0.75 and 0.60, respectively. Semi-quantitative analysis using SUVratio achieved a sensitivity and specificity of 1.0 and 0.8, respectively. The increase of specificity for central component infection was statistically significant (P = 0.05).
Conclusions
[18F]FDG PET/CT reliably predicts the presence of DRI in LVAD recipients. Semi-quantitative analysis may increase the specificity of [18F]FDG PET/CT for the analysis of central device component infection and should be considered in equivocal cases after visual analysis.
Keywords: LVAD, device-related infection, [18F]FDG PET/CT, semi-quantitative analysis
Graphical Abstract
Graphical Abstract.
Introduction
Left ventricular assist device (LVAD) therapy increases survival and quality of life in patients with advanced heart failure, with median 5-year survival now approaching 50%.1 However, device-related infections (DRIs) occur frequently, with an incidence of 18.1% during the first year after implantation and 11.9% per year in the following years.1 Timely diagnosis of these infections is crucial since they can be life-threatening if they reach the central device components, especially when this is complicated by bloodstream infection, which carries an in-hospital mortality rate of up to 50%.2–4 However, establishing the presence, extent, and severity of DRI can be difficult because conventional imaging modalities such as echocardiography and CT are hampered by device-related artefacts.5,6
Several studies assessed the diagnostic performance of [18F]-fluorodeoxyglucose positron emission tomography combined with computed tomography ([18F]FDG PET/CT) for diagnosing driveline and/or central LVAD component infections7–15 and two meta-analyses combined their results.15,16 Visual analysis of [18F]FDG PET/CT has a high sensitivity for establishing LVAD infections and a high but variable specificity, with a pooled sensitivity of 0.95 [95% confidence interval (CI): 0.89–0.97] and a pooled specificity of 0.91 (95% CI: 0.54–0.99).16 This variable specificity might be caused by differences in scan procedures or specific technical issues. Furthermore, semi-quantitative analysis might increase the specificity of [18F]FDG PET/CT. So far, only one study investigated the role of semi-quantitative analysis for diagnosing infections of both driveline and central LVAD components,9 while two studies focused on driveline infections alone.7,10 The results of these studies were inconclusive regarding the additional value of semi-quantitative analysis. Therefore, we evaluated whether factors that may affect [18F]FDG PET/CT quality can affect the accuracy of visual analysis and whether quantification of FDG uptake around the LVAD and driveline improves the specificity and overall diagnostic accuracy of [18F]FDG PET/CT.
Methods
Patients
In this retrospective, dual-centre study, all consecutive patients that underwent [18F]FDG PET/CT for assessment of suspected LVAD and/or driveline infections in two medical centres in the Netherlands were included from December 2012, which is the date electronic records were first used at both hospitals, until 31 August 2020. Initial suspicion of infection was based on clinical history and presentation. Presenting symptoms of patients were recorded alongside general demographic data and information about the LVAD (e.g. implantation date, indication for implantation, and type/brand of LVAD). Other implanted cardiac materials (prosthetic valves or cardiac implanted electronic devices) were not routinely recorded, except when these showed signs of infection. Findings from clinical and laboratory investigations such as inflammatory markers, blood cultures, cultures from exit site swabs, and findings during surgery whenever performed were recorded, together with information about the use of intravenous or oral (suppressive) antibiotics at the time of the [18F]FDG PET/CT, including the duration of their use in days. If patients underwent [18F]FDG PET/CT scans during multiple episodes of suspected infection, the latter scans were only included if there were recurring symptoms after a symptom-free period of at least 1 month and after treatment for the earlier episode was fully completed (minimum interval between consecutive [18F]FDG PET/CTs was 2 months). Particular care was taken to avoid [18F]FDG PET/CT scans that were performed to establish treatment effect. All [18F]FDG PET/CT scans were performed on PET/CT systems that were calibrated according to EANM/EARL standards. The concomitant low-dose CT was most commonly non-enhanced and was used for anatomic orientation and attenuation correction. The study was approved and the need for informed consent was waived by the Local Medical Ethics Review Committees of both centres due to the non-WMO (Dutch law on studies involving human subjects) nature of this study using retrospective data: protocol nr M19.223017 (UMCG)/MEC-2019-0613 (EMC).
[18F]FDG PET/CT protocol
The protocol for [18F]FDG PET/CT preparation that was followed for each scan was documented. This included the duration of the fasting period before [18F]FDG PET/CT, whether an HFLC (high fat and low carbohydrate) diet had been used prior to the scan, patient blood glucose levels at the time of FDG injection, the injected FDG activity, and the used vendor type of PET/CT camera system.
Final diagnosis
Infections of the driveline and those of the LVAD central device components were evaluated as two separate diagnoses since their [18F]FDG PET/CT interpretation, treatment, and prognosis are different. The possible outcomes were the absence of DRI, driveline infection, infection of central device components, and infection of both driveline and central device components. If surgery was performed, macroscopic signs of infection during surgery and evidence from cultures and molecular diagnostics on swabs in the affected areas or from removed tissues were considered the gold standard for the diagnosis. If surgery was not performed, the final clinical diagnosis was established by the treating physician within the multidisciplinary LVAD team, which included cardiologists, thoracic surgeons, engineers specialized in LVAD technique and maintenance, medical microbiologists, and infectious disease specialists, with radiologists and nuclear medicine physicians consulted on a case-by-case basis. All clinical findings, including swabs from driveline exit sites, blood cultures, findings from imaging (including PET/CT, echocardiography, and diagnostic CT whenever performed), and the outcome during a minimum of 4 months of follow-up, were combined into a post hoc composite gold standard, an approach similar to that often used for the diagnosis of endocarditis.17,18 In case the final diagnosis of either driveline or central device component infection remained uncertain even after obtaining all available clinical information and follow-up, these patients were excluded from further analyses, see Table 1.
Table 1.
Overview of the patient characteristics
| Patient characteristics | |
|---|---|
| Age (mean, standard deviation) years | 56 years (11.5) |
| Gender | |
| Male | 37 (84%) |
| Female | 7 (16%) |
| BMI (Median, IQR) | 27.1 (24.1–30.1) |
| Diabetes, n (%) | 20 (34%) |
| Days since LVAD implantation: median (IQR; range) | Overall: 267 (136.5–617.5; 24–1548) |
| Heartmate II: 179 (132–669; 30–1548) | |
| Heartmate III: 304 (138–616; 24–1279) | |
| Presenting symptoms (n = 59) | |
| Local symptoms (erythema, pain, discharge, skin breakdown at driveline exit site) | 30 (51%) |
| ȃOf whom driveline infection confirmed (n = 34) | 27 (79%) |
| ȃOf whom central device infection confirmed (n = 8) | 2 (25%) |
| Systemic symptoms (malaise, fever, elevated inflammatory markers) | 31 (53%) |
| ȃOf whom driveline infection confirmed (n = 34) | 10 (29%) |
| ȃOf whom central device infection confirmed (n = 8) | 7 (88%) |
| HFLC diet (n = 59) | |
| ȃYes | 27 (46%) |
| ȃNo | 6 (10% |
| ȃUnknown | 26 (44%) |
| Myocardial suppression (n = 59) | |
| ȃGood | 49 (83%) |
| ȃReasonable | 3 (5%) |
| ȃPoor | 7 (12%) |
| Blood glucose level at PET/CT | |
| ȃ<11 mmol/L (198 mg/dL) | 56 (95%) |
| ȃ≥11 mmol/L | 3 (5%) |
| ȃLeucocytes <4 or >10 × 109/L | 15 (26%) |
| ȃCRP ≥5 mg/L (n = 57) | 51 (90%) |
| Duration of IV antibiotics (median, IQR) days | 5 (0–11) |
| Use of oral antibiotic suppression therapy, n (%) | 8 (14%) |
| Final diagnosis (n = 59), n (%) | |
| Driveline infected (n = 58, 1 uncertain diagnosis excluded) | 34 (59%) |
| ȃconfirmation through surgery/histopathology (n = 34) | 9 (26%) |
| ȃconfirmation through local cultures (n = 34) a | 28 (82%) |
| ȃbased on clinical judgment and follow-up (n = 34) | 1 (3%) |
| Central DRI (n = 53, 6 uncertain diagnoses excluded) | 8 (13%) |
| ȃconfirmation through surgery/histopathology (n = 8) | 3 (38%) |
| ȃconfirmation through local cultures (n = 8)a | 4 (50%) |
| ȃbased on clinical judgment and follow-up (n = 8) | 1 (13%) |
| Combined driveline infection and central DRI (of those with DRI, n = 38) | 2 (5%) |
| ȃconfirmation through surgery/histopathology (n = 2) | 2 (100%) |
| Cultured pathogen | |
| Driveline infection (n = 34) | |
| ȃBlood cultures | S. aureus (3), E. cloacae (2), E. faecium (1), C. acnes (1), S. dysgalactiae (1), negative (26) |
| ȃCultures exit site/removed tissue | S. aureus (16), P. aeruginosa (4), E. cloacae (2), H. parainfluenzae (1), K. oxytoca (1), S. hominis (1), M. chelonae (1), P. mirabilis (1), S. dysgalactiae (1), negative (6) |
| Central device component infection (n = 8) | |
| ȃBlood cultures | E. faecalis (2), S. aureus (2), S. lugdunensis (1), negative (3) |
| ȃCultures of removed tissue | S. aureus (2), S. Epidermidis, C. Acnes, Candida (1), negative (1), not available (4) |
These included both driveline exit site and deep driveline cultures, whenever obtained.
Visual analysis
[18F]FDG PET/CT visual analysis and standardized uptake value (SUV) calculations were performed using Syngo.via VB30 (Siemens Healthineers, Knoxville, TN, USA). Scans were analysed through consensus reading by two experienced nuclear medicine physicians, both of whom were blinded to the clinical context of the patients. Visual evaluation and interpretation of [18F]FDG PET/CT were performed according to EANM guidelines and were based on the FDG uptake pattern, intensity, and extension of any FDG-avid lesions around the driveline and/or the central LVAD components, including nearby soft tissue lesions and fluid collections. Both attenuation-corrected (AC) and uncorrected (NAC) images were used for the analyses.19
Semi-quantitative analysis
After manual delineation of volumes of interest (VOI), the maximum standardized uptake value (SUVmax) was measured at six predefined areas of interest in each patient. These regions comprised three areas alongside the peripheral driveline tract: driveline exit site, suprafascial driveline tract, and subfascial driveline, and three areas around the central device components: intrathoracic driveline, inflow canula/pump housing, and the outflow tract (Figures 1–3). Reference regions were manually drawn as spherical VOIs in the thoracic aorta and liver. The VOI in the aorta excluded the aortic vascular wall, and it was verified that the liver function tests were normal at the time of [18F]FDG PET/CT in the included patients.
Figure 1.
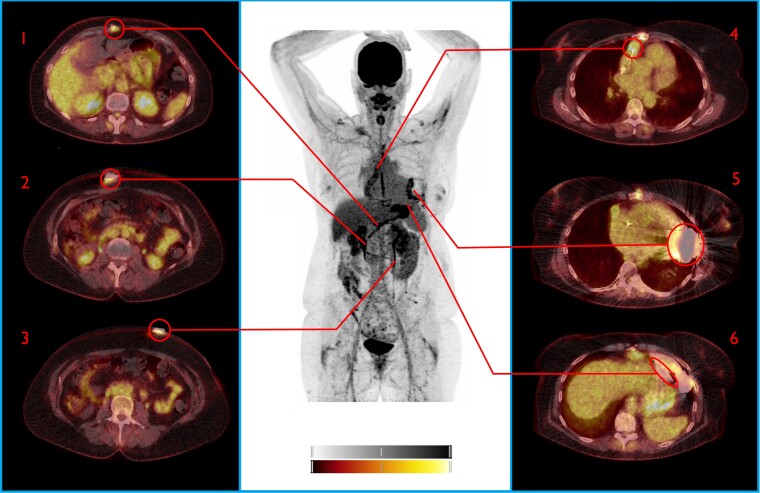
[18F]FDG PET/CT (true negative both visually and by SQ analysis). This patient presented with wounds on his lower legs due to peripheral arterial insufficiency without further symptoms, but blood cultures showed Streptococcus gordonii. In absence of local symptoms and repeated negative cultures after, this positive blood culture was explained as contamination. FDG PET/CT showed no evidence of LVAD DRI. Asymptomatic increased FDG uptake at the sternum was explained as postoperative reactive uptake in a patient with impaired wound healing (complicated LVAD implantation 6 months before). 1: Subfascial driveline, 2: Suprafascial driveline, 3: Driveline exit site, 4: Outflow tract 5: Pump housing, 6: Intrathoracic part driveline. Note the increased FDG uptake at the sternum (#). Colour scales: 0–5 SUV.
Figure 2.
[18F]FDG PET/CT (true positive both visually and by SQ analysis). This patient was admitted for a cardiac decompensation and elevated infection parameters in absence of fever. Patient had been treated for a Staphylococcus aureus driveline infection 2 months prior, which had initially responded well to Cefuroxime and debridement of the driveline. FDG PET/CT demonstrated advanced infection affecting the whole LVAD (earlier, only the driveline was affected). Patient underwent urgent heart transplantation and infection of the device was confirmed during surgery. 1: Subfascial driveline, 2: Suprafascial driveline, 3: Driveline exit site, 4: Outflow tract 5: Pump housing, 6: Intrathoracic part driveline. NB: Note the residual FDG uptake below the insertion of the driveline after surgical debridement and shortening of the driveline 2 months before (#). Colour scales: 0–5 SUV.
Figure 3.
[18F]FDG PET/CT Driveline infection. Central device components false positive by visual analysis, true negative by SQ analysis. This patient presented with pain and discharge at driveline exit site, without further symptoms. FDFG uptake at driveline was homogenous and minimal, while central device components were FDG-avid, suspect for infection. Staphylococcus aureus was cultured from the driveline exit site and this was treated with oral antibiotics with good initial effect. Three months later, minimal surgical debridement of the driveline exist site was performed due to ongoing local irritation, still without any signs of systemic infection. A repeat [18F]FDG PET/CT 11 months after this episode showed an almost identical FDG uptake pattern around the central device component FDG uptake, confirming that this uptake was reactive. SUV ratio (liver/thorax) correctly identified this increased FDG uptake as too little to indicate infection. 1: Subfascial driveline, 2: Suprafascial driveline, 3: Driveline exit site, 4: Outflow tract 5: Pump housing, 6: Intrathoracic part driveline. NB: Note the increased FDG uptake around the driveline and the heterogeneous uptake around the pump housing and outflow tract (also visible on NAC images). Colour scales: 0–5 SUV.
The highest SUVmax value alongside the driveline and the highest value around the central device components were used for the evaluation of the diagnostic accuracy of the semi-quantitative analysis. The standardized uptake value ratio (SUVratio) was calculated by dividing the highest SUVmax value alongside the driveline and the highest SUVmax value around the central device components by the mean activity in both reference regions separately (SUVratio-bloodpool and SUVratio-liver). All calculations were performed on EANM Research Ltd (EARL)-reconstructed images.18
Potential confounders
Factors that could potentially affect the assessment of either driveline infection or infections of central device components were identified for further analysis. These included patient age, gender, BMI, diabetes mellitus, type of LVAD, the interval between LVAD implantation and PET/CT, type of PET/CT system, patient preparation using an HFLC diet and the duration of IV antibiotic use prior to [18F]FDG PET/CT.
Statistics
For demographic data, continuous variables are presented as mean ± SD, while categorical variables are reported as frequencies. We used logistic regression to evaluate the accuracy of visual analysis and semi-quantitative analysis diagnosis of driveline and/or central device infections. Potential confounders were assessed using univariate logistic regression for effect on visual analysis and final diagnosis. For semi-quantitative analyses receiver operating characteristics (ROC) analysis was performed to determine the optimum threshold values to maximize sensitivity. McNemar’s test was used to compare the sensitivity and specificity of visual analysis with those of semi-quantitative analysis. For all statistical analyses, a two-tailed P-value ≤0.05 was considered statistically significant. Analyses were performed using IBM® SPSS 26 (IBM Corp., NY, USA).
Results
Patient characteristics
In total, 44 patients underwent a total of 70 [18F]FDG PET/CT scans because of a clinical suspicion of LVAD and/or driveline infection. Eight scans were excluded because they had been performed to establish treatment effect and three because non-attenuation-corrected images were unavailable for these patients. This left 41 patients and 59 scans for analysis. Of these, 17 patients (22 scans) were evaluated in Medical Centre 1, while the remainder of 24 patients (37 scans) were evaluated in Medical Centre 2. The implanted LVAD system was either the Heartmate III (32/41 or 78% of patients) or the Heartmate II (9/41 or 22% of patients). None of the patients had LVAD infections prior to their inclusion in the study. Further demographic data can be found in Table 1.
[18F]FDG PET/CT protocol
Both medical centres used a fasting period of at least 6 h as standard. However, while in Medical Centre 1 the 24-h HFLC diet was standard since October 2016, in Medical Centre 2 this was only formalized at the beginning of 2020.14 The HFLC diet was used to prepare patients for [18F]FDG PET/CT in 27 of 59 scans (46%). Six patients had not followed the diet and in 26 instances, the use of the diet could not be verified. The PET/CT systems used included the Biograph mCT40, mCT64, and the Biograph 128 VISION (Siemens Healthineers, KN, USA) for 13, 20, and 26 scans, respectively. [18F]FDG PET/CT was performed 60 min after the injection of FDG in both centres. The average injected activity was 263 or 2.9 MBq/kg per patient, in accordance with EANM guidelines.20
Final diagnosis
Isolated driveline infection was confirmed in 32 of 59 (54%) cases, while in one additional case the diagnosis could neither be confirmed nor excluded; this patient was treated empirically with antibiotics. Isolated infection of central device components was established in 6 of 53 (11%) episodes, with an additional six indeterminate cases that were also treated empirically. Two patients (4%) had a combined infection of both driveline and central device components (see also Table 1). All patients not diagnosed with infection remained infection free during follow-up, supporting the rejected diagnosis of infection.
Visual analysis
Visual analysis of [18F]FDG PET/CT reliably predicted the presence of driveline infections [OR: 11.3 (95% CI: 3.3–39.4); P < 0.001 by logistic regression]. The corresponding sensitivity and specificity were 0.79 and 0.71, respectively. When only AC images were used, diagnostic accuracy was essentially unchanged, with a sensitivity and specificity of 0.82 and 0.71, respectively. The visual analysis could not reliably predict infection of central device components by logistic regression, though it showed a trend towards significance [odds ratio (OR): 4.50 (95% CI: 0.82–24.83); P = 0.08]. Sensitivity and specificity of visual analysis were 0.75 and 0.60, respectively, when both AC and NAC images were used. When only AC images were analysed, this increased sensitivity to 1.0, but this came at the cost of specificity, which then dropped to 0.27. Analysis of FDG uptake patterns showed that heterogeneous and even (multi-)focal FDG uptake around the central device components were not always predictive of infection: of the 22 patients with heterogeneous uptake in this area, only 2 were diagnosed with central device component infection and of the 19 patients with (multi-)focal uptake, only 6 had an infection of the central device components.
Semi-quantitative analysis
Semi-quantitative analysis using SUVmax reliably predicted driveline infections (OR: 4.18 [95% CI: 1.81–9.65], P = 0.001). ROC curve analysis showed that the optimum balance between sensitivity and specificity was achieved using a SUVmax cut-off value of 3.53, resulting in a sensitivity and specificity of 0.94 and 0.83, respectively. This constituted an improvement compared to visual analysis, but the difference was not statistically significant for either sensitivity: P = 0.12 or specificity: P = 0.38. For suspected central device component infection, semi-quantitative analysis using SUVmax reliably predicted infection [OR 1.95 (95% CI: 1.19–3.18), P = 0.008]; as did SUVratio-bloodpool [OR: 3.51 (95% CI: 1.42–8.70) P = 0.007] and SUVratio-liver [OR: 14.38 (95% CI: 2.18–94.60) P = 0.006]. SUVratio-liver achieved an AUC of 0.95, with a sensitivity and specificity of 1.0 and 0.80 respectively, at a cut-off value of 2.45. SUVratio-bloodpool achieved an AUC of 0.91 and a sensitivity and specificity of 0.89 and 0.83, respectively, at a cut-off value of 3.04. The AUC of SUVmax alone was 0.80 and at its optimum cut-off value of 5.14 it achieved a sensitivity and specificity of 0.75 and 0.80, respectively. The differences in sensitivity between semi-quantitative and visual analysis were statistically non-significant for central device component infections: SUVmax: P = 0.50, SUVratio-bloodpool: P = 1.0 and SUVratio-liver: P = 1.0, respectively. The improvement of specificity however was statistically significant for SUVratio-bloodpool: P = 0.03 and SUVratio-liver: P = 0.05, while SUVmax showed a statistical trend (P = 0.08.) The corresponding ROC curves for both driveline and central device components are shown in Figure 4.
Figure 4.
ROC curves [18F]FDG PET/CT for infection of the LVAD driveline and central device components.
Potential confounders for [18F]FDG PET/CT
Of the evaluated potential confounders, only the interval between LVAD implantation and [18F]FDG PET/CT showed a statistically significant effect on the visual analysis of [18F]FDG PET/CT. [18F]FDG PET/CT was performed within 30 days after implantation in only two cases and both were correctly identified as negative for driveline infection and infection of central device components by both visual analysis and semi-quantitative analysis. However, in the 12 scans performed within 90 days after LVAD implantation, the risk of driveline infection was unexpectedly lower than in scans performed 90 days or more after LVAD implantation: 27 vs. 64%; OR 0.21 (95% CI: 0.05–0.83), P = 0.03 by logistic regression. The chance of finding driveline infection by [18F]FDG PET/CT in this group was decreased as well, but this difference was not significant: OR: 0.41 (95% CI: 0.11–1.47), P = 0.17, implying a slightly confounding effect. The effect of a HFLC diet on [18F]FDG PET/CT visual analysis could not be established by logistic regression, due to insufficient cases per group. Use of this diet did however significantly decrease the chance of myocardial physiological FDG uptake on PET/CT: OR: 0.125 (95% CI: 0.02–0.92), P = 0.04. For a full overview of all potential confounders evaluated by logistic regression see Supplementary material online, supplemental data file.
Discussion
In this study the diagnostic accuracy of [18F]FDG PET/CT for LVAD DRIs was evaluated in 41 patients and 59 episodes of suspected infection, making it one of the largest cohorts for this indication to date.7–15 Additionally, infections involving the driveline and infections involving central device components were stratified, the assessors were blinded to the clinical context of patients and evaluation was performed in accordance with EANM recommendations, using both AC and NAC images. Furthermore, we included semi-quantitative analyses performed in accordance with EARL recommendations and it is the first study in which the majority of the [18F]FDG PET/CT scans were performed to evaluate LVADs of the newest generation (Heartmate III), which are increasingly used in clinical practice around the world.
[18F]FDG PET/CT showed good sensitivity and specificity for diagnosing infections of the driveline, both with visual and semi-quantitative analysis. For the diagnosis of central device component infection, visual analysis achieved moderate sensitivity and specificity. The use of non-attenuation-corrected images was pivotal for an accurate visual assessment, in particular for achieving sufficient specificity. The marked difference in diagnostic accuracy between AC and NAC images could be explained by the presence of substantial amounts of metal in the LVAD pump housing which causes scatter artefacts. In newer PET/CT systems, reconstruction algorithms are available that can significantly reduce metal-related artefacts. In this retrospective cohort, however, these were not always available, and the potential impact of artefact-reducing algorithms on the diagnosis of LVAD DRI is currently unknown.
Semi-quantitative analysis using a target-to-background ratio (either liver or aortic bloodpool) achieved similar sensitivity compared to visual analysis for central device component infection and higher specificity. The semi-quantitative analysis could not be directly compared to other studies, as standardization according to EARL was not described in any of these studies, while the only study in which SUVratio was evaluated did not use either the liver or bloodpool as their reference region.7,9,10
The diagnostic performance of visual analysis of [18F]FDG PET/CT for both driveline and central device components in this study was comparatively low compared to current literature, in which overall sensitivity and specificity were 95 and 91%, respectively.7–16 As an explanation for this, firstly, in our study the assessors were blinded to the patients’ clinical context, while in about half of earlier studies, assessors were not (fully) blinded, which may have led to observer bias. Secondly, reactive uptake around the insertion of the pump in the left ventricle and the outflow tract are expected findings in the presence of an LVAD and this presents a significant diagnostic challenge, as distinguishing between sterile inflammation and infection at these locations proved difficult by visual analysis, even while using NAC images. This was due in part to the finding that reactive FDG uptake surrounding these central device components and the outflow tract, in particular was often heterogeneous, in some instances even (multi-)focal. An example of this is shown in Figure 3. The semi-quantitative analysis quantifies the absolute FDG uptake in a suspected lesion and does not account for heterogeneity. If this heterogeneity is a confounder, it might explain the additional specificity achieved by semi-quantitative analysis for this indication. Therefore, when [18F]FDG PET/CT is evaluated visually for suspected central LVAD component DRI, it is important to be extra conservative when heterogeneous FDG uptake is seen around pump housing and outflow tract, as this does not necessarily indicate infection.
Although the study period covers quite a long time period (2012–2020), the different camera systems used were from one manufacturer and did not show a confounding effect by univariate logistic regression for either driveline or central LVAD infection (data not shown). Furthermore, for quantitative analysis, all scans were reconstructed according to standardized EARL guidelines, as stated in the methods.
Recommendation for implementation of [18F]FDG PET/CT in suspected DRI in LVAD patients
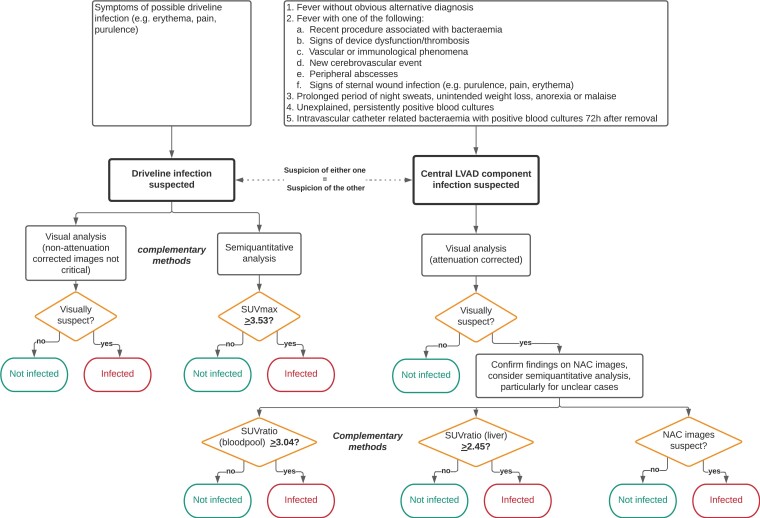
Based on the findings in this study, visual and semi-quantitative analysis of [18F]FDG PET/CT could be complementary methods for the evaluation of driveline infections and central device component infections. We propose a tentative flow chart for deciding which method of assessment is most suitable for various parts of the evaluation according to our findings in Figure 5. Future prospective studies would be invaluable for further validation of our results, and in particular the cut-off values we found for semi-quantitative analysis.
Figure 5.
Proposed flowchart [18F]FDG PET/CT evaluation for DRI LVAD based on our results.
Limitations
This study had a retrospective design which makes it prone to inclusion bias, though care was taken to include every consecutive patient with a suspicion of infection. A general limitation of studies investigating DRI refers to the gold standard for the diagnosis, i.e. direct culture and molecular diagnostics using 16S PCR of the suspected device parts. This gold standard for DRI is frequently unattainable because surgery is not always performed. While exit site swabs and blood cultures may give an indication for device infection, they are often insufficient to fully ascertain the diagnosis, in particular when infection of central device components is suspected. Findings during surgery were considered the true gold standard for the diagnosis whenever available, and when these were unavailable, all clinical findings, such as blood cultures, driveline exit site swabs, results from other available imaging modalities, and outcomes during follow-up were considered together to avoid reliance on [18F]FDG PET/CT alone.
For most patients in this series, [18F]FDG PET was combined with a low-dose, non-enhanced CT. A combination of PET/CT-Angiography would allow for better detection of anatomical lesions, which can be of great value for the diagnosis of LVAD DRI, increasing the specificity of the scans. Therefore this is highly recommended for the evaluation of vascular structures and all related prosthetic materials/devices whenever possible. Furthermore, the effect of (non-)adherence to the HFLC diet on [18F]FDG PET/CT accuracy could not be statistically verified in our study due to limited case numbers in this group. The HFLC diet was not officiated in one of the participating centres until some years ago. In clinical practice, it was often followed nevertheless, but this was not always documented in patient records. This also explains the high rate of effective myocardial suppression in our cohort as shown in Table 1. Because physiological myocardial uptake leads to decreased evaluability of the left ventricle wall and because central device component infections have a well-known risk of concurrent endocarditis, we recommend using this diet for all cases of suspected LVAD DRI.
Conclusion
[18F]FDG PET/CT may help identify LVAD infections when both normal and abnormal FDG uptake patterns surrounding these devices are correctly weighed. Visual analysis and semi-quantitative analysis complement each other to reliably establish the presence of driveline infections. For central device component infection, visual analysis achieves moderate sensitivity and specificity. Using both AC and NAC images is pivotal for achieving sufficient specificity for this indication, while semi-quantitative analysis may be of additive value for further increasing [18F]FDG PET/CT specificity for these infections.
Supplementary Material
Acknowledgements
This work was supported in part by an unconditional grant from PUSH (a collaboration between Siemens Healthineers and the UMCG). The sponsor had no role in the conceptualization, writing, or publication of the article.
Contributor Information
Derk ten Hove, Department of Nuclear Medicine and Molecular Imaging, University of Groningen, University Medical Center Groningen, Hanzeplein 1, 9713GZ, Groningen, The Netherlands; Department of Medical Microbiology and Infection Prevention, University of Groningen, University Medical Center Groningen, Hanzeplein 1, 9713GZ, Groningen, The Netherlands.
Ali R Wahadat, Department of Radiology and Nuclear Medicine, Erasmus Medical Center, Doctor Molewaterplein 40, 3015 GD Rotterdam, South Holland, The Netherlands; Department of Cardiology, Erasmus Medical Center, Doctor Molewaterplein 40, 3015 GD Rotterdam, South Holland, The Netherlands; Department of Cardiology, HagaZiekenhuis, Els Borst-Eilersplein 275, 2545 AA The Hague, South Holland, The Netherlands.
Riemer H J A Slart, Department of Nuclear Medicine and Molecular Imaging, University of Groningen, University Medical Center Groningen, Hanzeplein 1, 9713GZ, Groningen, The Netherlands; Department of Biomedical Photonic Imaging, Faculty of Science and Technology, University of Twente, attn. BFD/TNW Carré 3033, P.O. Box 217, 7500AE, Enschede, Overijssel, The Netherlands.
Marjan Wouthuyzen-Bakker, Department of Medical Microbiology and Infection Prevention, University of Groningen, University Medical Center Groningen, Hanzeplein 1, 9713GZ, Groningen, The Netherlands.
Gianclaudio Mecozzi, Department of Cardiothoracic Surgery, University of Groningen, University Medical Center Groningen, Hanzeplein 1, 9713GZ, Groningen, The Netherlands.
Kevin Damman, Department of Cardiology, University of Groningen, University Medical Center Groningen, Hanzeplein 1, 9713GZ, Groningen, The Netherlands.
Hester Witteveen, Department of Cardiology, University of Groningen, University Medical Center Groningen, Hanzeplein 1, 9713GZ, Groningen, The Netherlands.
Kadir Caliskan, Department of Cardiology, Erasmus Medical Center, Doctor Molewaterplein 40, 3015 GD Rotterdam, South Holland, The Netherlands.
Olivier C Manintveld, Department of Cardiology, Erasmus Medical Center, Doctor Molewaterplein 40, 3015 GD Rotterdam, South Holland, The Netherlands.
Bhanu Sinha, Department of Medical Microbiology and Infection Prevention, University of Groningen, University Medical Center Groningen, Hanzeplein 1, 9713GZ, Groningen, The Netherlands.
Ricardo P J Budde, Department of Radiology and Nuclear Medicine, Erasmus Medical Center, Doctor Molewaterplein 40, 3015 GD Rotterdam, South Holland, The Netherlands.
Andor W J M Glaudemans, Department of Nuclear Medicine and Molecular Imaging, University of Groningen, University Medical Center Groningen, Hanzeplein 1, 9713GZ, Groningen, The Netherlands.
Supplementary data
Supplementary data are available at European Heart Journal - Cardiovascular Imaging online.
Funding
None declared.
Data availability
The data that support the findings of this study are available from the corresponding author, D.t.H., upon reasonable request.
References
- 1. Molina EJ, Shah P, Kiernan MS, Cornwell WK III, Copeland H, Takeda Ket al. The society of thoracic surgeons intermacs 2020 annual report. Ann Thorac Surg 2021;111:778–92. [DOI] [PubMed] [Google Scholar]
- 2. Kirklin JK, Naftel DC, Pagani FD, Kormos RL, Stevenson LW, Blume EDet al. Seventh INTERMACS annual report: 15,000 patients and counting. J Heart Lung Transplant [Internet] 2015;34:1495–504. [DOI] [PubMed] [Google Scholar]
- 3. Aggarwal A, Gupta A, Kumar S, Baumblatt JA, Pauwaa S, Gallagher Cet al. Are blood stream infections associated with an increased risk of hemorrhagic stroke in patients with a left ventricular assist device? ASAIO J 2012;58:509–13. [DOI] [PubMed] [Google Scholar]
- 4. Trachtenberg BH, Cordero-Reyes AM, Aldeiri M, Alvarez P, Bhimaraj A, Ashrith Get al. Persistent blood stream infection in patients supported with a continuous-flow left ventricular assist device is associated with an increased risk of cerebrovascular accidents. J Card Fail [Internet] 2015;21:119–25. [DOI] [PubMed] [Google Scholar]
- 5. Lesicka A, Feinman JW, Thiele K, Andrawes MN. Echocardiographic artifact induced by HeartWare left ventricular assist device. Anesth Analg 2015;120:1208–11. [DOI] [PubMed] [Google Scholar]
- 6. Shroff GS, Ocazionez D, Akkanti B, Vargas D, Garza A, Gupta Pet al. CT Imaging of complications associated with continuous-flow left ventricular assist devices (LVADs). semin ultrasound. CT MRI [Internet] 2017;38:616–28. [DOI] [PubMed] [Google Scholar]
- 7. Avramovic N, Dell’Aquila AM, Weckesser M, Milankovic D, Vrachimis A, Sindermann JRet al. Metabolic volume performs better than SUVmax in the detection of left ventricular assist device driveline infection. Eur J Nucl Med Mol Imaging 2017;44:1870–7. [DOI] [PubMed] [Google Scholar]
- 8. Dell’Aquila AM, Mastrobuoni S, Alles S, Wenning C, Henryk W, Schneider SRBet al. Contributory role of fluorine 18-fluorodeoxyglucose positron emission tomography/computed tomography in the diagnosis and clinical management of infections in patients supported with a continuous-flow left ventricular assist device. Ann Thorac Surg 2016;101:87–94. [DOI] [PubMed] [Google Scholar]
- 9. Dell’aquila AM, Avramovic N, Mastrobuoni S, Motekallemi A, Wisniewski K, Scherer Met al. Fluorine-18 fluorodeoxyglucose positron emission tomography/computed tomography for improving diagnosis of infection in patients on CF-LVAD: longing for more insights. Eur Heart J Cardiovasc Imaging 2018;19:532–43. [DOI] [PubMed] [Google Scholar]
- 10. Kanapinn P, Burchert W, Körperich H, Körfer J. 18F-FDG PET/CT-imaging of left ventricular assist device infection: a retrospective quantitative intrapatient analysis. J Nucl Cardiol 2019;26:1212–21. [DOI] [PubMed] [Google Scholar]
- 11. Kim J, Feller ED, Chen W, Liang Y, Dilsizian V. FDG PET/CT for early detection and localization of left ventricular assist device infection: impact on patient management and outcome. JACC Cardiovasc Imaging 2019;12:722–9. [DOI] [PubMed] [Google Scholar]
- 12. Bernhardt AM, Pamirsad MA, Brand C, Reichart D, Tienken M, Barten MJet al. The value of fluorine-18 deoxyglucose positron emission tomography scans in patients with ventricular assist device specific infections. Eur J Cardio-Thoracic Surg 2017;51:1072–7. [DOI] [PubMed] [Google Scholar]
- 13. De Vaugelade C, Mesguich C, Nubret K, Camou F, Greib C, Dournes Get al. Infections in patients using ventricular-assist devices: comparison of the diagnostic performance of 18 F-FDG PET/CT scan and leucocyte-labeled scintigraphy. J Nucl Cardiol 2019;26:42–55. [DOI] [PubMed] [Google Scholar]
- 14. Akin S, Muslem R, Constantinescu AA, Manintveld OC, Birim O, Brugts JJet al. 18F-FDG PET/CT in the diagnosis and management of continuous flow left ventricular assist device infections: A case series and review of the literature ASAIO J 2018;64:e11–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tam MC, Patel VN, Weinberg RL, Hulten EA, Aaronson KD, Pagani FDet al. Diagnostic accuracy of FDG PET/CT in suspected LVAD infections. JACC Cardiovasc Imaging 2020;13:1191–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ten Hove D, Treglia G, Slart RHJA, Damman K, Wouthuyzen-Bakker M, Postma DFet al. The value of 18F-FDG PET/CT for the diagnosis of device-related infections in patients with a left ventricular assist device: a systematic review and meta-analysis. Eur J Nucl Med Mol Imaging 2021;48:241–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Swart LE, Gomes A, Scholtens AM, Sinha B, Tanis W, Lam MGEHet al. Improving the diagnostic performance of 18F-fluorodeoxyglucose positron-emission tomography/computed tomography in prosthetic heart valve endocarditis. Circulation 2018;138:1412–27. [DOI] [PubMed] [Google Scholar]
- 18. Gomes A, Glaudemans AWJM, Touw DJ, van Melle JP, Willems TP, Maass AHet al. Diagnostic value of imaging in infective endocarditis: a systematic review. Lancet Infect Dis 2017;17:1–14. [DOI] [PubMed] [Google Scholar]
- 19. Slart RHJA, Glaudemans AWJM, Gheysens O, Lubberink M, Kero T, Dweck MRet al. ; 4Is cardiovascular imaging: a joint initiative of the European association of cardiovascular imaging (EACVI); European association of nuclear medicine (EANM). procedural recommendations of cardiac PET/CT imaging: standardization in inflammatory-, infective-, infiltrative-, and innervation (4Is)-related cardiovascular diseases: a joint collaboration of the EACVI and the EANM. Eur J Nucl Med Mol Imaging 2021;48:1016–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Boellaard R, Delgado-Bolton R, Oyen WJG, Giammarile F, Tatsch K, Eschner Wet al. FDG PET/CT: EANM procedure guidelines for tumour imaging: version 2.0. Eur J Nucl Med Mol Imaging 2015;42:328–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, D.t.H., upon reasonable request.