ABSTRACT
Background
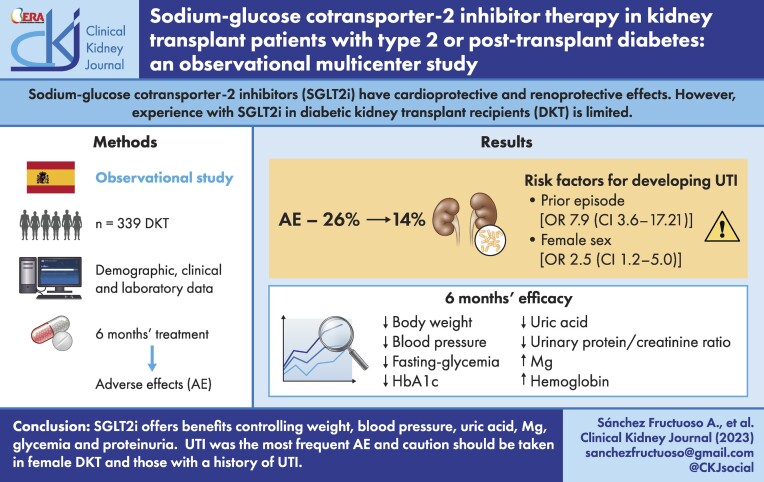
Sodium–glucose cotransporter-2 inhibitors (SGLT2is) have cardioprotective and renoprotective effects. However, experience with SGLT2is in diabetic kidney transplant recipients (DKTRs) is limited.
Methods
This observational multicentre study was designed to examine the efficacy and safety of SGLT2is in DKTRs. The primary outcome was adverse effects within 6 months of SGLT2i treatment.
Results
Among 339 treated DKTRs, adverse effects were recorded in 26%, the most frequent (14%) being urinary tract infection (UTI). In 10%, SGLT2is were suspended mostly because of UTI. Risk factors for developing a UTI were a prior episode of UTI in the 6 months leading up to SGLT2i use {odds ratio [OR] 7.90 [confidence interval (CI) 3.63–17.21]} and female sex [OR 2.46 (CI 1.19–5.03)]. In a post hoc subgroup analysis, the incidence of UTI emerged as similar in DKTRs treated with SGLT2i for 12 months versus non-DKTRs (17.9% versus 16.7%). Between baseline and 6 months, significant reductions were observed in body weight [−2.22 kg (95% CI −2.79 to −1.65)], blood pressure, fasting glycaemia, haemoglobin A1c [−0.36% (95% CI −0.51 to −0.21)], serum uric acid [−0.44 mg/dl (95% CI −0.60 to −0.28)] and urinary protein:creatinine ratio, while serum magnesium [+0.15 mg/dl (95% CI 0.11–0.18)] and haemoglobin levels rose [+0.44 g/dl (95% CI 0.28–0.58]. These outcomes persisted in participants followed over 12 months of treatment.
Conclusions
SGLT2is in kidney transplant offer benefits in terms of controlling glycaemia, weight, blood pressure, anaemia, proteinuria and serum uric acid and magnesium. UTI was the most frequent adverse effect. According to our findings, these agents should be prescribed with caution in female DKTRs and those with a history of UTI.
Keywords: post-transplant diabetes mellitus, SGLT2 inhibitors, type 2 diabetes
Graphical Abstract
Graphical Abstract.
INTRODUCTION
Diabetes mellitus (DM) is an important cause of end-stage renal disease worldwide [1], and this has led to a steady increase in diabetic individuals among kidney transplant recipients (KTRs) [2]. According to the latest data from the US transplant registry, almost 47% of patients on the kidney transplant waiting list have diabetes and the trend was for this proportion to increase [3]. Further, 15–30% of KTRs without diabetes develop persistent hyperglycaemia following transplant, known as post-transplant diabetes mellitus (PTDM) [4–6]. This situation gives rise to a high prevalence of individuals with both a kidney transplant and pre-existing diabetes or PTDM.
In KTRs, diabetes has been associated with a 2- to 4-fold increased risk of cardiac events, as well as infectious complications and lower patient survival [2, 4, 7, 8]. Good diabetes management is therefore essential to prevent poor outcomes.
Evidence concerning the efficacy and safety of glucose‐lowering agents in KTRs is limited. At a consensus meeting held in Vienna in September 2013 [5], it was agreed that the data available were inadequate to recommend a hierarchy of anti-glycaemic agents in this setting. More recently, a systematic review of the available evidence [6] was also unable to draw any valid conclusions regarding specific recommendations for glucose-lowering therapy in these patients. Over many years, the only therapeutic strategy available to transplant nephrologists for the management of proteinuric diabetic renal disease has been renin–angiotensin–aldosterone system blockade. However, in the last few years, sodium–glucose cotransporter-2 inhibitors (SGLT2is) have shown a clear kidney protective effect in terms of delaying diabetic kidney disease progression and reducing albuminuria in non-transplanted patients [9, 10]. A cardioprotective effect has also been described for these drugs [9–15]. However, KTRs were excluded from these trials.
Experience with SGLT2i treatment in KTRs with diabetes is limited. This is likely because treatment in non-transplanted diabetic patients has been linked to an increased risk of renal dysfunction and urinary tract infection (UTI) [6, 16]. However, the information on this issue is still scarce and reports exist of only one small clinical trial [17], a few case series [18–20], three prospective descriptive studies [21–23] and three retrospective studies [24–26]. The results of these investigations suggest that SGLT2i treatment is effective at lowering haemoglobin A1c (HbA1c), reducing body weight and preserving kidney function without serious adverse events. However, such a small number of reported cases means there is insufficient statistical power to draw valid conclusions on which to base recommendations.
According to the information available, there are only three clinical trials under way investigating the use of SGLTis in KTRs [27–29] and estimated completion dates are mid-2024. In the meantime, prospective studies could help explore the safety, tolerability and efficacy of SGLT2is in KTRs with diabetes. The aim of the present study was to describe experience with the use of these drugs in diabetic persons with a transplanted kidney.
MATERIALS AND METHODS
This was a multicentre observational study conducted in KTRs with pre-existing type 2 diabetes or PTDM at 18 participating centres across Spain. Participants were recruited over the period January 2021 to March 2022 inclusively.
Our main objective was to assess the incidence of UTI and/or mycoses in diabetic KTRs in response to SGLT2i treatment. Data were compiled regarding episodes of UTI experienced 6 months before treatment onset and 6 months after treatment onset. When available, UTI data for 1-year after treatment onset were also considered.
Secondary outcomes were changes produced in the following analytical data: haemoglobin level, estimated glomerular filtration rate (eGFR) using the Chronic Kidney Disease Epidemiology Collaboration equation [30], urinary albumin:creatinine ratio (UACR) and/or urinary protein:creatinine ratio (UPCR), glycaemia (fasting plasma glucose, HbA1c) and lipid metabolism (serum triglycerides, high-density lipoproteins and total cholesterol).
Standard demographic, clinical and laboratory data (including medication details) and complications were compiled from medical records 6 months prior to the start of SGLT2i treatment, at baseline and 6 months thereafter. When available, 12-month follow-up data were also considered. We also examined cardiovascular disease and microvascular complications, hospitalizations and reasons for admission and mortality. More information about the study protocol may be found in the Supplementary Material.
The study protocol was approved by a central review board (Ethics Committee of Hospital Clínico San Carlos, 16 December 2020). While SGLT2i treatment has not been specifically approved for use in KTRs with pre-existing type 2 DM or PTDM, in our patients treatment was prescribed based on evidence from clinical trials indicating that SGLT2is are cardio- and nephroprotective. Criteria for treatment were based on the recommendations of the American Diabetes Association [type 2 diabetes with poor glycaemic control (main reason HbA1c ≥7.5% in 109 cases) and/or cardiovascular risk (main reason in 94 patients) and/or kidney risk factors (136 patients had an eGFR <60 ml/min/1.73 m2 and/or UACR >300 mg/g and/or UPCR >300 mg/g) [31]. Written informed consent and the criteria for SGLT2i treatment fulfilled by participants were clearly detailed in their clinical records. Effectively this was a prerequisite for entering any patient information in the database created for this study.
In a post hoc subanalysis, we compared the incidence of UTI at the centre providing the most participants (n = 84) with that recorded in a reference group of non-diabetic KTRs from the outpatient clinic seen over the same time period. Reference patients were matched 1 to 1 with the study population, using age, sex, number of grafts and time post-transplant and kidney function (eGFR) as matching criteria.
Statistical analysis
Categorical variables are provided as absolute and relative frequencies. Depending on their distribution, continuous variables are described as the mean and standard deviation (SD) or median and interquartile range (IQR).
To compare continuous variables, we used parametric (paired Student's t-test and repeated measures analysis of variance with Bonferroni post hoc analysis) and non-parametric (Wilcoxon or Friedman) tests. Median differences were calculated using the Hodges–Lehmann estimator. For categorical variables, significant differences were assessed using the McNemar test for comparisons between visits or chi-squared test between patient subsets. To compare absolute differences in means and percentages at 6 months versus baseline, a repeated measures general linear model was constructed, providing means and percentages along with their differences adjusted for sex and age and their 95% confidence intervals (CIs). Asymmetric variables were normalized through their log transformation. Significance was set at P < .05.
To identify factors possibly associated with UTI, we conducted a univariate analysis including the variables sex, age, type of diabetes, time post-transplant, SGLT2i and UTI previous to SGLT2i treatment onset. A logistic regression model was constructed adjusted by backward stepwise regression based on maximum likelihood estimators including variables with a P-value <.15 in the univariate analysis. Odds ratios (ORs) and their significance were calculated for each variable according to criteria for entry (P < .05) and removal (P > .10).
All statistical tests were performed on an intention-to-treat basis using SPSS version 25 (IBM, Armonk, NY, USA).
Sample size calculation
Sample size was calculated according to a reported expected incidence of UTI and/or mycoses of 13.4% per year in diabetic non-transplanted patients [9]. For a 95% CI and accuracy of 95%, this yielded a figure of 179 patients. Considering a 10% increase to account for losses to follow-up, this gave a minimum sample size of 206 patients. To avoid bias in patient selection, each centre was required to provide data pertaining to every KTR receiving SGLT2i treatment and giving their informed consent during the study period.
RESULTS
The baseline characteristics of the 339 patients finally included are provided in Table 1. This table shows that 134 (39.5%) participants had been diagnosed with type 2 diabetes before transplant and the rest had developed PTDM.
Table 1:
Patient characteristics (N = 339).
| Variable | Values |
|---|---|
| KTR age (years), mean (SD) | 61.6 (9.9) |
| Male, n (%) | 250 (73.7) |
| Time after transplantation (months), median (IQR) | 72.3 (27.3–141.8) |
| Pre-KT type 2 DM, n (%) | 134 (39.5) |
| Pre-KT type 2 DM duration (years), median (IQR) | 16.9 (12.2–23.0) |
| PTDM, n (%) | 205 (60.5) |
| PTDM duration (months), median (IQR) | 47.5 (17.1–104.5) |
| Pre-KT coronary disease, n (%) | 62 (18.3) |
| Pre-KT stroke, n (%) | 21 (6.2) |
| Pre-KT peripheral vascular disease, n (%) | 34 (10) |
| Immunosuppression, n (%) | |
| Tacrolimus | 298 (87.9) |
| Cyclosporine | 10 (2.9) |
| Mycophenolic acid | 270 (79.6) |
| Everolimus | 31 (9.1) |
| Sirolimus | 32 (9.4) |
| Prednisone | 194 (57.2) |
| Antidiabetic agents, n (%) | |
| Long-acting insulin | 172 (50.7) |
| Short-acting insulin | 94 (26.8) |
| Metformin | 112 (33.0) |
| DPP-4i | 124 (36.6) |
| GLP-1 RA | 44 (13.0) |
| Others | 42 (12.4) |
| SGLT2i, n (%) | |
| Canaglifozin | 64 (18.9) |
| Empaglifozin | 193 (56,9) |
| Dapaglifozin | 81 (23.9) |
| Ertuglifozin | 1 (0.3) |
DPP-4i, dipeptidyl peptidase 4 inhibitor; GLP-1 RA, glucagon-like peptide 1 receptor agonist.
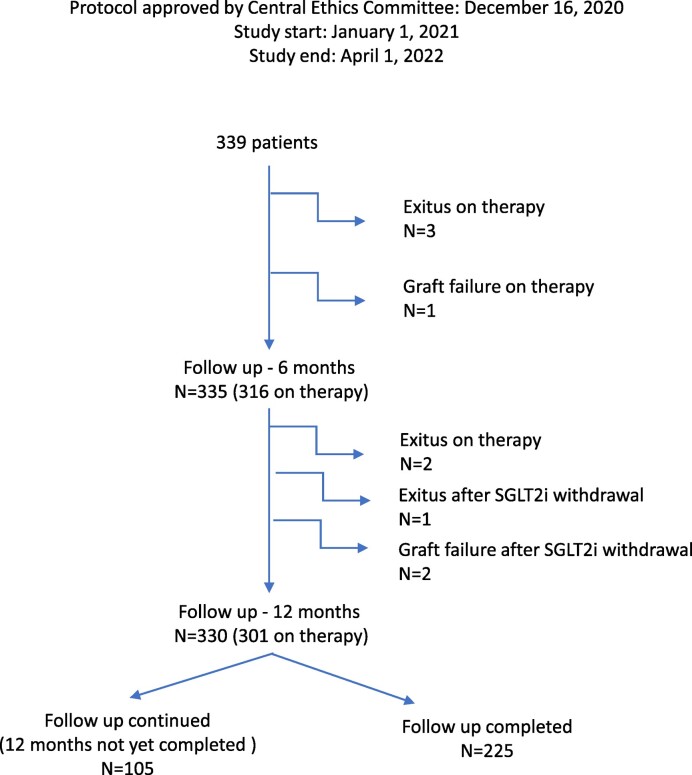
Patient flow is described in Fig. 1. The most frequently used SGLT2i was empagliflozin [n = 193 (56.9%)], followed by dapagliflozin [n = 81 (23.9%)] and canagliflozin [n = 64 (18.9%)].
Figure 1:
Flow of patients included in this study.
Adverse effects and safety
During the 6 months leading up to the onset of SGLT2i treatment, 46 patients (13.6%) had one or more UTI episodes. In the following 6 months, 35 (10.3%) patients developed a UTI, which was more frequently observed in those who had had an episode in the 6 months prior to treatment (35.6 versus 6.5%; P < .001). Also, UTI was more prevalent in women [18.5% versus 8.5% in men; P = .015]. No differences were detected in the rates of participants developing a UTI when these were stratified by SGLT2i received (canagliflozin group 8.1%, empagliflozin group 13.0%, dapagliflozin group 9.1%; P = .463). Neither were differences in UTI observed by age, time post-transplant or diabetes type. Our multivariate regression analysis adjusted for age and time post-transplant revealed that patients experiencing a UTI 6 months before initiating SGLT2i treatment [OR 7.90 (CI 3.63–17.21)] and women [OR 2.46 (CI 1.19–5.03)] had a greater risk of developing a UTI within 6 months of this treatment. In patients followed for 1 year, 13 more UTI episodes were seen. In the UTI subanalysis, incidences at 12 months were similar in DKTRs treated with SGLT2i and the reference group of non-DKTRs (17.9% versus 16.7%).
Throughout follow-up extending to up to 1 year, adverse effects were recorded in 88 (26%) patients: UTI was the most frequent [n = 48 (14%)], followed by polyuria [N = 16 (4.7%)], acute kidney injury [AKI; n = 6 (1.8%)], genital mycosis [n = 5 (1.5%)], hypoglycaemia [n = 4 (1.2%)], diarrhoea [n = 2 (0.6%)], weight loss [n = 2 (0.6%)] and other [n = 4 (1.2%)]. AKI was more frequent in patients with a baseline eGFR <40 ml/min/1.73 m2 (6.2%) versus ≥40 ml/min/1.73 m2 (0.7%; P = .003). No case was reported of ketoacidosis or bone fracture.
The drug had to be suspended in 40 patients (11.8%): definitively in 34 and temporarily in 6. Reasons for definitive SGLT2i suspension were UTI [n = 10 (3%)], AKI [n = 5 (1.5%)], genital mycosis [n = 3 (0.9%)], diarrhoea [n = 2 (0.6%)], weight loss [n = 2 (0.6%)] and other [n = 12 (3.5%)].
During follow-up, 46 patients were admitted to hospital because of 55 events. In order of frequency these were 28 episodes of infection (11 UTIs and 8 respiratory infections), 10 episodes of cardiovascular complications, 5 episodes affecting the gut, 2 episodes of AKI and 10 other episodes. No patient suffered acute rejection.
Three cases of kidney graft loss were recorded, one related to SGLT2i treatment (in a patient with recurrent urinary candidiasis who developed fungal pyelonephritis). Over the year of follow-up, six patients died of causes unrelated to SGLT2i, as judged by the investigator (three due to severe acute respiratory syndrome coronavirus 2, one brain haemorrhage, one acute myocardial infarction and one lung cancer).
Results at 6 months
As shown in Table 2, significant reductions were observed in body weight, blood pressure, fasting glucose and HbA1c levels, along with significant increases in serum magnesium and haemoglobin levels. We also detected a significant improvement in blood uric acid levels, which fell from 6.27 mg/dl (SD 1.48) to 5.85 mg/dl (SD 1.42). In patients with an eGFR <60 ml/min/1.73 m2, a significant reduction was also seen [from 6.61 (SD 1.44) at baseline to 6.16 mg/dl (SD 1.46) at 6 months; P < .001].
Table 2:
Pairwise comparisons between data recorded at baseline and at 6 months post-SGLT2i onset on an intention-to-treat basis.
| Characteristics | Baseline | 6 months of SGLT2i | Baseline versus 6 months (95% CI) | P-value |
|---|---|---|---|---|
| Body weight (kg), mean (95% CI) (n = 309) | 81.5 (79.4–83.6) | 79.3 (77.2–81.4) | −2.22 (−2.79 to −1.65)a | <.001d |
| SBP (mmHg), mean (95% CI) (n = 312) | 137 (135–139) | 132 (130–134) | −4.63 (−6.73 to −2.52)a | <.001d |
| DPB (mmHg), mean (95% CI) (n = 312) | 75.8 (74.5–77.1) | 73.6 (72.3–74.8) | −2.24 (−3.49 to −1.00)a | <.001d |
| Haemoglobin (g/dl), mean (95% CI) (n = 319) | 13.3 (13.1–13.5) | 13.8 (13.6–14.0) | 0.44 (0.29–0.58)a | <.001d |
| Fasting glycaemia (mg/dl), mean (95% CI) (n = 328) | 148 (142–154) | 133 (129–138) | −14.5 (−20.0 to −9.0)a | <.001d |
| HbA1c (%), mean (95% CI) (n = 294) | 7.56 (7.41–7.71) | 7.20 (7.05–7.35) | −0.36 (−0.51 to −0.21)a | <.001d |
| eGFR (ml/min/1.73 m2), mean (95% CI) (n = 327) | 58.4 (56.2–60.6) | 56.2 (54.0–58.5) | −2.13 (−3.26 to −1.0)a | <.001d |
| Total cholesterol (mg/dl), mean (95% CI) (n = 305) | 164 (159–168) | 159 (155–164) | −4.19 (−8.26 to −0.133)a | .043d |
| HDL cholesterol (mg/dl), mean (95% CI) (n = 268) | 49.1 (47.2–51.0) | 48.7 (46.7–50.8) | −0.36 (−1.48–0.76)a | .569d |
| Triglycerides (mg/dl), median (IQR) (n = 295) | 182 (170–193) | 186 (173–200) | 4.26 (−5.30–13.81)a | .860d |
| Serum uric acid (mg/dl), mean (95% CI) (n = 282) | 6.18 (5.98–6.38) | 5.74 (5.55–5.93) | −0.44 (−0.60 to −0.28)a | <.001d |
| Serum magnesium (mg/dl), mean (95% CI) (n = 208) | 1.61 (1.57–1.66) | 1.76 (1.72–1.80) | 0.15 (0.18–0.11)a | <.001d |
| UPC (mg/g), median (IQR) (n = 230) | 164 (82–430) | 160 (80–342) | −26 (−47 to −10)b | .006e |
| Baseline UPCR <300 mg/g, median (IQR) (n = 157) | 100 (60–174) | 110 (63–187) | −5 (−5–18)b | .226e |
| Baseline UPCR ≥300 mg/g, median (IQR) (n = 73) | 760 (454–1594) | 534 (285–1092) | −248 (−392 to −161)b | .001e |
| UACR (mg/g), median (IQR) (n = 108) | 80 (19–210) | 48 (10–171) | −16 (−30 to −5)b | .001e |
| Tacrolimus dose (mg/kg/day), mean (95% CI) (n = 285) | 0.043 (0.039–0.047) | 0.042 (0.038–0.046) | −0.001 (−0.002–0.0004)a | .222d |
| FENa (%), median (IQR) (n = 120) | 1.19 (0.81–1.76) | 1.44 (1.02–1.96) | 0.20 (0.07–0.33)b | .053e |
| Glycosuria (mg/dl), median (IQR) (n = 298) | 0 (0–150) | 1000 (500–1000) | 560 (500–650)b | <.001e |
| Tacrolimus (ng/ml), mean (95% CI) (n = 281) | 7.01 (6.73–7.29) | 6.86 (6.58–7.15) | −0.15 (−0.44–0.15)a | .340d |
| Mycophenolate (mg/day), mean (95% CI) (n = 257) | 828 (788–869) | 827 (787–866) | −1.52 (−14.91–11.86)a | .823d |
| Prednisone treatment, n (%) (n = 335) | 59.9 (53.8–66.0) | 59.8 (53.7–65.9) | −0.1 (−2.4–2.2)c | .935d |
| Prednisone dose (mg/day), mean (95% CI) (n = 177) | 5.01 (4.61–5.42) | 4.77 (4.50–5.03) | −0.25 (−0.55–0.05)c | .098d |
| Antidiabetic drugs, n (%) | ||||
| Long-acting insulin (n = 334) | 50.3 (44.1–56.5) | 46.1 (39.9–52.3) | −4.2 (−7.0 to −1.4)c | .003d |
| Short-acting insulin (n = 330) | 26.7 (21.1–32.2) | 24.6 (19.2–30.1) | −2.0 (−4.4–0.3)c | .094d |
| Metformin (n = 333) | 35.2 (29.5–41.0) | 36.7 (30.8–42.5) | 1.4 (−2.6–5.4)c | .480d |
| DPP-4i (n = 332) | 35.2 (29.2–41.2) | 33.7 (27.8–39.6) | −1.4 (−5.7–2.8)c | .509d |
| GLP-1 RA (n = 332) | 13.5 (9.3–17.6) | 14.5 (10.2–18.8) | 1.0 (−2.1–4.1)c | .528d |
| Antihypertensives, mean (95% CI) (n = 327) | 2.22 (2.04, 2.40) | 2.11 (1.94, 2.29) | −0.10 (−0.20 to −0.09) | .033d |
| ACEIs, n (%) (n = 327) | 20.3 (15.1–25.6) | 21.1 (15.8–26.4) | 0.8 (−1.6–3.2)c | .539d |
| ARBs, n (%) (n = 327) | 40.1 (33.9–46.2) | 37.5 (31.3–43.6) | −2.6 (−5.8–0.6)c | .106d |
| MBRs, n (%) (n = 327) | 9.7 (6.0–13.4) | 10.6 (6.9–14.4) | 0.9 (−1.1–3.0)c | .370d |
| Diuretics, n (%) (n = 327) | 26.4 (21.3–31.5) | 20.7 (15.9–25.6) | −5.7 (−9.1 to −2.3)c | .017d |
SBP, systolic blood pressure; DBP, diastolic blood pressure; FENa, fractional excretion of sodium; DPP-4i, dipeptidyl peptidase 4 inhibitor; GLP-1 RA, glucagon-like peptide 1 receptor agonist; ACEIs, angiotensin-converting enzyme inhibitors; ARBs, angiotensin II receptor blockers; MRBs, mineralocorticoid receptor blockers.
Difference between means (95% CI) adjusted by age and sex.
Unadjusted difference between medians (95% CI).
Difference between percentages (95% CI) adjusted by age and sex.
P-values were adjusted for sex and age (except eGFR).
For non-parametric data, P-values were calculated on log-transformed values and these were then adjusted by age and sex.
As an outcome of SGLT2i treatment, a significant increase was observed in glycosuria and fractional excretion of sodium.
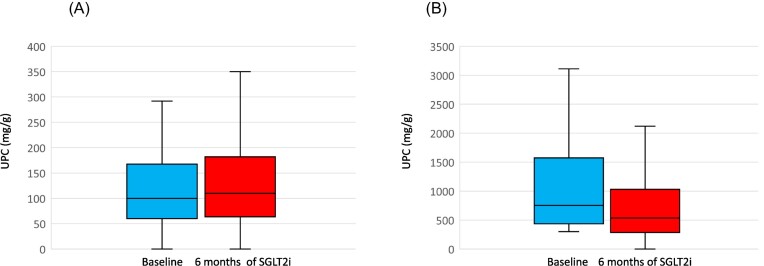
The eGFR also decreased significantly, albeit slightly, from 58.4 ml/min (SD 20.0) to 56.2. We also observed a significant yet clinically non-meaningful decline in the UPCR, from a median of 164 mg/g (IQR 82–430) to 160 (IQR 80–347). When patients were stratified by a baseline UPCR lower or higher than 300 mg/g, we noted that improvement occurred in subjects showing baseline ratios ≥300 mg/g (Fig. 2) [from 760 mg/g (IQR 454–1594) to 534 (IQR 285–1092); P < .001]. Although only measured in 108 participants, the UACR improved in these individuals, as shown in Table 2.
Figure 2:
Median (IQR) UPCR recorded at baseline and 6 months post-SGLT2i treatment onset in patients (A) with a baseline UPCR <300 mg/g (n = 173) (P = .229) and (B) those with a baseline UPCR ≥300 mg/g (n = 73) (P < .001).
When participants with pre-existing type 2 DM (Table 3) and those with PTDM (Table 4) were analysed separately, no differences were detected in terms of analytical variables or the glucose-lowering, immunosuppressive or antihypertensive medication received. Neither were differences observed between these groups of participants in the given SGLT2i taken (Table 5).
Table 3:
Pairwise comparisons between data recorded at baseline and at 6 months post-SGLT2i onset on an intention-to-treat basis in individuals with pretransplant type 2 DM.
| Characteristics | Baseline | 6 months of SGLT2i | Baseline versus 6 months (95% CI) | P-value |
|---|---|---|---|---|
| Body weight (kg), mean (95% CI) (n = 121) | 81.8 (78.1–85.6) | 79.7 (75.9–83.4) | −2.1 (−2.9 to −1.4)a | <.001d |
| SBP (mmHg), mean (95% CI) (n = 124) | 140 (137–144) | 135 (131–138) | −5.8 (−9.1 to −2.5)a | .001d |
| DPB (mmHg), mean (95% CI) (n = 124) | 75.0 (72.9–77.2) | 72.5 (70.5–74.6) | −2.5 (−4.7 to −0.3)a | .027d |
| Haemoglobin (g/dl), mean (95% CI) (n = 123) | 13.2 (12.8–13.6) | 13.5 (13.1–13.9) | 0.32 (0.07–0.59)a | .013d |
| Fasting glycaemia (mg/dl), mean (95% CI) (n = 128) | 159 (148–171) | 145 (134–155) | −14.4 (−26.5 to −2.3) a | .020d |
| HbA1c (%), mean (95% CI) (n = 111) | 7.74 (7.48–8.01) | 7.39 (7.12–7.67) | −0.35 (−0.61 to −0.09)a | .010d |
| eGFR (ml/min/1.73 m2), mean (95% CI) (n = 128) | 57.3 (54.1–60.5) | 55.4 (52.1–58.7) | −1.93 (−3.62 to −0.25)a | .025 |
| Total cholesterol (mg/dl), mean (95% CI) (n = 120) | 161 (151–170) | 148 (140–155) | −12.8 (−20.5 to −5.1)a | .001d |
| HDL cholesterol (mg/dl), mean (95% CI) (n = 106) | 51.9 (47.8–56.0) | 49.9 (46.0–53.8) | −1.97 (−3.77 to −0.16)a | .033d |
| Triglycerides (mg/dl), median (IQR) (n = 116) | 173 (153–194) | 185 (158–212) | 11.9 (−7.3–31.1)a | .221d |
| Serum uric acid (mg/dl), mean (95% CI) (n = 106) | 5.97 (5.61–6.34) | 5.57 (5.21–5.94) | − 0.40 (−0.64 to −0.15)a | .002d |
| Serum magnesium (mg/dl), mean (95% CI) (n = 84) | 1.60 (1.51–1.69) | 1.77 (1.68–1.86) | 0.17 (0.09–0.24)a | <.001d |
| UPCR (mg/g), median (IQR) (n = 92) | 205 (98–470) | 187 (108–336) | −23.0 (−57.5–0.5)b | .060e |
| Baseline UPCR <300 mg/g, median (IQR) (n = 62) | 130 (70–210) | 150 (77–210) | 10.5 (−5.0–28.0)b | .168e |
| Baseline UPCR ≥300 mg/g, median (IQR) (n = 30) | 700 (470–1632) | 470 (285–1116) | −230 (−525 to −114)b | .002e |
| UACR (mg/g), median (IQR) (n = 47) | 90 (28–238) | 45 (14–167) | −34.4 (−53.7 to −18.4)b | <.001e |
| Tacrolimus dose (mg/kg/day), mean (95% CI) (n = 116) | 0.040 (0.036–0.044) | 0.039 (0.035–0.043) | −0.001 (−0.002–0.001)a | .329d |
| FENa (%), median (IQR) (n = 49) | 1.25 (0.90–1.78) | 1.58 (1.18–2.19) | 0.23 (0.07–0.43)b | .010e |
| Glycosuria (mg/dl), median (IQR) (n = 116) | 1.0 (0–300) | 1000 (1000–1000) | 675 (510–750)b | <.001e |
| Tacrolimus level (ng/ml), mean (95% CI) (n = 117) | 7.16 (6.66–7.66) | 7.14 (5.23–8.97) | −0.03 (−1.90–1.85)a | .977d |
| Mycophenolate dose (mg/day), mean (95% CI) (n = 114) | 810 (758–862) | 808 (754–862) | −2.6 (−16.7–11.5)a | .717d |
| Prednisone treatment, n (%) (n = 131) | 63.7 (52.8–74.7) | 61.9 (50.8–72.9) | −1.9 (−6.6–2.9)c | .438d |
| Prednisone dose (mg/day), mean (95% CI) (n = 73) | 4.82 (3.86–5.77) | 4.49 (4.04–4.91) | −0.34 (−1.06–0.38)c | .353d |
| Antidiabetic drugs, n (%) | ||||
| Long-acting insulin (n = 132) | 79.9 (71.0–88.8) | 80.8 (72.1–89.4) | 0.9 (2.9 to −4.7)c | .644d |
| Short-acting insulin (n = 129) | 59.2 (48.2–70.3) | 55.2 (44.2–66.3) | −4.0 (−8.4–0.4)c | .073d |
| Metformin (n = 131) | 31.9 (21.8–41.9) | 34.2 (23.9–44.6) | 2.4 (−3.5–8.2)c | .426d |
| DPP-4i (n = 130) | 30.1 (19.7–40.6) | 29.2 (19.0–39.4) | −0.9 (−7.7–5.8)c | .783d |
| GLP-1 RA (n = 130) | 15.7 (7.2–24.1) | 18.2 (9.4–27.0) | 2.6 (−3.0–8.1)c | .368d |
| Antihypertensives, n (%) | .374d | |||
| ACEIs (n = 128) | 21.9 (12.6–31.3) | 21.9 (12.6–31.2) | 0.01 (−2.9– 2.9)c | .995d |
| ARBs (n = 128) | 34.5 (23.3–45.6) | 37.6 (26.3–48.9) | 3.1 (−1.4–17.2)c | .172d |
| MBRs (n = 127) | 7.9 (1.0–14.9) | 9.6 (2.6–16.6) | 1.7 (−2.4–5.8)c | .412d |
| Diuretics (n = 128) | 29.9 (19.9–39.8) | 27.2 (17.4–37.0) | −2.7 (−8.4–3.1)c | .359d |
SBP, systolic blood pressure; DBP, diastolic blood pressure; FENa, fractional excretion of sodium; DPP-4i, dipeptidyl peptidase 4 inhibitor; GLP-1 RA, glucagon-like peptide 1 receptor agonist; ACEIs, angiotensin-converting enzyme inhibitors; ARBs, angiotensin II receptor blockers; MRBs, mineralocorticoid receptor blockers.
Difference between means (95% CI) adjusted by age and sex.
Unadjusted difference between medians (95% CI).
Difference between percentages (95% CI) adjusted by age and sex.
P-values were adjusted for sex and age (except eGFR).
For non-parametric data, P-values were calculated using the Wilcoxon test.
Table 4:
Pairwise comparisons between data recorded at baseline and at 6 months post-SGLT2i onset on an intention-to-treat basis in individuals with post-transplant diabetes mellitus.
| Characteristics | Baseline | 6 months of SGLT2i | Baseline versus 6 months (95% CI) | P-value |
|---|---|---|---|---|
| Body weight (kg), mean (95% CI) (n = 188) | 81.8 (78.6–83.7) | 78.9 (76.4–81.4) | −2.22 (−3.00 to −1.43)a | <.001d |
| SBP (mmHg), mean (95% CI) (n = 188) | 135 (133–137) | 131 (128–133) | −3.93 (−6.70 to −1.15)a | .006d |
| DPB (mmHg), mean (95% CI) (n = 188) | 76.2 (74.6–77.9) | 74.0 (72.4–75.6) | −2.23 (−3.80 to −0.66)a | .006d |
| Haemoglobin (g/dl), mean (95% CI) (n = 196) | 13.4 (13.2–13.6) | 13.9 (13.7–14.1) | 0.46 (0.28–0.65)a | <.001d |
| Fasting glycaemia (mg/dl), mean (95% CI) (n = 200) | 142 (136–148) | 128 (123–133) | −14.4 (−20.1 to −8.7)a | <.001d |
| HbA1c (%), mean (95% CI) (n = 183) | 7.45 (7.29–7.65) | 7.01 (6.92–7.28) | −0.37 (−0.55 to −0.19)a | <.001d |
| eGFR (ml/min/1.73 m2), mean (95% CI) (n = 199) | 59.0 (56.1–62.0) | 57.1 (54.0–60.1) | −1.98 (−3.44 to −0.51)a | .009 |
| Total cholesterol (mg/dl), mean (95% CI) (n = 185) | 165 (160–170) | 165 (160–170) | −0.37 (−5.15–4.44)a | .880d |
| HDL cholesterol (mg/dl), mean (95% CI) (n = 162) | 47.9 (45.8–49.9) | 48.3 (45.9–50.7) | 0.46 (−1.00–1.92)a | .537d |
| Triglycerides (mg/dl), median (IQR) (n = 179) | 186 (170–201) | 185 (171–199) | −0.69 (−11.49–10.11)a | .899d |
| Serum uric acid (mg/dl), mean (95% CI) (n = 176) | 6.27 (6.03–6.51) | 5.81 (5.58–6.03) | −0.46 (−0.67 to −0.25)a | <.001d |
| Serum magnesium (mg/dl), mean (95% CI) (n = 124) | 1.62 (1.58–1.67) | 1.75 (1.71–1.80) | 0.13 (0.09–0.17)a | <.001d |
| UPC (mg/g), median (IQR) (n = 138) | 140 (70–396) | 133 (70–358) | −29.0 (−57.0 to −8.0)b | <.001e |
| Baseline UPCR <300 mg/g, median (IQR) (n = 95) | 97 (60–144) | 94 (60–160) | 3.0 (−11.0–20.0)b | .641e |
| Baseline UPCR ≥300 mg/g, median (IQR) (n = 43) | 838 (430–1600) | 607 (279–1088) | −258 (−505 to −139)b | <.001e |
| UACR (mg/g), median (IQR) (n = 61) | 56 (14–190) | 50 (9–255) | −3.30 (−17.0–2.80)b | .339e |
| Tacrolimus dose (mg/kg/day), mean (95% CI) (n = 169) | 0.048 (0.039–0.047) | 0.048 (0.040–0.055) | −0.001 (−0.002–0.001)a | .543d |
| FENa (%), median (IQR) (n = 71) | 1.15 (0.75–1.76) | 1.33 (0.93–1.96 | 0.17 (−0.007–0.35)b | .06e |
| Glycosuria (mg/dl), median (IQR) (n = 182) | 0 (0–100) | 1000 (300–1000) | 675 (500–600)b | <.001e |
| Tacrolimus level (ng/ml), mean (95% CI) (n = 164) | 6.86 (6.52–7.19) | 6.96 (6.58–7.34) | 0.11 (−0.26–0.47)a | .567d |
| Mycophenolate dose (mg/day), mean (95% CI) (n = 143) | 845 (784–905) | 844 (787–901) | −0.55 (−21.3–20.2)a | .959d |
| Prednisone treatment, n (%) (n = 204) | 57.2 (49.8–64.7) | 58.3 (50.8–65.7) | 1.0 (−1.6–3.6) | .438d |
| Prednisone dose (mg/day), mean (95% CI) (n = 104) | 4.97 (4.61–5.33) | 4.82 (4.48–5.17) | −0.14 (−0.39–0.11)c | .262d |
| Antidiabetic drugs, n (%) | ||||
| Long-acting insulin (n = 202) | 33.7 (26.5–40.9) | 26.9 (20.0–33.8) | −6.80 (−10.60 to −3.00)c | <.001d |
| Short-acting insulin (n = 201) | 10.2 (5.4–15.0) | 9.3 (4.6–13.9) | −0.9 (−3.7–2.0)c | .557d |
| Metformin (n = 202) | 37.3 (30.2–44.3) | 36.7 (29.6–43.8) | −0.5 (−6.2–5.1)c | .850d |
| DPP-4i (n = 202) | 38.6 (31.2–46.1) | 36.6 (29.2–43.9) | −2.0 (−7.6–3.6)c | .474d |
| GLP-1 RA (n = 202) | 11.4 (6.9–16.0) | 11.8 (7.2–16.4) | 0.4 (−3.5–4)c | .985d |
| Antihypertensives, n (%) | ||||
| ACEIs (n = 199) | 20.8 (14.3–27.2) | 21.9 (15.3–28.4) | 1.1 (−2.3–4.5)c | .532d |
| ARBs (n = 199) | 42.1 (34.5–49.7) | 36.7 (29.3–44.1) | −5.4 (−9.7 to −1.1)c | .014 d |
| MBRs (n = 200) | 10.0 (5.5–14.5) | 10.7 (6.2–15.3) | 0.7 (−1.7–3.1)c | .556d |
| Diuretics (n = 199) | 24.4 (18.5–30.4) | 17.5 (11.9–23.0) | −7.0 (−11.3 to −2.7)c | .002d |
SBP, systolic blood pressure; DBP, diastolic blood pressure; FENa, fractional excretion of sodium; DPP-4i, dipeptidyl peptidase 4 inhibitor; GLP-1 RA, glucagon-like peptide 1 receptor agonist; ACEIs, angiotensin-converting enzyme inhibitors; ARBs, angiotensin II receptor blockers; MRBs, mineralocorticoid receptor blockers.
Difference between means (95% CI) adjusted by age and sex.
Unadjusted difference between medians (95% CI).
Difference between percentages (95% CI) adjusted by age and sex.
P-values were adjusted for sex and age (except eGFR).
For non-parametric data, P-values were calculated using the Wilcoxon test.
Table 5:
Pairwise comparisons between data recorded at baseline and at 6 months post-SGLT2i onset on an intention-to-treat basis stratified by SGLT2i prescribed.
| Characteristics | Baseline | 6 months of SGLT2i |
P-value baseline versus 6 months |
P-value,a,b SGLT2i |
|---|---|---|---|---|
| Body weight (kg), mean (95% CI) | .334 | |||
| Canagliflozin (n = 62) | 85.6 (81.4–90.0) | 83.2 (80.0–87.5) | <.001 | |
| Empagliflozin (n = 170) | 84.0 (80.4–86.6) | 81.8 (79.2–84.3) | <.001 | |
| Dapagliflozin (n = 76) | 81.4 (76.1–83.8) | 79.9 (76.1–83.8) | <.001 | |
| SBP (mmHg), mean (95% CI) | .650 | |||
| Canagliflozin (N = 62) | 137 (133–140) | 131 (127–135) | .01 | |
| Empagliflozin (m = 172) | 138 (136–141) | 133 (132–136) | .01 | |
| Dapagliflozin (N = 77) | 133 (130–137) | 130 (127–134) | .141 | |
| DPB (mmHg), mean (95% CI) | .993 | |||
| Canagliflozin (N = 62) | 75.9 (73.4–78.4) | 73.5 (71.1–76.0) | .034 | |
| Empagliflozin (n = 172) | 77.0 (75.5–78.5) | 74.7 (73.2–76.1) | .003 | |
| Dapagliflozin (n = 77) | 74.8 (72.5–77.0) | 72.6 (70.4–74.8) | .063 | |
| Haemoglobin (g/dl), mean (95% CI) | .318 | |||
| Canagliflozin (n = 60) | 13.8 (13.3–14.2) | 14.1 (13.7–14.5) | .021 | |
| Empagliflozin (N = 182) | 13.6 (13.3–13.8) | 14.0 (13.7–14.2) | <.001 | |
| Dapagliflozin (n = 76) | 13.3 (13.0–13.7) | 13.9 (13.6–14.3) | <.001 | |
| Fasting glycaemia (mg/dl), mean (95% CI) | .343 | |||
| Canagliflozin (n = 62) | 147 (136–158) | 129 (120–139) | .009 | |
| Empagliflozin (n = 187) | 149 (142–155) | 139 (134–145) | .002 | |
| Dapagliflozin (n = 78) | 149 (140–159) | 133 (124–141) | .001 | |
| HbA1c (%), mean (95% CI) | .075 | |||
| Canagliflozin (n = 56) | 7.50 (7.20–7.80) | 7.05 (6.75–7.35) | .002 | |
| Empagliflozin (n = 164) | 7.53 (7.35–7.70) | 7.32 (7.15–7.50) | .022 | |
| Dapagliflozin (n = 73) | 7.50 (7.24–7.76) | 6.96 (6.70–7.22) | <.001 | |
| eGFR (ml/min/1.73 m2), mean (95% CI) | .607 | |||
| Canagliflozin (n = 62) | 58.5 (53.5–63.4 | 55.4 (50.3–60.6) | .027 | |
| Empagliflozin (n = 187) | 59.1 (56.2–62.0) | 57.4 (54.4–60.3) | .032 | |
| Dapagliflozin (n = 77) | 55.9 (51.4–60.4) | 54.5 (49.8–59.1) | .088 | |
| Total cholesterol (mg/dl), mean (95% CI) | .794 | |||
| Canagliflozin (n = 60) | 167 (158–177) | 162 (153–171) | .241 | |
| Empagliflozin (n = 170) | 155 (149–160) | 151 (145–156) | .075 | |
| Dapagliflozin (n = 74) | 158 (150–166) | 156 (148–164) | .647 | |
| HDL cholesterol (mg/dl), mean (95% CI) | .565 | |||
| Canagliflozin (n = 47) | 46.7 (42.3–51.1) | 47.0 (42.4–51.6) | .809 | |
| Empagliflozin (n = 149) | 45.4 (42.9–47.8) | 45.3 (42.7–47.9) | .900 | |
| Dapagliflozin (n = 71) | 48.1 (44.5–51.7) | 46.9 (43.2–50.7) | .202 | |
| Triglycerides (mg/dl), median (IQR) | .654 | |||
| Canagliflozin (n = 54) | 174 (149–200) | 172 (145–199) | .779 | |
| Empagliflozin (n = 167) | 178 (164–193) | 180 (165–199) | .742 | |
| Dapagliflozin (n = 73) | 180 (158–202) | 189 (166–213) | .242 | |
| Serum uric acid (mg/dl), mean (95% CI) | .908 | |||
| Canagliflozin (n = 52) | 6.08 (5.68–6.49) | 5.93 (5.54–6.32) | .397 | |
| Empagliflozin (n = 157) | 6.32 (6.09–6.55) | 5.87 (5.65–6.91) | <.001 | |
| Dapagliflozin (n = 72) | 6.29 (5.94–6.63) | 5.82 (5.49–6.15) | .001 | |
| Serum magnesium (mg/dl), mean (95% CI) | .839 | |||
| Canagliflozin (n = 22) | 1.61 (1.50–1.72) | 1.74 (1.63–1.85) | .001 | |
| Empagliflozin (n = 130) | 1.61 (1.56–1.65) | 1.75 (1.71–1.80) | <.001 | |
| Dapagliflozin (n = 76) | 1.64 (1.57–1.71 | 1.80 (1.73–1.87) | <.001 | |
| UPCR (mg/g), median (IQR) | .222 | |||
| Canagliflozin (n = 47) | 140 (80–345) | 130 (70–249) | .056 | |
| Empagliflozin (n = 128) | 174 (75–426) | 160 (77–348) | .027 | |
| Dapagliflozin (n = 54) | 198 (95–618) | 185 (98–490) | .100 | |
| Baseline UPCR <300 mg/g, median (IQR) | .386 | |||
| Canagliflozin (n = 35) | 100 (68–170) | 110 (66–170) | .815 | |
| Empagliflozin (n = 87) | 101 (51–178) | 110 (59–210) | .293 | |
| Dapagliflozin (n = 34) | 112 (70–171) | 113 (72–183) | .241 | |
| Baseline UPC ≥300 mg/g, median (IQR) | .072 | |||
| Canagliflozin (n = 12) | 1680 (447–3945) | 602 (275–2350) | .028 | |
| Empagliflozin (n = 41) | 709 (430–1423) | 520 (273–919) | <.001 | |
| Dapagliflozin (n = 20) | 820 (524–1334) | 699 (411–1093) | .006 | |
| UAC (mg/g), median (IQR) | .900 | |||
| Canagliflozin (n = 42) | 90 (14–163) | 50 (10–175) | .037 | |
| Empagliflozin (n = 38) | 77 (20–215) | 41 (12–151) | .054 | |
| Dapagliflozin (n = 27) | 82 (28–360) | 50 (10–210) | .091 |
SBP, systolic blood pressure; DBP, dyastolic blood pressure.
For parametric data, P-values were calculated in a repeated measures general linear model.
For non-parametric data, P-values were calculated on log-transformed values and these were then adjusted by age and sex.
Results at 12 months
Currently we have 12-month follow-up data for 225 patients (Table 6). These data reveal maintained improvements with respect to baseline levels in most of the variables examined at 6 months, including blood pressure, haemoglobin, fasting glycemia, HbA1c, serum uric acid and magnesium, UPCR (if baseline ≥300 mg/g), UACR, fractional excretion of sodium and glycosuria. No differences were observed between the 6- and 12-month follow-up visits in the variables examined except body weight, which continued to decline (P = .001).
Table 6:
Comparison between baseline data and 6 and 12 months post-SGLT2i treatment.
| Characteristics | Baseline | 6 months post-SGLT2i | 12 months post-SGLT2i | P-value* |
|---|---|---|---|---|
| Body weight (kg), mean (SD) (n = 194) | 83.9 (17.6) | 81.8 (17.2) | 80.6 (17.4) | <.001a,b,c |
| SBP (mmHg), mean (SD) (n = 198) | 137.2 (15.7) | 133.0 (15.9) | 132.0 (14.9) | <.001a,b |
| DBP (mmHg), mean (SD) (n = 198) | 76.7 (9.8) | 74.2 (9.8) | 74.5 (10.0) | <.001a,b |
| Haemoglobin (g/dl), mean (SD) (n = 218) | 13.6 (1.7) | 14.1 (1.6) | 14.2 (1.5) | <.001a,b |
| Fasting glycaemia (mg/dl), mean (SD) (n = 224) | 152.8 (42.2) | 135.2 (37.2) | 139.1 (50.0) | <.001a,b |
| HbA1c (%), mean (SD) (n = 188) | 7.61 (1.18) | 7.12 (0.94) | 7.14 (0.99) | <.001a,b |
| eGFR (ml/min/1.73 m2), mean (SD) (n = 225) | 60.2 (20.2) | 58.5 (20.9) | 58.8 (21.2) | .01a |
| Uric acid (mg/dl), mean (SD) (n = 188) | 6.20 (1.41) | 5.79 (1.30) | 5.70 (1.26) | <.001a,b |
| Magnesium (mg/dl), mean (SD) (n = 138) | 1.61 (0.27) | 1.76 (0.25) | 1.79 (0.27) | <.001a,b |
| UPCR (mg/g), median (IQR) (n = 152) | 156 (80–380) | 156 (90–370) | 150 (90–407) | .320 |
| Baseline UPCR <300 mg/g, median (IQR) (n = 132) | 156 (80–380) | 159 (90–370) | 150 (90–407) | .119 |
| Baseline UPCR ≥300 mg/g, median (IQR) (n = 49) | 750 (390–1410) | 520 (270–950) | 440 (230–700) | .001a,b |
| UACR (mg/g), median (IQR) (n = 62) | 82 (28–253) | 50 (18–210) | 50 (17–180) | <.001a,b |
| FENa (%), median (IQR) (n = 78) | 1.07 (0.76–1.63) | 1.34 (0.93–1.82) | 1.29 (0.92–1.96) | .002a,b |
| Glycosuria (mg/dl), median (IQR) (n = 201) | 0 (0–150) | 1000 (500–1000) | 1000 (500–1000) | <.001a,b |
| Tacrolimus dose (mg/kg/day), mean (95% CI) (n = 167) | 0.0394 (0.0282) | 0.0389 (0.0266) | 0.0388 (0.0279) | |
| Tacrolimus level (ng/ml), mean (SD) (n = 192) | 6.92 (2.00) | 6.86 (2.00) | 7.14 (2.10) | .717 |
| Mycophenolic acid dose (mg/day), mean (SD) (n = 177) | 856 (298) | 854 (288) | 846 (292) | .822 |
| Prednisone treatment, n (%) (n = 224) | 111 (55) | 105 (52.0) | 104 (51.5) | .092 |
| Prednisone dose (mg/day), mean (SD) (n = 186) | 4.73 (1.81) | 4.67 (1.78) | 4.64 (1.78) | .430 |
| Antidiabetic drugs, n (%) | ||||
| Insulin (n = 224) | 118 (52.7) | 110 (49.1) | 105 (46.9) | .009b |
| Long-acting insulin (n = 224) | 117 (52.2) | 108 (48)2) | 105 (46.9) | .026b |
| Short-acting insulin (n = 224) | 65 (29.0) | 59 (26.3) | 57 (25.5) | .100 |
| Metformin (n = 224) | 74 (33.0) | 88 (39.3) | 92 (41.8) | <.002a,b |
| DPP-4i ( n = 221) | 89 (40.3) | 86 (38.9) | 83 (37.6) | .528 |
| GLP-1 RA (n = 221) | 26 (11.8) | 29 (13.1) | 34 (15.4) | .255 |
SBP, systolic blood pressure; DBP, diastolic blood pressure; FENa, fractional excretion of sodium; DPP-4i, dipeptidyl peptidase 4 inhibitor; GLP-1 RA, glucagon-like peptide 1 receptor agonist.
Manova test; Bonferroni post hoc analysis.
P < .05 baseline versus 6 months post-SGLT2i.
P < .05 basal versus 12 months post-SGLT2i.
P < .05 6 versus 12 months post-SGLT2i.
To assess adherence to SGLT2i treatment, we revised the pharmacy fill database of the centre with the largest number of participating patients. All medications were withdrawn. In two individuals there were 7- and 10-day delays in collecting one of the monthly supplies. This database pertaining to the Madrid Community [Módulo Único de Prescripción (MUP)] provides prescription details for all individuals living in this region.
DISCUSSION
The pathogenesis of diabetic nephropathy is multifactorial such that different structural, physiological, haemodynamic and inflammatory alterations cause progressive kidney damage. Increased activity of the SGLT2 transporter plays a key role in triggering many of these pathophysiological abnormalities. Glucose hyperreabsorption in the proximal tubule involves massive energy consumption, which drastically increases oxygen demand, leading to ischaemia. In addition, this hyperreabsorption saturates normal glucose oxidation pathways, prompting the use of other pathways, the formation of advanced glycosylation products and the production of free oxygen radicals (reviewed in DeFronzo et al. [32]). Inhibition of SGLT2 reverses many of these disturbances and can be a useful strategy to prevent this damage. In KTRs, this is especially important, as these individuals have a reduced functioning renal mass and may also have been subjected to other causes of damage such as ischaemia–reperfusion injury, rejection, etc. Furthermore, a cardioprotective effect of SGLT2i has been described in type 2 diabetic patients [9–16]. We should also mention that kidney transplant, regardless of the presence or not of diabetes, is linked to a high rate of cardiovascular mortality [8] because of conditions inherent to the transplant itself (impaired renal function, viral infections, etc.) and the consequences of immunosuppressive therapy.
To the best of our knowledge, this is the observational study examining SGLT2i treatment in KTRs with the largest number of participants. Our results show that the use of SGLT2i in diabetic KTRs is fairly safe. It is well known that immunosuppressive treatment is related to a high infection risk and that the glycosuria induced by SGLT2i may promote bacterial and fungal growth. In our study, the rate of UTI was ≈14%, similar to that observed in other studies in diabetic KTRs treated with these drugs [17, 24], yet somewhat higher than the rates provided in the literature for non-transplanted individuals with diabetes [9, 10, 12]. We should also consider that UTI is a frequent complication in KTRs [33], the prevalence of which varies considerably between studies and locations, from 7% to 80% [34]. Further, having diabetes has been shown to increase the risk of UTI in KTRs [33]. We observed no significant difference in infection rates between before and after initiating SGLT2i treatment. Neither did our subanalysis reveal a higher incidence of UTI in non-diabetic KTRs versus treated diabetic KTRs. The identified risk factors for UTI were having had a prior UTI before SGLT2i treatment and female sex. We also recorded a 1.5% rate of genital mycotic infections, similar to the figure reported by others [17]. Notwithstanding, one woman with a history of genital mycotic infections lost her kidney graft to fungal pyelonephritis. This points to a need to carefully consider these drugs in female KTRs with a previous history of UTI.
Because of their diuretic effects, SGLT2is may reduce intravascular volume status. In our patient cohort, treatment led to AKI in 1.5% of participants, which prompted drug suspension. While a mild significant reduction in eGFR was observed after 6 months of treatment, by 12 months the eGFR had stabilized. This initial decline in eGFR followed by kidney function stabilization has also been observed in clinical trials conducted in non-transplanted diabetic subjects [9, 10, 17]. Further, it is well known that kidney function declines gradually after transplantation. In a large cohort of KTRs, GFR decreased by an average of 1.66 ml/min/1.73 m2/year [35]. As we had no control arm, we cannot attribute the decrease in eGFR observed to the use of SGLT2i, as declining kidney function could be part of the natural course of the transplant itself.
The reduction in proteinuria observed in response to therapy, especially in patients with high baseline values likely reflecting a more established diabetic nephropathy, is consistent with the findings of clinical trials performed in non-transplanted subjects with diabetes [9, 10]. Proteinuria is a clear risk factor for kidney allograft loss and death [36]. In fact, a recent Korean retrospective study showed that SGLT2i treatment produces a decrease in proteinuria and improves graft and patient survival [25]. Although we have no information on whether proteinuria in our patients was attributable or not to diabetic kidney disease, treatment was able to improve proteinuria and this effect persisted at 1 year. In the Dapagliflozin in Patients with Chronic Kidney Disease (DAPA-CKD) study [10], dapagliflozin treatment improved proteinuria even in non-diabetic patients with kidney disease. Hence these agents could be beneficial in KTRs with proteinuria related or not to their diabetes.
As reported by others [17, 19–21, 23], treatment was able to improve glycaemic control, involving reductions in levels of both fasting glucose and HbA1c. Other interesting effects also reported by others in DKTRs treated with SGLT2i were weight loss [17, 19, 21, 23, 24] and improved blood pressure control [21, 23] and haemoglobin levels [17, 18, 21, 22].
Following SGLT2i treatment, we also observed a decrease in serum uric acid concentrations. Post-kidney transplant hyperuricaemia is common, with a reported prevalence of 15.5–84% [37]. Hyperuricemia is an independent predictor of the development and progression of diabetic kidney disease, atherosclerosis, hypertension and cardiovascular disease [38]. A meta-analysis including 62 randomized controlled trials associated SGLT2i treatment with a significant reduction in serum uric acid levels [39]. However, this dramatic reduction was not observed in CKD patients (eGFR<60 ml/min/1.73 m2) [39]. Here we observed an improvement in blood uric acid levels even in patients with a baseline eGFR below this threshold. While we are unaware of the impact that normalization of uric acid levels could have on DKTRs, we feel that this is a positive finding of our study.
Another interesting finding was an increase in serum magnesium levels. This has also been observed in non-transplanted diabetic patients [40, 41]. Low serum magnesium is the most frequently described electrolyte disturbance in renal transplantation (≈25%) [42], tacrolimus treatment being the major risk factor for its development. In KTRs, hypomagnesemia is difficult to treat and oral magnesium supplementation is ineffective [43]. This disturbance has been linked to the development and progression of diabetes, CKD and hypertension, along with cardiovascular risk (reviewed in Rodelo-Haad et al. [44]). Accordingly, it remains to be established whether the increase in serum magnesium induced by SGLT2is could have a beneficial impact and SGLT2is could be used to treat KTRs with hypomagnesemia.
The main limitation of the present study was that it was an observational study of clinical practice and we had no control arm. This was partly because we believe that KTRs should benefit from this treatment and have ethical concerns about depriving patients of this treatment. Our intention was to use these drugs in all participants with poor glycaemic control and/or high renal or cardiovascular risk factors. A control arm of untreated DKTRs (which at the moment would be retrospective) could introduce bias, as these patients may be in a worse clinical situation. Another limitation was that our median follow-up time was short. We nevertheless consider that in KTRs the most worrying complications (infection, renal function decline, acute rejection, etc.) usually develop early on. Among the strengths of this study, we should highlight its large sample size; the systematic collection of data before, at baseline and 6 and 12 months post-SGLT2i treatment onset; and the recording of adverse events, hospitalizations and cardiovascular events. Finally, while we did not include an objective measure of adherence to the new treatment, the presence of elevated glycosuria suggests that participants did comply with the medication regimen. Moreover, pharmacy SGLT2i prescription fills were adequate at the centre recruiting the largest number of participants.
In conclusion, the use of SGLT2i in DKTRs offers benefits in terms of control of glycaemia, weight, blood pressure, anaemia, proteinuria, serum magnesium and serum uric acid, provoking few adverse effects. Nevertheless, these agents should be carefully considered in female KTRs and in those with a history of recurrent UTI.
Supplementary Material
ACKNOWLEDGEMENTS
The authors thank Oscar García Ruiz for creating the database for this study and the coordinators of SENTRA of the Spanish Society of Nephrology (Marta Crespo Arias and Emilio Rodrigo) for help with recruiting the participating centres.
APPENDIX
Participating investigators:
Ana I. Sánchez Fructuoso, Isabel Pérez Flores, Nancy Daniela Valencia Morales and Clara García Carro (Nephrology Department, Hospital Clínico San Carlos IdSSC, Complutense University, Madrid, Spain);
Andrea Bedia Raba and Sofia Zárraga (Nephrology Department, Hospital de Cruces, Bilbao, Spain);
Eduardo Banegas Deras and Natalia Ridao Cano (Nephrology Department, Hospital Central de Asturias, Oviedo, Spain);
Luis Alberto Vigara Sánchez and Auxiliadora Mazuecos Blanca (Nephrology Department, Hospital Puerta del Mar, Cádiz, Spain);
Rosalía Valero San Cecilio and Covadonga López del Moral Cuesta (Nephrology Department, Hospital Marqués de Valdecilla, Santander, Spain);
Antonio Franco Esteve and Noelia Balibrea Lara (Nephrology Department, Hospital General de Alicante, Alicante, Spain);
Leonidas L. Cruzado Vega and Alba Santos García (Nephrology Department, Hospital General de Elche, Elche, Spain);
Eva Gavela Martínez and Asunción Sancho Calabuig (Nephrology Department, Hospital Peset, Valencia, Spain);
María E. González Garcia and María Ovidia López (Nephrology Department, Hospital La Paz, Madrid, Spain);
Pablo Saurdy Coronado and Inmaculada Lorenzo (Nephrology Department, Complejo Hospitalario de Albacete, Albacete, Spain);
Domingo Hernández Marrero (Nephrology Department, Hospital Carlos Haya, Malaga, Spain);
Isabel Beneyto Castello (Nephrology Department, Hospital La Fe, Valencia, Spain);
Javier Paul Ramos (Nephrology Department, Hospital Miguel Servet, Zaragoza, Spain);
Adriana Sierra Ochoa and Carlos Arias Cabrales (Nephrology Department, Hospital del Mar, Barcelona, Spain);
Carmen Facundo Molas and Luis Guirado Perich (Nephrology Department, Fundación Puigvert, Barcelona, Spain);
Francisco González Roncero (Nephrology Department, Hospital Virgen del Rocio, Sevilla, Spain);
Armando Torres Ramírez and Nuria Sanchez Dorta (Hospital Universitario de Canarias, Tenerife, Spain) and
Secundino Cigarrán Guldris (Nephrology Department, Hospital Da Costa, Lugo, Spain).
Contributor Information
Ana I Sánchez Fructuoso, Nephrology Department, Hospital Clínico San Carlos IdSSC, Complutense University, Madrid, Spain.
Andrea Bedia Raba, Nephrology Department, Hospital de Cruces, Bilbao, Spain.
Eduardo Banegas Deras, Nephrology Department, Hospital Central de Asturias, Oviedo, Spain.
Luis A Vigara Sánchez, Nephrology Department, Hospital Puerta del Mar, Cádiz, Spain.
Rosalía Valero San Cecilio, Nephrology Department, Hospital Marqués de Valdecilla, Santander, Spain.
Antonio Franco Esteve, Nephrology Department, Hospital General de Alicante, Alicante, Spain.
Leonidas Cruzado Vega, Nephrology Department, Hospital General de Elche, Elche, Spain.
Eva Gavela Martínez, Nephrology Department, Hospital Peset, Valencia, Spain.
María E González Garcia, Nephrology Department, Hospital La Paz, Madrid, Spain.
Pablo Saurdy Coronado, Nephrology Department, Complejo Hospitalario de Albacete, Albacete, Spain.
Nancy D Valencia Morales, Nephrology Department, Hospital Clínico San Carlos IdSSC, Complutense University, Madrid, Spain.
Sofía Zarraga Larrondo, Nephrology Department, Hospital de Cruces, Bilbao, Spain.
Natalia Ridao Cano, Nephrology Department, Hospital Central de Asturias, Oviedo, Spain.
Auxiliadora Mazuecos Blanca, Nephrology Department, Hospital Puerta del Mar, Cádiz, Spain.
Domingo Hernández Marrero, Nephrology Department, Hospital Carlos Haya, Malaga, Spain.
Isabel Beneyto Castello, Nephrology Department, Hospital La Fe, Valencia, Spain.
Javier Paul Ramos, Nephrology Department, Hospital Miguel Servet, Zaragoza, Spain.
Adriana Sierra Ochoa, Nephrology Department, Hospital del Mar, Barcelona, Spain.
Carmen Facundo Molas, Nephrology Department, Fundación Puigvert, Barcelona, Spain.
Francisco González Roncero, Nephrology Department, Hospital Virgen del Rocio, Sevilla, Spain.
Armando Torres Ramírez, Hospital Universitario de Canarias, Tenerife, Spain.
Secundino Cigarrán Guldris, Nephrology Department, Hospital Da Costa, Lugo, Spain.
Isabel Pérez Flores, Nephrology Department, Hospital Clínico San Carlos IdSSC, Complutense University, Madrid, Spain.
FUNDING
None declared.
CONFLICT OF INTEREST STATEMENT
None declared.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Koye DN, Magliano DJ, Nelson RGet al. The global epidemiology of diabetes and kidney disease. Adv Chronic Kidney Dis 2018;25:121–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lim WH, Wong G, Pilmore HLet al. Long-term outcomes of kidney transplantation in people with type 2 diabetes: a population cohort study. Lancet Diabetes Endocrinol 2017;5:26–33. [DOI] [PubMed] [Google Scholar]
- 3. Hart A, Lentine KL, Smith JMet al. OPTN/SRTR 2019 annual data report: kidney. Am J Transplant 2021;21:21–137. [DOI] [PubMed] [Google Scholar]
- 4. Shivaswamy V, Boerner B, Larsen J. Post-transplant diabetes mellitus: causes, treatment, and impact on outcomes. Endocr Rev 2016;37:37–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sharif A, Hecking M, de Vries APet al. Proceedings from an international consensus meeting on posttransplantation diabetes mellitus: recommendations and future directions. Am J Transplant 2014;14:1992–2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chowdhury TA, Wahba M, Mallik Ret al. Association of British Clinical Diabetologists and Renal Association guidelines on the detection and management of diabetes post solid organ transplantation. Diabet Med 2021;38:e14523. [DOI] [PubMed] [Google Scholar]
- 7. Porrini E, Diaz JM, Moreso Fet al. Prediabetes is a risk factor for cardiovascular disease following renal transplantation. Kidney Int 2019;96:1374–80. [DOI] [PubMed] [Google Scholar]
- 8. Ying T, Shi B, Kelly PJet al. Death after kidney transplantation: an analysis by era and time post-transplant. J Am Soc Nephrol 2020;31:2887–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Perkovic V, Jardine MJ, Neal Bet al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med 2019;380:2295–306. [DOI] [PubMed] [Google Scholar]
- 10. Heerspink HJL, Stefánsson BV, Correa-Rotter Ret al. Dapaglifozin in patients with chronic kidney disease. N Engl J Med 2020;383:1436–46. [DOI] [PubMed] [Google Scholar]
- 11. Jiang Y, Yang P, Fu Let al. Comparative cardiovascular outcomes of SGLT2 inhibitors in type 2 diabetes mellitus: a network meta-analysis of randomized controlled trials. Front Endocrinol 2022;13:802992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Neal B, Perkovic V, Mahaffey KWet al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med 2017;377:644–57. [DOI] [PubMed] [Google Scholar]
- 13. Wiviott SD, Raz I, Bonaca MPet al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2019;380:347–57. [DOI] [PubMed] [Google Scholar]
- 14. Cannon CP, Pratley R, Dagogo-Jack Set al. Cardiovascular outcomes with ertugliflozin in type 2 diabetes. N Engl J Med 2020;383:1425–35. [DOI] [PubMed] [Google Scholar]
- 15. Zinman B, Wanner C, Lachin JMet al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 2015;373:2117–28. [DOI] [PubMed] [Google Scholar]
- 16. Hecking M, Jenssen T.. Considerations for SGLT2 inhibitor use in post-transplantation diabetes. Nat Rev Nephrol 2019;5:525–6. [DOI] [PubMed] [Google Scholar]
- 17. Halden TAS, Kvitne KE, Midtvedt Ket al. Efficacy and safety of empagliflozin in renal transplant recipients with posttransplant diabetes mellitus. Diabetes Care 2019;42:1067–74. [DOI] [PubMed] [Google Scholar]
- 18. Rajasekeran H, Kim JS, Cardella CJet al. Use of canagliflozin in kidney transplant recipients for the treatment of type 2 diabetes: a case series. Diabetes Care 2017;40:e75–6. [DOI] [PubMed] [Google Scholar]
- 19. Alkindi F, Al-Omary HL, Hussain Qet al. Outcomes of SGLT2 inhibitors use in diabetic renal transplant patients. Transplant Proc 2020;52:175–8. [DOI] [PubMed] [Google Scholar]
- 20. Attallah N, Yassine L.. Use of empagliflozin in recipients of kidney transplant: a report of 8 cases. Transplant Proc 2019;51:3275–80. [DOI] [PubMed] [Google Scholar]
- 21. Schwaiger E, Burghart L, Signorini Let al. Empagliflozin in posttransplantation diabetes mellitus: a prospective, interventional pilot study on glucose metabolism, fluid volume, and patient safety. Am J Transplant 2019;19:907–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mahling M, Schork A, Nadalin Set al. Sodium-glucose cotransporter 2 (SGLT2) inhibition in kidney transplant recipients with diabetes mellitus. Kidney Blood Pressure Res 2019;44:984–92. [DOI] [PubMed] [Google Scholar]
- 23. Shah M, Virani Z, Rajput Pet al. Efficacy and safety of canagliflozin in kidney transplant patients. Indian J Nephrol 2019;29:278–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Song CC, Brown A, Winstead Ret al. Early initiation of sodium-glucose linked transporter inhibitors (SGLT-2i) and associated metabolic and electrolyte outcomes in diabetic kidney transplant recipients. Endocrinol Diabetes Metab 2020;4:e00185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lim JH, Kwon S, Jeon Yet al. The efficacy and safety of SGLT2 inhibitor in diabetic kidney transplant recipients. Transplantation 2022;106:e404–12. [DOI] [PubMed] [Google Scholar]
- 26. Lemke A, Brokmeier HM, Leung SBet al. Sodium-glucose cotransporter 2 inhibitors for treatment of diabetes mellitus after kidney transplantation. Clin Transplant 2022;36:e14718. [DOI] [PubMed] [Google Scholar]
- 27. University Health Network Toronto . Efficacy, Mechanisms and Safety of SGLT2 Inhibitors in Kidney Transplant Recipients (INFINITI2019). https://www.clinicaltrials.gov/ct2/show/NCT04965935?cond=Post-transplant±Diabetes±Mellitus&draw=2&rank=8 (7 October2022, date last accessed).
- 28. Duke University . CardioRenal Effects of SGLT2 Inhibition in Kidney Transplant Recipients (CREST-KT). https://clinicaltrials.gov/ct2/show/NCT04906213 (7 October2022, date last accessed).
- 29. University of Sao Paulo General Hospital Brazil . Effect of adding dapagliflozin to allograft dysfunction of renal transplanted patients. https://www.clinicaltrials.gov/ct2/show/NCT04743453?term=dapaglifozin&cond=allograft+disfunction&draw=2&rank=1.
- 30. Levey AS, Stevens LA, Schmid CHet al. A new equation to estimate glomerular filtration rate. Ann Intern Med 2009;150:604–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. American Diabetes Association Professional Practice Committee . Pharmacologic approaches to glycemic treatment: standards of medical care in diabetes—2022. Diabetes Care 2022;45(Suppl 1):S125–43. [DOI] [PubMed] [Google Scholar]
- 32. DeFronzo RA, Reeves WB, Awad AS.. Pathophysiology of diabetic kidney disease: impact of SGLT2 inhibitors. Nat Rev Nephrol 2021;17:319–34. [DOI] [PubMed] [Google Scholar]
- 33. Vidal E, Torre-Cisneros J, Blanes Met al. Bacterial urinary tract infection after solid organ transplantation in the RESITRA cohort. Transplant Infect Dis 2012;14:595–603. [DOI] [PubMed] [Google Scholar]
- 34. Hollyer I, Ison MG.. The challenge of urinary tract infections in renal transplant recipients. Transplant Infect Dis 2018;20:e12828. [DOI] [PubMed] [Google Scholar]
- 35. Gill JS, Tonelli M, Mix CHet al. The change in allograft function among long-term kidney transplant recipients. J Am Soc Nephrol 2003;14:1636–42. [DOI] [PubMed] [Google Scholar]
- 36. Fernández-Fresnedo G, Plaza JJ, Sánchez-Plumed Jet al. Proteinuria: a new marker of long-term graft and patient survival in kidney transplantation. Nephrol Dial Transplant 2004;19:47–51. [DOI] [PubMed] [Google Scholar]
- 37. Clive DM. Renal transplant-associated hyperuricemia and gout. J Am Soc Nephrol 2000;11:974–9. [DOI] [PubMed] [Google Scholar]
- 38. Kanbay M, Afsar B, Siriopol Det al. Relevance of uric acid and asymmetric dimethylarginine for modeling cardiovascular risk prediction in chronic kidney disease patients. Int Urol Nephrol 2016;48:1129–36. [DOI] [PubMed] [Google Scholar]
- 39. Zhao Y, Xu L, Tian Det al. Effects of sodium-glucose co-transporter 2 (SGLT2) inhibitors on serum uric acid level: a meta-analysis of randomized controlled trials. Diabetes Obes Metab 2018;20:458–62. [DOI] [PubMed] [Google Scholar]
- 40. Wang KM, Li J, Bhalla Vet al. Canagliflozin, serum magnesium and cardiovascular outcomes—analysis from the CANVAS Program. Endocrinol Diabetes Metab 2021;4:e00247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Toto RD, Goldenberg R, Chertow GMet al. Correction of hypomagnesemia by dapagliflozin in patients with type 2 diabetes: a post hoc analysis of 10 randomized, placebo-controlled trials. J Diabetes Complications 2019;33:107402. [DOI] [PubMed] [Google Scholar]
- 42. Beilhack G, Lindner G, Funk GCet al. Electrolyte disorders in stable renal allograft recipients. Swiss Med Wkly 2020;150:w20366. [DOI] [PubMed] [Google Scholar]
- 43. Van Laecke S, Nagler EV, Taes Yet al. The effect of magnesium supplements on early posttransplantation glucose metabolism: a randomized controlled trial. Transpl Int 2014;27:895–902. [DOI] [PubMed] [Google Scholar]
- 44. Rodelo-Haad C, Pendón-Ruiz de Mier MV, Díaz-Tocados JMet al. The role of disturbed Mg homeostasis in chronic kidney disease comorbidities. Front Cell Dev Biol 2020;8:543099. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.