ABSTRACT
Background
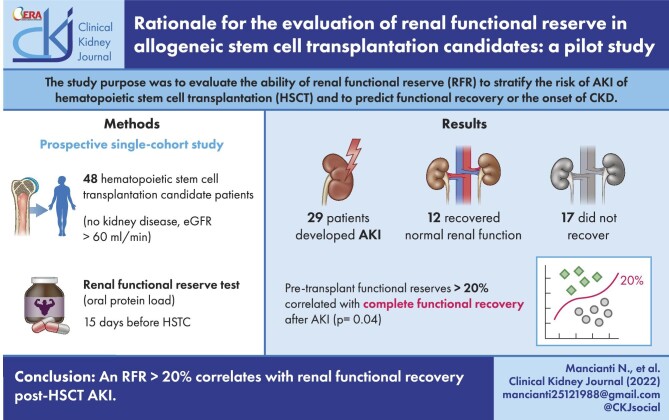
The main purpose of our study was to evaluate the ability of renal functional reserve (RFR) to stratify the risk of acute kidney injury (AKI) occurrence within 100 days of hematopoietic stem cell transplantation (HSCT) and to predict any functional recovery or the onset of chronic kidney disease. A secondary aim was to identify the clinical/laboratory risk factors for the occurrence of AKI.
Methods
The study design is prospective observational. We enrolled 48 patients with normal basal glomerular filtration rate (bGFR) who underwent allogenic HSCT. A multiparameter assessment and the Renal Functional Reserve Test (RFR-T) using an oral protein load stress test were performed 15 days before the HSCT.
Results
Different RFRs corresponded to the same bGFR values. Of 48 patients, 29 (60%) developed AKI. Comparing the AKI group with the group that did not develop AKI, no statistically significant difference emerged in any characteristic related to demographic, clinical or multiparameter assessment variables except for the estimated GFR (eGFR). eGFR ≤100 mL/min/1.73 m2 was significantly related to the risk of developing AKI (Fisher’s exact test, P = .001). Moreover, RFR-T was lower in AKI+ patients vs AKI– patients, but did not allow statistical significance (28% vs 40%). In AKI patients, RFR >20% was associated with complete functional recovery (one-sided Fisher’s exact test, P = .041). The risk of failure to recover increases significantly when RFR ≤20% (odds ratio = 5.50, 95% confidence interval = 1.06–28.4).
Conclusion
RFR identifies subclinical functional deterioration conditions essential for post-AKI recovery. In our cohort of patients with no kidney disease (NKD), the degree of pre-HSCT eGFR is associated with AKI risk, and a reduction in pre-HSCT RFR above a threshold of 20% is related to complete renal functional recovery post-AKI. Identifying eGFR first and RFR second could help select patients who might benefit from changes in transplant management or early nephrological assessment.
Keywords: acute kidney injury, chronic kidney disease, hematopoietic stem cell transplantation, renal functional reserve
Graphical Abstract
Graphical Abstract.
INTRODUCTION
Hematopoietic stem cell transplantation (HSCT) is a highly effective treatment for myelo- and lymphoproliferative disorders and bone marrow failure. Despite this success, acute kidney injury (AKI) and chronic kidney disease (CKD) remain the Achilles’ heel of HSCT. The incidence of AKI in HSCT studies is reported to be from 15% to 60% [1]. Post-HSCT-AKI is defined within the first 100 days after transplantation. Most cases of AKI develop 10–40 days after HSCT [2–4]. The incidence of AKI is higher with myeloablative compared with nonmyeloablative allogeneic HSCT [5]. Recent estimates suggest that nearly 30% of subjects undergoing allogenic HSCT will develop CKD [6, 7]. Studies reported that AKI is a risk factor for CKD and influences HSCT patients’ prognosis. In the study by Ando and colleagues, previous AKI was the factor most relevantly associated with CKD development after HSCT (odds ratio 9.92) [7]. The need for dialysis in the early post-HSCT stages occurs in 5% of patients; in these patients, the mortality rate is exceptionally high (>80%) [5]. Many factors are involved in the development of AKI, including sepsis, nephrotoxic, graft versus host disease (GVHD), veno-occlusive disease (VOD), tumor lysis syndrome, cytokine engraftment/storm syndrome and thrombotic microangiopathy (TMA) and can lead to kidney damage [8]. These factors affect the renal compartment at various levels: vascular, glomerular and tubulointerstitial. There are no HSCT scores able to predict which patients will develop renal damage and which will not. The score currently in use is a prognostic stratification index limited to the transplant outcome [the Hematopoietic Cell Transplantation–Comorbidity Index (HCT-CI) or European Society for Blood and Marrow Transplantation Risk Score] [9–11]. The HCT-CI score captures the prevalence and magnitude of various organ impairments before HSCT and provides prognostic information. From the renal point of view, it only considers whether the patient is affected by CKD. There are also no factors to discriminate which patients will resolve their kidney damage and which will develop CKD. Though HSCT candidate patients are subjected to careful clinical selection, the routine evaluation of risk factors/comorbidities does not help identify the subset of patients with the highest risk of AKI. Another challenge of post-transplant AKI is the differential diagnosis of the underlying causes. Transplant patients are prone to bleeding complications that hinder the availability of renal biopsy, meaning that these patients are often undertreated or are undergoing non-personalized therapy. An improved nephrological assessment of patients before HSCT should be of paramount importance for a correct stratification of the population that could develop AKI/CKD. Ordinarily, nephrological evaluation is limited to the glomerular filtration rate (GFR) value. The most used endogenous marker for assessing glomerular function is creatinine. Any GFR evaluation method has its advantages and disadvantages in terms of accuracy and cost. However, none of them considers the renal functional reserve (RFR), although the presence of normal GFR does not exclude the presence of kidney disease: in early stages, basal GFR (bGFR) is normal while RFR is reduced or absent [12]. The kidney adapts to the loss of some nephrons by compensating in the remaining normal nephrons, masking the initial functional deficit picture [13]. Furthermore, serum creatinine and the bGFR estimate are late indicators of renal failure: renal function is reduced by 50% before increased creatinine [14]. While numerous studies have focused on the exact measurement of GFR through complex methods, such as inulin clearance or radioisotope tracers, there are few data on the methodologies for assessing RFR. Human organs have innate mechanisms to adapt to increased demand for work. An example is heart function: at rest, cardiac output is approximately 5.0 L/min; however, during exercise, cardiac output can double or even triple [15]. Similarly, renal reserve is a physiological variable with peculiar characteristics in different renal contexts. In healthy subjects, the kidneys usually function at about 75% of their maximum capacity, adapting their functioning according to metabolic demands [16]. Some animal species show a marked functional dynamism in their GFR. Bears, while hibernating, can reduce their bGFR by 70%, while seals can increase it up to three times after a large intake of fish [17]. The absence of RFR is associated with a state of single nephron hyperfiltration, which seems to be a factor contributing to the progression of renal failure [18]. Subjects with a reduction in RFR show a ‘sub-clinical deterioration’ with increased kidney susceptibility even in the presence of a slight renal damage. Sharma et al. described a standard protocol for a “kidney stress test” to assess RFR using oral protein loads adjusted to the patient's body weight (1–1.2 g/kg), named the Renal Functional Reserve Test (RFR-T) [19]. The RFR-T could be useful in specific clinical situations (e.g. nephrological study of potential living kidney donors, before surgery, etc.) [20, 21]. The cardiac stress test has now entered the clinical practice as a fundamental utility tool for cardiac reserve evaluation. On the contrary, the RFR-T is still struggling to enter clinical practice.
Objectives of the study
Our study's main purpose was to evaluate the ability of RFR-T to stratify the risk of AKI occurrence within 100 days post-HSCT and to predict functional recovery. Follow-up was extended by an additional 3 months in patients who developed AKI, the minimum duration for delineating the onset of CKD, following Kidney Disease: Improving Global Outcomes (KDIGO) guidelines [22]. A secondary aim was to identify the clinical/laboratory risk factors for the onset of AKI.
MATERIALS AND METHODS
Study design and participants
The study design is prospective observational. We enrolled 48 patients with normal bGFR, candidates for allogenic HSCT for hematological malignancies as well as non-malignant hematological diseases. Stem cell sources could be either bone marrow or peripheral blood stem cells. The patients were recruited from the outpatient clinic of the Stem Cell Transplant and Cellular Therapy Unit, University Hospital, Siena (Italy) between April 2017 and March 2020.
Ethical approval
All procedures performed in studies involving human participants were following the ethical standards of the Institutional Research Committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. An ethics committee procedure was not required as the patient tests were in-depth nephrological analyses already known, coded, and free of possible side effects. Informed consent was obtained from all individual participants included in the study.
Eligibility
Inclusion criteria were age ≥18 years and estimated GFR (eGFR) ≥60 mL/min/1.73 m2 Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI). Autologous transplant candidates were excluded due to the lower impact of renal complications. Exclusion criteria were known kidney disease, clinically manifest cardiovascular disease, psychiatric disorders and inability to follow study instructions. Further exclusion criteria were current treatment with non-steroidal anti-inflammatory drugs, angiotensin-converting enzyme inhibitors or angiotensin receptor blockers, as these drugs could influence GFR. All participants were informed about the objectives of the study. This study was performed under the ethical principles of the Declaration of Helsinki.
Procedures
The overall assessment and the RFR-T were performed approximately 15 days before HSCT to avoid excessive latency times, leading to results that could not coincide with the clinical reality at the time of HSCT. In addition to a careful history of the risk factors, a multiparametric assessment was performed as follows:
Blood tests: creatinine, complete blood count, albumin. Variables were measured using standard laboratory techniques unless otherwise indicated.
Cardiovascular risk parameters: blood pressure, ankle-brachial index, body mass index, waist circumference, carotid intima-media thickness.
Renal assessment: markers of glomerular (albuminuria, urine sediment) and tubular damage (urinary alpha 1 microglobulin, urinary pH), morphological-vascular data (renal B-mode ultrasound and Doppler evaluation including intraparenchymal resistance index and semi-quantitative renal perfusion). The urine sediment was performed with manual microscopy technique. It was reported as pathological in the presence of cellular and non-cellular casts or endogenous and drug-related crystals.
RFR-T was assessed using an oral protein load stress test consisting of an acute protein load (1–1.2 g/kg) through lyophilized products (Prother®) and 10 mL/kg oral hydration. The Prother® product used for our study consisted of calcium caseinate. The lyophilized protein protocol for RFR measurement was developed by Sharma et al. using Protein-up Deutera®, also containing calcium caseinate. This protocol is comparable to tests using meat or amino acid intravenous (i.v.) infusion [19, 23]. A bioimpedance measurement was performed on all patients before RFR-T to check the euvolemia status at the time of the test. The urine volume was replaced with equal amounts of oral water. GFR after protein load [stress glomerular filtration rate (sGFR)] and bGFR were measured with endogenous creatinine clearance (CrCl) corrected for body surface area (DuBois formula). Urinary creatinine and serum creatinine (sCr) were measured by the enzymatic method. Blood samples were taken at predefined time points with respect to oral protein load (–120, –60, 120, 180, 240 min). bGFR was calculated with the mean of the two baseline measurements before the protein load (–120 and –60 min) of creatinine clearance. The sGFR was the maximum creatinine clearance achieved among the values measured after protein load. The protocol timing, developed according to previous studies, considers the trend of the GFR peak after protein loading [19–21]. Patients remained supine in a quiet room for at least 60 min before starting the RFR-T and throughout the test. RFR was defined as the difference between the maximum value of sGFR and bGFR expressed as a percentage; normal RFR was defined as >20%. Figure 1 shows the test protocol. AKI was defined according to KDIGO guidelines as an increase in sCr ≥0.3 mg/dL within 48 h or ≥50% within 7 days in the 100 days following the HSCT and is assigned Stages 1–3: Stage 1 = sCr increase to 1.5–1.9 times baseline; Stage 2 = sCr increase to 2.0–2.9 times baseline; Stage 3 = sCr increase ≥3.0 times baseline. CKD was defined as GFR <60 mL/min/1.73 m2 for ≥3 months since the AKI event. Recovery of renal function was defined as an eGFR at discharge ≥90% of baseline eGFR [24, 25]. Follow-up was extended by a further 3 months from the AKI event to verify functional recovery or evolution to CKD as defined by the KDIGO guidelines.
Figure 1:

RFR-T.
Conditioning regimens
Standard (MAC): 12.8 mg/kg busulfan (BU), 120 mg/kg cyclopho-sphamide (CY) or BU 12.8 mg/kg, fludarabine (FLU) 150 mg/m2.
Haploidentical transplantation: thiotepa (THIO) 10 mg/kg, BU 9.6 mg/kg, FLU 150 mg/m2.
Reduced intensity conditioning (RIC): rituximab 500 mg/m2, THIO 12 mg/kg, CY 60 mg/kg,
FLU 60 mg/m2 or THIO 10 mg/kg, BU 6.4 mg/kg, FLU 150 mg/m2.
Sequential conditioning regimen (SCR): clofarabine 150 mg/m2, FLU 150 mg/m2, CY 29 mg/kg, melphalan 110 mg/m2.
Patients submitted to match-related donor (MRD) or match-unrelated donor (MUD) transplants also received rabbit thymoglobulin 2.5 and 5 mg/kg i.v., respectively.
GVHD prophylaxis
MRD (MAC or RIC regimens): cyclosporin A (CYA) 3 mg/kg i.v. starting on Day 0, methotrexate (MTX) (15 mg/m2) on Day +1 and 10 mg/m2 on Days +3, +6. MUD: same prophylaxis with one additional administration of MTX 10 mg/m2 on Day +11. For haploidentical transplantation: CYA 1 mg/kg/day i.v. increased at 3 mg/kg at the moment of oral administration, mycophenolate mofetil 30 mg/kg/day from Day +1 to Day +28, CY 50 mg/kg/day i.v. at Days +3 and +5.
Supportive care
All patients were given prophylactically aciclovir, fluconazole and sulfamethoxazole plus trimethoprim at the usual dosage. In the case of cytomegalovirus PCR ≥10 000 copies 10 mg/kg of ganciclovir was initiated. Neutropenic fever was treated with broad-spectrum antibiotics along with our microbiological susceptibility profile. Red blood cell transfusions were performed for hemoglobin values <8 g/dL. Also, platelet transfusions were administered to maintain a platelet count >20 × 109/L.
Statistical analysis
Clinical and anthropometric variables were statistically described as: mean ± standard deviation (SD) for normally distributed quantitative data; median and interquartile range (IQR) for non-normally distributed data; frequency count and percentage for qualitative data. Normal distribution of the data was verified using the Shapiro–Wilk test. Univariate associations between AKI and anthropometric variables, cardiovascular risk factors, indicators of RFR-T and sCr were studied with Student's t-test or Mann–Whitney test, for normally or non-normally distributed quantitative variables, respectively, and Fisher’s exact test or Chi-square test for dichotomous or polytomous qualitative variables, respectively. The null hypothesis that an RFR >20% does not lead to a statistically significant increase in renal recovery after AKI was tested using the one-sided Fisher’s exact test, which also allows for greater study power. The Pearson correlation coefficient was calculated to assess the association between quantitative variables. A quantitative assessment of potential risk factors associated with AKI was carried out using binary logistic regression. The odds ratio was also estimated for statistically significant risk factors, dichotomizing according to the median where they were quantitative. Two groups of AKI patients with and without normalization of post-renal function were also statistically compared for RFR-T and sCr levels, pre- and post-AKI, using the Student's t-test, if the levels were normally distributed, or otherwise with the Mann–Whitney test. Statistical analysis was performed using RStudio 2022.02., always selecting a significance level of 95% (P < .05).
RESULTS
Table 1 shows demographic characteristics, hematological diseases, comorbidities and HSCT data of the 48 patients studied, for the AKI+ and AKI– groups. No significant difference was observed between the two groups. Twenty-nine out of 48 patients (60%) developed AKI within 100 days of HSCT (AKI+ group); 66% of patients developed AKI by Day 30, 34% by Day 31–60 and the remaining 10% by Day 61–90. Of these, 23 had AKI Stage 1, 5 had AKI stage 2 and 1 had AKI stage 3. No case required hemodialysis. Nineteen out of 48 patients (40%) did not develop AKI (AKI– group). The results of the multiparametric assessment of the 48 patients studied, the AKI+ and AKI– groups, are summarized in Table 2. No significant difference was observed between the two groups, except for the eGFR value, which showed significantly lower values in the group that demonstrated AKI, despite the low statistical power. Patients with eGFR values ≤100 mL/min/1.73 m2 had a significantly higher risk of AKI than patients with eGFR >100 mL/min/1.73 m2 (odds ratio = 9.8, 95% confidence interval = 2.5–38.8, Fisher’s exact test, P = .001) (Fig. 2). Table 3 shows the results of RFR-T in the 48 patients studied, for the AKI+ and AKI– groups. An apparently large, but not statistically significant (possibly due to the low sample size), difference was observed in normal RFR between AKI+ (mean 28.2%, SD 43.3%) and AKI– group (mean 40.3%, SD 25.1%). Table 4 shows the results of post-HSCT nephrological follow-up in the AKI+ group. Pearson correlation coefficient r = 0.258 between bGFR and RFR gave a non-statistically significant association between the two variables (P = .077). Of the 29 AKI patients, 12 recovered normal renal function while 17 did not recover and developed CKD. Our pilot study found a statistically significant correlation between functional reserves >20% with complete functional recovery after AKI (one-sided Fisher’s exact test, P = .041), but further studies with larger sample sizes are needed for confirmation (Fig. 3). An increase of statistical power was also explored, taking RFR as a continuous variable (data not reported), but neither Student's t-test nor the Mann–Whitney test for independent data provided statistically significant differences. This would seem to confirm the existence of a threshold effect, but once again it could simply depend on the low sample size.
Table 1:
Association between demographic/HSCT data and AKI events.
| N = 48 | AKI+ (n = 29) | AKI– (n = 19) | |
|---|---|---|---|
| Age (years), mean ± SD | 51 (±12) | 52 (±13) | 49 (±10) |
| Male, n (%) | 24 (50) | 16 (55) | 8 (42) |
| Female, n (%) | 24 (50) | 13 (45) | 11 (58) |
| Comorbidities, n (%) | |||
| Diabetes | 4 (8) | 3 (10) | 1 (5) |
| Hypertension | 5 (10) | 3 (10) | 2 (11) |
| Hematological diseases, n (%) | |||
| AML | 25 (52) | 15 (52) | 10 (53) |
| ALL | 11 (23) | 5 (17) | 6 (32) |
| CLL | 1 (2) | 1 (3) | 0 (0) |
| MDS | 1 (3) | 1 (17) | 0 (0) |
| PCL | 1 (3) | 1 (17) | 0 (0) |
| NHL | 3 (6) | 2 (7) | 1 (5) |
| BAL | 1 (2) | 1 (3) | 0 (0) |
| AA | 1 (2) | 1 (3) | 0 (0) |
| HSCT data | |||
| Stem cell source | |||
| BMSC, n (%) | 10 (21) | 3 (10) | 7 (37) |
| PBSC, n (%) | 38 (79) | 26 (90) | 12 (63) |
| Donor type | |||
| MRD, n (%) | 8 (17) | 5 (17) | 3 (16) |
| MUD, n (%) | 28 (58) | 19 (66) | 9 (47) |
| HAPLO, n (%) | 12 (25) | 5 (17) | 7 (37) |
| Conditioning regimens | |||
| MAC, n (%) | 31 (65) | 17 (59) | 14 (74) |
| RIC, n (%) | 16 (33) | 12 (41) | 4 (21) |
| SCR, n (%) | 1 (2) | 0 (0) | 1 (5) |
AML, acute myeloblastic leukemia; ALL, acute lymphoblastic leukemia; CLL, chronic lymphocytic leukemia; MDS, myelodysplastic syndromes; PCL, plasma cell leukemia; NHL, non-Hodgkin's lymphoma; BAL, biphenotypic acute leukaemia; AA, aplastic anemia; BMSC, bone marrow stem cell transplantation; PBSC, peripheral blood stem cell transplantation; HAPLO, haploidentical transplant.
Table 2:
Association between laboratory and clinical data and AKI events.
| Characteristics | No. of patients (n = 48) | AKI+ (n = 29) | AKI– (n = 19) | P-value |
|---|---|---|---|---|
| Body mass index (kg/m2), mean ± SD | 24 (±3) | 23 (±6) | 24 (±3) | .505 |
| Body surface area (m2), mean ± SD | 1.7 (±0.1) | 1.7 (±0.7) | 1.7 (±0.6) | 1.000 |
| Abdominal circumference (cm), mean ± SD | 92 (±11) | 93 (±44) | 92 (± 36) | 1.000 |
| Hemoglobin (g/dL), mean ± SD | 11 (±2) | 11 (±4) | 11 (±3) | 1.000 |
| Albumin (g/dl), mean ± SD | 4 (±0.4) | 4 (±0.4) | 4 (±0.5) | 1.000 |
| eGFR baseline (CKD-EPI) (mL/min/1.73 m2), mean ± SD | 97 (±14) | 93 (±15) | 103 (±11) | .016 |
| Pathological urinary sediment, n (%) | 4 (8) | 3 (10) | 1 (5) | .424 |
| Albuminuria | 2 (4) | 1 (3) | 1 (5) | 1.000 |
| Urinary α1m (>12 mg/L), n (%) | 18 (38) | 12 (41) | 6 (32) | .593 |
| ABI <1, n (%) | 4 (8) | 1 (3) | 3 (16) | .513 |
| CIMT >0.9 mm, n (%) | 3 (6) | 2 (7) | 1 (5) | .593 |
| RI, mean ± SD | 0.64 (±0.06) | 0.65 (±0.06) | 0.62 (±0.06) | .097 |
| SQP, mean ± SD | 0.58 (±0.05) | 0.58 (±0.04) | 0.58 (±0.05) | 1.000 |
BSA, body surface area; α1m, alpha 1-microglobulin; ABI, ankle-brachial index; CIMT, carotid intima-media thickness; eGFR, estimated glomerular filtration rate; RI, Doppler-based resistive index; SQP, semi-quantitative renal perfusion. Bold based on statistical significance.
Figure 2:
AKI risk with pre-HSCT eGFR ≤100 mL/min/1.73 m2.
Table 3:
Association between renal stress test, HSCT data and AKI events.
| Renal stress test data | No. of patients (n = 48) | AKI+ (n = 29) | AKI– (n = 19) | P-value |
|---|---|---|---|---|
| Basal creatinine clearance mL/min/1.73 m2, mean ± SD | 105 (±22) | 104 (±23) | 107 (±21) | .800 |
| RFR (% increase), mean ± SD | 33 (±34) | 28 (±25) | 40 (±43) | .227 |
| RFR (mL/min/1.73 m2), mean ± SD | 35 (±43) | 28 (±25) | 46 (±60) | .156 |
Table 4:
AKI data.
| AKI data (n = 29) | |
|---|---|
| AKI post-HSCT (within 100 days post-HSCT), n (%) | 29 (60) |
| Post-HSCT day on which the AKI occurred, median (IQR) | 27 (14–80) |
| sCr pre-AKI (mg/dL), mean ± SD | 0.8 (±0.2) |
| sCr AKI (mg/dL), mean ± SD | 1.6 (±0.6) |
| AKI stage (KDIGO criteria), n (%) | |
| 1 | 23 (80) |
| 2 | 5 (17) |
| 3 | 1 (3) |
| Renal function normalization post-AKI, n (%) | 12 (41) |
Figure 3:
Renal functional recovery post-AKI and RFR ≤20%. RF, renal function.
DISCUSSION
We believe that the lack of studies on this topic necessitates a close evaluation of our limited data. Our two population groups were homogeneous in hematological diseases and HSCT data (stem cell source, donor type and preconditioning strategies). In our experience, 60% of the patients enrolled in the study developed AKI. In another 2021 single-center retrospective study of 616 patients, 64% developed AKI. Our result is in line with the evidence from the literature [26, 27]. Hingorani and colleagues [3] found that AKI most commonly occurs between 10 and 30 days post-HSCT, as in our experience. The variables chosen in our multiparametric approach were known as potentially contributing to AKI development in some specific conditions [28–35]. No parameters predicted post-HSCT AKI except eGFR. This finding is already described in the literature in both the general population and those at risk of post-surgical AKI. In the meta-analysis of Grams and colleagues, the correlation between eGFR and AKI risk was significant, also when analyzing patients with normal renal function [35]. Similarly, in the study by Mokhtar et al., preoperative eGFR is the strongest predictor of postoperative AKI in subjects undergoing non-emergent cardiac surgery [36]. On the other hand, the measured CrCl was not predictive of AKI. CrCl is slightly higher than true GFR because creatinine is secreted by the proximal tubule (as well as being filtered by the glomerulus). Further secretion of the proximal tubule falsely elevates the CrCl, causing an overestimated GFR by approximately 10%. In our study, CrCl overestimates the GFR respect eGFR by 8% (105 vs 97 mL/min/1.73 m2) [37–39]. Therefore, the urinary creatinine value is a variability factor in calculating the measured clearance that does not allow for fixed correction factors. On the other hand, this variability factor is not present in the CKD-EPI estimation formula, which does not consider tubular excretion of creatinine. There is evidence in the literature that eGFR is more reliable than measured CrCl in AKI prediction [40, 41]. The eGFR is an easily performed test that could help the clinician select AKI-risk patients; however, it does not provide information regarding the prognosis regarding the damage. On the contrary, the RFR could provide information on the possible evolution of the damage by configuring a prognostic element. The increase in GFR following oral protein load shows differences between patients undergoing post-HSCT AKI and subjects maintaining post-HSCT normal renal function: the median % increase of basal GFR (RFR) in the AKI+ population was 28 vs 40 in AKI– subjects. Unsurprisingly, a dynamic parameter looks promising in detecting subclinical renal frailty conditions that could predispose to AKI. A growing body of evidence has been published about an association between RFR and susceptibility to kidney injury, particularly following kidney transplantation [20] and post-cardiac surgery [18, 21]. The broadest experience is from Ronco and colleagues [18], who showed on 110 cardiac surgery patients that the preoperative RFR was highly predictive of postoperative AKI (area under the curve 0.83), with a >10-fold increase in risk. A multiparametric assessment failed to achieve such a prediction. Failure to achieve the statistical significance of AKI concerning RFR could be for the mild degree of the majority of AKI cases (Stage 1); therefore, conditions of mild transient damage. Our study showed that RFR was significantly related to functional recovery (one-sided Fisher’s exact test, P = .041). A conserved pre-HSCT RFR above a threshold of 20% is related to complete renal functional recovery post-AKI (Fig. 3). The RFR threshold of 20% is a clinical choice. The optimal statistical choice, with reference to the receiver operating chatacteristic curve and the related Youden index (maximum of sensitivity + specificity), would have been a value of about 23%. Using the statistically optimal 23% threshold, we would have a P-value of .020. The bilateral Fisher's exact test with RFR >20% gives a P-value of .06, which goes down to .025 when setting the threshold to 23%. Using the clinical choice of RFR >20%, it seems plausible to consider as a null hypothesis that this does not lead to a statistically significant increase in kidney recovery after AKI. Furthermore, the one-sided Fisher’s exact test, compared with the two-sided one, guarantees greater statistical power, which is important in this pilot study of limited sample size. To our knowledge, pre-damage RFR has never been evaluated in this context: our pilot study seems to be the first experience in these terms. Identifying the presence of a substantial reduction RFR can predict which of the patients most susceptible to AKI will most easily undergo functional recovery. These data provide accurate prognostic information for a patient who is a candidate for medical or surgical treatment. The high nephrological risk of the HSCT procedure strongly suggests a tight collaboration between hematologists and nephrologists to assess the nephrological pre-HSCT risk of the patient; in this procedure, our preliminary data suggest a possible pivotal role for RFR-T. A definite assessment of renal risk could be considered in defining the HSCT procedure to minimize renal risk by customizing the conditioning regimens of the transplant.
CONCLUSIONS
Kidney injury is becoming a serious clinical challenge in the era of widespread HSCT availability. A “bedside” assessment to estimate the risk of AKI pre-HSCT could be the measurement of the eGFR. Baseline eGFR ≤100 mL/min/1.73 m2 is related to the risk of developing post-HSCT AKI. An RFR >20% correlates with renal functional recovery post-HSCT-AKI. In patients with an eGFR ≤100 mL/min/1.73 m2, it might be rational to evaluate the RFR to select the group at the most significant risk of evolution into CKD. RFR could be the missing link between AKI and CKD development.
Limitations
Several limitations warrant discussion. The small number of patients from a single medical center is a limitation. Renal histological investigation of AKI was not performed due to hemorrhagic diathesis secondary to thrombocytopenia related to incomplete hematopoietic recovery. Finally, we did not re-examine the RFR after HSCT to check for any subclinical decline in renal function.
ACKNOWLEDGEMENTS
We thank medical students Corinne Caldei and Uberto Percudani for their valuable contribution to data collection and bibliographic research. We are also grateful to the staff of the Nephrology Unit for their practical contribution.
Contributor Information
Nicoletta Mancianti, Dipartimento di Emergenza-Urgenza e dei Trapianti, UOC Nefrologia, Dialisi e Trapianti, Azienda Ospedaliera Universitaria Senese, Siena, Italy.
Andrea Guarnieri, Dipartimento di Emergenza-Urgenza e dei Trapianti, UOC Nefrologia, Dialisi e Trapianti, Azienda Ospedaliera Universitaria Senese, Siena, Italy.
Mariapia Lenoci, Dipartimento Innovazione, Sperimentazione e Ricerca Clinica e Traslazionale, UOC Terapie Cellulari e Officina Trasfusionale, Azienda Ospedaliera Universitaria Senese, Siena, Italy.
Francesca Toraldo, Dipartimento Innovazione, Sperimentazione e Ricerca Clinica e Traslazionale, UOC Terapie Cellulari e Officina Trasfusionale, Azienda Ospedaliera Universitaria Senese, Siena, Italy.
Domenica Paola Salvo, Dipartimento di Emergenza-Urgenza e dei Trapianti, UOC Nefrologia, Dialisi e Trapianti, Azienda Ospedaliera Universitaria Senese, Siena, Italy.
Massimo Belluardo, Dipartimento di Emergenza-Urgenza e dei Trapianti, UOC Nefrologia, Dialisi e Trapianti, Azienda Ospedaliera Universitaria Senese, Siena, Italy.
Ernesto Iadanza, Dipartimento di Biotecnologie Mediche, Università di Siena, Siena, Italy.
Fabio Ferretti, Dipartimento di Scienze Mediche, Chirurgiche e Neuroscienze, Azienda Ospedaliera Universitaria Senese, Siena, Italy.
Giuseppe Marotta, Dipartimento Innovazione, Sperimentazione e Ricerca Clinica e Traslazionale, UOC Terapie Cellulari e Officina Trasfusionale, Azienda Ospedaliera Universitaria Senese, Siena, Italy.
Guido Garosi, Dipartimento di Emergenza-Urgenza e dei Trapianti, UOC Nefrologia, Dialisi e Trapianti, Azienda Ospedaliera Universitaria Senese, Siena, Italy.
FUNDING
The study was entirely supported by institutional funding.
AUTHORS’ CONTRIBUTIONS
N.M. and A.G. conceived the study, collected data and wrote the article. M.L. and F.T. collected data and critically revised the manuscript. E.I. and F.F. designed the analysis and revised the manuscript. D.P.S., M.B. and G.M. contributed to the ultrasound data and supervised the analysis. G.G. reviewed the manuscript and final approval.
DATA AVAILABILITY STATEMENT
The data underlying this article will be shared on reasonable request to the corresponding author.
CONFLICT OF INTEREST STATEMENT
The authors have no conflicts of interest to declare. The results presented in this paper have not been published previously in whole or part, except in abstract format.
REFERENCES
- 1. Ando M, Mori J, Ohashi Ket al. A comparative assessment of the RIFLE, AKIN and conventional criteria for acute kidney injury after hematopoietic SCT. Bone Marrow Transplant 2010;45:1427–34. [DOI] [PubMed] [Google Scholar]
- 2. Parikh CR, McSweeney PA, Korular Det al. Renal dysfunction in allogeneic hematopoietic cell transplantation. Kidney Int 2002;62:566–73. [DOI] [PubMed] [Google Scholar]
- 3. Hingorani SR, Guthrie K, Batchelder Aet al. Acute renal failure after myeloablative hematopoietic cell transplant: incidence and risk factors. Kidney Int 2005;67:272–7. [DOI] [PubMed] [Google Scholar]
- 4. Shingai N, Morito T, Najima Yet al. Early-onset acute kidney injury is a poor prognostic sign for allogeneic SCT recipients. Bone Marrow Transplant 2015;50:1557–62. [DOI] [PubMed] [Google Scholar]
- 5. Renaghan AD, Jaimes EA, Malyszko Jet al. Acute kidney injury and CKD associated with hematopoietic stem cell transplantation. Clin J Am Soc Nephrol 2020;15:289–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kersting S, Hené RJ, Koomans HAet al. Chronic kidney disease after myeloablative allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant 2007;13:1169–75. [DOI] [PubMed] [Google Scholar]
- 7. Ando M, Ohashi K, Akiyama Het al. Chronic kidney disease in long-term survivors of myeloablative allogeneic haematopoietic cell transplantation: prevalence and risk factors. Nephrol Dial Transplant 2010;25:278–82. [DOI] [PubMed] [Google Scholar]
- 8. Krishnappa V, Gupta M, Manu Get al. Acute kidney injury in hematopoietic stem cell transplantation: a review. Int J Nephrol 2016;2016:5163789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gratwohl A. The EBMT risk score. Bone Marrow Transplant 2012;47:749–56. [DOI] [PubMed] [Google Scholar]
- 10. Sorror ML. How I assess comorbidities before hematopoietic cell transplantation. Blood 2013;121:2854–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zhu X, Huang L, Zheng Cet al. European Group for Blood and Marrow Transplantation risk score predicts the outcome of patients with acute leukemia receiving single umbilical cord blood transplantation. Biol Blood Marrow Transplant 2017;23:2118–26. [DOI] [PubMed] [Google Scholar]
- 12. Fliser D, Zeier M, Nowack Ret al. Renal functional reserve in healthy elderly subjects. J Am Soc Nephrol 1993;3:1371–7. [DOI] [PubMed] [Google Scholar]
- 13. Hostetter TH, Olson JL, Rennke HGet al. Hyperfiltration in remnant nephrons: a potentially adverse response to renal ablation. J Am Soc Nephrol 2001;12:1315–25. [DOI] [PubMed] [Google Scholar]
- 14. Liu KD, Brakeman PR.. Renal repair and recovery. Crit Care Med 2008;36:S187–92. [DOI] [PubMed] [Google Scholar]
- 15. Agostoni P, Vignati C, Gentile Pet al. Reference values for peak exercise cardiac output in healthy individuals. Chest 2017;151:1329–37. [DOI] [PubMed] [Google Scholar]
- 16. Koopman MG, Koomen GC, Krediet RTet al. Circadian rhythm of glomerular filtration rate in normal individuals. Clin Sci (Colch) 1989;77:105–11. [DOI] [PubMed] [Google Scholar]
- 17. Stenvinkel P, Jani AH, Johnson RJ.. Hibernating bears (Ursidae): metabolic magicians of definite interest for the nephrologist. Kidney Int 2013;83:207–12. [DOI] [PubMed] [Google Scholar]
- 18. Sharma A, Mucino MJ, Ronco C.. Renal functional reserve and renal recovery after acute kidney injury. Nephron Clin Pract 2014;127:94–100. [DOI] [PubMed] [Google Scholar]
- 19. Sharma A, Zaragoza JJ, Villa Get al. Optimizing a kidney stress test to evaluate renal functional reserve. Clin Nephrol 2016;86:18–26. [DOI] [PubMed] [Google Scholar]
- 20. Rook M, Hofker HS, van Son WJet al. Predictive capacity of pre-donation GFR and renal reserve capacity for donor renal function after living kidney donation. Am J Transplant 2006;6:1653–9. [DOI] [PubMed] [Google Scholar]
- 21. Husain-Syed F, Ferrari F, Sharma Aet al. Preoperative renal functional reserve predicts risk of acute kidney injury after cardiac operation. Ann Thorac Surg 2018;105:1094–101. [DOI] [PubMed] [Google Scholar]
- 22. Khwaja A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin Pract 2012;120:c179–c84. [DOI] [PubMed] [Google Scholar]
- 23. Mansy H, Patel D, Tapson JS.et al. Four methods to recruit renal functional reserve. Nephrol Dial Transplant 1987;2:228–32. [PubMed] [Google Scholar]
- 24. Xu J, Xu X, Shen Bet al. Evaluation of five different renal recovery definitions for estimation of long-term outcomes of cardiac surgery associated acute kidney injury. BMC Nephrol 2019;20:427.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bucaloiu ID, Kirchner HL, Norfolk ERet al. Increased risk of death and de novo chronic kidney disease following reversible acute kidney injury. Kidney Int 2012;81:477–85. [DOI] [PubMed] [Google Scholar]
- 26. Kanduri SR, Cheungpasitporn W, Thongprayoon Cet al. Incidence and mortality of acute kidney injury in patients undergoing hematopoietic stem cell transplantation: a systematic review and meta-analysis. QJM 2020;113:621–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Abramson MH, Gutgarts V, Zheng Jet al. Acute kidney injury in the modern era of allogeneic hematopoietic stem cell transplantation. Clin J Am Soc Nephrol 2021;16:1318–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Taplitz RA, Kennedy EB, Bow EJet al. Antimicrobial prophylaxis for adult patients with cancer-related immunosuppression: ASCO and IDSA clinical practice guideline update. J Clin Oncol 2018;36:3043–54. [DOI] [PubMed] [Google Scholar]
- 29. Kovesdy CP, Furth SL, Zoccali Cet al. Obesity and kidney disease: hidden consequences of the epidemic. J Nephrol 2017;30:1–10. [DOI] [PubMed] [Google Scholar]
- 30. Schefold JC, Filippatos G, Hasenfuss Get al. Heart failure and kidney dysfunction: epidemiology, mechanisms and management. Nat Rev Nephrol 2016;12:610–23. [DOI] [PubMed] [Google Scholar]
- 31. Karkouti K, Grocott HP, Hall Ret al. Interrelationship of preoperative anemia, intraoperative anemia, and red blood cell transfusion as potentially modifiable risk factors for acute kidney injury in cardiac surgery: a historical multicentre cohort study. Can J Anesth 2015;62:377–84. [DOI] [PubMed] [Google Scholar]
- 32. Wiedermann CJ, Wiedermann W, Joannidis M.. Causal relationship between hypoalbuminemia and acute kidney injury. World J Nephrol 2017;6:176–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bullen AL, Katz R, Lee AKet al. The SPRINT trial suggests that markers of tubule cell function in the urine associate with risk of subsequent acute kidney injury while injury markers elevate after the injury. Kidney Int 2019;96:470–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ninet S, Schnell D, Dewitte Aet al. Doppler-based renal resistive index for prediction of renal dysfunction reversibility: a systematic review and meta-analysis. J Crit Care 2015;30:629–35. [DOI] [PubMed] [Google Scholar]
- 35. Grams ME, Sang Y, Ballew SHet al. CKD Prognosis Consortium. A meta-analysis of the association of estimated GFR, albuminuria, age, race, and sex with acute kidney injury. Am J Kidney Dis 2015;66:591–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Cong N, Tian T, Hu Bet al. Development of a preoperative scorecard for prediction of acute kidney injury following cardiac surgery. J Card Surg 2021;36:3474–5. [DOI] [PubMed] [Google Scholar]
- 37. Carrie BJ, Golbetz HV, Michaels ASet al. Creatinine: an inadequate filtration marker in glomerular diseases. Am J Med 1980;69:177–82. [DOI] [PubMed] [Google Scholar]
- 38. Shahbaz H, Gupta M. Creatinine clearance. 2022. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 Jan. [PubMed] [Google Scholar]
- 39. Shemesh O, Golbetz H, Kriss JPet al. Limitations of creatinine as a filtration marker in glomerulopathic patients. Kidney Int 1985;28:830–8. [DOI] [PubMed] [Google Scholar]
- 40. Iwasaki Y, Sawada T, Kijima H.et al. Estimated glomerular filtration rate is superior to measured creatinine clearance for predicting postoperative renal dysfunction in patients undergoing pancreatoduodenectomy. Pancreas 2010;39:20–5. [DOI] [PubMed] [Google Scholar]
- 41. Nguyen TV, Goldfarb DS.. The older adult patient and kidney function. Consult Pharm 2012;27:431–44. doi: 10.4140/TCP.n.2012.431 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.