ABSTRACT
Acute kidney injury is a common and important complication following hematopoietic stem cell transplantation. In the nephrology community, acute kidney injury is no longer viewed as a simple temporary and potentially reversible decline in kidney clearance as acute kidney injury imposes a risk for immediate and future complications. Therefore, stratifying patients for the risk of acute kidney injury following stem cell transplantation would be very helpful to optimize peri-stem cell transplant management and could potentially improve outcomes in this patient population. In the current issue of CKJ, Mancianti et al. report on the testing of the kidney's functional reserve in patients planned for stem cell transplantation and demonstrate that stem cell transplant candidates with a preserved kidney response on a protein load had a higher chance of full kidney recovery after an episode of acute kidney injury. In this editorial, we discuss the kidney's functional reserve test and its limitations.
Keywords: acute kidney injury, hematopoietic stem cell transplantation, kidney function test
Acute kidney injury (AKI) is a common complication in cancer patients and even more so in patients undergoing stem cell transplantation (SCT) [1]. The incidence of AKI following SCT varies depending on the definition of AKI, type of chemotherapeutic conditioning regimen (myeloablative versus nonmyeloablative), and type of transplant (allogeneic versus autologous) [2]. In a study by Zager et al. analyzing 272 patients after myeloablative SCT (89% allogeneic, 11% autologous), 53% of patients developed AKI (defined as a doubling of serum creatinine) and approximately half of these patients required dialysis [3]. Allogeneic myeloablative SCT has been identified by several studies to be associated with a greater incidence of severe AKI (73% versus 47%) and a 4-fold greater need for dialysis (12% versus 3%) compared with patients undergoing nonmyeloablative SCT [4, 5]. In the last decades, the incidence of AKI after SCT is decreasing, potentially because of the use of lower dose conditioning regimens [6, 7].
AKI following SCT has been associated with higher all-cause and nonrelapse mortality [8, 9], and there is a relation between the severity of AKI and the mortality risk regardless of transplant type. Studies have consistently shown extremely high (>80%) mortality rates in those patients requiring acute dialysis [3, 4, 6, 10]. Stratifying patients for the risk of AKI following SCT would be very helpful to optimize peri-SCT management and could potentially improve outcomes in this patient population.
Already, four decades ago, the documentation of the increase of glomerular filtration (GFR) rate after a protein load was advocated as a measure of a recruitable reserve function of the kidney, previously called the renal functional reserve (RFR) [11]. Loss of this functional reserve is thought to reveal subclinical kidney damage and nephron loss. The mechanism behind the stimulatory effect of an oral protein load or an intravenous administration of amino acids on the GFR has not been fully elucidated. It entails a temporary increase of both the renal plasma flow and the GFR. Several theories have been put forward to explain this transient hyperemia of the kidney. For detailed information, we refer the reader to some excellent reviews on this topic [12–14]. We can conclude that upon the appearance of amino acids in the portal circulation, an evolutionary preserved feedforward reflex is initiated that includes humoral factors such as glucagon and the antidiuretic hormone [15]. Both hormones cooperate to increase the urea clearance as well as the urinary concentration grade. In this way, nitrogenous waste products are dispensed faster while urinary fluid losses are being limited. The proximal tubule reabsorbs the higher load of filtered amino acids with the aid of sodium-coupled cotransporters. In the thick ascending loop of Henle, the sodium chloride reabsorption is stimulated by both glucagon and the antidiuretic hormone. In response to the resulting lower sodium chloride concentration in the tubular fluid reaching the macula densa, the glomerulotubular feedback induces dilatation of the afferent arteriole. Experimentally, paracrine substances such as nitric oxide and vasodilating prostaglandins play a facilitating role. The vasodilation of the afferent arteriole is indispensable to substantiate the increase of the GFR. When all the elements mentioned here are in place, a diminished or abolished kidney response after a protein meal implies already maximally dilated preglomerular arterioles. Theoretically, these kidneys will demonstrate a diminished autoregulation and a higher susceptibility to hemodynamic perturbations, hence the rationale for performing a RFR test in the clinical situations enumerated in Table 1.
Table 1:
Potential indications for testing the reserve function of the kidney
| Low GFR | Normal GFR | High GFR |
|---|---|---|
|
Known reduced kidney mass
- solitary kidney - congenital anomalies of the kidney and the urinary tract |
Prior to kidney mass reducing interventions
- kidney donation - nephrectomy |
Enhanced kidney clearance
- obesity - diabetes - burns - sepsis |
|
Suspected renal frailty of the kidney
- before high-risk pregnancy - before high-risk major surgery (e.g. cardiac surgery) - before chemotherapy (e.g. HSCT) - during nephrotoxic treatments - assessment of renal recovery after AKI - cardiorenal syndrome - known cardiovascular disease (hypertension, diabetes) - systemic sclerosis - the aging kidney |
||
In this issue of CKJ, Mancianti et al. report on the testing of the RFR in patients planned for SCT. In this pilot study, the investigators aimed to evaluate the ability of the RFR test to foretell the risk of AKI and to predict recovery of kidney function after AKI occurred. Forty-eight patients without kidney disease (defined as an estimated GFR >60 mL/min/1.73 m²) were included in this study and followed for >100 days after the SCT procedure with an additional 3 months of follow-up if AKI had developed. The RFR procedure used in this paper followed the protocol described by Sharma et al. [1]. In brief, patients were invited ∼15 days before the SCT to undergo a RFR test. This test requires 6 hours in total. The administration of a protein load (time zero) is scheduled after 2 hours. Baseline creatinine clearance is calculated as a mean of the 2-hourly collections before this time zero. At time zero, a commercially available preparation of a liquid whey protein isolate (PROther®) is consumed. For the next 4 hours, the renal creatinine clearances are documented. The peak creatinine clearance is identified as the maximal response. The difference between the maximal and the baseline creatinine clearance constitutes the functional response of the kidney. In the current study, the RFR test was incorporated in a broad nephrological assessment comprising a blood sample analysis, urinary sediment analysis and urine albuminuria measurement as well as an ultrasound examination of the kidneys complemented with doppler evaluation of intraparenchymal resistive index and semiquantitative renal perfusion. The authors could show that candidates for SCT with an eGFR >100 mL/min/1.7 m2 were less sensitive to AKI with a sensitivity of 85% and a specificity of 65%. Comparing the patients with AKI versus non-AKI, the authors documented a higher functional response in the non-AKI group. However, this did not reach statistical significance. Furthermore, patients showing a preserved kidney's response on a protein load had a higher chance of full kidney recovery after an AKI episode with a sensitivity of 70% and a specificity of 64%.
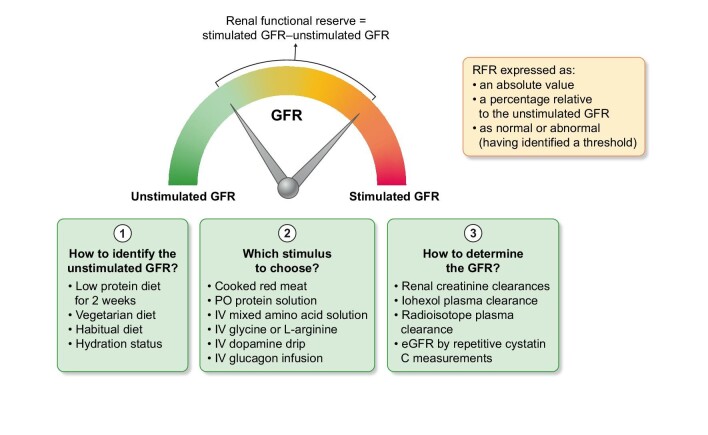
We applaud the authors for the tremendous effort they performed in a patient population at high risk for AKI. However, before RFR testing can be recommended in this setting, several issues have to be addressed. Although RFR testing has been proposed as a measure of frailty of the kidneys for several decades, testing is not widely performed. Why is that? The most obvious reasons are time and money. But more fundamental concerns have been expressed. Forty years after its conception, doubt remains on some practicalities, the interpretation, the intra- and inter-person variability, and the validity of the RFR test. Let us briefly zoom in on some of these practical issues (Fig. 1) [14]. A first issue concerns the definition of baseline GFR as the intensity of our kidney's excretory function fluctuates from day to day and from hour to hour. Ideally, patients are prepped 2 weeks before the RFR test by following a low protein or a vegetarian diet. In this way, a resting or real baseline GFR can be obtained. When subjects are highly hydrated the RFR is blunted due to a suppressed antidiuretic hormone. A second issue concerns the amount, type, and composition of amino acids used to stimulate the kidney during RFR testing. Maximally stimulating the kidney is traditionally seen after eating a substantial quantity of cooked red meat. Alternative protein servings may or may not elicit the same response [16, 17]. Infusion of mixed amino acid solutions as well as some single amino acids (such as glycine or L-arginine) also increases the GFR. Dopamine has been extensively documented as an additional stimulatory factor. Finally, issues concerning repeated GFR measurements have been raised. Although measuring real-time GFR might soon become feasible [18], for the moment, timed creatinine clearances or plasma decay curves of injected iohexol or radioisotopes are the only available possibilities. These methods come with their own imperfections and caveats. Recently, a paper was published showing that hourly follow-up of plasma cystatin C might also reflect the functional response of the kidney [19]. In this study, RFR testing was performed by hourly plasma cystatin C measurements compared with simultaneous creatinine clearance and 99technetium diethylenetriaminepentaacetatic acid-measured GFR measurements in 19 adult patients with chronic kidney disease (CKD) stages 3 and 21 adult patients with CKD stage 4. The authors demonstrated that there was a good agreement between the different methods [19]. In conjunction with this paper, we regret that Mancianti et al. omitted measuring cystatine C in their study protocol. Recently, the serum creatinine/cystatine c ratio emerges as a valuable marker for sarcopenia in cancer patients [20]. A lower ratio reveals a subset of patients wherein the eGFR calculations based on the serum creatinine overestimates true kidney function. Obviously, these patients will be more susceptible to AKI. Moreover, a diminished eGFRcystatine c/eGFRcreatinine ratio can suggest a selective glomerular hypofiltration syndrome [21]. Recently, this aberration was associated with a higher incidence of contrast associated AKI in a large population of patients scheduled for elective coronary intervention [21].
Figure 1:
Issues concerning the testing of the reserve function of the kidney.
Can the cumbersome RFR test be finetuned to increase its use? Several papers advocate ultrasound of the kidney with doppler to assess the drop in renal vascular resistance coinciding with the kidney's functional response [22]. However, others have refuted this method [23]. But the main criticism persists. Is a test that extends over 6 hours, needing dedicated supervision and multiple blood and urinary samples, more predictive than the single point multiparametric assessment as proposed by Mancianti et al.? As previously mentioned, the maximal increase of the GFR in a RFR test mainly depends on the amount and the distensibility of the preglomerular arterioles. In this way, a lower RFR identifies kidneys that are vulnerable to renal hypoperfusion. In a population scheduled for elective cardiac surgery, a lower RFR test preoperatively could successfully identify patients at risk for AKI with an area under the receiver operating characteristic curve of 0.83 (95% confidence interval, 0.70 to 0.96) [24]. In comparison, SCT recipients are exposed to a myriad of nonhemodynamic perturbations associated with the intense conditioning regimens and complications such as sepsis, hepatic sinusoidal obstruction syndrome, and medication-induced kidney injury. This is probably the reason why the testing of the RFR in this nonhomogeneous population shows less predictive power.
Finally, can the peri-SCT therapy or intervention be substantially modified to prevent AKI once vulnerable patients have been identified? Why not implement this alternative protocol to all patients? (Unless the alternative treatment arm is less effective, of course.) In this case, patients must have a say in it and be able to outweigh the greater risk of AKI in favor of a better cure rate.
In conclusion, this pilot study by Mancianti et al. directs our attention to a delicate patient population undergoing SCT in the treatment of a hematologic malignancy. During this period, these patients show a high risk of complications with AKI being one of them. The risk prediction in this setting offered by testing the functional response of the kidney after a protein load is weak at most. Offering a multiparameter nephrological assessment is already a firm step forward. In the advent of AKI, the consulting nephrologist has the biomarkers she/he needs to better accompany the patient during kidney recovery and follow-up.
Contributor Information
Bart De Moor, Department of Nephrology, Jessa Ziekenhuis, Hasselt, Belgium; Biomedical Research Institute, Department of Immunology and Infection, UHasselt, Diepenbeek, Belgium.
Ben Sprangers, Biomedical Research Institute, Department of Immunology and Infection, UHasselt, Diepenbeek, Belgium; Department of Nephrology, Ziekenhuis Oost Limburg, Genk, Belgium.
CONFLICT OF INTEREST STATEMENT
B.S. is member of the CKJ editorial board. The other author has no disclosures.
REFERENCES
- 1. Renaghan AD, Jaimes EA, Malyszko Jet al. Acute kidney injury and CKD associated with hematopoietic stem cell transplantation. Clin J Am Soc Nephrol 2020;15:289–97. 10.2215/CJN.08580719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hingorani S. Renal complications of hematopoietic-cell transplantation. N Engl J Med 2016;374:2256–67. 10.1056/NEJMra1404711. [DOI] [PubMed] [Google Scholar]
- 3. Zager RA, O'Quigley J, Zager BKet al. Acute renal failure following bone marrow transplantation: a retrospective study of 272 patients. Am J Kidney Dis 1989;13:210–6. 10.1016/S0272-6386(89)80054-X. [DOI] [PubMed] [Google Scholar]
- 4. Parikh CR, McSweeney PA, Korular Det al. Renal dysfunction in allogeneic hematopoietic cell transplantation. Kidney Int 2002;62:566–73. 10.1046/j.1523-1755.2002.00455.x. [DOI] [PubMed] [Google Scholar]
- 5. Parikh CR, Schrier RW, Storer Bet al. Comparison of ARF after myeloablative and nonmyeloablative hematopoietic cell transplantation. Am J Kidney Dis 2005;45:502–9. 10.1053/j.ajkd.2004.11.013. [DOI] [PubMed] [Google Scholar]
- 6. Canet E, Lengline E, Zafrani Let al. Acute kidney injury in critically ill allo-HSCT recipients. Bone Marrow Transplant 2014;49:1121–2. 10.1038/bmt.2014.100. [DOI] [PubMed] [Google Scholar]
- 7. Gooley TA, Chien JW, Pergam SAet al. Reduced mortality after allogeneic hematopoietic-cell transplantation. N Engl J Med 2010;363:2091–101. 10.1056/NEJMoa1004383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ando M, Mori J, Ohashi Ket al. A comparative assessment of the RIFLE, AKIN and conventional criteria for acute kidney injury after hematopoietic SCT. Bone Marrow Transplant 2010;45:1427–34. 10.1038/bmt.2009.377. [DOI] [PubMed] [Google Scholar]
- 9. Parikh CR, Yarlagadda SG, Storer Bet al. Impact of acute kidney injury on long-term mortality after nonmyeloablative hematopoietic cell transplantation. Biol Blood Marrow Transplant 2008;14:309–15. 10.1016/j.bbmt.2007.12.492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Parikh CR, Sandmaier BM, Storb RFet al. Acute renal failure after nonmyeloablative hematopoietic cell transplantation. J Am Soc Nephrol 2004;15:1868–76. 10.1097/01.ASN.0000129981.50357.1C. [DOI] [PubMed] [Google Scholar]
- 11. Bosch JP, Saccaggi A, Lauer Aet al. Renal functional reserve in humans. Effect of protein intake on glomerular filtration rate. Am J Med 1983;75:943–50. 10.1016/0002-9343(83)90873-2. [DOI] [PubMed] [Google Scholar]
- 12. Palsson R, Waikar SS. Renal functional reserve revisited. Adv Chronic Kidney Dis 2018;25:e1–e8. 10.1053/j.ackd.2018.03.001. [DOI] [PubMed] [Google Scholar]
- 13. Jufar AH, Lankadeva YR, May CNet al. Renal functional reserve: from physiological phenomenon to clinical biomarker and beyond. Am J Physiol - Regul Integr Comp Physiol 2020;319:R690–702. 10.1152/ajpregu.00237.2020. [DOI] [PubMed] [Google Scholar]
- 14. De Moor B, Vanwalleghem JF, Swennen Qet al. Haemodynamic or metabolic stimulation tests to reveal the renal functional response: requiem or revival? Clin Kidney J 2018;11:623–54. 10.1093/ckj/sfy022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bankir L, Roussel R, Bouby N. Protein- and diabetes-induced glomerular hyperfiltration: role of glucagon, vasopressin, and urea. J Physiol Renal Physiol 2015;309:F2–F23. 10.1152/ajprenal.00614.2014. [DOI] [PubMed] [Google Scholar]
- 16. Castellino P, Levin R, Shohat Jet al. Effect of specific amino acid groups on renal hemodynamics in humans. Am J Physiol 1990;258:F992–997. [DOI] [PubMed] [Google Scholar]
- 17. Claris-Appiani A, Assael BM, Tirelli ASet al. Lack of glomerular hemodynamic stimulation after infusion of branched-chain amino acids. Kidney Int 1988;33:91–94. 10.1038/ki.1988.14. [DOI] [PubMed] [Google Scholar]
- 18. Rizk DV, Meier D, Sandoval RMet al. A novel method for rapid bedside measurement of GFR. J Am Soc Nephrol 2018;29:1609–13. 10.1681/ASN.2018020160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Christiadi D, Simpson C, O'Brien Ket al. Cystatin C kidney functional reserve: a simple method to predict outcome in chronic kidney disease. Nephrol Dial Transplant 2022;37:1118–24. 10.1093/ndt/gfab188. [DOI] [PubMed] [Google Scholar]
- 20. Tlemsani C, Durand JP, Raynard Bet al. Relationship between the creatinine/cystatin C ratio and muscle mass measured by CT-scan in cancer patients. Clin Nutr ESPEN 2022;51:412–8. 10.1016/j.clnesp.2022.07.010. [DOI] [PubMed] [Google Scholar]
- 21. Zhang LW, Luo MQ, Xie XWet al. Shrunken pore syndrome: a new and more powerful phenotype of renal dysfunction than chronic kidney disease for predicting contrast-associated acute kidney injury. J Am Heart Assoc 2023;12:e027980. 10.1161/JAHA.122.027980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Samoni S, Villa G, De Rosa Set al. The relationship between intra-parenchymal renal resistive index variation and renal functional reserve in healthy subjects. J Nephrol 2021;34:403–9. 10.1007/s40620-020-00786-1. [DOI] [PubMed] [Google Scholar]
- 23. Damianaki A, Brito W, Garessus Jet al. Contrast-enhanced ultrasound and protein shakes are no alternatives for inulin clearance and meat to assess renal functional reserve in humans. Kidney Blood Press Res 2022;47:664–73. 10.1159/000527313. [DOI] [PubMed] [Google Scholar]
- 24. Husain-Syed F, Ferrari F, Sharma Aet al. Preoperative renal functional reserve predicts risk of acute kidney injury after cardiac operation. Ann Thorac Surg 2018;105:1094–101. 10.1016/j.athoracsur.2017.12.034. [DOI] [PubMed] [Google Scholar]