ABSTRACT
Background
Trimethoprim-sulfamethoxazole (TMP-SMX) can cause hyperkalemia by reducing renal potassium excretion. We assessed the risk of hyperkalemia after initiating TMP-SMX versus amoxicillin and determined if this risk is modified by a patient's baseline kidney function [estimated glomerular filtration rate (eGFR)].
Methods
We conducted a population-based cohort study in Ontario, Canada involving adults ≥66 years of age newly treated with TMP-SMX (n = 58 999) matched 1:1 with those newly treated with amoxicillin (2008–2020). The primary outcome was a hospital encounter with hyperkalemia defined by a laboratory serum potassium value ≥5.5 mmol/L within 14 days of antibiotic treatment. Secondary outcomes included a hospital encounter with acute kidney injury (AKI) and all-cause hospitalization. Risk ratios (RRs) were obtained using a modified Poisson regression.
Results
A hospital encounter with hyperkalemia occurred in 269/58 999 (0.46%) patients treated with TMP-SMX versus 80/58 999 (0.14%) in those treated with amoxicillin {RR 3.36 [95% confidence interval (CI) 2.62–4.31]}. The absolute risk of hyperkalemia in patients treated with TMP-SMX versus amoxicillin increased progressively with decreasing eGFR (risk difference of 0.12% for an eGFR ≥60 ml/min/1.73 m2, 0.42% for eGFR 45–59, 0.85% for eGFR 30–44 and 1.45% for eGFR <30; additive interaction P < .001). TMP-SMX versus amoxicillin was associated with a higher risk of a hospital encounter with AKI [RR 3.15 (95% CI 2.82–3.51)] and all-cause hospitalization [RR 1.43 (95% CI 1.34–1.53)].
Conclusions
The 14-day risk of a hospital encounter with hyperkalemia was higher in patients newly treated with TMP-SMX versus amoxicillin and the risk was highest in patients with a low eGFR.
Keywords: acute kidney injury, chronic kidney disease, hyperkalemia, trimethoprim, trimethoprim-sulfamethoxazole
KEY LEARNING POINTS.
What is already known about this subject?
Trimethoprim-sulfamethoxazole (TMP-SMX) is an antibiotic that can cause hyperkalemia by reducing potassium excretion by the kidneys.
Previous observational studies reporting a higher risk of hyperkalemia in patients prescribed TMP-SMX versus other antibiotics used administrative diagnosis codes to assess hyperkalemia.
Administrative diagnosis codes for hyperkalemia are insensitive, raising concerns about accurate outcome ascertainment.
What this study adds?
After controlling for potential confounders, new treatment with oral TMP-SMX versus amoxicillin was associated with a 3-fold higher risk of a hospital encounter with hyperkalemia.
Outcome of hyperkalemia was ascertained using laboratory serum potassium values, which is reflective of current clinical practice.
The risk of hyperkalemia associated with TMP-SMX was assessed across four estimated glomerular filtration rate (eGFR) categories and the risk was highest in patients with a low eGFR.
What impact this may have on practice or policy?
Given the growing number of adults with chronic kidney disease globally and their predispositions to electrolyte disorders and adverse drug outcomes due to poor renal clearance, the study findings have the potential to inform antibiotic prescribing.
INTRODUCTION
Trimethoprim-sulfamethoxazole (TMP-SMX) is a common antibiotic used to treat bacterial infections of the urinary tract, skin and soft tissues [1–4]. TMP-SMX is primarily excreted by the kidneys and can cause hyperkalemia through inhibition of amiloride-sensitive channels in the renal collecting ducts, reducing potassium excretion by the kidneys [5, 6].
Large observational studies report a higher risk of hyperkalemia in patients prescribed TMP-SMX versus other antibiotics. However, hyperkalemia in these studies was assessed solely with administrative database diagnosis codes [7–9] or with a combination of diagnosis codes and a primary care record showing an elevated potassium level [10]. The sensitivity of diagnosis codes to identify hyperkalemia is <15%, raising concerns about accurate outcome ascertainment [11]. Prior studies also did not adequately examine the risk of TMP-SMX–induced hyperkalemia in patients with chronic kidney disease, a growing segment of the population at risk for adverse drug events from renally excreted drugs.
We conducted a population-based cohort study of older adults who received an outpatient prescription for TMP-SMX versus amoxicillin. The primary outcome was a hospital encounter (emergency department visit or a hospital admission) with hyperkalemia assessed with laboratory measurements of serum potassium. Secondary outcomes were a hospital encounter with acute kidney injury (AKI) , all-cause hospitalization and all-cause mortality. Prespecified subgroup analyses were conducted by the baseline category of estimated glomerular filtration rate (eGFR), sex, evidence of a urine culture before the dispense date and whether the TMP-SMX dose was appropriate for the patient's level of eGFR [4, 5].
MATERIALS AND METHODS
Study design and setting
We designed a matched new-user, active-comparator, retrospective, population-based cohort study in Ontario, Canada (2008–2020) using provincial linked administrative healthcare databases housed at ICES (ices.on.ca). Ontario residents have universal access to outpatient and inpatient healthcare services. Residents ≥65 years of age additionally have universal coverage for prescription drugs [12]. Emigration from the province would be the only reason for lost follow-up and the rate is <0.5%/year [13]. The use of data in this study was authorized under Section 45 of Ontario's Personal Health Information Protection Act, which does not require review by a research ethics board. We have reported this observational study following the Strengthening the Reporting of Observational studies in Epidemiology and REporting of studies Conducted using Observational Routinely collected Data specific to pharmacoepidemiological research guidelines (Supplementary Table 1) [14, 15].
Data sources
We ascertained study drug exposure, covariates and outcomes using linked administrative healthcare databases. We ascertained vital information from the Registered Persons Database, which contains demographic information on all Ontario residents. We obtained data on prescription records from the Ontario Drug Benefit (ODB) database, which contains highly accurate records on medication dispensing (the overall error rate is <1%) [12]. Prescriber data were ascertained from the ICES Physician Database and the ODB database. Patient comorbidities were ascertained using the Canadian Institute for Health Information Discharge Abstract Database (CIHI-DAD) and the Ontario Health Insurance Plan (OHIP) database. The CIHI-DAD contains information on diagnoses and procedures during acute inpatient encounters. The OHIP database contains claims information for all physician services. Emergency department visits and hospitalization data were ascertained from the National Ambulatory Care Reporting System and CIHI-DAD, respectively. Laboratory measurements for serum potassium and creatinine were obtained from the Ontario Laboratories Information System, a provincial electronic repository of laboratory results from hospitals and community and public health laboratories [16]. These datasets were linked using unique encoded identifiers and analyzed at ICES.
Study cohort
The study cohort comprised adults ≥66 years of age with a new outpatient prescription for TMP-SMX or amoxicillin between 1 January 2008 and 17 December 2020. The prescription dispense date served as the date of cohort entry (i.e. the index date). We restricted the cohort to patients with baseline measurements of both serum potassium and creatinine taken in the 365- to 7-day period before the index date. Serum creatinine measurements were standardized using isotope dilution mass spectrometry. We grouped patients by four prespecified eGFR categories: ≥60, 45–59, 30–44 and <30 ml/min/1.73 m2 [17]. We calculated eGFR with the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation using the patient's most recent serum creatinine measurement obtained in the 365- to 7-day period before the index date [18]. To ensure we were studying new antibiotic prescriptions, we excluded those who received TMP-SMX or amoxicillin in the 180-day period before the index date and excluded patients who received other (nonstudy) antibiotics in the 180 days leading up to and including the index date [19]. To ensure study antibiotics were initiated in an outpatient setting, we excluded those with a hospital discharge or an emergency department visit in the 2 days before or on the index date. We also excluded patients who received potassium binders or had evidence of kidney failure (defined as receipt of chronic dialysis or a kidney transplant) in the 180-day period before the index date and excluded those with evidence of hyperkalemia (defined as a laboratory serum potassium value ≥5.5 mmol/L) in the 365-day period before the index date.
Exposure and comparator groups
The exposure group comprised outpatients newly treated with oral TMP-SMX. The comparator group comprised outpatients newly treated with oral amoxicillin (amoxicillin alone or in combination with clavulanic acid). Amoxicillin was chosen as an active comparator because it is commonly prescribed to treat similar infections as TMP-SMX but is not associated with hyperkalemia [7, 10]. Patients in the exposed and comparator groups were matched 1:1 on a priori selected baseline characteristics as described in the Statistical analysis section.
Outcomes
The primary outcome was a hospital encounter (an emergency department visit or a hospital admission) with hyperkalemia, defined by a laboratory serum potassium value ≥5.5 mmol/L. Three prespecified secondary outcomes were a hospital encounter with AKI, all-cause hospitalization and all-cause mortality. AKI was defined as an increase in serum creatinine concentration from the baseline value by either ≥0.3 mg/dl (≥26.5 μmol/L) or ≥50% or receipt of acute dialysis. As with serum potassium, the baseline serum creatinine value was the most recent measurement obtained in the 365- to 7-day period before the index date. Coding definitions for all-cause hospitalization and all-cause mortality are provided in Supplementary Table 2.
Patients were followed for 14 days after the index date to assess all study outcomes. This time frame reflects the typical antibiotic prescription duration and the period of acute exposure [8–10]. Hyperkalemia, when it occurs, is typically observed 5–10 days after TMP-SMX initiation [20, 21].
Baseline characteristics
Patient characteristics, including demographics, comorbidities, coprescriptions, healthcare use and laboratory measurements, were obtained from our study databases as described above. Coding definitions for these characteristics are provided in Supplementary Table 3. We examined comorbidities in the 5-year period before the index date, coprescriptions in the 180-day period before the index date and healthcare visits and laboratory measurements in the 365-day period before the index date; for laboratory measurements, the most recent measurement before the index date was used. Data on diagnostic tests such as urine cultures, chest X-rays, sputum collections, wound swabs and blood smears were examined in the 7-day period as well as 365-day period before the index date.
Statistical analysis
We used both standard and propensity score matching to address confounding in this study. We matched new users of TMP-SMX and amoxicillin 1:1 on the following variables: eGFR category (≥60, 45–59, 30–44 or <30 ml/min/1.73 m2), sex, presence of a urine culture in the 7 days up to and including the index date and the logit of the propensity score for the predicted probability of newly receiving TMP-SMX [within ±0.2 standard deviations (SD)]. We matched on sex and receipt of a urine culture to increase the probability that patients in the two groups received the study antibiotics for similar reasons and to facilitate subgroup analyses by sex and potential indication. The propensity score was derived from a multivariable logistic regression model with 146 measured baseline characteristics chosen a priori, including demographics, comorbidities, coprescriptions, healthcare use and laboratory measurements (see Supplementary Table 4 for a list of variables) [22]. Comorbidities and coprescriptions that may affect a patient's potassium level were carefully considered and included in the logistic model [23–25].
We summarized continuous variables as means and SDs or medians and interquartile ranges (IQRs; 25th–75th percentile) as appropriate and categorical variables as number and percentages. We used standardized differences to evaluate between-group differences on baseline characteristics in both the unmatched and matched cohorts [26]. Standardized differences >10% were considered meaningful. We estimated the risks of the primary and secondary outcomes in the matched cohort in both relative and absolute terms using risk ratios (RRs) and risk differences (RDs), respectively. Absolute risk was additionally expressed as the number needed to harm (1/RD). We estimated RRs and 95% confidence intervals (CIs) for the outcomes using modified Poisson regression models, using generalized estimating equations to account for the correlation induced by matching.
We tested for additive and multiplicative interaction for the primary outcome of hyperkalemia in the following four prespecified subgroups: eGFR category (≥60, 45–59, 30–44 and <30 ml/min/1.73 m2, sex (female or male), presence of a urine culture (absent or present) and appropriate TMP-SMX dose reduction for the level of eGFR (no or yes). A single-strength TMP-SMX tablet contains 80 mg TMP and a double-strength TMP-SMX tablet contains 160 mg TMP [5]. An appropriate daily dose for a patient's level of kidney function was defined as a TMP dose ≤320 mg for an eGFR ≥30 ml/min/1.73 m2, a TMP dose ≤160 mg for an eGFR of 15–29 ml/min/1.73 m2 and a TMP dose ≤80 mg for an eGFR <15 ml/min/1.73 m2 [4, 5].
Additional analyses
The primary outcome of hyperkalemia was further assessed using more severe definitions: a serum potassium level ≥6.0 mmol/L and ≥6.5 mmol/L. We calculated the E-value to evaluate the potential impact of unmeasured confounding on the risk estimate [27]. Briefly, an E-value indicates the strength of association that an unmeasured confounder needs to have with both the exposure and outcome to entirely explain the observed association between the two variables [28]. For example, an E-value of 6 would suggest that an observed association could be explained away by an unmeasured confounder that was associated with both the exposure and the outcome by an RR of 6-fold each. The higher the E-value the less likely the chance the observed association is due to unmeasured confounding. We performed all analyses with SAS version 9.4 (SAS Institute, Cary, NC, USA). In all outcome analyses we interpreted two-tailed P-values <.05 as statistically significant.
RESULTS
Study cohort
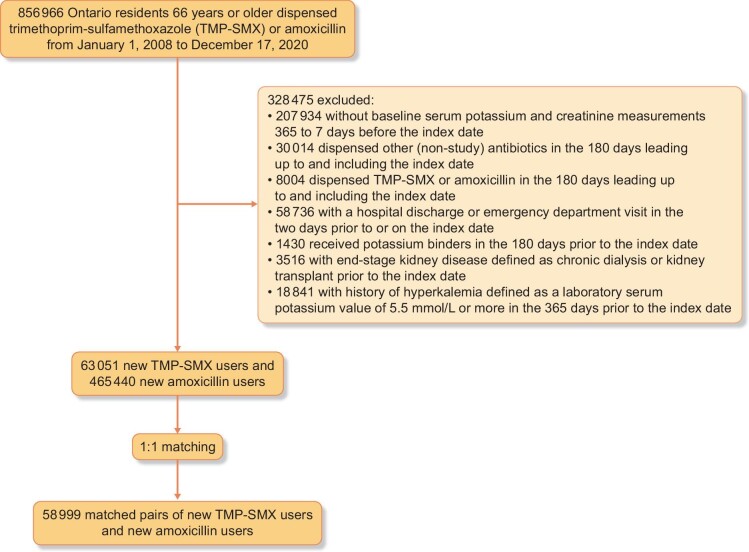
Cohort selection is presented in Fig. 1. We identified 63 051 new TMP-SMX users and 465 440 new amoxicillin users between 2008 and 2020. Before matching, TMP-SMX users were on average older;, more likely to be female; more likely to have a history of dementia, urinary tract infection, pneumonia and urinary retention; and were more likely to have coprescriptions for loop diuretics and angiotensin II receptor blockers (Table 1 and Supplementary Table 4). TMP-SMX users had a lower mean eGFR than amoxicillin users, a higher Charlson comorbidity score and a greater number of coprescriptions. The full set of 146 characteristics is shown in Supplementary Table 4. A total of 58 999 TMP-SMX users were successfully matched 1:1 with 58 999 amoxicillin users (93.6% of TMP-SMX users). After matching, all characteristics were well balanced between the two groups (all standardized differences <10%; Table 1 and Supplementary Table 4).
Figure 1:
Cohort derivation.
Table 1:
Baseline characteristics of new TMP-SMX users and amoxicillin users before and after matching (2008–2020).
| Before matching | After matching | |||||
|---|---|---|---|---|---|---|
| Characteristics | TMP-SMX (n = 63 051) | Amoxicillin (n = 465 440) | Standardized differencea, % | TMP-SMX (n = 58 999) | Amoxicillin (n = 58 999) | Standardized differencea, % |
| Age (years), mean (SD) | 78.33 (8.6) | 75.07 (7.6) | 40 | 78.29 (8.6) | 78.49 (8.8) | 2 |
| Female, n (%) | 43 169 (68.5) | 250 699 (53.9) | 30 | 40 103 (68.0) | 40 103 (68.0) | 0 |
| Year of cohort entryb, n (%) | ||||||
| 2008–2011 | 828 (1.2) | 3243 (0.7) | 5 | 768 (1.2) | 517 (0.9) | 3 |
| 2012–2014 | 8048 (12.8) | 53 326 (11.5) | 4 | 7548 (12.8) | 6895 (11.7) | 3 |
| 2015–2017 | 27 123 (43.0) | 210 009 (45.1) | 4 | 25 302 (42.9) | 26 634 (45.2) | 5 |
| 2018–2020 | 27 052 (42.9) | 198 862 (42.7) | 0 | 25 381 (43.0) | 24 953 (42.3) | 1 |
| Rural residence, n (%) | 10 051 (15.9) | 48 131 (10.3) | 17 | 9148 (15.5) | 9152 (15.5) | 0 |
| Neighborhood income quintilec, n (%) | ||||||
| 1 (lowest income) | 14 593 (23.1) | 92 240 (19.8) | 2 | 13 666 (23.2) | 13 782 (23.4) | 0 |
| 2 | 13 232 (21.0) | 95 111 (20.4) | 8 | 12 408 (21.0) | 12 480 (21.2) | 0 |
| 3 | 12 237 (19.4) | 92 687 (19.9) | 1 | 11 616 (19.7) | 11 535 (19.6) | 0 |
| 4 | 11 454 (18.2) | 88 510 (19.0) | 1 | 10 710 (18.2) | 10 654 (18.1) | 0 |
| 5 (highest income) | 11 324 (18.0) | 95 836 (20.6) | 2 | 10 599 (18.0) | 10 548 (17.9) | 0 |
| Long-term care residence, n (%) | 11 497 (18.2) | 22 417 (4.8) | 43 | 10 398 (17.6) | 10 289 (17.4) | 1 |
| Prescriber specialtyd, n (%) | ||||||
| General/family medicine | 49 919 (79.2) | 247 076 (53.1) | 57 | 47 073 (79.8) | 47 379 (80.3) | 1 |
| Nurse practitioner | 3665 (5.8) | 10 743 (2.3) | 18 | 3277 (5.6) | 3247 (5.5) | 0 |
| Urology | 2829 (4.5) | 2090 (0.4) | 27 | 2235 (3.8) | 1987 (3.4) | 2 |
| Internal medicine | 429 (0.7) | 2447 (0.5) | 3 | 414 (0.7) | 445 (0.8) | 1 |
| Nephrology | 179 (0.3) | 428 (0.1) | 4 | 174 (0.3) | 169 (0.3) | 0 |
| Dental | 10 (0.0) | 33 416 (7.2) | 39 | 10 (0.0) | 11 (0.0) | 4 |
| Other | 3997 (6.3) | 15 782 (3.4) | 14 | 3795 (6.4) | 4287 (7.3) | 4 |
| Comorbidities in prior 5 yearse, n (%) | ||||||
| Skin and soft tissue infection | 27 516 (43.6) | 181 789 (39.1) | 9 | 25 676 (43.5) | 25 796 (43.7) | 0 |
| Diabetes mellitus | 17 797 (28.2) | 132 318 (28.4) | 0 | 16 772 (28.4) | 16 977 (28.8) | 1 |
| Cancer | 10 756 (17.1) | 69 191 (14.9) | 6 | 10 007 (17.0) | 10 172 (17.2) | 1 |
| Urinary tract infection | 10 680 (16.9) | 26 789 (5.8) | 36 | 9624 (16.3) | 9291 (15.7) | 2 |
| Congestive heart failure | 9369 (14.9) | 51 309 (11.0) | 12 | 8879 (15.0) | 9132 (15.5) | 1 |
| Joint infection (septic arthritis) | 9289 (14.7) | 65 841 (14.1) | 2 | 8680 (14.7) | 8716 (14.8) | 0 |
| Pneumonia | 4802 (7.6) | 19 402 (4.2) | 14 | 4464 (7.6) | 4554 (7.7) | 0 |
| Chronic liver disease | 2804 (4.4) | 23 311 (5.0) | 3 | 2657 (4.5) | 2743 (4.6) | 0 |
| Other infection | 23 013 (36.5) | 172 556 (37.1) | 1 | 21 733 (36.8) | 22 167 (37.6) | 2 |
| Charlson Comorbidity Index, mean (SD)f | 0.9 (1.5) | 0.5 (1.2) | 23 | 0.8 (1.5) | 0.9 (1.5) | 1 |
| Coprescriptions in prior 180 daysg, n (%) | ||||||
| Glucocorticoid | 17 243 (27.3) | 120 428 (25.9) | 3 | 16 186 (27.4) | 16 443 (27.9) | 1 |
| Beta blockers | 16 452 (26.1) | 113 776 (24.4) | 4 | 15 538 (26.3) | 15 709 (26.6) | 1 |
| Calcium channel blocker | 16 404 (26.0) | 121 195 (26.0) | 0 | 15 513 (26.3) | 15 891 (26.9) | 1 |
| Angiotensin-converting enzyme inhibitor | 12 243 (19.4) | 99 717 (21.4) | 5 | 11 607 (19.7) | 11 958 (20.3) | 2 |
| Angiotensin II receptor blocker | 9134 (14.5) | 87 499 (18.8) | 12 | 8762 (14.9) | 9140 (15.5) | 2 |
| Loop diuretics | 9068 (14.4) | 40 814 (8.8) | 18 | 8511 (14.4) | 8695 (14.7) | 1 |
| Thiazide diuretics | 7774 (12.3) | 55 730 (12.0) | 1 | 7283 (12.3) | 7237 (12.3) | 0 |
| Nonsteroidal anti-inflammatory drug (excluding aspirin) | 5958 (9.4) | 45 955 (9.9) | 2 | 5540 (9.4) | 5418 (9.2) | 1 |
| Prednisone | 3237 (5.1) | 17 974 (3.9) | 6 | 3030 (5.1) | 3162 (5.4) | 1 |
| Antiarrhythmic agent | 2167 (3.4) | 12 656 (2.7) | 4 | 2071 (3.5) | 2211 (3.7) | 1 |
| Chemotherapeutic drugs | 1416 (2.2) | 8130 (1.7) | 4 | 1332 (2.3) | 1300 (2.2) | 1 |
| Tacrolimus | 163 (0.3) | 1237 (0.3) | 0 | 151 (0.3) | 161 (0.3) | 0 |
| Antiretroviral therapy | 63 (0.1) | 632 (0.1) | 0 | 62 (0.1) | 63 (0.1) | 0 |
| Cyclosporine | 27 (0.0) | 166 (0.0) | 6 | 26 (0.0) | 33 (0.1) | 4 |
| Healthcare visits in prior year, median (IQR) | ||||||
| General/family medicine visits | 9 (5–15) | 7 (4–12) | – | 9 (5–15) | 9 (5–15) | – |
| Hospitalizations | 0 (0–1) | 0 (0–1) | – | 0 (0–1) | 0 (0–1) | – |
| Emergency department visits | 0 (0–2) | 0 (0–1) | – | 0 (0–2) | 0 (0–2) | – |
| Diagnostic tests in prior 7 days, n (%) | ||||||
| Urine culture | 32 463 (51.5) | 38 160 (8.2) | 107 | 29 183 (49.5) | 29 183 (49.5) | 0 |
| Wound swab | 2788 (4.4) | 5521 (1.2) | 19 | 2435 (4.1) | 2664 (4.5) | 2 |
| Chest X-ray | 1907 (3.0) | 21 721 (4.7) | 9 | 1879 (3.2) | 2041 (3.5) | 2 |
| Sputum collection | 79 (0.1) | 318 (0.1) | 0 | 76 (0.1) | 87 (0.1) | 0 |
| Blood smear | 29 (0.0) | 108 (0.0) | 19 | 29 (0.0) | 26 (0.0) | 2 |
| Laboratory measurementsh | ||||||
| eGFR (ml/min/1.73 m2), mean (SD)i | 66 (19) | 70 (18) | 19 | 66 (19) | 66 (19) | 0 |
| eGFR categories (ml/min/1.73 m2), n (%) | ||||||
| ≥60 | 40 393 (64.1) | 335 855 (72.2) | 17 | 37 048 (62.8) | 37 048 (62.8) | 0 |
| 45–59 | 13 251 (21.0) | 83 466 (17.9) | 8 | 12 734 (21.6) | 12 734 (21.6) | 0 |
| 30–44 | 7171 (11.4) | 35 508 (7.6) | 13 | 7011 (11.9) | 7011 (11.9) | 0 |
| <30 | 2236 (3.5) | 10 611 (2.3) | 7 | 2206 (3.7) | 2206 (3.7) | 0 |
| Serum potassium (mmol/L), mean (SD) | 4.4 (0.4) | 4.4 (0.4) | 10 | 4.4 (0.4) | 4.4 (0.4) | 0 |
The difference between the groups divided by the pooled SD; a value >10% is interpreted as a meaningful difference.
The prescription dispense date was the date of cohort entry (i.e. the index date).
Income was categorized into fifths of average neighborhood income on the index date.
Information on prescriber specialty was available for 61 028 (96.8%) TMP-SMX users and 311 982 (67.0%) amoxicillin users before matching and 56 978 (96.6%) TMP-SMX users and 57 525 (97.6%) amoxicillin users after matching.
Comorbidities were assessed in the 5-year period before the index date. Information on hyperaldosteronism is not presented due to the limited number of patients with this comorbidity.
Charlson Comorbidity Index was calculated based on hospitalization data during the 5 years preceding the index date. For each patient, the index considers hospitalizations with the comorbidities of interest (acute myocardial infarction, congestive heart failure, peripheral vascular disease, cerebrovascular disease, dementia, chronic lung disease, rheumatic disease, peptic ulcer disease, mild and moderate/severe liver disease, diabetes mellitus with and without complications, hemiplegia/paraplegia, renal disease, cancer and metastatic solid tumor and acquired immunodeficiency syndrome/human immunodeficiency virus). It assigns a point score (1, 2, 3 or 6) for each comorbidity and sums them to generate an overall score of disease burden. The final risk scores range between 0 and 13, with higher values associated with higher mortality. Patients without a history of hospitalization received a score of 0.
Coprescriptions were examined in the 180-day period before the index date.
Laboratory measurements were examined in the 365-day period prior to the index date; the most recent value before the index date was used.
eGFR was calculated based on an individual's most recent serum creatinine measurement found in the 365 to 7 days prior to the index date, using the CKD-EPI equation: 141 × min([serum creatinine concentration in μmol/L/88.4]/ĸ, 1)α × max([serum creatinine concentration in μmol/L/88.4]/ĸ, 1)−1.209 × 0.993Age × 1.018 (if female) × 1.159 (if African American); ĸ = 0.7 if female and 0.9 if male; α = −0.329 if female and −0.411 if male; min = the minimum of serum creatinine concentration/ĸ or 1; max = the maximum of serum creatinine concentration/ĸ or 1. Information on race was not available in our data sources and all patients were assumed not to be of African Canadian race/ethnicity; African Canadians comprised <5% of the population of Ontario in 2006.
The median prescription duration for TMP-SMX was 7 days (IQR 5–7) and for amoxicillin it was 7 days (IQR 7–10). When examined by eGFR category, the median daily dose of TMP-SMX was 320 mg (IQR 320–320) in patients in the three highest eGFR categories (≥60, 45–59 and 30–44 ml/min/1.73 m2) and it was 320 mg (IQR 160–320) in patients with an eGFR <30 ml/min/1.73 m2 (Table 2).
Table 2:
Daily dose of TMP across eGFR categories.
| eGFR category (ml/min/1.73 m2) | n | Mean (SD) | Median (IQR) | Patients receiving a higher than appropriate daily TMP dosea, n (%) |
|---|---|---|---|---|
| ≥60 | 37 048 | 313.80 (111.49) | 320 (320–320) | 515 (1.4) |
| 45–59 | 12 734 | 304.46 (80.59) | 320 (320–320) | 165 (1.3) |
| 30–44 | 7011 | 284.53 (101.37) | 320 (300–320) | 97 (1.4) |
| <30 | 2206 | 252.08 (86.64) | 320 (160–320) | 1379 (62.5) |
A TMP-SMX tablet contains 80 mg TMP and a TMP-SMX double-strength tablet contains 160 mg TMP. An appropriate dose for a patient's eGFR was defined as a daily TMP dose ≤320 mg/day for eGFR ≥30 ml/min/1.73 m2, ≤160 mg/day for eGFR 15–29 and ≤80 mg/day for eGFR <15.
Primary outcome: a hospital encounter with hyperkalemia
The primary outcome results are presented in relative and absolute terms in Table 3. The risk of a hospital encounter with hyperkalemia was higher in patients treated with TMP-SMX [269/58 999 (0.46%)] versus amoxicillin [80/58 999 (0.14%)]; the RR was 3.36 (95% CI 2.62–4.31) and the RD was 0.32% (CI 0.26–0.38).
Table 3:
Risk of a hospital encounter with hyperkalemia and other outcomes within 14 days of initiating TMP-SMX versus amoxicillin.
| Event, n (%) | |||||
|---|---|---|---|---|---|
| Outcome | TMP-SMX (n = 58 999) | Amoxicillin (n = 58 999) | Risk difference, % (95% CI)c | Number needed to harm (95% CI) | RR (95% CI)c |
| Primary outcome | |||||
| Hyperkalemiaa | 269 (0.46) | 80 (0.14) | 0.32 (0.26–0.38) | 313 (263–385) | 3.36 (2.62–4.31) |
| Secondary outcomes | |||||
| AKIb | 1328 (2.25) | 422 (0.72) | 1.54 (1.40–1.67) | 65 (60–71) | 3.15 (2.82–3.51) |
| All-cause hospitalization | 2264 (3.84) | 1581 (2.68) | 1.16 (0.96–1.36) | 86 (74–104) | 1.43 (1.34–1.53) |
| All-cause mortality | 467 (0.79) | 427 (0.72) | 0.07 (−0.03–0.17) | Not significant | 1.09 (0.96–1.25) |
Hyperkalemia was defined as a serum potassium level ≥5.5 mmol/L.
AKI was defined as an increase in serum creatinine concentration from the baseline value of ≥0.3 mg/dl (≥26.5 μmol/L) or an increase of ≥50%, or receipt of acute dialysis. The baseline serum creatinine was the most recent value 365 to 7 days prior to the index date.
Risk differences and RRs were estimated within the matched cohort.
Secondary outcomes
The secondary outcome results are presented in relative and absolute terms in Table 3. The risk of a hospital encounter with AKI was higher in patients treated with TMP-SMX [1328/58 999 (2.25%)] versus amoxicillin [422/58 999 (0.72%)]; the RR was 3.15 (CI 2.82–3.51) and the RD was 1.54% (CI 1.40–1.67). The risk of all-cause hospitalization was higher in patients treated with TMP-SMX [2264/58 999 (3.84%)] versus amoxicillin [1581/58 999 (2.68%)]; the RR was 1.43 (CI 1.34–1.53) and the RD was 1.16% (CI 0.96–1.36). There was no significant difference in the risk of all-cause mortality between patients treated with TMP-SMX versus amoxicillin [RR 1.09 (CI 0.96–1.25)].
Subgroup analyses
The results of the subgroup analyses are presented in Table 4. The absolute risk of hyperkalemia in patients treated with TMP-SMX versus amoxicillin increased progressively with decreasing eGFR [at an eGFR ≥60 ml/min/1.73 m2, the RD was 0.12% (CI 0.06–0.18); at an eGFR of 45–59, the RD was 0.42% (CI 0.27–0.56); at an eGFR of 30–44, the RD was 0.85% (CI 0.58–1.11); and at an eGFR <30, the RD was 1.45% (CI 0.80–2.12); additive interaction P < .0001]. The relative risk of hyperkalemia in patients treated with TMP-SMX versus amoxicillin was not significantly modified by the eGFR category (multiplicative interaction P = .13).
Table 4:
Risk of a hospital encounter with hyperkalemia within 14 days of initiating TMP-SMX versus amoxicillin: subgroup analysis by eGFR categories, sex, presence of urine culture before antibiotic prescription and appropriate dose adjustment for kidney function.
| Subgroup | Exposure | n | Event, n (%) | Risk difference, % (95% CI) b | Additive interaction P-value | RR (95% CI)b | Multiplicative interaction P-value | |
|---|---|---|---|---|---|---|---|---|
| eGFR (ml/min/1.73 m2) | ≥60 | TMP-SMX | 37 048 | 81 (0.22) | 0.12 (0.06–0.18) | <.0001 | 2.25 (1.52–3.33) | .13 |
| Amoxicillin | 37 048 | 36 (0.10) | Reference | Reference | ||||
| 45–59 | TMP-SMX | 12 734 | 69 (0.54) | 0.42 (0.27–0.56) | 4.31 (2.50–7.43) | |||
| Amoxicillin | 12 734 | 16 (0.13) | Reference | Reference | ||||
| 30–44 | TMP-SMX | 7011 | 76 (1.08) | 0.85 (0.58–1.11) | 4.47 (2.66–7.52) | |||
| Amoxicillin | 7011 | 17 (0.24) | Reference | Reference | ||||
| <30 | TMP-SMX | 2206 | 43 (1.95) | 1.45 (0.80–2.12) | 3.91 (2.02–7.58) | |||
| Amoxicillin | 2206 | 11 (0.50) | Reference | Reference | ||||
| Sex | Female | TMP-SMX | 40 103 | 148 (0.37) | 0.24 (0.18–0.31) | .0013 | 2.96 (2.15–4.07) | .24 |
| Amoxicillin | 40 103 | 50 (0.12) | Reference | Reference | ||||
| Male | TMP-SMX | 18 896 | 121 (0.64) | 0.48 (0.35–0.61) | 4.03 (2.70–6.02) | |||
| Amoxicillin | 18 896 | 30 (0.16) | Reference | Reference | ||||
| Urine culture within 7 days before or on the day of antibiotic initiation | Absent | TMP-SMX | 29 816 | 152 (0.51) | 0.36 (0.27–0.45) | .22 | 3.38 (2.43–4.70) | .97 |
| Amoxicillin | 29 816 | 45 (0.15) | Reference | Reference | ||||
| Present | TMP-SMX | 29 183 | 117 (0.40) | 0.28 (0.20–0.36) | 3.34 (2.29–4.88) | |||
| Amoxicillin | 29 183 | 35 (0.12) | Reference | Reference | ||||
| Appropriate dose reduction per kidney functiona | No | TMP-SMX | 2156 | 35 (1.62) | 1.17 (0.55–1.78) | .0052 | 3.50 (1.73–7.07) | .90 |
| Amoxicillin | 2156 | 10 (0.46) | Reference | Reference | ||||
| Yes | TMP-SMX | 56 843 | 234 (0.41) | 0.29 (0.23–0.35) | 3.34 (2.56–4.36) | |||
| Amoxicillin | 56 843 | 70 (0.12) | Reference | Reference | ||||
A TMP-SMX tablet contains 80 mg TMP and a TMP-SMX double-strength tablet contains 160 mg TMP. An appropriate dose for a patient's eGFR was defined as a daily TMP dose ≤320 mg/day for eGFR ≥30 ml/min/1.73 m2, ≤160 mg/day for eGFR 15–<30 and ≤80 mg/day for eGFR <15.
Risk differences and RRs were estimated within the matched cohort.
The absolute risk of hyperkalemia in patients treated with TMP-SMX versus amoxicillin was higher in males than females (additive interaction P = .0013); however, the relative risk was not significantly modified by sex (multiplicative interaction P = .24). Neither the absolute risk nor the relative risk of hyperkalemia in patients treated with TMP-SMX versus amoxicillin was modified in patients with evidence of a urine culture (additive and multiplicative interaction P > .05). The absolute risk of hyperkalemia in patients treated with TMP-SMX versus amoxicillin was higher in those prescribed a higher-than-recommended TMP-SMX dose for their eGFR than those prescribed an appropriate dose (additive interaction P = .0052); however, the relative risk was not significantly modified (multiplicative interaction P = .90).
Additional analyses
The risk of hyperkalemia associated with TMP-SMX versus amoxicillin was similar using more severe definitions of hyperkalemia at a serum potassium level ≥6.0 mmol/L and ≥6.5 mmol/L (Supplementary Table 5). The calculated E-value for the RR and lower CI for the association between TMP-SMX and the primary outcome of hyperkalemia was 6.18 and 4.68 (Supplementary Fig. 1).
DISCUSSION
In this real-world cohort study of older adults, we found that new treatment with TMP-SMX versus amoxicillin was associated with a higher risk of a hospital encounter with hyperkalemia within 14 days. The difference in the risk of hyperkalemia in patients treated with TMP-SMX versus amoxicillin increased progressively across lower eGFR categories, from 0.12% (1/834) among patients with an eGFR ≥60 ml/min/1.73 m2 to 1.45% (1/69) among those with an eGFR <30 ml/min/1.73 m2. TMP-SMX versus amoxicillin use was also associated with a higher risk of a hospital encounter with AKI and all-cause hospitalization within 14 days. The difference in the risk of hyperkalemia with TMP-SMX versus amoxicillin use was higher in males than females and in those who received a higher-than-recommend daily dose of TMP for their level of eGFR [4, 5].
Our matched cohort of 117 998 older adults newly treated with TMP-SMX or amoxicillin provides robust estimates for the risk of a hospital encounter with hyperkalemia. The observed risk is supported by the known putative mechanism of reduced renal potassium excretion with TMP-SMX [6]. Our findings confirm the results of large cohort studies that examined the risk of hyperkalemia associated with TMP-SMX [7–9]. Whereas other studies primarily used database diagnosis codes to define hyperkalemic events (which have low sensitivity), we defined hyperkalemia using elevated serum potassium concentrations from laboratory measurements [11]. This is a more accurate way to define hyperkalemia and likely explains the 2-fold greater 14-day incidence of hyperkalemia in TMP-SMX users in our study compared with prior studies [10]. Similarly, our study used increasing concentrations of serum creatinine to define AKI rather than database codes, which likely explains the 5-fold higher 14-day incidence of AKI in the TMP-SMX users in our study compared with other studies [10, 29].
As with all renally cleared drugs, clinicians can consider alternate, non-renally cleared antibiotics guided by local microbiologic data when treating patients with chronic kidney disease. When TMP-SMX is prescribed to patients with diminished kidney function, strategies to mitigate renal dosing errors, such as featuring the information from this study within computerized order-entry systems with dosing prompts, should also be considered [30, 31].
Our study has several strengths. We conducted a population-based cohort study in the most populous province of Canada, a region with universal health insurance coverage. We included all older adults with valid study antibiotic prescriptions. We had an appropriately short duration of follow-up of 14 days to best capture outcome events relevant to the acute antibiotic exposure [20, 21]. Using laboratory measurements, we overcame the limitation of relying on diagnosis codes to ascertain study outcomes, which may substantively underestimate the true event rate. Our previous validation of International Classification of Diseases, Tenth Revision codes for hyperkalemia and AKI showed these codes have poor sensitivity when measured against laboratory-based definitions [11, 29].
Our study also has limitations. As with any observational study, our findings remain subject to residual confounding, including confounding by indication. To minimize these confounding effects, we employed a new-user, active-comparator design and used propensity score matching to balance comparison groups on baseline health, including on microbiology and radiologic investigations [32]. Moreover, the observed E-value of 6.18 for the estimated RR and 4.68 for the lower CI suggest that substantial unmeasured confounding would be needed to nullify the observed association or its 95% CI [28].
Our study only included adults ≥66 years of age and thus the generalizability of the study results to younger patients is uncertain. Older adults are of a population of interest for investigating adverse drug events, as they are predisposed to such outcomes [33]. Also, the follow-up serum potassium measurements in this study were done in routine care rather than as part of a research protocol where all participants would have a measurement at a fixed time point in follow-up.
In conclusion, treatment with TMP-SMX associates with a 3-fold higher risk of a hospital encounter with hyperkalemia in older adults. This risk is greater among patients with a lower eGFR and those who received a higher-than-recommended daily dose of TMP-SMX. Our findings have the potential to inform prescribing practice in clinical settings that warrant consideration of TMP-SMX use, especially among patients with diminished kidney function.
Supplementary Material
Contributor Information
Y Joseph Hwang, Department of Medicine, Johns Hopkins University School of Medicine, Baltimore, MD, USA.
Flory T Muanda, ICES, Toronto, Ontario, Canada; Department of Epidemiology and Biostatistics, Western University, London, Ontario, Canada.
Eric McArthur, ICES, Toronto, Ontario, Canada.
Matthew A Weir, ICES, Toronto, Ontario, Canada; Department of Epidemiology and Biostatistics, Western University, London, Ontario, Canada; Department of Medicine, Western University, London, Ontario, Canada.
Jessica M Sontrop, ICES, Toronto, Ontario, Canada; Department of Epidemiology and Biostatistics, Western University, London, Ontario, Canada.
Ngan N Lam, Division of Nephrology, University of Calgary, Calgary, Alberta, Canada.
Amit X Garg, ICES, Toronto, Ontario, Canada; Department of Epidemiology and Biostatistics, Western University, London, Ontario, Canada; Department of Medicine, Western University, London, Ontario, Canada.
ACKNOWLEDGEMENTS
The study sponsors had no role in the design and conduct of the study; collection, management, analysis and interpretation of the data; preparation, review or approval of the manuscript; and decision to submit the manuscript for publication.
FUNDING
This study was supported by the ICES Western Site. ICES is funded by an annual grant from the Ontario Ministry of Health and Long-Term Care (MOHLTC). Core funding for ICES Western is provided by the Academic Medical Organization of Southwestern Ontario (AMOSO), the Schulich School of Medicine and Dentistry (SSMD), Western University and the Lawson Health Research Institute (LHRI). The research was conducted by members of the ICES Kidney, Dialysis and Transplantation team at the ICES Western facility, who are supported by a grant from the Canadian Institutes of Health Research (CIHR). The opinions, results and conclusions are those of the authors and are independent from the funding sources. No endorsement by ICES, AMOSO, SSMD, LHRI, CIHR or the MOHLTC is intended or should be inferred. F.T.M. was supported by a Canadian Institutes of Health Research (CIHR) and MITACS postdoctoral award. A.G. was supported by the Dr Adam Linton Chair in Kidney Health Analytics and a CIHR Clinician Investigator Award.
AUTHORS’ CONTRIBUTIONS
Y.J.H., F.T.M. and A.X.G. conceived and designed the study. All authors contributed to the acquisition, analysis or interpretation of data. Y.J.H. drafted the manuscript. All authors contributed to critical revision of the manuscript for important intellectual content. E.M. performed statistical analysis. All authors read and approved the submitted manuscript.
DATA AVAILABILITY STATEMENT
The data set from this study is held securely coded from ICES. Although data sharing agreements prohibit ICES from making the data set publicly available, access may be granted to those who meet prespecified criteria for confidential access, available at https://www.ices.on.ca/DAS.
CONFLICT OF INTEREST STATEMENT
All authors have declared no competing interests. Results presented in this article have not been published in whole or part.
REFERENCES
- 1. Gupta K, Hooton TM, Naber KGet al. International clinical practice guidelines for the treatment of acute uncomplicated cystitis and pyelonephritis in women: a 2010 update by the Infectious Diseases Society of America and the European Society for Microbiology and Infectious Diseases. Clin Infect Dis 2011;52:e103–20. doi:10.1093/cid/ciq257. [DOI] [PubMed] [Google Scholar]
- 2. Drekonja DM, Rector TS, Cutting Aet al. Urinary tract infection in male veterans: treatment patterns and outcomes. JAMA Intern Med 2013;173:62. doi:10.1001/2013.jamainternmed.829. [DOI] [PubMed] [Google Scholar]
- 3. Stevens DL, Bisno AL, Chambers HFet al. Practice guidelines for the diagnosis and management of skin and soft tissue infections: 2014 update by the Infectious Diseases Society of America. Clin Infect Dis 2014;59:e10–52. doi:10.1093/cid/ciu296. [DOI] [PubMed] [Google Scholar]
- 4. May DB. Trimethoprim-sulfamethoxazole: an overview. In: UpToDate. Hooper D.C., Mitty J. (Eds.). 2021. https://www.uptodate.com/contents/trimethoprim-sulfamethoxazole-an-overview?search=tmpsmx&source=search_result&selectedTitle=1∼150&usage_type=default&display_rank=1#H7 (19 November 2021, date last accessed). [Google Scholar]
- 5. U.S. Food and Drug Administration . BactrimTM sulfamethoxazole and trimethoprim DS (double strength) tablets and tablets USP. June2013. https://www.accessdata.fda.gov/drugsatfda_docs/label/2013/017377s068s073lbl.pdf (19 November 2021, date last accessed). [Google Scholar]
- 6. Muto S, Tsuruoka S, Miyata Yet al. Effect of trimethoprim-sulfamethoxazole on Na and K+ transport properties in the rabbit cortical collecting duct perfused in vitro. Nephron Physiol 2006;102:51–60. doi:10.1159/000089682. [DOI] [PubMed] [Google Scholar]
- 7. Lam N, Weir MA, Juurlink DNet al. Hospital admissions for hyperkalemia with trimethoprim-sulfamethoxazole: a cohort study using health care database codes for 393,039 older women with urinary tract infections. Am J Kidney Dis 2011;57:521–3. doi:10.1053/j.ajkd.2010.11.006. [DOI] [PubMed] [Google Scholar]
- 8. Antoniou T, Gomes T, Juurlink DNet al. Trimethoprim-sulfamethoxazole–induced hyperkalemia in patients receiving inhibitors of the renin-angiotensin system: a population-based study. Arch Intern Med 2010;170:1045. doi:10.1001/archinternmed.2010.142. [DOI] [PubMed] [Google Scholar]
- 9. Antoniou T, Gomes T, Mamdani MMet al. Trimethoprim-sulfamethoxazole induced hyperkalaemia in elderly patients receiving spironolactone: nested case-control study. BMJ 2011;343:d5228. doi:10.1136/bmj.d5228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Crellin E, Mansfield KE, Leyrat Cet al. Trimethoprim use for urinary tract infection and risk of adverse outcomes in older patients: cohort study. BMJ 2018;360:k341. doi:10.1136/bmj.k341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fleet JL, Shariff SZ, Gandhi Set al. Validity of the International Classification of Diseases 10th revision code for hyperkalaemia in elderly patients at presentation to an emergency department and at hospital admission. BMJ Open 2012;2:e002011. doi:10.1136/bmjopen-2012-002011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Levy AR, O'Brien BJ, Sellors Cet al. Coding accuracy of administrative drug claims in the Ontario Drug Benefit database. Can J Clin Pharmacol 2003;10:67–71. [PubMed] [Google Scholar]
- 13. Statistics Canada . Table 17-10-0022-01. Estimates of interprovincial migrants by province or territory of origin and destination, annual. 29 September2021. https://www150.statcan.gc.ca/t1/tbl1/en/tv.action?pid=1710002201 (10 January 2022, date last accessed). [Google Scholar]
- 14. von Elm E, Altman DG, Egger Met al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann Intern Med 2007;147:573–7. [DOI] [PubMed] [Google Scholar]
- 15. Langan SM, Schmidt SA, Wing Ket al. The reporting of studies conducted using observational routinely collected health data statement for pharmacoepidemiology (RECORD-PE). BMJ 2018;363:k3532. doi:10.1136/bmj.k3532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Iskander C, McArthur E, Nash DMet al. Identifying Ontario geographic regions to assess adults who present to hospital with laboratory-defined conditions: a descriptive study. CMAJ Open 2019;7:E624–9. doi:10.9778/cmajo.20190065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Levey AS, Eckardt KU, Dorman NMet al. Nomenclature for kidney function and disease: report of a Kidney Disease: Improving Global Outcomes (KDIGO) Consensus Conference. Kidney Int 2020;97:1117–29. doi:10.1016/j.kint.2020.02.010. [DOI] [PubMed] [Google Scholar]
- 18. Levey AS, Stevens LA, Schmid CHet al. A new equation to estimate glomerular filtration rate. Ann Intern Med 2009;150:604–12. doi:10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ray WA. Evaluating medication effects outside of clinical trials: new-user designs. Am J Epidemiol 2003;158:915–20. doi:10.1093/aje/kwg231. [DOI] [PubMed] [Google Scholar]
- 20. Greenberg S, Reiser IW, Chou SYet al. Trimethoprim-sulfamethoxazole induces reversible hyperkalemia. Ann Intern Med 1993;119:291–5. [DOI] [PubMed] [Google Scholar]
- 21. Alappan R. Hyperkalemia in hospitalized patients treated with trimethoprim-sulfamethoxazole. Ann Intern Med 1996;124:316. doi:10.7326/0003-4819-124-3-199602010-00006. [DOI] [PubMed] [Google Scholar]
- 22. Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res 2011;46:399–424. doi:10.1080/00273171.2011.568786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Adelborg K, Nicolaisen SK, Hasvold Pet al. Predictors for repeated hyperkalemia and potassium trajectories in high-risk patients—a population-based cohort study. PLoS One 2019;14:e0218739. doi:10.1371/journal.pone.0218739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Thomsen RW, Nicolaisen SK, Hasvold Pet al. Elevated potassium levels in patients with chronic kidney disease: occurrence, risk factors and clinical outcomes—a Danish population-based cohort study. Nephrol Dial Transplant 2018;33:1610–20. doi:10.1093/ndt/gfx312. [DOI] [PubMed] [Google Scholar]
- 25. Thomsen RW, Nicolaisen SK, Hasvold Pet al. Elevated potassium levels in patients with congestive heart failure: occurrence, risk factors, and clinical outcomes: a Danish population-based cohort study. J Am Heart Assoc 2018;7:e008912. doi:10.1161/JAHA.118.008912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Austin PC. Using the standardized difference to compare the prevalence of a binary variable between two groups in observational research. Commun Stat Simul Comput 2009;38:1228–34. doi:10.1080/03610910902859574. [Google Scholar]
- 27. Mathur M, Ding P, Riddell Cet al. E-value calculator. 2020. https://www.evalue-calculator.com/ (1 November 2021, date last accessed). [Google Scholar]
- 28. VanderWeele TJ, Ding P.. Sensitivity analysis in observational research: introducing the E-value. Ann Intern Med 2017;167:268–74. doi:10.7326/M16-2607. [DOI] [PubMed] [Google Scholar]
- 29. Hwang YJ, Shariff SZ, Gandhi Set al. Validity of the international classification of diseases, tenth revision code for acute kidney injury in elderly patients at presentation to the emergency department and at hospital admission. BMJ Open 2012;2:e001821. doi:10.1136/bmjopen-2012-001821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tawadrous D, Shariff SZ, Haynes RBet al. Use of clinical decision support systems for kidney-related drug prescribing: a systematic review. Am J Kidney Dis 2011;58:903–14. doi:10.1053/j.ajkd.2011.07.022. [DOI] [PubMed] [Google Scholar]
- 31. Erler A, Beyer M, Petersen JJet al. How to improve drug dosing for patients with renal impairment in primary care - a cluster-randomized controlled trial. BMC Fam Pract 2012;13:91. doi:10.1186/1471-2296-13-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Yoshida K, Solomon DH, Kim SC.. Active-comparator design and new-user design in observational studies. Nat Rev Rheumatol 2015;11:437–41. doi:10.1038/nrrheum.2015.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Evans RS, Lloyd JF, Stoddard GJet al. Risk factors for adverse drug events: a 10-year analysis. Ann Pharmacother 2005;39:1161–8. doi:10.1345/aph.1E642. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data set from this study is held securely coded from ICES. Although data sharing agreements prohibit ICES from making the data set publicly available, access may be granted to those who meet prespecified criteria for confidential access, available at https://www.ices.on.ca/DAS.