ABSTRACT
Avoiding end-stage kidney disease in patients with anti-neutrophil cytoplasmic antibody–associated vasculitis (AAV) has a high therapeutic priority. Although renal response is a crucial measure to capture clinically relevant changes, clinal trials have used various definitions and no well-studied key surrogate markers to predict renal outcome in AAV exist. Differences in clinical features and histopathologic and therapeutic approaches will influence the course of kidney function. Its assessment through traditional surrogates (i.e. serum creatinine, glomerular filtration rate, proteinuria, hematuria and disease activity scores) has limitations. Refinement of these markers and the incorporation of novel approaches such as the assessment of histopathological changes using cutting-edge molecular and machine learning mechanisms or new biomarkers could significantly improve prognostication. The timing is favourable since large datasets of trials conducted in AAV are available and provide a valuable resource to establish renal surrogate markers and, likely, aim to investigate optimized and tailored treatment approaches according to a renal response score. In this review we discuss important points missed in the assessment of kidney function in patients with AAV and point towards the importance of defining renal response and clinically important short- and long-term predictors of renal outcome.
Keywords: ANCA vasculitis, ESKD, kidney function, outcome, renal response
THE SCOPE OF THE PROBLEM: SEVERE KIDNEY DISEASE IMPACTS ON PROGNOSIS
Anti-neutrophil cytoplasmic antibody (ANCA)-associated vasculitis (AAV) is a rare systemic autoimmune disease affecting small to medium-sized vessels and is characterized by autoantibodies against the major target of the neutrophil proteins, leucocyte proteinase 3 (PR3) and myeloperoxidase (MPO) [1]. AAV includes three clinically distinct groups: granulomatosis with polyangiitis (GPA), microscopic polyangiitis (MPA) and eosinophilic granulomatosis with polyangiitis (EGPA) [2, 3]. There is a 2.7-fold increased all-cause mortality risk among AAV patients compared with the general population [4]. Although cardiovascular disease and infections are important contributors to premature death [5, 6], the initial presence and the severity of kidney dysfunction are the most important predictors of mortality [7–9].
The frequency and severity of kidney involvement differ according to the clinical phenotype and ANCA serology. Despite refinement of approaches to induce and maintain remission in recent years, the rate of end-stage kidney disease (ESKD) remains high [1, 10]. Patients with systemic forms of MPA and GPA, characterized by multi-organ involvement, present with MPO- and PR3-ANCA and with kidney involvement in approximately 90–100% and 50–80%, respectively [1, 7, 11]. In contrast, EGPA patients have a substantially lower rate of kidney involvement (≈25%); however, differences according to ANCA positivity (predominantly MPO-ANCA) can be observed [7, 12]. Typically, AAV patients present with the clinical picture of rapidly progressive glomerulonephritis and face a rapid decline in kidney function, necessitating immediate therapy initiation to preserve kidney function and avoid the immediate threat of ESKD. Still, a subset of predominantly MPO-ANCA-positive patients may follow a slowly progressive course of kidney function deterioration, frequently associated with irreversible kidney lesions at their first presentation [13].
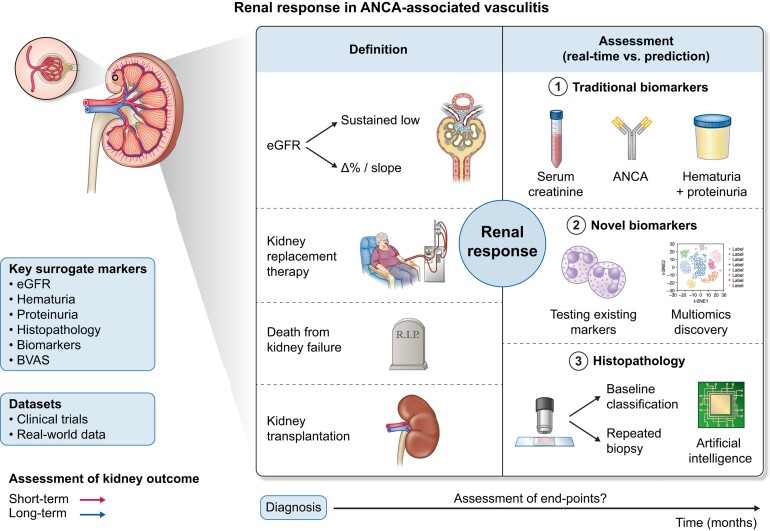
It is well recognized that kidney function impairment at the first presentation is associated with a transition from acute kidney injury to chronic kidney disease (CKD). Thus, halting progression of damage due to active vasculitis and thereby achieving kidney function recovery is a key therapeutic goal. A tool to quantify renal response is crucial not only to assess therapeutic response, but also to provide guidance for future clinical trials. However, the association of the extent of recovery with short- and long-term outcomes requires accurate definitions. This review raises the question: How do we define renal response in AAV Fig. 1? It becomes obvious that the focus should be turned towards defining standardized surrogate kidney markers associated with better long-term kidney and mortality outcomes. An ongoing project by the American College of Rheumatology (ACR) and European Alliance of Associations for Rheumatology (EULAR) is developing consensus response definitions for clinical trials but is not focusing on kidney responses [14].
Figure 1:
Definition and assessment of renal response in AAV. A schematic overview of surrogate markers of renal injury and potential renal recovery prediction, including differences in the definition (‘hard’ endpoints such as kidney replacement therapy or death from kidney failure) and assessment with novel and established methods).
WHAT IS THE CURRENT DEFINITION OF RENAL RESPONSE IN AAV AND WHAT IMPORTANT POINTS ARE MISSED?
Various definitions of clinical response have been used in AAV clinical trials [15]. Stabilization of serum creatinine level and resolution of renal hematuria are considered as markers of control of kidney inflammation. The Kidney Disease: Improving Global Outcomes (KDIGO) 2021 Clinical Practice Guideline for the Management of Glomerular Diseases [16] defines renal remission as stable or improved estimated glomerular filtration rate (eGFR), while the presence of hematuria and proteinuria may be considered as markers of active disease or chronic parenchymal damage (i.e. in the case of proteinuria). While this definition provides a rough direction, it is not known which extent of kidney function recovery and course are associated with favorable outcomes. In this regard, landmark clinical trials in AAV used different study definitions to describe renal response after initiation of remission induction therapy. In a secondary analysis of the Rituximab for the Treatment of Wegener's Granulomatosis and Microscopic Polyangiitis (RAVE) trial focusing on AAV patients with kidney involvement, renal remission was defined as stabilization or improvement in serum creatinine and resolution of hematuria [17], while most of the previous or later trials investigated changes in eGFR from baseline to assess renal response after therapy initiation [18–21]. Importantly, most of the trials did not look at renal remission as a primary endpoint, and renal response is usually defined as changes in serum creatinine level (i.e. using predefined cut-offs) or hematuria rather than eGFR-based measures.
Time points of outcome assessment
Assessment of endpoints vary between studies. A 3- to 6-month assessment of disease activity is typically used to investigate the efficacy of remission-inducing agents, and this should pick up differences in the speed of action of therapeutic agents, although earlier remission has been shown to reduce long-term ESKD and mortality risks [22]. One such agent is rituximab (RTX), and the general belief is that the onset of action of RTX is slower as compared with cyclophosphamide (CYC) or glucocorticoids (GCs) [23], particularly due to the missing effect on certain T cell–mediated pathways. However, GCs in combination with RTX have similar clinical efficacy in AAV patients with kidney involvement [19, 24] compared with GCs in combination with CYC and followed by azathioprine (AZA), and some patients did respond to RTX monotherapy [25]. Moreover, early GC withdrawal might be similarly effective in remission induction as the standard of care in patients with severe AAV [26]. In the ADVOCATE trial (NCT02994927), patients with kidney disease and eGFR <30 ml/min/1.73 m2 at baseline who received avacopan had a slightly delayed kidney function recovery immediately after therapy initiation as compared with the prednisone group. However, the same group experienced greater improvements in eGFR at both 26 and 52 weeks of follow-up [21]. This observation challenges the view that high doses of GCs are needed to abrogate inflammatory pathways leading to worsening of kidney function. It is important to note that C5aR1, which is the target of avacopan, is expressed by several key mediators of inflammation in AAV, including monocytes, macrophages and neutrophils [27], and this might explain the superior kidney function recovery potential of this agent. Thus far, it remains unclear whether the immediate or long-lasting action of a certain agent has sustained effects on kidney outcomes and further exploratory studies are needed.
Relevance of kidney function recovery
Baseline kidney function is an important subject when discussing outcome. Many randomized clinical trials have excluded AAV patients with more severe kidney function impairment at baseline. The RAVE trial excluded patients with a serum creatinine ≥4 mg/dl (353.6 µmol/l), but found in those with kidney disease a similar improvement of mean eGFR (RTX: +8 ml/min/1.73 m2; CYC/AZA: +7 ml/min/1.73 m2) after 18 months of follow-up [28]. In contrast, the RITUXVAS trial enrolled AAV patients with more advanced renal dysfunction [19]. In this study, a median GFR increase of 19 and 15 ml/min/1.73 m2 was observed at month 12 in patients who received either RTX (two pulses of CYC were given in this group) or CYC/AZA. Similarly, patients with more severe kidney dysfunction in the ADVOCATE trial had a greater improvement in mean eGFR in both study groups as compared with those with better kidney function [21]. This points towards the possibility of a more pronounced kidney function recovery in those with a more active/severe disease presentation at baseline and that the recovery potential might be predicted by acute histologic lesions. Importantly, age plays a crucial role in the prediction of kidney function recovery in those with acute presentation and recovery is more pronounced in pediatric patients and young adults [29]. Nevertheless, an exact definition of a clinically meaningful extent of kidney function recovery after remission induction therapy is lacking. Kidney function changes observed in landmark induction trials in AAV are summarized in Table 1.
Table 1:
Summary of kidney function changes observed in landmark induction therapy trials in AAV.
| Clinical trial (AAV patients involved) | Treatment (patients with renal disease) | Outcome measure | Outcome measure at baseline | Time point of outcome assessment | Outcome measure at end of follow-up |
|---|---|---|---|---|---|
| CYCLOPS (n = 149) [81] | Pulse versus daily oral CYC | eGFR (ml/min/1.73 m2) | 32 (15–52) versus 29 (18–48) | 3 months | 45 (28–64) versus 44 (30–63) |
| 6 months | 40 (28–60) versus 50 (37–64) | ||||
| MYCYC (n = 140) [20] | MMF versus CYC (n = 81) | eGFR (ml/min/1.73 m2) | 51 (29–92) versus 51 (31–79) | 18 months | 68 ± 4 versus 68 ± 4 |
| RITAZAREMa (n = 188) [82] | RTX; reduced versus standard-dose GC (n = 88) | Serum creatinine (µmol/l) | 92.5 (37.1–472) | 4 months | 97.3 (42–542) |
| RITUXVAS (n = 44) [19] | RTX versus CYC/AZA (n = 44) | eGFR (ml/min/1.73 m2) | 20 (5–44) versus 12 (9–33) | 12 months | 39 (20–45) versus 27 (12–47) |
| RAVE (n = 102)b [28] | RTX versus CYC/AZA (n = 51 each group) | eGFR (ml/min/1.73 m2) | 41.4 ± 3.3 versus 50.4 ± 3.3c | 18 months | 49 ± 3.4 versus 57 ± 3.4c |
| MEPEX (n = 137) [83] | IV MP versus PLEX | Serum creatinine (µmol/l) | 718 (498–1566) versus 754 (500–1669) | 12 monthsd | 198 (172–225) versus 199 (177–224) |
| PEXIVAS (n = 704) [30] | PLEX versus non-PLEX (n = 691) | Serum creatinine (µmol/l) | 327 (206–491) versus 336 (209–495) | n.d. | n.d. |
| Reduced versus standard-dose GC (n = 691) | 320 (190–480) versus 335 (219–502) | n.d. | n.d. | ||
| ADVOCATEe (n = 330) [21] | Prednisone versus avacopan (n = 265) | eGFR (ml/min/1.73 m2) | 45.6 ± 2.36 versus 44.6 ± 2.42c | Week 26 | +2.9 ± 1.03 versus +5.8 ± 1.04f |
| Week 52 | +4.1 ± 1.03 versus +7.3 ± 1.05f | ||||
| UACR (mg/g) | 312.2 (11–5367) versus 432.9 (20–6461) | Week 26 | −70 ± 9.5% versus −63 ± 9.7%g | ||
| Week 52 | −77 ± 9.6% versus −74 ± 9.8%g | ||||
| MCP-1:creatinine ratio (pg/mg) | 947.8 (160–6525) versus 983.8 (138–6145) | Week 26 | −64 ± 5.7% versus −67 ± 5.9%g | ||
| Week 52 | −71 ± 5.9% versus −73 ± 6.0%g |
IV: intravenous; MMF: mycophenolate mofetil; MP: methylprednisolone; n.d.: no data reported; UACR: urinary albumin:creatinine ratio.
Data are presented as median (range) or mean ± standard deviation unless stated otherwise.
AAV patients with relapsing disease.
Patients who had at least one major renal BVAS item.
Mean ± SEM.
In patients who had renal recovery.
In patients with renal disease at baseline based on BVAS.
Change from baseline, least square mean ± standard error of the mean.
Percent change from baseline, least square mean ± standard error of the mean.
Severity of kidney disease and long-term outcome
It seems obvious that patients with more severe and acute kidney disease may benefit from a more aggressive therapeutic approach, such as the addition of plasma exchange (PLEX). In the PEXIVAS trial (NCT00987389) [30], the median serum creatinine levels were 3.7 mg/dl (327 µmol/l) and 3.8 mg/dl (336 µmol/l) in the PLEX versus the control group, respectively. Approximately 30% of the patients had a serum creatinine level ≥5.7 mg/dl (500 µmol/l) or underwent dialysis. ESKD was a part of the primary composite outcome (together with death) and occurred in 28.4% and 31.0% of the cases [30]. The primary endpoint was assessed after almost 3 years on average of follow-up, but PLEX has an immediate effect on antibody titers and a therapeutic effect might be seen at an earlier stage, i.e. at 3 months. Indeed, the smaller MEPEX trial (NCT01408836) included patients with a serum creatinine ≥5.7 mg/dl (500 µmol/l) at baseline and found a beneficial effect of PLEX on ESKD at 3 and 12 months [21]. A retrospective study from the French Vasculitis Study Group aimed to refine the indication for PLEX and found the degree of kidney function impairment and MPA predictors of response. The addition of histologic parameters indicated that especially patients with a crescentic class on kidney biopsy would benefit from the addition of PLEX [31], which is in line with our understanding that cellular crescents are linked with acuity of kidney involvement.
Key point: The definition of renal response in AAV varies among clinical trials. No standardized, accepted model to define renal outcome prediction exists. Thus the impact of kidney function recovery (extent) and the ideal time point of assessment of early renal response (3 months versus 6 or 12 months) on long-term renal outcome remains unclear.
WHICH KIDNEY SURROGATE MARKERS ARE USED TO ASSESS RENAL RESPONSE IN AAV AND WHAT ARE THE LIMITATIONS?
Traditional serum markers: creatinine and GFR
Although a rapid rise in serum creatinine predicts chronic kidney damage in AAV [32], the use of serum creatinine has limitations. It is notably variable within individuals and is influenced by different factors, thus repeated measurements to verify abnormal results are often required. Moreover, serum creatinine is considered as a late indicator of kidney function impairment. The estimation of GFR is more accurate since it includes other variables and should be used rather than serum creatinine alone [33]. In accordance, a recent international consensus suggested well-defined definitions on kidney failure outcomes, including GFR-based surrogates (i.e. sustained low GFR or sustained percent decline in GFR) to predict progression to ESKD [34]. While these outcome definitions are based on a broad consensus, the course of kidney function might be highly variable between CKD populations with different disease phenotypes in addition to interindividual variability. However, the proposed GFR-based surrogates for kidney failure have not been extensively tested in large cohorts of patients with AAV and thus need validation and perhaps adaptation. Additionally, the median time to nadir serum creatinine in patients with ANCA-associated glomerulonephritis is 88 days and 50% of patients continue to experience improvement in serum creatinine beyond 3 months, a time point for initiation of maintenance therapy [35]. The assessment point for renal function recovery remains undefined.
Traditional urinary markers: hematuria
Hematuria is a characteristic feature of kidney involvement in AAV. Despite its diagnostic and clinical importance, studies on its utility for the prognostication of renal outcome are discordant. Some observational studies have reported an association between persistent hematuria and higher risk of renal relapses [36] or worsening of kidney function [37], while other authors have found no relationship with kidney function at 1 year after remission induction therapy [38]. Whether hematuria is a sign of ongoing glomerular inflammation or just a consequence of chronic damage is an open question [39, 40] and needs to be addressed in prospective studies. Studies using repeated kidney biopsies in the case of persistent or worsening hematuria are essential to answer these questions. A small study with interval biopsies after a median time of 130 days indicated low agreement with clinically suspected active disease, which was found in only 41% of patients [41]. More important information might be related to changes in the severity of hematuria [i.e. percentage of dysmorphic red blood cells (RBCs)], and additional studies in which the quantity of hematuria as part of the renal response and its association with the renal outcome are analyzed are needed. For this aim, however, other main challenges such as the definition of hematuria or the standardization of the method used for urine assessment need to be defined. As essential nephrological investigations, automated (blood cell counting or urine flow cytometry) or microscopic (light, including phase contrast microscopy) evaluations can be used for urinalysis, whereas microscopic assessment (ideally performed by a nephrologist) is essential to identify dysmorphic RBCs [42, 43] or RBC casts. Nevertheless, the availability of urine microscopy is limited in some centers and standardized nephrological training programs on its evaluation are often missing. Consequently, efforts to standardize hematuria assessment approaches (i.e. using microscopic examination) and accurate definitions of renal hematuria are needed to provide better accuracy.
Traditional urinary markers: proteinuria
According to the KDIGO 2021 Clinical Practice Guideline for the Management of Glomerular Diseases [16], persistent proteinuria is considered as a marker of chronic kidney damage in patients with AAV. In patients with CKD, the severity of proteinuria is associated with worse renal outcome, irrespective of baseline eGFR, [44] and is a strong predictor of ESKD [45]. In AAV, the degree of proteinuria might vary on a larger scale [46], but observational studies have found a positive correlation between baseline proteinuria and renal outcome [47, 48]. On the other hand, de Joode et al. [49] reported no significant association between persistent proteinuria (as assessed at baseline and 6 months after remission induction) and ESKD. In accordance, an analysis of the Wegener's Granulomatosis Etanercept Trial and RAVE trial data also found no significant effect of persistent proteinuria at the time of remission on eGFR slopes [36]. Nevertheless, differences in the extent of proteinuria (both unselective and selective) according to histopathological classification and the presence or absence of immune complexes were observed [50, 51]. This association might be explained in part by the proportion of normal glomeruli. However, the tubular toxicity of proteinuria might directly contribute to interstitial fibrosis and tubular atrophy [52], and consequently to worse renal outcome. This might also explain—at least in part—the relationship between the extent of proteinuria and histopathological classification observed in AAV. As such, simultaneous consideration of changes in proteinuria and histopathology (as discussed below) might serve as a reliable surrogate marker in assessing renal response and consequently in prognostication of renal outcome. Nevertheless, early blockade of glomerular inflammation and consequent resolution of proteinuria might also be a more precise marker of renal response if the clinically significant reduction is defined properly.
Birmingham Vasculitis Activity Score (BVAS)
The BVAS is a validated tool for assessing disease activity of various systemic vasculitides and consists of scored items divided into nine organ systems [53, 54]. The renal items include the presence of hypertension, proteinuria (>1+ assessed by urine protein spot), hematuria (≥10 RBCs/high-power field), serum creatinine divided into three groups (1.41–2.82, 2.83–5.64 and ≥5.66 mg/dl) and an increase in serum creatinine by >30% or a decrease in creatinine clearance by >25%, all related to active vasculitis. Changes in at least one major BVAS renal item are usually used to either describe kidney involvement or define renal relapse in clinical trials recruiting AAV patients. Nevertheless, it does not provide reliable information on the activity of the inflammatory processes at the tissue level and its use in assessing renal response might be limited in various clinical scenarios (i.e. in the setting of acute kidney injury, in the presence of chronic tissue damage or if extrarenal hematuria is present) due to its semiquantitative analysis.
Key point: Traditional serum and urinary markers are widely used as surrogate markers to define renal response in AAV, however, several significant limitations in their use exist. Further efforts towards validation and standardization are warranted. BVAS misses key aspects of kidney involvement in AAV, negating its use in the assessment of renal response.
CAN THE ANCA TITER AND NEWER BLOOD AND URINE BIOMARKERS REVEAL RENAL RESPONSE IN AAV PATIENTS?
ANCA
Both blood and urine biomarkers reflecting immunological and inflammatory processes in the kidney might be important alternatives to assess renal response in AAV. Although ANCA has an undisputed diagnostic value in AAV, its role as a response biomarker in the assessment of disease relapse risk is debated. A meta-analysis found that persistent positive or increasing ANCA levels are modestly associated with future disease relapses [55]. Nevertheless, patients with PR3-ANCA positivity (either persistent or reappearance) treated with RTX had a higher risk of relapse while the absence of PR3-ANCA is highly associated with a relapse-free status [56, 57]. Interestingly, an increase in PR3-ANCA titer specifically predicted disease relapse in patients with baseline kidney involvement or alveolar hemorrhage [58]. Accordingly, in their analysis of 166 AAV patients (104 with renal involvement and 62 with non-renal disease), Kemna et al. [59] observed an 11-fold higher risk for subsequent relapse associated with an ANCA increase in those with kidney disease, while it was less predictive in patients with non-renal disease. Whether these observations may determine the pathogenicity of ANCA in the kidney or are the consequence of a more severe vasculitic disease phenotype remains unresolved. Moreover, no studies thus far have investigated the course of ANCA titers and the impact on kidney function during follow-up. Notably, ANCA titers can also be influenced by antibody detection methodology, including assay type, detection limit or epitope masking [58, 60], which may have a significant effect on the utility of ANCA as a response biomarker. Therefore, rising ANCA titers may necessitate close clinical follow-up, but it is not clear whether its persistence or changes of its level after remission induction might serve as a marker of renal response.
Novel markers: urinary sCD163 and monocyte chemoattractant protein-1 (MCP-1)
Several promising markers, such as complement components and urinary proteins and chemokines, have been investigated in recent years [60]. One of these marker molecules is urinary soluble CD163 (sCD163), a membrane protein localized on the surface of monocytes and macrophages, which was shown to be associated with the activity of renal vasculitis [61] and also indicated renal relapse with an excellent discrimination [62]. Moreover, the addition of the T cell activation marker sCD25, both in urine and serum, helped to identify patients with early renal damage, as some patients might remain undetected if urinary sCD163 is measured alone [63]. As monocytes play a crucial role in substantiating inflammation in the kidney [64], further biomarkers reflecting their function have been evaluated in AAV. Among others, monocyte chemoattractant protein-1 (MCP-1), a potent chemotactic factor for monocytes, was shown to be a useful marker to identify kidney involvement and assess therapy response [65]. In a recent analysis, most of the AAV patients who developed ESKD had distinct differences in the MCP-1 gene (A/A genotype at -2518) associated with higher urinary MCP-1 levels at baseline [66]. Nevertheless, these markers need further validation, especially in the setting of renal response, which first requires an understanding of their role in the development of kidney disease in AAV.
The future of biomarker discovery in AAV
Significant developments in biomarker discovery, such as omics-based strategies, are currently arising; nevertheless, data with a focus on AAV are yet limited. Proteomics approaches have identified distinct dysregulated pathways in AAV [67, 68], and gene expression signatures using transcriptomics-predicted clinical outcomes are examples of the potential of ‘omics’ approaches [69], but data from well-defined clinical cohorts in AAV are currently missing. Modern approaches will eventually lead to a new era of precision medicine, shaping our understanding of how specific therapies promote renal response in patients with AAV.
Key point: The impact of changes in ANCA titers on kidney function over time is unknown. Novel urinary markers (i.e. sCD163 and MCP-1) provide promising alternatives to assess the activity of renal vasculitis, while their role as renal response markers needs to be further validated. Cutting-edge omics-based technologies could enable a new era of biomarker discovery in AAV.
CAN A KIDNEY TISSUE SPECIMEN PREDICT KIDNEY FUNCTION RECOVERY?
Kidney biopsy results inform about the potential of kidney function recovery in most cases. Analysis of 55 patients with PR3-ANCA and 74 patients with MPO-ANCA vasculitis revealed that patients with PR3-ANCA vasculitis more frequently have acute lesions, i.e. a focal form of crescentic glomerulonephritis and glomerular necrosis, while patients with MPO-ANCA vasculitis present with more severe damage at the time of initial diagnosis, characterized by diffuse crescentic glomerulonephritis, glomerulosclerosis and interstitial fibrosis [70]. The latter also explains the speed of kidney function decline and subsequent recovery in some patients with MPO-ANCA vasculitis, presenting with a slowly progressive form of ANCA-glomerulonephritis that is characterized by a renal-limited form of vasculitis and a predominance of glomerulosclerosis at the time of biopsy. Glomerulosclerosis represents irreversible damage, but despite the predominance of these lesions, most patients in this study experienced a response to therapy, with an improvement in eGFR ≥25% at 6 months reported in 73%. This was accompanied by a significant reduction in proteinuria over time [13].
Histopathologic prediction scores were established in recent years. The Berden score focuses on histology and incorporates the percent of normal glomeruli, the percent of crescents and the percent of glomerulosclerosis (for classes, see Table 2). Long-term prognosis is excellent in patients with a focal class, intermediate in those with either a crescentic or mixed class and worst in those with a sclerotic class. Notably, kidney function at baseline is similar in patients with a crescentic or sclerotic class, while marked differences are observed in terms of kidney function recovery [71]. A small study on repeated protocol kidney biopsies revealed a progression of the Berden score in almost all investigated cases, suggesting ongoing disease activity despite initiation of immunosuppressive therapy [72]. Sequential assessment of the Berden classification indicated a class switch in 50%, with a histologic progression reported in 30% [41]. Consequently, implementation of protocol biopsies might be a useful tool to determine therapy response. Glomerular histopathological changes do not occur in isolation, and severe glomerulosclerosis is usually accompanied by extensive tubulointerstitial damage [interstitial fibrosis/tubular atrophy (IF/TA)]. The addition of these changes did not add additional value when assessed by Berden et al. [73].
Table 2:
Summary of different risk scores used to predict outcomes of patients with ANCA glomerulonephritis.
| Score | |||
|---|---|---|---|
| Risk groups | Berden [73] | Brixa [74] | MCCSb,c [75] |
| Focal (≥50% normal glomeruli) eGFR at presentation: 50 ± 29 ml/min/1.73 m2 | Low risk (0), kidney survival at 3 years: 100% | Minimal (0–1) eGFR at baseline: 48.3 ml/min/1.73 m2Renal recovery: 83.8% | |
| eGFR at 1 year: 61 ± 24 ml/min/1.73 m2 | |||
| Crescentic (≥50% cellular crescents) eGFR at presentation: 18 ± 16 ml/min/1.73 m2eGFR at 1 year: 37 ± 21 ml/min/1.73 m2 | Intermediate risk (2–7), kidney survival at 3 years: 96% | Mild (2–4) eGFR at baseline: 29.2 ml/min/1.73 m2Renal recovery: 68.5% | |
| Mixed (<50% normal, cellular crescents, globally sclerotic, each) eGFR at presentation: 27 ± 19 ml/min/1.73 m2eGFR at 1 year: 38 ± 21 ml/min/1.73 m2 | High risk (8–11), kidney survival at 3 years: 77% | Moderate (5–7) eGFR at baseline: 23.7 ml/min/1.73 m2Renal recovery: 52.4% | |
| Sclerotic (≥50% globally sclerotic glomeruli) eGFR at presentation: 19 ± 12 ml/min/1.73 m2eGFR at 1 year: 20 ± 16 ml/min/1.73 m2 | Severe (≥8) eGFR at baseline: 18.5 ml/min/1.73 m2Renal recovery: 39.3% | ||
ANCA renal risk score comprises percentage of normal glomeruli [N0 (>25%, 0 points), N1 (10–25%, 4 points), N2 (<10%, 6 points)], tubular atrophy/interstitial fibrosis [T0 (≤25%, 0 points), T1 (>25%)] and kidney function at the time of diagnosis (eGFR) [G0 (>15 ml/min/1.73 m2), G1 (≤15 ml/min/1.73 m2)].
MCCS: (a) global and segmental glomerulosclerosis; (b) tubular atrophy; (c) interstitial fibrosis (<10% = 0; 10–25% = 1; 26–50% = 2; ≥50% = 3; each category a–c), (d) arteriosclerosis (intimal thickening ≥ media thickness = 1).
Definition of renal recovery: independence of kidney replacement therapy (for those in whom this therapy was initiated, as improvement of eGFR to values ≥30 ml/min/1.73 m2 (if severe renal disease at diagnosis), improvement of renal function (if non-severe renal disease at diagnosis) or sustained eGFR ≥30 ml/min/1.73 m2.
In contrast, the Brix score and the Mayo Clinic Chronicity Score (MCCS) both found a value of IF/TA in prognostication. The Brix score incorporates the percent of normal glomeruli, percent of IF/TA and baseline eGFR and subdivides the groups into low, medium and high risk to develop kidney failure. At 3 years of follow-up, patients in the high-risk group develop ESKD more frequently compared with the other groups [74]. Tubulointerstitial changes are weighted the most in the MCCS, which accurately predicts renal recovery. Baseline kidney function differs between the four different categories [75] (see Table 2 for further information). Despite differences in weighting and assessed items, all of these scores uniformly report that chronic and irreversible histopathological changes are important in predicting long-term renal survival. A refinement of these scores in combination with clinical surrogate markers, ideally incorporating machine learning algorithms, and implementation of novel approaches such as deep learning–based molecular morphometrics [76] will allow further improvement in prognostication at baseline.
Key point: The association between histopathological changes and renal outcome is well established; nevertheless, further refinement of the histopathologic scores is needed and under way. Validation of repeated protocol kidney biopsies as markers of renal response in well-defined cohorts is necessary.
What is the practical value of better predictors of renal response and what are the target endpoints?
Outcome prediction can eventually lead to refinement of induction therapy. In patients with the potential for kidney function recovery (high percentage of cellular crescents, younger age, non-oliguric/anuric kidney function, PR3-ANCA positive) [70, 77], greater immunosuppression might be initiated. This warrants further studies in larger cohorts, incorporating novel therapeutic approaches that hold great promise in leading to better eGFR recovery. In the long-term, every little bit of eGFR improvement counts, as patients need to live with the consequences of CKD and its sequelae. Demographic and laboratory parameters and histology need to be assessed in a large sample with discovery and replication cohorts to estimate the value to predict eGFR in the short and long term. Accordingly, recent analysis of major studies in lupus nephritis have provided evidence that the determination of well-established key surrogate markers can predict favorable long-term renal outcome and consequently open the way to better clinical trial designs [78]. Aside from the traditional endpoints, such as death from kidney failure, kidney transplantation or renal relapse, newer hard endpoints need to point towards eGFR recovery potential (i.e. eGFR slope based on validation in AAV cohorts) of a novel drug, which ideally needs to be assessed only in patients without renal relapse during follow-up. In those with a critical impairment of kidney function, the avoidance of a further decline in eGFR needs to be the target of continuous follow-up. Long-term maintenance strategies and eventually the addition of nephroprotective substances such as sodium–glucose cotransporter 2 inhibitors [79] after successful induction therapy need to be considered in such cases.
In conclusion: do we need to act?
Although renal response is a crucial measure and avoiding ESKD is one of the main goals in the therapy of AAV with kidney disease, no standardized, internationally accepted definition for key surrogate markers to predict renal outcome exist. As a prerequisite to further study renal outcome in AAV, a common definition of surrogate markers in outcome prediction is required. Therefore, establishing reliable markers that are clinically relevant, responsive to change and easily accessible by physicians is a high priority. Moreover, a more precise definition of renal response of different therapeutic agents with immediate or longer-lasting action while distinguishing changes in short-term kidney function on long-term renal outcome need to be extensively investigated. As expected, the occurrence of a renal relapse portends a risk factor to develop ESKD during follow-up [80].
Large datasets of trials conducted in AAV have become available recently, while large real-world data in registries (i.e. FAIRVASC) have been established and provide a valuable resource to establish renal surrogate markers and likewise aim to investigate optimized and tailored treatment approaches according to a renal response score. In addition, using novel approaches such as machine learning might further improve our arsenal of clinically relevant surrogate markers. These determinants need to provide useful information for prediction of good long-term renal outcomes and may help to shape kidney-related outcomes as relevant endpoints in future clinical trials of AAV. Therefore, the answer is crystal clear for us: yes, we need to act, and Box 1 summarizes a research agenda for future endeavors.
Box 1:
Research agenda for studies assessing renal response in patients with AAV
| Topic | Recommendations |
|---|---|
| Determine key renal surrogate markers for the assessment of early renal response/kidney function recovery in AAV | Test whether serum creatinine or eGFR can be used as a renal surrogate marker. |
| Test whether proteinuria can be used as a renal surrogate marker. | |
| Standardize and test whether hematuria/urine sediment can be used as a renal surrogate marker. | |
| Test whether ANCA (use standardized assays) or newer biomarkers (i.e. urinary sCD163 or MCP-1) can be used as a renal surrogate marker. | |
| Use novel molecular (i.e. multi-omics) approaches to identify new targets for kidney surrogate marker evaluation. | |
| Determine the role of kidney histopathology in combination with renal surrogate markers for the assessment of renal response in AAV | Test whether the incorporation of baseline histopathological changes can improve the predictive value of renal surrogate markers. |
| Develop clinical studies using protocolized/repeated kidney biopsies. | |
| Use novel cutting-edge approaches (i.e. machine learning) to identify further histopathological patterns associated with better clinical outcome. | |
| Determine key time points for assessing early renal response in AAV | Complete analysis on different time point assessments of early renal response associated with long-term renal outcome. |
| Determine key definitions of outcomes for long-term renal outcome (ESKD) in AAV | Test traditional renal endpoints (kidney replacement therapy for at least 90 days, death from kidney failure, kidney transplantation) to improve kidney clinical trial outcome measurements. |
| Test eGFR-based renal endpoints (sustained low or percent decline in GFR) using the Chronic Kidney Disease Epidemiology Collaboration formula to improve kidney clinical trial outcome measurements. |
Contributor Information
Balazs Odler, Division of Nephrology, Department of Internal Medicine, Medical University of Graz, Graz, Austria; Department of Medicine, University of Cambridge, Cambridge, UK.
Annette Bruchfeld, Department of Clinical Science, Intervention and Technology, Division of Renal Medicine Karolinska Institutet, Stockholm, Sweden; Department of Health, Medicine and Caring Sciences, Linköpings Universitet, Linköping, Sweden.
Jennifer Scott, Trinity Health Kidney Center, Trinity Translational Medicine Institute, Trinity College Dublin, Dublin, Ireland.
Duvuru Geetha, Division of Nephrology, Department of Medicine, School of Medicine, Johns Hopkins University, Baltimore, MD, USA.
Mark A Little, Trinity Health Kidney Center, Trinity Translational Medicine Institute, Trinity College Dublin, Dublin, Ireland.
David R W Jayne, Department of Medicine, University of Cambridge, Cambridge, UK.
Andreas Kronbichler, Department of Medicine, University of Cambridge, Cambridge, UK.
FUNDING
B.O. is a postdoctoral research fellow at the University of Cambridge supported by the Austrian Science Fund. This research was funded in whole or in part by the Austrian Science Fund (grant J 4664-B to B.O.). For the purpose of open access, the author has applied a CC BY public copyright licence to any author accepted manuscript version arising from this submission. J.S. is a Wellcome-HRB Irish Clinical Academic Training Fellow, supported by the Wellcome Trust and the Health Research Board (grant 203930/B/16/Z), the Health Service Executive, National Doctors Training and Planning and the Health and Social Care, Research and Development Division, Northern Ireland.
DATA AVAILABILITY STATEMENT
No new data were generated or analysed in support of this research.
CONFLICT OF INTEREST STATEMENT
B.O. has received speaker fees and travel support from Otsuka. A.B. is member of the CKJ editorial board and has received consulting and speaker fees from AstraZeneca, Bayer, ChemoCentryx, Fresenius, Merck/MSD and Vifor. D.G. has received consulting fees from ChemoCentryx, GlaxoSmithKline and Aurinia. D.J. has received consultancy or lecture fees from Amgen, AstraZeneca, Bristol-Myers Squibb, ChemoCentryx, CSL Vifor, Novartis, Otsuka, Roche and Takeda. A.K. is member of the CKJ editorial board and has received consulting fees from Vifor Pharma, Otsuka, Delta 4, UriSalt and Catalyst Biosciences. The other authors declared no conflicts of interest related to this work.
AUTHORS’ CONTRIBUTIONS
B.O. and A.K. drafted the initial version. The manuscript was critically reviewed and modified by all co-authors, who have approved the final draft.
REFERENCES
- 1. Kitching AR, Anders HJ, Basu Net al. ANCA-associated vasculitis. Nat Rev Dis Primers 2020;6:71. [DOI] [PubMed] [Google Scholar]
- 2. Jennette JC, Falk RJ, Bacon PAet al. 2012 revised International Chapel Hill Consensus Conference Nomenclature of Vasculitides. Arthritis Rheumat 2013;65:1–11. [DOI] [PubMed] [Google Scholar]
- 3. Almaani S, Fussner LA, Brodsky Set al. ANCA-associated vasculitis: an update. J Clin Med 2021;10:1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tan JA, Choi HK, Xie Het al. All-cause and cause-specific mortality in patients with granulomatosis with polyangiitis: a population-based study. Arthritis Care Res 2019;71:155–63. [DOI] [PubMed] [Google Scholar]
- 5. Wallace ZS, Fu X, Harkness Tet al. All-cause and cause-specific mortality in ANCA-associated vasculitis: overall and according to ANCA type. Rheumatology (Oxford) 2020;59:2308–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Little MA, Nightingale P, Verburgh CAet al. Early mortality in systemic vasculitis: relative contribution of adverse events and active vasculitis. Ann Rheum Dis 2010;69:1036–43. [DOI] [PubMed] [Google Scholar]
- 7. Sinico RA, Di Toma L, Radice A.. Renal involvement in anti-neutrophil cytoplasmic autoantibody associated vasculitis. Autoimmun Rev 2013;12:477–82. [DOI] [PubMed] [Google Scholar]
- 8. Booth AD, Almond MK, Burns Aet al. Outcome of ANCA-associated renal vasculitis: a 5-year retrospective study. Am J Kidney Dis 2003;41:776–84. [DOI] [PubMed] [Google Scholar]
- 9. Solans-Laqué R, Fraile G, Rodriguez-Carballeira Met al. Clinical characteristics and outcome of Spanish patients with ANCA-associated vasculitides: impact of the vasculitis type, ANCA specificity, and treatment on mortality and morbidity. Medicine (Baltimore) 2017;96:e6083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Moiseev S, Novikov P, Jayne Det al. End-stage renal disease in ANCA-associated vasculitis. Nephrol Dial Transplant 2017;32:248–53. [DOI] [PubMed] [Google Scholar]
- 11. Millet A, Pederzoli-Ribeil M, Guillevin Let al. Antineutrophil cytoplasmic antibody-associated vasculitides: is it time to split up the group? Ann Rheum Dis 2013;72:1273–9. [DOI] [PubMed] [Google Scholar]
- 12. Kronbichler A, Shin JI, Lee KHet al. Clinical associations of renal involvement in ANCA-associated vasculitis. Autoimmun Rev 2020;19:102495. [DOI] [PubMed] [Google Scholar]
- 13. Trivioli G, Gopaluni S, Urban MLet al. Slowly progressive anti-neutrophil cytoplasmic antibody-associated renal vasculitis: clinico-pathological characterization and outcome. Clin Kidney J 2021;14:332–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Quinn KA, Monti S, Christensen Ret al. An international Delphi exercise to identify items of importance for measuring response to treatment in ANCA-associated vasculitis. Semin Arthritis Rheum 2022;55:152021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Monti S, Quinn KA, Christensen Ret al. Use and reporting of outcome measures in randomized trials for anti-neutrophil cytoplasmic antibody-associated vasculitis: a systematic literature review. Semin Arthritis Rheum 2020;50:1314–25. [DOI] [PubMed] [Google Scholar]
- 16. KDIGO 2021 clinical practice guideline for the management of blood pressure in chronic kidney disease. Kidney Int 2021;99(3 Suppl):S1–87. [DOI] [PubMed] [Google Scholar]
- 17. Geetha D, Hruskova Z, Segelmark Met al. Rituximab for treatment of severe renal disease in ANCA associated vasculitis. J Nephrol 2016;29:195–201. [DOI] [PubMed] [Google Scholar]
- 18. de Groot K, Harper L, Jayne DRet al. Pulse versus daily oral cyclophosphamide for induction of remission in antineutrophil cytoplasmic antibody-associated vasculitis: a randomized trial. Ann Intern Med 2009;150:670–80. [DOI] [PubMed] [Google Scholar]
- 19. Jones RB, Tervaert JW, Hauser Tet al. Rituximab versus cyclophosphamide in ANCA-associated renal vasculitis. N Engl J Med 2010;363:211–20. [DOI] [PubMed] [Google Scholar]
- 20. Jones RB, Hiemstra TF, Ballarin Jet al. Mycophenolate mofetil versus cyclophosphamide for remission induction in ANCA-associated vasculitis: a randomised, non-inferiority trial. Ann Rheum Dis 2019;78:399–405. [DOI] [PubMed] [Google Scholar]
- 21. Jayne DRW, Merkel PA, Schall TJet al. Avacopan for the treatment of ANCA-associated vasculitis. N Engl J Med 2021;384:599–609. [DOI] [PubMed] [Google Scholar]
- 22. Gopaluni S, Flossmann O, Little MAet al. Effect of disease activity at three and six months after diagnosis on long-term outcomes in antineutrophil cytoplasmic antibody-associated vasculitis. Arthritis Rheumatol 2019;71:784–91 [DOI] [PubMed] [Google Scholar]
- 23. Kronbichler A, Jayne DR.. Con: Should all patients with anti-neutrophil cytoplasmic antibody-associated vasculitis be primarily treated with rituximab? Nephrol Dial Transplant 2015;30:1075–81. [DOI] [PubMed] [Google Scholar]
- 24. Specks U, Merkel PA, Seo Pet al. Efficacy of remission-induction regimens for ANCA-associated vasculitis. N Engl J Med 2013;369:417–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Farrah TE, Prendecki M, Hunter RWet al. Glucocorticoid-free treatment of severe ANCA-associated vasculitis. Nephrol Dial Transplant 2021;36:739–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pepper RJ, McAdoo SP, Moran SMet al. A novel glucocorticoid-free maintenance regimen for anti-neutrophil cytoplasm antibody-associated vasculitis. Rheumatology (Oxford) 2019;58:260–8. [DOI] [PubMed] [Google Scholar]
- 27. Stewart BJ, Ferdinand JR, Young MDet al. Spatiotemporal immune zonation of the human kidney. Science 2019;365:1461–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Geetha D, Specks U, Stone JHet al. Rituximab versus cyclophosphamide for ANCA-associated vasculitis with renal involvement. J Am Soc Nephrol 2015;26:976–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Calatroni M, Consonni F, Allinovi Met al. Prognostic factors and long-term outcome with ANCA-associated kidney vasculitis in childhood. Clin J Am Soc Nephrol 2021;16:1043–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Walsh M, Merkel PA, Peh CAet al. Plasma exchange and glucocorticoids in severe ANCA-associated vasculitis. N Engl J Med 2020;382:622–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Nezam D, Porcher R, Grolleau Fet al. Kidney histopathology can predict kidney function in ANCA-associated vasculitides with acute kidney injury treated with plasma exchanges. J Am Soc Nephrol 2022;33:628–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hogan SL, Falk RJ, Chin Het al. Predictors of relapse and treatment resistance in antineutrophil cytoplasmic antibody-associated small-vessel vasculitis. Ann Intern Med 2005;143:621–31. [DOI] [PubMed] [Google Scholar]
- 33. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl 2013;3:1–150. [DOI] [PubMed] [Google Scholar]
- 34. Levin A, Agarwal R, Herrington WGet al. International consensus definitions of clinical trial outcomes for kidney failure: 2020. Kidney Int 2020;98:849–59. [DOI] [PubMed] [Google Scholar]
- 35. Oomatia A, Moran SM, Kennedy Cet al. Prolonged duration of renal recovery following ANCA-associated glomerulonephritis. Am J Nephrol 2016;43:112–9. [DOI] [PubMed] [Google Scholar]
- 36. Rhee RL, Davis JC, Ding Let al. The utility of urinalysis in determining the risk of renal relapse in ANCA-associated vasculitis. Clin J Am Soc Nephrol 2018;13:251–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lv L, Chang DY, Li ZYet al. Persistent hematuria in patients with antineutrophil cytoplasmic antibody-associated vasculitis during clinical remission: chronic glomerular lesion or low-grade active renal vasculitis? BMC Nephrol 2017;18:354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Chen TK, Murakami C, Manno RLet al. Hematuria duration does not predict kidney function at 1 year in ANCA-associated glomerulonephritis. Semin Arthritis Rheum 2014;44:198–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Geetha D, Seo P, Ellis Cet al. Persistent or new onset microscopic hematuria in patients with small vessel vasculitis in remission: findings on renal biopsy. J Rheumatol 2012;39:1413–7. [DOI] [PubMed] [Google Scholar]
- 40. Magrey MN, Villa-Forte A, Koening CLet al. Persistent hematuria after induction of remission in Wegener granulomatosis: a therapeutic dilemma. Medicine (Baltimore) 2009;88:315–21. [DOI] [PubMed] [Google Scholar]
- 41. Chapman GB, Farrah TE, Chapman FAet al. Utility of interval kidney biopsy in ANCA-associated vasculitis. Rheumatology (Oxford) 2022;61:1966–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sayer J, McCarthy MP, Schmidt JD.. Identification and significance of dysmorphic versus isomorphic hematuria. J Urol 1990;143:545–8. [DOI] [PubMed] [Google Scholar]
- 43. Fogazzi GB, Delanghe J.. Microscopic examination of urine sediment: phase contrast versus bright field. Clin Chim Acta 2018;487:168–73. [DOI] [PubMed] [Google Scholar]
- 44. Turin TC, James M, Ravani Pet al. Proteinuria and rate of change in kidney function in a community-based population. J Am Soc Nephrol 2013;24:1661–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Wright JT, Bakris G, Greene Tet al. Effect of blood pressure lowering and antihypertensive drug class on progression of hypertensive kidney disease: results from the AASK trial. JAMA 2002;288:2421–31. [DOI] [PubMed] [Google Scholar]
- 46. Hilhorst M, Wilde B, van Breda Vriesman Pet al. Estimating renal survival using the ANCA-associated GN classification. J Am Soc Nephrol 2013;24:1371–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Neumann I, Kain R, Regele Het al. Histological and clinical predictors of early and late renal outcome in ANCA-associated vasculitis. Nephrol Dial Transplant 2005;20:96–104. [DOI] [PubMed] [Google Scholar]
- 48. Franssen CF, Stegeman CA, Oost-Kort WWet al. Determinants of renal outcome in anti-myeloperoxidase-associated necrotizing crescentic glomerulonephritis. J Am Soc Nephrol 1998;9:1915–23. [DOI] [PubMed] [Google Scholar]
- 49. de Joode AA, Sanders JS, Stegeman CA.. Renal survival in proteinase 3 and myeloperoxidase ANCA-associated systemic vasculitis. Clin J Am Soc Nephrol 2013;8:1709–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Tampe D, Korsten P, Ströbel Pet al. Proteinuria indicates decreased normal glomeruli in ANCA-associated glomerulonephritis independent of systemic disease activity. J Clin Med 2021;10;1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Haas M, Eustace JA.. Immune complex deposits in ANCA-associated crescentic glomerulonephritis: a study of 126 cases. Kidney Int 2004;65:2145–52. [DOI] [PubMed] [Google Scholar]
- 52. Zeisberg M, Neilson EG.. Mechanisms of tubulointerstitial fibrosis. J Am Soc Nephrol 2010;21:1819–34. [DOI] [PubMed] [Google Scholar]
- 53. Mukhtyar C, Lee R, Brown Det al. Modification and validation of the Birmingham Vasculitis Activity Score (version 3). Ann Rheum Dis 2009;68:1827–32. [DOI] [PubMed] [Google Scholar]
- 54. Suppiah R, Mukhtyar C, Flossmann Oet al. A cross-sectional study of the Birmingham Vasculitis Activity Score version 3 in systemic vasculitis. Rheumatology (Oxford) 2011;50:899–905. [DOI] [PubMed] [Google Scholar]
- 55. Tomasson G, Grayson PC, Mahr ADet al. Value of ANCA measurements during remission to predict a relapse of ANCA-associated vasculitis—a meta-analysis. Rheumatology (Oxford) 2012;51:100–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. van Dam LS, Dirikgil E, Bredewold EWet al. PR3-ANCAs predict relapses in ANCA-associated vasculitis patients after rituximab. Nephrol Dial Transplant 2021;36:1408–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. McClure ME, Wason J, Gopaluni Set al. Evaluation of PR3-ANCA status after rituximab for ANCA-associated vasculitis. J Clin Rheumatol 2019;25:217–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Fussner LA, Hummel AM, Schroeder DRet al. Factors determining the clinical utility of serial measurements of antineutrophil cytoplasmic antibodies targeting proteinase 3. Arthritis Rheumatol 2016;68:1700–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Kemna MJ, Damoiseaux J, Austen Jet al. ANCA as a predictor of relapse: useful in patients with renal involvement but not in patients with nonrenal disease. J Am Soc Nephrol 2015;26:537–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Morris AD, Rowbottom AW, Martin FLet al. Biomarkers in ANCA-associated vasculitis: potential pitfalls and future prospects. Kidney360 2021;2:586–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. O'Reilly VP, Wong L, Kennedy Cet al. Urinary soluble CD163 in active renal vasculitis. J Am Soc Nephrol 2016;27:2906–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Moran SM, Scott J, Clarkson MRet al. The clinical application of urine soluble CD163 in ANCA-associated vasculitis. J Am Soc Nephrol 2021;32:2920–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Dekkema GJ, Abdulahad WH, Bijma Tet al. Urinary and serum soluble CD25 complements urinary soluble CD163 to detect active renal anti-neutrophil cytoplasmic autoantibody-associated vasculitis: a cohort study. Nephrol Dial Transplant 2019;34:234–42. [DOI] [PubMed] [Google Scholar]
- 64. Brunini F, Page TH, Gallieni Met al. The role of monocytes in ANCA-associated vasculitides. Autoimmun Rev 2016;15:1046–53. [DOI] [PubMed] [Google Scholar]
- 65. Tam FW, Sanders JS, George Aet al. Urinary monocyte chemoattractant protein-1 (MCP-1) is a marker of active renal vasculitis. Nephrol Dial Transplant 2004;19:2761–8. [DOI] [PubMed] [Google Scholar]
- 66. Jönsson N, Erlandsson E, Gunnarsson Let al. Monocyte chemoattractant protein-1 in antineutrophil cytoplasmic autoantibody-associated vasculitis: biomarker potential and association with polymorphisms in the MCP-1 and the CC chemokine receptor-2 gene. Mediators Inflamm 2018;2018:6861257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Everts-Graber J, Martin KR, Thieblemont Net al. Proteomic analysis of neutrophils in ANCA-associated vasculitis reveals a dysregulation in proteinase 3-associated proteins such as annexin-A1 involved in apoptotic cell clearance. Kidney Int 2019;96:397–408. [DOI] [PubMed] [Google Scholar]
- 68. Prikryl P, Satrapova V, Frydlova Jet al. Mass spectrometry-based proteomic exploration of the small urinary extracellular vesicles in ANCA-associated vasculitis in comparison with total urine. J Proteomics 2021;233:104067. [DOI] [PubMed] [Google Scholar]
- 69. McKinney EF, Lee JC, Jayne DRet al. T-cell exhaustion, co-stimulation and clinical outcome in autoimmunity and infection. Nature 2015;523:612–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Vizjak A, Rott T, Koselj-Kajtna Met al. Histologic and immunohistologic study and clinical presentation of ANCA-associated glomerulonephritis with correlation to ANCA antigen specificity. Am J Kidney Dis 2003;41:539–49. [DOI] [PubMed] [Google Scholar]
- 71. van Daalen EE, Wester Trejo MAC, Göçeroğlu Aet al. Developments in the histopathological classification of ANCA-associated glomerulonephritis. Clin J Am Soc Nephrol 2020;15:1103–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Hruskova Z, Honsova E, Berden AEet al. Repeat protocol renal biopsy in ANCA-associated renal vasculitis. Nephrol Dial Transplant 2014;29:1728–32. [DOI] [PubMed] [Google Scholar]
- 73. Berden AE, Ferrario F, Hagen ECet al. Histopathologic classification of ANCA-associated glomerulonephritis. J Am Soc Nephrol 2010;21:1628–36. [DOI] [PubMed] [Google Scholar]
- 74. Brix SR, Noriega M, Tennstedt Pet al. Development and validation of a renal risk score in ANCA-associated glomerulonephritis. Kidney Int 2018;94:1177–88. [DOI] [PubMed] [Google Scholar]
- 75. Moura MC, Fervenza FC, Specks Uet al. Kidney biopsy chronicity grading in antineutrophil cytoplasmic antibody associated vasculitis. Nephrol Dial Transplant 2022;37:1710–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Zimmermann M, Klaus M, Wong MNet al. Deep learning-based molecular morphometrics for kidney biopsies. JCI Insight 2021;6:e144779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Hauer HA, Bajema IM, Van Houwelingen HCet al. Determinants of outcome in ANCA-associated glomerulonephritis: a prospective clinico-histopathological analysis of 96 patients. Kidney Int 2002;62:1732–42. [DOI] [PubMed] [Google Scholar]
- 78. Kostopoulou M, Fanouriakis A, Cheema Ket al. Management of lupus nephritis: a systematic literature review informing the 2019 update of the joint EULAR and European Renal Association-European Dialysis and Transplant Association (EULAR/ERA-EDTA) recommendations. RMD Open 2020;6:e001263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Säemann M, Kronbichler A.. Call for action in ANCA-associated vasculitis and lupus nephritis: promises and challenges of SGLT-2 inhibitors. Ann Rheum Dis 2022;81:614–7. [DOI] [PubMed] [Google Scholar]
- 80. Wester Trejo MAC, Floßmann O, Westman KWet al. Renal relapse in antineutrophil cytoplasmic autoantibody-associated vasculitis: unpredictable, but predictive of renal outcome. Rheumatology (Oxford) 2019;58:103–9. [DOI] [PubMed] [Google Scholar]
- 81. De Groot K, Rasmussen N, Bacon PAet al. Randomized trial of cyclophosphamide versus methotrexate for induction of remission in early systemic antineutrophil cytoplasmic antibody-associated vasculitis. Arthritis Rheum 2005;52:2461–9. [DOI] [PubMed] [Google Scholar]
- 82. Smith RM, Jones RB, Specks Uet al. Rituximab as therapy to induce remission after relapse in ANCA-associated vasculitis. Ann Rheum Dis 2020;79:1243–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Jayne DR, Gaskin G, Rasmussen Net al. Randomized trial of plasma exchange or high-dosage methylprednisolone as adjunctive therapy for severe renal vasculitis. J Am Soc Nephrol 2007;18:2180–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No new data were generated or analysed in support of this research.