ABSTRACT
Asymptomatic hyperuricaemia (HU) is considered a pathogenic factor in multiple disease contexts, but a causative role is only proven for the crystalline form of uric acid in gouty arthritis and urate nephropathy. Epidemiological studies document a robust association of HU with hypertension, cardiovascular disease (CVD) and CKD progression, but CKD-related impaired uric acid (UA) clearance and the use of diuretics that further impair UA clearance likely accounts for these associations. Interpreting the available trial evidence is further complicated by referring to xanthine oxidase inhibitors as urate-lowering treatment, although these drugs inhibit other substrates, so attributing their effects only to HU is problematic. In this review we provide new mechanistic insights into the biological effects of soluble and crystalline UA and discuss clinical evidence on the role of asymptomatic HU in CKD, CVD and sterile inflammation. We identify research areas with gaps in experimental and clinical evidence, specifically on infectious complications that represent the second common cause of death in CKD patients, referred to as secondary immunodeficiency related to kidney disease. In addition, we address potential therapeutic approaches on how and when to treat asymptomatic HU in patients with kidney disease and where further interventional studies are required.
Keywords: asymptomatic hyperuricemia, cardiovascular disease, chronic kidney disease, gout, infection, uric acid
Graphical Abstract
Graphical Abstract.
INTRODUCTION
Kidney diseases are a global health problem with high morbidity and mortality [1]. In chronic kidney disease (CKD), the gradual decline of excretory kidney function is associated with an increasing retention of water-soluble solutes and metabolites, some of which are used as biomarkers such as creatinine or urea, while others are referred to as ‘uraemic toxins’ [2]. One of these metabolites is uric acid (UA), a purine nucleotide breakdown product [2]. As UA clearance declines CKD stage G3–5, hyperuricemia (HU) develops [3]. Other causes of HU include genetic variants in urate transporters, dietary factors and the use of certain drugs, including diuretics and immunosuppressant agents, that impair kidney UA clearance [3–9]. HU (defined by serum UA levels >6.5) has a documented causative role in gouty arthritis, acute urate nephropathy, UA kidney stones and UA urolithiasis [10–12], hence urate-lowering therapies (ULTs) are recommended in patients with these disorders. Of note, all aforementioned disorders involve UA in its crystalline form, i.e. monosodium urate (MSU) crystals [11, 13, 14]. The pathogenic role of MSU crystals has been extensively reported in experimental and clinical studies documenting the pro-inflammatory effects of MSU crystals [15], which mainly relate to their corpuscular nature, because various other crystals or microcrystals can activate the NLRP3 inflammasome [16, 17].
In contrast, soluble UA/HU has been implicated as a potential risk factor for numerous other disorders, including metabolic syndrome, cardiovascular disease (CVD) and hypertension, mainly based on epidemiological data that report robust associations with the presence of HU [18–20]. So far, experimental data have remained conflicting and a subject of debate as to whether asymptomatic HU is linked to these disorders by causation [18, 21]. Here we take a closer look at the data mentioned in this context and discuss a series of problematic assumptions and misinterpretations. Just to give a first example, the biological effects of allopurinol or febuxostat are generally attributed to their urate-lowing effect, although xanthine oxidase inhibitors (XOIs) block the effects of other substrates of this enzyme, e.g. the production of reactive oxygen species (ROS) [22]. Thus the effects of allopurinol do not necessarily prove functions of UA. In addition, we discuss the more recently discovered role of soluble UA/asymptomatic HU as a suppressor of innate immunity. We discuss how CKD-related HU may contribute to secondary immunodeficiency related to kidney disease (SIDKD), characterized by impaired host defence to pathogens, poor vaccine response and attenuated sterile inflammation [23]. In this review we highlight new mechanistic insights into the differential effects of soluble versus crystalline UA and provide clinical evidence on the role of asymptomatic HU in CKD, CVD and SIDKD and potential therapeutic approaches on when and how to treat asymptomatic HU in patients with kidney disease.
MECHANISTIC INSIGHTS ON THE EFFECTS OF SOLUBLE VERSUS CRYSTALLINE UA
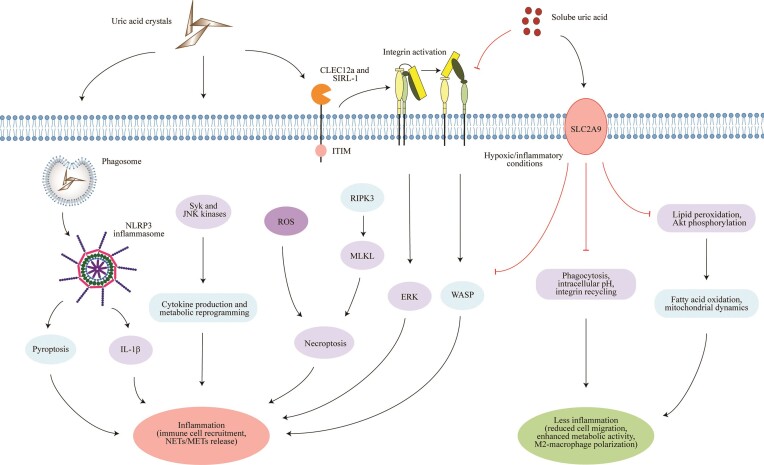
Soluble UA is a substrate for the formation of MSU crystals, a process promoted by UA supersaturation in biological fluids. A subnormal pH promotes this process [24, 25]. Crystalline UA, like many other crystals or microparticles [16], elicits an inflammatory response characterized by the secretion of pro-inflammatory mediators, the recruitment of leucocytes from the bloodstream into the inflamed tissue and neutrophil extracellular trap (NET) release, e.g. in joints, soft tissues or the kidney [16, 26, 27]. Mechanistically, in macrophages primed by pro-inflammatory signals, MSU crystals lead to direct activation of the NLRP3 inflammasome complex, resulting in the production of interleukin 1β (IL-1β), leading to an inflammatory cascade (Fig. 1) [15, 28]. Crystalline UA can also directly activate unprimed (or primed) macrophages, likely through a signalling pathway that involves the Syk and c-Jun N-terminal kinases, leading to cytokine production and metabolic reprogramming [29]. Similar results have been reported in neutrophils exposed to MSU crystals [30]. Crystalline UA can directly bind to plasma membrane lipids, leading to the aggregation of lipid raft domains in the membrane that in turn activate the Syk kinase [31]. In addition, UA crystals can also interact with inhibitory receptors such as CLEC12a and SIRL-1 on the cell surface of neutrophils (Fig. 1) [32, 33], resulting in integrin (CD18) activation [34], which enhances the migratory capacity of immune cells, as well as ROS production, but at the same time the release of NETs via a RIPK3–MLKL-mediated cell death pathway [35]. Thus MSU crystals undoubtedly exhibit pro-inflammatory effects. Additional mechanistic insights on the pro-inflammatory effects of MSU crystals have been discussed in detail elsewhere [10, 14, 28].
Figure 1:
The differential effects of soluble versus crystalline UA on innate immune cells and tubular epithelial cells. Crystalline UA (known as MSU crystals in gouty arthritis) triggers activation of the NLRP3 inflammasome complex following phagocytosis in macrophages, which results in the production of IL-1β, leading to an inflammatory cascade. Crystalline UA can also directly activate macrophages, likely through a signaling pathway that involves the Syk and JNK kinases, leading to cytokine production and metabolic reprogramming. UA crystals can also interact with receptors such as CLEC12a and SIRL-1 on the cell surface of immune cells and trigger the release of ROS and NETs via a RIPK3–MLKL-mediated cell death pathway (necroptosis), but also macrophage extracellular traps. In contrast, soluble UA suppresses these pro-inflammatory responses in activated cells. For example, soluble UA inhibits the pro-inflammatory function of human CD14+ blood monocytes through SLC2A9-mediated intracellular uptake of soluble UA. In macrophages, soluble UA reduces the production of ROS, and the release of IL-1β and IL-6 under hypoxic/inflammatory conditions, while enhancing fatty acid oxidation and mitochondrial dynamics, similar to that observed in tubular epithelial cells. In neutrophils, soluble UA impairs the β2 integrin activity and internalization/recycling by regulating intracellular pH and cytoskeletal dynamics that alter the migratory and phagocytic capacity of neutrophils. Soluble UA is known to scavenge ROS under hydrophilic conditions, thereby inhibiting lipid peroxidation as well as Akt phosphorylation in endothelial cells and modulating the activity of extracellular superoxide dismutase in atherosclerotic vessels. Akt, protein kinase B (PKB); CLEC12a, C-type lectin domain family 12 member A; ERK, extracellular signal-regulated kinase; IL-1β, interleukin-1β; JNK, c-Jun N-terminal kinase; METs, macrophage extracellular traps; MLKL, mixed lineage kinase domain-like protein; NETs, neutrophil extracellular traps; NLRP3, NOD-, LRR- and pyrin domain-containing protein 3; RIPK3, receptor interacting serine/threonine kinase-3; ROS, reactive oxygen species; Syk, spleen tyrosine kinase; SLC2A9, glucose transporter 9; SIRL-1, signal inhibitory receptor on leukocytes-1; WASP, Wiskott–Aldrich syndrome protein.
In contrast, published data on the pathophysiological role of soluble UA remained controversial. Reasons for the discrepancies include the preparation method through prewarming to solubilize UA for in vitro studies that created erroneous results because of UA microcrystal contaminants [36–42] and the inappropriate animal models of asymptomatic HU that did not reach clinically relevant serum UA levels [37, 43–45]. Recent evidence has now overcome these issues and suggests that soluble UA seems to have the opposite effects by acting as an immunoregulator in innate immune cells, similar to other CKD-related proteins and solutes [46–48]. For example, soluble UA suppresses the pro-inflammatory functions of activated human CD14+ blood monocytes isolated from healthy individuals through an SLC2A9-mediated intracellular uptake of soluble UA in vitro (Fig. 1), which was consistent with an impaired inflammatory function of human blood monocytes isolated from patients with CKD G2–4 or end-stage kidney disease (ESKD) [49]. In macrophages, soluble UA reduces the production of ROS and the release of IL-1β and IL-6 under hypoxic/inflammatory conditions, while enhancing fatty acid oxidation and mitochondrial dynamics [50]. Similar effects were also observed in tubular epithelial cells [50]. Thus soluble UA has anti-inflammatory effects by acting as an antioxidant and by shifting the polarization of macrophages toward an anti-inflammatory M2-like phenotype via metabolic reprogramming [50]. Soluble UA can also scavenge ROS under hydrophilic conditions, thereby inhibiting lipid peroxidation [51], as well as inhibit Akt phosphorylation in endothelial cells and modulate the activity of extracellular superoxide dismutase in atherosclerotic vessels. Recent evidence supports that XO activation, but not soluble UA itself, induces ROS and pro-inflammatory cytokine release in human macrophages [22], as a first indication that XO inhibition with so-called ULTs has biological effects unrelated to UA. Additionally, Ma et al. [52] found that exposure of human blood neutrophils to soluble UA leads to impaired β2 (CD18) integrin activity and recycling/internalization in vitro (to a number of inflammatory stimuli), causing reduced adhesion and chemotaxis by regulating intracellular pH. This mirrored the reduced in vitro chemotaxis seen in blood neutrophils isolated from patients with CKD and ESKD [52]. Whether soluble UA has immunoregulatory effects on the adaptive immunity promoted by T and B lymphocytes is currently unknown.
Taken together, soluble and crystalline UA have opposite effects on the immune system. While crystalline UA triggers sterile inflammation, soluble UA counteracts this process via its immunoregulatory and antioxidative effects.
ASYMPTOMATIC HU AND ITS CLINICAL IMPLICATIONS
Asymptomatic versus symptomatic HU in CKD progression
HU is common among patients with CKD and increases in severity with the decline of excretory kidney function. Whether asymptomatic HU contributes to the progression of CKD has remained a subject of debate for years [53]. Results from two Mendelian randomization studies found no causal effect of HU on estimated glomerular filtration rate (eGFR) levels or CKD risk; hence reducing serum UA levels is unlikely to reduce the risk of CKD progression [54, 55]. Experimental data using a novel transgenic animal model of HU showed that even robust and persistent asymptomatic HU alone (serum UA levels >12 mg/dl) does not cause kidney injury or accelerate the progression of CKD (Fig. 2) [56], confirming the conclusion of the Mendelian randomization studies. Although a meta-analysis of all single-centre studies support the benefit from urate-lowering drugs such as allopurinol on the progression of unselected forms of CKD at an odds ratio (OR) of 0.9, this meta-analysis of all well-powered, multicentre, randomized controlled trials (RCTs), including the Controlled Trial of Slowing of Kidney Disease Progression from the Inhibition of Xanthine Oxidase (CKD-FIX) [57] and Preventing Early Renal Loss in Diabetes (PERL) [58] studies, did not find ULTs delay CKD progression (at an OR of 1) and hence disproved a causal link between asymptomatic HU and the progression of CKD [59].
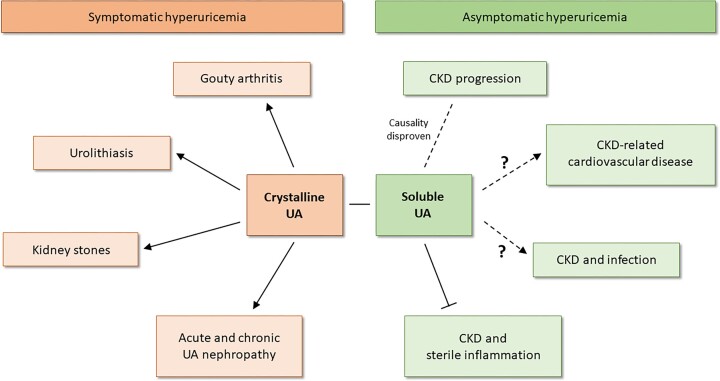
Figure 2:
The clinical implications of asymptomatic versus symptomatic HU. Experimental and clinical evidence implies that HU has a causative role in gouty arthritis, urolithiasis and kidney stones as well as in acute and chronic UA nephropathy. All diseases are caused by crystalline UA, which triggers pro-inflammatory responses in the kidney and joints, referred to symptomatic HU. In contrast, soluble UA/asymptomatic HU has now been shown to not cause kidney injury and contribute to the progression of CKD unless UA crystallizes in the kidney. Confirmation comes from large multicentre RCTs with ULT that have disproven a causal relationship of asymptomatic HU with CKD progression. Currently the role of asymptomatic HU in CKD-related CVD and the contribution to the acquired or secondary immunodeficiency (increased risk for infection) in patients with CKD is not well understood. Some recent findings indicate that soluble UA impairs the phagocytic capacity of human neutrophils and that HU patients with kidney disease are at a higher infection risk. Further studies are needed to clarify this. More recent experimental and clinical evidence indicates that soluble UA/asymptomatic HU suppresses sterile forms of inflammation by impairing immune cell functions, e.g. in the context of gouty arthritis in advanced CKD.
However, the story is different when UA precipitates inside the kidney. A low urine pH (≤5.5) promotes the precipitation of UA crystals. Various factors contribute to the urinary acidification that range from hydration status, age, obesity and dietary acid load (intake of foods high in fructose, protein and purine such as red meat, seafood and grains) to certain medications such as probenecid, sulfinpyrazone, losartan, benzbromarone and salicylic acid [56, 60–65]. However, this urinary acidification does not generally occur as a result of kidney dysfunction [63]. Such UA crystal deposits cause tubular obstruction, inflammation, infiltration and activation of macrophages and interstitial fibrosis [56], referred to as chronic urate nephropathy [66], similar to other crystalline nephropathies [67]. Diagnostic kidney biopsies or autopsies occasionally show medullary UA crystal deposits that are surrounded by granuloma-like macrophage infiltrates [68]. The finding of such UA granulomas in CKD kidneys raises a chicken-and-egg dilemma on the causality among persistent HU, UA crystalluria and CKD [69]. Indeed, lowering urinary pH was sufficient to turn asymptomatic HU into chronic UA crystalline nephropathy (symptomatic HU) [56]. Experimental data clarified that medullary UA crystal granulomas develop secondary to chronic UA crystal nephropathy and interstitial fibrosis (Fig. 2) [56], probably related to tubular crystal plugs and extratubulation [70] that remained invisible with standard pathology and were only detectable on frozen unfixed kidney sections, as observed in human kidney biopsy samples [56, 68]. The current scientific evidence shows that only HU with UA crystalluria drives the progression of CKD [56]. Thus, identifying UA crystals in the urine could serve as an additional diagnostic parameter as to whether CKD/ESKD patients with HU (but without oligoanuria) need to receive ULT.
Asymptomatic HU in CVDs
The association of HU with CVDs raises the question of causality. The general concept implies that HU is a risk factor for hypertension, atrial fibrillation, heart failure, coronary artery disease and cardiovascular death [71]. Many epidemiological studies conclude from such associations that the serum UA level is a marker for predicting future cardiovascular outcomes in hyperuricaemic patients with established CVD [71–77]. However, CKD is frequently not considered as a confounding factor for HU and CVD. Indeed, prospective studies report that the impaired clearance of numerous metabolites in CKD increases the risk for accelerated vascular aging, cardiovascular morbidity and mortality [78–81]. In addition to traditional risk factors such as hypertension, dyslipidaemia, smoking and hyperglycaemia, other mechanisms contribute to the development of CVDs in CKD [81], including persistent systemic inflammation, FGF-23-related left ventricular hypertrophy and accelerated vascular calcification [82–85]. The Canakinumab Anti-Inflammatory Thrombosis Outcome Study (CANTOS) with stable post-myocardial infarction patients with high-sensitivity C-reactive protein reported a therapeutic benefit of the IL-1β inhibitor canakinumab that was larger in CKD patients with an eGFR <60 ml/min/1.73 m2 than in those with an eGFR >60 ml/min/1.73 m2 [86]. However, further interventional studies are needed to firmly establish the pathophysiological mechanisms and potential treatment options for inflammation in patients with CKD (Fig. 2). Furthermore, CKD-related uraemic toxins [87] as well as haemodynamic alterations may also contribute to cardiac damage due to vascular calcification of central arterial vessels and left ventricular hypertrophy associated with a decrease in coronary perfusion [88–91]. Thus, despite numerous systematic reviews, meta-analyses and Mendelian randomization studies, convincing evidence of a causative role of asymptomatic HU in CVD independent from underlying CKD is lacking [55].
Asymptomatic HU in infection
Patients with kidney dysfunction, especially those on dialysis, show an increasing susceptibility for serious bacterial and viral infections, including coronavirus disease 2019 (COVID-19), influenza, tuberculosis, pneumonia and sepsis [92–97]. In addition, they frequently show poor vaccine responses [23], which together significantly contributes to overall mortality. SIDKD has been linked to an abnormal immunophenotype associated with persistent inflammation and immune paralysis in CKD, but precise data are sparse, as a definition of SIDKD did not exist [23]. Information on the relationship between HU and infectious complications in patients with kidney disease are also sparse (Fig. 2). Prospective cohort studies with patients in intensive care units reported that elevated serum UA levels may be associated with acute kidney injury in early sepsis [98] and COVID-19 [99]. Whether HU is associated with a higher mortality in patients at dialysis initiation was investigated in a multicentre prospective observational study in Japan. They found that the infection-related mortality was significantly higher in hyperuricaemic patients (mean serum UA levels 9.1 mg/dl) with CKD stage G5 compared with CKD stages G1–4 {hazard ratio [HR] 2.74 for G5 versus 1.73 for G1, 1.41 for G3 and 1.36 for G4 [95% confidence interval (CI) 1.36–5.52], P = .005} [100]. A possible mechanistic explanation for the increased risk of infections in CKD and dialysis patients is immune cell dysfunction [23]. Recent evidence indicates that soluble UA can affect the phagocytic capacity of human neutrophils by impairing the cytoskeleton of innate immune cells, limiting membrane dynamics more generally, which may explain the impaired migratory capability and host defence of neutrophils in CKD and ESKD patients [52]. However, further studies are needed to clarify the pathophysiological effects of asymptomatic HU in SIDKD, especially whether targeting UA can improve host defence in CKD/ESKD.
Asymptomatic HU during sterile inflammation
SIDKD also implies an attenuation of non-infectious forms of inflammation, such as the autoimmune disease activity that decreases as CKD progresses. In addition, clinical studies including the National Health and Nutrition Examination Survey and German CKD studies show that nearly all patients with CKD/ESKD have significant and persistent HU, while the rates of acute gout attacks are low (Fig. 2) [101–103]. UA crystals can also persist in the joint fluid between gout attacks without triggering an inflammatory response [104, 105] and patients are sometimes observed to have normal serum UA levels at the time of an acute gout attack [106], suggesting that the inflammatory potential of UA crystals may be modulated by other physiological factors. One mechanistic explanation besides physiochemical processes that influence UA crystallization might be the immunoregulatory property of soluble UA. Indeed, experimental evidence using a transgenic mouse model of HU with and without kidney dysfunction now suggests that asymptomatic HU suppresses sterile inflammation, including acute gouty arthritis, by impairing immune cell functions [49, 52]. This process was partially reversible by specifically targeting UA via urate-depleting therapy with rasburicase [52]. Confirmation comes from a recent study in an uricase-deficient larval zebrafish model of HU and acute gouty arthritis [107]. Hence asymptomatic HU may contribute to the acquired immunodeficiency observed in CKD/ESKD patients [23] and could serve as an explanation for several unexplained clinical phenomena. For example, a sudden reduction of HU upon initiation of ULT with allopurinol, pegloticase and febuxostat can elicit acute gout attacks [108], probably because of a sudden decrease in the negative regulator of sterile inflammation, soluble UA. However, further studies are needed to better understand the role of asymptomatic HU in gouty arthritis and beyond.
WHEN AND HOW TO TREAT HU IN CKD: EVIDENCE FROM CLINICAL TRIALS
According to the traditional paradigm, profound HU in patients with advanced CKD should cause gouty arthritis in the vast majority of CKD patients. However, the prevalence of gouty arthritis is no more than 24% in those with an eGFR <60 ml/min/1.73 m2 [103]. Currently, quality evidence to guide appropriate gout management in hyperuricaemic patients with CKD is lacking, owing at least in part to the exclusion of people with CKD from trials of gout therapies, failure to report results stratified by kidney function and inconsistencies in the outcome measures used and reported [109–111]. The Gout, Hyperuricaemia and Crystal-Associated Disease Network has summarized some evidence on how to treat gout flares and use ULT in CKD patients in a consensus statement [109]. Published literature on non-steroidal anti-inflammatory drugs indicates potential kidney-related adverse effects in people with CKD due to impaired clearance of colchicine in CKD patients [112–114], thus recommendations for the use of colchicine in CKD remain largely empirical. On the other hand, corticosteroids have been generally accepted as safe in most people with gout flares and concomitant CKD (Fig. 3). IL-1 inhibition with anakinra or canakinumab has not been investigated in RCTs in patients with gout and CKD in which the results are presented according to kidney function. On the other hand, case series and case reports are reassuring [112]. Data show that the clearance of anakinra is directly related to kidney function in people without gouty arthritis and that the drug is not cleared by dialysis [115]. In patients with an eGFR <30 ml/min/1.73 m2, anakinra should be administered every other day [115]. By comparison, canakinumab is a human immunoglobulin G with a large molecular size (≈150 kDa), so not much renal excretion is expected [116].
Figure 3:
The schematic illustrates how to manage asymptomatic and symptomatic HU in patients with CKD according to the current clinical evidence. In CKD patients with asymptomatic HU, ULT is not beneficial and therefore not recommended to slow down CKD progression. This accounts also for CKD and ESKD patients with cardiovascular complications; due to the lack of RCTs with ULT in these patients, causality has not been proven. Data on asymptomatic HU and infectious complication are sparse because experimental and clinical evidence are needed to identify the physiological effects of UA and to test whether ULT improves host defence in CKD/ESKD patients. In CKD patients with symptomatic HU, patients with gout flares and for gout prophylaxis should be treated with corticosteroids or IL-1 inhibitors (e.g. anakinra, canakinumab) as well as with ULT (e.g. allopurinol, febuxostat, benzbromarone). This also applies to ESKD patients with gouty arthritis. Caution should be exercised when giving colchicine and ULT due to potential side effects (e.g. allopurinol hypersensitivity syndrome, recurrence of acute gout attacks). Patients with CKD/ESKD should be examined for the presence of UA crystals in the urine and the urine pH measured to identify patients with chronic UA nephropathy. Treatment with ULT in these patients is currently not recommended due to the lack of RCTs.
Generally, ULT for prophylaxis of gouty arthritis has to be considered carefully in patients with CKD, as efficacy declines and the risk of adverse events increases. For example, the XO inhibitor allopurinol increases the risk for allopurinol hypersensitivity syndrome in patients with an eGFR <30 ml/min/1.73 m2 [117], while using febuxostat has been more accepted in CKD patients because it is mainly metabolized in the liver and not dependent on kidney excretory function [118] (Fig. 3). The uricosuric drug benzbromarone is effective even in those with an eGFR <30 ml/min/1.73 m2 and data on pegloticase (although largely understudied) suggest similar efficacy and safety in those with and without kidney disease [119]. As the frequency of gouty arthritis flares decreases in advanced CKD and ESKD, patients with mild HU or normouricaemia and no flares will not require ULT [120]. In ESKD patients, dialysis reduces serum UA levels to some degree, thus specific ULT is no longer required [121]. However, this should not be generalized, because serum UA concentrations less often reached the target UA concentration in haemodialysis (HD) patients compared with peritoneal dialysis [122]. The data for the use of allopurinol and febuxostat in HD patients are mainly limited to case reports and case series [123–125]. For allopurinol, information about the effect of HD on plasma concentrations of oxypurinol (the active metabolite of allopurinol) indicates that it is effectively dialyzed and suggests that allopurinol should be given after HD [123]. Nevertheless, care should be taken on appropriate dosing of XO inhibitors in gout management in CKD and ESKD patients, owing to the risk of hypersensitivity reactions and the occurrence of sudden gout attacks due to a rapid drop in serum UA levels.
In regard to treating asymptomatic HU in CKD, well-powered, multicentre, RCTs [57, 58] have disproved a causal link between asymptomatic HU and the progression of CKD [59]. This conclusion was consistent with two Mendelian randomized studies [54, 55] and in vivo data [56], as mentioned above; thus the use of ULT in patients with asymptomatic HU and CKD, at least not with the purpose of attenuating CKD progression, is not supported by the best available scientific evidence (Fig. 3). It is plausible to speculate that patients with chronic UA crystal nephropathy may benefit from ULT; however, clinical trials are needed to address this.
Several clinical trials have been conducted to evaluate the effects of ULT on cardiovascular outcomes [71]. While a small, double-blind, placebo-controlled RCT showed a significant reduction in carotid intima–media thickness by allopurinol in patients with recent ischaemic stroke [126], the PRIZE study, a multicentre, open-label, blinded-endpoint RCT, did not indicate a delay of carotid atherosclerosis progression after febuxostat treatment in patients with asymptomatic HU [127]. Similarly, several RCTs that have evaluated the effect of ULT on hypertension in CKD patients have not noted any significant blood pressure–lowering effect compared with controls [128–130]. No clinical improvements after ULT were also noted in CKD patients with heart failure in the Oxypurinol Therapy for Congestive Heart Failure (OPT-CHF) and Effects of Xanthine Oxidase Inhibition in Hyperuricemic Heart Failure Patients: the Xanthine Oxidase Inhibition for Hyperuricemic Heart Failure Patients (EXACT-HF) studies [131, 132]. The Febuxostat versus Allopurinol Streamlined Trial (FAST) evaluated the cardiovascular risk between allopurinol and febuxostat treatment in patients with gout and found no evidence of increased death with febuxostat versus allopurinol during the median follow-up period of 48 months [all-cause mortality: 3.5% febuxostat versus 5.7% allopurinol; HR 0.75 (95% CI 0.59–0.95)] [133]. In the Cardiovascular Safety of Febuxostat and Allopurinol in Patients with Gout and Cardiovascular Morbidities (CARES) trial, the risk of cardiovascular events did not differ in those on febuxostat with normal, mild or moderate kidney impairment [134]. Of note, all-cause mortality and cardiovascular mortality were higher with febuxostat than with allopurinol in the CARES trial [134]. However, CKD patients with an eGFR <30 ml/min/1.73 m2 were excluded in all trials. Furthermore, the Febuxostat for Cerebral and caRdiorenovascular Events prEvEntion stuDy (FREED) reported cerebral, cardiovascular and renal events, and all deaths occurred in 23.3% and 28.7% in the febuxostat and non-febuxostat groups during the median follow-up period of 35 months (P = .02), mainly driven by a difference in worsening proteinuria [135]. Combination therapy with an XO inhibitor and a uricosuric drug might be an alternative therapeutic approach, although uricosuric medications are usually not considered in ESKD patients. Further trials are needed to address this (Fig. 3). From this standpoint, the efficacy of ULT on clinical cardiovascular outcomes in patients with ESKD remains to be addressed, thus treatment recommendations for asymptomatic HU should be avoided.
CONCLUDING REMARKS
While crystalline UA triggers an inflammatory response, soluble UA acts as an immunoregulator by attenuating sterile inflammation, which provides an explanation for several previously unresolved clinical observations in the context of gouty arthritis as mentioned above. In this manner, HU may contribute to SIDKD, among other factors. In addition, experimental and clinical evidence indicate that asymptomatic HU is not causally linked to the progression of CKD, therefore treatment recommendations with ULT should be avoided. This is different in symptomatic HU, where urinary UA crystals cause chronic UA nephropathy, which subsequently progresses to advanced CKD. Whether patients with chronic UA nephropathy would benefit from ULT is currently unknown because such CKD patients have not been included in RCTs with ULT so far (Box 1). Although several prospective studies propose an association of asymptomatic HU and cardiovascular complications, appropriate experimental and clinical evidence to prove causality for the beneficial use of ULT in CKD-related CVD is currently lacking. Furthermore, infectious complications in CKD/ESKD patients remain a large clinical problem, and more awareness and research are needed to advance our understanding of the pathophysiology of SIDKD and to develop better preventive and therapeutic strategies. Although experimental data point towards a role for soluble UA in this context by impairing the phagocytic capacity of neutrophils, further scientific evidence is needed to validate the use of ULT as a treatment approach to improve host defence.
Box 1:
Proposed research priorities in CKD/ESKD patients with comorbidities
Are IL-1 inhibitors and ULT a safe option to prevent CKD progression in chronic UA nephropathy?
What is the role of UA in the association between CVD and CKD/ESKD?
Would ULT be beneficial to prevent cardiovascular events in CKD/ESKD patients?
What are the pathophysiological effects of asymptomatic HU in SIDKD related to infection?
Could targeting UA with ULT improve host defence and reduce severe infections in CKD/ESKD?
To what extent do the effects of XO inhibitors relate to their urate-lowering effect versus the blockade of other XO substrates?
How should colchicine be used in people with ESKD on dialysis?
Overall, asymptomatic HU is not as bad as previously thought, at least in the context of CKD progression and sterile inflammation, but further research is needed to clarify a potential role in CVDs, infectious-related complications and beyond, e.g. metabolic syndrome, in CKD/ESKD.
Contributor Information
Hans-Joachim Anders, Division of Nephrology, Department of Medicine IV, Hospital of the Ludwig-Maximilians University, Munich, Germany.
Qiubo Li, Division of Nephrology, Department of Medicine IV, Hospital of the Ludwig-Maximilians University, Munich, Germany.
Stefanie Steiger, Division of Nephrology, Department of Medicine IV, Hospital of the Ludwig-Maximilians University, Munich, Germany.
FUNDING
S.S. was supported by the Deutsche Forschungsgemeinschaft (STE2437/4-1, TRR332 Project A7). Q.L. was supported by the China Scholarship Council. H.J.A. received funding from the Deutsche Forschungsgemeinschaft (TRR332 Project A7, AN372/30-1).
AUTHORS’ CONTRIBUTIONS
S.S. designed the concept and drafted the work. S.S., Q.L. and H.-J.A. wrote the manuscript, S.S. and Q.L. prepared the figures, and all contributing authors read and revised the manuscript.
DATA AVAILABILITY STATEMENT
No new data were generated or analysed in support of this research.
CONFLICT OF INTEREST STATEMENT
S.S. received funding from Eleva. H.J.A. received consultancy or lecture fees from Boehringer Ingelheim, Bayer, GlaxoSmithKline, AstraZeneca, Novartis, Otsuka, Janssen, Kezar, Sanofi, Vifor, Keza, Variant Bio and PreviPharma. Q.L. declares no conflicts of interest.
REFERENCES
- 1. Jager KJ, Kovesdy C, Langham Ret al. A single number for advocacy and communication-worldwide more than 850 million individuals have kidney diseases. Kidney Int 2019;96:1048–50. [DOI] [PubMed] [Google Scholar]
- 2. Vanholder R, De Smet R, Glorieux Get al. Review on uremic toxins: classification, concentration, and interindividual variability. Kidney Int 2003;63:1934–43. [DOI] [PubMed] [Google Scholar]
- 3. So A, Thorens B, Uric acid transport and disease. J Clin Invest 2010;120:1791–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Vitart V, Rudan I, Hayward Cet al. SLC2A9 is a newly identified urate transporter influencing serum urate concentration, urate excretion and gout. Nat Genet 2008;40:437–42. [DOI] [PubMed] [Google Scholar]
- 5. Enomoto A, Kimura H, Chairoungdua Aet al. Molecular identification of a renal urate anion exchanger that regulates blood urate levels. Nature 2002;417:447–52. [DOI] [PubMed] [Google Scholar]
- 6. Matsuo H, Takada T, Ichida Ket al. Common defects of ABCG2, a high-capacity urate exporter, cause gout: a function-based genetic analysis in a Japanese population. Sci Transl Med 2009;1:5ra11. [DOI] [PubMed] [Google Scholar]
- 7. Kottgen A, Albrecht E, Teumer Aet al. Genome-wide association analyses identify 18 new loci associated with serum urate concentrations. Nat Genet 2013;45:145–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Work WP. Adjustable dissection and surgical table as an aid to teaching. Laryngoscope 1988;98:238–41. [DOI] [PubMed] [Google Scholar]
- 9. Ben Salem C, Slim R, Fathallah Net al. Drug-induced hyperuricaemia and gout. Rheumatology (Oxford) 2017;56:679–88. [DOI] [PubMed] [Google Scholar]
- 10. Dalbeth N, Choi HK, Joosten LABet al. Gout. Nat Rev Dis Primers 2019;5:69. [DOI] [PubMed] [Google Scholar]
- 11. Kim S, Chang Y, Yun KEet al. Development of nephrolithiasis in asymptomatic hyperuricemia: a cohort study. Am J Kidney Dis 2017;70:173–81. [DOI] [PubMed] [Google Scholar]
- 12. Barbar T, Jaffer Sathick I. Tumor lysis syndrome. Adv Chronic Kidney Dis 2021;28:438–446.e1. [DOI] [PubMed] [Google Scholar]
- 13. Chonchol M, Shlipak MG, Katz Ret al. Relationship of uric acid with progression of kidney disease. Am J Kidney Dis 2007;50:239–47. [DOI] [PubMed] [Google Scholar]
- 14. Desai J, Steiger S, Anders HJ, Molecular pathophysiology of gout. Trends Mol Med 2017;23:756–68. [DOI] [PubMed] [Google Scholar]
- 15. Martinon F, Petrilli V, Mayor Aet al. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature 2006;440:237–41. [DOI] [PubMed] [Google Scholar]
- 16. Mulay SR, Anders HJ. Crystallopathies. N Engl J Med 2016;375:e29. [DOI] [PubMed] [Google Scholar]
- 17. Franklin BS, Mangan MS, Latz E, Crystal formation in inflammation. Annu Rev Immunol 2016;34:173–202. [DOI] [PubMed] [Google Scholar]
- 18. Feig DI, Kang DH, Johnson RJ. Uric acid and cardiovascular risk. N Engl J Med 2008;359:1811–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Johnson RJ, Bakris GL, Borghi Cet al. Hyperuricemia, acute and chronic kidney disease, hypertension, and cardiovascular disease: report of a scientific workshop organized by the National Kidney Foundation. Am J Kidney Dis 2018;71:851–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. King C, Lanaspa MA, Jensen Tet al. Uric acid as a cause of the metabolic syndrome. Contrib Nephrol 2018;192:88–102. [DOI] [PubMed] [Google Scholar]
- 21. Sattui SE, Singh JA, Gaffo AL. Comorbidities in patients with crystal diseases and hyperuricemia. Rheum Dis Clin North Am 2014;40:251–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ives A, Nomura J, Martinon Fet al. Xanthine oxidoreductase regulates macrophage IL1β secretion upon NLRP3 inflammasome activation. Nat Commun 2015;6:6555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Steiger S, Rossaint J, Zarbock Aet al. Secondary immunodeficiency related to kidney disease (SIDKD)—definition, unmet need, and mechanisms. J Am Soc Nephrol 2022;33:259–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Martillo MA, Nazzal L, Crittenden DB. The crystallization of monosodium urate. Curr Rheumatol Rep 2014;16:400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chhana A, Lee G, Dalbeth N. Factors influencing the crystallization of monosodium urate: a systematic literature review. BMC Musculoskelet Disord 2015;16:296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mulay SR, Steiger S, Shi Cet al. A guide to crystal-related and nano- or microparticle-related tissue responses. FEBS J 2020;287:818–32. [DOI] [PubMed] [Google Scholar]
- 27. Shi C, Kim T, Steiger Set al. Crystal clots as therapeutic target in cholesterol crystal embolism. Circ Res 2020;126:e37–52. [DOI] [PubMed] [Google Scholar]
- 28. So AK, Martinon F. Inflammation in gout: mechanisms and therapeutic targets. Nat Rev Rheumatol 2017;13:639–47. [DOI] [PubMed] [Google Scholar]
- 29. Cobo I, Cheng A, Murillo-Saich Jet al. Monosodium urate crystals regulate a unique JNK-dependent macrophage metabolic and inflammatory response. Cell Rep 2022;38:110489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tatsiy O, Mayer TZ, de Carvalho Oliveira Vet al. Cytokine production and NET formation by monosodium urate-activated human neutrophils involves early and late events, and requires upstream TAK1 and Syk. Front Immunol 2019;10:2996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ng G, Sharma K, Ward SMet al. Receptor-independent, direct membrane binding leads to cell-surface lipid sorting and Syk kinase activation in dendritic cells. Immunity 2008;29:807–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Fernandes MJ, Naccache PH. The role of inhibitory receptors in monosodium urate crystal-induced inflammation. Front Immunol 2018;9:1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Neumann K, Castineiras-Vilarino M, Hockendorf Uet al. Clec12a is an inhibitory receptor for uric acid crystals that regulates inflammation in response to cell death. Immunity 2014;40:389–99. [DOI] [PubMed] [Google Scholar]
- 34. Barabe F, Gilbert C, Liao Net al. Crystal-induced neutrophil activation VI. Involvement of FcγRIIIB (CD16) and CD11b in response to inflammatory microcrystals. FASEB J 1998;12:209–20. [DOI] [PubMed] [Google Scholar]
- 35. Desai J, Foresto-Neto O, Honarpisheh Met al. Author correction: Particles of different sizes and shapes induce neutrophil necroptosis followed by the release of neutrophil extracellular trap-like chromatin. Sci Rep 2018;8:6663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Crisan TO, Cleophas MCP, Novakovic Bet al. Uric acid priming in human monocytes is driven by the AKT-PRAS40 autophagy pathway. Proc Natl Acad Sci USA 2017;114:5485–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Braga TT, Forni MF, Correa-Costa Met al. Soluble uric acid activates the NLRP3 inflammasome. Sci Rep 2017;7:39884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sautin YY, Nakagawa T, Zharikov Set al. Adverse effects of the classic antioxidant uric acid in adipocytes: NADPH oxidase-mediated oxidative/nitrosative stress. Am J Physiol Cell Physiol 2007;293:C584–96. [DOI] [PubMed] [Google Scholar]
- 39. Khosla UM, Zharikov S, Finch JLet al. Hyperuricemia induces endothelial dysfunction. Kidney Int 2005;67:1739–42. [DOI] [PubMed] [Google Scholar]
- 40. Park JH, Jin YM, Hwang Set al. Uric acid attenuates nitric oxide production by decreasing the interaction between endothelial nitric oxide synthase and calmodulin in human umbilical vein endothelial cells: a mechanism for uric acid-induced cardiovascular disease development. Nitric Oxide 2013;32:36–42. [DOI] [PubMed] [Google Scholar]
- 41. Choi YJ, Yoon Y, Lee KYet al. Uric acid induces endothelial dysfunction by vascular insulin resistance associated with the impairment of nitric oxide synthesis. FASEB J 2014;28:3197–204. [DOI] [PubMed] [Google Scholar]
- 42. Kanellis J, Watanabe S, Li JHet al. Uric acid stimulates monocyte chemoattractant protein-1 production in vascular smooth muscle cells via mitogen-activated protein kinase and cyclooxygenase-2. Hypertension 2003;41:1287–93. [DOI] [PubMed] [Google Scholar]
- 43. Crisan TO, Cleophas MC, Oosting Met al. Soluble uric acid primes TLR-induced proinflammatory cytokine production by human primary cells via inhibition of IL-1Ra. Ann Rheum Dis 2016;75:755–62. [DOI] [PubMed] [Google Scholar]
- 44. Kim SM, Lee SH, Kim YGet al. Hyperuricemia-induced NLRP3 activation of macrophages contributes to the progression of diabetic nephropathy. Am J Physiol Renal Physiol 2015;308:F993–1003. [DOI] [PubMed] [Google Scholar]
- 45. Yang Z, Xiaohua W, Lei Jet al. Uric acid increases fibronectin synthesis through upregulation of lysyl oxidase expression in rat renal tubular epithelial cells. Am J Physiol Renal Physiol 2010;299:F336–46. [DOI] [PubMed] [Google Scholar]
- 46. Rossaint J, Oehmichen J, Van Aken Het al. FGF23 signaling impairs neutrophil recruitment and host defense during CKD. J Clin Invest 2016;126:962–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Cohen G, Rudnicki M, Horl WH. Isolation of modified ubiquitin as a neutrophil chemotaxis inhibitor from uremic patients. J Am Soc Nephrol 1998;9:451–6. [DOI] [PubMed] [Google Scholar]
- 48. Ottonello L, Gnerre P, Bertolotto Met al. Leptin as a uremic toxin interferes with neutrophil chemotaxis. J Am Soc Nephrol 2004;15:2366–72. [DOI] [PubMed] [Google Scholar]
- 49. Ma Q, Honarpisheh M, Li Cet al. Soluble uric acid is an intrinsic negative regulator of monocyte activation in monosodium urate crystal-induced tissue inflammation. J Immunol 2020;205:789–800. [DOI] [PubMed] [Google Scholar]
- 50. Gnemmi V, Li Q, Ma Qet al. Asymptomatic hyperuricemia promotes recovery from ischemic organ injury by modulating the phenotype of macrophages. Cells 2022;11:626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Stinefelt B, Leonard SS, Blemings KPet al. Free radical scavenging, DNA protection, and inhibition of lipid peroxidation mediated by uric acid. Ann Clin Lab Sci 2005;35:37–45. [PubMed] [Google Scholar]
- 52. Ma Q, Immler R, Pruenster Met al. Soluble uric acid inhibits beta2 integrin-mediated neutrophil recruitment in innate immunity. Blood 2022;139:3402–17. [DOI] [PubMed] [Google Scholar]
- 53. Sato Y, Feig DI, Stack AGet al. The case for uric acid-lowering treatment in patients with hyperuricaemia and CKD. Nat Rev Nephrol 2019;15:767–75. [DOI] [PubMed] [Google Scholar]
- 54. Jordan DM, Choi HK, Verbanck Met al. No causal effects of serum urate levels on the risk of chronic kidney disease: a Mendelian randomization study. PLoS Med 2019;16:e1002725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Li X, Meng X, Timofeeva Met al. Serum uric acid levels and multiple health outcomes: umbrella review of evidence from observational studies, randomised controlled trials, and Mendelian randomisation studies. BMJ 2017;357:j2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Sellmayr M, Hernandez Petzsche MR, Ma Qet al. Only Hyperuricemia with crystalluria, but not asymptomatic hyperuricemia, drives progression of chronic kidney disease. J Am Soc Nephrol 2020;31:2773–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Badve SV, Pascoe EM, Tiku Aet al. Effects of allopurinol on the progression of chronic kidney disease. N Engl J Med 2020;382:2504–13. [DOI] [PubMed] [Google Scholar]
- 58. Doria A, Galecki AT, Spino Cet al. Serum urate lowering with allopurinol and kidney function in type 1 diabetes. N Engl J Med 2020;382:2493–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Steiger S, Ma Q, Anders HJ. The case for evidence-based medicine for the association between hyperuricaemia and CKD. Nat Rev Nephrol 2020;16:422. [DOI] [PubMed] [Google Scholar]
- 60. Bell DS. Beware the low urine pH—the major cause of the increased prevalence of nephrolithiasis in the patient with type 2 diabetes. Diabetes Obes Metab 2012;14:299–303. [DOI] [PubMed] [Google Scholar]
- 61. Pazos Perez F. Uric acid renal lithiasis: new concepts. Contrib Nephrol 2018;192:116–24. [DOI] [PubMed] [Google Scholar]
- 62. Kamel KS, Cheema-Dhadli S, Halperin ML. Studies on the pathophysiology of the low urine pH in patients with uric acid stones. Kidney Int 2002;61:988–94. [DOI] [PubMed] [Google Scholar]
- 63. Menezes CJ, Worcester EM, Coe FLet al. Mechanisms for falling urine pH with age in stone formers. Am J Physiol Renal Physiol 2019;317:F65–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Maalouf NM, Cameron MA, Moe OWet al. Low urine pH: a novel feature of the metabolic syndrome. Clin J Am Soc Nephrol 2007;2:883–8. [DOI] [PubMed] [Google Scholar]
- 65. Manish KC, Leslie SW. Uric acid nephrolithiasis. In: StatPearls. Treasure Island, FL: StatPearls, 2022. [PubMed] [Google Scholar]
- 66. Bjornstad P, Maahs DM, Roncal CAet al. Role of bicarbonate supplementation on urine uric acid crystals and diabetic tubulopathy in adults with type 1 diabetes. Diabetes Obes Metab 2018;20:1776–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Anders HJ, Suarez-Alvarez B, Grigorescu Met al. The macrophage phenotype and inflammasome component NLRP3 contributes to nephrocalcinosis-related chronic kidney disease independent from IL-1-mediated tissue injury. Kidney Int 2018;93:656–69. [DOI] [PubMed] [Google Scholar]
- 68. Ayoub I, Almaani S, Brodsky Set al. Revisiting medullary tophi: a link between uric acid and progressive chronic kidney disease? Clin Nephrol 2016;85:109–13. [DOI] [PubMed] [Google Scholar]
- 69. Nickeleit V, Mihatsch MJ. Uric acid nephropathy and end-stage renal disease—review of a non-disease. Nephrol Dial Transplant 1997;12:1832–8. [DOI] [PubMed] [Google Scholar]
- 70. Klinkhammer BM, Djudjaj S, Kunter Uet al. Cellular and molecular mechanisms of kidney injury in 2,8-dihydroxyadenine nephropathy. J Am Soc Nephrol 2020;31:799–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Saito Y, Tanaka A, Node Ket al. Uric acid and cardiovascular disease: a clinical review. J Cardiol 2021;78:51–7. [DOI] [PubMed] [Google Scholar]
- 72. Wang J, Qin T, Chen Jet al. Hyperuricemia and risk of incident hypertension: a systematic review and meta-analysis of observational studies. PLoS One 2014;9:e114259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Verdecchia P, Schillaci G, Reboldi Get al. Relation between serum uric acid and risk of cardiovascular disease in essential hypertension. The PIUMA study. Hypertension 2000;36:1072–8. [DOI] [PubMed] [Google Scholar]
- 74. Kojima S, Sakamoto T, Ishihara Met al. Prognostic usefulness of serum uric acid after acute myocardial infarction (the Japanese Acute Coronary Syndrome Study). Am J Cardiol 2005;96:489–95. [DOI] [PubMed] [Google Scholar]
- 75. Anker SD, Doehner W, Rauchhaus Met al. Uric acid and survival in chronic heart failure: validation and application in metabolic, functional, and hemodynamic staging. Circulation 2003;107:1991–7. [DOI] [PubMed] [Google Scholar]
- 76. Niskanen LK, Laaksonen D E, Nyyssonen Ket al. Uric acid level as a risk factor for cardiovascular and all-cause mortality in middle-aged men: a prospective cohort study. Arch Intern Med 2004;164:1546–51. [DOI] [PubMed] [Google Scholar]
- 77. Fang J, Alderman MH. Serum uric acid and cardiovascular mortality the NHANES I epidemiologic follow-up study, 1971–1992. JAMA 2000;283:2404–10. [DOI] [PubMed] [Google Scholar]
- 78. Matsushita K, Blecker S, Pazin-Filho Aet al. The association of hemoglobin A1c with incident heart failure among people without diabetes: the atherosclerosis risk in communities study. Diabetes 2010;59:2020–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. van der Velde M, Matsushita K, Coresh Jet al. Lower estimated glomerular filtration rate and higher albuminuria are associated with all-cause and cardiovascular mortality. A collaborative meta-analysis of high-risk population cohorts. Kidney Int 2011;79:1341–52. [DOI] [PubMed] [Google Scholar]
- 80. Gansevoort RT, Correa-Rotter R, Hemmelgarn BRet al. Chronic kidney disease and cardiovascular risk: epidemiology, mechanisms, and prevention. Lancet 2013;382:339–52. [DOI] [PubMed] [Google Scholar]
- 81. Jankowski J, Floege J, Fliser Det al. Cardiovascular disease in chronic kidney disease: pathophysiological insights and therapeutic options. Circulation 2021;143:1157–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Kielstein JT, Veldink H, Martens-Lobenhoffer Jet al. Unilateral nephrectomy causes an abrupt increase in inflammatory mediators and a simultaneous decrease in plasma ADMA: a study in living kidney donors. Am J Physiol Renal Physiol 2011;301:F1042–6. [DOI] [PubMed] [Google Scholar]
- 83. Tan K, Sethi SK. Biomarkers in cardiorenal syndromes. Transl Res 2014;164:122–34. [DOI] [PubMed] [Google Scholar]
- 84. Sparks MA, Crowley SD, Gurley SBet al. Classical renin-angiotensin system in kidney physiology. Compr Physiol 2014;4:1201–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Agharazii M, St-Louis R, Gautier-Bastien Aet al. Inflammatory cytokines and reactive oxygen species as mediators of chronic kidney disease-related vascular calcification. Am J Hypertens 2015;28:746–55. [DOI] [PubMed] [Google Scholar]
- 86. Ridker PM, MacFadyen JG, Glynn RJet al. Inhibition of interleukin-1β by canakinumab and cardiovascular outcomes in patients with chronic kidney disease. J Am Coll Cardiol 2018;71:2405–14. [DOI] [PubMed] [Google Scholar]
- 87. Fujii H, Goto S, Fukagawa M. Role of uremic toxins for kidney, cardiovascular, and bone dysfunction. Toxins 2018;10:202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Guerin AP, London GM, Marchais SJet al. Arterial stiffening and vascular calcifications in end-stage renal disease. Nephrol Dial Transplant 2000;15:1014–21. [DOI] [PubMed] [Google Scholar]
- 89. London GM, Guerin AP. Influence of arterial pulse and reflected waves on blood pressure and cardiac function. Am Heart J 1999;138:220–4. [DOI] [PubMed] [Google Scholar]
- 90. Gauthier-Bastien A, Ung RV, Lariviere Ret al. Vascular remodeling and media calcification increases arterial stiffness in chronic kidney disease. Clin Exp Hypertens 2014;36:173–80. [DOI] [PubMed] [Google Scholar]
- 91. Raggi P. Cardiovascular disease: coronary artery calcification predicts risk of CVD in patients with CKD. Nat Rev Nephrol 2017;13:324–6. [DOI] [PubMed] [Google Scholar]
- 92. Kronbichler A, Gauckler P, Windpessl Met al. COVID-19: implications for immunosuppression in kidney disease and transplantation. Nat Rev Nephrol 2020;16:365–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. James MT, Quan H, Tonelli Met al. CKD and risk of hospitalization and death with pneumonia. Am J Kidney Dis 2009;54:24–32. [DOI] [PubMed] [Google Scholar]
- 94. Goffin E, Candellier A, Vart Pet al. COVID-19-related mortality in kidney transplant and haemodialysis patients: a comparative, prospective registry-based study. Nephrol Dial Transplant 2021;36:2094–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Moran E, Baharani J, Dedicoat Met al. Risk factors associated with the development of active tuberculosis among patients with advanced chronic kidney disease. J Infect 2018;77:291–5. [DOI] [PubMed] [Google Scholar]
- 96. Maizel J, Deransy R, Dehedin Bet al. Impact of non-dialysis chronic kidney disease on survival in patients with septic shock. BMC Nephrol 2013;14:77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Steiger S, Ehreiser L, Anders Jet al. Biological drugs for systemic lupus erythematosus or active lupus nephritis and rates of infectious complications. Evidence from large clinical trials. Front Immunol 2022;13:999704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Akbar SR, Long DM, Hussain Ket al. Hyperuricemia: an early marker for severity of illness in sepsis. Int J Nephrol 2015;2015:301021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Chauhan K, Pattharanitima P, Piani Fet al. Prevalence and outcomes associated with hyperuricemia in hospitalized patients with COVID-19. Am J Nephrol 2022;53:78–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Yoshida H, Inaguma D, Koshi-Ito Eet al. Extreme hyperuricemia is a risk factor for infection-related deaths in incident dialysis patients: a multicenter prospective cohort study. Ren Fail 2020;42:646–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Juraschek SP, Kovell LC, Miller ER 3rdet al. Association of kidney disease with prevalent gout in the United States in 1988–1994 and 2007–2010. Semin Arthritis Rheum 2013;42:551–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Jing J, Kielstein JT, Schultheiss UTet al. Prevalence and correlates of gout in a large cohort of patients with chronic kidney disease: the German Chronic Kidney Disease (GCKD) study. Nephrol Dial Transplant 2015;30:613–21. [DOI] [PubMed] [Google Scholar]
- 103. Krishnan E. Reduced glomerular function and prevalence of gout: NHANES 2009–10. PLoS One 2012;7:e50046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Bardin T, Richette P. Definition of hyperuricemia and gouty conditions. Curr Opin Rheumatol 2014;26:186–91. [DOI] [PubMed] [Google Scholar]
- 105. Dalbeth N, House ME, Aati Oet al. Urate crystal deposition in asymptomatic hyperuricaemia and symptomatic gout: a dual energy CT study. Ann Rheum Dis 2015;74:908–11. [DOI] [PubMed] [Google Scholar]
- 106. Zhang WZ. Why does hyperuricemia not necessarily induce gout? Biomolecules 2021;11:280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Linnerz TSYJ, Rolland L, Astin JWet al. Uricase-deficient larval zebrafish with elevated urate levels demonstrate suppressed acute inflammatory response to monosodium urate crystals and prolonged crystal persistence. Genes 2022;13:2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Becker MA, Schumacher HR Jr, Wortmann RLet al. Febuxostat compared with allopurinol in patients with hyperuricemia and gout. N Engl J Med 2005;353:2450–61. [DOI] [PubMed] [Google Scholar]
- 109. Stamp LK, Farquhar H, Pisaniello HLet al. Management of gout in chronic kidney disease: a G-CAN Consensus Statement on the research priorities. Nat Rev Rheumatol 2021;17:633–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Stamp LK, Morillon MB, Taylor WJet al. Variability in the reporting of serum urate and flares in gout clinical trials: need for minimum reporting requirements. J Rheumatol 2018;45:419–24. [DOI] [PubMed] [Google Scholar]
- 111. Stewart S, Tallon A, Taylor WJet al. How flare prevention outcomes are reported in gout studies: a systematic review and content analysis of randomized controlled trials. Semin Arthritis Rheum 2020;50:303–13. [DOI] [PubMed] [Google Scholar]
- 112. Pisaniello HL, Fisher MC, Farquhar Het al. Efficacy and safety of gout flare prophylaxis and therapy use in people with chronic kidney disease: a Gout, Hyperuricemia and Crystal-Associated Disease Network (G-CAN)-initiated literature review. Arthritis Res Ther 2021;23:130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Tang KS, Shah AD. Nonsteroidal anti-inflammatory drugs in end-stage kidney disease: dangerous or underutilized? Expert Opin Pharmacother 2021;22:769–77. [DOI] [PubMed] [Google Scholar]
- 114. Wason S, Mount D, Faulkner R. Single-dose, open-label study of the differences in pharmacokinetics of colchicine in subjects with renal impairment, including end-stage renal disease. Clin Drug Investig 2014;34:845–55. [DOI] [PubMed] [Google Scholar]
- 115. Yang BB, Baughman S, Sullivan JT. Pharmacokinetics of anakinra in subjects with different levels of renal function. Clin Pharmacol Ther 2003;74:85–94. [DOI] [PubMed] [Google Scholar]
- 116. Chakraborty A, Tannenbaum S, Rordorf Cet al. Pharmacokinetic and pharmacodynamic properties of canakinumab, a human anti-interleukin-1beta monoclonal antibody. Clin Pharmacokinet 2012;51:e1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Chung WH, Chang WC, Stocker SLet al. Insights into the poor prognosis of allopurinol-induced severe cutaneous adverse reactions: the impact of renal insufficiency, high plasma levels of oxypurinol and granulysin. Ann Rheum Dis 2015;74:2157–64. [DOI] [PubMed] [Google Scholar]
- 118. Kim SH, Lee SY, Kim JMet al. Renal safety and urate-lowering efficacy of febuxostat in gout patients with stage 4–5 chronic kidney disease not yet on dialysis. Korean J Intern Med 2020;35:998–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Farquhar H, Vargas-Santos AB, Pisaniello HLet al. Efficacy and safety of urate-lowering therapy in people with kidney impairment: a GCAN-initiated literature review. Rheumatol Adv Pract 2021;5:rkaa073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Ohno I, Ichida K, Okabe Het al. Frequency of gouty arthritis in patients with end-stage renal disease in Japan. Intern Med 2005;44:706–9. [DOI] [PubMed] [Google Scholar]
- 121. Arenas MD, Soriano R, Andres Met al. Serum urate levels of hemodialyzed renal patients revisited. J Clin Rheumatol 2021;27:e362–6. [DOI] [PubMed] [Google Scholar]
- 122. Yeo E, Palmer SC, Chapman PTet al. Serum urate levels and therapy in adults treated with long-term dialysis: a retrospective cross-sectional study. Intern Med J 2019;49:838–42. [DOI] [PubMed] [Google Scholar]
- 123. Wright DF, Doogue MP, Barclay MLet al. A population pharmacokinetic model to predict oxypurinol exposure in patients on haemodialysis. Eur J Clin Pharmacol 2017;73:71–8. [DOI] [PubMed] [Google Scholar]
- 124. Alvarez-Nemegyei J, Cen-Piste JC, Medina-Escobedo Met al. Factors associated with musculoskeletal disability and chronic renal failure in clinically diagnosed primary gout. J Rheumatol 2005;32:1923–7. [PubMed] [Google Scholar]
- 125. Rutherford E, Stewart G, Houston JGet al. An open-label dose-finding study of allopurinol to target defined reduction in urate levels in hemodialysis patients. J Clin Pharmacol 2017;57:1409–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Higgins P, Walters MR, Murray HMet al. Allopurinol reduces brachial and central blood pressure, and carotid intima-media thickness progression after ischaemic stroke and transient ischaemic attack: a randomised controlled trial. Heart 2014;100:1085–92. [DOI] [PubMed] [Google Scholar]
- 127. Tanaka A, Taguchi I, Teragawa Het al. Febuxostat does not delay progression of carotid atherosclerosis in patients with asymptomatic hyperuricemia: a randomized, controlled trial. PLoS Med 2020;17:e1003095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Goicoechea M, de Vinuesa SG, Verdalles Uet al. Effect of allopurinol in chronic kidney disease progression and cardiovascular risk. Clin J Am Soc Nephrol 2010;5:1388–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Siu YP, Leung KT, Tong MKet al. Use of allopurinol in slowing the progression of renal disease through its ability to lower serum uric acid level. Am J Kidney Dis 2006;47:51–9. [DOI] [PubMed] [Google Scholar]
- 130. Kimura K, Hosoya T, Uchida Set al. Febuxostat therapy for patients with stage 3 CKD and asymptomatic hyperuricemia: a randomized trial. Am J Kidney Dis 2018;72:798–810. [DOI] [PubMed] [Google Scholar]
- 131. Hare JM, Mangal B, Brown Jet al. Impact of oxypurinol in patients with symptomatic heart failure. Results of the OPT-CHF study. J Am Coll Cardiol 2008;51:2301–9. [DOI] [PubMed] [Google Scholar]
- 132. Givertz MM, Anstrom KJ, Redfield MMet al. Effects of xanthine oxidase inhibition in hyperuricemic heart failure patients: the Xanthine Oxidase Inhibition for Hyperuricemic Heart Failure Patients (EXACT-HF) Study. Circulation 2015;131:1763–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Mackenzie IS, Ford I, Nuki Get al. Long-term cardiovascular safety of febuxostat compared with allopurinol in patients with gout (FAST): a multicentre, prospective, randomised, open-label, non-inferiority trial. Lancet 2020;396:1745–57. [DOI] [PubMed] [Google Scholar]
- 134. White WB, Saag KG, Becker MAet al. Cardiovascular safety of febuxostat or allopurinol in patients with gout. N Engl J Med 2018;378:1200–10. [DOI] [PubMed] [Google Scholar]
- 135. Kojima S, Matsui K, Hiramitsu Set al. Febuxostat for Cerebral and CaRdiorenovascular Events PrEvEntion StuDy. Eur Heart J 2019;40:1778–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No new data were generated or analysed in support of this research.