Abstract
The situation of secondary hyperparathyroidism (SHPT) in chronic kidney disease patients not on dialysis (ND-CKD) is probably best characterised by the Kidney Disease: Improving Global Outcomes Chronic Kidney Disease–Mineral and Bone Disorder Update 2017 guideline 4.2.1 stating that the optimal parathyroid hormone levels are not known in these stages. Furthermore, new caution became recommended with regard to the routine use of active vitamin D analogues in early CKD stages and moderate SHPT phenotypes, due to their potential risks for hypercalcaemia and hyperphosphataemia aggravation. Nevertheless, there is still a substantial clinical need to prevent the development of parathyroid gland autonomy, with its associated consequences of bone and vascular damage, including fracture risks and cardiovascular events. Therefore we now attempt to review the current guideline-based and clinical practice management of SHPT in ND-CKD, including their strengths and weaknesses, favouring individualised approaches respecting calcium and phosphate homeostasis. We further comment on extended-release calcifediol (ERC) as a new differential therapeutic option now also available in Europe and on a potentially novel understanding of a required vitamin D saturation in more advanced CKD stages. There is no doubt, however, that knowledge gaps will remain unless powerful randomised controlled trials with hard and meaningful endpoints are performed.
Keywords: chronic kidney disease, CKD-MBD, parathyroid hormone, secondary hyperparathyroidism
 Watch the video of this contribution at https://academic.oup.com/ndt/pages/author_videos
Watch the video of this contribution at https://academic.oup.com/ndt/pages/author_videos
INTRODUCTION: SHPT FROM THE GUIDELINE PERSPECTIVE
Biochemical abnormalities, including calcium, phosphate, parathyroid hormone (PTH) and alkaline phosphatase (ALP) activity, represent one integral part of chronic kidney disease–mineral and bone disorder (CKD-MBD) [Grading of Recommendations Assessment, Development and Evaluation (GRADE) evidence 1C] [1]. Monitoring these serum levels, beginning with CKD G3a, has always been recommended in the Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD clinical practice guidelines [2, 3]. Their deviations have all been frequently associated with hard kidney and/or cardiovascular outcomes, including increased mortality [2,3]. Guidelines also recommend that therapeutic decisions should be based on trends rather than on single laboratory values, taking into account all available CKD-MBD parameters and assessments (evidence 1C) [2, 3]. Since high PTH levels [secondary hyperparathyroidism (SHPT)] are pathophysiologically closely related to the body's vitamin D status, among other factors, guidelines also suggest that 25-hydroxy vitamin D [25(OH)D; calcidiol] levels might be measured (evidence 2C) [3]. Moreover, it is also recommended that clinical laboratories inform clinicians of the actual PTH assay method in use (evidence 1B) [3]. In fact, it is well known that PTH measurement is subject of important intermethod variability owing to pre-analytical sampling errors, antibody specificity, interference of various PTH fragments and post-translational PTH forms, as well as the lack of homogeneous standardization [4–6].
Whereas in patients with CKD G5D, it is suggested to maintain intact PTH (iPTH) levels in the wide range of approximately two to nine times the upper normal limit (UNL) for the assay (evidence 2C), in patients with CKD G3a–G5 not on dialysis, the optimal PTH level to prevent or attenuate CKD progression, decrease cardiovascular events and/or improve survival is not known. Thus the 2017 KDIGO CKD-MBD guidelines suggest that patients with levels of iPTH progressively increasing or persistently above the UNL for the assay should be evaluated for modifiable factors (evidence 2C) [3]. However, since the PRIMO (NCT00497146) and OPERA (NCT00796679) studies [7, 8] failed to demonstrate improvements in clinically relevant outcomes but demonstrated increased risks of hypercalcaemia, the 2017 guideline update no longer recommends routine use of calcitriol or its analogues in adults with CKD G3a–G5 (evidence 2C) [3]. However, it should be emphasized that the PRIMO and OPERA [7, 8] studies focussed on cardiovascular endpoints and were not specifically targeted at PTH control or any other hard- or patient-centred outcomes. The fact that active vitamin D increases the risk of hypercalcaemia is well recognized, and was recently confirmed in these patients, even excluding these two studies [9]. Nevertheless, this 2017 recommendation represented a major paradigm shift with regard to previous clinical practice. Consequently, we will analyse the current status of the controversial change on the use of vitamin D derivatives in non-dialysis CKD patients with SHPT in light of the new available information.
SHPT MANAGEMENT APPROACHES IN ND-CKD OVER 20 YEARS
The 2003 Kidney Disease Outcomes Quality Initiative (KDOQI) guidelines provided arguable opinion-based ranges for iPTH dependent on CKD stages [10], and therapy with an active oral vitamin D sterol was indicated in patients with CKD G3–G4 when serum levels of 25(OH)D were >30 ng/ml (75 nmol/l) and plasma levels of iPTH were simply above the target range for subsequent CKD stages (Table 1).
Table 1:
Evolution of guidelines on the treatment and therapy of abnormal PTH levels in non-dialysis CKD patients.
| KDOQI 2003 [10] | KDIGO 2009 [2] | KDIGO 2017 [3] |
|---|---|---|
| Guideline 8A.1 | Guideline 4.2.2 | Guideline 4.1.4 |
| In patients with CKD stages 3 and 4, therapy with an active oral vitamin D sterol (calcitriol, alfacalcidol or doxercalciferol) is indicated when serum levels of 25(OH)D are >30 ng/ml (75 nmol/L) and plasma levels of iPTH are above the target range for the CKD stage a | In patients with CKD stages 3–5 not on dialysis, in whom serum PTH is progressively rising and remains persistently above the ULN for the assay despite correction of modifiable factors, we suggest treatment with calcitriol or vitamin D analogues (2C) | In patients with CKD stages 3a–5 not on dialysis, we suggest calcitriol and vitamin D analogues not be routinely used (2C). It is reasonable to reserve their use for patients with CKD stages 4–5 with severe and progressive hyperparathyroidism (not graded) |
aCKD stage 3: 35–70 pmol/l (opinion); CKD stage 4: 70–110 pmol/l (opinion); CKD stage 5: 150–300 pmol/l (evidence).
As already indicated above, the results of the PRIMO and OPERA studies [7, 8] led to the revision and reformulation of previous KDOQI and KDIGO statements [2, 10], now considering it reasonable to reserve their use for patients with CKD G4–G5 with severe and progressive SHPT (Table 1) [2, 3].
However, due to missing hard endpoints or threshold data, a clearcut definition of ‘severe and progressive SHPT’ could not be provided and there had been a controversial and differentiated discussion among the work group members about this last statement before a consensus was reached [3]. In fact, the primary goal of the PRIMO and OPERA studies was not the biochemical control of SHPT, but the potential prevention of left ventricular hypertrophy development [7, 8]. Furthermore, study participants only had moderate SHPT, comparably quite high doses of paricalcitol were used in these trials (2 and 1 µg/day, respectively) and a significant percentage of patients received calcium-based phosphate binders (Table 2) [7, 8]. These reasons could probably explain the high incidence of hypercalcaemic episodes. Furthermore, this study design may have led to ‘oversuppression’ of PTH secretion on the one hand and to fibroblast growth factor 23 (FGF23) overstimulation on the other hand, with FGF23’s potential for negative myocardial remodelling, and thus the lack of efficacy to improve the cardiovascular study endpoint of left ventricular hypertrophy amelioration [11].
Table 2:
| Characteristics | PRIMO (Multinational) | OPERA (Chinese) | Comments |
|---|---|---|---|
| n | 227 (115/112) | N = 60 (30/30) | |
| eGFR (ml/min/1.73 m2) | 31 (24–43) | 19.7 (16–30.6) | Opera mean (IQR) |
| LVH | Mild moderate or no LVH | Moderate to severe LVH | |
| Diagnosis | MRI/echo | Echo | MRI (primary endpoint) |
| Initial dosing | Mostly 2 µg paricalcitol/day | Mostly 1µg paricalcitol/day (2 µg if >500 pg/ml) | 48 weeks PRIMO; 52 weeks OPERA |
| iPTH absolute basal values (pg/ml) | Paricalcitol group: 100 (66–174); placebo group: 106 (71–153) | Paricalcitol group: 156 (108–235) Placebo group: 129 (121–176) Post-paricalcitol: 51 (37–78) | |
| ΔLVMI (g/m2) | Paricalcitol group 0.34 g/m2 (−0.14–0.83) versus placebo group −0.07 g/m2 (−0.55–0.42 g/m2); adjusted least squares mean (95% CI) | Paricalcitol group −2.59 g/m2 (−6.13–0.32) versus placebo group −4.85 g/m2 (−9.89–1.10); median (IQR) | NS |
| Hypercalcaemia | 23% | 43.3 versus 3.3% | OPERA 70% on calcium-based phosphate binders |
| Other | Lower hospitalization rate | Lower hospitalization rate (no CV events in paricalcitol group) | Underpowered for events (OPERA 0 versus 6) |
IQR: interquartile range; LVMI: left ventricular mass index; MRI: magnetic resonance image; NSL not significant.
SHPT IN ND-CKD: CURRENT COHORTS
Some consider that future response to treatments aiming to control PTH may be compromised by the delay induced by the guideline update [12, 13]. In fact, it has recently been shown that increased iPTH before dialysis inception predicts a higher PTH level 9–12 months later and a greater use of anti-parathyroid treatments [14]. Untreated SHPT results in progressively increasing PTH levels, as observed in randomized clinical trials in placebo-treated patients [15–17], and parathyroid hyperplasia with progressive SHPT due to delayed treatment is accompanied by progressive reduction in sensitivity to calcium and vitamin D regulation and the consequent risk of treatment resistance later in the disease course [18]. Therefore, either excessive suppression of PTH, possibly causing low turnover bone disease, or insufficient SHPT control resulting in hyperparathyroid bone disease and osteitis fibrosa should be avoided to improve the long-term bone (in terms of fractures) and cardiovascular outcomes.
Interestingly, the independent effects and potential interactions of SHPT, hyperphosphataemia and hypercalcaemia on CKD progression and registered cardiovascular outcomes were also recently analysed in the Spanish NEFRONA low-risk CKD G3–G5 cohort [19]. Authors found that SHPT and hyperphosphataemia (as defined by the KDOQI guideline targets) and higher iPTH and/or phosphate levels as continuous variables were independently associated with an increased risk of both CKD progression and/or cardiovascular events (a trend for SHPT in the fully adjusted model). These results offer support for the claim that iPTH levels higher than those previously recommended by the KDOQI for non-dialysis CKD patients were indeed associated with clinically significant hard outcomes [12, 19]. Although these interesting results are flawed by the observational nature of the study and that an important impact on clinical practice and guidelines is out of the question [12], they do underline the need to improve, or at least better define, the control of SHPT in non-dialysis patients in order to improve outcomes. Moreover, these results stress the need to establish cut-off targets for safe upper PTH levels in non-dialysis patients and whether reservation of active vitamin D analogues only for severe SHPT is exceedingly cautious [12]. It should also be considered that optimal PTH targets may be quite different depending on whether bone, renal or cardiovascular parameters are taken into account [12, 20, 21].
A REMINDER: IN ND-CKD THE OPTIMAL PTH LEVELS ARE NOT KNOWN
In any case, it cannot be forgotten that a certain degree of SHPT may represent an adaptive clinical response and therefore some recent clinical guidelines underline that clinicians should neither wait until severe SHPT is present nor aim to completely normalize PTH levels in CKD non-dialysis patients [12, 13]. The presence of bone hyporesponsiveness to PTH in CKD [22, 23] and the phosphaturic properties of PTH explain, at least in part, this last recommendation. On the other hand, PTH is recognized as an uraemic toxin, increases FGF23 and is independently associated with fractures, atrial fibrillation, cardiovascular events, progression to dialysis, more rapid decline in residual renal function, healthcare resource utilization and death in observational studies [24–28]. PTH is also associated with arrhythmias and cardiovascular events in normocalcaemic and/or hypercalcaemic primary HPT [29, 30] and a significant risk for nephrocalcinosis and graft failure censored for death in renal transplant patients [31, 32].
HOW TO ADDRESS SHPT IN ND-CKD IN CLINICAL PRACTICE
Since the current KDIGO 2017 guidelines do not indicate target values for PTH or for serum calcium and phosphate, simply suggesting to pursue normal-range values, clinicians lack solid reference biomarkers to tailor their therapeutic choices to the individual patient. Alternatively, bone biomarkers could be evaluated to determine bone lesions and SHPT in renal patients. However, a major problem is represented by the renal excretion of most of these biomarkers, so that only a few [like bone-specific alkaline phosphatase (BALP), intact purine nucleoside phosphorylase (PNP) and tartrate-resistant acid phosphatase 5b (TRAP5b)] are generally considered sufficiently reliable in renal insufficiency. Also, combinations of these biomarkers (i.e. PTH and ALP or bone-specific ALP) seem to better reflect bone activity (versus isolated parathyroid activity) [33, 34]. Moreover, isolated ALP is linearly associated with survival, as opposed to the U-shaped curve of survival versus PTH [35]. In any case, no recommended reference range is available for these bone biomarkers that is useful for daily clinical decisions.
Importantly, besides biochemical parameters, in more recent years there is growing attention to the issue of bone fragility and fractures, while their prevalence increases along with estimated glomerular filtration range (eGFR) reduction and carries significant morbidity and mortality burdens [36]. Accordingly, beyond metabolic balance, mechanical competence of bone deserves new appreciation when treating SHPT [37]. With this complex scenario, the diagnostic and therapeutic approach to SHPT in the nephrologic community is frequently empirical, with therapeutic dilemmas in non-dialysis CKD patients among the available choices within two categories of drugs: phosphate binders and vitamin D compounds. Not surprisingly, in daily clinical practice, diagnostic and therapeutic strategies appear to be suboptimal. For example, a recent survey in the USA [38] used a large electronic health records database that included >50 million patients and spanned from 2010 to 2019 to identify incident cohorts of CKD patients in stages 3–5. In these cohorts, the rate of laboratory testing for PTH, calcium, phosphate and vitamin D, although increasing in the three worsening CKD stages, was limited to 26, 46 and 41%, respectively, for PTH and to 34, 38 and 25%, respectively, for vitamin D, while higher prevalences were observed for phosphate (54, 74 and 73%, respectively), calcium (94, 94 and 87%, respectively) and ALP (88, 86 and 73%, respectively). Along with worsening renal failure, abnormal values became progressively more frequent and, in particular, averaged pretreatment values of PTH were high: 154, 221 and 352 pg/ml in the three CKD stages, respectively, which suggests a reactive more than a preventive and proactive intervention. Therapeutic strategies in this study were reported as rates per 100 person-years (PY) due to follow-up variability and included mainly cholecalciferol as native vitamin D (rates: 20, 30 and 50% per 100 PY in the three CKD stages, respectively) and calcitriol as active vitamin D (with an average rate limited to <25% per 100 PY). Phosphate binders were almost exclusively calcium-based drugs in stages 3 and 4 and, among non-calcium based, included sevelamer, but only in stage 5. Rates of treatment with any binder increased from 5 to 12 to 55% per 100 PY, respectively. Taken together, these diagnostic and therapeutic patterns, in particular in the two earlier stages of renal failure, appear to be suboptimal for prevalence rates and mainly focused on SHPT suppression, which is considered when PTH levels increase without any attempt at prevention. However, we now know that the target of therapy should include not only biochemical targets, but also osteopenia and osteoporosis.
For this reason, it is interesting to compare the results of this study with the observation by Portales-Castillo et al. [39], who analysed the prescriptive patterns of osteoporosis medications in CKD stages 2–5 patients who were diagnosed as osteoporotic by dual-energy X-ray absorptiometry scan. The average attention of the nephrologist to the specific aspect of bone disease is thus described. In this population, among the anti-osteoporosis drugs, only anti-resorptives were prescribed, mainly in CKD stages 2–3 (71%) and less frequently in stages 4–5 (52%). This reduction is due to the current restrictions on the use of bisphosphonates in more advanced stages of CKD. Notably, compared with the previous study, prescription rates of calcium supplements or vitamin D were sensibly higher and stable in the two different levels of renal insufficiency (87 and 89%, respectively), along with lower PTH values (average 65 pg/ml in CKD 2–3 and 92 pg/ml in CKD 4–5 non-vitamin D), which points to empirical therapeutic choices aimed at counteracting the biochemical effects of anti-resorptive therapies (namely hypocalcaemia) or as adherence to standard anti-osteoporotic therapy. Ideally, instead, therapy should be based on more precise knowledge of bone disease obtained with bone biopsy. In any case, it is necessary to underline that the clinical efficacy of these strategies is not supported by specific randomized controlled trial (RCT) data, in particular in more advanced stages of renal insufficiency [40, 41], given the risk of excessive bone turnover suppression. A further issue to consider, when deciding therapy for SHPT is the complex link between bone and vessels. In fact, severe suppression of PTH may result in reduced buffering capacity by bone cells with regard to excessive calcium and phosphate loads, which may favour extraosseous and specifically vascular calcification [42], although PTH or bone turnover suppression per se is not always associated with negative outcomes [41].
PERSONALISED MEDICINE IN SHPT
Thus personalising treatment of CKD-MBD represents the new therapeutic challenge. Besides considering age, sex, race and comorbidities, traditional and novel markers should be evaluated aimed at recognising possibly different patient phenotypes, as suggested for example by Block et al. [43] in a prevalent population of dialysis patients. In this study, the contemporary occurrence of either low, within or above empirically identified reference range values for the three most frequently assayed biomarkers (namely, calcium, phosphate and PTH) allowed identification of 36 possible CKD-MBD phenotypes, with possibly different cardiovascular or any-cause mortality risk. As an example, having the three parameters all in the categories identified as ‘high’ (PTH >600 pg/ml, calcium >10.2 mg/dl and phosphate >5.5 mg/dl) carried significantly greater risk of death and of pooled cardiovascular hospitalization or death as compared with the reference phenotype (PTH 150–300 pg/ml, calcium 8.2–10.2 mg/dl and phosphate 3.5–5.5 mg/dl) [43]. Notably, PTH >600 pg/ml alone was not associated with any adverse risk if calcium and phosphate were well controlled in this analysis.
Also, there is evidence in incident dialysis patients that changes over time of these same parameters may have significant implications in the clinical outcome of a patients [44]. As illustrated by Vervloet [45] in a commentary, besides therapeutic interventions, a number of other factors (e.g. inflammation, microbiota etc.) may be responsible for modifications occurring during follow-up in the concentration of MBD biomarker serum levels, independent of the therapies adopted for SHPT. For this reason, prospective observational studies examining the trajectory of biomarkers are warranted, but, to the best of our knowledge, no similar studies are available in the early phases of CKD, which are the phases where SHPT starts. Recently a retrospective clinical observation in CKD stages 3–4 clearly demonstrated that the abnormalities of mineral metabolism accelerate their development ∼5 years before patients reach end-stage kidney disease [46], thus suggesting a time frame for prompt therapeutic choices. Clinical research and large databases are necessary to evaluate the complexity of SHPT development in CKD and to select the best available therapy for the individual patient. Also, new therapeutic strategies, if available, deserve exploration.
EXTENDED-RELEASE CALCIFEDIOL (ERC) AS A FUTURE OPTION?
As already pointed out above, prevention of early autonomy of SHPT and good PTH control at the time of transfer of ND-CKD patients into the dialysis stage appears to be of great importance. Both native vitamin supplementation (due to its limited effect on PTH levels) and active vitamin D treatment (due to its inherent risk of hypercalcaemia and hyperphosphataemia) have been suboptimal options in this regard.
ERC was approved in the USA in 2016 and may become an interesting alternative treatment option for ND-CKD patients in stages G3–G4 who develop progressive SHPT. ERC is an orally administered prohormone of calcitriol in an extended (or prolonged) release formulation. This formulation creates specific pharmacokinetic properties—as a consequence, a slow and steady release of calcifediol over its full intestinal transport confers a steady low-level uptake of the prohormone [47, 48]. Therefore ERC does not cause peaks of 25(OH)D and calcitriol levels, avoiding the induction and upregulation of 24-hydroxylases and thus overcoming rapid inactivation and degradation [47], especially of subsequently and constantly rising calcitriol concentrations [48]. By this kind of substrate availability, endogenous calcitriol remains in charge of vitamin D receptor activation in target tissues to a much greater degree than otherwise possible in these stages of CKD, effectively contributing to suppressing PTH synthesis and secretion by the parathyroid glands.
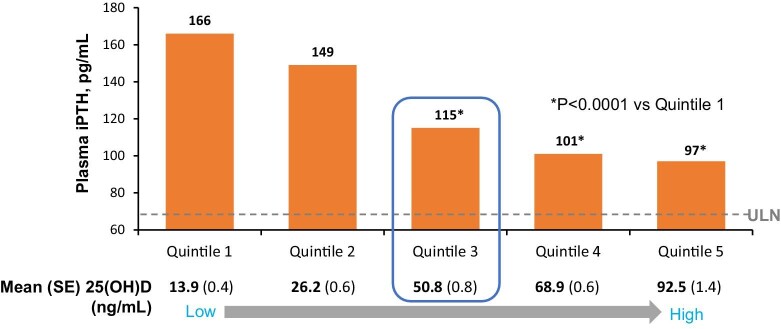
The efficacy and safety of oral ERC in patients with CKD stages G3–G4 was demonstrated in phase 3 clinical trials [48]. A total of 429 patients with established SHPT and accompanying vitamin D insufficiency were treated with 30 μg ERC or placebo daily for 12 weeks, followed by potential up-titration to 60 μg plus an open-label extension study. In this protocol, one-third of patients achieved the primary endpoint of a ≥30% reduction in PTH after 6 months [49]. In the open-label extension phase of the study, placebo patients were switched to ERC and showed a decrease in serum PTH levels of the same magnitude as those under active treatment in the RCTs. ERC treatment appeared to be stable and effective for the whole 12 months of observations under these trial settings. A key and critical point was the requirement that mean serum total 25(OH)D levels had to increase to ≥50 ng/ml in order to demonstrate full efficacy to suppress SHPT (and bone turnover biomarkers; Figure 1) [50]. In this study, along with increasing 25(OH)D quintiles, 24,25-dihydroxyvitamin D [24,25(OH)2D] levels increased as well, so that the 24,25(OH)2 D:25(OH)D ratio increased. These data suggest that a more complete evaluation of vitamin D therapy could be obtained by assaying all the three metabolites.
Figure 1:
Plasma iPTH levels at weeks 20–26 as a function of post-treatment calcidiol [25(OH)D] quintile. Calcidiol levels >50 ng/ml were associated with significant reductions in iPTH. Adapted from Strugnell et al. [47]. SE: standard error.
Concerning safety, treatment-emergent adverse events were not different between the ERC and placebo arms of the phase 3 trials. In particular, no overt events of hypercalcaemia and hyperphosphataemia were observed, which seems to distinguish ERC from recent observations in trials such as PRIMO and OPERA with active vitamin D analogues [7, 8]. Even elevations of 25(OH)D serum levels to as high as 92.5 ng/ml over a 6-month period did not show safety signals [45]. Emerging real-world data support the findings of the randomised controlled phase 3 trials on ERC in US routine clinical practice settings. Retrospective analyses from 18 US nephrology clinics including patients in CKD stages G3–G4 with a history of SHPT and vitamin D insufficiency presented data on subsets receiving either ERC (n = 174), active vitamin D or its analogues (n = 55) and nutritional vitamin D (n = 147) [51]. ERC lowered PTH serum levels by >30% in more than one-third of exposed subjects without an impact on calcium and phosphate levels, while patients treated with active vitamin D analogues had small but statistically significant increases in serum calcium levels. Nutritional vitamin D was more commonly used in earlier CKD, making data less comparable.
Nevertheless, and despite promising biochemical endpoint data on SHPT progression in ND-CKD patients, it needs to be emphasised that there are no hard endpoint data available on ERC with regard to meaningfully ameliorating the clinical disease burden (fractures, cardiovascular events etc.) of affected patients.
UNANSWERED QUESTIONS AND DATA GAPS
It remains undisputable that stable and good control of SHPT in CKD stages G3–G4 enables a smoother entry into stage G5D. As already briefly pointed out above, a Dialysis Outcomes and Practice Patterns Study (a prospective observational study program in selected centres worldwide) report recently analysed incident dialysis patients for 1 year, grouped according to their baseline PTH levels, and revealed a lesser pill burden (vitamin D analogues, calcimimetics) for the individual patient and an improved economic impact with regard to prescriptions for PTH management the better their initial PTH control [14].
Nevertheless, there is still significant uncertainty concerning PTH target levels and dynamics, when to start intervention and with what intensity and aim. ERC possesses the potential to better enable PTH control in non-dialysis stages, but what we might have learned from the above-mentioned trials is that we may have aimed for too low 25(OH)D serum levels in the past. One elephant in the room may thus be the question, how achieving such 25(OH)D levels by increasing native vitamin D3 supplementation impacts on PTH measures? As an estimate, this would probably require doses close to or >4000 IU/day, which would go beyond the upper levels of safety as recommended by the Institute of Medicine in 2011 in the majority of patients [52, 53]. Or is this unique formulation of ERC the only way and ultimate clue to detour vitamin D–degrading feedback mechanisms by 24-hydroxylases? Head-to-head comparisons could be a viable approach to find an answer.
Above all, the key issue is how to best prevent and improve any morbidity and mortality outcomes in CKD patients, including fractures, cardiovascular risks and death. The possible modulatory effect of ALP on the association of PTH with unfavourable outcomes could be evaluated in trials with combined ALP and PTH targets. However, straightforward study designs are difficult to construct in this context, because even significant mismanagement of SHPT development in CKD stages G3–G4 will see its consequences and endpoints after 5–10 years, or even later. It seems imperative to strictly demand performances of such hard outcome RCTs, with endpoints including fracture protection, impact on CKD-MBD management in CKD stage 5D, cardiovascular events and cardiovascular and all-cause mortality.
For the time being, we may be limited to keep on relying on biological plausibilities and surrogate parameters when making treatment decisions in these ND-CKD stages. Such parameters will then include avoidance of overt hypercalcaemia and hyperphosphataemia, vitamin D and PTH (trends) serum levels and possibly control of (high turnover) bone biomarkers. Prospective large and long-term registry approaches are certainly inferior and suboptimal data resources when compared with RCTs, especially with regard to potential cause-and-effect relationships, but they will still provide some valuable information when started in the earliest stages of progressive CKD.
CONCLUSIONS
There is still no uniform approach towards the management of SHPT in ND-CKD in 2022 [3,54]. Safety issues (hypercalcaemia, hyperphosphataemia) must be weighed against the necessity of efficient prevention of severe and progressive SHPT development and parathyroid gland autonomy. Current guidelines favour a combined look at the key biomarkers of CKD-MBD (calcium, phosphate, PTH, ALP) and at parameter trends when making treatment decisions. ERC might become an additional useful tool for controlling SHPT prior to the start of dialysis, while the general importance of the optimal vitamin D setting and supply remains to be better understood and determined in all stages of CKD. Such an understanding may then pave the way to also better define the optimal PTH level in ND-CKD than is currently expressed in KDIGO guideline 4.2.1.
ACKNOWLEDGEMENTS
The CKD-MBD Working Group is an official body of the European Renal Association.
Contributor Information
Markus Ketteler, Department of General Internal Medicine and Nephrology, Robert-Bosch-Krankenhaus, Stuttgart, Germany.
Jordi Bover, Nephrology Department, University Hospital Germans Trias i Pujol, Badalona, Catalonia, Spain; REMAR-IGTP Group, Germans Trias i Pujol Research Institute, Can Ruti Campus, Badalona, Catalonia, Spain.
Sandro Mazzaferro, Department of Translational and Precision Medicine, Sapienza University of Rome, Rome, Italy.
FUNDING
No financial support has been received for this article.
AUTHORS’ CONTRIBUTIONS
All three authors contributed equally to data research, data interpretation and writing of this manuscript.
DATA AVAILABILITY STATEMENT
The data underlying this research will be shared upon reasonable request to the corresponding author.
CONFLICT OF INTEREST STATEMENT
M.K. has received advisory and/or lecture fees from Amgen and Vifor Pharma. S.M. has received lecture fees from Vifor Pharma. J.B. has received advisory and/or lecture fees from Amgen, AbbVie, Sanofi, Rubió and Vifor Fresenius Renal Pharma.
REFERENCES
- 1. Moe S, Drüeke T, Cunningham Jet al. Kidney Disease: Improving Global Outcomes (KDIGO) definition, evaluation, and classification of renal osteodystrophy: a position statement from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int 2006;69:1945–53. 10.1038/sj.ki.5000414 [DOI] [PubMed] [Google Scholar]
- 2. Kidney Disease: Improving Global Outcomes CKD-MBD Work Group Kidney Disease: Improving Global Outcomes CKD-MBD Work Group . KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of chronic kidney disease-mineral and bone disorder (CKD-MBD). Kidney Int Suppl 2009;113:S1–130. [DOI] [PubMed] [Google Scholar]
- 3. Kidney Disease: Improving Global Outcomes CKD-MBD Update Work Group Kidney Disease: Improving Global Outcomes CKD-MBD Update Work Group . KDIGO 2017 clinical practice guideline update for the diagnosis, evaluation, prevention, and treatment of chronic kidney disease-mineral and bone disorder (CKD-MBD). Kidney Int Suppl 2017;7:1–59. 10.1016/j.kisu.2017.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Souberbielle JC, Boutten A, Carlier MCet al. Inter-method variability in PTH measurement: implication for the care of CKD patients. Kidney Int 2006;70:345–50. 10.1038/sj.ki.5001606 [DOI] [PubMed] [Google Scholar]
- 5. White CA, Sarabia S, Collier CPet al. Parathyroid hormone measurement in chronic kidney disease: impact of inter-method variability on mineral bone disease assessment. Clin Biochem 2021;94:62–6. 10.1016/j.clinbiochem.2021.04.018 [DOI] [PubMed] [Google Scholar]
- 6. Kritmetapak K, Pongchaiyakul C.. Parathyroid hormone measurement in chronic kidney disease: from basics to clinical implications. Int J Nephrol 2019;2019:5496710. 10.1155/2019/5496710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Thadhani R, Appelbaum E, Pritchett Yet al. Vitamin d therapy and cardiac structure and function in patients with chronic kidney disease: the PRIMO randomized controlled trial. JAMA 2012;307:674–84. 10.1001/jama.2012.120 [DOI] [PubMed] [Google Scholar]
- 8. Wang AY, Fang F, Chan Jet al. Effect of paricalcitol on left ventricular mass and function in CKD—the OPERA trial. J Am Soc Nephrol 2014;25:175–86. 10.1681/ASN.2013010103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cozzolino M, Bernard L, Csomor PA.. Active vitamin D increases the risk of hypercalcaemia in non-dialysis chronic kidney disease patients with secondary hyperparathyroidism: a systematic review and meta-analysis. Clin Kidney J 2021;14:2437–43. 10.1093/ckj/sfab091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. National Kidney Foundation National Kidney Foundation . K/DOQI clinical practice guidelines for bone metabolism and disease in chronic kidney disease. Am J Kidney Dis 2003;42(4 Suppl 3):S1–201. 10.1016/S0272-6386(03)00905-3 [DOI] [PubMed] [Google Scholar]
- 11. Faul C, Amaral AP, Oskouei Bet al. FGF23 induces left ventricular hypertrophy. J Clin Invest 2011;121:4393–408. 10.1172/JCI46122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ureña-Torres P, Troya MI, Dauvernge Met al. Independent effects of parathyroid hormone and phosphate levels on hard outcomes in non-dialysis patients: food for thought. Nephrol Dial Transplant 2022;37:613–6. 10.1093/ndt/gfab308 [DOI] [PubMed] [Google Scholar]
- 13. Torregrosa V, Bover J, Rodriguez Met al. Recomendaciones de la Sociedad Española de Nefrología para el manejo de las alteraciones del metabolismo mineral en los pacientes con enfermedad renal crónica 2021 (SEN-MM). Nefrolog í a 2022; https://doi.org/10.1016/j.nefro.2022.03.007. [PubMed] [Google Scholar]
- 14. Tabibzadeh N, Karaboyas A, Robinson BMet al. The risk of medically uncontrolled secondary hyperparathyroidism depends on parathyroid hormone levels at haemodialysis initiation. Nephrol Dial Transplant 2021;36:160–9. 10.1093/ndt/gfaa195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Westerberg PA, Sterner G, Ljunggren Öet al. High doses of cholecalciferol alleviate the progression of hyperparathyroidism in patients with CKD stages 3-4: results of a 12-week double-blind, randomized, controlled study. Nephrol Dial Transplant 2018;33:466–71. 10.1093/ndt/gfx059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Coyne D, Acharya M, Qiu Pet al. Paricalcitol capsule for the treatment of secondary hyperparathyroidism in stages 3 and 4 CKD. Am J Kidney Dis 2006;47:263–76. 10.1053/j.ajkd.2005.10.007 [DOI] [PubMed] [Google Scholar]
- 17. Bover J, Gunnarsson J, Csomor Pet al. Impact of nutritional vitamin D supplementation on parathyroid hormone and 25-hydroxyvitamin D levels in non-dialysis chronic kidney disease: a meta-analysis. Clin Kidney J 2021;14:2177–86. 10.1093/ckj/sfab035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ketteler M, Ambühl P.. Where are we now? Emerging opportunities and challenges in the management of secondary hyperparathyroidism in patients with non-dialysis chronic kidney disease. J Nephrol 2021;34:1405–18. 10.1007/s40620-021-01082-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bozic M, Diaz-Tocados JM, Bermudez-Lopez Met al. Independent effects of secondary hyperparathyroidism and hyperphosphataemia on chronic kidney disease progression and cardiovascular events: an analysis from the NEFRONA cohort. Nephrol Dial Transplant 2022;37:663–72. 10.1093/ndt/gfab184 [DOI] [PubMed] [Google Scholar]
- 20. Cailleaux PE, Ostertag A, Metzger Met al. Longitudinal bone loss occurs at the radius in CKD. Kidney Int Rep 2021;6:1525–36. 10.1016/j.ekir.2021.03.874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. van Ballegooijen AJ, Reinders I, Visser Met al. Parathyroid hormone and cardiovascular disease events: a systematic review and meta-analysis of prospective studies. Am Heart J 2013;165:655–664.e5. 10.1016/j.ahj.2013.02.014 [DOI] [PubMed] [Google Scholar]
- 22. Evenepoel P, Bover J, Ureña Torres P.. Parathyroid hormone metabolism and signaling in health and chronic kidney disease. Kidney Int 2016;90:1184–90. 10.1016/j.kint.2016.06.041 [DOI] [PubMed] [Google Scholar]
- 23. Bover J, Arana C, Ureña Pet al. Hyporesponsiveness or resistance to the action of parathyroid hormone in chronic kidney disease. Nefrolog í a 2021;41:514–28. 10.1016/j.nefro.2020.12.019 [DOI] [PubMed] [Google Scholar]
- 24. Duque EJ, Elias RM, Moysés RMA.. Parathyroid hormone: a uremic toxin. Toxins 2020;12:189. 10.3390/toxins12030189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lavi-Moshayoff V, Wasserman G, Meir Tet al. PTH increases FGF23 gene expression and mediates the high-FGF23 levels of experimental kidney failure: a bone parathyroid feedback loop. Am J Physiol Renal Physiol 2010;299:F882–9. 10.1152/ajprenal.00360.2010 [DOI] [PubMed] [Google Scholar]
- 26. Geng S, Kuang Z, Peissig PLet al. Parathyroid hormone independently predicts fracture, vascular events, and death in patients with stage 3 and 4 chronic kidney disease. Osteoporos Int 2019;30:2019–25. 10.1007/s00198-019-05033-3 [DOI] [PubMed] [Google Scholar]
- 27. Arase H, Yamada S, Tanaka Set al. Association between plasma intact parathyroid hormone levels and the prevalence of atrial fibrillation in patients with chronic kidney disease—the Fukuoka Kidney Disease Registry Study. Circ J 2020;84:1105–11. 10.1253/circj.CJ-19-1201 [DOI] [PubMed] [Google Scholar]
- 28. Lee YJ, Okuda Y, Sy Jet al. Association of mineral bone disorder with decline in residual kidney function in incident hemodialysis patients. J Bone Miner Res 2020;35:317–25. 10.1002/jbmr.3893 [DOI] [PubMed] [Google Scholar]
- 29. Beysel S, Caliskan M, Kizilgul Met al. Parathyroidectomy improves cardiovascular risk factors in normocalcemic and hypercalcemic primary hyperparathyroidism. BMC Cardiovasc Disord 2019;19:106. 10.1186/s12872-019-1093-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Pepe J, Cipriani C, Sonato Cet al. Cardiovascular manifestations of primary hyperparathyroidism: a narrative review. Eur J Endocrinol 2017;177:R297–308. 10.1530/EJE-17-0485 [DOI] [PubMed] [Google Scholar]
- 31. Gwinner W, Suppa S, Mengel Met al. Early calcification of renal allografts detected by protocol biopsies: causes and clinical implications. Am J Transplant 2005;5:1934–41. 10.1111/j.1600-6143.2005.00938.x [DOI] [PubMed] [Google Scholar]
- 32. Roodnat JI, van Gurp EA, Mulder PGet al. High pretransplant parathyroid hormone levels increase the risk for graft failure after renal transplantation. Transplantation 2006;82:362–7. 10.1097/01.tp.0000228923.75739.88 [DOI] [PubMed] [Google Scholar]
- 33. Mazzaferro S, Tartaglione L, Rotondi Set al. News on biomarkers in CKD-MBD. Semin Nephrol 2014;34:598–611. 10.1016/j.semnephrol.2014.09.006 [DOI] [PubMed] [Google Scholar]
- 34. Bover J, Ureña-Torres P, Cozzolino Met al. The non-invasive diagnosis of bone disorders in CKD. Calcif Tissue Int 2021;108:512–27. 10.1007/s00223-020-00781-5 [DOI] [PubMed] [Google Scholar]
- 35. Kalantar-Zadeh K, Kuwae N, Regidor Det al. Survival predictability of time-varying indicators of bone disease in maintenance hemodialysis patients. Kidney Int 2006;70:771–80. 10.1038/sj.ki.5001514 [DOI] [PubMed] [Google Scholar]
- 36. Pimentel A, Ureña-Torres P, Zillikens MCet al. Fractures in patients with CKD-diagnosis, treatment, and prevention: a review by members of the European Calcified Tissue Society and the European Renal Association of Nephrology Dialysis and Transplantation. Kidney Int 2017;92:1343–55. 10.1016/j.kint.2017.07.021 [DOI] [PubMed] [Google Scholar]
- 37. McNerny EMB, Nickolas TL.. Bone quality in chronic kidney disease: definitions and diagnostics. Curr Osteopor Rep 2017;15:207–13. 10.1007/s11914-017-0366-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wetmore JB, Ji Y, Ashfaq Aet al. Testing patterns for CKD-MBD abnormalities in a sample US population. Kidney Int Rep 2021;6:1141–50. 10.1016/j.ekir.2020.12.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Portales-Castillo IA, Aksu C, Zhao Set al. Prescription patterns of osteoporosis medications in patients with advanced CKD: a retrospective cohort study. Kidney Med 2021;3:1112–5. 10.1016/j.xkme.2021.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Haarhaus M, Evenepoel P, European Renal Osteodystrophy (EUROD) workgroup . European Renal Osteodystrophy (EUROD) workgroup, Chronic Kidney Disease Mineral and Bone Disorder (CKD-MBD) working group of the European Renal Association–European Dialysis and Transplant Association (ERA-EDTA). Differentiating the causes of adynamic bone in advanced chronic kidney disease informs osteoporosis treatment. Kidney Int 2021;100:546–58. [DOI] [PubMed] [Google Scholar]
- 41. Khairallah P, Nickolas TL.. Management of osteoporosis in CKD. Clin J Am Soc Nephrol 2018;13:962–9. 10.2215/CJN.11031017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kurz P, Monier-Faugere MC, Bognar Bet al. Evidence for abnormal calcium homeostasis in patients with adynamic bone disease. Kidney Int 1994;46:855–61. 10.1038/ki.1994.342 [DOI] [PubMed] [Google Scholar]
- 43. Block GA, Kilpatrick RD, Lowe KAet al. CKD-mineral and bone disorder and risk of death and cardiovascular hospitalization in patients on hemodialysis. Clin J Am Soc Nephrol 2013;8:2132–40. 10.2215/CJN.04260413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Lamina C, Kronenberg F, Stenvinkel Pet al. Association of changes in bone mineral parameters with mortality in haemodialysis patients: insights from the ARO cohort. Nephrol Dial Transplant 2020;35:478–87. 10.1093/ndt/gfz060 [DOI] [PubMed] [Google Scholar]
- 45. Vervloet MG. Chronic kidney disease-mineral and bone disorder: changing insights form changing parameters? Nephrol Dial Transplant 2020;35:385–9. 10.1093/ndt/gfz113 [DOI] [PubMed] [Google Scholar]
- 46. Isakova T, Cai X, Lee Jet al. Longitudinal evolution of markers of mineral metabolism in patients with CKD: the Chronic Renal Insufficiency Cohort (CRIC) Study. Am J Kidney Dis 2020;75:235–44. 10.1053/j.ajkd.2019.07.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Petkovich M, Melnick J, White Jet al. Modified-release oral calcifediol corrects vitamin d insufficiency with minimal CYP24A1 upregulation. J Steroid Biochem Mol Biol 2015;148:283–9. 10.1016/j.jsbmb.2014.11.022 [DOI] [PubMed] [Google Scholar]
- 48. Sprague SM, Strugnell SA, Bishop CW.. Extended-release calcifediol for secondary hyperparathyroidism in stage 3–4 chronic kidney disease. Expert Rev Endocrinol Metab 2017;12:289–301. [DOI] [PubMed] [Google Scholar]
- 49. Sprague SM, Crawford PW, Melnick JZet al. Use of extended-release calcifediol to treat secondary hyperparathyroidism in stages 3 and 4 chronic kidney disease. Am J Nephrol 2016;44:316–25. 10.1159/000450766 [DOI] [PubMed] [Google Scholar]
- 50. Strugnell SA, Sprague SM, Ashfaq Aet al. Rationale for raising current clinical practice guideline target for serum 25-hydroxyvitamin D in chronic kidney disease. Am J Nephrol 2019;49:284–93. 10.1159/000499187 [DOI] [PubMed] [Google Scholar]
- 51. Fadda G, Germain MJ, Broumand Vet al. Real-world assessment: clinical effectiveness and safety of extended-release calcifediol. Am J Nephrol 2021;52:798–807. 10.1159/000518545 [DOI] [PubMed] [Google Scholar]
- 52. Institute of Medicine; Food and Nutrition Board; Committee to Review Dietary Reference Intakes for Vitamin D and Calcium Institute of Medicine; Food and Nutrition Board; Committee to Review Dietary Reference Intakes for Vitamin D and Calcium. et al. Dietary Reference Intakes for Vitamin D and Calcium. Washington, DC: National Academies Press, 2011. https://www.ncbi.nlm.nih.gov/books/NBK56070/. [Google Scholar]
- 53. Marckmann P, Agerskov H, Thineshkumar Set al. Randomized controlled trial of cholecalciferol supplementation in chronic kidney disease patients with hypovitaminosis D. Nephrol Dial Transplant 2012;27:3523–31. 10.1093/ndt/gfs138 [DOI] [PubMed] [Google Scholar]
- 54. Mazzaferro S, Goldsmith D, Larsson TEet al. Vitamin D metabolites and/or analogs: which D for which patient? Curr Vasc Pharmacol 2014;12:339–49. 10.2174/15701611113119990024 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data underlying this research will be shared upon reasonable request to the corresponding author.