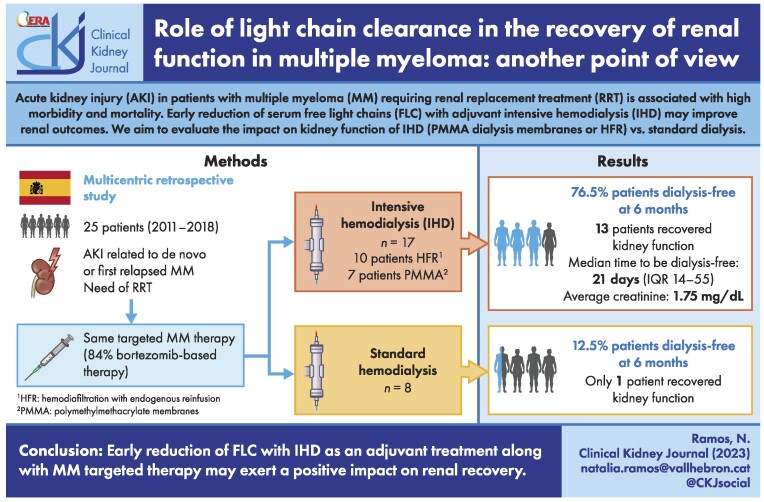
ABSTRACT
Background
Acute kidney injury (AKI) in patients with multiple myeloma (MM) requiring renal replacement treatment (RRT) is associated with high morbidity and mortality. Early reduction of serum free light chains (FLC) using both targeted therapy against MM and intensive hemodialysis (IHD) may improve renal outcomes. We evaluated the effectiveness of two different RRT techniques on renal recovery in an MM patient population: standard dialysis procedure vs IHD with either polymethylmethacrylate (PMMA) or hemodiafiltration with endogenous reinfusion (HFR).
Methods
This was a multicentric retrospective study with severe AKI related to MM, between 2011 and 2018. Twenty-five consecutive patients with AKI secondary to MM requiring RRT were included. Patients that underwent IHD received six dialysis sessions per week during the first 14 days (PMMA vs HFR). All patients were diagnosed with de novo MM or first relapsed MM. Primary outcome was renal recovery defined as dialysis-free at 6 months follow-up.
Results
A total of 25 patients were included. Seventeen patients received IHD and eight standard dialysis. All patients were treated with targeted therapy, 84% bortezomib-based. Of the 25 patients included, 14 (56%) became dialysis independent. We observed a higher proportion of patients who received IHD in the group who recovered kidney function compared with those who remained in HD (92.9% vs 36.4%, P = .007). In our study, the use of IHD to remove FLC had a statistically significant association with renal recovery compared with the standard dialysis group (P = .024).
Conclusion
Early reduction of FLC with IHD as an adjuvant treatment along with MM-targeted therapy may exert a positive impact on renal recovery.
Keywords: acute kidney injury, free light chains, hemodialysis, multiple myeloma, renal function recovery
Graphical Abstract
Graphical Abstract.
INTRODUCTION
Multiple myeloma (MM) represents approximately 10% of all hematologic neoplasms. Between 4% and 10% of patients with MM require renal replacement treatment (RRT) at the moment of diagnosis [1–3], and renal function recovery is associated with a significant increase in patient's overall survival [4]. Patients who remained on dialysis had an increased risk of death (15%–30%) within the first months from diagnosis [2, 3]. These patients treated with classic chemotherapy had an overall survival less than 12 months. With the use of proteasome inhibitor agents, survival has been greatly increased, but life expectancy still remains lower in patients with renal impairment at the moment of diagnosis. Dimopoulos et al. studied the impact of bortezomib treatment in 83 patients with severe kidney failure [estimated glomerular filtration rate (eGFR) <30 mL/min/1.73 m2] and newly diagnosed MM [3]. Fifteen of the 31 (48%) patients—all of whom were on dialysis at diagnosis—became dialysis independent. In addition, the study suggested that the use of bortezomib-based triplets was associated with a higher probability of dialysis discontinuation [4, 5].
Between 20% and 50% of patients with MM have some degree of renal impairment at diagnosis. Light-chain cast nephropathy, also known as myeloma kidney, is the first cause of kidney injury in MM patients and is characterized by tubular atrophy and tubular-interstitial fibrosis [6]. The risk factors of severe acute kidney injury (AKI) described include the type of light chain involved and its serum levels, the time of MM evolution and the use of loop diuretics (e.g. furosemide) [1, 7, 8]. Renal function recovery depends on the severity of AKI, the fibrosis degree in the renal biopsy, the quantity of the deposit of light chains and how quickly free light chains (FLC) are removed [1].
Previous studies have shown that a reduction higher than 50% in serum levels of light chains within the first 21 days after diagnosis of MM increases the probability of renal function recovery [9–12]. Prompt initiation of targeted therapy against MM seems to be the main factor which increases patient survival achieving renal function recovery rate between 30% and 60% [1, 5, 13]. However, the persistence of light chains over time increases the exposure and therefore worsens the renal prognosis. Different depuration strategies to remove light chains from the blood have been used with contradictory results [14]. Hutchinson tested different high permeability membranes. HCO-1100 Theralite® (Gambro) was the most effective one. However, polymethylmethacrylate's (BK-F Toray PMMA) effectiveness has been reported at 88% and 73% for kappa and lambda chains removal, respectively [10]. For hemodiafiltration with endogenous reinfusion (HFR), there are reported removal rates of 54% and 36.7% for kappa and lambda, respectively [12]. The reduction of circulating light chains by IHD (intensive hemodialysis) as a supportive treatment of early targeted therapy in selected patients with AKI secondary to MM may improve the renal outcome, with a positive impact in patient survival. Thus, the aim of our study is to evaluate the impact of intensive hemodialysis with PMMA and hemodiafiltration with endogenous reinfusion (HFR) on renal survival in patients with severe AKI due to MM.
MATERIALS AND METHODS
This is a retrospective, multicenter study of 25 consecutive patients that developed severe AKI requiring RRT secondary to cast nephropathy from three Spanish University hospitals (Vall d'Hebron University Hospital, HUVH; Hospital del Mar, HM; Hospital 12 Octubre, HDO) between June 2011 and December 2018 (Supplementary data, Table S1). The diagnosis of AKI was based on the Acute Kidney Injury Network (AKIN) classification and dialysis was indicated as per protocol in each center. We defined as probable light chain cast nephropathy when the following conditions were met: patients had a diagnosis of MM, serum FLC levels over 500 mg/L, urinary excretion of albumin inferior to 30% [15] and AKI persistence after correction of reversible factors (dehydration, nephrotoxic drugs or hypercalcemia). We performed renal biopsy in patients with no contraindication (n = 11/25, 44%) to confirm the diagnosis. Patients were diagnosed from a de novo MM or a first relapse.
The RRT used in these patients were based on either conventional hemodialysis therapy or IHD, which was done with PMMA or HFR according to the clinical protocol of each center. At HUVH, HFR was used for kappa light chain whereas PMMA was used for lambda light chain due to distinct molecular weights (22.5 kDa and 45 kDa, respectively). At HM, all patients were treated with HFR, whereas at HDO all patients received standard dialysis. In all patients treated with IHD serum FLC levels were higher than 1000 mg/L. Renal outcomes were collected at 6 months’ follow up.
This study was performed in accordance with the principles of the Helsinki Declaration. The present study was approved by Ethics Committee of Hospital Valle de Hebron [PR (AG) 02/2022].
IHD protocol
In all patients treated with IHD, 12 consecutive dialysis sessions were performed divided in two cycles (six sessions each one) with a 2-day break after the sixth session. Patients did two cycles unless FLC fell below 1000 mg/L or the patient's kidney function recovered, defined as a permanent dialysis-independency with an eGFR [Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI)] >10 mL/min/1.73 m2, in which case IHD was stopped earlier. Either a PMMA membrane or HFR were used. Each session lasted 3 h and 4 h, respectively. Blood flow and dialysate flow prescription were 300 mL/min and 500 mL/min, respectively.
The referent hematologist of each center decided the MM targeted therapy considering the International Myeloma Working Group (IMWG) and Grupo Español de Mieloma recommendations.
Standard dialysis in MM patients was indicated according to the nephrologist criteria of each center.
Data collection
Baseline and follow-up data were obtained from medical records of the three participating centers. Baseline renal function was measured using the CKD-EPI formula in 6 previous months (if it was available) and serum creatinine and eGFR at 6 months’ follow-up in patients that recovered renal function without need of dialysis. In Hospital Vall d'Hebron, serum FLC levels were measured by immunonephelometric assay (Freelite, The Binding Site, San Diego, CA, USA) several times: before dialysis sessions on Days 1 and 4, at the end of each cycle and 21 days after dialysis started.
Outcome measures
The primary outcome was the percentage dialysis independence within the first 6 months defined as eGFR >10 mL/min/1.73 m2 based on serum creatinine using CKD-EPI formula. The secondary outcome was detecting the clinical characteristics, that may play a role in determining renal prognosis at the moment of AKI secondary to MM. The clinical characteristics collected were age (years), sex (male), previous CKD, baseline FLC levels, whether the diagnosis was de novo or first relapse, type of target MM treatment, time to MM target therapy initiation (days), hematological response after first cycle (% of patients with complete response or very good partial response), FLC reduction by Day 21, serum creatinine (mg/dL), proteinuria (mg/day), presence of diuresis (>500 mL/day) or presence of hypercalcemia define by level upper 10.5 mg/dL.
Statistics
Descriptive statistics are presented as mean ± standard deviation (SD), or median and interquartile range (IQR) for continuous variables, and percentages for categorical variables. We made univariate comparisons using Fischer's exact test for categorical variables, and Student's t-test or Mann–Whitney–Wilcoxon for continuous variables. Persistent dialysis survival was measured from the first dialysis session using Kaplan–Meier curves, and compared between groups using the Log-rank test. After Neperian logarithm transformation to obtain normality of proteinuria levels and time to chemo-immunotherapy initiation, two logistic regressions analysis were performed to identify independent predictors of renal function recovery. A two-sided P-value <.05 was considered statistically significant. Statistical analyses were performed using SPSS version 25.0.
RESULTS
Between 2011 and 2018, 25 consecutive patients with dialysis-dependent AKI related to MM were analyzed. Median age was 70.7 (SD = 11.6) years and 52% (n = 13) of patients were men. All patients presented with AKI stage 3 and needed RRT. Baseline serum FLC in all patients was 8700 (IQR 3870–11 875). In the group of patients that remained in hemodialysis it was 13 000 (IQR 4280–19 654) and in the group on hemodialysis independence it was 8011 (IQR 3757–11 019). Main patients baseline characteristics are detailed in Table 1. Nineteen patients (76%) had an MM de novo at the time of AKI diagnosis.
Table 1:
Main characteristics of IHD and standard hemodialysis groups.
| n = 25 | IHD (n = 17) | Standard hemodialysis (n = 8) | P-value | |
|---|---|---|---|---|
| Age, years (mean, SD) | 70.7 (11.6) | 67.24 (11.7) | 77.8 (6.4) | .025 |
| >65 years (%, n) | 76.5 (19) | 70.6 (12) | 87,5 (7) | .624 |
| Gender, male (%, n) | 52 (13) | 64.7 (11) | 25 (2) | .097 |
| Previous CKD (GFR <60 mL/min) (%, n) | 44 (11) | 35.3 (6) | 62.5 (5) | .389 |
| De novo (%, n) | 76 (19) | 76.5 (13) | 75 (6) | 1 |
| FLC kappa/lambda (%, n) | 56(14)/44(11) | 75 (6)/25(2) | 47.1(8)/52.9(9) | .234 |
| Baseline serum FLC, mg/L (median, IQR) | 9084 (4470–11 985) | 8700 (4470–11 498) | 9609 (2347–13 216) | .788 |
| Bortezomib-based therapy (%, n) | 84 (21) | 82.4 (14) | 100 (8) | .527 |
| Time to MM target therapy initiation, days (median, IQR) | 5 (2.25–21.5) | 4 (2.25–7.5) | 26 (1.75–30.75) | .149 |
| Hematological response after first cycle (%, n) | 40 (10) | 47.1 (7) | 37.5 (3) | 1 |
| MM response (CR/VGPR) (%, n) | 44 (11) | 52.9 (9) | 25 (2) | .234 |
| FLC reduction by Day 21 (%, n) | .138 | |||
| <50% | 15.8 (3) | 11.8 (2) | 50 (1) | |
| 50–75% | 21.1 (4) | 17.6 (3) | 50 (1) | |
| >75% | 63.2 (12) | 70.6 (12) | 0 | |
| Serum creatinine, mg/dL (mean, SD) | 6.7 (2.8) | 6.75 (2.84) | 6.8 (3.01) | .658 |
| Dialysis independence (%, n) | 56 (14) | 76.5 (13) | 12.5 (1) | .007 |
| Proteinuria, mg/day (median, IQR) | 3400 (1350–6000) | 4950 (3075 –7045) | 1350 (0.260–2590) | .009 |
| Ca (mg/dL) (mean, SD) | 10.3 (2.18) | 10.9 (2.31) | 8.9 (0.99) | .03 |
| Ca >10.5 mg/dL (%, n) | 36 (9) | 47.1 (8) | 12.5 (1) | .182 |
| Diuresis >500 mL/day (%, n) | 62.5 (15) | 76.5 (13) | 28.6 (2) | .061 |
CR: complete response; VGPR: very good partial response: Ca: calcium.
Renal biopsy was performed in 11 patients (44%). Cast nephropathy was found in 10 of the patients (Supplementary data, Table S2).
We found a significant difference in the proteinuria degree at the time of AKI diagnosis between patients that underwent IHD and standard dialysis (median 4950 mg/g vs 1350 mg/g, respectively).
We compared the main clinical, demographic and immunological characteristics among groups according to whether they became dialysis-free or not (Table 2). Notably, there was no significant difference among baseline serum monoclonal light chains isotypes (P = .165) between the two groups.
Table 2:
Main patient baseline characteristics of the dialysis-dependent patients and independent patients.
| n = 25 | Hemodialysis dependent (n = 11) | Hemodialysis independent (n = 14) | P-value | |
|---|---|---|---|---|
| Age, years (mean, SD) | 70.7 (11.6) | 76.3 (6.6) | 65.9 (12.9) | .02 |
| >65 years (%, n) | 76.5 (19) | 90.9 (10) | 61.5 (9) | .166 |
| Gender, male (%, n) | 52 (13) | 36.4 (4) | 64.3 (9) | .238 |
| Previous CKD (GFR <60 mL/min) (%, n) | 44 (11) | 45.5 (5) | 42.9 (6) | 1 |
| De novo (%, n) | 76 (19) | 81.8 (9) | 71.4 (10) | .232 |
| FLC kappa/lambda (%, n) | 56 (14)/44 (11) | 54.6 (6)/45.5 (5) | 57.1 (8)/42.9 (6) | .165 |
| Baseline serum FLC, mg/L (median, IQR) | 9084 (4470–11 985) | 9940 (5862–14 302) | 8011 (3757–11 019) | .136 |
| Bortezomib-based chemotherapy (%, n) | 84 (21) | 90.9 (10) | 78.6 (11) | .604 |
| Time to MM target therapy initiation, days (median, IQR) | 5 (2.25–21.5) | 26 (2.5–41.75) | 4 (2.5–7) | .064 |
| Hematological response after first (at least PR) cycle (%, n) | 40 (10) | 45.5 (5) | 35.7 (5) | .697 |
| MM response (≥VGRP) (%, n) | 44 (11) | 36.4 (4) | 50 (7) | .689 |
| Involved FLC reduction by day 21 (%, n) | .138 | |||
| <50 | 15.8 (3) | 40 (2) | 7.1 (1) | |
| 50–75 | 21.1 (4) | 0 (0) | 28.6 (4) | |
| >75 | 63.2 (12) | 60 (3) | 64.3 (9) | |
| IHD (%, n) | 68 (17) | 36.4 (4) | 92.9 (13) | .007 |
| Number of IHD sessions | 15 | 12 | ||
| Serum creatinine, mg/dL (mean, SD) | 6.7 (2.8) | 7.5 (3.5) | 6 (2.95) | .215 |
| Proteinuria, mg/day (median, IQR) | 3400 (1350–6000) | 1775 (0.850–3075) | 5500 (3950–8200) | .002 |
| Kidney biopsy (%, n) | 44 (11) | 45.5 (5) | 42.9 (6) | 1 |
| Ca (mg/dL) (mean, SD) | 10.3 (2.18) | 9.2 (1.0) | 11.15 (2.5) | .027 |
| Ca >10.5 mg/dL (%, n) | 36 (9) | 18.2 (2) | 50 (7) | .208 |
| Diuresis >500 mL/day (%, n) | 62.5 (15) | 27.3 (3) | 85.7 (12) | .01 |
| Deaths (%, n) | 64 (16) | 81.8 (9) | 50 (7) | .208 |
CR: complete response; VGPR: very good partial response; PR: partial response; Ca: calcium.
Twenty-one patients (84%) received a bortezomib-based regimen with no significant difference between groups. The majority of patients in treatment with bortezomib had a diagnostic de novo of MM (Supplementary data, Table S3). Ten patients were treated with two drugs and 11 with three drugs in target MM treatment. The median follow-up was 14.5 months (IQR 6–25).
IHD characteristics
Seventeen patients underwent IHD in HUVH and HM. The main characteristics of IHD and standard hemodialysis groups are shown in Supplementary data, Table S2. Seven patients used PMMA dialyzer and seven HFR. Three patients started dialysis with HFR but had to switch to PMMA due to technical failure due to light chains aggregates. During IHD sessions no complications related to albumin depletion occurred. The median reduction of FLC was 75.6% at Day 21. However, this information was only available in the intensive dialysis group.
Renal function recovery
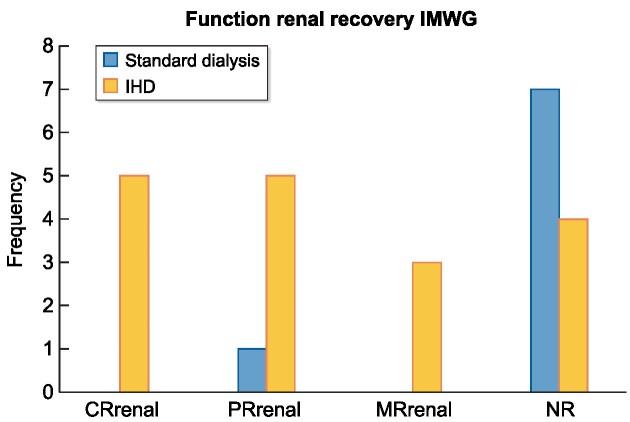
Independence from dialysis occurred in 14 patients (56%), after a median period of 21 days (IQR 14–55). Renal function recovery according to IMWG criteria is shown in Fig. 1. The average creatinine at 6 months of follow-up was 1.75 mg/dL. Patients with IHD regimen evidenced more renal function recovery compared with standard dialysis (P = .007).
Figure 1:
Frequency of patients according to IMWG criteria. CRrenal: complete renal response [best creatine clearance (CrCl): >60 mL/min]; PRrenal: partial renal response (CrCl: 30–59 mL/min); MRrenal: minor renal response (CrCl 15–29 mL/min); NR: no response.
We evidenced a higher proportion of renal recovery in patients under 65 years but it was not statically significant. In the univariate analysis the use of IHD (P = .007), diuresis maintenance (P = .01) and a major degree of proteinuria (P = .002) were associated with renal recovery (Table 2). We observed that in the group of patients that achieved dialysis independence time to treatment initiation was shorter, although it did not reach statistical significance (P = .064).
In the univariable analysis calcium levels were higher in patients with hemodialysis independence (P = .027), however this significant difference was lost in the multivariate adjusted analysis.
Surprisingly, proteinuria had also a reverse relationship to what we would expect to find. Patients with major degree of proteinuria presented better renal outcome, the opposite of what has been previously published [9]. Thus, we surmise that the proteinuria data may be in part related to the volume of the diuresis. For this reason, this variable was not been included in the multivariate analysis.
Furthermore, we performed a logistic regression analysis to assess the independent risk factors for renal recovery, and we evidenced that the group of patients that underwent IHD had a statistically significant higher proportion of renal recovery function than the group of patients treated with standard dialysis regimen (P = .014) (Table 3). This analysis was adjusted by age, Napierian logarithm transformation of time of initiation of target MM treatment, calcium levels and the presence of diuresis. As mentioned above, proteinuria was not included in the analysis.
Table 3:
Predictors of renal recovery.
| β (95% CI) | HR | R | R2 | P-value | |
|---|---|---|---|---|---|
| IHD | −3.09 (1.85–260.65) | 0.045 | 0.329 | 0.446 | .014 |
Independent predictors of renal recovery, adjusted by age, diuresis, calcium, Neperian logarithm of time to MM target therapy initiation.
CI: confidence interval.
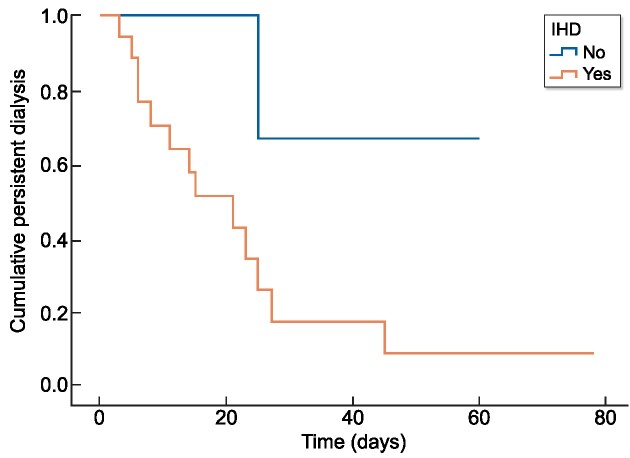
There was a statistically significant association between dialysis independence and the use of IHD as is shown in Fig. 2 (P = .045). In the Cox analysis, we observed a positive effect of FLC removal by IHD in renal recovery (P = .024) (Table 4). In this case we also adjusted the model with the same variables described in logistic regression.
Figure 2:
Actuarial survival curves of persistent dialysis according to IHD.
Table 4:
Cox analysis of impact of IHD.
| HR (95% CI) | P-value | |
|---|---|---|
| IHD | 0.183 (0.06–0.918) | .024 |
Independent predictors of renal recovery, adjusted by age, diuresis, calcium, Neperian logarithm of time to MM target therapy initiation.
P is in bold type due to be less than 0.05.
CI: confidence interval.
Hematological response and overall survival
At 3 months of follow-up there were no differences between the two groups (P = .689) regarding the hematological response at 3 months, overall mortality and overall survival. We also did not find differences in hematological response after the first cycle of target MM treatment in order to evaluate a rapid and deep hematologic response among groups.
DISCUSSION
We performed a multicentric retrospective study that included 25 patients with severe AKI related to MM. All of them required treatment with hemodialysis. IHD was performed with the intention of early reduction of FLC or received a standard dialysis according to the regular clinical practice of each center. The aim of the study was to evaluate the clinical impact of early removal of FLC. We observed that patients who received IHD achieved better renal outcome. In addition, the presence of preserved diuresis and an early initiation of targeted therapy against plasma cells may also play an important role in a better renal prognosis.
During the last decade, previously published studies have suggested than FLC reduction may improve renal survival [10, 11]. Until the use of new agents such as the proteasome inhibitor bortezomib, patients with dialysis-dependent AKI due to multiple myeloma had a poor overall survival [9]. The use of bortezomib-based treatments achieved up to 50% of dialysis independence with a medium time to event of 217 days [5, 16]. In our study dialysis independence was reached at a medium time of 21 days. We hypothesize that if circulating FLC can be quickly decreased by IHD, persistent kidney damage could be avoided and this would help an early recovery of renal function.
Dimopoulos et al. only explained renal response with total of patients according to the requirement of dialysis, and data about the degree of renal function in the follow-up were not shown [5]. In our study, patients that became dialysis independent had medium creatinine at 6 months follow-up of 1.75 mg/dL. We want to highlight the importance of recovering renal function besides acquiring dialysis independence as the absence of recovery is associated with superimposed cardiovascular morbidity and mortality [17, 18].
Regarding the use of IHD, there is scarce evidence supporting this regime and it is controversial. Hutchinson et al. used IHD with high cut-off membranes, and in non-control studies seemed to improve renal function recovery when FLC reduction was started within the first 21 days after the diagnosis [1, 10, 11]. More recently two studies, EuLITE (NCT00700531) and MYRE (NCT01208818), showed that the renal recovery rate was not achieved as reported from previous studies [19, 20]. Despite minor difference in scheme of IHD between the two studies, at 3 months these patients were not able to obtain better renal recovery rates than in group of standard dialysis. The MYRE study seemed to show a better renal evolution at 6 and 12 months but a statistically significant difference could not be demonstrated [19, 20]. This conclusion is in agreement with a recent metanalysis about the use of high cut-off. Tarragón et al. revised five studies where the use of these membranes was not associated with renal recovery despite of achieving FLC reduction [21]. Moreover, IHD with high cut-off membranes was associated with more albumin loss, FLC removal failure due to aggregates and higher costs [22, 23].
Other types of membranes have shown good results in efficient FLC removal [24–26]. Sens et al. demonstrated that IHD with PMMA membranes is associated with effective reduction of FLC and renal recovery [24]. Afterwards, in a previous retrospective study comparing two cohorts of patients who underwent IHD with PMMA, the hematological response was identified as a positive prognostic variable for renal recovery, independent of IHD [27].
In our study, we were not able to find any difference between hematological response and the use of bortezomib. In patients dependent on dialysis, FLC level below 500 mg/dL after the first cycle of chemotherapy has been described as an independent factor for renal recovery [20, 28]. Heavy amounts of Bence Jones proteinuria (>2 g/day) have been associated with increased risk of cast nephropathy [9]. In addition, it was surprising that the group without renal function recovery had a lower degree of proteinuria. Diuresis was less preserved in the group of patients who remained dialysis dependent (three patients, 27.3%). Since the amount of proteinuria in 24 h depends on the volume of diuresis, an explanation for finding less proteinuria in the group with dialysis dependence could be its lower output diuresis. Another fact is that patients who did not undergo IHD, this parameter was not controlled, and the moment of urine collection was not homogeneous. Taking all of this together, it seems that the patients’ diuresis output could reflect the degree of kidney damage. According to this, two studies had been published evaluating the prognosis value of renal biopsy. In the subgroup of patients that responded to MM treatment, the number of casts was an independent factor for renal recovery [29, 30]. In our study, the group with better renal outcome maintained preserved diuresis. It would be reasonable to think that the number of casts might be in part related to the volume of diuresis. To the best of our knowledge, there are no studies focused on oliguria as a risk factor for poor renal prognosis in MM patients. Taking this into account, in the future maintaining the diuresis in MM patients may be a cornerstone in the treatment.
On the other hand, despite the fact that the group of patients with better renal outcome are younger than patients that remain on hemodialysis, there were no differences between proportion of patients older than 65 years between groups. In fact, the age of our patients did not affect in the recovery of renal function.
Another factor to consider is the presence of previous chronic kidney disease. This data were only available in half of the patients since these patients were not previously followed up for any known kidney pathology. Thus, we should probably assume they had previous normal kidney function.
Renal biopsy should be performed whenever possible, but we considered that it should not delay chemo-immunotherapeutic treatment. Clinical suspicion, high FLC levels and less than 25% of urinary albumin excretion strongly suggest cast nephropathy as a cause of AKI [15]. In this group of patients, the most important prognostic factor is speed and depth of response to targeted MM therapy. We did not find differences in the quality of hematologic response, but there were better renal outcomes when prompt treatment was started. To improve the renal and patient prognosis of AKI-related MM it is recommended to consider this situation a medical urgency [31]. The main objective of FLC removal is to decrease the tumor burden as soon as possible, and IHD is a therapy intended to give support during the downtime of targeted MM therapy.
Our study has important limitations. First, this was a retrospective study where variables were not controlled and the sample size is small. Another limitation is diuresis data, since proteinuria was collected at diagnosis but not at the same time, and some patients were anuric. Although not significant, the difference regarding the time on target therapy in the IHD group (4 days) vs the standard HD group (26 days) is also a limitation of our study. In some cases, FLC levels during the first weeks after diagnosis were not available in medical records, especially in patients that performed standard dialysis, probably because this does not change the therapeutic attitude. In addition, severe AKI associated with MM is a rare pathology, and for this reason the scientific contrasted evidence-based knowledge has been scarce. We think that the lack of evidence is the main reason for treating patients in the past with HFR for FLC removal instead of PMMA membranes. After observed FLC removal failure due to aggregates, we switch to PMMA membranes. Another limitation is that in only 44% of patients was a renal biopsy done, so we cannot exclude other types of associated monoclonal gammopathy such as amyloidosis, vascular nephropathy, severe interstitial fibrosis or tubular atrophy.
Importantly, the achievement of dialysis independence improves quality of patients’ lives and reduces mortality. This also leads to a significant decrease in health costs secondary to RRT.
In conclusion, the role of FLC removal in severe AKI patients with MM still remains controversial, and further studies are required. We found a statistical significance between the use of IHD regime and renal recovery in myeloma kidney. Patients who reached dialysis independence (14 out of 25) did it at a median time of 21 days, which is significantly earlier than reported. We postulate that this may be in part related to the prompt initiation of antimyeloma therapy in combination with IHD. To our knowledge, this is the first study that correlates diuresis maintenance and renal recovery.
Supplementary Material
ACKNOWLEDGEMENTS
We would like to thank sincerely the investigators who participated in this trial.
Contributor Information
Natàlia Ramos Terrades, Department of Nephrology, Hospital Vall d'Hebron, Barcelona, Spain.
Alicia Senin, Hospital Duran i Reynalds, ICO, Hospitalet, Spain.
Maria A Azancot, Department of Nephrology, Hospital Vall d'Hebron, Barcelona, Spain.
Mercedes Gironella, Department of Hematology, Hospital Vall d'Hebron, Barcelona, Spain.
Nestor Toapanta, Department of Nephrology, Hospital Vall d'Hebron, Barcelona, Spain.
Sheila Bermejo, Department of Nephrology, Hospital Vall d'Hebron, Barcelona, Spain.
Lucia Martin, Department of Hematology, Hospital Vall d'Hebron, Barcelona, Spain.
Fernando Caravaca-Fontán, Department of Nephrology, Hospital 12 de Octubre, Madrid, Spain.
Clara Cuellar, Department of Hematology, Hospital 12 de Octubre, Madrid, Spain.
Joaquin Martínez-Lopez, Department of Hematology, Hospital 12 de Octubre, Madrid, Spain.
Eva Rodríguez, Department of Nephrology, Hospital del Mar, Barcelona, Spain.
Oriol Bestard, Department of Nephrology, Hospital Vall d'Hebron, Barcelona, Spain.
Maria Jose Soler, Department of Nephrology, Hospital Vall d'Hebron, Barcelona, Spain.
FUNDING
This research has not received any funding.
AUTHORS’ CONTRIBUTIONS.
N.R.T. contributed to the research idea and study design. N.R.T., A.S., F.C.-F., C.C., J.M.-L. and E.R. were responsible for data collection. N.R.T., A.S., M.A.A., M.J.S. and O.B. contributed to data analysis. Each author contributed in critical revision during manuscript drafting.
DATA AVAILABILITY STATEMENT
The datasets generated and analysed during the current study are available from the corresponding authors on reasonable request.
CONFLICT OF INTEREST STATEMENT
M.J.S. is former Editor-in-Chief of CKJ and reports honorarium for conferences, consulting fees and advisory boards from AstraZeneca, NovoNordsik, Fresenius, Vifor, Bayer, Mundipharma, Ingelheim Lilly, Jansen, ICU Medical, Travere Therapeutics and Boehringer outside the submitted work. S.B. reports honorarium for conferences, consulting fees and advisory boards from AstraZeneca, Boehringer and Mundipharma outside the submitted work. N.R.T., A.S., M.A.A., M.G, N.T., L.M., F.C.-F, C.C., J.M.-L., E.R. and O.B. declare no conflicts of interest.
REFERENCES
- 1. Hutchison CA, Bladé J, Cockwell Pet al. Novel approaches for reducing free light chains in patients with myeloma kidney. Nat Rev Nephrol 2012;8:234–43. 10.1038/nrneph.2012.14. [DOI] [PubMed] [Google Scholar]
- 2. Haynes RJ, Read S, Collins GPet al. Presentation and survival of patients with severe acute kidney injury and multiple myeloma: a 20-year experience from a single centre. Nephrol Dial Transplant 2010;25:419–26. [DOI] [PubMed] [Google Scholar]
- 3. Dimopoulos MA, Sonneveld P, Leung Net al. International Myeloma Working Group recommendations for the diagnosis and management of myeloma-related renal impairment. J Clin Oncol 2016;34:1544–57. 10.1200/JCO.2015.65.0044. [DOI] [PubMed] [Google Scholar]
- 4. Leung N, Gertz MA, Zeldenrust SRet al. Improvement of cast nephropathy with plasma exchange depends on the diagnosis and on reduction of serum free light chains. Kidney Int 2008;73:1282–8. 10.1038/ki.2008.108. [DOI] [PubMed] [Google Scholar]
- 5. Dimopoulos MA, Roussou M, Gavriatopoulou Met al. Bortezomib-based triplets are associated with a high probability of dialysis independence and rapid renal recovery in newly diagnosed myeloma patients with severe renal failure or those requiring dialysis. Am J Hematol 2016;91:499–502. 10.1002/ajh.24335. [DOI] [PubMed] [Google Scholar]
- 6. Dimopoulos MA, Terpos E, Chanan-Khan Aet al. Renal impairment in patients with multiple myeloma: a consensus statement on behalf of the International Myeloma Working Group. J Clin Oncol 2010;28:4976–84. 10.1200/JCO.2010.30.8791. [DOI] [PubMed] [Google Scholar]
- 7. Yadav P, Cockwell P, Cook Met al. Serum free light chain levels and renal function at diagnosis in patients with multiple myeloma. BMC Nephrol 2018;19:178. 10.1186/s12882-018-0962-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Roussou M, Kastritis E, Migkou Met al. Treatment of patients with multiple myeloma complicated by renal failure with bortezomib-based regimens. Leuk Lymphoma 2008;49:890–5. 10.1080/10428190801930506. [DOI] [PubMed] [Google Scholar]
- 9. Hutchison CA, Batuman V, Behrens Jet al. The pathogenesis and diagnosis of acute kidney injury in multiple myeloma. Nat Rev Nephrol 2011;8:43–51. 10.1038/nrneph.2011.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hutchison CA, Cockwell P, Reid Set al. Efficient removal of immunoglobulin free light chains by hemodialysis for multiple myeloma: in vitro and in vivo studies. J Am Soc Nephrol 2007;18:886–95. 10.1681/ASN.2006080821. [DOI] [PubMed] [Google Scholar]
- 11. Hutchison CA, Cockwell P, Stringer Set al. Early reduction of serum-free light chains associates with renal recovery in myeloma kidney. J Am Soc Nephrol 2011;22:1129–36. 10.1681/ASN.2010080857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Testa A, Dejoie T, Lecarrer Det al. Reduction of free immunoglobulin light chains using adsorption properties of hemodiafiltration with endogenous reinfusion. Blood Purif 2010;30:34–6. 10.1159/000316684. [DOI] [PubMed] [Google Scholar]
- 13. Chanan-Khan AA, Kaufman JL, Mehta Jet al. Activity and safety of bortezomib in multiple myeloma patients with advanced renal failure: a multicenter retrospective study. Blood 2007;109:2604–6. 10.1182/blood-2006-09-046409. [DOI] [PubMed] [Google Scholar]
- 14. Clark WF, Stewart AK, Rock GAet al. Plasma exchange when myeloma presents as acute renal failure: a randomized, controlled trial. Ann Intern Med 2005;143:777–84. 10.7326/0003-4819-143-11-200512060-00005. [DOI] [PubMed] [Google Scholar]
- 15. Leung N, Gertz M, Kyle RAet al. Urinary albumin excretion patterns of patients with cast nephropathy and other monoclonal gammopathy-related kidney diseases. Clin J Am Soc Nephrol 2012;7:1964–8. 10.2215/CJN.11161111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ying WZ, Wang PX, Aaron KJet al. Immunoglobulin light chains activate nuclear factor-κB in renal epithelial cells through a Src-dependent mechanism. Blood 2011;117:1301–7. 10.1182/blood-2010-08-302505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Matsushita K, Coresh J, Sang Yet al. Estimated glomerular filtration rate and albuminuria for prediction of cardiovascular outcomes: a collaborative meta-analysis of individual participant data. Lancet Diabetes Endocrinol 2015;3:514–25. 10.1016/S2213-8587(15)00040-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Levey AS, de Jong PE, Coresh Jet al. The definition, classification, and prognosis of chronic kidney disease: a KDIGO Controversies Conference report. Kidney Int 2011;80:17–28. 10.1038/ki.2010.483. [DOI] [PubMed] [Google Scholar]
- 19. Hutchison CA, Cockwell P, Moroz Vet al. High cutoff versus high-flux haemodialysis for myeloma cast nephropathy in patients receiving bortezomib-based chemotherapy (EuLITE): a phase 2 randomised controlled trial. Lancet Haematol 2019;6:e217–28. 10.1016/S2352-3026(19)30014-6. [DOI] [PubMed] [Google Scholar]
- 20. Bridoux F, Carron PL, Pegourie Bet al. Effect of high-cutoff hemodialysis vs conventional hemodialysis on hemodialysis independence among patients with myeloma cast nephropathy: a randomized clinical trial. JAMA 2017;318:2099–110. 10.1001/jama.2017.17924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tarragón B, Ye N, Gallagher Met al. Effect of high cut-off dialysis for acute kidney injury secondary to cast nephropathy in patients with multiple myeloma: a systematic review and meta-analysis. Clin Kidney J 2021;14:1894–900. 10.1093/ckj/sfaa220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Harding S, Provot F, Beuscart JBet al. Aggregated serum free light chains may prevent adequate removal by high cut-off haemodialysis. Nephrol Dial Transplant 2011;26:1438. [DOI] [PubMed] [Google Scholar]
- 23. Berni Wennekers A, Martín Azara MP, Dourdil Sahun Vet al. Thirteen treated of acute renal failure secondary to multiple myeloma with high cut off filters. Nefrologia 2016;36:418–26. [DOI] [PubMed] [Google Scholar]
- 24. Sens F, Chaintreuil D, Jolivot Aet al. Effectiveness of IHD with adsorptive PMMA membrane in myeloma cast nephropathy: a cohort study. Am J Nephrol 2017;46:355–63. 10.1159/000481461. [DOI] [PubMed] [Google Scholar]
- 25. Fabbrini P, Finkel K, Gallieni Met al. Light chains removal by extracorporeal techniques in acute kidney injury due to multiple myeloma: a position statement of the Onconephrology Work Group of the Italian Society of Nephrology. J Nephrol 2016;29:735–46. 10.1007/s40620-016-0347-9. [DOI] [PubMed] [Google Scholar]
- 26. Santoro A, Grazia M, Mancini E.. The double polymethylmethacrylate filter (DELETE system) in the removal of light chains in chronic dialysis patients with multiple myeloma. Blood Purif 2013;35 Suppl 2:5–13. 10.1159/000350837. [DOI] [PubMed] [Google Scholar]
- 27. Hudier L, Decaux O, Haddj-Elmrabet Aet al. Intensive haemodialysis using PMMA dialyser does not increase renal response rate in multiple myeloma patients with acute kidney injury. Clin Kidney J 2018;11:230–5. 10.1093/ckj/sfx079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bridoux F, Leung N, Belmouaz Met al. Management of acute kidney injury in symptomatic multiple myeloma. Kidney Int 2021;99:570–80. 10.1016/j.kint.2020.11.010. [DOI] [PubMed] [Google Scholar]
- 29. Ecotière L, Thierry A, Debiais-Delpech Cet al. Prognostic value of kidney biopsy in myeloma cast nephropathy: a retrospective study of 70 patients. Nephrol Dial Transplant 2016;31:64–72. [DOI] [PubMed] [Google Scholar]
- 30. Royal V, Leung N, Troyanov Set al. Clinicopathologic predictors of renal outcomes in light chain cast nephropathy: a multicenter retrospective study. Blood 2020;135:1833–46. 10.1182/blood.2019003807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wirk B. Renal failure in multiple myeloma: a medical emergency. Bone Marrow Transplant 2011;46:771–83. 10.1038/bmt.2011.8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated and analysed during the current study are available from the corresponding authors on reasonable request.