Abstract
Background
Bexagliflozin is a sodium‐glucose cotransporter 2 (SGLT2) inhibitor. A pilot study has shown that bexagliflozin can decrease dependence on exogenous insulin in cats with diabetes mellitus (DM).
Objective
To evaluate the safety and effectiveness of bexagliflozin as a monotherapy for DM in previously untreated cats.
Animals
Eighty‐four client‐owned cats.
Methods
Historically controlled prospective open‐label clinical trial. Cats were dosed PO with 15 mg bexagliflozin once daily for 56 days, with a 124‐day extension to evaluate safety and treatment effect durability. The primary endpoint was the proportion of cats experiencing a decrease in hyperglycemia and improvement in clinical signs of hyperglycemia from baseline on day 56.
Results
Of 84 enrolled cats, 81 were evaluable on day 56, and 68 (84.0%) were treatment successes. Decreases in mean serum glucose, fructosamine, and β‐hydroxybutyrate (β‐OHB) concentrations were observed, and investigator assessments of cat neurological status, musculature, and hair coat quality improved. Owner evaluations of both cat and owner quality of life were favorable. The fructosamine half‐life in diabetic cats was found to be 6.8 days. Commonly observed adverse events included emesis, diarrhea, anorexia, lethargy, and dehydration. Eight cats experienced serious adverse events, 3 of which led to death or euthanasia. The most important adverse event was euglycemic diabetic ketoacidosis, diagnosed in 3 cats and presumed present in a fourth.
Conclusion and Clinical Importance
Bexagliflozin decreased hyperglycemia and observed clinical signs in cats newly diagnosed with DM. As a once‐daily PO medication, bexagliflozin may simplify management of DM in cats.
Keywords: feline, field study, fructosamine half‐life, hyperglycemia, SGLT2
Abbreviations
- ANOVA
analysis of variance
- BGC
blood glucose curve
- CI
confidence interval
- DM
diabetes mellitus
- FDA
Food and Drug Administration
- FPL
feline pancreatic lipase
- IGF‐1
insulin‐like growth factor 1
- IVP
investigational veterinary product
- SAE
serious adverse event
- SDMA
symmetrical dimethylarginine
- SGLT2
sodium‐glucose cotransporter 2
- T2DM
type 2 diabetes mellitus
- T4
thyroxine
- VeDDRA
Veterinary Dictionary for Drug Regulatory Activities
- β‐OHB
β‐hydroxybutyrate
1. INTRODUCTION
Active (concentrative) reuptake of glucose by the kidney is principally performed by sodium‐glucose cotransporter 2 (SGLT2), an integral membrane protein responsible for the recovery of >90% of glucose from the glomerular filtrate of healthy animals. 1 , 2 Mice and humans genetically deficient for SGLT2 activity exhibit good health and do not experience hypoglycemia despite severe glucosuria. 2 , 3
Pharmacological inhibitors of SGLT2 have been found to improve glycemic control in humans with type 2 diabetes mellitus (T2DM), a disease characterized by insulin resistance. 4 Administration of SGLT2 inhibitors to healthy humans results in a loss to urine of approximately half of the glomerular flux of glucose, with the remainder reabsorbed by the increased compensatory action of sodium‐glucose cotransporter 1. 5 , 6 Additional benefits of SGLT2 inhibitor treatment reported in studies of humans include weight loss, a decrease in systolic blood pressure, decreased susceptibility to major adverse cardiovascular events, and a decrease in the rate of progression of chronic kidney disease. 7 , 8 Inhibition of SGLT2 in healthy and diabetic humans does not result in hypoglycemia although episodes of hypoglycemia have been observed with coadministration of other antidiabetic agents, particularly insulin, and sulfonylureas. 8
Diabetes mellitus (DM) in cats shares some attributes with T2DM in humans. Both diseases are associated with obesity and respond to changes in diet. Important causal genetic factors have not been identified for either disease in either species, with the exception of a few rare alleles. 9 , 10 , 11 , 12 In both diseases, but more prominently in the cat, pancreatic amyloidosis is observed. 13 The amyloid deposits result from the aggregation and precipitation of amylin, a β‐cell peptide hormone co‐secreted with insulin. 13 , 14 , 15 , 16 , 17 In cats, DM is associated with amyloidosis of the exocrine pancreas as well as a destruction of β‐cells that is more typical of advanced disease in humans. 13 , 15 , 16 , 17 Cats with DM usually are presented in a state of severe disease, with severe hyperglycemia and a disease progression similar to that of late‐stage T2DM in humans. 18 In both species, diabetes also is observed with acromegaly, a condition associated with marked insulin resistance. 19 , 20
A pilot study has reported the utility of bexagliflozin as an adjunct to insulin for the management of DM in cats poorly controlled by insulin alone. 21 Bexagliflozin was found to decrease the insulin dose requirement and decrease mean blood glucose concentration measured by a serial inpatient sampling every 2 hours for 10 hours. 21 However, additional studies have been needed to establish whether bexagliflozin can be used to treat diabetes in cats newly diagnosed with the disease.
Our principal hypothesis was that bexagliflozin veterinary tablets, 15 mg, would be safe and effective for the management of DM in cats when administered once daily as monotherapy.
2. MATERIALS AND METHODS
2.1. Animals
The study enrolled client‐owned cats that had been newly diagnosed with DM. To be eligible a cat was required to exhibit: (i) 2 separate fasting (≥6 hours) blood glucose concentrations >250 mg/dL, (ii) glucosuria, (iii) a serum fructosamine concentration >358 μmol/L, and (iv) documented history of ≥1 of the following clinical signs: polyuria, polydipsia, polyphagia, or weight loss. Cats that were pregnant, lactating, in estrus, or intended for breeding were excluded, as were cats with heart failure, advanced chronic kidney disease (International Renal Interest Society stage 3 or 4), hyperthyroidism, hypertension, neoplasia, a history of feline idiopathic cystitis, major infectious processes (other than treatable acute urinary tract infections), or any other conditions that would, in the opinion of the clinician, interfere with obtaining or monitoring blood samples, treatment administration or assessment of effectiveness of the investigational veterinary product (IVP). Cats exhibiting inappetence in the previous week, or with serum with β‐hydroxybutyrate (β‐OHB) concentration ≥37.0 mg/dL were not eligible. Cats with β‐OHB concentration >25.0 mg/dL but <37.0 mg/dL could be enrolled if they were not inappetent and had no history of either renal disease or acidosis. Cats with insulin‐like growth factor 1 (IGF‐1) higher than the upper limit of the laboratory reference interval (92 nmol/L) could be enrolled but were excluded from the endpoint analysis. Cats with a history of urinary tract surgery or with planned elective surgeries requiring general anesthesia during the study period were ineligible, with the exception of dental surgery after day 56. Cats that had a history of ongoing pharmacologic management of DM were excluded. Previously treated diabetic cats that were determined to be in remission, had not received insulin for at least 3 months, and met the enrollment criteria could be enrolled, but none of the cats in the study met these criteria. Cats with baseline alkaline phosphatase or alanine aminotransferase activity 3 times the upper limit of normal or with a feline pancreatic lipase (FPL) immunoassay concentration >5.3 μg/L also were excluded from the study. If an enrolled cat was determined to have an increased IGF‐1 concentration at screening or had received corticosteroids or progestogens between day 0 and 56, the cat was evaluated for safety but not effectiveness. Enrollment continued until at least 80 cases evaluable for the effectiveness endpoint had been enrolled in the study.
2.2. Study design
The trial was a prospective, open‐labeled, historically controlled, multisite pivotal clinical field study conducted at 27 private practices in the United States and Canada between July 1, 2019, and March 22, 2021, in compliance with regulatory guidance and following a statement of concurrence with the protocol design by the United States Food and Drug Administration (FDA) Center for Veterinary Medicine. The first visit occurred when the owner initially presented the cat for treatment. If the owner provided informed consent and the cat met the eligibility criteria, the owner was instructed to return to the clinic with the cat for a baseline in‐clinic 8‐hour blood glucose curve (BGC) evaluation consisting of 5 glucometer measurements at 2‐hour intervals. At the end of the visit, the owner was provided with IVP and instructions for dosing. Clinic visits were scheduled at 14, 28, 56, 84, 112, 140, and 180 days (with a target window of ±3 days) after treatment initiation for blood and urine collection and owner and investigator assessment of effectiveness, tolerability, and safety. All scheduled visits included an in‐clinic 8‐hour BGC evaluation. Treatment was initiated immediately after the collection of the initial sample for the day 0 BGC and consisted of bexagliflozin veterinary tablets, 15 mg, administered PO once daily. It was recommended, but not required, that the cat consume a low‐carbohydrate diet while enrolled in the study.
Eight‐hour BGC sampling was conducted at every visit starting with day 0. Blood glucose concentration was measured using a validated glucometer (AlphaTRAK 2, Zoetis).
History and physical examination were completed during all scheduled visits and, if possible, upon withdrawal from the study if the cat was removed from the study. Blood pressure, presence of polyuria, polydipsia, or polyphagia, body weight, and body condition score (using the Purina body condition score chart for cats) were assessed and recorded.
Blood samples for CBC, serum chemistry analysis, fructosamine, specific pancreatic lipase, symmetric dimethylarginine (SDMA), and β‐OHB concentration were collected at screening and every scheduled visit after initiation of treatment. Urine samples also were collected at each visit for routine urinalysis, including urine ketones and bacterial culture. Cystocentesis was the preferred method of urine sampling. Serum measurements of total T4 and IGF‐1 were performed at the screening visit only. Samples were submitted to a single commercial laboratory for any given analyte. However, measurement of fructosamine concentration was complicated somewhat by a change in business organization of the central laboratory during the study that resulted in sample analysis at a different site with a different reference interval. The site change took place after all cats had completed the day 56 endpoint visit. Analyses that included both sites also were expressed as a percent of the reference interval, but no changes in conclusions resulted.
At each visit, the investigator assessed hair coat quality, muscle mass, and neurological status, assigning a rating of 0 to excellent, 1 to good, 2 to fair, and 3 to poor. The specific criteria for the scoring system are presented in the Supporting Information. The P values for the change from day 0 were calculated using the Fisher‐Freeman‐Halton test. Significance thresholds were not assigned because the results were not prespecified endpoints subject to a false discovery rate correction. On day 56, before the visit, the owners were requested to complete a questionnaire that asked them to evaluate the clinical signs of polyuria, polydipsia, and polyphagia. Each clinical sign was scored as significantly improved, somewhat improved, unchanged, or worsened, compared to day 0. If the owner had not completed the questionnaire before visiting the site, the owner was asked to complete it before interacting with the investigator or other staff who might provide feedback on the condition of the cat.
On days 0 and 56, owners were asked to complete a quality of life questionnaire adapted from a previously validated instrument developed to evaluate life quality for owners of cats with diabetes. 22 Questions referring to insulin were reworded to reflect a PO agent as described in the Supporting Information. The score was calculated as the arithmetic average of individual scores constructed by multiplying the owner‐ascribed importance of a response by the frequency with which the response occurred. Lower scores reflect greater adverse impact of the disease on owner quality of life. 22 Importance was scored as “very important” (4), “important” (3), “moderately important” (2), “low importance” (1), or “not at all important” (0), whereas frequency was scored as “all the time” (−3), “often” (−2), “occasionally” (−1), or “rarely” (0). The P values were determined by repeated measures analysis of variance (ANOVA), with visit as the repeated measure.
Effectiveness was determined at the day 56 visit and was based on the change from baseline in serum fructosamine concentration, mean BGC results over 8 hours, change in body weight, and owner assessments of clinical signs.
2.3. Statistical analysis
Statistical analyses were performed using SAS 9.4 (SAS Institute, Cary, North Carolina). Each cat was classified as a treatment success or failure on day 56. Success was defined as attainment of glycemic control (mean of 8 hour in‐clinic BGC < 250 mg/dL or serum fructosamine concentration <358 μmol/L) and improvement from baseline of at least 1 clinical sign (polyuria, polydipsia, polyphagia, or weight loss). Body weight was deemed to have improved if the weight on day 56 was ≥ the weight at baseline.
The safety population included all cats that received at least 1 dose of bexagliflozin. The effectiveness population consisted of cats that had documented baseline and day 56 assessments and that were without a presumptive diagnosis of acromegaly or clinically relevant protocol deviations. Cats receiving corticosteroids or progestogens before day 56 were to be assessed for safety, but excluded from the effectiveness population. Cases from sites enrolling a single cat did not qualify for inclusion in the analysis of effectiveness.
Animals withdrawn from the study for any reason, including owner dissatisfaction of any sort, were considered treatment failures. All cats in the effectiveness population that were not defined as a treatment success were considered treatment failures.
The null hypothesis was that the lower bound of the 2‐sided 90% confidence interval (CI) calculated by the Clopper‐Pearson exact test for the proportion of successes would be ≤66%. The analysis was performed using the SAS FREQ procedure. An enrollment of approximately 80 cats without increased IGF‐1 concentration was determined to be sufficient for determining the effectiveness and safety of bexagliflozin. A minimum sample of 50 evaluable cases provided >90% power to reject the hypothesis that the response rate was <66%, given a hypothesized response rate of 80% and an alpha of 10%.
Descriptive statistics (mean, SD, minimum, median, and maximum) were calculated for body weight, in‐clinic blood glucose concentrations (mean BGC concentrations), and central laboratory fructosamine, serum glucose, and β‐OHB concentrations at each visit.
3. RESULTS
3.1. Effectiveness
A total of 206 cats were screened at 17 sites for enrollment, of which 122 were considered ineligible and 84 were enrolled, received at least 1 dose of IVP, and were assessed for safety (Figure S2). Of the 84 animals enrolled, 81 were effectiveness‐evaluable (2 had increased IGF‐1 concentration at baseline and 1 was the sole cat enrolled at the study site); 43 were male (51.2%) and 41 were female (48.8%). At enrollment, the median age was 10.8 years (range, 3.0‐18.5 years) and the median weight was 5.4 kg (range, 3.3‐11.3 kg).
Sixty‐eight of the 81 effectiveness‐evaluable cats were considered treatment successes (84.0%). The lower bound of the 2‐sided 90% CI by the Clopper‐Pearson exact test was 75.70%. Hence, the null hypothesis was rejected and the primary objective of the study was met.
Of the 13 cats that were considered treatment failures, 6 cats were removed from the study before day 56 after adverse events, 5 of which were serious adverse events (SAEs). Four cats did not meet the glycemic control criteria, but met the criteria for improvement in clinical signs, and 3 cats met the glycemic control criteria but did not meet the criteria for improvement in clinical signs. No cat failed by both glycemic control and clinical sign criteria.
Among the 75 effectiveness‐evaluable cats remaining in the study at the effectiveness endpoint visit on day 56, 9 cats did not meet the serum fructosamine concentration target but did meet the mean blood glucose concentration target and were classified as glycemic control successes. Four cats did not meet the fructosamine target or the mean blood glucose concentration target and were classified as treatment failures.
3.1.1. Blood glucose curves
The mean blood glucose concentration measured during the BCGs decreased rapidly during the 8 hours after initiation of treatment (Figure 1). At subsequent visits, the decrease in mean blood glucose concentration was maintained. The mean of the 5 blood glucose concentration determinations was 281.5 mg/dL (95% CI, 264.6‐298.5) on day 0, 143.7 mg/dL (95% CI 129.2‐158.3) on day 56, and 133.7 mg/dL (95% CI 122.7‐144.7) on day 180.
FIGURE 1.
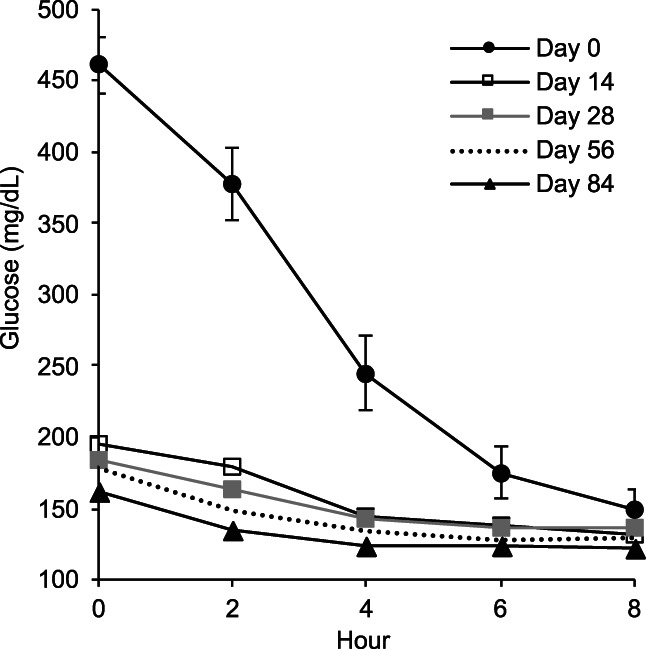
Blood glucose curves for days 0, 14, 28, 56, and 84. Mean glucose concentration in mg/dL with 95% confidence interval (CI) for day 0 curve. (CI suppressed for other days to facilitate data visualization).
3.1.2. Blood glucose concentration
The mean blood glucose concentration measured by the central laboratory (Figure 2A) was 439.8 mg/dL (95% CI, 424.2‐455.4) on day 0, 161.4 mg/dL (95% CI, 132.1‐161.5) on day 56, and 144.4 mg/dL (95% CI, 126.1‐162.6) on day 180. On day 56, the blood glucose concentration was within the laboratory normal reference interval (65‐155 mg/dL) for 43 of 75 cats (57.3%).
FIGURE 2.
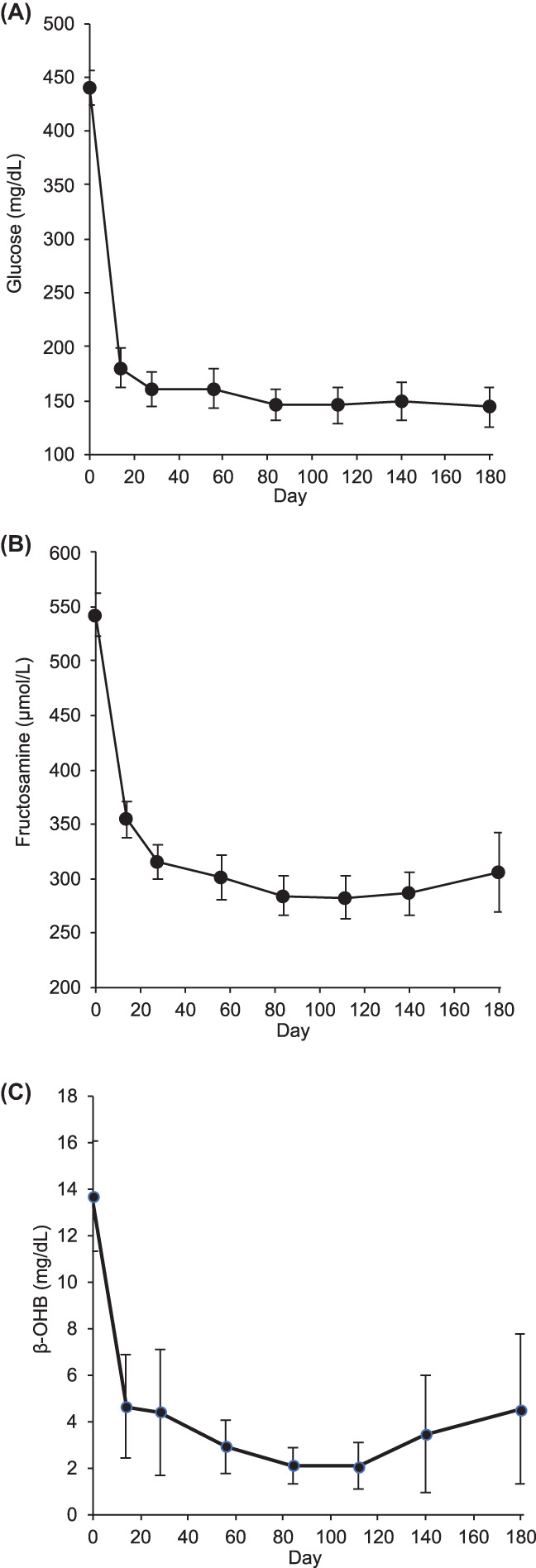
Serum glucose (A), fructosamine (B), and β‐hydroxybutyrate (β‐OHB) (C) as a function of time, plotted as means ± 95% confidence intervals.
3.1.3. Serum fructosamine concentration
The mean serum fructosamine concentration (Figure 2B) decreased from 542.5 μmol/L (95% CI, 523.0‐562.0) on day 0 to 301.3 μmol/L (95% CI, 280.4‐322.2) on day 56 and 305.6 μmol/L (95% CI, 269.1‐342.0) on day 180. The fructosamine concentration was within the laboratory normal reference interval (154‐275 μmol/L) for 34 of 75 cats (45.3%) on day 56.
3.1.4. Serum β‐OHB concentration
The β‐OHB concentration (Figure 2C) dropped rapidly from a baseline of 13.7 mg/dL (95% CI, 11.3‐16.1) to 2.91 mg/dL (95% CI, 1.8‐4.1) on day 56 and 3.21 mg/dL (95% CI, 1.3‐7.5) on day 180. On day 56, 51 of 75 cats (68.0%) were within the laboratory normal reference interval (≤1.9 mg/dL).
3.1.5. Body weight
Of the 75 cats completing the day 56 visit, 43 (57.3%) either maintained or gained weight from day 0 to day 56. A significant increase (uncorrected for multiplicity) was observed in mean body weight from day 0 to day 84 and at all subsequent visits (Figure 3).
FIGURE 3.
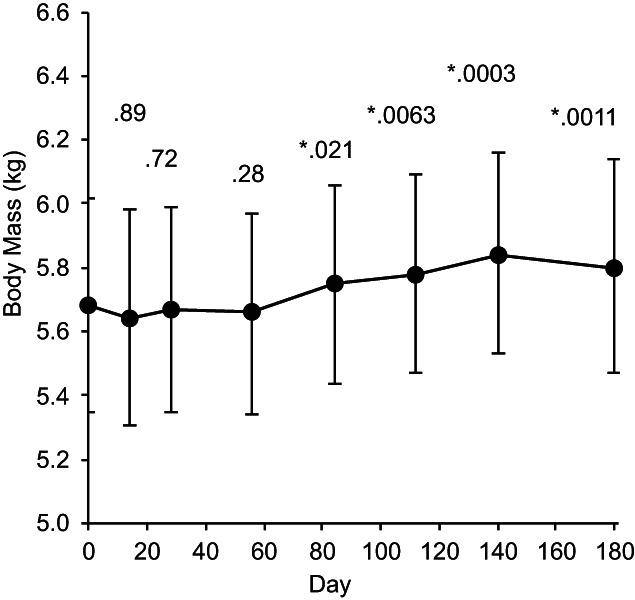
Body mass as a function of time, plotted as means ± 95% confidence intervals with P values for difference from day 0. *P < .05.
3.1.6. Clinical signs
Of the 75 effectiveness‐evaluable cats, 68 (90.7%) were found to have improved by at least 1 clinical sign of hyperglycemia (Table 1). Compared to baseline, the owner‐assessed overall quality of life of the cat indicated improvement in 65 cats (86.7%), no change in 9 (12.0%), and worsening in 1 (1.3%). The latter was the consequence of missing information that resulted in the assignment of the least favorable score to all owner assessments according to the prespecified analysis.
TABLE 1.
Owner assessment of clinical signs on day 56 compared to day 0.
| Variable | Significantly improved | Somewhat improved | Unchanged | Worsened | Success | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | n | % | |
| Polyphagia | 26 | 34.67 | 21 | 28.00 | 24 | 32.00 | 4 | 5.33 | 47 | 62.67 |
| Polydipsia | 33 | 44.00 | 28 | 37.33 | 12 | 16.00 | 2 | 2.67 | 61 | 81.33 |
| Polyuria | 29 | 38.67 | 27 | 36.00 | 17 | 22.67 | 2 | 2.67 | 56 | 74.67 |
3.1.7. Investigator evaluations
Mean investigator evaluation scores are plotted in Figure 4A (neurological signs), Figure 4B (musculature), and Figure 4C (hair coat quality). Initial scores were favorable for neurological signs and less improvement accordingly was seen. Musculature and hair coat quality showed more improvement with time in study.
FIGURE 4.
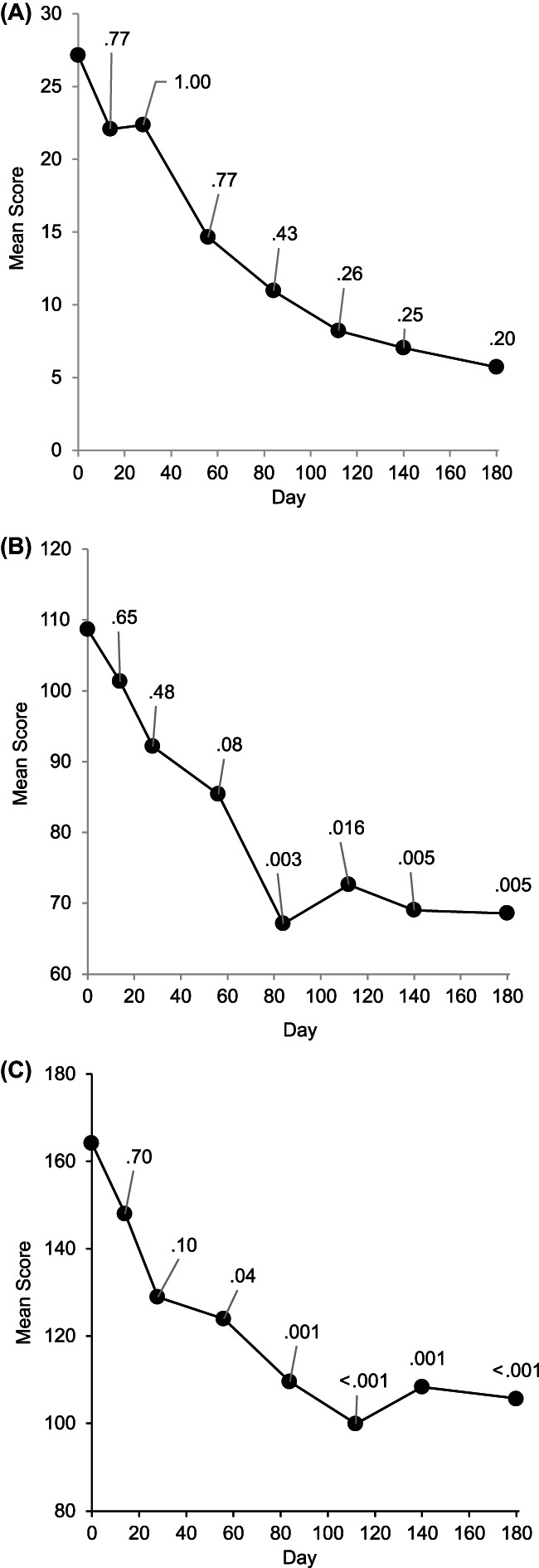
Mean investigator scores (multiplied by 100) for neurological status (A), musculature (B), and coat quality (C) as a function of time, with P values for difference from day 0 by Fisher‐Freeman‐Halton test.
3.1.8. Owner quality of life
Analysis of quality‐of‐life survey data was conducted as an exploratory endpoint. The change in score for all questions is summarized in Table S1. The mean score increased from day 0 to day 56, indicating an overall improvement in the owner quality of life (P < .0001, uncorrected for multiplicity).
3.1.9. Evaluation of fructosamine half‐life
The rapid and sustained action of bexagliflozin allowed the half‐life of serum fructosamine to be estimated as 6.8 days in diabetic cats (Supporting Information). The residual contribution of the initial fructosamine concentration to the result measured on day 56 was predicted to be 0.3%.
3.2. Safety
There were 84 cats included in the safety analysis population. Over the 6‐month study duration, 75 of the 84 cats (89.3%) in the safety analysis population accounted for 559 investigator‐reported adverse events, of which 524 were considered nonserious and 35, affecting 8 cats, were considered SAEs. Of the 559 adverse events, the investigators considered 93 to be unrelated to exposure to bexagliflozin, 263 to have an unknown relationship to exposure, 187 to have a possible relationship to exposure, and 16 to have a probable relationship to exposure. Twelve of the 84 enrolled cats (14.3%) were withdrawn before completing the 6‐month study and 70 cats were evaluated within the 3‐day window for the final 180 day visit, with 2 cats evaluated outside the prescribed window for evaluation.
Adverse events reported for ≥5% of the enrolled cats over the study duration are listed in Table 2 by the Veterinary Dictionary for Drug Regulatory Activities (VeDDRA) preferred term. The most common adverse events were vomiting, diarrhea, anorexia, lethargy, and dehydration. The majority appeared to be sporadic but some were associated with concurrent illness. All occurrences of vomiting and diarrhea were considered nonserious by investigators.
TABLE 2.
Number and percentage of enrolled animals having at least 1 adverse event, by VeDDRA preferred term.
| VeDDRA preferred term | n | % |
|---|---|---|
| Emesis | 42 | 50 |
| Diarrhea | 32 | 38 |
| Anorexia | 31 | 37 |
| Lethargy | 17 | 20 |
| Dehydration | 16 | 19 |
| Elevated pancreatic enzymes | 15 | 18 |
| Weight loss | 13 | 16 |
| Increased blood urea nitrogen or creatinine | 12 | 14 |
| Diabetes mellitus a | 10 | 12 |
| Urinary tract infection b | 10 | 12 |
| Elevated liver enzymes | 8 | 10 |
| Elevated symmetrical dimethylarginine | 6 | 7 |
| Hypokalaemia | 6 | 7 |
| Haematuria | 5 | 6 |
| Otitis externa | 5 | 6 |
| Bacterial skin infection | 4 | 5 |
| Elevated aspartate aminotransferase | 4 | 5 |
| Hypercalcaemic condition | 4 | 5 |
| Hypochloraemia | 4 | 5 |
| Hypoglycaemia | 4 | 5 |
| Inappropriate urination | 4 | 5 |
| Myopathy | 4 | 5 |
| Proteinuria | 4 | 5 |
Note: Each affected cat counted once for a given term over the 180 day course of the study. Events were classified by investigators using the Veterinary Dictionary for Drug Regulatory Activities (VeDDRA).
Indicates an investigator assessment of poor glycemic control.
All urinary tract infections were of bacterial origin.
Eight cats experienced SAEs while enrolled, and 3 of those died or were euthanized. The 3 deaths were attributed to weight loss with anemia, an unknown cause, and hepatic lipidosis. Brief summaries of the histories are presented in the Supporting Information.
Four of the 8 cats with SAEs experienced anorexia and lethargy and 3 of the 4 cats with anorexia and lethargy were diagnosed with diabetic ketoacidosis (DKA), which occurred in the absence of acute hyperglycemia. The events affecting the remaining 4 cats were observed in 1 cat each and included: poor glycemic control, constipation, and pancreatitis; dehydration and weight loss; weight loss and anemia; and dehydration with presumed DKA and pyelonephritis. One cat with diagnosed DKA experienced a progressive increase in transaminase activity, hypokalemia, and anemia despite supportive care and was euthanized. The cause of death was determined to be hepatic lipidosis.
All but 1 of the 8 cats were withdrawn from the study as a result of the SAEs. A clustering of events close to initiation of treatment was observed, with 4 of the 8 cats receiving a total of 2, 3, 3, and 5 doses before cessation of treatment. Anorexia and lethargy were present in 3 of the 4 cats, and 2 had underlying infections (urinary tract infection and presumed pyelonephritis). Because DKA can emerge in the setting of diabetes with concurrent disease, a specific etiology cannot be ascribed, but at minimum the possibility of the co‐occurrence of DKA with other diseases should be considered in the setting of bexagliflozin treatment, and anorexia or lethargy appearing shortly after initiation of treatment should be promptly investigated. In our study, DKA was diagnosed or presumed present on days 2, 3, 4, and 31. One of the cats died and the remainder were transitioned to insulin treatment.
No episodes of symptomatic hypoglycemia were observed in the study. The lowest serum glucose concentration was 54 mg/dL, recorded at a day 28 visit. No clinical signs of hypoglycemia were present.
4. DISCUSSION
In our study, bexagliflozin veterinary tablets, 15 mg, decreased blood glucose and serum fructosamine concentrations, and decreased clinical signs of DM in newly diagnosed diabetic cats when administered PO once daily. Treatment was successful for 84% of enrolled cats and measures of owner quality of life improved. Bexagliflozin was well‐tolerated by most cats with SAEs reported for 8 cats.
Management of diabetes in cats using bexagliflozin incurred minimal risk of hypoglycemia, with no symptomatic episodes of hypoglycemia observed. Nonclinical studies have shown that bexagliflozin does not produce hypoglycemia in healthy animals at doses of up to 9 tablets per day for 182 days. A low risk of hypoglycemia is consistent with reports that rodents and humans homozygous for null mutations in the gene encoding SGLT2 are euglycemic. 2 , 3
The action of bexagliflozin was apparent after administration of the first dose, as detected by a prompt and reproducible decrease in blood glucose concentration measured over the course of an 8‐hour, 5‐sample evaluation. Durable effects on mean blood glucose concentration and mean fructosamine concentration were observed over the 6‐month span of the study. Serum β‐OHB concentration decreased for the study population as a whole.
Decrease in the clinical signs of hyperglycemia was scored by a composite endpoint of 4 categorical outcomes, 3 of which were based on subjective owner assessments of the change in polydipsia, polyuria, or polyphagia from baseline and 1 of which was based on investigator measurement of body weight. The number of cats that were classified as achieving treatment success was higher for polydipsia and polyuria than for polyphagia and weight gain.
Improvement in the clinical signs of hyperglycemia was expected to be confounded by their overlap with the mechanistic consequences of SGLT2 inhibition. Upon administration to healthy animals, bexagliflozin produces severe glucosuria with compensatory increases in food and water consumption, creating the clinical impression of an animal with severe DM. Although an initial increase in clinical signs would be expected in diabetic cats, for the animals in our study the decrease in blood glucose concentration induced by SGLT2 inhibition apparently resulted in a lower glucose efflux overall, with attendant improvement in the clinical signs.
Polyphagia was the clinical sign least likely to show improvement, consistent with previous studies in other species indicating that the caloric wasting induced by SGLT2 inhibition frequently gives rise to compensatory hyperphagia.
Investigator ratings showed that neurological signs, musculature, and coat quality improved throughout the study, whereas owner quality of life, as reflected by responses to a previously validated instrument 22 adapted for this context, improved by the end of the study.
Two cats presented at screening were found to have increased IGF‐1 concentrations suggestive of acromegaly, after enrollment. Acromegaly in cats is caused by growth hormone overproduction from an anterior pituitary adenoma, which causes increased IGF‐1 expression and insulin‐resistant DM. Because SGLT2 inhibitors have an insulin‐independent mechanism of action, they may be useful for the treatment of acromegaly‐associated DM, and a small study in humans has supported this view. 20 Because of the distinct character of the DM attributable to acromegaly, cats with high IGF‐1 were not included in the effectiveness evaluation. However, both cats with high baseline IGF‐1 met the glycemic and clinical sign criteria for success and both cats remained enrolled for the 6‐month duration of the study.
The SAE most plausibly attributed to bexagliflozin treatment in our study was DKA, a rare complication of SGLT2 inhibitor treatment in humans. 23 , 24 The risk of ketoacidosis in human diabetic patients is increased by decreased carbohydrate intake, alcohol abuse, intercurrent illness, medication nonadherence, dehydration, or surgery. 25 , 26 In patients treated with SGLT2 inhibitors, recognition of ketoacidosis may be delayed because the characteristic high blood glucose concentration associated with ketoacidosis is masked by inhibitor action. 22 In our study, ketoacidosis was diagnosed in 3 cats and presumed present in another cat and in all cases was observed in the context of good glycemic control. In humans, the corresponding clinical condition often is described as euglycemic DKA. Clinical signs in our study included anorexia and dehydration, and some of the cats had concurrent illness. All but 1 responded to treatment with fluids, insulin, and dextrose and the death of the nonresponding cat was accompanied by signs of hepatic and renal failure.
In its physiological form, ketosis is a nonpathological response associated with fat catabolism in response to depletion of glycogen reserves, the principal stores of which are found in liver and muscle. Adaptive ketosis can be induced by restriction of carbohydrate intake in humans, but the most effective dietary manipulation in rodents is restriction of both carbohydrate and protein, presumably because restriction of dietary protein decreases the conversion of amino acids into glucose via gluconeogenesis. 27 Fat catabolism in both rodents and humans is antagonized by insulin, which suppresses lipolysis in adipocytes and effectively terminates the acidotic state.
In healthy rats, the SGLT2 inhibitor dapagliflozin has been reported to produce ketosis by a mechanism that requires dehydration. 28 Administration of dapagliflozin resulted in a decrease in blood glucose concentration of approximately 25 mg/dL and a corresponding decrease in plasma insulin concentration of approximately 50%. 28 In rats that had water withheld, plasma β‐OHB concentration increased 8‐fold and bicarbonate concentration decreased by 30%. 28 The role of dehydration in the induction of ketoacidosis appeared to be indirect, and mediated by increases in corticosterone and catecholamines. 28 Infusion of glucose overcame the dapagliflozin‐mediated hypoglycemia and resulted in an increase in insulin production and suppression of adipocyte lipolysis. 28
Osmotic diuresis associated with glucosuria produces mild volume contraction and may increase the risk of dehydration‐dependent ketonemia. Although a dehydration‐dependent increase in glucocorticoids and catecholamines represents 1 mechanism for initiation, other precipitating factors might account for susceptibility to ketoacidosis.
Experiments with healthy mice have shown that ketonemia associated with fasting or occurring in the context of administration of SGLT2 inhibitors has little dependence on glucagon. 29 Whether these conclusions apply to diabetic cats treated with SGLT2 inhibitors is uncertain.
Appropriate reduction of risk of ketoacidosis in bexagliflozin treatment is an important objective. The dapagliflozin rat model identifies access to water as an important factor, and diet may be another. Provision of a diet with a consistently high proportion of metabolizable energy in the form of carbohydrates should be considered, because restriction of carbohydrate intake is a well‐documented method to induce adaptive ketosis in humans. In healthy rats, β‐adrenergic blockade nearly completely suppresses dehydration‐dependent ketonemia, 28 and this approach may have utility for the management of cats with ketosis secondary to bexagliflozin administration.
The analysis of adverse events and clinically relevant laboratory test abnormalities in our study has produced evidence that bexagliflozin and insulin exhibit complementary patterns of drug liability. Bexagliflozin exposure produces minimal risk of hypoglycemia, but may be associated with increased risk of DKA (see comparability discussion below). Insulin can quell the latter but poses a substantial risk of hypoglycemia.
No adverse drug experiences in our study were the result of exposure to owners or members of the owners' households, consistent with the favorable safety profile for SGLT2 inhibitors observed in clinical experimentation using humans to date. An evaluation of cases of SGLT2 inhibitor overdosage referred to United States poison control centers has shown that serious drug reactions are rare. 30 In addition, nonclinical studies have shown that high multiples of the pharmacologically effective dose are well‐tolerated in multiple species and thus there is likely also little risk to nonhuman members of the household.
Our study had some limitations. One limitation was the unblinded design and the absence of a comparator arm managed by twice‐daily insulin injection. Assessment of improvement in clinical signs of hyperglycemia relied on owner evaluations, and a central adjudication system was not created to evaluate improvement in signs. An endpoint measuring clinical remission was not included.
Although the relative degree of glycemic control and the proportion of adverse reactions cannot be directly compared, published studies suggest comparisons favorable to bexagliflozin. In studies reporting the effects of traditionally manufactured protamine zinc insulin 31 and recombinant human protamine zinc insulin, 32 mean serum fructosamine concentration decreased from 598 to 419 μmol/L and from 505 to 375 μmol/L, respectively, over 45 days, whereas in our study, a decrease from 543 to 301 μmol/L was observed over 56 days (Figure 2B). Extended safety data were not reported in detail for either insulin study, but regulatory documents report an FDA analysis for the second study that showed that of 176 cats enrolled, 7 died or were euthanized before day 45 and 14 died or were euthanized during the extended safety evaluation (an additional 136 days). 33 Thus, 21 of 176 cats died or were euthanized over 181 days compared to 3 out of 84 over 180 days in our study. In the insulin study, 4 cats were diagnosed with DKA during the initial 45‐day effectiveness evaluation period. 33 If there were no additional DKA events in the study, the proportion of cats experiencing DKA would be approximately half that seen in our study (in which 4 cats with known or presumed DKA were found out of 84 over 180 days).
Some studies have evaluated insulin glargine management of DM in cats with generally favorable results but with smaller numbers of enrolled animals, either 13, 34 24, 35 or 13 cats. 36 Serum fructosamine concentrations decreased from 556 to 458, 568 to 543, and 604 to 366 μmol/L, respectively, and tabulations of adverse events were not reported. 34 , 35 , 36 Insulin glargine appears to have several advantages over insulins approved for veterinary use but a definitive study has yet to be reported.
Bexagliflozin represents a new option for the management of DM in cats newly diagnosed with the disease. It favorably affects owner quality of life, provides excellent glycemic control, and facilitates owner compliance by providing an easily administered, once‐daily PO tablet formulation. The principal risk attributable to bexagliflozin treatment may be DKA. Other common adverse effects such as vomiting, diarrhea, anorexia, and lethargy are typically mild and self‐limiting.
CONFLICT OF INTEREST DECLARATION
Thomas J. Dupree was employed by and owned stock in IncreVet. Michael J. Hadd and J. Catharine Scott‐Moncrieff consulted for IncreVet. Stephen E. Bienhoff and Jennifer Ogne‐Stevenson were employed by Argenta, which was contracted by IncreVet for study advice and execution. Susan E. Little and Samuel Geller were study investigators and were compensated by IncreVet for their participation.
OFF‐LABEL ANTIMICROBIAL DECLARATION
Authors declare no off‐label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
This study was conducted following a protocol reviewed and accepted through a formal letter of concurrence from the Center for Veterinary Medicine, Food and Drug Administration.
HUMAN ETHICS APPROVAL DECLARATION
Authors declare human ethics approval was not needed for this study.
Supporting information
Data S1. Supporting Information.
ACKNOWLEDGMENT
Funding provided by IncreVet, Inc. The authors thank Al Collinson, Brian Connelly, Bert Mileski, and Brian Seed for their contributions to the study.
Hadd MJ, Bienhoff SE, Little SE, et al. Safety and effectiveness of the sodium‐glucose cotransporter inhibitor bexagliflozin in cats newly diagnosed with diabetes mellitus. J Vet Intern Med. 2023;37(3):915‐924. doi: 10.1111/jvim.16730
REFERENCES
- 1. Hummel CS, Lu C, Loo DD, et al. Glucose transport by human renal Na+/D‐glucose cotransporters SGLT1 and SGLT2. Am J Physiol Cell Physiol. 2011;300:C14‐C21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Vallon V, Platt KA, Cunard R, et al. SGLT2 mediates glucose reabsorption in the early proximal tubule. J Am Soc Nephrol. 2011;22:104‐112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Santer R, Calado J. Familial renal glucosuria and SGLT2: from a mendelian trait to a therapeutic target. Clin J Am Soc Nephrol. 2010;5:133‐141. [DOI] [PubMed] [Google Scholar]
- 4. American Diabetes Association . 9. Pharmacologic approaches to glycemic treatment: standards of medical Care in Diabetes‐2019. Diabetes Care. 2019;42:S90‐S102. [DOI] [PubMed] [Google Scholar]
- 5. Rieg T, Masuda T, Gerasimova M, et al. Increase in SGLT1‐mediated transport explains renal glucose reabsorption during genetic and pharmacological SGLT2 inhibition in euglycemia. Am J Physiol Renal Physiol. 2014;306:F188‐F193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Vrhovac I, Balen Eror D, Klessen D, et al. Localizations of Na(+)‐D‐glucose cotransporters SGLT1 and SGLT2 in human kidney and of SGLT1 in human small intestine, liver, lung, and heart. Pflugers Arch. 2015;467:1881‐1898. [DOI] [PubMed] [Google Scholar]
- 7. Scheen AJ. Sodium‐glucose cotransporter type 2 inhibitors for the treatment of type 2 diabetes mellitus. Nat Rev Endocrinol. 2020;16:556‐577. [DOI] [PubMed] [Google Scholar]
- 8. van Baar MJB, van Ruiten CC, Muskiet MHA, van Bloemendaal L, IJzerman RG, van Raalte DH. SGLT2 inhibitors in combination therapy: from mechanisms to clinical considerations in type 2 diabetes management. Diabetes Care. 2018;41:1543‐1556. [DOI] [PubMed] [Google Scholar]
- 9. Balmer L, O'Leary CA, Menotti‐Raymond M, et al. Mapping of diabetes susceptibility loci in a domestic cat breed with an unusually high incidence of diabetes mellitus. Genes (Basel). 2020;11:1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Florez JC, Hirschhorn J, Altshuler D. The inherited basis of diabetes mellitus: implications for the genetic analysis of complex traits. Annu Rev Genomics Hum Genet. 2003;4:257‐291. [DOI] [PubMed] [Google Scholar]
- 11. Forcada Y, Boursnell M, Catchpole B, et al. A genome‐wide association study identifies novel candidate genes for susceptibility to diabetes mellitus in non‐obese cats. PLoS One. 2021;16:e0259939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Samaha G, Wade CM, Beatty J, Lyons LA, Fleeman LM, Haase B. Mapping the genetic basis of diabetes mellitus in the Australian Burmese cat (Felis catus). Sci Rep. 2020;10:19194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Westermark P, Andersson A, Westermark GT. Islet amyloid polypeptide, islet amyloid, and diabetes mellitus. Physiol Rev. 2011;91:795‐826. [DOI] [PubMed] [Google Scholar]
- 14. Cooper GJ, Willis AC, Clark A, Turner RC, Sim RB, Reid KB. Purification and characterization of a peptide from amyloid‐rich pancreases of type 2 diabetic patients. Proc Natl Acad Sci U S A. 1987;84:8628‐8632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jotha‐Mattos L, Vieira AB, Castelo M, et al. Amyloidogenesis of feline amylin and plasma levels in cats with diabetes mellitus or pancreatitis. Domest Anim Endocrinol. 2021;74:106532. [DOI] [PubMed] [Google Scholar]
- 16. Westermark P, Wernstedt C, Wilander E, Hayden DW, O'Brien TD, Johnson KH. Amyloid fibrils in human insulinoma and islets of Langerhans of the diabetic cat are derived from a neuropeptide‐like protein also present in normal islet cells. Proc Natl Acad Sci U S A. 1987;84:3881‐3885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zini E, Lunardi F, Zanetti R, et al. Endocrine pancreas in cats with diabetes mellitus. Vet Pathol. 2016;53:136‐144. [DOI] [PubMed] [Google Scholar]
- 18. Baral RM, Little, S.E . Endocrinology. In: Little SE, ed. The Cat: Clinical Medicine and Management. Philadelphia, PA: Elsevier Saunders; 2012. [Google Scholar]
- 19. Niessen SJ, Forcada Y, Mantis P, et al. Studying cat (Felis catus) diabetes: beware of the acromegalic imposter. PLoS One. 2015;10:e0127794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zaina A, Grober Y, Abid A, Arad E, Golden E, Badarny S. Sodium glucose cotransporter 2 inhibitors treatment in acromegalic patients with diabetes—a case series and literature review. Endocrine. 2021;73:65‐70. [DOI] [PubMed] [Google Scholar]
- 21. Benedict SL, Mahony OM, McKee TS, Bergman PJ. Evaluation of bexagliflozin in cats with poorly regulated diabetes mellitus. Can J Vet Res. 2022;86:52‐58. [PMC free article] [PubMed] [Google Scholar]
- 22. Niessen SJ, Powney S, Guitian J, et al. Evaluation of a quality‐of‐life tool for cats with diabetes mellitus. J Vet Intern Med. 2010;24:1098‐1105. [DOI] [PubMed] [Google Scholar]
- 23. Peters AL, Buschur EO, Buse JB, Cohan P, Diner JC, Hirsch IB. Euglycemic diabetic ketoacidosis: a potential complication of treatment with sodium‐glucose cotransporter 2 inhibition. Diabetes Care. 2015;38:1687‐1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kaze AD, Zhuo M, Kim SC, Patorno E, Paik JM. Association of SGLT2 inhibitors with cardiovascular, kidney, and safety outcomes among patients with diabetic kidney disease: a meta‐analysis. Cardiovasc Diabetol. 2022;21:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Burke KR, Schumacher CA, Harpe SE. SGLT2 inhibitors: a systematic review of diabetic ketoacidosis and related risk factors in the primary literature. Pharmacotherapy. 2017;37:187‐194. [DOI] [PubMed] [Google Scholar]
- 26. Liu J, Li L, Li S, et al. Sodium‐glucose co‐transporter‐2 inhibitors and the risk of diabetic ketoacidosis in patients with type 2 diabetes: a systematic review and meta‐analysis of randomized controlled trials. Diabetes Obes Metab. 2020;22:1619‐1627. [DOI] [PubMed] [Google Scholar]
- 27. Bielohuby M, Menhofer D, Kirchner H, et al. Induction of ketosis in rats fed low‐carbohydrate, high‐fat diets depends on the relative abundance of dietary fat and protein. Am J Physiol Endocrinol Metab. 2011;300:E65‐E76. [DOI] [PubMed] [Google Scholar]
- 28. Perry RJ, Rabin‐Court A, Song JD, et al. Dehydration and insulinopenia are necessary and sufficient for euglycemic ketoacidosis in SGLT2 inhibitor‐treated rats. Nat Commun. 2019;10:548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Capozzi ME, Coch RW, Koech J, et al. The limited role of glucagon for ketogenesis during fasting or in response to SGLT2 inhibition. Diabetes. 2020;69:882‐892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Schaeffer SE, DesLauriers C, Spiller HA, Aleguas A, Baeza S, Ryan ML. Retrospective review of SGLT2 inhibitor exposures reported to 13 poison centers. Clin Toxicol (Phila). 2018;56:204‐208. [DOI] [PubMed] [Google Scholar]
- 31. Nelson RW, Lynn RC, Wagner‐Mann CC, Michels GM. Efficacy of protamine zinc insulin for treatment of diabetes mellitus in cats. J Am Vet Med Assoc. 2001;218:38‐42. [DOI] [PubMed] [Google Scholar]
- 32. Nelson RW, Henley K, Cole C, the PZIR Clinical Study Group . Field safety and efficacy of protamine zinc recombinant human insulin for treatment of diabetes mellitus in cats. J Vet Intern Med. 2009;23:787‐793. [DOI] [PubMed] [Google Scholar]
- 33. DailyMed . PROZINC—Protamine Zinc Recombinant Human Insulin Injection Package Insert for Cats. Bethesda, MD: U.S. National Library of Medicine. Available from: https://dailymed.nlm.nih.gov/dailymed/fda, keyword prozinc. [Google Scholar]
- 34. Weaver KE, Rozanski EA, Mahony OM, Chan DL, Freeman LM. Use of glargine and lente insulins in cats with diabetes mellitus. J Vet Intern Med. 2006;20:234‐238. [DOI] [PubMed] [Google Scholar]
- 35. Marshall RD, Rand JS, Morton JM. Treatment of newly diagnosed diabetic cats with glargine insulin improves glycaemic control and results in higher probability of remission than protamine zinc and lente insulins. J Feline Med Surg. 2009;11:683‐691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Linari G, Fleeman L, Gilor C, Giacomelli L, Fracassi F. Insulin glargine 300 U/ml for the treatment of feline diabetes mellitus. J Feline Med Surg. 2022;24:168‐176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Link KR, Rand JS. Changes in blood glucose concentration are associated with relatively rapid changes in circulating fructosamine concentrations in cats. J Feline Med Surg. 2008;10:583‐592. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Supporting Information.


