Abstract
Background
Echocardiographic reference intervals have not been reported for North American whippets, or for whippets that have undergone pet‐level athletic training.
Objectives
To develop normal echocardiographic reference intervals for North American whippets and investigate differences in echocardiographic parameters based on athletic conditioning in pet whippets engaged in competitive sports.
Animals
One‐hundred healthy North American whippets.
Methods
Dogs were examined at national shows between 2005 and 2009. Echocardiographic reference intervals were constructed and the effect of athletic conditioning on parameters of structure and function was assessed.
Results
Two dimensional, M‐mode, Doppler and tissue Doppler reference ranges for healthy North American whippets are presented. Measures of left ventricular (LV) chamber diameter were larger in conditioned whippets (N = 25) and remained significantly larger than in unconditioned whippets (N = 16) when normalized for weight using allometric equations. Calculated LV mass was higher in conditioned dogs than in unconditioned dogs, and this difference persisted when LV mass was normalized by weight. Mitral E velocity was higher in conditioned dogs than in unconditioned dogs, whereas E/A and measures related to systolic function were not different.
Conclusions and Clinical Importance
Pet whippets in peak athletic condition have larger hearts than do less conditioned whippets, but measures of systolic function are similar. Whippet pet athletes may show eccentric LV hypertrophy at peak condition. Normal values for cardiac size and function in North American whippets might be considered abnormal if population‐specific whippet reference intervals are not used in analysis.
Keywords: athletic, canine, dog, echocardiography, reference values
Abbreviations
- a′
peak late diastolic myocardial velocity determined by tissue Doppler imaging
- AoLTvel
peak aortic systolic velocity, left apical view
- AoSax
aortic diameter in short axis
- AoSCvel
peak aortic systolic velocity, subcostal view
- Avel
mitral valve peak A wave velocity
- dLVvol
left ventricular diastolic volume
- e′
peak early diastolic myocardial velocity determined by tissue Doppler imaging
- E/A
mitral valve E wave to A wave ratio
- EF
ejection fraction
- EPSS
E‐point septal separation
- Evel
mitral valve peak E wave velocity
- FS
left ventricular fractional shortening
- IVSd
interventricular septal thickness in diastole
- IVSdN
interventricular septal thickness in diastole indexed for body weight
- IVSs
interventricular septal thickness in systole
- LA
AoLax: left atrial to aortic diameter ratio in long axis
- LA
AoSax: left atrial to aortic diameter ratio in short axis
- LADLax
left atrial diameter in long axis
- LADLaxN
left atrial diameter in long axis indexed for body weight
- LADSax
left atrial diameter in short axis
- LV
left ventricular
- LVIDd
left ventricular diameter in diastole
- LVIDdN
left ventricular diameter in diastole indexed for body weight
- LVIDs
left ventricular diameter in systole
- LVIDsN
left ventricular diameter in systole indexed for body weight
- LVmass
calculated left ventricular mass
- LVWd
left ventricular wall thickness in diastole
- LVWdN
left ventricular wall thickness in diastole indexed for body weight
- LVWs
left ventricular wall thickness in systole
- MMVD
myxomatous mitral valve disease
- PVvel
peak pulmonary valve systolic velocity
- s′
peak systolic myocardial velocity determined by tissue Doppler imaging
- sLVvol
left ventricular systolic volume
- SV
stroke volume
1. INTRODUCTION
As a breed, whippets are typically physically active and may participate in racing, agility and other performance events as well as functioning as pet companions. The acknowledged risk of adult‐onset myxomatous mitral valve disease (MMVD) in whippets 1 , 2 as well as recent concerns about the effect of diet on cardiac function 3 , 4 have increased interest in echocardiographic screening of at‐risk dogs for evidence of subclinical MMVD or changes suggestive of cardiomyopathy.
Reliable reference intervals are essential when reviewing echocardiographic data for evidence of mild abnormalities. Previous studies of sighthounds including western European whippets, Australian whippets, greyhounds, Italian greyhounds and salukis 5 , 6 , 7 , 8 , 9 , 10 as well as large studies of various breeds of normal dogs 11 have indicated that weight or body surface area‐based normal echocardiographic reference ranges for dogs in general may not be applicable to sighthounds, presumably because of sighthounds' distinctive body conformation, degree of athleticism, or both. Other methods of adjusting measurements for body size have been suggested 12 , 13 and some have been applied to whippets. 5
Some studies have indicated that purposeful athletic conditioning (eg, for endurance or racing events or as treadmill training) results in cardiac hypertrophy that might be misdiagnosed as abnormal in a healthy dog, 14 , 15 , 16 but it is currently unclear if the degree of athletic training in casual athletes (ie, pet animals that regularly participate in organized athletic events) is sufficient to cause physiologic hypertrophy to an extent that might be misdiagnosed as abnormal.
Our aims were to develop normal echocardiographic reference intervals for healthy North American whippets and to investigate possible differences in echocardiographic parameters based on athletic conditioning in pet whippets engaged in competitive sports.
2. MATERIALS AND METHODS
2.1. Patient population
Our study was an observational cross‐sectional study that was approved by the Institutional Animal Care and Use Committee at the University of Wisconsin School of Veterinary Medicine. All dog owners provided informed consent. Dogs included in the study were part of a healthy population screened at the American Whippet Club National Specialty as participants or spectators between 2005 and 2009. The National Specialty includes show, lure coursing, agility, endurance and obedience events, and subjects examined included dogs with variable degrees of athletic conditioning. Genetic background regarding racing or show breeding lines 5 , 7 was not investigated. Dogs were submitted for examination by their owners without regard to age or breeding status. Over the course of the events of 2005‐2009, 341 dogs were examined. Dogs chosen for further analysis to develop normal reference intervals included only clinically healthy dogs with no or trivial valvular regurgitation identified by echocardiography. Only the first examination of any dog that had undergone multiple examinations over the study time frame was included for further analysis. Complete diet histories were not obtained. The time frame chosen for review was limited to years preceding recent reports of diet‐related cardiac disease in dogs.
Based on previous studies suggesting that athletic conditioning may influence some cardiac parameters, 5 , 7 , 14 , 15 , 17 dogs were allocated to 4 conditioning levels based on owner assessment of their own dogs. Owners were asked to subjectively identify their dogs' athletic conditioning level as “peak” physical condition currently competing in athletic events (C1), “good” physical condition but not currently competing (C2), “pet‐level” condition (daily walks and play but not training, C3) or “non‐athletic” (C4).
2.2. Examination
Heart rate was recorded by auscultation before echocardiographic examination. All echocardiograms were recorded by a single echocardiographer (VLF, a board‐certified veterinary cardiologist) using an Acuson Cypress System (Siemens Medical Solutions USA, Inc., Mountain View, California) with 7V3c (2‐dimensional images) and 3V2c (Doppler signals) phased array sector probes. Dogs were restrained in right and left lateral recumbency in the presence of the owner or handler on a table with a cut‐out to allow placement of the ultrasound probe on the lowermost side of the thorax. A lead 2 ECG tracing was recorded simultaneously for measurement purposes. The echocardiographer was unaware of age, auscultation results, clinical history and any findings available from examinations in previous years. Echocardiographic, color‐ and spectral Doppler images were stored on optical discs for later off‐line analysis by a single trained observer blinded to condition status (LJW). All measurements were recorded as the mean of 3 consecutive cardiac cycles.
Images were obtained in the same sequence, according to the recommendations of the Echocardiography Committee of the Specialty of Cardiology, American College of Veterinary Internal Medicine 18 for all dogs. In each view, the visible valves were screened for anatomic abnormalities or regurgitation jets. Dogs with any valvular regurgitation considered more than trivial were excluded from further analysis.
Measurements were obtained from both 2‐dimensional (2‐D) and M‐mode images. Details of measurement abbreviations, the order of and methods by which the values were obtained, calculated values and formulae used are presented in Table 1. Left ventricular M‐mode measurements were obtained at the level of the chordae tendineae by the leading‐edge to leading‐edge method. End‐diastolic measurements were obtained using image frames at the onset of the QRS complex, and end‐systolic measurements were obtained at the point of maximal septal systolic excursion. Volumetric measurements were obtained using a monoplane Simpson's method of discs by tracing the endocardial border of the chamber in question using the right parasternal long‐axis 2‐D images optimized for LV inflow. 7 Left ventricular mass and LV mass/kg were estimated using M‐mode measures of interventricular septum in diastole (IVSd), left ventricular wall in diastole (LVWd) and left ventricular internal diameter in diastole (LVIDd) according to previously described methods. 19
TABLE 1.
Echocardiographic measurements obtained from 100 North American whippets in the sequence in which they were obtained.
| Parameter | Abbreviation | Units | Method |
|---|---|---|---|
| Right parasternal long axis four‐chamber view (2‐D) | |||
| LV diastolic volume | dLVvol | mL | Modified SMOD, monoplane, inner edge area at end‐diastole |
| LV systolic volume | sLVvol | mL | Modified SMOD, monoplane, inner edge area at peak systole |
| LA end‐systolic diameter | LADLax | cm | Inflow view, diameter measured midway between MV annulus and pulmonary vein parallel to MV annulus 13 |
| LA end‐systolic area | LAA | cm2 | Area traced using SMOD, inner edge method in basilar view excluding the pulmonary vein 13 |
| Ao systolic diameter | AoLax | cm | Outflow view, diameter measured at valve hinge points during systole |
| Right parasternal short axis view (chordal level, M‐mode) | |||
| IVS thickness in diastole | IVSd | cm | Leading edge to leading edge method at end‐diastole |
| LV diameter in diastole | LVIDd | cm | Leading edge to leading edge method at end‐diastole |
| LV wall thickness in diastole | LVWd | cm | Leading edge to leading edge method at end‐diastole |
| IVS thickness in systole | IVSs | cm | Leading edge to leading edge method at end‐systole |
| LV diameter in systole | LVIDs | cm | Leading edge to leading edge method at end‐systole |
| LV wall thickness in systole | LVWs | cm | Leading edge to leading edge method at end‐systole |
| E‐point to septal separation | EPSS | cm | Leading edge to leading edge method at maximal E wave excursion of MV |
| Right parasternal short axis view (at heart base, 2‐D) | |||
| Ao diameter in short axis | AoSax | cm | Inner edge to inner edge, midpoint of right coronary cusp to opposite wall at point of left coronary cusp/noncoronary cusp convergence at early diastole 33 |
| LA diameter in short axis | LASax | cm | Extension of Ao diameter line from Ao wall to inner edge of opposite LA wall at early diastole 33 |
| PV peak systolic velocity | PVvel | m/s | Pulsed waved Doppler, sample volume placed just distal to PV leaflets |
| Subcostal view | |||
| Ao peak systolic velocity | AoSCvel | m/s | Measured using continuous wave Doppler with 2‐D guidance |
| Left apical four‐chamber view (2‐D) | |||
| MV peak E wave velocity | Evel | m/s | Pulsed waved Doppler, sample volume placed at tips of open mitral valve in diastole |
| MV peak A wave velocity | Avel | m/s | Pulsed waved Doppler, sample volume placed at tips of open mitral valve in diastole |
| Left apical 3‐chamber view (2‐D) | |||
| Peak Ao systolic velocity | AoLTvel | m/s | Pulsed waved Doppler, sample volume placed just distal to Ao valve leaflets/Ao sinuses |
| TDI e′ velocity | e′ | m/s | Tissue Doppler, sample volume in basal segment of LV wall at lateral MV annulus, mid‐diastole 34 |
| TDI a′ velocity | a′ | m/s | Tissue Doppler, sample volume in basal segment of LV wall at lateral MV annulus, end‐diastole 34 |
| TDI s′ velocity | s′ | m/s | Tissue Doppler, sample volume in basal segment of LV wall at lateral MV annulus, mid‐systole 34 |
| Calculated parameters | |||
| Ejection fraction | EF | % | EF = (dLVvol − sLVvol/dLVvol) × 100 |
| Fractional shortening | FS | % | FS = (LVIDd − LVIDs/LVIDd) × 100 |
| Left atrial to aortic diameter, long axis | LA:AoLax | Using LADLax and AoLax (2‐D, right parasternal long axis) | |
| Left atrial to aortic diameter short axis | LA:AoSax | Using LASax and AoSax (2‐D, right parasternal short axis) | |
| E/A | E/A | Ratio of peak E wave velocity to peak A wave velocity (mitral inflow) | |
| LV mass (calculated) | LVmass | g | LVmass = 1.04 [LVIDd + LVWd + IVSd 3 ] − 13.6 g, using M‐mode measurements obtained from right parasternal short axis view at level of chordae tendineae 19 |
| LV mass/kg | LVmass/kg | g/kg | Calculated LVmass/kg body weight |
| LV diastolic volume/kg (mL/kg) | dLVvol/kg | mL/kg | LV diastolic volume by monoplane SMOD (right parasternal long axis view) per kg body weight |
| LV systolic volume/kg (mL/kg) | sLVvol/kg | mL/kg | LV systolic volume by monoplane SMOD (right parasternal long axis view) per kg body weight |
| Stroke volume | SV | mL | SV = dLVvol − sLVvol (2‐D, right parasternal long axis) |
| Stroke volume/kg | SV/kg | mL/kg | Calculated SV/kg body weight |
Note: References are provided when applicable.
Abbreviations: Ao, aortic valve; IVS, interventricular septum; LA, left atrium; LV, left ventricle; MV, mitral valve; PV, pulmonic valve; SMOD, Simpson's method of discs; TDI, tissue Doppler imaging; 2‐D, two‐dimensional imaging.
2.3. Statistical methods
Commercial software programs (Prism 9, GraphPad Software LLC, San Diego, California and MedCalc Statistical Software, MedCalc Software bvba, Ostend, Belgium) were used for data analysis. Descriptive statistics were generated for population characteristics and normal dataset distribution was assessed using a Shapiro‐Wilk test. Multiple regression was used to explore the effects of conditioning (C1 vs “not C1” categorization), age, body weight and sex (without regard to neuter status) on selected echocardiographic measures of cardiac structure and function. Ninety‐five percent reference intervals were generated using the “robust method,” as recommended by Clinical Laboratory Standards Institute guidelines when the reference sample does not exceed 120 subjects. 20 Ninety percent confidence intervals were generated for reference limits using a bootstrapping technique.
Selected linear measurements (left atrial diameter in long axis [LADLax], LVIDd, left ventricular internal diameter in systole [LVIDs], IVSd, LVWd) were normalized (indexed) to body weight by dividing by body weight (kg) b . The constant b was specific to each measurement and was generated by allometric scaling or nonlinear regression using the power equation, Y = ax b , where a represents the proportionality constant, b represents the scaling exponent, Y is the linear echocardiographic measurement, and x is body weight (kg). 12
To further investigate the effects of high‐level athletic conditioning, dogs were separated into 2 subgroups based on condition score; “conditioned” dogs included dogs categorized by their owners as C1 (n = 27), and “unconditioned” dogs (n = 16) included dogs categorized as C3 or C4. Selected parameters were compared between these groups using an unpaired t test or Mann‐Whitney U test, as appropriate. No adjustment was made for multiple comparisons. Significance for all statistical tests was set at P < .05.
3. RESULTS
Results from examinations of 100 whippets (56 female or spayed female, 44 male or castrated male) were assessed. Population characteristics are presented in Table 2. At the time of examination, dogs were assessed by their owners as being in Condition 1 (C1, n = 27), Condition 2 (C2, n = 55), Condition 3 (C3, n = 14) or Condition 4 (C4, n = 2). Two dogs did not have a condition score recorded. Echocardiographic results (2‐D, M‐mode, Doppler, calculated values) are presented in Table 3, including number of measurements available, mean, SD, median, range and calculated 95% reference limits with 90% confidence intervals. Measurements obtained from sub‐optimal images were excluded from analysis; not all values were available for all dogs.
TABLE 2.
Population characteristics of 100 normal North American whippets.
| Clinical data | N | Mean | SD | Median | Min‐Max | Other |
|---|---|---|---|---|---|---|
| Age (years) a | 100 | 3.3 | 2.2 | 2.7 | .4‐9.0 | IQR = 1.75‐4.40 |
| Body weight (kg) a | 100 | 15.2 | 2.4 | 14.7 | 9.8‐22.2 | IQR = 13.3‐16.6 |
| Heart rate (bpm) a | 100 | 99.0 | 21.9 | 90.0 | 60.0‐160.0 | IQR = 80.0‐110.0 |
| Sex (female/male) | 100 | 56 female, 44 male |
Abbreviation: IQR, interquartile range.
Reject normality (Shapiro‐Wilk test).
TABLE 3.
Reference intervals (95% RI, generated using “robust” method [n = 100] reporting lower limit and upper limit, each with 90% confidence intervals) for measured and calculated echocardiographic variables in normal North American whippets.
| Echo measurement | N | Mean | SD | Median | Min‐Max | 95% reference intervals |
|---|---|---|---|---|---|---|
| Lower limit (90% CI) − upper limit (90% CI) | ||||||
| 2‐D/M‐MODE | ||||||
| LADLax (cm) | 99 | 3.47 | .33 | 3.46 | 2.64‐4.34 | 2.81 (2.71‐2.90), 4.14 (4.03‐4.23) |
| LASax (cm) | 96 | 2.62 | .35 | 2.60 | 1.71‐3.56 | 1.89 (1.79‐1.98), 3.30 (3.20‐3.41) |
| AoLax (cm) | 99 | 1.70 | .14 | 1.70 | 1.34‐2.04 | 1.43 (1.40‐1.47), 1.98 (1.93‐2.01) |
| AoSax (cm) | 96 | 2.04 | .20 | 2.02 | 1.52‐2.57 | 1.64 (1.59‐1.71), 2.42 (2.35‐2.47) |
| LAA (cm2) | 99 | 9.82 | 1.82 | 9.61 | 5.82‐14.26 | 6.12 (5.69‐6.60), 13.42 (12.85‐13.92) |
| LA:AoLax | 98 | 2.05 | .19 | 2.04 | 1.53‐2.55 | 1.67 (1.61‐1.72), 2.43 (2.38‐2.48) |
| LA:AoSax | 96 | 1.28 | .17 | 1.27 | .82‐1.62 | .95 (.90‐1.00), 1.62 (1.57‐1.67) |
| IVSd (cm) a | 97 | 1.02 | .14 | 1.00 | .75‐1.39 | .72 (.69‐.77), 1.28 (1.23‐1.32) |
| LVIDd (cm) | 97 | 3.73 | .36 | 3.75 | 2.69‐4.66 | 3.02 (2.90‐3.12), 4.44 (4.34‐4.55) |
| LVWd (cm) | 97 | .88 | .13 | .87 | .63‐1.22 | .61 (.57‐.64), 1.13 (1.09‐1.17) |
| IVSs (cm) a | 97 | 1.23 | .17 | 1.20 | .84‐1.94 | .88 (.81‐.94), 1.56 (1.49‐1.62) |
| LVIDs (cm) | 97 | 2.88 | .38 | 2.84 | 1.93‐3.89 | 2.10 (2.00‐2.21), 3.63 (3.51‐3.75) |
| LVWs (cm) | 97 | 1.13 | .17 | 1.11 | .73‐1.6 | .77 (.73‐.82), 1.47 (1.42‐1.52) |
| LVmass (g) | 97 | 121.3 | 31.9 | 119.4 | 60.5‐207.5 | 56.0 (47.7‐64.4), 183.7 (173.9‐193.0) |
| EPSS (cm) a | 90 | .43 | .15 | .42 | .22‐.89 | .12 (.08‐.16), .72 (.66‐.77) |
| DOPPLER | ||||||
| PVvel (m/s) a | 88 | .97 | .21 | .93 | .56‐1.91 | .51 (.42‐.61), 1.35 (1.25‐1.45) |
| AoSCvel (m/s) | 81 | 1.74 | .27 | 1.73 | 1.03‐2.41 | 1.19 (1.11‐1.29), 2.29 (2.20‐2.38) |
| AoLTvel (m/s) | 97 | 1.50 | .26 | 1.47 | .99‐2.19 | .96 (.89‐1.03), 1.99 (1.90‐2.08) |
| Evel(m/s) | 97 | .79 | .14 | .80 | .40‐1.29 | .52 (.47‐.57), 1.06 (1.02‐1.11) |
| Avel (m/s) a | 97 | .39 | .12 | .38 | .16‐.72 | .14 (.11‐.17), .62 (.58‐.66) |
| E/A a | 97 | 2.22 | .74 | 2.16 | 1.05‐4.78 | .67 (.42‐.87), 3.62 (3.36‐3.88) |
| e′ (m/s) a | 99 | .19 | .05 | .18 | .07‐.37 | .07 (.05‐.09), .29 (.27‐.30) |
| a′ (m/s) a | 99 | .12 | .11 | .09 | .05‐.89 | .05, .53 b |
| s′ (m/s) a | 99 | .14 | .04 | .14 | .08‐.25 | .07 (.05‐.08), .21 (.2‐.22) |
| Volume/function | ||||||
| FS (%) a | 96 | 23.2 | 5.7 | 23.0 | 10.0‐43.0 | 11.1 (9.2‐13.2), 34.0 (32.1‐35.9) |
| EF (%) a | 99 | 59.0 | 7.9 | 59.9 | 33.8‐82.2 | 43.9 (41.2‐46.8), 75.5 (73.0‐78.1) |
| dLVvol (mL) a | 99 | 52.0 | 11.4 | 50.9 | 30.0‐80.8 | 27.9 (24.3‐30.9), 73.5 (69.6‐77.2) |
| sLVvol (mL) a | 99 | 21.7 | 8.0 | 20.1 | 7.8‐53.5 | 3.9 (1.1‐6.9), 36.3 (32.9‐39.3) |
| SV (mL) | 99 | 30.3 | 6.0 | 29.7 | 16.4‐53.5 | 18.0 (16.0‐20.1), 42.2 (40.1‐44.1) |
| Indexed parameters | ||||||
| LADLaxN (cm/kg.391) | 99 | 1.21 | .09 | 1.20 | .86‐1.41 | 1.03 (1.00‐1.06), 1.39 (1.36‐1.42) |
| IVSDdN (cm/kg.222) a | 97 | .56 | .07 | .55 | .43‐.75 | .40 (.38‐.42), .70 (.67‐.72) |
| LVWdN (cm/kg.335) a | 97 | .35 | .05 | .35 | .26‐.47 | .25 (.24‐.26), .45 (.43‐.47) |
| LVIDdN (cm/kg.332) | 97 | 1.52 | .12 | 1.52 | 1.17‐1.81 | 1.28 (1.24‐1.32), 1.77 (1.73‐1.80) |
| LVIDsN (cm/kg.433) | 97 | .89 | .10 | .90 | .61‐1.15 | .69 (.66‐.72), 1.09 (1.06‐1.12) |
| LVmass/kg (g/kg) a | 97 | 8.00 | 1.73 | 7.69 | 4.75‐11.73 | 4.4 (3.96‐4.86), 11.41 (10.85‐11.92) |
| dLVvol/kg (mL/kg) | 99 | 3.42 | .49 | 3.40 | 2.39‐4.65 | 2.42 (2.29‐2.57), 4.38 (4.23‐4.52) |
| sLVvol/kg (mL/kg) a | 99 | 1.42 | .42 | 1.33 | .48‐3.07 | .47 (.33‐.64), 2.19 (2.02‐2.35) |
| SV/kg (mL/kg) | 99 | 2.00 | .30 | 2.00 | 1.25‐2.81 | 1.4 (1.32‐1.50), 2.6 (2.51‐2.68) |
Note: Not all values were available for all dogs.
Abbreviations: Ao, aortic valve; IVS, interventricular septum; LA, left atrium; LV, left ventricle; MV, mitral valve; PV, pulmonic valve; SMOD, Simpson's method of discs; TDI, tissue Doppler imaging; 2‐D, 2‐dimensional imaging.
Reject normality (Shapiro‐Wilk test).
Confidence intervals could not be calculated.
Assessment of the influence of age, sex, weight, and C1 status is presented in Table 4. Sex had no apparent effect on any measured or calculated echocardiographic values. Age was not associated with any linear or volumetric echocardiographic measurements but was associated with several indirect measures of function. A weak but significant negative association was found between age and E wave velocity (Evel; P = .0005; r = −0.35), a weak but positive association of age with A wave velocity (Avel; P = .009; r = 0.27) and a moderate negative association between age and E wave to A wave ratio (E/A; P < .0001; r = 0.42). Weight was positively associated with measures of left atrial, aortic and left ventricular dimensions and volumes; these associations were negated by indexing to body weight by use of allometric equations (Table 5) or by normalizing the values by comparison to aortic size (left atrial to aorta in the long axis [LA:AoLax], left atrial to aorta in the short axis [LA:AoSax]) or on a per kilogram (kg) basis. The categorization of C1 (yes/no) showed a weak but positive association with calculated LVmass (P = .03; r = 0.18) and this association was retained when LVmass was normalized on a per kg basis (P = .04; r = 0.21).
TABLE 4.
Association of peak athletic conditioning (Condition 1 status, n = 27), body weight, age and sex with selected echocardiographic measures of structure, volume and function in normal North American whippets.
| N | Condition 1 status (yes/no) | Body weight (kg) | Age (mo) | Sex (M/F) | |
|---|---|---|---|---|---|
| Structural measures | |||||
| LADLax (cm) | 99 | NS | r = .40, P < .0001 | NS | NS |
| LASax (cm) | 96 | NS | r = .28, P = .03 | NS | NS |
| AoLax (cm) | 99 | NS | r = .37, P < .0001 | NS | NS |
| AoSax (cm) | 96 | NS | r = .41, P < .0001 | NS | NS |
| LA:AoLax | 98 | NS | NS | NS | NS |
| LA:AoSax | 96 | NS | NS | NS | NS |
| IVSd (cm) a | 97 | NS | NS | NS | NS |
| LVIDd (cm) | 97 | NS | r = .42, P < .0001 | NS | NS |
| LVWd (cm) | 97 | NS | NS | NS | NS |
| IVSs (cm) a | 97 | NS | NS | NS | NS |
| LVIDs (cm) | 97 | NS | r = .28, P = .002 | NS | NS |
| LVWs (cm) | 97 | NS | NS | NS | NS |
| LVIDdN (cm/kg332) | 97 | NS | NS | NS | NS |
| LVIDsN (cm/kg433) | 97 | NS | NS | NS | NS |
| LVmass (g) | 97 | r = .18, P = .03 | r = .34, P < .0001 | NS | NS |
| LVmass/kg a (g/kg) | 97 | r = .21, P = .04 | NS | NS | NS |
| EPSS (cm) a | 90 | NS | NS | NS | NS |
| Volume measures | |||||
| dLVvol (mL) a | 99 | NS | r = .50, P < .0001 | NS | NS |
| sLVvol (mL) a | 99 | NS | r = .36, P = .0001 | NS | NS |
| dLVvol/kg (mL/kg) | 99 | NS | NS | NS | NS |
| sLVvol/kg (mL/kg) | 99 | NS | NS | NS | NS |
| SV (mL) | 99 | NS | r = .46, P < .0001 | NS | NS |
| SV/kg | 99 | NS | NS | NS | NS |
| Functional measures | |||||
| EF (%) a | 99 | NS | NS | NS | NS |
| FS (%) a | 96 | NS | NS | NS | NS |
| Evel (m/s) | 97 | NS | NS | r = −.35, P = .0005 | NS |
| Avel (m/s) a | 97 | NS | NS | r = .27, P = .009 | NS |
| E/A | 97 | NS | NS | r = −.42, P < .0001 | NS |
Note: The r value is presented to estimate the strength of the association when significant (P < .05, bold).
Abbreviations: Ao, aortic valve; IVS, interventricular septum; LA, left atrium; LV, left ventricle; MV, mitral valve; NS, association was not significant; PV, pulmonic valve; SMOD, Simpson's method of discs; TDI, tissue Doppler imaging; 2‐D, 2‐dimensional imaging.
Reject normality (Shapiro‐Wilk test).
TABLE 5.
Results of the linear regression analyses describing how log10 of selected linear chamber measurements relate to log10 body weight in 100 healthy North American whippets.
| Echo measurement | a | SE of Y estimate | Scaling exponent b | SE of b | R 2 |
|---|---|---|---|---|---|
| IVSd (n = 97) | .55 | .100 | .222 | .085 | .07 |
| LVIDd (n = 97) | 1.51 | .064 | .332 | .054 | .28 |
| LVWd (n = 97) | .35 | .109 | .335 | .092 | .12 |
| LVIDs (n = 97) | .89 | .089 | .433 | .076 | .26 |
| LADLax (n = 99) | 1.20 | .061 | .391 | .052 | .37 |
Note: The constants derived permit the calculation normalized echocardiographic linear measurements for any body weight (in kg) using the equation: measured linear dimension (in cm)/body weight (in kg) b , where b is the scaling exponent respective to each index. All correlations were statistically significant (P < .05).
Abbreviations: Ao, aortic valve; IVS, interventricular septum; LA, left atrium; LV, left ventricle; MV, mitral valve; NS, association was not significant; PV, pulmonic valve; SMOD, Simpson's method of discs; TDI, tissue Doppler imaging; 2‐D, 2‐dimensional imaging.
Results of comparisons between the “conditioned” and “unconditioned” groups are presented in Table 6. “Conditioned” dogs were younger and had lower heart rate on examination than did “unconditioned” dogs, but weight did not differ between the groups. No difference was found in measures of left atrial diameter (LADLax, LA:AoSax, LA:AoLax) between the groups. Measures of left ventricular chamber diameter (LVIDd, LVIDs, E‐point septal separation [EPSS]) were significantly larger in “conditioned” dogs, and left ventricular diameters normalized for weight using the allometric equations (LVIDdN and LVIDsN) remained significantly larger than those of “unconditioned” dogs. Regardless of conditioning status, LVIDd and LVIDs were frequently above a published reference range for dogs of unspecified breed and similar weight 21 (Figure 1). The IVSd was significantly higher (P = .04) in “conditioned” dogs but this difference was not significant for IVSdN. Left ventricular volume in systole and diastole tended to be higher in “conditioned” dogs, but the differences were not significant. Calculated LVmass was higher in “conditioned” dogs vs “unconditioned” dogs (P = .007; Figure 2) and this difference persisted when LVmass was normalized by weight (P = .002). Among the functional measurements examined, Evel was higher in “conditioned” dogs (P = .005), whereas E/A and measures related to systolic function (ejection fraction [EF], fractional shortening [FS], tissue Doppler imaging of peak systolic velocity [s′]) did not differ.
TABLE 6.
Comparison of selected echocardiographic values between “conditioned” dogs and “unconditioned” dogs.
| Parameter | Conditioned dogs | Unconditioned dogs | P value a | ||
|---|---|---|---|---|---|
| Median [range] N | Mean (SD) | Median [range] N | Mean (SD) | ||
| Age (mo) | 30 [11‐70] 27 | 33 (14) | 62 [24‐104] 16 | 64 (28) | <.0001 |
| Weight (kg) | 15.2 [11.6‐20.4] 27 | 15.4 (2.5) | 14.7 [12.0‐19.0] 16 | 15.0 (2.2) | NS |
| HR (bpm) b | 90 [60‐160] 27 | 97 (22) | 110 [70‐150] 16 | 110 (20) | .013 |
| 2‐D/M‐mode measurements | |||||
| LADLax b | 3.62 [2.78‐4.14] 27 | 3.58 (.37) | 3.33 [2.64‐4.34] 16 | 3.41 (.37) | NS |
| LA:AoSax | 1.30 [1.12‐1.57] 26 | 1.32 (.12) | 1.22 [.98‐1.44] 15 | 1.24 (.13) | NS |
| LA:AoLax | 2.07 [1.58‐2.46] 27 | 2.08 (.20) | 2.00 [1.53‐2.55] 16 | 2.04 (.23) | NS |
| IVSd | 1.02 [.79‐1.39] 25 | 1.05 (.14) | .92 [.80‐1.27] 16 | .96 (.13) | .036 |
| LVWd | .90 [.67‐1.19) 25 | .90 (.12) | .88 [.65‐1.19] 16 | .87 (.16) | NS |
| LVIDd | 3.85 [3.14‐4.57] 25 | 3.83 (.35) | 3.52 [3.12‐4.27] 16 | 3.54 (.29) | .01 |
| LVIDs | 2.98 [2.33‐3.71] 25 | 2.99 (.39) | 2.68 [2.12‐3.26] 16 | 2.66 (.31) | .007 |
| EPSS | .44 [.23‐.74] 25 | .47 (.15) | .33 [.22‐.57] 13 | .34 (.10) | .007 |
| dLVvol | 50.9 [34.4‐78.5] 27 | 53.4 (12.5) | 42.9 [35.4‐73.0] 16 | 47 (10) | NS |
| sLVvol b | 20.0 [14.9‐40.2] 27 | 22.2 (7.4) | 17.3 [11.5‐37.7] 16 | 19.2 (7) | NS |
| LVmass b | 132.6 [80.8‐207.5] 25 | 132.7 (33.2) | 97.1 [69.5‐176.1] 16 | 104.4 (31.1) | .007 |
| Indexed values | |||||
| LADLaxN b | 1.25 [1.01‐1.34] 27 | 1.23 (.08) | 1.19 [.86‐1.38] 16 | 1.19 (.12) | NS |
| IVSdN b | .55 [.44‐.73] 25 | .57 (.08) | .53 [.45‐.68] 16 | .53 (.07) | NS |
| LVIDdN b | 1.53 [1.31‐1.81] 25 | 1.55 (.11) | 1.44 [1.28‐1.62] 16 | 1.45 (.10) | .004 |
| LVWdN b | .36 [.29‐.45] 25 | .36 (.04) | .35 [.27‐.47] 16 | .35 (.06) | NS |
| LVIDsN b | .94 [.74‐1.10] 25 | .91 (.10) | .82 [.61‐1.07] 16 | .83 (.10) | .016 |
| LVmass/kg | 8.62 [5.91‐11.28] 25 | 8.58 (1.52) | 6.68 [4.75‐10.03] 16 | 6.94 (1.56) | .002 |
| Functional measures | |||||
| Evel | .84 [.50‐1.11] 26 | .83 (.14) | .72 [.59‐.83] 16 | .72 (.08) | .005 |
| E/A | 2.2 [1.2‐3.6] 26 | 2.2 (.6) | 1.9 [1.1‐3.8] 16 | 2.1 (.8) | NS |
| EF b | 60.7 [38.8‐68.2] 27 | 58.8 (7.3) | 60.3 [48.4‐69.7] 16 | 60.0 (6.9) | NS |
| FS b | 21.0 [10.0‐39.0] 25 | 22.1 (6.0) | 24.5 [14.0‐43.0] 16 | 24.9 (6.0) | NS |
| s′ | .13 [.08‐.20] 27 | .13 (.03) | .14 [.09‐.20] 16 | .14 (.03) | NS |
Note: Abbreviations and LV mass calculations appear in Table 1. Significant P values (<.05) appear in bold.
Abbreviation: NS, no significance.
Compares mean (SD) if normal distribution or median [range] if non‐normal distribution.
Reject normality (Shapiro‐Wilk).
FIGURE 1.
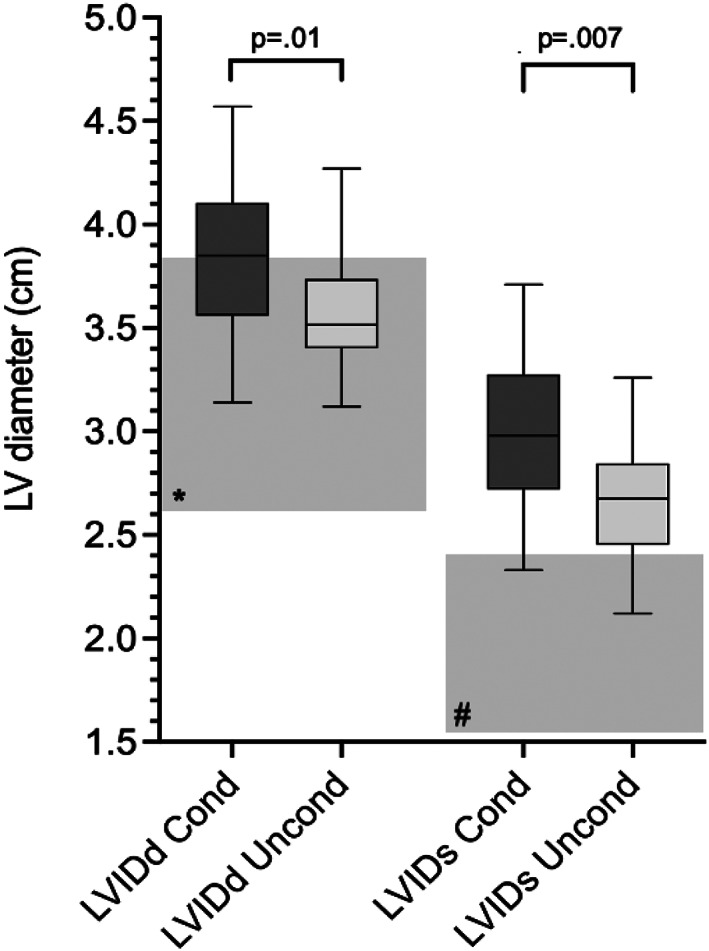
Box and whiskers plot comparing left ventricular diameter in diastole and systole between “conditioned” (n = 25) and “unconditioned” (n = 16) dogs (Mann‐Whitney U test, P < .05). Line: median value, box: 25th and 75th percentile, whiskers: range. The shaded boxes indicate the reference interval for dogs of nonspecified breed within a similar weight range (11.8‐22.7 kg) in *diastole and #systole. 21 LV, left ventricular; LVIDd, left ventricular diameter in diastole; LVIDs, left ventricular diameter in systole; Cond, “conditioned” dogs; Uncond, “unconditioned” dogs.
FIGURE 2.
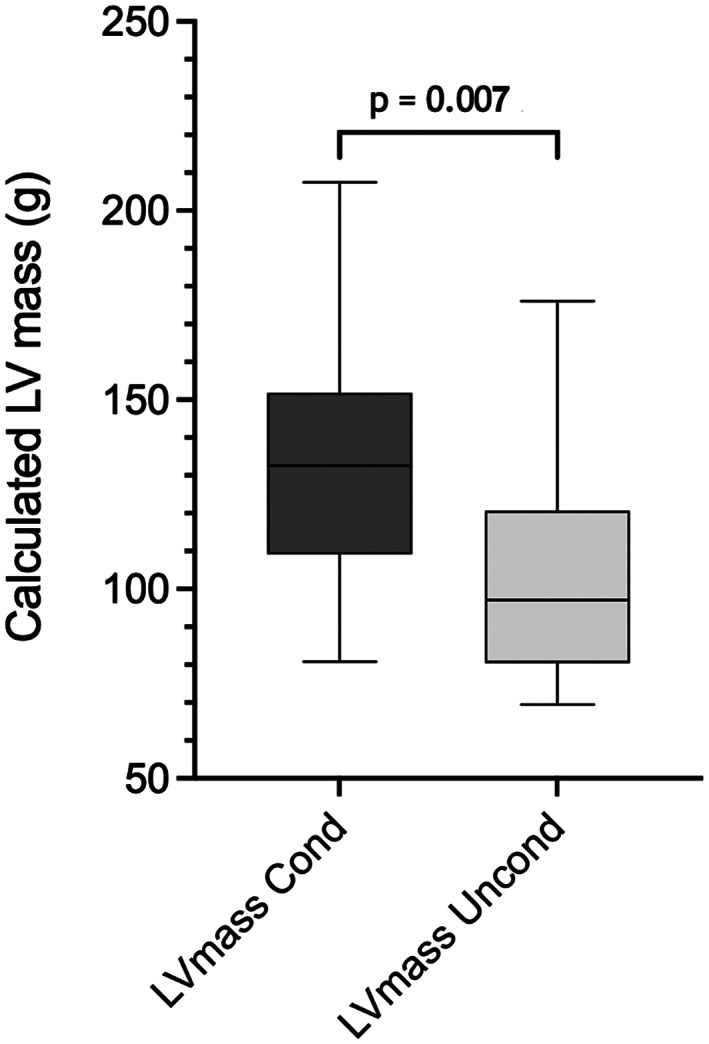
Box and whiskers plot comparing calculated left ventricular mass between “conditioned” (Cond, n = 25) and “unconditioned” (Uncond, n = 16) dogs (Students t test, P < .05). Line: median value, box: 25th and 75th percentile, whiskers: range.
4. DISCUSSION
Multiple studies have established breed‐related differences in normal echocardiographic values in dogs, particularly sighthounds. 5 , 6 , 7 , 8 , 9 , 11 , 22 , 23 , 24 Previous studies of whippets have involved populations in Europe 5 , 7 , 11 , 23 and Australia 6 and have rendered population‐specific reference ranges and indexed values. Indexing of echocardiographic variables, whether on a per kg basis, by body surface area or by use of allometric equations derived for application to all breeds 12 , 13 has allowed development of reference intervals applicable over a wide range of body weights. Breed‐specific reference intervals and indexed values have been advocated for echocardiographic measurements and calculated values. This recommendation is especially important when comparing echocardiographic measurements of chamber size in a breed where adult body weight may vary by 100% in a healthy population or in single breed populations that may differ because of local genetic breeding pools. The population of healthy whippets in our study was drawn from whippets bred and living in North America and ranged in weight from 9.8 to 22.2 kg. The mean weight of these dogs was significantly higher than that of dogs reported in previous studies drawn from European whippet populations, 5 , 7 , 23 emphasizing the need for population‐specific indexed reference intervals when weight is used in the calculations. As in previous studies, the dogs in our study had left ventricular chamber and wall dimensions generally larger than those published for dogs of nonspecified breeds. 11 , 21 The coefficients of allometric equations developed for these North American whippets (Table 5) differ from those developed in European whippets 5 and from coefficients developed for application over a range of breeds. 11 , 12 , 13 Our results provide reference intervals for echocardiographic parameters of cardiac size and function in North American whippets and add indexed values for selected commonly used parameters as well as additional information on measurements not previously available in whippets.
We did not find independent associations between sex and any of the variables assessed. This finding contrasts with previous studies, 5 , 7 in which males tended to have some values that were higher than observed in females. In those studies, the sex association did not persist when values were indexed, again supporting use of indexed normal values when assessing cardiac size in this breed. Age distribution in the dogs of our study showed a predominance of younger animals, as would be expected in a healthy population traveling to national specialty events that include both show and performance trials. Nonetheless, increasing age was found to be significantly associated with measures reflecting decreased LV early filling velocities (decreased mitral Evel, increased mitral Avel, and decreased E/A in the absence of changes in preload). The negative association between age and Evel, and age and E/A and the positive association of age and Avel are similar to the associations reported previously in 2 different studies of dogs of various breeds 25 , 26 and consistent with previous reports in people where age was the most important determinant of Doppler‐assessed LV early filling in healthy subjects, so much so that decade‐specific reference ranges for these values may be used in people. 27 , 28 Although parameters associated with LV early filling decreased with age, no dog, regardless of age, had an E/A ratio < 1.0, a value considered to indicate impaired myocardial relaxation. 25 , 29 In contrast to other studies of dogs of multiple breeds, 25 many whippets in our study had E/A ≥ 2.0 (53/97, 55%) and E/A values ≥2.0 were more common in the younger dogs (38/56 [68%] dogs ≤3 years vs 14/41 [34%] dogs >3 years of age). Increased E/A ratio because of a predominance of early diastolic filling may reflect so‐called “supernormal” diastolic function in these healthy dogs. Similar findings are seen in athletic people at rest, wherein an E/A ratio > 2 is expected, with predominance of the early diastolic phase of filling. 30
Previous studies have confirmed that whippets as a breed have increased LV dimensions and volumes on a body weight basis but found little 5 or no 7 difference between dogs purpose‐bred for racing vs dogs bred for show events. Those studies did not investigate the effect of degree of athletic conditioning at the time of examination. The effect of conditioning was examined in 2 ways in our study. The categorization of C1 (yes/no) showed a weak but positive association with calculated LVmass (P = .03; r = 0.18) and this association was retained when LVmass was normalized on a per kg basis (P = .04; r = 0.21), but C1 status was not associated with other measures of structure or function examined when compared to all other (C2, C3, and C4) dogs as a group. The calculation of LV mass is an approximation based on studies of people 19 and, although calculated LVmass may not be precisely accurate, it provides a basis by which the effect of body weight in conditioned animals can be examined. This method utilizes familiar and easily attained echocardiographic measurements and allows comparison to other canine athletes. Both conditioned and unconditioned dogs had higher LVmass/kg values than untrained sled dogs in a previous study, but similar measurements to those obtained from sled dogs after endurance training. 14 These findings may reflect a genetic component to left ventricular mass in whippets regardless of training compared to non‐purebred sled dogs, a population that reflects more genetic heterogeneity. The calculation of LVmass includes measures of LV diameter, IVS thickness and LVW thickness in diastole. The influence of each of these component parameters individually did not reach significance in our analysis, but taken together, the calculated values indicated a difference. This first analysis of association used dogs of all conditioning categories, and the inclusion of C2 dogs (dogs in “good but not peak” condition) may have obscured true differences and weakened associations. Direct comparison of groups that were more different in degree of conditioning (the second analysis, “conditioned” vs “unconditioned” dogs) eliminated the mixed conditioning of group C2 and allowed for better differentiation of echocardiographic findings. This analysis investigated differences in structure and function between dogs considered to be in “peak” athletic condition and dogs considered to be “unconditioned.”
As might be expected, currently competing “conditioned” dogs were younger as a group than were “unconditioned” dogs. Resting heart rate was lower in “conditioned” dogs, and lower resting heart rates in conditioned human and canine athletes have been reported. 14 , 31 , 32 When “conditioned” dogs were compared to “unconditioned” dogs, LVIDdN, LVIDsN and LVmass/kg were higher in “conditioned” dogs. These findings suggest that the observed larger normalized LV diameter in systole and diastole and higher LV mass in “conditioned” dogs reflect true LV hypertrophy less evident in “unconditioned” dogs, rather than differences in body weight. In addition, the higher LVIDdN, LVIDsN, and EPSS but similar IVSdN and LVWdN suggest that the LV hypertrophy in “conditioned” dogs was eccentric, consistent with typical whippet training that involves various running events. The lack of significantly higher IVS and LVW thickness in the “conditioned” dogs may reflect an actual difference in this breed vs greyhounds 15 or, given a tendency toward thicker walls in the “conditioned” group, may reflect a milder degree of conditioning in these pet animals compared to greyhounds more rigorously trained for racing.
The eccentric LV hypertrophy, accentuated measures of LV filling and unchanged systolic function at rest in the “conditioned” dogs compared to “unconditioned” dogs are typical of human and canine athletes. 14 , 15 , 30 In studies of human athletes, the degree of LV enlargement in athletes depended upon genetic background, but also body surface area, age, sex and type of sport. The “conditioned” dogs in our study were pet dogs and not bred exclusively for racing, and thus specific genetic “race‐bred” background was less likely to have contributed to the changes seen. These “conditioned” dogs were pets competing in running or agility events rather than “professional” dogs trained for competitive track racing, but showed eccentric LV hypertrophy consistent with that seen in endurance athletes. 30 The similar volumetric measurements at rest between “conditioned” and “unconditioned” dogs while resting heart rates were lower in “conditioned” dogs were supportive of higher cardiac output “reserve,” wherein the potential difference in cardiac output between rest and exercise is expected to be higher in athletes. 14 The higher E velocity in “conditioned” dogs also is consistent with the rapid early diastolic filling needed to achieve higher ventricular volumes. 30
Whippets generally tend to have larger cardiac dimensions for their size than dogs of other breeds. This finding, in combination with modifications of LV dimensions that may occur because of athletic conditioning in competitive pet dogs, may lead to overdiagnosis of LV dilatation if nonbreed specific weight‐normalized reference intervals are used. Specifically, the upper limits of the range of “normal” LVIDdN and LVIDsN in “conditioned” dogs of our study may fall outside of generic reference ranges for dogs. Use of breed‐specific ventricular measurement reference ranges normalized to body weight is of particular importance when screening dogs for evidence of cardiomyopathy in a population of athletic dogs that may display eccentric LV hypertrophy at peak condition even when not used as “professional” racing animals.
Our study has strengths and limitations. It is the only study of whippets thus far that has attempted to examine the effect of conditioning on the echocardiographic findings of this athletic breed and served to develop echocardiographic reference ranges for a North American population of whippets. The study population did not include any dogs with definable cardiac abnormalities other than trivial valvular regurgitation, eliminating the possible effects of valvular regurgitation on findings. Our population was drawn specifically from a population examined before recent reports of increased risk of abnormal cardiac findings related to specialty meat‐based non‐grain diets, 4 and the values developed in our study can be used as reference values when diet‐related or other changes in cardiac size and function are suspected.
Limitations of our study include the imprecise characterization of athletic conditioning. The owners were requested to select a conditioning level, but no attempt was made to standardize the amount of athletic training each dog had experienced. Personal bias by the owners may have obscured differences among groups, especially between C1 and C2 dogs. To maximize the difference in athletic conditioning, C1 dogs were directly compared to dogs not currently in training (C3 and C4), excluding the C2 dogs because that group contained a wide range of previous athletes, well‐conditioned but not competing dogs and pet animals considered by their owners to be in “good but not great” condition; categorization of these dogs was problematic. The study group was drawn from healthy dogs made available by their owners or breeders for examination; unknown biases and motives of the owners and breeders in dog submission likely affected some characteristics of the population, particularly age. The overall number of dogs included in this analysis was relatively small, especially for subgroup comparisons, which may have affected reference intervals and limited ability to detect the true strength of the influence of C1 status using multiple regression. Because no accepted standards exist on how to generate reference intervals for echocardiography data in healthy dogs, we elected to use standards adopted by the Clinical Laboratory Standards Institute and the American Society of Veterinary Clinical Pathology. 13 , 20 , 35 Our reference intervals consist of central 95% reference values of the data set (dependent on Gaussian versus non‐Gaussian distribution), each with 90% confidence intervals around the upper and lower limits. The latter help define the degree of uncertainty around the reference limits.
Many additional dogs were imaged, but our stringent requirement for exclusion of all but the most trivial valvular regurgitation and use of only the first examination per dog decreased the number of examinations eligible for inclusion. In some dogs, valvular regurgitation considered “mild” may not have reflected true disease but still required exclusion from analysis. Because of the large number of measurements gathered from each dog and limitations of imaging in some dogs, every animal was missing at least 1 of the recorded variables. The weight range of this single‐breed population was relatively narrow, conceivably limiting the strength of associations documented, but these indexed values still are helpful to distinguish normal from abnormal in this population. We used echocardiographic views commonly available and frequently used when screening outwardly healthy dogs for subclinical cardiac disease. Volume measures and estimated ejection fractions using a modified Simpson's method of discs were obtained using right parasternal long axis views only (monoplane) rather than a biplane measurement. A previous study 7 found volume estimates from the right parasternal long axis view to be slightly smaller than those obtained from a left apical view, but the difference was judged not to be clinically relevant. Left ventricular mass estimates were based on methods validated in people, but do provide a basis for comparison between animals with different degrees of athletic conditioning as well as with previously published reports. Although every effort was made to obtain images according to published standards, the calculation of LVmass might have been impacted if any of the contributing values were inaccurate.
Our study provides echocardiographic reference intervals for cardiac structure and function for normal North American whippets and provides values indexed for body weight for the most frequently used measurements pertaining to cardiac chamber size and volume. These values may help distinguish normal dogs of this athletic breed from dogs with myocardial or other abnormalities. Whippets considered to be in peak athletic condition have larger hearts than do less conditioned whippets, but measures of systolic function are similar. Whippet athletes in peak condition have eccentric LV hypertrophy greater than would be expected based on body weight.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
OFF‐LABEL ANTIMICROBIAL DECLARATION
The authors declare no off‐label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
Approved by the University of Wisconsin School of Veterinary Medicine (#V01414‐0‐03‐09).
HUMAN ETHICS APPROVAL DECLARATION
The authors declare human ethics approval was not needed for this study.
ACKNOWLEDGMENT
This research was partially supported by The Whippet Health Foundation and The American Kennel Club. The authors also thank the pet owners who allowed their dogs to participate in this study.
Stepien RL, Kellihan HB, Visser LC, Wenholz L, Luis Fuentes V. Echocardiographic values for normal conditioned and unconditioned North American whippets. J Vet Intern Med. 2023;37(3):844‐855. doi: 10.1111/jvim.16691
REFERENCES
- 1. Stepien RL, Kellihan HB, Fuentes VL. Prevalence and diagnostic characteristics of non‐clinical mitral regurgitation murmurs in North American whippets. J Vet Cardiol. 2017;19:317‐324. doi: 10.1016/j.jvc.2017.04.004 [DOI] [PubMed] [Google Scholar]
- 2. Stern JA, Hsue W, Song K‐H, Ontiveros ES, Luis Fuentes V, Stepien RL. Severity of mitral valve degeneration is associated with chromosome 15 loci in whippet dogs. PLoS ONE. 2015;10(10):e0141234. doi: 10.1371/journal.pone.0141234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kaplan JL, Stern JA, Fascetti AJ, et al. Taurine deficiency and dilated cardiomyopathy in golden retrievers fed commercial diets. PLoS ONE. 2018;13:e0209112. doi: 10.1371/journal.pone.0209112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Adin D, Freeman L, Stepien R, et al. Effect of type of diet on blood and plasma taurine concentrations, cardiac biomarkers, and echocardiograms in 4 dog breeds. J Vet Intern Med. 2021;35:771‐779. doi: 10.1111/jvim.16075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bavegems V, Duchateau L, Sys SU, et al. Echocardiographic reference values for whippets. Vet Radiol Ultrasound. 2007;48:230‐238. doi: 10.1111/j.1740-8261.2007.00234.x [DOI] [PubMed] [Google Scholar]
- 6. Della Torre PK, Kirby AC, Church DB, et al. Echocardiographic measurements in greyhounds, whippets and Italian greyhounds—dogs with a similar conformation but different size. Aust Vet J. 2000;78:49‐55. doi: 10.1111/j.1751-0813.2000.tb10361.x [DOI] [PubMed] [Google Scholar]
- 7. Seckerdieck M, Holler P, Smets P, Wess G. Simpson's method of discs in salukis and whippets: echocardiographic reference intervals for end‐diastolic and end‐systolic left ventricular volumes. J Vet Cardiol. 2015;17:271‐281. doi: 10.1016/j.jvc.2015.08.002 [DOI] [PubMed] [Google Scholar]
- 8. Giraut S, Häggström J, Koskinen LLE, Lohi H, Wiberg M. Breed‐specific reference ranges for standard echocardiographic measurements in salukis. J Small Anim Pract. 2019;60:374‐378. doi: 10.1111/jsap.12975 [DOI] [PubMed] [Google Scholar]
- 9. Page A, Edmunds G, Atwell R. Echocardiographic values in the greyhound. Aust Vet J. 1993;70:361‐364. doi: 10.1111/j.1751-0813.1993.tb00808.x [DOI] [PubMed] [Google Scholar]
- 10. Snyder PS, Sato T, Atkins CE. A comparison of echocardiographic indices of the nonracing, healthy greyhound to reference values from other breeds. Vet Radiol Ultrasound. 1995;36:387‐392. [Google Scholar]
- 11. Esser LC, Borkovec M, Bauer A, Häggström J, Wess G. Left ventricular M‐mode prediction intervals in 7651 dogs: population‐wide and selected breed‐specific values. J Vet Intern Med. 2020;34:2242‐2252. doi: 10.1111/jvim.15914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cornell CC, Kittleson MD, Torre PD, et al. Allometric scaling of M‐mode cardiac measurements in normal adult dogs. J Small Anim Pract. 2004;18:311‐321. doi: 10.1111/j.1939-1676.2004.tb02551.x [DOI] [PubMed] [Google Scholar]
- 13. Visser LC, Ciccozzi MM, Sintov DJ, Sharpe AN. Echocardiographic quantitation of left heart size and function in 122 healthy dogs: a prospective study proposing reference intervals and assessing repeatability. J Vet Intern Med. 2019;33:1909‐1920. doi: 10.1111/jvim.15562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Stepien RL, Hinchcliff KW, Constable PD, Olson J. Effect of endurance training on cardiac morphology in Alaskan sled dogs. J Appl Physiol. 1998;85:1368‐1375. doi: 10.1007/bf00423288 [DOI] [PubMed] [Google Scholar]
- 15. Lonsdale RA, Labuc RH, Robertson ID. Echocardiographic parameters in training compared with non‐training greyhounds. Vet Radiol Ultrasound. 1998;39:325‐330. doi: 10.1111/j.1740-8261.1998.tb01615.x [DOI] [PubMed] [Google Scholar]
- 16. Wyatt HL, Mitchell JH. Influences of physical training on the heart of dogs. Circ Res. 1974;35:883‐889. doi: 10.1161/01.res.35.6.883 [DOI] [PubMed] [Google Scholar]
- 17. Jacobson JH, Boon JA, Bright JM. An echocardiographic study of healthy Border Collies with normal reference ranges for the breed. J Vet Cardiol. 2013;15:123‐130. [DOI] [PubMed] [Google Scholar]
- 18. Thomas WP, Gaber CE, Jacobs GJ, et al. Recommendations for standards in transthoracic two‐dimensional echocardiography in the dog and cat. J Vet Intern Med. 1993;7:247‐252. [DOI] [PubMed] [Google Scholar]
- 19. Devereux RB, Reichek N. Echocardiographic determination of left ventricular mass in man. Circulation. 1977;55:613‐618. doi: 10.1161/01.cir.55.4.613 [DOI] [PubMed] [Google Scholar]
- 20. Horn PS, Pesce AJ. Reference intervals: an update. Clin Chim Acta. 2003;334:5‐23. doi: 10.1016/s0009-8981(03)00133-5 [DOI] [PubMed] [Google Scholar]
- 21. Boon JA. Veterinary Echocardiography. 2nd ed. West Sussex, UK: Wiley‐Blackwell; 2011. [Google Scholar]
- 22. Vollmar A. Echocardiographic measurements in the Irish wolfhound, reference values for the breed. J Am Anim Hosp Assoc. 1999;35:271e7‐271e277. [DOI] [PubMed] [Google Scholar]
- 23. Wess G, Bauer A, Kopp A. Echocardiographic reference intervals for volumetric measurements of the left ventricle using the Simpson's method of discs in 1331 dogs. J Vet Intern Med. 2021;35:724‐738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Dutton E, Cripps P, Harris J, et al. Echocardiographic reference intervals in healthy UK deerhounds and prevalence of preclinical dilated cardiomyopathy: a prospective, longitudinal study. J Vet Cardiol. 2022;40:142‐155. [DOI] [PubMed] [Google Scholar]
- 25. Schober KE, Fuentes VL. Effects of age, body weight, and heart rate on transmitral and pulmonary venous flow in clinically normal dogs. Am J Vet Res. 2001;62:1447‐1454. doi: 10.2460/ajvr.2001.62.1447 [DOI] [PubMed] [Google Scholar]
- 26. Garncarz M, Parzeniecka‐Jaworska M, Czopowicz M. The influence of age, gender and weight on transthoracic echocardiographic evaluation of transmitral and left ventricular outflow tract diastolic parameter sin healthy dogs. Pol J Vet Sci. 2018;22:43‐49. doi: 10.24425/pjvs.2018.125606 [DOI] [PubMed] [Google Scholar]
- 27. Benjamin EJ, Levy D, Anderson KM, et al. Determinants of Doppler indexes of left ventricular diastolic function in normal subjects (the Framingham heart study). Am J Cardiol. 1992;70:508‐515. doi: 10.1016/0002-9149(92)91199-e [DOI] [PubMed] [Google Scholar]
- 28. Spirito P, Maron BJ. Influence of aging on Doppler echocardiographic indices of left ventricular diastolic function. Br Heart J. 1988;59:672‐679. doi: 10.1136/hrt.59.6.672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Oh JK, Appleton CP, Hatle LK, Nishimura RA, Seward JB, Tajik AJ. The noninvasive assessment of left ventricular diastolic function with two‐dimensional and Doppler echocardiography. J Am Soc Echocardiogr. 1997;10:246‐270. doi: 10.1016/s0894-7317(97)70062-2 [DOI] [PubMed] [Google Scholar]
- 30. D'Andrea A, Formisano T, Riegler L, et al. Exercise for cardiovascular disease prevention and treatment, from molecular to clinical, part 1. Adv Exp Med Biol. 2017;999:21‐41. doi: 10.1007/978-981-10-4307-9 [DOI] [PubMed] [Google Scholar]
- 31. Constable PD, Hinchcliff KW, Olson J, Hamlin RL. Athletic heart syndrome in dogs competing in a long‐distance sled race. J Appl Physiol. 1994;76(1):433‐438. doi: 10.1152/jappl.1994.76.1.433 [DOI] [PubMed] [Google Scholar]
- 32. Huang G, Shi X, Davis‐Brezette JA, et al. Resting heart rate changes after endurance training in older adults: a meta‐analysis. Med Sci Sports Exerc. 2005;37:1381‐1386. doi: 10.1249/01.mss.0000174899.35392.0c [DOI] [PubMed] [Google Scholar]
- 33. Hansson K, Häggström J, Kvart C, et al. Left atrial to aortic root indices using two‐dimensional and M‐mode echocardiography in cavalier King Charles spaniels with and without left atrial enlargement. Vet Radiol Ultrasound. 2002;43:568‐575. [DOI] [PubMed] [Google Scholar]
- 34. Chetboul V, Athanassiadis N, Carlos C, et al. Assessment of repeatability, reproducibility, and effect of anesthesia on determination of radial and longitudinal left ventricular velocities via tissue Doppler imaging in dogs. Am J Vet Res. 2004;65:909‐915. [DOI] [PubMed] [Google Scholar]
- 35. Friedrichs KR, Harr KE, Freeman KP, et al. ASVCP reference interval guidelines: determination of de novo reference intervals in veterinary species and other related topics. Vet Clin Pathol. 2012;41(4):441‐453. doi: 10.1111/vcp.12006 [DOI] [PubMed] [Google Scholar]


