Abstract
Background
The behavior of the comprehensive circulating renin‐angiotensin system (RAS) in dogs with myxomatous mitral valve disease (MMVD) before to the onset of congestive heart failure remains largely unexplored.
Hypothesis/Objectives
The classical and alternative RAS activity and aldosterone concentrations will be significantly higher in dogs with American College of Veterinary Internal Medicine (ACVIM) stage B2 MMVD compared to normal dogs and dogs with ACVIM stage B1 MMVD.
Animals
One‐hundred seventeen client‐owned dogs (normal = 60; B1 = 31; B2 = 26).
Methods
Prospective observational study. Angiotensin peptides (AP) and aldosterone concentrations were measured using liquid chromatography and mass spectrometry. Angiotensin converting enzymes 1 and 2 (ACE, ACE2) and renin activity surrogates were calculated from AP concentrations. Equilibrium dialysis (ED) and immediate protease inhibition (PI) methods of AP quantification were compared in 14 healthy dogs.
Results
Core RAS activity and aldosterone concentrations did not differ among the 3 groups. However, the balance between the alternative and classical RAS differed, with dogs with stage B2 MMVD having significantly higher ACE2 activity surrogate (ACE2surr) when compared to normal dogs (adjusted P = .02; ratio of medians for ACE2surr [B2:normal], 1.89; 95% confidence interval [CI]: 1.4‐2.6). The ED and PI methods of AP quantification were highly correlated (AngI, r = .9, P < .0001; AngII, r = .8, P = .001).
Conclusions and Clinical Importance
Circulating alternative RAS activity, specifically the surrogate measure of ACE2 activity, was increased in dogs with stage B2 MMVD as compared to normal dogs. Equilibrium dialysis results are analogous to immediate protease inhibition in dogs.
Keywords: angiotensin converting enzyme 2, equilibrium dialysis, heart failure, neurohormones, protease inhibition, urine aldosterone to creatinine ratio
Abbreviations
- AA2surr
surrogate of adrenal responsiveness to angiotensin II
- ACE2surr
surrogate measure of angiotensin‐converting enzyme 2 activity
- ACEi
angiotensin converting enzyme inhibitor
- ACEsurr
surrogate measure of angiotensin‐converting enzyme activity
- ACVIM
American College of Veterinary Internal Medicine
- ALTsurr
surrogate measure of alternative renin‐angiotensin‐system activity
- Ang
angiotensin
- ANOVA
analysis of variance
- AP
angiotensin peptide
- CI
confidence Interval
- CSU‐VTH
Colorado State University Veterinary Teaching Hospital
- ED
equilibrium dialysis
- IQR
interquartile range
- LAmax:Ao
left atrium to aortic ratio obtained from right parasternal long axis 2D‐image
- LVIDdN
normalized left ventricular internal dimension in diastole
- MAS
MAS1 proto‐oncogene
- MMVD
myxomatous mitral valve disease
- PI
protease inhibition
- RAAS
renin angiotensin aldosterone system
- RAS
renin angiotensin system
- Reninsurr
surrogate measure of renin activity
- TR‐PG
Tricuspid regurgitation pressure gradient
- UAldo:C
urine aldosterone to creatinine ratio
- UCD‐VMTH
University of California, Davis Veterinary Medical Teaching Hospital
- WCI/WRI
width of the confidence interval to width of the reference interval ratio
1. INTRODUCTION
Myxomatous mitral valve disease (MMVD) is the most common cause of cardiac morbidity and mortality in dogs. 1 , 2 Activation of the circulating renin‐angiotensin‐aldosterone system (RAAS) accompanies the progression of MMVD 3 , 4 and likely contributes to disease progression via increased sodium and water retention, vasoconstriction, and pathologic remodeling of the myocardium. Blockade of the RAAS using angiotensin‐converting enzyme inhibitors (ACEi) alone and in combination with spironolactone has been shown to improve survival in dogs with heart failure caused by MMVD (ACVIM stage C). 2 , 5 , 6 , 7 , 8 , 9 , 10 , 11 , 12 However, in earlier stages of MMVD (stages B1 and B2), RAAS inhibition has not been shown to delay the time to congestive heart failure or prolong survival. 13 , 14 , 15 The reasons for this lack of effect remain unknown and may include (a) inefficiency of current RAAS inhibitors in suppressing tissue RAAS, (b) underpowered studies or inadequate RAAS inhibitor dosage or duration, or (c) RAAS activation of insufficient magnitude to adversely affect disease progression.
The renin‐angiotensin‐system (RAS) can be divided into classical and alternative arms (Figure 1). Key components of the classical RAS are angiotensin I and II (AngI, AngII), renin and angiotensin‐converting enzyme (ACE), and the angiotensin II type‐1 receptor. This arm of the RAS stimulates aldosterone release and is responsible for the classical effects of RAS described above. The alternative RAS, including ACE2, the AngII metabolite Ang1,7, and its G‐protein coupled receptor, Mas (Mas1 protooncogene), is a counter‐regulatory arm of this system that counteracts the vasoconstrictive and profibrotic actions of AngII. Angiotensin 1,5 (Ang1,5) arises from the degradation of Ang1,7 by ACE and both Ang1,7 and Ang1,5 concentrations reflect activity of the alternative arm of the RAS.
FIGURE 1.
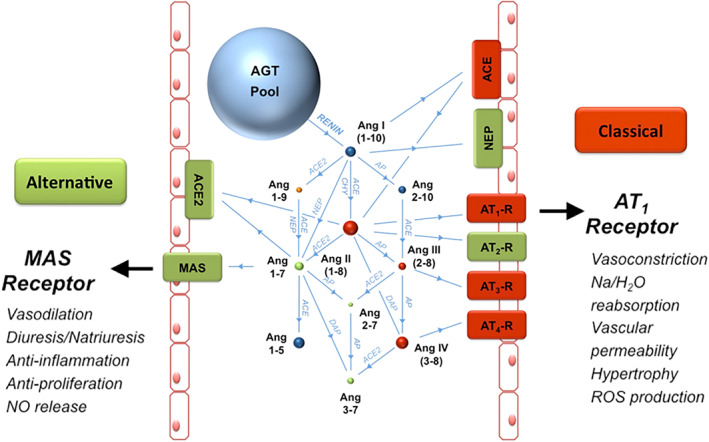
The classical and alternative renin‐angiotensin systems illustrated using the RAS Fingerprint from Attoquant diagnostics (hypothetical patient). Spheres show relative concentrations of angiotensinogen (AGT) and angiotensin (Ang) peptides. Blue spheres signify that the angiotensin peptide's action is inert and orange spheres indicate uncertain activity. Red spheres and boxes indicate key players of the classical RAS that facilitate vasoconstrictive and profibrotic effects whereas green spheres and boxes indicate key players of the alternative RAS that facilitate vasodilatory and anti‐fibrotic actions. Enzymes are shown as blue connecting lines between peptides. ACE, angiotensin‐converting enzyme; ACE2, angiotensin‐converting enzyme 2; AGT, angiotensinogen; Ang, angiotensin; AP, aminopeptidase; AT1‐R, angiotensin II type‐1 receptor; AT2‐R, angiotensin II type‐2 receptor; AT3‐R, angiotensin III receptor; AT4‐R, angiotensin IV receptor; CHY, chymase; DAP, aspartyl aminopeptidase; NEP, neutral endopeptidase (neprilysin); NO, nitric oxide; ROS, reactive oxygen species.
Liquid chromatography and mass spectrometry assays, such as the RAS Fingerprint from Attoquant Diagnostics (Figure 1), are improving our understanding of how the enzymes and angiotensin peptides (AP) of this system change during disease progression and treatment. This assay can be performed by 2 methods: immediate protease inhibition (PI) of the blood sample or simply using untreated serum or plasma. The former method provides an instantaneous picture representing actual concentrations of RAS peptides at the moment the sample was taken. The latter approach uses the concept of equilibrium dialysis (ED) where serum or plasma is incubated ex vivo at 37°C for 1 hour. Because angiotensin metabolizing enzymes remain active in the sample and exceed the concentrations of metabolites, an equilibrium of downstream metabolites is reached, where formation and degradation rates of peptides are equal. This situation represents the potential of an individual's RAS and allows for estimation of enzyme activity. The ED approach is more clinically practical and is the approach that has been reported in the veterinary literature thus far. 16 , 17 , 18 , 19 , 20
We sought to characterize the comprehensive circulating RAAS (RAS Fingerprint and aldosterone) in normal dogs and to determine the prevalence and magnitude of RAAS activation in dogs with stage B1 and B2 MMVD. Secondary aims were to evaluate week‐to‐week variation in RAS peptide concentrations in healthy dogs and compare peptide quantification from samples obtained with PI and samples obtained without PI (the latter using ED).
2. MATERIALS AND METHODS
2.1. Healthy dogs
Client‐owned apparently healthy dogs were recruited at the Colorado State University Veterinary Teaching Hospital (CSU‐VTH). Dogs of all ages, body weight, and neuter status were accepted with an aim to achieve a balance between dogs older than and younger than 6 years of age. There were no restrictions regarding type of diet and feeding schedule. Dogs were excluded if they had a history of preexisting cardiac, endocrine, respiratory, urinary, or renal disease. Additional exclusion criteria included history of polyuria or polydipsia, cough, treatment with pimobendan, ACEi, spironolactone, angiotensin receptor blocker, corticosteroid, sympathomimetic, beta‐blocker, or mineralocorticoid. Dogs documented to have a heart murmur or systemic hypertension also were excluded from the healthy dog group.
2.2. Myxomatous mitral valve disease
Client‐owned dogs diagnosed with stage B1 and B2 MMVD were prospectively enrolled through the CSU‐VTH and University of California Davis Veterinary Medical Teaching Hospital (UCD‐VMTH) cardiology services. Additional inclusion criteria included body weight <20 kg and age >5 years. Any neuter status was accepted. There were no restrictions regarding type of diet and feeding schedule. Dogs were excluded if they had a history of preexisting endocrine, respiratory, urinary, or renal disease. Additional exclusion criteria included history of polyuria or polydipsia, cough, treatment with pimobendan, ACEi, spironolactone, angiotensin receptor blocker, corticosteroid, sympathomimetic, beta‐blocker, or mineralocorticoid. Dogs were excluded if diagnostic testing identified concurrent heart disease including severe pulmonary hypertension, congenital defect, other acquired or secondary heart disease or if they had proteinuria, a serum creatinine concentration > 2.0 mg/dL, or systolic blood pressure > 180 mmHg. Dogs with 1+ proteinuria and urine specific gravity <1.040 and all dogs with ≥2+ proteinuria were screened by a urine protein to creatinine ratio and dogs with a ratio > 0.5 were excluded. Dogs with increased tricuspid regurgitation pressure gradients (TR‐PG) were excluded if >60 mm Hg.
Physical examination findings, diet, timing of sample collection relative to last meal, and patient demeanor (calm or nervous) were recorded. Dogs were gently restrained for systolic blood pressure measurement in right or left lateral recumbency, a cuff with a width of 30% to 40% of the distal forelimb radius was placed on the forelimb. At least 3 measurements within 10% of each other were averaged. Sedation with butorphanol was allowed after the blood sample and blood pressure measurement were obtained.
2.3. Blood and urine collection
Blood collection was performed with the dog gently restrained in a sitting position or lateral recumbency. Serum samples were allowed to clot at room temperature for 30 minutes before centrifugation at 3000g for 10 minutes at 4°C. Serum was divided with 1 aliquot submitted to the CSU‐VTH or the UCD‐VMTH Clinical Pathology Laboratory for serum biochemistry analysis and 2 aliquots stored at −80°C and sent out within 4 months for RAS Fingerprint analysis by ED. Blood was collected in an EDTA tube and submitted to the CSU‐VTH or the UCD‐VMTH Clinical Pathology Laboratory for CBC. A subset of normal dogs returned 7 to 14 days after their initial visit for a second blood collection to measure week‐to‐week variation and differences between PI and ED analyses. Serum was collected as described above. Additionally, 1.3 mL of whole blood was collected in a lithium heparin tube. Protease inhibitor provided by Attoquant Diagnostics was thawed and re‐suspended by 1 minute of vortexing and 60 μL of protease inhibitor was transferred to a microcentrifuge tube suspended in an ice bath. Within 1 minute after collection in the lithium heparin tube, 1.2 mL of blood was transferred to the microcentrifuge tube containing protease inhibitor and the tube was capped and inverted several times and then centrifuged at 3000g for 10 minutes at 4°C. Plasma was transferred to 2 cryovials, stored at −80°C, and sent out within 4 months for RAS Fingerprint analysis. Estimated plasma volume was calculated using Duarte's formula. 21 Urine was collected by voiding or cystocentesis. One aliquot was submitted to the CSU‐VTH or UCD‐VMTH Clinical Pathology Laboratory for urinalysis and 1 aliquot was centrifuged at 3000g for 10 minutes at 4°C and the aliquot stored at −80°C.
2.4. RAS fingerprint and serum aldosterone analysis (equilibrium and immediately stabilized)
Serum was incubated for 1 hour at 37°C to establish equilibrium concentrations before stabilization with an inhibitor cocktail. The immediately stabilized plasma samples (see above) were thawed on ice. Both the serum and plasma samples were spiked with stable isotope‐labeled internal standards for each angiotensin metabolite and aldosterone at a concentration of 200 pg/mL. Angiotensin peptides and aldosterone then were quantified using high‐performance liquid chromatography‐mass spectrometry. Details of the method are presented in Supporting Information.
Surrogates for enzyme activity, a surrogate for adrenal responsiveness, and a surrogate for the balance of the alternative to classical enzyme activity (ALTsurr) were determined from the equilibrium concentrations as follows:
Surrogate measure of ACE activity (ACEsurr)— AngII/AngI
Surrogate measure of ACE2 activity (ACE2surr)—Ang1,5/AngII
Surrogate measure of renin activity (reninsurr)—AngI + AngII
Surrogate measure of adrenal responsiveness (AA2surr)—Aldo/AngII
Surrogate measure of alternative RAS activity (ALTsurr)—(Ang1,7 + Ang1,5)/(Ang1 + AngII+Ang1,7 + Ang1,5)
The calculated enzyme surrogates (renin, ACE, and ACE2) have been shown to correlate with directly measured enzyme activity. 22 , 23 , 24 The surrogate measure of adrenal responsiveness (AA2surr; the aldosterone to AngII ratio), assesses the responsiveness of the adrenal gland in releasing aldosterone in response to AngII. 25
2.5. Urine aldosterone to creatinine ratio (UAldo:C)
Two to 5 mL of urine was centrifuged and transferred to a −80°C freezer, within 1 hour of collection and stored for future analysis. A radioimmunoassay was used to measure urine aldosterone concentration and was performed according to the manufacturer's directions (Aldosterone Active Radioimmunoassay, Beckman Coulter Inc., Brea, California) at a veterinary diagnostic laboratory (Michigan State University Veterinary Diagnostic Laboratory, Lansing, MI). This assay measures free aldosterone and the aldosterone 18β‐G metabolite (after acid hydrolysis to aldosterone). Urine creatinine concentration was determined using a standard colorimetric assay at the same laboratory.
2.6. Echocardiogram
A complete echocardiogram was performed by a cardiologist or supervised cardiology resident using either a Philips Epiq7 or CVx machine. Standard echocardiographic 2‐dimensional, M‐mode, and Doppler images were obtained from both right and left parasternal windows. Echocardiographic variables analyzed and included in the analysis were left atrial‐to‐aortic ratio (LAmax:Ao; measured on the 2D right parasternal long axis view), 26 normalized left ventricular internal dimension in diastole (LVIDdN; measured on the left ventricular m‐mode), 27 and presence of an estimated TR‐PG > 35 mm Hg (evaluated as a categorical variable, yes/no). At least 3 measurements for each variable were obtained and averaged. MMVD was confirmed based on thickening and insufficiency of the mitral valve leaflets. Dogs were classified as stage B1 or B2 based on ACVIM guidelines using the left atrial‐to‐aortic ratio measured at the right parasternal short axis 28 and the LVIDdN as described above. 2
2.7. Ethics approval
The study was approved by the clinical trials review boards at Colorado State University and the University of California, Davis. Owner permission was obtained by signed consent before enrolling patients.
2.8. Statistical analysis
Power analysis was based on data obtained from a prior study. 16 The difference in serum AngII in normal dogs at baseline and approximately 4 hours after IV furosemide administration mimics mild to moderate RAAS activation and was 23.0 pmol/L with a SD of 38.9 pmol/L. Using these data and an alpha of .05 and power of .80, it was determined that at least 45 dogs per group would be ideal. When creating reference intervals, sample sizes >55 are recommended to increase precision of the upper and lower limits. 29 Keeping budget constraints in mind, enrollment targets for the normal and MMVD groups were set at 60 each. The dogs with MMVD were further divided into 2 groups (B1 and B2) and analyses were performed with 3 groups (normal, B1, and B2). This approach decreased statistical power but was considered to have more potential for clinically relevant hypothesis generation. It was decided a priori to not analyze these data as 2 groups (normal and B1/B2). Normality was assessed using histograms and QQ plots, and non‐normally distributed variables were log‐transformed before analysis. Normal distribution after transformation was confirmed by inspection of histograms and residual diagnostic plots. Medians and interquartile ranges (IQR) are reported as summary statistics and for group comparisons.
Reference intervals were determined from the 60 normal dogs and represent the central 95% of the study sample's values with the 2.5th and 97.5th quantiles serving as the lower and upper limits, respectively. The 90% confidence intervals (CI) for upper and lower limits of the reference interval and the width of the CI/width of the reference interval (WCI/WRI) were calculated using a robust bootstrap method for obtaining the 5th and 95th quantiles from 1000 ranked bootstrap samples for each of the upper and lower bounds
After log transformation, serum AP and aldosterone concentrations, surrogates, and the UAldo:C were compared among the 3 groups using analysis of variance (ANOVA). Welch's ANOVA p values were reported in all cases, regardless of variance. Analytes with results below the lower limit of quantification were halved and this result was used in the analysis. Differences in age, time between blood sample and last meal, sex (M, F, SF, MN), and demeanor (calm or nervous) were determined using ANOVA or a Chi‐squared test. The Pearson correlation coefficient was used to measure the association between RAAS analytes and these variables. Variables that differed significantly among groups and found to correlate with the RAAS analytes were handled as possible confounders and were used as covariates in a linear regression model.
Echocardiographic variables (LAmax:Ao, LVIDdN, pulmonary hypertension [y/n]), serum biochemical variables (serum potassium and creatinine concentrations), and estimated plasma volume were compared among the 3 groups using ANOVA. The association between the RAAS analytes and echocardiographic variables (LAmax:Ao, LVIDdN, pulmonary hypertension [y/n]), serum biochemical variables (serum potassium and creatinine concentrations), and estimated plasma volume was evaluated using the Pearson correlation coefficient (continuous variables) or a t test (pulmonary hypertension [y/n]) using a pooled or Satterthwaite method depending on equality of variances.
The Pearson correlation coefficient was used to measure the association between PI samples and samples analyzed using the ED (nonprotease inhibited) approach. Strength of correlation was described as very high, high, moderate, low, and negligible for r values of .9 to 1.0, .7 to .089, .5 to .69, .3 to .49, and 0 to .29, respectively. Week‐to‐week variation for each analyte was evaluated by calculating the week‐to‐week coefficient of variation for each dog. A paired t test also was used to compare group differences between the 2 timepoints. The coefficients of variation for several measured analytes (AngI and II, aldosterone, Ang1,5, and Ang1,7) were compared between dogs classified as either normal or nervous using a t test and the pooled or Satterthwaite method, depending on equality of variances.
3. RESULTS
3.1. Dogs
One‐hundred thirty‐two dogs were enrolled in the study. Fifteen dogs were excluded (11 had proteinuria with UP:C > 0.5 with 2 of these receiving prohibited drugs; 2 were inadvertently enrolled twice, 1 was in heart failure, and 1 normal dog had marked activation of RAAS without any clinically apparent reason and was determined statistically to be an outlier for all APs and aldosterone), leaving 117 dogs included in the analysis cohort. Dogs in the normal group were heavier and younger than dogs in the B1 and B2 groups (P < .0001; Table 1). The number of dogs determined to be calm or nervous during blood pressure measurement and venipuncture did not differ between groups. Median (IQR) time between the last meal and blood sampling differed significantly between groups and was 5.2 hours (3.2‐14.5), 7.8 hours (3.8‐15), and 9.7 hours (6.0‐16.6) for the normal, B1, and B2 groups, respectively (P = .04).
TABLE 1.
Median (interquartile range) values for demographic, clinical, and echocardiographic variables for all dogs
| Parameter | Normal (N = 60) | B1 (N = 31) | B2 (N = 26) | Reference interval | P value |
|---|---|---|---|---|---|
| Age (year) | 4.5 (2.7‐7.0) | 10.6 (8.8‐11.7) a | 11.0 (9.8‐12.1) a | — | <.0001 |
| Weight (kg) | 20.7 (8.4‐29.3) | 6.2 (4.9‐9.0) a | 8.0 (6.1‐9.5) a | — | <.0001 |
| Meal to sample time (hours) | 5.2 (3.2‐14.5) | 7.8 (3.8‐15.0) | 9.7 (6.0‐16.6) a | — | .02 |
| Blood pressure (mm Hg) | 124 (115‐138) | 146 (128‐155) a | 140 (122‐146) | 110‐160 mmHg | .002 |
| ePV (dL/g) | 2.7 (2.3‐3.0) | 2.9 (2.6‐3.4) | 3.1 (2.8‐3.6) a | — | .0004 |
| Potassium (mmol/L) | 4.4 (4.3‐4.7) | 4.7 (4.4‐5.0) a | 4.4 (4.3‐4.7) | 3.6‐4.8 mmol/L | .04 |
| Creatinine (mg/dL) | 1.0 (0.8‐1.2) | 0.9 (0.7‐1.1) | 0.9 (0.7‐1.0) | .8‐1.5 mg/dL | .05 |
| LAmax:Ao | 2.0 (1.9‐2.1) | 2.3 (2.2‐2.5) a | 3.0 (2.8‐3.3) a , b | <2.6 | <.0001 |
| LVIDdN | 1.4 (1.3‐1.5) | 1.5 (1.4‐1.6) a | 1.9 (1.8‐2.0) a , b | <1.7 | <.0001 |
Abbreviations: AePV, estimated plasma volume; LAmax:Ao, left atrium to aortic ratio (left atrium measured at maximal dimension from the two‐dimensional right parasternal long axis view); LVIDdN, left ventricular internal dimension in diastole normalized for body weight.
Differs significantly from the normal group.
Differs significantly from the B1 group.
3.2. Blood pressure, echocardiography, serum biochemistry, CBC, and urinalysis results
All variables except for LAmax:Ao were normally distributed. Abnormalities incidentally detected in the minimum database included 12 dogs with mild (normal = 5, B1 = 1, B2 = 6) and 1 dog (B1 group) with a marked increase in ALP activity and 13 dogs with mild increases in ALT activity (normal = 3, B1 = 4, B2 = 6). Seven dogs had mildly increased BUN concentration (normal = 3, B1 = 3, B2 = 1). Only 1 dog (B1 group) had a mildly increased serum creatinine concentration (1.7 mg/dL). Three dogs were isosthenuric (normal = 2, B2 = 1) and 4 dogs were hyposthenuric (normal = 3, B2 = 1). Serum potassium concentration was significantly higher in the B1 group compared to the normal group (Table 1). Systolic blood pressure was significantly higher in the B1 group compared to the normal group (P = .002). Estimated plasma volume was significantly higher in the B2 group compared to the normal group (P = .0004) and LAmax:Ao and LVIDdN differed significantly among groups as expected (P < .0001).
Correlations between analytes (AP and aldosterone concentrations, surrogates, and the UAldo:C) and clinicopathologic and echocardiographic variables were evaluated in all 117 dogs as a single cohort (Table 2). All variables were non‐normally distributed. Negligible correlations were found between LAmax:Ao and Ang1,5 (r = .2, P = .03) and Ang1,7 (r = .2, P = .01). A low, negative correlation was found between LAmax:Ao and LVIDdN and ACEsurr (r = −.3, P = .004 and r = −.3, P = .005, respectively). Both LAmax and LVIDdN had low or negligible correlation with ACE2surr (r = .3, P = .0004 and r = .2, P = .02). The LAmax:Ao had low correlation with ALTsurr (r = .3, P = .006). Correlation between serum creatinine concentration and ACEsurr (r = .2, P = .02) was negligible. Negligible to low, negative correlations were found between serum creatinine concentration and Ang1,5, ACE2surr, ALTsurr, and UAldo:C (r = −.2, P = .04; r = − .3, P = .0001; r = −.3, P = .003; r = −.4, P < .0001, respectively). Negligible correlation was found between serum potassium concentration and ACEsurr (r = .2, P = .04). Estimated plasma volume did not have a significant correlation with any of the measured or calculated RAAS variables. Seventeen dogs had estimated TR‐PG > 35 mm Hg (B1 = 10 and B2 = 7). Serum aldosterone concentration was significantly lower (median [IQR]: 12 pmol/L [5.0‐48.2] vs 44.8 pmol/L [17.9‐86.6]) and ALTsurr significantly higher (0.42 [0.36‐0.54] vs 0.37 [0.30‐0.46]) in dogs with an increased TR‐PG (P = .01 and .03, respectively).
TABLE 2.
Correlation between age, mean to sample time, left atrial to aortic ratio, normalized diastolic left ventricular internal dimension, tricuspid regurgitation pressure gradient and angiotensin peptide and aldosterone concentrations, enzyme surrogates, adrenal function surrogate, and alternative renin‐angiotensin system surrogate in 19 normal dogs
| Person correlation coefficient; P value | t test P value | ||||
|---|---|---|---|---|---|
| Analyte | Age | Meal to sample time (h) | LAmax: Ao | LVIDdN | TR‐PG >35 mmHg (y/n) |
| AngII (pmol/L) | −0.006; .9 | 0.1; .2 | −0.4; .7 | −0.07; .5 | .74 |
| Ang1,7 (pmol/L) | 0.2; .01 | 0.1; .2 | 0.2; .01 | 0.1; .2 | .35 |
| AngI (pmol/L) | 0.1; .2 | 0.2; .03 | 0.2; .1 | 0.1; .3 | .74 |
| AngIII (pmol/L) | 0.09; .3 | 0.3; .003 | 0.04; .6 | 0.9; .3 | .49 |
| Ang1,5 (pmol/L) | 0.2; .05 | 0.2; .06 | 0.2; .03 | 0.1; .3 | .39 |
| AngIV (pmol/L) | 0.009; .9 | 0.2; .04 | 0.02; .8 | 0.03; .7 | .31 |
| Aldo (pmol/L) | −0.1; .1 | 0.1; .2 | −0.1; .2 | 0.2; .08 | .01 |
| AA2 ([pmol/L]/[pmol/L]) | −0.1; .1 | 0.05; .6 | −0.1; .3 | 0.1; .2 | .01 |
| Reninsurr pmol/L | 0.07; .4 | 0.2; .05 | 0.09; .4 | 0.04; .7 | .7 |
| ACEsurr ([pmol/L]/[pmol/L]) | −0.1; .07 | −0.1; .3 | −0.3; .0004 | −0.3; .005 | .97 |
| ACE2surr ([pmol/L]/[pmol/L]) | 0.3; .006 | 0.1; .3 | 0.3; .0004 | 0.2; .02 | .12 |
| ALTsurr ([pmol/L]/[pmol/L]) | 0.3; .005 | 0.03; .7 | 0.3; .006 | 0.1; .2 | .03 |
| UAldo:C (μg/g) | 0.2; .1 | 0.08; .5 | 0.1; .2 | 0.1; .2 | .6 |
Note: Bold values denote statistical significance at the P < 0.05 level.
Abbreviations: AA2, adrenal function surrogate (Aldo/AngII); ACEsurr, angiotensin‐converting enzyme surrogate (AngII/AngI); ACE2surr, surrogate measure of angiotensin‐converting enzyme 2 activity (Ang1,5/AngII); Aldo, aldosterone; ALTsurr, surrogate measure of alternative RAS activity (Ang1,7 + Ang1,5)/(Ang1 + AngII+Ang1,7 + Ang1,5); Ang, angiotensin; Reninsurr, surrogate measure of renin activity (AngI + AngII); LAmax:Ao: left atrium to aortic ratio (left atrium measured at maximal dimension from the two‐dimensional right parasternal long axis view); LVIDdN, left ventricular internal dimension in diastole normalized for body weight; TR‐PG: tricuspid regurgitation pressure gradient.
3.3. RAS fingerprint and serum aldosterone and UAldo: C analysis
Reference intervals were determined for all RAAS analytes measured in the 60 healthy dogs. The WCI/WRI of the lower limit of the reference interval was <0.2 for all RAAS analytes whereas the WCI/WRI of the upper limit was >0.2 for all RAAS analytes. (Table 3)
TABLE 3.
Ninety five percent confidence intervals and reference intervals for angiotensin peptide and aldosterone concentrations, enzyme surrogates, adrenal function surrogate, alternative renin‐angiotensin system surrogate, and urine aldosterone to creatinine ratio from 60 normal dogs
| Analyte | 95% CI | Reference interval | 90% CI of Lower limit | 90% CI of Upper limit | WCI/WRI Lower limit | WCI/WRI Upper limit |
|---|---|---|---|---|---|---|
| AngII (pmol/L) | 14.1‐226.3 | 17.2‐218.1 | 9.5‐26.7 | 158.1‐264.3 | 0.08 | 0.53 |
| Ang1,7 (pmol/L) | 6.2‐160.2 | 7.0‐160.7 | 5.4‐9.9 | 97.9‐242.7 | 0.03 | 0.94 |
| AngI (pmol/L) | 27.5‐299.6 | 28.9‐284.9 | 23.0‐37.5 | 179.9‐381.2 | 0.06 | 0.79 |
| AngIII (pmol/L) | 1.0‐25.6 | 1.0‐23.7 | 1.0‐1.0 | 12.7‐34.5 | 0 | 0.96 |
| Ang1,5 (pmol/L) | 11.2‐287.0 | 13.6‐278.3 | 7.9‐21.0 | 162.3‐413.5 | 0.05 | 0.95 |
| AngIV (pmol/L) | 1.0‐28.8 | 1.1‐29.9 | 1.0‐2.1 | 20.8‐43.7 | 0.04 | 0.80 |
| AA2 ([pmol/L]/[pmol/L]) | 0.07‐4.0 | 0.1‐3.7 | 0.03‐0.1 | 2.5‐4.9 | 0.03 | 0.67 |
| Reninsurr pmol/L | 45.3‐501.4 | 49.0‐481.3 | 32.5‐67.6 | 367.0‐581.6 | 0.08 | 0.50 |
| ACEsurr ([pmol/L]/[pmol/L]) | 0.3‐1.7 | 0.3‐1.9 | 0.3‐0.4 | 1.4‐3.0 | 0.07 | 1.24 |
| ACE2surr ([pmol/L]/[pmol/L]) | 0.3‐2.0 | 0.3‐2.3 | 0.3‐0.4 | 1.6‐4.0 | 0.09 | 1.20 |
| ALTsurr ([pmol/L]/[pmol/L]) | 0.2‐0.5 | 0.2‐0.5 | 0.2‐0.3 | 0.5‐0.6 | 0.19 | 0.34 |
| Aldo (pmol/L) | 5.0‐186.4 | 5.0‐184.4 | 5.0‐5.0 | 154.8‐219 | 0 | 0.36 |
| UAldo:C (μg/g) | 0.2‐1.2 | 0.2‐1.2 | 0.1‐0.2 | 1.0‐1.3 | 0.04 | 0.30 |
Abbreviations: AA2, adrenal function surrogate (Aldo/AngII); ACEsurr, surrogate measure of angiotensin‐converting enzyme activity (AngII/AngI); ACE2surr, surrogate measure of angiotensin‐converting enzyme 2 activity (Ang1,5/AngII); Aldo, aldosterone; ALTsurr: surrogate measure of alternative RAS activity (Ang1,7 + Ang1,5)/(Ang1 + AngII+Ang1,7 + Ang1,5); Ang, angiotensin; Reninsurr, surrogate measure of renin activity (AngI + AngII); WCI/WRI, ratio of the width of the confidence interval to the width of the reference interval.
Results were below the lower limit of quantification in 8% of measurements of directly quantified AP and aldosterone concentrations (normal: 29/420, B1: 22/217, and B2 17/182). No significant differences among groups were noted in the classical RAAS peptides (AngI, II, III, IV, Aldo), Ang1,5, and UAldo:C (Figure 2 and Table 4). Angiotensin 1‐7 and ACEsurr were significantly higher in the B2 group when compared to the normal group (P = .03 in both cases; Table 4). The ACE2surr was significantly higher in the B2 group when compared to dogs in the normal and B1 groups (P = .0005; Tables 4 and S1). The ALTsurr was significantly higher in the B2 dogs when compared to normal dogs (P = .001; Tables 4 and S1). Age and time between last meal and blood sampling were correlated with a few RAAS analytes (Table 2). As such, comparisons of RAAS analytes among groups were repeated after controlling for age and time between the last meal and blood sampling. After this adjustment, ACE2surr remained significantly different between the B2 and normal groups (adjusted P = .02; Tables 4 and S1).
FIGURE 2.
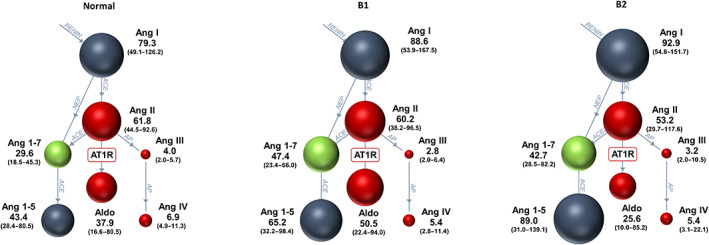
The renin‐angiotensin system (RAS) Fingerprint from 60 normal dogs, 31 dogs with ACVIM stage B1, and 26 dogs with ACVIM stage B2 myxomatous mitral valve disease. Spheres show relative concentrations of angiotensin peptides. Median (interquartile range) concentrations of angiotensin peptides are shown next to the spheres (pmol/L). ACE, angiotensin‐converting enzyme; ACE2, angiotensin‐converting enzyme 2; Aldo, aldosterone; Ang, angiotensin; AP, aminopeptidase; AT1R, angiotensin type‐1 receptor; DAP, aspartyl aminopeptidase; NEP, neutral endopeptidase (neprilysin).
TABLE 4.
Median (interquartile range) of angiotensin peptide and aldosterone concentrations, enzyme surrogates, adrenal function surrogate, alternative renin‐angiotensin system surrogate, and urine aldosterone to creatinine ratio from all dogs
| Analyte | Normal | B1 | B2 | Unadjusted P value | Adjusted P value |
|---|---|---|---|---|---|
| AngII (pmol/L) | 61.8 (44.5‐92.6) | 60.2 (38.2‐96.5) | 53.2 (29.7‐117.6) | .9 | .7 |
| Ang1,7 (pmol/L) | 29.6 (18.5‐45.3) | 47.4 (23.4‐66.0) a | 42.7 (28.5‐82.2) a | .03 | .3 |
| AngI (pmol/L) | 79.3 (49.1‐126.2) | 88.5 (53.9‐167.5) | 92.9 (54.8‐151.7) | .5 | .8 |
| AngIII (pmol/L) | 4.0 (1.2‐5.7) | 2.8 (1.0‐6.4) | 3.2 (1.0‐10.5) | .3 | .1 |
| Ang1,5 (pmol/L) | 43.4 (28.4‐80.1) | 65.2 (32.2‐98.4) | 89.0 (31.0‐139.1) | .1 | .3 |
| AngIV (pmol/L) | 6.9 (4.9‐11.3) | 5.4 (2.8‐11.4) | 5.4 (3.1‐22.1) | .5 | .5 |
| Aldo (pmol/L) | 37.9 (16.6‐80.5) | 50.5 (22.4‐94.0) | 25.6 (5.0‐86.2) | .2 | .5 |
| AA2 ([pmol/L]/[pmol/L]) | 0.6 (0.3‐1.2) | 0.72 (0.3‐1.5) | 0.4 (0.2‐1.4) | .3 | .3 |
| Reninsurr pmol/L | 147.1 (91.0‐220.5) | 156.7 (94.9‐250.3) | 155.8 (99.2‐260.2) | .9 | .9 |
| ACEsurr ([pmol/L]/[pmol/L]) | 0.8 (0.6‐1.0) | 0.7 (0.5‐1.0) | 0.6 (0.4‐0.8) a | .03 | .1 |
| ACE2surr ([pmol/L]/[pmol/L]) | 0.8 (0.5‐1.2) | 0.9 (0.6‐1.3) | 1.6 (0.9‐2.0) a , b , c | .0005 | .02 |
| ALTsurr ([pmol/L]/[pmol/L]) | 0.34 (0.29‐0.42) | 0.40 (0.33‐0.48) | 0.43 (0.38‐0.54) a | .001 | .08 |
| UAldo:C (μg/g) | 0.47 (0.34‐0.62) | 0.49 (0.42‐0.91) | 0.93 (0.32‐1.2) | .07 | .5 |
Note: Bold values denote statistical significance at the P <<0.05 level.
Abbreviations: ACEsurr, angiotensin‐converting enzyme surrogate (AngII/AngI); ACE2surr, surrogate measure of angiotensin‐converting enzyme 2 activity (Ang1,5/AngII); AA2, adrenal function surrogate (Aldo/AngII); Aldo, aldosterone; ALTsurr, surrogate measure of alternative RAS activity (Ang1,7 + Ang1,5)/(Ang1 + AngII+Ang1,7 + Ang1,5); Ang, angiotensin; Reninsurr, surrogate measure of renin activity (AngI + AngII).
Differs significantly from the normal group.
Differs significantly from the B1 group.
Differs significantly from the normal group after controlling for age and meal to sample time (in multiple regression model).
3.4. Week‐to‐week variation in RAS fingerprint and aldosterone
Nineteen healthy dogs returned 1 week (median, 7 days; range, 4‐10 days) after enrollment and initial blood sampling. Median week‐to‐week coefficients of variation for the RAAS analytes and calculated surrogates ranged from 10.4% to 93.5% (Table 5). The ALTsurr had the lowest coefficient of variation (median, 10.4%; range, 0.9%‐27.2%). No analyte differed significantly between weeks 1 and 2. The coefficients of variation for the RAAS analytes did not differ between dogs classified as calm vs those classified as nervous.
TABLE 5.
Median (range) of week‐to‐week coefficient of variation in angiotensin peptide and aldosterone concentrations, enzyme surrogates, adrenal function surrogate, and alternative renin‐angiotensin system surrogate in 19 normal dogs
| Analyte | Week‐to‐week CV (%) | Paired t test P value week 1 vs week 2 | Paired t test: mean difference; SD of difference |
|---|---|---|---|
| AngII (pmol/L) | 37.8 (3.8‐89.3) | .6 | .08; .7 |
| Ang1,7 (pmol/L) | 29.0 (7.6‐95.6) | .4 | .2; .8 |
| AngI (pmol/L) | 28.9 (4.2‐101.1) | .8 | .05; .7 |
| AngIII (pmol/L) | 93.5 (13.9‐133.5) | 1 | .01; 1.6 |
| Ang1,5 (pmol/L) | 54.8 (3.1‐93.8) | .8 | .07; .9 |
| AngIV (pmol/L) | 41.5 (4.6‐127.9) | .6 | .1; 1.0 |
| Aldo (pmol/L) | 65.4 (14.0‐107.6) | .07 | .4; .9 |
| AA2 ([pmol/L]/[pmol/L]) | 51.8 (6.3‐117.3) | .06 | .5; 1.0 |
| Reninsurr pmol/L | 29.7 (1.5‐95.7) | .8 | .03; .7 |
| ACEsurr ([pmol/L]/[pmol/L]) | 14.9 (0.1‐61.7) | 1 | .001; .3 |
| ACE2surr ([pmol/L]/[pmol/L]) | 22.4 (0.1‐80.6) | .8 | .03; .5 |
| ALTsurr ([pmol/L]/[pmol/L]) | 10.4 (0.9‐27.2) | .3 | .05; .2 |
Abbreviations: AA2, adrenal function surrogate (Aldo/AngII); ACEsurr, angiotensin‐converting enzyme surrogate (AngII/AngI); ACE2surr, surrogate measure of angiotensin‐converting enzyme 2 activity (Ang1,5/AngII); Aldo, aldosterone; ALTsurr, surrogate measure of alternative RAS activity (Ang1,7 + Ang1,5)/(Ang1 + AngII+Ang1,7 + Ang1,5); Ang, angiotensin; Reninsurr, surrogate measure of renin activity (AngI + AngII).
3.5. Protease inhibition vs equilibrium dialysis
A second blood sample was immediately protease inhibited in 14 healthy dogs. Two APs (AngI and AngII) were measurable (above the lower limit of quantification) after PI of the blood sample. Correlation between RAS quantification via PI and ED for AngI (r = .9, P < .0001) and AngII (r = .8, P = .0006) was very high and high, respectively. The ACEsurr (AngI + AngII) measured by these 2 methods had high correlation (r = .7, P = .005).
4. DISCUSSION
We characterized the comprehensive circulating RAS activity and aldosterone concentrations in normal dogs and dogs with stage B1 and B2 MMVD. The circulating classical RAS (AngI, AngII, reninsurr, ACEsurr) and aldosterone did not differ among these 3 groups. The balance between the circulating alternative (Ang1,7, Ang1,5, and ACE2) and classical RAS, however, did differ with dogs at stage B2 MMVD having significantly higher estimated ACE2 activity when compared to normal dogs. The week‐to‐week intra‐dog coefficient of variation for measured RAS analytes was high, yet the balance of the alternative to classical RAS peptides (ALTsurr) remained relatively stable from week to week (median, 10.4%; range, 0.9%‐27.2%). We also showed that the less cumbersome ED method of AP quantification was highly correlated with quantification using immediate PI. Ninety‐five percent CI for serum concentrations of APs, aldosterone, and enzyme surrogates in normal dogs are presented to facilitate future comparisons and study design.
The circulating, classical RAS activity and aldosterone concentrations did not differ significantly between normal dogs and dogs with stage B1 and B2 MMVD. Before correction for the covariates of age and meal‐to‐sample time, Ang1,7 was significantly higher in both the B1 and B2 groups as compared to the normal group, the ALTsurr was significantly higher in the B2 group as compared to the normal group, and the ACEsurr was significantly lower in the B2 group as compared to the normal group. These differences did not persist after correcting for the 2 covariates. The ACE2surr was significantly higher in the B2 group when compared to both B1 and normal, and the difference between the B2 and normal groups persisted after correcting for covariates (Tables 4 and S1). The covariation of age and meal‐to‐sample time with several analytes may have arisen, in part, from the study design. Healthy dogs were recruited from the Community Practice Service and from students, staff, and faculty at the CSU VMTH and were seen at weekend morning clinics whereas dogs with B1 and B2 MMVD were recruited as they presented to our cardiology clinics. Dogs that fit the clinical inclusion criteria for the healthy group tended to be younger. The correction for these covariates in the statistical plan may be viewed as excessively stringent, because only a few variables covaried. Regardless, this analysis strengthened the finding that increased ACE2 activity accompanied the progression of MMVD in this cohort. The significant changes in the ALTsurr and Ang1,7 concentrations before the correction is a compelling finding that warrants further study. Whereas the ACEsurr and AngII concentrations were negatively correlated with LAmax:Ao and LVIDDn, the alternative peptides and surrogates (Ang1,7, Ang1,5, ACE2surr, and ALTsurr) were positively correlated with these measures of left heart size (Table 2). This finding supported a shift in the RAS towards increased ACE2 activity and accumulation of alternative RAS peptides as the severity of MMVD increased in this cohort.
Increased circulating ACE2 has been associated with worse severity of structural and functional abnormalities, and is an independent predictor of adverse clinical events in humans with heart failure 30 , 31 , 32 , 33 and mortality in patients with acquired aortic stenosis. 34 Angiotensin‐converting enzyme 2 catalyzes the conversion of AngI and II to Ang1,9, Ang1,7, and Ang1,5 (Figure 1). Of the resulting metabolites, Ang1,7 is the best studied and has been found to act at the Mas receptor to counteract the vasoconstrictive, prohypertrophic, and profibrotic actions of the classical RAS. 35 Angiotensin‐converting enzyme 2 is expressed in the myocardium and is usually tissue bound, conferring its protective effects locally at the tissue level. 36 Enhanced circulating ACE2 likely implies higher cleavage from tissues by the protease ADAM17 (also called tumor necrosis factor‐alpha converting enzyme or TACE) resulting in less tissue ACE2 to mitigate local tissue effects of the classical RAS. Angiotensin II itself induces ADAM17 cleavage of ACE2, creating a vicious cycle of RAS activation and unchecked effects of the classical RAS on tissues. 37 The correlation between increased plasma ACE2 concentration and markers of ADAM17 activation has been demonstrated in people with severe acute respiratory syndrome coronavirus 2 (COVID‐19). 38 Although increased unbound ACE2 likely creates a more favorable balance between the alternative and classical RAS in the circulation, it likely reflects a less favorable tissue RAAS environment where the profibrotic and proinflammatory effects of AngII meet little opposition. Additional mechanisms of increased circulating ACE2 concentrations include increased cellular expression and decreased clearance.
Changes in tissue RAS during disease and the impact of treatment on this local system remain largely unexplored in veterinary medicine. Whether the increase in circulating ACE2 in these dogs with stage B2 MMVD reflects higher tissue AngII or ADAM17 activity or both cannot be confirmed from these data, but the hypothesis warrants further investigation. Whether ACE2surr or ALTsurr or both could serve as biomarkers for MMVD disease severity also remains undetermined. The week‐to‐week intra‐dog coefficient of variation for measured individual RAAS analytes was high, which is likely reflective of the many variables that affect renin release. The RAS Fingerprint assay coefficient of variation is <10% within reference intervals and < 15% when close to the lower limit of quantification for all covered analytes. Considering this and given that dogs were screened for systemic and cardiovascular disease, the disparity noted for the individual analytes may reflect variations in stress level, timing of the sample with the most recent meal, hydration, and dietary sodium intake. The ALTsurr ([Ang1,7 + Ang1,5]/[Ang1 + AngII+Ang1,7 + Ang1,5]), a ratio of alternative to classical RAS peptides, remained relatively stable (median, 10.4%; range, 0.9%‐27.2%). This lower variation of the ALTsurr suggested that the balance of alternative vs classical enzyme activity (i.e., ACE2 vs ACE) remained relatively stable as compared to the AP concentrations. This surrogate may serve as a more reliable indicator of a patient's RAS activity and may have prognostic value.
Neither serum aldosterone concentration nor the UAldo:C differed among groups. The UAldo:C did not change in concert with serum aldosterone concentration and increased with advancing disease whereas serum aldosterone concentration was highest in the B1 group and lowest in the B2 group. The spot blood aldosterone concentrations varied widely in normal dogs, likely reflecting variations in hydration status, dietary sodium intake, and level of excitement. Urine aldosterone concentration represents hours of aldosterone secretion and may be a better indicator of blood aldosterone concentration. However, day‐to‐day variation in the UAldo:C is high, 39 limiting the utility of this biomarker. Systolic blood pressure mirrored the change in the serum aldosterone concentration and AA2surr. This increase in circulating aldosterone concentration is a possible mechanism behind the increased blood pressure in the B1 group.
The RAAS reference intervals calculated from the 60 normal dogs had precise lower limits, but the more clinically relevant upper limits were imprecise (Table 3). It has been recommended that reference interval determination in veterinary species should aim to include at least 120 subjects, 40 yet it is likely this number is higher for many of the RAAS peptides with high WCI/WRI values. The most precise upper limits were noted for ALTsurr and UAldo:C. Overall RAAS activity in the normal dogs was higher when compared to previously reported results from a cohort of 6 healthy, middle‐aged, purpose‐bred male Beagles. 16 This difference may be explained by the lack of acclimation in pet dogs as compared to trained research dogs, environmental and dietary differences in owned vs research dogs, breed‐related differences in RAS, or small sample size. Regardless of the imprecision of these reference intervals, the 95% CI still should prove useful for future power calculations, study design development, and comparisons.
Quantification of AngI and AngII after immediate PI was highly correlated with the ED approach. Immediate PI of the blood sample provides a snapshot of a patient's circulating AP concentrations at the moment the sample was taken. In these dogs, as is typical in humans, 22 only AngI and II concentrations were high enough to be quantified after PI. The ED approach takes a routinely handled serum or plasma sample and incubates it at body temperature for 1 hour. Because angiotensinogen concentrations markedly exceed those of downstream metabolites, there is constant formation of AngI. Because angiotensin metabolizing enzymes are still active in the sample, stable equilibrium concentrations of downstream metabolites are established, where formation and degradation rates of peptides become equal. The ED approach therefore estimates the capacity of a patient's RAS and allows for assessment of relative enzyme activities. In effect, the ALTsurr reflects the balance (alternative vs classical) of a patient's RAS.
Our study had strengths and limitations. Although 117 dogs is a relatively large sample size for a veterinary study, division of the MMVD dogs into B1 and B2 groups decreased the statistical power. The planned analysis using 3 groups was decided a priori and thought to have the best potential for generating clinically relevant hypotheses. Age and time between the last meal and blood sampling were correlated with several RAAS analytes. However, a stringent statistical approach was used to correct for this covariation. Ideally, dogs would have been age‐matched and the timing between sample collection and the last meal more strictly controlled. Whether this was true covariation or related to study design is not known. Despite using a robust approach to determining reference intervals, the WCI/WRI exceeded 0.2 for all upper limits, indicating that the sample size of 60 normal dogs was inadequate and a larger sample size is needed. Only normal dogs were included in the week‐to‐week variation evaluation and comparison of PI and ED methods, and additional studies are needed to determine if these findings also apply to diseased dogs. Although systemic health screening was thorough, comorbidities not evident on physical examination, blood tests, urinalysis, or echocardiography may have been missed and may have influenced RAAS activity. Lastly, the exact etiology of an increased TR‐PG was not definitively determined and may not have been caused by a postcapillary increase in pulmonary artery pressures in every case.
In conclusion, the comprehensive circulating RAAS was evaluated in a large study sample of healthy dogs and dogs with stage B1 and B2 MMVD. The ACE2surr was increased in dogs with stage B2 MMVD compared to normal dogs and may indicate cleavage of ACE2 from the tissues and a less favorable RAS environment in the tissues as this disease progresses. The reference intervals for individual RAAS peptide concentrations were determined, albeit with imprecise upper limits. Although week‐to‐week variation in RAAS peptide concentrations was high, the ratio of alternative to classical RAAS was relatively stable. High correlation was found between PI samples and samples analyzed using the more clinically practical ED approach. The ALT and ACE2 surrogates, measured by the ED approach, are clinically feasible indicators of RAS dysregulation. Additional studies evaluating whether these biomarkers have an independent association with cardiovascular outcomes, such as heart failure or death, are warranted.
CONFLICT OF INTEREST DECLARATION
Marisa K. Ames has received consulting fees, research support, and honoraria from Ceva Sante Animale/Ceva Animal Health, LLC; Oliver Domenig is a founding member and employee of Attoquant Diagnostics; Brian A. Scansen has received travel support and honoraria from Ceva Animal Health, LLC; Hillary H. Hammond, Nuen Tsang Yang, Machelle D. Wilson, Erin Sunshine, Kaitlyn Brunk, and Allison Masters do not have a conflict of interest.
OFF‐LABEL ANTIMICROBIAL DECLARATION
Authors declare no off‐label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
Approvals from Clinical Review Boards from University of California, Davis and Colorado State University.
HUMAN ETHICS APPROVAL DECLARATION
Authors declare human ethics approval was not needed for this study.
Supporting information
Data S1. Supporting Information.
Table S1. The ratio of the two medians and the confidence interval around this ratio is shown below for variables that differed significantly prior to controlling for the covariates of age and time between the last meal and blood sampling. ACEsurr: surrogate measure of angiotensin converting enzyme activity (AngII/AngI); ACE2surr: surrogate measure of angiotensin converting enzyme 2 activity (Ang 1,5/AngII); AA2surr: surrogate of adrenal responsiveness to angiotensin II (Aldo/AngII); Aldo: aldosterone; ALTsurr: surrogate measure of alternative renin‐angiotensin system activity (Ang1,7 + Ang1,5)/(Ang1 + AngII+Ang1,7 + Ang1,5). *Differs significantly between groups prior to controlling for covariates. †Differed significantly between groups after controlling for covariates.
ACKNOWLEDGMENT
This study was funded by a gift from Ceva Sante Animale. Kaitlyn Brunk was supported by a stipend from the Veterinary Summer Scholars program at Colorado State University, which receives funding from Boehringer‐Ingelheim. The authors thank Yvonne Li and C. J. Florian for providing technical support.
Hammond HH, Ames MK, Domenig O, et al. The classical and alternative circulating renin‐angiotensin system in normal dogs and dogs with stage B1 and B2 myxomatous mitral valve disease. J Vet Intern Med. 2023;37(3):875‐886. doi: 10.1111/jvim.16687
REFERENCES
- 1. Fox PR. Pathology of myxomatous mitral valve disease in the dog. J Vet Cardiol. 2012;14(1):103‐126. doi: 10.1016/j.jvc.2012.02.001 [DOI] [PubMed] [Google Scholar]
- 2. Keene BW, Atkins CE, Bonagura JD, et al. ACVIM consensus guidelines for the diagnosis and treatment of myxomatous mitral valve disease in dogs. J Vet Intern Med. 2019;33(3):1127‐1140. doi: 10.1111/jvim.15488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Adin D, Kurtz K, Atkins C, Papich MG, Vaden S. Role of electrolyte concentrations and renin‐angiotensin‐aldosterone activation in the staging of canine heart disease. J Vet Intern Med. 2020;34(1):53‐64. doi: 10.1111/jvim.15662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Larouche‐Lebel É, Loughran KA, Oyama MA, et al. Plasma and tissue angiotensin‐converting enzyme 2 activity and plasma equilibrium concentrations of angiotensin peptides in dogs with heart disease. J Vet Intern Med. 2019;33(4):1571‐1584. doi: 10.1111/jvim.15548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bernay F, Bland JM, Häggström J, et al. Efficacy of spironolactone on survival in dogs with naturally occurring mitral regurgitation caused by myxomatous mitral valve disease. J Vet Intern Med. 2010;24(2):331‐341. doi: 10.1111/j.1939-1676.2009.0467.x [DOI] [PubMed] [Google Scholar]
- 6. Coffman M, Guillot E, Blondel T, et al. Clinical efficacy of a benazepril and spironolactone combination in dogs with congestive heart failure due to myxomatous mitral valve disease: the BEnazepril spironolactone STudy (BESST). J Vet Intern Med. 2021;35(4):1673‐1687. doi: 10.1111/jvim.16155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Amberger C, Chetboul V, Bomassi E, Rougier S, Woehrlé F, Thoulon F. Comparison of the effects of imidapril and enalapril in a prospective, multicentric randomized trial in dogs with naturally acquired heart failure. J Vet Cardiol. 2004;6(2):9‐16. doi: 10.1016/S1760-2734(06)70053-4 [DOI] [PubMed] [Google Scholar]
- 8. Ettinger SJ, Benitz AM, Ericsson GF, et al. Effects of enalapril maleate on survival of dogs with naturally acquired heart failure. The long‐term investigation of veterinary Enalapril (LIVE) study group. J Am Vet Med Assoc. 1998;213(11):1573‐1577. [PubMed] [Google Scholar]
- 9. BENCH Group . The effect of benazepril on survival times and clinical signs of dogs with congestive heart failure: results of a multicenter, prospective, randomized, double‐blinded, placebo‐controlled, long‐term clinical trial. J Vet Cardiol. 1999;1(1):7‐18. doi: 10.1016/S1760-2734(06)70025-X [DOI] [PubMed] [Google Scholar]
- 10. The COVE Study Group . Controlled clinical evaluation of enalapril in dogs with heart failure: results of the Cooperative Veterinary Enalapril Study Group. J Vet Intern Med. 1995;9(4):243‐252. doi: 10.1111/j.1939-1676.1995.tb01075.x [DOI] [PubMed] [Google Scholar]
- 11. Besche B, Chetboul V, Lachaud Lefay MP, Grandemange E. Clinical evaluation of imidapril in congestive heart failure in dogs: results of the EFFIC study. J Small Anim Pract. 2007;48(5):265‐270. doi: 10.1111/j.1748-5827.2006.00170.x [DOI] [PubMed] [Google Scholar]
- 12. The IMPROVE Study Group . Acute and short‐term hemodynamic, echocardiographic, and clinical effects of enalapril maleate in dogs with naturally acquired heart failure: results of the Invasive Multicenter PROspective Veterinary Evaluation of Enalapril study. J Vet Intern Med. 1995;9(4):234‐242. doi: 10.1111/j.1939-1676.1995.tb01074.x [DOI] [PubMed] [Google Scholar]
- 13. Borgarelli M, Ferasin L, Lamb K, et al. DELay of appearance of sYmptoms of canine degenerative mitral valve disease treated with spironolactone and benazepril: the DELAY Study. J Vet Cardiol. 2020;27:34‐53. doi: 10.1016/j.jvc.2019.12.002 [DOI] [PubMed] [Google Scholar]
- 14. Kvart C, Häggström J, Pedersen HD, et al. Efficacy of enalapril for prevention of congestive heart failure in dogs with myxomatous valve disease and asymptomatic mitral regurgitation. J Vet Intern Med. 2002;16(1):80‐88. [PubMed] [Google Scholar]
- 15. Atkins CE, Keene BW, Brown WA, et al. Results of the veterinary enalapril trial to prove reduction in onset of heart failure in dogs chronically treated with enalapril alone for compensated, naturally occurring mitral valve insufficiency. J Am Vet Med Assoc. 2007;231(7):1061‐1069. doi: 10.2460/javma.231.7.1061 [DOI] [PubMed] [Google Scholar]
- 16. Potter BM, Ames MK, Hess A, Poglitsch M. Comparison between the effects of torsemide and furosemide on the renin‐angiotensin‐aldosterone system of normal dogs. J Vet Cardiol. 2019;26:51‐62. doi: 10.1016/j.jvc.2019.11.003 [DOI] [PubMed] [Google Scholar]
- 17. Huh T, Larouche‐Lebel É, Loughran KA, Oyama MA. Effect of angiotensin receptor blockers and angiotensin‐converting enzyme 2 on plasma equilibrium angiotensin peptide concentrations in cats with heart disease. J Vet Intern Med. 2021;35(1):33‐42. doi: 10.1111/jvim.15948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Larouche‐Lebel É, Loughran KA, Huh T, Oyama MA. Effect of angiotensin receptor blockers and angiotensin converting enzyme 2 on plasma equilibrium angiotensin peptide concentrations in dogs with heart disease. J Vet Intern Med. 2021;35(1):22‐32. doi: 10.1111/jvim.16025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Adin D, Atkins C, Domenig O, et al. Renin‐angiotensin aldosterone profile before and after angiotensin‐converting enzyme‐inhibitor administration in dogs with angiotensin‐converting enzyme gene polymorphism. J Vet Intern Med. 2020;34(2):600‐606. doi: 10.1111/jvim.15746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ward JL, Guillot E, Domenig O, Ware WA, Yuan L, Mochel JP. Circulating renin‐angiotensin‐aldosterone system activity in cats with systemic hypertension or cardiomyopathy. J Vet Intern Med. 2022;36(3):897‐909. doi: 10.1111/jvim.16401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kobayashi M, Girerd N, Duarte K, et al. Estimated plasma volume status in heart failure: clinical implications and future directions. Clin Res Cardiol. 2021;110(8):1159‐1172. doi: 10.1007/s00392-020-01794-8 [DOI] [PubMed] [Google Scholar]
- 22. Pavo N, Goliasch G, Wurm R, et al. Low‐ and high‐renin heart failure phenotypes with clinical implications. Clin Chem. 2018;64(3):597‐608. doi: 10.1373/clinchem.2017.278705 [DOI] [PubMed] [Google Scholar]
- 23. Zoufaly A, Poglitsch M, Aberle JH, et al. Human recombinant soluble ACE2 in severe COVID‐19. Lancet Respir Med. 2020;8(11):1154‐1158. doi: 10.1016/S2213-2600(20)30418-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Guo Z, Poglitsch M, Cowley D, et al. Effects of Ramipril on the aldosterone/renin ratio and the aldosterone/angiotensin II ratio in patients with primary Aldosteronism. Hypertension. 2020;76(2):488‐496. doi: 10.1161/HYPERTENSIONAHA.120.14871 [DOI] [PubMed] [Google Scholar]
- 25. Burrello J, Buffolo F, Domenig O, et al. Renin‐angiotensin‐aldosterone system triple‐a analysis for the screening of primary aldosteronism. Hypertension. 2020;75(1):163‐172. doi: 10.1161/HYPERTENSIONAHA.119.13772 [DOI] [PubMed] [Google Scholar]
- 26. Visser LC, Ciccozzi MM, Sintov DJ, Sharpe AN. Echocardiographic quantitation of left heart size and function in 122 healthy dogs: a prospective study proposing reference intervals and assessing repeatability. J Vet Intern Med. 2019;33(5):1909‐1920. doi: 10.1111/jvim.15562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cornell CC, Kittleson MD, Della Torre P, et al. Allometric scaling of M‐mode cardiac measurements in normal adult dogs. J Vet Intern Med. 2004;18(3):311‐321. doi: 10.1892/0891-6640(2004)182.0.co;2 [DOI] [PubMed] [Google Scholar]
- 28. Hansson K, Häggström J, Kvart C, Lord P. Left atrial to aortic root indices using two‐dimensional and M‐mode echocardiography in cavalier king Charles spaniels with and without left atrial enlargement. Vet Radiol Ultrasound. 2002;43(6):568‐575. doi: 10.1111/j.1740-8261.2002.tb01051.x [DOI] [PubMed] [Google Scholar]
- 29. Braun JP, Concordet D, Geffré A, Bourges Abella N, Trumel C. Confidence intervals of reference limits in small reference sample groups. Vet Clin Pathol. 2013;42(3):395‐398. doi: 10.1111/vcp.12065 [DOI] [PubMed] [Google Scholar]
- 30. Epelman S, Shrestha K, Troughton RW, et al. Soluble angiotensin‐converting enzyme 2 in human heart failure: relation with myocardial function and clinical outcomes. J Card Fail. 2009;15(7):565‐571. doi: 10.1016/j.cardfail.2009.01.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Epelman S, Tang WH, Chen SY, Van Lente F, Francis GS, Sen S. Detection of soluble angiotensin‐converting enzyme 2 in heart failure: insights into the endogenous counter‐regulatory pathway of the renin‐angiotensin‐aldosterone system. J Am Coll Cardiol. 2008;52(9):750‐754. doi: 10.1016/j.jacc.2008.02.088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Almengló C, Couselo‐Seijas M, Agra RM, et al. Soluble angiotensin‐converting enzyme levels in heart failure or acute coronary syndrome: revisiting its modulation and prognosis value. J Mol Med (Berl). 2021;99(12):1741‐1753. doi: 10.1007/s00109-021-02129-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Úri K, Fagyas M, Mányiné Siket I, et al. New perspectives in the renin‐angiotensin‐aldosterone system (RAAS) IV: circulating ACE2 as a biomarker of systolic dysfunction in human hypertension and heart failure. PLoS One. 2014;9(4):e87845. doi: 10.1371/journal.pone.0087845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ramchand J, Patel SK, Kearney LG, et al. Plasma ACE2 activity predicts mortality in aortic stenosis and is associated with severe myocardial fibrosis. JACC Cardiovasc Imaging. 2020;13(3):655‐664. doi: 10.1016/j.jcmg.2019.09.005 [DOI] [PubMed] [Google Scholar]
- 35. Santos RAS, Sampaio WO, Alzamora AC, et al. The ACE2/angiotensin‐(1–7)/MAS Axis of the renin‐angiotensin system: focus on angiotensin‐(1–7). Physiol Rev. 2018;98(1):505‐553. doi: 10.1152/physrev.00023.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lambert DW, Yarski M, Warner FJ, et al. Tumor necrosis factor‐alpha convertase (ADAM17) mediates regulated ectodomain shedding of the severe‐acute respiratory syndrome‐coronavirus (SARS‐CoV) receptor, angiotensin‐converting enzyme‐2 (ACE2). J Biol Chem. 2005;280(34):30113‐30119. doi: 10.1074/jbc.M505111200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Patel VB, Clarke N, Wang Z, et al. Angiotensin II induced proteolytic cleavage of myocardial ACE2 is mediated by TACE/ADAM‐17: a positive feedback mechanism in the RAS. J Mol Cell Cardiol. 2014;66:167‐176. doi: 10.1016/j.yjmcc.2013.11.017 [DOI] [PubMed] [Google Scholar]
- 38. Wang K, Gheblawi M, Nikhanj A, et al. Dysregulation of ACE (angiotensin‐converting enzyme)‐2 and renin‐angiotensin peptides in SARS‐CoV‐2 mediated mortality and end‐organ injuries. Hypertension. 2022;79(2):365‐378. doi: 10.1161/HYPERTENSIONAHA.121.18295 [DOI] [PubMed] [Google Scholar]
- 39. Ames MK, Vaden SL, Atkins CE, et al. Prevalence of aldosterone breakthrough in dogs receiving renin‐angiotensin system inhibitors for proteinuric chronic kidney disease. J Vet Intern Med. 2022;36(6):2088‐2097. doi: 10.1111/jvim.16573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Friedrichs KR, Harr KE, Freeman KP, et al. ASVCP reference interval guidelines: determination of de novo reference intervals in veterinary species and other related topics. Vet Clin Pathol. 2012;41(4):441‐453. doi: 10.1111/vcp.12006 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Supporting Information.
Table S1. The ratio of the two medians and the confidence interval around this ratio is shown below for variables that differed significantly prior to controlling for the covariates of age and time between the last meal and blood sampling. ACEsurr: surrogate measure of angiotensin converting enzyme activity (AngII/AngI); ACE2surr: surrogate measure of angiotensin converting enzyme 2 activity (Ang 1,5/AngII); AA2surr: surrogate of adrenal responsiveness to angiotensin II (Aldo/AngII); Aldo: aldosterone; ALTsurr: surrogate measure of alternative renin‐angiotensin system activity (Ang1,7 + Ang1,5)/(Ang1 + AngII+Ang1,7 + Ang1,5). *Differs significantly between groups prior to controlling for covariates. †Differed significantly between groups after controlling for covariates.


