Abstract
Emerging research indicates the importance of gut microbiota in mediating the relationship between meat intake and human health outcomes. We aimed to assess the state of available scientific literature on meat intake and gut microbiota in humans (PROSPERO, International Prospective Register of Systematic Reviews, CRD42020135649). We first conducted a scoping review to identify observational and interventional studies on this topic. Searches were performed for English language articles using PubMed, Cochrane Library, Scopus, and CINAHL (Cumulated Index to Nursing and Allied Health Literature) databases from inception to August 2021 and using keywords related to meat (inclusive of mammalian, avian, and aquatic subtypes) and gut microbiota. Of 14,680 records, 85 eligible articles were included in the scoping review, comprising 57 observational and 28 interventional studies. One prospective observational study and 13 randomized controlled trials (RCTs) were identified in adults without diagnosed disease. We included the 13 RCTs, comprising 18 comparisons, in the systematic review to assess the effects of higher and lower intakes of total meat and meat subtypes on the gut microbiota composition. The bacterial composition was differentially affected by consuming diets with and without meat or with varied meat subtypes. For example, higher meat intake tended to decrease population sizes of genera Anerostipes and Faecalibacterium, but it increased the population size of Roseburia across studies. However, the magnitude and directionality of most microbial responses varied, with inconsistent patterns of responses across studies. The data were insufficient for comparison within or between meat subtypes. The paucity of research, especially among meat subtypes, and heterogeneity of findings underscore the need for more well-designed prospective studies and full-feeding RCTs to address the relationships between and effects of consuming total meat and meat subtypes on gut microbiota, respectively.
Keywords: red meat, poultry, fish, seafood, animal-based protein sources, dietary pattern
Statement of Significance.
This scoping and systematic review summarizes the current state of knowledge on meat intake and gut microbiota, and identifies gaps and priorities for future research.
Introduction
Meat is an integral part of most individuals’ dietary patterns, which affects their health. Since the 1960s, the total amount of meat consumed has increased by 40% among Americans and by 100% globally [1]. The meat types consumed by Americans (as of 2017) include 51% red meat, 42% poultry, and 7% fish and shellfish [2]. Historically, meat-related research has focused on the nutrients in meat and their influence on traditional health indices (e.g., cardiometabolic diseases and risk factors). This body of literature presents inconsistent and controversial health impacts of meat intake. Importantly, accumulating research indicates that the influence of diet on human health may be mediated, in part, by the gut microbiota. Knowing how meat intake influences gut microbiota can provide novel insights into understanding the impact of meat intake on human health outcomes. However, how the gut microbiota is impacted by meat intake is not well-studied.
Notably, “meat” is not a single food, but is defined by the American Meat Science Association (AMSA) as including mammalian, avian, aquatic, and other sources of flesh and related tissue foods [3]. However, the term “meat” is used inconsistently among and between observational and interventional studies and in regulatory, scientific, or public settings [4]. For example, the 2015–2020 Dietary Guidelines for Americans identifies meats as mammalian sources, including “beef, goat, lamb, pork, and game meat (e.g., bison, moose, elk, and deer),” separate from other muscle food categories such as poultry and seafood [5]. The varied uses of the term “meat” add to the inconsistency and controversy of research evidence on meat intake and health outcomes [6]. Our review addresses the inconsistency by adhering to the AMSA definition of meat by including mammalian, avian, and aquatic sources (i.e., red meat, white meat, and fish/seafood).
Over the past decade, there has been an exponential increase in the number of studies on human gut microbiota with fast-advancing sampling and sequencing technologies. In contrast, few systematic reviews have synthesized meat consumption and gut microbiota–related outcomes in humans. A rigorous, unbiased, systematic assessment of research literature is needed to evaluate the current evidence on the effects of consuming mammalian, avian, and aquatic meats on the gut microbiota. Although another systematic review included research using animal models [7], findings in the gut microbiota from animal models (murine in particular) may have limited transferability into humans because of the differences in gut and microbial physiologies and metabolisms [8, 9]. Therefore, this review aimed to assess the influence of consuming total meat and meat subtypes on the gut microbiota in humans. Given the large body of microbial literature, a scoping review was conducted to identify the types and extent of available research evidence, followed by a systematic review to chronicle and synthesize evidence from relevant studies.
Methods
Research question
Our research question comprised 2 parts: 1) What are the current state of knowledge and gaps in the literature on meat intake and human gut microbiota? 2) What are the effects of meat consumption on the gut microbiota in healthy human adults? To assess the state of knowledge, we conducted a scoping review to chronicle available literature without regard to reporting results. Based on the findings from the scoping review, we conducted a systematic review using suitable literature to assess the influence of meat intake on the gut microbiota. The systematic review presents the results and assesses the quality of the included articles.
The primary outcomes of the systematic review included changes or differences in the gut microbiota composition at the community (alpha and beta diversity metrics) and taxonomic levels (bacterial abundances). The primary outcomes were compared between the groups of participants who consumed diets with higher and lower amounts of meats or no meat. A priori subgroup analyses included comparisons 1) between vegetarian and vegan diets; 2) among subtypes of meats (e.g., red meat, white meat, and fish/seafood; processed or unprocessed), 3) between different meat subtypes and nonmeat, and 4) between higher and lower intakes of total meat and meat subtypes.
Inclusion and exclusion criteria
This systematic review was registered in the PROSPERO registry (https://www.crd.york.ac.uk/PROSPERO/) before the formal screening of search results commenced (CRD42020135649). The conduct of this review follows the PRISMA [10] and the PRISMA extension for scoping reviews [11] guidelines.
The Population, Investigated Condition, Comparison, Outcome, and Study Type criteria defining our research questions for the scoping review and systematic review are presented in Supplemental Tables S1 and S2, respectively. Relevant articles that met all of the following criteria were included in the scoping review (Supplemental Table S1): 1) is a peer-reviewed primary research article; 2) is published in English; 3) includes human population; 4) has a comparison between higher and lower dietary intakes of meat (in whole food form), with or without control of overall diets; and 5) includes outcomes on the gut microbiota composition. Articles were excluded if they did not meet any one of the listed inclusion criteria. Review articles and gray literature were excluded. Articles from the scoping review that met all of the additional criteria were included in the systematic review (Supplemental Table S2): 1) is a randomized controlled trial (RCT) and 2) includes human adults without diagnosed disease and with stable health status (e.g., not pregnant). Because of the selection process, reference lists of included articles from the scoping or systematic reviews were not searched.
Search strategy
The initial search was conducted in 4 scientific databases, including PubMed, Cochrane Library, Scopus, and CINAHL (EBSCO, Cumulated Index to Nursing and Allied Health Literature), on January 14, 2020. An updated search was conducted on August 17, 2021, in all databases (e.g., with entry dates from 14 January, 2020, to 17 August, 2021, in PubMed). A complete list of search strategies developed by a research librarian (JBR) is included (Supplemental Document 1). Briefly, keywords and alternative terms for meat, gut microbiota, and gut microbial metabolites were used in combinations to identify relevant articles in each database. Definitions of total meat and meat subtypes used in this review are listed in Table 1 [3, 5]. Searches were restricted to articles published in English from inception to August 2021. Trial registries were searched through results generated from the Cochrane Library.
TABLE 1.
Terminology of meat subtypes.
| Meat subtypes | Definitions |
|---|---|
| Meat | “Skeletal muscle and its associated tissues derived from mammalian, avian, reptilian, amphibian, and aquatic species harvested for human consumption,” including “edible offal consisting of organs and non-skeletal muscle tissues” [3]. |
| Red meat | “All forms of beef, pork, lamb, veal, goat, and nonbird game (e.g., venison, bison, and elk)” [5]. |
| White meat/poultry | “Domestic avian species that includes chickens, turkeys, geese, ducks, guinea, squab and in some cases ratites (ostrich, emu, rhea)” [3]. |
| Fish/seafood | “Any form of animal sea life regarded as food by humans,” which predominantly includes fish (e.g., “salmon, tilapia, and catfish”) and shellfish (i.e., “various species of mollusks, crustaceans, and echinoderms”) [3]. |
| Unprocessed meat | Meats with “minimal processing,” which includes “any process where raw, uncooked meat products have not been significantly altered compositionally and contain no added ingredients, but may have been reduced in size by fabrication, mincing, grinding, and/or a meat recovery system” [3]. |
| Processed meat | Meat that is “preserved by smoking, curing, salting, and/or the addition of chemical preservatives” [5]. For the purpose of this review, salting during home cooking or table use is not considered processing. |
Article screening and data extraction
Records of identified articles were uploaded to the Rayyan systematic review management tool (Rayyan Systems Inc, https://www.rayyan.ai/) for screening. After removing duplicates (n = 10,543), articles from the initial search (n = 10,297) were screened independently by pairs of investigators (YW, CNU, REB, and CMC) and crosschecked to reach an agreement through 2 stages: 1) potential eligibility assessment based on publication titles and abstracts and 2) confirmation of eligibility based on full-text assessment of qualified abstracts. To increase the efficiency of the process, results from the updated search (from 14 January, 2020, to date) were first screened by 1 investigator to exclude clearly irrelevant records (e.g., studies not in human populations; secondary research; and non–peer-reviewed gray literature, such as reports, government documents, and white papers). A second investigator screened the full texts of the remaining results for eligibility. Information was extracted and crosschecked by pairs of investigators (YW, CNU, REB, and CMC) from each of the included articles. The extracted information primarily included article publication details (title, author, and year), study design, dietary interventions (meat consumption), gut microbial composition, and key gut microbial metabolites and cardiometabolic disease risk factors, if reported. The authors of these articles were not contacted as the purpose of the review was to assess the current state of literature based on published findings and additional information was not needed. During the screening and extraction processes, disagreements between pairs of investigators were discussed and resolved by a third investigator.
Risk of bias assessment
The risk of bias of articles in the systematic review was assessed independently and crosschecked by 2 investigators (YW and CNU) using the Cochrane Risk of Bias Assessment Tool 2 [12]. The Cochrane tool assesses each article based on 5 major domains tailored for crossover and parallel RCTs. The 5 major domains that were considered were as follows: 1) the study randomization process, and for crossover RCT, the period and carryover effects; 2) effects of assignment to intervention; 3) missing outcome data; 4) outcome measurement; and 5) selection of results reporting. An overall judgment of low, some concerns, or high risk of bias was given for each article.
Results
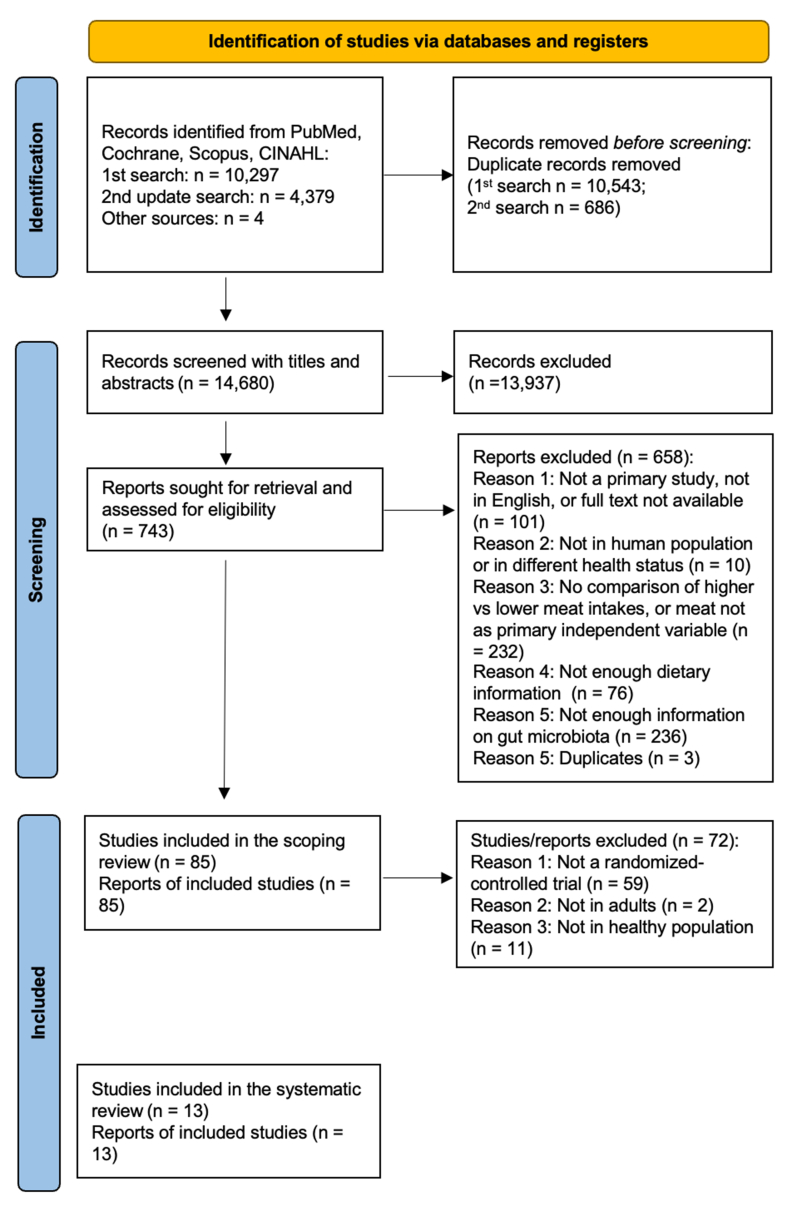
The search and screening process identified 85 and 13 articles suitable for the scoping and systematic reviews, respectively (Figure 1).
FIGURE 1.
PRISMA flow diagram of the search and screening process.
Scoping review
Table 2 presents the basic characteristics of the 85 studies included in the scoping review ([13], [14], [15], [16], [17], [18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41], [42], [43], [44], [45], [46], [47], [48], [49], [50], [51], [52], [53], [54], [55], [56], [57], [58], [59], [60], [61], [62], [63], [64], [65], [66], [67], [68], [69], [70], [71], [72], [73], [74], [75], [76], [77], [78], [79], [80], [81], [82], [83], [84], [85], [86], [87], [88], [89], [90], [91], [92], [93], [94], [95], [96], [97]). In all observational studies, comparisons of higher and lower meat intakes were based on various dietary patterns, instead of being meat-specific, which further limits the potential for causal inference between meat intake and gut microbiota. Overall, there was insufficient evidence from eligible prospective cohort studies for a systematic review of total meat intake and gut microbiota. Therefore, we proceeded with the 13 eligible RCTs to assess the causal relationships between total meat and meat subtype intakes and gut microbiota in healthy adults with stable health status.
TABLE 2.
Summary of the articles in the scoping review1.
| Study type/Health status | Total number of studies | Age | Study design | ||||
|---|---|---|---|---|---|---|---|
| Observational study | Total | ≥18 y | <18 y | NR | Cross-sectional cohort | Prospective cohort | Other (retrospective, case-control) |
| All | 57 [13–69] | 47 [13–40, 48, 49, 51, 54–59, 61–69] | 7 [41–47] | 3 [50, 52, 53] | 47 [13–27, 29–40, 43–53, 55–58, 60, 63, 67–69] | 6 [28, 41, 42, 59, 61, 62] | 4 [54, 64–66] |
| Healthy | 39 [13–47, 63, 67–69] | 32 [13–40, 63, 67–69] | 7 [41–47] | 0 | 36 [13–27, 29–40, 43–47, 63, 67–69] | 3 [28, 41, 42] | 0 |
| With condition/disease2 | 10 [54–59, 61, 64–66] | 10 [54–59, 61, 64–66] | 0 | 0 | 4 [55–58] | 2 [59, 61] | 4 [54, 64–66] |
| NR health status | 8 [48–53, 60, 62] | 5 [48, 49, 51, 60, 62] | 0 | 3 [50, 52, 53] | 7 [48–53, 60] | 1 [62] | 0 |
| Interventional study | Total | ≥18 y | <18 y | NR | Crossover RCT | Parallel RCT | Other (incomplete randomization)4 |
| All | 28 [70–97] | 26 [70–93, 96, 95] | 2 [94, 95] | 0 | 12 [70, 71, 73, 74, 79, 82, 84–88, 96] | 13 [76–78, 80, 81, 83, 89–95] | 3 [72, 75, 97] |
| Healthy | 17 [71–74, 84–95, 97] | 15 [71–74, 84–93, 97] | 2 [94, 95] | 0 | 8 [71, 73, 74, 84–88] | 7 [89–95] | 2 [72, 97] |
| With condition/disease2 | 10 [70, 75–83]3 | 10 [70, 75–83] | 0 | 0 | 3 [70, 79, 82] | 6 [76–78, 80, 81, 83] | 1 [75] |
| NR health status | 1 [96] | 1 [96] | 0 | 0 | 1 [96] | 0 | 0 |
Age categories were divided based on reported subject inclusion, subject age range, and group mean age of each study. The age category “<18” also includes studies that recruited subjects aged both <18 and >18 y. The number of studies is reported in each cell, with references of studies included in square brackets.
With condition/disease: Ten observational studies had cohorts with certain diseases or health conditions [[54], [55], [56], [57], [58], [59], 61, [64], [65], [66]], including reactive hypoglycemia [54], cirrhosis [55], type 2 diabetes [58], metabolic syndrome [61], colorectal cancer [64], intestinal diseases (colon carcinoma, inflammatory bowel disease [56], Crohn’s disease or ulcerative colitis, irritable bowel syndrome [56, 65, 66]), or pregnancy [57, 59]. Among interventional studies, 10 studies were in cohorts with the following diseases or health conditions: Crohn’s disease [75], type 2 diabetes [76, 77], multiple sclerosis [78], chronic kidney disease [79], obesity with insulin resistance [80] or metabolic syndrome [81], chronic gastrointestinal disease [70], ischemic heart disease [82], or pregnancy [83].
One study [70] included all healthy subjects, except for 1 subject with chronic gastrointestinal disease.
One study [75] had all participants receive the same intervention (pre vs. post), thus the study was not categorized as crossover or parallel RCT; 2 studies [72, 97] included partial randomization, in which the intervention group was compared with a nonrandomly assigned comparison group. NR, not reported; RCT, randomized controlled trial.
Systematic review
The 13 RCTs generated 18 comparisons (Table 3), including 12 primary comparisons on higher and lower total meat intakes based on data from 10 RCTs [73, 74, [84], [85], [86], [87], [88], [89], [90], [91]] and 6 secondary comparisons among meat subtypes based on data from 5 RCTs [71, 85, 87, 92, 93]. Adults with stable health status have relatively more stable gut microbiota composition with less influence from growth and confounding disease conditions [98]. As a result, the 13 RCTs in healthy adults included 8 crossover [71, 73, 74, [84], [85], [86], [87], [88]] and 5 parallel RCTs [[89], [90], [91], [92], [93]].
TABLE 3.
Characteristics and gut microbiota outcomes of included comparisons by subgroups1.
| Study (year) | Comparison diet 1 | Comparison diet 2 | Study type | Diet length | Dietary control | Alpha diversity |
Phylum | Class | Order | Family | Genus | Species | Other | Major differences in gut microbiota outcomes | Effects? (p<0.05) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Primary comparisons: meat vs. nonmeat | |||||||||||||||
| Lang et al. 2018 [87] | Red meat2, 3 | Nonmeat2, 3 | CO, Yes | 4 wk | F | N | N | NR | NR | NR | Y | — | — | 3 OTUs differed when high and low amounts of saturated fat were combined | Yes |
| White meat2, 3 | CO, Yes | 4 wk | F | N | N | NR | NR | NR | Y | — | — | 1 OTU differed when high and low amounts of saturated fat were combined | Yes | ||
| van Faassen et al. 1987 [85] | Western omnivorous2, 3 | Vegetarian2, 3 | CO, NS | 20 d | F | — | — | N | — | N | N | — | N2, 4 | No difference | No |
| Vegan2, 3 | CO, NS | 20 d | F | — | — | N | — | N | Y | — | N2, 4 | Omnivorous diet ↑ counts of genus Lactobacilli | Yes14 | ||
| Kohnert et al. 2021 [91] | Omnivorous4, 5 | Vegan4, 5 | PL | 4 wk | C | N2, 4 | NR | NR | NR | NR | Y4, 5 | Y4, 5 | — | Differences in several genera and species (Table 4) | Yes |
| Kahleova et al. 2020 [89] | Habitual omnivorous4, 5 | Vegan4, 5 | PL | 16 wk | C | Y | Y4, 5 | N | N | Y4, 5 | Y4, 5 | Y4, 5 | — | Differences in alpha diversity and bacterial abundance (Table 4) | Yes |
| Pagliai et al. 2020 [74] | Mediterranean4, 5 | Vegetarian4, 5 | CO10, No | 12 wk | C | Y4, 5 | N4, 5 | Y4, 5 | Y4, 5 | Y4, 5 | Y4, 5 | — | — | Differences in alpha diversity and bacterial abundance from phylum to genus levels (Table 4) | Yes |
| Total % of significant out of measured (%) – meat vs. nonmeat | 40 | 25 | 25 | 50 | 50 | 86 | 100 | N/A | N/A | 86 | |||||
| Proportion of significant out of measured (counts) – meat vs. nonmeat | 2/5 | 1/4 | 1/4 | 1/2 | 2/4 | 6/7 | 2/2 | N/A | N/A | 6/7 | |||||
| Primary comparisons: higher vs. lower meat within omnivorous habitual/basal diets | |||||||||||||||
| Hess et al. 2018 [84] | Red meat4, 5 | Mushroom4, 5 | CO, Yes | 10 d | P2 | — | Y | NR | NR | NR | Y | — | — | Red meat ↓ phylum Bacteroidetes and ↑ phylum Firmicutes | Yes |
| Foerster et al. 2014 [86] | Red meat4, 5 | Whole grain4, 5 | CO, Yes | 3 wk | P2 | — | — | — | — | — | — | Y | Y13 | Whole grain ↑ DGGE band number and species Collinsella aerofaciens; red meat ↓ species Clostridium sp | Yes |
| Crimarco et al. 2020 [88] | Animal-based meat2, 6 | Plant-based meat2, 6 | CO, No | 8 wk | P1 | — | NR | NR | NR | NR | Y4, 5 | Y4, 5 | — | Differences in bacterial abundance (Table 4) | Yes |
| McKenna et al. 2021 [90] | Higher red meat2, 4, 7 | Lower red meat2, 4, 7 | PL11 | 10 wk | P2 | N | NR | NR | Y2, 4 | Y2, 4 | Y2, 4 | — | — | Differences in bacterial abundance (Table 4) | Yes |
| Windey et al. 2012 [73] | Higher meat2, 8 | Lower meat2, 8 | CO, No | 2 wk | C | — | — | — | — | — | — | — | N13 | No difference in DGGE band numbers | No |
| Total % of significant out of measured (%) – higher vs. lower meat | 0 | 100 | 0 | 100 | 100 | — | 100 | N/A | N/A | 80 | |||||
| Proportion of significant out of measured (counts) – higher vs. lower meat | 0/1 | 1/1 | 0 | 1/1 | 1/1 | 3/3 | 2/2 | N/A | N/A | 4/5 | |||||
| Total % of significant out of measured (%) – primary comparisons | 33 | 40 | 25 | 67 | 60 | 90 | 100 | N/A | N/A | 83 | |||||
| Proportion of significant out of measured (counts) – primary comparisons | 2/6 | 2/5 | 1/4 | 2/3 | 3/5 | 9/10 | 4/4 | N/A | N/A | 10/12 | |||||
| Secondary comparisons: meat subtypes | |||||||||||||||
| Lang et al. 2018 [87] | Red meat2, 3 | White meat2, 3 | CO, Yes | 4 wk | F | N | N | NR | NR | NR | Y | — | — | Several OTUs differed at low or high amounts of saturated fat, but no difference when both amounts were combined. | Yes |
| Schmedes et al. 2019 [71] | Lean seafood4, 5 | Non-seafood meat4, 5 | CO, Yes | 4 wk | P1 | — | N | NR | NR | NR | Y | — | — | Non-seafood diet ↓ genus group Clostridium cluster IV | Yes |
| Bratlie et al. 2021 [93] | Cod2 | Nonfish habitual Meat2 | PL | 8 wk | P2 | — | NR | NR | Y | NR | NR | NR | — | Cod/Salmon ↓ order-level bacterial counts of Bacteroidales, Clostridiales, and Selenomonadales | Yes |
| Salmon2 | PL | 8 wk | P2 | — | NR | NR | Y | NR | NR | NR | — | Yes | |||
| Meslier et al. 2020 [92] | Mediterranean (higher fish)2, 4, 9 | Habitual omnivorous (higher nonfish meat)2, 4, 9 | PL | 8 wk | P1 | — | NR | NR | NR | NR | NR | Y2, 4 | — | Differences in beta diversity and several species (Table 4) | Yes |
| Total % of significant out of measured (%) – secondary comparisons | 0 | 0 | 0 | 100 | 0 | 100 | 100 | N/A | N/A | 100 | |||||
| Proportion of significant out of measured (counts) – secondary comparisons | 0/1 | 0/2 | 0 | 2/2 | 0 | 2/2 | 1/1 | N/A | N/A | 5/5 | |||||
| Secondary comparison: vegetarian vs. vegan | |||||||||||||||
| van Faassen et al. 1987 [85] | Vegetarian2, 4, 9 | Vegan2, 4, 9 | CO, NS | 20 d | F | — | — | N | — | N | Y | — | N12 | Vegetarian diet ↑ counts of genus Enterococci and Lactobacilli | Yes |
| Total % of significant out of measured (%) – all comparisons | 29 | 29 | 20 | 80 | 50 | 92 | 100 | N/A | N/A | 89 | |||||
| Proportion of significant out of measured (counts) - all comparisons | 2/7 | 2/7 | 1/5 | 4/5 | 3/6 | 12/13 | 5/5 | N/A | N/A | 16/18 | |||||
| Secondary comparison not available: unprocessed vs. processed meat | |||||||||||||||
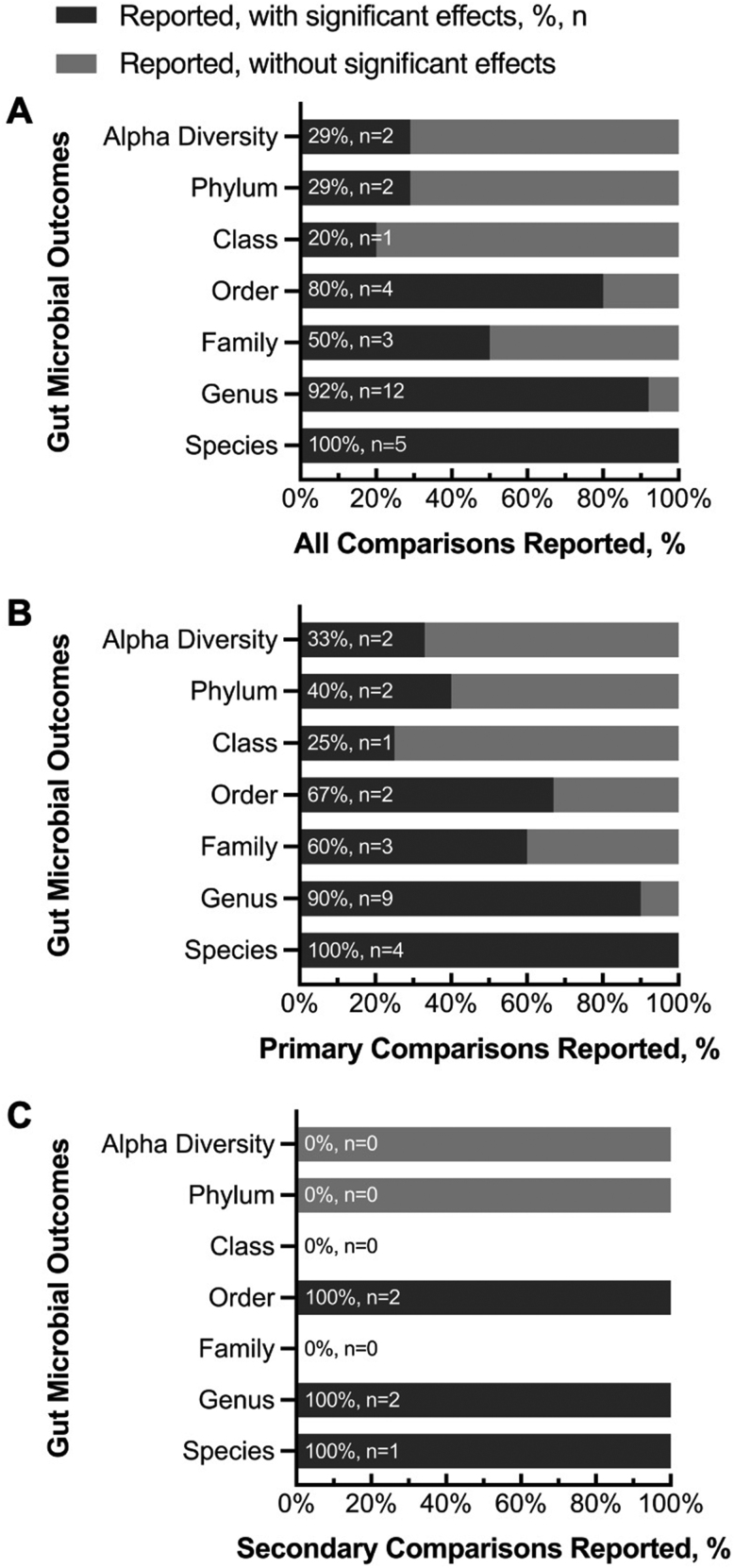
All vegetarian diets are lacto-ovo vegetarian that included dairy and eggs. Vegan diet did not contain any animal-based products. Meat, if not specified, refers to mixed meat subtypes. Category “Study type” includes CO and PL RCTs, as well as the washout period for crossover RCTs (Yes for existence, No for no washout, and NS for not specified or not reported). Category “Length” is the length of intervention of each study arm (d, day; wk, week). Category “Dietary control” includes full control (F) that provided all study foods; partial control that provided some but not all study foods, either with dietary guidance on basal diet (P1) or with uncontrolled habitual diet (P2) and counseling only (C) that did not provide any study foods except for dietary guidance. Categories “Alpha diversity” through “Other”: bacterial abundance at taxonomic levels such as phylum, class, order, family, genus, and species, or in other forms of measurement (e.g., OTU, operational taxonomic unit). Y, significant changes or differences were observed; N, no significant change or difference was observed; NR, outcomes were evaluable using methods reported by the study authors, but no data were reported at the specific taxonomic level; Em dash (-), outcomes and data likely were not measured based on study description, and thus were not reported for the selected comparisons; N/A, not applicable. Results were considered significant only when the authors of the included studies reported the statistical significance of the outcome measures. Category “Effects”: overall effects of diet on gut microbiota. Summary of Table 3: Among the primary comparisons, 83% of the comparisons showed significant effects of meat intake on the gut microbiota (Figure 2B). Although global bacterial composition measured using alpha diversity metrics was reported in 50% of primary comparisons, only 33% of them showed significant effects of meat intake on the gut microbiota composition. In contrast, among 83% of primary comparisons that reported bacterial abundances at the genus level, 90% of them showed significant effects. Only 4 of the 12 primary comparisons reported outcomes at the species level, but all 4 comparisons suggested significant effects. A similar hierarchical pattern of findings was observed among the secondary comparisons. Two of 2 comparisons (100%) at the genus level and 1 of 1 comparison at the species level showed significant effects, but zero of 1 comparison showed effects for alpha diversity metrics (Figure 2C).
Difference in post-intervention values between groups.
Each diet was consumed with high vs. low amounts of saturated fat.
Within-group pre–post changes.
Difference in between-group changes.
Pre- and post-intervention samples combined.
Comparisons were made among baseline vs. 1-wk intervention vs. post-intervention within and between the groups.
Samples were collected during the diet intervention period without a clearly specified timepoint.
Comparisons were made among baseline vs. 4-wk intervention vs. 8-wk intervention within and between the groups.
Cointervention with energy restriction.
Cointervention with resistance training.
Counts of total aerobes and anaerobes.
Denaturing gradient gel electrophoresis (DGGE) bandclass counts.
Results from this study were obtained via the cultivation-dependent method, which could potentially miss capturing significant gut microbial outcomes compared with more advanced techniques.
The characteristics of the 13 RCTs in the systematic review are summarized in Table 3 (with details presented in Supplemental Tables S3 and S4), including 1) author, publication year, and country of study; 2) study length; 3) comparison groups; 4) type of study meat; 5) study diets; 6) use of prebiotics, probiotics, or antibiotics; 7) cointervention; 8) sample size estimation; 9) participant characteristics; 10) fecal sample collection timepoint, collection methods, and storage; 11) microbiota composition processing and analysis methods; and 12) study’s funding source and potential conflict of interest. The experimental designs of RCTs are provided in Table 3 and Supplemental Tables S3A and S4 and are summarized in the footnote of Supplemental Table S3A. Microbiome analysis methods used in RCTs are described in Supplemental Table S3B, including a summary in the footnote.
Type of study meat
Studies used either food substitution with total meat and meat subtypes or dietary patterns to compare higher and lower intakes of total meat and meat subtypes (Table 3). The primary comparisons include 12 comparisons from 10 studies [73, 74, [84], [85], [86], [87], [88], [89], [90], [91]] to compare higher and lower total meat intakes. Among the primary comparisons, 7 comparisons from 5 studies [74, 85, 87, 89, 91] were performed between a diet with meat (red meat, white meat, or mixed meat subtypes) and a diet without meat (vegan or vegetarian) (Table 3, meat compared with nonmeat). The other 5 comparisons from 5 studies either substituted meat (red meat or mixed meat subtypes) with plant-based foods [73, 84, 86, 88] or increased the amount of meat intake without dietary substitution [90], with uncontrolled or minimally controlled habitual diet (Table 3, higher compared with lower meat within omnivorous habitual/basal diets).
Five comparisons from 4 studies [71, 87, 92, 93] were included as secondary comparisons (Table 3, meat subtypes). These comparisons substituted meat with other meat subtypes with or without changes to overall dietary patterns. Specifically, 3 comparisons from 2 studies [71, 93] substituted fish or seafood with non-seafood meat; 1 comparison [92] compared Mediterranean diet with higher fish-meat intake to a habitual omnivorous diet with higher non–fish-meat intake. Only 1 comparison [87] was between red and white meat. We also documented 1 comparison [85] of vegetarian and vegan diets based on data from RCTs, but this comparison was not used because it was not covered in our a priori search strategies.
Summary of study outcomes on gut microbiota
Overall, the effects of consuming meat on the gut microbiota were more frequently detected at the lower taxonomic levels (genus and species) than at the higher taxonomic levels (phylum and class) or in overall community diversity (e.g., Shannon’s and inverse Simpson’s indices; Table 3). Significant changes or differences in the gut microbiota composition were reported at the genus (12 of 13 comparisons) and species (5 of 5 comparisons, Figure 2A) levels. In contrast, significant effects on gut microbiota composition were reported for alpha diversity in only 2 of 7 comparisons (Figure 2A). The percentages of primary and secondary comparisons showing study-reported significant effects of meat intake on the gut microbiota are presented in Figure 2 and Table 3, with a summary in the footnote of Table 3.
FIGURE 2.
Percentages of (A) all, (B) primary, and (C) secondary comparisons that reported gut microbial outcomes at the specified levels, with (darker gray) or without (lighter gray) significant effects of diet on gut microbiota. The effects refer to whether significant changes or differences in microbiota composition were reported by authors of the included studies. The percentages and numbers of studies showing any significant effects out of the total number of studies that reported the specific outcome were denoted in percentages and n.
The types and directionality of bacterial taxa responses varied (Table 3, Table 4). Complementing the summary provided in Table 3, Table 4 presents the types and directionality of bacterial differences per taxonomic level for all studies. Although most comparisons did not show any significant effects of higher intake of total meat and meat subtypes on the phyla Bacteroidota (formerly Bacteroidetes [99]), Bacillota (formerly Firmicutes [99]), and Pseudomonadota (formerly Proteobacteria [99]), seemingly consistent directions of changes or differences in members of the phylum Bacteroidota were observed in 2 primary comparisons [84, 89]. After consuming red meat or mushroom in an otherwise uncontrolled diet for 10 d, abundances of bacteroidota and bacillota were lower and higher, respectively [84]. Consuming a controlled vegan diet or a habitual omnivorous diet for 16 wk did not influence the observed increase in bacteroidota over time [89].
Table 4.
Gut microbiota outcomes reported in studies1.
| Study author, year | n | Comparison diet 1 | Comparison diet 2 | Alpha diversity | Beta diversity | Phylum | Class | Order | Family | Genus | Species | Other/notes | Significant effects on gut microbiota? |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| van Faassen et al. 1987 [85] | 12 | Post-omnivorous | Post-vegan | — | — | — | Clostridia:↔ | — | Enterobacteriaceae:↔ | Anaerobes:Bifidobacteria (count):↔Bacteroides (count):↔Aerobes:Enterococci (count):↔Lactobacilli (count):↑ | — | Mean counts of bacteria:Total anaerobes:↔Total aerobes:↔ | Yes |
| 12 | Post-omnivorous | Post-vegetarian | — | — | — | Clostridia:↔ | — | Enterobacteriaceae:↔ | Anaerobes:Bifidobacteria (count):↔Bacteroides (count):↔Aerobes:Enterococci (count):↔Lactobacilli (count):↔ | — | Mean counts of bacteria:Total anaerobes:↔Total aerobes:↔ | No | |
| 12 | Post-vegetarian | Post-vegan | — | — | — | Clostridia:↔ | — | Enterobacteriaceae:↔ | Anaerobes:Bifidobacteria (count):↔Bacteroides (count):↔Aerobes:Enterococci (count):↑Lactobacilli (count):↑ | — | Mean counts of bacteria:Total anaerobes:↔Total aerobes:↔ | Yes | |
| Windey 2012 et al. [73] | 20 | Higher meat | Lower meat | — | — | — | — | — | — | — | — | Among 64 bandclasses, no difference was detected in the allocation of bandclass between diets. | No |
| Foerster et al. 2014 [86] | 20 | Post-red meat | Pre-red meat | — | — | — | — | — | — | — | Clostridium sp.: ↓ | Band appearance of bacteria:↔ band diversity (total number of bands)↑ 5 bands appearance↓ 3 bands appearance | Yes |
| 20 | Post-whole grain | Pre-whole grain | — | — | — | — | — | — | — | Collinsella aerofaciens: ↑ | Band appearance of bacteria:↑ band diversity (total number of bands)↑ 5 bands appearance↓ 3 bands appearance | ||
| Lang et al. 2018 [87] | 109 | By protein sources (1) Red meat(2) White meat (3) Non-meat | By amounts of saturated fat(1) High saturated fat(2) Low saturated fat | Shannon diversity index:↔ among protein sources or between high vs low amounts of saturated fat | ↔unweighted UniFrac distance: no clustering by diet | F/B ratio (log2):↔ by diet or saturated fat | NR | NR | NR | 2 reported genera (Coprococcus and Ruminococcus) differed by protein sources, but pairwise comparisons between protein sources were not reported.57 genera differed by saturated fat amounts. | — | 19 OTUs responded to protein source at both high and low saturated fat amounts (protein-sensitive, but not specific to protein sources). Differentially abundant OTUs: [high and low amounts of saturated fat combined] 3 between red meat vs non-meat, 1 between white meat vs non-meat, 0 between red meat vs white meat; [low saturated fat] 115, 125, 130 OTUs, respectively; [high saturated fat] 203, 198, 240 OTUs, respectively. | Yes |
| Hess et al. 2018 [84] | 32 | Post-Red meat (5d average) | Post-Mushroom (5d average) | — | — | Bacteroidetes:↓Firmicutes:↑ | NR | NR | NR | Bacteroides:↓Parabacteroides:↓Coprococcus:↓Anaerostipes:↓Sutterella:↓Dorea:↑02d06:↑ | — | Taxa (phylum and genus) with at least 0.05% relative abundance were reported. Relative abundances were log-transformed and controlled for false discovery rate with p<0.004. | Yes |
| Schmedes et al. 2018 [71] | 19 | Post-lean seafood | Pre-lean seafood | — | — | Firmicutes:↔Bacteroidetes:↔F/B ratio:↔ | — | — | — | Clostridium cluster IV:↔ | — | The following results were described and not reported in details: the dataset included "42 bacterial OTU groups at the genera level, 8 bacterial OTU groups at the family level, 2 bacterial OTU groups at the order level, 1 bacterial OTU group at the class level, and 1 bacterial OTU group at the domain level”. | Yes |
| 19 | Post-non-seafood | Pre-non-seafood | — | — | Firmicutes:↔(p=0.1)Bacteroidetes:↔(p=0.1)F/B ratio:↔(p=0.1) | NR | NR | NR | Clostridium cluster IV:↓ (greater reduction than lean-seafood group) | — | |||
| Pagliai et al. 2020 [74] | 23 | Post-Mediterranean diet | Pre-Mediterranean diet | Richness; inverse Simpson’s, Gini-Simpson’s, Shannon’s indices; evenness, dominance:Phylum-level:↔Class-level:↔Order-level:↔Family-level:↔Genus-level:↔ | (1) Weighted Unifrac (PCoA)(2) Bray-Curtis dissimilarity metrics(3) Principle component analysis (PCA) & co-inertia analysis (CIA):No clearly separated clustering by diet.LEfSe tool: no discriminating characteristics by diet.PERMANOVA: no difference in microbiota composition by diet at any taxonomic level. | F/B ratio:↔F/P ratio:↔Other phyla:↔ | Betaproteobacteria:↓ (p=0.05) | Burkholderiales:↓ (p=0.05) | Alcaligenaceae:↓ (p=0.05) | Enterorhabdus:↑Lachnoclostridium:↑Parabacteroides:↓Parasutterella:↓ (p=0.05) | — | — | Yes |
| 23 | Post-vegetarian diet | Pre-vegetarian diet | Richness; inverse Simpson’s, Gini-Simpson’s, Shannon’s indices; evenness, dominance:Phylum-level:↔Class-level:↔Order-level:↔Family-level:↔Genus-level:↔ | F/B ratio:↔F/P ratio:↔Euryarchaeota:↑ (abundance median increased from 0 to 1)Other phyla:↔ | Methanobacteria:↑ (abundance median increased from 0 to 1) | Methanobacteriales:↑ (abundance median increased from 0 to 1) | Clostridiaceae_1:↓Methanobacteriaceae:↑ (abundance median increased from 0 to 1) | Anaerostipes:↑Clostridium_sensu_stricto_1:↓Methanobrevibacter:↑ (abundance median increased from 0 to 1)Odoribacter:↓Streptococcus:↑Tyzzerella_3: abundance median=0, interquartile range ↑ | — | — | |||
| 23 | Pre-Post change of Mediterranean diet | Pre-Post change of vegetarian diet | Order-level richness: median=1, interquartile range↑Genus-level richness:↓All other parameters:↔ | F/B ratio:↔F/P ratio:↔Other phyla:↔ | — | — | Clostridiaceae_1:↑ | Anaerostipes:↓Clostridium_sensu_stricto_1:↑Enterorhabdus:↑Slackia: abundance median=0, interquartile range ↑Veillonella:↑ | — | — | |||
| Crimarco et al. 2020 [88] | 36 | Animal diet | Plant diet | — | Bray-Curtis dissimilarity:↔ | NR | NR | NR | NR | Volcano plot of taxa with between-group difference in abundance:Lachnospiraceae sp: mixedRuminococcus sp.:↑Roseburia sp.:↑Ruminococcaceae sp.:↓ | Volcano plot of taxa with between-group difference in abundance:H. parainfluenzae:↓ | — | Yes |
| 36 | Post-animal | Pre-animal | — | — | — | — | — | — | — | — | |||
| 36 | Post-plant | Pre-plant | — | — | — | — | — | — | — | — | |||
| Kahleova et al. 2020 [89] | 84 | Post-omnivorous | Pre-omnivorous | Abundance-weighted phylogenetic diversity measure:↑ | — | ↔ | — | — | — | Methanobrevibacter (read counts and %):↑Eubacterium (read counts):↑ | Bacteroides fragilis (read counts and %):↓ | — | Yes |
| 84 | Post-vegan | Pre-vegan | Abundance-weighted phylogenetic diversity measure: ↔ | — | Bacteroidetes (read counts):↑Proteobacteria (%):↓ | — | — | — | Bifidobacterium (read counts):↑Anaerostipes (read counts):↑ | Faecalibacterium prausnitzii (read counts&%):↑Bacteroides fragilis (read counts and %):↓ | — | ||
| 168 | Post-pre change in omnivorous | Post-pre change in vegan | Abundance-weighted phylogenetic diversity measure:↑ | — | ↔ | — | — | — | ↔ | Faecalibacterium prausnitzii (%):↓Bacteroides fragilis (read counts and %):↑ | — | ||
| Meslier et al. 2020 [92] | 62 | Post-Mediterranean diet (wk4) | Post-omnivorous diet (wk4) | — | Separate diversity (N-integrative supervised analysis)Dissimilarity by adherence to MedD diet 0-4wk | NR | NR | NR | NR | NR | 77 species differed at week 4, primarily:↓ species:Ruthenibacterium lactatiformans, Flavonifractor plautii, Parabacteroides merdae, Ruminococcus torques, Ruminococcus Gnavus, Streptococcus thermophilusi, Streptococcus thermophilus↑ species: 5 members of Faecalibacterium prausnitzii and 7 members of taxa Roseburia and Lachnospiraceae |
— | Yes |
| 62 | Post-Mediterranean diet (wk8) | Post-omnivorous diet (wk8) | — | 44 species differed at week 8. Information on detailed species was not extracted. | — | ||||||||
| 32 | Post-omnivorous diet (wk4/wk8) | Pre-omnivorous diet | — | 14 species differed at week 4 compared to baseline, 19 differed at week 8 compared to week 4. Information on detailed species was not extracted. | — | ||||||||
| 30 | Post- Mediterranean diet (wk4/wk8) | Pre- Mediterranean diet | — | 69 species differed at week 4 compared to baseline, 17 differed at week 8 compared to week 4. Information on detailed species was not extracted. | — | ||||||||
| Kohnert et al. 2021 [91] | 53 | Post-pre change in omnivorous | Post-pre change in vegan | NR | Bray-Curtis dissimilarity: no clustering. | NR | NR | NR | NR | ↑ generaBacteroides, Clostridium, Faecalibacterium, Roseburia↓ genera:Bacteroides, Blautia, Dialister, Faecalibacterium, Ruminococcus | Differences in species were observed at ASV level.Information on detailed species was not extracted. | Unweighted UniFrac distances showed two separate clusters, but not clearly between two diets. | Yes |
| 27 | Post-omnivorous | Pre-omnivorous | Chao1, Shannon, Inverse Simpson, Fisher’s index:↔ | Bray-Curtis dissimilarity: no clustering | NR | NR | NR | NR | ↑ genera:Alistipes, Bacteroides, Blautia, Clostridium, Faecalibacterium (unspecified species), Megamonas, Roseburia, Ruminococcus↓ genera:Bacteroides, Bifidobacterium, Blautia, Dialister, Faecalibacterium, Gemminger, Phascolarctobacterium, Prevotella, Ruminococcus, Sutterella | Yes | |||
| 26 | Post-vegan | Pre-vegan | Chao1, Shannon, Inverse Simpson, Fisher’s index:↔ | Bray-Curtis dissimilarity: no clustering | NR | NR | NR | NR | ↑ genera:Alistipes, Bacteroides, Blautia, Coprococcus, Dialister, Dorea, Faecalibacterium (unspecified species), Phascolarctobacterium, Ruminococcus↓ genera:Akkermansia, Bacteroides, Bifidobacterium, Clostridium, Coprococcus, Faecalibacterium, Roseburia, Ruminococcus | Yes | |||
| McKenna et al. 2021 [90] | 28 | wk1-higher red meat (high) | Pre-high | Faith’s phylogenetic diversity and Shannon: ↔ |
Unweighted UniFrac: significant between-group differenceWeighted UniFrac:↔DEICODE metrics:↔ | NR | NR | Betaproteobacteriales:↑ | — | ↓ genera:Akkermansia, unclassified genus of family Eggerthellaceae, Ruminococcaceae UCG-010, Megasphaera, Veillonella↑ genera:Catenibacterium | — | — | Yes |
| 28 | Post-high | wk1-high | — | — | ↓ genera:Catenibacterium↑ genera:Akkermansia, unclassified genus of family Eggerthellaceae, Veillonella | — | — | ||||||
| 28 | Post-high | Pre-high | Betaproteobacteriales:↑ | — | Megasphaera:↓ | — | — | ||||||
| 22 | wk1-Lower red meat (MOD) | Pre-MOD | Faith’s phylogenetic diversity and Shannon: ↔ |
Unweighted UniFrac: significant between-group differenceWeighted UniFrac:↔DEICODE metrics:↔ | NR | NR | Mollicutes RF39:↓ | Lachnospiraceae:↑ | ↓ genera:Streptococcus↑ genera:Coprococcus 2, Holdemanella, Acidaminococcus | — | — | ||
| 22 | Post-MOD | wk1-MOD | Mollicutes RF39:↑ | — | ↓ genera:Acidaminococcus, Holdemanella, Catenibacterium↑ genera:Veillonella | — | — | ||||||
| 22 | Post-MOD | Pre-MOD | — | Lachnospiraceae:↑ | ↓ genera:Blautia, Catenibacterium↑ genera:Coprococcus 2 | — | — | ||||||
| 50 | Post-MOD | Post-high | Faith’s phylogenetic diversity and Shannon: ↔ |
Unweighted UniFrac: significant between-group differenceWeighted UniFrac:↔DEICODE metrics:↔ | NR | NR | — | Ruminococcaceae:↓ | ↓ genera:HoldemanellaI, Acidaminococcus↑ genera:Megasphaera, metagenome | — | — | ||
| 50 | wk1-MOD | wk1-high | Mollicutes RF39:↓ | — | ↑ genera:Ruminiclostridium 5, Oscillibacter, Megasphaera, metagenome, Lactobacillus, Veillonella, uncultured genus of family Eggerthellaceae | — | — | ||||||
| 50 | Pre-MOD | Pre-high | Betaproteobacteriales:↑ | Enterobacteriaceae:↑ | ↓ genera:[Eubacterium] eligens group, Ruminococcaceae UCG-013, Coprococcus 2, Holdemanella, Acidaminococcus, Slackia, uncultured genus of family Eggerthellaceae↑ genera:Streptococcus, Catenibacterium, metagenome, Dielma | — | — | ||||||
| Bratlie et al. 2021 [93] | 9 | Post-cod | Pre-cod | — | No clear separation between diets pre-intervention.Trend of separation between diets post-intervention, especially between post-salmon and post-control. | NR | NR | Post-Cod & Post-Salmon compared to Post-Control (bacterial counts):Bacteroidales:↓Clostridiales:↓Selenomonadales:↓ | NR | NR | NR | — | Yes |
| 13 | Post-salmon | Pre-salmon | |||||||||||
| 11 | Post-control | Pre-control |
Higher abundance (↑), lower abundance (↓), or comparable abundances (↔). NR, data measured but not reported; OTU, operational taxonomic unit; ASV, amplicon sequence variant.
There was limited consistency in bacterial taxa responses at the lower taxonomic levels when effects were detected. At the genus level, which was the most frequently reported taxonomic level by the included studies, 28 genera were reported to be affected by meat intake, with only 4 of these genera observed more than once (Bacteroides, Anaerostipes, Ruminococcus, and Roseburia; Table 5). Higher meat consumption, including presumably self-chosen omnivorous diets before vegan or vegetarian diets, appeared to reduce Anaerostipes abundance as reported in 3 studies [74, 84, 89]. The abundance of Bacteroides was reduced in 1 study [84] after consuming red meat compared with mushroom but not different as reported in 2 other studies [85, 91]. Similarly, the abundance of Roseburia was increased by higher meat intake in 2 studies [88, 91] but reduced in another study [92]. Although 3 studies [87, 88, 91] reported meat-related changes or differences in Ruminococcus abundance, the results were not clearly reported or were inconsistent for a comparison between higher and lower meat intakes. At the species level, Faecalibacterium prausnitzii was reported in 2 studies [89, 92] to have a lower abundance with higher meat intake (Table 5).
TABLE 5.
Genera and species that were affected by meat intake as reported by at least 2 studies.
| Bacteria | Study | Higher meat intake | Lower meat intake | Higher vs lower1 |
|---|---|---|---|---|
| Genera | ||||
| Bacteroides | van Faassen et al. 1987 [85] | Post-omnivorous | Post-vegan | ↔ |
| Post-omnivorous | Post-vegetarian | ↔ | ||
| Post-vegetarian | Post-vegan | ↔ | ||
| Hess et al. 2018 [84] | Post-red meat | Post-mushroom | ↓ | |
| Kohnert et al. 2021 [91] | Post-pre-omnivorous | Post-pre, post-, or pre-vegan | ↔2 | |
| Anaerostipes | Hess et al. 2018 [84] | Post-red meat | Post-mushroom | ↓ |
| Kahleova et al. 2020 [89] | Pre-vegan | Post-vegan | ↓ | |
| Pagliai et al. 2020 [74] | Post-pre-Mediterranean | Post-pre-vegetarian | ↓ | |
| Pre-vegetarian | Post-vegetarian | ↓ | ||
| Ruminococcus | Lang et al. 2018 [87] | Red/white meat | Nonmeat | Differed3 |
| Crimarco et al. 2020 [88] | Animal-based diet | Plant-based diet | ↑ | |
| Kohnert et al. 2021 [91] | Post-pre-omnivorous | Post-pre-vegan | ↓ | |
| Post-omnivorous | Pre-omnivorous | ↔2 | ||
| Pre-vegan | Post-vegan | ↔2 | ||
| Roseburia | Crimarco et al. 2020 [88] | Animal-based diet | Plant-based diet | ↑ |
| Meslier et al. 2020 [92] | Post-omnivorous (higher nonfish meat) | Post-Mediterranean diet (higher fish) | ↓ | |
| Kohnert et al. 2021 [91] | Post-pre-omnivorous | Post-pre-vegan | ↑ | |
| Post-omnivorous | Pre-omnivorous | ↑ | ||
| Pre-vegan | Post-vegan | ↑ | ||
| Species | ||||
| Faecalibacterium prausnitzii | Kahleova et al. 2020 [89] | Post-pre-omnivorous | Post-pre-vegan | ↓ |
| Pre-vegan | Post-vegan | ↓ | ||
| Meslier et al. 2020 [92] | Post-omnivorous (higher nonfish meat) | Post-Mediterranean diet (higher fish) | ↓ | |
Higher abundance (↑), Lower abundance (↓), or comparable abundances (↔) in the higher meat intake group compared to the lower meat intake group.
Amplicon sequence variant of the genus or species was reported as both enriched and depleted in the comparison by the study author.
Authors reported that the abundance of the genus differed among the red meat, white meat, and non-meat groups, but did not report the pair-wise difference between groups.
Subgroup analysis for heterogeneity exploration
To investigate potential contributors to the heterogeneity in reported outcomes, our a priori subgroup analyses included the following: 1) lacto-ovo vegetarian diet compared with the vegan diet; 2) comparisons between meat subtypes, including i) unprocessed compared with processed meat and ii) red meat compared with white meat compared with fish/seafood; 3) meat compared with nonmeat (plant-based foods or vegetarian/vegan diet), including i) red meat compared with nonmeat, ii) white meat compared with nonmeat, and iii) fish/seafood compared with nonmeat; and 4) higher and lower meat intakes (mixed meat subtypes). We also included additional subgroup analyses based on the type and length of studies, type of study diets, and levels of dietary control. The categorization of studies based on the listed subgroups is presented in Table 3.
Insufficient comparisons were available to compare specific meat subtypes, with only 1 comparison [87] available between red meat and white meat and 3 comparisons [71, 93] between mixed seafood subtypes and mixed non-seafood meat subtypes. No comparison was available between unprocessed and processed meat, red meat and fish/seafood, or white meat and fish/seafood. Among the 12 primary comparisons on higher and lower meat intakes, comparisons were between red meat (4 comparisons [84, 86, 87, 90]), white meat (1 comparison [87]), or mixed meat subtypes (7 comparisons [73, 74, 85, 88, 89, 91]) and plant-based foods or diets (Table 3). Although 4 comparisons [84, 86, 87, 90] used red meat and 3 [84, 86, 87] of them compared red meat with plant-based foods (e.g., mushroom and whole grains), only 1 of them [87] controlled basal dietary intake besides the intervention foods.
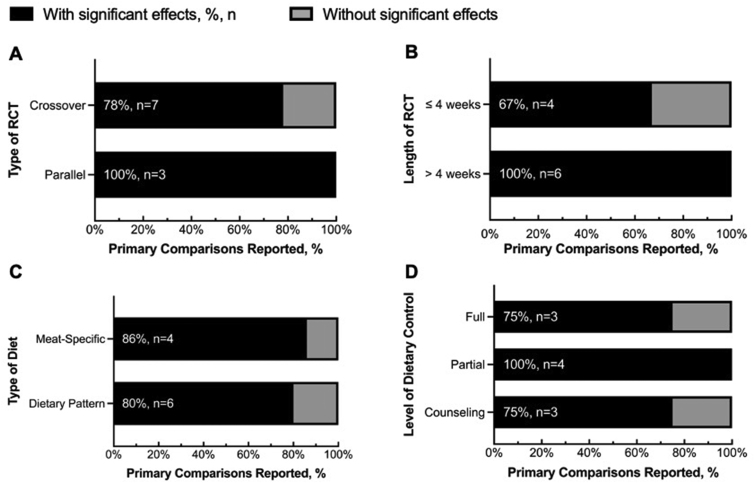
The effects of meat consumption on the gut microbiota were observed in studies utilizing various experimental design features (Figure 3). The proportions of outcomes showing and not showing effects of meat consumption on the gut microbiota are comparable between the crossover and parallel RCTs (Figure 3A) or between studies with meat-specific diets and those with dietary patterns (Figure 3C). At least 67% of comparisons across different study lengths and levels of dietary control consistently showed some effects (Figure 3B, D). The direction of changes and specific differences in the gut microbiota composition for each comparison showed limited consistency across studies (Table 4).
FIGURE 3.
Percentages of primary comparisons that did vs. did not report significant effects of diet on gut microbiota based on the (A) type of randomized controlled trial (RCT), (B) length of RCT, (C) type of diet, and (D) level of dietary control. The partial level of dietary control (D) encompasses the studies in which partial foods were provided with or without control over basal dietary intake, as well as studies in which 1 group was controlled and 1 group followed a habitual diet. The significant effects refer to whether significant changes or differences in microbiota composition were reported by authors of the included studies. The percentages and numbers of studies showing any significant effects out of the total number of studies with the specific study characteristics were denoted in percentages and n.
Risk of bias assessments
Crossover and parallel RCTs were assessed separately for the intention-to-treat effects on the gut microbiota with selected experimental and comparator groups. Among the 13 studies, none was rated low risk, 11 were rated with some concerns, and 2 were rated high risk of bias (Table 6) [71, 73, 74, [84], [85], [86], [87], [88], [89], [90], [91], [92], [93]]. The risk of bias is mainly generated from 1) period and carryover effects, 2) deviations from the intended interventions (effect of assignment to intervention), and 3) selection of the reported results. The 2 studies [73, 74] rated high risk of bias were crossover RCTs without washout periods between interventions, of which the one [74] with cointervention of energy restriction showed significant effects on the gut microbiota.
TABLE 6.
Cochrane risk of bias.
| Study (year) | Domain 1a: risk of bias arising from the randomization process | Domain S: risk of bias arising from period and carryover effects | Domain 2: risk of bias due to deviations from the intended interventions (effect of assignment to intervention) | Domain 3: risk of bias due to missing outcome data | Domain 4: risk of bias in the measurement of the outcome | Domain 5: risk of bias in the selection of the reported result | Overall risk of bias1 |
|---|---|---|---|---|---|---|---|
| Crossover RCTs | |||||||
| van Faassen et al. (1987) [85] | Some concerns | Low | Low | Low | Low | Some concerns | Some concerns |
| Windey et al. (2012) [73] | Low | High | Some concerns | Low | Low | Some concerns | High |
| Foerster et al. (2014) [86] | Low | Some concerns | Low | Low | Low | Some concerns | Some concerns |
| Lang et al. (2018) [87] | Some concerns | Some concerns | Some concerns | Low | Low | Some concerns | Some concerns |
| Hess et al. (2018) [84] | Some concerns | Some concerns | Some concerns | Low | Low | Some concerns | Some concerns |
| Schmedes et al. (2019) [71] | Low | Low | Some concerns | Low | Low | Some concerns | Some concerns |
| Pagliai et al. (2020) [74] | Low | High | Some concerns | Low | Low | Some concerns | High |
| Crimarco et al. (2020) [88] | Low | Low | Low | Low | Low | Some concerns | Some concerns |
| Parallel RCTs | |||||||
| Kahleova et al. (2020) [89] | Low | N/A | Low | Low | Low | Some concerns | Some concerns |
| Meslier et al. (2020) [92] | Low | N/A | Low | Low | Low | Some concerns | Some concerns |
| Kohnert et al. (2021) [91] | Low | N/A | Low | Low | Low | Some concerns | Some concerns |
| McKenna et al. (2021) [90] | Low | N/A | Low | Low | Low | Some concerns | Some concerns |
| Bratlie et al. (2020) [93] | Low | N/A | Some concerns | Low | Low | Some concerns | Some concerns |
For the description of overall risk of bias, “some concerns” refers to “the study is judged to raise some concerns in ≥1 domain for this result but not to be at high risk of bias for any domain”; “high” refers to “the study is judged to have some concerns for multiple domains in a way that substantially lowers confidence in the result.” N/A, not applicable; RCT, randomized controlled trial.
Discussion
The scoping review documents both observational and experimental research assessing the influence of meat consumption on the gut microbiota. Most observational research used cross-sectional designs. The paucity of meticulously designed prospective cohort studies precluded systematically assessing associations between meat intake and gut microbiota. Experimental research, primarily conducted with apparently healthy adults and using RCTs with crossover or parallel intervention designs, suggests that changes and differences in gut microbiota composition due to meat consumption are more detectable at lower taxonomic levels (e.g., genus and species) than higher taxonomic or community levels. Caution is warranted against drawing firm conclusions regarding the effects of meat intake on gut microbiota due to inconsistencies among the levels of dietary control, sources of meat, and specificity of bacterial composition.
Current state of knowledge (scoping review)
To our knowledge, our scoping and systematic review is among the first systematic reviews pertinent to meat intake and gut microbiota in humans. Our scoping review provides a comprehensive overview of the current state of knowledge and clearly identifies where research gaps exist. Compared with interventional studies, the number of observational studies more than doubled, whereas the health status and ages of participants in these studies were not always clearly described. Most observational research did not focus on meat intake but more broadly on dietary patterns with or without meat. Importantly, there is a paucity of prospective cohorts especially in healthy humans and RCTs, in particular crossover RCTs, in children and adolescents. In addition, the degree of meat processing (including the use of meat extenders) and cooking methods were not well documented by most studies. Given that most Americans and the global population consume meat, the influence of consuming total meat and specific meat subtypes on the gut microbiota composition across different age groups requires investigation.
Limitations and gaps in the current literature (systematic review)
Our systematic review included 13 RCTs in healthy adults with stable health status to assess the effects of meat intake on the gut microbiota. Initially, we planned to include both prospective cohort and RCT studies in healthy human adults with stable health status in our systematic review. However, only 1 eligible prospective cohort [25] was identified, which was insufficient for a systematic review. The findings from our systematic review implicate that the consumption of total meat and meat subtypes affects the gut microbiota composition, but the directionality and interpretation of these changes should be cautiously discussed, considering the inconsistencies across studies.
To investigate potential sources of heterogeneity, our a priori subgroup analyses based on the sources of meat did not yield any meaningful results because of the inadequate number of comparisons per subgroup. Gathering available literature, the number of comparisons between red meat and nonmeat intakes (4 comparisons [84, 86, 87, 90]) may potentially qualify for a subgroup review. However, only 1 of these 4 comparisons fully controlled participants’ basal dietary intake, and their comparator foods ranged from high-fiber plant-based foods (e.g., mushroom and whole grains) to mixed nonmeat foods or lower amounts of red meat. Comparing meat to different comparator foods was shown to result in differential cardiometabolic effects in a meta-analysis of RCTs [100]. For example, consuming red meat led to greater reductions in LDL and HDL cholesterol concentrations when fish was the comparator and to greater reductions in triglycerides when carbohydrates were the comparat but lesser reductions in triglycerides when high-quality plant-based protein foods were the comparator [100]. It remains questionable how much of the dietary effects on the gut microbiota were attributable to red meat intake when the participant’s dietary intake was not fully controlled and the comparator food was not specific, highlighting the importance of the “instead of what” question inherent to all nutrition science inquiries [101].
There is a particular paucity of research on the degree of meat processing. Of all red meat and poultry consumed by the US population, 55% were red meat and 45% were poultry, among which 32% of total red meat and 14% of total poultry were consumed in processed form (NHANES 2015–2018) [102]. Only ∼10% of the US adults who are beef consumers choose lean and unprocessed forms [103]. Accumulating observational research indicates that consuming processed meat is associated with a higher risk for cardiometabolic diseases compared with consuming unprocessed meat [104]. However, our search did not identify any available study comparing intakes of unprocessed and processed meats. High-temperature cooking methods (e.g., grilling) also produce carcinogens from meat that may increase the risk for certain cancers (e.g., colorectal cancer) [105, 106]. However, among the 4 studies [84, 86, 87, 90] that reported using meat in unprocessed form, only 1 study [90] briefly described the cooking method (Supplemental Table S4).
Although the study designs varied among studies, our post hoc subgroup analyses based on selected study characteristics (i.e., study type, length of diet, type of diet, and level of dietary control) did not suggest any specific sources of heterogeneity. Other subgroup analyses by demographic and anthropometric criteria may be considered but were not possible due to reasons such as unclear age cut-offs or an insufficient number of comparisons (male compared with female). The mean group ages of the study participants were between 18 and 65 y, whereas healthy older adults aged >65 y were rarely studied. Aging shifts the gut microbiota composition by reducing Bacteroides, which contributes to healthy aging [107]. Most studies have more female than male participants included in one or both of their intervention arms, whereas gut microbiota composition and responses can differ by sex [108, 109]. The racial diversity was inconsistently reported among studies (6 of 13 studies [71, 74, 85, [87], [88], [89]]), with most participants being Caucasian. Collectively, these limitations underscore the importance of future research being inclusive of age, sex, and racial diversity.
Noteworthy, we observed a lack of a priori power calculation based on the microbiota outcome in almost all RCTs. Only 1 [89] of the 13 RCTs reported the estimation of sample size based on outcomes related to gut microbiota. The lack of a priori power calculation could be due to not only a lack of pilot data or challenge of estimating the sample size for microbiota outcomes [110] but also that the gut microbiota were secondary or exploratory outcomes. Each study’s primary outcome is presented in Supplemental Table S3A (column “sample size estimation”). Although an adequate sample size is important for detecting statistical significance in outcomes of interest, the limited comparability and lack of research evidence restrict meaningful sample size estimation. Age, sex, and race are important participant characteristics to consider, which contribute to interpersonal variability in the gut microbiota but remain challenging to be addressed or adjusted for. One RCT [87] assessed how individual biological and anthropometric characteristics altered gut microbiota relative abundances. Genera such as Akkermansia and Haemophilus are positively and negatively associated with age, respectively; race and sex were found to influence community diversity and dozens of bacterial taxa abundances [87]. However, individual traits were not adjusted for when assessing diet-induced effects [87]. Three studies [84, 86, 89] reported statistical adjustments of potential confounders (e.g., age, sex, BMI, and race). Studies also attempted to control for differences in individual traits by recruiting within the same race (e.g., Caucasian [71, 74, 85]) or the same sex (e.g., male [85]). However, they missed considering other potential confounders such as the use of medications (e.g., antibiotics) or supplementations (e.g., pre- or probiotics), as well as intakes of certain dietary components (e.g., fiber) with a known impact on the gut microbiota. Nevertheless, data from these RCTs can serve as preliminary pilot data for future research studying the effects of specific meat subtypes on the gut microbiota.
The analysis and reporting of data were not standardized, thereby limiting the comparability of findings among studies. For instance, 2 of the 12 primary comparisons [73, 85] did not suggest any effect of meat consumption on the gut microbiota. Yet, these 2 studies [73, 85] differ from others in both study design (i.e., no washout [73]) and molecular analysis methods (i.e., denaturing gradient gel electrophoresis [73] and cell culture [85]). Compared with more recent techniques such as 16S rRNA amplicon and shotgun sequencing, cell culture captures a small fraction of the bacterial profile, and denaturing gradient gel electrophoresis does not provide phylogenetic characterization without further analysis [111]. For community-level diversity measures, operational taxonomic unit (OTU)-based Shannon’s index and Bray–Curtis dissimilarity were used most frequently for measuring alpha and beta diversity, respectively, in the RCTs (Table 4), but they tend to be less sensitive in detecting differences than phylotype-based approaches (e.g., weighted UniFrac distance) [112, 113]. The type of metrics used for alpha and beta diversity was not consistent either, which requires careful consideration based on the meaning and ecological relevance of the indices or metrics [114]. Biases in assessing the bacterial composition are introduced when different sample collection, processing, and analysis methods are chosen [[115], [116], [117]], whereas they were generally not justified by the study authors. In the 2 comparisons [71, 93] that compared fish/seafood with a mixture of non–fish/seafood meat subtypes, changes in bacterial abundances were detected in both studies but were reported at different taxonomic levels for different taxa. Recent findings argue against using the relative abundance of bacteria compared with absolute abundance for potentially misleading reporting [118]. Among the comparisons with significant effects, either mean change values or post-intervention values were used for between-group comparisons. In line with the aforementioned inconsistencies, all studies were rated as having some concerns for or high risk of bias. Although it was impractical to completely blind subjects regarding the type of diet in RCTs studying the effects of meat intake at the whole food level, in 3 crossover RCTs without a washout period between 2 diet interventions [73, 74, 88], the effects of the first diet on the following diet were not adequately assessed or discussed in 2 studies [73, 74]. The lack of washout period and repeated measures for baseline assessments and comparisons before each intervention leads to a high risk of bias from period and carryover effects in these 2 studies [73, 74]. Future studies on diet and gut microbiota should follow standardized protocols that are comparable with those reported in the previous studies, with justified and comprehensive reporting to improve research reproducibility [119].
The connections behind changes in the gut microbiota and host health outcomes warrant further research. Ten [71, 74, [86], [87], [88], [89], [90], [91], [92], [93]] of the 13 RCTs measured clinical health outcomes besides gut microbiota, and 9 [71, 74, 86, 87, [89], [90], [91], [92], [93]] of the 10 studies conducted correlation analyses to explore potential concurrence. Among the genera and species reported to be affected by meat intake (Table 5), Anaerostipes was shown to be positively associated with LDL cholesterol and total cholesterol [74]. However, the functions and clinical relevance of bacteria and their metabolites remain largely unclear. Bacteria with no expression of metabolic activities may not be as clinically relevant as those producing clinically relevant metabolites, but the metabolic activities of bacteria can also be altered by the environment (e.g., diet) [118]. Identified correlations between gut microbiota and health outcomes should be further validated with functional analyses, which, however, requires expensive molecular and analysis methods with higher resolution (e.g., metagenomics and multiomic approaches) [118], and is currently limited by the availability of functional databases [120].
Potential mechanisms of meat–microbiota interactions
The effects of meat intake on the gut microbiota and host health are complex. Mechanistically, meat may influence gut microbiota by providing abundant nutrients (e.g., protein, fat, choline, and iron) available after host digestion and absorption for further metabolism, influencing the host health directly through bacterial components (e.g., LPS entering the systemic circulation) or indirectly through generating metabolites. Although these nutrients are not unique to meat, it is unclear how the matrix (e.g., combination of dietary components) and processing (e.g., cooking and addition of dietary emulsifiers) of meat may influence the health effects of consumption on gut microbiota [121]. Potential health-impacting gut microbiota–derived metabolites include secondary bile acids (e.g., deoxycholic and lithocholic acids) [122], SCFAs and branched-chained fatty acids [122], and trimethylamine and trimethylamine-N-oxide (TMAO) [123], as well as ammonia, hydrogen sulfide, polyamine, and indolic and phenolic compounds [124]. However, inconsistencies in the effects of meat intake on these metabolites (e.g., secondary bile acids [85, 92, 93], SCFAs and branched-chained fatty acids [71, 73, 74, 84, 86, 92, 93], and TMAO [71, 88, 92]) were observed in RCTs included in this review. Assessments of purported plausible mechanisms to explain the effects of meat intakes on the gut microbiota and host health outcomes are beyond the scope and focus of this review.
The effects of meat intake on the gut microbiota are further complexed by interactions with and the effects of nonmeat components within a dietary pattern [[125], [126], [127]]. Purported confounding dietary factors may include different fiber intakes [128] between higher and lower meat intake groups [84, 89, 91, 92], saturated fat with overriding effects on gut microbiota independent of meat subtypes or protein sources [87], or components (e.g., 3,3-dimethyl-1-butanol in olive oil) [129, 130] of a healthy dietary pattern that counteracts certain effects of meat intake. Therefore, it is important to specify meat subtypes and control the overall dietary intake (including acutely co-consumed foods and broader dietary patterns) when studying meat and gut microbiota and to interpret changes in the gut microbiota considering their functional impacts.
Strengths and limitations
Our scoping and systematic reviews provide a comprehensive summary and overview of the current scientific literature on meat intake and gut microbiota in humans. By focusing on RCTs in the systematic review, we provided informative guidance on features of study design and reporting of results for conducting the human gut microbiota study with dietary interventions. However, our review focuses on summarizing and synthesizing research evidence for gut microbial changes related to meat intake and not on mechanisms. Although we observed the effects of dietary modifications on the gut microbiota, the biological significance of these effects is limited by associations but not causal relationships to health indices. The findings of results were determined by authors of the studies, which are prone to the bias of results’ reporting. Furthermore, adequate data are not available for quantitative analysis of outcome measures, but a quantitative assessment and meta-analysis of the gut microbiota outcomes remain implausible.
Conclusions
The prominence of meat consumed in the United States and globally underscores the importance of knowing how it relates to and affects the human gut microbiota as an important diet-health mediator. The main intentions of this research were to provide a scoping review and a systematic review to inform meat industry stakeholders, nutrition and health researchers, and policymakers about the current state of literature and directions for future research. The main findings from this review are as follows: 1) a limited number of RCTs (particularly in older adults) and prospective cohort studies have been conducted to understand the relationships between meat intake and gut microbiota in healthy adults; 2) inconsistencies among studies were observed for the types of bacteria affected and the magnitude and directionality of bacterial responses; 3) the experimental designs and reporting of results were inconsistent among studies, highlighting the need for well-designed, full-feed RCTs; 4) the current evidence is not adequate for studying the heterogeneity of results based on meat subtypes, the degree of processing (including degree of meat extension), or method of cooking; and 5) the directionality and health implications of changes or differences in the gut microbiota warrant future research. Our findings will hopefully shed light on areas that have not been well explored and spur new research.
Recommendations for Future Research
Based on the findings from our scoping review and systematic review, future research may consider the following 3 high-priority recommendations:
-
1)
Full-feeding RCTs are warranted to assess the effects of consuming specific meat subtypes, with specified degrees of meat processing and dietary patterns, on the gut microbiota in humans.
Rationale
Our findings indicate a paucity of research assessing meat subtypes with full-feeding dietary interventions. Our review identified only 1 study in white meat [87], 2 studies in fish/seafood [71, 93], and 4 studies in red meat [84, 86, 87, 90]. None of the studies assessed how the degree of meat processing (e.g., unprocessed or processed meat) or methods of cooking (e.g., baking or grilling) affects the gut microbiota. We identified and excluded 1 RCT [131] comparing the effects of processed meat and nitrate-added drinking water on the gut microbiota because it did not provide a comparison between higher and lower meat intakes and no changes in gut microbiota abundance were reported; it is also unlikely that nitrate by itself transits to the colon [132]. Although the level of dietary control (i.e., full-feed, partial-feed, and dietary counseling) did not seem to influence whether any diet-induced effects can be observed in the gut microbiota, when meat was consumed within different dietary patterns or minimally controlled habitual diets, it was implausible to assess meat-specific effects. There also seems to be a trend of more studies showing dietary effects on the gut microbiota when the diet intervention was >4 wk. However, the selection of 4 wk to categorize and group studies was based on the observations of included study durations and not on an established definition of short- and long-term studies. Further, the 2 studies that showed “no effects” either used a cultivation-based method that is prone to miss capturing statistically significant findings in the gut microbiota [85] or did not include a washout period between 2 interventions to avoid any carryover effects [73]. Thus, the recommended length of intervention should be determined based on the expected time needed for both microbial and nonmicrobial outcomes of interest to respond to a diet intervention. Studies should also be powered based on the gut microbiota outcomes of interest a priori. Additional suggestions are presented elsewhere [119]. Nevertheless, data from these RCTs can be used for sample size estimation based on gut microbiota outcomes in future studies.
-
2)
There is a need for more prospective observational studies on gut microbiota with total meat and meat subtypes as a priori independent variables among adults without diagnosed disease. A standardized definition of total meat and meat subtypes should be used and reported.
Rationale
Only 1 prospective observational study on meat intake and gut microbiota in healthy adults was identified through our comprehensive and systematic search of available English peer-reviewed literature. Most observational studies were cross-sectional (47 of 57 studies) with a weak level of temporality (i.e., exposure happens before the outcome occurs). Meat was studied as part of a habitual dietary pattern with inconsistent groupings of meat [133], making it challenging to draw consistent conclusions on the associations between meat and gut microbiota based on observational evidence. Furthermore, most of our identified observational studies had a small sample size. However, current national databases such as the NHANES [134] or the Human Microbiome Project [[135], [137]] provide data on either dietary intake or gut microbiota composition, but not both. Further research collecting data on both dietary intake and gut microbiota with larger cohorts is warranted.
-
3)
Upon addressing recommendations 1 and 2, systematic reviews and meta-analyses are needed to assess the effects of meat intake on gut microbiota–derived metabolites and health outcomes (e.g., cardiometabolic risk factors), as well as the relationships between meat-induced gut microbiota compositional changes and health outcomes.
Rationale
It remains unclear how the effects of meat consumption on gut microbiota would impact the host’s health. Although gut microbiota–derived metabolites and health indices other than gut microbiota were also assessed in the included RCTs, their conclusions generally only describe effect or no-effect regarding diet and gut microbiota. Six [71, 86, [88], [89], [90], [91]] of the 13 RCTs associated results in the gut microbiota with potential health implications, but only 1 [91] of them summarized their findings with directional terms (e.g., harmful or beneficial). Our systematic review documents secondary outcomes of gut microbiota–derived metabolites that were also measured and reported in the included RCTs (Supplemental Table S5). We specifically selected metabolites that are associated with health outcomes (e.g., cardiometabolic disease risk factors). We also documented whether a correlational analysis was conducted between changes in gut microbiota composition and these metabolites or cardiometabolic health outcomes (Supplemental Table S5). However, this was not the focus and intention of our current review. Systematic reviews and meta-analyses with targeted focus on dietary intake and gut microbiota are lacking. Based on our brief search on PubMed, the few systematic reviews published previously on diet and gut microbiota are mostly qualitative or have a broader scope of focus on nutrition, including diet and nutrition in general [137, 138], probiotic supplementation [139], oat intake [140], and a specific nutrient in both humans and animals [7]. None of them included any of the 13 RCTs identified and included in our systematic review. Importantly, both the current and previous reviews emphasize the need for future research to address the differences in individual responses, the influence of dietary patterns and processing and cooking methods, and health implications and mechanisms related to microbial changes.
Author disclosures
During the time when this review was conducted, WWC received funding for research grants, travel, or honoraria for scientific presentations or consulting services from the following organizations: US NIH, USDA (Hatch Funding), Pork Checkoff, National Pork Board, Beef Checkoff, North Dakota Beef Commission, National Cattlemen’s Beef Association, Foundation for Meat and Poultry Research and Education, American Egg Board, Whey Protein Research Consortium, National Dairy Council, Barilla Group, Mushroom Council, and the National Chicken Council. JBR received grant support from the National Chicken Council and Whey Protein Research Consortium. REB is currently employed by ADM; research presented in this article was conducted in a former role and has no connection with ADM. YW, CNU, CMC, T-WLC, SRL, and MT report no conflicts of interest. The funder and the aforementioned other organizations had no role in the design and conduct of the review, analysis, interpretation of the results, and writing of the manuscript.
Acknowledgments
The authors’ responsibilities were as follows – YW and WWC: conceptualization and methodology; JBR and YW: literature searches; YW, CNU, REB, and CMC: article screening and data extraction; YW: original draft writing and preparation; YW, WWC, T-WLC, SRL, MT, JBR, REB, and CNU: draft review and editing; WWC, T-WLC, SRL, and MT: supervision; YW: project administration; and YW, REB, and WWC: funding acquisition. All authors read and approved the final manuscript.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.advnut.2022.10.005.
Funding
Funded by the Beef Checkoff.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Food Balances (2010-) [Internet]. Food and Agriculture Organization of the United Nations. [Cited on December 24, 2021]. Available at https://www.fao.org/faostat/en/#data/FBS.
- 2.U.S. Per Capita Availability of Red Meat, Poultry, and Seafood on the Rise [Internet] U.S. Department of Agriculture Economic Research Service; December 2019. https://www.ers.usda.gov/amber-waves/2019/december/us-per-capita-availability-of-red-meat-poultry-and-seafood-on-the-rise/ [Cited on December 24, 2021]. Available at. [Google Scholar]
- 3.Seman D.L., Boler D.D., Carr C.C., Dikeman M.E., Owens C.M., Keeton J.T., et al. Meat science lexicon, Meat Musc. Biol. 2018;2:1–15. [Google Scholar]
- 4.O’Connor L.E., Gifford C.L., Woerner D.R., Sharp J.L., Belk K.E., Campbell W.W. Dietary meat categories and descriptions in chronic disease research are substantively different within and between experimental and observational studies: a systematic review and landscape analysis. Adv Nutr. 2020;11:41–51. doi: 10.1093/advances/nmz072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dietary Guidelines for Americans, 2020–2025 [Internet] 9th Edition. U.S. Department of Agriculture and U.S. Department of Health and Human Services; December 2020. DietaryGuidelines.gov Available at. [Google Scholar]
- 6.Richi E.B., Baumer B., Conrad B., Darioli R., Schmid A., Keller U. Health risks associated with meat consumption: a review of epidemiological studies. Int J Vitam Nutr Res. 2015;85:70–78. doi: 10.1024/0300-9831/a000224. [DOI] [PubMed] [Google Scholar]
- 7.Albracht-Schulte K., Islam T., Johnson P., Moustaid-Moussa N. Systematic review of beef protein effects on gut microbiota: implications for health. Adv Nutr. 2021;12:102–114. doi: 10.1093/advances/nmaa085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nguyen T.L.A., Vieira-Silva S., Liston A., Raes J. How informative is the mouse for human gut microbiota research? Dis Model Mech. 2015;8:1–16. doi: 10.1242/dmm.017400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Park J.C., Im S.H. Of men in mice: the development and application of a humanized gnotobiotic mouse model for microbiome therapeutics. Exp Mol Med. 2020;52:1383–1396. doi: 10.1038/s12276-020-0473-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Page M.J., McKenzie J.E., Bossuyt P.M., Boutron I., Hoffmann T.C., Mulrow C.D., et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tricco A.C., Lillie E., Zarin W., O’Brien K.K., Colquhoun H., Levac D., et al. PRISMA extension for scoping reviews (PRISMA-ScR): checklist and explanation. Ann Intern Med. 2018;169:467–473. doi: 10.7326/M18-0850. [DOI] [PubMed] [Google Scholar]
- 12.Sterne J.A.C., Savović J., Page M.J., Elbers R.G., Blencowe N.S., Boutron I., et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. doi: 10.1136/bmj.l4898. [DOI] [PubMed] [Google Scholar]
- 13.Wu G.D., Compher C., Chen E.Z., Smith S.A., Shah R.D., Bittinger K., et al. Comparative metabolomics in vegans and omnivores reveal constraints on diet-dependent gut microbiota metabolite production. Gut. 2016;65:63–72. doi: 10.1136/gutjnl-2014-308209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Moraes A.C.F., Fernandes G.R., da Silva I.T., Almeida-Pititto B., Gomes E.P., Pereira A.D., et al. Enterotype may drive the dietary-associated cardiometabolic risk factors. Front Cell Infect Microbiol. 2017;7:47. doi: 10.3389/fcimb.2017.00047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kabeerdoss J., Devi R.S., Mary R.R., Ramakrishna B.S. Faecal microbiota composition in vegetarians: comparison with omnivores in a cohort of young women in southern India. Br J Nutr. 2012;108:953–957. doi: 10.1017/S0007114511006362. [DOI] [PubMed] [Google Scholar]
- 16.Ramadass B., Rani B.S., Pugazhendhi S., John K.R., Ramakrishna B.S. Faecal microbiota of healthy adults in south India: comparison of a tribal and a rural population. Indian J Med Res. 2017;145:237–246. doi: 10.4103/ijmr.IJMR_639_14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shikany J.M., Demmer R.T., Johnson A.J., Fino N.F., Meyer K., Ensrud K.E., et al. Association of dietary patterns with the gut microbiota in older, community-dwelling men. Am J Clin Nutr. 2019;110:1003–1014. doi: 10.1093/ajcn/nqz174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Genoni A., Christophersen C.T., Lo J., Coghlan M., Boyce M.C., Bird A.R., et al. Long-term Paleolithic diet is associated with lower resistant starch intake, different gut microbiota composition and increased serum TMAO concentrations. Eur J Nutr. 2020;59:1845–1858. doi: 10.1007/s00394-019-02036-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Das B., Ghosh T.S., Kedia S., Rampal R., Saxena S., Bag S., et al. Analysis of the gut microbiome of rural and urban healthy Indians living in sea level and high altitude areas. Sci Rep. 2018;8 doi: 10.1038/s41598-018-28550-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Losasso C., Di Cesare A., Mastrorilli E., Patuzzi I., Cibin V., Eckert E.M., et al. Assessing antimicrobial resistance gene load in vegan, vegetarian and omnivore human gut microbiota. Int J Antimicrob Agents. 2018;52:702–705. doi: 10.1016/j.ijantimicag.2018.07.023. [DOI] [PubMed] [Google Scholar]
- 21.Wu W.K., Chen C.C., Liu P.Y., Panyod S., Liao B.Y., Chen P.C., et al. Identification of TMAO-producer phenotype and host–diet–gut dysbiosis by carnitine challenge test in human and germ-free mice. Gut. 2019;68:1439–1449. doi: 10.1136/gutjnl-2018-317155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.De Filippis F., Pellegrini N., Vannini L., Jeffery I.B., La Storia A., Laghi L., et al. High-level adherence to a Mediterranean diet beneficially impacts the gut microbiota and associated metabolome. Gut. 2016;65:1812–1821. doi: 10.1136/gutjnl-2015-309957. [DOI] [PubMed] [Google Scholar]
- 23.Ruengsomwong S., La-Ongkham O., Jiang J., Wannissorn B., Nakayama J., Nitisinprasert S. Microbial community of healthy Thai vegetarians and non-vegetarians, their core gut microbiota, and pathogen risk. J Microbiol Biotechnol. 2016;26:1723–1735. doi: 10.4014/jmb.1603.03057. [DOI] [PubMed] [Google Scholar]
- 24.Wang F., Yu T., Huang G., Cai D., Liang X., Su H., et al. Gut microbiota community and its assembly associated with age and diet in Chinese centenarians. J Microbiol Biotechnol. 2015;25:1195–1204. doi: 10.4014/jmb.1410.10014. [DOI] [PubMed] [Google Scholar]
- 25.Liszt K., Zwielehner J., Handschur M., Hippe B., Thaler R., Haslberger A.G. Characterization of bacteria, Clostridia and Bacteroides in faeces of vegetarians using qPCR and PCR-DGGE fingerprinting. Ann Nutr Metab. 2009;54:253–257. doi: 10.1159/000229505. [DOI] [PubMed] [Google Scholar]
- 26.Zimmer J., Lange B., Frick J.S., Sauer H., Zimmermann K., Schwiertz A., et al. A vegan or vegetarian diet substantially alters the human colonic faecal microbiota. Eur J Clin Nutr. 2012;66:53–60. doi: 10.1038/ejcn.2011.141. [DOI] [PubMed] [Google Scholar]
- 27.Luongo D., Coppola A., Treppiccione L., Bergamo P., Sorrentino A., Ferrocino I., et al. Modulation of the cytokine profile in Caco-2 cells by faecal lactobacilli and bifidobacteria from individuals with distinct dietary habits. Cytokine. 2017;90:80–87. doi: 10.1016/j.cyto.2016.11.007. [DOI] [PubMed] [Google Scholar]
- 28.Zhang J., Guo Z., Lim A.A.Q., Zheng Y., Koh E.Y., Ho D., et al. Mongolians core gut microbiota and its correlation with seasonal dietary changes. Sci Rep. 2014;4:5001. doi: 10.1038/srep05001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ferrocino I., Di Cagno R., De Angelis M., Turroni S., Vannini L., Bancalari E., et al. Fecal microbiota in healthy subjects following omnivore, vegetarian and vegan diets: culturable populations and rRNA DGGE profiling. PLoS One. 2015;10 doi: 10.1371/journal.pone.0128669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Trefflich I., Jabakhanji A., Menzel J., Blaut M., Michalsen A., Lampen A., et al. Is a vegan or a vegetarian diet associated with the microbiota composition in the gut? Results of a new cross-sectional study and systematic review. Crit Rev Food Sci Nutr. 2020;60:2990–3004. doi: 10.1080/10408398.2019.1676697. [DOI] [PubMed] [Google Scholar]
- 31.Hippe B., Zwielehner J., Liszt K., Lassl C., Unger F., Haslberger A.G. Quantification of butyryl CoA:acetate CoA-transferase genes reveals different butyrate production capacity in individuals according to diet and age. FEMS Microbiol Lett. 2011;316:130–135. doi: 10.1111/j.1574-6968.2010.02197.x. [DOI] [PubMed] [Google Scholar]
- 32.O’Keefe S.J.D., Chung D., Mahmoud N., Sepulveda A.R., Manafe M., Arch J., et al. Why do African Americans get more colon cancer than native Africans? J Nutr. 2007;137:175S. doi: 10.1093/jn/137.1.175S. –82S. [DOI] [PubMed] [Google Scholar]
- 33.Seura T., Fukuwatari T. Japanese diet score is associated with gut microbiota composition in young Japanese adults. J Nutr Sci Vitaminol (Tokyo) 2019;65:414–420. doi: 10.3177/jnsv.65.414. [DOI] [PubMed] [Google Scholar]
- 34.Zhang J., Zheng Y., Guo Z., Qiao J., Gesudu Q., Sun Z., et al. The diversity of intestinal microbiota of Mongolians living in Inner Mongolia, China. Benef Microbes. 2013;4:319–328. doi: 10.3920/BM2013.0028. [DOI] [PubMed] [Google Scholar]
- 35.Bajaj J.S., Idilman R., Mabudian L., Hood M., Fagan A., Turan D., et al. Diet affects gut microbiota and modulates hospitalization risk differentially in an international cirrhosis cohort. Hepatology. 2018;68:234–247. doi: 10.1002/hep.29791. [DOI] [PubMed] [Google Scholar]
- 36.De Filippis F., Pasolli E., Tett A., Tarallo S., Naccarati A., De Angelis M., et al. Distinct genetic and functional traits of human intestinal Prevotella copri strains are associated with different habitual diets. Cell Host Microbe. 2019;25:444–453. doi: 10.1016/j.chom.2019.01.004. e3. [DOI] [PubMed] [Google Scholar]
- 37.Garcia-Mantrana I., Selma-Royo M., Alcantara C., Collado M.C. Shifts on gut microbiota associated to Mediterranean diet adherence and specific dietary intakes on general adult population. Front Microbiol. 2018;9:890. doi: 10.3389/fmicb.2018.00890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mitsou E.K., Kakali A., Antonopoulou S., Mountzouris K.C., Yannakoulia M., Panagiotakos D.B., et al. Adherence to the Mediterranean diet is associated with the gut microbiota pattern and gastrointestinal characteristics in an adult population. Br J Nutr. 2017;117:1645–1655. doi: 10.1017/S0007114517001593. [DOI] [PubMed] [Google Scholar]
- 39.Federici E., Prete R., Lazzi C., Pellegrini N., Moretti M., Corsetti A., et al. Bacterial composition, genotoxicity, and cytotoxicity of fecal samples from individuals consuming omnivorous or vegetarian diets. Front Microbiol. 2017;8:300. doi: 10.3389/fmicb.2017.00300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang X., Gibson G.R., Costabile A., Sailer M., Theis S., Rastall R.A. Prebiotic supplementation of in vitro fecal fermentations inhibits proteolysis by gut bacteria, and host diet shapes gut bacterial metabolism and response to intervention. Appl Environ Microbiol. 2019;85:e02749. doi: 10.1128/AEM.02749-18. 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vallès Y., Artacho A., Pascual-García A., Ferrús M.L., Gosalbes M.J., Abellán J.J., et al. Microbial succession in the gut: directional trends of taxonomic and functional change in a birth cohort of Spanish infants. PLoS Genet. 2014;10 doi: 10.1371/journal.pgen.1004406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bai J., Hu Y., Bruner D.W. Composition of gut microbiota and its association with body mass index and lifestyle factors in a cohort of 7–18 years old children from the American Gut Project. Pediatr Obes. 2019;14 doi: 10.1111/ijpo.12480. [DOI] [PubMed] [Google Scholar]
- 43.De Filippo C., Cavalieri D., Di Paola M., Ramazzotti M., Poullet J.B., Massart S., et al. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc Natl Acad Sci U S A. 2010;107:14691–14696. doi: 10.1073/pnas.1005963107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.La-Ongkham O., Nakphaichit M., Leelavatcharamas V., Keawsompong S., Nitisinprasert S. Distinct gut microbiota of healthy children from two different geographic regions of Thailand. Arch Microbiol. 2015;197:561–573. doi: 10.1007/s00203-015-1089-0. [DOI] [PubMed] [Google Scholar]
- 45.Nakayama J., Yamamoto A., Palermo-Conde L.A., Higashi K., Sonomoto K., Tan J., et al. Impact of westernized diet on gut microbiota in children on Leyte island. Front Microbiol. 2017;8:197. doi: 10.3389/fmicb.2017.00197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Matijašić B.B., Obermajer T., Lipoglavšek L., Grabnar I., Avguštin G., Rogelj I. Association of dietary type with fecal microbiota in vegetarians and omnivores in Slovenia. Eur J Nutr. 2014;53:1051–1064. doi: 10.1007/s00394-013-0607-6. [DOI] [PubMed] [Google Scholar]
- 47.Markiewicz L.H., Honke J., Haros M., Świątecka D., Wróblewska B. Diet shapes the ability of human intestinal microbiota to degrade phytate – in vitro studies. J Appl Microbiol. 2013;115:247–259. doi: 10.1111/jam.12204. [DOI] [PubMed] [Google Scholar]
- 48.Maclennan R., Jensen O.M. Dietary fibre, transit-time, faecal bacteria, steroids, and colon cancer in two Scandinavian populations. Report from the International Agency for Research on Cancer Intestinal Microecology Group. Lancet. 1977;2:207–211. [PubMed] [Google Scholar]
- 49.Goldberg M.J., Smith J.W., Nichols R.L. Comparison of the fecal microflora of Seventh-Day Adventists with individuals consuming a general diet. Implications concerning colonic carcinoma. Ann Surg. 1977;186:97–100. doi: 10.1097/00000658-197707000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Drasar B.S., Montgomery F., Tomkins A.M. Diet and faecal flora in three dietary groups in rural northern Nigeria. J Hyg (Lond). 1986;96:59–65. doi: 10.1017/s0022172400062537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liao M., Xie Y., Mao Y., Lu Z., Tan A., Wu C., et al. Comparative analyses of fecal microbiota in Chinese isolated Yao population, minority Zhuang and rural Han by 16sRNA sequencing. Sci Rep. 2018;8:1142. doi: 10.1038/s41598-017-17851-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Drasar B.S., Crowther J.S., Goddard P., Hawksworth G., Hill M.J., Peach S., et al. The relation between diet and the gut microflora in man. Proc Nutr Soc. 1973;32:49–52. doi: 10.1079/pns19730014. [DOI] [PubMed] [Google Scholar]
- 53.Aries V.C., Crowther J.S., Drasar B.S., Hill M.J., Ellis F.R. The effect of a strict vegetarian diet on the faecal flora and faecal steroid concentration. J Pathol. 1971;103:54–56. doi: 10.1002/path.1711030108. [DOI] [PubMed] [Google Scholar]
- 54.Quercia S., Turroni S., Fiori J., Soverini M., Rampelli S., Biagi E., et al. Gut microbiome response to short-term dietary interventions in reactive hypoglycemia subjects. Diabetes Metab Res Rev. 2017;33:e2927. doi: 10.1002/dmrr.2927. [DOI] [PubMed] [Google Scholar]
- 55.Cox I.J., Idilman R., Fagan A., Turan D., Ajayi L., Le Guennec A.D., et al. Metabolomics and microbial composition increase insight into the impact of dietary differences in cirrhosis. Liver Int. 2020;40:416–427. doi: 10.1111/liv.14256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bamola V.D., Ghosh A., Kapardar R.K., Lal B., Cheema S., Sarma P., et al. Gut microbial diversity in health and disease: experience of healthy Indian subjects, and colon carcinoma and inflammatory bowel disease patients. Microb Ecol Health Dis. 2017;28 doi: 10.1080/16512235.2017.1322447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tang M., Frank D.N., Tshefu A., Lokangaka A., Goudar S.S., Dhaded S.M., et al. Different gut microbial profiles in sub-Saharan African and South Asian women of childbearing age are primarily associated with dietary intakes. Front Microbiol. 2019;10:1848. doi: 10.3389/fmicb.2019.01848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Katsidzira L., Ocvirk S., Wilson A., Li J., Mahachi C.B., Soni D., et al. Differences in fecal gut microbiota, short-chain fatty acids and bile acids link colorectal cancer risk to dietary changes associated with urbanization among Zimbabweans. Nutr Cancer. 2019;71:1313–1324. doi: 10.1080/01635581.2019.1602659. [DOI] [PubMed] [Google Scholar]
- 59.Barrett H.L., Gomez-Arango L.F., Wilkinson S.A., McIntyre H.D., Callaway L.K., Morrison M., et al. A vegetarian diet is a major determinant of gut microbiota composition in early pregnancy. Nutrients. 2018;10:890. doi: 10.3390/nu10070890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.García-Vega Á.S., Corrales-Agudelo V., Reyes A., Escobar J.S. Diet quality, food groups and nutrients associated with the gut microbiota in a nonwestern population. Nutrients. 2020;12:2938. doi: 10.3390/nu12102938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wu X., Unno T., Kang S., Park S. A Korean-style balanced diet has a potential connection with Ruminococcaceae enterotype and reduction of metabolic syndrome incidence in Korean adults. Nutrients. 2021;13:495. doi: 10.3390/nu13020495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yu D., Nguyen S.M., Yang Y., Xu W., Cai H., Wu J., et al. Long-term diet quality is associated with gut microbiome diversity and composition among urban Chinese adults. Am J Clin Nutr. 2021;113:684–694. doi: 10.1093/ajcn/nqaa350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Veca R., O’Dea C., Burke J., Hatje E., Kuballa A., Katouli M. A comparative study of the adherent-invasive Escherichia coli population and gut microbiota of healthy vegans versus omnivores. Microorganisms. 2020;8:1165. doi: 10.3390/microorganisms8081165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shen W., Sun J., Li Z., Yao F., Lin K., Jiao X. Food intake and its effect on the species and abundance of intestinal flora in colorectal cancer and healthy individuals. Korean J Intern Med. 2021;36:568–583. doi: 10.3904/kjim.2019.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tap J., Störsrud S., Le Nevé B., Cotillard A., Pons N., Doré J., et al. Diet and gut microbiome interactions of relevance for symptoms in irritable bowel syndrome. Microbiome. 2021;9:74. doi: 10.1186/s40168-021-01018-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bolte L.A., Vich Vila A., Imhann F., Collij V., Gacesa R., Peters V., et al. Long-term dietary patterns are associated with pro-inflammatory and anti-inflammatory features of the gut microbiome. Gut. 2021;70:1287–1298. doi: 10.1136/gutjnl-2020-322670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Trefflich I., Dietrich S., Braune A., Abraham K., Weikert C. Short- and branched-chain fatty acids as fecal markers for microbiota activity in vegans and omnivores. Nutrients. 2021;13:1808. doi: 10.3390/nu13061808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shi J., Zhao D., Zhao F., Wang C., Zamaratskaia G., Li C. Chicken-eaters and pork-eaters have different gut microbiota and tryptophan metabolites. Sci Rep. 2021;11 doi: 10.1038/s41598-021-91429-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jia W., Zhen J., Liu A., Yuan J., Wu X., Zhao P., et al. Long-term vegan meditation improved human gut microbiota. Evid Based Complement Alternat Med. 2020 doi: 10.1155/2020/9517897. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.David L.A., Maurice C.F., Carmody R.N., Gootenberg D.B., Button J.E., Wolfe B.E., et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature. 2014;505:559–563. doi: 10.1038/nature12820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Schmedes M., Brejnrod A.D., Aadland E.K., Kiilerich P., Kristiansen K., Jacques H., et al. The effect of lean-seafood and non-seafood diets on fecal metabolites and gut microbiome: results from a randomized crossover intervention study. Mol Nutr Food Res. 2019;63 doi: 10.1002/mnfr.201700976. [DOI] [PubMed] [Google Scholar]
- 72.Zhang C., Björkman A., Cai K., Liu G., Wang C., Li Y., et al. Impact of a 3-months vegetarian diet on the gut microbiota and immune repertoire. Front Immunol. 2018;9:908. doi: 10.3389/fimmu.2018.00908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Windey K., De Preter V., Louat T., Schuit F., Herman J., Vansant G., et al. Modulation of protein fermentation does not affect fecal water toxicity: a randomized cross-over study in healthy subjects. PLoS One. 2012;7 doi: 10.1371/journal.pone.0052387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pagliai G., Russo E., Niccolai E., Dinu M., Di Pilato V., Magrini A., et al. Influence of a 3-month low-calorie Mediterranean diet compared to the vegetarian diet on human gut microbiota and SCFA: the CARDIVEG study. Eur J Nutr. 2020;59:2011–2024. doi: 10.1007/s00394-019-02050-0. [DOI] [PubMed] [Google Scholar]
- 75.Marlow G., Ellett S., Ferguson I.R., Zhu S., Karunasinghe N., Jesuthasan A.C., et al. Transcriptomics to study the effect of a Mediterranean-inspired diet on inflammation in Crohn’s disease patients. Hum Genomics. 2013;7:24. doi: 10.1186/1479-7364-7-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Balfegó M., Canivell S., Hanzu F.A., Sala-Vila A., Martínez-Medina M., Murillo S., et al. Effects of sardine-enriched diet on metabolic control, inflammation and gut microbiota in drug-naïve patients with type 2 diabetes: a pilot randomized trial. Lipids Health Dis. 2016;15:78. doi: 10.1186/s12944-016-0245-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Candela M., Biagi E., Soverini M., Consolandi C., Quercia S., Severgnini M., et al. Modulation of gut microbiota dysbioses in type 2 diabetic patients by macrobiotic Ma-Pi 2 diet. Br J Nutr. 2016;116:80–93. doi: 10.1017/S0007114516001045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Saresella M., Mendozzi L., Rossi V., Mazzali F., Piancone F., LaRosa F., et al. Immunological and clinical effect of diet modulation of the gut microbiome in multiple sclerosis patients: a pilot study. Front Immunol. 2017;8:1391. doi: 10.3389/fimmu.2017.01391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Di Iorio B.R., Rocchetti M.T., De Angelis M., Cosola C., Marzocco S., Di Micco L., et al. Nutritional therapy modulates intestinal microbiota and reduces serum levels of total and free indoxyl sulfate and P-cresyl sulfate in chronic kidney disease (Medika study) J Clin Med. 2019;8:1424. doi: 10.3390/jcm8091424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Basciani S., Camajani E., Contini S., Persichetti A., Risi R., Bertoldi L., et al. Very-low-calorie ketogenic diets with whey, vegetable, or animal protein in patients with obesity: a randomized pilot study. J Clin Endocrinol Metab. 2020;105:2939–2949. doi: 10.1210/clinem/dgaa336. [DOI] [PubMed] [Google Scholar]
- 81.Roager H.M., Licht T.R., Poulsen S.K., Larsen T.M., Bahl M.I. Microbial enterotypes, inferred by the Prevotella-to-Bacteroides ratio, remained stable during a 6-month randomized controlled diet intervention with the new Nordic diet. Appl Environ Microbiol. 2014;80:1142–1149. doi: 10.1128/AEM.03549-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Djekic D., Shi L., Brolin H., Carlsson F., Särnqvist C., Savolainen O., et al. Effects of a vegetarian diet on cardiometabolic risk factors, gut microbiota, and plasma metabolome in subjects with ischemic heart disease: a randomized, crossover study. J Am Heart Assoc. 2020;9 doi: 10.1161/JAHA.120.016518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Urwin H.J., Miles E.A., Noakes P.S., Kremmyda L.S., Vlachava M., Diaper N.D., et al. Effect of salmon consumption during pregnancy on maternal and infant faecal microbiota, secretory IgA and calprotectin. Br J Nutr. 2014;111:773–784. doi: 10.1017/S0007114513003097. [DOI] [PubMed] [Google Scholar]
- 84.Hess J., Wang Q., Gould T., Slavin J. Impact of Agaricus bisporus mushroom consumption on gut health markers in healthy adults. Nutrients. 2018;10:1402. doi: 10.3390/nu10101402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.van Faassen A., Bol J., van Dokkum W., Pikaar N.A., Ockhuizen T., Hermus R.J. Bile acids, neutral steroids, and bacteria in feces as affected by a mixed, a lacto-ovovegetarian, and a vegan diet. Am J Clin Nutr. 1987;46:962–967. doi: 10.1093/ajcn/46.6.962. [DOI] [PubMed] [Google Scholar]
- 86.Foerster J., Maskarinec G., Reichardt N., Tett A., Narbad A., Blaut M., et al. The influence of whole grain products and red meat on intestinal microbiota composition in normal weight adults: a randomized crossover intervention trial. PLoS One. 2014;9 doi: 10.1371/journal.pone.0109606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lang J.M., Pan C., Cantor R.M., Tang W.H.W., Garcia-Garcia J.C., Kurtz I., et al. Impact of individual traits, saturated fat, and protein source on the gut microbiome. mBio. 2018;9:e01604–18. doi: 10.1128/mBio.01604-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Crimarco A., Springfield S., Petlura C., Streaty T., Cunanan K., Lee J., et al. A randomized crossover trial on the effect of plant-based compared with animal-based meat on trimethylamine-N-oxide and cardiovascular disease risk factors in generally healthy adults: Study With Appetizing Plantfood-Meat Eating Alternative Trial (SWAP-MEAT) Am J Clin Nutr. 2020;112:1188–1199. doi: 10.1093/ajcn/nqaa203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kahleova H., Rembert E., Alwarith J., Yonas W.N., Tura A., Holubkov R., et al. Effects of a low-fat vegan diet on gut microbiota in overweight individuals and relationships with body weight, body composition, and insulin sensitivity. A randomized clinical trial. Nutrients. 2020;12:2917. doi: 10.3390/nu12102917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.McKenna C.F., Salvador A.F., Hughes R.L., Scaroni S.E., Alamilla R.A., Askow A.T., et al. Higher protein intake during resistance training does not potentiate strength, but modulates gut microbiota, in middle-aged adults: a randomized control trial. Am J Physiol Endocrinol Metab. 2021;320:E900–E913. doi: 10.1152/ajpendo.00574.2020. [DOI] [PubMed] [Google Scholar]
- 91.Kohnert E., Kreutz C., Binder N., Hannibal L., Gorkiewicz G., Müller A., et al. Changes in gut microbiota after a four-week intervention with vegan vs. meat-rich diets in healthy participants: a randomized controlled trial. Microorganisms. 2021;9:727. doi: 10.3390/microorganisms9040727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Meslier V., Laiola M., Roager H.M., De Filippis F., Roume H., Quinquis B., et al. Mediterranean diet intervention in overweight and obese subjects lowers plasma cholesterol and causes changes in the gut microbiome and metabolome independently of energy intake. Gut. 2020;69:1258–1268. doi: 10.1136/gutjnl-2019-320438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bratlie M., Hagen I.V., Helland A., Erchinger F., Ø Midttun, Ueland P.M., et al. Effects of high intake of cod or salmon on gut microbiota profile, faecal output and serum concentrations of lipids and bile acids in overweight adults: a randomised clinical trial. Eur J Nutr. 2021;60:2231–2248. doi: 10.1007/s00394-020-02417-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Krebs N.F., Sherlock L.G., Westcott J., Culbertson D., Hambidge K.M., Feazel L.M., et al. Effects of different complementary feeding regimens on iron status and enteric microbiota in breastfed infants. J Pediatr. 2013;163:416–423. doi: 10.1016/j.jpeds.2013.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Qasem W., Azad M.B., Hossain Z., Azad E., Jorgensen S., Castillo San Juan S., et al. Assessment of complementary feeding of Canadian infants: effects on microbiome and oxidative stress, a randomized controlled trial. BMC Pediatr. 2017;17:54. doi: 10.1186/s12887-017-0805-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Reddy B.S., Weisburger J.H., Wynder E.L. Effects of high risk and low risk diets for colon carcinogenesis on fecal microflora and steroids in man. J Nutr. 1975;105:878–884. doi: 10.1093/jn/105.7.878. [DOI] [PubMed] [Google Scholar]
- 97.Tanes C., Bittinger K., Gao Y., Friedman E.S., Nessel L., Paladhi U.R., et al. Role of dietary fiber in the recovery of the human gut microbiome and its metabolome. Cell Host Microbe. 2021;29:394–407. doi: 10.1016/j.chom.2020.12.012. e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Rodríguez J.M., Murphy K., Stanton C., Ross R.P., Kober O.I., Juge N., et al. The composition of the gut microbiota throughout life, with an emphasis on early life. Microb Ecol Health Dis. 2015;26 doi: 10.3402/mehd.v26.26050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Oren A., Garrity G.M.Y. Valid publication of the names of forty-two phyla of prokaryotes. Int J Syst Evol Microbiol. 2021;71(10):005056. doi: 10.1099/ijsem.0.005056. [DOI] [PubMed] [Google Scholar]
- 100.Guasch-Ferré M., Satija A., Blondin S.A., Janiszewski M., Emlen E., O’Connor L.E., et al. Meta-analysis of randomized controlled trials of red meat consumption in comparison with various comparison diets on cardiovascular risk factors. Circulation. 2019;139:1828–1845. doi: 10.1161/CIRCULATIONAHA.118.035225. [DOI] [PubMed] [Google Scholar]
- 101.Gardner C. Instead of what,” and repeated 4-year interval change regarding red meat and T2D: increasing causal inference in nutritional epidemiology through methodological advances. Am J Clin Nutr. 2021;113:497–498. doi: 10.1093/ajcn/nqaa385. [DOI] [PubMed] [Google Scholar]
- 102.O’Connor L.E., Wambogo E.A., Herrick K.A., Parsons R., Reedy J. A standardized assessment of processed red meat and processed poultry intake in the US population aged ≥2 years using NHANES. J Nutr. 2022;152:190–199. doi: 10.1093/jn/nxab316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.An R., Nickols-Richardson S., Alston R., Shen S., Clarke C. Total, fresh, lean, and fresh lean beef consumption in relation to nutrient intakes and diet quality among U.S. adults. Nutrients. 2019;11:563. doi: 10.3390/nu11030563. 2005-[2016] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Micha R., Michas G., Mozaffarian D. Unprocessed red and processed meats and risk of coronary artery disease and type 2 diabetes – an updated review of the evidence. Curr Atheroscler Rep. 2012;14:515–524. doi: 10.1007/s11883-012-0282-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.John E.M., Stern M.C., Sinha R., Koo J. Meat consumption, cooking practices, meat mutagens, and risk of prostate cancer. Nutr Cancer. 2011;63:525–537. doi: 10.1080/01635581.2011.539311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Tabatabaei S.M., Fritschi L., Knuiman M.W., Boyle T., Iacopetta B.J., Platell C., et al. Meat consumption and cooking practices and the risk of colorectal cancer. Eur J Clin Nutr. 2011;65:668–675. doi: 10.1038/ejcn.2011.17. [DOI] [PubMed] [Google Scholar]
- 107.Wilmanski T., Diener C., Rappaport N., Patwardhan S., Wiedrick J., Lapidus J., et al. Gut microbiome pattern reflects healthy ageing and predicts survival in humans. Nat Metab. 2021;3:274–286. doi: 10.1038/s42255-021-00348-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Haro C., Rangel-Zúñiga O.A., Alcalá-Díaz J.F., Gómez-Delgado F., Pérez-Martínez P., Delgado-Lista J., et al. Intestinal microbiota is influenced by gender and body mass index. PLoS One. 2016;11 doi: 10.1371/journal.pone.0154090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.de la Cuesta-Zuluaga J., Kelley S.T., Chen Y., Escobar J.S., Mueller N.T., Ley R.E., et al. Age- and sex-dependent patterns of gut microbial diversity in human adults. mSystems. 2019;4:e00261. doi: 10.1128/mSystems.00261-19. 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Debelius J., Song S.J., Vazquez-Baeza Y., Xu Z.Z., Gonzalez A., Knight R. Tiny microbes, enormous impacts: what matters in gut microbiome studies? Genome Biol. 2016;17:217. doi: 10.1186/s13059-016-1086-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Rezasoltani S., Ahmadi Bashirzadeh D., Nazemalhosseini Mojarad E., Asadzadeh Aghdaei H., Norouzinia M., Shahrokh S. Signature of gut microbiome by conventional and advanced analysis techniques: advantages and disadvantages. Middle East J Dig Dis. 2020;12:5–11. doi: 10.15171/mejdd.2020.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Singh G., Brass A., Cruickshank S.M., Knight C.G. Distinct microbial communities in the murine gut are revealed by taxonomy-independent phylogenetic random forests. bioRxiv. 2019 [Google Scholar]
- 113.Schloss P.D., Westcott S.L. Assessing and improving methods used in operational taxonomic unit-based approaches for 16S rRNA gene sequence analysis. Appl Environ Microbiol. 2011;77:3219–3226. doi: 10.1128/AEM.02810-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Hill T.C.J., Walsh K.A., Harris J.A., Moffett B.F. Using ecological diversity measures with bacterial communities. FEMS Microbiol Ecol. 2003;43:1–11. doi: 10.1111/j.1574-6941.2003.tb01040.x. [DOI] [PubMed] [Google Scholar]
- 115.Birtel J., Walser J.C., Pichon S., Bürgmann H., Matthews B. Estimating bacterial diversity for ecological studies: methods, metrics, and assumptions. PLoS One. 2015;10 doi: 10.1371/journal.pone.0125356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Nearing J.T., Comeau A.M., Langille M.G.I. Identifying biases and their potential solutions in human microbiome studies. Microbiome. 2021;9:113. doi: 10.1186/s40168-021-01059-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Fouhy F., Clooney A.G., Stanton C., Claesson M.J., Cotter P.D. 16S rRNA gene sequencing of mock microbial populations- impact of DNA extraction method, primer choice and sequencing platform. BMC Microbiol. 2016;16:123. doi: 10.1186/s12866-016-0738-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Cani P.D. Human gut microbiome: hopes, threats and promises. Gut. 2018;67:1716–1725. doi: 10.1136/gutjnl-2018-316723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Johnson A.J., Zheng J.J., Kang J.W., Saboe A., Knights D., Zivkovic A.M. A guide to diet-microbiome study design. Front Nutr. 2020;7:79. doi: 10.3389/fnut.2020.00079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Dias C.K., Starke R., Pylro V.S., Morais D.K. Database limitations for studying the human gut microbiome. PeerJ Comput Sci. 2020;6:e289. doi: 10.7717/peerj-cs.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ward R.E., Benninghoff A.D., Hintze K.J. Food matrix and the microbiome: considerations for preclinical chronic disease studies. Nutr Res. 2020;78:1–10. doi: 10.1016/j.nutres.2020.02.012. [DOI] [PubMed] [Google Scholar]
- 122.Zeng H., Umar S., Rust B., Lazarova D., Bordonaro M. Secondary bile acids and short chain fatty acids in the colon: a focus on colonic microbiome, cell proliferation, inflammation, and cancer. Int J Mol Sci. 2019;20:1214. doi: 10.3390/ijms20051214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Sharma V., Sharma V., Shahjouei S., Li J., Chaudhary D., Khan A., et al. At the intersection of gut microbiome and stroke: a systematic review of the literature. Front Neurol. 2021;12 doi: 10.3389/fneur.2021.729399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Zhao J., Zhang X., Liu H., Brown M.A., Qiao S. Dietary protein and gut microbiota composition and function. Curr Protein Pept Sci. 2019;20:145–154. doi: 10.2174/1389203719666180514145437. [DOI] [PubMed] [Google Scholar]
- 125.Krishnan S., O’Connor L.E., Wang Y., Gertz E.R., Campbell W.W., Bennett B.J. Adopting a Mediterranean-style eating pattern with low, but not moderate, unprocessed, lean red meat intake reduces fasting serum trimethylamine N-oxide (TMAO) in adults who are overweight or obese. Br J Nutr. 2021;128:1–21. doi: 10.1017/S0007114521004694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Chen M.L., Yi L., Zhang Y., Zhou X., Ran L., Yang J., et al. Resveratrol attenuates trimethylamine-N-oxide (TMAO)-induced atherosclerosis by regulating TMAO synthesis and bile acid metabolism via remodeling of the gut microbiota. mBio. 2016;7:e02210–e02215. doi: 10.1128/mBio.02210-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Wang Z., Roberts A.B., Buffa J.A., Levison B.S., Zhu W., Org E., et al. Non-lethal inhibition of gut microbial trimethylamine production for the treatment of atherosclerosis. Cell. 2015;163:1585–1595. doi: 10.1016/j.cell.2015.11.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Makki K., Deehan E.C., Walter J., Bäckhed F. The impact of dietary fiber on gut microbiota in host health and disease. Cell Host Microbe. 2018;23:705–715. doi: 10.1016/j.chom.2018.05.012. [DOI] [PubMed] [Google Scholar]
- 129.Roberts A.B., Gu X., Buffa J.A., Hurd A.G., Wang Z., Zhu W., et al. Development of a gut microbe–targeted nonlethal therapeutic to inhibit thrombosis potential. Nat Med. 2018;24:1407–1417. doi: 10.1038/s41591-018-0128-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Simó C., García-Cañas V. Dietary bioactive ingredients to modulate the gut microbiota-derived metabolite TMAO. New opportunities for functional food development. Food Funct. 2020;11:6745–6776. doi: 10.1039/d0fo01237h. [DOI] [PubMed] [Google Scholar]
- 131.Sinha R., Zhao N., Goedert J.J., Byrd D.A., Wan Y., Hua X., et al. Effects of processed meat and drinking water nitrate on oral and fecal microbial populations in a controlled feeding study. Environ Res. 2021;197 doi: 10.1016/j.envres.2021.111084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Florin T.H.J., Neale G., Cummings J.H. The effect of dietary nitrate on nitrate and nitrite excretion in man. Br J Nutr. 1990;64:387–397. doi: 10.1079/bjn19900040. [DOI] [PubMed] [Google Scholar]
- 133.Gifford C.L., O’Connor L.E., Campbell W.W., Woerner D.R., Belk K.E. Broad and inconsistent muscle food classification is problematic for dietary guidance in the. U.S. Nutrients. 2017;9:1027. doi: 10.3390/nu9091027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.National Health and, Nutrition Examination Survey Questionnaire [Internet]. Centers for Disease Control and Prevention (CDC). National Center for Health Statistics (NCHS). Hyattsville, MD: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention.
- 135.Human Microbiome Project Consortium A framework for human microbiome research. Nature. 2012;486(7402):215–221. doi: 10.1038/nature11209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Human Microbiome Project Consortium Structure, function and diversity of the healthy human microbiome. Nature. 2012;486(7402):207–214. doi: 10.1038/nature11234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Frame L.A., Costa E., Jackson S.A. Current explorations of nutrition and the gut microbiome: a comprehensive evaluation of the review literature. Nutr Rev. 2020;78:798–812. doi: 10.1093/nutrit/nuz106. [DOI] [PubMed] [Google Scholar]
- 138.Willson K., Situ C. Systematic review on effects of diet on gut microbiota in relation to metabolic syndromes. J Clin Nutr Metab. 2017;1:2. [Google Scholar]
- 139.Kristensen N.B., Bryrup T., Allin K.H., Nielsen T., Hansen T.H., Pedersen O. Alterations in fecal microbiota composition by probiotic supplementation in healthy adults: a systematic review of randomized controlled trials. Genome Med. 2016;8:52. doi: 10.1186/s13073-016-0300-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Valido E., Stoyanov J., Bertolo A., Hertig-Godeschalk A., Zeh R.M., Flueck J.L., et al. Systematic review of the effects of oat intake on gastrointestinal health. J Nutr. 2021;151:3075–3090. doi: 10.1093/jn/nxab245. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.