Abstract
The ramosus (rms) mutation (rms1) of pea (Pisum sativum) causes increased branching through modification of graft-transmissible signal(s) produced in rootstock and shoot. Additional grafting techniques have led us to propose that the novel signal regulated by Rms1 moves acropetally in shoots and acts as a branching inhibitor. Epicotyl interstock grafts showed that wild-type (WT) epicotyls grafted between rms1 scions and rootstocks can revert mutant scions to a WT non-branching phenotype. Mutant scions grafted together with mutant and WT rootstocks did not branch despite a contiguous mutant root-shoot system. The primary action of Rms1 is, therefore, unlikely to be to block transport of a branching stimulus from root to shoot. Rather, Rms1 may influence a long-distance signal that functions, directly or indirectly, as a branching inhibitor. It can be deduced that this signal moves acropetally in shoots because WT rootstocks inhibit branching in rms1 shoots, and although WT scions do not branch when grafted to mutant rootstocks, they do not inhibit branching in rms1 cotyledonary shoots growing from the same rootstocks. The acropetal direction of transport of the Rms1 signal supports previous evidence that the rms1 lesion is not in an auxin biosynthesis or transport pathway. The different branching phenotypes of WT and rms1 shoots growing from the same rms1 rootstock provides further evidence that the shoot has a major role in the regulation of branching and, moreover, that root-exported cytokinin is not the only graft-transmissible signal regulating branching in intact pea plants.
The term “apical dominance” is often used to describe the control of lateral branching and was developed from the observation that lateral bud outgrowth is promoted following shoot decapitation. However, tissues outside the shoot apical region clearly can have a major impact on lateral branching (e.g. Hosokawa et al., 1990; Napoli et al., 1999; Beveridge, 2000a). For example, in the dad1 branching mutant of petunia, a small wild-type (WT) internode interstock is able to revert a mutant scion to WT branching phenotype (Napoli, 1996). Apical dominance, or the control exerted by the apical bud and surrounding young and expanding tissues on axillary bud outgrowth, is, therefore, only one component of the branching control system in intact plants.
Early studies demonstrated that exogenous auxin could inhibit bud outgrowth caused by removal of the shoot apex (Thimann and Skoog, 1933). Snow (1937) and later Morris (1977) suggested that inhibitory effects of one shoot on the growth of another could not be directly attributed to auxin, as auxin did not travel from a dominant to a subordinate shoot. Such experiments have been the basis of the notion that a second substance is necessary for auxin to act. Growth inhibitors such as ethylene and abscisic acid are not promising candidates in this regard. Romano et al. (1993) showed that reduced ethylene level or response did not influence the effectiveness of increased auxin at modifying branching in transgenic plants. Likewise, mutants deficient in abscisic acid synthesis do not show increased branching (Cornish and Zeevaart, 1988; de Bruijn et al., 1993).
Sachs and Thimann (1967) and, more recently, Bangerth (1994), Li et al. (1995), Blaz̆ková et al. (1999), and Kotov and Kotova (2000) propose that auxin may regulate branching via interaction with another hormone, cytokinin. Evidence in support of this model has been largely derived from studies with decapitated plants. As yet, the model has not been tested widely in intact systems. Evidence from ramosus- (rms) increased branching mutants of pea (Pisum sativum) indicates novel signals may be involved in branching control in intact plants (Beveridge, 2000a). A model that includes roles for additional long-distance signals reduces the complexity inherent in attempts to explain how cytokinin and auxin alone can affect so many developmental processes (Beveridge, 2000b).
Grafting and endogenous auxin and cytokinin analyses have provided evidence that Rms1 controls a novel graft-transmissible substance. Grafting rms1 scions to WT rootstocks restores the scion to a WT branching phenotype (Beveridge et al., 1997b). In addition to the rootstock, Rms1 also acts in the shoot, as WT scions do not branch when grafted to rms1 rootstocks. It is unlikely that the graft-transmissible signal is cytokinin because rms1 plants have reduced xylem sap cytokinin content (Beveridge et al., 1997b) and cytokinins are thought to act as branching stimulators and not inhibitors. Likewise, auxin or an auxin precursor is a poor candidate for this long-distance signal because the indole-3-acetic acid content of rms1 shoots is not depleted (Beveridge et al., 1997b). Furthermore, in comparison with WT shoots, rms1 mutant shoots do not exhibit a reduced capacity for polar indole-3-acetic acid transport (Beveridge et al., 2000).
Recent decapitation, grafting, and auxin application studies have shown that the unidentified mobile substance(s) regulated by Rms1 influence auxin inhibition of branching following decapitation (Beveridge et al., 2000). Decapitated rms1 plants have a greatly reduced response to applied auxin, but this response is restored in an rms1 scion grafted to a WT rootstock (Beveridge et al., 2000). Like much of the evidence from studies with WT plants (e.g. Sachs and Thimann, 1967; Bangerth, 1994), evidence that the signal regulated by Rms1 affects auxin action has been drawn from experiments with exogenous auxin and decapitated plants. We do not yet know whether the signal regulated by Rms1 also modulates endogenous auxin signaling in intact plants (Beveridge et al., 2000).
Many of the experimental systems that have provided evidence for the involvement of long-distance signals, particularly auxin, in branching regulation have used decapitation to induce branching (e.g. Thimann and Skoog, 1933; Snow, 1937; Sachs and Thimann, 1967; Morris, 1977; Bangerth, 1994; Li et al., 1995; Kotov and Kotova, 2000). In contrast, branching in rms mutant plants occurs in the presence of vigorous main shoot tip growth. In this study we have designed a series of complex grafting experiments to reveal further information on the nature of the Rms1 signal. Three new grafting techniques are described for pea: epicotyl interstock, two-rootstock, and two-rootstock interstock grafts, together with Y grafts previously described by Beveridge and Murfet (1996). These experiments provide evidence for the site of action of the Rms1 gene, its putative function (stimulation or inhibition of branching), and direction of signal transport in intact plants.
RESULTS
Interstock Grafting
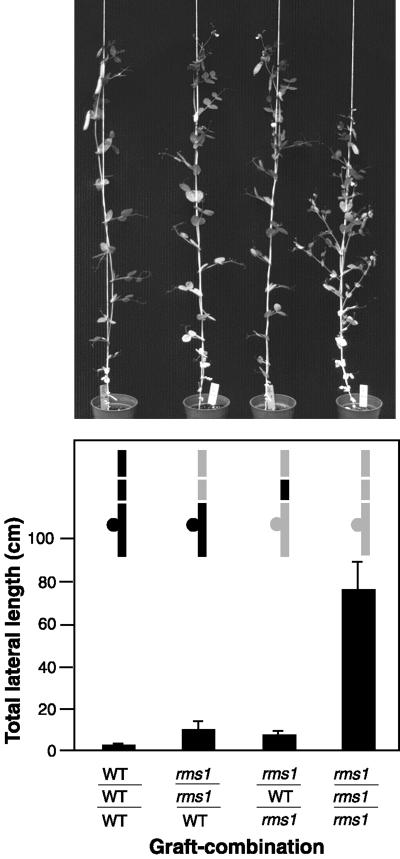
A WT (cv Weitor) epicotyl interstock of 5 to 10 mm in length, grafted between an rms1-2 scion and rootstock, almost completely inhibited lateral branching in the mutant scion (Fig. 1). As in ungrafted plants, lateral branching was profuse in rms1-2 self-grafted plants and almost absent in WT self-grafted plants (Fig. 1). Branching was inhibited approximately 10-fold in rms1-2 scions grafted to rms1-2 interstocks and WT rootstocks. In the combination rms1-2/WT/rms1-2 (notation: scion/interstock/rootstock), WT interstocks caused a similar 10-fold reduction in total lateral length (TLL) of mutant scions (Fig. 1). There was no significant difference among the TLLs of scions of rms1-2/WT/rms1-2, rms1-2/rms1-2/WT grafts, and cv Weitor self-grafted plants (Fig. 1, P > 0.05). Similar results were obtained with a different allele, rms1-1, and its corresponding WT, cv Parvus (data not shown). Grafted plants with inverted epicotyl interstocks did not survive.
Figure 1.
Branching phenotype of rms1-2 and cv Weitor interstock grafted plants. Top, Phenotype of 60-d-old plants; bottom, TLL in centimeters of 55-d-old plants. Inset is a diagram of the graft combinations; shaded areas indicate mutant tissue, black areas indicate WT tissue, and horizontal white lines indicate graft unions. Data shown are means + se; n = 10 to 13.
Y Grafting
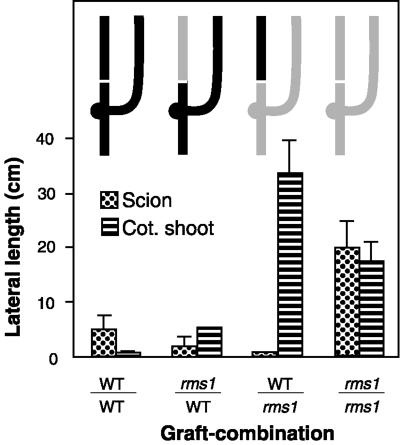
Plants with shoots of two different genotypes growing from the same rootstock were generated as described by Beveridge and Murfet (1996). This involved performing a single epicotyl graft and permitting an additional shoot to grow from the cotyledonary node of the rootstock. Shoots of rms1-2 and cv Weitor self-grafted plants exhibited profuse branching and an absence of lateral bud release, respectively (Fig. 2). In the rms1-2/WT combination, branching was inhibited in WT cotyledonary shoots and in rms1 scions (notation: scion/rootstock with the rootstock bearing a cotyledonary shoot; Fig. 2). In the WT/rms1-2 combination, WT and mutant shoots growing on the same rms1-2 rootstock exhibited vastly different branching phenotypes (Fig. 2). WT scions remained unbranched, but did not inhibit branching in rms1-2 cotyledonary shoots. Mutant cotyledonary shoots of these WT/rms1-2 Y-grafted plants exhibited similar, if not greater, lateral lengths than those observed in the cotyledonary shoots of mutant self-grafted plants (Fig. 2). When the lateral lengths of scion and cotyledonary shoots were combined (TLL), there was no significant difference between WT/rms1-2 Y-grafted and rms1-2 self-grafted plants (Table I, P > 0.05). On a different genetic background, branching was also inhibited in cv Parvus scions and profuse in the rms1-1 cotyledonary shoots of cv Parvus/rms1-1 plants (data not shown). For the graft combination WT/rms1, although total stem length and number of leaves expanded were slightly reduced in cv Weitor scions compared with rms1-2 cotyledonary shoots (Table I), the reverse was true for cv Parvus and rms1-1 shoots (data not shown). In both genetic backgrounds, only 10% of seedlings of the rms1/WT graft combination produced a vigorous cotyledonary shoot, whereas approximately 50% of seedlings of other graft combinations produced two vigorous shoots (data not shown; only data from plants with two vigorous shoots are reported).
Figure 2.
Lateral length in centimeters of scions and cotyledonary shoots of 46-d-old rms1-2 and cv Weitor Y-grafted plants. Inset is a diagram of the graft-combinations; shaded areas indicate mutant tissue, black areas indicate WT tissue, and horizontal white lines indicate graft unions. Data shown are means + se; n = 6 to 12, except for graft-combination rms1-2/WT, where only two plants had a cotyledonary shoot.
Table I.
TLL, total stem length (TL), and no. of leaves expanded (LE) of the scion and cotyledonary shoot of 46-d-old rms1-2 and Weitor Y-grafted plants
| Graft Combination | TLL | Scion
|
Cotyledonary Shoot
|
n | ||
|---|---|---|---|---|---|---|
| TL | LE | TL | LE | |||
| cm | node | cm | node | |||
| WT/WT | 57.6 ± 21.2 | 28.5 ± 4.2 | 12.5 ± 0.9 | 25.8 ± 2.4 | 11.4 ± 0.4 | 6 |
| rms1/WT | 27.0 ± 14.5 | 20.5 ± 7.5 | 12.0 ± 1.0 | 22.0 ± 2.0 | 11.0 ± 0.0 | 2 |
| WT/rms1 | 343.6 ± 83.8 | 19.4 ± 1.5 | 10.4 ± 0.4 | 29.2 ± 1.0 | 11.5 ± 0.3 | 10 |
| rms1/rms1 | 374.5 ± 60.6 | 26.2 ± 0.9 | 11.4 ± 0.4 | 25.1 ± 1.4 | 11.7 ± 0.3 | 12 |
TLL is the total length of all the laterals on both shoots. Data are means ± se. Lateral lengths of each shoot are shown in Figure 2.
Two-Rootstock Grafts
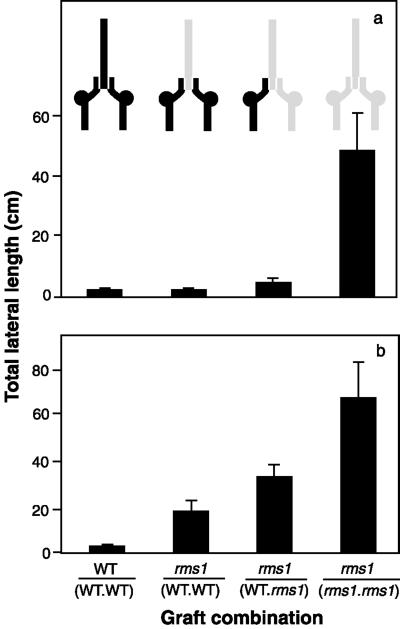
Plants with rootstocks of two different genotypes were obtained by grafting a wedge-cut scion between the oblique cut surfaces of two adjacent rootstocks. As expected, branching was profuse in rms1-2 self-grafted plants and almost absent in WT self-grafted plants (Fig. 3a). An 11-fold reduction in TLL was observed when rms1-2 scions were grafted with two cv Weitor rootstocks or to one cv Weitor and one rms1-2 rootstock (Fig. 3a). There was no significant difference among the TLLs of rms1-2/(rms1-2.WT), rms1-2/(WT.WT), and WT self-grafted plants [notation: scion/(rootstock.rootstock); Fig. 3a, P > 0.05].
Figure 3.
TLL (in centimeters) of rms1 and WT two-rootstock grafted plants. a, Forty-nine-d-old rms1-2 and cv Weitor plants. b, Forty-eight-d-old rms1-1 and cv Parvus plants. Inset is a diagram of the graft-combinations; shaded areas indicate mutant tissue, black areas indicate WT tissue, and vertical white lines indicate graft unions. Data shown are means + se; n = 6 to 14.
Similar trends were observed in two-rootstock studies performed with the rms1-1 mutant and its progenitor, cv Parvus (Fig. 3b), except that cv Parvus rootstocks did not inhibit branching in rms1-1 scions to the same extent as cv Weitor rootstocks in rms1-2 scions. Although reduced about 3-fold, bud outgrowth in rms1-1 scions grafted to two cv Parvus rootstocks was still greater than in self-grafted cv Parvus plants (Fig. 3b). Grafting rms1-1 scions with rms1-1 and cv Parvus rootstocks reduced bud outgrowth about 2-fold, resulting in a phenotype intermediate between rms1-1 self-grafted plants and plants of rms1-1/(WT.WT) combination (Fig. 3b).
Two-Rootstock Epicotyl Interstock Grafts
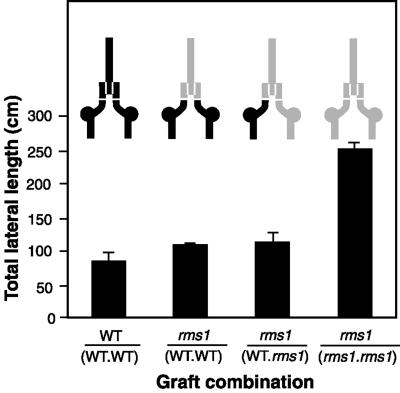
To determine whether lateral movement of regulatory substances occurred from one rootstock to another, two-rootstock epicotyl interstock grafts were performed. This technique involved insertion of spacer epicotyl interstocks between each rootstock and the scion to separate WT (cv Weitor) rootstocks from the adjacent rms1-2 rootstock and scion (Fig. 4; the spacer interstocks were all genotype rms1-2 except for WT self grafts). Plants were grown under a natural photoperiod of about 12 h, stimulating some bud release even in WT plants and thus poising plants closer to a branching threshold. As a consequence, bud outgrowth occurred at several nodes of plants of all graft combinations (Fig. 4 and data not shown). As with two-rootstock rms1-2 and cv Weitor grafts described above, there was no significant difference among TLLs of rms1-2 scions interstock-grafted to rms1-2 and cv Weitor rootstocks; rms1-2 scions interstock-grafted to two cv Weitor rootstocks; and scions of cv Weitor self-grafted plants (Fig. 4).
Figure 4.
TLL (in centimeters) of scions of 51-d-old rms1-2 and cv Weitor two-rootstock interstock grafted plants. Inset is a diagram of the graft combinations; shaded areas indicate mutant tissue, black areas indicate WT tissue, and horizontal and vertical white lines indicate graft unions. Data shown are means + se; n = 6, except WT self-grafts where n = 3.
DISCUSSION
Using two rms1 mutant alleles from different genetic backgrounds we have demonstrated that a small section of WT epicotyl interstock tissue can revert rms1 scions to a WT phenotype, that two shoots growing from the same rootstock can exhibit different branching phenotypes, and that grafting rms1 scions to WT and rms1 rootstocks leads to a substantial or absolute inhibition of branching in the mutant scion.
WT epicotyl tissue inserted between an rms1 scion and rootstock can inhibit axillary bud release and subsequent growth down to the level caused by an entire WT rootstock (Fig. 1). The small quantity (5–10 mm) of epicotyl tissue required indicates that the signal regulated by Rms1 may be highly active or that the response threshold may be very low. It is also possible that the epicotyl is the only site of Rms1 signal production because epicotyl tissue has been included in rootstock and scion of all grafting experiments with rms1. As it is yet to be determined whether Rms1 acts in roots, it is important to make a distinction between root and rootstock. Hypocotyl grafts between rms1 and WT seedlings have been attempted, but success rates have been too low to yield meaningful results.
Hypocotyl interstock grafting studies have been performed with the increased branching mutant, dad1 of petunia (Napoli, 1996). As with rms1, a tiny piece of WT interstock can revert a dad1 mutant scion to WT phenotype. In this case, adventitious dad1 roots forming on the mutant scion negate the inhibitory effect of the WT hypocotyl interstock. This indicates that a contiguous dad1 root-shoot system is required for branch promotion and that Dad1 may block movement of a branching stimulus from root to shoot.
In contrast with dad1, a contiguous rms1 mutant root-shoot system did not result in promotion of branching in rms1 scions grafted to rms1 and WT rootstocks (Figs. 3 and 4). Branching inhibition occurred even when the WT (cv Weitor) rootstock was separated from the adjacent mutant rootstock and scion by mutant epicotyl interstocks (Fig. 4). Thus, in the two-rootstock graft combination rms1/(rms1.WT), branching is probably not inhibited by lateral movement of substances from the WT epicotyl that in turn influence signals moving from the rms1 rootstock to the mutant scion. As the relative quantity of mutant and WT tissue does not always correlate with the extent of bud outgrowth, the simplest explanation is that Rms1 regulates a signal that acts as a branching inhibitor. The alternative is that rms1 plants exhibit to down-regulate a branching stimulus. If this is the case, the level of this branching stimulus is below the threshold required to promote branching in plants of graft combinations with rms1 scions and WT root tissue and/or a tiny section of WT epicotyl tissue.
In Y grafting studies, a non-branching WT scion was unable to inhibit branching in an rms1 mutant cotyledonary shoot (Fig. 2). Thus, the signal regulated by Rms1 does not move basipetally in shoots (down the WT scion) or cannot move basipetally and acropetally in shoots (up the mutant cotyledonary shoot). As WT rootstocks alone can inhibit branching in rms1 scions (Fig. 3), any signal regulated by Rms1 can move acropetally in mutant shoots. It is, therefore, most likely that the signal regulated by Rms1 moves acropetally in the shoot, but not basipetally.
The Y grafting studies have also shown that two shoots growing from the same rootstock can exhibit entirely different branching phenotypes (Fig. 2). Mutant rms1 cotyledonary shoots from rms1 rootstocks undergo bud release and subsequent outgrowth, whereas the axillary buds of WT scions grafted to the same rootstock remain inhibited. This indicates that root-derived substances do not control shoot architecture independently of shoot processes. If, as suggested above, Rms1 regulates the level or transport of a signal that moves acropetally in shoots, this signal is probably not xylem-translocated cytokinin for a number of reasons. First, cytokinin concentration in the root xylem sap is lower in rms1 plants than in WT plants (Beveridge et al., 1997b). Second, rms1 and WT scions grafted to rms1 rootstocks have the same root-cytokinin source and yet differ in branching phenotype. The highly branched phenotype of rms1 shoots compared with non-branching WT shoots on the same rootstock is unlikely to be due simply to genotypic differences in cytokinin metabolism or transport, as a complete inhibition of branching in rms1 scions can be achieved by grafting to WT rootstocks (Beveridge et al., 1997b).
Morris (1977) studied shoot growth and radiolabeled auxin transport in pea plants with dominant and subordinate cotyledonary shoots generated by decapitation of young seedlings. The subordinate shoot was relatively slow growing and had reduced basipetal auxin transport. Decapitation of the dominant shoot led to restoration of a high rate of growth and auxin transport in the subordinate shoot, and auxin application to the decapitated dominant shoot nearly eliminated this effect. Nevertheless, apically applied radiolabeled auxin did not enter the subordinate shoot. Using the same system, Li and Bangerth (1999) also argue that auxin transport from the dominant shoot inhibits growth of the subordinate shoot by reducing its ability to transport auxin. Our two-shoot Y grafting studies differ from the two-shoot studies described by Morris (1977) and Li and Bangerth (1999) because both shoots of the Y-grafted plants here exhibited vigorous shoot tip growth (Table I and data not shown). Furthermore, our study investigated effects on lateral bud outgrowth rather than shoot tip growth.
Non-branching intact WT shoots could not inhibit branching in adjacent rms1 shoots even though both shoot tips were intact. We have recently shown that reduced polar auxin transport is not the cause of bud outgrowth in rms1 shoots (Beveridge et al., 2000). Grafting studies, including auxin application to decapitated WT and rms1 plants, have shown that the signal regulated by Rms1 is required for the inhibition of bud growth by exogenous auxin following decapitation. As a consequence, if endogenous auxin does control branching in intact pea shoots, then it may directly or indirectly require the acropetally transported signal regulated by Rms1.
In conclusion, the simplest hypothesis to account for our results is that Rms1 controls the level of a novel graft-transmissible substance that moves acropetally in shoots and acts as a branching inhibitor. It may do so directly or indirectly through down-regulation of a branching stimulus. In an alternate manner, the Rms1 signal could be cast as a second messenger for auxin. However, before we can be confident of a link between auxin action and Rms1 action, we must determine if endogenous auxin is required for the Rms1 signal to regulate branching in intact and decapitated plants. Cloning of branching genes in pea and/or other species and the identification of the signals involved will be integral steps toward understanding the mode of action of Rms1 and the signal(s) it regulates.
MATERIALS AND METHODS
Plant Material and Growth Conditions
The mutant lines used in this study were derived from two cultivars, both of which exhibit a tall growth habit. Two rms1 alleles, rms1-1 and rms1-2, and their respective progenitors, cv Parvus and cv Weitor, were utilized (Arumingtyas et al., 1992; Beveridge et al., 1997b and refs. therein). Unless otherwise stated, plants were grown under long days with the natural daylength extended to 18 h with weak incandescent light. Plants were grown in 2-L pots in a 2:1 mixture of pasteurized peat:sand potting mix and perlite or in Premium Blend potting mix (7:2:1 pine bark:peat blend:sand). Nutrient was supplied through Osmocote (Scotts, Europe) and/or as solution (Flowfeed EX7, Grow Force, Australia) applied weekly.
Plants were grafted before macroscopic axillary bud development had occurred, usually when 7 d old. Plants were covered by 1.25-L plastic bottles (with the bases removed) for several days after grafting. Cotyledonary shoots were regularly removed (unless otherwise stated) to ensure the development of the graft union and growth of the scion. Nodes were numbered acropetally from the first scale leaf and lateral lengths were measured from the leaf axil to the apex, to the accuracy of 1 mm. TLL was the sum of the lengths of all laterals emerging from nodes along the main stem or cotyledonary shoot, as described.
Interstock Grafts
A variation of epicotyl wedge grafts described by Beveridge et al. (1994) was used to perform epicotyl interstock grafts. Epicotyls were cut to approximately 5 to 10 mm in length with a wedge at the basipetal end and a slit at the acropetal end. Maintaining the orientation found in intact plants, this epicotyl interstock was wedge grafted between a scion and a rootstock in the epicotyl and held in place with small elastic bands. In some experiments, the interstock was wedge grafted to the rootstock, but left intact until wedge grafted to the scion 1 week later. Only vigorous plants were included in the analysis (approximately 50%–60%). Graft notation: scion/interstock/rootstock.
Y Grafts
Y grafts were performed as described by Beveridge and Murfet (1996). The first or second cotyledonary bud that enlarged after grafting was allowed to form into a cotyledonary shoot. This resulted in a plant with a rootstock and cotyledonary shoot of the same genotype connected to a scion of the same or different genotype. Only plants with vigorous scions and cotyledonary shoots were included in the analysis (usually about 50%). Plants were grown under natural short day conditions (12-h photoperiod, 23°C/18°C day/night) to encourage lateral branching. Graft notation: scion/rootstock and cotyledonary shoot.
Two-Rootstock Grafts
Rootstocks were planted in 2-L pots orientated such that the plumules would grow side by side. At d 7 the two rootstocks were severed in the epicotyl and a diagonal slice was made in each. An elastic band was placed over the two rootstocks, creating a slit at the junction of the two cut surfaces. The wedge cut scion was placed into the slit, bringing the scion into contact with the cut surface of both rootstocks. Roots were examined after shoot measurements were undertaken and only plants with two substantial rootstocks were included in the analysis (approximately 50%). Graft notation: scion/(rootstock.rootstock).
Two-Rootstock Interstock Grafts
Two-rootstock interstock grafts are a combination of interstock and two-rootstock grafts whereby the rootstocks are physically separated from the scion by epicotyl interstocks. Interstocks of the same genotype as the scion were grafted to both rootstocks as described for interstock grafts. The scion was grafted between the interstocks as described for two-rootstock grafts.
ACKNOWLEDGMENTS
We thank Brian Kaddatz and Kathy Crew for technical assistance and Lyn Jessup for preparation of the figures.
Footnotes
This work was supported by the Australian Research Council.
LITERATURE CITED
- Arumingtyas EL, Floyd RS, Gregory MJ, Murfet IC. Branching in Pisum: inheritance and allelism tests with 17 ramosus mutants. Pisum Genet. 1992;24:17–31. [Google Scholar]
- Bangerth F. Response of cytokinin concentration in the xylem exudate of bean (Phaseolus vulgaris L.) plants to decapitation and auxin treatment, and relationship to apical dominance. Planta. 1994;194:439–442. [Google Scholar]
- Beveridge CA. Long-distance signalling and a mutational analysis of branching in pea. Plant Growth Regul. 2000a;32:193–203. [Google Scholar]
- Beveridge CA. The ups and downs of signalling between root and shoot. New Phytol. 2000b;147:413–416. doi: 10.1046/j.1469-8137.2000.00729.x. [DOI] [PubMed] [Google Scholar]
- Beveridge CA, Murfet IC. The gigas mutant in pea is deficient in the floral stimulus. Physiol Plant. 1996;96:637–645. [Google Scholar]
- Beveridge CA, Murfet IC, Kerhoas L, Sotta B, Miginiac E, Rameau C. The shoot controls zeatin riboside export from pea roots: evidence from the branching mutant rms4. Plant J. 1997a;11:339–345. [Google Scholar]
- Beveridge CA, Ross JJ, Murfet IC. Branching in pea: action of genes Rms3 and Rms4. Plant Physiol. 1996;110:859–865. doi: 10.1104/pp.110.3.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beveridge CA, Symons GM, Murfet IC, Ross JJ, Rameau C. The rms1 mutant of pea has elevated indole-3-acetic acid levels and reduced root sap zeatin riboside content but increased branching controlled by graft transmissible signal(s) Plant Physiol. 1997b;115:1251–1258. [Google Scholar]
- Beveridge CA, Symons GM, Turnbull CGN. Auxin inhibition of decapitation-induced branching is dependent on graft-transmissible signals regulated by genes Rms1 and Rms2. Plant Physiol. 2000;123:689–697. doi: 10.1104/pp.123.2.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaz̆ková J, Krekule J, Machác̆ková I, Procházka S. Auxin and cytokinins in the control of apical dominance in pea: a differential response due to bud position. Plant Physiol. 1999;154:691–696. [Google Scholar]
- Cornish K, Zeevaart JAD. Phenotypic expression of wild-type tomato and three wilty mutants in relation to abscisic acid accumulation in roots and leaflets of reciprocal grafts. Plant Physiol. 1988;87:190–194. doi: 10.1104/pp.87.1.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Bruijn SM, Buddendorf CHJJ, Vreugdenhil D. Characterization of the ABA-deficient Pisum sativum “wilty” mutant. Acta Bot Neerl. 1993;42:491–503. [Google Scholar]
- Hosokawa Z, Shi L, Prasad TK, Cline MG. Apical dominance control in Ipomea nil: the influence of the shoot apex, leaves and stem. Ann Bot. 1990;65:547–556. [Google Scholar]
- Kotov AA, Kotova LM. The contents of auxins and cytokinins in pea internodes as related to the growth of lateral buds. J Plant Physiol. 2000;156:438–448. [Google Scholar]
- Li C-J, Bangerth F. Autoinhibition of indoleacetic acid transport in the shoots of two-branched pea (Pisum sativum) plants and its relationship to correlative dominance. Physiol Plant. 1999;106:415–420. [Google Scholar]
- Li CJ, Guevara E, Gerrera J, Bangerth F. Effect of apex excision and replacement by 1-naphthylacetic acid on cytokinin concentration and apical dominance in pea plants. Physiol Plant. 1995;94:465–469. [Google Scholar]
- Morris DA. Transport of exogenous auxin in two-branched dwarf pea seedlings (Pisum sativum L.): some implications for polarity and apical dominance. Planta. 1977;136:91–96. doi: 10.1007/BF00387930. [DOI] [PubMed] [Google Scholar]
- Napoli CA. Highly branched phenotype of the petunia dad1-1 mutant is reversed by grafting. Plant Physiol. 1996;111:27–37. doi: 10.1104/pp.111.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Napoli CA, Beveridge CA, Snowden KC. Reevaluating concepts of apical dominance and the control of axillary bud outgrowth. Curr Topics Dev Biol. 1999;44:127–169. doi: 10.1016/s0070-2153(08)60469-x. [DOI] [PubMed] [Google Scholar]
- Romano CP, Cooper ML, Klee HJ. Uncoupling auxin and ethylene effects in transgenic tobacco and Arabidopsis plants. Plant Cell. 1993;5:181–189. doi: 10.1105/tpc.5.2.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachs T, Thimann KV. The role of auxins and cytokinins in the release of buds from apical dominance. Am J Bot. 1967;45:136–144. [Google Scholar]
- Snow R. On the nature of correlative inhibition. New Phytol. 1937;36:283–300. [Google Scholar]
- Thimann KV, Skoog F. On the inhibition of bud development and other functions of growth substances in Vicia faba. Proc Roy Soc B. 1933;114:317–339. [Google Scholar]