Giant-cell tumor of bone (GCTB) is an intermediate type of primary bone tumor characterized by locally aggressive growth with metastatic potential.7 These tumors typically affect young adults and the female population. Unlike most primary bone tumors that occur in the metaphysis, GCTBs occur in the meta-epiphyseal region of long bones, adjacent to the joint. The most common localizations are the knee joint, hip, wrist, and shoulder. Despite metastases being rare, GCTB growth destroys the bone close to the joint, making definitive treatment very demanding.1,12
GCTB is receptor activator of nuclear factor kappa B ligand (RANKL) positive, so the use of denosumab, a RANKL inhibitor, has been approved. Also, particularly in cases where surgical resection is likely to be problematic, the neoadjuvant therapy denosumab has shown objective benefits.14
The elbow is a rare location of musculoskeletal tumors. Currently, limb-salvage surgery and the reconstruction of bone defects have achieved overall good outcomes and acceptance.13 Limb-salvage options such as arthrodesis or osteocartilaginous allograft has been reported to have limited functional outcomes and high revision and failure rates.10 On the contrary, elbow replacement has reported promising overall clinical outcomes and high long-term survivorship rates.4 Also, the replacement replaces the bone defect without the need for an allograft.2
Case report
An otherwise healthy 46-year-old male presented to a local emergency department with elbow pain after falling off a bike. Injuries included left-sided elbow swelling and hematoma. X-ray imaging showed an olecranon AO type B, Schatzker A fracture (Fig. 1).
Figure 1.
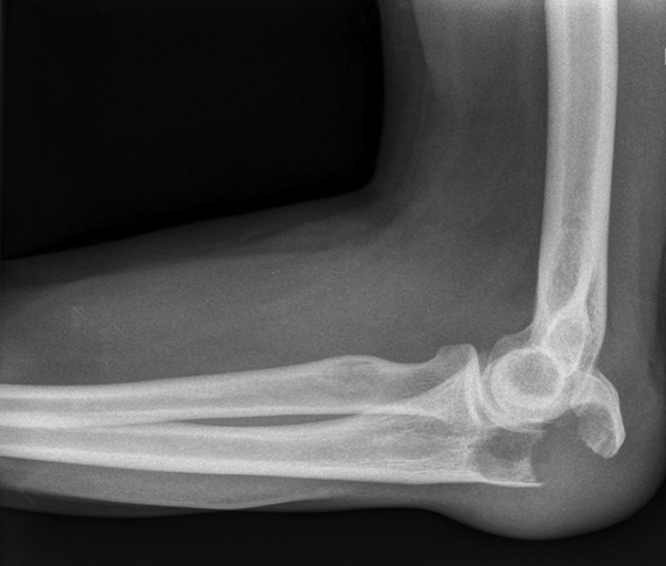
X-ray image of the elbow in the lateral projection after the accident.
He was hospitalized and indicated for osteosynthesis of the fracture, an open reduction and tension band wiring—cerclage of the olecranon (Fig. 2). After 3 days and without any postoperative complication, the patient was released.
Figure 2.
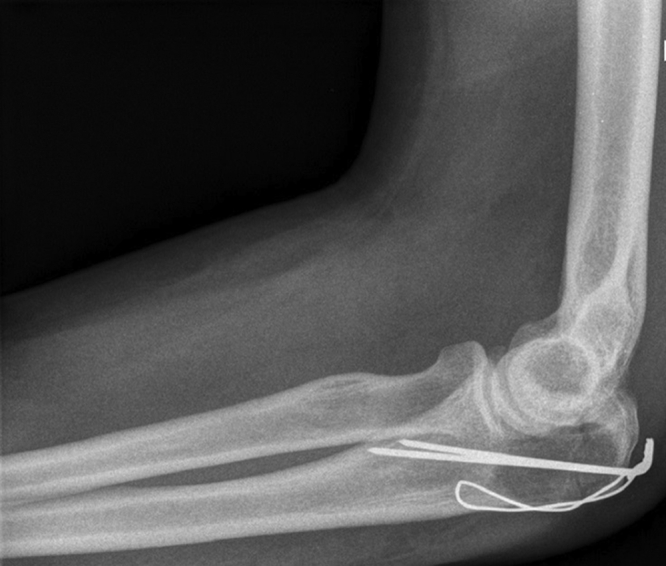
X-ray of the elbow in the lateral projection after the tension band wiring.
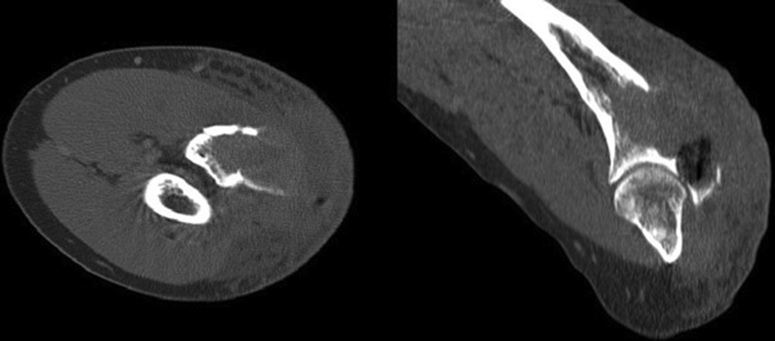
He regularly attended the local trauma department and after 6 months was indicated for hardware removal. Intraoperatively, there was unexpected bleeding and a bone cyst was identified which was primarily in the proximal ulna. A tissue sample was taken for histological examination during the hardware removal procedure. The histopathology report described an aneurysmal bone cyst. At his 2-week follow-up, the patient developed a progression of elbow swelling and pain. Computed tomography (CT) scans revealed a 78 mm osteolytic lesion of the proximal ulna with an extraosseous component (Fig. 3).
Figure 3.
Computed tomography scan of the elbow before the neoadjuvant therapy, osteolytic lesion of the proximal ulna with an extraosseous component.
The patient was referred to our clinic, First Orthopedic Department of St. Anne’s University Hospital in Brno. Considering the extraosseous component and the osteolysis, the Musculoskeletal Tumor Committee recommended a fresh biopsy. A new tissue sample of bone and extraosseous mass was taken from the ulna, respecting the principles of the open incisional biopsy. The new histopathology report described a GCTB with a secondary aneurysmatic bone cyst. According to the Campanacci grading system for GCTB, this was a Grade III lesion, described as aggressive. A CT scan of the chest was completed as part of the oncological screening; no lung metastases were found.
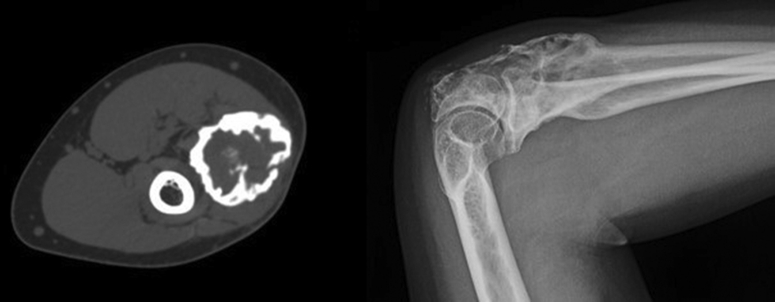
The use of neoadjuvant denosumab for 3 months was recommended. In terms of dosing, a 120 mg Xgeva subcutaneous injection every 4 weeks and 2 loading doses in the first month of the therapy were used. After the end of the neoadjuvant therapy, the CT scan and X-rays showed a calcified rim surrounding the soft tissue component (Fig. 4).
Figure 4.
Radiographic examination after the denosumab neoadjuvant therapy: Left, computed tomography scan in the transversal plane; Right, X-ray image of elbow in the lateral projection.
Resection of the proximal ulna and tumorous total elbow replacement surgical procedure was scheduled. From the dorsal approach to elbow, we removed the proximal 11 cm of ulna, sparing the neurovascular structures. We preserved the surrounding muscles and unattached the tendon of the triceps brachii muscle from the olecranon. Anterior transposition of the ulnar nerve was performed. A biopsy sample was taken from the diaphysis of the remaining ulna to exclude margin infiltration. Next, we resected the radial head. After the preparation and rasping of the proximal ulna and humeral cavity (Fig. 5), we implanted the MUTARS (ImplantCast, Dallas, TX, USA) total elbow uncemented modular components. Before the final reduction and the locking of the hinge system, a Trevira attachment tube was used (Fig. 6). Finally, reduction and attachment of the triceps brachii tendon on the Trevira tube and reconstruction of the soft tissues were performed. The final histology report described a 100% loss of osteoclasts in the removed bone and the resection was adequate.
Figure 5.
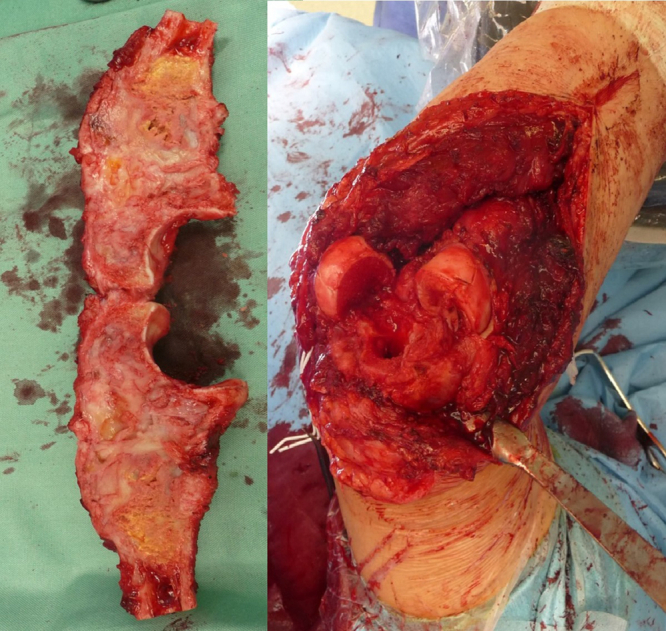
Photo documentation during the procedure: Left, distal ulna tumor resection; Right, bone defect and preparation of the cavities.
Figure 6.
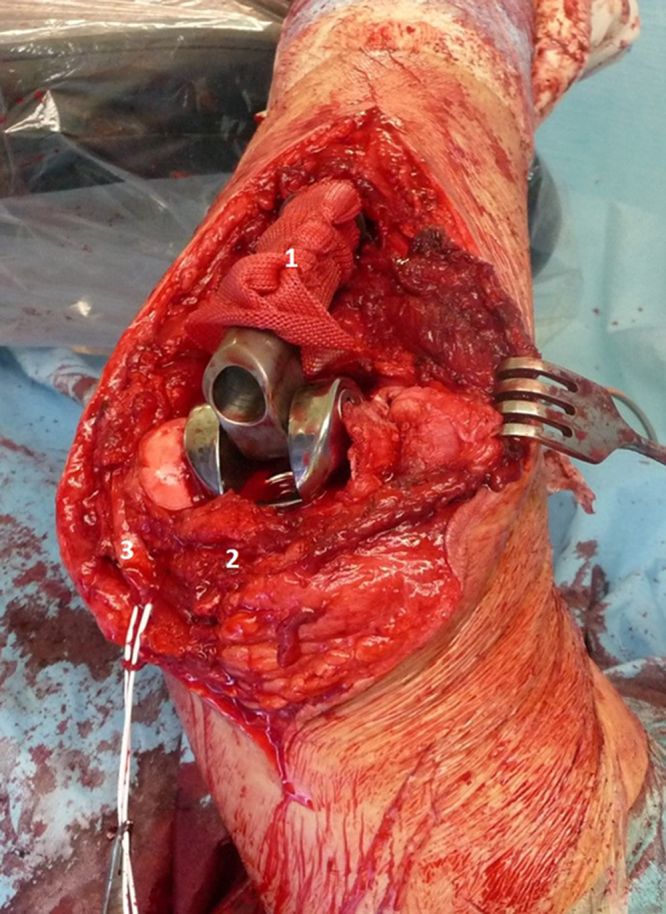
Implantation of the TU-TEP and Trevira attachment tube application: (1) Trevira attachment tube, (2) triceps brachii, and (3) ulnar nerve.
The patient was discharged 6 days after surgery without any perioperative complications. A hinged elbow brace was used for the first 4 postoperative weeks and the patient was then hospitalized for intensive specialized rehabilitation. In the post-surgical rehabilitation management, the patient was allowed to do passive movement of the elbow for the first 4 weeks, which was then increased to allow active movement gradually for the next 3 months. The scheduled follow-ups were quarterly for the first 2 years, including radiographic examination.
At 1 year after the surgery, he was free of pain or swelling, achieving full elbow extension and 110° of elbow flexion, while the Musculoskeletal Tumor Society score was 25. The X-ray images showed an adequate implant position and no signs of aseptic loosening.
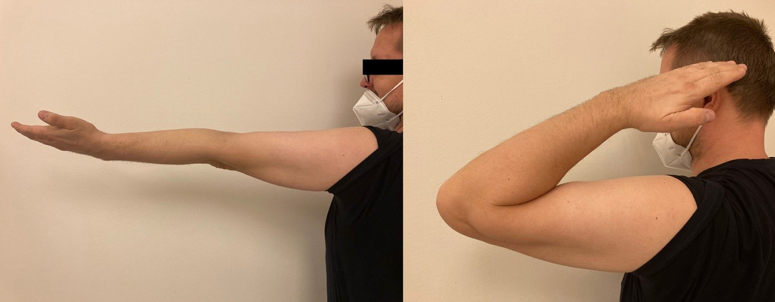
At the last follow-up appointment, 3 years after surgery, the Musculoskeletal Tumor Society functional score was 27, achieving full elbow extension and 120° of elbow flexion, supination of 75°, and pronation of 75° (Fig. 7). Furthermore, there were no signs of aseptic loosening or local recurrence of the tumor in the radiographic results (Fig. 8). The patient achieved almost full extremity activity and was instructed to avoid heavy lifting or sporting activities with the arm.
Figure 7.
Elbow extension and flexion, 3 years after the surgery.
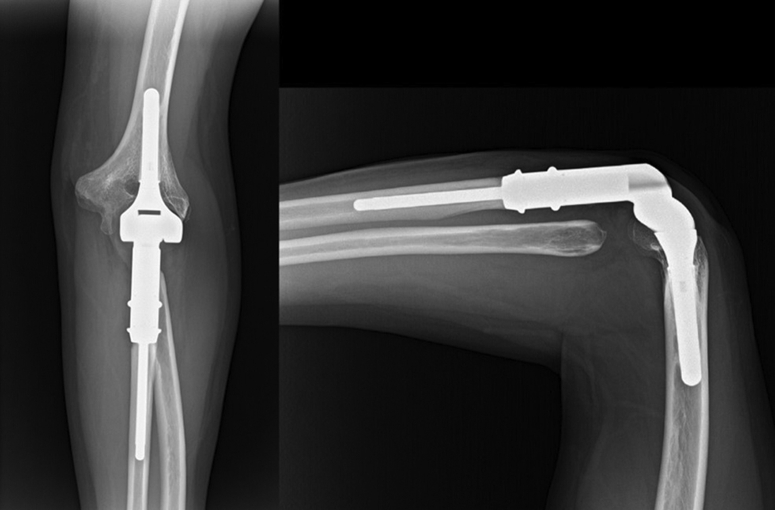
Figure 8.
X-ray images at the 3-year follow-up.
Discussion
Our case describes a misdiagnosed elbow bone tumor. Bone tumors of the elbow have a low incidence (1%) when considering all primary bone tumors. Due to the rarity, elbow tumors are easily missed.16 On the X-ray after the accident there was a visible lucency around the fracture which was overlooked. As a result, the patient underwent an intralesional tension band wiring. This led to tumor progression, complicated the following resection and increased the risk of tumor recurrence. GCBT shows high local recurrence rates; due to the aggressive character of the tumor and the extraosseous component in our case, wide resection was indicated.
In the current case, GCTB was radiographically classified as Campanacci Grade III, described as aggressive. A grade 3 lesion signifies indistinct borders, cortical destruction, and extraosseous promotion.1 The use of denosumab is indicated in cases when radical resection cannot be performed, as in our case, as well as when local recurrence occurs and for the control of metastatic disease.3 Numerous studies demonstrate the beneficial effect of denosumab in the case of advanced GCTB. Denosumab is a fully human monoclonal antibody that binds the cytokine RANKL, a mediator of the function, formation, and survival of osteoclasts. A reduction in tumor size, a calcified rim around the tumor and tumor soft tissue components were observed after 3 months of therapy to facilitate en bloc resection.6 According to the literature, the risk of GCTB recurrence is still relatively high after the use of denosumab.18
Histologically, denosumab causes a reduction of osteoclast-like giant cell formation which decreases bone destruction. Denosumab targets multinucleated osteoclastic cells rather than neoplastic stromal cells, which persist and continue to proliferate. Neoplastic stromal cell persistence could be associated with the appearance of GCTB local recurrence. In advance, sunitinib, a platelet-derived growth factor receptor A inhibitor, could become an effective addition to denosumab, resulting in the disappearance of both multinuclear giant cells and neoplastic stromal cells.11
After proximal ulna resection, we needed to restore the bone defect and spare joint motion. Elbow osteocartilaginous allograft reconstructions have a high failure rate in the mid-term and degenerative changes such as arthrosis or bone resorption and infection also routinely occur.10 Another option could be an allograft-prosthetic composite reconstruction associated with difficulties in terms of optimal positioning, contact, fixation, and compression across the host-graft junction.15 We prefer those biological reconstructions in patients younger than 20 years. Total elbow replacement for tumors in the last decade showed a good outcome score and a tolerable survival rate. This presents an effective method, with an acceptable level of complications and restores the defect without the need for bone-to-bone healing.2,16 Considering the patient's age, we preferred total elbow arthroplasty with modular ImplantCast MUTARS.
Soft tissue reconstruction is key for appropriate functional results. Especially when an extensive bone resection is performed, the reconstruction of the attached tendons is crucial. The use of attachment tubes in previous studies showed disputable outcomes. Studies on megaprostheses claim the use of attachment tubes with optimal results.5,9 On the contrary, a higher infection rate has been described for implants with Trevira reconstruction.17 We tend to use the Trevira tube for anatomical reinsertion of the remaining soft tissue after resection, recording adequate results.
Another significant factor for acceptable results in oncologic orthopedics is diagnosis and treatment in specialty musculoskeletal centers.8 Due to the lack of awareness of health care providers, interventions in tumor surgery are commonly provided by many general orthopedic surgeons. Inappropriately treated bone tumors, as in the case reported here, acquire worse resectability and subsequently have higher recurrence and mortality rates. Even biopsy led to complications, nondiagnostic results and worse outcomes when it was performed in a referring institution instead of a specialty center.
Conclusion
Elbow is a rare location of bone tumors. Our case demonstrates a misdiagnosis that led to tumor progression. The use of denosumab had a beneficial effect on tumor grading, which allowed a safe complete resection. Total elbow replacement restored the bone defect and offered an excellent functional outcome in combination with attachment tube soft tissue reconstruction.
Disclaimers
Funding: No funding was disclosed by the authors.
Conflicts of interest: The authors, their immediate families, and any research foundation with which they are affiliated have not received any financial payments or other benefits from any commercial entity related to the subject of this article.
Patient consent: Obtained.
Footnotes
Institutional review board approval was not required for this case report.
References
- 1.Campanacci M., Baldini N., Boriani S., Sudanese A. Giant-cell tumor of bone. J Bone Joint Surg Am. 1987;69:106–114. [PubMed] [Google Scholar]
- 2.Casadei R., De Paolis M., Drago G., Romagnoli C., Donati D. Total elbow arthroplasty for primary and metastatic tumor. Orthop Traumatol Surg Res. 2016;102:459–465. doi: 10.1016/j.otsr.2015.12.026. [DOI] [PubMed] [Google Scholar]
- 3.Chawla S., Henshaw R., Seeger L., Choy E., Blay J.-Y., Ferrari S., et al. Safety and efficacy of denosumab for adults and skeletally mature adolescents with giant cell tumor of bone: interim analysis of an open-label, parallel-group, phase 2 study. Lancet Oncol. 2013;14:901–908. doi: 10.1016/S1470-2045(13)70277-8. [DOI] [PubMed] [Google Scholar]
- 4.Davey M.S., Hurley E.T., Gaafar M., Molony D., Mullett H., Pauzenberger L. Long-term outcomes of total elbow arthroplasty: a systematic review of studies at 10-year follow-up. J Shoulder Elbow Surg. 2021;30:1423–1430. doi: 10.1016/j.jse.2020.11.014. [DOI] [PubMed] [Google Scholar]
- 5.Donati F., Di Giacomo G., D’Adamio S., Ziranu A., Careri S., Rosa Ma, et al. Silver-coated hip megaprosthesis in oncological limb savage surgery. Biomed Res Int. 2016;2016:1–6. doi: 10.1155/2016/9079041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Errani C., Tsukamoto S., Mavrogenis A.F. How safe and effective is denosumab for bone giant cell tumor? Int Orthop. 2017;41:2397–2400. doi: 10.1007/s00264-017-3536-9. [DOI] [PubMed] [Google Scholar]
- 7.Fletcher CDM, World Health Organization, International Agency for Research on Cancer . WHO classification of tumors of soft tissue and bone. 4th ed. IARC Press; Lyon: 2013. [Google Scholar]
- 8.Goedhart L.M., Leithner A., Jutte P.C. Organization of bone sarcoma care: a cross-sectional European study. Orthop Surg. 2020;12:1030–1035. doi: 10.1111/os.12716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gosheger G., Hillmann A., Lindner N., Rödl R., Hoffmann C., Bürger H., et al. Soft tissue reconstruction of megaprostheses using a trevira tube. Clin Orthop. 2001;393:264–271. doi: 10.1097/00003086-200112000-00030. [DOI] [PubMed] [Google Scholar]
- 10.Kharrazi F.D., Busfield B.T., Khorshad D.S., Hornicek F.J., Mankin H.J. Osteoarticular and total elbow allograft reconstruction with severe bone loss. Clin Orthop. 2008;466:205–209. doi: 10.1007/s11999-007-0011-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mahdal M., Neradil J., Mudry P., Paukovcekova S., Staniczkova Zambo I., Urban J., et al. New target for precision medicine treatment of giant-cell tumor of bone: sunitinib is effective in the treatment of neoplastic stromal cells with activated PDGFRβ signaling. Cancers. 2021;13:3543. doi: 10.3390/cancers13143543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mavrogenis A.F., Igoumenou V.G., Megaloikonomos P.D., Panagopoulos G.N., Papagelopoulos P.J., Soucacos P.N. Giant cell tumor of bone revisited. SICOT-J. 2017;3:54. doi: 10.1051/sicotj/2017041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ruggieri P., Mavrogenis A.F., Mercuri M. Quality of life following limb-salvage surgery for bone sarcomas. Expert Rev Pharmacoecon Outcomes Res. 2011;11:59–73. doi: 10.1586/erp.10.91. [DOI] [PubMed] [Google Scholar]
- 14.Rutkowski P., Gaston L., Borkowska A., Stacchiotti S., Gelderblom H., Baldi G.G., et al. Denosumab treatment of inoperable or locally advanced giant cell tumor of bone - Multicenter analysis outside clinical trial. Eur J Surg Oncol. 2018;44:1384–1390. doi: 10.1016/j.ejso.2018.03.020. [DOI] [PubMed] [Google Scholar]
- 15.Sanchez-Sotelo J., Wagner E.R., Houdek M.T. Allograft-prosthetic composite reconstruction for massive proximal humeral bone loss in reverse shoulder arthroplasty. JBJS Essent Surg Tech. 2018;8:e3. doi: 10.2106/JBJS.ST.17.00051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Savvidou O.D., Koutsouradis P., Chloros G.D., Papanastasiou I., Sarlikiotis T., Kaspiris A., et al. Bone tumors around the elbow: a rare entity. EFORT Open Rev. 2019;4:133–142. doi: 10.1302/2058-5241.4.180086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schmolders J., Koob S., Schepers P., Kehrer M., Frey S.P., Wirtz D.C., et al. Silver-coated endoprosthetic replacement of the proximal humerus in case of tumor—is there an increased risk of periprosthetic infection by using a trevira tube? Int Orthop. 2017;41:423–428. doi: 10.1007/s00264-016-3329-6. [DOI] [PubMed] [Google Scholar]
- 18.Tsukamoto S., Mavrogenis A.F., Tanaka Y., Kido A., Kawaguchi M., Errani C. Denosumab does not decrease local recurrence in giant cell tumor of bone treated with en bloc resection. Orthopedics. 2021;44:326–332. doi: 10.3928/01477447-20211001-09. [DOI] [PubMed] [Google Scholar]