Correction to: Cellular & Molecular Immunology 10.1038/s41423-023-00983-5, published online 24 February 2023
In this article the legends for all the figures were inadvertently omitted, the figures should have appeared as shown below.
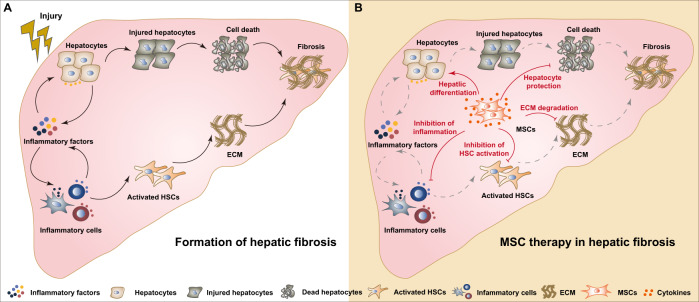
Fig. 1.
The role of MSCs in hepatic fibrosis formation and therapy. A, In the process of chronic inflammatory injury of liver, the synergistic effect from inflammation and the death of hepatocytes promotes the formation of hepatic fibrosis; B, The mechanisms of MSC therapy in hepatic fibrosis, including hepatic differentiation, hepatocyte protection, inhibition of HSC activation, ECM degradation and inflammation inhibition
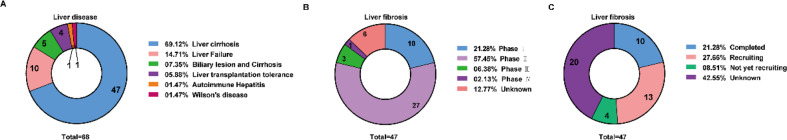
Fig. 2.
Clinical trials of MSCs in liver diseases. A, An overall of clinical trials based on MSCs classified by liver disease type. B, Clinical trials of liver cirrhosis based on MSCs classified by the clinical phase. C, Clinical trials of liver cirrhosis based on MSCs classified by status. The data showed the number and percentage
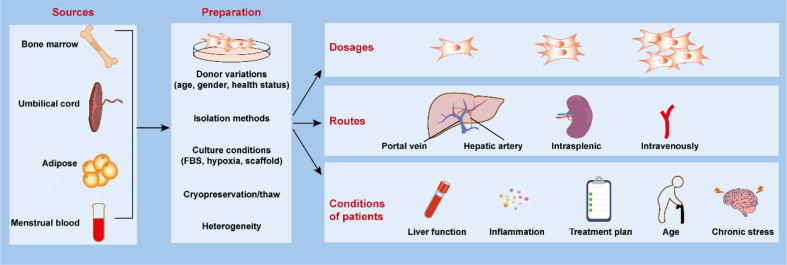
Fig. 3.
The parameters need to be standardized precisely in clinical application of MSCs in liver cirrhosis. Currently, MSCs used in clinical trials for liver cirrhosis treatment are obtained from different sources (bone marrow, umbilical cord, adipose tissue, and menstrual blood). During the process of MSC preparation, there are various imprecise parameters, including donor seleciton, isolation methods, culture conditions, cryopreservation and thaw, and cell heterogeneity. MSCs are transferred at different dosages and through different routes. All of the above parameters would affect the therapeutic effect of MSCs and developing the best standard for clinical application of MSCs is essential for the successful clinical translation of MSCs in the future
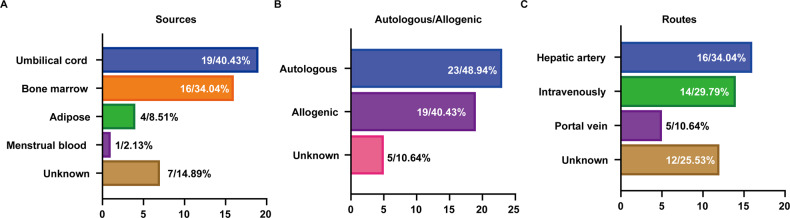
Fig. 4.
Clinical trials of liver cirrhosis based on MSCs application. A, Clinical trials of liver cirrhosis based on different sources of MSCs. B, Clinical trials of liver cirrhosis based on autologous and allogeneic transplantation of MSCs. C, Clinical trials of liver cirrhosis based on transplantation routes of MSCs. The data showed the number and percentage of corresponding clinical trials
The original article has been corrected.
Contributor Information
Yufang Shi, Email: shiyufang2@gmail.com.
Zhipeng Han, Email: hanzhipeng0311@126.com.