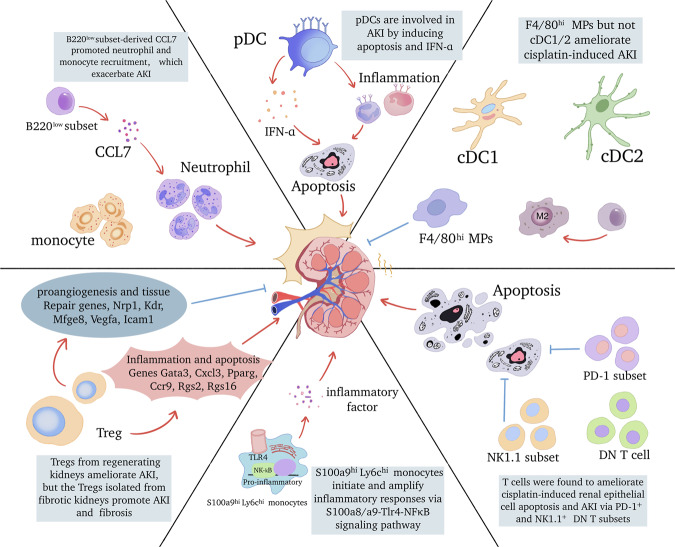
Acute kidney injury (AKI) is a frequent complication affecting hospitalized patients in medical wards and usually arises from ischemia‒reperfusion injury (IRI), sepsis, and nephrotoxic drugs. Excess infiltration and activation of immune cells in the kidney induce a continuous inflammatory response, which is characterized by substantial cytotoxicity that ultimately exacerbates kidney damage [1]. The role of individual immune cell types in AKI has been mostly investigated in previous years. However, despite the successful results in experimental studies, treatments targeting a broad immune response have yielded minor improvements in patient outcomes, in part due to the imprecise understanding of immune cell heterogeneity. Recently, the focus of AKI research has shifted toward gaining insight into the heterogeneity and dynamic changes in immune cells with novel experimental approaches such as single-cell analysis, thereby redefining AKI pathophysiology.
Neutrophils, which are primary effector cells of the innate immune system, become activated and are recruited to the kidneys during AKI; however, their mere presence does not necessarily indicate a causal role, given the inconsistent results of neutrophil blockade or depletion in various rodent studies. Neutrophils appear to function primarily as inflammatory indicators and amplifiers of AKI. These cells contribute substantially to kidney injury by obstructing the microvasculature and secreting toxic molecules, such as proteases, oxidants, cytokines, and neutrophil extracellular traps. To date, neutrophils have been viewed as a largely homogenous population. However, recent research has identified a novel Siglec-F-expressing subset of neutrophils that contributes to renal fibrosis in murine and human kidneys [2]. This study showed that the Ly-6G+ neutrophil population that coexpressed the eosinophil-specific surface marker Siglec-F was increased with kidney inflammation and fibrosis progression. Siglec-F+ neutrophils produce more profibrotic factors and collagen I than conventional neutrophils. TGF-β1 and GM-CSF may induce Siglec-F expression in neutrophils. Although this research focuses on the role of neutrophil subsets in chronic kidney disease (CKD), the findings suggest that specific neutrophil subsets can be generated by renal microenvironments and that different neutrophil subsets exhibit diverse biological functions. This may partially explain the inconsistent results from the aforementioned depletion studies. Because single-cell studies of neutrophils in kidney disease are still lacking, further investigation of neutrophil profiling in AKI is necessary.
Macrophages participate in all phases of the injury and recovery process of AKI. The heterogeneity of macrophages has long been known. Typically, macrophages can be classified as kidney resident macrophages (KRMs) and infiltrating monocyte-derived macrophages. Functionally, macrophages polarize to a proinflammatory phenotype (M1) or an anti-inflammatory phenotype (M2). However, the mechanism for this shift in phenotype remains unclear and appears to be influenced by the intrarenal microenvironment. Recent research has identified a pro-resolving macrophage characterized by increased expression of cyclooxygenase 2 (COX-2) [3]. Signaling through myeloid COX-2 induces the polarization of KRMs to a pro-resolving phenotype via the E-type prostanoid receptor (EP) 4. Conversely, EP3, another member of the prostaglandin receptor family, plays an opposing role in myeloid cells by amplifying the necro-inflammatory cascade and exacerbating kidney injury [4]. Advances in single-cell RNA sequencing (scRNA-seq) have enabled detailed analysis of macrophages and revealed that macrophages may be a more heterogeneous population than previously thought. For instance, Yao et al. mapped the macrophage subsets of the kidney, spleen, and blood during the acute stage of IRI [5]. They identified S100a9hiLy6chi monocytes as the earliest blood-originated responders to renal injury. These monocytes were recruited to the kidney, initiating and amplifying renal inflammation via the S100a8/a9-TLR4-NFκB signaling pathway. This study also observed S100A8/A9+ macrophage infiltration in clinical kidney samples, and the degree of infiltration correlated with the extent of kidney damage. Targeting S100a8/a9 signaling significantly reduced macrophage infiltration and improved renal function.
DCs exert a crucial effect on tissue homeostasis. Based on their phenotype, origin, and functions, DCs are broadly categorized into two subtypes: myeloid DCs (mDCs) and plasmacytoid DCs (pDCs). We recently reported that pDCs participate in acute kidney injury through type I interferon secretion using DTRBDCA2 transgenic mice [6]. pDCs promote kidney inflammation and tubular apoptosis in ischemia- and cisplatin-induced AKI. Kidney DCs and macrophages are thought to share partly overlapping phenotypes and functions and are collectively referred to as mononuclear phagocytes (MPs). Salei et al. identified four subsets of MPs (the main conventional DC1 (cDC1) and cDC2 subtypes, as well as CD64-expressing F4/80hi and CD11bhi cells) in AKI using Clec9a-Cre-mediated fate mapping [7]. They found that resident renal MHCII+ F4/80hi DCs responded to AKI by downregulating MHCII expression, possibly as a response to prostaglandin E2 (PGE2). These cells contribute to inflammatory cell recruitment. A further study used several transgenic mouse models and showed that the intrinsic potency associated with the renoprotective effects of CD11c+ cell was specifically attributed to CD64+ MPs [8]. Cisplatin treatment induced more severe kidney injury in Clec9acreRosaiDTR mice, which showed the protective effect of CD11c+ cells with a history of Clec9a expression. The loss of cDC1 and cDC2 had no impact on the severity of cisplatin-induced AKI, as indicated in Xcr1Venus-DTR and Clec9acreIrf4fl/fl mice. However, the Clec9acreCd64iDTR mouse model, which permits DT-mediated depletion of F4/80hi MPs, showed increased susceptibility to cisplatin-induced AKI, strongly indicating that F4/80hi MPs are the key immune cell subset in AKI.
Significant headway has also been made in the study of lymphoid cell subsets. B220low B-cell subsets were discovered in post-AKI kidneys. Siglec-G-deficient mice, which exhibit increased B220low B cells, suffered worsened kidney injury in response to different models of AKI [9]. Further research revealed that the exacerbation of AKI was induced by B220low subset-derived CCL7, which promoted neutrophil and monocyte recruitment. CD4- CD8- double-negative (DN) αβ T cells were shown to protect against ischemic AKI. Sadasivam et al. recently identified two subsets of these DN T cells in mouse and human AKI: the programmed cell death protein-1 receptor (PD-1+) and natural killer 1.1 (NK1.1+) subsets. Instead of the NK1.1+ subset and CD8+ T cells, PD-1+ subsets are the main responders to ischemic AKI [10]. Furthermore, Gong et al. found that cisplatin treatment decreased PD-1+ and NK1.1 subsets in kidney DN T cells [11]. The adoptive transfer of DN T cells attenuated kidney dysfunction and structural damage in cisplatin-induced AKI. It is essential to note that cisplatin treatment led to the expansion of total DN T cells, despite the reduction in PD-1+ and NK1.1+ subsets, necessitating caution regarding undiscovered subsets. A new class of innate lymphoid cells (ILCs), including ILC1s, ILC2s, and ILC3s, have been identified as participants in AKI. Endogenous ILC2s have no discernible effect on IRI, and no differences were observed in the severity of kidney injury among wild-type, ILC2-deficient, and ILC2-depleted mice. Expansion by IL-33 and IL-233, as well as the adoptive transfer of ILC2s, resulted in renoprotection in IRI. The mechanism relies on the production of Areg and the induction of M2 macrophages. Recently, a novel subset of ILC-regulatory innate lymphoid cells (ILCregs) was discovered in the kidney [12]. These cells exerted immunosuppressive effects on innate immune cells via the secretion of IL-10 and transforming growth factor-β in IRI.
The heterogeneity of Tregs in AKI has recently been extensively studied. Initially, Tregs were believed to limit inflammation and cellular injury in various models of AKI. However, Duraes et al performed comprehensive scRNA-Seq analyses on isolated kidney immune cells and found distinctive gene expression patterns of Tregs in regenerating and fibrotic kidneys [13]. Specifically, Tregs from regenerating kidneys expressed genes associated with angiogenesis and tissue repair (including Nrp1, Kdr, Mfge8, Vegfa, and Icam1), suggesting that these cells play a crucial role in promoting tissue regeneration after injury. On the other hand, Tregs from fibrotic kidneys expressed genes linked to proinflammatory responses and apoptosis (including Gata3, Cxcl3, Pparg, Ccr9, Rgs2, and Rgs16), suggesting their contribution to tissue damage and fibrosis. These findings underscore the plasticity of immune cells in specific kidney disease contexts. Given that AKI increases the risk of developing CKD and that the AKI-to-CKD transition significantly impacts kidney prognosis and patient quality of life, it is imperative to conduct further research on the plasticity and dynamics of immune cells during various stages of AKI.
New technology has emerged and may help us elucidate the crosstalk between the kidney and the immune system and reveal how the kidney environment re-educates immune cells toward different profiles during AKI. Using the 10× Genomics Visium Spatial Gene Expression solution, Dixon et al. mapped the spatiotemporal transcriptional changes in the female mouse kidney [14]. During renal injury and repair, there is a significant increase in the frequency of interactions between injured proximal tubule cells and T cells and macrophages, which persist for up to six weeks. Notably, a marked increase in the F4/80 signal surrounding Kim-1-positive tubules at six weeks postinjury was also observed. Another study conducted a single-cell dissection of the epithelial-immune cell interplay and identified three novel subtypes of proximal tubule cells (PTC-S1-new/PTC-S2-new/PTC-S3-new). Monocytes and macrophages interact closely with injured PTCs, influencing AKI progression mainly through CXCL and TNF signaling [15]. These findings suggest an intricate interplay between immune and intrinsic renal cells.
In summary, AKI is accompanied by the infiltration of various immune cells and a highly responsive immune response, which collectively establish a complicated microenvironment within the kidney (Fig. 1). By using transgenic rodent models and new omics techniques, the dynamics, heterogeneity, and plasticity of immune cells during distinct stages of AKI have been elucidated. Additionally, the crosstalk between intrinsic renal cells and immune cells has been verified. With the recognition of immune cell diversity in AKI progression, exploring the therapeutic potential of targeted immune cells for AKI therapy is a promising strategy.
Fig. 1.
Immune cell diversity and biology in the pathogenesis and repair of AKI
Acknowledgements
This study was supported by grants from the Shanghai Sailing Program (No: 20YF1423400 to B.D.), the National Natural Science Foundation of China (No: 81870462, 82070789 to F.D.; No: 82200747 to B.D.; No: 32002153 to S.W.), the Shanghai Huangpu Industry Support Grant (XK2020002 to F.D.), the Collaborative Innovation Center for Clinical and Translational Science by Chinese Ministry of Education & Shanghai (CCTS-2022206 to F.D.), the Biomaterials & Regenerative Medicine Institute Cooperative Research Project (2022LHB01 to F.D.), the Clinical Research Program of 9th People’s Hospital (JYLJ202218 to F.D.), and the Postdoctoral Scientific Research Foundation of Shanghai Ninth People’s Hospital, Shanghai Jiao Tong University School of Medicine (DengBo-2021-Scientific Research Start-up Funds to B.D.)
Author contributions
BD and SW wrote the manuscript. PZ drew the picture. FD designed and performed the final proofreading of the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
These authors contributed equally: Bo Deng, Sutian Wang.
References
- 1.Jang HR, Rabb H. Immune cells in experimental acute kidney injury. Nat Rev Nephrol. 2015;11:88–101. doi: 10.1038/nrneph.2014.180. [DOI] [PubMed] [Google Scholar]
- 2.Ryu S, Shin JW, Kwon S, Lee J, Kim YC, Bae YS, et al. Siglec-F-expressing neutrophils are essential for creating a profibrotic microenvironment in renal fibrosis. J Clin Invest. 2022;132:e156876. doi: 10.1172/JCI156876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pan Y, Cao S, Terker AS, Tang J, Sasaki K, Wang Y, et al. Myeloid cyclooxygenase-2/prostaglandin E2/E-type prostanoid receptor 4 promotes transcription factor MafB-dependent inflammatory resolution in acute kidney injury. Kidney Int. 2022;101:79–91. doi: 10.1016/j.kint.2021.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Leng J, Zhao W, Guo J, Yu G, Zhu G, Ge J, et al. E-prostanoid 3 receptor deficiency on myeloid cells protects against ischemic acute kidney injury via breaking the auto-amplification loop of necroinflammation. Kidney Int. 2023;103:100–14. doi: 10.1016/j.kint.2022.08.019. [DOI] [PubMed] [Google Scholar]
- 5.Yao W, Chen Y, Li Z, Ji J, You A, Jin S, et al. Single Cell RNA sequencing identifies a unique inflammatory macrophage subset as a druggable target for alleviating acute kidney injury. Adv Sci. 2022;9:e2103675. doi: 10.1002/advs.202103675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Deng B, Lin Y, Chen Y, Ma S, Cai Q, Wang W, et al. Plasmacytoid dendritic cells promote acute kidney injury by producing interferon-alpha. Cell Mol Immunol. 2021;18:219–29. doi: 10.1038/s41423-019-0343-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Salei N, Rambichler S, Salvermoser J, Papaioannou NE, Schuchert R, Pakalniskyte D, et al. The kidney contains ontogenetically distinct dendritic cell and macrophage subtypes throughout development that differ in their inflammatory properties. J Am Soc Nephrol. 2020;31:257–78. doi: 10.1681/ASN.2019040419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Salei N, Ji X, Pakalniskyte D, Kuentzel V, Rambichler S, Li N, et al. Selective depletion of a CD64-expressing phagocyte subset mediates protection against toxic kidney injury and failure. Proc Natl Acad Sci USA. 2021;118:e2022311118. doi: 10.1073/pnas.2022311118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Inaba A, Tuong ZK, Riding AM, Mathews RJ, Martin JL, Saeb-Parsy K, et al. CCL7 augments neutrophil and monocyte recruitment, exacerbating acute kidney injury. J Immunol. 2020;205:1376–84. doi: 10.4049/jimmunol.2000454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sadasivam M, Noel S, Lee SA, Gong J, Allaf ME, Pierorazio P, et al. Activation and proliferation of PD-1(+) kidney double-negative T cells is dependent on nonclassical MHC Proteins and IL-2. J Am Soc Nephrol. 2019;30:277–92. doi: 10.1681/ASN.2018080815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gong J, Noel S, Hsu J, Bush EL, Arend LJ, Sadasivam M, et al. TCR(+)CD4(-)CD8(-) (double negative) T cells protect from cisplatin-induced renal epithelial cell apoptosis and acute kidney injury. Am J Physiol Ren Physiol. 2020;318:F1500–F1512. doi: 10.1152/ajprenal.00033.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cao Q, Wang R, Wang Y, Niu Z, Chen T, Wang C, et al. Regulatory innate lymphoid cells suppress innate immunity and reduce renal ischemia/reperfusion injury. Kidney Int. 2020;97:130–42. doi: 10.1016/j.kint.2019.07.019. [DOI] [PubMed] [Google Scholar]
- 13.do Valle Duraes F, Lafont A, Beibel M, Martin K, Darribat K, Cuttat R, et al. Immune cell landscaping reveals a protective role for regulatory T cells during kidney injury and fibrosis. JCI Insight. 2020;5:e130651. doi: 10.1172/jci.insight.130651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dixon EE, Wu H, Muto Y, Wilson PC, Humphreys BD. Spatially resolved transcriptomic analysis of acute kidney injury in a female murine model. J Am Soc Nephrol. 2022;33:279–89. doi: 10.1681/ASN.2021081150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang M, Wu L, Deng Y, Peng F, Wang T, Zhao Y, et al. Single cell dissection of epithelial-immune cellular interplay in acute kidney injury microenvironment. Front Immunol. 2022;13:857025. doi: 10.3389/fimmu.2022.857025. [DOI] [PMC free article] [PubMed] [Google Scholar]