Abstract
Key Clinical Message
The differential diagnosis of post‐COVID‐19 syndrome is important in patients with symptoms of biliary obstruction. This patient had severe COVID‐19 who underwent ERCP and mimicked cholangiocarcinoma.
Abstract
Patients with severe coronavirus disease 2019 (COVID‐19) manifest liver injuries with pathological changes because of lowered blood oxygen saturation, cardiac malfunction, hepatotoxic drugs during treatment, and cellular injury. This paper reports a patient with a history of severe COVID‐19 who mimics cholangiocarcinoma after undergoing endoscopic retrograde cholangiopancreatography (ERCP). It was shown that differential diagnosis of post‐COVID‐19 syndrome is greatly important mostly in patients with symptoms of biliary obstruction.
Keywords: cholangiocarcinoma, common bile duct (CBD), COVID‐19, critical illness
1. INTRODUCTION
Although the main impacts of COVID‐19 (coronavirus disease 2019) are considered in the pulmonary system, recent studies highlight the liver as the mainly affected organ. Studies show that a significant percentage of COVID‐19 patients suffer from hepatic injury, especially patients with critical or severe forms of COVID‐19. Major reported pathological changes consist dilatation in the sinusoidal system, infiltration of lymphocytic cells in the sinusoidal system, multifocal hepatic necrosis, Kupffer cell hyperstimulation, and cholestasis. 1 Some fundamental mechanisms have been described for a hepatic malfunction that important causes include lowered blood oxygen saturation due to pneumonia, compromise of cardiac function, direct potential hepatotoxicity from therapeutic drugs (e.g., non‐steroidal anti‐inflammatory drugs (NSAIDs), hydroxychloroquine, and azithromycin), and cellular injury due to direct viral invasion, and micro and macrovascular changes from hypercoagulability. 2 Aberration in liver function test (LFT) occurs in 14%–53% of COVID‐19 patients. 3 Most of these abnormalities in the LFT return to normal condition without specific treatment. Nevertheless, hepatic injuries occur when the LFT is thrice or higher than the normal limit, which may need attentive management. 2 A recent meta‐analysis showed that liver vascular thrombosis and portal inflammation occurred in 29.4% and 13.2% of patients with a severe form of COVID‐19. 4 This study also showed that progressive ischemic injury in the liver biliary system might rapidly develop in some patients with severe illness, similar to the symptoms seen in the primary sclerosing cholangitis. This unique entity of liver injury occurs after a few weeks to months of the first diagnosis of the infection caused by SARS‐CoV‐2 and dictates possibly serious consequences. 5
Cholangiocarcinoma as the second most common primary hepatobiliary malignancy, accounts for approximately 15% of liver cancers. Many clinical conditions such as primary sclerosing cholangitis, viral and bacterial pyogenic cholangitis, acquired immunodeficiency syndrome autoimmune and inflammatory diseases, sarcoidosis, chemotherapy‐induced sclerosis, and carcinoid tumors could mimic cholangiocarcinoma. 6 According to the previous studies, the most common findings of hepatobiliary system involvement in COVID‐19 infection were hepatic steatosis (HS), portal inflammatory infiltrate, Kupffer cell hyperstimulation, and cholestasis. 1 However, there were very rare case reports about infection with COVID‐19 that could mimic all clinical symptoms of cholangiocarcinoma. Here, we described a case with a history of severe COVID‐19 presented with jaundice, elevated liver enzyme, and alkaline phosphatase (ALP) undergoing endoscopic retrograde cholangiopancreatography (ERCP). The findings of primary ERCP were similar to cholangiocarcinoma but the architects of the bile duct system came back to nearly normal, 2 months after the insertion of a common bile duct (CBD) stent.
2. CASE PRESENTATION
A woman aged 54 years was referred to Firoozgar Hospital of Tehran, Iran for the evaluation and management of her elevated liver enzymes and ALP. She had a 6‐week history of intermittent cough, myalgia, and general weakness. Her past medical history showed a positive PCR test for COVID‐19 2 months earlier. Her initial lab test showed leukocytosis with a WBC count of 13 × 109/L, ALP of 1816 U/L (normal range: 110–390 U/L), alanine aminotransferase (ALT) of 375 U/L, aspartate aminotransferase (AST) of 367 U/L, total bilirubin of 10/9 mg/dL, and direct bilirubin of 5.4 mg/dL. Her liver ultrasound analysis showed dilatation of the intra and extrahepatic bile duct and with dilated CBD of 10 mm. The patient underwent an ERCP procedure whose findings confirmed her severe diffuse dilatation in the left and right portions of the hepatic and intrahepatic ducts suggestive of cholangiocarcinoma (Figure 1). After sphincterotomy and balloon dilation, CBD stent and brush cytology of the stenosis site were also performed. Two months later, a repeated ERCP showed mild dilatation in the left and right portions of the intrahepatic duct and a successful stent removal was done. Intestinally, the diameter of CBD was near standard and the whole portion of CBD had an irregular appearance (Figure 2). It should be noticed that the result of brushed cytology showed only moderate to no specific inflammation.
FIGURE 1.
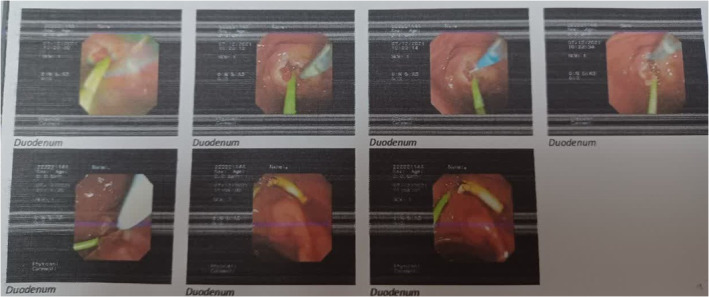
ERCP results before sphincterotomy and balloon dilation. Endoscopic retrograde cholangiopancreatography (ERCP) results before insertion of a common bile duct (CBD) stent.
FIGURE 2.
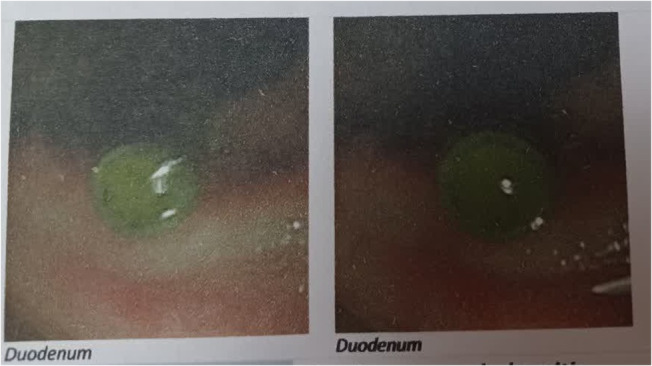
ERCP results after sphincterotomy and balloon dilation. Endoscopic retrograde cholangiopancreatography (ERCP) results after the insertion of a common bile duct (CBD) stent.
3. DISCUSSION
In this case study, the clinical findings were significantly compatible with cholangiocarcinoma that was improved after COVID‐19 recovery. The basic pathophysiology in cases with COVID‐19 and biliary duct involvement is more likely multifactorial and even though not entirely understood. Ischemic cholangiopathy and altered bile composition or bile duct ischemia are the two mechanisms responsible for bile duct involvement in critical illness patients. 7 After the virus spreads from the gastrointestinal tract to the blood through the portal tract system, it passes to the bile duct system. ACE2's presence is moderately low in hepatocytes that are primarily located in upper levels of cholangiocytes. There are limited histological data about the mentioned patients but the examination of liver biopsies shows varying degrees of portal fibrosis and large duct obstruction. 8 Evidence shows that the stimulation of a prothrombotic state by SARS‐CoV‐2 subsequently causes endothelial damage. These changes expose the tissue factor and collagen in the subendothelial matrix that triggers the coagulation cascade and subsequently results in the generation of thrombin. When these events are accompanied by platelet aggregation, it results in blood clots in hepatic arterial branches that supply the epithelium of the bile duct. On the other hand, there is a higher susceptibility of the intrahepatic biliary epithelium to ischemia since there is a single blood source through the hepatic arteries. 9 In one case study, secondary sclerosing cholangitis (SSC) was diagnosed in a severely ill patient induced by COVID‐19 that demonstrates a rare ailment specifically developed in the setting of SARS‐CoV‐2 infection. 7
Marchi et al 10 reported a patient with severe pneumonia induced by COVID‐19 that suffered from abdominal pain of cytomegalovirus (CMV)‐induced duodenitis along with pancreatitis and bleeding.
The imaging findings of a large series of case reports that evaluated 17 patients with COVID‐19‐associated SSC reported that all patients had intrahepatic bile duct structures on magnetic resonance cholangiopancreatography (MRCP) and beaded appearance of bile ducts. 11
One study reported a male aged 68 years with a severe form of COVID‐19 and a large hepatic lesion and multiple floating thrombi in the splenic infarcts and aorta, who was suspected of intrahepatic cholangiocarcinoma. However, a liver biopsy showed an inflammatory lesion described by plasma cellular infiltrates and portal‐based confluent lymphohistiocytosis. It was hypothesized that the embolic sprouting of floating aortic thrombi caused splenic ischemic lesions. 12
A retrospective study on 12 patients that recovered from severe COVID‐19 with serum ALP of three times upper than the normal limit showed that 92% (11/12) of them had a beaded appearance of intrahepatic ducts in MRCP. Unfortunately, five patients who experienced constant jaundice, liver insufficiency, and frequent bacterial cholangitis had been referred for liver transplantation. 13
4. CONCLUSION
This study confirmed that the severe form of COVID‐19 has potential effects for producing progressive biliary injury and long‐term hepatic morbidity that mimics cholangiocarcinoma. Physicians should be aware of this differential diagnosis as a longstanding manifestation of critical COVID‐19, especially in patients with jaundice, elevated liver enzymes, and symptoms of biliary obstruction.
AUTHOR CONTRIBUTIONS
Mahmoodreza Khonsari: Data curation; investigation; project administration; supervision; visualization; writing – review and editing. Kamal Boostani: Conceptualization; investigation; supervision; validation; writing – review and editing. Farahnoosh Farnood: Data curation; resources; visualization; writing – original draft; writing – review and editing.
FUNDING INFORMATION
This research received no specific grant from any funding agency in the public, commercial, or not‐for‐profit sectors.
CONFLICT OF INTEREST STATEMENT
The authors declare that they have no conflict of interest.
CONSENT
Written informed consent was obtained from the patient to publish this report in accordance with the journal's patient consent policy.
Khonsari M, Boostani K, Farnood F. Post‐COVID‐19 syndrome mimicking cholangiocarcinoma: A case report. Clin Case Rep. 2023;11:e7449. doi: 10.1002/ccr3.7449
Contributor Information
Kamal Boostani, Email: kamal_boostani@yahoo.com.
Farahnoosh Farnood, Email: farnoodkidney@gmail.com.
DATA AVAILABILITY STATEMENT
The data obtained will be available from the corresponding authors upon request.
REFERENCES
- 1. Moreira JLS, Barbosa SMB, Vieira JG, Chaves NCB, Gonçalves Júnior J. Liver histopathological changes and COVID‐19: what does literature have to tell us? Dig Liver Dis. 2022;54(3):296‐298. doi: 10.1016/j.dld.2022.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Li Y, Xiao SY. Hepatic involvement in COVID‐19 patients: pathology, pathogenesis, and clinical implications. J Med Virol. 2020;92(9):1491‐1494. doi: 10.1002/jmv.25973 [DOI] [PubMed] [Google Scholar]
- 3. Zhang Y, Zheng L, Liu L, Zhao M, Xiao J, Zhao Q. Liver impairment in COVID‐19 patients: a retrospective analysis of 115 cases from a single Centre in Wuhan city, China. Liver Int. 2020;40(9):2095‐2103. doi: 10.1111/liv.14455 [DOI] [PubMed] [Google Scholar]
- 4. Díaz LA, Idalsoaga F, Cannistra M, et al. High prevalence of hepatic steatosis and vascular thrombosis in COVID‐19: a systematic review and meta‐analysis of autopsy data. World J Gastroenterol. 2020;26(48):7693‐7706. doi: 10.3748/wjg.v26.i48.7693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bethineedi LD, Suvvari TK. Post COVID‐19 cholangiopathy – a deep dive. Dig Liver Dis. 2021;53(10):1235‐1236. doi: 10.1016/j.dld.2021.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tyson GL, El‐Serag HB. Risk factors for cholangiocarcinoma. Hepatology. 2011;54(1):173‐184. doi: 10.1002/hep.24351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Edwards K, Allison M, Ghuman S. Secondary sclerosing cholangitis in critically ill patients: a rare disease precipitated by severe SARS‐CoV‐2 infection. BMJ Case Reports. 2020;13(11):e237984. doi: 10.1136/bcr-2020-237984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Shih AR, Hatipoglu D, Wilechansky R, et al. Persistent cholestatic injury and secondary sclerosing cholangitis in COVID‐19 patients. Arch Pathol Lab Med. 2022;146(10):1184‐1193. doi: 10.5858/arpa.2021-0605-SA [DOI] [PubMed] [Google Scholar]
- 9. Moreira JLS, Barbosa SMB, Gonçalves Júnior J. Pathophysiology and molecular mechanisms of liver injury in severe forms of COVID‐19: an integrative review. Clin Res Hepatol Gastroenterol. 2021;45(6):101752. doi: 10.1016/j.clinre.2021.101752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Marchi G, Vianello A, Crisafulli E, et al. Cytomegalovirus‐induced gastrointestinal bleeding and pancreatitis complicating severe Covid‐19 pneumonia: a paradigmatic case. Mediterr J Hematol Infect Dis. 2020;12(1):e2020060. doi: 10.4084/mjhid.2020.060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ghafoor S, Germann M, Jüngst C, Müllhaupt B, Reiner CS, Stocker D. Imaging features of COVID‐19‐associated secondary sclerosing cholangitis on magnetic resonance cholangiopancreatography: a retrospective analysis. Insights Imaging. 2022;13(1):128. doi: 10.1186/s13244-022-01266-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ugolotti MC, Pedrazzini M, Silini EM, et al. Vascular liver injury mimicking an intrahepatic cholangiocarcinoma in a COVID‐19 patient. J Med Virol. 2021;93(4):1940‐1942. doi: 10.1002/jmv.26803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Faruqui S, Okoli FC, Olsen SK, et al. Cholangiopathy after severe COVID‐19: clinical features and prognostic implications. Am J Gastroenterol. 2021;116(7):1414‐1425. doi: 10.14309/ajg.0000000000001264 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data obtained will be available from the corresponding authors upon request.


