Abstract
Background
Recent preclinical and clinical studies have shed light on the possible impact of sex and estrous/menstrual cycle on ketamine’s antidepressant action but with incongruous results. The preclinical studies that have shown the effects of ovarian sex hormones have not done so in animal models of depression. Thus, the aim of the present study is to scrutinize the acute behavioral responses to a subanesthetic dose of S-ketamine in males vs females and in different estrous phases in free-cycling females in a well-powered translational approach.
Methods
We evaluated the behavioral sensitivity to 20 mg/kg S-ketamine (i.p.) in male and female Flinders Sensitive Line rats (FSLs) and their counterpart Flinders Resistant Line rats (FRLs) subjected to the open field and forced swim tests. Female rats were disaggregated into different estrous phases, and the behavioral outcomes were compared.
Results
Acute administration of S-ketamine had robust antidepressant-like effects in FSLs. Within our study power, we could not detect sex– or estrous cycle–specific different antidepressant-like responses to S-ketamine in FSLs. Fluctuations in the levels of ovarian sex hormones across different estrous cycles did not behaviorally affect S-ketamine’s rapid-acting antidepressant mode of action. No sex-related or estrous cycle–related impact on behavioral despair was observed even among FRLs and saline-treated FSLs.
Conclusions
We conclude that physiological oscillations of estrogen and progesterone levels neither amplify nor diminish the behavioral antidepressant-like effect of S-ketamine. In addition, fluctuations of ovarian sex hormones do not predispose female animals to exhibit enhanced or reduced depressive-like and anxiety-like behaviors.
Keywords: S-ketamine, sex-specific difference, estrous cycle, depression, Flinders Sensitive Line rats
Significance Statement.
While holding great promise for treating the subgroups of patients with depression who do not adequately respond to antidepressants, discrepancies among the findings of preclinical studies, as well as a dearth of disaggregation of the outcome of clinical studies in males and females and of females at different stages of the menstrual cycle, have limited personalized prescription of ketamine. Here, in a translational approach, the behavioral sensitivity to an acute subanesthetic dose of ketamine in freely cycling female and male Flinders Sensitive Line rats was extensively investigated. Moreover, the baseline depressive/anxiety-like behaviors were assessed during physiological fluctuations of ovarian sex hormones. Disaggregation of findings of preclinical neuropsychiatric research, especially on depressive and anxiety-related disorders by sex and estrous cycle, may provide new insight into the etiology and pathophysiology of brain disorders and improved therapeutic approaches.
INTRODUCTION
Major depressive disorder is a debilitating mental illness and a leading cause of death by suicide, which carries a significant burden with a lifetime prevalence of more than 20% (Otte et al., 2016; Hasin et al., 2018). The prevalence, symptomatology, and outcome of major depressive disorder are influenced by sex, and women are twice as likely to be struck with depression (Otte et al., 2016). There is also evidence pointing to sex-specific responses to antidepressants (Khan et al., 2005; Keers and Aitchison, 2010).
Ketamine, due to its rapid onset of action in the alleviation of depressive symptoms followed by a sustained effect, has gained much interest compared with other conventional antidepressants. Emerging evidence suggests that fluctuations in the level of steroid ovarian sex hormones can affect the behavioral responses to ketamine (Sarkar and Kabbaj, 2016; Dossat et al., 2018; Schoepfer et al., 2019). Despite these facts, we have not yet fully delved into the underlying mechanisms leading to such differences. So far, scarce research and resources have mainly focused on exploring some possible sex-related contributing factors in both clinical and preclinical studies.
Clinical studies have revealed that the profile of side effects experienced by women differs from those of men (Zhang et al., 2013; Derntl et al., 2019; Freeman et al., 2019). Although there are few studies to rely on, it seems the difference in response to ketamine between men and women as well as women premenopause and postmenopause is slight (Coyle and Laws, 2015; Freeman et al., 2019). However, preclinical studies fail to replicate all the findings of clinical studies and have reported sex-specific and estrous cycle–specific differences in ketamine’s antidepressant effect, relying on behavioral despair to measure depressive-like behaviors (Carrier and Kabbaj, 2013; Franceschelli et al., 2015; Sarkar and Kabbaj, 2016; Dossat et al., 2018; Ponton et al., 2022; Saland et al., 2022). Nevertheless, most preclinical studies have not used animal models of depression (Ponton et al., 2022), which could be a potential deviation from real life and a source of discrepancies.
Here, in a translational preclinical approach, we attempted to determine whether there are possible differences in the behavioral response to a subanesthetic dose of S-ketamine in a selectively bred genetic animal model of depression, both between the sexes and across estrous cycles in females.
The Flinders Sensitive Line rats (FSLs) resemble depressed individuals in some behavioral phenotypes, and they exhibit face validity, constructive validity, and predictive validity as an animal model of depression. The FSLs demonstrate exaggerated passive behaviors upon an exposure to stress (e.g., increased immobility in the forced swim test [FST]) compared with their counterparts, the Flinders Resistant Line rats (FRLs), which is considered a sign of behavioral despair. For detailed information about this selectively bred genetic animal model of depression, see (Overstreet et al., 2005; Overstreet and Wegener, 2013).
MATERIALS AND METHODS
Animals
Adult (8- to 11-week-old) female (n = 90 FSLs, n = 26 FRLs) and male (n = 23 FSLs, n = 12 FRLs) FSLs and their control counterpart FRLs obtained from the breeding facility at the Translational Neuropsychiatry Unit were used in the study. The animals were housed in pairs in a room with a controlled temperature of 22°C ± 2°C and humidity of 60% while having ad libitum access to food and water. The rats were kept on a 12-hour-light/-dark cycle (photoperiod 6:00 am to 6:00 pm). All cages were enriched with a metallic shelter, Pura crinkle paper nesting material, and a wooden stick. All animal experiments were performed during the light cycle, and no animal was handled prior to experimentation. All rats were randomly allocated to different groups, and the experimenters were semi-blinded. The animals were acclimatized one-half an hour before the initiation of the experiment in the room. All animal procedures were approved by the Danish National Committee for Ethics in Animal Experimentation (ID: 2021-15-0201-01010), and no animal met the criteria of the humane endpoints during the study. Data are reported according to the ARRIVE guidelines (see the Supplementary Materials).
Drugs and Administration
One hour before subjecting the animals to the FST, either S-ketamine HCl (Pfizer, Ballerup, Denmark) provided by the hospital pharmacy of the Central Denmark Region at a single dose of 20 mg/kg (Silote et al., 2021) or saline as a control was i.p. administered. In-house experiments have consistently replicated the antidepressant-like effect of S-ketamine in FSLs at doses of 15 and 20 mg/kg (i.p.) without any confounding effects on locomotion.
Open Field Test
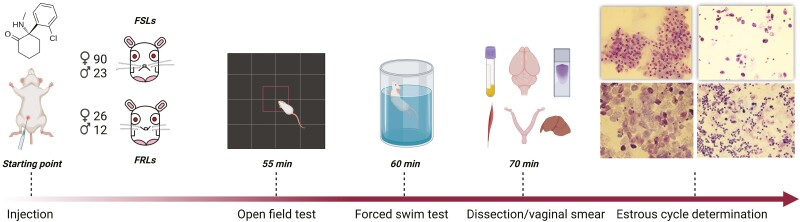
Five minutes before the FST, the animals were put in a square 1-m2 arena under a light intensity of 25–30 lux in the center and 10–20 lux in the periphery, and their behavior was live-tracked and analyzed/reanalyzed using EthoVision XT16 (Noldus Information Technology, Wageningen, Netherlands) having 3 points (nose, center, and tail) detected. The total distance moved (TDM), velocity, and time spent in the center (TSC) were measured (Figure 1).
Figure 1.
Timeline of the experiment.
Forced Swim Test
The animals were forced to swim for 7 minutes in a cylinder (24 cm diameter and 60 cm height) filled with 40 cm tap water at a temperature of 25°C ± 1°C. The behavior of the animals (struggling, swimming, and immobility) was video-recorded for 7 minutes and later analyzed/scored manually in 5-second time bins by 2 blinded independent experimenters (Figure 1).
Sample Collection
Animals were decapitated, and brains were removed and snap-frozen immediately in 2-methyl butane (isopentane) precooled with dry ice. The trunk blood sample was collected in K3-EDTA–coated collecting tubes and centrifuged later at 3800 rpm for 15 minutes at 4°C to extract plasma. Then a part of the left median lobe of the liver and the uterus were dissected. The uterus was kept in 4% paraformaldehyde, and the brain and the liver tissue were stored at −80°C for future analyses.
Estrous Cycle
To determine the estrous cycle of female animals, vaginal lavages diluted in 50 µL sterile saline were obtained from the vaginal canal and pipetted 4 to 5 times (Ajayi and Akhigbe, 2020) right after the animal was decapitated. Drops were introduced on a glass slide, air-dried, and stained with 0.01% crystal violet. The slide was overlaid with a coverslip and the addition of 1 drop of glycerol and was immediately visually examined at 40×, 100×, and 200× magnification under microscopy (Nikon Eclipse Ni 1.4NA) with an Olympus DP73 camera, newCAST software package (Visiopharm, Hørsholm, Denmark). Proestrus was characterized by the predominant presence of clusters of round nucleated epithelial cells, estrus with the predominance of cornified squamous (agranular) epithelial cells often packed in clusters, diestrus with having the hallmark of leukocyte infiltration and almost empty space lacking the cornified squamous epithelial cells, and metestrus with a crowded presentation of infiltrated leukocytes with the presence of both nucleated and agranular epithelial cells. Two independent researchers evaluated the slides, and in case of discrepancies, a third researcher tried to reevaluate. The estrous cycle was also confirmed by visual inspection of the uterus (Ajayi and Akhigbe, 2020) (data not shown).
Statistical Analysis
Statistical analyses were performed using GraphPad Prism (v. 9.4.1) and Jamovi (v. 2.2.5). For comparisons between more than 2 groups, 1-way ANOVA was used, and for comparing 2 groups, unpaired t tests were carried out. Where necessary, data were log-transformed to fulfil the assumption of homoscedasticity. In the case of normally distributed data, data were shown by mean ± SD, and in the case of application of nonparametric tests, data were illustrated as median ± 95% CI. The power (1-β) of the study was a priori set to 0.8, and the effect size was calculated and reported using G*Power (version 3.1.9.4, Heinrich Heine Universität, Düsseldorf, NRW, Germany). With a calculated effect size of 1.077, the sample size of 12 was required. P < .05 was considered significant (*P < .05, **P < .01, ***P < .001, ****P < .0001).
RESULTS
Locomotor and Thigmotactic Behavior
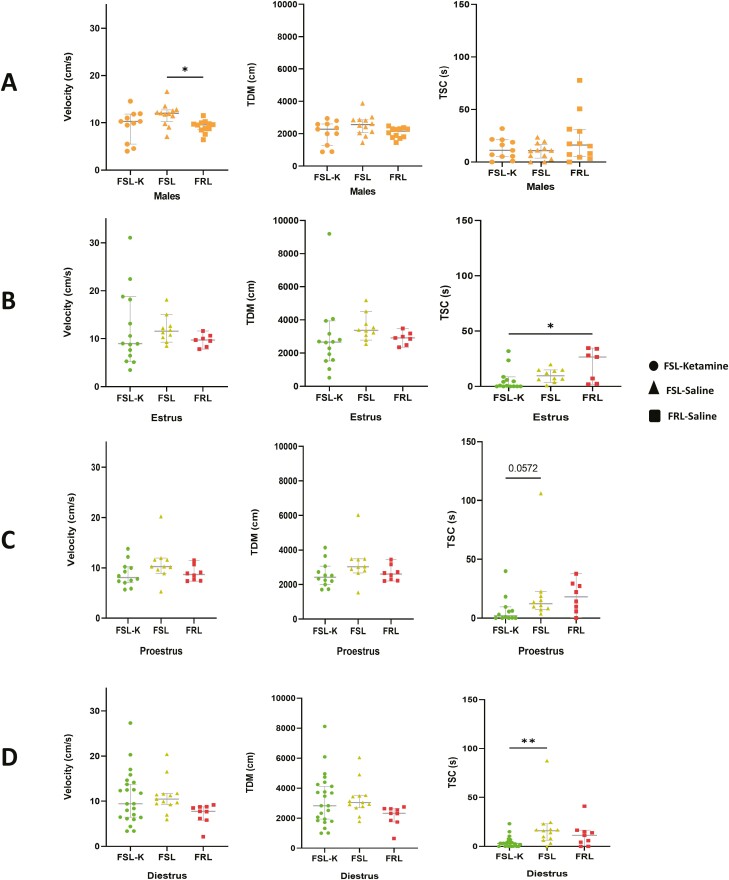
S-ketamine–treated male FSLs and both saline-treated male FSLs and FRLs exhibited no significant differences in TDM or TSC (Figure 2A). When comparing these 3 groups in terms of velocity, male FRLs were significantly slower than male FSLs receiving saline (P < .05), but there was no significant difference between S-ketamine–treated male FSLs and saline-injected male FSLs (Figure 2A). No significant differences were observed in velocity, TDM, or TSC between females during various estrous cycle phases (Figure 2B–D), between female and male FSLs treated with either S-ketamine or saline, and or between male and female FRLs (Figure 3A–C). Administration of S-ketamine did not significantly alter the locomotion and anxiety-like behaviors nor did the different phases of the estrous cycle. The only difference was seen when comparing TSC in the diestrus and estrus, where ketamine-treated FSLs were significantly less explorative than saline-receiving FSLs (P < .01) and FRLs (P < .05), respectively (Figure 2B and D).
Figure 2.
(A) Locomotor activity (velocity, total distance moved [TDM], and time spent in center [TSC]) in male Flinders Sensitive and Resistant Line rats (FSLs and FRLs, respectively). Orange represents the male population in all figures. Circle dots represent FSLs treated with S-ketamine, triangle dots represent FSLs treated with saline, and square dots represent FRLs treated with saline. (B) Locomotor activity (velocity, TDM, and TSC) in female FSLs and FRLs across the estrus cycle. Circle dots represent FSLs treated with S-ketamine, triangle dots represent FSLs treated with saline, and square dots represent FRLs treated with saline. (C) Locomotor activity (velocity, TDM, and TSC) in female FSLs and FRLs across proestrus cycle. Circle dots represent FSLs treated with S-ketamine, triangle dots represent FSLs treated with saline, and square dots represent FRLs treated with saline. (D) Locomotor activity (velocity, TDM, and TSC) in female FSLs and FRLs across diestrus cycle. Circle dots represent FSLs treated with S-ketamine, triangle dots represent FSLs treated with saline, and square dots represent FRLs treated with saline.
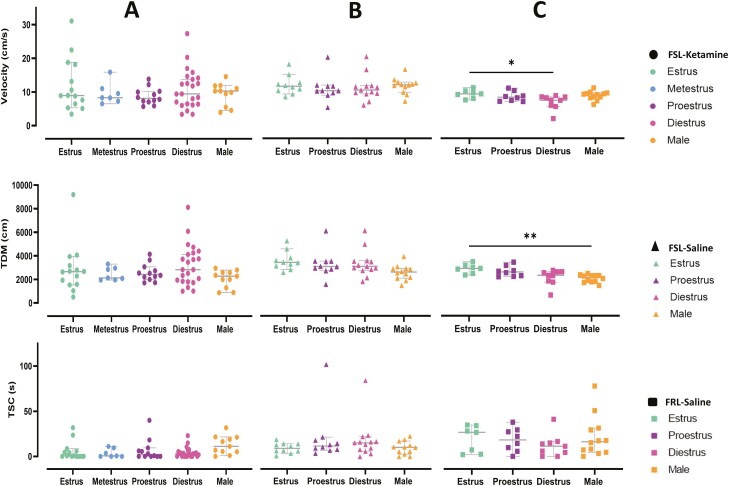
Figure 3.
Scatter plots on locomotor activity (velocity, total distance moved (TDM), and time spent in center [TSC]) comparing the effect of S-ketamine between different sexes and across different estrous cycles in females in different populations (S-ketamine–treated Flinders Sensitive Line rats [FSLs], saline-treated FSLs, and saline-treated Flinders Resistant Line rats [FRLs]). Circle dots represent FSLs treated with S-ketamine, triangle dots represent FSLs treated with saline, and square dots represent FRLs treated with saline. Orange represents males, green estrus, blue metestrus, purple proestrus, and pink represents diestrus. Same animals are included in the analysis.
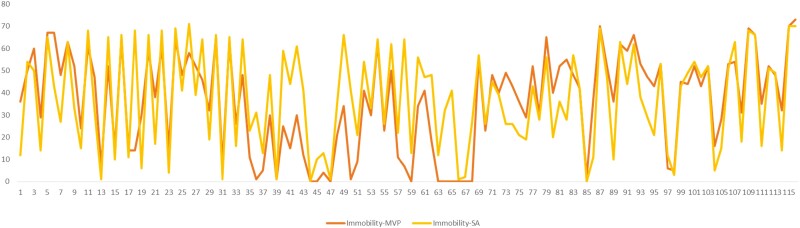
Comparison of Scoring
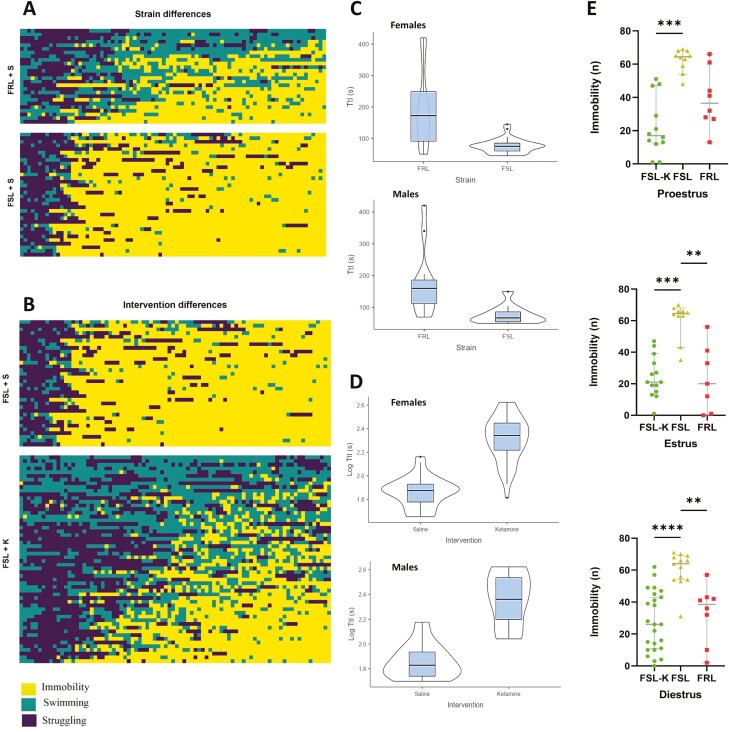
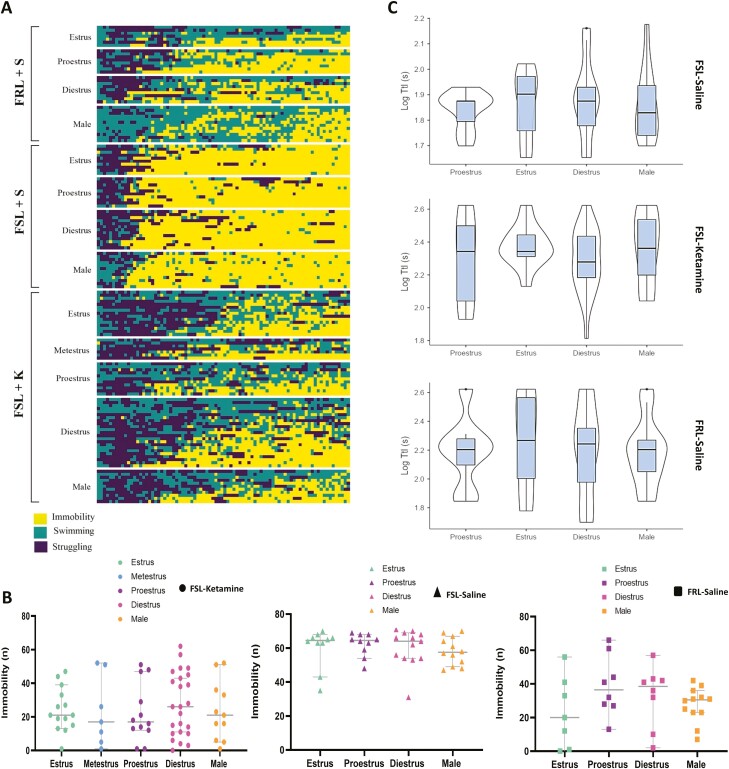
Frequency/time of immobility, struggling, and swimming were scored by 2 independent researchers and the scoring pattern compared. The scorers followed the same scoring pattern (Figure 4). The swimming, immobility, and struggling patterns of each animal are depicted in Figures 5A–B and 6A.
Figure 4.
Comparison of immobility scoring pattern by 2 independent scorers using the same method (5-second time bins).
Figure 5.
(A) A heatmap showing the strain difference among Flinders Sensitive and Resistant Line rats (FSLs and FRLs, respectively) in terms of behavioral despair by depicting the immobility, struggling, and swimming pattern. Yellow represents immobility, green represents swimming, and dark purple represents struggling. (B) A heatmap exhibiting the acute antidepressant effect of S-ketamine compared with saline in FSLs relying on behavioral despair through an illustration of the immobility, struggling, and swimming patterns. Yellow represents immobility, green represents swimming, and dark purple represents struggling. (C–D) Violin plots depicting comparisons of time to immobility (TtI) in different strains and across different sexes and interventions. (E) Strain difference between FSLs and FRLs and the acute antidepressant effect of S-ketamine across different estrous cycles. Circle dots represent FSLs treated with S-ketamine, triangle dots represent FSLs treated with saline, and square dots represent FRLs treated with saline. The y-axis is the frequency of immobility (n).
Figure 6.
(A) A comparison of immobility, struggling, and swimming patterns across strains, interventions, sexes, and estrous cycles at a glance. Yellow represents immobility, green represents swimming, and dark purple represents struggling. (B) Scatter plots of comparisons of immobility frequency (behavioral despair) between different sexes and across different estrous cycles in females. Circle dots represent Flinders Sensitive Line rats (FSLs) treated with S-ketamine, triangle dots represent FSLs treated with saline, and square dots represent Flinders Resistant Line rats (FRLs) treated with saline. Orange represents males, green estrus, blue metestrus, purple proestrus, and pink represents diestrus. The y-axis is the frequency of immobility (n). Same animals are included in the analysis. (C) Violin plots depicting comparisons of time to immobility (TtI) in different strains, sexes, estrous cycles, and interventions.
Assessment of Depressive-Like Behaviors Based on Despair
A strain difference (P < .01) between FSLs and FRLs was observed for immobility between male animals and females at all different estrous cycles except proestrus (P = .0692) (Figure 5A and E). Administration of a single subanesthetic dose of S-ketamine (20 mg/kg, i.p.) 1 hour before the behavioral test significantly reduced immobility time in the FST (P < .001 [estrus and proestrus], P < .0001 [diestrus]) (Figure 5B and E). No significant difference in the acute antidepressant response to ketamine was observed between genetically depressed male and female rats within our study power (P = .9673) (Figure 6B). Given the power of our study, we could not detect any significant difference in ketamine’s rapid antidepressant effect among the estrous cycles (estrus, metestrus, proestrus, diestrus) (P = .9673) (Figure 6B). No baseline differences in immobility were observed across estrous cycles or between sexes (P = .8132) (Figure 6B). S-ketamine–treated animals demonstrated more heterogenous immobility scores and showed a higher range of within-group variation, while the saline-treated group and nondepressed control counterparts were more homogenous (for a perspective on the immobility, struggling, and swimming patterns as well as the impact of the intervention, sex, and estrous cycles, see Figure 6A) [For the effects of treatment and estrous cycles/sex in the FSLs analyzed by 2-way ANOVA, please refer to the Supplementary Materials].
Time to Immobility (TtI)
TtI was defined as the time required to reach a period of 2 consecutive 5-second immobility bins. TtI was significantly lower in FSLs compared with FRLs in both male and female rats (P < .001, P < .001, respectively) (Figure 6C), and treatment with S-ketamine significantly enhanced TtI in both sexes (P < .001) (Figure 6C). The estrous cycle did not influence TtI in FRLs (P = .958), FSLs receiving saline, (P = .894), or FSLs treated with S-ketamine (P = .378) (Figure 6C).
DISCUSSION
In the present study, first, we confirmed previous studies showing that S-ketamine has antidepressant-like effects in FSLs when administered at 20 mg/kg i.p. (Silote et al., 2021). We chose this subanesthetic dose of S-ketamine as we could observe robust and consistent reproducible antidepressant-like effect in the FSLs. We aimed to investigate the potential impact of sex and estrous cycle on this phenomenon, but in the current study we could not detect any sex-specific or estrous-cycle–specific differences in antidepressant-like responses to an acute subanesthetic dose of S-ketamine in this genetic animal model of depression. S-ketamine’s rapid-acting antidepressant action (administered at 20 mg/kg, i.p.) under physiological conditions (freely cycling females) is not affected by the fluctuation of estrogen or progesterone in the FSL animal model of depression. We conclude that physiological oscillations in the levels of estrogen and progesterone across various estrous cycles neither amplify nor diminish the behavioral antidepressant-like effect of S-ketamine in the FSL animal model of depression. In addition, such fluctuations of ovarian sex hormones do not predispose female animals to exhibit enhanced or reduced depressive-like behaviors.
Increased immobility observed in the FST cannot be attributed to a possible difference in the basal locomotor activity as the animals failed to show any significant differences in TDM. Moreover, similarity in thigmotactic measures refutes the presence of anxiety-like behaviors as a confounding variable to the FST results. Most preclinical studies, however, have used lower doses of different formulations of ketamine ranging from 1.5 to 10 mg/kg. Since, unlike such studies, we could not detect any significant differences in despair and depression-like phenotype at the baseline, the probable impact of the chosen dose on this observation seems negligible, consistent with our previous report (Eskelund et al., 2016). In line with our findings, Chang and colleagues did not observe any difference between sexes in terms of acute antidepressant response to low-dose R-ketamine (3, 10 mg/kg) in a lipopolysaccharide ( LPS)-induced animal model of depression. However, they did not consider the estrous cycle (Chang et al., 2018). Using different ketamine enantiomers, it appears that the stressed (depression-like) phenotype of the animals has played a crucial role in the lack of behavioral antidepressant-like response to ketamine in the different sexes. Our results align with the only human trial reporting treatment response between men and women, both premenopausal and postmenopausal (Freeman et al., 2019).
Nevertheless, some reports indicate the susceptibility of female rats to respond to lower doses of racemic ketamine (2.5 mg/kg), which was abolished after ovariectomizing the female rats (Carrier and Kabbaj, 2013). Dossat and colleagues (Dossat et al., 2018) have moved a step further to explore whether this effect is estrous cycle dependent. C57BL6 male and female mice responded to 3 mg/kg racemic ketamine one-half an hour after injection, while only female mice in the proestrus phase exhibited an antidepressant-like effect of 1.5 mg/kg racemic ketamine. After administering estrogen receptor agonists, female mice in the diestrus phase responded to 1.5 mg/kg ketamine (Dossat et al., 2018). However, it is noteworthy to mention they failed to detect any acute sex-related different behavioral sensitivity to ketamine (Dossat et al., 2018).
Zanos and colleagues have also reported a greater chronic antidepressant potency of racemic ketamine in female C57BL/6J mice undergoing chronic social defeat stress and highlighted a threefold higher level of racemic hydroxynorketamine in the brains of female mice after 24 hours compared with male mice (Zanos et al., 2016). However, male and female mice responded to 10 and 30 mg/kg of ketamine (Zanos et al., 2016).
Ovarian sex hormones, especially estrogen, alter the CYP enzymes responsible for the metabolism of ketamine (CYP2B6, CYP2C9, and CYP3A4), thereby potentially influencing the effects of ketamine. Nevertheless, we have only investigated the acute antidepressant effect of S-ketamine, and this matter may need to be considered when ketamine is chronically administered in preclinical settings. A study reports higher plasma and brain concentrations of racemic ketamine and its metabolite, norketamine, in female compared with male Sprague Dawley rats one-half an hour after the treatment (Saland and Kabbaj, 2018). The effect of circulating ovarian sex hormones, and therefore estrous cycles, however, was reported to be insignificant (Saland and Kabbaj, 2018). On the contrary, Chang and colleagues did not observe any sex-specific differences in the plasma and brain levels of R-ketamine and its metabolites in female and male LPS-treated mice (Chang et al., 2018). The fact that the doses administered, ketamine enantiomers, and the depression-like phenotype of the animals differed among studies could affect the outcome and be possible sources of discrepancy, but the extent of the influence exerted by each parameter calls for further research.
It is worth mentioning that although FSLs have demonstrated face, construct, and predictive validity as a rodent model of depression, they do not mimic all symptoms of depression (Overstreet and Wegener, 2013). The FSLs were also introduced by selective breeding, and therefore, potential caveats in regard to use of selectively bred animal models should be kept in mind.
In connection to the FST, despite its popularity in screening of antidepressants and evaluation of depressive-like behaviors, concerns have been raised. Researchers should therefore be mindful of false-negative and false-positive results. It is also based on a stress paradigm, and a combination of different behavioral tests, wherever possible, can increase the robustness of the data (Armario, 2021).
In conclusion, we have demonstrated that the acute antidepressant-like effect of 20 mg/kg S-ketamine is sex and estrous cycle independent in the FSL model of depression and was not confounded by altered locomotion and anxiety. We also conclude that ovarian sex hormones do not leave a distinct impact on depressive-like behaviors in either FSLs or FRLs. Such an endeavor highlights the importance of striving to disaggregate findings of both clinical and preclinical neuropsychiatric research, especially depressive disorders, by sex and different states of hormonal contents to explore possible diversities in responses to treatments.
Supplementary Materials
Information on the study characteristics according to the ARRIVE 2.0 guidelines and a 2-way ANOVA of the effects of treatment and sex/estrous cycle in the FSLs are available at IJNP online.
Acknowledgments
S.A. thanks Amanda Eskelund for providing useful suggestions regarding the experimental design.
This work was supported by Aarhus University (PhD scholarship).
Contributor Information
Shokouh Arjmand, Translational Neuropsychiatry Unit, Department of Clinical Medicine, Aarhus University, Aarhus, Denmark; Department of Biomedicine, Aarhus University, Aarhus, Denmark.
Marie Vadstrup Pedersen, Translational Neuropsychiatry Unit, Department of Clinical Medicine, Aarhus University, Aarhus, Denmark.
Nicole R Silva, Department of Biomedicine, Aarhus University, Aarhus, Denmark; Translational Neuropsychiatry Unit, Department of Clinical Medicine, Aarhus University, Aarhus, Denmark.
Anne M Landau, Translational Neuropsychiatry Unit, Department of Clinical Medicine, Aarhus University, Aarhus, Denmark; Department of Nuclear Medicine and PET Center, Department of Clinical Medicine, Aarhus University and Hospital, Aarhus, Denmark.
Sâmia Joca, Department of Biomedicine, Aarhus University, Aarhus, Denmark.
Gregers Wegener, Translational Neuropsychiatry Unit, Department of Clinical Medicine, Aarhus University, Aarhus, Denmark.
Interest Statement
None.
Data Availability
The raw data underlying this article will be shared on reasonable request to the corresponding author.
References
- Ajayi AF, Akhigbe RE (2020) Staging of the estrous cycle and induction of estrus in experimental rodents: an update. Fertil Res Pract 61:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armario A (2021) The forced swim test: historical, conceptual and methodological considerations and its relationship with individual behavioral traits. Neurosci Biobehav Rev 128:74–86. [DOI] [PubMed] [Google Scholar]
- Carrier N, Kabbaj M (2013) Sex differences in the antidepressant-like effects of ketamine. Neuropharmacology 70:27–34. [DOI] [PubMed] [Google Scholar]
- Chang L, Toki H, Qu Y, Fujita Y, Mizuno-Yasuhira A, Yamaguchi JI, Chaki S, Hashimoto K (2018) No sex-specific differences in the acute antidepressant actions of (R)-ketamine in an inflammation model. Int J Neuropsychopharmacol 21:932–937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyle CM, Laws KR (2015) The use of ketamine as an antidepressant: a systematic review and meta-analysis. Hum Psychopharmacol Clin Exp 30:152–163. [DOI] [PubMed] [Google Scholar]
- Derntl B, Hornung J, Sen ZD, Colic L, Li M, Walter M (2019) Interaction of sex and age on the dissociative effects of ketamine action in young healthy participants. Front Neurosci 13:616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dossat AM, Wright KN, Strong CE, Kabbaj M (2018) Behavioral and biochemical sensitivity to low doses of ketamine: influence of estrous cycle in C57BL/6 mice. Neuropharmacology 130:30–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskelund A, Budac DP, Sanchez C, Elfving B, Wegener G (2016) Female Flinders Sensitive Line rats show estrous cycle-independent depression-like behavior and altered tryptophan metabolism. Neuroscience 329:337–348. [DOI] [PubMed] [Google Scholar]
- Franceschelli A, Sens J, Herchick S, Thelen C, Pitychoutis PM (2015) Sex differences in the rapid and the sustained antidepressant-like effects of ketamine in stress-naïve and “depressed” mice exposed to chronic mild stress. Neuroscience 290:49–60. [DOI] [PubMed] [Google Scholar]
- Freeman MP, Papakostas GI, Hoeppner B, Mazzone E, Judge H, Cusin C, Mathew S, Sanacora G, Iosifescu D, DeBattista C, Trivedi MH, Fava M (2019) Sex differences in response to ketamine as a rapidly acting intervention for treatment resistant depression. J Psychiatr Res 110:166–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasin DS, Sarvet AL, Meyers JL, Saha TD, Ruan WJ, Stohl M, Grant BF (2018) Epidemiology of adult DSM-5 major depressive disorder and its specifiers in the United States. JAMA Psychiatry 75:336–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keers R, Aitchison KJ (2010) Gender differences in antidepressant drug response. Int Rev Psychiatry 22:485–500. [DOI] [PubMed] [Google Scholar]
- Khan A, Brodhead AE, Schwartz KA, Kolts RL, Brown WA (2005) Sex differences in antidepressant response in recent antidepressant clinical trials. J Clin Psychopharmacol 25:318–324. [DOI] [PubMed] [Google Scholar]
- Otte C, Gold SM, Penninx BW, Pariante CM, Etkin A, Fava M, Mohr DC, Schatzberg AF (2016) Major depressive disorder. Nat Rev Dis Prim 21:1–20. [DOI] [PubMed] [Google Scholar]
- Overstreet DH, Friedman E, Mathé AA, Yadid G (2005) The Flinders Sensitive Line rat: a selectively bred putative animal model of depression. Neurosci Biobehav Rev 29:739–759. [DOI] [PubMed] [Google Scholar]
- Overstreet DH, Wegener G (2013) The flinders sensitive line rat model of depression-25 years and still producing. Pharmacol Rev 65:143–155. [DOI] [PubMed] [Google Scholar]
- Ponton E, Turecki G, Nagy C, Blvd L (2022) Sex differences in the behavioral, molecular, and structural effects of ketamine treatment in depression. Int J Neuropsychopharmacol 25:75–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saland SK, Kabbaj M (2018) Sex differences in the pharmacokinetics of low-dose ketamine in plasma and brain of male and female rats. J Pharmacol Exp Ther 367:393–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saland SK, Wilczak K, Voss E, Lam TKT, Kabbaj M (2022) Sex- and estrous-cycle dependent dorsal hippocampal phosphoproteomic changes induced by low-dose ketamine. Sci Rep 121:1–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar A, Kabbaj M (2016) Sex differences in effects of ketamine on behavior, spine density, and synaptic proteins in socially isolated rats. Biol Psychiatry 80:448–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoepfer KJ, Strong CE, Saland SK, Wright KN, Kabbaj M (2019) Sex- and dose-dependent abuse liability of repeated subanesthetic ketamine in rats. Physiol Behav 203:60–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silote GP, Gatto MC, Eskelund A, Guimarães FS, Wegener G, Joca SRL (2021) Strain-, sex-, and time-dependent antidepressant-like effects of cannabidiol. Pharmaceuticals 14:1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanos P, et al. (2016) NMDAR inhibition-independent antidepressant actions of ketamine metabolites. Nature 533:481–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Lu C, Zhang J, Hu L, Song H, Li J, Kang L (2013) Gender differences in abusers of amphetamine-type stimulants and ketamine in southwestern China. Addict Behav 38:1424–1430. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The raw data underlying this article will be shared on reasonable request to the corresponding author.