Abstract
Purpose:
To evaluate the use of Scheimpflug tomography in corneal densitometry (CD) in comparing the stages of keratoconic eyes.
Methods:
Keratoconic (KC) corneas (stages 1–3 classified according to the topographic parameters) were examined using the Scheimpflug tomographer (Pentacam, Oculus) using the CD software. CD was measured over three different depths (anterior stromal layer [120 μm], posterior stromal layer [60 μm], and middle stromal layer between these two layers), and concentric annular zones (0.0 to 2.0, 2.0 to 6.0, 6.0 to 10.0, and 10.0 to 12.0 mm diameter area).
Results:
The study participants were divided into three groups: keratoconus (KC) stage 1 (KC1) with 64 participants, keratoconus stage 2 (KC2) with 29 participants, and keratoconus stage 3 (KC3) with 36 participants. Comparing CD of all three layers (anterior, central, and posterior) of the cornea over different circular annuli (0–2, 2–6, 6–10, and 10–12 mm) revealed a significant difference in the 6–10 mm annulus between all groups and in all layers (P = 0.3, 0.2, and 0.2, respectively). Area under curve (AUC) was done. It revealed that the central layer showed the highest specificity (93.8%) in comparing KC1 and KC2, whereas CD in the anterior layer between KC2 and KC3 had the highest specificity (86.2%).
Conclusion:
CD showed increased values in the anterior corneal layer and in the annulus 6–10 mm more than other locations in all stages of KC.
Keywords: Corneal densitometry, keratoconus, Scheimpflug imaging
Keratoconus (KC) is a degenerative noninflammatory eye disease characterized by progressive corneal ectasia with increasing irregular astigmatism, which can be complicated by possible scarring or hydrops.[1] The diagnosis of early KC is considered a challenge and remains a significant area of research. Scheimpflug tomography is the diagnostic method of choice, which is attributed to its increased sensitivity and specificity for KC screening.[2-4] Corneal densitometry (CD) software is implemented in the Scheimpflug corneal tomography system (Pentacam, Oculus), providing numerical values on CD and transparency.[5] It has been implemented in different conditions, such as postrefractive surgery,[6,7] infectious keratitis,[8] corneal dystrophies,[9] corneal graft surgery,[10,11] and evaluating the cross-linking result in patients with KC.[12,13]
KC has been reported to affect corneal transparency. The cornea maintains its transparency through the regular spacing of collagen fibers and its small uniform orthogonal pattern.[14]
KC histopathological changes show disturbance of the balance of corneal extracellular matrix and keratocyte cells leading to apical scarring.[15]
The aim of the present study is to evaluate CD changes with the Scheimpflug tomography system in different stages of KC.
Methods
This study was a retrospective cross-sectional study that included all patients who attended and sought laser refractive surgery at the Center for Corneal and Refractive Surgeries, Sohag, Egypt, between October 2020 and October 2021 and had a diagnosis of KC.
The patients’ records were evaluated. The patients’ data were extracted, including the demographic data (age, sex, and laterality) and clinical data (uncorrected visual acuity [UCVA], best-corrected visual acuity [BCVA] in decimal notion, sphere, cylinder along with slit-lamp biomicroscopic examination). Corneal tomography data were evaluated using the Oculus Pentacam system (Oculus Optikgerate GmbH, Wetzlar, Germany). The recorded corneal tomography findings were keratometry, pachymetry, elevation, as well as tomographic indices and patterns. All patients had their contact lens wear discontinued for at least 3 weeks for rigid contact lenses and 1 week for soft contact lenses before the assessment. They were divided into four groups according to the grading system of the Modified Krumeich Classification of KC.[16]
Exclusion criteria included: previous ocular surgery, corneal opacity, and pellucid marginal degeneration. Patients with pregnancy or connective tissue diseases were also excluded.
The corneal densitometric evaluation was done by documenting changes in corneal transparency through backward light scattering measured by the same device. All measurements were performed in a dark room by one single physician (A.M.).
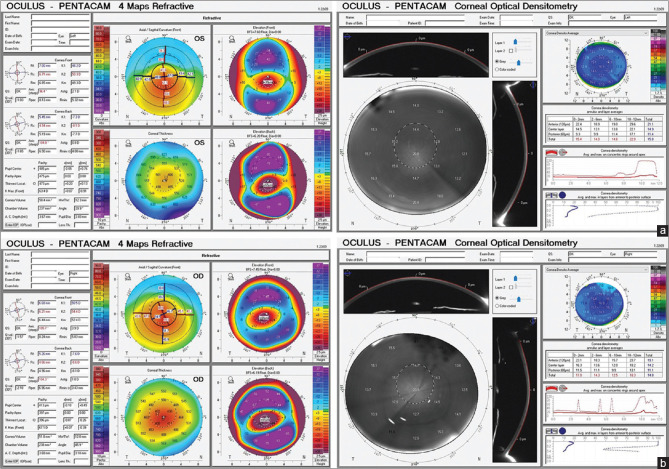
The standardized Scheimpflug densitometry is expressed in gray scale units (GSUs), which evaluates backward light scatter on a scale of 0 (minimum scatter; maximum transparency) to 100 (maximum scatter; minimum transparency). Device software allows the operator to analyze the CD over a 12-mm corneal area at various concentric zones (0.0 to 2.0, 2.0 to 6.0, 6.0 to 10.0, and 10.0 to 12.0 mm) and stromal depths of the cornea (anterior stromal layer [120 μm], posterior stromal layer [60 mm], and middle stromal layer between these two layers) [Fig. 1].
Figure 1.
(a) Four Map printout Corneal Topography of a case of KC grade 2 (Left), Corneal Optical Densitometry printout of the same eye (Right). (b) 4 Map printout Corneal Topography of a case of KC grade 3 (Left), Corneal Optical Densitometry printout of the same eye (Right)
The study adhered to the tenants of the Helsinki Declaration with the approval of the Ethical Committee of the local health authorities under institutional review board (IBR) registration number: Soh-Med-22-06-20.
Statistical analyses
Data were analyzed using STATA version 14.2 (Stata Statistical Software: Release 14.2 College Station, TX: StataCorp LP.). Quantitative data were represented as mean, standard deviation, median, and range. Data were analyzed using the ANOVA test with Bonferroni post hoc if data were normally distributed. When the data were not normally distributed, the Kruskal–Wallis test for the comparison of three or more groups and the Mann–Whitney test was used to compare two groups. Qualitative data were presented as numbers and percentages and compared using the Chi-square test. Roc curve analysis was used to calculate sensitivity, specificity, positive predicted value, and negative predictive value. Pearson’s correlation tests were performed to examine the correlations if the data were normal distribution, and Spearman’s correlation tests were used if the data were not normally distributed. Graphs were produced using Excel or STATA program. P value was considered significant if it was less than 0.05.
Results
Our study included 129 patients (60 males and 69 females) with an average age of 24.5 ± 7 years. The participants were divided into three categories: KC stage 1 (KC1) with 64 participants, KC stage 2 (KC2) with 29 participants, and KC stage 3 (KC3) with 36 participants. All three groups were age- and sex-matched. Their demographic data and topographic characteristics are listed in Table 1. Cones in the KC participants were mostly located paracentral (112 eyes [86.8%]). There was a clinically significant difference in all tomographic parameters between the three groups.
Table 1.
Demographic and corneal topographic parameters in the studied population
| Parameter Mean±SD Median (range) | KC 1 n=64 | KC 2 n=29 | KC 3 n=36 | P | P1 | P2 | P3 |
|---|---|---|---|---|---|---|---|
| Age/year | 25.41±7.34 | 25.38±7.00 | 22.78±6.69 | ||||
| 25 (10:43) | 25 (14:43) | 21.5 (12:36) | 0.19 | 0.80 | 0.08 | 0.18 | |
| Gender | |||||||
| Female | 30 (46.88%) | 17 (58.62%) | 22 (61.11%) | 0.32 | 0.29 | 0.17 | 0.84 |
| Male | 34 (53.13%) | 12 (41.38%) | 14 (38.89%) | ||||
| K flat (D) | 43.26±2.13 43.5 (36.3:48.0) | 46.37±1.56 46.3 (43.5:49) | 52.65±4.80 51.9 (44.6:64.8) | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| K steep (D) | 45.40±1.79 45.65 (40.7:48.9) | 50.62±1.12 50.4 (48.4:52.5) | 57.51±5.28 56.4 (45.1:72.9) | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| K mean (D) | 44.31±1.85 44.3 (39.9:48.4) | 48.39±1.05 48.3 (46.2:50.3) | 55.05±4.70 53.85 (48.7:68.6) | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| Thinnest corneal location (µm) | 480.64±44.29 478.5 (393:575) | 448.55±34.88 448 (385:551) | 402.06±64.20 412.5 (132:527) | <0.0001 | 0.01 | <0.0001 | 0.001 |
| Pachy apex (µm) | 489.95±44.86 489.5 (400:581) | 458.03±35.86 457 (393:578) | 412.61±65.86 420 (143:534) | <0.0001 | 0.02 | <0.0001 | 0.001 |
| K max (D) | 49.05±3.48 48.55 (44.3:59.9) | 55.00±3.74 54.8 (42.6:63.4) | 66.68±8.68 64 (55:88) | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| Keratometric astigmatism (D) | 1.85±1.51 1.5 (0:8) | 3.64±2.10 4.3 (0.2:8.4) | 4.65±2.61 4.6 (0.7:9.3) | 0.0001 | 0.0001 | 0.0001 | 0.13 |
| Back corneal elevation (µm) | 36.78±21.38 33 (8:128) | 53.86±21.84 48 (32:147) | 44.72±16.61 39 (15:83) | 0.0001 | 0.0001 | 0.01 | 0.03 |
| Front corneal elevation (µm) | 18.48±13.66 14.5 (3:68) | 26.52±14.16 25 (12:83) | 16.82±7.79 15.1 (12.2:60.4) | 0.0001 | 0.0004 | 0.55 | 0.0001 |
| Corneal densitometric average | 14.72±2.01 14.1 (11.8:23.1) | 14.81±1.55 14.5 (11.4:18.0) | 16.82±7.79 15.1 (12.2:60.4) | 0.03 | 0.41 | 0.01 | 0.12 |
P compared in the three groups, P1 compared KC 1 and KC 2, P2 compared KC 1 and KC 3, P3 compared KC 2 and KC 3. KC, keratoconus
Corneal densitometry
Comparing the CD of all three layers (anterior, central, and posterior) of the cornea over different circular annuli (0–2, 2–6, 6–10, and 10–12 mm) revealed a significant difference in the 6–10 mm annulus between all groups and in all layers (P = 0.03, 0.02, 0.02, respectively). On comparing KC1 and KC3, there was also a significant difference in the 6–10 annulus in all layers (P = 0.03, 0.02, 0.02, respectively). In addition, there is a more significant difference in the anterior corneal layer in the 0–2 annulus between all groups and between KC1 and KC3 (P = 0.005, 0.006) [Table 2].
Table 2.
Corneal densitometry parameters in all layers
| Parameter Mean±SD Median (range) | KC 1 n=64 | KC 2 n=29 | KC 3 n=36 | P | P1 | P2 | P3 |
|---|---|---|---|---|---|---|---|
| A. Corneal densitometry in the anterior corneal layer in the studied population | |||||||
| CD 0–2 A | 18.59±3.16 17.7 (14.8:33.8) | 18.7±2.78 18.3 (14.8:33.8) | 20.57±2.85 19.65 (17.8:32.4) | 0.005 | 1.00 | 0.006 | 0.04 |
| CD 2–6 A | 17.15±3.63 16 (13.7:32) | 16.18±2.04 16 (11.9:21) | 17.47±3.82 16.35 (14.5:37.5) | 0.29 | 0.61 | 1.00 | 0.40 |
| CD 6–10 A | 18.23±6.70 16.1 (12.6:46.5) | 16.48±3.95 15.7 (11.8:27.7) | 15.38±2.79 14.7 (11.4:24.1) | 0.03 | 0.43 | 0.03 | 1.00 |
| CD 10–12 A | 31.84±9.43 31.2 (15.2:58.0) | 26.99±9.37 26.4 (10.7:51.4) | 28.15±10.90 26.25 (15.1:72.7) | 0.051 | 0.09 | 0.22 | 1.00 |
| Total A | 19.95±4.28 19.4 (12.2:37.5) | 18.38±2.80 18.0 (12.1:23.7) | 18.68±3.07 18.15 (15.0:31.4) | 0.09 | 0.17 | 0.30 | 1.00 |
| B. Corneal densitometry in the central layer of the cornea in the studied population | |||||||
| CD 0–2 C | 13.88±1.85 13.45 (11.1:22) | 13.48±1.32 13.3 (11.5:16.5) | 13.94±1.66 13.94 (11:19) | 0.50 | 0.89 | 1.00 | 0.86 |
| CD 2–6 C | 12.58±2.17 11.95 (10.1:20.7) | 11.79±1.09 11.7 (9.9:14.5) | 12.13±1.27 11.8 (10.7:17.1) | 0.12 | 0.14 | 0.68 | 1.00 |
| CD 6–10 C | 13.53±4.43 12.1 (9.8:33.3) | 12.22±2.26 11.6 (9.4:17.8) | 11.54±1.73 11.05 (9.3:16.4) | 0.02 | 0.27 | 0.02 | 1.00 |
| CD 10–12 C | 20.56±4.60 20.1 (12:32.2) | 18.83±5.16 18.2 (8.5:32.4) | 19.61±5.65 19.25 (11:36.1) | 0.28 | 0.38 | 1.00 | 1.00 |
| Total C | 14.31±2.42 13.65 (11.4:24.8) | 13.30±1.53 13.1 (10.2:16.1) | 13.13±1.44 12.85 (11:17.7) | 0.008 | 0.08 | 0.02 | 1.00 |
| C. Corneal densitometry in the posterior layer of the cornea in the studied population | |||||||
| CD 0-2 P | 10.47±0.95 10.35 (8.7:13.4) | 10.31±0.84 10.2 (8.9:12.3) | 11.05±6.70 10.2 (6.3:43.6) | 0.57 | 1.00 | 1.00 | 1.00 |
| CD 2-6 P | 9.85±1.28 9.7 (8:16) | 9.56±0.69 9.4 (8.4:11.7) | 10.21±2.32 9.8 (8.5:23) | 0.25 | 1.00 | 0.80 | 0.30 |
| CD 6-10 P | 10.69±2.37 10 (8.2:21.8) | 10.16±1.52 9.6 (7.9:13.5) | 9.58±1.13 9.4 (7.8:13) | 0.02 | 0.65 | 0.02 | 0.68 |
| CD 10-12 P | 14.19±2.67 14.45 (9.5:21.2) | 14.23±3.05 14.4 (7.8:22.6) | 14.62±3.20 14.4 (7.4:20.8) | 0.76 | 1.00 | 1.00 | 1.00 |
| Total P | 10.89±1.31 10.5 (8.8:16.3) | 10.6±1.03 10.6 (8.8:12.8) | 10.67±1.86 10.3 (8.9:20.1) | 0.59 | 1.00 | 1.00 | 1.00 |
P compared in the three groups, P1 compared KC 1 and KC 2, P2 compared KC 1 and KC 3, P3 compared KC 2 and KC 3. KC, keratoconus
On evaluating the total central corneal layer, there was a clinically significant difference between all groups; KC1 and KC2; and KC1 and KC3; KC3 (P = 0.083, 0.08, 0.02, respectively). There was a significant difference between KC2 and KC3 groups in the central 0–2 annulus in the anterior layer with P = 0.004 [Fig. 1a and b].
For detecting the cutoff point of the values of CD with the highest sensitivity and specificity [Table 3], AUR was done, revealing that the central layer showed the highest specificity (93.8%) on comparing KC1 and KC2. The CD in the anterior layer between KC2 and KC3 had the highest specificity (86.2%) [Fig. 2a and b].
Table 3.
Cutoff points between different groups
| Parameter | Cutoff point | AUC (95% CI) | Sensitivity | Specificity |
|---|---|---|---|---|
| A. Cutoff points of different corneal density parameters to differentiate between KC 1 and KC 2 | ||||
| Total A | ≤19.2 | 0.60 (0.50:0.70) | 69.0 | 51.6 |
| Total C | ≤12.2 | 0.62 (0.51:0.71) | 31.0 | 93.8 |
| Total P | ≤10 | 0.55 (0.44:0.65) | 37.9 | 78.1 |
| Total corneal densitometry | ≤12.9 | 0.60 (0.49:0.70) | 31.0 | 93.8 |
| B. Cutoff points of different corneal density parameters to differentiate between KC 2 and KC 3 | ||||
| Total A | >21.4 | 0.51 (0.39:0.64) | 5.56 | 86.21 |
| Total C | ≤13.5 | 0.54 (0.41:0.66) | 72.22 | 41.38 |
| Total P | ≤10.4 | 0.54 (0.42:0.67) | 58.3 | 55.2 |
| Total | ≤15.6 | 0.51 (0.39:0.64) | 86.1 | 24.1 |
AUC, area under the curve; KC, keratoconus
Figure 2.
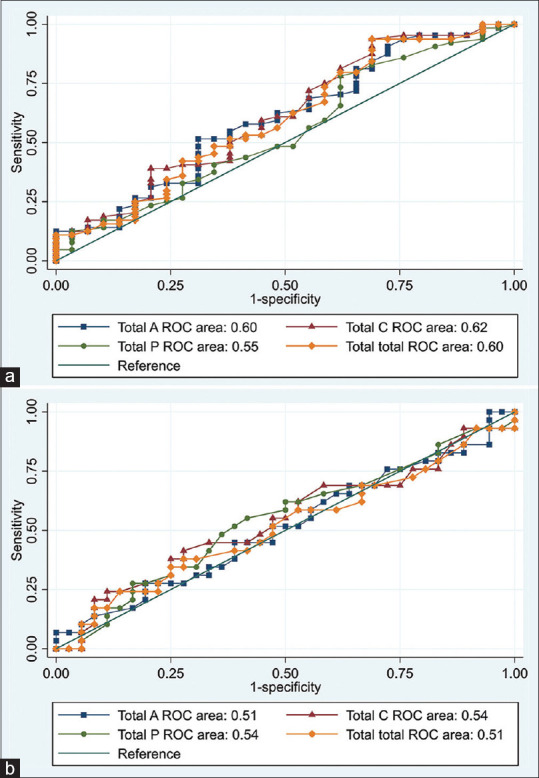
(a) Receiver operating characteristic (ROC) curve of different corneal density parameters to differentiate between KC 1 and KC 2. (b) ROC curve of different corneal density parameters to differentiate between KC 2 and KC 3
Running correlation studies on CD in all layers and variable parameters, the KC1 group did not reveal significant correlations with any of the following: maximum K, keratometric astigmatism, thinnest corneal location, and anterior or posterior corneal elevation.
Yet, in the group of the KC2, a significant correlation was found between K max and anterior corneal elevation, on the one hand, and total CD, on the other hand [Fig. 3a]. In the KC3 group, a significant correlation was found between total CD, on one hand, and thinnest corneal thickness and the anterior corneal elevation, on the other hand [Fig. 3b] [Table 4].
Figure 3.
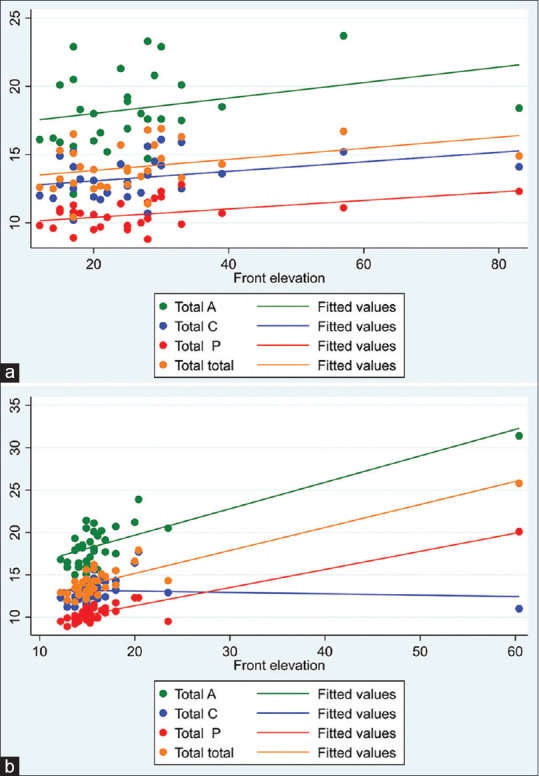
(a) Correlation between front elevation and corneal density parameters in KC 2. (b) Correlation between front corneal elevation and corneal density parameters in KC 3
Table 4.
Correlation between different parameters and corneal density parameters in KC 2
| Parameter | K max | Keratometric astigmatism | Thinnest corneal location | Back corneal elevation | Front corneal elevation | |||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|
||||||
| r | P | r | P | r | P | r | P | r | P | |
| Total A | 0.37 | 0.049 | −0.34 | 0.07 | 0.18 | 0.36 | 0.13 | 0.50 | 0.38 | 0.04 |
| Total C | 0.36 | 0.052 | −0.34 | 0.07 | 0.09 | 0.63 | 0.13 | 0.50 | 0.40 | 0.03 |
| Total P | 0.35 | 0.06 | −0.19 | 0.33 | 0.07 | 0.73 | 0.14 | 0.46 | 0.38 | 0.04 |
| Total total | 0.39 | 0.04 | −0.34 | 0.07 | 0.13 | 0.49 | 0.17 | 0.37 | 0.43 | 0.02 |
| A. Correlation between different parameters and corneal density parameters in KC 3 | ||||||||||
| Total A | 0.11 | 0.53 | −0.17 | 0.32 | −0.42 | 0.01 | 0.07 | 0.68 | 0.53 | 0.001 |
| Total C | −0.25 | 0.14 | −0.12 | 0.49 | 0.25 | 0.15 | −0.17 | 0.31 | 0.47 | 0.004 |
| Total P | 0.24 | 0.15 | −0.22 | 0.19 | −0.66 | <0.0001 | −0.08 | 0.66 | 0.61 | 0.0001 |
| Total | 0.18 | 0.29 | −0.14 | 0.41 | −0.55 | 0.0005 | 0.04 | 0.82 | 0.63 | <0.0001 |
KC, keratoconus
Discussion
With the vast advent of the Scheimpflug camera use in the refractive surgery domain, diverse uses have evolved, including measuring the back corneal scatter, which is known as CD.[14]
The main advantage of the Scheimpflug camera use in densitometry is the noninvasive ability to collect objective values of corneal clarity over different layers and annuli. It allows accurate follow-ups in corneal diseases and postsurgical comparisons with high repeatability.[17,18]
Healthy corneas were reported to decrease the scatter from anterior to posterior layers[5] as our studied KC population.
KC can compromise corneal clarity due to disarrangement in corneal histology caused by KC, thus altering densitometry parameters.[19]
Garzon et al.[5] did not report any correlation between CD with keratometry in healthy corneas. One of the early reports that reported change in densitometry in keratoconic eyes was in 2014 by Lopes et al.[20]
Yet they did not correlate their findings with topometric values. Our results revealed a clinically significant correlation between maximum keratometry and anterior corneal elevation in the KC2 group. In the KC3 group, CD correlated with the thinnest corneal thickness and anterior corneal elevation. Thus, the higher the anterior corneal elevation is, the more densitometric changes occur in all stages.
Shen et al.[21] results revealed that CD values of the anterior (0–2 and 2–6 mm zones) significantly correlated with the severity of KC. Yet a study by Ahsounah et al.[22] reported changes in all layers of the cornea as regards sensitometry in all groups when compared with a control group.
In the current study, most of the increased values in CD were reported in the annulus 6–10 mm as most of the KC cones were paracentral. Also, all differences were most significant in the anterior layer. The posterior corneal layer did not show any difference between the stages of KC, playing no role in discrimination.
These results can be related to what was reported in the KC pathology; the anterior cornea is affected early in the disease course as the basal epithelial cell layer shows the first changes leading to its disappearance and thinning of epithelial layer[23] along with breaks in the Bowman layer and thickened sub-basal nerve plexus.[15,24] CD values showed irrelevant numbers in the periphery 10–12 mm, which can be explained that the pathology is far from this zone.
One point that needs to be taken into consideration is that the repeatability of the densitometry by the Pentacam worsens by the severity of the disease as was discussed by Kreps et al.[25]
We are aware that our study has limitations, such as not including a healthy control group. Yet, our concern was to document the difference between KC groups. In addition, we aimed at finding out the CD correlations with tomographic parameters along with the cutoff points in each layer that could be used with ease when knowing exact figures (by detecting the specificity and sensitivity levels). Further studies are required between KC suspects and diagnosed KC groups, which would add substantial data to the topic.
Conclusion
In conclusion, CD over different annuli and through the corneal layers is an evolving, reliable, and accessible tool in grading KC with definite correlations with anterior corneal elevation, keratometric astigmatism, and thinnest corneal thickness according to the staging of the disease. Further studies are warranted before implementing CD as an anticipated tool for grading KC.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Sykakis E, Karim R, Evans JR, Bunce C, Amissah-Arthur KN, Patwary S, et al. Corneal collagen cross-linking for treating keratoconus. Cochrane Database Syst Rev. 2015:CD010621. doi: 10.1002/14651858.CD010621.pub2. doi:10.1002/14651858. CD010621.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.De Sanctis U, Loiacono C, Richiardi L, Turco D, Mutani B, Grignolo FM, et al. Sensitivity and specificity of posterior corneal elevation measured by Pentacam in discriminating keratoconus/subclinical keratoconus. Ophthalmology. 2008;115:1534–9. doi: 10.1016/j.ophtha.2008.02.020. [DOI] [PubMed] [Google Scholar]
- 3.Swartz T, Marten L, Wang M. Measuring the cornea:The latest developments in corneal topography. Curr Opin Ophthalmol. 2007;18:325–33. doi: 10.1097/ICU.0b013e3281ca7121. [DOI] [PubMed] [Google Scholar]
- 4.Tejwani S, Shetty R, Kurien M, Dinakaran S, Ghosh A, Sinha Roy A. Biomechanics of the cornea evaluated by spectral analysis of waveforms from ocular response analyzer and Corvis-ST. PLoS One. 2014;9:e97591. doi: 10.1371/journal.pone.0097591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Garzon N, Poyales F, Illarramendi I, Mendicute J, Jáñez Ó, Caro P, et al. Corneal densitometry and its correlation with age, pachymetry, corneal curvature, and refraction. Int Ophthalmol. 2017;37:1263–8. doi: 10.1007/s10792-016-0397-y. [DOI] [PubMed] [Google Scholar]
- 6.Cennamo G, Forte R, Aufiero B, La Rana A. Computerized scheimpflug densitometry as a measure of corneal optical density after excimer laser refractive surgery in myopic eyes. J Cataract Refract Surg. 2011;37:1502–6. doi: 10.1016/j.jcrs.2011.03.037. [DOI] [PubMed] [Google Scholar]
- 7.Rozema JJ, Trau R, Verbruggen KH, Tassignon MJ. Backscattered light from the cornea before and after laser-assisted subepithelial keratectomy for myopia. J Cataract Refract Surg. 2011;37:1648–54. doi: 10.1016/j.jcrs.2011.03.043. [DOI] [PubMed] [Google Scholar]
- 8.Otri AM, Fares U, Al-Aqaba MA, Dua HS. Corneal densitometry as an indicator of corneal health. Ophthalmology. 2012;119:501–8. doi: 10.1016/j.ophtha.2011.08.024. [DOI] [PubMed] [Google Scholar]
- 9.Elflein HM, Hofherr T, Berisha-Ramadani F, Weyer V, Lampe C, Beck M, et al. Measuring corneal clouding in patients suffering from mucopolysaccharidosis with the Pentacam densitometry programme. Br J Ophthalmol. 2013;97:829–33. doi: 10.1136/bjophthalmol-2012-302913. [DOI] [PubMed] [Google Scholar]
- 10.Bhatt UK, Fares U, Rahman I, Said DG, Maharajan SV, Dua HS. Outcomes of deep anterior lamellar keratoplasty following successful and failed 'big bubble'. Br J Ophthalmol. 2012;96:564–9. doi: 10.1136/bjophthalmol-2011-300214. [DOI] [PubMed] [Google Scholar]
- 11.Koh S, Maeda N, Nakagawa T, Nishida K. Quality of vision in eyes after selective lamellar keratoplasty. Cornea. 2012;31(Suppl 1):S45–9. doi: 10.1097/ICO.0b013e318269c9cd. [DOI] [PubMed] [Google Scholar]
- 12.Gutierrez R, Lopez I, Villa-Collar C, Gonzalez-Meijome JM. Corneal transparency after cross-linking for Keratoconus:1-year follow-up. J Refract Surg. 2012;28:781–6. doi: 10.3928/1081597X-20121011-06. [DOI] [PubMed] [Google Scholar]
- 13.Greenstein SA, Fry KL, Bhatt J, Hersh PS. Natural history of corneal haze after collagen cross-linking for keratoconus and corneal ectasia:Scheimpflug and biomicroscopic analysis. J Cataract Refract Surg. 2010;36:2105–14. doi: 10.1016/j.jcrs.2010.06.067. [DOI] [PubMed] [Google Scholar]
- 14.Jester JV, Moller-Pedersen T, Huang J, Sax CM, Kays WT, Cavangh HD, et al. The cellular basis of corneal transparency:Evidence for 'corneal crystallins'. J Cell Sci. 1999;112:613–22. doi: 10.1242/jcs.112.5.613. [DOI] [PubMed] [Google Scholar]
- 15.Rabinowitz YS. Keratoconus. Surv Ophthalmol. 1998;42:297–319. doi: 10.1016/s0039-6257(97)00119-7. [DOI] [PubMed] [Google Scholar]
- 16.Colin J, Velou S. Current surgical options for keratoconus. J Cataract Refract Surg. 2003;29:379–86. doi: 10.1016/s0886-3350(02)01968-5. [DOI] [PubMed] [Google Scholar]
- 17.Smith GT, Brown NA, Shun-Shin GA. Light scatter from the central human cornea. Eye (Lond) 1990;4:584–8. doi: 10.1038/eye.1990.81. [DOI] [PubMed] [Google Scholar]
- 18.Dhubhghaill SN RJ, Jongenelen S, Ruiz Hidalgo I, Zakaria N, Tassignon MJ. Normative values for corneal densitometry analysis by Scheimpflug optical assessment. Invest Ophthalmol Vis Sci. 2014;55:162–8. doi: 10.1167/iovs.13-13236. [DOI] [PubMed] [Google Scholar]
- 19.Rozema JJ KC, Bral N, Tassignon MJ. Changes in forward and backward light scatter in keratoconus resulting from corneal cross-linking. Asia-Pacific J Ophthalmol. 2013;2:5–19. doi: 10.1097/APO.0b013e3182729df0. [DOI] [PubMed] [Google Scholar]
- 20.Lopes B, Ramos I, Ambrósio RJC. Corneal densitometry in keratoconus. Cornea. 2014;33:1282–6. doi: 10.1097/ICO.0000000000000266. [DOI] [PubMed] [Google Scholar]
- 21.Shen Y, Han T, Jhanji V, Shang J, Zhao J, Li M, et al. Correlation between corneal topographic, densitometry, and biomechanical parameters in keratoconus eyes. Transl Vis Sci Technol. 2019;8:12. doi: 10.1167/tvst.8.3.12. doi:10.1167/tvst.8.3.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ahsounah MA, Abou-Samra WA, Khalaf M, Farag RK. Distribution of Corneal Densitometry in Different Grades of Keratoconus. EJO (MOC) 2021;2:91–102. [Google Scholar]
- 23.Mathew JH, Goosey JD, Bergmanson JP. Quantified histopathology of the keratoconic cornea. Optom Vis Sci. 2011;88:988–97. doi: 10.1097/OPX.0b013e31821ffbd4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ucakhan OO KA, Ylmaz N, Ozkan M. In vivo confocal microscopy findings in keratoconus. Eye Contact Lens. 2006;32:183–91. doi: 10.1097/01.icl.0000189038.74139.4a. [DOI] [PubMed] [Google Scholar]
- 25.Kreps EO, Jimenez-Garcia M, Issarti I, Claerhout I, Koppen C, Rozema JJ. Repeatability of the pentacam HR in various grades of keratoconus. Am J Ophthalmol. 2020;219:154–62. doi: 10.1016/j.ajo.2020.06.013. [DOI] [PubMed] [Google Scholar]