Abstract
A 23-year-old patient presented with complaints of redness, pain, photophobia, and blurred vision in the right eye 15 days after she received the third dose of BNT162b2 vaccination. Ocular examination revealed 2+ cellular reactions in the anterior chamber and mutton fat keratic precipitate with no vitritis or retinal alterations. Active uveitis findings regressed with corticosteroid and cycloplegic eye drops. We present a case of unilateral granulomatous anterior uveitis following the BNT162b2 vaccination, with no etiologic factor in uveitis work-up and no previous history of uveitis before vaccination. This report demonstrates a potential causal association of coronavirus disease 2019 (COVID-19) vaccine with granulomatous anterior uveitis.
Keywords: BNT162b2, COVID-19, COVID-19 vaccine, granulomatous anterior uveitis, mutton fat keratic precipitate
Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) is a novel coronavirus that was first identified in Wuhan, central China, and is responsible for the coronavirus disease 2019 (COVID-19) pandemic.[1] The US Food and Drug Administration (FDA) has approved the BNT162b2 (Pfizer-BioNTech) mRNA-based vaccine for the prevention of COVID-19 in persons ≥16 years old. Various local and systemic reactions have been observed following the spread of COVID-19 vaccines.
The local side effects of COVID-19 vaccines include pain, erythema, and swelling[2] and the systemic reactions include myocarditis,[3] vaccine-induced immune thrombotic thrombocytopenia, and cerebral venous sinus thrombosis.[4] Ocular adverse effects of COVID-19 vaccinations include abducens nerve palsy, new-onset Graves’ disease, thrombosis, acute macular neuroretinopathy, central serous retinopathy, multiple evanescent white dot syndrome, and Vogt–Koyanagi–Harada disease reactivation.[5]
Here, we report a case of unilateral granulomatous anterior uveitis following the third dose of the BNT162b2 COVID-19 vaccine.
Case Report
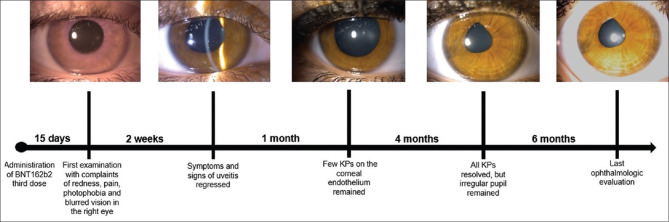
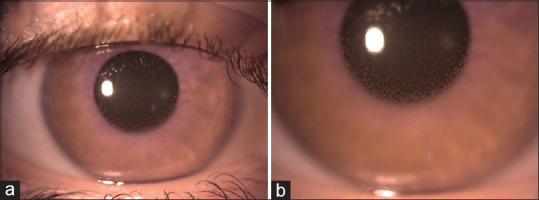
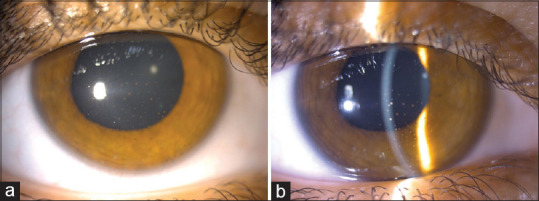
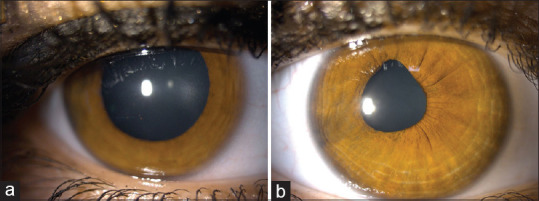
A 23-year-old female patient presented to our clinic with the complaint of redness, pain, photophobia, and blurred vision in the right eye (RE). The patient had no history of uveitis and any systemic disease. She mentioned that she received the third dose of BNT162b2 vaccination 15 days ago. Her best-corrected visual acuity (BCVA) was 20/50 in the RE and 20/20 in the left eye (LE). Slit-lamp examination of the RE revealed 2 + cellular reaction in the anterior chamber (AC) and granulomatous keratic precipitates (KPs) on the central and, mainly, inferior corneal endothelium [Fig. 1a and b]. Fundus examination, spectral domain optical coherence tomography, and fluorescein angiography were normal. The intraocular pressure was 15 mmHg for the RE and 14 mmHg for the LE. Detailed ophthalmologic examination of the LE was normal. Based on the ophthalmologic examination, intermediate and posterior uveitis were excluded and the patient was diagnosed with granulomatous anterior uveitis. Blood analyses were normal. The whole serum serological tests (antibodies against Toxoplasma gondii, human immunodeficiency virus, cytomegalovirus, herpes simplex virus type 1 and 2, varicella zoster virus, syphilis, etc.) were negative. Although the patient did not have any signs of sarcoidosis or tuberculosis, she was refererred to the pulmonary medicine department for evaluation of systemic diseases that may cause granulomatous uveitis. Computed tomography of the thorax was normal, and the tuberculin skin test resulted in 4 mm. Angiotensin-converting enzyme and lysozyme levels were normal. No infectious or noninfectious etiological factors were found to cause granulomatous anterior uveitis. The patient was started on prednisolone acetate eye drops every hour a day, as well as cyclopentolate 1% eye drops twice a day. Cytomegalovirus, herpes simplex virus, varicella zoster virus, Epstein–Barr virus, and coronavirus polymerase chain reaction (PCR) were negative in aqueous humor sampling. One week later, 2 + cellular reactions in AC and granulomatous KP were observed in the RE. Symptoms and signs of uveitis regressed and corneal granulomatous KPs decreased 2 weeks after the presentation [Fig. 2a and b]. There were no signs of active anterior uveitis, and the BCVA was 20/20 in the RE. Topical corticosteroid therapy was tapered and the patient was followed up monthly. Two months later, there were a few KPs on the corneal endothelium and the pupil was irregular [Fig. 3a]. Six months later, there was no KP and active inflammation; nevertheless, the pupil was irregular [Fig. 3b]. The patient was followed for 1 year and showed no relapse; however, the peaked pupil persisted at the last visit [Fig. 4].
Figure 1.
Anterior segment photo of the right eye showing the mutton fat keratic precipitates at presentation (a and b)
Figure 2.
Two weeks later, the mutton fat keratic precipitates regressed significantly (a and b)
Figure 3.
Two months later, a few KPs were present (a). In the last visit, there was no KP on the corneal endothelium; nevertheless, the pupil was irregular (b). KPs = keratic precipitates
Figure 4.
Timeline of the patient with anterior segment photographs during 1-year follow-up
Discussion
Uveitis has been reported after vaccinations for hepatitis B virus, human papillomavirus, influenza virus, bacillus Calmette–Guerin, varicella virus, measles–mumps–rubella, yellow fever, hepatitis A virus, and typhoid.[6] Although the pathogenesis of vaccine-induced uveitis remains unclear, different mechanisms have been hypothesized, including molecular mimicry secondary to a close resemblance between the vaccine peptide fragments and uveal self-peptides, inflammatory damage induced by adjuvants such as aluminum salts, direct viral infection (applicable to live and attenuated vaccines), and delayed-type hypersensitivity with the deposition of immune complexes.[7]
COVID-19 pandemic has accelerated the development of new vaccines. BNT162b2 is a lipid nanoparticle–formulated, nucleoside-modified RNA vaccine that encodes a prefusion stabilized, membrane-anchored SARS-CoV-2 full-length spike protein; nevertheless, it does not contain any live virus and aluminum salts. It has been suggested that the strong antibody and cell-mediated immune response induced by mRNA vaccines to fight SARS-CoV-2 infection may cause inflammatory clinical entities such as uveitis.[8] Adjuvants in mRNA vaccine can stimulate the innate immunity through Toll-Like-Receptors (TLRs) 3, 7, 8, and 9 and components of the inflammasome.[9] The patient presented with acute granulomatous anterior uveitis. At the last visit, the patient had mild iris atrophy and irregular pupil, which are common in viral uveitis. Viral anterior uveitis was initially considered in this patient. However, all serologic tests and aqueous humor PCR analysis of the patient were negative. Furthermore, good response to topical steroid therapy strengthens the possibility of a vaccine-induced autoimmune reaction. Autoimmune reaction in the uveal tissue via molecular mimicry as a result of an adaptive humoral and poly-specific cellular immune response against epitopes may be the potential mechanism for post-vaccine uveitis in this patient. Furthermore, the onset of uveitis after the third dose of the vaccine may be due to the stronger inflammation caused by the accumulated immune complexes.
Vaccine-induced uveitis is often anterior, mild, and responds well to topical corticosteroids.[7] In a recently published multinational case series investigating ocular inflammatory events after COVID-19 vaccines, it was reported that the mean time interval between vaccine and onset of uveitis was 7 days (range: 1–30 days), anterior uveitis cases with non-granulomatous KP were most common, and the number of events reported after the BNT162b2 vaccine was higher.[10,11] In addition, although more than half of the cases have a history of previous ocular inflammation,[10,11] some patients have had ocular inflammatory events for the first time after COVID-19 vaccination.[8,10,11] Reactivation has been reported after COVID-19 vaccines in cases with a previous history of viral uveitis,[12] whereas no case of granulomatous anterior uveitis that occurred for the first time after vaccination has been reported. In this case, 2 weeks between the onset of ocular symptoms and vaccination, no previous history of uveitis, negative test results in aqueous humor sampling, no systemic disease that may cause granulomatous uveitis, and favorable response to topical steroid treatment suggest that mRNA-based COVID-19 vaccination was the cause of anterior uveitis. Bradford–Hill criteria include nine aspects when inferring causality between events: strength, consistency, specificity, temporality, biological gradient, plausibility, coherence, experiment, and analogy.[13] We consider that the most likely contribution of vaccination to the occurrence of uveitis is based on a clear temporal relationship, as in previously reported cases.[8,10] According to Naranjo’s algorithm, the association of the COVID-19 vaccine with uveitis is “possible” in this case (the score of Naranjo’s algorithm was 4).
This report has some limitations. The patient was carefully investigated for granulomatous anterior uveitis and all possible etiologies were ruled out. Moreover, the patient was managed well only with topical steroid therapy. We consider that there is a possible causality between uveitis and vaccine. Since this report is a retrospective case report, it has a lack of possibility to establish cause-to-effect relationships.
Conclusion
This is an unusual case of unilateral granulomatous anterior uveitis after COVID-19 vaccine, with no etiologic factor for granulomatous uveitis and no previous history of uveitis. Based on the temporal association between vaccination and the onset of ocular symptoms, we consider that the vaccination is the probable cause of uveitis by triggering an autoimmune reaction through molecular mimicry. Future studies are needed to explore the association. Physicians should be warned about the possibility of ocular inflammation after COVID-19 vaccination.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Shereen MA, Khan S, Kazmi A, Bashir N, Siddique R. COVID-19 infection:Emergence, transmission, and characteristics of human coronaviruses. J Adv Res. 2020;24:91–8. doi: 10.1016/j.jare.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kaur RJ, Dutta S, Bhardwaj P, Charan J, Dhingra S, Mitra P, et al. Adverse events reported from COVID-19 vaccine trials:A systematic review. Indian J Clin Biochem. 2021;36:427–39. doi: 10.1007/s12291-021-00968-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Singh B, Kaur P, Cedeno L, Brahimi T, Patel P, Virk H, et al. COVID-19 mRNA vaccine and myocarditis. Eur J Case Rep Intern Med. 2021;8:002681. doi: 10.12890/2021_002681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sharifian-Dorche M, Bahmanyar M, Sharifian-Dorche A, Mohammadi P, Nomovi M, Mowla A. Vaccine-induced immune thrombotic thrombocytopenia and cerebral venous sinus thrombosis post COVID-19 vaccination;A systematic review. J Neurol Sci. 2021;428:117607. doi: 10.1016/j.jns.2021.117607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ng X le, Betzler BK, Testi I, Ho SL, Tien M, Ngo WK, et al. Ocular adverse events after COVID-19 vaccination. Ocul Immunol Inflamm. 2021;29:1216–24. doi: 10.1080/09273948.2021.1976221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Benage M, Fraunfelder FW. Vaccine-associated uveitis. Mo Med. 2016;113:48–52. [PMC free article] [PubMed] [Google Scholar]
- 7.Cunningham ET, Moorthy RS, Fraunfelder FW, Zierhut M. Vaccine-associated uveitis. Ocul Immunol Inflamm. 2019;27:517–20. doi: 10.1080/09273948.2019.1626188. [DOI] [PubMed] [Google Scholar]
- 8.Renisi G, Lombardi A, Stanzione M, Invernizzi A, Bandera A, Gori A. Anterior uveitis onset after bnt162b2 vaccination:Is this just a coincidence? Int J Infect Dis. 2021;110:95–7. doi: 10.1016/j.ijid.2021.07.035. [DOI] [PubMed] [Google Scholar]
- 9.Watad A, de Marco G, Mahajna H, Druyan A, Eltity M, Hijazi N, et al. Immune-mediated disease flares or new-onset disease in 27 subjects following mRNA/DNA SARS-CoV-2 vaccination. Vaccines (Basel) 2021;9:435. doi: 10.3390/vaccines9050435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Testi I, Brandão-de-Resende C, Agrawal R, Pavesio C, Steeples L, Balasubramaniam B, et al. Ocular inflammatory events following COVID-19 vaccination:A multinational case series. J Ophthalmic Inflamm Infect. 2022;12:4. doi: 10.1186/s12348-021-00275-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rabinovitch T, Ben-Arie-Weintrob Y, Hareuveni-Blum T, Shaer B, Vishnevskia-Dai V, Shulman S, et al. Uveitis after the BNT162b2 mRNA vaccination against SARS-CoV-2 infection. Retina. 2021;41:2462–71. doi: 10.1097/IAE.0000000000003277. [DOI] [PubMed] [Google Scholar]
- 12.Bolletta E, Iannetta D, Mastrofilippo V, de Simone L, Gozzi F, Croci S, et al. Uveitis and other ocular complications following COVID-19 vaccination. J Clin Med. 2021;10:5960. doi: 10.3390/jcm10245960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.HILL AB. The environment and disease:Association or causation? Proc R Soc Med. 1965;58:295–300. [PMC free article] [PubMed] [Google Scholar]