Abstract
Pediatric rhegmatogenous retinal detachment (RRD) is an issue of debate regarding its surgical outcomes and prognosis because of diagnosis delay, more complex etiological factors, and a higher prevalence of postoperative complications. This meta-analysis aims to evaluate the anatomical and visual outcomes of pediatric RRD and the factors that influence the treatment results. This is the first meta-analysis on this subject. We searched the relevant publications in the electronic databases of PubMed, Scopus, and Google Scholar. Eligible studies were included in the analysis. Anatomical success after one surgery and the final rates of success were estimated. Subgroup analysis was performed to find the rate of success in patients with different prognostic factors. This meta-analysis showed that the total rate of success after one surgery was about 64%, which implies that performing the first surgery was enough to get anatomical reattachment in most of the patients. The final anatomical rate of success was about 84%. In terms of visual acuity, the pooled results revealed statistically significant (P < 0.001) improvement in postoperative vision, with a 0.42 reduction in log of minimum angle of resolution (logMAR). The final rate of success was significantly lower in eyes with proliferative vitreoretinopathy (PVR) (about 25% lower in eyes with PVR, P < 0.001) and in the presence of congenital anomalies (about 36% lower in congenital cases, P = 0.008). Myopic RRD had a significantly better anatomical success rate. In conclusion, this study shows that there is a high chance of anatomical success after pediatric RRD treatment. The presence of PVR and congenital anomalies was associated with a poorer prognosis.
Keywords: Meta-analysis, pediatric rhegmatogenous retinal detachment, proliferative vitreoretinopathy, rate of success, surgical success rate
Retinal detachment occurs due to the separation of the neurosensory retina from the underlying retinal pigment epithelium and is a cause of ocular morbidities.[1] Rhegmatogenous retinal detachment (RRD) is the most common form of retinal detachment with an approximate incidence of 1 per 10,000 each year.[2] Pediatric RRD accounts for 3%–12% of all RRD cases.[3] In the pediatric population, RRD can be quite different in clinical presentations, predisposing conditions, and treatment outcomes compared to adult RRD.[4] Due to factors like delayed diagnosis, frequent association with proliferative vitreoretinopathy (PVR), and the possibility of amblyopia, pediatric RRD usually has a worse outcome than RRD in adults.[5]
RRD is the result of various risk factors.[6] More than 40% of pediatric RRD cases occur due to ocular trauma.[7] Other predisposing factors include high myopia (>−6 D), history of ocular surgery, uveitis, retinopathy of prematurity (ROP), hereditary vitreoretinopathies, and structural abnormalities like Stickler syndrome, familial exudative vitreoretinopathy (FEVR), and Marfan syndrome.[4,8]
Different types of retinal breaks can be found in eyes with RRD, including horseshoe tears, giant retinal tears, atrophic holes, dialysis, and retinoschisis.[9] The type of retinal break can influence final visual and surgical outcomes.[10] In a previous study of pediatric RRD cases, a larger percentage of patients with atrophic holes and dialysis had final reattachment compared to those with giant retinal tears and tractional retinal tears.[11,12]
The main treatment approach for pediatric RRD is surgery, including scleral buckling (SB), pars plana vitrectomy (PPV), and combined SB/PPV with or without lensectomy.[13] The surgical treatment approach is at the discretion of the surgeon based on certain findings of examination like PVR. In eyes without advanced PVR, SB is usually the first procedure. Primary vitrectomy is often performed in eyes with advanced PVR, opaque media, or a posterior tear.[7,8,14] Different types of tamponade, like silicone oil or gas, may be utilized in vitrectomy. Adherence to the position after the application of gas tamponade is quite difficult for young children. The use of silicone oil resolves this issue, but may lead to a worse visual and anatomical rate of success (RS) and more postoperative complications.[14]
Achieving retinal reattachment might require multiple surgeries[15] and despite that, in some cases, treatment is not successful.[16] The RS of retinal detachment surgery is usually evaluated based on visual outcome, defined as final best-corrected visual acuity, and anatomical success, defined as final persistent retinal reattachment.[17] In some cases, the visual outcome can remain unfavorable, despite successful retinal reattachment.[18]
Many factors can lead to a lower visual and anatomical RS in pediatric RRD. The interval between retinal detachment and surgery is longer in children because of delayed diagnosis, which comes as a result of the difficulty in expressing symptoms. Macula-off RRD and PVR are also more commonly reported in this age group.[19] In older children, congenital anomalies are less likely to cause RRD. Also, anatomical and visual RSs are higher in older children.[20]
Pediatric RRD is a challenge for ophthalmologists, especially when it comes to surgical outcomes and prognosis, because of later diagnosis, more complex etiological factors, and higher prevalence of postoperative complications.[19] Although many studies have been conducted to investigate the outcomes of pediatric RRD, the primary anatomical and visual outcomes, as well as factors affecting them have been quite different in various studies. This meta-analysis aims to glean data from various studies to evaluate anatomical and visual outcomes of pediatric RRD and the influencing factors. This is the first meta-analysis of this subject.
Methods
The PRISMA (Preferred Reporting Items for Systematic reviews and Meta-Analyses) protocol was considered a guideline to perform the study. In this meta-analysis of pediatric studies, those studies that defined pediatric population as “patients under 18 years of age” were included.
Search strategy and eligibility criteria
Relevant publications released by February 2021 were searched in the electronic databases of PubMed, Scopus, and Google Scholar. The Mesh terms and text keywords searched were “rhegmatogenous retinal detachment” AND “Pediatric OR children OR pediatric” AND “scleral buckling OR scleral buckling procedure OR vitrectomy OR vitrectomies” AND retinopexy. Studies were included if the participants were <18 years old (pediatric population), were published in English, were published before February 2021, and reported the rate of final surgical success, defined as persisting retinal reattachment at the last follow-up (at least 6 months after the last surgery) and after silicone oil removal (where applicable). Case reports and studies with unclear methodology, outcomes, and information were excluded. Two authors (SH and DM) searched and investigated the publications and selected eligible studies. After reviewing full-text articles, relevant studies which met the eligibility criteria were selected. The selection process is shown in Fig. 1.
Figure 1.
Flow diagram of studies assessed for the review
Data extraction and outcomes
A relevant checklist was designed for data extraction. Publication year, country, sample size, method of design, mean (standard deviation [SD]) of age and gender of participants, etiology, common postsurgery complications, type of break and probable risk factors, and other relevant data were extracted. The surgical procedure was categorized into SB and vitrectomy or both.
The RS was measured as the rate of retinal reattachment. The anatomical success after one surgery is defined as retinal reattachment after a single surgery (PPV, SB, or combined), and the final RS is defined as persisting retinal reattachment at the last follow-up (at least 6 months postoperatively) after silicone oil removal following single or multiple surgeries. The anatomical success after one surgery and the final RS in total and etiological subgroups like trauma, high myopia (>−6 D), congenital and developmental anomalies (including ROP, persistent fetal vasculature, Marfan syndrome, Stickler syndrome, CHARGE (coloboma, heart defects, atresia choanae, growth retardation, genital abnormalities, and ear abnormalities) syndrome, microspherophakia, buphthalmos [congenital glaucoma], FEVR (Familial Exudative Vitreoretinopathy), morning glory syndrome, juvenile retinoschisis, microphthalmos, atopic dermatitis, and uveal coloboma), and history of lensectomy were extracted. Also, the final RS was considered in age groups (<10 and 10–18 years old) and eyes with PVR (grade C or worse). For investigating the influence of etiology on the outcome, final anatomical RS in cases involving trauma, high myopia, congenital problems, and eyes with a history of lensectomy was compared with other groups. Also, the final anatomical RS was compared in eyes with and without PVR. Age-group comparisons were also performed to assess the effect of age on anatomical RS. In all subgroup analyses, the studies with adequate information were included; thus, the number of studies included in the subgroup analysis was not the same.
Pre- and postoperative final visual acuities (VAs) were compared to investigate the functional success of the surgery. VA was analyzed in studies that reported the information on the log of minimum angle of resolution (logMAR) scale. Functional success was defined as a significant change in the mean of VA (logMAR scale) after the last surgery.
Risk of bias
The quality of the included studies was assessed by two independent investigators using the Mixed Methods Appraisal Tool (MMAT)[21] checklist, which is a guideline for observational studies. The risk of bias was found to be low.
Statistical analysis
STATA 16 (StataCorp LP, College Station, TX, USA) was used to perform the analysis. As a first step, heterogeneity was assessed through the I2 heterogeneity statistic. I2 is an index for calculating the percentage of the variability in estimation that is due to heterogeneity.[22] An I2 of 25%–50% indicates a low degree of heterogeneity, 50%–75% indicates moderate heterogeneity, and >75% indicates high heterogeneity.[23] In this study, an I2 >50% was considered remarkable heterogeneity. The fixed effect model was utilized for homogenous studies, and the random effect model was used in heterogeneous studies for estimations, confidence intervals (CIs), and tests. In random effect modes, the restricted maximum likelihood method was regarded as the estimation method. Continuous variables were categorized to perform subgroup analyses. The cutoff points were selected based on the information from the studies. For instance, in most studies, the RS in children <10 years of age was reported separately, thus the best chosen cutoff for age was 10. Forest plots were created for revealing the CIs for each study. All pooled estimates (total RS and the RS differences) were calculated by weighting each study based on the standard error of the reported results.
Although most studies claimed that their method was retrospective, the method parts revealed that they were historical cohort studies. So, the risk rate was considered the effect size to show the RS. Risk difference (the difference in risk rates in two groups) was used as the effect size to compare the RSs in subgroups. P value <0.05 was considered significant.
Results
Fig. 1 illustrates the research steps toward study results.[24] In the end, a total of 28 publications were found eligible to be included in the meta-analysis. Table 1 shows the characteristics of included studies.
Table 1.
Characteristics of the included studies
| Study | Country | Study design | No. of patients (eyes) | Reattachment rate after one surgery | Final reattachment rate | Preoperative BCVA (logMAR) | Postoperative BCVA (logMAR) |
|---|---|---|---|---|---|---|---|
| Akabane et al., 2001[4] | Japan | A retrospective study | Female: 8 patients (11 eyes) Male: 20 patients (21 eyes) | - | Final rate: 30 eyes (93.8%) | - | 16 eyes: no change in postoperative visual acuity 16 eyes: improved visual acuity of more than two Snellen lines In 3/32 eyes (9.4%), postoperative visual acuity was less than 20/200 |
| Butler, 2015[41] | UK | A retrospective survey | 15 (15 eyes) | - | Final rate: 13/15 (86.6%) | At least 6/12 or better in 1/15 (6.6%) | At least 6/12 or better in Visual improvement occurred in 8/15 (53.3%), remained unchanged in 5/15 (33.3%) worsened in 2/15 (13.3%) |
| Sadeh et al., 2001[27] | Israel | A retrospective review | 16 eyes | - | All operated eyes (100%) | 6/20 or better: 5/11 eyes (46%)Three eyes with attached macula on presentation, VA ≥6/20: 2 (67%)Eight eyes with detached macula on presentation, VA ≥6/20: 3 (38%), 6/60 or worse: 3 (38%) | |
| Weinberg et al., 2003[26] | USA | Retrospective survey | 34 (39 eyes) | - | 31 of the 39 eyes (79%) | Mean: 3.4/200 Median: 20/400 | |
| Yokoyama et al., 2004[28] | Japan | Retrospective review | 49 (55 eyes) | From 43 eyes without PVR: 40 eyes (93%), from 12 eyes with PVR: 3 eyes (25%) | 43 eyes from 43 without PVR (100%)/five eyes from 12 PVR-positive eyes (42%) without silicone oil Complete retinal reattachment in the absence of silicone oil: 48 of the 55 eyes (87%) | Median: 0.3 | Median: 0.7 |
| Chang et al., 2005[12] | Taiwan | Review | 146 (152 eyes) | Eight patients | 119 eyes (78.3%) | - | - |
| Wang et al., 2005[14] | Taiwan | Retrospective survey | 278 (296 eyes) | 214 eyes (72%) | 250 eyes (85%) | 28.6/200 | 68.1/200 |
| Chen et al., 2006[36] | Taiwan | Retrospective study | 32 (35 eyes) | 24 eyes | 80% (totally) (n=25) | - | Congenital anomalies: five eyes: 0.4-1.0, four eyes: 0.1-0.3, one eye: counting fingers-0.1, five eyes: no LP, not available in one eye Trauma: three eyes: 0.4-1.0, four eyes: 0.1 and 0.3, one eye: between counting fingers and 0.1 |
| Gonzales et al., 2008[11] | USA | Retrospective study | 45 patients (46 eyes) | 24 eyes (52%) | 88% | Counting fingers (median) | 20/40 or better: 7 (21) 20/50–20/200: 8 (23) 20/400 to CF: 6 (18) HM to LP: 7 (21) NLP: 6 (18) |
| Wadhwa et al., 2008[5] | India | Retrospective interventional case series | 230 eyes (216 patients) | - | Complete: 204 (88.7%) | - | <4/200: 86 ≥4/200: 138 |
| Wang et al., 2009[44] | Taiwan | Retrospective study | 111 eyes (107 patients) | 90 eyes/111 | 101 eyes | High myopia group: hm (hand motion), 20/20 Extreme myopia group: LP, 20/60 Totally VA ≥20/200: 42 eyes (both extreme and high myopia groups) | ≥20/200: 81 eyes |
| Cheema et al., 2009[37] | Saudi Arabia | Retrospective chart review | 20 patients | - | 17/20 (85%) | Mean=2.146 logMAR | Improvement (the number was not mentioned) |
| Soheilian et al., 2009[31] | Iran | Retrospective case series | 108 patients (127 eyes) | - | Complete: 88 (70.9) | VA >20/40: 11 (8.6) 20/40 ≤ VA ≤ 20/200: 45 (35.4) 20/200 > VA ≥ 5/200: 18 (14.3) VA <5/200: 53 (41.7) | VA >20/40: 14 (11.0) 20/40 ≤ VA ≤ 20/200: 33 (26.0) 20/200 > VA ≥ 5/200: 24 (18.9) VA <5/200: 56 (44.1) |
| Oono et al., 2012[25] | Japan | Retrospective study | 44 (48 eyes) | 40 (83%) | 96% (46 eyes) | Trauma: 0.22 ± 0.55 Myopia: 0.34 ± 0.84 Congenital: 1.51 ± 1.51 Atopic dermatitis: 0.88 ± 1.06 Others: 0.61 ± 1.09 | Trauma: 0.04 ± 0.42 Myopia: 0.24 ± 0.67 Congenital: 1.23 ± 1.36 Atopic dermatitis: 0.36 ± 0.84 Others: 0.54 ± 1.34 |
| Al-Zaaidi et al., 2013[45] | Saudi Arabia | Retrospective chart review | 166 eyes (148 patients) | 106 (63.8%) eyes | 134 (80.4%) eyes | VA ranged from 20/20 in two (1.2%) eyes to LP in 27 (16.27%) eyes | 68 (50.4%) eyes: no change 46 (34.1%) eyes: VA improved 21 (15.5%): VA decreased |
| Rahimi et al., 2014[46] | Iran | Retrospective, noncomparative, interventional case series | 77 eyes (77 patients) | - | 62.3% (n=48) | Negative RAPD: n=40, 52% Positive RAPD: 34 patients (44.2%) | Final BCVA could not be assessed in four patients and the rate of functional visual loss at the last examination was 48.6% (n=34 out of 70 eyes with available data) |
| Imaizumi et al., 2014[47] | Japan | Multicenter study | 10 eyes of nine children | - | Eight eyes (80%) | Hand motion | 0.1 |
| Errera et al., 2015[8] | United Kingdom | Retrospective consecutive case series | 99 patients (104 eyes) | 76/104 (73%) | 98/104 (94%) | - | - |
| Yokoyama, 2004[28] | Japan | Case report | Three eyes (three patients) | - | - | 0.3 (BCVA) with slight hyperopic astigmatism in the right eye 1.2 (BCVA) 0.2 (BCVA) | Unchanged (0.4) 0.8 0.6 |
| Sin et al., 2017[30] | China | Retrospective study | 37 (39 eyes) | 27 (69.2%) | 32 (82.1%) | 32 (logMAR: 1.05 ± 0.79) | 36 (logMAR: 0.93 ± 0.86) |
| Sen, 2016[42] | India | Retrospective, observational, consecutive case series | 15 patients (16 eyes) | 11 eyes (68.7%) | 14/16 (87.5%) eyes | 1.19 ± 0.77 (BCVA) | 0.86 ± 0.83 logMAR |
| Fong et al., 2016[18] | China | Retrospective, consecutive case series | 47 (49 eyes) | 65.30% | 85.7% | 0.97 ± 0.78 | 0.91 ± 1.18 |
| Huang et al., 2019[33] | Taiwan | Retrospective study | 86 (86 eyes) | Trauma: 41.7% Myopia: 68.8% Congenital: 50% Previous ocular surgeries: 20% | Trauma: 70.8% Myopia: 87.5% Congenital: 50% Previous ocular surgeries: 60% | Trauma: 1.9 Myopia: 1.4 Congenital: 2 Previous surgery: 2.2 | Trauma: 1.9 Myopia: 1.1 Congenital: 2 Previous surgery: 2.3 |
| Tsai et al., 2019[34] | Singapore | Retrospective case review | 152 (171 eyes) | 96 (60.7%) | 137 (86.7) | 1.46 ± 1.16 (BCVA logMAR) | 0.99 ± 0.58 (<8 years old), 0.81 ± 1.12 (≥8 years old) |
| Yaşa et al., 2018[43] | Turkey | Retrospective study | 57 patients | - | Anatomical success: 72%, open-globe trauma: 25/36 (69%), closed-globe trauma: 16/21 (76%) | NLP: 4 (7), LP/HM: 35 (61), 1/200-19/200: 8 (14), 20/200-20/50: 4 (7), ≥20/40: 1 (2) | NLP: 5 (9), LP/HM: 27 (46), 1/200–19/200: 14 (25), 20/200–20/50: 8 (14), ≥20/40: 3 (5) |
| Smith et al., 2019[32] | USA | Retrospective, interventional, case series | 191 (212) | 119/183 (65%) | 1 month 115/143 (80%), 3 months 124/161 (77%), 6 months 123/154 (80%), 12 months 133/165 (81%) | Mean: 1.77 (SD: 1.03), (n=137) | 1 month: 1.24 (0.78), (n=91), 3 months: 1.27 (0.88), (n=100), 6 months: 1.30 (0.86), (n=101), 12 months: 1.11 (0.85), (n=105) |
| Abdullatif, 2020[35] | Egypt | Retrospective, interventional, case series | 25 patients (29 eyes) | 55.2% (16 eyes) | 24 eyes (82.7%) | - | 1.5± 0.9 SD (logMAR) Better than 20/200: (11 eyes) 37.9% |
| Ghoraba et al., 2020[29] | Egypt | Retrospective review | 72 patients (and eyes) | - | 44/72 eyes (61.11%) | Perception of light: 24 (33.3%) Hand motion: 27 (37.5%) CF at 20 cm: 0 (0%) CF at 50 cm: 5 (6.9%) CF at 1 m: 8 (11.1%) CF at 2 m: 1 (1.4%) 0.5: 5 (6.9%) 0.1: 1 (1.4%) 0.2: 1 (1.4%) VA not assessed: 0 (0%) | NLP: 7 (5%) PL: 15 (10.6%) HM: 30 (21.3%) CF at 50 cm: 2 (1.4%) CF at 1 m: 26 (18.4%) CF at 2 m: 8 (5.7%) 0.05: 17 (12%) 0.1: 6 (4.3%) 0.3: 4 (2.8%) 0.4: 3 (2.1%) 0.5: 4 (2.8%) 0.6: 0 (0%) 0.9: 0 (0%) |
BCVA=best-corrected visual acuity, logMAR=log of minimum angle of resolution, LP=light perception, PVR=proliferative vitreoretinopathy, SD=standard deviation, VA = visual acuity, RAPD = Relative afferent pupillary defect
The pooled mean age was 11.75 years (95% CI: 9.79–13.71). In total, 76% (95% CI: 74%–78%) of patients were male. The pooled follow-up time was 10.19 months (95% CI: 5.95–14.44). In eight studies, the mean and SD of the number of surgeries were reported. Merging these data, the mean number of surgeries was 1.61 (95% CI: 1.31–1.90).
The most frequent type of break was round retinal hole (40%, 95% CI: 28%–0.52%) followed by tear (26%, 95% CI: 14%–38%), dialysis (8%, 95% CI: 6%–11%) and giant tear (7%, 95% CI: 4%–9%). About 37% (95% CI: 24%–49%) of holes and tears were in relation to lattice degeneration. A macular hole was detected in 2% (95% CI: 1%–3%) of the patients.
Considering the publications with both traumatic and myopic etiologies, trauma and high myopia were the cause of RRD in 29% and 38% of the patients, respectively. The total incidence of ROP and FEVR was 7% (95% CI: 3%–11%) and 7% (95% CI: 1%–13%), respectively.
The operations were divided into three categories: SB, PPV, or both. In the first operation, mostly PPV was done (43%), followed by a scleral buckle (37%) and both (29%). Silicone oil was the most common tamponade in the surgeries (83%, 95% CI: 75%–92%). In other patients, gas was used as the tamponade (except for two patients in one study, in whom the type of tamponade was not mentioned in the article).
Anatomical success after one surgery and final RS
Fig. 2a illustrates the anatomical success after one surgery. Nineteen studies were eligible to be included in the analysis. The highest and lowest rates of anatomical success after one surgery were those of Oono et al.[25] with RS = 83%, n = 48 and Weinberg et al.[26] with RS = 46%, n = 39, respectively. The most accurate study (the narrowest CI for RS) belonged to Wang et al.[14] with RS = 72%, n = 296. The total RS was about 64% (95% CI: 59%–70.94%), which implies that in most of the patients, the first surgery was enough to get anatomical success.
Figure 2.
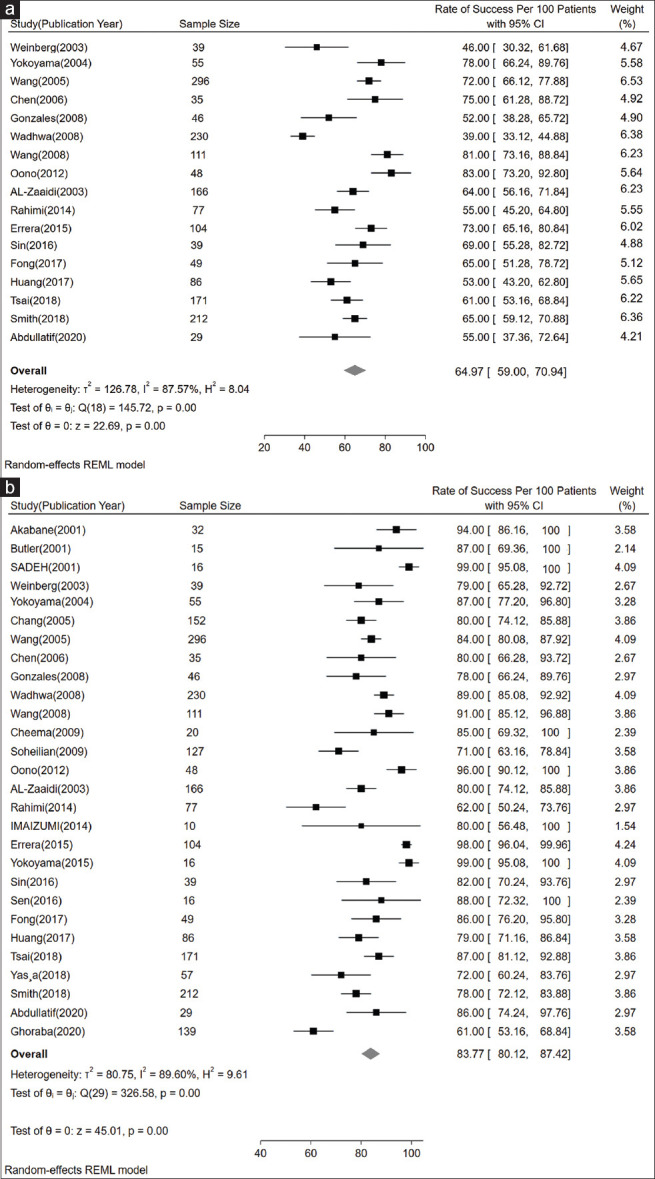
(a) Forest plot showing pooled primary rate of success per 100 patients based on 17 studies. (b) Forest plot showing pooled final rate of success per 100 patients based on 28 studies
The final anatomical RSs of 28 studies are illustrated in Fig. 2b; all of the participants in Sadeh et al.[27] (n = 16) and Yokoyama et al.[28] (n = 16) achieved complete retinal attachment. Among studies, including failed retinal reattachments, the highest and the lowest final RS belonged to Errera et al.[8] with RS = 98%, n = 104, and Ghoraba et al.[29] with RS = 61%, n = 139, respectively. The most accurate results (the narrowest CI for RS) were reported by Errera et al.[8] with RS = 98%, n = 104. The merged final anatomical RS was about 84% (95% CI: 80.12%–87.42%). Thus, it is expected that at least 80% of eyes achieve anatomical reattachment with 95% certainty.
The merged final RS for patients younger than 10 was 74% (95% CI: 59%–90%). In 10–18-year-old patients, the final RS was 83% (95% CI: 76%–90%). Fig. 3 shows the pooled rate differences in patients younger and older than 10. There was no significant difference between the RSs of these two groups.
Figure 3.
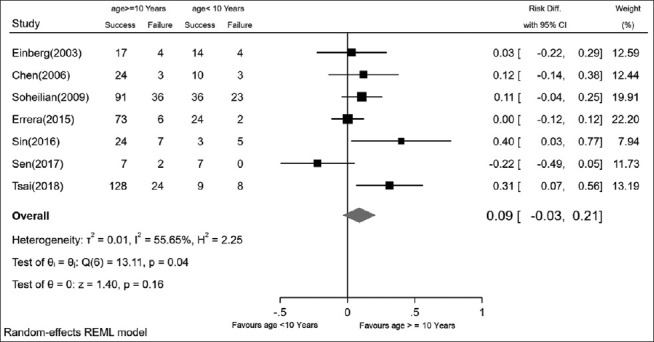
The pooled rate differences in patients younger and older than 10 years
Final anatomical RS in traumatic and myopic RRD
A total of 15 studies on traumatic RRD were included in the meta-analysis. Four studies reported a 100% rate of final success in traumatic RRD.[18,25,27,30] Among other publications, Errera et al.[8] reported the highest RS = 95% (95% CI: 89.12%–100%) and Ghoraba et al.[29] reported the lowest RS = 61% (95% CI: 53.16%–68.84%). Oono et al.[25] and Sin et al.[30] reported the lowest CI. The total RS in traumatic RRD was 82.39% (95% CI: 74.52%–90.25%). Comparison of the final RS between traumatic and other groups is shown in Fig. 4a. Two studies solely contained traumatic patients, so the comparisons between traumatic and nontraumatic eyes were not applicable in these two studies. Therefore, 13 studies were included in the comparison. In seven studies (47%), traumatic RRD had higher RS, but only in one study, a significant difference was detected.[12] In total, the RS difference between traumatic and non-traumatic RRD was not significant (RS difference = 2%, 95% CI: −4% to 8%).
Figure 4.
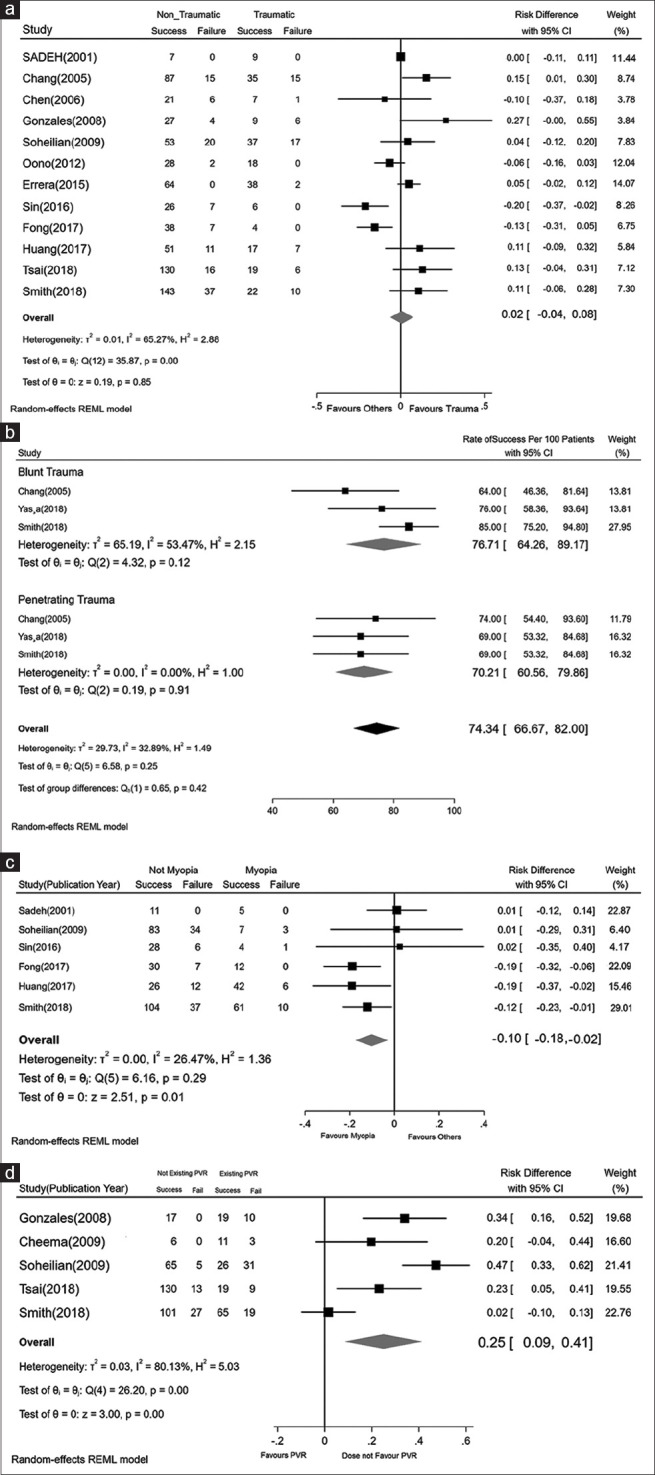
(a) The pooled rate differences in traumatic and nontraumatic RRD eyes. (b) The pooled rate differences in blunt and penetrating traumatic RRD eyes. (c) The pooled rate differences in myopic and nonmyopic RRD eyes. (d) The pooled rate differences in eyes with and without PVR. PVR = proliferative vitreoretinopathy, RRD = rhegmatogenous retinal detachment
Fig. 4b shows the results of four studies that separately reported the RS in blunt and penetrating traumatic RRD. Although the RS in the blunt trauma group was about 6% higher, there was no significant difference in RS between the groups (P = 0.350).
The total RS in myopic RRD was estimated by analyzing eight eligible studies. Sadeh et al.[27] and Fong et al.[18] reported 100% anatomical success in myopic RRD. Among other publications, Gurler et al.[17] reported the highest RS = 92% (95% CI: 76.32%–100%) and Soheilian et al.[31] reported the lowest RS = 70% (95% CI: 42.56%–97.44%). Merging the studies shows that the total RS was 88.68% (95% CI: 81.17%–96.2%). Fig. 4c shows the rate difference between myopic RRD and other groups. The rate difference in the three studies was statistically significant,[18,32,33] and in total, high myopia was significantly related to better anatomical success and an increase in the RS by about 10% (95% CI: 2%–18%) in comparison to other etiologies.
Final anatomical RS in RRD cases with PVR, congenital and developmental cases, and in patients with a history of lensectomy
Twelve studies reported the RS in pediatric RRD due to congenital and developmental anomalies.[8,12,18,25,26,30-36] The RS ranged from 10% to 99%. The total RS was 55.24% (95% CI: 37.50%–72.92%). A statistically significant difference (P = 0.008) was detected in RRD cases due to congenital anomalies compared to noncongenital ones. The RS in noncongenital patients was 36% (CI: 15%–55%) higher.
Five studies with a reported RS in eyes with PVR were included in the analysis. The RS in eyes with PVR ranged from 45% (95% CI: 31. 28%–58.72%) to 78% (95% CI: 75.84%–8.16%) in the studies of Soheilian et al.[31] and Cheema et al.,[37] respectively. The pooled final reattachment rate in eyes with PVR was 67.87% (95% CI: 55.81%–79.91%). In eyes without PVR, the RS was 92.44% (95% CI: 85.71%–99.17%).
Fig. 4d shows the comparison of RS between eyes with and without PVR. The highest RS difference was reported by Soheilian et al.[31] In total, a statistically significant difference (P < 0.001) was detected in eyes with PVR in comparison to those without PVR. The RS in patients without PVR was 25% (95% CI: 9%–41%) higher.
Lensectomy combined with reattachment operation was performed in about 29% of the eyes (95% CI: 18%–40%). Four studies[11,26,34,37] reported RS considering the history of lensectomy. History of lensectomy could be found in 19% of pediatric RRD patients (95% CI: 11%–28%). The RS in patients with a history of lensectomy was about 74% (95% CI: 62%–85%). Although the RS in eyes without a history of lensectomy was about 9% (95% CI: −3% to 22%) higher, there was no significant difference in RS between eyes with and without a history of lensectomy (P = 0.150).
Comparing the final anatomical RS in traumatic and myopic RRD patients
Fig. 5 shows a comparison between RS in traumatic and myopic RRD patients. As it is illustrated, none of the studies reported a significant difference between the RS of these two subtypes of RRD, and in total, there was no significant difference between RS in patients with traumatic and myopic etiology (RD = 0.04, P = 0.54).
Figure 5.
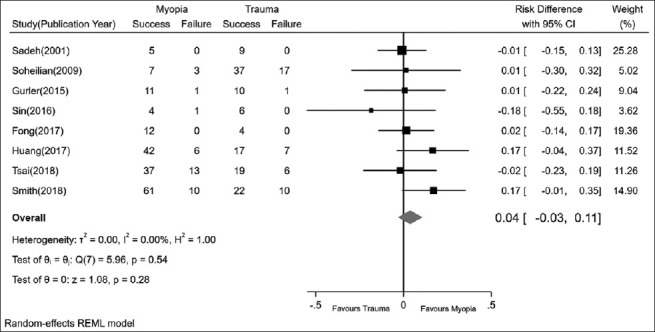
The pooled rate differences in traumatic and myopic RRD eyes. RRD = rhegmatogenous retinal detachment
VA improvement
Eight studies considered changes in the mean pre- and postoperative VA. In three studies,[18,25,30] there was no statistically significant difference between pre-and postoperative VA. The pooled results revealed statistically significant (P < 0.01) changes between pre- and postsurgery VA, with a 0.42 reduction in logMAR (95% CI: −0.57 to − 0.26) [Fig. 6].
Figure 6.
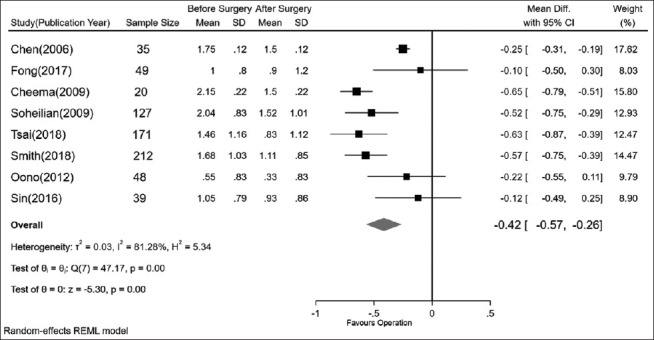
The comparison of visual acuity (mean of logMAR) pre- and postsurgery. logMAR = log of minimum angle of resolution
Discussion
We identified 28 studies describing the outcome of RRD treatment in the pediatric group. The total anatomical success rate was 84%. Based on the pooled data analysis, the mean number of surgeries was 1.61; however, results revealed that successful reattachment was achievable after the first procedure in 64% of patients.
We found that high myopia and trauma were the leading causes of pediatric RRD (67%), followed by other causes such as FEVR and ROP categorized as congenital. In between-group comparisons, only RRDs in cases with congenital ocular anomalies had a significantly lower RS than the other groups and the cause of retinal detachment did not seem to have a significant impact on the final treatment outcome in the other two groups (as there was no significant difference between the 82.39% RS in traumatic RRD patients and the 88.68% RS in myopic RRD group). Compared to traumatic cases and those with congenital ocular anomalies, myopic RRD occurs in relatively normal eyes, which allows for better anatomical reattachment, and also the risk of PVR is much lower than in the other two mentioned groups. On the other hand, myopic RRD patients are usually older than congenital cases, so they can cooperate better for postoperative care and positioning.[34] Eyes with congenital or developmental anomalies often have abnormal vitreoretinal junction and stronger vitreoretinal adhesion. As they often present at a younger age, there are also problems of delayed diagnosis, PVR formation, difficulty in positioning, and visual rehabilitation in the postoperative period;[30] altogether, these make the results of this study justified.
In the traumatic RRD group, RS was slightly lower in patients with a history of penetrating trauma; the difference, however, was not significant in comparison to patients with blunt trauma. Due to the small number of trauma studies that met the criteria of this meta-analysis, the results should be interpreted with caution. According to the literature, the risk of PVR is higher in perforation than in rupture, which might explain this finding in our research.[38]
The PPV (either alone or combined with SB) has been the preferred surgical method for pediatric RRD. In 29% of patients, lensectomy was performed at the time of PPV. Comparing different surgical approaches was not possible, thus this study could not define whether the type of surgical procedure can change the anatomical RS. Dividing pediatric RRD patients based on their crystalline lens status revealed that this factor did not have a significant impact on the RS. Nineteen percent of patients had a history of lensectomy before the retinal detachment, and even though they had a slightly lower RS than phakic patients (74% vs. 83%), the difference was not significant. Comparison of the results to those from adults showed that the phakic pediatric patients had RSs comparable to adults (83% in pediatric RRD vs. 79.6% for PPV and 88.6% for SB in adults); however, the RS in aphakic/pseudophakic patients was found to be lower (74% in pediatric RRD vs. 90.5% for PPV and 88.7% for SB in adults).[39]
There was limited data on PVR management in the studies; therefore, we were unable to evaluate the efficacy of different treatment modalities in the outcome of RRD cases with PVR. Nevertheless, pooled data analysis revealed that the presence of PVR had been associated with a lower RS in pediatric RRD, as expected.
Aside from the anatomical RS, this study showed that the treatment yielded a significant improvement in VA in pediatric RRD patients. One should not overlook the great impact of amblyopia and its treatment on the visual outcome in this age group. Thus, this visual gain (or loss) should be interpreted judiciously as the data on the quality of amblyopia therapy is very limited.
The most limiting factor of this study was that the included studies were heterogeneous in terms of the definitions of characteristics. Also, there was a lack of information for analysis. Therefore, many studies were excluded when the analysis was performed on VA improvement, lens status, and PVR management. Even though many studies have reported the type of break in RRD, the role of this categorization in the final pediatric RRD outcome remains unclear. Another major limitation of this study was its inability to analyze and compare the surgical complications of different study groups. Even though we could report the overall rate of complications such as ocular hypertension (23%), cataract (10%), epiretinal membrane (16%), and band keratopathy (4%), making a comparison between different study groups was not possible due to lack of sufficient data. The data about diplopia and strabismus was not included in the study, as in the pediatric group, suppression and amblyopia occur in no time and the squint could be a result of vision loss, and not necessarily the surgical procedure itself. The data about the cystoid macular edema was also very limited, probably due to paraclinical exam difficulty in this age group.
Conclusion
In conclusion, this study shows that there is a high chance of anatomical success after pediatric RRD treatment. The presence of PVR and congenital anomalies was associated with a poorer prognosis. Although VA improvement was reported in most studies, the impact of amblyopia and its treatment should not be overlooked in this regard. Further and more organized studies are needed to evaluate the RS of different surgical approaches as well as the rate of long-term complications.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Mitry D, Charteris DG, Yorston D, Fleck BW, Wright A, Campbell H, et al. Rhegmatogenous retinal detachment in Scotland:Research design and methodology. BMC Ophthalmol. 2009;9:2. doi: 10.1186/1471-2415-9-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Feltgen N, Walter P. Rhegmatogenous retinal detachment--An ophthalmologic emergency. Dtsch Arztebl Int. 2014;111:12–21. doi: 10.3238/arztebl.2014.0012. quiz 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Soliman MM, Macky TA. Pediatric rhegmatogenous retinal detachment. Int Ophthalmol Clin. 2011;51:147–71. doi: 10.1097/IIO.0b013e31820099c5. [DOI] [PubMed] [Google Scholar]
- 4.Akabane N, Yamamoto S, Tsukahara I, Ishida M, Mitamura Y, Yamamoto T, et al. Surgical outcomes in juvenile retinal detachment. Jpn J Ophthalmol. 2001;45:409–11. doi: 10.1016/s0021-5155(01)00361-6. [DOI] [PubMed] [Google Scholar]
- 5.Wadhwa N, Venkatesh P, Sampangi R, Garg S. Rhegmatogenous retinal detachments in children in India:Clinical characteristics, risk factors, and surgical outcomes. J AAPOS. 2008;12:551–4. doi: 10.1016/j.jaapos.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 6.Polkinghorne PJ, Craig JP. Northern New Zealand rhegmatogenous retinal detachment study:Epidemiology and risk factors. Clin Exp Ophthalmol. 2004;32:159–63. doi: 10.1111/j.1442-9071.2004.00003.x. [DOI] [PubMed] [Google Scholar]
- 7.Fivgas GD, Capone A., Jr Pediatric rhegmatogenous retinal detachment. Retina. 2001;21:101–6. doi: 10.1097/00006982-200104000-00001. [DOI] [PubMed] [Google Scholar]
- 8.Errera MH, Liyanage SE, Moya R, Wong SC, Ezra E. Primary scleral buckling for pediatric rhegmatogenous retinal detachment. Retina. 2015;35:1441–9. doi: 10.1097/IAE.0000000000000480. [DOI] [PubMed] [Google Scholar]
- 9.Mitry D, Singh J, Yorston D, Siddiqui MA, Wright A, Fleck BW, et al. The predisposing pathology and clinical characteristics in the Scottish retinal detachment study. Ophthalmology. 2011;118:1429–34. doi: 10.1016/j.ophtha.2010.11.031. [DOI] [PubMed] [Google Scholar]
- 10.Williamson TH, Shunmugam M, Rodrigues I, Dogramaci M, Lee E. Characteristics of rhegmatogenous retinal detachment and their relationship to visual outcome. Eye (Lond) 2013;27:1063–9. doi: 10.1038/eye.2013.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gonzales CR, Singh S, Yu F, Kreiger AE, Gupta A, Schwartz SD. Pediatric rhegmatogenous retinal detachment:Clinical features and surgical outcomes. Retina. 2008;28:847–52. doi: 10.1097/IAE.0b013e3181679f79. [DOI] [PubMed] [Google Scholar]
- 12.Chang PY, Yang CM, Yang CH, Huang JS, Ho TC, Lin CP, et al. Clinical characteristics and surgical outcomes of pediatric rhegmatogenous retinal detachment in Taiwan. Am J Ophthalmol. 2005;139:1067–72. doi: 10.1016/j.ajo.2005.01.027. [DOI] [PubMed] [Google Scholar]
- 13.Read SP, Aziz HA, Kuriyan A, Kothari N, Davis JL, Smiddy WE, et al. Retinal detachment surgery in a pediatric population:Visual and anatomic outcomes. Retina. 2018;38:1393–402. doi: 10.1097/IAE.0000000000001725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang NK, Tsai CH, Chen YP, Yeung L, Wu WC, Chen TL, et al. Pediatric rhegmatogenous retinal detachment in East Asians. Ophthalmology. 2005;112:1890–5. doi: 10.1016/j.ophtha.2005.06.019. [DOI] [PubMed] [Google Scholar]
- 15.Nagpal M, Chaudhary P, Wachasundar S, Eltayib A, Raihan A. Management of recurrent rhegmatogenous retinal detachment. Indian J Ophthalmol. 2018;66:1763–71. doi: 10.4103/ijo.IJO_1212_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Narala R, Nassiri N, Kim C, Mehregan C, Padidam S, Abrams GW. Outcomes of repeat pars plana vitrectomy after failed surgery for proliferative vitreoretinopathy. Retina. 2018;38(Suppl 1):S49–59. doi: 10.1097/IAE.0000000000002000. [DOI] [PubMed] [Google Scholar]
- 17.Gurler B, Coskun E, Öner V, Comez A, Erbagci I. Clinical characteristics and surgical outcomes of pediatric rhegmatogenous retinal detachment. Int Ophthalmol. 2016;36:521–5. doi: 10.1007/s10792-015-0158-3. [DOI] [PubMed] [Google Scholar]
- 18.Fong AH, Yip PP, Kwok TY, Tsang CW. A 12-year review on the aetiology and surgical outcomes of paediatric rhegmatogenous retinal detachments in Hong Kong. Eye (Lond) 2016;30:355–61. doi: 10.1038/eye.2015.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rumelt S, Sarrazin L, Averbukh E, Halpert M, Hemo I. Paediatric vs adult retinal detachment. Eye (Lond) 2007;21:1473–8. doi: 10.1038/sj.eye.6702511. [DOI] [PubMed] [Google Scholar]
- 20.Sodhi A, Leung LS, Do DV, Gower EW, Schein OD, Handa JT. Recent trends in the management of rhegmatogenous retinal detachment. Surv Ophthalmol. 2008;53:50–67. doi: 10.1016/j.survophthal.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 21.Hong QN, Fàbregues S, Bartlett G, Boardman F, Cargo M, Dagenais P, et al. The Mixed Methods Appraisal Tool (MMAT) version 2018 for information professionals and researchers. Education for information. 2018;34:285–91. [Google Scholar]
- 22.Higgins JP. Cochrane handbook for systematic reviews of interventions. Version 5.1. 0 [updated March 2011] The Cochrane Collaboration. 2011. Available from:www. Cochrane-handbook.org .
- 23.Zhou R, Sun Y, Chen H, Sha S, He M, Wang W. Laser trabeculoplasty for open-angle glaucoma:A systematic review and network meta-analysis. Am J Ophthalmol. 2021;229:301–13. doi: 10.1016/j.ajo.2020.07.046. [DOI] [PubMed] [Google Scholar]
- 24.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement:An updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. doi:10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oono Y, Uehara K, Haruta M, Yamakawa R. Characteristics and surgical outcomes of pediatric rhegmatogenous retinal detachment. Clin Ophthalmol. 2012;6:939–43. doi: 10.2147/OPTH.S31765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weinberg DV, Lyon AT, Greenwald MJ, Mets MB. Rhegmatogenous retinal detachments in children:Risk factors and surgical outcomes. Ophthalmology. 2003;110:1708–13. doi: 10.1016/S0161-6420(03)00569-4. [DOI] [PubMed] [Google Scholar]
- 27.Sadeh AD, Dotan G, Bracha R, Lazar M, Loewenstein A. Characteristics and outcomes of paediatric rhegmatogenous retinal detachment treated by segmental scleral buckling plus an encircling element. Eye (Lond) 2001;15:31–3. doi: 10.1038/eye.2001.8. [DOI] [PubMed] [Google Scholar]
- 28.Yokoyama T, Kato T, Minamoto A, Sugihara A, Imada M, Kuwabara R, et al. Characteristics and surgical outcomes of paediatric retinal detachment. Eye (Lond) 2004;18:889–92. doi: 10.1038/sj.eye.6701341. [DOI] [PubMed] [Google Scholar]
- 29.Ghoraba HH, Mansour HO, Abdelhafez MA, El Gouhary SM, Zaky AG, Heikal MA, et al. Comparison between pars plana vitrectomy with and without encircling band in the treatment of pediatric traumatic rhegmatogenous retinal detachment. Clin Ophthalmol. 2020;14:3271–7. doi: 10.2147/OPTH.S275778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sin HPY, Yip WWK, Chan VCK, Young AL. Etiologies and surgical outcomes of pediatric retinal detachment in Hong Kong. Int Ophthalmol. 2017;37:875–83. doi: 10.1007/s10792-016-0287-3. [DOI] [PubMed] [Google Scholar]
- 31.Soheilian M, Ramezani A, Malihi M, Yaseri M, Ahmadieh H, Dehghan MH, et al. Clinical features and surgical outcomes of pediatric rhegmatogenous retinal detachment. Retina. 2009;29:545–51. doi: 10.1097/IAE.0b013e318194fd1a. [DOI] [PubMed] [Google Scholar]
- 32.Smith JM, Ward LT, Townsend JH, Yan J, Hendrick AM, Cribbs BE, et al. Rhegmatogenous retinal detachment in children:Clinical factors predictive of successful surgical repair. Ophthalmology. 2019;126:1263–70. doi: 10.1016/j.ophtha.2018.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huang YC, Chu YC, Wang NK, Lai CC, Chen KJ, Hwang YS, et al. Impact of etiology on the outcome of pediatric rhegmatogenous retinal detachment. Retina. 2019;39:118–26. doi: 10.1097/IAE.0000000000001908. [DOI] [PubMed] [Google Scholar]
- 34.Tsai ASH, Wong CW, Lim L, Yeo I, Wong D, Wong E, et al. Pediatric retinal detachment in an asian population with high prevalence of myopia:Clinical characteristics, surgical outcomes, and prognostic factors. Retina. 2019;39:1751–60. doi: 10.1097/IAE.0000000000002238. [DOI] [PubMed] [Google Scholar]
- 35.Abdullatif AM, Macky TA, Mortada HA. Extended internal limiting membrane peeling in complex pediatric rhegmatogenous retinal detachment. Ophthalmologica. 2021;244:223–8. doi: 10.1159/000512194. [DOI] [PubMed] [Google Scholar]
- 36.Chen SN, Jiunn-Feng H, Te-Cheng Y. Pediatric rhegmatogenous retinal detachment in taiwan. Retina. 2006;26:410–4. doi: 10.1097/01.iae.0000238546.51756.cd. [DOI] [PubMed] [Google Scholar]
- 37.Cheema RA, Al-Khars W, Al-Askar E, Amin YM. Pediatric retinal detachment in the Eastern Province of Saudi Arabia:Experience of a tertiary care hospital. Ann Saudi Med. 2009;29:361–4. doi: 10.4103/0256-4947.55165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cardillo JA, Stout JT, LaBree L, Azen SP, Omphroy L, Cui JZ, et al. Post-traumatic proliferative vitreoretinopathy:The Epidemiologfic profile, onset, risk factors, and visual outcome. Ophthalmology. 1997;104:1166–73. doi: 10.1016/s0161-6420(97)30167-5. [DOI] [PubMed] [Google Scholar]
- 39.Sun Q, Sun T, Xu Y, Yang XL, Xu X, Wang BS, et al. Primary vitrectomy versus scleral buckling for the treatment of rhegmatogenous retinal detachment:A meta-analysis of randomized controlled clinical trials. Curr Eye Res. 2012;37:492–9. doi: 10.3109/02713683.2012.663854. [DOI] [PubMed] [Google Scholar]
- 40.Lv Z, Li Y, Wu Y, Qu Y. Surgical complications of primary rhegmatogenous retinal detachment:A meta-analysis. PLoS One. 2015;10:e0116493. doi: 10.1371/journal.pone.0116493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Butler TK, Kiel AW, Orr GM. Anatomical and visual outcome of retinal detachment surgery in children. Br J Ophthalmol. 2001;85:1437–9. doi: 10.1136/bjo.85.12.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sen P, Shaikh SI, Sreelakshmi K. Rhegmatogenous retinal detachment in paediatric patients after pars plana vitrectomy and sutured scleral-fixated intraocular lenses. Eye (Lond) 2018;32:345–51. doi: 10.1038/eye.2017.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yaşa D, Erdem ZG, Ürdem U, Demir G, Demircan A, Alkın Z. Pediatric traumatic retinal detachment:Clinical features, prognostic factors, and surgical outcomes. J Ophthalmol. 2018;2018:9186237. doi: 10.1155/2018/9186237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang NK, Chen YP, Lai CC, Chen TL, Yang KJ, Kuo YH, et al. Paediatric retinal detachment:Comparison of high myopia and extreme myopia. Br J Ophthalmol. 2009;93:650–5. doi: 10.1136/bjo.2008.145920. [DOI] [PubMed] [Google Scholar]
- 45.Al-Zaaidi S, Al-Rashaed S, Al-Harthi E, Al-Kahtani E, Abu El-Asrar AM. Rhegmatogenous retinal detachment in children 16 years of age or younger. Clin Ophthalmol. 2013;7:1001–14. doi: 10.2147/OPTH.S40056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rahimi M, Bagheri M, Nowroozzadeh MH. Characteristics and outcomes of pediatric retinal detachment surgery at a tertiary referral center. J Ophthalmic Vis Res. 2014;9:210–4. [PMC free article] [PubMed] [Google Scholar]
- 47.Imaizumi A, Kusaka S, Noguchi H, Shimomura Y, Sawaguchi S. Efficacy of short-term postoperative perfluoro-n-octane tamponade for pediatric complex retinal detachment. Am J Ophthalmol. 2014;157:384–9.e2. doi: 10.1016/j.ajo.2013.10.002. [DOI] [PubMed] [Google Scholar]


