Abstract
The tumor microenvironment (TME) is a complex system composed of many cell types and an extracellular matrix (ECM). During tumorigenesis, cancer cells constantly interact with cellular components, biochemical cues, and the ECM in the TME, all of which make the environment favorable for cancer growth. Emerging evidence has revealed the importance of substrate elasticity and biomechanical forces in tumor progression and metastasis. However, the mechanisms underlying the cell response to mechanical signals—such as extrinsic mechanical forces and forces generated within the TME—are still relatively unknown. Moreover, having a deeper understanding of the mechanisms by which cancer cells sense mechanical forces and transmit signals to the cytoplasm would substantially help develop effective strategies for cancer treatment. This review provides an overview of biomechanical forces in the TME and the intracellular signaling pathways activated by mechanical cues as well as highlights the role of mechanotransductive pathways through mechanosensors that detect the altering biomechanical forces in the TME.
Keywords: Biomechanical forces, Cancer progression, Extracellular matrix (ECM), Tumor microenvironment (TME)
INTRODUCTION
Tumor formation and progression can be affected by genetic alterations in tumor cells and the repositioning of components of the tumor microenvironment (TME) through reciprocal dynamic crosstalk (1). The TME consists of tumor cells, stromal fibroblasts, endothelial cells, immune cells, and non-cellular components of the extracellular matrix, such as collagen, fibronectin, and hyaluronan (2). Mutual biochemical and biophysical interactions between cellular and non-cellular components result in a unique TME, which determines the disease outcome. The cellular components contribute to tumor growth by creating a unique environment in terms of oxygen supply, the availability of metabolites, and pH (3). Further, the interaction of tumor cells with the non-cellular components accelerates carcinogenesis and disease progression.
The physical forces within the TME play critical roles in leading the physiological and pathological processes of cancer (4). These forces have been known as critical components of the TME, and emerging evidence suggests that mechanical forces affect tumor behavior, including cell division, survival, and migration (5). As solid tumors grow, biomechanical forces may be generated as a result of an altered architecture within the TME. With an increasing number of cancer and noncancerous cells, the pressure inside the tumor increases, and the signals of mechanical forces are transferred to cancer cells, thus leading to mechanotransduction and cancer progression (6). There are many types of stress in the TME that can be loaded onto cancer cells, including substrate rigidity, fluid shear stress, hydrostatic pressure, tensile forces, and compressive forces (7).
In this review, we summarize some key biomechanical force changes that occur in the TME and describe how these changes generate pathophysiological forces. We also focus on how these biomechanical forces influence cancer progression (Fig. 1).
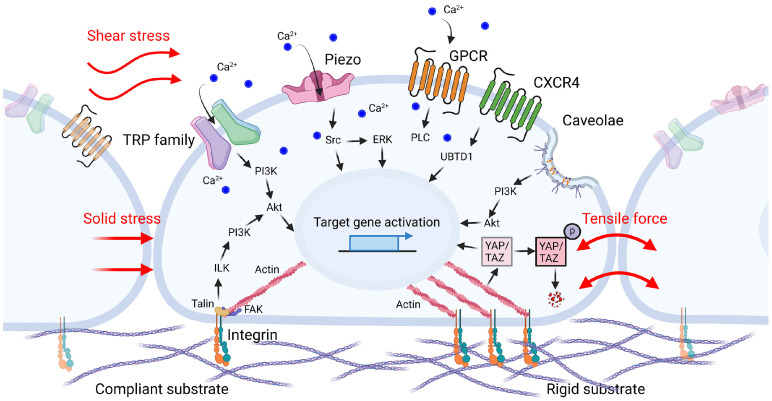
Fig. 1.
Mechanical forces and mechanosensors in cancer cells. As cancer progresses in the TME, the substrate rigidity of the ECM is elevated as well as the solid stress and tensile force are enhanced. Shear stress is also induced by vascular leakage and interstitial fluid flow. Altered microenvironment induced mechanical forces can be perceived by mechanosensors such as integrin, TRP family, Piezo, GPCRs, and caveolae on the membrane of cancer cells. The extrinsic signals detected by these sensors are converted into biochemical signals which promote migration and metastasis of cancer cell through signal transduction cascade.
SUBSTRATE RIGIDITY (STIFFNESS) OF ECM IS ALTERED DURING CANCER PROGRESSION
The extracellular matrix (ECM) has an important function within the TME, as it comprises up to 60% of the tumor mass in most solid cancers (8). The ECM is composed of proteoglycans, glycoproteins, and fibrous proteins such as collagen, fibronectin, elastin, and laminin, which provide biochemical signals and mechanical support to maintain cellular components (9). Throughout the development of cancer, excessive cell proliferation and abnormal ECM accumulation affect tissue stiffness (10). It is generally known that the stiffness of solid tumors is much higher than that of normal tissues. For example, normal mammary glands have a modulus of elasticity of less than 200 Pa, while breast cancers have a modulus of elasticity of more than 4 kPa (11, 12). Similarly, normal liver tissue has a stiffness of 300 to 600 Pa whereas liver cancer has a stiffness of 1.6 to 20 kPa (13). Cancer cells secrete significant amounts of ECM during tumorigenesis, which stiffens their TME as a result of increased fiber cross-linking (14). Alterations of ECM composition and substrate elasticity contributes to changes in the cytoskeletal structure of cancer cells, thus promoting metastasis (15).
Matrix stiffness and density also alter tumor cell behavior by promoting the activation of focal adhesion proteins, including integrin clustering, thereby strengthening the connection between the ECM and cytoskeleton. Integrin clustering triggers the recruitment of focal adhesion signaling, which further triggers focal adhesion signaling molecules, such as FAK, Src, and paxillin, thereby leading to signaling cascades and cytoskeletal reorganization through the small GTPases Rac, Rho, and Ras. It also promotes tumor formation by increasing cytoskeletal tension via ERK and Rho/ROCK signaling (11, 16). Pang et al. (17) demonstrated that increased ECM stiffness contributes to a malignancy in hepatocellular carcinoma by modulating the response through the β1 integrin/FAK/Rho GTPase signal transduction pathway as well as the activation of transforming growth factor-β1. ECM stiffness can also regulate miRNA transcription. Le et al. reported that rigid substrates modulate cell contractility by downregulating miR-203 through the ROBO1/Rac/FAK signaling pathway (18). Research has also found that ECM stiffness is related to the transforming growth factor-β (TGFβ) (19) and Hippo pathways (20, 21). Studies have shown that TGFβ increases actin polymerization in colorectal cancer cells, thus leading to the activation of the RhoA/LIMK/cofilin1 pathway, which regulates the actin cytoskeleton (22). Taken together, these reports suggest that cancer development is potentiated when ECM stiffness is increased by ECM remodeling or cell-cell adhesion through FAK, Rho, and integrin.
MECHANICAL FORCES REGULATING CANCER METASTASIS AND MIGRATION
In addition to the altered rigidity of the ECM, physical stimuli also induce changes in the TME, which affect tumor growth and metastasis (23). This section describes the types of physical forces that affect tumorigenesis and development—such as solid stress, fluid shear stress, and tensile force—which are generated as the tumor grows, and discusses the potential mechanotransductive signaling induced by biomechanical forces that alter tumor cell fate (Fig. 1).
Solid stress (compression force)
Solid stresses, such as compression forces, are present in solid tumor tissues. Since cancer cells proliferate rapidly in a limited space, the core of a solid tumor experiences higher stress than its border areas. Solid stress can affect cancer cell growth by either directly compressing cancer cells or indirectly compressing blood or lymphatic vessels, which can hinder cancer cell growth and induce apoptosis while increasing invasiveness and metastatic potential (24, 25). The solid pressure inside the tumor ranges from 45 to 120 mmHg, while the lymphatic or vascular pressure is from 6 to 17 mmHg (26). Walsh et al. (27) showed that the human colon cancer cell line SW620 proliferated more rapidly in response to a modest increase in pressure of 15 mmHg via the FAK/c-Src mechanism. Meanwhile, in Kalli’s study, a pressure of 4 mmHg was applied to the pancreatic cancer cell lines MIA PaCa-2 and BxPC-3 for 16 h using a transmembrane pressure device (28). Their results showed that solid stress increased the migration ability of cells by activating the PI3K/Akt/CREB1 pathway and upregulating the expression of GDF15 to promote cell migration. Overall, these results revealed that stress generated by the solid components of tumors affects cancer cell migration and promotes tumor progression.
Fluid shear stress
Cancer cells secrete vascular endothelial growth factors (VEGF) and other angiogenic factors during tumorigenesis, which results in disorganized angiogenesis and lymphangiogenesis (29). The formation of hyperpermeable tumor vessels increases both red blood cell concentration and blood viscosity due to the enhanced leakage of blood plasma into the interstitial space (30, 31). Moreover, as mentioned above in the section on solid stress, blood and lymphatic vessels are compressed, which increases the geometric resistance to flow. These abnormalities in the tumor microenvironment affect blood flow, interstitial fluid pressure, and fluid shear stress (6). During intravasation, transport through blood or lymphatic flow, and extravasation, cancer cells experiences frictional forces from adjacent cells and hydrodynamic flow (32). Fluid shear stress, which is induced by interstitial, lymphatic, or blood flow, is important for vascular remodeling and regulates tumor cell growth, metastasis, and transport. The shear stress of the interstitial flow range is approximately 0.02-2 dyne/cm2, while blood flow has a higher velocity. Blood flow can produce greater fluid shear at 1-4 dyne/cm2 in narrow vessels and at 4-30 dyne/cm2 in larger vessels (23). During metastasis, cancer cells, cancer stem-like cells (CSCs), or circulating tumor cells (CTCs) gradually leave circulation because of blood shear stress and successfully develop metastatic tumors (24). Lee et al. (33) simulated an interstitial fluid (0.05 dyne/cm2) using a microfluidic device to demonstrate the role of fluid shear stress in stimulating YAP1 to promote cancer cell migration. This process was induced by ROCK-triggered YAP1 activation via the LIMK-cofilin signaling axis (33). Triantafillu et al. (34) showed that, when CSCs were maintained within the circulation of breast cancer cells, a fluid shear stress of 20 dyne/cm2 mimicking blood flow promoted the expression of CD24, EpCaM, Oct-4, Nanog, and CSC markers. These results provide strong evidence indicating that shear stress might be a critical factor for cancer progression in the tumor microenvironment and therefore highlight the need to consider biomechanical forces when simulating in vitro tumor models.
Tensile force (stretch force)
Cancer cells are subjected to irregular growth, compressive stress from the external ECM, and internal tension from the surrounding tumor tissue. This can be visualized as a ball filling with an air; as the inside of the ball expands, the outside of ball stretches through interactions with its surroundings (24). Several studies have shown that tensile stress potentially contributes to cancer metastasis. Wang et al. (35) reported that cancer cell proliferation and migration were increased when breast cancer cells (MCF-7, MDA-MB-231, 4T1.2) were exposed to uniaxial cyclic strain or 10% static stretching at 10% and 0.3 Hz for 48 h. Moreover, the transplantation of cyclic stretch-conditioned 4T1.2 cells into BALB/c mice was shown to lead to a significant increase in tumor growth on days 8 and 11 compared to the transplantation of unstretched control cells. Tensile forces increase the lateral membrane tension of cells by activating mechanically gated ion channels known as stretch-activated channels (SACs) (36). The tensile force on the plasma membrane can induce the opening of mechanically gated ion channels, such as Piezo1/2, thus triggering Ca2+ signaling (37). Jiang et al. (38) reported that applying 20% surface elongation to human osteosarcoma cells (MG63 and U2) using a computer-controlled vacuum stretcher increased Piezo1 protein expression in the stretched group. They also found that Piezo1 shRNA could block osteosarcoma invasion in nude mice. These results suggest that the stretch force itself can affect cancer cell proliferation, migration, and invasion, in the same way that other mechanical forces in the TME influence cancer cell progression.
SENSORS ON CELLS AS TRANSDUCERS OF EXTRINSIC PHYSICAL CUES
External mechanical stimuli—such as the ECM, solid stress, fluid shear stress, and tensile force—are recognized based on the mechanosensitive machinery of cells, and they influence cell behavior through an intracellular cascade (Fig. 1). The conversion of mechanical stimuli into biochemical cascades consists of the following steps: (1) mechanotransmission, (2) mechanosensing, and (3) mechanosignaling (37). Cellular mechanosensing is based on force-induced conformational changes in various mechanosensitive proteins in cancer cells, which activate signaling pathways by opening transmembrane channels or changing their affinity for binding partners (39). In this section, we discuss how mechanosensors such as integrins, G-protein coupled receptors (GPCRs), ion channels, and caveolae perceive and transmit signals into cells and affect cellular behavior (Table 1).
Table 1.
Mechanosensors and their signaling in tumor microenvironment
| Mechanosensors | Functions | Interactions | Cells | Downstream | References |
|---|---|---|---|---|---|
| Cell adhesion molecules (Integrin) | |||||
| ITGβ1 | Malignant | Stiffness (10 kPa) | Human hepatocarcinoma cell (HepG2) | ITGβ1/FAK/GTPase | (17) |
| ITGβ1 | Invasive, angiogenesis | Stiffness (130-4,020 Pa) | Mouse mammary carcinoma cell (4T1) | ILK/PI3K/Akt | (40) |
| αvβ3 | Adhesion, migration, invasion | Shear stress (1.84 dyne/cm2, 2 h) | Human breast cancer cell (MDA-MB-231) | PI3K/Akt, NF-kB | (41) |
| α6β4 | Proliferation, migration | Shear stress (15 dyne/cm2, 12 h) | Human colon cancer (SW480) | β-catenin/PI3K/Rac1 | (74) |
| Transient receptor potential (TRP) family | |||||
| TRPV2 | Migration | Stretch (micropipette suction force, 10 mm H2O) | Human fibrosarcoma (HT1080) | Ca2+ influx, PI3K | (48) |
| TRPV4 | Metastasis | Stretch (micropipette suction force, elasticity, about 2-400 Pa) | Mouse breast cancer cell (4T07) | (49) | |
| Piezo | |||||
| Piezo1 | Metastasis | Shear stress (0.05 dyne/cm2) | Human prostate cancer cell (PC3) | Piezo1-Src-YAP | (54) |
| Piezo1 | Proliferation | Stiffness (5,000 Pa) | Human glioblastoma | Piezo1-integrin-FAK | (58) |
| Piezo1 | Invasion, migration | Compression (400 Pa) | Human breast cancer cell (MDA-MB-231) | Piezo1-Src-ERK | (55) |
| Piezo1 | Invasion | Stretch | Human osteosarcoma (MG63, U2) | (38) | |
| GPCRs | |||||
| OGR1 (GPR68, Gq/11 coupled receptor) | Ca2+ release | Disturbed shear stress (4 s, 60 Hz, 2 Pa) | Human breast cancer cell (MDA-MB-231) | PLC activation, Ca2+ release | (64) |
| OGR1 (GPR68, Gq receptor) | Migration | Stiffness (0.2 kPa), stretch (10-60%, 1 h) | Human medulloblastoma cell (DAOY), human osteosarcoma (MG63) | Ca2+ release | (65) |
| CXCR4 | Proliferation | Stiffness (12 kPa) | Human hepatocellular carcinoma (Hep3B, Huh7) | CXCR4/UBTD1/YAP | (66) |
| Caveolae | |||||
| Caveolin-1, Cavin-1 | Invasive | Pressure (osmotic stress, 440 mOsmol/kg; pressure, 30 mmHg) | Human glioblastoma (U118) | (72) | |
| Caveolin-1 | Migration, invasion | Shear stress (1.8-4 dyne/cm2) | Human breast cancer cell (MDA-MB-231) | PI3K/Akt/mTOR | (73) |
Integrin
Integrins can sense ECM stiffness, and cell-ECM interactions in normal and pathological conditions are primarily mediated through integrins, which regulate cell behaviors such as motility and migration (36). Using mechanical forces to strengthen ligand-integrin-cytoskeletal connections modulates cytoskeletal organization and activates intracellular signaling pathways to transmit mechanical and chemical signals. Pang et al. (40) demonstrated that increased ECM stiffness in breast cancer cells activates integrin-linked kinase (ILK) and enhances cancer cell invasiveness and angiogenesis through PI3K/Akt regulation, which eventually promotes tumor cell dissemination. Recent studies have shown that the physical deformation of membranes by mechanical forces, such as fluid shear stress, can induce integrin activation. For example, exposure to shear stress of 1.84 dynes/cm2 for 2 h has been shown to upregulate the expression of matrix metalloproteinase-2 (MMP-2), MMP-9, and αvβ3 integrin in MDA-MB-231 cells (41). Shear stress has also been shown to induced the sustained activation of p85, a regulatory subunit of PI3K and Akt, thus suggesting that αvβ3 integrin activation modulates downstream pathways for MDA-MB-231 cell adhesion, migration, and invasion. Similarly, breast cancer cells on a rigid 3D matrix increase Rho signaling through integrin-mediated mechanotransduction, which leads the disruption of epithelial adhesion and polarity and inducing invasion (11). Moreover, collagen-binding integrin α2β2 and kindlin2 have been found to be required for invadopodia and the invasion of fibrosarcoma cells or mesenchymal-like carcinoma cells in high-density fibrillar collagen (42). Integrin-mediated mechanotransductive pathway in cancer may not be limited to the activation of Rho or FAK signaling. In the adhesion and spreading of cells via integrins, mechanical forces transmitted by the cytoskeleton can act on the nucleus to activate the myocardin-related transcription factor (MRTF)-serum response factor (SRF) complex and modulate chromatin organization to induce epigenetic silencing via polycomb repressive complex 2 (43). Future studies should investigate how this process promotes tumorigenesis to target integrin-dependent mechanotransduction in cancer.
TRP family
Transient receptor potential (TRP) family proteins are a major group of Ca2+ channels that trigger the activation of specific intracellular cascades through changes in ion flux in response to various extracellular cues, including biochemical factors, pH, heat, and physical stimuli (44). The TRP family comprises more than 30 cation channels in various tissues. According to the sequence homology, the TRP superfamily can be divided into seven subfamilies: vanilloid (TRPV), ankyrin (TRPA), canonical (TRPC), melastatin (TRPM), mucolipin (TRPML), NOMPC (TRPN), and TRPP (polycystin) (45). Among them, TRPV channels have been shown to be associated with various types of human cancers. The major Ca2+-triggered pathways include the CAMKII, NF-κB, calpain, and calcineurin pathways, which are involved in cancer progression via cell proliferation, differentiation, and apoptosis (46, 47). The TRPV subfamily consists of TRPV members 1-6, which are further divided into two subgroups: TRPV1-4 and TRPV5-6. TRPV2 and TRPV4 are both sensitive to membrane stretching and play important roles in tissues subjected to high mechanical stress induced by solid tumors in the TME. Nagasawa and Kojima (48) investigated the effect of local mechanical force on TRPV2 localization in the human fibrosarcoma cell line, HT1080. Mechanical stress applied using a glass pipette locally activates PI3-kinase and induces the translocation of TRPV2, which leads to increased Ca2+ levels (48). Lee et al. (49) found that when pressure (400 Pa/s for 200 s) was applied using a glass pipette, TRPV4 modified the cytoskeleton of breast cancer cells through the Ca2+-dependent activation of Akt and downregulation of E-cadherin cell cortex protein. Since the remodeling of Ca2+ homeostasis in cancer cells may significantly contribute to tumor progression, TRPV—as a physical stimuli-induced Ca2+ channel— should be considered to be an important mechanosensor for propagating cancer signaling.
Piezo
Piezo channels, along with the TRP family, are mechanosensitive cation channels that detect mechanical signals. When activated, they increase cytosolic Ca2+ concentrations through rapid Ca2+ influx into the extracellular space, thus converting mechanical stimuli into intracellular signals. The two main variants of Piezo channels are Piezo1 and Piezo2. Piezo1 is a cation-selective channel that senses changes in the stiffness of the environment without requiring a helper protein for its activity. Dalghi et al. (50) found Piezo1 to be widely expressed in the murine urinary tract, including the kidney, ureter, bladder, and urethra, suggesting it plays a role as a mechanical sensor that is sensitive to wall tension and urine flow. Several studies have demonstrated that mechanical forces, including wall shear stress or stretch, significantly increase Piezo1 activity in human prostate cancer compared to normal tissue (51-53). Recently, Kim et al. (54) showed that fluid shear stress (0.05 dyne/cm2) increased the expression of Piezo1 in human prostate cancer cells (PC3, 22Rv1, and DU145) and played an important role in promoting cancer metastasis through the Piezo1-Src-YAP axis. In another study, Luo et al. (55) showed that, in breast cancer cells (MDA-MB-231), mechanical activation with compression (400 Pa) induces calcium influx through Piezo1 and stimulates downstream signaling pathways, such as Src and ERK, to form actin-based protrusions referred to as invadopodia. The invadopodia support the further invasion and migration of cancer cells by degrading the surrounding ECM via MMPs accumulated in these projections (55). Piezo2 plays a crucial role in proprioception and is also an important regulator of tumor angiogenesis and vascular permeability (39, 56). Although the Piezo2 channel has yet to be examined in cancer progression to the extent that Piezo1 has, Pardo-Pastor et al. (57) reported that Piezo2-mediated Ca2+ influx in metastatic breast cancer cells in the brain (MDA-MB-231-BrM2) activates RhoA modulating the formation and orientation of stress fibers and focal adhesions, thus bringing Fyn kinase to the cell leading edge and subsequently inducing calpain activation. In conclusion, they showed that the Piezo2-initiated signal transduction pathway affects various characteristics of cancer cell invasion and metastasis. Thus, Piezo senses various mechanical forces, including stiffness (58), compression (55), shear stress (54), and stretch, and it modulates the metastasis, proliferation, migration, and invasion of cancer.
G-protein coupled receptors (GPCRs)
The G-protein-coupled receptor (GPCRs) superfamily is the largest family of cell surface signaling receptors, which is encoded by more than 800-1,000 genes in the human genome (59). GPCRs—also called 7-transmembrane receptors—are structures that cross the cell membrane seven times and are linked to G proteins, Gα, Gβ, and Gγ, which bind to the guanine nucleotide GDP. Gα subunits can be further classified into four classes: Gαs, Gαi/o, Gαq/11, and Gα12/13 (60, 61). GPCRs regulate cell proliferation, survival, angiogenesis, immune cell evasion, migration, invasion, and metastasis (62). Although they have mainly been investigated in terms of their chemosensory function, several studies have shown that they can also serve as mechanical sensors when stimulated directly by mechanical forces (63).
It has been reported that the proton-sensing ovarian cancer G-protein coupled receptor 1 (OGR1, also known as GPR68) is activated by mechanical stress on the cellular membrane, such as membrane elongation. According to Xu et al. (64), human breast cancer cells (MDA-MB-231) showed little increase in intracellular calcium by GPR68 at pH 7.4 or higher, whereas the response of GPR68 to stimulation was maximal at pH 6.5 or lower. Moreover, when disturbed shear stress is applied at various pH levels, OGR1 increases phospholipase C (PLC) activation and Ca2+ release via the Gαq/11 receptor (64). Wei et al. (65) also demonstrated that a 10-60% stretch stress for 1 h in human medulloblastoma, DAOY, or human osteosarcoma MG63 activated OGR1, and that cytosolic Ca2+ levels were increased from intracellular Ca2+ stores by Gq-coupled receptors. Chemokine receptors have been linked to organ-specific metastases in various malignancies. One chemokine receptor—C-X-C chemokine receptor type 4 (CXCR4)—is the best-known chemokine receptor involved in proliferation, survival, and migration, and it is aberrantly expressed and involved in metastasis in many cancers (59). Yang et al. (66) reported that increased matrix stiffness (12 kPa) in hepatocellular carcinoma (HCC) markedly upregulates the expression of CXCR4, decreases the level of ubiquitin domain-containing protein 1 (UBTD1), and decreases the expression of YAP target genes involved in the proliferation, EMT, and stemness of HCC cells. These results suggest that GPCRs, such as OGR1 (GPR68) and CXCR4, play a role in chemokine sensing and regulate cancer progression when stimulated and activated by mechanical forces.
Caveolae
Caveolae are small (550-100 nm) plasma membrane invaginations that were first identified using electron microscopy in 1953 (67). Caveolae, which are primarily present in the plasma membrane, were initially assumed to be immobile (68); however, later studies established them as dynamic structures (69). Caveolae appear to mediate endocytosis, transcytosis, and potocytosis as well as support the uptake and intracellular delivery of bacteria, bacterial toxins, and viruses. They are composed of two essential structural proteins: caveolin (caveolin-1,2,3) and cavin (cavin-1,2,3,4). The caveolar neck contains EHD2 and Pacsin2 that binds Dynamin2 and the N-terminus of caveolin (70). Caveolae flattening is followed by caveolae disassembly, as indicated by the release of caveolin-1 and cavin-1. Numerous studies have implicated caveolin-1 in the regulation of tumor growth and several parameters related to cancer growth. Previous studies have focused on caveolin transcriptomic changes in tumors. However, recent findings have highlighted the need to investigate the mechanobiology of caveolae (67, 71). In another study, Pu et al. (72) showed that glioblastoma cell lines (U118) upregulated MMP and EMT markers in a caveolin-1 and cavin-1-dependent manner in response to osmotic pressure and increased their invasive potential. Moreover, Yang et al. (73) provided evidence for the important functional role of caveolin-1 in promoting invadopodia formation and tumor metastasis by sensing shear stress. In breast cancer cells (MDA-MB-231), low shear stress (1.8-4 dyne/cm2) exposure induced caveolin-1 activation and the PI3K/Akt/mTOR signaling cascade, increased MT1-MMP expression and trafficking, cytoskeletal reorganization, invasion formation, and ECM degradation, ultimately promoting tumor cell motility and metastasis (73). The role of caveolae in mechanical forces in cancer has yet to be well investigated. There is also an ongoing debate as to whether caveolin-1 promotes tumor function or has an antitumor function. Nonetheless, recent studies have suggested that some aspects of cancer progression may be controlled by the caveolae through physical cues.
CONCLUSION
During tumor progression, the architecture and microenvironment are gradually altered, followed by dynamic changes in the physical cues and forces surrounding the tumor. Biomechanical forces in TME affect cancer progression and metastasis. Here, we summarize the biomechanical forces applied to the TME and the mechanosensors on cells that receive stimuli from the TME. Physical cues—including rigid substrates or forces such as solid stress, fluid shear stress, and tensile force—stimulate cancer cells and various surface receptors or channels, as the mechanosensors of cancer cells perceive these stimuli and therefore propagate signal transduction. Recent research detailing the complexities of the TME and its biophysical requirements highlights the need to further elucidate its biological, biochemical, and biophysical aspects. Understanding the mechanisms underlying the mechanical properties of the TME can provide a new approach to cancer treatment, and this perspective is expected to promote the development of innovative therapeutic strategies.
ABBREVIATIONS
TME, tumor microenvironment; ECM, extracellular matrix; GTPase, guanosin triphosphatase; FAK, focal adhesion kinase; ERK, extracellular signal-regulated kinase; ROCK, Rho-associated protein kinase; ROBO1, roundabout homolog 1; TGFb, transforming growth factor-β; LIMK, Lim kinase; PI3K, phosphoinositide 3-kinase; CREB1, CAMP responsive element binding protein 1; GDF15, growth/differentiation factor 15; VEGF, vascular endothelial growth factor; CSC, cancer stem-like cell; CTC, circulating tumor cell; YAP1, Yes associated protein1; EpCaM, Epithelial cellular adhesion molecule; SAC, stretch-activated channel; GPCR, G-protein coupled receptor; ILK, integrin-linked kinase; MMP, matrix metalloproteinase; MRTF, Myocardin-related transcription factor; SRF, serum response factor; TRP, transient receptor potential; CAMKII, Ca2+/calmodulin-dependent protein kinase II; NF-κB, nuclear factor κB; OGR1, ovarian cancer G-protein coupled receptor 1; PLC, phospholipase C; CXCR4, C-X-C chemokine receptor type 4; HCC, hepatocellular carcinoma; UBTD1, ubiquitin domain-containing protein 1; EMT, epithelial-mesenchymal transition; EHD2, EH-domain containing 2; mTOR, mammalian target of rapamycin; MT1-MMP, membrane type 1 matrix metalloproteinase.
Funding Statement
ACKNOWLEDGEMENTS This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korean government (Grant No. 2020R1A2C2011617) and a Chung-Ang University Research Grant in 2021.
Footnotes
CONFLICTS OF INTEREST
The authors have no conflicting interests.
REFERENCES
- 1.Mansouri S, Heylmann D, Stiewe T, et al. Cancer genome and tumor microenvironment: reciprocal crosstalk shapes lung cancer plasticity. Elife. 2022;11:e79895. doi: 10.7554/eLife.79895.c0c5ba511710412b8bb64f4e1d4f7c41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jahanban-Esfahlan R, Seidi K, Banimohamad-Shotorbani B, Jahanban-Esfahlan A, Yousefi B. Combination of nanotechnology with vascular targeting agents for effective cancer therapy. J Cell Physiol. 2018;233:2982–2992. doi: 10.1002/jcp.26051. [DOI] [PubMed] [Google Scholar]
- 3.Brown JM, Wilson WR. Exploiting tumour hypoxia in cancer treatment. Nat Rev Cancer. 2004;4:437–447. doi: 10.1038/nrc1367. [DOI] [PubMed] [Google Scholar]
- 4.Moraes C, Sun Y, Simmons CA. (Micro) managing the mechanical microenvironment. Integr Biol (Camb) 2011;3:959–971. doi: 10.1039/c1ib00056j. [DOI] [PubMed] [Google Scholar]
- 5.Ladoux B, Mège RM. Mechanobiology of collective cell behaviours. Nat Rev Mol Cell Biol. 2017;18:743–757. doi: 10.1038/nrm.2017.98. [DOI] [PubMed] [Google Scholar]
- 6.Jain RK, Martin JD, Stylianopoulos T. The role of mechanical forces in tumor growth and therapy. Annu Rev Biomed Eng. 2014;16:321–346. doi: 10.1146/annurev-bioeng-071813-105259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jang I, Beningo KA. Integrins, CAFs and mechanical forces in the progression of cancer. Cancers. 2019;11:721. doi: 10.3390/cancers11050721.bb608f665d9442e183d901b930dfa535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Henke E, Nandigama R, Ergün S. Extracellular matrix in the tumor microenvironment and its impact on cancer therapy. Front Mol Biosci. 2020;6:160. doi: 10.3389/fmolb.2019.00160.9037d43c1e9148bbb71ee0ec94b00c8f [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Theocharis AD, Skandalis SS, Gialeli C, Karamanos NK. Extracellular matrix structure. Adv Drug Deliv Rev. 2016;97:4–27. doi: 10.1016/j.addr.2015.11.001. [DOI] [PubMed] [Google Scholar]
- 10.Huang J, Zhang L, Wan D, et al. Extracellular matrix and its therapeutic potential for cancer treatment. Signal Transduct Targeted Ther. 2021;6:153. doi: 10.1038/s41392-021-00544-0.667ad3620dca4d3db49ba213c192ee03 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Paszek MJ, Zahir N, Johnson KR, et al. Tensional homeostasis and the malignant phenotype. Cancer Cell. 2005;8:241–254. doi: 10.1016/j.ccr.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 12.Shieh AC. Biomechanical forces shape the tumor microenvironment. Ann Biomed Eng. 2011;39:1379–1389. doi: 10.1007/s10439-011-0252-2. [DOI] [PubMed] [Google Scholar]
- 13.Yu H, Mouw JK, Weaver VM. Forcing form and function: biomechanical regulation of tumor evolution. Trends Cell Biol. 2011;21:47–56. doi: 10.1016/j.tcb.2010.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Levental KR, Yu H, Kass L, et al. Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell. 2009;139:891–906. doi: 10.1016/j.cell.2009.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hynes RO. The extracellular matrix: not just pretty fibrils. Science. 2009;326:1216–1219. doi: 10.1126/science.1176009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Northey JJ, Przybyla L, Weaver VM. Tissue force programs cell fate and tumor aggression. Cancer Discov. 2017;7:1224–1237. doi: 10.1158/2159-8290.CD-16-0733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pang M, Teng Y, Huang J, Yuan Y, Lin F, Xiong C. Substrate stiffness promotes latent TGF-beta1 activation in hepatocellular carcinoma. Biochem Biophys Res Commun. 2017;483:553–558. doi: 10.1016/j.bbrc.2016.12.107. [DOI] [PubMed] [Google Scholar]
- 18.Le LT, Cazares O, Mouw JK, et al. Loss of miR-203 regulates cell shape and matrix adhesion through ROBO1/Rac/FAK in response to stiffness. J Cell Biol. 2016;212:707–719. doi: 10.1083/jcb.201507054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kanematsu A, Marui A, Yamamoto S, et al. Type I collagen can function as a reservoir of basic fibroblast growth factor. J Control Release. 2004;99:281–292. doi: 10.1016/j.jconrel.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 20.Noguchi S, Saito A, Nagase T. YAP/TAZ signaling as a molecular link between fibrosis and cancer. Int J Mol Sci. 2018;19:3674. doi: 10.3390/ijms19113674.dc0a121e92454994b70b47217f16ad4e [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Scott KE, Fraley SI, Rangamani P. A spatial model of YAP/TAZ signaling reveals how stiffness, dimensionality, and shape contribute to emergent outcomes. Proc Natl Acad Sci U S A. 2021;118:e2021571118. doi: 10.1073/pnas.2021571118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sousa-Squiavinato ACM, Rocha MR, Barcellos-de-Souza P, de Souza WF, Morgado-Diaz JA. Cofilin-1 signaling mediates epithelial-mesenchymal transition by promoting actin cytoskeleton reorganization and cell-cell adhesion regulation in colorectal cancer cells. Biochim Biophys Acta Mol Cell Res. 2019;1866:418–429. doi: 10.1016/j.bbamcr.2018.10.003. [DOI] [PubMed] [Google Scholar]
- 23.Huang Q, Hu X, He W, et al. Fluid shear stress and tumor metastasis. Am J Cancer Res. 2018;8:763–777. [PMC free article] [PubMed] [Google Scholar]
- 24.Tian BR, Lin WF, Zhang Y. Effects of biomechanical forces on the biological behavior of cancer stem cells. J Cancer. 2021;12:5895–5902. doi: 10.7150/jca.60893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stylianopoulos T. The solid mechanics of cancer and strategies for improved therapy. J Biomech Eng. 2017;139 doi: 10.1115/1.4034991. 10.115/1.4034991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Helmlinger G, Netti PA, Lichtenbeld HC, Melder RJ, Jain RK. Solid stress inhibits the growth of multicellular tumor spheroids. Nat Biotechnol. 1997;15:778–783. doi: 10.1038/nbt0897-778. [DOI] [PubMed] [Google Scholar]
- 27.Walsh MF, Woo RK, Gomez R, Basson MD. Extracellular pressure stimulates colon cancer cell proliferation via a mechanism requiring PKC and tyrosine kinase signals. Cell Prolif. 2004;37:427–441. doi: 10.1111/j.1365-2184.2004.00324.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kalli M, Minia A, Pliaka V, Fotis C, Alexopoulos LG, Stylianopoulos T. Solid stress-induced migration is mediated by GDF15 through Akt pathway activation in pancreatic cancer cells. Sci Rep. 2019;9:978. doi: 10.1038/s41598-018-37425-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Carmeliet P. VEGF as a key mediator of angiogenesis in cancer. Oncology. 2005;69:4–10. doi: 10.1159/000088478. [DOI] [PubMed] [Google Scholar]
- 30.Less JR, Posner MC, Skalak TC, Wolmark N, Jain RK. Geometric resistance and microvascular network architecture of human colorectal carcinoma. Microcirculation. 1997;4:25–33. doi: 10.3109/10739689709148315. [DOI] [PubMed] [Google Scholar]
- 31.Sun C, Jain RK, Munn LL. Non-uniform plasma leakage affects local hematocrit and blood flow: implications for inflammation and tumor perfusion. Ann Biomed Eng. 2007;35:2121–2129. doi: 10.1007/s10439-007-9377-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bacac M, Stamenkovic I. Metastatic Cancer Cell. Ann Rev Pathol Mech. 2008;3:221–247. doi: 10.1146/annurev.pathmechdis.3.121806.151523. [DOI] [PubMed] [Google Scholar]
- 33.Lee HJ, Diaz MF, Price KM, et al. Fluid shear stress activates YAP1 to promote cancer cell motility. Nat Commun. 2017;8:14122. doi: 10.1038/ncomms14122.8e8007f0521c4fb5861ef0ff47661f28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Triantafillu UL, Park S, Klaassen NL, Raddatz AD, Kim Y. Fluid shear stress induces cancer stem cell-like phenotype in MCF7 breast cancer cell line without inducing epithelial to mesenchymal transition. Int J Oncol. 2017;50:993–1001. doi: 10.3892/ijo.2017.3865. [DOI] [PubMed] [Google Scholar]
- 35.Wang Y, Goliwas KF, Severino PE, et al. Mechanical strain induces phenotypic changes in breast cancer cells and promotes immunosuppression in the tumor microenvironment. Lab Invest. 2020;100:1503–1516. doi: 10.1038/s41374-020-0452-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ross TD, Coon BG, Yun S, et al. Integrins in mechanotransduction. Curr Opin Cell Biol. 2013;25:613–618. doi: 10.1016/j.ceb.2013.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Montagner M, Dupont S. Mechanical forces as determinants of disseminated metastatic cell fate. Cells. 2020;9:250. doi: 10.3390/cells9010250.3281c4daccd94572ba303ef543d55335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jiang L, Zhao YD, Chen WX. The function of the novel mechanical activated ion channel Piezo1 in the human osteosarcoma cells. Med Sci Monit. 2017;23:5070–5082. doi: 10.12659/MSM.906959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li X, Wang J. Mechanical tumor microenvironment and transduction: cytoskeleton mediates cancer cell invasion and metastasis. Int J Biol Sci. 2020;16:2014–2028. doi: 10.7150/ijbs.44943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pang MF, Siedlik MJ, Han S, Stallings-Mann M, Radisky DC, Nelson CM. Tissue stiffness and hypoxia modulate the integrin-linked kinase ILK to control breast cancer stem-like cells. Cancer Res. 2016;76:5277–5287. doi: 10.1158/0008-5472.CAN-16-0579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhao F, Li L, Guan L, Yang H, Wu C, Liu Y. Roles for GP IIb/IIIa and alphavbeta3 integrins in MDA-MB-231 cell invasion and shear flow-induced cancer cell mechanotransduction. Cancer Lett. 2014;344:62–73. doi: 10.1016/j.canlet.2013.10.019. [DOI] [PubMed] [Google Scholar]
- 42.Artym VV, Swatkoski S, Matsumoto K, et al. Dense fibrillar collagen is a potent inducer of invadopodia via a specific signaling network. J Cell Biol. 2015;208:331–350. doi: 10.1083/jcb.201405099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Le HQ, Ghatak S, Yeung CYC, et al. Mechanical regulation of transcription controls polycomb-mediated gene silencing during lineage commitment. Nat Cell Biol. 2016;18:864–875. doi: 10.1038/ncb3387. [DOI] [PubMed] [Google Scholar]
- 44.Vangeel L, Voets T. Transient receptor potential channels and calcium signaling. Cold Spring Harb Perspect Biol. 2019;11:a035048. doi: 10.1101/cshperspect.a035048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pedersen SF, Owsianik G, Nilius B. TRP channels: an overview. Cell Calcium. 2005;38:233–252. doi: 10.1016/j.ceca.2005.06.028. [DOI] [PubMed] [Google Scholar]
- 46.Santoni G, Farfariello V, Amantini C. TRPV channels in tumor growth and progression. Adv Exp Med Biol. 2011;704:947–967. doi: 10.1007/978-94-007-0265-3_49. [DOI] [PubMed] [Google Scholar]
- 47.Yang D, Kim J. Emerging role of transient receptor potential (TRP) channels in cancer progression. BMB Rep. 2020;53:125–132. doi: 10.5483/BMBRep.2020.53.3.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nagasawa M, Kojima I. Translocation of TRPV2 channel induced by focal administration of mechanical stress. Physiol Rep. 2015;3:e12296. doi: 10.14814/phy2.12296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lee WH, Choong LY, Mon NN, et al. TRPV4 regulates breast cancer cell extravasation, stiffness and actin cortex. Sci Rep. 2016;6:27903. doi: 10.1038/srep27903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dalghi MG, Clayton DR, Ruiz WG, et al. Expression and distribution of PIEZO1 in the mouse urinary tract. Am J Physiol Renal Physiol. 2019;317:F303–F321. doi: 10.1152/ajprenal.00214.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hegarty PK, Watson RW, Coffey RN, Webber MM, Fitzpatrick JM. Effects of cyclic stretch on prostatic cells in culture. J Urol. 2002;168:2291–2295. doi: 10.1016/S0022-5347(05)64373-X. [DOI] [PubMed] [Google Scholar]
- 52.Hoyt K, Castaneda B, Zhang M, et al. Tissue elasticity properties as biomarkers for prostate cancer. Cancer Biomark. 2008;4:213–225. doi: 10.3233/CBM-2008-44-505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wadhera P. An introduction to acinar pressures in BPH and prostate cancer. Nat Rev Urol. 2013;10:358–366. doi: 10.1038/nrurol.2013.86. [DOI] [PubMed] [Google Scholar]
- 54.Kim OH, Choi YW, Park JH, et al. Fluid shear stress facilitates prostate cancer metastasis through Piezo1-Src-YAP axis. Life Sci. 2022;308:120936. doi: 10.1016/j.lfs.2022.120936. [DOI] [PubMed] [Google Scholar]
- 55.Luo M, Cai G, Ho KKY, et al. Compression enhances invasive phenotype and matrix degradation of breast cancer cells via Piezo1 activation. BMC Mol Cell Biol. 2022;23:1. doi: 10.1186/s12860-021-00401-6.c05d1e93a4ba44149544ed06481292ad [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.De Felice D, Alaimo A. Mechanosensitive piezo channels in cancer: focus on altered calcium signaling in cancer cells and in tumor progression. Cancers (Basel) 2020;12:1780. doi: 10.3390/cancers12071780.7c13a1d9fe114fe592d0282a358a3449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pardo-Pastor C, Rubio-Moscardo F, Vogel-Gonzalez M, et al. Piezo2 channel regulates RhoA and actin cytoskeleton to promote cell mechanobiological responses. Proc Natl Acad Sci U S A. 2018;115:1925–1930. doi: 10.1073/pnas.1718177115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chen X, Wanggou S, Bodalia A, et al. A feedforward mechanism mediated by mechanosensitive ion channel piezo1 and tissue mechanics promotes glioma aggression. Neuron. 2018;100:799–815. doi: 10.1016/j.neuron.2018.09.046. [DOI] [PubMed] [Google Scholar]
- 59.Chaudhary PK, Kim S. An insight into GPCR and G-proteins as cancer drivers. Cells. 2021;10:3288. doi: 10.3390/cells10123288.7302865e4a9043199bfb6c086dd28fee [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rosenbaum DM, Rasmussen SG, Kobilka BK. The structure and function of G-protein-coupled receptors. Nature. 2009;459:356–363. doi: 10.1038/nature08144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Venkatakrishnan AJ, Deupi X, Lebon G, Tate CG, Schertler GF, Babu MM. Molecular signatures of G-protein-coupled receptors. Nature. 2013;494:185–194. doi: 10.1038/nature11896. [DOI] [PubMed] [Google Scholar]
- 62.O'Hayre M, Degese MS, Gutkind JS. Novel insights into G protein and G protein-coupled receptor signaling in cancer. Curr Opin Cell Biol. 2014;27:126–135. doi: 10.1016/j.ceb.2014.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Balkwill F. Cancer and the chemokine network. Nat Rev Cancer. 2004;4:540–550. doi: 10.1038/nrc1388. [DOI] [PubMed] [Google Scholar]
- 64.Xu J, Mathur J, Vessieres E, et al. GPR68 senses flow and is essential for vascular physiology. Cell. 2018;173:762–775. doi: 10.1016/j.cell.2018.03.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wei WC, Bianchi F, Wang YK, Tang MJ, Ye H, Glitsch MD. Coincidence detection of membrane stretch and extracellular pH by the proton-sensing receptor OGR1 (GPR68) Curr Biology. 2018;28:3815–3823. doi: 10.1016/j.cub.2018.10.046. [DOI] [PubMed] [Google Scholar]
- 66.Yang N, Chen T, Wang L, et al. CXCR4 mediates matrix stiffness-induced downregulation of UBTD1 driving hepatocellular carcinoma progression via YAP signaling pathway. Theranostics. 2020;10:5790–5801. doi: 10.7150/thno.44789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lamaze C, Torrino S. Caveolae and cancer: a new mechanical perspective. Biomed J. 2015;38:367–379. doi: 10.4103/2319-4170.164229. [DOI] [PubMed] [Google Scholar]
- 68.Thomsen P, Roepstorff K, Stahlhut M, van Deurs B. Caveolae are highly immobile plasma membrane microdomains, which are not involved in constitutive endocytic trafficking. Mol Biol Cell. 2002;13:238–250. doi: 10.1091/mbc.01-06-0317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Boucrot E, Howes MT, Kirchhausen T, Parton RG. Redistribution of caveolae during mitosis. J Cell Sci. 2011;124:1965–1972. doi: 10.1242/jcs.076570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Buwa N, Mazumdar D, Balasubramanian N. Caveolin1 tyrosine-14 phosphorylation: role in cellular responsiveness to mechanical cues. J Membr Biol. 2020;253:509–534. doi: 10.1007/s00232-020-00143-0. [DOI] [PubMed] [Google Scholar]
- 71.Wang Z, Wang N, Liu P, et al. Caveolin-1, a stress-related oncotarget, in drug resistance. Oncotarget. 2015;6:37135–37150. doi: 10.18632/oncotarget.5789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pu W, Qiu J, Nassar ZD, et al. A role for caveola-forming proteins caveolin-1 and CAVIN1 in the pro-invasive response of glioblastoma to osmotic and hydrostatic pressure. J Cell Mol Med. 2020;24:3724–3738. doi: 10.1111/jcmm.15076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yang H, Guan L, Li S, et al. Mechanosensitive caveolin-1 activation-induced PI3K/Akt/mTOR signaling pathway promotes breast cancer motility, invadopodia formation and metastasis in vivo. Oncotarget. 2016;7:16227–16247. doi: 10.18632/oncotarget.7583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Avvisato CL, Yang X, Shah S, et al. Mechanical force modulates global gene expression and beta-catenin signaling in colon cancer cells. J Cell Sci. 2007;120:2672–2682. doi: 10.1242/jcs.03476. [DOI] [PubMed] [Google Scholar]