Abstract
Cancer immunotherapy has been acknowledged as a new paradigm for cancer treatment, with notable therapeutic effects on certain cancer types. Despite their significant potential, clinical studies over the past decade have revealed that cancer immunotherapy has low response rates in the majority of solid tumors. One of the key causes for poor responses is known to be the relatively low immunogenicity of solid tumors. Because most solid tumors are immune desert ‘cold tumors’ with antitumor immunity blocked from the onset of innate immunity, combination therapies that combine validated T-based therapies with approaches that can increase tumor-immunogenicity are being considered as relevant therapeutic options. This review paper focuses on immunogenic cell death (ICD) as a way of enhancing immunogenicity in tumor tissues. We will thoroughly review how ICDs such as necroptosis, pyroptosis, and ferroptosis can improve anti-tumor immunity and outline clinical trials targeting ICD. Finally, we will discuss the potential of ICD inducers as an adjuvant for cancer immunotherapy.
Keywords: Cancer immunotherapy, Cold tumor, Ferroptosis, Hot tumor, Immunogenic cell death, Necroptosis, Pyroptosis
INTRODUCTION
To improve the efficacy of cancer immunotherapy, previous studies have primarily focused on ways for maintaining the activity of tumor-infiltrating T lymphocytes (TIL) by alleviating the immunosuppressive tumor microenvironment (TME) such as hypoxia, low PH, inhibitory immune checkpoints, and T-cell suppressors (1, 2). These approaches presuppose the initiation of adaptive immunity after successful T-cell priming by antigen-presenting cells (APCs). However, substantial activation of adaptive immunity cannot be anticipated because a large number of solid tumors are cool tumors with limited immune cell infiltration and immunological responses (3). Indeed, cold tumors with low immune scores show poor responses to T-cell-based cancer immunotherapy, including immune checkpoint blockers (ICBs). Therefore, many immuno-oncologists pay attention to the transition of cold tumors into hot tumors before T-cell therapy (4, 5). To create a tumor microenvironment (TME) favorable for antitumor immunity, various approaches have been attempted to activate innate immunity by increasing intratumoral immunogenicity using oncolytic viruses (6), thermo-active and photo-active nanoparticles (7, 8), and the like. In particular, it has been revealed that new controllable types of lytic cell death can inflame tumor tissues, attempts to activate antitumor immunity by inducing immunogenic cell death (ICD) in tumors have attracted great interest (9, 10). Here, we comprehensively review what damage-associated molecular patterns (DAMPs) as key effectors of ICD are and how they activate antitumor immunity. We also introduce functional mechanisms of controllable lytic cell death such as necroptosis, pyroptosis, and ferroptosis. Therapeutic potential of ICD is also discussed by introducing clinical trials using ICD in cancer treatment.
DAMPs IN ICD
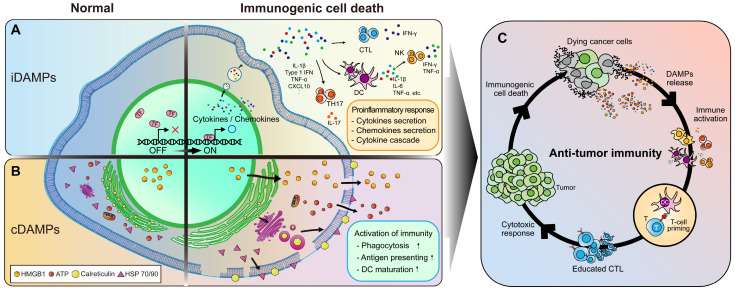
As an important mechanism of our immune system, immune cells are activated by recognizing exposure to non-self antigens or specific patterns released from damaged cells (11). Indeed, dying tumor cells undergoing ICD release intracellular danger signals called damage associated molecular patterns (DAMPs) and/or expose them on the cell surface, thus enhancing intratumoral immune responses and serving as important triggers for antitumor immunity (12). DAMPs can directly or indirectly modulate phagocytosis and antigen presenting activity of macrophages and dendritic cells. They can also create a pro-inflammatory environment in which innate immunity can work within the tumor (13). DAMPs can be subclassed into constitutive DAMPs (cDAMPs) and inducible DAMPs (iDAMPs) based on their expression and function (Fig. 1) (14).
Fig. 1.
Role of DAMPs in ICD. (A) Induction and role of iDAMPs during ICD. (B) Induction and role of cDAMPs during ICD. (C) Activation of antitumor immunity by DAMPs.
Constitutive DAMPs
It is known that cDAMPs have their own intracellular functions in normal conditions. However, when cells are damaged, cDAMPs are released extracellularly or loaded into the plasma membrane to play as alarm molecules to stimulate innate immune cells. Macrophages and dendritic cells have receptors that can recognize cDAMPs. They are activated by programmed ligand-receptor signaling. Recent studies have shown that release of cDAMPs from dying cancer cells can activate intratumoral immune responses. cDAMPs are emerging as a major effector of TME remodeling supporting antitumor immunity (Fig. 1A) (15).
High mobility group box 1 (HMGB1): HMGB1, a member of the high mobility group (HMG) subfamily, is a non-histone nuclear protein that has important roles in chromatin structure and transcriptional regulation (16). During ICD, HMGB1 can be passively diffused through a ruptured cell membrane and/or actively secreted by vesicular transport activated through cellular stress signals. Released HMGB1 is recognized by pattern-recognition receptors (PRRs), which can then activate various immune cells (17). In particular, released HMGB1 can bind to TLR4 of macrophages and activate the NK-kB signaling pathway, thereby increasing the expression of inflammatory cytokines and enhancing phagocytic activity (18). The HMGB1/PI3K/Akt/mTOR axis is also known to improve antigen-presenting ability by maturing macrophages and dendritic cells (19). On the other hand, HMGB1 can bind to receptors on vascular endothelial cells to increase expression of ICAM-1 and VCAM-1, thus enhancing the adhesion and migration of immune cells on blood vessels and promoting infiltration of immune cells into tumor tissues (20). Therefore, HMGB1 released from ICD can create a pro-inflammatory environment in which phagocytosis and antigen presentation activity by innate immunity are improved.
Calreticulin (CRT): Under normal conditions, CRT is located in the lumen of the endoplasmic reticulum (ER). It acts as a chaperone protein. CRT is also involved in homeostasis such as regulating calcium signals and assisting in the assembly and loading of MHC class I molecules (21). However, when ICD is induced, CRT translocates from the ER to the plasma membrane where it is exposed on the cell surface, which is called ecto-CRT with immunological functions (22). Induction of ICD results in translocation of CRT before phosphatidylserine exposure, which can be rapidly recognized by antigen-presenting cells (APC) like DCs and macrophages, triggering phagocytosis followed by antigen presentation and T cell priming. It has been reported that when cancer cells are treated with anthracycline, an ICD inducer, CRT is exposed more rapidly (23). Consistently, phagocytosis of DCs can be effectively suppressed when CRT is knocked-down or its function is inhibited by antibody treatment (24). Collectively, ICD-induced membrane exposure of CRT might act as an ‘eat me’ signal for immune cells and increase phagocytosis by APCs.
Heat shock proteins (HSPs): HSPs are chaperone proteins that can preserve cells by preventing protein misfolding and inhibiting apoptotic signalings. HSPs are markedly upregulated to inhibit apoptosis under stressful conditions such as oxidative stress, elevated temperature, and exposure to pro-inflammatory cytokines. HSPs are classified into six types (HSP40, HSP60, HSP70, HSP90, and HSP100) according to their molecular weights. HSP70 and HSP90 have high levels in cancer cells. These HSPs show strong immunogenicity when exposed to the cell surface (25). Furthermore, exposed HSP is known to promote secretion of cytokines such as interferon-γ (IFN-γ) known to be important for anticancer immunity by binding to scavenger receptors of antigen-presenting cells (APCs), such as CD91 and LOX1 (26). However, it has been reported that HSPs can be exposed to the extracellular space even under normal conditions. HSP-gp96 has also been reported to have immunosuppressive effects via regulatory T-cells in mouse skin graft transplantation (27, 28). Therefore, depending on the HSP, its function in immune response might be different. Further studies are needed to classify HSPs as DAMPs.
Extracellular adenosine triphosphate (eATP): ATP is known as a fundamental energy storage molecule that drives almost all cellular functions. Like other cDAMPs, ATP is also released into the extracellular space as a danger signal called eATP when ICD is induced. It has been reported that eATP can promote chemotaxis to immune cells by stimulating formation of NLRP3 inflammasome and inducing secretion of IL-1β, a caspase 1-mediated inflammatory cytokine (29). Various pathways by which eATP is released have been studied. Recent studies have demonstrated that eATP can also be secreted by dying cancer cells through the ER-Golgi secretion pathway. eATPs released by dying cancer cells as ‘find me’ signals can bind to P2Y2R of macrophages (to stimulate phagocytic activity to eliminate cancer cells) or P2X7R (mainly expressed in mast cells, macrophages, and DCs) to promote IFN-γ expression and CD8+ T-cell priming. In addition, neutrophils are known to secrete eATPs to recruit other immune cells (30). When ICD was induced in EL4 and EG7 cells by oxaliplatin, eATPs are released and activation signal of immune cells is amplified. Furthermore, inhibition of ATP production or treatment with apyrase, an ATP-diphosphohydrolase, can effectively neutralize such enhanced immune cell responses (31). Although eATP itself can activate antitumor immunity, eADP and AMP metabolized by CD39 and CD37 expressed in cancer cells and various stromal cells in the tumor microenvironment (TME) can effectively suppress the activity of cytotoxic T lymphocytes (CTLs), the main effector cells of antitumor immunity (32). Therefore, depending on the expression of CD39 and CD37 in cancer tissues, eATP can act as a double-edged sword for antitumor immunity.
Inducible DAMPs
Various intracellular signaling pathways activated during the early stage of cell death can promote transcriptional expression levels of cytokines and chemokines that support and recruit immune cells. These cytokines and chemokines are called iDAMPs to distinguish from cDAMPs. iDAMPs are necessary for maintaining a pro-inflammatory environment in which innate immunity is harmoniously activated (15) (Fig. 1B). It has been shown that a synergistic effect of iDAMPs induced by intracellular signal transduction pathways and cDAMPs released is very important for the activation of antitumor immunity (Fig. 1C). Thus, lytic cell death accompanied by an induction of iDAMPs is evaluated as a valuable ICD.
Tumor necroptosis factor α (TNF-α): TNF-α serves as a central mediator of autoimmunity, allergy, and microbial infection. In the past, TNF-α inhibitors have been mainly studied because TNF-α is overexpressed in autoimmune disease to exacerbate the disease (33). As the inflammatory environment created by ICD in tumor tissue becomes important for enhancing antitumor immunity, recent research studies have focused on developing new strategies to utilize TNF-α as an adjuvant for cancer immunotherapy. Macrophages express and secrete TNF-α through NF-κB and JNK signaling by recognizing cDAMPs. Secreted TNF-α can induce tumor necroptosis through tumor necroptosis factor receptor 1 (TNFR1) and amplify immune responses through tumor necroptosis factor receptor 2 (TNFR2) (34). It has been reported that the therapeutic effect of anti-PD-1 can be enhanced when tumors are treated with TNF-α armed adenoviruses (35). In addition, combined administration of recombinant mutated human TNF-α that specifically targets tumor vasculature (RGD-rmhTNF-α) and doxorubicin can increase tumor vascular permeability and antitumor efficacy (36). However, studies have shown that high concentrations of TNF-α released from tumors can cause vascular bleeding and contribute to the growth of cancer cells (37, 38). Therefore, in order to use TNF-α as a cancer immunotherapeutic agent, more studies are required to confirm its safety.
Interleukin 1 (IL-1) family: There are two types of IL-1, IL-1α and IL-1β. IL-1α can be produced and secreted by most cell types including epithelial cells as well as immune cells. On the other hand, IL-1β is mainly produced by immune cells. It acts as a pro-inflammatory cytokine alarm (39). Toll-like receptors (TLRs) of macrophages can form a complex with myeloid differentiation primary response 88 (MyD88) by binding to cDAMPs released from dying cells, leading to pro-IL-1β production through NF-κB signaling (40). In addition, NLRP3 activated by ICDs can form NLRP3 inflammasome together with pro-caspase 1. The inflammasome can convert pro-IL-1β to IL-1β (41). Consistently, neutrophil recruitment is significantly reduced when ICD is induced in mice lacking MyD88 and interleukin-1 receptor (IL-1R) proteins important for TLR signaling (42). Thus, TLR signaling and NLRP3 signaling can cooperatively regulate IL-1 expression at transcriptional and post-translational levels to enhance immune responses.
Interferons (IFNs): IFNs are known as multifunctional cytokines involved in various phases of innate and adaptive immunity, including immune responses to infectious pathogens (43). As cancer immunotherapy has recently received attention, roles of interferons in anti-tumor immunity have been emphasized. IFNs can be classified as type 1 IFNs (IFN-α, IFN-β, IFN-κ, etc.), type 2 IFN (IFN-γ), and type 3 IFN (IFN-λ). Dying cancer cells can release their nucleic acids (particularly single-stranded RNA) into the TME to activate TLR3 and induce the expression of type 1 and type 2 IFNs in surrounding cells that are still viable. Secreted IFNs can bind to interferon-α/β receptor (IFNAR) of their own cells (autocrine) or nearby immune cells (paracrine) to generate additional IFNs (44, 45). Especially, it is known that IFN-γ has multiple functions in cancer immunogenicity. IFN-γ can upregulate MHC class I, transporter associated with antigen-presenting (TAP)1/TAP2, and low molecular weight protein (LMP)2/LMP7 components of antigen-presenting machinery (46, 47). Additionally, IFN-γ can inhibit immunosuppressive factors transforming growth factor-β (TGF-β) and prostaglandin E2 (PGE2), thereby reducing the function of regulatory T-cells (48). Moreover, IFN-γ can enhance T-cell infiltration by increasing cytokines such as C-X-C motif chemokine ligand (CXCL) 9/10 and suppress tumor angiogenesis by downregulating vascular endothelial growth factor (VSVG) (49). Indeed, doxorubicin-induced ICD in tumors can increase the antitumor effect. This effect is neutralized by anti-IFN treatment (50).
CONTROLLABLE ICD PATHWAY
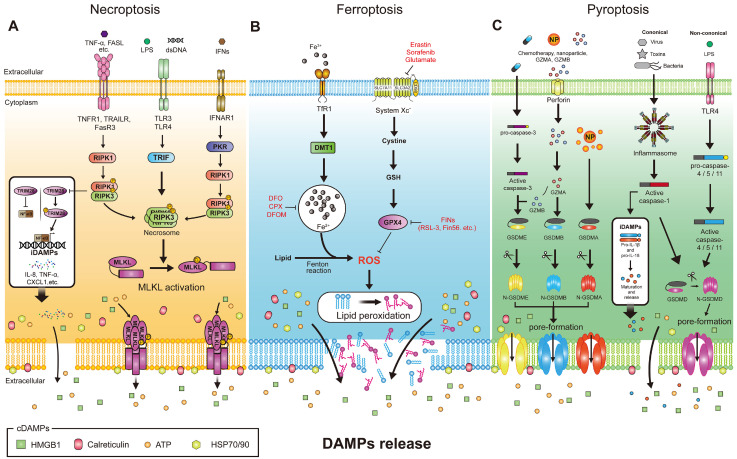
Lytic cell death has been recognized as an uncontrollable and detrimental cell death induced by severe conditions such as hypoxia, disease conditions, and traumatic injury (51). However, recent studies have reported new controllable lytic cell deaths and the possibility to increase immunogenicity by inducing mild lytic cell death in tumors (52). Such immunogenic cell death (ICD) initiated by specific external stimuli can activate innate or adapted immune cells to recognize cancer cells by releasing and/or exposing various DAMPs to the cell surface (53). Mechanisms of necroptosis, pyroptosis, and ferroptosis, which are ICDs that are attracting attention as controllable lytic cell death, and their potential to enhance antitumor immunity will be introduced next (Fig. 2).
Fig. 2.
The molecular machinery of controllable ICDs. (A) The signaling pathway of necroptosis. (B) The signaling pathway of ferroptosis. (C) The signaling pathway of pyroptosis.
Necroptosis
Necroptosis is one of the caspase-independent lytic cell deaths regulated by receptor-interacting protein kinases 3 (RIPK3) or receptor-interacting protein kinases (RIPK1) and mixed lineage kinase domain like pseudokinase (MLKL) (54) (Fig. 2A). Initiation of necroptosis is triggered by specific death receptors of the TNF receptor superfamily (TNFR, Fas, and DR4/DR5 that can recognize TNF, Fas ligand, and TRAIL (TNFSF10), respectively) or induced by Toll-like receptor 3 (TLR3) and Toll-like receptor 4 (TLR4) which can bind to viral RNA and lipopolysaccharide (LPS) from bacteria (55). The mechanism of TNF signaling is fairly well known. In TNFR signaling, trimerized TNF can bind to TNFR 1, RIPK1, TRADD with death domain (DD), TNF receptor-associated factor 2 (TRAF2), and cIAP1/2 are recruited to form Complex I (56). RIP1 deubiquitylated by CYLD can bind to FADD and TRADD to form complex II. FADD then recruits caspase-8 (apoptosis via activation of caspase cascade) and inactivates RIPK1 and RIPK3 to induce apoptosis, whereas inhibition of caspase-8 can suppress this pathway and lead to necroptosis. Therefore, inhibition of caspase-8 is required for the induction of necroptosis through TNF signaling (57). When caspase8 is inhibited, RIP homotypic interaction motif (RHIM) domains of RIPK1 and RIPK3 are phosphorylated to form complexes called necrosomes that can recruit MLKL to phosphorylate and oligomerize (58). Translocation of MLKL to the plasma membrane can induce TRPM7-mediated increases in cytoplasmic Ca2+ and ROS, intracellular acidification, and ATP depletion, resulting in plasma membrane rupture and initiation of necroptosis accompanied by the release of DAMPs (54, 59). Therefore, necroptosis is known as an immunogenic cell death that can contribute to antitumor immunity by inducing activation of the immune system with morphological characteristics of leakage of intracellular substances (60).
Many studies have reported that necroptosis of cancer cells can mediate intratumoral immune responses by promoting interactions between immune cells through the release of cDAMPs and iDAMPs. RIPK1/RIPK3 activated during necroptosis can inhibit tripartite motif protein 28 (TRIM28), which then suppresses the production of inflammatory cytokines. Accordingly, enhanced release of cDAMPs and iDAMPs during necroptosis is thought to enhance immuno-anticancer effects (61). In HCC patients, increased RIPK1, RIPK3, and MLKL-p levels were positively correlated with increases of intratumoral CD3+ and CD8+ T-lymphocytes, with the expression of RIP3 having a positive effect on chemotherapy (62, 63). In addition, AAV-induced expression of pro-necroptotic RIPK3 can promote necroptosis in the TME, enhance neoantigen presentation activity, and induce tumor-specific CD8+ T-cell priming, resulting in improved therapeutic efficacy of immune checkpoint blocker (64). Furthermore, it has been reported that combination therapy of radiotherapy, chemotherapy, and hyperthermia with zVAD-fmk, a necroptosis inducer, can increase macrophage activation, retardation of tumor growth, and immune cell infiltration into tumors through the release of DAMPs in melanoma (65). These reports suggest that necroptosis by activation of RIPK1/RIPK3 in the TME can induce maturation of APCs through the release of DAMPs and promote strong antitumor immunity by inducing tumor-specific CD8+ T-cells.
Ferroptosis
Ferroptosis is an iron-dependent form of regulated cell death that occurs through peroxidation of polyunsaturated fatty acids (PUFAs), which are abundant in phospholipids (66) (Fig. 2C). The central axis of ferroptosis regulation, the system Xc-/glutathione (GSH)/glutathione peroxidase 4 (GPX4) pathway, is well known as a major target for inducing ferroptosis. System Xc- is a cystine-glutamate antiporter composed of SLC7A11 (xCT) and SLC3A2 (4F2hc) that can transport intracellular glutamate and extracellular cystine. Cystine is transported from the extracellular space in the form of oxidized disulfide, reduced to cysteine within cells, and used for the synthesis of GSH, an antioxidant (67). The antioxidant enzyme GPX4 uses GSH as a cofactor and acts as a catalyst to reduce lipid peroxides that are toxic to cells (68). Inhibition of System Xc- can reduce GSH synthesis, resulting in inactivation of GPX4. Thus, ferroptosis can be induced by inactivating a major protective mechanism of cell membranes against damage by lipid peroxidation.
Main pathways inducing ferroptosis can be divided into a canonical pathway by inhibiting GSH and GPX4 and a non-canonical pathway by increasing intracellular labile iron pool (LIP) (69). First, class I FINs (Ferroptosis Inducing agents) including erastin, sulfasalazine, and glutamate known to inhibit system Xc- can induce depletion of GSH and inactivate GPX4 (70, 71) while class II FINs that directly target GPX4, such as RSL3, can inhibit enzyme activity through covalent binding with GPX4 (72). Class III FINs include FIN56, which depletes the antioxidant coenzyme Q10 (CoQ 10) with depletion of GPX4 (73). Class IV FIN can induce lipid peroxidation by directly increasing iron oxide or by forming free radicals through Fenton reaction of ferrous iron (Fe2+), a reduced state of LIP (74).
Interestingly, since cancer cells are much more sensitive to iron depletion than normal cells due to their high dependence on iron for cell growth, a new therapeutic approach using ferroptosis for cancers resistant to conventional anticancer drugs has been proposed (75-77). Many studies have suggested that induction of ferroptosis can be used for treating aggressive cancers such as breast cancer (78), prostate cancer (79), kidney cancer (80), pancreatic cancer (81), and acute myeloid leukemia (AML) (82) that are resistant to conventional anticancer drugs (83).
Ferroptosis has also been reported as an immunogenic cell death accompanied by the release of DAMPs (HMGB1, ATP) that can trigger inflammatory and immune responses during cell death (84, 85). It has been demonstrated that 1-steaoryl-2-15-HpETE-sn-glycero-3-phosphatidylethanolamine (SAPE-OOH), a major eat-me signal expressed on the surface of ferroptotic cells, can directly interact with TLR2 in macrophages, allowing ferroptotic cells to be effectively eliminated through phagocytosis (86). Recovery of CD8+ cytotoxic T cell function within the TME is a key factor determining responsiveness of cancer immunotherapy (87). Efimova et al. have demonstrated the possibility that ferroptosis could stimulate the adaptive immune system through results showing that DAMPs released during early ferroptosis can increase phenotypic maturation and cancer cell phagocytosis of bone marrow-derived dendritic cells (BMDCs) (88). In a colon cancer model, upregulation of OTUD1, a deubiquitinase of IREB2, can activate IREB2-TFRC signal. An increase in intracellular iron concentration by activation of TFRC induced ferroptosis. As a result, ICD by upregulation of OTUD1 was accompanied by the release of DAMPs and enhanced T-cell responses to cancer cells (89). Consequently, ferroptosis inducers can promote antitumor immune responses by inducing ICD and enhancing the efficacy of cancer treatment via combined administration with cancer immunotherapeutic agents (90). In addition, a recent study has shown that IFN-γ secreted by the activation of CD8+ T-cells by ICB can induce ferroptosis by suppressing the expression of SLC3A2 and SLC7A11 in tumor cells (91). In another study, inhibition of SLC7A11 expression by radiotherapy showed a synergistic effect with PD-L1/PD-1 or CTLA4 blockade (92). Based on these studies, the great potential of ferroptosis in cancer treatment has emerged and ferroptosis inducers have been rapidly developed in recent years. Since ferroptosis can induce ICD as one of the regulated cell deaths, it can be combined with cancer immunotherapeutic drugs. Accordingly, induction of ferroptosis can be a new strategy to improve response rate to cancer immunotherapy (93).
Pyroptosis
Pyroptosis is a programmed cell death that occurs in response to a pathogen infection (Fig. 2B). Pyroptosis is characterized by leakage of intracellular substances through cell swelling, disruption of the plasma membrane, and the release of many pro-inflammatory factors including interleukin (IL)-1β, IL-18, ATP, and HMGB1 (94-96). It has been known that pyroptosis is induced in a caspase-1-dependent manner in macrophages by pathogen infection (97). However, pyroptosis has emerged as a potential regulated cell death for cancer treatment recently (98).
Gasdermins, inflammasomes, and pro-inflammatory cytokines are all essential components of the pyroptotic cell death pathway. Targeting this pathway could be an effective therapeutic approach for cancer immunotherapy. Pyroptosis is divided into a canonical pathway in which caspase-1 is activated by the inflammasome and a non-canonical pathway in which caspase-4/-5 (caspase-11 in mouse) is mediated by LPS or lipid A (99, 100).
The canonical pyroptosis pathway is initiated by pattern-recognition receptors (PRR) such as nucleotide-binding and oligomerization domain (NOD)-like receptors (NLRP1, NLRC4, and NLRP3), AIM2 and TRIM family (Pyrin/TRIM20) that can recognize dsDNA, pathogen-associated molecular patterns (PAMPs), and DAMPs to assemble inflammasome (101, 102). The inflammasome is a protein complex containing pro-caspase-1 and apoptosis-associated speck-like protein containing a CARD domain (ASC), an adaptor protein. It is assembled in response to stimuli to activate caspase-1 (103). Caspase-1, also known as IL-1β converting enzyme, plays two main roles in the canonical pyroptosis pathway. First, caspase-1 cleaves the hinge region between N- and C-terminal domains of gasdermin D (GSDMD) and inserts a GSDMD N-terminal domain into cell membrane, creating a pore with a diameter of about 15 nm (104). Second, caspase-1 cleaves and activates inflammatory cytokines pro-IL-1β and pro-IL-18. In contrast, in the non-canonical pathway, caspase-4/-5/-11 is activated by direct binding of LPS through the CARD domain to cleave GSDMD (105).
The gasdermin (GSDM) superfamily consists of GSDMA, GSDMB, GSDMC, GSDMD, GSDME, and Pejvakin (PJVK). GSDMD and GSDME have been studied as major factors in pyroptosis (106, 107). All GSDMs except for PJVK contain a pore-forming N-terminal domain and an autoinhibitory C-terminal domain (108). GSDME is activated by caspase-3. ASC can initiate GSDME-dependent pyroptosis by independently activating caspase-8 in the absence of caspase-1/-11 (109, 110).
Pyroptosis is a cell death with highly immunogenicity that can induce local inflammation and immune cell infiltration. Therefore, inflammatory response induced by pyroptosis can suppress cancer cell proliferation by alleviating immunosuppression of the tumor microenvironment (TME) and activating antitumor immunity (111). A recent study has shown that GSDME-mediated pyroptosis by caspase-3 in melanoma can induce activation of DCs through release of HMGB1 and enhance antitumor immune responses in a T-cell-dependent manner (95). Additionally, exogenous activation of pyroptosis appears to induce potent antitumor activity. IFN-γ-induced GSDMB is cleaved by granzyme A of cytotoxic lymphocytes, causing pyroptosis. Anti-PD-1 treatment can induce effective tumor reduction in GSDMB-expressing mice (112).
Furthermore, Wang et al. have developed nanoparticles (NPs) capable of inducing pyroptosis by GSDMA in a recent study. Although pyroptosis occurs in only a fraction of the tumor using these NPs, all tumor cells are regressed by immune cells. ICB treatment showed a synergistic effect due to increased CD4+ and CD8+ T-cells in the tumor by pyroptosis (113). As a result of in vitro and in vivo as well as clinical data evaluating pyroptosis potential, CD4+ and CD8+ T-cells, NK cells, macrophages, and DCs showed positive correlations, indicating that pyroptosis could activate inflammatory tumor immune microenvironment. Moreover, cancer immunotherapy responders had a high pyroptosis potential, suggesting that pyroptosis could be a biomarker for ICB treatment (114). These results indicate that success of ICB treatment depends on activity of antitumor T-cells in the TME. ICB alone might not be sufficient to increase the number of antitumor CD8+ T-cells (115). Consequently, it is expected that ICBs can be more effective by combining them with inducers of pyroptosis that can increase tumor-infiltrating T-cells.
CLINICAL TRIALS RELATED TO ICD
The antitumor efficacy of ICD has been confirmed both in vitro or in vivo. Clinical studies related to ICD are still being actively conducted (Table 1). A total of 13 clinical trials are in progress or have been completed from the perspective of cell death in tumor tissue and cancer immunotherapy (5 completed, 8 in progress). Among them, two completed clinical trials were conducted directly targeting ICD. First, a phase 2 clinical trial (NCT01666444) was conducted to find a drug that could enhance the antitumor immune response effect of pegylated liposomal doxorubicin (PLD) in patients with recurrent epithelial ovarian cancer. In this study, the efficacy and safety of PLD alone or in combination with VTX-2337 (motolimod), a selective small molecule agonist for Toll-like receptor 8 (TLR8) involved in immune system regulation, were compared. VTX-2337 is primarily used to stimulate the immune system by activating TLR8, thereby activating NK cells and increasing the production of IFN-γ and antibody-dependent cellular cytotoxicity (ADCC) (116, 117). Therefore, this study hypothesized that administration of VTX-2337 to ovarian cancer patients could activate immune cells in the tumor and enhance the antitumor effect of PLD by inducing an immune response caused by TLR8. As a result, although the combination of PLD and VTX-2337 showed superior tolerability, no significant results were observed in overall survival or progression-free survival. Clinical results were not significantly improved either. However, it was confirmed that VTX-2337 activated the immune response by increasing the expression of inflammatory cytokines and chemokines in plasma (118).
Table 1.
Clinical trials targeting ICD
| Conditions | NCT number | Trial title | Treatment | Phases | Enrollment |
|---|---|---|---|---|---|
| Metastatic colorectal cancer | NCT03186326 | Standard Chemotherapy vs Immunotherapy in 2nd Line Treatment of MSI Colorectal Metastatic Cancer | FOLFOX, FOLFIRI, Avelumab, Panitumumab, Cetuximab, Bevacizumab, Aflibercept | Phase 2 | 132 |
| NCT03388190 | METIMMOX: Colorectal Cancer METastasis – Shaping Anti tumor IMMunity by OXaliplatin | FLOX, Nivolumab | Phase 2 | 80 | |
| NCT03721653 | FOLFOXIRI + Bev + Atezo vs FOLFOXIRI + Bev as First-line Treatment of Unresectable Metastatic Colorectal Cancer Patients | FOLFOXIRI, Bevacizumab, Atezolizumab | Phase 2 | 201 | |
| NCT04262687 | Chemotherapy and Immunotherapy as Treatment for MSS Metastatic Colorectal Cancer With High Immune Infiltrate (POCHI) | Capecitabine, Oxaliplatin, Bevacizumab, Pembrolizumab | Phase 2 | 55 | |
| Ovarian cancer | NCT01637532 | Feasibility of the Combination of Chemotherapy (Carbo/Caelyx or Carbo/Doxorubicin) With Tocilizumab (mAb IL-6R) and Peg Intron in Patients With Recurrent Ovarian Cancer | Carboplatin and caelyx or doxorubicin, Tocilizumab and interferon alpha 2-b | Phase 1/Phase 2 | 21 |
| NCT01666444 | VTX-2337 and Pegylated Liposomal Doxorubicin (PLD) in Patients With Recurrent or Persistent Epithelial Ovarian, Fallopian Tube or Primary Peritoneal Cancer | VTX-2337, Pegylated liposomal doxorubicin | Phase 2 | 297 | |
| NCT04072263 | Adoptive T Cell Therapy in Patients With Recurrent Ovarian Cancer | Tumor-infiltrating lymphocytes, Interferon alfa 2A, Carboplatin, Paclitaxel | Phase 1/Phase 2 | 12 | |
| Breast cancer | NCT03164993 | Atezolizumab Combined With Immunogenic Chemotherapy in Patients With Metastatic Triple negative Breast Cancer | Atezolizumab, Pegylated liposomal doxorubicin, Cyclophosphamide | Phase 2 | 75 |
| NCT03409198 | Phase IIb Study Evaluating Immunogenic Chemotherapy Combined With Ipilimumab and Nivolumab in Breast Cancer | Ipilimumab, Nivolumab, Pegylated liposomal doxorubicin, Cyclophosphamide | Phase 2 | 82 | |
| Endometrial cancer | NCT03276013 | Pembrolizumab in Combination With Doxorubicin in Advanced, Recurrent or Metastatic Endometrial Cancer | Doxorubicin, Pembrolizumab | Phase 2 | 48 |
| B-cell lymphoma | NCT03321643 | Atezolizumab, Gemcitabine, Oxaliplatin, and Rituximab in Treating Patients With Relapsed or Refractory Transformed Diffuse Large B-Cell Lymphoma | Atezolizumab, Gemcitabine, Oxaliplatin, Rituximab | Phase 1 | 24 |
| Non-small cell lung cancer | NCT04043195 | Nivolumab and Ipilimumab in Combination With Immunogenic Chemotherapy for Patients With Advanced NSCLC | Oxaliplatin, Nivolumab, Ipilimumab | Phase 1/Phase 2 | 30 |
| Uveal melanoma | NCT04463368 | Isolated Hepatic Perfusion in Combination With Ipilimumab and Nivolumab in Patients With Uveal Melanoma Metastases | Melphalan, Ipilimumab, Nivolumab | Phase 1 | 18 |
Another phase 1/2 clinical trial (NCT01637532) was conducted to determine the combination of standard chemotherapy and immunotherapy by confirming the feasibility of combining carboplatin and doxorubicin, chemotherapeutic agents that could induce ICD, with tocilizumab, an anti-IL-6R antibody in recurrent ovarian cancer patients. IL-6 promoted the development of bone marrow-derived suppressor cells (MDSC) with immunosuppressive functions (119), enhanced tumor cell survival, and increased resistance to chemotherapy in ovarian cancer (120). Therefore, this study hypothesized that blocking IL-6R with tocilizumab could inhibit the IL-6 pathway, thereby eliminating drug resistance and immunosuppressive responses and resulting in enhanced ICD and antitumor responses. Although results have not been completely published yet, drug resistance, dose-limiting toxicity (DLT), and treatment-related death did not occur. It was confirmed that IL-6 activity was clearly blocked when the highest dose of 8 mg/kg tocilizumab was administered (121). As such, some clinical trials are being conducted to evaluate the combination of ICD-inducing chemotherapeutic agents with immunotherapy. However, many clinical trials are also being conducted to predict whether antitumor responses could be induced through specific ICD biomarkers identified in vitro. A recent clinical trial (NCT029 21854) is investigating whether ICD markers such as HMGB1, HSP70, HSP90, and CRT can be detected in serum after non-small cell lung cancer (NSCLC) patients are treated with radiation therapy and cisplatin, a chemotherapeutic agent (122). If DAMPs released by lytic cell death from tumor tissue can be effectively detected in the serum, it is expected to be used as an important biomarker for determining application of cancer immunotherapeutic drugs after ICD induction. In this way, many clinical trials related to ICD are still actively undergoing. These clinical trial results will enable us to better understand interactions between ICD, tumor, and immune response and achieve a practical therapeutic approach to activate anticancer immunity in tumors.
CONCLUDING REMARKS
As characteristics of cold tumors have been studied, the activity of innate immunity in TME has been recognized as an important target for the activation of systemic antitumor immunity. In this regard, necroptosis, ferroptosis, and pyroptosis are different forms of controllable lytic cell death with potential to create an inflammatory environment in immune desert by releasing sufficient DAMPs during cancer cell death. Therefore, they are garnering attention as critical ICDs that can transfer cold tumors into hot tumors by imparting immunogenicity and activating antitumor immunity.
Although necroptosis, ferroptosis, and pyroptosis all offer a great deal of potential as ICDs for the treatment of cancer, cancer cell lines can exhibit very different sensitivity levels to these ICDs. Indeed, there are differences in the availability of enzymes that can affect the beginning and development of cell death. Expression levels of essential regulatory proteins involved in each kind of cell death can also be different. Differences in oxidative stress responses can also influence susceptibility to ferroptosis. Namely, sensitivity to various types of cell death can be influenced by genetic heterogeneity of cancer cells. Therefore, to effectively use ICD inducers as adjuvants for immunotherapy, it is necessary to determine the sensitivity to each type of cell death according to cancer type and patient and select the optimal ICD inducer based on this. As the opposite point of view, there also appears to be a connection between different types of cell death. In certain cases, it is difficult to discern the death phenotype. It has been shown that apoptosis-inducing agents can induce necroptosis and/or pyroptosis depending on the cancer cell. Therefore, if the link between apoptosis and ICDs is identified, existing efficient apoptosis agents might be repurposed for ICD induction.
Negative effects of normal tissue injury and multiple inflammatory responses should always be addressed when using ICD inducers. Tumor-specific drug delivery using carriers such as antibody-drug conjugates (ADCs) and nano-delivery systems can be very useful in reducing these side effects. In addition, tumor-specific ICD drug delivery can effectively eliminate not only primary tumor cancer cells, but also metastatic cancer cells, by triggering systemic anti-tumor immunity. Higher levels of anticancer treatment effectiveness are predicted in the near future as a result of the development of ADCs and/or nano-delivery systems that can load ICD inducers targeting necroptosis, ferroptosis, and pyroptosis.
In conclusion, ICD represents a promising strategy for treating cancer. This field has advanced significantly over the past decade. Several ongoing clinical trials are evaluating the efficacy of ICDs in various cancer types. Outcomes of these trials will provide important insight into the potential of this approach. Despite some challenges, the future of cancer treatments targeting ICD is promising. Continued research and innovation in this field will lead to new and more effective treatments for cancer patients.
Funding Statement
ACKNOWLEDGEMENTS This work was supported by INHA UNIVERSITY Research Grant.
Footnotes
CONFLICTS OF INTEREST
The authors have no conflicting interests.
REFERENCES
- 1.Swann JB, Smyth MJ. Immune surveillance of tumors. J Clin Invest. 2007;117:1137–1146. doi: 10.1172/JCI31405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Verma NK, Wong BHS, Poh ZS, et al. Obstacles for T-lymphocytes in the tumour microenvironment: therapeutic challenges, advances and opportunities beyond immune checkpoint. EBioMedicine. 2022;83:104216. doi: 10.1016/j.ebiom.2022.104216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang M, Wang S, Desai J, Trapani JA, Neeson PJ. Therapeutic strategies to remodel immunologically cold tumors. Clin Transl Immunology. 2020;9:e1226. doi: 10.1002/cti2.1226.2dea8cbb86c348f8bf8ac816c4d45a10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Duan Q, Zhang H, Zheng J, Zhang L. Turning cold into hot: firing up the tumor microenvironment. Trends Cancer. 2020;6:605–618. doi: 10.1016/j.trecan.2020.02.022. [DOI] [PubMed] [Google Scholar]
- 5.Zhang J, Huang D, Saw PE, Song E. Turning cold tumors hot: from molecular mechanisms to clinical applications. Trends Immunol. 2022;43:523–545. doi: 10.1016/j.it.2022.04.010. [DOI] [PubMed] [Google Scholar]
- 6.Kaufman HL, Kohlhapp FJ, Zloza A. Oncolytic viruses: a new class of immunotherapy drugs. Nat Rev Drug Discov. 2015;14:642–662. doi: 10.1038/nrd4663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li K, Zhang Z, Mei Y, et al. Targeting the innate immune system with nanoparticles for cancer immunotherapy. J Mater Chem B. 2022;10:1709–1733. doi: 10.1039/D1TB02818A. [DOI] [PubMed] [Google Scholar]
- 8.Bazak R, Houri M, El Achy S, Kamel S, Refaat T. Cancer active targeting by nanoparticles: a comprehensive review of literature. J Cancer Res Clin Oncol. 2015;141:769–784. doi: 10.1007/s00432-014-1767-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rosenbaum SR, Wilski NA, Aplin AE. Fueling the fire: inflammatory forms of cell death and implications for cancer immunotherapy. Cancer Discov. 2021;11:266–281. doi: 10.1158/2159-8290.CD-20-0805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu M, Wang L, Xia X, et al. Regulated lytic cell death in breast cancer. Cell Biol Int. 2022;46:12–33. doi: 10.1002/cbin.11705. [DOI] [PubMed] [Google Scholar]
- 11.Chen GY, Nunez G. Sterile inflammation: sensing and reacting to damage. Nat Rev Immunol. 2010;10:826–837. doi: 10.1038/nri2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Krysko DV, Garg AD, Kaczmarek A, Krysko O, Agostinis P, Vandenabeele P. Immunogenic cell death and DAMPs in cancer therapy. Nat Rev Cancer. 2012;12:860–875. doi: 10.1038/nrc3380. [DOI] [PubMed] [Google Scholar]
- 13.Ahmed A, Tait SWG. Targeting immunogenic cell death in cancer. Mol Oncol. 2020;14:2994–3006. doi: 10.1002/1878-0261.12851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Serrano-Del Valle A, Anel A, Naval J, Marzo I. Immunogenic cell death and immunotherapy of multiple myeloma. Front Cell Dev Biol. 2019;7:50. doi: 10.3389/fcell.2019.00050.5b1d0ae9069f43dab824049eb445a43e [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Legrand AJ, Konstantinou M, Goode EF, Meier P. The diversification of cell death and immunity: memento mori. Mol Cell. 2019;76:232–242. doi: 10.1016/j.molcel.2019.09.006. [DOI] [PubMed] [Google Scholar]
- 16.Bustin M. Regulation of DNA-dependent activities by the functional motifs of the high-mobility-group chromosomal proteins. Mol Cell Biol. 1999;19:5237–5246. doi: 10.1128/MCB.19.8.5237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lui G, Wong CK, Ip M, et al. HMGB1/RAGE signaling and pro-inflammatory cytokine responses in non-HIV adults with active pulmonary tuberculosis. PLoS One. 2016;11:e0159132. doi: 10.1371/journal.pone.0159132.cde6aba2678342458596ad80b36c2c9b [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li L, Lu YQ. The regulatory role of high-mobility group protein 1 in sepsis-related immunity. Front Immunol. 2020;11:601815. doi: 10.3389/fimmu.2020.601815.34657a372f49429fbe883e0c50c4f1e3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ge Y, Huang M, Yao YM. The effect and regulatory mechanism of high mobility group box-1 protein on immune cells in inflammatory diseases. Cells. 2021;10:1044. doi: 10.3390/cells10051044.d5a0c75fc44d45e0965eec6be9831aa7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Klune JR, Dhupar R, Cardinal J, Billiar TR, Tsung A. HMGB1: endogenous danger signaling. Mol Med. 2008;14:476–484. doi: 10.2119/2008-00034.Klune. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pandya UM, Egbuta C, Abdullah Norman TM, et al. The biophysical interaction of the danger-associated molecular pattern (DAMP) calreticulin with the pattern-associated molecular pattern (PAMP) lipopolysaccharide. Int J Mol Sci. 2019;20:408. doi: 10.3390/ijms20020408.ea26aac3bf024ba5b3e4c9e177b8e6b1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Obeid M, Tesniere A, Panaretakis T, et al. Ecto-calreticulin in immunogenic chemotherapy. Immunol Rev. 2007;220:22–34. doi: 10.1111/j.1600-065X.2007.00567.x. [DOI] [PubMed] [Google Scholar]
- 23.Wemeau M, Kepp O, Tesniere A, et al. Calreticulin exposure on malignant blasts predicts a cellular anticancer immune response in patients with acute myeloid leukemia. Cell Death Dis. 2010;1:e104. doi: 10.1038/cddis.2010.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Obeid M, Tesniere A, Ghiringhelli F, et al. Calreticulin exposure dictates the immunogenicity of cancer cell death. Nat Med. 2007;13:54–61. doi: 10.1038/nm1523. [DOI] [PubMed] [Google Scholar]
- 25.Zunino B, Rubio-Patino C, Villa E, et al. Hyperthermic intraperitoneal chemotherapy leads to an anticancer immune response via exposure of cell surface heat shock protein 90. Oncogene. 2016;35:261–268. doi: 10.1038/onc.2015.82. [DOI] [PubMed] [Google Scholar]
- 26.Murshid A, Gong J, Calderwood SK. The role of heat shock proteins in antigen cross presentation. Front Immunol. 2012;3:63. doi: 10.3389/fimmu.2012.00063.420455f61bb74b219a8fe758deb8a931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van Eden W, van der Zee R, Prakken B. Heat-shock proteins induce T-cell regulation of chronic inflammation. Nat Rev Immunol. 2005;5:318–330. doi: 10.1038/nri1593. [DOI] [PubMed] [Google Scholar]
- 28.Kovalchin JT, Mendonca C, Wagh MS, Wang R, Chandawarkar RY. In vivo treatment of mice with heat shock protein, gp 96, improves survival of skin grafts with minor and major antigenic disparity. Transpl Immunol. 2006;15:179–185. doi: 10.1016/j.trim.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 29.Amores-Iniesta J, Barbera-Cremades M, Martinez CM, et al. Extracellular ATP Activates the NLRP3 inflammasome and is an early danger signal of skin allograft rejection. Cell Rep. 2017;21:3414–3426. doi: 10.1016/j.celrep.2017.11.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Venereau E, Ceriotti C, Bianchi ME. DAMPs from cell death to new life. Front Immunol. 2015;6:422. doi: 10.3389/fimmu.2015.00422.23c7a5ffba8b496fb670d7868e926991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ghiringhelli F, Apetoh L, Tesniere A, et al. Activation of the NLRP3 inflammasome in dendritic cells induces IL-1beta-dependent adaptive immunity against tumors. Nat Med. 2009;15:1170–1178. doi: 10.1038/nm.2028. [DOI] [PubMed] [Google Scholar]
- 32.Kepp O, Bezu L, Yamazaki T, et al. ATP and cancer immunosurveillance. EMBO J. 2021;40:e108130. doi: 10.15252/embj.2021108130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nyboe Andersen N, Pasternak B, Friis-Moller N, Andersson M, Jess T. Association between tumour necrosis factor-alpha inhibitors and risk of serious infections in people with inflammatory bowel disease: nationwide Danish cohort study. BMJ. 2015;350:h2809. doi: 10.1136/bmj.h2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Parameswaran N, Patial S. Tumor necrosis factor-alpha signaling in macrophages. Crit Rev Eukaryot Gene Expr. 2010;20:87–103. doi: 10.1615/CritRevEukarGeneExpr.v20.i2.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cervera-Carrascon V, Siurala M, Santos JM, et al. TNFa and IL-2 armed adenoviruses enable complete responses by anti-PD-1 checkpoint blockade. Oncoimmunology. 2018;7:e1412902. doi: 10.1080/2162402X.2017.1412902.d89957be564a46b29a243aac324e3904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jiang C, Niu J, Li M, Teng Y, Wang H, Zhang Y. Tumor vasculature-targeted recombinant mutated human TNF-alpha enhanced the antitumor activity of doxorubicin by increasing tumor vessel permeability in mouse xenograft models. PLoS One. 2014;9:e87036. doi: 10.1371/journal.pone.0087036.8bd34818776249cda38c7f80e195f21e [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Egberts JH, Cloosters V, Noack A, et al. Anti-tumor necrosis factor therapy inhibits pancreatic tumor growth and metastasis. Cancer Res. 2008;68:1443–1450. doi: 10.1158/0008-5472.CAN-07-5704. [DOI] [PubMed] [Google Scholar]
- 38.Cruceriu D, Baldasici O, Balacescu O, Berindan-Neagoe I. The dual role of tumor necrosis factor-alpha (TNF-alpha) in breast cancer: molecular insights and therapeutic approaches. Cell Oncol (Dordr) 2020;43:1–18. doi: 10.1007/s13402-019-00489-1. [DOI] [PubMed] [Google Scholar]
- 39.Zhang W, Borcherding N, Kolb R. IL-1 signaling in tumor microenvironment. Adv Exp Med Biol. 2020;1240:1–23. doi: 10.1007/978-3-030-38315-2_1. [DOI] [PubMed] [Google Scholar]
- 40.Chen L, Zheng L, Chen P, Liang G. Myeloid differentiation primary response protein 88 (MyD88): the central hub of TLR/IL-1R signaling. J Med Chem. 2020;63:13316–13329. doi: 10.1021/acs.jmedchem.0c00884. [DOI] [PubMed] [Google Scholar]
- 41.Nakamura K, Smyth MJ. Targeting cancer-related inflammation in the era of immunotherapy. Immunol Cell Biol. 2017;95:325–332. doi: 10.1038/icb.2016.126. [DOI] [PubMed] [Google Scholar]
- 42.Chen CJ, Kono H, Golenbock D, Reed G, Akira S, Rock KL. Identification of a key pathway required for the sterile inflammatory response triggered by dying cells. Nat Med. 2007;13:851–856. doi: 10.1038/nm1603. [DOI] [PubMed] [Google Scholar]
- 43.Platanias LC. Mechanisms of type-I- and type-II-interferon-mediated signalling. Nat Rev Immunol. 2005;5:375–386. doi: 10.1038/nri1604. [DOI] [PubMed] [Google Scholar]
- 44.Zhu Y, An X, Zhang X, Qiao Y, Zheng T, Li X. STING: a master regulator in the cancer-immunity cycle. Mol Cancer. 2019;18:152. doi: 10.1186/s12943-019-1087-y.73bd097f8b674b2b81d296eeb06cdb46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vacchelli E, Sistigu A, Yamazaki T, Vitale I, Zitvogel L, Kroemer G. Autocrine signaling of type 1 interferons in successful anticancer chemotherapy. Oncoimmunology. 2015;4:e988042. doi: 10.4161/2162402X.2014.988042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang S, Kohli K, Black RG, et al. Systemic interferon-gamma increases MHC class I expression and T-cell infiltration in cold tumors: results of a phase 0 clinical trial. Cancer Immunol Res. 2019;7:1237–1243. doi: 10.1158/2326-6066.CIR-18-0940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dovhey SE, Ghosh NS, Wright KL. Loss of interferon-gamma inducibility of TAP1 and LMP2 in a renal cell carcinoma cell line. Cancer Res. 2000;60:5789–5796. [PubMed] [Google Scholar]
- 48.Gangaplara A, Martens C, Dahlstrom E, et al. Type I interferon signaling attenuates regulatory T cell function in viral infection and in the tumor microenvironment. PLoS Pathog. 2018;14:e1006985. doi: 10.1371/journal.ppat.1006985.352cb6df548545caacd04633bbe41048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Coughlin CM, Salhany KE, Gee MS, et al. Tumor cell responses to IFNgamma affect tumorigenicity and response to IL-12 therapy and antiangiogenesis. Immunity. 1998;9:25–34. doi: 10.1016/S1074-7613(00)80585-3. [DOI] [PubMed] [Google Scholar]
- 50.Sistigu A, Yamazaki T, Vacchelli E, et al. Cancer cell-autonomous contribution of type I interferon signaling to the efficacy of chemotherapy. Nat Med. 2014;20:1301–1309. doi: 10.1038/nm.3708. [DOI] [PubMed] [Google Scholar]
- 51.Karsch-Bluman A, Feiglin A, Arbib E, et al. Tissue necrosis and its role in cancer progression. Oncogene. 2019;38:1920–1935. doi: 10.1038/s41388-018-0555-y. [DOI] [PubMed] [Google Scholar]
- 52.Koren E, Fuchs Y. Modes of Regulated cell death in cancermodes of regulated cell death in cancer. Cancer Discov. 2021;11:245–265. doi: 10.1158/2159-8290.CD-20-0789. [DOI] [PubMed] [Google Scholar]
- 53.Galluzzi L, Vitale I, Aaronson SA, et al. Molecular mechanisms of cell death: recommendations of the nomenclature committee on cell death 2018. Cell Death Differ. 2018;25:486–541. doi: 10.1038/s41418-017-0012-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cai Z, Jitkaew S, Zhao J, et al. Plasma membrane translocation of trimerized MLKL protein is required for TNF-induced necroptosis. Nat Cell Biol. 2014;16:55–65. doi: 10.1038/ncb2883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kaiser WJ, idharan H, Sr, Huang C, et al. Toll-like receptor 3-mediated necrosis via TRIF, RIP3, and MLKL. J Biol Chem. 2013;288:31268–31279. doi: 10.1074/jbc.M113.462341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhang Y, Chen X, Gueydan C, Han J. Plasma membrane changes during programmed cell deaths. Cell Res. 2018;28:9–21. doi: 10.1038/cr.2017.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Günther C, Martini E, Wittkopf N, et al. Caspase-8 regulates TNF-α-induced epithelial necroptosis and terminal ileitis. Nature. 2011;477:335–339. doi: 10.1038/nature10400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Berghe TV, Linkermann A, Jouan-Lanhouet S, Walczak H, Vandenabeele P. Regulated necrosis: the expanding network of non-apoptotic cell death pathways. Nat Rev Mol Cell Biol. 2014;15:135–147. doi: 10.1038/nrm3737. [DOI] [PubMed] [Google Scholar]
- 59.Murphy JM, Czabotar PE, Hildebrand JM, et al. The pseudokinase MLKL mediates necroptosis via a molecular switch mechanism. Immunity. 2013;39:443–453. doi: 10.1016/j.immuni.2013.06.018. [DOI] [PubMed] [Google Scholar]
- 60.Aaes TL, Kaczmarek A, Delvaeye T, et al. Vaccination with necroptotic cancer cells induces efficient anti-tumor immunity. Cell Rep. 2016;15:274–287. doi: 10.1016/j.celrep.2016.03.037.12e7c9eabdcf4753a2c5424185446077 [DOI] [PubMed] [Google Scholar]
- 61.Park HH, Kim HR, Park SY, et al. RIPK3 activation induces TRIM28 derepression in cancer cells and enhances the anti-tumor microenvironment. Mol Cancer. 2021;20:107. doi: 10.1186/s12943-021-01399-3.9649dec1a70f41ebb05ee81d7275fc5f [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nicolè L, Sanavia T, Cappellesso R, et al. Necroptosis-driving genes RIPK1, RIPK3 and MLKL-p are associated with intratumoral CD3+ and CD8+ T cell density and predict prognosis in hepatocellular carcinoma. J Immunother Cancer. 2022;10:e004031. doi: 10.1136/jitc-2021-004031.0a285e23f19c4f498713d63cb388c4d4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Koo GB, Morgan MJ, Lee DG, et al. Methylation-dependent loss of RIP3 expression in cancer represses programmed necrosis in response to chemotherapeutics. Cell Res. 2015;25:707–725. doi: 10.1038/cr.2015.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Snyder AG, Hubbard NW, Messmer MN, et al. Intratumoral activation of the necroptotic pathway components RIPK1 and RIPK3 potentiates antitumor immunity. Sci immunol. 2019;4:eaaw2004. doi: 10.1126/sciimmunol.aaw2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Werthmöller N, Frey B, Wunderlich R, Fietkau R, Gaipl US. Modulation of radiochemoimmunotherapy-induced B16 melanoma cell death by the pan-caspase inhibitor zVAD-fmk induces anti-tumor immunity in a HMGB1-, nucleotide- and T-cell-dependent manner. Cell Death Dis. 2015;6:e1761. doi: 10.1038/cddis.2015.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jiang X, Stockwell BR, Conrad M. Ferroptosis: mechanisms, biology and role in disease. Nat Rev Mol Cell Biol. 2021;22:266–282. doi: 10.1038/s41580-020-00324-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lewerenz J, Klein M, Methner A. Cooperative action of glutamate transporters and cystine/glutamate antiporter system Xc-protects from oxidative glutamate toxicity. J Neurochem. 2006;98:916–925. doi: 10.1111/j.1471-4159.2006.03921.x. [DOI] [PubMed] [Google Scholar]
- 68.Brigelius-Flohé R, Maiorino M. Glutathione peroxidases. Biochim Biophys Acta. 2013;1830:3289–3303. doi: 10.1016/j.bbagen.2012.11.020. [DOI] [PubMed] [Google Scholar]
- 69.Feng H, Stockwell BR. Unsolved mysteries: how does lipid peroxidation cause ferroptosis? PLoS Biol. 2018;16:e2006203. doi: 10.1371/journal.pbio.2006203.255652d9cc1b4e7c880c131bf61438c6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dixon SJ, Lemberg KM, Lamprecht MR, et al. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell. 2012;149:1060–1072. doi: 10.1016/j.cell.2012.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Louandre C, Ezzoukhry Z, Godin C, et al. Iron‐ dependent cell death of hepatocellular carcinoma cells exposed to sorafenib. Int J Cancer. 2013;133:1732–1742. doi: 10.1002/ijc.28159. [DOI] [PubMed] [Google Scholar]
- 72.Yang WS, iRamaratnam R, Sr, Welsch ME, et al. Regulation of ferroptotic cancer cell death by GPX4. Cell. 2014;156:317–331. doi: 10.1016/j.cell.2013.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yang WS, Kim KJ, Gaschler MM, Patel M, Shchepinov MS, Stockwell BR. Peroxidation of polyunsaturated fatty acids by lipoxygenases drives ferroptosis. Proc Natl Acad Sci U S A. 2016;113:E4966–E4975. doi: 10.1073/pnas.1603244113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gaschler MM, Andia AA, Liu H, et al. FINO2 initiates ferroptosis through GPX4 inactivation and iron oxidation. Nat Chem Biol. 2018;14:507–515. doi: 10.1038/s41589-018-0031-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Manz DH, Blanchette NL, Paul BT, Torti FM, Torti SV. Iron and cancer: recent insights. Ann N Y Acad Sci. 2016;1368:149–161. doi: 10.1111/nyas.13008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Whitnall M, Howard J, Ponka P, Richardson DR. A class of iron chelators with a wide spectrum of potent antitumor activity that overcomes resistance to chemotherapeutics. Proc Natl Acad Sci U S A. 2006;103:14901–14906. doi: 10.1073/pnas.0604979103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chen SJ, Kuo CC, Pan HY, Tsou TC, Yeh SC, Chang JY. Desferal regulates hCtr1 and transferrin receptor expression through Sp1 and exhibits synergistic cytotoxicity with platinum drugs in oxaliplatin-resistant human cervical cancer cells in vitro and in vivo. Oncotarget. 2016;7:49310–49321. doi: 10.18632/oncotarget.10336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wang W, Green M, Choi JE, et al. CD8+ T cells regulate tumour ferroptosis during cancer immunotherapy. Nature. 2019;569:270–274. doi: 10.1038/s41586-019-1170-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bordini J, Morisi F, Elia AR, et al. Iron induces cell death and strengthens the efficacy of antiandrogen therapy in prostate cancer models. Clin Cancer Res. 2020;26:6387–6398. doi: 10.1158/1078-0432.CCR-20-3182. [DOI] [PubMed] [Google Scholar]
- 80.Zou Y, Palte MJ, Deik AA, et al. A GPX4-dependent cancer cell state underlies the clear-cell morphology and confers sensitivity to ferroptosis. Nat Commun. 2019;10:1617. doi: 10.1038/s41467-019-09277-9.30381869b6f34533b2be4a86cf0a583a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gout P, Buckley A, Simms C, Bruchovsky N. Sulfasalazine, a potent suppressor of lymphoma growth by inhibition of the xc− cystine transporter: a new action for an old drug. Leukemia. 2001;15:1633–1640. doi: 10.1038/sj.leu.2402238. [DOI] [PubMed] [Google Scholar]
- 82.Yu Y, Xie Y, Cao L, et al. The ferroptosis inducer erastin enhances sensitivity of acute myeloid leukemia cells to chemotherapeutic agents. Mol Cell Oncol. 2015;2:e1054549. doi: 10.1080/23723556.2015.1054549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hassannia B, Vandenabeele P, Berghe TV. Targeting ferroptosis to iron out cancer. Cancer Cell. 2019;35:830–849. doi: 10.1016/j.ccell.2019.04.002. [DOI] [PubMed] [Google Scholar]
- 84.Wen Q, Liu J, Kang R, Zhou B, Tang D. The release and activity of HMGB1 in ferroptosis. Biochem Biophys Res Commun. 2019;510:278–283. doi: 10.1016/j.bbrc.2019.01.090. [DOI] [PubMed] [Google Scholar]
- 85.Ye F, Chai W, Xie M, et al. HMGB1 regulates erastin-induced ferroptosis via RAS-JNK/p38 signaling in HL-60/NRAS(Q61L) cells. Am J Cancer Res. 2019;9:730–739. [PMC free article] [PubMed] [Google Scholar]
- 86.Luo X, Gong HB, Gao HY, et al. Oxygenated phosphatidylethanolamine navigates phagocytosis of ferroptotic cells by interacting with TLR2. Cell Death Differ. 2021;28:1971–1989. doi: 10.1038/s41418-020-00719-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Raskov H, Orhan A, Christensen JP, Gögenur I. Cytotoxic CD8+ T cells in cancer and cancer immunotherapy. Br J Cancer. 2021;124:359–367. doi: 10.1038/s41416-020-01048-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Efimova I, Catanzaro E, Van der Meeren L, et al. Vaccination with early ferroptotic cancer cells induces efficient antitumor immunity. J Immunother Cancer. 2020;8:e001369. doi: 10.1136/jitc-2020-001369.2a503d3f5540469a8ba20ba437a6ab07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Song J, Liu T, Yin Y, et al. The deubiquitinase OTUD1 enhances iron transport and potentiates host antitumor immunity. EMBO Rep. 2021;22:e51162. doi: 10.15252/embr.202051162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Jiang Z, Lim S-O, Yan M, et al. TYRO3 induces anti-PD-1/PD-L1 therapy resistance by limiting innate immunity and tumoral ferroptosis. J Clin Invest. 2021;131:e139434. doi: 10.1172/JCI139434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wang W, Green M, Choi JE, et al. CD8(+) T cells regulate tumour ferroptosis during cancer immunotherapy. Nature. 2019;569:270–274. doi: 10.1038/s41586-019-1170-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lang X, Green MD, Wang W, et al. Radiotherapy and immunotherapy promote tumoral lipid oxidation and ferroptosis via synergistic repression of SLC7A11 ferroptosis connects radiotherapy and immunotherapy. Cancer Discov. 2019;9:1673–1685. doi: 10.1158/2159-8290.CD-19-0338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wang H, Cheng Y, Mao C, et al. Emerging mechanisms and targeted therapy of ferroptosis in cancer. Mol Ther. 2021;29:2185–2208. doi: 10.1016/j.ymthe.2021.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Aglietti RA, Dueber EC. Recent Insights into the molecular mechanisms underlying pyroptosis and gasdermin family functions. Trends Immunol. 2017;38:261–271. doi: 10.1016/j.it.2017.01.003. [DOI] [PubMed] [Google Scholar]
- 95.Erkes DA, Cai W, Sanchez IM, et al. Mutant BRAF and MEK inhibitors regulate the tumor immune microenvironment via pyroptosis. Cancer Discov. 2020;10:254–269. doi: 10.1158/2159-8290.CD-19-0672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Yang D, He Y, Muñoz-Planillo R, Liu Q, Núñez G. Caspase-11 requires the pannexin-1 channel and the purinergic P2X7 pore to mediate pyroptosis and endotoxic shock. Immunity. 2015;43:923–932. doi: 10.1016/j.immuni.2015.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Fink SL, Cookson BT. Caspase‐1‐dependent pore formation during pyroptosis leads to osmotic lysis of infected host macrophages. Cell Microbiol. 2006;8:1812–1825. doi: 10.1111/j.1462-5822.2006.00751.x. [DOI] [PubMed] [Google Scholar]
- 98.Yu P, Zhang X, Liu N, Tang L, Peng C, Chen X. Pyroptosis: mechanisms and diseases. Signal Transduct Target Ther. 2021;6:1–21. doi: 10.1038/s41392-021-00507-5.995383e34d6140f9a9190ce2dddc3c75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Platnich JM, Muruve DA. NOD-like receptors and inflammasomes: a review of their canonical and non-canonical signaling pathways. Arch Biochem Biophys. 2019;670:4–14. doi: 10.1016/j.abb.2019.02.008. [DOI] [PubMed] [Google Scholar]
- 100.Shi J, Zhao Y, Wang Y, et al. Inflammatory caspases are innate immune receptors for intracellular LPS. Nature. 2014;514:187–192. doi: 10.1038/nature13683. [DOI] [PubMed] [Google Scholar]
- 101.Hornung V, Ablasser A, Charrel-Dennis M, et al. AIM2 recognizes cytosolic dsDNA and forms a caspase-1-activating inflammasome with ASC. Nature. 2009;458:514–518. doi: 10.1038/nature07725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Chae JJ, Wood G, Masters SL, et al. The B30. 2 domain of pyrin, the familial Mediterranean fever protein, interacts directly with caspase-1 to modulate IL-1β production. Proc Natl Acad Sci U S A. 2006;103:9982–9987. doi: 10.1073/pnas.0602081103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lu A, Magupalli Venkat G, Ruan J, et al. Unified polymerization mechanism for the assembly of ASC-dependent inflammasomes. Cell. 2014;156:1193–1206. doi: 10.1016/j.cell.2014.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Liu X, Zhang Z, Ruan J, et al. Inflammasome-activated gasdermin D causes pyroptosis by forming membrane pores. Nature. 2016;535:153–158. doi: 10.1038/nature18629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kayagaki N, Stowe IB, Lee BL, et al. Caspase-11 cleaves gasdermin D for non-canonical inflammasome signalling. Nature. 2015;526:666–671. doi: 10.1038/nature15541. [DOI] [PubMed] [Google Scholar]
- 106.Shi J, Zhao Y, Wang K, et al. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature. 2015;526:660–665. doi: 10.1038/nature15514. [DOI] [PubMed] [Google Scholar]
- 107.Wang Y, Gao W, Shi X, et al. Chemotherapy drugs induce pyroptosis through caspase-3 cleavage of a gasdermin. Nature. 2017;547:99–103. doi: 10.1038/nature22393. [DOI] [PubMed] [Google Scholar]
- 108.Ding J, Wang K, Liu W, et al. Pore-forming activity and structural autoinhibition of the gasdermin family. Nature. 2016;535:111–116. doi: 10.1038/nature18590. [DOI] [PubMed] [Google Scholar]
- 109.Jiang M, Qi L, Li L, Li Y. The caspase-3/GSDME signal pathway as a switch between apoptosis and pyroptosis in cancer. Cell Death Discov. 2020;6:1–11. doi: 10.1038/s41420-020-00349-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Aizawa E, Karasawa T, Watanabe S, et al. GSDME-dependent incomplete pyroptosis permits selective IL-1α release under caspase-1 inhibition. iScience. 2020;23:101070. doi: 10.1016/j.isci.2020.101070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Zhang Z, Zhang Y, Xia S, et al. Gasdermin E suppresses tumour growth by activating anti-tumour immunity. Nature. 2020;579:415–420. doi: 10.1038/s41586-020-2071-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Zhou Z, He H, Wang K, et al. Granzyme A from cytotoxic lymphocytes cleaves GSDMB to trigger pyroptosis in target cells. Science. 2020;368:eaaz7548. doi: 10.1126/science.aaz7548. [DOI] [PubMed] [Google Scholar]
- 113.Wang Q, Wang Y, Ding J, et al. A bioorthogonal system reveals antitumour immune function of pyroptosis. Nature. 2020;579:421–426. doi: 10.1038/s41586-020-2079-1. [DOI] [PubMed] [Google Scholar]
- 114.Li S, Chen P, Cheng B, et al. Pyroptosis predicts immunotherapy outcomes across multiple cancer types. Clin Immunol. 2022;245:109163. doi: 10.1016/j.clim.2022.109163. [DOI] [PubMed] [Google Scholar]
- 115.Pauken KE, Wherry EJ. Overcoming T cell exhaustion in infection and cancer. Trends Immunol. 2015;36:265–276. doi: 10.1016/j.it.2015.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Lu H, Dietsch GN, Matthews MA, et al. VTX-2337 is a novel TLR8 agonist that activates NK cells and augments ADCC. Clin Cancer Res. 2012;18:499–509. doi: 10.1158/1078-0432.CCR-11-1625. [DOI] [PubMed] [Google Scholar]
- 117.Dietsch GN, Lu H, Yang Y, et al. Coordinated activation of toll-like receptor8 (TLR8) and NLRP3 by the TLR8 agonist, VTX-2337, ignites tumoricidal natural killer cell activity. PLoS One. 2016;11:e0148764. doi: 10.1371/journal.pone.0148764.76249c953a2e4007813d22b488c7aa4a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Monk BJ, Brady MF, Aghajanian C, et al. A phase 2, randomized, double-blind, placebo-controlled study of chemo-immunotherapy combination using motolimod with pegylated liposomal doxorubicin in recurrent or persistent ovarian cancer: a gynecologic oncology group partners study. Ann Oncol. 2017;28:996–1004. doi: 10.1093/annonc/mdx049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9:162–174. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Coward J, Kulbe H, Chakravarty P, et al. Interleukin-6 as a therapeutic target in human ovarian cancer. Clin Cancer Res. 2011;17:6083–6096. doi: 10.1158/1078-0432.CCR-11-0945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Dijkgraaf EM, Santegoets SJ, Reyners AK, et al. A phase I trial combining carboplatin/doxorubicin with tocilizumab, an anti-IL-6R monoclonal antibody, and interferon-alpha2b in patients with recurrent epithelial ovarian cancer. Ann Oncol. 2015;26:2141–2149. doi: 10.1093/annonc/mdv309. [DOI] [PubMed] [Google Scholar]
- 122.Birmpilis AI, Paschalis A, Mourkakis A, et al. Immunogenic cell death, damps and prothymosin alpha as a putative anticancer immune response biomarker. Cells. 2022;11:1415. doi: 10.3390/cells11091415.a0d252a316844a5fb71c302cd1054f61 [DOI] [PMC free article] [PubMed] [Google Scholar]