Abstract
The endoplasmic reticulum (ER) stress response is a signal transduction pathway activated by the perturbation of normal ER metabolism. We used the maize (Zea mays) floury-2 (fl2) mutant and soybean (Glycine max) suspension cultures treated with tunicamycin (Tm) to investigate the ER stress response as it relates to phospholipid metabolism in plants. Four key phospholipid biosynthetic enzymes, including DG kinase and phosphatidylinositol (PI) 4-phosphate 5-kinase were up-regulated in the fl2 mutant, specifically in protein body fractions where the mutation has its greatest effect. The third up-regulated enzyme, choline-phosphate cytidylyltransferase, was regulated by fl2 gene dosage and developmental signals. Elevated accumulation of the fourth enzyme, PI 4-kinase, was observed in the fl2 endosperm and soybean cells treated with Tm. The activation of these phospholipid biosynthetic enzymes was accompanied by alterations in membrane lipid synthesis and accumulation. The fl2 mutant exhibited increased PI content in protein body membranes at 18 d after pollination and more than 3-fold higher triacylglycerol accumulation in the endosperm by 36 d after pollination. Incorporation of radiolabeled acetate into phospholipids in soybean culture cells increased by about 30% with Tm treatment. The coordinated regulation of ER stress related proteins and multiple components of phospholipid biosynthesis is consistent with signaling through a common pathway. We postulate that the plant ER stress response has an important role in general plant metabolism, and more specifically in integrating the synthesis of protein and lipid reserves to allow proper seed formation.
Seed development requires coordination between formation of a pre-emergent embryo and synthesis of the major starch, lipid, and protein storage reserves. Regulation of storage reserve synthesis is complex because of a need to keep the individual components in their proper ratios for the development of mature, viable seeds that will desiccate and subsequently germinate properly. Little is known about how these processes are regulated or how seeds adapt to perturbations in the metabolic pathways essential for their growth and maturation. Because the endoplasmic reticulum (ER) is the site for processing of secretory proteins and formation of membrane and storage lipids, it is likely to play a key role in coordinating the metabolism of proteins and lipids during seed development.
One of the simplest systems for exploring intracellular communication between protein and phospholipid biosynthesis is the formation of protein bodies in maize (Zea mays). These membrane-bound organelles arise directly from the ER as ordered aggregates of storage proteins within the ER lumen. Much attention has been given to the packaging of storage proteins within protein bodies (Okita and Rogers, 1996), yet little is known about vesiculation of the membrane to form the limiting boundaries of the organelles. Because maize protein bodies represent terminal vesicles, they do not require additional complex sorting signals or docking proteins. Nevertheless, the transition from flattened ER cisternae to distinct spherical protein bodies must involve some changes in membrane organization in response to storage protein accumulation during seed fill.
A more dramatic alteration in ER membranes is seen in the endosperm of the maize mutant, floury-2 (fl2). Protein bodies derived from the ER are deeply convoluted in this mutant and remain clustered near nuclei rather than dispersed throughout the endosperm (Zhang and Boston, 1992). In addition, ER-resident molecular chaperones that are essential for proper protein folding and secretion increase markedly (Fontes et al., 1991; Marocco et al., 1991; Li and Larkins, 1996; Wrobel et al., 1997). The primary defect in the fl2 mutant is a single point mutation in a 22-kD α-zein storage protein that prevents the signal peptide of the zein from being cleaved (Coleman et al., 1995). Instead, the signal peptide remains attached and anchors the zein to the membrane (Gillikin et al., 1997). Because the mutant zein gene is expressed specifically in the seed, the rest of the maize plant is phenotypically normal.
The chaperone induction and changes in membrane phenotype previously observed in the fl2 endosperm are similar to characteristics of mammalian and yeast cells during ER stress. Accumulation of unfolded proteins in the ER triggers a well-defined signal transduction pathway in yeast called the unfolded protein response (UPR; for review, see Chapman et al., 1998; Kaufman, 1999). The most upstream component of the UPR identified to date is a yeast transmembrane protein kinase named Ire1p (Cox et al., 1993; Mori et al., 1993). This Ser/Thr kinase is activated by dimerization and phosphorylation in response to unfolded protein accumulation from the ER lumen (Shamu and Walter, 1996). Subsequent signal transduction through a series of intermediates leads to transcriptional induction of a number of ER resident proteins, including the molecular chaperones, binding protein (BiP), and protein disulfide isomerase (PDI; for molecular chaperone review, see Gething and Sambrook, 1992; Boston et al., 1996). Recent evidence from mammalian research is suggestive that BiP is constitutively bound to Ire1p and that the release of BiP following introduction of ER stress is the signal for Ire1p activation (Bertolotti et al., 2000). BiP dissociation likely occurs because of its higher affinity for unfolded proteins than Ire1p. UPR components appear to be conserved from yeast to mammalian systems (Foti et al., 1999), and the existence of an IRE1 homolog in Arabidopsis (N. Koizumi and M.J. Chrispeels, personal communication) suggests that a similar signal transduction pathway is likely to be present in plants.
Several lines of evidence suggest a connection between phospholipid biosynthesis and UPR signaling. Overexpression of integral membrane proteins is correlated with an increase in phospholipid biosynthesis, proliferation of ER, and induction of the UPR (Chapman et al., 1998 and refs. therein). Such membrane proliferation is presumably a cellular accommodation to depletion of free chaperones and/or proteins involved in import into the ER and represents a perceived need to increase membrane surface area. The stimulus for phospholipid biosynthesis has been suggested by Cox et al. (1997) to be transduced through the same Ire1p-mediated signaling cascade that controls molecular chaperone production.
The fl2-mediated induction of ER stress occurs in a tissue devoted primarily to synthesis of storage reserves and gives us the means to determine whether or not protein and lipid metabolism are coordinated during protein body formation in seeds. Cell cultures provide an additional system to investigate the ER stress response in plants without the pleiotropic effects of the fl2 mutation. A number of pharmacological agents, including tunicamycin (Tm), an inhibitor N-linked glycosylation of proteins, can be used to induce an ER stress response in cell cultures. Tm is a potent inducer of the ER stress response (Watowich and Morimoto, 1988; Lee, 1992) and has been shown to increase mRNA and protein accumulation of ER-resident molecular chaperones in plants (D'Amico et al., 1992; Denecke et al., 1995; Wrobel, 1996).
In this study we report that four key phospholipid biosynthetic enzymes are up-regulated in fl2 protein bodies during early kernel development. Levels of phosphatidylinositol (PI) in fl2 protein body membranes and total triacylglycerols in the fl2 endosperm are increased when compared with their normal counterparts. In addition, expression levels of PI 4-kinase and overall phospholipid synthesis are elevated in soybean (Glycine max) cells in which an ER stress response is chemically induced. The data presented here provide new insights into coordination of the ER stress response and phospholipid metabolism in plants.
RESULTS
Enzymes Assayed
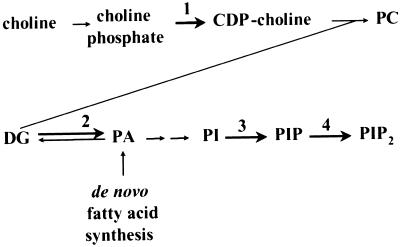
We assayed protein bodies of normal and fl2 maize for activities of enzymes that are believed to be involved at regulatory or rate-limiting steps in phospholipid biosynthesis (Fig. 1). Choline-phosphate cytidylyltransferase (CCT, enzyme 1), is considered to be the rate-limiting step in the biosynthesis of phosphatidylcholine (PC), the major phospholipid component of most eukaryotic membranes (for a review of plant phospholipid metabolism, see Ohlrogge and Browse, 1995). The lipid kinases diacylglycerol (DG) kinase (enzyme 2), PI 4-kinase (enzyme 3), and PI 4-phosphate 5-kinase (PIP 5-kinase, enzyme 4) are enzymes that catalyze the biosynthesis of phosphatidic acid (PA), PI 4-phosphate (PIP), and PI 4,5-bisphosphate (PIP2), respectively. Although these phospholipids are minor components of most membranes, they have been implicated in the regulation of vesicle trafficking and other essential signaling pathways (De Camilli et al., 1996; Roth, 1999).
Figure 1.
Abbreviated pathway showing reactions of phospholipid metabolism assayed in this study. Bold arrows highlight reactions catalyzed by CCT (1), DG kinase (2), PI 4-kinase (3), and PIP 5-kinase (4).
CCT Activity Increases with fl2 Gene Dosage
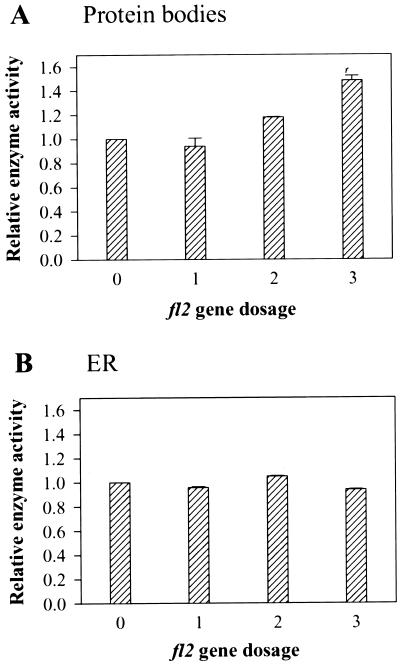
To determine if CCT activity was affected by the fl2 mutation we analyzed enzyme activity associated with protein bodies of normal and fl2 kernels. Because of the triploid nature of maize endosperm, a gene dosage series ranging from zero to three copies of a given gene can be generated by reciprocal crosses between homozygous parental lines. Figure 2 shows the effects of fl2 gene dosage on CCT activity at 18 d after pollination (DAP). Although a single copy of the fl2 gene had no significant effect on the enzyme activity associated with protein bodies, two and three copies resulted in increases of about 1.2- and 1.5-fold, respectively. This dosage effect correlates well with our previous findings that visible morphological effects of fl2 on protein bodies increase minimally with one copy of the mutant gene, and become progressively more severe with the introduction of two and three copies (Zhang and Boston, 1992). Levels of BiP RNA (Boston et al., 1991) and protein (Zhang and Boston, 1992) also increase incrementally with each dose of the fl2 gene (Boston et al., 1991; Wrobel et al., 1997). CCT activities in corresponding ER fractions were not significantly different regardless of fl2 gene dosage (Fig. 2B).
Figure 2.
Relative CCT activity of protein body fractions in relation to fl2 gene dosage. CCT activity is expressed as fold induction over activity in normal endosperm. A, Protein body fractions (mean normal value was 0.237 nmol min−1 mg−1 protein). B, ER fractions. Proteins were extracted from kernels harvested 18 DAP with the triploid endosperm genotypes +/+/+ (0 doses), +/+/fl2 (one dose), +/fl2/fl2 (two doses), and fl2/fl2/fl2 (three doses) and were fractionated by centrifugation through Suc gradients. Bars represent means of three separate experiments ± se
CCT Activity Changes during Seed Development
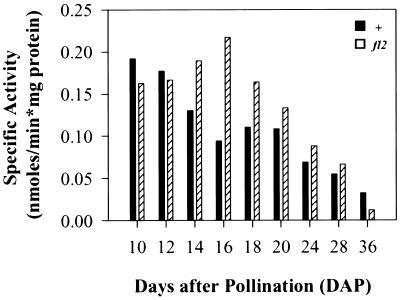
To obtain a profile of the differences in CCT activity associated with normal and fl2 protein bodies during seed development we assayed endosperm at developmental stages from 10 to 36 DAP. During early seed fill, CCT activity was high in normal and fl2 maize (Fig. 3). As the seed matured, CCT activity remained high in fl2 protein bodies, but dropped in normal ones, with the largest differences appearing at 14 to 18 DAP. At later stages of endosperm maturation, CCT activity dropped overall and differences between normal and fl2 protein bodies decreased.
Figure 3.
CCT activities of protein body fractions during endosperm development. Black bars, Normal protein bodies; hatched bars, fl2 protein bodies. Data from a representative experiment are shown. Similar trends were seen in four independent experiments.
Lipid Kinase Activities Increase in fl2 Protein Bodies
The activities of enzymes previously implicated in vesicle formation, DG kinase, PI 4-kinase, and PIP 5-kinase, were assayed in protein bodies from kernels harvested 18 and 28 DAP. An increase in each of these activities in protein body fractions was observed in fl2 relative to normal maize. Table I shows the averages of duplicate values obtained from a single experiment. Because considerable variation was observed in the total amount of radioactivity incorporated among independently isolated protein body fractions, the results of five separate experiments were analyzed as the relative increase in enzyme activity in fl2 versus normal for each independent experiment. These results are shown in Table I as mean ratios ± se. To determine if differences in lipid kinase activities at 18 DAP were specific to protein bodies, ER-enriched fractions from the Suc gradients were assayed. No significant differences were observed (data not shown). By 28 DAP, the mean lipid kinase activities tended to be higher in fl2 protein bodies than in normal protein bodies at the same developmental stage, but the increases were not significant.
Table I.
DG, PI, and PIP kinase activities of normal (+) and fl2 protein bodies
| DAP | Product | Activitya
|
Ratio (fl2/+) | Mean Ratio ± seb | |
|---|---|---|---|---|---|
| + | fl2 | ||||
| 18 | PA | 0.59 | 1.30 | 2.2 | 3.2 ± 0.45 |
| PIP | 0.24 | 0.49 | 2.0 | 2.9 ± 0.70 | |
| PIP2 | 0.01 | 0.03 | 3.0 | 2.5 ± 0.33 | |
| 28 | PA | 0.40 | 0.55 | 1.4 | 1.6 ± 0.27 |
| PIP | 0.14 | 0.19 | 1.4 | 1.6 ± 0.43 | |
| PIP2 | 0.01 | 0.02 | 2.0 | 1.8 ± 0.57 | |
Specific activities (nmol min−1 mg−1 protein) represent an average of duplicate reactions from one experiment.
Final column (far right) shows mean of ratios (fl2/+) from five separate experiments ± se.
PI 4-Kinase Expression Increases in fl2 Protein Bodies
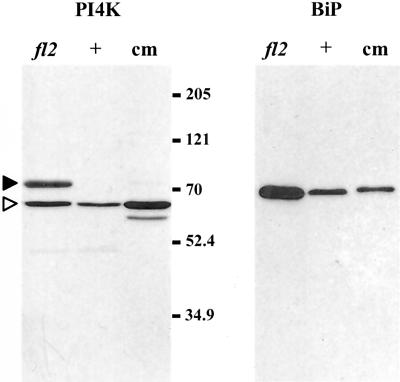
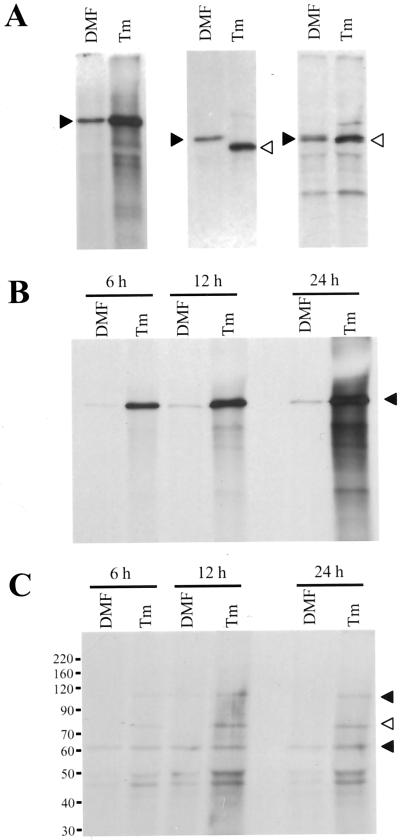
To determine if up-regulation of lipid kinases was due, at least in part, to an increase in enzyme amounts, we investigated the accumulation of PI 4-kinase by immunoblot analysis. Protein body fractions from normal and fl2 endosperm and a microsomal membrane fraction from carrot cells grown in suspension culture were subjected to SDS-PAGE as described in “Materials and Methods.” A band of approximately 65 kD was detected in maize and carrot (Fig. 4). This band most likely corresponds to the low-Mr PI 4-kinase that has been previously described by Westergren et al. (1999) to have PI 4-kinase activity in spinach. A second cross-reacting band with an apparent molecular mass of 77 kD was prominent in fl2 samples, but not in samples from carrot microsomes unless the blots were exposed for much longer times (data not shown). Both cross-reacting proteins were much more abundant in fl2 protein bodies than in normal ones and were not seen in identical blots probed with an equal concentration of preimmune serum (data not shown). The isoforms of PI 4-kinase have not been investigated in detail in maize, but the existence of multiple isoforms has been reported in spinach, carrot, and Arabidopsis (Stevenson et al., 1998; Westergren et al., 1999; Xue et al., 1999). A duplicate immunoblot probed with anti-BiP antibody is shown for comparison.
Figure 4.
Immunoblot analysis of proteins from fl2 and normal (+) protein bodies. Samples contained equal amounts of protein (150 μg) from protein bodies; equivalency was confirmed by staining duplicate gels with Coomassie Brilliant Blue (data not shown). Protein (100 μg) from carrot microsomes (cm) was used as a positive control. Left, Immunoblot probed with anti-PI4K antibody. Arrowheads mark major cross-reacting species (white arrowhead, 65-kD band; black arrowhead, 77-kD band). Right, Immunoblot of a duplicate membrane probed with anti-BiP antibody. Sizes and relative mobilities of prestained protein markers are indicated to the right of the PI4K immunoblot.
PI 4-Kinase Expression Increases with Chemical Induction of the ER Stress Response
The common feature between the fl2 mutation and chemical treatment of cell cultures with Tm is induction of an ER stress response. We investigated the expression of PI4-kinase in response to chemical induction of an ER stress response as a way of verifying that the effect on lipid metabolism is directly due to the ER stress response and not due to pleiotropic effects of the mutation. Soybean suspension cultures incubated with Tm showed a strong induction of the molecular chaperones BiP and PDI compared with control cultures, as judged by immunoblot analysis of whole-cell extracts (Fig. 5, A and B). PI4K immunoblots of the same samples also showed increases in response to chemical induction (Fig. 5C). Modest increases in molecular chaperone and PI4-kinase accumulation were observed over the time course of these experiments in control cells treated only with the N, N-dimethylformamide solvent. Similar results were observed with untreated soybean cultures, however, suggesting that the abundance of these proteins also changes with culture age (data not shown).
Figure 5.
Immunoblot analysis of proteins from soybean cells treated with Tm. Proteins were extracted from whole cells as described in “Materials and Methods” and equal protein loading was confirmed by staining of duplicate gels with Coomassie Brilliant Blue (data not shown). A, Immunoblot probed with anti-BiP antibody. The arrowhead indicates BiP cross-reacting band of about 75 kD. B, Immunoblot probed with anti-PDI antibody. The arrowhead indicates cross-reacting PDI doublet. The slight shift in mobility of PDI in Tm-treated cells represents the inhibition of glycosylation. C, Immunoblot probed with anti-PI4K antibody. The arrowhead indicates a 65-kD cross-reacting band.
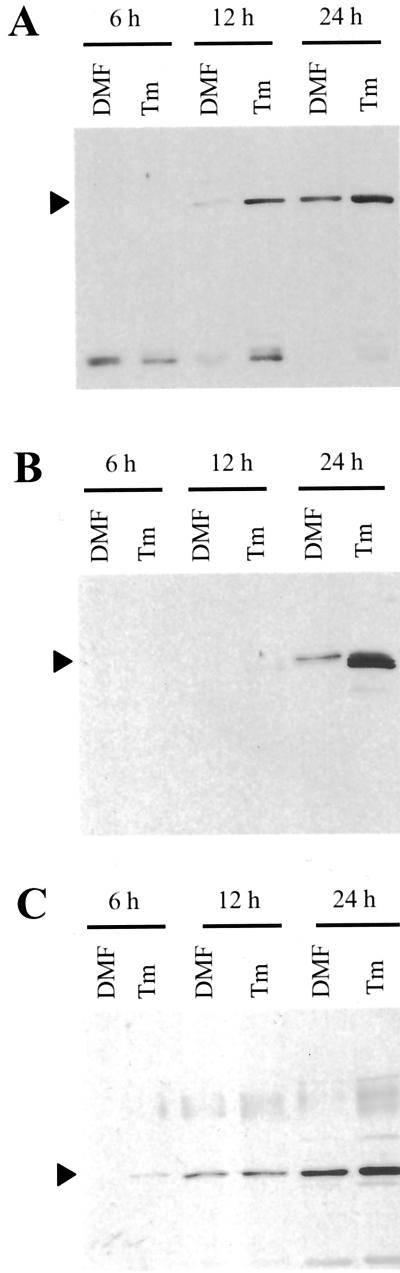
One limitation to immunoblot analysis is that the observed signal represents the combined accumulation of protein produced before and after the application of the stress-inducing compound. To overcome this limitation we performed short-term radiolabeling and immunoprecipitation of treated soybean cells to determine the effects of the Tm treatment on protein synthesis. Immunoprecipitation from whole-cell extracts revealed a strong induction of the ER stress response by Tm as shown by increased synthesis of the chaperones BiP, PDI, and calreticulin (Fig. 6A). Immunoprecipitation of newly synthesized proteins from ER-enriched fractions showed induction of BiP and PI4K expression in as little as 6 h after Tm treatment (Fig. 6, B and C). The multiple cross-reacting proteins (known active forms are designated by the arrowheads to the right of the figure) all increase in response to chemical treatment.
Figure 6.
Immunoprecipitation analysis of soybean cell cultures treated with Tm. A, Protein from whole-cell extracts labeled in vivo was immunoprecipitated with anti-BiP antibody (left), anti-calreticulin antibody (center), or anti-PDI antibody (right). Cells were treated with Tm for 12 h prior to a 30-min incubation with [35S] protein labeling mix. Arrowheads designate major cross-reacting bands. Calreticulin and PDI cross-reacting species are both glycosylated and show a shift to the non-glycosylated form with Tm treatment (white arrowheads). B, Protein from ER-enriched fractions immunoprecipitated with anti-BiP antibody. C, Protein from ER-enriched fractions immunoprecipitated with anti-PI4K antibody. Sizes and relative mobilities of prestained markers are indicated to the left of the figure. Black arrowheads designate bands similar in size to previously characterized PI4-kinases and the white arrowhead indicates band of similar size to the 77-kD band observed in maize (Fig. 4).
Normal and fl2 Protein Body Membranes Differ in Composition
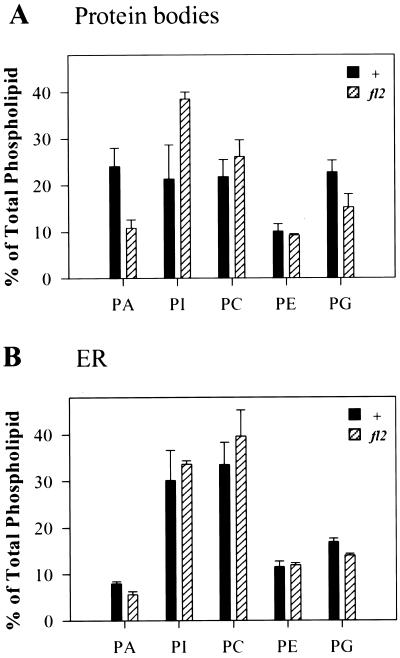
The differences in activities of lipid metabolic enzymes suggested that the phospholipid composition of fl2 protein bodies may differ from that of normal protein bodies. To investigate whether or not membranes were affected by the fl2 mutation we quantified the amounts of the major phospholipids from protein body and ER fractions. Protein body membranes from the fl2 mutant contained much lower levels of PA and higher levels of PI than normal protein body membranes (Fig. 7A). Amounts of PA, PI, PC, phosphatidylethanolamine, and phosphatidylglycerol in ER did not differ significantly between normal and fl2 samples (Fig. 7B). PIP and PIP2 were not detected in this assay.
Figure 7.
Phospholipid content of normal and fl2 protein body and ER fractions separated by Suc density gradient centrifugation. PA, PI, PC, phosphatidylethanolamine (PE), and PG are expressed as percentages of total polar lipids in the sample as quantified by gas chromatography. A, Protein body profile. B, ER profile. Means are from three independent experiments ± se. Black bars, Normal; hatched bars, fl2.
Triaglycerol Accumulation Increases in fl2 Endosperm
Martiniello et al. (1978) compared seed quality traits between 142 normal inbred lines and their fl2 counterparts and noted that the fl2 lines displayed, on average, 32% higher oil content. In normal maize inbreds, 78% of total lipid in mature maize kernels, mostly in the form of triaglycerol, is found in the embryo, whereas only 17% is contained in the endosperm (Weber, 1978). Because fl2 is an endosperm-specific trait we questioned whether or not the reported increases in total seed lipid resulted from an increase in triaglycerol accumulation specifically within the endosperm. To test this possibility, endosperm and embryo were dissected from fl2 and normal kernels harvested at 18, 28, and 36 DAP and were dried for 2 d to obtain accurate dry weights prior to lipid extraction and analysis. As shown in Table II, endosperm triaglycerol levels were greatly affected by the mutation. At 18 DAP, triaglycerol levels were 1.3-fold higher in fl2 and by 36 DAP, they were more than 3-fold higher in fl2 than in normal endosperm. In contrast, triaglycerol content in the fl2 embryos was not significantly different from the content of normal embryos. In endosperm and embryo, total PL content in fl2 was similar to that of normal seeds.
Table II.
Triacylglycerol (TG) and total polar lipid (PL) levels of normal (+) and fl2 endosperm and embryo
| DAP | Endosperm
|
Embryo
|
|||
|---|---|---|---|---|---|
| + | fl2 | + | fl2 | ||
| TG | |||||
| 18 | 4.5 ± 1.10 | 6.0 ± 1.30 | 160 ± 7.5 | 170 ± 8.6 | |
| 28 | 1.7 ± 0.53 | 3.2 ± 0.63 | 79 ± 8.0 | 89 ± 10 | |
| 36 | 1.4 ± 0.27 | 4.6 ± 0.41 | 81 ± 8.4 | 73 ± 4.3 | |
| PL | |||||
| 18 | 1.30 ± 0.11 | 1.30 ± 0.17 | 5.2 ± 1.30 | 4.5 ± 1.1 | |
| 28 | 0.27 ± 0.07 | 0.38 ± 0.02 | 2.1 ± 0.22 | 2.6 ± 0.4 | |
| 36 | 0.11 ± 0.01 | 0.17 ± 0.02 | 2.0 ± 0.03 | 2.8 ± 0.9 | |
Endosperm and embryo lipid levels are expressed as milligrams of lipid per gram of dry wt and are means of three experiments ± se.
Acetate Incorporation into Lipid Species Increases in Treated Soybean Cells
If induction of ER stress does indeed lead to increased phospholipid biosynthesis, we might expect to see this reflected as an increase in acetate incorporation into these species in induced cell cultures. Investigation of lipid synthesis in soybean cells revealed that overall incorporation of acetate into various lipid species was up-regulated with induction of the ER stress response by Tm (Table III). An increase in acetate incorporation of about 30% was seen in response to treatment with Tm for 12 and 24 h. TLC separation of the radiolabeled compounds revealed a general overall increase in the synthesis of each of the major phospholipids (data not shown) as opposed to specific increases in any one phospholipid species.
Table III.
Increases in acetate incorporation into lipid species after incubation of soybean cells with Tm for 12 and 24 h
| Replicates | 12 h | 24 h |
|---|---|---|
| 1 | 1.35 | 1.64 |
| 2 | 1.20 | 1.30 |
| 3 | 1.43 | 1.00 |
| 4 | 1.47 | 1.26 |
| Average ± se | 1.36 ± 0.06 | 1.30 ± 0.13 |
Increases in acetate incorporation (counts per minute) are expressed as ratios of Tm treated/DMF treated.
DISCUSSION
The ER mediates synthesis and storage of proteins, phospholipids, and triacylglycerols in the developing seed. Our findings suggest that phospholipid metabolism and protein production in protein bodies are coordinately regulated through a common ER pathway that shares many characteristics of the ER stress response. Examination of phospholipid metabolism in the fl2 mutant revealed alterations in enzyme activities and the phospholipid composition of protein body membranes. Given that PC is a major structural phospholipid of ER and protein body membranes, it is not surprising that signals eliciting the need for increased membrane biogenesis would result in enhanced activity of CCT, the rate-limiting enzyme in PC synthesis. The greatest differences in CCT activity were seen from 14 to 18 DAP. It has been previously shown that at 14 and 18 DAP, mRNA and protein of the major zeins decrease in the fl2 mutant when compared with normal maize (Jones, 1978; Lopes et al., 1994). Together with our observations, these data suggest that the reduction in zein accumulation in the fl2 mutant coincides with the increases in CCT activity.
Because the products of the DG, PI, and PIP kinase reactions represent relatively minor membrane components, we suggest that the increases in their activities are less likely to be needed for membrane biogenesis and instead are related to the roles these phospholipids play in secretory vesicle formation. PA and polyphosphoinositides are involved in secretory vesicle formation in mammalian and yeast cells and are essential for vesicle trafficking (Roth, 1999). These lipids are necessary for recruiting proteins to the vesicle surface for vesicle budding, docking, and fusion signals (De Camilli et al., 1996). For example, in yeast the SEC14 gene encodes a PI transfer protein that is necessary for the formation of secretory vesicles. The mutation in sec14, however, can be suppressed by overexpressing PI 4-kinase (Hama et al., 1999). In our studies, increases in PI 4-kinase activity were accompanied by increases in the amount of PI 4-kinase protein (Figs. 4–6). These increases are suggestive of regulation at the level of transcription and/or translation. Because more than one cross-reacting band was observed in samples displaying an ER stress response, further studies are needed to determine which of the cross-reacting proteins is responsible for the enzyme activity measured in vitro. However, all bands were increased as a result of the ER stress response induced by the fl2 mutation or chemical treatment.
It is likely that protein body formation in maize is controlled in a manner similar to vesicle budding and that the fl2 mutant zein may interfere with this process. Investigation of fl2 endosperm morphology reveals deeply invaginated protein bodies that are clustered near nuclei rather than being dispersed throughout the cell (Zhang and Boston, 1992). If the fl2 mutation perturbs the process of protein body formation, then the seed might be expected to increase PA and/or PI production to facilitate normal protein body formation under the stress imposed by accumulation of the mutant zein in the ER membrane. Despite the fact that higher DG kinase activity was observed in fl2 protein bodies, PA levels were significantly lower than those of normal protein body membranes (Fig. 5A). It is interesting that the decrease in PA was accompanied by a concomitant increase in PI (and enhanced PI 4-kinase and 5-kinase activities). Taken together, these observations lead us to suggest that the fl2 mutation causes an enhanced flux of phospholipid metabolites through the phosphoinositide pathway, at the expense of net PA accumulation.
Previous studies have identified a number of specialized ER subdomains with differing morphological and biochemical properties such as non-random localization of mRNAs, unequal distribution of molecular chaperones, and localized assembly and packaging of proteins into protein bodies (Okita and Rogers, 1996). Thus, the concept of protein bodies as protein-filled extensions of the ER as a whole may be overly simplified. We hypothesize that the fl2 mutant zein specifically perturbs the regions of the ER involved in protein body biogenesis.
In addition to mediating the synthesis of phospholipids and seed storage proteins, the ER controls production of triacylglycerols that are stored as oil bodies. With the exception of DG acyltransferase, the final enzyme in triacylglycerol synthesis, all other steps of the triacylglycerol pathway are shared with phospholipid biosynthetic pathways (Ohlrogge and Browse, 1995). Thus, signals leading to increased phospholipid synthesis during seed development may concomitantly give rise to increased triacylglycerol accumulation in the seed (assuming DG acyltransferase activity is not limiting). Our results demonstrate that the fl2-specific increase in triacylglycerol levels was largely confined to the endosperm where fl2 is expressed, as opposed to the embryo, the primary site of oil storage in the maize kernel. Moreover, increases in triacylglycerol content in fl2 endosperm are in agreement with the previously documented increase in total oil content in fl2 kernels averaged over 142 inbred lines (Martiniello et al., 1978). These results suggest that the triacylglycerol content of crop species may be influenced by the ER stress response pathway during seed development.
Taken together, the results presented here demonstrate that the fl2 mutation affects lipid biosynthesis, as well as the storage protein composition of maize seeds. Chemical induction of the ER stress response in soybean cell cultures showed similar results. In both cases the ER stress response in plants lead to increases in molecular chaperone levels and lipid biosynthesis. In maize kernels, ER stress specifically leads to alterations in protein body membrane composition and morphology and increases in phospholipid biosynthetic enzyme activities. Future studies will be directed toward understanding the underlying mechanisms that coordinate the integrated regulation of protein and lipid metabolism in plants.
MATERIALS AND METHODS
Plant Materials
Maize (Zea mays) inbred W64A (normal) and its near isogenic mutant, W64Afloury-2 (fl2), were grown at the North Carolina Central Crops Research Station (Clayton) in the summers of 1998 and 1999. Maize kernels were harvested at specific DAP, frozen in liquid nitrogen, and stored at −80°C.
Soybean (Glycine max) cell cultures were grown according to Abusteit et al. (1985) and were passaged into fresh media weekly. Cultures were treated 4 d after passage with Tm (Calbiochem, La Jolla, CA) as previously described (Wrobel et al., 1997). Control cultures for the Tm samples were treated with an equal amount of its solvent N, N-dimethylformamide.
Fractionation of ER and Protein Bodies
All centrifugation and extraction steps were performed at 0°C to 4°C. Buffer A [100 mm {Tricine N-[2-hydroxy-1,1-Bis(hydroxymethyl)ethyl]glycine}-NaOH, pH 7.5, 1 mm EDTA, and 10 mm KCl] was used for enzyme activity assays. Buffer B (10 mm Tris-HCl [pH 8.5 at 25°C], 10 mm KCl, and 5 mm MgCl2) was used for immunoblot and lipid analyses. Homogenization was carried out in buffer A made 20% (w/v) Suc and 10 mm dithiothreitol or buffer B made 7.2% (w/v) Suc and 10 mm dithiothreitol as noted below for specific procedures. For maize protein extractions, endosperm was removed from kernels and was ground in buffer (1:2, w/v) with a mortar and pestle. Homogenates were subjected to centrifugation at 300g for 10 min to remove cell debris. Supernatants were applied to discontinuous Suc gradients (Larkins and Hurkman, 1978) prepared as 2-mL steps of 2.0, 1.5, and 1.0 m Suc in buffer A or B and mixed at the 2.0 m/1.5 m interface by gently moving a curved Pasteur pipette through the interface six to 10 times. Gradients were subjected to centrifugation at 156,000g for 30 min at 4°C in a swinging bucket rotor. ER and protein bodies were collected from the 1.0 m/1.5 m and 1.5 m/2.0 m interfaces, respectively. Cellular fractions were subjected to centrifugation at 5,000g for 10 min in a tabletop centrifuge (protein bodies) or 100,000g for 15 min in a fixed angle rotor (ER).
An alternative method of fractionation was used to obtain protein for the CCT activity assay. After centrifugation at 300g to remove cell debris, supernatant was subjected to a 5,000g centrifugation for 10 min to separate protein bodies (5,000g pellet) from ER and other membranes (5,000g supernatant). Supernatant was subjected to centrifugation at 100,000g for 15 min in a fixed angle rotor. Resulting protein body and ER pellets from either method were resuspended in homogenization buffer and were quantified for protein using the Bio-Rad Protein Assay Kit I, with a bovine γ-globulin protein standard (Bio-Rad, Hercules, CA).
CCT Activity Assay
Protein bodies prepared in buffer A (50 μg in 25 μL of buffer) were added to an equal volume of CCT reaction buffer (Kinney et al., 1987) containing 4 mm [methyl-14C]choline-phosphate (50 mCi mmol−1, NEN Life Science Products, Boston). Reactions were incubated in a water bath at 30°C for 15 min and were stopped by heating at 95°C for 1.5 min. Product (cytidine diphosphate-choline) and substrate (choline-phosphate) were separated by thin-layer chromatography (TLC) on 60Å silica plates (Whatman, Ann Arbor, MI) with a developing solvent of equal parts 95% (v/v) ethanol and 2% (w/v) NH4OH. Bands were visualized with a Bioscan System 500 imaging scanner, scraped, and quantified by liquid scintillation spectroscopy in a liquid scintillation analyzer (Packard TRI-CARB 2100TR, Packard Instrument Company, Meriden, CT).
Lipid Kinase Activity Assay
Protein body and ER fractions from Suc gradients made with buffer A were resuspended in 30 mm Tris-HCl (pH 7.2 at 25°C) and were assayed for endogenous lipid kinase activities. Reactions of 20 μg of protein from protein body fractions or 2 μg of protein from ER fractions in 30 mm Tris-HCl (pH 7.2), 7.5 mm MgCl2, 1 mm NaMolybdate, 0.01% (v/v) Triton X-100, and 0.9 mm [γ-32P]ATP (0.2 Ci mmol−1; 50-μL total reaction volume) were incubated for 10 min at room temperature and stopped with 1.5 mL of chloroform:methanol (1:2, v/v). Lipid products were extracted and analyzed by TLC as previously described (Cho et al., 1995).
Immunoblotting
Protein bodies from Suc gradients made with buffer B were washed twice by dilution in buffer B followed by a 5,000g centrifugation at 4°C for 10 min. The final pellet was resuspended in buffer B containing 0.15 m NaCl, made 1% (v/v) Triton X-100, and were allowed to mix on a Nutator (Innovative Medical Systems, Ivyland, PA) for 1 h at 4°C. Prior to fractionation through 8% (w/v; PI 4-kinase) or 10% (w/v; BiP) SDS-polyacrylamide gels, one volume SDS-PAGE sample buffer (Laemmli, 1970) was added and samples were boiled for 5 min. For soybean cells, protein was extracted by grinding cells in 2× SDS-PAGE sample buffer (4 mL g−1) using a mortar and pestle. Samples were boiled for 5 min and were subjected to centrifugation at 16,000g for 2 min prior to fractionation through 8% (w/v; PI-4 kinase) or 10% (w/v; BiP and PDI) SDS-polyacrylamide gels.
The PI 4-kinase immunoblots were probed with antibodies raised against a recombinant protein encoding the C-terminal one-third of AtPI4Kα as described by Stevenson et al. (1998) except that proteins were transferred in a submerged system in 10 mm CAPS [3-(cyclohexylamino)propanesulfonic acid], pH 11, and 20% (v/v) methanol for 1.5 h at 40V to Immobilon-P polyvinylidene difluoride membrane (Millipore, Bedford, MA). Proteins for BiP immunoblots were transferred in a similar manner and the membranes were probed with monoclonal antiserum raised against spinach BiP (ID9, StressGen Biotechnologies, Victoria, BC, Canada; 1:10,000 in Tris-buffered saline [TBS]) and goat anti-mouse IgG conjugated to horseradish peroxidase (Bio-Rad; 1:30,000 dilution in TBS). Three low-Mr proteins are often seen in immunoblot analysis of soybean cells, but are not recognized by the monoclonal BiP antibody in maize or in immunoprecipitations from soybean cells. The expression of these proteins does not seem to follow the BiP expression pattern with various treatments (data not shown). Proteins for PDI blots were transferred as described above and the membranes were probed with polyclonal antiserum raised against recombinant castor bean PDI (Coughlan et al., 1996; 1:5,000 dilution in TBS) and goat anti-rabbit IgG conjugated to horseradish peroxidase (Bio-Rad; 1:30,000 dilution in TBS). Membranes were blocked with 5% (w/v) non-fat dry milk in TBS prior to incubation with primary antibody and cross-reacting material was visualized by chemiluminescent detection according to Stevenson et al. (1998).
Protein Labeling
At the designated times after treatment, 1-mL aliquots of soybean suspension culture cells were transferred to a 24-well tissue culture plate (Becton-Dickinson, Lincoln Park, NJ). Cells were incubated with Easytag Expre35S35S -protein labeling mix (NEN Life Science Products) for 30 min and were washed according to the protocol described by Malik et al. (1999) prior to being frozen in liquid nitrogen and kept at −80°C until analysis.
ER-enriched fractions for immunoprecipitation were prepared by grinding the frozen cells in buffer A made 20% (w/v) Suc using a mortar and pestle with a small amount of glass beads. Cellular debris was removed by a 2,000g centrifugation for 2 min and the supernatant was applied to a discontinuous Suc gradient prepared as a 0.6-mL step of 1.5 m Suc and a 1.0-mL step of 1.0 m Suc, both in buffer A. The remaining fractionation steps were identical to those described above for fractionation of ER from maize.
Immunoprecipitation
All immunoprecipitation steps were conducted at 0°C to 4°C. Radiolabeled cells were resuspended at a ratio of 2 mL g−1 in lysis buffer consisting of 20 mm sodium phosphate buffer (pH 7.5), 500 mm NaCl, 0.1% (w/v) SDS, 1% (v/v) NP-40, 0.02% (w/v) sodium azide, and 0.5% (w/v) sodium deoxycholate and were disrupted using a microtube sample pestle (Research Products International, Mt. Prospect, IL) with a small amount of glass beads. After centrifugation at 16,000g for 2 min, the resulting supernatant was saved for analysis. In an alternate manner, ER-enriched fractions were obtained from soybean cells by Suc gradient fractionation as described above.
As a clearing step, 25 μL of a 50% (v/v) slurry of Protein-A agarose (Life Technologies, Rockville, MD) in TBS was added to each sample (40 μg of protein per incubation diluted to 200 μL with lysis buffer). After clearing, proteins were incubated with antibodies against spinach BiP (1D9, StressGen Biotechnologies; 1 μL), calreticulin (Coughlan et al., 1997; 1 μL), or the C-terminal one-third of AtPI4Kα (Stevenson et al., 1998; 8 μL) for 4 h at 4°C on a Nutator, followed by incubation with Protein-A agarose on a Nutator for 1 h at 4°C. After washing the beads three times with lysis buffer, we resuspended the samples in 20 μL of 2× SDS-PAGE sample buffer, boiled them for 5 min, and then separated proteins by SDS-PAGE. Gels were incubated with En3Hance autoradiography enhancer (NEN Life Science Products) for 1 h and in 5% (v/v) glycerol (4°C) for 30 min at room temperature. Gels were dried under vacuum onto filter paper (Whatman, Clifton, NJ) and were exposed to x-ray film at −80°C.
Lipid Analysis
Endosperm was homogenized in buffer B, left undisturbed for 15 min on ice to allow starch to settle, and then decanted prior to a low-speed centrifugation at 80g for 5 min and fractionation through Suc gradients as described above for separation of ER and protein bodies. Fractions from the gradients were diluted to the refractive index of the homogenization buffer and were subjected to centrifugation at 5,000g in a tabletop centrifuge (protein bodies) or 100,000g in a fixed angle rotor (ER). ER and protein body pellets were resuspended in buffer B prior to extraction of the lipids into the organic phase by overnight incubation in chloroform:methanol (2:1, v/v) at −20°C.
For total PL and triacylglycerol analysis, five to seven kernels of each phenotype were dissected. Endosperm and embryos were separated from each other and were placed in a drying oven (80°C) for 2 d prior to measuring of dry weights. Dried samples were pulverized with a mallet and lipids were extracted in chloroform:methanol (2:1, v/v) overnight at −20°C. Particulates were removed from the lipid extract by vacuum filtration through an solid-phase extraction cartridge with a 60-μm exclusion size (Alltech Associates, Deerfield, IL).
Lipid extracts were evaporated under nitrogen at 50°C, resuspended in 100 μL of chloroform:methanol (2:1), and applied to a 60-Å silica gel plate (Whatman). Lipid species were resolved by TLC with a developing solvent of petroleum ether:diethyl ether:acetic acid (80:20:1, v/v) to separate total PL and triacylglycerol or chloroform:methanol:concentrated NH4OH (60:30:1.5, v/v) to separate individual phospholipid species. All lipids separated by TLC were visualized with 2,7-dichlorofluorescein in 95% (w/v) ethanol and were identified by comigration with known standards. The regions of the TLC plate corresponding to individual lipid species were extracted with hexane and quantified by gas chromatography (Browse et al., 1986).
[14C]Acetate Incorporation
An aliquot of cells was removed at the designated time after treatment with Tm and was incubated with shaking at room temperature for 2 h with [14C]-acetic acid (NEN Life Science Products) at a concentration of 0.5 μCi mL−1. After labeling, cells were harvested by centrifugation at 5,000g and were frozen at −80°C until analysis.
Labeled cells were thawed on ice and rinsed with ice-cold 5% (w/v) TCA. Lipids were extracted, dried under nitrogen, resuspended, and resolved by TLC as described above. Bands were visualized and quantified with an imaging scanner (500, Bioscan System, Washington, DC).
ACKNOWLEDGMENTS
We are grateful to Nozomu Koizumi and Maarten J. Chrispeels for sharing unpublished data. Special thanks to Bill Novitzky for technical assistance with the TLC and GC analyses, Bonnie Sheldon for sharing her soybean cell cultures, Weibing Xing for implementing the CCT enzyme activity assay, Jeff Gillikin for sharing his protein fractionation expertise, and members of the Boston, Boss, and Dewey laboratories for helpful discussions.
Footnotes
This work was supported by the U.S. Department of Energy (grant no. DE–FG02–00ER150065 to R.S.B., R.E.D., and W.F.B.), by the National Science Foundation (grant nos. MCB96–04285 [to W.F.B.], IBN–9513582 [to R.E.D.], and MCB93–17303 [to R.S.B.]), by the North Carolina Agricultural Research Service (to W.F.B., R.S.B., and R.E.D.), and by the National Science Foundation for Interdisciplinary Research Training Group on Transgenic Plant Technology for Laboratory and Field Applications (fellowship no. BIR–9420689 to K.J.S.).
LITERATURE CITED
- Abusteit EO, Corbin FT, Schmitt DP, Burton JW, Worsham AD, Thompson L., Jr Absorption, translocation and metabolism of metribuzin in diploid and tetraploid soybean (Glycine max) plants and cell cultures. Weed Sci. 1985;33:618–628. [Google Scholar]
- Bertolotti A, Zhang Y, Hendershot LM, Harding HP, Ron D. Dynamic interaction of BiP and ER stress transducers in the unfolded-protein response. Nat Cell Biol. 2000;2:326–332. doi: 10.1038/35014014. [DOI] [PubMed] [Google Scholar]
- Boston RS, Fontes EBP, Shank BB, Wrobel RL. Increased expression of the maize immunoglobulin binding protein homolog b-70 in three zein regulatory mutants. Plant Cell. 1991;3:497–505. doi: 10.1105/tpc.3.5.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boston RS, Viitanen PV, Vierling E. Molecular chaperones and protein folding in plants. Plant Mol Biol. 1996;32:191–222. doi: 10.1007/BF00039383. [DOI] [PubMed] [Google Scholar]
- Browse J, McCourt PJ, Somerville CR. Fatty acid composition of leaf lipids determined after combined digestion and fatty acid methyl ester formation from fresh tissue. Anal Biochem. 1986;152:141–145. doi: 10.1016/0003-2697(86)90132-6. [DOI] [PubMed] [Google Scholar]
- Chapman R, Sidrauski C, Walter P. Intracellular signaling from the endoplasmic reticulum to the nucleus. Annu Rev Cell Dev Biol. 1998;14:459–485. doi: 10.1146/annurev.cellbio.14.1.459. [DOI] [PubMed] [Google Scholar]
- Cho MH, Tan Z, Erneux C, Shears SB, Boss WF. The effects of mastoparan on the carrot cell plasma membrane polyphosphoinositide phospholipase C. Plant Physiol. 1995;107:845–856. doi: 10.1104/pp.107.3.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman CE, Lopes MA, Gillikin JW, Boston RS, Larkins BA. A defective signal peptide in the maize high-lysine mutant floury-2. Proc Natl Acad Sci USA. 1995;92:6828–6831. doi: 10.1073/pnas.92.15.6828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coughlan SJ, Hastings C, Winfrey RJ., Jr Molecular characterization of plant endoplasmic reticulum: identification of protein disulfide-isomerase as the major reticuloplasmin. Eur J Biochem. 1996;235:215–224. doi: 10.1111/j.1432-1033.1996.00215.x. [DOI] [PubMed] [Google Scholar]
- Coughlan SJ, Hastings C, Winfrey R., Jr Cloning and characterization of the calreticulin gene from Ricinus communis L. Plant Mol Biol. 1997;34:897–911. doi: 10.1023/a:1005822327479. [DOI] [PubMed] [Google Scholar]
- Cox JS, Chapman RE, Walter P. The unfolded protein response coordinates the production of endoplasmic reticulum protein and endoplasmic reticulum membrane. Mol Biol Cell. 1997;8:1805–1814. doi: 10.1091/mbc.8.9.1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox JS, Shamu CE, Walter P. Transcriptional induction of genes encoding endoplasmic reticulum resident proteins requires a transmembrane protein kinase. Cell. 1993;73:1197–1206. doi: 10.1016/0092-8674(93)90648-a. [DOI] [PubMed] [Google Scholar]
- D'Amico L, Valsasina B, Daminati MG, Fabbrini MS, Nitti G, Bollini R, Ceriotti A, Vitale A. Bean homologs of the mammalian glucose-regulated proteins: induction by tunicamycin and interaction with newly synthesized seed storage proteins in the endoplasmic reticulum. Plant J. 1992;2:443–455. doi: 10.1111/j.1365-313x.1992.00443.x. [DOI] [PubMed] [Google Scholar]
- De Camilli P, Emr SD, McPherson PS, Novick P. Phosphoinositides as regulators in membrane traffic. Science. 1996;271:1533–1539. doi: 10.1126/science.271.5255.1533. [DOI] [PubMed] [Google Scholar]
- Denecke J, Carlsson LE, Vidal S, Hoglund AS, Ek B, van Zeijl MJ, Sinjorgo KM, Palva ET. The tobacco homolog of mammalian calreticulin is present in protein complexes in vivo. Plant Cell. 1995;7:391–406. doi: 10.1105/tpc.7.4.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontes EBP, Shank BB, Wrobel RL, Moose SP, OBrian GR, Wurtzel ET, Boston RS. Characterization of an immunoglobulin binding protein homolog in the maize floury-2 endosperm mutant. Plant Cell. 1991;3:483–496. doi: 10.1105/tpc.3.5.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foti DM, Welihinda A, Kaufman RJ, Lee AS. Conservation and divergence of the yeast and mammalian unfolded protein response: activation of specific mammalian endoplasmic reticulum stress element of the grp78/BiP promoter by yeast Hac1. J Biol Chem. 1999;274:30402–30409. doi: 10.1074/jbc.274.43.30402. [DOI] [PubMed] [Google Scholar]
- Gething MJ, Sambrook J. Protein folding in the cell. Nature. 1992;355:33–44. doi: 10.1038/355033a0. [DOI] [PubMed] [Google Scholar]
- Gillikin JW, Zhang F, Coleman CE, Bass HW, Larkins BA, Boston RS. A defective signal peptide tethers the floury-2 zein to the endoplasmic reticulum membrane. Plant Physiol. 1997;114:345–352. doi: 10.1104/pp.114.1.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hama H, Schnieders EA, Thorner J, Takemoto JY, DeWald DB. Direct involvement of phosphatidylinositol 4-phosphate in secretion in the yeast Saccharomyces cerevisiae. J Biol Chem. 1999;274:34294–34300. doi: 10.1074/jbc.274.48.34294. [DOI] [PubMed] [Google Scholar]
- Jones RA. Effects of floury-2 locus on zein accumulation and RNA metabolism during maize endosperm development. Biochem Genet. 1978;16:27–38. doi: 10.1007/BF00484382. [DOI] [PubMed] [Google Scholar]
- Kaufman RJ. Stress signaling from the lumen of the endoplasmic reticulum: coordination of gene transcriptional and translational controls. Genes Dev. 1999;13:1211–1233. doi: 10.1101/gad.13.10.1211. [DOI] [PubMed] [Google Scholar]
- Kinney AJ, Clarkson DT, Loughman BC. The regulation of phosphatidylcholine biosynthesis in rye (Secale cereale) roots: stimulation of the nucleotide pathway by low temperature. Biochem J. 1987;242:755–759. doi: 10.1042/bj2420755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Larkins BA, Hurkman WJ. Synthesis and deposition of zein protein bodies in maize endosperm. Plant Physiol. 1978;62:256–263. doi: 10.1104/pp.62.2.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee AS. Mammalian stress response: induction of the glucose-regulated protein family. Curr Opin Cell Biol. 1992;4:267–273. doi: 10.1016/0955-0674(92)90042-b. [DOI] [PubMed] [Google Scholar]
- Li CP, Larkins BA. Expression of protein disulfide isomerase is elevated in the endosperm of the maize floury-2 mutant. Plant Mol Biol. 1996;30:873–882. doi: 10.1007/BF00020800. [DOI] [PubMed] [Google Scholar]
- Lopes MA, Coleman CE, Kodrzycki R, Lending CR, Larkins BA. Synthesis of an unusual α-zein protein is correlated with the phenotypic effects of the floury2 mutation in maize. Mol Gen Genet. 1994;245:537–547. doi: 10.1007/BF00282216. [DOI] [PubMed] [Google Scholar]
- Malik MK, Slovin JP, Hwang CH, Zimmerman JL. Modified expression of a carrot small heat shock protein gene, hsp17.7, results in increased or decreased thermotolerance double dagger. Plant J. 1999;20:89–99. doi: 10.1046/j.1365-313x.1999.00581.x. [DOI] [PubMed] [Google Scholar]
- Marocco A, Santucci A, Cerioli S, Motto M, Di Fonzo N, Thompson R, Salamini F. Three high-lysine mutations control the level of ATP-binding HSP70-like proteins in the maize endosperm. Plant Cell. 1991;3:507–515. doi: 10.1105/tpc.3.5.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martiniello P, Lorenzoni C, Stanca AM, Maggiore T, Gentinetta E, Salamini F. Seed quality differences between normal, floury-2 and opaque-2 maize inbreds. Euphytica. 1978;27:411–417. [Google Scholar]
- Mori K, Ma W, Gething M-J, Sambrook J. A transmembrane protein with a cdc2+/CDC28-related kinase activity is required for signaling from the ER to the nucleus. Cell. 1993;74:743–756. doi: 10.1016/0092-8674(93)90521-q. [DOI] [PubMed] [Google Scholar]
- Ohlrogge J, Browse J. Lipid biosynthesis. Plant Cell. 1995;7:957–970. doi: 10.1105/tpc.7.7.957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okita TW, Rogers JC. Compartmentation of proteins in the endomembrane system of plant cells. Annu Rev Plant Physiol Plant Mol Biol. 1996;47:327–350. doi: 10.1146/annurev.arplant.47.1.327. [DOI] [PubMed] [Google Scholar]
- Roth MG. Lipid regulators of membrane traffic through the Golgi complex. Trends Cell Biol. 1999;9:174–179. doi: 10.1016/s0962-8924(99)01535-4. [DOI] [PubMed] [Google Scholar]
- Shamu CE, Walter P. Oligomerization and phosphorylation of the Ire1p kinase during intracellular signaling from the endoplasmic reticulum to the nucleus. EMBO J. 1996;15:3028–3039. [PMC free article] [PubMed] [Google Scholar]
- Stevenson JM, Perera IY, Boss WF. A phosphatidylinositol 4-kinase pleckstrin homology domain that binds phosphatidylinositol 4-monophosphate. J Biol Chem. 1998;273:22761–22767. doi: 10.1074/jbc.273.35.22761. [DOI] [PubMed] [Google Scholar]
- Watowich SS, Morimoto RI. Complex regulation of heat shock- and glucose-responsive genes in human cells. Mol Cell Biol. 1988;8:393–405. doi: 10.1128/mcb.8.1.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber EJ. The lipids of corn germ and endosperm. J Am Oil Chem Soc. 1978;56:637–641. [Google Scholar]
- Westergren T, Ekblad L, Jergil B, Sommarin M. Phosphatidylinositol 4-kinase associated with spinach plasma membranes: isolation and characterization of two distinct forms. Plant Physiol. 1999;121:507–516. doi: 10.1104/pp.121.2.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrobel R. Expression of molecular chaperones in endoplasmic reticulum of maize endosperm. PhD thesis. Raleigh, NC: North Carolina State University; 1996. [Google Scholar]
- Wrobel RL, OBrian GR, Boston RS. Comparative analysis of BiP gene expression in maize endosperm. Gene. 1997;204:105–113. doi: 10.1016/s0378-1119(97)00529-5. [DOI] [PubMed] [Google Scholar]
- Xue HW, Pical C, Brearley C, Elge S, Muller-Rober B. A plant 126-kDa phosphatidylinositol 4-kinase with a novel repeat structure: cloning and functional expression in baculovirus-infected insect cells. J Biol Chem. 1999;274:5738–5745. doi: 10.1074/jbc.274.9.5738. [DOI] [PubMed] [Google Scholar]
- Zhang F, Boston RS. Increases in binding protein (BiP) accompany changes in protein body morphology in three high-lysine mutants of maize. Protoplasma. 1992;171:142–152. [Google Scholar]