Abstract
Background and Aims
Hepatocellular carcinoma (HCC) is one of the commonest causes of cancer‐related death worldwide. Whether gender is an independent factor for HCC survival is debatable. We studied the influence of gender on the clinical characteristics of HCC and on survival.
Methods
The study cohort comprised patients with HCC seen in our department from 1988 to 2021. Clinical data were prospectively collected. We studied and compared demography, HCC characteristics, and survival between females and males. Survival analysis was censored on October 31, 2015.
Results
There were 1716 HCC patients. 343 (20.0%) were females. Females were significantly older at diagnosis (median 69 vs 62 years, P < 0.001). More females were diagnosed via regular HCC surveillance (37.9% vs 29.6%, P = 0.003). Hence, as expected, females had less‐advanced HCC at diagnosis with smaller median tumor diameter (30 vs 39.5 mm, P = 0.038), lower frequency of portal vein tumor thrombus (19.4% vs 33.4%, P < 0.001), less distant metastases (7.7% vs 11%, P = 0.043), and earlier Barcelona Clinic Liver Cancer (BCLC) stages (0/A, 39.7% vs 28.4%, P < 0.001). On multivariable analysis, HCC diagnosis via surveillance but not female gender was an independent predictor of improved HCC survival.
Conclusions
In this large cohort of multi‐ethnic Asian patients, females with HCC were significantly more adherent to surveillance and hence presented with less advanced HCC with correspondingly better overall survival than males. The gender difference in survival is likely due to females having better adherence to HCC surveillance. Surveillance to diagnose early‐stage HCC remains crucial in improving outcomes.
Keywords: adherence, gender, hepatocellular carcinoma, surveillance, survival
Our study has shown that better overall survival in females with hepatocellular carcinoma (HCC) compared to males is most likely due to the fact that females have better adherence to HCC surveillance. This reinforces the importance of HCC surveillance in at‐risk individuals.
Introduction
Hepatocellular carcinoma (HCC) is the sixth most common cancer and the third leading cause of cancer‐related mortality worldwide. 1 The incidence of HCC is higher in males than females, with a reported ratio of 4 to 1. 2 Aside from well‐known etiological differences in chronic liver disease, the differences in characteristics and prognosis of HCC between males and females remain poorly understood. A previous study reported a better survival rate in female patients with HCC than their male counterparts. 3 Gender‐related hormonal effect on the growth of HCC has been hypothesized but it has yet to be proven. Whether gender is an independent factor for HCC survival remains debatable. The aim of this study was to evaluate the influence of gender on clinical characteristics of HCC and on survival and to identify factors that may explain the differences if present.
Patients and methods
We have been prospectively enrolling patients with HCC who are seen in our Department of Gastroenterology and Hepatology Singapore General Hospital, a tertiary hospital in Singapore, into a Research Electronic Data Capture (REDCap) database. Our department also runs a regular program for HCC surveillance in at‐risk patients. Patients who were enrolled in the database between 1988 and August 2021 were included in our study. Clinical data were prospectively collected. We compared demography, clinical and tumor characteristics, and survival between male and female patients with HCC. Survival census was done on October 31, 2015 with input from our National Registry of Deaths.
The study was approved by the institutional review board of Singapore General Hospital in accordance with the Declarations of Helsinki and Istanbul, with a waiver of informed consent.
Data collected included patient demographics, etiology of chronic liver disease, Child‐Pugh status, and laboratory studies at the time of HCC diagnosis, mode of HCC diagnosis, HCC‐related variables, and treatment modalities.
The etiology was classified as hepatitis B virus (HBV) if the HBV surface antigen was positive, or anti‐HBV core total antibody positive in the absence of other risk factors for HCC, as hepatitis C virus (HCV) if anti‐HCV IgG antibody or HCV RNA was positive and as alcohol if the consumption of alcohol exceeded 60 g/day for at least 5 years in both male and female patients.
Severity of underlying liver cirrhosis was classified based on Child‐Pugh status at the time of HCC diagnosis.
Mode of HCC diagnosis was considered as “surveillance” if the HCC was diagnosed during regular surveillance of at‐risk patients for HCC. It is defined as “symptomatic” when the patient was not under HCC surveillance and presented with symptoms. Barcelona Clinic Liver Cancer (BCLC) system which incorporates a patient's physical functional status and liver functional status, as well as tumor characteristics based on imaging, was used to stage HCC in our study population.
Treatment modalities were classified into three categories, curative, non‐curative, and best supportive care. Curative treatment modalities included liver transplantation, surgical resection, and radiofrequency or microwave ablation. Non‐curative treatment modalities included transhepatic arterial chemoembolization (TACE), Ytrium‐90 selective internal radiation therapy (Y90‐SIRT), stereotactic body radiation therapy (SBRT), and systemic therapy. If the patient did not receive any specific HCC treatment, it was deemed as the best supportive care.
A survival census was performed with the National Registry of Death on October 31, 2015. Under the law, all deaths of local residents must be reported to the National Registry of Deaths.
Statistical analysis
Continuous variables were presented as median with interquartile range (IQR) and compared using the Mann–Whitney U test. Categorical variables were presented as frequencies and percentages and compared using the Chi‐square test. Survival census was performed on October 31, 2015. Survival duration was defined from the date of HCC diagnosis to the date of death as recorded in the National Registry of Deaths or censored on October 31, 2015 if the patient was still alive. The survival probability was calculated using Kaplan–Meier method and compared using the log‐rank test. To factor in the possibility of lead‐time bias, which represents the apparently improved survival due to earlier HCC diagnosis in the course of the disease, we subtracted the calculated lead‐time from the survival duration for patients in the surveillance group. The lead time was calculated based on the formula, T = 3 × HCC doubling time × log(d1/d0)/log2, which was proposed by Schwartz et al. 4 T is the lead time in days, d0 and d1 are the median tumor diameters of the surveillance group and the symptomatic group respectively from the survival analysis cohort. We calculated the lead time with an assumption of three different HCC doubling times, 60, 90, and 120 days, in keeping with the ranges used in previous studies. 5 , 6 , 7 Univariate and multivariable Cox proportional hazard regression analyses were performed to identify factors associated with overall survival. A two‐tailed P value of 0.05 or less was considered statistically significant. All statistical analyses were performed using SPSS version 28 (IBM).
Results
Patient characteristics
A total of 1716 patients with HCC between 1988 and August 2021 were included in our study, 80% were males. Patients' baseline demography and characteristics are shown in Table 1. Females were significantly older at HCC diagnosis (median age of 69 vs 62, P < 0.001). Females had a significantly higher frequency of non‐viral liver disease (33.8% vs 24.0%, P < 0.001). In terms of specific etiology, autoimmune liver disease was more common (2.0% vs 0.2%, P < 0.001), and alcohol was less common (0.9% vs 5.8%, P < 0.001) in females. There was no difference in the surveillance status for viral and non‐viral liver disease (Table 2). There was also no difference in the distribution of Child‐Pugh status between the two genders.
Table 1.
Clinical characteristics of males and females with HCC
| Patient characteristics | Overall (n = 1716) | Male (n = 1373) | Female (n = 343) | P value |
|---|---|---|---|---|
| Age, median (IQR) | 63 (55–71) | 62 (54–69) | 69 (61–76) | <0.001 |
| Ethnicity | ||||
| Chinese | 1505 (87.7%) | 1197 (87.2%) | 308 (89.8%) | 0.26 |
| Malay | 130 (7.6%) | 105 (7.6%) | 25 (7.3%) | |
| Indian | 45 (2.6%) | 41 (3.0%) | 4 (1.2%) | |
| Others | 36 (2.1%) | 30 (2.2%) | 6 (1.7%) | |
| Eastern cooperative oncology group (ECOG) status | ||||
| 0 | 728 (45.7%) | 572 (44.7%) | 156 (49.8%) | 0.10 |
| 1 | 437 (27.4%) | 363 (28.4%) | 74 (23.6%) | |
| 2 | 308 (19.3%) | 255 (19.9%) | 53 (16.9%) | |
| 3 | 98 (6.2%) | 75 (5.9%) | 23 (7.3%) | |
| 4 | 21 (1.3%) | 14 (1.1%) | 7 (2.2%) | |
| Etiology of underlying liver disease | ||||
| HBV | 1137 (66.3%) | 930 (67.7%) | 207 (60.3%) | |
| HCV | 78 (4.5%) | 64 (4.7%) | 14 (4.1%) | |
| HBV/HCV | 56 (3.3%) | 50 (3.6%) | 6 (1.7%) | |
| Alcohol | 83 (4.8%) | 80 (5.8%) | 3 (0.9%) | |
| Autoimmune | 9 (0.5%) | 2 (0.2%) | 7 (2%) | |
| Cryptogenic/NASH | 353 (20.5%) | 247 (17.9%) | 106 (30.9%) | |
| Viral | 1271 (74.1%) | 1044 (76%) | 227 (66.2%) | <0.001 |
| Non‐viral | 445 (25.9%) | 329 (24.0%) | 116 (33.8%) | |
| HBV related | 1193 (69.5%) | 980 (71.4%) | 213 (62.1%) | <0.001 |
| Non‐HBV related | 523 (30.5%) | 393 (28.6%) | 130 (37.9%) | |
| Autoimmune | 9 (0.5%) | 2 (0.2%) | 7 (2%) | <0.001 |
| Non‐autoimmune | 1707 (99.5%) | 1371 (99.8%) | 336 (98%) | |
| Alcohol | 83 (4.8%) | 80 (5.8%) | 3 (0.9%) | < 0.001 |
| Non‐alcohol | 1633 (95.2%) | 1293 (94.2%) | 340 (99.1%) | |
| HCC diagnosis | ||||
| Surveillance | 535 (31.3%) | 406 (29.6%) | 129 (37.9%) | 0.003 |
| Symptomatic | 1177 (68.7%) | 966 (70.4%) | 211 (62.1%) | |
| Ascites | ||||
| None | 1142 (67.8%) | 922 (68.4%) | 220 (65.5%) | 0.21 |
| Mild/controlled | 272 (16.2%) | 207 (15.4%) | 65 (19.3%) | |
| Severe/uncontrolled | 270 (16%) | 219 (16.2%) | 51 (15.2%) | |
| Encephalopathy | ||||
| None | 1638 (97.2%) | 1308 (97%) | 330 (97.6%) | 0.32 |
| Grade I/II | 39 (2.3%) | 34 (2.5%) | 5 (1.5%) | |
| Grade III/IV | 9 (0.5%) | 6 (0.4%) | 3 (0.9%) | |
| Child‐Pugh status | ||||
| A | 899 (54.2%) | 720 (54.1%) | 179 (54.6%) | 0.73 |
| B | 567 (34.2%) | 452 (34.0%) | 115 (35.1%) | |
| C | 192 (11.6%) | 158 (11.9%) | 34 (10.4%) | |
Table 2.
Comparison of patient characteristics based on the mode of HCC diagnosis
| Patient characteristics | Overall (n = 1712) | Surveillance (n = 535) | Symptomatic (n = 1177) | P value |
|---|---|---|---|---|
| Age, median (IQR) | 63 (55–71) | 63 (56–70) | 63 (55–71) | 0.992 |
| Eastern cooperative oncology group (ECOG) status | ||||
| 0–1 | 1163 (73.1%) | 475 (94.4%) | 688 (63.3%) | <0.001 |
| 2–4 | 427 (26.9%) | 28 (5.6%) | 399 (36.7%) | |
| Etiology of underlying liver disease | ||||
| HBV | 1134 (66.2%) | 351 (65.6%) | 783 (66.5%) | |
| HCV | 77 (4.5%) | 31 (5.8%) | 46 (3.9%) | |
| HBV/HCV | 56 (3.3%) | 7 (1.3%) | 49 (4.2%) | |
| Alcohol | 83 (4.8%) | 34 (6.4%) | 49 (4.2%) | |
| Autoimmune | 9 (0.5%) | 5 (0.9%) | 4 (0.3%) | |
| Cryptogenic/NASH | 353 (20.6%) | 107 (20%) | 246 (20.9%) | |
| HBV related | 1190 (69.5%) | 358 (66.9%) | 832 (70.7%) | 0.116 |
| Non‐HBV related | 522 (30.5%) | 177 (33.1%) | 345 (29.3%) | |
| Viral | 1267 (74.0%) | 389 (72.7%) | 878 (74.6%) | 0.409 |
| Non‐viral | 445 (26.0%) | 146 (27.3%) | 299 (25.4%) | |
| Ascites | ||||
| None | 1140 (67.8%) | 447 (85.3%) | 693 (59.8%) | <0.001 |
| Mild or severe | 542 (32.2%) | 77 (14.7%) | 465 (40.2%) | |
| Encephalopathy | ||||
| None | 1636 (97.1%) | 510 (97.5%) | 1126 (97.0%) | 0.784 |
| Grade I/II | 39 (2.3%) | 11 (2.1%) | 28 (2.4%) | |
| Grade III/IV | 9 (0.5%) | 2 (0.4%) | 7 (0.6%) | |
| Child‐Pugh status | ||||
| A | 898 (54.2%) | 393 (76.3%) | 505 (44.2%) | <0.001 |
| B or C | 759 (45.8%) | 122 (23.7%) | 637 (55.8%) | |
Clinical presentation and tumor characteristics
Significantly more females were diagnosed with HCC via surveillance compared to males (37.9% vs 29.6%, P = 0.003). As a result, females had less advanced HCC at diagnosis. The differences in HCC characteristics between the two genders are shown in Table 3. Females had a higher frequency of solitary HCC (60.8% vs 50.7%, P < 0.001) as well as smaller median tumor size (30.0 vs 39.5 mm, P < 0.001) when compared with males. Females also had a lower frequency of portal vein tumor invasion (19.4% vs 33.4%, P < 0.001) and extrahepatic involvement (5.6% vs 8.6%, P = 0.048). Similarly, significantly more females were in BCLC stages 0/A (39.7% vs 28.4%, P < 0.001). Alpha‐fetoprotein (AFP) concentration at the time of HCC diagnosis did not differ significantly between the two genders (Table 3).
Table 3.
Comparison of HCC characteristics between genders
| Tumor characteristic | Overall (n = 1716) | Male (n = 1373) | Female (n = 343) | P value |
|---|---|---|---|---|
| Numbers of lesion | ||||
| Single | 890 (52.7%) | 685 (50.7%) | 205 (60.8%) | <0.001 |
| Multiple/diffuse | 799 (47.3%) | 667 (49.3%) | 132 (39.2%) | |
| Tumor diameters (mm), median (IQR) | 36 (20–71) | 39.5 (20–78) | 30 (17–56.5) | 0.038 |
| Portal vein invasion | 480 (30.6%) | 419 (33.4%) | 61 (19.4%) | <0.001 |
| Lymph node involvement | 129 (8%) | 111 (8.6%) | 18 (5.6%) | 0.048 |
| Distant metastases | 174 (10.4%) | 148 (11.0%) | 26 (7.7%) | 0.043 |
| AFP, median (IQR) | 79 (8–4123.5) | 82 (8–544) | 56 (7–1905.5) | 0.57 |
| BCLC | ||||
| 0/A | 468 (30.7%) | 348 (28.4%) | 120 (39.7%) | <0.001 |
| B/C/D | 1058 (69.3%) | 876 (71.6%) | 182 (60.3%) | |
| Treatment | ||||
| Curative | 534 (31.1%) | 411 (29.9%) | 123 (35.9%) | 0.12 |
| Non‐curative | 390 (22.7%) | 321 (23.4%) | 69 (20.1%) | |
| Best supportive care | 510 (29.7%) | 410 (29.9%) | 100 (29.2%) | |
Comparison of clinical and tumor characteristics of patients with HCC diagnosed via surveillance versus symptomatic presentation
As surveillance was the main factor affecting survival in our study populations, we compared the baseline clinical and HCC characteristics of patients with HCC diagnosed via surveillance versus symptomatic presentation. Patients who had HCC diagnosed via surveillance had significantly better Eastern Cooperative Oncology Group (ECOG) status (ECOG 0–1 94.4% vs 63.3%, P < 0.001) at the time of HCC diagnosis. Surveillance cases also had significantly better Child‐Pugh status (Child‐Pugh A, 76.3% vs 44.2%, P < 0.001) and were free of ascites at presentation (85.3% vs 59.8%, P < 0.001) (Table 2). In terms of tumor characteristics, HCCs in the surveillance group were less advanced at presentation, as evidenced by significantly smaller tumor size (23 vs 56 mm, P < 0.001), less portal vein invasion (6.9% vs 42.4%, P < 0.001), and less extrahepatic involvement (0.5% vs 14.9%, P < 0.001) (Table 4). Patients who had HCC diagnosed via surveillance also had significantly better BCLC stage (BCLC 0/A, 69.7% vs 12.2%, P < 0.001) and hence were significantly more likely to receive curative treatment (72.0% vs 19.2%, P < 0.001) (Table 4).
Table 4.
Comparison of HCC characteristics based on the mode of HCC diagnosis
| Tumor characteristic | Overall (n = 1712) | Surveillance (n = 535) | Symptomatic (n = 1177) | P value |
|---|---|---|---|---|
| Numbers of lesion | ||||
| Single | 889 (52.7%) | 367 (69.5%) | 522 (45.0%) | <0.001 |
| Multiple/diffuse | 799 (47.3%) | 161 (30.5%) | 638 (55.0%) | |
| Tumor diameters (mm), median (IQR) | 36 (20–71) | 23 (16–38) | 56 (29–100) | <0.001 |
| Portal vein invasion | 480 (30.6%) | 36 (6.9%) | 444 (42.4%) | <0.001 |
| Lymph node involvement | 129 (8%) | 11 (2.1%) | 118 (10.8%) | <0.001 |
| Distant metastases | 174 (10.4%) | 3 (0.5%) | 171 (14.9%) | <0.001 |
| AFP, median (IQR) | 79 (8–4139) | 10.5 (4–46.2) | 520 (18–12 000) | <0.001 |
| BCLC | ||||
| 0/A | 467 (30.6%) | 341 (69.7%) | 126 (12.2%) | <0.001 |
| B/C/D | 1058 (69.4%) | 148 (30.3%) | 910 (87.8%) | |
| Treatment | ||||
| Curative | 532 (37.2%) | 350 (72.0%) | 182 (19.2%) | <0.001 |
| Non‐curative | 390 (27.2%) | 101 (20.8%) | 289 (30.6%) | |
| Best supportive care | 510 (35.6%) | 35 (7.2%) | 475 (50.2%) | |
Treatment and survival analysis
More females underwent HCC treatment with curative intent compared with males, but this was not statistically significant (35.9% vs 29.9%, P = 0.053) (Table 3).
As the survival census was performed on October 31, 2015, patients who were enrolled in the database after this date were excluded from the survival analysis. A total of 1270 patients were included in the survival analysis (Fig. 1).
Figure 1.
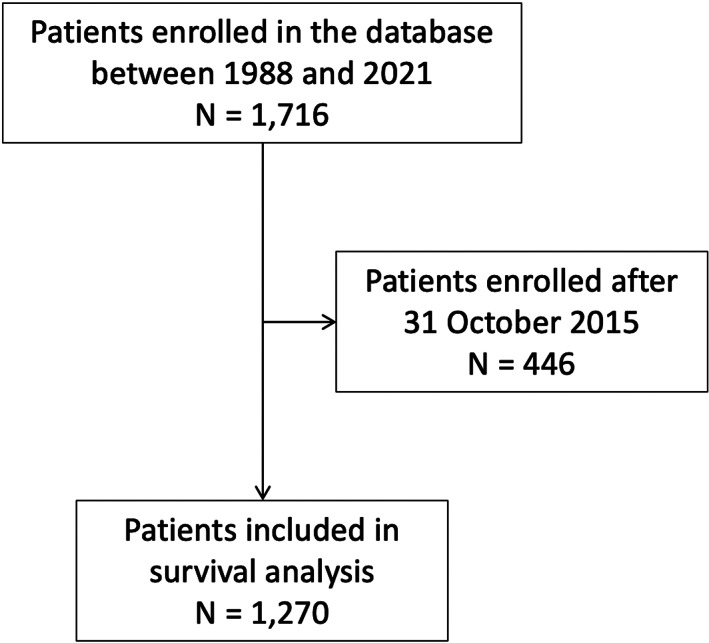
Flowchart of study population with survival census on October 31, 2015.
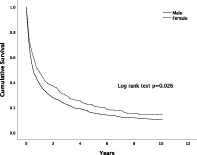
The overall median survival of HCC was significantly higher in females than in males (10.1 vs 6.1 months, P = 0.03) (Fig. 2). The 1‐, 3‐ and 5‐year survival rates were 48%, 29%, and 21% in females compared to 39%, 23%, and 16% in males respectively (Table 5).
Figure 2.
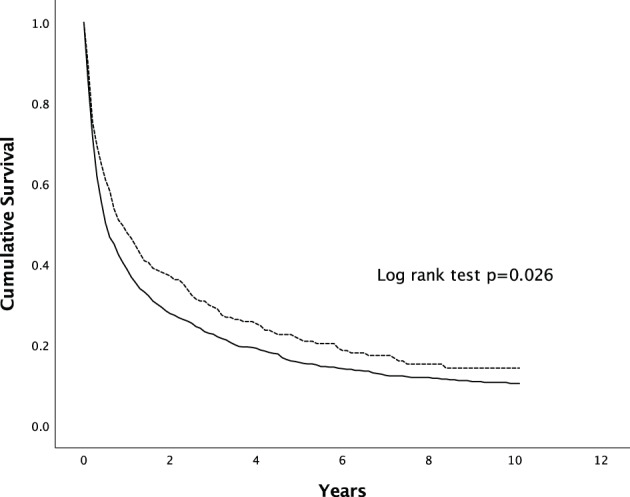
Overall survival of males and females with HCC. The difference in survival was statistically significant (P = 0.026 by Kaplan–Meier, log‐rank test).  , Male;
, Male;  , Female.
, Female.
Table 5.
1, 3, 5‐year survival
| 1 year (%) | 3 years (%) | 5 years (%) | P value | |
|---|---|---|---|---|
| Overall | ||||
| Male | 39 | 23 | 16 | 0.026 |
| Female | 48 | 29 | 21 | |
| Surveillance group | ||||
| Male | 53 | 30 | 22 | 0.836 |
| Female | 57 | 34 | 20 | |
| Symptomatic group | ||||
| Male | 21 | 9 | 5 | 0.056 |
| Female | 27 | 14 | 10 | |
In univariate analysis, the female gender was associated with better survival when compared to the male. However, this was not statistically significant in multivariable analysis. Multivariable Cox regression analysis after adjusting for gender and age showed that the main predictors for improved survival were HCC diagnosis via surveillance, Child‐Pugh status A, BCLC 0/A, and HCC with less advanced features (Table 6). When survival was analyzed according to the treatment modality, there was no statistically significant difference between the two genders (Table 7).
Table 6.
Univariate and multivariable Cox regression analysis on overall survival
| Univariate | Multivariable | |||||
|---|---|---|---|---|---|---|
| Variable | Hazard ratio | Confidence interval | P value | Hazard ratio | Confidence interval | P value |
| Female | 0.84 | 0.72–0.98 | 0.026 | 0.96 | 0.79–1.17 | 0.695 |
| Age | 1.01 | 1.01–1.02 | <0.001 | 1.01 | 0.99–1.01 | 0.086 |
| Surveillance | 0.73 | 0.62–0.86 | <0.001 | 0.81 | 0.67–0.99 | 0.042 |
| Child‐Pugh A | 0.36 | 0.32–0.41 | <0.001 | 0.58 | 0.49–0.68 | <0.001 |
| BCLC 0/A | 0.23 | 0.19–0.27 | <0.001 | 0.40 | 0.21–0.50 | <0.001 |
| Single lesion | 0.49 | 0.43–0.55 | <0.001 | 0.78 | 0.67–0.91 | 0.002 |
| Absence of metastasis | 0.33 | 0.27–0.39 | <0.001 | 0.56 | 0.46–0.69 | <0.001 |
| Absence of portal vein tumor thrombosis | 0.26 | 0.22–0.29 | <0.001 | 0.41 | 0.35–0.49 | <0.001 |
| Non‐viral etiology | 0.93 | 0.79–1.08 | 0.327 | – | – | – |
Table 7.
Comparison of survival between males and females stratified by treatment modality
| 1 year (%) | 3 years (%) | 5 years (%) | P value | |
|---|---|---|---|---|
| Curative ‡ | ||||
| Male | 87 | 67 | 50 | 0.310 |
| Female | 92 | 73 | 57 | |
| Non‐curative † | ||||
| Male | 39 | 13 | 5 | 0.178 |
| Female | 43 | 19 | 16 | |
| Best supportive care | ||||
| Male | 13 | 1 | 0 | 0.114 |
| Female | 17 | 3 | 0 | |
Non‐curative: TACE, Y90‐SIRT, SBRT, or systemic therapy.
Curative: Resection, radiofrequency ablation, or liver transplant.
As HCC diagnosis via surveillance was a predictor for survival, we performed statistical analysis to adjust for possible lead‐time bias. The median diameter of HCC from the survival analysis cohort was 57 mm in the symptomatic group, and 28 mm in the surveillance group. Based on these tumor diameters and HCC doubling times of 60, 90, and 120 days, the calculated lead time bias in our populations corresponded to 184, 277, and 369 days respectively. Survival benefit remained significant in females after adjustment of HCC doubling time of 60 days (P = 0.036). However, the survival benefit with HCC doubling times of 90 and 120 days decreased to near significance (Table 8).
Table 8.
Differences in survival after adjustment for lead time
| Median survival (Days) | P value | |||
|---|---|---|---|---|
| Doubling times (Days) | Estimated lead times (Days) | Male | Female | |
| 60 | 184 | 169 | 260 | 0.036 |
| 90 | 277 | 164 | 251 | 0.052 |
| 120 | 369 | 159 | 248 | 0.057 |
When the survival was analyzed according to the mode of diagnosis, the median survival was higher in patients with HCC diagnosed through surveillance in comparison to the symptomatic group (14.0 vs 5.6 months, P < 0.001) (Fig. 3). When females were compared with males according to the mode of HCC diagnosis, there was no significant difference in survival in between the two genders, as shown in Figures 4, 5.
Figure 3.
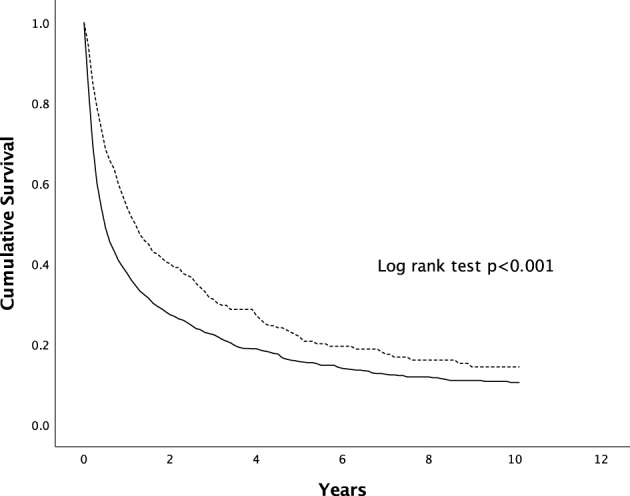
Survival of patients with HCC diagnosed via surveillance versus symptomatic presentation. The difference in survival was statistically significant (P < 0.001 by Kaplan–Meier, log‐rank test).  , surveillance;
, surveillance;  , symptomatic.
, symptomatic.
Figure 4.
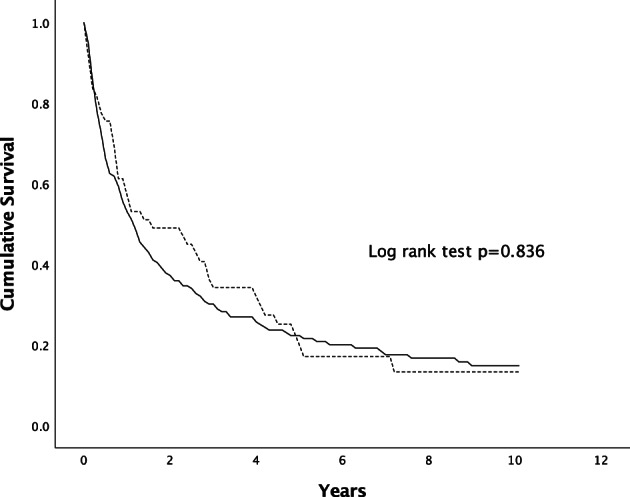
Survival of patients with HCC diagnosed via surveillance. The difference in survival was statistically not significant (P = 0.836 by Kaplan–Meier, log‐rank test).  , Male;
, Male;  , Female.
, Female.
Figure 5.
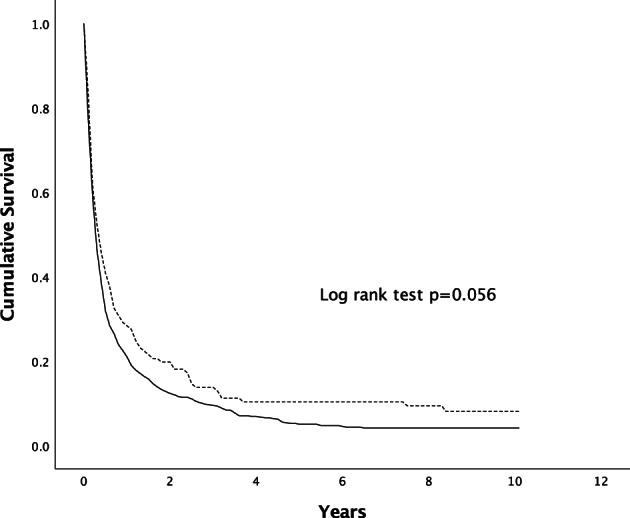
Survival of patients with HCC diagnosed via symptomatic presentation. The difference in survival was statistically not significant (P = 0.056 by Kaplan–Meier, log‐rank test). , Male;
, Male;  , Female.
, Female.
Discussion
This large cohort study of multi‐ethnic Asian patients across three decades demonstrated several clinical differences between males and females with HCC. Importantly, females diagnosed with HCC had better survival than their male counterparts. However, this advantage in survival disappeared when the analysis is adjusted according to the mode of HCC diagnosis, that is, via HCC surveillance versus symptomatic HCC disease. This suggests that the better survival of females with HCC may be due to more of them being diagnosed via surveillance rather than HCCs in females being less aggressive compared to males.
Our study confirmed the predilection of HCC for males, 8 with a male‐to‐female ratio of 4 to 1. Studies have hypothesized sex hormones as the potential biological factors in the pathogenesis of HCC, with the protective effect of estrogen against HCC development and increased risk with testosterone. 9 , 10 Traditional factors driving this male predominance in HCC include higher prevalence of HBV infection in males as well as gender differences in high‐risk lifestyle behavior with heavier alcohol consumption and smoking among males. 11 Nevertheless, this male predominance may diminish in the near future with the control of HBV and HCV and the increasing prevalence of NAFLD especially in females. 12
In this study, females were diagnosed at a significantly older age than males. It is well known that chronic HBV infection confers a higher risk of HCC as the HBV itself is oncogenic, and hence HCC may develop at a younger age regardless of underlying cirrhosis status. 13 This age difference may be due to a significantly higher proportion of males with HBV as the underlying etiology of their HCC. Indeed, HCC patients with HBV were significantly younger at diagnosis compared with those with non‐HBV causes (data not shown). Notably, other workers have shown that females were also older at diagnosis in both areas with high 14 or low 15 , 16 , 17 prevalence of HBV infection, suggesting there are other additional factors contributing to the age difference.
Our study demonstrated that females with HCC had better overall survival than males. This finding has been reported previously, with the majority of the studies involving Western populations and only two studies being in Asian cohorts. 3 , 14 , 15 , 16 , 17 , 18 We have also shown that a significantly higher proportion of females had HCC diagnosed during regular HCC surveillance. However, only two of the aforementioned studies looked at HCC surveillance status. 16 , 18 Rich et al. reported survival superiority in females with HCC, and more HCCs were diagnosed via surveillance in females but this was not statistically significant. 18 In the study by Farinati et al. more HCCs were detected through surveillance among females. 16 This is similar to our study where there was no survival advantage in females when a comparison was made based on HCC surveillance status. In the two studies involving Asian populations, both reported better survival in female patients with HCC but did not look at the patients' HCC surveillance status. 3 , 14 Thus our study is the first to demonstrate that better survival in Asian females with HCC is likely due to better adherence to HCC surveillance. Nevertheless, it should be mentioned that although the Asian study by Tangkijvanich et al. did not look at HCC surveillance status, gender was not an independent predictor of survival, and the independent factors, such as tumor stage at initial diagnosis, could have been related to surveillance. 14
Multivariable analysis confirmed that the detection of HCC at an early stage via surveillance was a predictor of improved survival. As a significantly higher proportion of females had HCC diagnosed during regular HCC surveillance, the tumor was detected at a significantly earlier BCLC stage, with significantly smaller tumor size and significantly lower incidences of portal vein tumor invasion and extrahepatic involvement. Being diagnosed at an earlier stage allows better therapeutic options, as evidenced by the trend of a higher proportion of women receiving curative treatment options in this study. Although Child‐Pugh status was also a predictor of better survival, there was no significant difference between the two genders in their Child‐Pugh status. Hence Child‐Pugh status did not account for the difference in survival between females and males. Although patients with non‐viral liver disease were significantly greater in females than in males, there was no difference in the surveillance status for viral and non‐viral liver disease. Hence, the discrepancy in the proportion of viral and non‐viral liver disease in females and males did not account for females undergoing more regular surveillance.
It can be argued the survival benefit seen in our female patients is due to a long lead‐time bias inherent in a surveillance population as the survival advantage diminished when we factored in the lead‐time duration of 90 and 120 days as opposed to a lead‐time duration of 60 days. However, surveillance is currently the only way to detect HCC at an early stage. Surgical treatment including resection and liver transplantation, as well as ablation, are the mainstay and only potentially curative treatments for patients with HCC, with 5‐year survival exceeding 70%. 19 These options are only possible with early detection of HCC. In other words, in a patient who is destined to develop HCC, being diagnosed at an early stage during HCC surveillance will allow better chances for curative treatment and correspondingly better survival. Indeed, a randomized controlled study by Zhang et al. showed that biannual surveillance versus non‐surveillance improved HCC survival. 20 The role of HCC surveillance has been well established and proven over the years, with improvement in the prognosis of HCC as well as overall survival in patients with cirrhosis. 21 , 22 , 23
To our knowledge, this is one of the largest studies to examine gender differences in HCC characteristics and prognosis and the only multi‐ethnic study in Asia. This is also the first Asian study to show that better survival in females with HCC is likely due to better adherence to a program of HCC surveillance.
One of the limitations of our study is lead‐time bias as that is often unavoidable in an observational study. As mentioned earlier, we addressed this by studying the effect of possible lead time bias with various HCC sojourn times as HCC is known to have various tumor doubling times. 24 Another limitation of our study is that we did not explore why females were more adherent to HCC surveillance. A recent study looking at HCC surveillance compliance showed that females were 2.5 times more likely to participate in HCC surveillance programs than males. 25 Female gender was also associated with a higher compliance rate with HCC surveillance in another study. 26 Common factors associated with higher compliance rates in these two studies included a family history of liver cancer, older age, lower household income, and higher education degree. The exact reasons for this healthcare‐seeking behavioral difference between females and males remain unclear and may be related to conformity to masculinity. 27 Owing to the retrospective design of our analysis, we were unable to identify the reasons for this gender difference in adherence rate in our population but were likely due to a combination of various socio‐economic factors as seen in the other studies.
In conclusion, the gender difference in HCC survival in our study was due to better adherence to a surveillance program among female patients. Thus, our study reinforces the importance of regular HCC surveillance to detect early‐stage HCC in patients at risk of developing HCC. More efforts are needed to improve our patients' adherence to a program of regular surveillance for HCC.
Declaration of conflict of interest: Chee‐Kiat Tan has been on advisory boards for Abbott Laboratories, AbbVie, Astellas, Bayer, Bristol‐Myers‐Squibb, Eisai, Gilead Sciences, Janssen, Light Sciences, MSD, Novartis, and Roche Diagnostics. Chee‐Kiat Tan has received research support from Abbott Laboratories, Bayer, Bristol‐Myers Squibb, Fujifilm, and Roche Diagnostics. George Boon‐Bee Goh has served as a consultant for Gilead and Boehringer Ingelheim. The other authors have no relevant conflicts of interest.
Author contribution: Wei‐Lun Liou and Chee‐Kiat Tan contributed to the study concept, data analysis, and manuscript writing. All authors contributed to the collection and maintenance of hepatocellular carcinoma database as well as reviewed and refined the manuscript.
Financial support: No financial support has been received for the work done.
References
- 1. Sung H, Ferlay J, Siegel RL et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021; 71: 209–49. [DOI] [PubMed] [Google Scholar]
- 2. Park J‐W, Chen M, Colombo M et al. Global patterns of hepatocellular carcinoma management from diagnosis to death: The BRIDGE study. Liver Int. 2015; 35: 2155–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lam CM, Yong JL, Chan AO et al. Better survival in female patients with hepatocellular carcinoma: oral contraceptive pills related? J. Clin. Gastroenterol. 2005; 39: 533–9. [DOI] [PubMed] [Google Scholar]
- 4. Schwartz M. A biomathematical approach to clinical tumor growth. Cancer. 1961; 14: 1272–94. [DOI] [PubMed] [Google Scholar]
- 5. Wong GL, Wong VW, Tan GM et al. Surveillance programme for hepatocellular carcinoma improves the survival of patients with chronic viral hepatitis. Liver Int. 2008; 28: 79–87. [DOI] [PubMed] [Google Scholar]
- 6. Cucchetti A, Trevisani F, Pecorelli A et al. Estimation of lead‐time bias and its impact on the outcome of surveillance for the early diagnosis of hepatocellular carcinoma. J. Hepatol. 2014; 61: 333–41. [DOI] [PubMed] [Google Scholar]
- 7. van Meer S, de Man RA, Coenraad MJ et al. Surveillance for hepatocellular carcinoma is associated with increased survival: results from a large cohort in The Netherlands. J. Hepatol. 2015; 63: 1156–63. [DOI] [PubMed] [Google Scholar]
- 8. El‐Serag HB. Hepatocellular carcinoma: an epidemiologic view. J. Clin. Gastroenterol. 2002; 35: S72–8. [DOI] [PubMed] [Google Scholar]
- 9. Mucci LA, Kuper HE, Tamimi R, Lagiou P, Spanos E, Trichopoulos D. Age at menarche and age at menopause in relation to hepatocellular carcinoma in women. BJOG. 2001; 108: 291–4. [DOI] [PubMed] [Google Scholar]
- 10. Tuo JY, Li HL, Wang J, Fang J, Tan YT, Xiang YB. Menstrual factors, reproductive history and liver cancer risk: findings from a prospective cohort study in Chinese women. Cancer Epidemiol. Biomark. Prev. 2022:EPI‐22‐0439; 31: 2046–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jackson SM, Marks MA, Katki HA et al. Sex disparities in the incidence of 21 cancer types: quantification of the contribution of risk factors. Cancer. 2022; 128: 3531–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Goh GB, Li JW, Chang PE, Chow KY, Tan CK. Deciphering the epidemiology of hepatocellular carcinoma through the passage of time: A study of 1,401 patients across 3 decades. Hepatol Commun. 2017; 1: 564–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Beasley RP. Hepatitis B virus: the major etiology of hepatocellular carcinoma. Cancer. 1988; 61: 1942–56. [DOI] [PubMed] [Google Scholar]
- 14. Tangkijvanich P, Mahachai V, Suwangool P, Poovorawan Y. Gender difference in clinicopathologic features and survival of patients with hepatocellular carcinoma. World J. Gastroenterol. 2004; 10: 1547–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Phipps M, Livanos A, Guo A et al. Gender matters: characteristics of hepatocellular carcinoma in women from a large, multicenter study in the United States. Am. J. Gastroenterol. 2020; 115: 1486–95. [DOI] [PubMed] [Google Scholar]
- 16. Farinati F, Sergio A, Giacomin A et al. Is female sex a significant favorable prognostic factor in hepatocellular carcinoma? Eur. J. Gastroenterol. Hepatol. 2009; 21: 1212–18. [DOI] [PubMed] [Google Scholar]
- 17. Dohmen K, Shigematsu H, Irie K, Ishibashi H. Longer survival in female than male with hepatocellular carcinoma. J. Gastroenterol. Hepatol. 2003; 18: 267–72. [DOI] [PubMed] [Google Scholar]
- 18. Rich NE, Murphy CC, Yopp AC et al. Sex disparities in presentation and prognosis of 1110 patients with hepatocellular carcinoma. Aliment. Pharmacol. Ther. 2020; 52: 701–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. European Association for the Study of the Liver , Electronic address: easloffice@easloffice.eu; European Association for the Study of the Liver . EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma [published correction appears in J Hepatol. 2019 Apr;70(4):817]. J. Hepatol. 2018; 69: 182–236. [DOI] [PubMed] [Google Scholar]
- 20. Zhang BH, Yang BH, Tang ZY. Randomized controlled trial of screening for hepatocellular carcinoma. J. Cancer Res. Clin. Oncol. 2004; 130: 417–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Singal AG, Pillai A, Tiro J. Early detection, curative treatment, and survival rates for hepatocellular carcinoma surveillance in patients with cirrhosis: a meta‐analysis. PLoS Med. 2014; 11: e1001624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Stravitz RT, Heuman DM, Chand N et al. Surveillance for hepatocellular carcinoma in patients with cirrhosis improves outcome. Am. J. Med. 2008; 121: 119–26. [DOI] [PubMed] [Google Scholar]
- 23. Yuen MF, Cheng CC, Lauder IJ, Lam SK, Ooi CG, Lai CL. Early detection of hepatocellular carcinoma increases the chance of treatment: Hong Kong experience. Hepatology. 2000; 31: 330–5. [DOI] [PubMed] [Google Scholar]
- 24. Sheu JC, Sung JL, Chen DS et al. Growth rate of asymptomatic hepatocellular carcinoma and its clinical implications. Gastroenterology. 1985; 89: 259–66. [DOI] [PubMed] [Google Scholar]
- 25. Cao M, Li H, Sun D et al. Assessment of the compliance, influencing factors, and yielding results of liver cancer screening in a high‐risk population: a cross‐sectional study. Cancer. 2022; 128: 3653–62. [DOI] [PubMed] [Google Scholar]
- 26. Guo LW, Zhang SK, Liu SZ et al. Compliance rate and impact factor analysis of liver cancer screening in urban areas of Henan Province. Zhonghua Zhong Liu Za Zhi. 2021; 43: 233–7. [DOI] [PubMed] [Google Scholar]
- 27. O'Brien R, Hunt K, Hart G. 'It's caveman stuff, but that is to a certain extent how guys still operate': men's accounts of masculinity and help seeking. Soc. Sci. Med. 2005; 61: 503–16. [DOI] [PubMed] [Google Scholar]


