Abstract
Objectives
Multicomponent exercise program have shown to improve function and cognition in older adults but studies on pre-frail older adults in the primary care setting are limited. This study aimed i) to evaluate impact of 6 months exercise (Ex) versus complementary effect of 3 months of cognitive stimulation therapy (CST) to 6 months of Ex (Ex+CST) on physical function, muscle mass and cognition versus control group at 3, 6 and 12 months ii) inflammatory biomarkers such as Interleukin-6 (IL-6) and Tumor Necrosis Factor Alpha (TNF-α).
Design
Cluster randomised control trial.
Setting & Intervention
Pre-frail older adults ≥ 65 years attending primary care clinic. Two intervention groups i) Ex 6 months ii) CST 3 months with Ex 6 months.
Measurements
At 0, 3, 6 and 12 months, questionnaires (on demographics, physical function, cognition, and depression) were administered and physical function assessment (gait speed, short physical performance battery (SPPB) test, handgrip strength, five times sit-to-stand (5x-STS)) was conducted. Muscle mass and its surrogates such as phase angle and body cell mass were measured using bioelectrical impedance analysis machine. Inflammatory biomarkers were measured at 0 and 3 months.
Results
Data from 190 participants was analysed at 3 months (111 control, 37 Ex and 41 Ex+CST). At 3 months, significant improvement in cognition was seen only in the Ex+CST group whereas improvements in depression, gait speed, SPPB and 5x-STS were seen in both the Ex and Ex+CST groups. At 6 months, the Ex+CST group improved in cognition and depression whereas improvement in frailty and muscle mass indices were seen in both the interventions groups. At 12 months, both the interventions groups had better perceived health, gait speed and less decline in muscle mass compared with control groups. Both the Ex and Ex+CST had significant association with TNF-α at 3 months (β −2.71 (95% CI −4.80–−0.62); p = 0.012 and β −1.74 (95% CI −3.43–−0.06); p = 0.043 respectively).
Conclusion
Combined Ex+CST had significant improvement in cognition whereas the intervention groups improved in depression, physical function, muscle mass, frailty, perceived health and TNF-α levels. With growing evidence of the benefits of multicomponent interventions at primary care level, incorporating it into mainstream care with action plans on long-term sustainability and scalability should be a priority for every country.
Electronic Supplementary Material
Supplementary material is available for this article at 10.1007/s12603-023-1928-7 and is accessible for authorized users.
Key words: Exercise, cognitive stimulation therapy, pre-frail, tumor necrosis factor alpha, interleukin 6, muscle mass, phase angle, body cell mass
Introduction
The global population is ageing rapidly with Asia-Pacific being at the forefront. Older adults 60 years old and above are expected to triple between 2010 and 2050 to 1.3 billion in the Asia-Pacific (1). Despite increase in life expectancy, healthspan continues to lag behind with an average of 9 years is spent in poor health (2). Many countries are in the process of developing action plans to reduce the gap between healthspan and lifespan (3). The World Health Organisation (WHO) public health framework for Healthy Aging recommends intervening at an early stage to prevent or delay frailty and consequent disability, as detailed in the most recent publication by WHO on Integrated care for Older People (ICOPE) (4).
Frailty is a state of decline in physiological reserve predisposing older adults to adverse outcomes when exposed to stressors (5). It is a dynamic state and may be reversible before the onset of disability (6). Pre-frailty is a transition state to frailty and is increasingly being recognised as a public health target for implementation of multi-domain intervention to delay the onset of functional decline and disability (7). The prevalence of pre-frailty varies between 35% to 53% (7–9). Multi-domain interventions such as nutrition, exercise and cognitive training have been shown to prevent progression of frailty and disability (10, 11). Primary care is the foundation of healthcare systems, often being the first point of contact for older adults and a core site for preventive healthcare. Many countries have introduced frailty screening in primary care using screening tools such as the FRAIL scale and Kihon checklist with the aim of early identification, management, and prevention (5, 12).
Inflammation is common both in aging and frailty. Elevated levels of pro-inflammatory cytokines such as C-reactive protein (CRP), Interleukin-6 (IL-6) levels, Growth Differentiation Factor-15 (GDF-15) and Tumor Necrosis Factor Alpha (TNF-α) are found in frailty, and are associated with declining physical function and mortality (13, 14). Conversely, anti-inflammatory cytokines such as IL-10 suppresses pro-inflammatory activity in various tissues and aging is associated with declining levels of IL-10 (15). Chronic inflammation is associated with decreased muscle mass and strength, disability, dementia, increased morbidity, and mortality (16). Studies on impact of exercise on pro-inflammatory and anti-inflammatory cytokines have shown mixed results depending on the intensity and duration of exercise (17, 18). There are limited studies on association of moderate intensity exercise on inflammation in pre-frail older adults (19, 20).
Multidomain interventions incorporating cognitive training have shown to be beneficial in improving cognition in pre-frail older adults (21). Multiple studies have shown the beneficial effects of cognitive stimulation therapy (CST) in improving global cognitive function mainly in persons with dementia (22). However, there is a paucity of literature on the impact of CST and exercise in combination on functional outcomes, cognition, muscle mass and inflammatory biomarkers in pre-frail older adults. As such, our study sets out to assess the impact of 6 months exercise (Ex) versus complementary effect of 3 months of cognitive stimulation therapy (CST) to 6 months of exercise (Ex+CST) on physical function, muscle mass indices and cognition at 3, 6 and 12 months (6 months post cessation of intervention). A secondary aim was to assess the differential effects of Ex and Ex+CST on inflammatory biomarkers.
Methods
Participants and Study Design
This was a cluster randomized control trial (NCT 03797352) conducted between 2019 to 2022 involving participants from two primary care clinics 5km apart in the Western region of Singapore. Participants attending Choa Chu Kang Polyclinic were allocated to control group and participants from Bukit Batok Polyclinic were allocated to exercise only (Ex) or exercise and CST (Ex + CST) groups (Figure 1). There was no blinding and allocation to Ex or Ex+CST group was not randomised within the same site. The allocation to Ex or Ex+CST was based on which research staff recruited them. Participants ≥ 65 years old, who were ambulatory and screened to be pre-frail based on FRAIL scale by coordinators in the primary care clinic and able to consent were invited to participate. Participation was voluntary. The inclusion criteria included age ≥ 65 years old, having the ability to follow instructions and participate in the intervention as deemed suitable by a primary care physician or trained members of the study team. Participants with a pacemaker or a defibrillator, liver or gastro-intestinal disease, end stage lung disease, cardiac disease, cancer undergoing active treatment, gout, underlying psychiatric conditions and nursing home residents were excluded.
Figure 1.
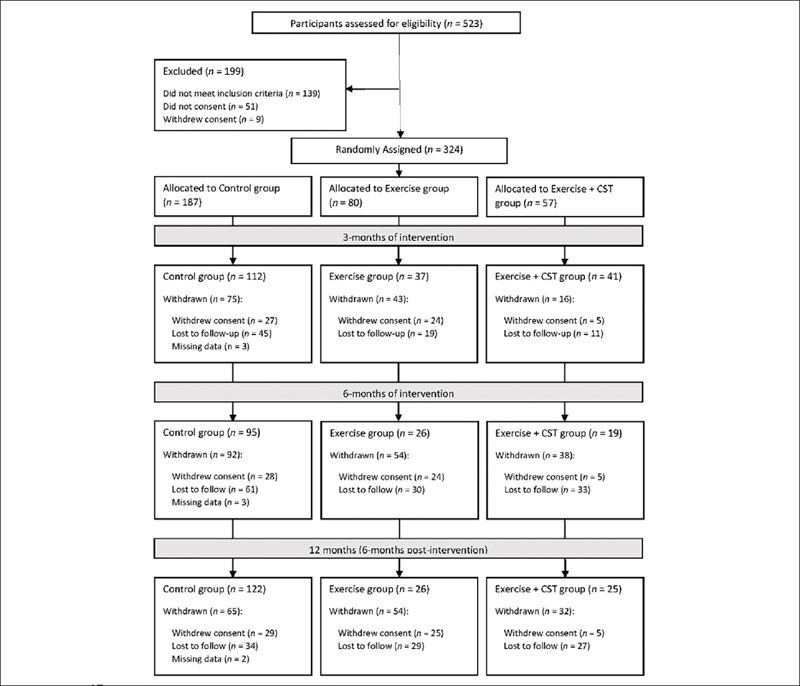
Participant screening, group allocation, and follow-up
Interventions
The control group received general health education advice. The intervention groups received 60 minutes twice a week multicomponent exercise program consisting of aerobic training, resistance training, dual task, and balance training. The study team members were trained to conduct CST by qualified trainers from St Louis University, Missouri, USA. The Ex+CST group received 30 minutes of CST twice a week for a total of 3 months in addition to exercise, followed by 3 more months of exercise only. Participants were divided into English and Mandarin speaking training groups (Figure 2). CST was conducted after the 60 minutes of exercise. The training was adapted from the CST manual which included games, food, current affairs, art and word association (23). The study lasted for 12 months. Participants were assessed at 3 months, and at the end of the 6-month intervention. A 6-month washout period was observed where participants received no interventions and were then assessed at the end of this period.
Figure 2.

Group Based Cognitive Stimulation Therapy
Demographics and Covariates
Trained study team members collected data on demographics, chronic diseases, medications, perceived health, frailty, physical activity levels, falls, cognition, and nutrition. Frailty was assessed using the five component FRAIL scale (Fatigue, Resistance, Aerobic, Illness and Loss of Weight) (24). Participants were considered pre-frail if they scored 1–2 out of maximum score of 5. Polypharmacy was defined as taking five or more long-term medications. The 3-item UCLA Loneliness Scale (UCLA-3) was used to assess whether participants were experiencing loneliness (25). The EuroQoL-Visual Analogue Scale (EQ-VAS) was used to understand participants’ perceived health (26). Physical activity level was assessed using the Rapid Physical Assessment (RAPA) tool (27). RAPA was developed to assess the quantity and intensity of physical activity in adults 50 years or older with a maximum score of 7. Score of 6–7 are considered active, 4–5 under-active regular, 3 under-active light activities, 2 under-active and 1 considered as sedentary (27). Lawton’s instrumental activities of daily living (IADL) scale was used to assess IADL and Katz activity of daily living (ADL) to assess ADL (28, 29). Participants who reported at least 1 fall in the past year were considered fallers (30). Cognition was assessed using the Montreal Cognitive Assessment (MoCA) (31). The Geriatric Depression Scale (GDS) was used to identify participants with depression where those with a score ≥ 5 were considered depressed (32). Nutritional status was evaluated using the Mini-Nutritional Assessment short-form (MNA-SF) (33). The MNA-SF is a 6-item screening questionnaire which has been validated as a sensitive rapid nutrition screening instrument . Participants were classified as normal nutritional status (12 – 14 points), at risk of malnutrition (8 – 11 points) and malnourished (0 – 7 points).
Physical Performance
Physical performance measures comprised of maximum handgrip strength (HGS), gait speed, and the Short Physical Performance Battery (SPPB) test. HGS was measured using Jamar hand dynamometer on the dominant hand with participant in a seated position and elbow flexed 90°. Low HGS was defined as < 28kg for males and < 18 kg for females based on the 2019 consensus by the Asian Working Group for Sarcopenia (AWGS) (34). The SPPB, with three components - balance, GS and 5 times sit-to-stand (5xSTS) timing, has a maximum score of 12 points (4 per component). Gait speed < 1.0m/s was considered slow gait.
Body Composition
Body composition was measured using the InBody S10 multi-frequency bioelectrical impedance analyser (BIA). Appendicular skeletal muscle (ASM), body cell mass and whole-body phase angle were measured as surrogate for muscle mass. Appendicular skeletal muscle index (ASMI) was derived from ASM divided by height square. Sarcopenia was diagnosed based on the 2019 AWGS criteria of gender specific cut offs for ASMI and either low HGS or poor physical performance.
Plasma Biomarkers
Non-fasting blood test was voluntary and only participants who agreed to have 15mls of blood drawn were invited. The TNF-α, IL-6, GDF-15, and IL-10 cytokines were measured by an accredited hospital-based laboratory. TNF-α cytokine was measured using Immunoenzymetric assay with a detection range between 1.0 – 498 pg./mL and IL-6 measured using the electrochemiluminescence immunoassay (ECLIA) with a detection range between 1.5 – 50 000 pg./mL. Enzyme-linked immunosorbent Assay was used to measure IL-10 with a detection range of 2.0 – 400.0 pg./mL and GDF-15 with a detection range of 2.0 –2400 pg./mL.
Ethics approval and informed consent
The study protocol including screening and intervention was approved by the National Healthcare Group (NHG) Domain Specific Review Board (DSRB) (Reference number: 2017/00035). Written consent was obtained from all participants.
Statistical analysis
IBM SPSS Version 28.0 was used for our data analysis. Categorical variables were presented as frequencies with percentages while continuous variables were presented as mean ± standard deviation in. χ2 test was used for significance testing of categorical variables. For continuous variables, normality assumption was tested using Sharpiro Wilk test. Significance testing for normally distributed variables with equal variances was carried out using one-way ANOVA whereas Welch test was used for those with unequal variances. The Kruskal-Wallis test was used when continuous variables were not normally distributed.
General Linear Model (GLM) was used to compare changes in continuous variables between groups adjusted for age, gender, ethnicity, education, hypertension, hyperlipidaemia, diabetes, physical activity, polypharmacy, cognition, IADL impairment and corresponding baseline values.
Mood’s median test was used to compare the median levels of plasma biomarkers. Quantile regression was also performed to compare changes in plasma biomarker levels between the groups adjusted for age, gender, ethnicity, education, hypertension, hyperlipidaemia, diabetes, physical activity, polypharmacy, cognition, IADL impairment and corresponding baseline values.
Results
A total of 324 participants were initially enrolled in the study just before the Covid-19 pandemic with 187 allocated to the control group and 137 to the intervention groups (Ex = 80; Ex + CST = 57). Full data for analysis was available for 190 participants at 3 months (112 control, 37 Ex and 41 Ex + CST), 140 at 6 months (95 control, 26 Ex and 19 Ex + CST) and 173 at 12 months (122 control, 26 Ex and 25 Ex + CST) (Figure 1). The intervention study was conducted during the peak of COVID-19 pandemic with multiple lockdowns resulting in significant numbers withdrawing consent after enrolment or lost to follow-up. Some participants missed 3- and 6-month assessments due to COVID-19 measures. The median adherence was 75% for Ex+CST and 67% for Ex group. There was no significant difference between the participants who dropped out before the 3 months evaluation and those who continued except for falls risk which was adjusted for in the final analysis (supplementary Table 1). There were significant differences in gender distribution where 73.7% of those in the Ex + CST group, 58.8% in the Ex group and 48.4% in the control group were females (Table 1). The mean age in the control group was the lowest (71.7 years) compared with Ex (73.4 years) and Ex + CST (72.6 years). The control group had the greatest proportion of participants with hyperlipidaemia (84.6%) versus Ex group (70.0%).
Table 1.
Baseline Characteristics
| Control n = 187 (57.7%) | Exercise n = 80 (24.7%) | Exercise + CST n = 57 (17.6%) | P-value | |
|---|---|---|---|---|
| Demographics | ||||
| Gender | 0.003 | |||
| Male | 96 (51.3) | 33 (41.3) | 15 (26.3) | |
| Female | 91 (48.7) | 47 (58.8) | 42 (73.7) | |
| Age (years) | 71.69 ± 4.99a,b | 73.39 ± 5.20a | 72.56 ± 5.06b | 0.037 |
| Ethnicity | 0.253 | |||
| Chinese | 150 (80.2) | 74 (92.5) | 46 (80.7) | |
| Malay | 18 (9.6) | 3 (3.8) | 4 (7.0) | |
| Indian | 19 (10.2) | 3 (3.8) | 7 (7.0) | |
| Others | 1 (0.5) | 0 (0.0) | 0 (0.0) | |
| Hypertension | 141 (75.4) | 55 (68.8) | 40 (70.2) | 0.661 |
| Hyperlipidaemia | 159 (85.0) | 56 (70.0) | 46 (80.7) | 0.043 |
| Diabetes | 104 (55.6) | 33 (41.3) | 33 (57.9) | 0.099 |
| Polypharmacy | 61 (32.6) | 24 (30.0) | 17 (29.8) | 0.899 |
| Living Alone | 14 (7.5) | 4 (5.0) | 6 (10.5) | 0.476 |
| Loneliness (UCLA-3) | 33 (17.6) | 9 (11.3) | 14 (24.6) | 0.125 |
| BMI (kg/m2) | 26.32 ± 4.68 | 25.34 ± 4.72 | 26.11 ± 4.58 | 0.291 |
| Education (years) | 7.29 ± 4.00 | 8.25 ± 4.22 | 8.39 ± 3.73 | 0.060 |
| Perceived Health (EQ-VAS) | 69.12 ± 15.31 | 70.31 ± 12.69 | 67.81 ± 13.79 | 0.604 |
| FRAIL Total | 1.24 ± 0.44 | 1.39 ± 0.59 | 1.30 ± 0.50 | 0.052 |
| Physical Activity (RAPA) | 3.51 ± 1.52a | 2.69 ± 1.41a,b | 3.39 ± 1.51b | <0.001 |
| At least 1 IADL Impairment | 37 (19.8) | 31 (38.8) | 14 (24.6) | 0.004 |
| At least 1 ADL Impairment | 32 (17.0) | 25 (31.3) | 15 (26.3) | 0.032 |
| Sarcopenia1 | 18 (9.6) | 16 (20.0) | 8 (14.0) | 0.057 |
| Falls in Last One Year ≥ 1 | 38 (20.2) | 22 (27.5) | 16 (28.1) | 0.322 |
| MoCA | 25.05 ± 3.42a,b | 26.55 ± 3.21a | 27.24 ± 2.38b | <0.001 |
| Depression | 56 (29.8) | 19 (23.8) | 12 (21.1) | 0.293 |
| Nutritional Status (MNA-SF) | 0.894 | |||
| Malnourished | 3 (1.6) | 1 (1.3) | 1 (1.8) | |
| At risk of malnourishment | 32 (17.1) | 15 (18.8) | 7 (12.3) | |
| Normal nutritional status | 152 (81.3) | 64 (80.0) | 49 (86.0) | |
| Physical Performance | ||||
| Handgrip Strength (kg) | 22.91 ± 7.08 | 21.99 ± 6.45 | 21.49 ± 6.78 | 0.363 |
| Gait Speed (m/s) | 0.94 ± 0.27 | 0.94 ± 0.33 | 0.97 ± 0.27 | 0.656 |
| 5x Chair Stand Time (s) | 12.89 ± 4.79 | 13.98 ± 5.41 | 13.30 ± 4.46 | 0.251 |
| Total SPPB Score | 9.78 ± 2.07 | 9.55 ± 2.13 | 9.75 ± 2.06 | 0.704 |
| Body Composition | ||||
| ASMI (kg/m2) | 6.86 ± 1.02 | 6.82 ± 1.50 | 6.71 ± 1.27 | 0.388 |
| Body Cell Mass (kg) | 25.82 ± 7.86 | 28.36 ± 7.74 | 26.86 ± 6.66 | 0.149 |
| 50khz-Trunk Phase Angle (θ) | 5.67 ± 2.42 | 6.04 ± 3.95 | 5.39 ± 2.06 | 0.445 |
Values presented as n (%) or mean ± SD; Bold indicates significance (p < 0.05); a.b. Values with common superscript are significantly different. Abbreviations: BMI, Body Mass Index; ADL; Activities of Daily Living; MoCA; Montreal Cognitive Assessment; SPPB, Short Physical Performance Battery; ASMI, Appendicular Skeletal Muscle Index. 1. Based on Asian Working Group for Sarcopenia (AWGS) 2019’s definition; 2 Adjusted for gender
Cognition, Depression and Quality of life
MoCA scores were significantly different amongst the groups with the score lowest in the control group (25.05 ± 3.42) and highest in the Ex + CST (27.24 ± 2.38, p <0.001) group. Those in Ex group had the greatest proportion of participants with at least 1 IADL (38.8%) and ADL (31.3%) impairments. After 3 months of intervention, only the Ex + CST group had significant improvements in MoCA score compared to the control group (1.83, 95% CI 1.24 – 2.42, p <0.001) while both Ex (−1.15, 95% CI −1.87 – 0.44, p <0.001) and Ex + CST (−1.55, 95% CI −2.23 – −0.88, p <0.001) groups saw significant improvements in GDS scores. After 6 months of intervention, improvements in MoCA and GDS scores were sustained only in the Ex + CST groups (1.67, 95% CI 0.56 – 2.78, p = 0.005 and −1.23, 95% CI −2.44 – −0.02, p = 0.010, respectively). However, there were only significant changes in perceived health in both Ex (4.79, 95% CI −0.35 – 9.93, p = 0.012) and Ex + CST (6.03, 1.19 – 10.87, p = 0.012) groups at 12 months (Table 2).
Table 2.
Mean changes in outcome variables from baseline to 3 months, 6 months, and 12 months respectively
| 0 Month – 3 Months | 0 Month – 6 Months | 0 Month – 12 Months | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control n = 111 (54.6%) | Exercise n = 37 (17.9%) | Exercise + CST n = 41 (27.5%) | p-value | Control n = 95 (67.4%) | Exercise n = 26 (18.4%) | Exercise + CST n = 19 (13.6%) | p-value | Control n = 122 (70.5%) | Exercise n = 26 (15.0%) | Exercise + CST n = 25 (14.5%) | p-value | |
| Cognition and Mental Health | ||||||||||||
| MoCA | 0.49a (0.13–0.85) | 1.67 (1.01–2.33) | 1.83a (1.24 – 2.42) | <0.001 | −0.03a (−0.52 – 0.58) | 1.71 (0.70 – 2.72) | 1.67a (0.56 – 2.78) | 0.005 | 0.31 (−0.15 – 0.76) | 0.51 (−0.40 – 1.41) | 1.03 (0.12 – 1.94) | 0.394 |
| GDS | 0.17a,b (−0.23 – 0.58) | −1.15a (−1.87 – 0.44) | −1.55b (−2.23 – −0.88) | <0.001 | 0.52a (−0.09 – 1.12) | −1.10 (−2.21 – 0.01) | −1.23a (−2.44 – −0.02) | 0.010 | 0.23 (−0.20 – 0.67) | 0.67 (−0.36 – 1.69) | 0.32 (−0.63 – 1.26) | 0.759 |
| Perceived Health (EQ-VAS) | −3.58 (−6.40 – −0.76) | 0.27 (−4.73 – 5.27) | 2.32 (−2.38 – 7.03) | 0.101 | −1.84 (−5.14 – 1.47) | 6.00 (−5.98 – 17.97) | 11.16 (−0.13 – 22.44) | 0.060 | −1.23a,b (−3.43 − 0.98) | 4.79a (−0.35 – 9.93) | 6.03b (1.19 – 10.87) | 0.012 |
| Physical Performance | ||||||||||||
| FRAIL Total | −0.15 (−0.29 – 0.02) | −0.33 (−0.59 – 0.07) | −0.40 (−0.64 – 0.16) | 0.199 | 0.02ab (−0.24 – 0.27) | −0.56a (−1.04 – −0.11) | −0.57b (−1.08 – −0.06) | 0.039 | −0.28 (−0.42 – −0.15) | −0.49 (−0.82 – −0.16) | −0.44 (−0.75 – −0.13) | 0.451 |
| HGS | −0.36 (−0.97 – 0.27) | 0.74 (−0.38 – 1.86) | 0.17 (−0.84 – 1.18) | 0.268 | −0.34 (−1.08 – 0.41) | 0.52 (−0.88 – 1.92) | 0.88 (−0.60 – 2.36) | 0.292 | −0.66 (−1.25 – −0.08) | −0.41 (−1.82 – 0.99) | −0.48 (−1.76 – 0.79) | 0.938 |
| Gait Speed | −0.01a,b (−0.04 – 0.03) | 0.17a (0.11 – 0.22) | 0.13b (0.07 – 0.18) | <0.001 | −0.01a,b (−0.06 – 0.04) | 0.20a (0.11 – 0.30) | 0.25b (0.14 – 0.35) | <0.001 | 0.02a,b (−0.04 – 0.05) | 0.20a (0.10 – 0.31) | 0.15b (0.05 – 0.24) | <0.001 |
| 5x STS Time | −0.27a,b (−0.88 – 0.35) | −1.87a (−2.93 – −0.82) | −2.24b (−3.22 – −1.27) | 0.002 | −0.25 (−1.04 – 0.54) | −1.06 (−2.46 – 0.33) | −2.23 (−3.73 – −0.74) | 0.077 | 0.80 (−0.04 – 1.64) | 0.88 (−0.99 – 2.76) | −1.03 (−2.78 – 0.72) | 0.162 |
| SPPB Total | 0.13a,b (−0.12 – 0.38) | 0.68a (0.25 – 1.12) | 0.65b (0.24 – 1.05) | 0.044 | −0.07 (−0.47 – 0.32) | 0.80 (0.08 – 1.52) | 0.75 (−0.04 – 1.54) | 0.059 | 0.07 (−0.22 – 0.36) | 0.29 (−0.38 – 0.96) | 0.44 (−0.19 – 1.07) | 0.551 |
| Body Composition | ||||||||||||
| ASMI | −0.04 (−0.14 – 0.07) | 0.05 (−0.16 – 0.25) | 0.12 (−0.04 – 0.28) | 0.277 | 0.10a,b (−0.27 – 0.47) | 1.73a (0.63 – 2.83) | 1.90b (0.62 – 3.17) | 0.002 | −2.68a,b (−3.06 – −2.31) | −0.30a (−1.42 – 0.82) | −0.35b (−1.12 – 0.42) | <0.001 |
| BCM | −0.23 (−0.66 – 0.20) | 0.22 (−0.51–0.96) | 0.20 (−0.36 – 0.77) | 0.419 | −0.03a,b (−1.08 – 1.02) | 2.38a (0.68 – 4.08) | 2.35b (0.70 – 4.00) | 0.026 | −0.27 (−0.70 – 0.16) | −0.91 (−1.80 – −0.16) | −0.34 (−1.07 – 0.39) | 0.468 |
| Phase Angle | −0.12 (−0.24 – 0.02) | 0.02 (−0.19 – 0.24) | 0.07 (−0.11 – 0.25) | 0.210 | −0.50a,b (−1.01 – 0.01) | 0.65a (−0.23 – 1.52) | 1.01b (0.09 – 1.93) | 0.010 | −0.30 (−0.41 – −0.18) | −0.17 (−0.43 – 0.09) | −0.11 (−0.33 – 0.12) | 0.323 |
| Physical Activity and Nutritional Status | ||||||||||||
| RAPA | −0.02a,b (−0.31 – 0.26) | 1.22a (0.71 – 1.73) | 1.06b (0.59 – 1.54) | <0.001 | 0.25a,b (−0.11 – 0.60) | 1.18a (0.53 – 1.83) | 1.30b (0.59 – 2.01) | 0.009 | −0.23a,b (−0.51 – 0.05) | 1.00a (0.35 - 1.64) | 0.53b (−0.09 – 1.14) | 0.002 |
| MNA | −0.14 (−0.41 – 0.14) | 0.30 (−0.20 – 0.79) | 0.45 (−0.01 – 0.90) | 0.084 | 0.22 (−0.10 – 0.53) | −0.09 (−0.67 – 0.50) | 0.64 (0.01 – 1.28) | 0.247 | 0.11 (−0.14 – 0.36) | 0.69 (0.11 – 1.27) | 0.52 (−0.02 – 1.06) | 0.146 |
Values present as mean (95% confidence interval); Bold indicates significance (p < 0.05); a.b. Values with common superscript are significantly different. MoCA, Montreal Cognitive Assessment; GDS, Geriatric Depression Scale; EQ-VAS, EuroQoL-Visual Analogue Scale; HGS, Handgrip Strength; STS, Sit-to-Stand; SPPB, Short Physical Performance Battery; ASMI, Appendicular Skeletal Muscle Index; BCM, Body Cell Mass; RAPA, Rapid Assessment of Physical Activity; MNA, Mini Nutritional Assessment. Adjusted for Age, Gender, Ethnicity, Education, Hypertension, Hyperlipidaemia, Diabetes Mellitus, Physical Activity, Polypharmacy, IADL Impairment, MoCA, Falls, Adherence and Baseline Values
Frailty, Sarcopenia and Physical Function
There were no significant differences in frailty score, sarcopenia, and physical function at baseline. Significant improvement in FRAIL total scores were seen in both the Ex and Ex + CST groups at 6 months (−0.56, 95% CI −1.04 – −0.11, p = 0.039 and −0.57, 95% CI −1.08 – −0.06, p = 0.039). At 3 months, participants in the Ex and Ex + CST group improved in all physical function measures except for HGS (gait speed: 0.17, 95% CI 0.11 – 0.22, p <0.001 and 0.13, 95% CI 0.07 – 0.18, p <0.01; 5x STS: −1.87, 95% CI −2.93 – −0.82, p = 0.002 and −2.24, 95% CI −3.22 – −1.27, p = 0.002; SPPB Total: 0.68, 95% CI 0.25 – 1.12, p 0.044 and 0.65, 95% CI 0.24 – 1.05, p = 0.044). Only gait speed significantly improved in both groups at 6 months (0.20, 95% CI 0.11 – 0.30 and 0.25, 95% CI 0.14 – 0.35, p <0.001) and remained significantly higher at 12 months (0.20, 95% CI 0.10 – 0.31, p <0.001 and 0.15, 95% CI 0.05 – 0.24, p <0.001) (Table 2).
Body Composition
There were no differences in muscle mass measures at baseline or at 3 months across the groups. At 6 months, both Ex and Ex + CST groups had significant improvements in ASMI (1.73, 95% CI 0.68 – 2.83, p = 0.002 and 1.90, 95% CI 0.62 – 3.17, p = 0.002), body cell mass (2.38, 95% CI −1.08 – 1.02, p = 0.026 and 2.35, 95% CI 0.70 – 4.00, p = 0.026) and phase angle (0.65, 95% CI −0.23 – 1.52, p = 0.010 and 1.01, 95% CI 0.09 – 1.93, p = 0.010). At 12 months, Ex and Ex+CST groups had significantly less decline in ASMI compared with control group.
Plasma Biomarkers
At baseline there were no significant differences in the plasma biomarker levels. After 3 months of exercise and CST interventions, only TNFα levels were significantly reduced in the Ex (B −2.71, 95% CI −4.80 – −0.62, p = 0.012) and Ex + CST (B −1.74, 95% CI −3.43 – −0.06, p = 0.043) groups as compared to the control group (Table 3).
Table 3.
Unadjusted and Adjusted Quantile Regression Models of Median Change in Plasma Biomarkers
| Biomarker | Group | Baseline Median (IQR)# | Unadjusted | Adjusted+ |
|---|---|---|---|---|
| Coefficient (95% CI) p-value | Coefficient (95% CI) p-value | |||
| IL6 (pg./mL) | Control | 3.20 (1.50) | Reference | |
| Exercise | 2.65 (2.10) | −0.20 (−1.63 – 1.23) p = 0.780 | 0.67 (−0.79 – 2.13) p = 0.360 | |
| Exercise + CST | 3.10 (2.30) | −0.10 (−1.33 – 1.13) p = 0.871 | 0.32 (−0.91 – 1.54) p = 0.602 | |
| IL10 (ng/mL) | Control | 2.39 (1.91) | Reference | |
| Exercise | 2.25 (1.48) | 0.31 (−0.50 – 1.12) p = 0.446 | 0.24 (−0.54 – 1.02) p = 0.543 | |
| Exercise + CST | 2.61 (1.57) | 0.53 (−0.17 – 1.23) p = 0.136 | 0.30 (−0.39 – 0.99) p = 0.385 | |
| TNF-α (pg./mL) | Control | 7.80 (3.80) | Reference | |
| Exercise | 7.40 (2.90) | −2.70 (−4.16 – −1.24) p <0.001 | −2.71 (−4.80 – −0.62) p = 0.012 | |
| Exercise + CST | 8.90 (3.80) | −0.70 (−1.93 – 0.53) p = 0.257 | −1.74 (−3.43 – −0.06) p = 0.043 | |
| GDF-15 (pg./mL) | Control | 943.15 (1083.90) | Reference | |
| Exercise | 907.60 (786.70) | 333.70 (−394.09 – 1061.49) p = 0.362 | 129.29 (−361.58 – 620.17) p = 0.597 | |
| Exercise + CST | 943.30 (933.40) | −35.70 (−662.52 – 591.52) p = 0.909 | −164.61 (−583.09 – 253.87) p = 0.431 | |
# No baseline differences were observed. + Adjusted for Age, Gender, Ethnicity, Education, Hypertension, Hyperlipidemia, Diabetes, Physical Activity, Polypharmacy, Cognition, IADL Impairment, Falls, Adherence and baseline values; IL-6, Interleukin 6; IL10, Interleukin 10E; TNF-α, Tumour Necrosis Factor-α; GDF-15, Growth Differentiation Factor-15.
Discussion
The aim of our study was to assess the impact of Ex and Ex + CST on physical function, muscle mass indices and cognition at 3, 6 and 12 months (6 months after cessation of intervention). Our study found that addition of CST to exercise interventions in pre-frail older adults in primary care setting had improved outcomes in cognitive and mental health domains at 3 and 6 months. Improvement in functional outcomes and muscle mass indices were evident in both intervention groups at 6 months. After discontinuation of intervention at 6 months, gait speed and perceived health were significantly higher in the intervention groups at 12 months. The findings from our study further supports the link between physical function and cognition. Both share a common pathway mediated by inflammation with common endpoint of disability and functional decline. Frailty accelerates disease expression in people with Alzheimer’s disease pathology with consequent disability (35). A systematic review reported multi-domain interventions to be superior to single domain in improving frailty status, muscle mass and strength and physical function (36). Since then, many studies have shown the benefits of multicomponent interventions in improving cognitive and physical function in community-dwelling pre-frail older adults (21, 37–39).
The Ex+CST group improved significantly in cognition, depression and physical function. To date, there are no studies on benefits of CST in pre-frail older adults. CST has largely been studied in persons with dementia (40) and has been found to be cost effective in improving QoL(41), function and cognition (42). Our study thus adds to the evidence of the impact that CST and physical exercise has on cognition in pre-frail older adults.
There are limited studies on outcomes data after discontinuation of intervention. Significant improvements were only seen in gait speed, perceived health, muscle mass and physical activity level. However, statistically significant change may not always correlate with clinically meaningful change. Using the latter, the change in SPPB would be considered significant at 6 months in the Ex and Ex+CST group and only in the Ex+CST group at 12 months (43). This adds to the imperative for sustained, long-term physical activity interventions and cognitive training programs in the primary care setting which is often the pillar for preventive population health. The intervention groups had significantly higher perceived health at 12 months. Poor self-rated health has been shown to be associated with increased mortality, frailty and 70% higher risk of slow gait over 4 years (44). Both the Ex and Ex+CST groups had significantly higher gait speed at 3, 6 and 12 months. Slow gait is recognised as the ‘sixth’ vital sign, a well-known harbinger of dementia and associated with adverse outcomes such as falls, fractures, social isolation, and mortality (45). It is not known if improvement in gait speed will delay or prevent poor outcomes.
The components of multi-domain interventions can be personalized based on screening tools such as the FRAIL scale which is one of the components for the Rapid Geriatric Assessment with assisted management pathway available in the EPIC electronic medical records and as a mobile application. Three-quarters of those reported fatigue had undiagnosed depression (12). People who have difficulty climbing one flight of stairs or walking 50 meters can be referred for multicomponent intervention and protein enriched diet. The ICOPE guidelines can be used to screen and manage for decline in intrinsic capacity which is a surrogate for underlying physiological reserve. It includes five key steps from screening for decline in intrinsic capacity, intervening to engaging communities and supporting caregivers (46). Various studies are in progress to determine if improvement in intrinsic capacity through implementation of multicomponent intervention can change the trajectory of frailty, one of which includes a large-scale prospective study, the INSPIRE ICOPE-CARE program in Occitania (47, 48).
Inflammation is a well-known hallmark of aging and potential mechanisms include mitochondrial dysfunction, immune senescence, microbiota composition changes, genetic susceptibility, sedentary lifestyle, obesity, and increased gut permeability (49). Inflammation is a key driver for many mental and physical health issues and non-communicable diseases (49). Exercise has shown to exert immuno-modulatory effect but studies on the effect of exercise on inflammation have shown mixed results due to varied study participants, type, duration and intensity of exercise (17). To complicate analysis, resistance training has also shown to increase IL-6, IL-10 and TNF-α levels (17). Exercise-induced reduction in visceral adipose tissue is thought to be mediated through IL-6 released during exercise (50). At 3 months, only lower TNF-α was associated with Ex and Ex+CST group. Elevated TNF-α can cause insulin resistance, beta-cell dysfunction and accelerate aging. GDF-15 is increasingly being recognised as biomarker of biological aging as well as mitochondrial dysfunction (51). It is both an anti-inflammatory and pro-inflammatory cytokine. Release of GDF-15 can be activated by various growth factors and cytokines, tissue injury, exercise, cancer, cardiovascular disease, cellular stress, exercise, and drugs like metformin (11). Very high levels are associated with mortality and poor physical function especially in diabetics, but moderately high levels may be protective possibly explained by the mitohormesis theory (51, 52). Effect of intervention on other inflammation biomarkers such as IL-6 and GDF-15 were not significant possibly due to timing of blood test as cytokines have different half-lives, short duration of intervention or varied exercise intensity (53). Nicklas et al showed elevation of IL-after 12 months of intervention (54) and Kleinert et al showed GDF-15 reached peak immediately after vigorous submaximal exercise (55).
Declining muscle mass and strength is prevalent in frailty, sarcopenia, and aging, and associated with increased mortality and morbidity (56). Muscle is considered as an endocrine organ, involved in glucose metabolism, insulin sensitivity and secretes myokines which regulates adipose, liver, brain, and muscle tissue function (57). All the muscle mass indices improved at 6 months in the intervention groups. Whole body phase angle is a well-known indicator for nutritional status, muscle size, quality and function, and predictor of mortality whereas body cell mass is a measure of metabolically active tissue and reliable indicator of muscle mass loss with aging (58–60). At 6 months, significant improvements were observed in ASMI, whole body phase angle and body cell mass but at 12 months, only differences in ASMI were significant in the intervention groups. Yamada et al recently reported that people who exercised regularly regardless of age or type of exercise had significantly higher phase angle value (61). While there are multiple studies on improvement in muscle mass with multicomponent exercise, there are no studies on improvement in phase angle or body cell mass in pre-frail older adults.
The main strengths of this study were the robust assessment measures, validation through inflammatory biomarkers and community dwelling participants from the primary care setting. There are however some limitations to mention. A large part of the study was conducted during the height of COVID-19 pandemic in 2020 which resulted in significant dropout rate which could lead to attrition bias and variable sample sizes. Despite the small sample size, significant benefits were seen in the Ex and Ex+CST groups which were not fully sustained after the discontinuation of the interventions at 12 months. Second, chronic disease, functional status and other demographic data collected through questionnaire may be subject to recall bias. Third, the baseline cognition for participants in the Ex+CST group was significantly higher which could have resulted in a ceiling effect. However, after excluding the dropouts, there were no significant differences in the Ex and Ex+CST groups at 3, 6 and 12 months as reflected in the supplementary Table 1. Fourth, we have no information on the intensity of the exercises or maximal heart rate achieved although it was reported to be of moderate intensity. Whilst the intervention provided 120 minutes of physical exercise per week, it did not reach the minimum recommendations by the World Health Organisation of at least 150 to 300 minutes of moderate intensity aerobic physical activity or at least 75–150 minutes of vigorous-intensity aerobic physical activity; or an equivalent combination of moderate- and vigorous-intensity activity throughout the week (62). Fifth, functional and cognitive improvements could be due to multiple interacting factors e.g., hearing, nutrition and environment, and causal inferences cannot be assumed. Lastly, while this was a cluster randomised trial, allocation to Ex and Ex+CST was not randomised. In addition, participants and assessors where not blinded.
Our study adds to the growing evidence of multi-component exercises which includes CST in improving physical function and cognition of pre-frail older adults in primary care which may help change the frailty trajectory. Policymakers should consider mandating frailty screening in primary care and social prescribing incorporating multi-component interventions to change frailty trajectory at the population level. Further research on design, implementation and longer-term impact of such programs including sustainability and scalability are needed.
Conclusion
Physical exercise with or without CST in pre-frail older adults in primary care improved depression, muscle mass indices, physical function, frailty, and perceived health. Significant improvement in cognition was only evident in the exercise supplemented by CST. Both groups had significant improvement of TNF-α levels at 3 months. With growing evidence of the benefits of multicomponent interventions at primary care level, incorporating it into mainstream care with action plans on long-term sustainability and scalability should be a priority for every country.
Electronic supplementary material
Supplementary Table 1. Baseline characteristics of participants who completed 3 months and 6 month follow-up respectively.
Funding: This research was funded by Ministry of Health of Singapore: Healthy Ageing Innovation Grant under National Innovation Challenge on Active and Confident Ageing (Award No: MOH/NIC/HAIG02/2017).
Conflict of interest: The authors declare no conflict of interest.
References
- 1.ESCAP U. Ageing in Asia and the Pacific: overview 2017 [Available from: https://www.unescap.org/resources/ageing-asia-and-pacific-overview.
- 2.Garmany A, Yamada S, Terzic A. Longevity leap: mind the healthspan gap. npj Regenerative Medicine. 2021;6(1):57. doi: 10.1038/s41536-021-00169-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Duque G. Community implementation of evidence-based interventions in geriatric medicine: Time to translate research into practice. Archives of Gerontology and Geriatrics. 2023;106:104914. doi: 10.1016/j.archger.2022.104914. [DOI] [PubMed] [Google Scholar]
- 4.Organisation WH. Integrated care for older people (ICOPE): guidance for person-centred assessment and pathways in primary care. 2019.
- 5.Ruiz JG, Dent E, Morley JE, Merchant RA, Beilby J, Beard J, et al. Screening for and Managing the Person with Frailty in Primary Care: ICFSR Consensus Guidelines. The journal of nutrition, health & aging. 2020;24(9):920–7. doi: 10.1007/s12603-020-1498-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xue Q-L, Bandeen-Roche K, Tian J, Kasper JD, Fried LP. Progression of Physical Frailty and the Risk of All-Cause Mortality: Is There a Point of No Return? Journal of the American Geriatrics Society. 2021;69(4):908–15. doi: 10.1111/jgs.16976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sezgin D, Liew A, O’Donovan MR, O’Caoimh R. Pre-frailty as a multi-dimensional construct: A systematic review of definitions in the scientific literature. Geriatric Nursing. 2020;41(2):139–46. doi: 10.1016/j.gerinurse.2019.08.004. [DOI] [PubMed] [Google Scholar]
- 8.Collard RM, Boter H, Schoevers RA, Oude Voshaar RC. Prevalence of frailty in community-dwelling older persons: a systematic review. Journal of the american geriatrics society. 2012;60(8):1487–92. doi: 10.1111/j.1532-5415.2012.04054.x. [DOI] [PubMed] [Google Scholar]
- 9.Merchant RA, Chen MZ, Tan LWL, Lim MY, Ho HK, van Dam RM. Singapore Healthy Older People Everyday (HOPE) Study: Prevalence of Frailty and Associated Factors in Older Adults. J Am Med Dir Assoc. 2017;18(8):734.e9–.e14. doi: 10.1016/j.jamda.2017.04.020. [DOI] [PubMed] [Google Scholar]
- 10.Apóstolo J, Cooke R, Bobrowicz-Campos E, Santana S, Marcucci M, Cano A, et al. Effectiveness of interventions to prevent pre-frailty and frailty progression in older adults: a systematic review. JBI Database of Systematic Reviews and Implementation Reports. 2018;16(1):140–232. doi: 10.11124/JBISRIR-2017-003382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jadczak AD, Makwana N, Luscombe-Marsh N, Visvanathan R, Schultz TJ. Effectiveness of exercise interventions on physical function in community-dwelling frail older people: an umbrella review of systematic reviews. JBI Database System Rev Implement Rep. 2018;16(3):752–75. doi: 10.11124/JBISRIR-2017-003551. [DOI] [PubMed] [Google Scholar]
- 12.Merchant RA, Hui RJY, Kwek SC, Sundram M, Tay A, Jayasundram J, et al. Rapid Geriatric Assessment Using Mobile App in Primary Care: Prevalence of Geriatric Syndromes and Review of Its Feasibility. Front Med (Lausanne) 2020;7:261. doi: 10.3389/fmed.2020.00261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xu Y, Wang M, Chen D, Jiang X, Xiong Z. Inflammatory biomarkers in older adults with frailty: a systematic review and meta-analysis of cross-sectional studies. Aging Clin Exp Res. 2022;34(5):971–87. doi: 10.1007/s40520-021-02022-7. [DOI] [PubMed] [Google Scholar]
- 14.Lu W-H, Gonzalez-Bautista E, Guyonnet S, Lucas A, Parini A, Walston JD, et al. Plasma inflammation-related biomarkers are associated with intrinsic capacity in community-dwelling older adults. Journal of Cachexia, Sarcopenia and Muscle.n/a(n/a). doi: 10.1002/jcsm.13163. [DOI] [PMC free article] [PubMed]
- 15.Dagdeviren S, Young Jung D, Friedline RH, Noh HL, Kim JH, Patel PR, et al. IL-10 prevents aging-associated inflammation and insulin resistance in skeletal muscle. The FASEB Journal. 2017;31(2):701–10. doi: 10.1096/fj.201600832R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pan L, Xie W, Fu X, Lu W, Jin H, Lai J, et al. Inflammation and sarcopenia: A focus on circulating inflammatory cytokines. Exp Gerontol. 2021;154:111544. doi: 10.1016/j.exger.2021.111544. [DOI] [PubMed] [Google Scholar]
- 17.Sellami M, Bragazzi NL, Aboghaba B, Elrayess MA. The Impact of Acute and Chronic Exercise on Immunoglobulins and Cytokines in Elderly: Insights From a Critical Review of the Literature. Frontiers in Immunology. 2021;12. doi: 10.3389/fimmu.2021.631873. [DOI] [PMC free article] [PubMed]
- 18.Bautmans I, Njemini R, Vasseur S, Chabert H, Moens L, Demanet C, et al. Biochemical changes in response to intensive resistance exercise training in the elderly. Gerontology. 2005;51(4):253–65. doi: 10.1159/000085122. [DOI] [PubMed] [Google Scholar]
- 19.Petrella M, Aprahamian I, Mamoni RL, De Vasconcellos Romanini CF, Lima NA, De Cássio Robello E, et al. The effect of a multicomponent exercise protocol (VIVIFRAIL©) on inflammatory profile and physical performance of older adults with different frailty status: study protocol for a randomized controlled trial. BMC Geriatrics. 2021;21(1). doi: 10.1186/s12877-021-02030-2. [DOI] [PMC free article] [PubMed]
- 20.Ferrer MD, Capó X, Martorell M, Busquets-Cortés C, Bouzas C, Carreres S, et al. Regular Practice of Moderate Physical Activity by Older Adults Ameliorates Their Anti-Inflammatory Status. Nutrients. 2018;10(11). doi: 10.3390/nu10111780. [DOI] [PMC free article] [PubMed]
- 21.Yu R, Tong C, Ho F, Woo J. Effects of a Multicomponent Frailty Prevention Program in Prefrail Community-Dwelling Older Persons: A Randomized Controlled Trial. J Am Med Dir Assoc. 2020;21(2):294e1–e10. doi: 10.1016/j.jamda.2019.08.024. [DOI] [PubMed] [Google Scholar]
- 22.Saragih ID, Tonapa SI, Saragih IS, Lee B-O. Effects of cognitive stimulation therapy for people with dementia: A systematic review and meta-analysis of randomized controlled studies. International Journal of Nursing Studies. 2022;128:104181. doi: 10.1016/j.ijnurstu.2022.104181. [DOI] [PubMed] [Google Scholar]
- 23.Aimee Spector BW, Charlote R.Stoner, Martin Orrell. Making a difference 1: An evidence-based group programme to offer Cognitive Stimulation Therapy (CST) to people living with dementia. 2020.
- 24.Morley JE, Malmstrom TK, Miller DK. A simple frailty questionnaire (FRAIL) predicts outcomes in middle aged African Americans. J Nutr Health Aging. 2012;16(7):601–8. doi: 10.1007/s12603-012-0084-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Russell D, Peplau LA, Ferguson ML. Developing a measure of loneliness. J Pers Assess. 1978;42(3):290–4. doi: 10.1207/s15327752jpa4203_11. [DOI] [PubMed] [Google Scholar]
- 26.Schrag A, Selai C, Jahanshahi M, Quinn NP. The EQ-5D—a generic quality of life measure—is a useful instrument to measure quality of life in patients with Parkinson’s disease. Journal of Neurology, Neurosurgery & Psychiatry. 2000;69(1):67–73. doi: 10.1136/jnnp.69.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Topolski TD, LoGerfo J, Patrick DL, Williams B, Walwick J, Patrick MB. The Rapid Assessment of Physical Activity (RAPA) among older adults. Prev Chronic Dis. 2006;3(4):A118. [PMC free article] [PubMed] [Google Scholar]
- 28.Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist. 1969;9(3):179–86. doi: 10.1093/geront/9.3_Part_1.179. [DOI] [PubMed] [Google Scholar]
- 29.Wallace M, Shelkey M. Katz Index of Independence in Activities of Daily Living (ADL) Urol Nurs. 2007;27(1):93–4. [PubMed] [Google Scholar]
- 30.Yoshida-Intern S. A Global Report on Falls Prevention Epidemiology of Falls. Geneva: WHO; 2007. [Google Scholar]
- 31.Dong Y, Lee WY, Basri NA, Collinson SL, Merchant RA, Venketasubramanian N, et al. The Montreal Cognitive Assessment is superior to the Mini-Mental State Examination in detecting patients at higher risk of dementia. International psychogeriatrics. 2012;24(11):1749–55. doi: 10.1017/S1041610212001068. [DOI] [PubMed] [Google Scholar]
- 32.Dias F, Teixeira AL, Guimarães HC, Barbosa MT, Resende EPF, Beato RG, et al. Accuracy of the 15-item Geriatric Depression Scale (GDS-15) in a community-dwelling oldest-old sample: the Pietà Study. Trends in psychiatry and psychotherapy. 2017;39(4):276–9. doi: 10.1590/2237-6089-2017-0046. [DOI] [PubMed] [Google Scholar]
- 33.Kaiser MJ, Bauer JM, Ramsch C, Uter W, Guigoz Y, Cederholm T, et al. Validation of the Mini Nutritional Assessment short-form (MNA-SF): a practical tool for identification of nutritional status. J Nutr Health Aging. 2009;13(9):782–8. doi: 10.1007/s12603-009-0214-7. [DOI] [PubMed] [Google Scholar]
- 34.Chen LK, Woo J, Assantachai P, Auyeung TW, Chou MY, Iijima K, et al. Asian Working Group for Sarcopenia: 2019 Consensus Update on Sarcopenia Diagnosis and Treatment. Journal of the American Medical Directors Association. 2020;21(3):300–7.e2. doi: 10.1016/j.jamda.2019.12.012. [DOI] [PubMed] [Google Scholar]
- 35.Wallace LMK, Theou O, Godin J, Andrew MK, Bennett DA, Rockwood K. Investigation of frailty as a moderator of the relationship between neuropathology and dementia in Alzheimer’s disease: a cross-sectional analysis of data from the Rush Memory and Aging Project. Lancet Neurol. 2019;18(2):177–84. doi: 10.1016/S1474-4422(18)30371-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dedeyne L, Deschodt M, Verschueren S, Tournoy J, Gielen E. Effects of multi-domain interventions in (pre)frail elderly on frailty, functional, and cognitive status: a systematic review. Clin Interv Aging. 2017;12:873–96. doi: 10.2147/CIA.S130794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Merchant RA, Tsoi CT, Tan WM, Lau W, Sandrasageran S, Arai H. Community-Based Peer-Led Intervention for Healthy Ageing and Evaluation of the ‘HAPPY’ Program. J Nutr Health Aging. 2021;25(4):520–7. doi: 10.1007/s12603-021-1606-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Izquierdo M, Merchant RA, Morley JE, Anker SD, Aprahamian I, Arai H, et al. International Exercise Recommendations in Older Adults (ICFSR): Expert Consensus Guidelines. J Nutr Health Aging. 2021;25(7):824–53. doi: 10.1007/s12603-021-1665-8. [DOI] [PubMed] [Google Scholar]
- 39.Shimada H, Makizako H, Doi T, Park H, Tsutsumimoto K, Verghese J, et al. Effects of Combined Physical and Cognitive Exercises on Cognition and Mobility in Patients With Mild Cognitive Impairment: A Randomized Clinical Trial. J Am Med Dir Assoc. 2018;19(7):584–91. doi: 10.1016/j.jamda.2017.09.019. [DOI] [PubMed] [Google Scholar]
- 40.Woods B, Aguirre E, Spector AE, Orrell M. Cognitive stimulation to improve cognitive functioning in people with dementia. Cochrane Database Syst Rev. 2012(2):CD005562. doi: 10.1002/14651858.CD005562.pub2. [DOI] [PubMed]
- 41.Orrell M, Yates L, Leung P, Kang S, Hoare Z, Whitaker C, et al. The impact of individual Cognitive Stimulation Therapy (iCST) on cognition, quality of life, caregiver health, and family relationships in dementia: A randomised controlled trial. PLoS Med. 2017;14(3):e1002269. doi: 10.1371/journal.pmed.1002269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen X. Effectiveness of cognitive stimulation therapy (CST) on cognition, quality of life and neuropsychiatric symptoms for patients living with dementia: A meta-analysis. Geriatr Nurs. 2022;47:201–10. doi: 10.1016/j.gerinurse.2022.07.012. [DOI] [PubMed] [Google Scholar]
- 43.Guralnik J, Bandeen-Roche K, Bhasin SAR, Eremenco S, Landi F, Muscedere J, et al. CLINICALLY MEANINGFUL CHANGE FOR PHYSICAL PERFORMANCE: PERSPECTIVES OF THE ICFSR TASK FORCE. Journal of Frailty & Aging. 2019:1–5. doi: 10.14283/jfa.2019.33. [DOI] [PMC free article] [PubMed]
- 44.Pilleron S, Le Goff M, Ajana S, Helmer C, Pérès K, Dartigues JF, et al. Self-Rated Health and Frailty in Older Adults from the Population-Based Three-City Bordeaux Cohort. Gerontology. 2022;68(7):755–62. doi: 10.1159/000518864. [DOI] [PubMed] [Google Scholar]
- 45.Merchant RA, Goh J, Chan YH, Lim JY, Vellas B. Slow Gait, Subjective Cognitive Decline and Motoric Cognitive RISK Syndrome: Prevalence and Associated Factors in Community Dwelling Older Adults. J Nutr Health Aging. 2021;25(1):48–56. doi: 10.1007/s12603-020-1525-y. [DOI] [PubMed] [Google Scholar]
- 46.Banerjee A, Sadana R. Integrated Care For Older People (ICOPE): From Guidelines To Demonstrating Feasibility. The Journal of Frailty & Aging. 2021;10(2):84–5. doi: 10.14283/jfa.2020.40. [DOI] [PubMed] [Google Scholar]
- 47.Tavassoli N, de Souto Barreto P, Berbon C, Mathieu C, de Kerimel J, Lafont C, et al. Implementation of the WHO integrated care for older people (ICOPE) programme in clinical practice: a prospective study. Lancet Healthy Longev. 2022;3(6):e394–e404. doi: 10.1016/S2666-7568(22)00097-6. [DOI] [PubMed] [Google Scholar]
- 48.Blancafort Alias S, Cuevas-Lara C, Martínez-Velilla N, Zambom-Ferraresi F, Soto ME, Tavassoli N, et al. A Multi-Domain Group-Based Intervention to Promote Physical Activity, Healthy Nutrition, and Psychological Wellbeing in Older People with Losses in Intrinsic Capacity: AMICOPE Development Study. Int J Environ Res Public Health. 2021;18(11). doi: 10.3390/ijerph18115979. [DOI] [PMC free article] [PubMed]
- 49.Furman D, Campisi J, Verdin E, Carrera-Bastos P, Targ S, Franceschi C, et al. Chronic inflammation in the etiology of disease across the life span. Nature Medicine. 2019;25(12):1822–32. doi: 10.1038/s41591-019-0675-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wedell-Neergaard AS, Lang Lehrskov L, Christensen RH, Legaard GE, Dorph E, Larsen MK, et al. Exercise-Induced Changes in Visceral Adipose Tissue Mass Are Regulated by IL-6 Signaling: A Randomized Controlled Trial. Cell Metab. 2019;29(4):844–55.e3. doi: 10.1016/j.cmet.2018.12.007. [DOI] [PubMed] [Google Scholar]
- 51.Wang D, Day EA, Townsend LK, Djordjevic D, Jørgensen SB, Steinberg GR. GDF15: emerging biology and therapeutic applications for obesity and cardiometabolic disease. Nature Reviews Endocrinology. 2021;17(10):592–607. doi: 10.1038/s41574-021-00529-7. [DOI] [PubMed] [Google Scholar]
- 52.Merchant RA, Chan YH, Duque G. GDF-15 Is Associated with Poor Physical Function in Prefrail Older Adults with Diabetes. Journal of Diabetes Research. 2023;2023:2519128. doi: 10.1155/2023/2519128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Docherty S, Harley R, McAuley JJ, Crowe LAN, Pedret C, Kirwan PD, et al. The effect of exercise on cytokines: implications for musculoskeletal health: a narrative review. BMC Sports Sci Med Rehabil. 2022;14(1):5. doi: 10.1186/s13102-022-00397-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nicklas BJ, Hsu FC, Brinkley TJ, Church T, Goodpaster BH, Kritchevsky SB, et al. Exercise training and plasma C-reactive protein and interleukin-6 in elderly people. J Am Geriatr Soc. 2008;56(11):2045–52. doi: 10.1111/j.1532-5415.2008.01994.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kleinert M, Clemmensen C, Sjøberg KA, Carl CS, Jeppesen JF, Wojtaszewski JFP, et al. Exercise increases circulating GDF15 in humans. Mol Metab. 2018;9:187–91. doi: 10.1016/j.molmet.2017.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.de Santana FM, Premaor MO, Tanigava NY, Pereira RMR. Low muscle mass in older adults and mortality: A systematic review and meta-analysis. Exp Gerontol. 2021;152:111461. doi: 10.1016/j.exger.2021.111461. [DOI] [PubMed] [Google Scholar]
- 57.Walowski CO, Braun W, Maisch MJ, Jensen B, Peine S, Norman K, et al. Reference Values for Skeletal Muscle Mass - Current Concepts and Methodological Considerations. Nutrients. 2020;12(3). doi: 10.3390/nu12030755. [DOI] [PMC free article] [PubMed]
- 58.Rondanelli M, Talluri J, Peroni G, Donelli C, Guerriero F, Ferrini K, et al. Beyond Body Mass Index. Is the Body Cell Mass Index (BCMI) a useful prognostic factor to describe nutritional, inflammation and muscle mass status in hospitalized elderly?: Body Cell Mass Index links in elderly. Clin Nutr. 2018;37(3):934–9. doi: 10.1016/j.clnu.2017.03.021. [DOI] [PubMed] [Google Scholar]
- 59.Mattiello R, Amaral MA, Mundstock E, Ziegelmann PK. Reference values for the phase angle of the electrical bioimpedance: Systematic review and meta-analysis involving more than 250,000 subjects. Clin Nutr. 2020;39(5):1411–7. doi: 10.1016/j.clnu.2019.07.004. [DOI] [PubMed] [Google Scholar]
- 60.Nunes JP, Ribeiro AS, Silva AM, Schoenfeld BJ, Dos Santos L, Cunha PM, et al. Improvements in Phase Angle Are Related With Muscle Quality Index After Resistance Training in Older Women. J Aging Phys Act. 2019;27(4):515–20. doi: 10.1123/japa.2018-0259. [DOI] [PubMed] [Google Scholar]
- 61.Yamada Y, Yoshida T, Murakami H, Kawakami R, Gando Y, Ohno H, et al. Phase angle obtained via bioelectrical impedance analysis and objectively measured physical activity or exercise habits. Scientific Reports. 2022;12(1):17274. doi: 10.1038/s41598-022-21095-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Organisation WH. WHO GUIDELINES ON PHYSICAL ACTIVITY AND SEDENTARY BEHAVIOUR 2020 [Available from: https://www.who.int/news-room/fact-sheets/detail/physical-activity. [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1. Baseline characteristics of participants who completed 3 months and 6 month follow-up respectively.


