Abstract
The COVID-19 pandemic has mandated people to use medical masks to protect the public. However the improper management of disposable mask waste has led to the increase of marine pollution, in terms of water quality, and the decline in aquatic microorganisms. The aim of this research was to investigate the impact of disposable mask waste on fresh water and microalgae biomass quality. Disposable masks (untreated or treated with Enterococcus faecalis) were placed in 10-L glass reactors containing fresh water or water containing algal Chlorella sp. and its growth supplements (Chlorella medium) (four 10-L reactors in total) and kept in controlled conditions for 3 months. Water and biomass yield quality were evaluated using water quality analysis, spectroscopy, scanning electron microscopy (SEM), and proximate lipid and protein analysis. Disposable masks, incubated in either fresh water or Chlorella medium, affected several water quality parameters such as chemical oxygen demand (COD), biological oxygen demand (BOD), dissolved oxygen (DO), and pH. Microplastic identification revealed that some fibers were present in the water following a 100-day treatment process. Fourier transform–infrared spectroscopy (FTIR) analysis was used to determine the change in important, organic functional groups and highlighted the disappearance of a peak at 1530 cm−1 corresponding to the primary protein (C–N) and the appearance of new peaks at 1651 cm−1 and 1270 cm−1 corresponding to methyl alcohol (CH2OH) and ketone (C = O), respectively. This indicated the detrimental effect of disposable mask fragmentation on the biomass quality. The SEM investigation has shown a damage to the surface membrane of Chlorella sp. cells. Altogether, disposable masks decreased the water quality and damaged microalgae by inhibiting their growth. Therefore, the disposable mask contaminated by various microbes, after being used by a human, may be one of the most dangerous hazards to the environment.
Keywords: Biomass production, Environmental sustainability, Environmental impact, Pandemic, Degradation
Introduction
The COVID-19 pandemic has led to a global surge in medical waste, particularly a single used mask (Benson et al. 2021; Sangkham 2020). As a consequence of the pandemic, billions tons of mask waste endanger the aquatic environment (Fred-Ahmadu et al. 2020). The handling of medical waste in both household and institutional settings, particularly of disposable masks, has not significantly decreased mask pollution in the environment (Klemeš et al. 2020). Medical mask waste in the terrestrial and aquatic environment has the potential to breakdown into micro- (< 5 mm) and nano- (< 1 μm) particles, which compound the risks associated with plastic pollution (Fred-Ahmadu et al. 2020). The buildup of mask waste in the environment, owing to inadequate mask waste management, poses a significant hazard to the ecosystem and marine life (Safiuddin and Salam 2020) . According to Hagemann et al., marine plastic pollution may lead to a 1 to 5% decrease in ecosystems diversity (Hagemann 2020). It harms marine ecosystems and affects the economies of coastal towns, fishing, and tourism to global cultural heritage sites (Benson et al. 2021).
The single used mask mostly composed of polymers for their production, including polystyrene (PS), polycarbonate, polypropylene, polyethylene, and polyester (Henneberry 2020). Disposable masks consist of three filter layers (outer, middle, and inner) that are made of a thin non-woven material-spun-bonded, non-slip, non-cellulose, and hydrophobic, with air permeability and a high bacterial filtering rate, for micro-denier fibers (< 1 to 10 microns) (Chellamani et al. 2013; Barycka et al. 2020). The outer layer of the mask comprised a non-woven polypropylene material that is waterproof and translucent to reflect aerosols from the environment. The inner layer is a soft white non-woven cotton layer, while the middle layer is a white polypropylene fiber (melt-blown) filter that repels bacteria and other particles from the outside and inside of the mask. The fiber density of the outer and inner layers is less than that of the middle layer (Barycka et al. 2020). Chellamani et al. (2013) noted that surgical masks provide greater protection than textile or microporous textile masks. Besides, surgical masks composed from polymer materials, are remarkably durable and resistant to natural deterioration (O’Dowd et al. 2020). The increase in production and use of disposable surgical masks, the lack of public awareness regarding their proper disposal, and the absence of processing techniques, have resulted in a greater accumulation of waste in the environment, particularly pollution in the oceans and freshwater ecosystems (Aragaw 2020) and, eventually, harmful to the organisms living in this aquatic system (Ma et al. 2020).
Microalgae are photosynthetic microorganisms and have been a source of protein-rich sustenance for humans (Garcia et al. 2017). Microalgae are capable of producing biomass via a photosynthetic mechanism that assimilates light, water, and carbon dioxide (Vigani et al. 2015; Molino et al. 2020). Microalgae biomass is abundant in protein (60.6%), lipids (5–10%), hydrocarbons (10–20%), antioxidants, vitamins A (beta-carotene), B1 (thiamine), B2 (riboflavin), B6 (pyridoxine), B9 (folic acid), B12 (cobalamin), C, E, minerals, iodine, potassium, iron, magnesium, and calcium (Sugiharto 2020). Microalgae are also capable of producing phytosterols and organic functional groups in the form of two polysaccharides composed of 37.8% rhamnose and 15.8% mannose. Both groups exhibit antitumor, anticancer, antioxidant, anti-inflammatory, anti-hypercholesterolemic, anti-diabetic, and anti-tumor activities. However, the presence of plastic debris, particularly during the COVID-19 pandemic, poses a significant danger to aquatic ecosystems, and the pollutants have an undeniable impact on the quality of the microalgae biomass.
Plastic waste, such as polymer-based disposable surgical masks, includes a variety of chemical additives, including stabilizers, plasticizers, heavy metals (cadmium, copper, titanium, zinc, etc.), bisphenol A, and phthalate (Campanale et al. 2020). This latter chemical is added during the plastic manufacturing process to boost the plastic’s tensile strength, which affects the material’s capacity to degrade (Asriza and Pitulima 2017). Plastic breakdown products in microplastics and the release of chemical compounds will have a negative impact on microorganism in the aquatic system (Koelmans et al. 2013). Multiple studies have shown that plastic debris may inhibit the development and quality of microalgae. Sadiq et al. (2011) studied the impact of TiO2 additions on the cells of the microalgae Chlorella sp. and Scenedesmus sp. for 24–72 h. The additives were able to affect multiple peaks representing functional groups in microalgae, such as stretching the peptide peaks, which indicated the presence of protein at a wavelength of 1800–1300 cm−1. The alcoholic group, namely the C–O, which is the main component of the green algae cell wall, also experienced stretching. This stretching indicated a reduction in protein and polysaccharide concentration as a result of additive assault.
Fu et al. (2019) investigated the impact of aged microplastic polyvinyl chloride (mPVC) and virgin mPVC with copper on the development of Chlorella vulgaris over 240 h; the study revealed that aged mPVC had a greater maximum growth inhibition ratio (IR) (35.26%) than virgin mPVC (IR = 28.2%), and that increasing concentrations of mPVC had more inhibitory impact. In addition, mPVC combined with copper could also limit Chlorella vulgaris development. It was discovered that Chlorella vulgaris cells were severely damaged and that superoxide dismutase and intracellular malonaldehyde were elevated. Tunali et al. (2020) and Aruoja et al. (2015) also studied the interaction between microplastic with varied concentrations of MPs and the combination of MPs with metals (Cu, Zn, Mn) on the growth and chlorophyll content of Chlorella vulgaris over a period of 20 days (El-Meihy et al. 2019). The findings demonstrated that the addition of microplastic considerably decreased the growth (28.86%) and chlorophyll-a content (9.2–21.3%) of Chlorella vulgaris. Other researchers (Hazeem et al. 2020) examined the harmful effects of PS at micro and nanosizes on Chlorella vulgaris microalgae throughout a 28-day period. The research revealed cell damage and aggregation formation, as well as diminished protein, lipid, nucleic acid, and polysaccharide peaks. These findings demonstrated that microplastics and their chemical additions negatively impacted the development of microalgae and the functional groups present in microalgae, resulting in a decline in the quality of their biomass.
Numerous reports have shown the existence of microplastic contamination in Indonesian watersheds, which negatively affects water quality. Dewata and Zainul (2015) reported a considerable change in COD and BOD parameters in Padang’s Batang Arau River downstream watershed. They concluded that waste degradation occurred mostly in the downstream watershed compared to the upstream watershed. Kumar et al. (2017a,b) also investigated water quality changes in the Ciliwung River watershed in Jakarta. The result showed that NO3 parameter varied from 6.07 to 13.34 mg/L, whereas the BOD and COD values increased from 7.65 to 11.41 mg/L and from 20.16 to 51.01 mg/L, respectively. Widodo et al. (2019) observed substantial sewage pollution with an increase in COD to 510.5 mg/L, and a total microbial coliform concentration between 24,000 and 240,000/mL, which exceeded the water quality requirement for all water classes. Luo et al. (2019) observed that although TSS and BOD levels decreased in the rivers, however, the DO value was often below 5 mg/L, indicating that the water was still contaminated and did not fulfill the minimum quality criteria. Buwono et al. (2021) revealed that the quantity of microplastics in the Brantas River was 5467 particles/m3, with more fiber present in upstream (39–49%) than downstream. It was observed that low DO conditions and metrics of pH, COD, BOD, TSS, and TDS were below the standard for water quality. This research aims to investigate the effect of microplastics—resulting from an increase in disposable masks owing to COVID-19—on the growth of Chlorella microalgae and the quality of fresh water utilized as water supply for daily human life.
Materials and methods
Materials
The masks utilized in this investigation were 3-ply earloop SENSI brand surgical face masks acquired from Arista Latino Company, Indonesia, manufactured according to SNI EN 14683: 2019; AC: 2019 and SNI 8483: 2018 standards (Indonesian National Standard for disposable mask characteristics).
Bacteria preparation
Enterococcus faecalis, a facultative anaerobe that resides in the human oral cavity, was chosen as the bacterium to simulate mask contamination, as it is comparable to a mask exposed to aerosol droplets from individuals making natural, active use of the material. The culture of bacteria was obtained from the Central Laboratory of the Faculty of Medicine at Universitas Semarang. Agar media in a petri dish containing a bacterial culture was kept in an anaerobic jar with an anaerobic kit added to prevent the entry of air, and then incubated in a carbon dioxide incubator at 37 °C. After 7 days of cultivation on agar media, Enterococcus faecalis was diluted to determine the concentration of Enterococcus faecalis bacteria in liquid medium by preparing a 0.5 McFarland solution, the standard developed for estimations of bacterial counts in suspension fluids. The objective of the McFarland turbidity standard is to adjust the turbidity of the suspension liquid such that the number of bacteria is within the defined range . The standard level of 0.5 McFarland is 1.5 × 108 bacterial cells per mL (Gayatri et al. 2019).
The first step in preparing a standard McFarland solution was to fill a 10-mL test tube with distilled water. Using a disposable 6-in Onemed cotton swab, bacteria from the agar medium were collected and placed in a test tube with Aqua Dest (distilled water). After the solution had become homogenous, its turbidity color was adjusted to the 0.5 McFarland standard as outlined in Gayatri et al. (2019). After the same turbidity was obtained, the solution was ready for the spraying procedure over the surgical mask.
Mask preparation
Out of four disposable surgical masks, two were left untreated, while the other two were sprayed with a solution containing Enterococcus faecalis in order to simulate used surgical masks that are discarded into the environment. Masks from each group were marked either with a red or yellow line. Two masks allocated to the treatment group were then sprayed with McFarland’s solution; both the front and back of the mask received five sprays each. The bacterial masks were then exposed to outside air for 7 days.
Medium and sample preparation
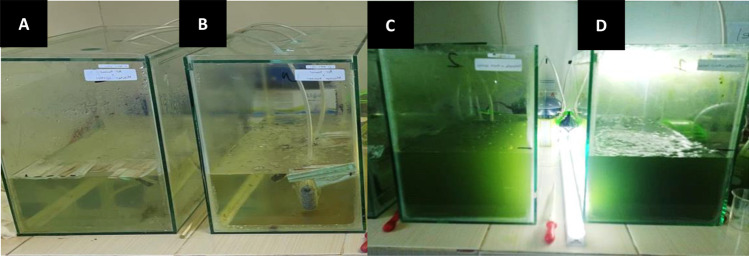
The media used in this investigation were freshwater medium (control) and freshwater medium containing microalgae Chlorella sp.; the masks were without bacteria (control) and masks contaminated with bacteria (bacterial masks). Hence, this research utilized four 40 × 20 × 30 cm glass reactors: reactor A (freshwater with a control mask); reactor B (freshwater with a bacterial mask); reactor C (Chlorella sp. with a control mask); and reactor D (Chlorella sp. with a bacterial mask). All reactors contained 10 L of freshwater (Fig. 1).
Fig. 1.
Experimental design and the appearance of reactors for 100 days of treatment. A Fresh water treated by control mask, B fresh water treated by a bacterial mask, C Chlorella sp. treated by control mask, and D Chlorella sp. treated by a bacterial mask
Chlorella sp. culture was provided by the C-BIORE Laboratory at Diponegoro University. Chlorella sp. cultures were inoculated into reactors C and D and supplemented with triple super phosphate (TSP) at 0.05 g/L and urea and NaHCO3, both at 0.1 g/L, which were subsequently administered every 5 days to promote Chlorella sp. development. The optical density (OD) of Chlorella sp. suspension was measured on days 0 and 100 using a spectrophotometer (Spectroquant Pharo 300 M) at a wavelength of 680 nm, and ultrapure water was used as a blank solution (Hadiyanto et al. 2021).
The four reactors were fitted with aerators for oxygen delivery and LED lamps (3000 lx) for light intensity, and the temperature and pH were regulated to range between 24 and 26 °C and 7–8, respectively. Surgical masks without bacterial treatments and with pre-prepared bacterial treatments were added to each reactor on day 0. The aerator aided in the stirring operation to ensure that the surgical mask was evenly distributed throughout the medium. The experiment lasted for 3 months (~ 100 days); pH, temperature, and cell density were recorded daily, and oxygen levels, carbon dioxide levels, and cell density (OD) were assessed every other week and compared between the samples.
Determination of growth rate of Chlorella sp.
The results of the OD Chlorella sp. in control and reactors C and D for 100 days were then applied in the equation to determine the growth rate μ (h − 1) with a growth time of t (h) in the exponential phase, as described by Fogg and Thake. (1987).
| 1 |
where x is the optical density and t is the cultivation time.
Microalgae Chlorella sp. biomass quality measurements
Analyses of the quality of the microalgae Chlorella sp., including proximate (lipid and protein) analysis, were performed before mask placement (control) and at the end of the experiment (3 months after mask placement). The proximate analysis began with the collection of Chlorella sp., and the wet biomass was then dried in an oven, at 30–35 °C for 24 h, to produce dry biomass.
Fourier-transform–infrared spectrometer (FTIR) analysis was performed using a Perkin Elmer-type Frontier instrument (MA, USA) by collecting the spectrum from 4000–400 cm−1 using the SNI 19–4370-2004 and ASTM D6288-89 methods on Chlorella sp. dry biomass based on ISO-846 and ISO-11266 standards (Lucas et al. 2008; Schmitt et al.1998).
A scanning electron microscopy (SEM) examination was conducted on dried Chlorella sp. biomass that was metalized with Au at room temperature (microscope model JSM-6510 LA, US). To study the presence of microorganisms on the material’s surface, images were acquired at original magnifications of × 1000, × 3000, × 5000, and × 10,000 according to ISO-846 and ISO-11266 (Lucas et al 2008).
Extracellular polymeric substances (EPS) evaluation of microalgae Chlorella sp.
The EPS assessment was performed on the material extracted from the Chlorella sp.–containing C and D treatment reactors after 3 months of culture. The technique for measuring EPS began with the calculation of the EPS yield contained in microalgae in the reactor using a ratio of 2:3 (v/v) microalgae to 96% alcohol. The experiment was conducted using 6-replicate 15-mL centrifuge tubes for each reactor. For this, 6 mL of microalgae was combined with 9 mL of 96% chilled alcohol in each of 15-mL centrifuge tubes, and the mixture was homogenized to guarantee consistency. The tubes were then refrigerated at 5 °C for 24 h during which the Chlorella biomass would drop to the bottom of the tube, while the EPS slime would remain floating nearer the surface. Pipette-collected EPS mucus (still semi-liquid) was then put on a petri plate and dried in an oven at 30–35 °C for 24 h. The weight of the dry EPS slime was used to compare the EPS production from reactors C and D.
Water quality analysis
The water quality in each of the four reactors was evaluated seven times each sample over a 3-month period (every other week). Analysis of pH, COD, BOD, and DO is helpful for determining whether the medium containing the mask undergoes biodegradation interactions or not. These measures would, therefore, reflect the water quality level and the amount of contamination induced by the presence of masks in Chlorella sp. and freshwater media.
Before (day 0) and after having a mask for 100 days, the quality of the water was compared. According to PDAM Environmental Quality Control Laboratory (2020), water quality analysis consists of pH parameters (SNI 06–6989.11–2004), COD (SNI 6989.2:2009), BOD (SNI 6989.72:2009), and DO (SNI 06–698914-2004). Analysis of pH was performed using the OHAUS ST-300 pH meter; analysis of COD and BOD employed the COD reactor and test tube heater reactor (25 Hole HANNA Instrument HI 839800), respectively, and analysis of DO utilized the Lutron DO-5510 DO meter.
Identification of microplastics
The identification of microplastics refers to measuring the presence of microplastic fibers from the masks after their breakdown in fresh water or Chlorella sp. media in all the four reactors. The examination was performed on a Stereo Zoom Microscope NSZ 606 (Shanghai, China) on the media samples from each reactor, at the same magnifications × 2000 and × 3000, in order to determine the quantity and length of microplastic fibers in each reactor. Using a pipette, samples were obtained from each medium and placed on the Sailbrand glass preparation slides with three drops from each reactor: 0.05 μL for the first drop and 0.15 μL each for the second and third drops. After acquiring the sufficient number of microplastic fibers with magnifications of × 2000 and × 3000 from each reactor, the mean and standard deviation (SD) values were calculated and compared between the reactors. The lengths of the microplastic fibers in each sample drop (in μm) were obtained using measurement tools supplied with the microscope.
Results and discussion
The impact of disposable masks on the water quality
Evaluation of chemical and physical parameters: pH, COD, BOD, and DO
The values of COD, BOD, DO, and pH in the freshwater reactors (A, B) and Chlorella sp. reactors (C, D) after 100 days of treatment with disposable control or Enterococcus faecalis treated masks are shown in Table 1. As seen in Table 1, the COD and BOD values rose considerably after 100 days of treatment, particularly in Chlorella sp. reactors. COD and BOD measurements reflect the quantity of oxygen required to chemically and physiologically oxidize the substances in the mask. Therefore, increased COD and BOD levels in the reactors suggested that the contaminated medium was becoming rich in both organic and inorganic matters. High COD and BOD levels have a negative influence on dissolved oxygen (DO) because microorganisms in the medium decrease the DO. The observed decrease in the DO confirmed that the quality of the medium was deteriorating.
Table 1.
Media pH after 100-day treatment
| Reactor | SNI COD |
COD (mg/L) | SNI BOD |
BOD (mg/L) | SNI DO |
DO (mg/L) | SNI pH |
pH | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| H0 | H100 | H0 | H100 | H0 | H100 | H0 | H100 | |||||
| A | 100 | 25 | 123.33 | 12 | 6 | 38.23 | 6.8–7 | 7.6 | 6.6 | 6.8–7 | 7.2 | 7.4 |
| B | 100 | 25 | 93.33 | 12 | 6 | 28.93 | 6.8–7 | 7.6 | 5.8 | 6.8–7 | 7.2 | 7.7 |
| C | – | 161 | 999 | – | 152.93 | 609.67 | – | 7.5 | 6.2 | 6–9 | 6.3 | 9.5 |
| D | – | 161 | 1003 | – | 152.93 | 666.33 | – | 7.5 | 5.7 | 6–9 | 6.3 | 9.7 |
(A) Fresh water treated by control mask, (B) Fresh water treated by a bacterial mask, (C) Chlorella sp. treated by control mask, (D) Chlorella sp. treated by a bacterial mask. SNI, Indonesian Standard H0; H100, days 0 and 100, respectively; H0, day 0 and H100 represents day 100
The addition of bacteria alters the microorganisms’ activity in the media. Enterococcus faecalis is a gram-positive, resistant bacterium that could be utilized to speed up the process of waste degradation, including the decomposition of disposable masks. In this investigation, Enterococcus faecalis was sprayed on a disposable mask and subsequently applied to freshwater. According to Wei et al. (2017), water is a possible habitat for Enterococcus faecalis transmission. Enterococcus faecalis thrives and proliferates via developing a biofilm on the mask’s surface (Dale et al. 2017). Enterococcus faecalis bacteria are facultative anaerobes, meaning they may grow in the presence of oxygen or in the absence of oxygen (Portela et al. 2014). Its interaction with disposable masks results in processes of oxidation, biosorption, and bioaccumulation. These processes are associated with the properties of Enterococcus faecalis itself. In order to hydrolyze disposable masks, oxygen and light must be present in the media during their chemical oxidation.
The presence of carbon and light sources in the medium aids photosynthesis, which generates glucose and oxygen for respiration. Aerobic respiration in Enterococcus faecalis generates greater amounts of carbon dioxide, water, and adenosine triphosphate (ATP) than anaerobic respiration. In addition, photosynthesis-derived glucose promotes bacterial growth and plays a role in anaerobic respiration in Enterococcus faecalis, where it is converted into lactic acid or carboxylic acid with one hydroxyl group, acetate (ester), and carbon dioxide (Pankratova et al. 2018).
Aerobic and anaerobic respiration and photosynthesis produce carbon sources, as seen in the diagrams above. Enterococcus faecalis grows and adapts more easily in an aerobic environment than in an anaerobic one (Portela et al. 2014); therefore, aerobic respiration will occur more often, ultimately increasing the mask oxidation process. Intriguingly, Enterococcus faecalis can continue to carry out the oxidation process even if there is less oxygen in the media (Portela et al. 2014).
After the mask undergoes chemical oxidation, it undergoes biodegradation. Where Enterococcus faecalis may also undertake bioabsorption and bioaccumulation is through the action of extracellular enzymes on the bacterial cell wall surface (Igiri et al. 2018). Gram-positive bacteria, such as Enterococcus faecalis, possess a thicker cell wall than gram-negative bacteria due to the presence of a cytoplasmic membrane layer, a polysaccharide layer, and 30 layers of peptidoglycan (Christita et al. 2018; Rohde 2019). Interestingly, in the presence of peptidoglycan as a cytoplasmic membrane protector, each layer of peptidoglycan includes anion-providing phosphate compounds like teichoic acid. Phosphate and teichoic acid in peptidoglycan may bind heavy metal cations to the cell walls from disposable masks (Rohde et al. 2019). In addition, Enterococcus faecalis colonies create EPS and form biofilms on the surface of disposable masks as a defensive reaction, particularly when heavy metals are present in the environment, and when environmental circumstances are hazardous. Other anionic groups, such as carboxyl, amine, and hydroxyl groups, have a role in binding cations and adsorbing metal additions in biofilms containing bacterial EPS (Irawati and Tahya 2021; Vijayaraghavan and Yun 2008). The hetero-aggregation process in this biofilm incorporates EPS from Enterococcus faecalis, disposable masks, and other microorganisms present in the media, such as microalgae Chlorella sp.
Based on the results of the study, the appearance of freshwater medium (left) and Chlorella sp. (right) can be seen in Fig. 1.
COD and BOD values
Based on the study of COD and BOD in freshwater medium, mask oxidation in reactor A depends only on light and oxygen in the medium to chemically oxidize the mask, followed by microorganisms native to the freshwater medium. In contrast, the COD and BOD readings of reactor B were lower than those of reactor A, indicating that reactor A was more contaminated than reactor B (Table 1). This could be explained as follows: Only chemical oxidation involving oxygen in the culture and photooxidation by light occurs in reactor A. Insufficient oxygen present in the culture would then be due to breakdown of inorganic and organic chemicals from the mask. This also inhibits the photosynthesis of natural bacteria, which may contribute to the mask’s oxidation. In the absence of other organisms to absorb mask additives, these chemicals accumulate, causing the COD and BOD levels of the medium to increase. In contrast, the addition of Enterococcus faecalis to reactor B accelerated the uptake of organic and inorganic waste components from disposable masks by bacterial cells. According to Zhu et al. (2020), heavy metal ions absorbed by bacterial cells have the ability to accumulate within microorganism cells. As energy sources, Enterococcus faecalis stores oxidation products in inorganic compounds derived from carbon, hydrogen, ammonia, nitrite, sulfur components, iron ions, and other heavy metals (Irawati and Tahya 2021). Moreover, according to Campanale et al. (2020), additive dyes are used into the production of disposable masks. The masks utilized in this investigation were disposable PP masks with a green front layer and white middle and inner layers. Campanale et al. stated that iron (Fe) may be employed as one of the additive dyes to produce green, red, black, and brown hues. Eslami et al. (2019) investigated the relationship between Enterococcus faecalis bacteria and textile waste. After 72 h of incubation, Enterococcus faecalis was able to absorb up to 99.26% of the red reactive dye. Additionally, Hossen et al. (2019) observed that Enterococcus faecalis could absorb 25% of black dye. These studies demonstrate that Enterococcus faecalis could absorb waste, hence decreasing the COD and BOD concentrations in the medium.
COD and BOD readings from reactors C and D differ (Table 1), which use a Chlorella microalgae medium. Although not statistically significant, the COD value in reactor D containing Chlorella sp. with a bacterial mask (1003 mg/L) was higher than in reactor C without the inclusion of Enterococcus faecalis bacteria in the mask (999 mg/L). Reactor D is reportedly more contaminated than reactor C. Through its extracellular enzymes and anion components in the Chlorella sp. cell wall (in the form of carboxyl, hydroxyl, sulfate, phosphate, etc.), Chlorella sp. aids in the binding and absorption of heavy metals. In the cell walls of Chlorella sp., proteins with high-affinity metal ion-binding properties are produced, termed metallothioneins (MTs) and phytochelatins (PCs) (Chatterjee et al. 2020). They contain 10–35% of the amino acid cysteine, which is much more than the 2% seen in other proteins. The capacity of the amino acid cysteine to bind metal ions extremely quickly is due to its thiol or sulfhydryl (–SH) groups, which readily interact with heavy metals and play a major role in the bioaccumulation of metals (Gutiérrez et al. 2018). Kurniawan and Aunurohim (2014) discovered that Chlorella sp. might decrease lead metal by up to 60%. Balzano et al. (2020) found that all Chlorella sp were capable of forming MTs to absorb diverse metals. It is unknown whether Enterococcus faecalis is a bacterium capable of producing MTs and PCs in a metal environment, but Enterococcus faecalis has a high concentration of teichoic acid in the peptidoglycan layer of its cell wall, which allows it to bind and absorb metals into its cells. The peptidoglycan in Enterococcus faecalis plays a crucial role in preventing cells from rupturing (Christita et al. 2018; Rohde et al. 2019). MTs are generated in all microorganisms, including gram-positive and gram-negative bacteria, according to Habjanič et al. (2020). Enterococcus faecalis is a gram-positive, Bacillus-type, Bacillus-class bacteria. The gram-positive bacterium Bacillus sp. is capable of producing MTs for metal bioaccumulation (Chatterjee et al. 2020). According to Aktan et al. (2013), Enterococcus faecalis is also a resistant gram-positive bacterium since it can absorb up to a concentration 1200 ppm of lead metal in the waste medium. It should be mentioned that the capacity of each microbe to accumulate heavy metals varies (Benhalima et al. 2020). Several investigations have shown that Chlorella sp. and Enterococcus faecalis may concurrently gather metal compounds from cell walls and transport them to vacuoles, which serve as storage sites for ions and metabolites, resulting in lower metal concentrations (detoxification) (Chatterjee et al. 2020).
Moreover, the contents of reactor D (Fig. 1) seem more green and cloudy compared to reactor C. The addition of Enterococcus faecalis on disposable masks to Chlorella medium alters the medium’s state. The high COD and BOD values in reactor D were caused by the large number of microorganisms involved in the hetero-aggregation process of the biofilm on the surface of the disposable masks. These microorganisms included Chlorella sp., Enterococcus faecalis, and natural organisms in the water and on the disposable masks themselves. In addition to the vast number of hydrophobic compounds produced by disposable face masks, the ability of all microorganisms to damage the surface of the masks in the biofilm and absorb metals from masks may expedite the process of chemical and biological degradation. According to their capacity, bacteria will continue to collect metals until their body cells can no longer endure metal exposure (Fahruddin et al. 2020). Chatterjee et al. (2020) stated that MT as a binding protein will continue to be produced as long as metal ions still available in the media are bound to the cell wall until the cell reaches saturation and enters the death phase. Abdelbary et al. (2019) showed that microorganisms would discharge (desorb) metal compounds from the cell at lower concentrations if there were an excess of them within the cell. Kurniawan and Aunurohim (2014) highlighted that Chlorella sp. has a high adsorption capacity, allowing it to retain more metal compounds than are released into the medium, hence inhibiting the development and production of Chlorella sp. biomass. The decline in Chlorella sp. biomass is also due to the formation of organic ammonium compounds (NH4+), which may harm Chlorella sp. by interfering with its metabolism and inducing the dead Chlorella sp. cells to settle at the reactor’s bottom (Kurniawan and Aunurohim 2014).
Due to the presence of metal compounds from degradation and residual metal compounds generated by microalgae and bacteria, this precipitate makes the medium optically cloudier and darker, as seen in Fig. 1 (reactor D). Additionally, the presence of a large quantity of these deposits disrupts the process of photosynthesis, causing bacteria to contend for an adequate amount of oxygen as energy. Additionally, dead Chlorella sp. cells may readily settle for an extended period of time without losing their ability to decompose disposable masks (Michalak et al. 2013). Abdelbary et al. (2019) reported that living or nonliving microorganism cells may adsorb metal compounds in particulates and fluids. Even more so than active cells, Aksu and Kutsal (1990) found that dead microorganism cells may rebuild and utilize biomass to absorb heavy metals. Biodeg and Sibi (2014) demonstrated that the biosorption rate of As3+ by a variety of microalgae was much greater when employing dead biomass as opposed to living cells. In the biodegradation of waste, microalgae are capable of producing new biomass to sustain themselves. It may be inferred that the greater the activity of microorganisms to decrease metal compounds, the more cells die and settle but also create new biomass in the form of acetyl coenzyme A (CoA), which plays a role in the production of proteins, carbohydrates, and lipids. The quantity of deposits in reactor D causes the COD and BOD readings to be greater than in reactor C. In addition to being cloudier and darker, owing to the accumulation of sediment, reactor D has a stronger odor of rotten eggs and more mucus due to the increased microbial activity. This pungent stench is the result of bacteria decomposing organic matter and releasing sulfide acid gas (H2S) and methane (CH4) (Yulianto et al. 2020).
Dissolved oxygen (DO) value
The quantity of dissolved oxygen (DO) in both fresh water and Chlorella sp. media impacts the decomposition and reduction of organic and inorganic components from disposable face masks. The photosynthesis of microorganisms, the environment, or an additional aerator may provide DO. The greater the DO value, the higher the water quality and the amount of oxygen present. In contrast, the lower the DO value, the more contaminated the environment. According to the findings of this investigation (Table 1), all reactors exhibited a reduction in DO. After 100 days of treatment, reactor A had the highest DO value at 6.6 mg/L, followed by reactor C at 6.2 mg/L, which reduced further when Enterococcus faecalis was introduced to either reactor B., which had a value of 5.8 mg/L, or reactor D, with 5.7 mg/L. This implies that the deterioration of disposable masks is now occurring. As a result of the release of hydrocarbon molecules and different additives, the DO concentration in the medium was reduced from its starting value. Díez-Montero et al. (2020) and Davis et al. (2003) found that DO levels might be enhanced in the presence of microorganisms in the medium in order for photosynthesis to create more oxygen. However, the inclusion of Enterococcus faecalis in freshwater medium (reactor B) and Chlorella sp. (reactor D) resulted in the lowest DO values. This indicated that the biodegradation process of disposable masks was accelerated, owing to the increased microbial activity in reactor D.
In reactor D, the high activity of microorganisms reduced DO and increased metabolic processes and carbon dioxide accumulation (as a result of aerobic respiration), as well as other compounds such as ammonium (NH4+) or suspended solids so that Enterococcus faecalis or Chlorella sp. was unable to absorb them completely. Chlorella sp. could then be released into the medium (Kazbar et al. 2019). Due to anaerobic deterioration, reactor D exhibited a cloudier and denser hue, as well as a distinct odor, as seen in Fig. 1. The DO value could also be impacted by the quantity of microbe cell bodies that have been deposited. A hazy hue lowers the amount of light entering a medium. Raso et al. (2012) revealed that the absence of oxygen poses a danger to the survival of microorganisms, owing to the high activity of aerobic respiration, which reduces photosynthetic activity. Martzoff (2013) explored this increase in respiratory activity, which lowered oxygen levels and necessitated a higher photon flux (light intensity) to sustain biomass production. According to Simatupang et al. (2015), the high concentration of waste caused by the biodegradation process might harm microorganism cells, resulting in a drop in oxygen concentration. Low DO content could then be generated. Díez-Montero et al. (2020) examined the interaction between Chlorella sp. microalgae and agricultural waste. They observed that, at nighttime, photosynthesis was reduced, and that excessive microbial respiration caused oxygen depletion and lowered microalgae biomass output. Alternatively, aerobic respiration activity creates new biomass as a type of defense, allowing microorganisms to survive and continue the subsequent biodegradation process.
Comparable to reactor B in Fig. 5, reactor B was cloudier than reactor A. In fact, the addition of Enterococcus faecalis to a freshwater environment accelerated the sediment-degrading process. Reactor B generated a lower DO value than reactor D. This was due to the fact that reactor B exclusively employed Enterococcus faecalis for the degrading process. Reactor D, in contrast, utilized two biosorbents, namely Chlorella sp. and Enterococcus faecalis. Therefore, the drop in DO and quantity of sediment caused by microbial activity in reactor D was larger than in reactor B. According to Díez-Montero et al. (2020), the quantity of microorganisms in the medium has a significant impact on the pace of waste breakdown. In accordance with (PP No. 82, 2001) governing the quality values of clean water from surface streams, the pH, COD, BOD, and DO levels in freshwater medium in this experiment did not satisfy the norm for the raw water threshold. This indicates that the presented bioremediation procedure to minimize disposable mask waste in a freshwater medium for 100 days was not sufficient to ensure that raw water was safe for human consumption.
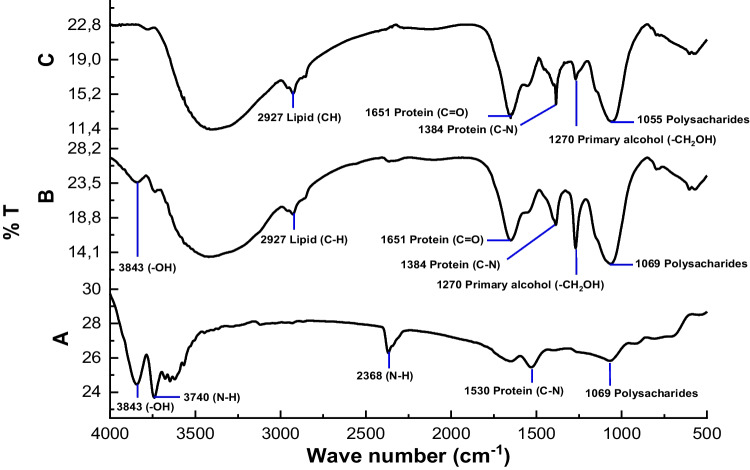
Fig. 5.
FTIR analysis of Chlorella sp. biomass. A Chlorella sp. control, B Chlorella sp. treated with control mask, and C Chlorella sp. treated with a bacterial mask
pH value
According to Table 1, the pH of the solution-containing Chlorella sp. rise as disposable masks degraded. Díez-Montero et al. (2020) observed that variations in pH result from microalgae’s photosynthetic activity after interacting with agricultural waste. The authors emphasized that the pH would rise if the photosynthetic process was inhibited. According to their study, the pH of Chlorella sp. cultures climbed to 9.6 at night. This is because light-dependent photosynthetic activity ceases at night. The same holds true for the findings of the pH in Chlorella media in Table 1 (reactors C and D), which increased relative to freshwater media when the photosynthetic process in Chlorella media began to slow down. Díez-Montero et al. (2020) observed that a high pH, owing to photosynthetic suppression, resulted in the buildup of inorganic and organic contents in Chlorella media. Hendrawan et al. (2021) also explained that ammonium (NH4+) buildup might impede photosynthesis. Chlorella sp. may directly use ammonium as a nitrogen source to produce protein cells. Some of them are oxidized in the media, hence elevating the pH. The suppression of photosynthesis also hinders the generation of nitrite and nitrate from ammonium, resulting in the precipitation of large quantities of ammonium. This accumulation also depletes oxygen, decreasing DO and elevating the pH value. In addition, the presence of Enterococcus faecalis enhanced the degradation of Chlorella sp. in reactor D relative to the other three reactors. According to Jayawardena et al. (2017), the concentration of MTs increases with pH. Changes in the structure of the cell wall of microorganisms and the binding of toxic chemicals as a result of the degradation of disposable masks could contribute to the increased concentration of MTs.
Identification microplastics in the medium
According to the microscopic results of fresh water and microalgae Chlorella sp. (Fig. 2), the presence of thin-fiber microplastics was a result from mask degradation. Visually, with a magnification of × 3000, the size of the mask fibers spread in 10 L of fresh water, and Chlorella sp. medium looks almost the same (Table 2). However, the number of microplastics that accumulated in the Chlorella sp. media (reactors C and D) was much higher than that in freshwater media (reactors A and B). Microalgae and Enterococcus faecalis bacteria, as well as natural microorganisms in the media, play a significant role in forming biofilms on the surface of the mask, thereby accelerating the hetero aggregation process on the surface of the mask. However, compared to the same media, the number of microplastics detected was higher without Enterococcus faecalis. This is possible due to the influence of the many microorganisms that release EPS to attach and form biofilms on the surface of the mask. The presence of Enterococcus faecalis bacteria increases the EPS secretion released so that the process of attaching bacteria to the surface of the mask is also getting stronger. In reactors B and D, the biofilm that adheres firmly to the surface of the mask speeds up the hetero aggregation process, which causes the mask fibers to get trapped in the biofilm stack, possibly, so they do not spread entirely in the media. Furthermore, the additive compounds released from the mask will be adsorbed in the cells and affect their growth, especially in Chlorella sp.
Fig. 2.
Microplastics identification by microscopy. A Fresh water treated by control mask, B fresh water treated by a bacterial mask, C Chlorella sp. treated by control mask, and D Chlorella sp. treated by a bacterial mask
Table 2.
Average sizes of microplastics from different reactors
| Reactor | Particles/mL | Particle size )* |
|---|---|---|
| A | 46.7 | 3.2892.517 |
| B | 30.0 | 4.5391.528 |
| C | 70.0 | 4.6503.000 |
| D | 66.7 | 4.5511.155 |
A) Fresh water treated by control mask, (B) Fresh water treated by a bacterial mask, (C) Chlorella sp. treated by control mask, (D) Chlorella sp. treated by a bacterial mask
* Microplastics sizes were measured by microscopy
The impact of a disposable mask on the quality of the biomass
Evaluation of Chlorella growth rate
The results of measuring the growth rate of Chlorella sp. control and Chlorella sp. treatment based on the formula in Eqs. (1)–(5) can be seen in Fig. 3. The results showed that, by adding Enterococcus faecalis bacteria to the mask, the reaction rate of Chlorella sp. in reactor D increased significantly compared to reactor C and Chlorella sp. control. This shows an increase in bacterial growth. However, the increase in OD could also be due to the development of Chlorella sp. Based on COD, BOD, DO, and pH data, reactor D has the most contaminated media; this shows that the degradation process occurs faster in reactor D with Enterococcus faecalis than in reactor C. In reactor D, some cells died due to their inability to tolerate toxic substances in their cells; others could be discharged back into the medium and formed sediment (Leong and Chang 2020). In addition to deposits of hazardous substances and leftover nutrients, the OD value could be also affected by deposits from the dead cells. As seen in Fig. 1, reactor D had a cloudier appearance, owing to the presence of numerous deposits. These deposits could increase the OD value in reactor D, even though it could have less biomass than in reactor C, resulting in worse Chlorella sp. development in reactor D. In addition, the mask damage in reactor D, which was greater than in reactor C (owing to its microbial activity), had a harmful impact on the proliferation of Chlorella sp. Song et al. (2020) described the presence of microplastic particles in the microalgae culture of Chlorella sp. capable of inhibiting its growth by increasing reactive oxygen species (ROS), such as oxygen and hydrogen peroxide, and triggerring stress and damage in algal cells. In the work by Hadiyanto et al. (2021), the microalgae Spirulina sp. reacted with PP-type microplastic, which is also the primary component of disposable masks. Their findings revealed that microplastics produced cell damage, which slowed the development of microalgae relative to untreated microalgae.
Fig. 3.
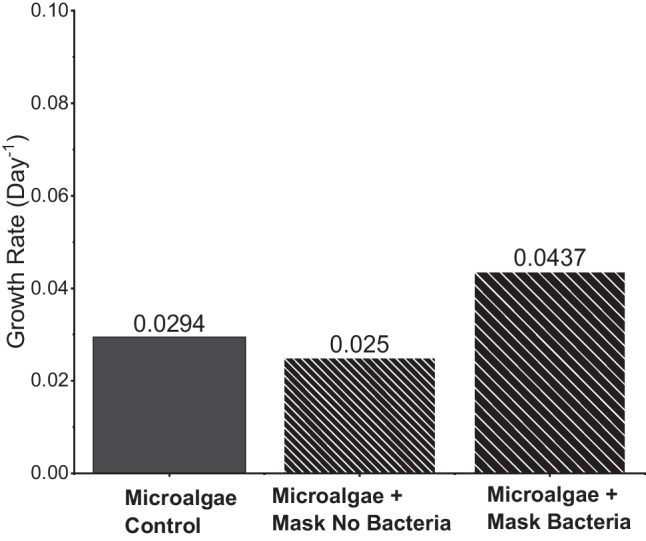
Growth rate of Chlorella sp. measured by spectrophotometry for 100-day treatment
Gram-positive Enterococcus faecalis bacteria produce lactic acid, which has probiotic properties. As a preventative step against the proliferation of inhibitory bacteria, the inclusion of probiotic bacteria to microalgae medium is helpful (Le Chevantonet al., 2013). Liu et al. (2009) found that the treatment of microalgae with bacteria had a substantial effect on the growth rate and cell density of microalgae, with 7 out of 7 bacteria able to boost algal development directly or indirectly. In the present investigation, the application of Enterococcus faecalis to a disposable mask led to increased Chlorella growth. As seen in Fig. 3, Chlorella growth rate was greatly enhanced with the presence of Enterococcus faecalis in comparison to the control or medium contaminated with masks without Enterococcus faecalis bacteria.
EPS determination
Microalgae cells produce a heterogeneous matrix that is expelled from the cells, particularly under stress caused by both external and internal conditions. The EPS (extracellular polymeric substances) term refers to the compounds that are discharged by microalgae into adjacent environments. EPS is composed of a complex blend of biopolymers of high molecular weights (HMW). In turn, EPS has a substantial effect on the physicochemical characteristics of algal aggregates, including surface charge, viscosity, flocculation, structure, and bond deposition qualities. EPS is a multifunctional natural biopolymer that may serve as an anticoagulant to protect cells from dehydration and harmful chemicals, as well as a stress-responsive energy and carbon sink. In addition to being able to shield the microalgae, EPS may also form a sheath inside the biofilm matrix that serves as a food source for decomposition bacteria (Xiao and Zheng 2016).
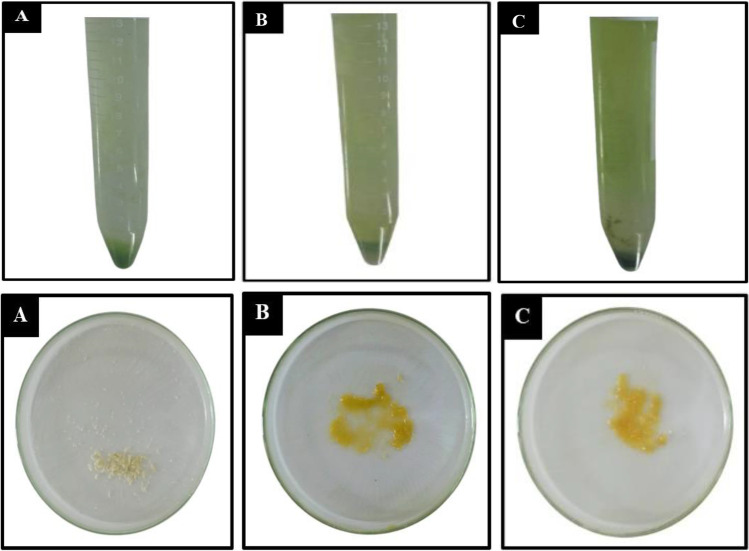
In this study, examination of EPS deposition demonstrated the contribution of Chlorella sp. to the biodegradation process, which is highly valuable for exploring the potential of Chlorella sp. in producing high-quality EPS and for establishing a mutually beneficial association between Chlorella sp. microalgae and Enterococcus faecalis bacteria. In addition to enhancing Chlorella sp. development, the presence of Enterococcus faecalis bacteria affects various elements of microalgae metabolism, including cell size, color, lipid content (De-Bashan et al. 2002), toxin generation, extracellular secretion, and cell aggregation (Gardes et al. 2011). As shown in Fig. 4 in the top panel that contains Chlorella sp., there was a change in color and thickness in the EPS tube after being given the mask treatment (Fig. 4B and 4C) (bacterial mask). Then, after the EPS was separated from the Chlorella sp media (Fig. 4 in bottom panel), as shown in Fig. 4A, EPS is still white in color; after treatment the EPS color mask looks yellow and thick (Fig. 4B and 4C). EPS weight was weighed and presented in Table 3; the observations of three Chlorella sp. treatments revealed variations in EPS results. EPS production in Fig. 4C is higher than in Fig. 4B, proving that the presence of Enterococcus faecalis could accelerate the biodegradation process, where both Chlorella sp. and Enterococcus faecalis would release EPS to form biofilms on the surface of disposable masks.
Fig. 4.
Chlorella sp. EPS apprearance and yield before (top panels) and after (bottom panels) treatment. A Chlorella sp. control, B Chlorella sp. treated by control mask, and C Chlorella sp. treated by a bacterial mask
Table 3.
Total EPS yields of Chlorella sp. before and after treatment
| Chlorella | EPS yield (mg/mL−1) |
|---|---|
| Control | 0.097 ± 0.112 |
| Treated by mask control | 0.324 ± 0.450 |
| Treated by a bacterial mask | 0.543 ± 0.550 |
FTIR analysis of organic structure changes in Chlorella biomass
FTIR analysis is an ideal method for monitoring changes in the Chlorella sp. biomass composition and in microplastics release from disposable masks. The strength of the peaks at certain wavelengths indicates the proportion of carbohydrates, lipids, and proteins in the Chlorella sp. biomass (Cheah and Chan 2022). Changes in the concentration of key chemicals in Chlorella sp. biomass offer evidence of Chlorella sp. cell destruction, indicating the influence of microplastics on aquatic environments. Bacterial contamination of microalgae medium may inhibit microalgae development and influence algal cell density, lipid content, and pigmentation (De-Bashan et al. 2002).
Based on the FTIR results in Fig. 5, the curve of the Chlorella sp. control biomass sample then undergoes significant changes in organic functional groups such as protein, polysaccharide, and lipid content after treatment with masks and bacteria. At a wavelength of 3843 cm−1, there was a decrease in the intensity of the hydroxyl groups (O–H) of the polysaccharide after the mask treatment (curve B), and it disappeared after the addition of bacteria to the mask (curve C). Another phenomenon is a significant increase in intensity in the alcoholic group (C–O) in carbohydrates at a wavelength of 1055 cm−1 and 1069 cm−1. These results made it possible to release exopolysaccharide substances in more significant quantities in the cell wall of Chlorella sp. as a response to cell protection due to the presence of a mask in the medium, especially in curve C due to the addition of bacteria. Furthermore, it highlights the loss of the secondary amide group (C–N) peak from the protein at a wavelength of 1530 cm−1 and the peak at 2368 cm−1 as the amine group (N–H) of the protein. However, a new peak indicates a carbonyl group (C = O) formation from a peptide bond at a wavelength of 1384 cm−1 and a primary amide group (C–N) from a protein at a wavelength of 1651 cm−1, and then follows the peak of the alkyl group (C–H, CH2) at a wavelength of 2927, which correlates with the presence of lipid formation after the mask and bacteria treatment as well as forming new peaks on curves B and C after mask treatment, which corresponds to methyl alcohol (CH2OH) and ketones (C = O) at a wavelength of 1270 cm−1.
The formation of amine, amide, and carbonyl peaks from the protein was due to the buildup of ammonium in the Chlorella sp. media. Due to inhibition of the process of photosynthesis (Díez-Montero et al. 2020), ammonium that accumulates, especially in Chlorella sp., given the bacterial mask treatment (curve C), will be utilized by Chlorella sp. as a nitrogen source to produce a higher protein peak intensity than the control mask treatment (curve B). The majority of peaks that emerged in the control reactors also appeared in Chlorella sp. treated with masks containing or lacking Enterococcus faecalis. Significantly, the mask containing Enterococcus faecalis negatively impacted the quality of the Chlorella sp. biomass.
SEM investigation of Chlorella biomass
SEM analysis is one technique that offers a morphological image of damaged Chlorella sp. cells. Morphological study of Chlorella sp. by SEM revealed distinct morphologies for each treatment (Fig. 6). In Fig. 6A, the round cell shape in the Chlorella sp. control biomass still looks good. The structure starts to break down after Chlorella sp gets threatened by the mask (Fig. 6B). It can be seen that the surface of the cell wall of Chlorella sp. formed several holes or cavities, indicating that there were some damaged cells. Cell damage increased after adding Entercoccus faecalis bacteria to the mask, where Entercoccus faecalis was a reducing microorganism that accelerated damage to the masks. In Fig. 6C, the surface of the cell wall of Chlorella sp. is like flaky and uneven. The round cell wall structure is broken, and many holes are formed due to the attack of the masks. In aquatic environments with limited sources of nutrition, bacteria will compete with microalgae and create harmful compounds that diminish microalgae yield, especially in Fig. 6C (Zhang et al. 2012). PP-containing particles from a disposable mask could adsorb and breach the cell wall of Chlorella sp., harming the organism. Damage to the Chlorella sp. cell wall could also be caused by the generation of ROS as a consequence of its contact with PP additives, which are produced as PP degrades in water. In addition, damage seemed to have increased in Chlorella sp. treated with a mask devoid of bacteria.
Fig. 6.
SEM analysis of Chlorella sp. biomass. A Chlorella sp. control, B Chlorella sp. treated with control mask, and C Chlorella sp. treated with a bacterial mask
Proximal analysis
To clarify the effect of contamination of disposable masks on the quality of Chlorella sp. biomass, the protein, fat, moisture, ash, and carbohydrate content in the biomass should be determined. Enterococcus faecalis, in aerobic circumstances, will convert glucose (carbohydrates) into acetic acid, acetate, and CO2 (Andrade 2018), which microalgae may use as a source of nutrition. Possible interactions between bacteria and algae include competition and mutualism. The interaction pattern of bacteria and microalgae is greatly impacted by environmental circumstances according to Zhang et al. (2012). The quality of Chlorella sp. biomass is affected by the symbiotic connection between microalgae and Enterococcus faecalis, which is determined by environmental conditions and nutrition availability. The findings of the proximal analysis (Table 4) differed with those of the FTIR analysis. According to proximal analysis, the content of lipids in Chlorella sp. treated with masks and Enterococcus faecalis bacteria was reduced when compared to the control and mask treatments without Enterococcus faecalis bacteria (Table 4).
Table 4.
Proximal analysis results of Chlorella sp. after 100 days
| Chlorella | Lipid (%) | Protein (%) |
|---|---|---|
| Control | 56.17 | 43.25 |
| Treated by mask control | 56.12 | 42.99 |
| Treated by a bacterial mask | 56.04 | 38.20 |
Conclusion
The breakdown of disposable masks in Chlorella media had several effects on both the water quality, in which Chlorella sp. thrives, and the development of the algae itself. The capacity of Chlorella sp. to form EPS influenced the effectiveness of biodegradation and the microalgae’s resistance to being poisoned by additives released from disposable masks during biodegradation. The generation of EPS by Chlorella sp. is impacted by environmental factors, particularly the presence of elements that induce stress, which boost its capacity to release EPS. The presence of Enterococcus faecalis bacteria on disposable masks contributed significantly to these phenomena. Furthermore, this research was able to prove that Enterococcus faecalis bacteria could boost Chlorella sp.’s EPS production. On the other hand, EPS is also able to protect the cell walls of microalgae, as evidenced by the fact that the FTIR analysis revealed intensity increase of polysaccharides, proteins, and lipids in Chlorella sp. biomass. which is treated with a mask, where natural microorganisms are able to maintain good growth and quality of biomass in Chlorella sp reactor that does not contain Enterococcus faecalis. In order for EPS polymers to play a larger role in the aquatic environment, including the removal of microplastic pollutants, further study is required to explore their potential to interact with microplastics in the formation of aggregations and flocs. Further studies are also required to evaluate the detailed interaction between EPS and Enterococcus faecalis and their effect on algae cell metabolism and nutrition profiles.
Author contribution
AK and HH: investigation, writing of the original draft, review, and editing; EH: collection of data; ID, FAJ, and WZP: investigation and collection of data.
Funding
This work was supported by Directorate of Higher Education, Ministry of Education, Culture, Research and Technology, Republic of Indonesia through Penelitian Kompetensi Dasar Nasional (PDKN) under a Contract Number: 345-13/UN7.6.1/PP/2022 of research project: Pemanfaatan biopolimer ExoPolySacharides(EPS) dari mikroalga untuk bioremediasi mikroplastik dalam sistem perairan.
Data availability
All data supporting the findings of this study are available within the article.
Declarations
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Abdelbary S, Elgamal MS, Farrag A (2019) Trends in heavy metals tolerance and uptake by Pseudomonas aeruginosa . Pseudomonas Aeruginosa - An Armory Within. Intechopen, ISBN: 78-1-83962-214-41–12. 10.5772/intechopen.85875
- Aksu Z, Kutsal T. A comparative study for biosorption characteristics of heavy metal ions with C. vulgaris. Environ Technol (United Kingdom) 1990;11(10):979–987. doi: 10.1080/09593339009384950. [DOI] [Google Scholar]
- Aktan Y, Tan S, Icgen B. Characterization of lead-resistant river isolate Enterococcus faecalis and assessment of its multiple metal and antibiotic resistance. Environ Monit Assess. 2013;185(6):5285–5293. doi: 10.1007/s10661-012-2945-x. [DOI] [PubMed] [Google Scholar]
- Andrade LM. Chlorella and spirulina microalgae as sources of functional foods, nutraceuticals, and food supplements: an overview. MOJ Food Process Technol. 2018;6(1):45–58. doi: 10.15406/mojfpt.2018.06.00144. [DOI] [Google Scholar]
- Aragaw TA. Surgical face masks as a potential source for microplastic pollution in the COVID-19 scenario. Mar Pollut Bull. 2020;159(October):111517. doi: 10.1016/j.marpolbul.2020.111517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aruoja V, Pokhrel S, Sihtmäe M, Mortimer M, Mädler L, Kahru A. Toxicity of 12 metal-based nanoparticles to algae, bacteria and protozoa. Environ Sci Nano. 2015;2(6):630–644. doi: 10.1039/c5en00057b. [DOI] [Google Scholar]
- Asriza RO, Pitulima J. Photodegradation of high density polyethylene containing oxo-biodegradation additives Fotodegradasi High Density Polyethylene Yang Mengandung Aditif Okso-Biodegradasi. J Chem Res. 2017;4(2):402–405. [Google Scholar]
- Barycka K, Szarpak L, Filipiak KJ, Jaguszewski M, Smereka J, Ladny JR, Turan O (2020) Comparative effectiveness of N95 respirators and surgical/face masks in preventing airborne infections in the era of SARS-CoV2 pandemic: A meta-analysis of randomized trials. PLoS ONE 15(12): e0242901. 10.1371/journal.pone.0242901 [DOI] [PMC free article] [PubMed]
- Balzano S, Sardo A, Blasio M, Chahine TB, Dell’Anno F, Sansone C, Brunet C. Microalgal metallothioneins and phytochelatins and their potential use in bioremediumtion. Front Microbiol. 2020;11(April):1–16. doi: 10.3389/fmicb.2020.00517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benhalima L, Amri S, Bensouilah M, Ouzrout R. Heavy metal resistance and metallothionein induction in bacteria isolated from seybouse river, Algeria. Appl Ecol Environ Res. 2020;18(1):1721–1737. doi: 10.15666/aeer/1801_17211737. [DOI] [Google Scholar]
- Benson NU, Bassey DE, Palanisami T. COVID pollution: impact of COVID-19 pandemic on global plastic waste footprint. Heliyon. 2021;7(2):e06343. doi: 10.1016/j.heliyon.2021.e06343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buwono NR, Risjani Y, Soegianto A. Distribution of microplastic in relation to water quality parameters in the Brantas River, East Java, Indonesia. Environ Technol Innov. 2021;24:101915. doi: 10.1016/j.eti.2021.101915. [DOI] [Google Scholar]
- Campanale C, Massarelli C, Savino I, Locaputo V, Uricchio VF (2020) A detailed review study on potential effects of microplastics and additives of concern on human health. Int J Environ Res Public Health 17(4):1212 10.3390/ijerph17041212 [DOI] [PMC free article] [PubMed]
- Chatterjee S, Kumari S, Rath S, Priyadarshanee M, Das S. Diversity, structure and regulation of microbial metallothionein: metal resistance and possible applications in sequestration of toxic metals. Metallomics. 2020;12(11):1637–1655. doi: 10.1039/d0mt00140f. [DOI] [PubMed] [Google Scholar]
- Chellamani KP, Veerasubramanian D, Vignesh Balaji RS. Surgical face masks: manufacturing methods and classification. J Acad Indus Res. 2013;2(6):320. [Google Scholar]
- Cheah YT, Chan DJC. (2022) A methodological review on the characterization of microalgal biofilm and its extracellular polymeric substances. J Appl Microbiol 132(5):3490–3514. 10.1111/jam.15455 [DOI] [PubMed]
- Christita M, Iwanuddin I, Kafiar Y, Supratman T, Mokodompit HS. Identifikasi Bakteri Pada Air Dari Lahan Bekas Tambang Nikel Di Halmahera Timur. Jurnal Wasian. 2018;5(1):35–42. doi: 10.20886/jwas.v5i1.4265. [DOI] [Google Scholar]
- de-Bashan LE, Bashan Y, Moreno M, Lebsky VK, Bustillos JJ (2002) Increased pigment and lipid content, lipid variety, and cell and population size of the microalgae Chlorella spp. when co-immobilized in alginate beads with the microalgae-growth-promoting bacterium Azospirillum brasilense. Can J Microbiol 48:514–521. 10.1139/w02-051 [DOI] [PubMed]
- Dale JL, Nilson JL, Barnes AMT, Dunny GM. Restructuring of Enterococcus faecalis biofilm architecture in response to antibiotic-induced stress. NPJ Biofilms Microbiomes. 2017;3(1):1–9. doi: 10.1038/s41522-017-0023-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis PA, Dent M, Parker J, Reynolds CS, Walsby AE. The annual cycle of growth rate and biomass change in Planktothrix spp in Blelham Tarn, English Lake District. Freshw Biol. 2003;48(5):852–867. doi: 10.1046/j.1365-2427.2003.01055.x. [DOI] [Google Scholar]
- Dewata I, Zainul R. Determination of pH-BOD-COD and degradation in Batang Arau Watersheds at Padang City. Chem Pharm REs. 2015;7(12):445–451. [Google Scholar]
- Díez-Montero R, Belohlav V, Ortiz A, Uggetti E, García-Galán MJ, García J. Evaluation of daily and seasonal variations in a semi-closed photobioreactor for microalgae-based bioremediumtion of agricultural runoff at full-scale. Algal Res. 2020;47(October 2019):101859. doi: 10.1016/j.algal.2020.101859. [DOI] [Google Scholar]
- El-Meihy RM, Abou-Aly HE, Youssef AM, Tewfike TA, El-Alkshar EA. Efficiency of heavy metals-tolerant plant growth promoting bacteria for alleviating heavy metals toxicity on sorghum. Environ Exp Bot. 2019;162(March):295–301. doi: 10.1016/j.envexpbot.2019.03.005. [DOI] [Google Scholar]
- Eslami H, Shariatifar A, Rafiee E, Shiranian M, Salehi F, Hosseini SS, Eslami G, Ghanbari R, Ebrahimi AA. Decolorization and biodegradation of reactive Red 198 Azo dye by a new Enterococcus faecalis–Klebsiella variicola bacterial consortium isolated from textile wastewater sludge. World J Microbiol Biotechnol. 2019;35(3):1–10. doi: 10.1007/s11274-019-2608-y. [DOI] [PubMed] [Google Scholar]
- Fahruddin F, Haedar N, Abdullah A, Wahab A, Rifaat R. Deteksi Unsur Logam Dengan Xrf Dan Analisis Mikroba Pada Limbah Air Asam Tambang Dari Pertambangan Di Lamuru - Kabupaten Bone. Jurnal Geocelebes. 2020;4(1):7. doi: 10.20956/geocelebes.v4i1.7831. [DOI] [Google Scholar]
- Fred-Ahmadu OH, Ayejuyo OO, Benson NU. Microplastics distribution and characterization in epipsammic sediments of tropical Atlantic Ocean, Nigeria. Reg Stud Mar Sci. 2020;38:101365. doi: 10.1016/j.rsma.2020.101365. [DOI] [Google Scholar]
- Fu D, Zhang Q, Fan Z, Qi H, Wang Z, Peng L. Aged microplastics polyvinyl chloride interact with copper and cause oxidative stress towards microalgae Chlorella vulgaris. Aquat Toxicol. 2019;216(September):105319. doi: 10.1016/j.aquatox.2019.105319. [DOI] [PubMed] [Google Scholar]
- Fogg, G.E. and Thake, B. (1987) Algae Cultures and Phytoplankton Ecology. 3rd Edition, The University of Winsconsins Press Ltd., London.
- García JL, de Vicente M, Galán B. Microalgae, old sustainable food and fashion nutraceuticals. Microb Biotechnol. 2017;10(5):1017–1024. doi: 10.1111/1751-7915.12800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gayatri KV, Soundhari C and Pavithra BP. (2019).Biofilm inhibitory effect of chlorella extracts on Pseudomonas aeruginosa. Int J Pharm Sci Res 10(4):1966–71. 10.13040/IJPSR.0975-8232.10(4).1966-71
- Gärdes A, Iversen MH, Grossart HP, Passow U, Ullrich MS (2011) Diatom-associated bacteria are required for aggregation of Thalassiosira weissflogii. ISME J. 5(3):436–45. 10.1038/ismej.2010.145 [DOI] [PMC free article] [PubMed]
- Gutiérrez JC, de Francisco P, Amaro F, Díaz S, Martín-González A (2018) Structural and functional diversity of microbial metallothionein genes. Microbial Diversity in the Genomic Era, January, 387–407. 10.1016/B978-0-12-814849-5.00022-8
- Hazeem LJ, Yesilay G, Bououdina M, Perna S, Cetin D, Suludere Z, Barras A, Boukherroub R. Investigation of the toxic effects of different polystyrene micro-and nanoplastics on microalgae Chlorella vulgaris by analysis of cell viability, pigment content, oxidative stress and ultrastructural changes. Mar Pollut Bull. 2020;156(May):111278. doi: 10.1016/j.marpolbul.2020.111278. [DOI] [PubMed] [Google Scholar]
- Habjanič J, Mathew A, Eberl L, Freisinger E. Deciphering the enigmatic function of Pseudomonas metallothioneins. Front Microbiol. 2020;11(July):1–10. doi: 10.3389/fmicb.2020.01709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagemann, H., 2020. Coronavirus Fears Prompt Suspensions of Bans on Single-Use Plastic Bags. https://www.npr.org/sections/coronavirus-live-updates/2020/04/13/832. Accessed 10 Sept 2022
- Hendrawan AKF, Afiati N, Rahman A (2021) Laju Nitrifikasi pada Bioremediumsi Air Limbah Organik Menggunakan Chlorella sp. dan Bakteri Nitrifikasi-Denitrifikasi. Jurnal Pengelolaan Sumberdaya Alam Dan Lingkungan. J Nat Resour Environ Management 11(2):309–323. 10.29244/jpsl.11.2.309-323
- Henneberry B. 2020. How Surgical Masks are Made, Tested and Used. https://www.thomasnet.com/articles/other/how-surgical-masks-are-made/Whataresurgical. Accessed June 15 2021.
- Hadiyanto H, Khoironi A, Dianratri I, Suherman S, Muhammad F, Vaidyanathan S. Interactions between polyethylene and polypropylene microplastics and Spirulina sp. microalgae in aquatic systems. Heliyon. 2021;7(8):e07676. doi: 10.1016/j.heliyon.2021.e07676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hossen MZ, Hussain ME, Hakim A, Islam K, Uddin MN, Azad AK. Biodegradation of reactive textile dye Novacron Super Black G by free cells of newly isolated Alcaligenes faecalis AZ26 and Bacillus spp obtained from textile effluents. Heliyon. 2019;5(7):e02068. doi: 10.1016/j.heliyon.2019.e02068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irawati W, Tahya CY (2021) Copper removal by Enterobacter cloacae strain IrSuk1, Enterobacter cloacae strain IrSuk4a, and Serratia nematodiphila strain IrSuk13 isolated from Sukolilo River-Indonesia. IOP Conf Ser: Mater Sci Eng 1053(1) 012038. 10.1088/1757-899x/1053/1/012038
- Igiri BE, Okoduwa SIR, Idoko GO, Akabuogu EP, Adeyi AO, Ejiogu IK (2018) Toxicity and Bioremediation of Heavy Metals Contaminated Ecosystem from Tannery Wastewater: A Review. J Toxicol 2018:2568038. 10.1155/2018/2568038 [DOI] [PMC free article] [PubMed]
- Jayawardena UA, Angunawela P, Wickramasinghe DD, Ratnasooriya WD, Udagama PV. Heavy metal–induced toxicity in the Indian green frog: biochemical and histopathological alterations. Environ Toxicol Chem. 2017;36(10):2855–2867. doi: 10.1002/etc.3848. [DOI] [PubMed] [Google Scholar]
- Kazbar A, Cogne G, Urbain B, Marec H, Le-Gouic B, Tallec J, Takache H, Ismail A, Pruvost J (2019) Effect of dissolved oxygen concentration on microalgal culture in photobioreactors, Algal Research,39: 101432. 10.1016/j.algal.2019.101432
- Klemeš JJ, Fan YV, Tan RR, Jiang P (2020) Minimising the present and future plastic waste, energy and environmental footprints related to COVID-19. Renew Sustain Energy Rev 127:109883. 10.1016/j.rser.2020.109883 [DOI] [PMC free article] [PubMed]
- Koelmans AA, Besseling E, Wegner A, Foekema EM. Plastic as a carrier of POPs to aquatic organisms: a model analysis. Environ Sci Technol. 2013;47(14):7812–7820. doi: 10.1021/es401169n. [DOI] [PubMed] [Google Scholar]
- Kumar P, Masago Y, Mishra BK, Jalilov S, Emam AR, Kefi M, Fukushi K (2017a) Current assessment and future outlook for water resources considering climate change and a population burst: a case study of Ciliwung River, Jakarta City, Indonesia. Water (Switzerland) 9(6):410. 10.3390/w9060410
- KurniawanAunurohim JI. Biosorpsi Logam Zn2+ dan Pb2+ oleh Mikroalga Chlorella sp. Jurnal Sains Dan Seni ITS. 2014;3(1):2337–3520. [Google Scholar]
- Kumar V, Kumar M, Sharma S, Prasad R (2017b) Probiotics in agroecosystem. Springer Nature Singapore Pte Ltd, p.1–537. 10.1007/978-981-10-4059-7
- Le Chevanton M, Garnier M, Bougaran G, Schreiber N, Lukomska E, Bérard, JB, Fouilland E, Bernard O, Cadoret J.-P (2013) Screening and selection of growth-promoting bacteria for Dunaliella cultures. Algal Res 2:212–222. 10.1016/j.algal.2013.05.003
- Leong YK, Chang JS. Bioremediumtion of heavy metals using microalgae: recent advances and mechanisms. Bioresour Technol. 2020;303(December 2019):122886. doi: 10.1016/j.biortech.2020.122886. [DOI] [PubMed] [Google Scholar]
- Luo P, Kang S, Zhou M, Lyu J, Aisyah S, Binaya M, Regmi RK, Nover D. Water quality trend assessment in Jakarta: a rapidly growing Asian megacity. PLoS ONE. 2019;14(7):1–17. doi: 10.1371/journal.pone.0219009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas N, Bienaime C, Belloy C, Queneudec M, Silvestre F, Nava-Saucedo JE (2008) Polymer biodegradation: mechanisms and estimation techniques. Chemosphere 73(4):429–42. 10.1016/j.chemosphere.2008.06.064 [DOI] [PubMed]
- Liu Y, Zhang W, Sileika T, Warta R, Cianciotto NP, Packman A. (2009) Role of bacterial adhesion in the microbial ecology of biofilms in cooling tower systems. Biofouling 25(3):241–53. 10.1080/08927010802713414 [DOI] [PMC free article] [PubMed]
- Ma Y, Lin X, Wu A, Huang Q, Li X, Yan J. Suggested guidelines for emergency treatment of medical waste during COVID-19: Chinese experience. Waste Dispos Sustain Energy. 2020;2(2):81–84. doi: 10.1007/s42768-020-00039-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martzoff A. (2013) Analyse systémique du métabolisme carboné et énergétique de Chlamydomonas reinhardtii (Thesis) University of Nantes
- Michalak I, Chojnacka K, Witek-Krowiak A. State of the art for the biosorption process - a review. Appl Biochem Biotechnol. 2013;170(6):1389–1416. doi: 10.1007/s12010-013-0269-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molino A, Iovine A, Casella P, Mehariya S, Chianese S, Cerbone A, Rimauro J, Musmarra D (2020) Microalgae characterization for consolidated and new application in human food, animal feed and nutraceuticals. Int J Environ Res Public Health 15(11):1–21. 10.3390/ijerph15112436 [DOI] [PMC free article] [PubMed]
- O’Dowd K, Nair KM, Forouzandeh P, Mathew S, Grant J, Moran R, Bartlett J, Bird J, Pillai SC (2020) Face masks and respirators in the fight against the COVID-19 pandemic: a review of current materials, advances and future perspectives. Materials 13(15):3363. 10.3390/ma13153363 [DOI] [PMC free article] [PubMed]
- Pankratova G, Leech D, Gorton L, Hederstedt L. Extracellular electron transfer by the gram-positive bacterium Enterococcus faecalis. Biochemistry. 2018;57(30):4597–4603. doi: 10.1021/acs.biochem.8b00600. [DOI] [PubMed] [Google Scholar]
- Portela CAF, Smart KF, Tumanov S, Cook GM, Villas-Bôas SG. Global metabolic response of Enterococcus faecalis to oxygen. J Bacteriol. 2014;196(11):2012–2022. doi: 10.1128/JB.01354-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raso S, van Genugten B, Vermuë M, Wijffels RH. Effect of oxygen concentration on the growth of Nannochloropsis sp. at low light intensity. J Appl Phycology. 2012;24(4):863–871. doi: 10.1007/s10811-011-9706-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohde M. The gram-positive bacterial cell wall. Microbiol Spectrum. 2019;7(3):1–21. doi: 10.1128/microbiolspec.gpp3-0044-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohde E, Pachler K, Gimona M (2019) Manufacturing and characterization of extracellular vesicles from umbilical cord–derived mesenchymal stromal cells for clinical testing. Cytotherapy 21(6):581–592. 10.1016/j.jcyt.2018.12.006 [DOI] [PubMed]
- Sangkham S. Face mask and medical waste disposal during the novel COVID-19 pandemic in Asia. Case Stud Chem Environ Eng. 2020;2(September):100052. doi: 10.1016/j.cscee.2020.100052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadiq IM, Dalai S, Chandrasekaran N, Mukherjee A. Ecotoxicity study of titania (TiO2) NPs on two microalgae species: Scenedesmus sp and Chlorella sp. Ecotoxicol Environ Saf. 2011;74(5):1180–1187. doi: 10.1016/j.ecoenv.2011.03.006. [DOI] [PubMed] [Google Scholar]
- Simatupang JB, Pratomo A, & Reja’i TS (2015) Struktur Komunitas Makroalga pada Daerah Litoral di Parairan Teluk Bakau Kecamatan Gunung Kijang Kabupaten Bintan. [Thesis].
- Sibi G (2014) Biosorption of arsenic by living and dried biomass of fresh water microalgae - potentials and equilibrium studies. J Bioremed Biodeg 05(06):249. 10.4172/2155-6199.1000249
- Safiuddin M, Salam MA (2020) Efficiency of surgical masks as a means of source control of SARS-CoV-2 and protection against COVID-19. 7: 179–189. 10.15739/irjpeh.20.025
- Schmitt J and Flemming H (1998) FTIR –Spectros-copy in Microbial and Material Analysis. Int Biodeterior Biodegrad 41:1–11. 10.1016/S0964-8305(98)80002-4
- Song C, Liu Z, Wang C, Li S, Kitamura Y. Different interaction performance between microplastics and microalgae: the bio-elimination potential of Chlorella sp. L38 and Phaeodactylum tricornutum MASCC-0025. Sci Total Environ. 2020;23:138146. doi: 10.1016/j.scitotenv.2020.138146. [DOI] [PubMed] [Google Scholar]
- Sugiharto Chlorella vulgaris dan Spirulina platensis : Kandungan Nutrisi dan Senyawa Bioaktifnya untuk Meningkatkan Produktivitas Unggas. Wartazoa. 2020;30(3):123–138. doi: 10.14334/wartazoa.v30i3.2523. [DOI] [Google Scholar]
- Tunali M, Uzoefuna EN, Tunali MM, Yenigun O. Effect of microplastics and microplastic-metal combinations on growth and chlorophyll a concentration of Chlorella vulgaris. Sci Total Environ. 2020;743:140479. doi: 10.1016/j.scitotenv.2020.140479. [DOI] [PubMed] [Google Scholar]
- Vijayaraghavan K, Yun YS. Bacterial biosorbents and biosorption. Biotechnol Adv. 2008;26(3):266–291. doi: 10.1016/j.biotechadv.2008.02.002. [DOI] [PubMed] [Google Scholar]
- Vigani M, Parisi C, Rodríguez-Cerezo E, Barbosa MJ, Sijtsma L, Ploeg M, Enzing C (2015) Food and feed products from micro-algae: Market opportunities and challenges for the EU. Trends Food Sci Technol 42(1):81–92. 10.1016/j.tifs.2014.12.004.
- Wei L, Wu Q, Zhang J, Guo W, Chen M, Xue L, Wang J, Ma L. Prevalence and genetic diversity of Enterococcus faecalis isolates from mineral water and spring water in China. Front Microbiol. 2017;8(JUN):1–8. doi: 10.3389/fmicb.2017.01109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widodo T, Budiastuti MTS, Komariah K. Water quality and pollution index in Grenjeng River Boyolali Regency, Indonesia. Caraka Tani: J Sustain Agric. 2019;34(2):150. doi: 10.20961/carakatani.v34i2.29186. [DOI] [Google Scholar]
- Xiao R, Zheng Y (2016) Overview of microalgal extracellular polymeric substances (EPS) and their applications. Biotechnol Adv 34(7):1225–1244. 10.1016/j.biotechadv.2016.08.004 [DOI] [PubMed]
- Yulianto R, Prihanto RL, Redjeki S, Studi P, Kimia T, Studi P, Industri T, Teknik F, Anyar G (2020) Penurunan Kandungan Cod Dan Bod Limbah Cair. 01(01):9–15. 10.33005/chempro.v1i01.27.
- Zhang X, Amendola P, Hewson JC, Sommerfeld M, Hu Q (2012) Influence of growth phase on harvesting of Chlorella zofingiensis by dissolved air flotation. Bioresour Technol 116: 477–484. 10.1016/j.biortech.2012.04.002 [DOI] [PubMed]
- Zhu X, Zhao W, Chen X, Zhao T, Tan L, Wang J. Growth inhibition of the microalgae Skeletonema costatum under copper nanoparticles with microplastic exposure. Mar Environ Res. 2020;158(May):105005. doi: 10.1016/j.marenvres.2020.105005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data supporting the findings of this study are available within the article.