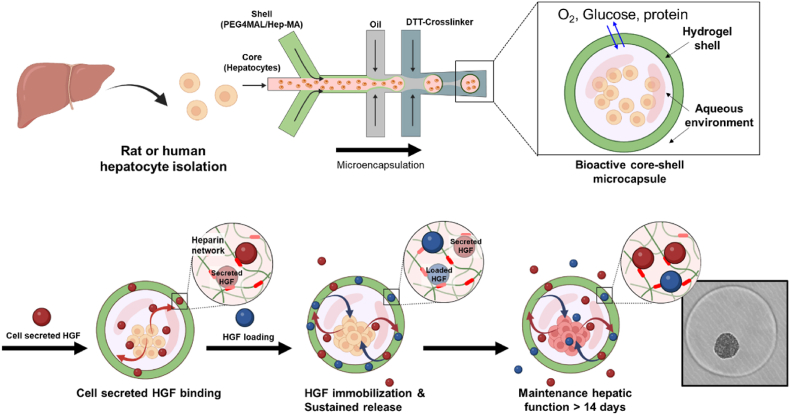
Abstract
The ability to maintain functional hepatocytes has important implications for bioartificial liver development, cell-based therapies, drug screening, and tissue engineering. Several approaches can be used to restore hepatocyte function in vitro, including coating a culture substrate with extracellular matrix (ECM), encapsulating cells within biomimetic gels (Collagen- or Matrigel-based), or co-cultivation with other cells. This paper describes the use of bioactive heparin-based core-shell microcapsules to form and cultivate hepatocyte spheroids. These microcapsules are comprised of an aqueous core that facilitates hepatocyte aggregation into spheroids and a heparin hydrogel shell that binds and releases growth factors. We demonstrate that bioactive microcapsules retain and release endogenous signals thus enhancing the function of encapsulated hepatocytes. We also demonstrate that hepatic function may be further enhanced by loading exogenous hepatocyte growth factor (HGF) into microcapsules and inhibiting transforming growth factor (TGF)-β1 signaling. Overall, bioactive microcapsules described here represent a promising new strategy for the encapsulation and maintenance of primary hepatocytes and will be beneficial for liver tissue engineering, regenerative medicine, and drug testing applications.
Keywords: Droplet microfluidics, Bioactive microcapsules, Hepatocyte spheroid, Heparin, Hepatocyte growth factor
Graphical abstract
Highlights
-
•
Heparin-containing bioactive microcapsules enable sustained release of inductive signal (growth factor) over the course of 2 weeks.
-
•
Bioactive microcapsules likely retain and release endogenous signals thus enhancing function of encapsulated hepatocytes
-
•
Loading exogenous hepatocyte growth factor (HGF) into microcapsules and inhibiting transforming growth factor (TGF)-β1 signaling further enhancing encapsulated hepatic function.
-
•
Loading inductive cues into heparin microcapsules can reduce the use of growth factor by several fold.
1. Introduction
Hepatocytes, the main epithelial cell type of the liver, are responsible for the majority of the liver's biochemical functions. The ability to maintain phenotype of primary hepatocytes is therefore beneficial for investigations of drug metabolism, toxicity, development of bioartificial livers, and tissue engineering fields [[1], [2], [3]]. Primary hepatocytes are known to lose viability and function when cultured in vitro. Several strategies are employed to preserve hepatocyte function in vitro, including the coating of culture substrates with extracellular matrix (ECM) proteins [4,5], entrapment within a biomimetic hydrogel (Collagen or Matrigel) [6,7], co-cultivation with other cell types [8,9], and formation of hepatocyte spheroids [[10], [11], [12]]. Among these approaches, formation of spheroids represents a relatively simple means of recapitulating 3D hepatic interactions and maintaining hepatic phenotype. Hepatocyte spheroids have been used in bio artificial liver assist devices [13], stirred suspension cultures [14], and transplantation [15]. A number of strategies for creating hepatocyte spheroids has been devised including hanging drop [10], micro-wells [11], as well as, spheroid cultures under stirring or rocking conditions [12]. Even with these reports, efficient and cost-effective culture protocols remain a challenge. Microencapsulation represents another approach for creating and cultivating spheroids using a number cell types including hepatocyte [16]. The benefits of microencapsulation are associated with the physical and biochemical properties of the capsule which may protect encapsulated cells against shear or mechanical damage in vitro, against immune response in vivo or may be used to deliver inductive/pro-survival signals.
Various methods have been developed to fabricate microcapsules, such as electrohydrodynamic co-jetting, micro-molding, photolithography, flow lithography microfluidics, soft lithography-based imprinting, and droplet microfluidics [[17], [18], [19]]. Droplet microfluidics offer the benefits of high throughput, structural complexity and narrow size distribution of microcapsules [20]. We have previously employed droplet microfluidics to fabricate core-shell microcapsule comprised of a poly(ethylene glycol) (PEG) hydrogel shell and an aqueous core. Both primary hepatocytes and embryonic stem cells formed spheroids and were cultured in microcapsules [21,22].
Hepatocyte cultures have been shown to benefit from cues presented on the culture substrate or produced by supporting cells in co-cultures [[23], [24], [25], [26]]. Hepatocyte growth factor (HGF) is a key signal for liver development and regeneration as well as for maintenance of hepatic phenotype [27,28]. However, HGF has a short half-life (<15 min) which may limit its bioavailability in vitro [29,30]. Heparin is a highly sulfated anionic polysaccharide and an ECM component with high affinity for a number of growth factors (GFs). Heparin may be functionalized and incorporated into hydrogels to enable sequestration and release of GFs [31]. Importantly, interactions with heparin have been shown to enhance the bioavailability of HGF [32]. Our laboratory has previously demonstrated long-term maintenance of hepatocytes sandwiched between heparin-containing gel layers or entrapped within heparin-based hydrogels [10,33]. We also previously demonstrated long-term release of HGF from macrogels containing hepatocytes [10]. Most recently, we incorporated heparin into microcapsules with a hydrogel shell and aqueous core for delivery of GFs to encapsulated stem cells [34].
In the present study, we wanted to assess the utility of bioactive heparin-containing microcapsules for cultivating primary hepatocytes. We fabricated heparin-based microcapsules using a droplet microfluidic device and characterized HGF loading and release. We demonstrated that hepatocytes organized into spheroids inside core-shell microcapsules and that signals such as HGF were secreted by the spheroids and retained inside the microcapsules. Retention of hepato-inductive signals inside microcapsules enhanced hepatic function of spheroids. We also identified optimal culture conditions where spheroids retained high levels of function over the course of two weeks.
While there have been a few previous reports, including from our group, on the subject of hepatocyte encapsulation [15,21,35], the present paper has multiple novel features. Ours is the first report that describes microcapsules capable of both organizing hepatocytes into spheroids and delivering GF molecules to induce and maintain hepatic phenotype. Furthermore, we demonstrate that bioactive microcapsules enhance hepatic phenotype in the absence of exogenous signals by amplifying endogenous/autocrine HGF signaling. These microcapsules may be used in the future for directing hepatic differentiation of stem cells and maintaining phenotype of primary or stem cell-derived hepatocytes for applications ranging from toxicology studies to to liver-directed cellular therapies.
2. Materials and methods
2.1. Materials
Sodium heparin (from porcine intestinal mucosa, 12 kDa) was purchased from Smithfield BioScience (Cincinnati, OH, USA). In the present study, we followed previously published protocols for synthesizing heparin−methacrylate (Hep-MA) [34] and heparin−thiol (Hep-SH) [31,36]. Four-arm polyethylene glycol(PEG)-maleimide (10 kDa, PEG4MAL) and four-arm PEG-thiol (10 kDa, PEG4SH) were purchased from Laysan Inc. (Arab, AL, USA). Dithiothreitol (DTT), 35 kDa PEG, triethanolamine (TEA), mineral oil, Span-80, PEG dimethacrylate (6 kDa, PEG-DMA), glucagon, hydrocortisone sodium succinate, and rabbit anti-phospho-Met Ab were purchased from Sigma-Aldrich (St. Louis, MO, USA). Epidermal growth factor (EGF), hepatocyte growth factor (HGF), and human HGF DuoSet ELISA were purchased from R&D Systems (Minneapolis, MN, USA). Aggrewell 400, optiprep densifier, HGF receptor c-met inhibitor (SU11274), and TGF-β1 inhibitor (A83-01) were purchased from STEMCELL Technologies (Vancouver, Canada). Metal enhanced 3,3′ Diaminobenzidine (DAB) substrate kit and Alexa Four 488 Phalloidin were obtained from Thermo Scientific Inc. (Rockford, IL, USA). Recombinant human insulin was obtained from Eli Lilly (Indianapolis, IN, USA). Phosphate-buffered saline (PBS) was purchased from Corning (Manassas, NY, USA). Dulbecco's modified Eagle's medium (DMEM), fetal bovine serum (FBS), penicillin/streptomycin were purchased from Gibco (Grand Island, NY, USA). Rat and human albumin ELISA kit, sheep anti-rat albumin Ab, and goat anti-human albumin Ab were purchased from Bethyl Laboratories (Montgomery, TX, USA). Mouse anti-Met Ab was purchased from Cell Signaling Technology (Danvers, MA, USA). Urea analysis kit and total bile acid assay kit were obtained from Bioassay Systems (Hayward, CA, USA), and Cell Biolabs Inc. (San Diego, CA, USA), respectively. The live/dead staining kit was obtained from Invitrogen (Waltham, MA, USA). P450-Glo CYP1A2 induction/inhibition assay system was purchased from Promega (Madison, WI, USA). Paraformaldehyde (PFA) was purchased from Election Microscopy Sciences (Hatfield, PA, USA). Alexa Fluor 546 (donkey anti-sheep, donkey anti-mouse, and donkey anti-goat) and Alexa Fluor 488 (donkey anti-rabbit) were purchased from Life Technologies (Carlsbad, CA, USA). DAPI was purchased from BD Bioscience (San Jose, CA, USA).
2.2. Generating core–shell microcapsule
We have previously described a polydimethylsiloxane (PDMS)-based droplet microfluidic system for stem cell encapsulation. Detailed protocols for fabricating the PDMS-based droplet microfluidic device used for cell encapsulation and for operating encapsulation system are provided elsewhere [34,37]. Briefly, the co-axial flow-focusing microfluidic device had channels of three heights: (1) 120 μm for the aqueous core channel, (2) 200 μm for aqueous shell channels, and (3) 300 μm for shielding oil, cross-linker oil, and serpentine channels. To generate microcapsule with liquid core and hydrogel shell, the following solutions were injected into the co-axial flow-focusing device: 1) 8% (w/v) PEG (35 kDa) and 17% (v/v) Optiprep densifier solution was injected into the core flow channel, 2) 4% (w/v) Hep-MA, 8% (w/v) PEG4MAL solution with 15 mM TEA was injected into the shell flow channel, 3) a mineral oil (with 0.5% span80) solution was injected into the continuous flow channel, and 4) a cross-linking emulsion (60 mM DTT dispersed in mineral oil with 3% Span-80) was injected into another flow channel. The flow rates in the core, shell, shielding oil, and crosslinker oil channels were 4, 4, 50, and 60 μL min−1, respectively. Devices and reagents were sterilized, and encapsulation was performed under sterile conditions. Microcapsules were collected, removed from the oil phase and placed into multi-well plates as described previously [37].
2.3. HGF binding to microcapsules
2.3.1. HGF loading and release studies
For HGF loading and release studies, 2000 Hep microcapsules were immersed in 2 mL of 20 or 100 ng mL−1 HGF solution for 1 h at 37 °C. Then, the capsules were collected and the residual solution was analyzed using a HGF ELISA kit. To establish the HGF release profile, 2000 microcapsules were placed into a 6-well plate containing 2 mL of 1x PBS. An aliquot of solution was collected at predetermined time points and analyzed for HGF. PEG microcapsules were prepared by substituting an equal amount of PEG-DMA for Hep-MA and were used as a bioinert alternative to bioactive Hep microcapsules [34].
2.3.2. Demonstrating HGF retention in core-shell microcapsule
To test HGF retention, Hep and PEG microcapsules were fabricated in the same manner and were soaked in 100 ng mL−1 HGF solution for 1 h at 37 °C as described in the previous section. The microcapsules were washed with PBS and stored in PBS at 37 °C for up to 3 days. The microcapsules were immunostained at 4 h and at 3-day time points using the following protocol. First, microcapsules were incubated with biotinylated anti-HGF antibody (diluted 1:2000) for 2 h at 25 °C and washed with PBS. Next, samples were incubated with avidin-(HRP) (diluted 1:200) for 1 h at 25 °C followed by washing with PBS. To visualize the presence of HGF, a DAB color reagent was diluted 1:10 in peroxide buffer and incubated with microcapsules for 10 min at 25 °C. The dark brown color due to HGF immunostaining was observed by bright-field microscopy [38].
2.4. Encapsulating hepatocytes, forming and culturing spheroids
All animal experiments were performed under the National Institutes of Health (NIH) guidelines for ethical care and use of laboratory animals with the approval of the Institutional Animal Care and Use Committee (IACUC) of the Mayo Clinic, Rochester, MN. Primary hepatocytes were isolated from adult female Lewis rats (Charles River Laboratories, Boston, MA, USA) weighing 125–200 g, using a two-step collagenase perfusion procedure as described previously [9,11]. Chimeric mice with humanized livers (cDNA- uPA+/−/SCID (uPA+/wt: B6; 129SvEv- Plau, SCID: C.B- 17/Icr- scid/scid Jcl)) were generated using a previously reported method [39,40]. Human hepatocytes were isolated from chimeric mice with high replacement index as defined previously [41] using standard cannulation and collagenase perfusion protocol. The cells were isolated in the Saito lab at USC, plated in T-75 flasks at the optimal seeding condition as determined previously [42] and shipped to Mayo Clinic Rochester. For hepatocyte encapsulation experiments, primary rat or human hepatocytes were re-suspended in the viscous core solution at a concentration of 50 × 106 cells mL−1 (which corresponds to ∼200 cells per capsule). The microcapsules carrying hepatocytes were collected into a 6 well-plate at a density of 2000 capsules/well and incubated at 37 °C with 5% CO2. The culture medium was changed every 48 h. The same encapsulation process was followed for Hep and PEG microcapsules. For unencapsulated spheroid control experiments, hepatocytes were seeded in Aggrewell 400 at a density of ∼200 cells/well according to manufacturer's instructions.
2.5. Assessing hepatocyte viability and spheroid formation after encapsulation
The viability of hepatocytes was determined by live/dead double-staining [34,37]. Stained cells were imaged using an inverted fluorescence microscope (IX83; Olympus, Center Valley, PA, USA) and cell viability was quantified by calculating the ratio of live cells (green) over the total number of cells (green and red) in the images. The formation of the hepatocyte spheroids in the microcapsules was imaged and the size of spheroids was analyzed with Image J software.
2.6. Analysis of hepatic function
Functionality of hepatocytes was assessed by ELISA to measure albumin secretion, a colorimetric assay to measure urea, and a fluorometric assay to measure bile acid production. For albumin ELISA, urea, and bile acid assays, cell culture supernatant was collected every other day and assayed according to manufacturer's instructions. In order to study the roles of HGF and TGF-β1 in hepatocytes carrying microcapsule cultivation, we administered c-met inhibitor (SU11274, 5 μM), a potent inhibitor of the HGF induced signaling pathway and TGF-β1 blockers (SB431542, 5 μM). CYP1A2 enzyme activity was evaluated with a P450-Glo CYP1A2 luminescence assay. Hepatocyte spheroids were induced with 100 μM omeprazole for 48 h. At this point CYP1A2-dependent conversion of Luciferin-1A2 to luciferin was evaluated by measuring luciferase-induced luminescence using UV/vis spectrophotometer (Synergy H1, BioTek, Winooski, VT, USA). For immunofluorescence staining, spheroids were fixed with 4% PFA, then permeabilized by immersion in 0.1% Triton X-100 for 30 min at 25 °C and subsequently blocked by immersing in 1% BSA for 1 h at 25 °C. After thorough washing with PBS, spheroids were incubated with primary mouse anti-Met Ab (1:1000 dilution) and rabbit anti-phospho-Met Ab (1:400 dilution) for 1 h at 25 °C. Subsequently, spheroids were washed thoroughly with PBS and incubated with secondary Abs for 1 h at 25 °C in the dark - 10 μg mL−1 Alexa Fluor 546 donkey anti-mouse Ab and 10 μg mL−1 Alexa Fluor 488 donkey anti-rabbit Ab. The spheroids were washed in PBS and mounted using a mounting medium with 1 μg mL−1 DAPI to stain for nuclei. To detect intracellular albumin, encapsulated or unencapsulated spheroids were fixed with 4% PFA and immersed in 30% sucrose for 24 h. The spheroids were then imbedded in an optimal cutting temperature (OCT) compound for 1 h, frozen, and sectioned into 10 μm thick slices using a cryostat instrument (Leica CM1950; Leica Biosystems Inc., Buffalo Grove, IL, USA). Subsequently, the sections were then permeabilized by immersion in 0.1% Triton X-100 and blocked by immersing in 1% BSA. After thorough washing with PBS, the sectioned spheroids were incubated with 2 μg mL−1 of sheep anti-rat albumin Ab for rat hepatocyte spheroid or 2 μg mL−1 of goat anti-human albumin Ab for human hepatocyte. The spheroid sections were then washed with PBS and incubated with 10 μg mL−1 Alexa Fluor 546 donkey anti-sheep Ab for rat hepatocyte spheroid or 10 μg mL−1 Alexa Fluor 546 donkey anti-goat Ab for human hepatocyte spheroid. For actin visualization, the spheroid sections were incubated with a 1:400 dilution of Alexa Four 488-conjugated phalloidin for 1 h. The samples were washed in PBS and stained with DAPI [33,43]. All stained samples were visualized and imaged using an inverted fluorescence microscope.
2.7. Characterizing hepatic gene expression by RT-PCR
For hepatic gene expression analysis, 2000 microcapsules containing hepatocyte spheroids were broken down by applying an electronic pestle for 5 min. Then total RNA was extracted and purified by using a RNeasy Plant Mini Kit (Qiagen, Valencia, CA, USA) following manufacturer's instructions. cDNA was synthesized with a QuantiTect Reverse Transcription Kit (Roche, Basel, Switzerland), and quantitative real-time RT-PCR was performed with the QuantStudio™ 5 System (Thermo Scientific Inc.) using universal SYBR Green Master (Roche) [34,44]. Relative gene expression was calculated using the comparative threshold cycle (ΔΔCT) method and normalized with glyceraldehyde 3-phophate dehydrogenase (GAPDH) as a housekeeping gene. The primer sequences used for RT-PCR are listed in Table S1.
2.8. Modeling dynamics of HGF secretion, uptake and release from microcapsules
We used COMSOL Multiphysics® software (Version 5.6, Burlington, MA) to model interactions between hepatocytes secreting HGF and heparin-containing microcapsules. Our model included geometry and cell number per microcapsule and assumed a constant secretion rate of 0.45 × 10−6 ng h−1 cell−1 for HGF [11]. The reaction kinetics HGF binding to heparin were modeled using a reversible kinetic expression: . Here, , , and are concentration of the GF, heparin, and GF-heparin complex. and are rate constants taken from literature to be 4.2 × 103 [1/MS] and 5.7 × 10−7 [1/S] [45]. To improve accuracy of the solver, the secretion-reaction-diffusion equations were non-dimensionalized prior to implementation in COMSOL. Microcapsules in a confined medium were simulated using experimental parameters of 3600 microcapsules (400 μm in diameter) distributed in 2 mL of medium. This led to a fluid layer of about 1.0 mm diameter surrounding each capsule with a no flux boundary condition at the imaginary interface between adjoining fluid layers. A cellular spheroid was simulated as an inert sphere of 100 μm diameter concentrically positioned inside the microcapsule. The secretion-reaction-diffusion equations for cellular production of HGF, HGF binding to heparin, and HGF diffusing out of capsules were solved using the 2D axisymmetric model in COMSOL. To avoid instability, a small diffusivity of 10−15 m2/s was used for heparin and GF-heparin complex in the medium and shell. Table S2 lists all the parameters used in the simulations.
2.9. Statistical analysis
All the experiments were performed at least in triplicate. All the data were presented as mean ± standard deviation (SD), unless stated differently. Statistical significance was declared when *p < 0.05, **p < 0.01 and ***p < 0.001 as determined by Student's t-test.
3. Results and discussion
This study explores the effects of endogenous and exogenous HGF on the function of hepatocyte spheroids cultured in heparin-containing bioactive microcapsules (Scheme 1).
Scheme 1.
Fabrication of bioactive heparin (Hep)-based microcapsules for maintenance of primary hepatocytes. The core (light pink stream) containing primary hepatocytes and shell (light green stream) containing PEG4MAL/Hep-MA flow co-axially and are then discretized into core-shell droplets at the junction with shielding oil (light gray stream). Droplets polymerize and acquire a hydrogel shell after interacting with cross-linker DTT (dark gray stream). Hep microcapsules retain and release hepatocyte-secreted endogenous signals thus enhancing function of encapsulated hepatocytes. Hepatic function was further enhanced by loading exogenous HGF into microcapsules and was maintained at a high level for over two weeks.
3.1. Fabricating bioactive core-shell microcapsules
Scheme 1 and Fig. S1 describe how bioactive core-shell microcapsules are fabricated using a co-axial flow-focusing microfluidic device. We have previously reported detailed protocols for fabricating and operating this encapsulation system [34,37]. Briefly, the core flow stream contained single cell suspension while the shell stream contained PEG4MAL and Hep-MA. The two aqueous streams were injected into the droplet microfluidic device. Aqueous droplets were formed when the aqueous dispersed phase was interjected by shielding oil (continuous phase), resulting in the formation of core-shell microcapsules. The exterior of the formed droplets was subsequently polymerized by the DTT crosslinker oil that reacted with PEG4MAL and Hep-MA present in the outer shell region via click chemistry and Michael-type addition reaction, respectively (Figs. S1A, C, D, and Supporting Video 1). These experimental conditions produce microcapsules with a ∼15 μm thick hydrogel shell and ∼380 ± 12 μm diameter [34]. Importantly, negatively charged heparin moieties were integrated into the hydrogel network upon cross-linking (Michael addition reaction) with DTT (see Fig. S1B), creating a Hep/PEG hybrid network [34]. Having fabricated heparin-containing bioactive microcapsules, we proceeded to investigate their interactions with HGF.
Supplementary video related to this article can be found at https://doi.org/10.1016/j.bioactmat.2023.05.009
The following are the supplementary data related to this article:
3.2. Characterizing HGF uptake, release and retention in bioactive microcapsule
As previously mentioned, HGF signaling plays a crucial role in liver development, regeneration and hepatic phenotype maintenance [27,28]. HGF is unstable in vitro but may be stabilized via interactions with heparin [23]. A central hypothesis of this paper was that HGF may incorporate into Hep microcapsules and may enhance phenotype of encapsulated hepatocyte spheroids. As a step toward testing this hypothesis, we investigated loading and release of HGF from microcapsules. Two types of microcapsules, Hep and PEG, were fabricated as described in section 2.3.1. and then soaked in 100 or 20 ng mL−1 HGF for 1 h. HGF remaining in the media was quantified by ELISA. This analysis (see Fig. 1A) showed that Hep microcapsules took up ∼2.7 times more HGF compared to PEG capsules in the 100 ng mL−1 soaking condition and while Hep microcapsules took up ∼2.8 times more HGF compared to PEG capsules in the 20 ng mL−1 soaking condition. While 90% of HGF taken up by PEG capsules was released within 1 h, it took 3 days to achieve the same level of HGF release from bioactive Hep microcapsules. This suggested that release from bioactive microcapsules was governed by affinity interactions with HGF and not by its diffusion. It is also worth noting that release profiles for 20 and 100 ng mL−1 HGF loading conditions were similar when normalized by the amount loaded (see Fig. 1B and Fig. S2).
Fig. 1.
Characterizing HGF loading, release, and retention. (A) Amount of HGF loaded into Hep and PEG microcapsules when using two different concentrations of HGF in solution. (B) Release of HGF from bioactive (Hep) and bioinert (PEG) microcapsules. Release profiles for capsules loaded using 100 or 20 ng ml−1 HGF. (C) Sequential loading of HGF using 100 ng ml−1 HGF. Note that no saturation was observed after 4 rounds of loading. (D) Release profiles for capsules loaded with different amount of HGF. (E) Incorporation of HGF into microcapsules was assessed by immunostaining with anti-HGF-biotin/avidin-HRP followed by exposure to DAB (brown color).
As may be seen from Fig. 1A, the amount of HGF loaded into microcapsules increased with HGF solution concentration. We wanted to assess the capacity of bioactive microcapsules for HGF and carried out sequential loading using a relatively high concentration of 100 ng mL−1. A given number of microcapsules (2000) was incubated in 2 mL of HGF solution for 1 h, then removed from the original solution, washed and incubated in a fresh HGF solution for 1 h. In this manner, microcapsules were loaded up to 4 times using the same solution of the same HGF concentration. As seen from Fig. 1C, sequential loading resulted in an increased retention of HGF in microcapsules with the amount of loaded HGF increasing as a function of the loading cycle. It is also worth noting that saturation of HGF loading was not observed after 4 loading cycles using 100 ng mL−1 HGF. The sponge-like properties of Hep microcapsules may be explained by 1) high affinity of heparin for HGF (dissociation constant of 0.14 nM) [45] and 2) much larger number of heparin molecules per capsule compared to HGF molecules. Each microcapsule contains ∼70 ng or 5.8 pmole of heparin (quantification described in Ref. [27]). For comparison, loading of HGF using high concentration (100 ng mL−1) resulted in 0.35 fmol HGF per capsule. This means that Hep microcapsules had four orders of magnitude more heparin molecules compared to HGF molecules.
In addition to HGF loading, we assessed the release profile for capsules containing different amounts of HGF. As seen in Fig. 1D, the release profiles were similar regardless of the loaded amount with sustained release occurring after two weeks. Uptake and release experiments highlight that bioactive microcapsules release HGF in a sustained manner, that release is governed by Hep/HGF affinity interactions, and that Hep microcapsules have a high capacity for taking up HGF molecules. We believe these to be key features contributing to enhanced hepatic phenotype in bioactive microcapsules.
In addition to characterizing release of HGF from microcapsules, we also wanted to demonstrate its retention in microcapsules. Microcapsules were stained with anti-HGF-HRP antibodies and then incubated with chromogenic reagent DAB to visualize the results. As seen from Fig. 1E, Hep microcapsules stained strongly for HGF 4 h and 3 days after loading. Conversely, no staining was observed in PEG microcapsules at either time point, confirming again that HGF was retained in bioactive (Hep) but not in bioinert (PEG) microcapsules. Taken together with HGF uptake and release studies, our immunostaining results support the notion that HGF strongly binds to and interacts with Hep microcapsules over the course of multiple days.
3.3. Encapsulating primary hepatocytes and forming spheroids
After characterizing GF loading into bioactive and bioinert microcapsules, we proceeded with hepatocyte encapsulation and spheroid formation experiments. A reader may remember that our microcapsules have an aqueous core and hydrogel shell and that single cells organize into spheroids when confined to such microcapsules. First, we used live/dead staining to assess viability of hepatocytes after going through the encapsulation process. As seen from images in Fig. 2A and quantification in Fig. 2B, the viability of hepatocytes was 96.5 ± 2.4% and 93.2 ± 3.2% before and after encapsulation. Thus, viability was not affected by the encapsulation process. As the next step, we evaluated the effects of inoculation cell concentration on the size and function of resultant spheroids. Previous studies demonstrated ∼80–100 μm diameter spheroids to be most functional [12,46]. We used 3 different cell inoculation concentrations low, medium and high (20, 50, and 100 × 106 cells mL−1) to assess spheroid size and hepatic function inside microcapsules. As shown in Figs. S3A and B, inoculation of 20 × 106 cells mL−1 (∼100 cells/capsule) resulted in 61.8 ± 9.4 μm diameter spheroids compared to 103.5 ± 6.2 μm and 91.4 ± 16.2 μm diameter spheroids formed with inputs of 50 × 106 cells mL−1 (∼200 cells/capsule) and 100 × 106 cells mL−1 (∼400 cells/capsule), respectively. We observed that medium and high inoculation concentrations resulted in similarly sized spheroids, but that high concentration was associated with loose single cells imbedded in the hydrogel shell. Albumin analysis (see Figs. S3C and D) revealed spheroids formed using medium inoculation concentrations to be most functional. It is likely that efficient conversion of single cells into spheroids is important and that single cells not incorporated into spheroids at high inoculation concentrations underwent apoptosis and adversely affected hepatic phenotype of spheroids. Based on these experiments, we proceeded to use the medium inoculation density of 50 × 106 cells mL−1 (∼200 cells/capsule) for all subsequent experiments described in this paper. In addition, we compared hepatocyte spheroid formation in microcapsules and in a commercial Aggrewell plate. This comparison (see Fig. 2C) revealed similar spheroid size but better spheroid uniformity inside microcapsules.
Fig. 2.
Assessing viability and spheroid formation of encapsulated hepatocytes. (A) Bright-field image and live/dead fluorescent image of encapsulated hepatocytes. (B) Comparison of viability before and after encapsulation of hepatocyte. (C) Size distribution of encapsulated hepatocyte spheroids in Hep capsule and bare spheroids generated in Aggrewell plates at day 2. Statistical analysis-number of samples = 30 (ns, p > 0.05; *, p ≤ 0.05).
3.4. Effects of HGF on hepatic function of encapsulated spheroids
As the next step, we wanted to assess hepatic phenotype of spheroids in Hep and PEG microcapsules. Morphology of spheroids was monitored over the course of 7 days using brightfield microscopy. As may be appreciated from a sequence of images in Fig. 3A, single hepatocytes organized into spheroids by day 2 of culture in both bioactive (Hep) and bioinert (PEG) microcapsules. However, by day 7 of culture, Hep microcapsules contained compact translucent spheroids with sharp edges and high viability while spheroids in PEG microcapsules began to disintegrate and had jagged edges with lower viability (see insets of Fig. 3A).
Fig. 3.
Phenotype maintenance of encapsulated spheroids. (A) Representative bright-field and live/dead fluorescent images of encapsulated hepatocytes in Hep and PEG microcapsule at different time points during cultures. HepImm and PEGImm refer to one-time loading of HGF into Hep and PEG microcapsules, respectively, after which time encapsulated spheroids were maintained in media without HGF. Based on the concentration of soluble HGF used for loading microcapsules, the “Imm” condition was further subdivided into “Imm-high” and “Imm-low” for 100 ng mL−1 and 20 ng mL−1 of HGF, respectively. HepSol-high refers to spheroids in Hep microcapsules cultured in media with 100 ng mL−1 of HGF exchanged every other day and PEGSol-low refers to spheroids in PEG microcapsules cultured in media with 20 ng mL−1 of HGF exchanged every other day. (B, C) ELISA analysis of hepatic albumin secretion and (D, E) urea synthesis at various time points during 9-day culture. Statistical analysis-number of samples = 3 (*, p ≤ 0.05; **, p ≤ 0.01; ***, p ≤ 0.001).
Beyond observing spheroid morphology, we wanted to assess hepatic function of spheroids and focused initially on synthesis of albumin and urea which are both hallmark indicators of liver metabolism. As seen from Fig. 3B, Hep microcapsules elicited 1.9-fold and 4.5-fold higher level of hepatic albumin production (328 ng/1 × 104 cells/day) at day 7, compared to PEG microcapsules (175 ng/1 × 104 cells/day) and Aggrewell plate (73 ng/1 × 104 cells/day) respectively. This result may be attributed to the fact that Hep microcapsules take up exogenous or endogenous signals and then release these biomolecules to encapsulated hepatocytes. Additional results described in the section below suggest that endogenous HGF is a key effector of hepatic function in bioactive microcapsules.
A goal of this study was to test a hypothesis that bioactive microcapsules may be used to deliver HGF to the encapsulated hepatocytes thereby improving hepatic phenotype. To begin assessing effects of HGF in microcapsules, we set up an experiment with five experimental groups: 1) one-time loading of 20 ng mL−1 HGF into Hep microcapsules, 2) one-time loading of 100 ng mL−1 HGF into Hep microcapsules, 3) bi-daily supplementation of 100 ng mL−1 HGF into media containing Hep microcapsules, 4) one-time loading of 20 ng mL−1 HGF into PEG microcapsules, and 5) bi-daily supplementation of 20 ng mL−1 HGF into media containing PEG microcapsules. One-time loading, or immobilization, was carried out for Hep and PEG microcapsules, and was denoted as HepImm and PEGImm, respectively. Based on the concentration of soluble HGF used for loading microcapsules, the HepImm condition was further subdivided into HepImm-high and HepImm-low for 100 ng mL−1 and 20 ng mL−1 of HGF, respectively. An experimental group of spheroids in PEG capsules exposed to bi-daily supplementation of 100 ng mL−1 HGF in the media was named PEGSol-high. As highlighted by the data in Fig. 3C, one-time loading of HGF into Hep microcapsules resulted in higher level of hepatic albumin production compared to Hep microcapsules without HGF, with improvement becoming more pronounced over time. For example, albumin levels were ∼1.4 and 1.6 times higher for HepImm-low and HepImm-high, respectively, compared to Hep at day 7. Albumin production for HepSol-high was similar to one-time loading of HGF into microcapsules (HepImm-high) group. Another interesting observation was that production of hepatic albumin in PEG capsule experimental groups, either PEGImm-low and or PEGSol-low, was significantly lower than in Hep microcapsules. In fact, albumin levels were similarly low for one-time loading or bi-daily supplementation of HGF. Urea production followed similar trends to that of albumin with higher levels observed in bioactive microcapsules compared to bioinert (PEG) capsules and Aggrewell plate (Fig. 3D and E).
Results in Fig. 3 are compelling for two reasons. First, hepatocyte spheroids in Hep microcapsules are much more functional compared to spheroids in PEG microcapsules or unencapsulated spheroids in a commercial 3D plate (Aggrewell). We posit that improved hepatic function is due, at least in part, to endogenous HGF signaling and describe results supporting this later in the paper. Second, one-time loading of exogenous HGF or its bi-daily supplementation in the media appears to have no effect on the function of spheroids in PEG microcapsules. Conversely, one-time loading of HGF into Hep microcapsules is sufficient to have a long-lasting effect on hepatic synthesis of albumin and urea. This observation suggests that HGF may be less active when present in solution and more active in Hep microcapsules which aligns with previous reports of enhanced stability and bioactivity of HGF when complexed with heparin [10,33].
It is important to note that improved hepatic function in Hep microcapsules is combined with lower cost. We estimate the cost adding HGF into media over the course of hepatic maintenance run to be $28 per well of a 6 well plate (2 mL of media per well). In comparison, one-time loading of HGF into Hep microcapsules costs $3.5 per well. Therefore, HGF immobilization approach is 8 times cheaper while offering comparable functionality to supplementation of HGF into media.
3.5. Assessing effects of HGF/TGF-β1 signaling on hepatic function in microcapsules
We hypothesized that enhanced hepatic function of bioactive microcapsules in the absence of exogenous HGF may be due to a positive feedback loop where a hepatocyte spheroid produces autocrine factors that bind to the hydrogel shell of a Hep microcapsule and are then released back to further stimulate encapsulated cells. This hypothesis was based on our previous observations that secreted signals, primarily HGF, act in an autocrine manner by enhancing phenotype of hepatocytes cultured in microfluidic devices [11]. To test this hypothesis, we added inhibitor of HGF receptor Met (iHGF, 5 μM SU11274) to culture media bathing Hep or PEG microcapsules. It may be appreciated from Fig. 4A that albumin production in PEG microcapsules was considerable lower than in Hep microcapsules and that treatment with iHGF decreased albumin levels for both culture types. Urea synthesis and bile acid production were also reduced in hepatocyte spheroids exposed to iHGF, albeit by a lower margin (see Fig. 4B and C). To further explore the importance of HGF signaling, we carried out RT-PCR analysis of Hgf and its receptor Met for rat hepatocyte spheroids in PEG and Hep microcapsules. Met is activated by binding of HGF, which induces the phosphorylation of two tyrosine residues (phospho-Met), tyrosine-1234 and tyrosine-1235 (Y1234/Y1235) of the catalytic loop of the kinase domain [47]. Inhibition of HGF/Met signaling may be achieved using Met inhibitors (e.g. SU11274) [48]. For RT-PCR analysis, we compared five experimental conditions for: 1) PEG-only microcapsules (PEG), 2) PEG microcapsules media supplemented with met inhibitor (PEG/iHGF), 3) Hep microcapsules (Hep), 4) Hep microcapsules media supplemented with Met inhibitor (Hep/iHGF), and 5) Hep microcapsules loaded with HGF (Hep/HGF). As seen from data in Fig. 4D, Hgf and Met expression were much higher for spheroids in Hep capsules than in PEG capsules, with highest expression values achieved for Hep capsules loaded with HGF.
Fig. 4.
Assessing the effects of endogenous HGF and TGF-β1 on the encapsulated hepatocyte spheroids. (A) Hepatic albumin secretion, (B) urea synthesis, and (C) bile acid production during 15 days of spheroid cultures in Hep or PEG microcapsule. Hepatic functions were downregulated by inhibition of HGF and enhanced by inhibition of TGF-β1. Statistical analysis-number of samples = 3 (ns, p > 0.05; *, p ≤ 0.05; **, p ≤ 0.01; ***, p ≤ 0.001). (D) RT-PCR analysis of Hgf and Met gene expression for different microcapsule conditions. (E) Immunofluorescence staining of encapsulated hepatocyte spheroids for Met and p-Met. (F) Retention of HGF in Hep microcapsules was assessed using immunostaining. Labeling with anti-HGF-biotin/avidin-HRP was followed by staining with DAB chromogenic agent that produced brownish color. Images were obtained after 7 days of culture in the presence or absence of HGF or TGF-β inhibitor. Intensity of HGF staining varied depending on culture conditions and was highest in cultures with TGF-β inhibitor. Description of experimental groups: iHGF refers to inhibiting HGF signaling, iTGF-β1 refers to inhibiting TGF-β1 signaling, and Hep/HGF condition refers to one-time incorporation of HGF into Hep microcapsule.
Beyond observing gene expression, we performed immunofluorescence staining for MET and phosphorylated (p)-MET for spheroids in Hep and PEG microcapsules (see Fig. 4E). These immunofluorescence images are compelling as they confirm high levels of MET expression in Hep microcapsules and also point to high levels of p-MET expression for spheroids in Hep microcapsules vs. low levels of p-MET in PEG microcapsules. These data further support the scientific premise of the paper that endogenous HGF signaling is an important driver of hepatic phenotype and that this signaling is enhanced in bioactive microcapsules.
It is well appreciated that TGF-β1 is a pro-fibrotic factor that has a detrimental effect on phenotype of multiple epithelial cell types, including the hepatocyte [49]. We have previously shown that functionality of microfluidic hepatocyte cultures may be improved by inhibiting TGF-β1 signaling [11,40,50] and hypothesized that such inhibition may be beneficial for encapsulated hepatocyte spheroids. Indeed, as may be seen from Fig. 4, supplementing media with TGF-β inhibitor (5 μM A83-01) resulted in a marked enhancement of albumin synthesis (2.3-fold higher at day 15) and significantly higher urea and bile acid production compared to encapsulated spheroids without the inhibitor. Beyond analysis of secreted factors, we carried out HGF immunostaining of microcapsules with spheroids cultured in the presence of HGF or TGF-β inhibitors. As highlighted by images in Fig. 4F, the highest intensity of HGF staining was observed in microcapsules cultured with TGF-β inhibitor, lower for cultures with neither HGF nor TGF-β inhibitors, and lowest in the presence of HGF inhibitor.
In summary, we demonstrate that endogenous signals contribute significantly to the phenotype of encapsulated hepatocyte spheroids, with HGF inhibitor degrading and TGF-β inhibitor improving hepatic phenotype. We also highlight the fact that Hep microcapsules bind endogenous and retain endogenous HGF.
3.6. Loading exogenous HGF while inhibiting TGF-β1 signaling to further enhance hepatic function of encapsulated spheroids
In the preceding sections, we demonstrated that hepatic phenotype is affected by endogenous as well as exogenous HGF and is improved by inhibiting TGF-β1 signaling. We hypothesized that loading bioactive microcapsules with HGF and supplementing culture media with TGF-β1 inhibitor will further enhance hepatic function of spheroids. To test this hypothesis, we compared four experimental conditions: 1) Hep microcapsules (control), 2) Hep microcapsules loaded with HGF (100 ng mL−1) – Hep/HGF, 3) Hep microcapsules media supplemented with TGF-β1 inhibitor – Hep/iTGF, and 4) Hep microcapsules loaded with HGF (100 ng mL−1) and media supplemented with TGF-β1 inhibitor – Hep/HGF/iTGF. The albumin ELISA results presented in Fig. 5A show that the combination condition elicited a higher level of albumin synthesis, compared with Hep microcapsules and Hep/HGF microcapsules. Specifically, albumin levels were 2 and 1.5 times higher for Hep/HGF/iTGF-β1 conditions (656 ng/1 × 104 cells/day) compared to Hep (328 ng/1 × 104 cells/day) and Hep/HGF (427 ng/1 × 104 cells/day) conditions at day 7. High levels of albumin production for combination conditions were maintained over the course of 2 weeks. The albumin levels for encapsulated rat hepatocyte spheroids comparable to or better than such well-established culture formats as micropatterned co-cultures (200 ng/1 × 104 cells/day) [38] and collagen gel sandwich (100 ng/1 × 104 cells/day) [51]. Higher levels of urea synthesis and bile acid production were also observed when providing exogenous HGF and inhibiting TGF-β1 signaling in bioactive microcapsules (Fig. 5B and C).
Fig. 5.
Improving function of rat hepatocyte spheroids by incorporating exogenous HGF into microcapsules. Hep/HGF condition refers to one-time incorporation of HGF into microcapsules and iTGF-β1 refers to inhibiting TGF-β1 signaling. 3D culture plate (Aggrewell) was used to form hepatocyte spheroids without microcapsules, also called bare spheroids. (A) Albumin secretion, (B) urea synthesis, and (C) bile acid production for three different culture conditions at different time points during 15-day culture. (D) CYP1A2 activity was assessed by adding omeprazole (Ome) into culture media. (E) RT-PCR analysis of key genes associated with maintenance (Albumin, Cyp1a2, and Hgf) or loss (TGF-β1) of hepatic phenotype. Dashed line represents gene expression of freshly isolated liver tissue (day 0). (F) Immunofluorescence staining for albumin expression of Hep microcapsules (top) and bare spheroids (bottom). Statistical analysis-number of samples = 3 (ns, p > 0.05; *, p ≤ 0.05; **, p ≤ 0.01; ***, p ≤ 0.001).
Moving beyond analysis of secreted factors, we also assessed activity of hepatic enzyme CYP1A2 - a member of cytochrome P450 family of enzymes responsible for metabolism of small molecule xenobiotics including drugs [42]. Encapsulated spheroids in optimal culture conditions (exogenous HGF loaded into microcapsules plus TGF-β1 inhibitor in the media) were exposed to omeprazole after which the activity of CYP1A2 was monitored using luciferase assay. As seen from Fig. 5D, treatment with omeprazole resulted in a 2-fold increase in activity of CYP1A2. For this experiment, we created a control where hepatocytes were organized into spheroids inside a commercial 3D culture (Aggrewell) plate. As seen from Fig. 5D, CYP1A2 activity was significantly lower for unencapsulated spheroids and was not induced by incubation with omeprazole. One may note high level of baseline activity for CYP1A2 in Hep microcapsules. This may be explained by presence in the culture media of glucocorticoids (hydrocortisone and dexamethasone) which are known to induce CYP1A2 via glucocorticoid receptor [52,53]. Much lower baseline levels for spheroids in commercial 3D plate (Aggrewell) are likely due to poor functionality and lower CYP expression levels. Thus, hepatocyte spheroids in Hep microcapsules retain CYP activity and may be useful for toxicity studies in the future.
In addition to characterizing enzyme activity, we carried out assessment of hepatic phenotype using RT-PCR analysis and immunofluorescence staining. RT-PCR analysis revealed higher levels of hepatic gene expression at day 7 of culture (Albumin, Cyp1a2, and Hgf) for optimal culture conditions (Hep/HGF/iTGF-β1) compared to other encapsulated conditions (Hep and Hep/HGF). Hepatic gene expression in microcapsules was benchmarked against spheroids in commercial 3D plate (Aggrewell) and freshly isolated rat liver tissue (dashed line). As seen from Fig. 5E, encapsulated spheroids had higher hepatic gene expression than comparator conditions.
Immunofluorescence staining (see Fig. 5F) highlighted that while the cytoskeletal structure of spheroids in Hep microcapsules and Aggrewell plate appeared to be similar after 7 days of culture, encapsulated spheroids contain much higher levels of albumin. This experiment corroborated ELISA and RT-PCR results for albumin, confirming that higher levels of this hepatic marker were expressed by spheroids in Hep microcapsules compared to bare spheroids.
3.7. Human hepatocyte spheroids cultured in bioactive microcapsules
Having optimized encapsulation and culture conditions for rat hepatocyte spheroids, we assessed the utility of these conditions for cultivation of human hepatocytes. These hepatocytes were isolated from mice with humanized livers [39,40], then encapsulated, cultured and analyzed as described in the preceding sections for rat hepatocytes. The results obtained from experiments using human hepatocytes showed a similar outcome to rat hepatocytes. Briefly, human hepatocytes formed spheroids inside a core-shell Hep microcapsules as seen from see Fig. S4A. Albumin synthesis at day 7, shown in Fig. S4B, was 1.3 times higher for Hep microcapsules loaded with HGF (HepImm) compared to Hep microcapsules alone. Once again, human hepatocyte spheroids in bioinert PEG capsules were not affected by one-time loading of HGF.
As the next step, we wanted to confirm that the combination culture condition (exogenous HGF, media supplement with TGF-β1 inhibitor) was optimal for human hepatocytes. As before, we tested three encapsulation culture conditions: 1) Hep microcapsule – without HGF and TGF-β1 inhibitor, 2) Hep/HGF - HGF loading without TGF-β1 inhibitor and 3) Hep/HGF/iTGF-β1 – the optimal condition with HGF and iTGF-β1. As seen from Fig. 6A, albumin secretion levels from the combination conditions (63.1 ng/1 × 104 cells/day) were 3.5 and 1.4 times higher compared to Hep microcapsules (18.3 ng/1 × 104 cells/day) and Hep/HGF microcapsules (45.7 ng/1 × 104 cells/day) at day 7, and were maintained over the course of 2 weeks (Fig. 6A). A similar pattern was observed for urea synthesis (Fig. 6B) and bile acid production (Fig. 6C).
Fig. 6.
Human hepatocyte spheroids in bioactive microcapsules. (A) Albumin secretion, (B) urea synthesis, and (C) bile acid production for three different culture conditions at different time points during 15-day culture. (D) CYP1A2 activity was assessed by adding omeprazole (Ome) into culture media. (E) RT-PCR analysis of hepatic gene expression. Dashed line represents gene expression of human hepatocytes prior to encapsulation (day 0). (F) Immunofluorescence staining for albumin expression of Hep microcapsules (top) and bare spheroids (bottom). Statistical analysis-number of samples = 3 (ns, p > 0.05; *, p ≤ 0.05; **, p ≤ 0.01; ***, p ≤ 0.001).
Baseline activity of CYP1A2 was significantly higher for encapsuled spheroids compared to unencapsulated spheroids (see Fig. 6D). Furthermore, incubation with omeprazole induced CYP1A2 activity of encapsulated spheroids but had no effect on bare spheroids. We also carried out hepatic gene expression analysis of encapsulated human hepatocyte in Hep microcapsule, unencapsulated hepatocyte spheroids in Aggrewell plates as well as human hepatocytes cultured as monolayers prior to their collection and encapsulation. As highlighted in Fig. 6E, encapsulated human hepatocytes under combination culture conditions (Hep/HGF/iTGF-β1) were more functional after 7 days of culture than bare spheroids in an Aggrewell plate and hepatocytes from monolayer cultures at the time of encapsulation (dashed line). The differences in hepatic phenotype were further corroborated by immunofluorescent staining with high levels of albumin observed for spheroids in Hep microcapsules and low levels for unencapsulated spheroids (see Fig. 6F). Overall, our results demonstrate successful encapsulation, spheroid formation and maintenance of human hepatocyte spheroids, and suggest that loading HGF and inhibiting TGF-β signaling enhanced phenotype of encapsulated spheroids.
3.8. Modeling interactions between a hepatocyte spheroid and a bioactive microcapsule
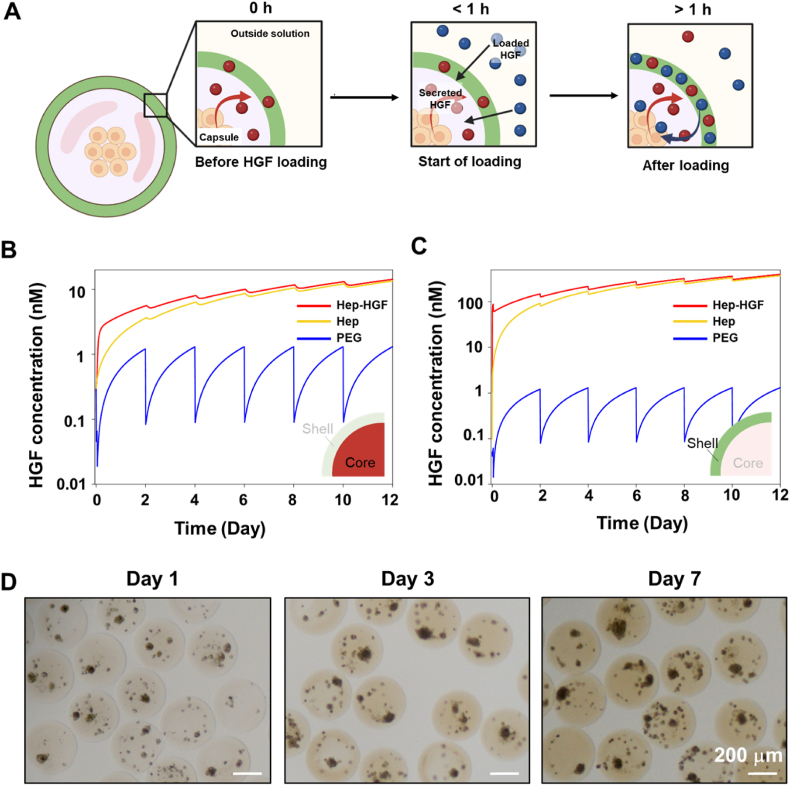
Our experimental results suggest an active role played by Hep microcapsules in sequestering and releasing secreted signals, including HGF. We observed that hepatocytes in Hep microcapsules have improved phenotype compared to hepatocytes in PEG capsules and that loading HGF into Hep capsules further enhanced hepatic phenotype while loading HGF into PEG capsules did not. How does endogenous HGF interact with Hep capsules, what are the levels of endogenous HGF inside a microcapsule next to a spheroid at different time points during culture and how do these levels compare to exogenous HGF loading? These questions are challenging to address experimentally, therefore, we set up a secretion-reaction-diffusion model that accounted for production of HGF by a hepatocyte spheroid, its affinity interactions with Hep microcapsules and its diffusion within and outside of capsules (see Fig. 7A for a schematic description of the model). We also modeled a scenario where HGF is both produced by cells and loaded into capsules exogenously. The final modeling scenario was bioinert PEG microcapsules that included endogenous and exogenous HGF as well as diffusion elements of the model but did not have affinity interactions of HGF with Hep. Parameters used for model construction are described in Table S2. We note that the majority of these parameters have been determined by us previously [28] with the exception of HGF binding constants and secretion rate that came from the work of others [35]. We would also like to note that the HGF secretion rate was assumed to be constant over time and the same for Hep and PEG capsules.
Fig. 7.
Modeling HGF secretion, loading, accumulation and release from microcapsules. (A) Scenarios being modeled include: Hep-HGF – where HGF is both secreted by a hepatocyte spheroid and also loaded exogenously into Hep microcapsule, Hep – where HGF is secreted by a spheroid but not loaded exogenously, PEG – where HGF is secreted by a spheroid into PEG microcapsule. (B,C) Average concentration of HGF inside microcapsules with and without HGF loading was plotted as a function of time for aqueous core and hydrogel shell. (D) Modeling prediction of HGF accumulation was confirmed experimentally by immunostaining for HGF at different time points during culture of encapsulated spheroids. The scenario evaluated here is Hep microcapsules without loading of exogenous HGF.
Our modeling results shown in Fig. 7B reveal several interesting insights. First, cell-secreted HGF is expected to reach much higher concentrations in the core of a bioactive Hep capsule compared to the core of a bioinert PEG capsule (6 nM vs 1 nM) after 48 h of culture in undisturbed media. Second, media exchange (see for example day 2) results in a dramatic decrease of HGF concentration in the core of a bioinert PEG capsule where it approaches zero and builds up again due to secretion and accumulation over the next 48 h. In comparison, HGF concentration in the core of a bioactive Hep capsule changes little in the process of media replacement. This is explained by high affinity of heparin for HGF [45] and high capacity of Hep capsules for binding HGF (5.8 pmol of heparin in a capsule) [34]. Third, levels of HGF continue to accumulate in Hep microcapsules over time while this does not occur in PEG capsules. Fourth, a scenario where endogenous production of HGF is combined with its exogenous loading at 100 ng mL−1 produces higher levels of HGF in the core compared to endogenous signaling alone at early time points (3.16 nM vs. 1.2 nM at 12 h), but this difference diminishes and becomes negligible by day 4 of culture. Fifth, a much higher concentration of HGF is contained in a Hep-based hydrogel shell than in the aqueous core of the microcapsule such that a hydrogel shell serves as a reservoir for these molecules (Fig. 7C). While our modeling helps explain why bioactive microcapsules enhance function of encapsulated hepatocytes, there are likely additional benefits of the microcapsules that are not captured by the model. For example, our model does not account for enhanced stability of HGF in Hep capsules or its low stability in PEG capsules. In addition, autocrine signaling may be associated with discontinuous phenotype switching where cells exposed to a certain threshold concentration of signal significantly increase production of the same signal [[54], [55], [56]] Thus, it is likely that HGF secretion rate is not constant and increases disproportionately in Hep microcapsules compared to PEG microcapsules.
To confirm the modeling prediction of HGF accumulation in bioactive microcapsules, we cultured rat hepatocyte spheroids in Hep microcapsules in the absence of exogenous HGF and immunostained microcapsules for HGF at different time points in culture. As shown in Fig. 7D, HGF signal increased over time, confirming model prediction of endogenous HGF accumulating in bioactive microcapsules.
4. Conclusions
This paper describes the use of bioactive Hep microcapsules for cultivation of hepatocyte spheroids. Bioactive microcapsules were shown to retain and release HGF over the course of several days. Hepatic function of encapsulated spheroids was significantly better in bioactive Hep microcapsules compared to biointert PEG microcapsules. The enhancement of hepatic function was shown to be dependent on endogenous HGF signaling. Loading exogenous HGF and supplementing media with TGF-β1 inhibitor further enhanced function of spheroids in bioactive microcapsules. The optimal culture conditions allowed maintenance of hepatic function for human and rat hepatocyte spheroids over the course of two weeks. Bioactive microcapsules described here hold significant promise as a method for culturing hepatocyte spheroids and may be used for disease modeling or drug testing. Additional applications include employing Hep microcapsules for hepatic differentiation of pluripotent stem cells and for maintaining phenotype of stem cell-derived hepatocytes.
Ethics approval and consent to participate
All animal experiments were performed under the National Institutes of Health (NIH) guidelines for ethical care and use of laboratory animals with the approval of the Institutional Animal Care and Use Committee (IACUC) of the Mayo Clinic, Rochester, MN.
CRediT authorship contribution statement
Kihak Gwon: Conceptualization, Methodology, Investigation, Formal analysis, Data curation, Writing – original draft. Daheui Choi: Methodology, Investigation, Formal analysis, Data curation, Writing – review & editing, Writing – review & editing. José M. de Hoyos-Vega: Methodology, Investigation, Data curation, Writing – review & editing. Harihara Baskaran: Software, Methodology, Data curation, Writing – review & editing. Alan M. Gonzalez-Suarez: Methodology, Investigation, Data curation. Seonhwa Lee: Writing – review & editing. Hye Jin Hong: Methodology, Investigation, Data curation. Kianna M. Nguyen: Methodology, Writing – review & editing. Ether Dharmesh: Methodology, Investigation. Go Sugahara: Formal analysis, Data curation. Yuji Ishida: Formal analysis, Data curation. Takeshi Saito: Methodology, Investigation. Gulnaz Stybayeva: Supervision, Project administration, Writing – review & editing. Alexander Revzin: Conceptualization, Funding acquisition, Resources, Project administration, Supervision, Writing – review & editing.
Declaration of competing interest
The authors declare no competing financial interest.
Acknowledgements
This study was supported in part by the Center for Regenerative Medicine and Cells to Cures Strategic Initiative at Mayo Clinic, J.W. Kieckhefer Foundation, Al Nahyan Foundation, and NIH (DK107255 and P30DK084567). Additional support was provided by an NIH Grant EB021911 to HB.
Footnotes
Peer review under responsibility of KeAi Communications Co., Ltd.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bioactmat.2023.05.009.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Ohashi K., Yokoyama T., Yamato M., Kuge H., Kanehiro H., Tsutsumi M., Amanuma T., Iwata H., Yang J., Okano T. Engineering functional two-and three-dimensional liver systems in vivo using hepatic tissue sheets. Nat. Med. 2007;13(7):880–885. doi: 10.1038/nm1576. [DOI] [PubMed] [Google Scholar]
- 2.Chan C., Berthiaume F., Nath B.D., Tilles A.W., Toner M., Yarmush M.L. Hepatic tissue engineering for adjunct and temporary liver support: critical technologies. Liver Transplant. 2004;10(11):1331–1342. doi: 10.1002/lt.20229. [DOI] [PubMed] [Google Scholar]
- 3.Allen J.W., Bhatia S.N. Engineering liver therapies for the future. Tissue Eng. 2002;8(5):725–737. doi: 10.1089/10763270260424097. [DOI] [PubMed] [Google Scholar]
- 4.Mooney D., Hansen L., Vacanti J., Langer R., Farmer S., Ingber D. Switching from differentiation to growth in hepatocytes: control by extracellular matrix. J. Cell. Physiol. 1992;151(3):497–505. doi: 10.1002/jcp.1041510308. [DOI] [PubMed] [Google Scholar]
- 5.Demetriou A.A., Whiting J.F., Feldman D., Levenson S.M., Chowdhury N.R., Moscioni A.D., Kram M., Chowdhury J.R. Replacement of liver function in rats by transplantation of microcarrier-attached hepatocytes. Science. 1986;233(4769):1190–1192. doi: 10.1126/science.2426782. [DOI] [PubMed] [Google Scholar]
- 6.Moghe P.V., Berthiaume F., Ezzell R.M., Toner M., Tompkins R.G., Yarmush M.L. Culture matrix configuration and composition in the maintenance of hepatocyte polarity and function. Biomaterials. 1996;17(3):373–385. doi: 10.1016/0142-9612(96)85576-1. [DOI] [PubMed] [Google Scholar]
- 7.Michalopoulos G.K., Bowen W.C., Zajac V.F., Beer‐Stolz D., Watkins S., Kostrubsky V., Strom S.C. Morphogenetic events in mixed cultures of rat hepatocytes and nonparenchymal cells maintained in biological matrices in the presence of hepatocyte growth factor and epidermal growth factor. Hepatology. 1999;29(1):90–100. doi: 10.1002/hep.510290149. [DOI] [PubMed] [Google Scholar]
- 8.Shimaoka S., Nakamura T., Ichihara A. Stimulation of growth of primary cultured adult rat hepatocytes without growth factors by coculture with nonparenchymal liver cells. Exp. Cell Res. 1987;172(1):228–242. doi: 10.1016/0014-4827(87)90109-1. [DOI] [PubMed] [Google Scholar]
- 9.Bhatia S.N., Yarmush M.L., Toner M. Controlling cell interactions by micropatterning in co‐cultures: hepatocytes and 3T3 fibroblasts. J. Biomed. Mater. Res.: An Official Journal of The Society for Biomaterials and The Japanese Society for Biomaterials. 1997;34(2):189–199. doi: 10.1002/(sici)1097-4636(199702)34:2<189::aid-jbm8>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 10.Kim M., Lee J.Y., Jones C.N., Revzin A., Tae G. Heparin-based hydrogel as a matrix for encapsulation and cultivation of primary hepatocytes. Biomaterials. 2010;31(13):3596–3603. doi: 10.1016/j.biomaterials.2010.01.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Choi J.H., Loarca L., De Hoyos-Vega J.M., Dadgar N., Loutherback K., Shah V.H., Stybayeva G., Revzin A. Microfluidic confinement enhances phenotype and function of hepatocyte spheroids. Am. J. Physiol. Cell Physiol. 2020;319(3):C552–C560. doi: 10.1152/ajpcell.00094.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brophy C.M., Luebke‐Wheeler J.L., Amiot B.P., Khan H., Remmel R.P., Rinaldo P., Nyberg S.L. Rat hepatocyte spheroids formed by rocked technique maintain differentiated hepatocyte gene expression and function. Hepatology. 2009;49(2):578–586. doi: 10.1002/hep.22674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Glorioso J.M., Mao S.A., Rodysill B., Mounajjed T., Kremers W.K., Elgilani F., Hickey R.D., Haugaa H., Rose C.F., Amiot B. Pivotal preclinical trial of the spheroid reservoir bioartificial liver. J. Hepatol. 2015;63(2):388–398. doi: 10.1016/j.jhep.2015.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Farzaneh Z., Najarasl M., Abbasalizadeh S., Vosough M., Baharvand H. Developing a cost-effective and scalable production of human hepatic competent endoderm from size-controlled pluripotent stem cell aggregates. Stem Cell. Dev. 2018;27(4):262–274. doi: 10.1089/scd.2017.0074. [DOI] [PubMed] [Google Scholar]
- 15.Dhawan A., Chaijitraruch N., Fitzpatrick E., Bansal S., Filippi C., Lehec S.C., Heaton N.D., Kane P., Verma A., Hughes R.D. Alginate microencapsulated human hepatocytes for the treatment of acute liver failure in children. J. Hepatol. 2020;72(5):877–884. doi: 10.1016/j.jhep.2019.12.002. [DOI] [PubMed] [Google Scholar]
- 16.Li W., Zhang L., Ge X., Xu B., Zhang W., Qu L., Choi C.-H., Xu J., Zhang A., Lee H. Microfluidic fabrication of microparticles for biomedical applications. Chem. Soc. Rev. 2018;47(15):5646–5683. doi: 10.1039/c7cs00263g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Seo K., Kim D., Sanchez S. Fabrication and applications of complex-shaped microparticles via microfluidics. Lab Chip. 2015;15(18):3622–3626. doi: 10.1039/c5lc90091c. [DOI] [PubMed] [Google Scholar]
- 18.Dendukuri D., Doyle P.S. The synthesis and assembly of polymeric microparticles using microfluidics. Adv. Mater. 2009;21(41):4071–4086. [Google Scholar]
- 19.Yeh J., Ling Y., Karp J.M., Gantz J., Chandawarkar A., Eng G., Blumling J., Iii, Langer R., Khademhosseini A. Micromolding of shape-controlled, harvestable cell-laden hydrogels. Biomaterials. 2006;27(31):5391–5398. doi: 10.1016/j.biomaterials.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 20.Daly A.C., Riley L., Segura T., Burdick J.A. Hydrogel microparticles for biomedical applications. Nat. Rev. Mater. 2020;5(1):20–43. doi: 10.1038/s41578-019-0148-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Siltanen C., Diakatou M., Lowen J., Haque A., Rahimian A., Stybayeva G., Revzin A. One step fabrication of hydrogel microcapsules with hollow core for assembly and cultivation of hepatocyte spheroids. Acta Biomater. 2017;50:428–436. doi: 10.1016/j.actbio.2017.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fattahi P., Rahimian A., Slama M.Q., Gwon K., Gonzalez-Suarez A.M., Wolf J., Baskaran H., Duffy C.D., Stybayeva G., Peterson Q.P. Core–shell hydrogel microcapsules enable formation of human pluripotent stem cell spheroids and their cultivation in a stirred bioreactor. Sci. Rep. 2021;11(1):1–13. doi: 10.1038/s41598-021-85786-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huh C.-G., Factor V.M., Sánchez A., Uchida K., Conner E.A., Thorgeirsson S.S. Hepatocyte growth factor/c-met signaling pathway is required for efficient liver regeneration and repair. Proc. Natl. Acad. Sci. USA. 2004;101(13):4477–4482. doi: 10.1073/pnas.0306068101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hui E.E., Bhatia S.N. Micromechanical control of cell–cell interactions. Proc. Natl. Acad. Sci. USA. 2007;104(14):5722–5726. doi: 10.1073/pnas.0608660104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Khetani S.R., Bhatia S.N. Microscale culture of human liver cells for drug development. Nat. Biotechnol. 2008;26(1):120–126. doi: 10.1038/nbt1361. [DOI] [PubMed] [Google Scholar]
- 26.Jones C.N., Tuleuova N., Lee J.Y., Ramanculov E., Reddi A.H., Zern M.A., Revzin A. Cultivating hepatocytes on printed arrays of HGF and BMP7 to characterize protective effects of these growth factors during in vitro alcohol injury. Biomaterials. 2010;31(23):5936–5944. doi: 10.1016/j.biomaterials.2010.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ijima H., Kubo T., Hou Y.-T. Primary rat hepatocytes form spheroids on hepatocyte growth factor/heparin-immobilized collagen film and maintain high albumin production. Biochem. Eng. J. 2009;46(2):227–233. [Google Scholar]
- 28.Semler E.J., Ranucci C.S., Moghe P.V. Mechanochemical manipulation of hepatocyte aggregation can selectively induce or repress liver‐specific function. Biotechnol. Bioeng. 2000;69(4):359–369. doi: 10.1002/1097-0290(20000820)69:4<359::aid-bit2>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 29.Cao X.-f., Jin S.-z., Sun L., Zhan Y.-b., Lin F., Li Y., Zhou Y.-l., Wang X.-m., Gao L., Zhang B. Therapeutic effects of hepatocyte growth factor-overexpressing dental pulp stem cells on liver cirrhosis in a rat model. Sci. Rep. 2017;7(1):1–12. doi: 10.1038/s41598-017-14995-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Appasamy R., Tanabe M., Murase N., Zarnegar R., Venkataramanan R., Van Thiel D., Michalopoulos G. Hepatocyte growth factor, blood clearance, organ uptake, and biliary excretion in normal and partially hepatectomized rats. Laboratory investigation; a journal of technical methods and pathology. 1993;68(3):270–276. [PubMed] [Google Scholar]
- 31.Tae G., Kim Y.-J., Choi W.-I., Kim M., Stayton P.S., Hoffman A.S. Formation of a novel heparin-based hydrogel in the presence of heparin-binding biomolecules. Biomacromolecules. 2007;8(6):1979–1986. doi: 10.1021/bm0701189. [DOI] [PubMed] [Google Scholar]
- 32.Kato Y., Liu K.X., Nakamura T., Sugiyama Y. Heparin‐hepatocyte growth factor complex with low plasma clearance and retained hepatocyte proliferating activity. Hepatology. 1994;20(2):417–424. [PubMed] [Google Scholar]
- 33.Foster E., You J., Siltanen C., Patel D., Haque A., Anderson L., Revzin A. Heparin hydrogel sandwich cultures of primary hepatocytes. Eur. Polym. J. 2015;72:726–735. [Google Scholar]
- 34.Gwon K., Hong H.J., Gonzalez-Suarez A.M., Slama M.Q., Choi D., Hong J., Baskaran H., Stybayeva G., Peterson Q.P., Revzin A. Bioactive hydrogel microcapsules for guiding stem cell fate decisions by release and reloading of growth factors. Bioact. Mater. 2022;15:1–14. doi: 10.1016/j.bioactmat.2021.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kukla D.A., Crampton A.L., Wood D.K., Khetani S.R. Microscale collagen and fibroblast interactions enhance primary human hepatocyte functions in three-dimensional models. Gene Expr. 2020;20(1):1. doi: 10.3727/105221620X15868728381608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gwon K., Kim E., Tae G. Heparin-hyaluronic acid hydrogel in support of cellular activities of 3D encapsulated adipose derived stem cells. Acta Biomater. 2017;49:284–295. doi: 10.1016/j.actbio.2016.12.001. [DOI] [PubMed] [Google Scholar]
- 37.Gwon K., Hong H.J., Gonzalez-Suarez A.M., Stybayeva G., Revzin A. Microfluidic fabrication of core-shell microcapsules carrying human pluripotent stem cell spheroids. JoVE. 2021;176 doi: 10.3791/62944. [DOI] [PubMed] [Google Scholar]
- 38.Shah S.S., Kim M., Cahill-Thompson K., Tae G., Revzin A. Micropatterning of bioactive heparin-based hydrogels. Soft Matter. 2011;7(7):3133–3140. [Google Scholar]
- 39.Kakuni M., Yamasaki C., Tachibana A., Yoshizane Y., Ishida Y., Tateno C. Chimeric mice with humanized livers: a unique tool for in vivo and in vitro enzyme induction studies. Int. J. Mol. Sci. 2014;15(1):58–74. doi: 10.3390/ijms15010058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tateno C., Yoshizane Y., Saito N., Kataoka M., Utoh R., Yamasaki C., Tachibana A., Soeno Y., Asahina K., Hino H., Asahara T., Yokoi T., Furukawa T., Yoshizato K. Am. J. Pathol. 2004;165(3):901–912. doi: 10.1016/S0002-9440(10)63352-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sugahara G., Ishida Y., Sun J., Tateno C., Saito T. Art of making artificial liver: depicting human liver biology and diseases in mice. Semin. Liver Dis. 2020;40(2):189–212. doi: 10.1055/s-0040-1701444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sugahara G., Ishida Y., Lee J.J., Li M., Tanaka Y., Eoh H., Higuchi Y., Saito T. Long-term cell fate and functional maintenance of human hepatocyte through stepwise culture configuration. FASEB (Fed. Am. Soc. Exp. Biol.) J. 2023;37(2) doi: 10.1096/fj.202201292RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.de Hoyos‐Vega J.M., Hong H.J., Loutherback K., Stybayeva G., Revzin A. A microfluidic device for long‐term maintenance of organotypic liver cultures. Advanced Materials Technologies. 2022;8(2) doi: 10.1002/admt.202201121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Choi D., Gwon K., Hong H.J., Baskaran H., Calvo-Lozano O., Gonzalez-Suarez A.M., Park K., de Hoyos-Vega J.M., Lechuga L.M., Hong J. Coating bioactive microcapsules with tannic acid enhances the phenotype of the encapsulated pluripotent stem cells. ACS Appl. Mater. Interfaces. 2022;14(23):27274–27286. doi: 10.1021/acsami.2c06783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang F., Zheng L., Cheng S., Peng Y., Fu L., Zhang X., Linhardt R.J. Comparison of the interactions of different growth factors and glycosaminoglycans. Molecules. 2019;24(18):3360. doi: 10.3390/molecules24183360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Glicklis R., Merchuk J.C., Cohen S. Modeling mass transfer in hepatocyte spheroids via cell viability, spheroid size, and hepatocellular functions. Biotechnol. Bioeng. 2004;86(6):672–680. doi: 10.1002/bit.20086. [DOI] [PubMed] [Google Scholar]
- 47.Li W., Dick A., Lu F., Zhang H., Sun H. Induction of MET receptor tyrosine kinase down-regulation through antibody-mediated receptor clustering. Sci. Rep. 2019;9(1):1–18. doi: 10.1038/s41598-018-36963-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang H., Feng Q., Chen W.-D., Wang Y.-D. HGF/c-MET: a promising therapeutic target in the digestive system cancers. Int. J. Mol. Sci. 2018;19(11):3295. doi: 10.3390/ijms19113295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lamouille S., Xu J., Derynck R. Molecular mechanisms of epithelial–mesenchymal transition. Nat. Rev. Mol. Cell Biol. 2014;15(3):178–196. doi: 10.1038/nrm3758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Haque A., Gheibi P., Gao Y., Foster E., Son K.J., You J., Stybayeva G., Patel D., Revzin A. Cell biology is different in small volumes: endogenous signals shape phenotype of primary hepatocytes cultured in microfluidic channels. Sci. Rep. 2016;6(1):1–15. doi: 10.1038/srep33980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hegde M., Jindal R., Bhushan A., Bale S.S., McCarty W.J., Golberg I., Usta O.B., Yarmush M.L. Dynamic interplay of flow and collagen stabilizes primary hepatocytes culture in a microfluidic platform. Lab Chip. 2014;14(12):2033–2039. doi: 10.1039/c4lc00071d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sato S., Shirakawa H., Tomita S., Tohkin M., Gonzalez F.J., Komai M. The aryl hydrocarbon receptor and glucocorticoid receptor interact to activate human metallothionein 2A. Toxicol. Appl. Pharmacol. 2013;273:90–99. doi: 10.1016/j.taap.2013.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vrzal R., Stejskalova L., Monostory K., Maurel P., Bachleda P., Pavek P., Dvorak Z. Dexamethasone controls aryl hydrocarbon receptor (AhR)-mediated CYP1A1 and CYP1A2 expression and activity in primary cultures of human hepatocytes. Chem. Biol. Interact. 2009;179:288–296. doi: 10.1016/j.cbi.2008.10.035. [DOI] [PubMed] [Google Scholar]
- 54.Eiselleova L., Matulka K., Kriz V., Kunova M., Schmidtova Z., Neradil J., Tichy B., Dvorakova D., Pospisilova S., Hampl A. A complex role for FGF-2 in self-renewal, survival, and adhesion of human embryonic stem cells. Stem Cell. 2009;27(8):1847–1857. doi: 10.1002/stem.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dvorak P., Dvorakova D., Koskova S., Vodinska M., Najvirtova M., Krekac D., Hampl A. Expression and potential role of fibroblast growth factor 2 and its receptors in human embryonic stem cells. Stem Cell. 2005;23(8):1200–1211. doi: 10.1634/stemcells.2004-0303. [DOI] [PubMed] [Google Scholar]
- 56.Savageau M.A., Coelho P.M., Fasani R.A., Tolla D.A., Salvador A. Phenotypes and tolerances in the design space of biochemical systems. Proc. Natl. Acad. Sci. USA. 2009;106(16):6435–6440. doi: 10.1073/pnas.0809869106. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.