Key Points
-
•
Combined deletion of pyruvate dehydrogenase kinase (PDK) 2 and 4 inhibits platelet function and arterial thrombosis.
-
•
PDK2/4 contributes to platelet biology by regulating pyruvate dehydrogenase phosphorylation and aerobic glycolysis in activated platelets.
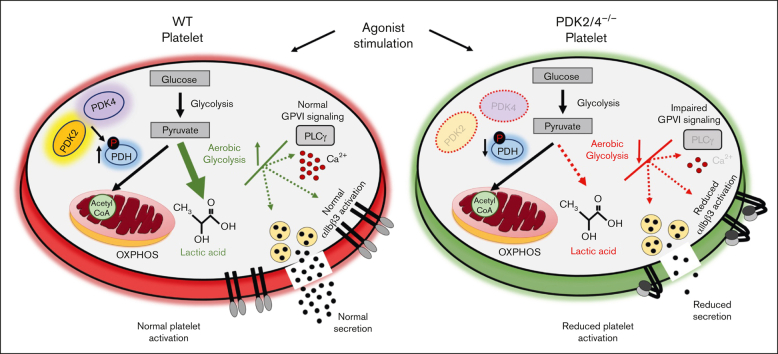
Visual Abstract
Abstract
Resting platelets rely on oxidative phosphorylation (OXPHOS) and aerobic glycolysis (conversion of glucose to lactate in the presence of oxygen) for their energy requirements. In contrast, platelet activation exhibits an increased rate of aerobic glycolysis relative to OXPHOS. Mitochondrial enzymes pyruvate dehydrogenase kinases (PDKs) phosphorylate the pyruvate dehydrogenase (PDH) complex to inhibit its activity, thereby diverting the pyruvate flux from OXPHOS to aerobic glycolysis upon platelet activation. Of 4 PDK isoforms, PDK2 and PDK4 (PDK2/4) are predominantly associated with metabolic diseases. Herein, we report that the combined deletion of PDK2/4 inhibits agonist-induced platelet functions, including aggregation, integrin αIIbβ3 activation, degranulation, spreading, and clot retraction. In addition, collagen-mediated PLCγ2 phosphorylation and calcium mobilization were significantly reduced in PDK2/4−/− platelets, suggesting impaired GPVI signaling. The PDK2/4−/− mice were less susceptible to FeCl3-induced carotid and laser-induced mesenteric artery thrombosis without any effect on hemostasis. In adoptive transfer experiments, thrombocytopenic hIL-4Rα/GPIbα-transgenic mice transfused with PDK2/4−/− platelets exhibited less susceptibility to FeCl3 injury–induced carotid thrombosis compared with hIL-4Rα/GPIbα-Tg mice transfused with WT platelets, suggesting a platelet-specific role of PDK2/4 in thrombosis. Mechanistically, the inhibitory effects of PDK2/4 deletion on platelet function were associated with reduced PDH phosphorylation and glycoPER in activated platelets, suggesting that PDK2/4 regulates aerobic glycolysis. Finally, using PDK2 or PDK4 single KO mice, we identified that PDK4 plays a more prominent role in regulating platelet secretion and thrombosis compared with PDK2. This study identifies the fundamental role of PDK2/4 in regulating platelet functions and identifies the PDK/PDH axis as a potentially novel antithrombotic target.
Introduction
Metabolic pathways are increasingly recognized as potential therapeutic targets in different cell types to treat numerous disorders.1, 2, 3, 4, 5 The role of metabolic regulatory mechanisms in modulating platelet activation is not well understood.6, 7, 8 Despite being anucleated, mitochondria and essential metabolites in platelets participate in different bioenergetic pathways to meet the energy requirements in both resting and high energy–driven activated states.9 It is now known that resting platelets rely on both aerobic glycolysis (conversion of glucose to lactate in the presence of oxygen, a phenomenon known as the Warburg effect) and oxidative phosphorylation (OXPHOS) for energy requirement, whereas platelet activation is characterized by a preferentially increased rate of aerobic glycolysis relative to OXPHOS.6,10, 11, 12
Mitochondrial pyruvate dehydrogenase (PDH) complex converts pyruvate (the end product of glycolysis) to acetyl coenzyme A, which is further oxidized via the tricarboxylic acid (TCA) cycle to produce ATP using the OXPHOS pathway. Upon platelet activation, the pyruvate dehydrogenase kinase (PDKs) phosphorylates the E1α subunit of PDH to inhibit its activity, which diverts the pyruvate flux from the TCA cycle to aerobic glycolysis (lactic acid production). Therefore, the PDK/PDH axis is an essential metabolic switch that regulates pyruvate flux during platelet activation. Four isozymes of PDK exist (PDK1-4) in humans and rodents. We recently reported that blocking the activity of PDKs using dichloroacetic acid (DCA) inhibits platelet functions in human and murine platelets.6 However, the specificity of DCA to inhibit PDKs and the underlying mechanisms by which PDKs regulate platelet functions remain unclear.
PDK2 and PDK4 have ubiquitous tissue distribution and are prominently associated with metabolic diseases, in contrast to PDK1 and PDK3.13, 14, 15 Therefore, we sought to examine the mechanistic role of PDK2/4 in platelet biology and arterial thrombosis. Here, we report that the combined genetic ablation of PDK2 and PDK4 inhibits a range of platelet functions, including agonist-induced platelet aggregation, integrin αIIbβ3 activation, and granule secretion. The PDK2/4−/− mice were less susceptible to arterial thrombosis without altering the hemostatic response. Mechanistically, impaired platelet functions and reduced susceptibility to experimental thrombosis in the PDK2/4−/− mice were attributed to the negative regulation of PDH phosphorylation and aerobic glycolysis in activated platelets. Finally, we demonstrated the platelet-specific role of PDK2/4 and the individual contributions of PDK2 and PDK4 in regulating platelet function and arterial thrombosis.
Materials and methods
Detailed information on the materials and methods used in the study is available in the online supplement.
Mice
We generated wild-type (WT) (PDK2/4+/+), double-knockout (PDK2/4−/−), and single-knockout mice (PDK2−/− and PDK4−/−) from heterozygous (PDK2+/−PDK4+/−) mice. The male and female mice used in the current study were on the C57BL/6J background and aged between 10 to 12 weeks. Mice were genotyped using the polymerase chain reaction according to the manufacturer’s protocols and as previously described.7 The mice were kept in standard animal housing conditions with controlled temperature and humidity, and they had ad libitum access to a standard chow diet and water. The University of Iowa Animal Care and Use Committee approved all experiments.
Statistical analysis
Data are represented as mean ± standard error (SEM) in all the figures. The statistical significance involving 2 groups was assessed using a Mann-Whitney U test. For more than 2 groups, significance was determined by 1-way ANOVA or 2-way ANOVA followed by Tukey or Sidak multiple comparisons test. P < .05 was considered to be statistically significant. GraphPad Prism software version 9 was used for statistical analysis.
Results
Combined genetic deletion of PDK2 and PDK4 impairs platelet function
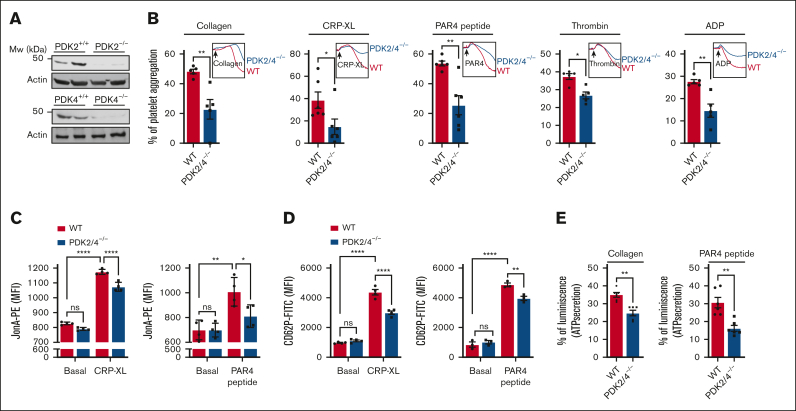
Using a polymerase chain reaction, we confirmed the global deletion of PDK2 and PDK4 (PDK2/4) (supplemental Figure 1A). In parallel, using western blot we confirmed the deletion of PDK2/4 in platelets (Figure 1A). The body weight, complete blood count (supplemental Table 1), and expression levels of significant platelet surface receptors, including integrin αIIbβ3, GPIb, and GPVI, were comparable between PDK2/4−/− and WT mice (supplemental Figure 1B). To determine the effects of combined deletion of PDK2/4 on platelet function, we evaluated the extent of platelet aggregation in PDK2/4−/− platelets stimulated by various agonists. The platelets from PDK2/4−/− mice exhibited significantly reduced aggregation upon stimulation with GPVI agonists [collagen or crosslinked collagen-related peptide (CRP-XL)] or G-protein-coupled receptor (GPCR) agonists (PAR4 peptide, thrombin, or ADP) compared to WT platelets (Figure 1B). The platelet aggregation defect in PDK2/4−/− platelets was not observed at higher concentrations of agonist stimulation, except in the case of PAR4 peptide stimulation (supplemental Figure 2A). Both GPVI and GPCR stimulation facilitate “inside-out” signaling in platelets that culminates in affinity upregulation of integrin αIIbβ3, leading to the formation of platelet aggregates. Consistent with the inhibition of platelet aggregation, JonA binding to activated integrin αIIbβ3 was significantly reduced in CRP-XL or PAR4 peptide-stimulated PDK2/4−/− platelets than in WT platelets (Figure 1C). The integrin αIIbβ3 expression level was comparable in CRP-XL or PAR4 peptide-stimulated WT and PDK2/4−/− platelets (supplemental Figure 2B). To investigate whether deletion of PDK2/4 affects apoptosis, we evaluated phosphatidylserine exposure (a marker of cellular apoptosis) on the platelet surface by measuring annexin V binding. No difference in annexin V binding was observed in resting or agonist-stimulated PDK2/4−/− platelets compared with WT platelets (supplemental Figure 2C).
Figure 1.
PDK2/4-double-deficient mice exhibit reduced platelet functions. (A)The deletion of PDK2 and PDK4 was confirmed using western blotting. (B) Platelet-rich plasma (PRP) from WT or PDK2/4−/− mice was stimulated with collagen (0.4 μg/mL), CRP-XL (0.05 μg/mL), PAR4 peptide (40 μM), thrombin (0.013 U/mL; washed platelelets were used), or ADP (0.13 μM). Results are expressed as the percent change in light transmission with respect to the blank (buffer without platelets), set at 100%. The representative aggregation curves are shown. Values are mean ± SEM, n = 5 to 6 mice per group. Statistical analysis: Mann-Whitney U test; ∗P < .05 and ∗∗P < .01. (C) The extent of integrin αIIbβ3 activation (using JonA binding), and (D) α-granule secretion (using P-selectin exposure) in WT and PDK2/4−/− platelets (in PRP) stimulated with CRP-XL (0.1 μg/mL) or PAR4 peptide (100 μM) was determined using flow cytometry. Values are mean ± SEM, n = 3 to 4 mice per group. Statistical analysis: 2-way ANOVA followed by Tukey multiple comparisons test; ∗P < .05; ∗∗P < .01 and ∗∗∗∗P < .0001. (E) ATP secretion from the dense granules of WT and PDK2/4−/− platelets (in PRP) after stimulation with collagen (0.8 μg/mL) or PAR4 peptide (70 μM) was measured using Lumi-aggregometry. Values are mean ± SE n = 6 mice per group. Statistical analysis: Mann-Whitney U test; ∗∗P < .01.
Next, we evaluated the effects of PDK2/4 deletion on granule secretion from platelets. Compared with WT platelets, a considerable reduction in the P-selectin exposure (a marker of α-granules) on the platelet surface was observed in PDK2/4−/− platelets after stimulation with CRP-XL or PAR4 peptide (Figure 1D). Furthermore, ATP release (a marker of dense granules) was inhibited in PDK2/4−/− platelets after stimulation with collagen or PAR4 peptide compared with WT platelets (Figure 1E). These findings suggest a fundamental role of PDK2/4 in regulating different aspects of platelet activation associated with “inside-out” signaling.
PDK2/4−/− platelets exhibit reduced outside-in signaling and in vitro thrombus formation
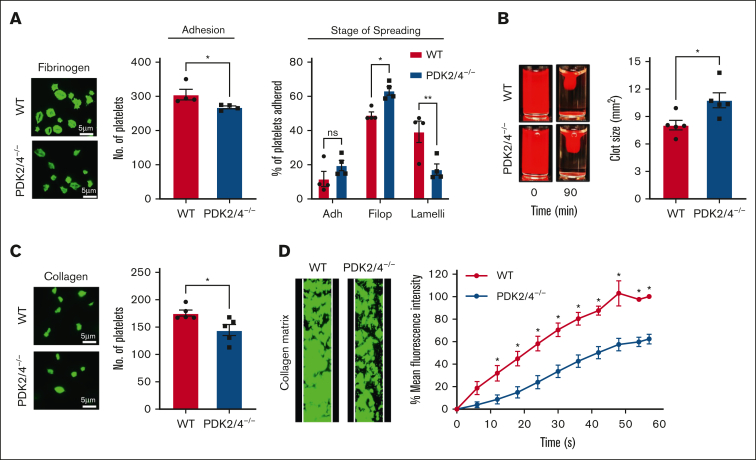
The binding of fibrinogen to integrin αIIbβ3 facilitates “outside-in” signaling in platelets, which enables platelet spreading and clot retraction, necessary for the stability of the growing thrombus. Therefore, we investigated the effects of PDK2/4 deletion on platelet spreading (on fibrinogen-coated coverslips) and fibrin clot retraction, regarded as the direct outcomes of “outside-in” signaling. For spreading, platelets were scored into 3 categories: adhered (but not spread), filopodium formation (in the process of spreading), or lamellipodium formation (fully spread).16, 17, 18 The percentage of platelets that adhered to fibrinogen but did not spread was comparable between WT and PDK2/4−/− platelets (Figure 2A). However, the extent of platelet spreading was significantly attenuated in PDK2/4−/− platelets as fewer platelets reached the lamellipodial stage, whereas a significant proportion of PDK2/4−/− platelets remained restricted to the intermediate filopodial stage compared with WT platelets (Figure 2A). Consistent with this, platelets from PDK2/4−/− mice exhibited significantly reduced fibrin clot retraction (determined by measuring the clot size) compared with WT platelets (Figure 2B). These findings suggest the ability of PDK2/4 to regulate bidirectional signaling across integrin αIIbβ3.
Figure 2.
PDK2/4−/− platelets exhibit reduced outside-in signaling and in vitro thrombus formation. (A) WT or PDK2/4−/− washed platelets (2 × 107 cells/mL) stained using Alexa-Fluor 488-conjugated phalloidin were stimulated with PAR4 peptide (70 μM) for 10 minutes and added onto fibrinogen-coated (100 μg/mL) coverslips for 120 minutes. Five images were captured of each sample at random locations. A representative image of platelet adhesion and spreading is shown. Spreading platelets were divided into 3 classes: adhered (Adh) but not spread, filopodia (Filop): spreading platelets and lamellipodia (Lamelli): fully spread. Results are expressed as a percentage of the total number of platelets adhered. Cumulative data of the number of platelets adhered in each sample is shown. Values are mean ± SEM, n = 4 mice per group. Statistical analysis: Mann-Whitney U test (adhesion) or 2-way ANOVA (stage of spreading) followed by Sidak multiple comparisons test; ∗P < .05 and ∗∗P < .01. (B) Clot retraction was measured for 90 minutes in PRP, supplemented with red blood cells, after adding 1 U/mL of thrombin. The left panel shows the representative images at 90 minutes, and the right panel shows the quantification of the clot size. Values are mean ± SEM, n = 5 mice per group. Statistical analysis: Mann-Whitney U test; ∗P < .05. (C) WT or PDK2−/− washed platelets (2 × 107 cells/mL) stained using Alexa-Fluor 488-conjugated phalloidin were added onto collagen-coated (100 μg/mL) coverslips for 45 minutes. Five images were captured of each sample at random locations. A representative image of platelet adhesion is shown. Cumulative data of the number of platelets adhered in each sample is shown. Values are mean ± SEM, n = 5 mice per group. Statistical analysis: Mann-Whitney U test; ∗P < .05. (D) DiOC6-loaded whole blood from WT or PDK2/4−/− mouse was perfused over a collagen-coated (100 μg/mL) matrix for 60 seconds at an arterial shear rate in a BioFlux Microfluidic flow chamber system. The left panel shows the representative image at the end of the assay, and the right panel shows the thrombus growth on the collagen matrix over time. Values are mean ± SEM, n = 3 mice per group. Statistical analysis: 2-way ANOVA followed by Sidak multiple comparisons test; ∗P < .05.
Furthermore, we observed that the adhesion of PDK2/4−/− platelets on collagen-coated coverslips was also inhibited compared with WT platelets (Figure 2C). Next, to determine whether combined deletion of PDK2/4 inhibits platelet activation in the presence of other blood cells, thrombus formation in vitro was examined using whole blood that was perfused over a collagen matrix at an arterial shear in a microfluidic flow chamber. The rate of thrombus growth and thrombus-covered surface were substantially reduced in whole blood from PDK2/4−/− mice compared with WT mice (Figure 2D).
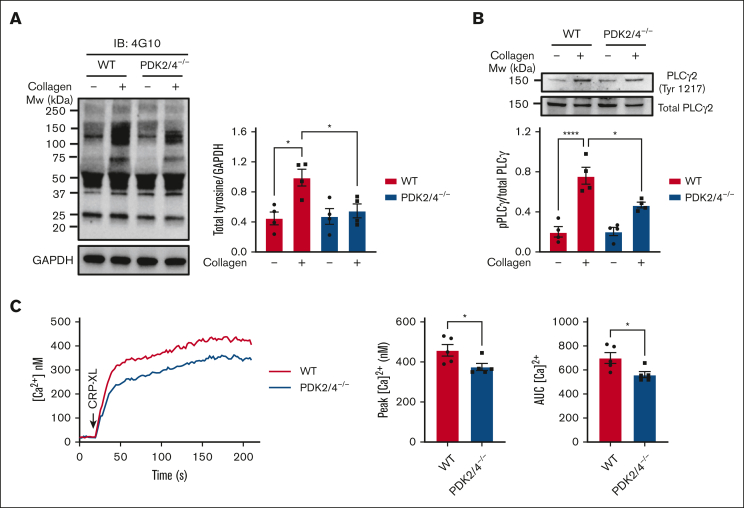
Combined deletion of PDK2/4 inhibits GPVI-mediated platelet signaling
We next examined whether ablation of PDK2/4 affects signaling upon platelet activation. We observed a negative regulation of collagen-induced total tyrosine phosphorylation, which suggests the ability of PDK2/4 to regulate the molecular events associated with GPVI signaling (Figure 3A). The upregulation of phospholipase Cγ2 is a characteristic feature that plays a central role in GPVI-mediated platelet signaling. PLCγ2 phosphorylation directly facilitates the elevation of cytosolic calcium, which further enables platelet secretion and integrin αIIbβ3 affinity upregulation. In comparison with WT controls, collagen-stimulated PDK2/4−/− platelets demonstrated reduced PLCγ2 phosphorylation at Tyr1217 (Figure 3B). Consistent with this, a PLCγ2-dependent rise in cytosolic calcium levels was reduced in CRP-XL–mediated PDK2/4−/− platelets compared with those in WT platelets (Figure 3C). No effect on PAR4 peptide-stimulated total tyrosine (supplemental Figure 3A) or protein kinase C (PKC) substrate phosphorylation (supplemental Figure 3B) was observed in PDK2/4−/− platelets.
Figure 3.
Platelets from PDK2/4 double-deficient mice exhibit impaired GPVI-mediated signaling. (A) Total tyrosine phosphorylation and (B) phospho-PLCγ2 phosphorylation (Tyr 1217) were measured in resting and collagen (25 μg/mL)–stimulated WT and PDK2/4−/− platelets. Representative western blots for total tyrosine phosphorylation and phospho-PLCγ2 (Tyr 1217) are shown. GAPDH or total PLCγ2 were used as loading controls. The bar graphs show densitometry analysis of immunoblots. Values are mean ± SEM, n = 4 mice per group. Statistical analysis: 2-way ANOVA followed by Tukey's multiple comparisons test; ∗P < .05 and ∗∗∗∗P < .0001. (C) Calcium mobilization was evaluated in Fura-2 LR loaded WT or PDK2/4−/− platelets washed platelets that were stimulated with CRP-XL (0.7 μg/mL) in the presence of 1.3 mM CaCl2. The left panel shows representative calcium mobilization after stimulation with CRP-XL. The middle and right panel shows the peak calcium level and the area under the curve (AUC), respectively. Values are mean ± SEM, n = 5 mice per group. Statistical analysis: Mann-Whitney U test; ∗P < .05.
PDK2/4−/− mice demonstrate reduced susceptibility toward arterial thrombosis without altering hemostasis
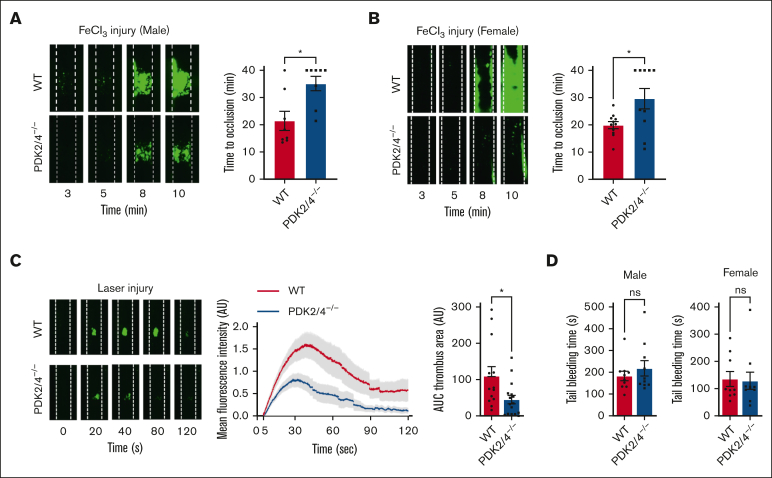
Given the inhibitory effects of combined PDK2/4 deletion on multiple aspects of platelet activation, we evaluated the role of PDK2/4 in regulating in vivo arterial thrombosis and hemostasis. Male WT and PDK2/4−/− mice were subjected to 5% FeCl3 injury–induced carotid artery thrombosis. The susceptibility to arterial thrombosis was significantly reduced in PDK2/4−/− mice, as suggested by a significantly prolonged occlusion time of the injured vessel compared with WT mice (Figure 4A). Previous studies have reported that sex-based differences in rodents affect platelet function and thrombosis. Male mice are more susceptible to thrombosis than females.19,20 This is mainly attributed to the cardioprotective roles of endogenous estrogens in female mice.21,22 Therefore, to rule out sex-based differences, we performed 5% FeCl3 injury–induced carotid artery thrombosis in female mice. Similar to male mice, the time to occlusion of the injured carotid vessel was prolonged in the PDK2/4−/− female mice compared with WT female mice (Figure 4B). In addition, we performed laser injury–induced mesenteric artery thrombosis to rule out that the observed effects on arterial thrombosis were not model-specific. The kinetics of thrombus growth (determined by measuring fluorescence intensity) and thrombus size (determined by measuring surface area) were significantly reduced in PDK2/4−/− mice than in WT mice (Figure 4C). Despite the effect on thrombosis, no effect on tail-bleeding time was observed between WT and PDK2/4−/− mice (both males and females) (Figure 4D), suggesting PDK2/4 deletion does not affect hemostasis.
Figure 4.
PDK2/4−/− mice are less susceptible to experimental arterial thrombosis. (A) A representative image of carotid artery thrombus (5% FeCl3 injury for 2 minutes) and the time to occlusion in WT and PDK2/4−/− male mice is shown. The time to occlusion was measured until 40 minutes, the cutoff point at which the experiment was terminated. Values are mean ± SEM, n = 8 vessels from 8 mice per group. Statistical analysis: Mann-Whitney U test; ∗P < .05. (B) A representative image of carotid artery thrombus (5% FeCl3 injury for 2 minutes) and the time to occlusion in WT and PDK2/4−/− female mice is shown. Values are mean ± SEM, n = 10 to 11 vessels from 6 mice per group. Statistical analysis: Mann-Whitney U test; ∗P < .05. (C) A representative image of laser injury–induced mesenteric artery thrombus in WT and PDK2/4−/− male mice as visualized by upright intravital microscopy. The mean fluorescence intensity over time and AUC (thrombus area) is shown. Values are mean ± SEM, n = 13 to 16 thrombi from 4 mice per group. Statistical analysis: Mann-Whitney U test; ∗P < .05. (D) The tail-bleeding time in WT and PDK2/4−/− male and female mice was determined by measuring the time taken for the initial cessation of bleeding after the tail transection. Values are mean ± SEM, n = 10 mice per group. Statistical analysis: Mann-Whitney U test.
Platelet-specific PDK2/4 regulates arterial thrombosis
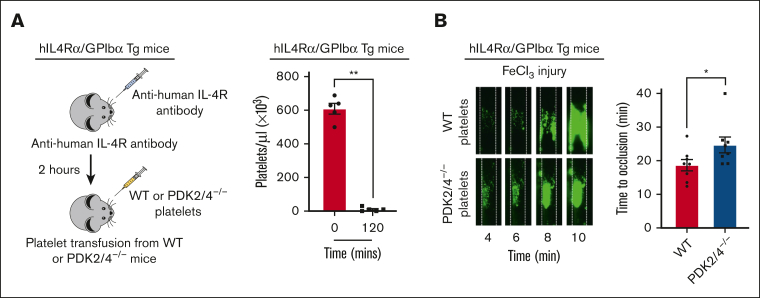
The PDK2/4−/− mice used in this study are global knockouts, and platelet-specific PDK-floxed mice (for all the isoforms) do not exist.6 To exclude the effects on thrombosis elicited by endothelium, neutrophils, and monocytes (known to interact with platelets to facilitate thrombus formation),23,24 we performed an adoptive transfer of PDK2/4−/− or WT platelets in thrombocytopenic hIL-4Rα/GPIbα transgenic (Tg) mice. The platelets from hIL-4Rα/GPIbα-Tg mice express a chimeric protein composed of the extracytoplasmic domain of the human interleukin-4 receptor (hIL-4Rα) fused to the transmembrane and cytoplasmic tail of GPIbα.25, 26, 27 Platelet depletion through the infusion of anti–hIL-4Rα does not cause hypothermia compared with that of anti-αIIbβ3 antibodies, and unlike anti-GPIbα antibodies, it does not target exogenous platelets that are transfused into the thrombocytopenic mice.26 As expected, the treatment with anti–hIL-4Rα antibody depleted the number of circulating platelets within 2 hours (Figure 5A), after which platelets were transfused from either WT or PDK2/4−/− mice. The time to vessel occlusion after 5% FeCl3 injury–induced carotid artery thrombosis was significantly prolonged in hIL-4Rα/GPIbα-Tg mice transfused with PDK2/4−/− platelets compared with hIL-4Rα/GPIbα-Tg mice transfused with WT platelets (Figure 5B). These findings delineate the platelet-specific role of PDK2/4 in regulating arterial thrombosis. Notably, we found that the effect of complete deletion of PDK2/4 on arterial thrombosis was more pronounced (62.5% mice did not occlude, Figure 4A) than the thrombocytopenic hIL-4Rα/GPIbα-Tg mice transfused with PDK2/4−/− platelets (12.5%, Figure 5B). We speculate that such a robust effect on arterial thrombosis in PDK2/4-/- mice might be due to a lack of PDK2/4 in other cell types in addition to platelets, such as neutrophils, which are known to exhibit a high rate of aerobic glycolysis and are known to contribute to thrombosis.
Figure 5.
Platelet-specific PDK2/4 contributes to arterial thrombosis. (A) The left panel demonstrates the schematic depicting the technique for generating mice with platelet-specific PDK2 and PDK4 deficiency. Thrombocytopenia was induced in hIL-4Rα/GPIbα-Tg mice by injecting antibodies against human IL-4R and platelet count was measured after 2 hours. N = 5 mice per group. Statistical analysis: Mann-Whitney U test; ∗∗P < .01. This was followed by the adoptive transfer of WT or PDK2/4−/− platelets into hIL-4Rα/GPIbα-Tg mice. (B) The left panel shows a representative image of carotid artery thrombus (5% FeCl3 injury for 3 minutes) as visualized by intravital microscopy in hIL-4Rα/GPIbα-Tg male mice transfused with WT or PDK2/4−/− platelets. The right panel shows the time to occlusion. The time to occlusion was measured until 40 minutes, the cutoff point at which the experiment was terminated. Values are mean ± SEM, n = 8 vessels from 4 mice per group. Data represents left and right carotid artery injury from each mouse. Statistical analysis: Mann-Whitney U test; ∗P < .05.
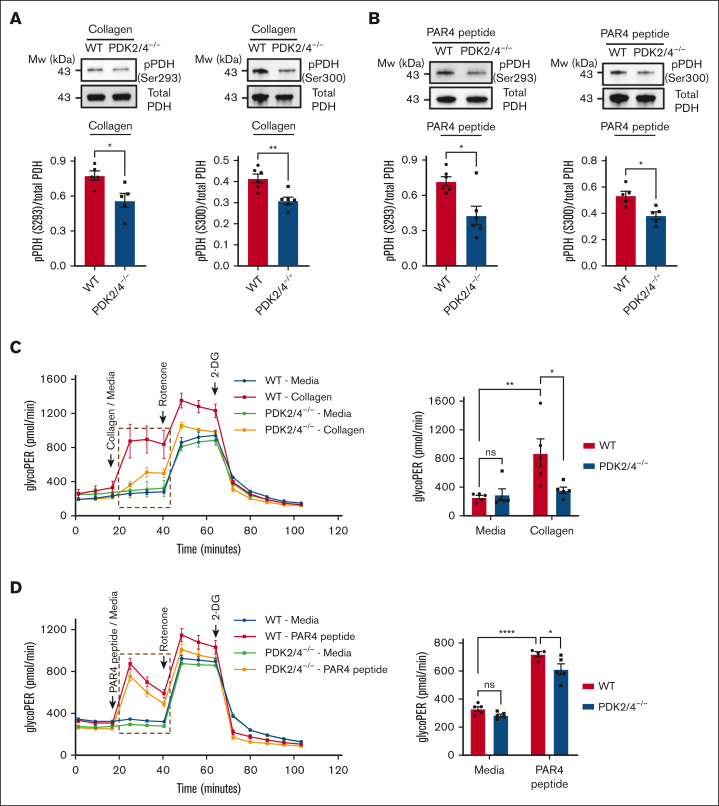
Combined deletion of PDK2/4 inhibits PDH phosphorylation and aerobic glycolysis in activated platelets
PDKs alter the activity of the PDH complex by phosphorylating their E1α subunit upon platelet activation, which enhances the pyruvate flux toward aerobic glycolysis relative to OXPHOS.6 Therefore, we examined whether deletion of PDK2/4 affects the phosphorylation state of PDH in activated platelets. Compared with WT platelets, PDK2/4−/− platelets exhibited significantly reduced collagen- (Figure 6A) and PAR4 peptide (Figure 6B)–stimulated PDH phosphorylation at Ser293 and Ser300. Next, we evaluated the effects of reduced PDH phosphorylation on aerobic glycolysis in PDK2/4−/− platelets. Using a Seahorse extracellular flux analyzer, we measured the total proton efflux rate (total PER) and glycolytic proton efflux rate (glycoPER) in resting and collagen or PAR4 peptide-stimulated WT and PDK2/4−/− platelets. The lactic acid production by aerobic glycolysis and CO2 generated via mitochondrial OXPHOS are the 2 primary sources of extracellular protons in live cells. They formulate the cells' total PER. GlycoPER is measured by subtracting the mitochondrial CO2-dependent acidification from the total PER, which directly estimates aerobic glycolysis and extracellular lactate production.28 In contrast, the oxygen consumption rate (OCR) estimates the level of mitochondrial OXPHOS.6 The extent of total PER after collagen- (supplemental Figure 4A) or PAR4 peptide (supplemental Figure 4B)–mediated activation was significantly inhibited in PDK2/4−/− platelets compared with WT platelets. In alignment with the inhibition of total PER and reduction of PDH phosphorylation in activated PDK2/4−/− platelets, the level of glycoPER in collagen (Figure 6C) or PAR4 peptide (Figure 6D) –stimulated PDK2/4−/− platelets was substantially reduced compared with WT platelets. This finding suggests a negative regulation of aerobic glycolysis and lactic acid production in activated PDK2/4−/− platelets compared with WT controls. Although there was a significant increase in the level of OCR in WT platelets upon collagen stimulation, no significant difference in the OCR between collagen-stimulated WT and PDK2/4−/− platelets was observed (supplemental Figure 4C). These findings suggest a key role of the PDK/PDH axis in regulating aerobic glycolysis during the transition of platelets from a resting to an activated state.
Figure 6.
Deletion of PDK2 and PDK4 inhibits PDH phosphorylation and aerobic glycolysis in activated platelets. PDH phosphorylation at Ser293 and Ser300 was measured in (A) collagen- (25 μg/mL) or (B) PAR4 peptide (70μM)–stimulated WT or PDK2/4−/− platelets. Representative western blots are shown. Total PDH was used as a loading control. The bar graphs show densitometry analysis of immunoblots. Values are mean ± SEM, n = 5 to 6 mice per group. Statistical analysis: Mann-Whitney U test; ∗P < .05 and ∗∗P < .01. The glycolytic proton efflux rate (glycoPER) was measured in WT or PDK2/4−/− platelets stimulated with (C) collagen (25 μg/mL) or (D) PAR4 peptide (50μM) using a Seahorse extracellular flux analyzer. The bar graph shows the quantified data (for the line graph values marked with a box). Values are mean ± SEM, with n = 4 to 5 mice per group. Statistical analysis: 2-way ANOVA followed by Tukey multiple comparisons test; ∗P < .05; ∗∗P < .01 and ∗∗∗∗P < .0001.
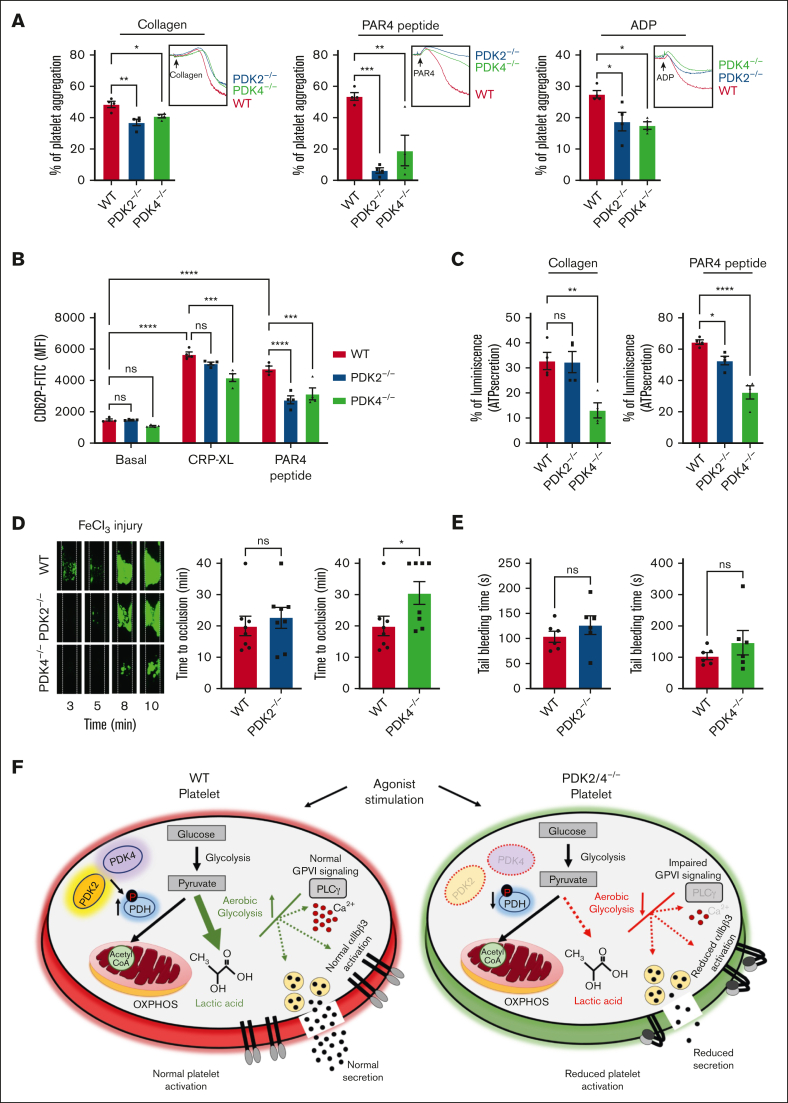
The role of PDK4 is indispensable for the regulation of arterial thrombosis
Given the wide range of inhibitory effects instigated by the combined deletion of PDK2/4 on platelet functions and thrombosis, we sought to dissect the individual contribution of these 2 PDK isoforms in platelets. The level of aggregation was significantly attenuated in collagen-, PAR4 peptide-, or ADP-stimulated platelets from PDK2−/− and PDK4−/− mice compared with WT platelets (Figure 7A). Platelets from PDK4−/− mice exhibited significantly reduced P-selectin exposure upon stimulation with either CRP-XL or PAR4 peptide compared with the WT control. In contrast, PDK2−/− platelets demonstrated a selective inhibition against PAR4 peptide–evoked P-selectin exposure but not against CRP-XL stimulation (Figure 7B). Similar results were observed with dense granule secretion (Figure 7C). These findings suggest a potentially important role of PDK4 in modulating platelet secretion in comparison to PDK2. Next, we investigated the individual contributions of PDK2 and PDK4 in regulating in vivo arterial thrombosis. The time to complete occlusion of the injured vessel was comparable between PDK2−/− and WT mice in the 5% FeCl3 injury–induced carotid thrombosis model. However, the susceptibility to arterial thrombosis was significantly reduced in PDK4−/− mice, as suggested by a significantly prolonged occlusion time exhibited by the injured carotid vessel compared to WT mice (Figure 7D). No effect of either PDK2 or PDK4 deletion on the tail-bleeding time was observed compared with WT mice (Figure 7E). These findings suggest a significant role of PDK4 compared with PDK2 in regulating arterial thrombosis in vivo. We also observed the expression of PDK1 and PDK3 in WT platelets. Their expression was unaltered by the deletion of PDK2 and PDK4 (supplemental Figure 5A). The treatment of PDK2/4−/− platelets with a global PDK inhibitor, DCA (25 mM), caused a further inhibition in the extent of collagen-stimulated PDK2/4−/− platelet aggregation (supplemental Figure 5B), suggesting that PDK1 or PDK3 (or both) might potentially contribute toward platelet activation in addition to PDK 2/4.
Figure 7.
PDK4 is the major determinant contributing to thrombosis in the FeCl3 injury–induced carotid thrombosis model. (A) Platelet-rich plasma (PRP) from WT or PDK2−/− or PDK4−/− mice was stimulated with collagen (0.4 μg/mL), PAR4 peptide (40 μM), or ADP (0.13 μM). Results are expressed as the percent change in light transmission with respect to the blank (buffer without platelets), set at 100%. The representative aggregation curves are shown. Values are mean ± SEM, n = 4 mice per group. Statistical analysis: 1-way ANOVA followed by Tukey's multiple comparisons test ∗P < .05; ∗∗P < .01 and ∗∗∗P < .001 (B) The extent of α-granule secretion (using P-selectin exposure) in WT, PDK2−/− and PDK4−/− platelets (in PRP) stimulated with CRP-XL (0.1 μg/mL) or PAR4 peptide(100 μM) was determined using flow cytometry. Values are mean ± SEM, n = 4 mice per group. Statistical analysis: 2-way ANOVA followed by Tukey's multiple comparisons test; ∗∗∗P < .001 and ∗∗∗∗P < .0001. (C) ATP secretion from the dense granules of WT, PDK2−/−, and PDK4−/− platelets (in PRP) after stimulation with collagen (0.8 μg/mL) or PAR4 peptide (70 μM) was measured using Lumi-aggregometry. Values are mean ± SE n = 4 mice per group. Statistical analysis: 1-way ANOVA followed by Tukey's multiple comparisons test ∗P < .05; ∗∗P < .01 and ∗∗∗∗P < .0001. (D) A representative image of carotid artery thrombus (5% FeCl3 injury for 2 minutes) as visualized by intravital microscopy in WT, PDK2−/−, and PDK4−/− male mice. Platelets were labeled ex vivo with calcein green. The time to occlusion is shown. These experiments were performed in the same setup with the same WT mice serving as a control group for both PDK2−/− and PDK4−/− mice. Values are mean ± SEM, n = 8 mice per group. Statistical analysis: Mann-Whitney U test. ∗P < .05 (E) The tail-bleeding time in WT, PDK2−/−, and PDK4−/− male mice was determined by the time taken for the initial cessation of bleeding after the tail transection. Values are mean ± SEM, n = 6 mice per group. Statistical analysis: Mann-Whitney U test. (F) A schematic depicting the role of PDK/PDH axis in regulating platelet activation.
Discussion
Platelets play a central role in coronary artery disease and the development of acute coronary syndromes,29 as confirmed by the beneficial effects of antiplatelet drugs, such as P2Y12 receptor antagonists (clopidogrel, prasugrel, and cangrelor) or cyclooxygenase inhibitor (aspirin). These antiplatelet drugs elicit their effects by targeting a specific key component or signaling pathway in platelets to prevent their activation.30 However, physiologically, platelet activation is the joint outcome of several molecular signaling pathways that become stimulated concomitantly through multiple agonist stimuli. The use of glycoprotein IIb/IIIa inhibitors (eptifibatide, abciximab, and tirofiban) blocks integrin αIIbβ3 (a common outcome downstream of all the platelet activation pathways) and is associated with an increased risk of bleeding that limits their long-term use.31 Therefore, novel therapeutic strategies are required that provide global protection against thrombotic events with minimal bleeding risk. All platelet agonist-stimulated pathways require energy to trigger a normal and sustained platelet activation response. Therefore, targeting metabolic pathways by blocking vital metabolic checkpoints in platelets might offer a potential alternative approach to preventing platelet activation, irrespective of the nature of the agonist and the stimulation of related signaling pathways. Here, using the mutant strains, we provide genetic evidence that mitochondrial PDK2/4 significantly contributes to platelet activation and thrombosis by regulating aerobic glycolysis.
Previously, we have reported that the DCA, a small-molecule inhibitor of PDKs, inhibits multiple aspects of platelet activation in human and murine platelets.6 Although this study suggests a role of PDKs in regulating platelet function, in our opinion, the findings reported herein are novel for the following reasons. First, the previously published study6 did not define the genetic or definitive role of individual PDKs in platelet biology. Second, key gaps remain in our understanding of the underlying mechanisms by which PDKs may regulate platelet function. Third, DCA inhibits all PDK isoforms and may have off-target effects. Because PDK2/4 isoforms are prominently associated with metabolic diseases, we used combined PDK2/4-deficient mice in this study. We provide genetic evidence for the first time that PDKs regulate multiple aspects of platelet activation, including aggregation, integrin αIIbβ3 activation, degranulation, spreading, and clot retraction upon stimulation with a variety of platelet agonists. Notably, we observed impaired platelet aggregation only at suboptimal agonist concentrations, and increasing the extent of agonist stimulation reversed these effects. Although activated platelets exhibit a preferentially glycolytic phenotype, remarkable metabolic flexibility exists in platelets that allow them to switch between OXPHOS, fatty acid oxidation, and glutaminolysis to support the bioenergetic demands in platelets,12,32, 33, 34 which can potentially overcome the effects of PDK2/4 deletion when challenged by higher agonist concentrations. The PDK2/4−/− mice demonstrated reduced susceptibility to arterial thrombosis in both laser- and FeCl3-induced injury models. These findings validated our premise that targeting mitochondrial enzymes might confer a broad range of protection against multiple platelet stimuli and is not restricted to a specific agonist and its related activation pathway.
We also investigated the underlying mechanisms by which PDK2/4 may contribute to platelet activation and, thereby, thrombosis. The transition of platelets from a quiescent state to an activated state is characterized by a dramatic shift in the energy requirement and the availability of ATP. Resting platelets use OXPHOS and aerobic glycolysis to maintain their baseline ATP requirements. In contrast, platelet activation is characterized by a predominantly increased rate of aerobic glycolysis relative to OXPHOS.12,32 Upon platelet activation, PDKs phosphorylate the PDH complex to inhibit its activity, which diverts the pyruvate flux toward aerobic glycolysis to generate lactic acid (relative to its restricted oxidation via the mitochondrial OXPHOS). Treatment with DCA has been reported to reduce the level of PDH phosphorylation upon platelet activation.8 In alignment with this, we found that combined genetic deletion of PDK2/4 downregulates PDH phosphorylation at the serine-293 and serine-300 residues35 in collagen or PAR4 peptide-stimulated platelets.
Furthermore, we observed that agonist-stimulated PDK2/4-deficient platelets exhibit reduced glycoPER, suggesting inhibition of aerobic glycolysis and lactic acid synthesis. These findings provide genetic evidence implicating the role of mitochondrial PDK/PDH axis in regulating aerobic glycolysis in platelets and could be 1 of the primary sources of ATP generation during platelet activation. A significant increase in the OCR was also observed in collagen-stimulated WT platelets, which was indistinguishable from the OCR demonstrated by collagen-stimulated PDK2/4−/− platelets. This outcome reinforces the understanding that platelets exhibit a preferentially glycolytic phenotype relative to OXPHOS upon activation.32 A similar scenario exists in various cancer36 and blood cells (T-cells, monocytes, macrophages, and dendritic cells),37, 38, 39 where glycolysis and OXPHOS cooperate to maintain the cellular energetic balance to meet the total ATP requirements of the cell. For instance, the inability of OXPHOS to meet the high energy requirement during the proliferation of cancer cells is compensated by the increased function of glycolysis.40 Despite aerobic glycolysis being a less efficient energy generation pathway (yields 2 ATP molecules per molecule of glucose) in contrast to OXPHOS (yields 36 ATP molecules per molecule of glucose), it exhibits an accelerated generation of ATP through rapid glucose metabolism.36,40 Previously, it was shown that the rate of ATP generation is 100× faster in aerobic glycolysis than that in OXPHOS.40 This favors the high energy–driven transformation of platelets from resting to an activated state to form a blood clot and prevent rapid blood loss. The reduced aerobic glycolysis in collagen-stimulated PDK2/4−/− platelets was associated with attenuated PLCγ2 phosphorylation and CRP-XL–stimulated calcium mobilization. This suggests a potential link between PDK2/4, aerobic glycolysis, and GPVI signaling in platelets. The possibility that aerobic glycolysis side products could be involved in PDK2/4–dependent GPVI signaling cannot be ruled out. Further metabolomic studies are required to identify the underlying mechanism by which aerobic glycolysis or its side products are linked to GPVI signaling in platelets.
PDK2/4 is expressed by other cell types, including endothelium, neutrophils, and monocytes. These cells play a pivotal role in forming a thrombus through their interaction with platelets.24,41 Using adoptive transfer experiments in thrombocytopenic hIL-4Rα/GPIbα-Tg mice that were transfused with PDK2/4−/− platelets or WT platelets, we provide evidence that platelet-specific PDK2/4 contributes to arterial thrombosis in vivo. We could not use platelet-specific PDK2/4 mice because PDK2/4 floxed mice do not exist. Though adoptive transfer experiments suggest a role of platelet PDK2/4 in modulating platelet functions, studies using mice with platelet-specific PDK2/4 deficiency are required to conclusively delineate the platelet-specific roles of PDKs in platelet biology.
Furthermore, using single-knockout mice, we further dissected the functional role of individual PDK2 and PDK4 in platelets. We provide evidence that although both PDK2 and PDK4 regulate agonist-induced platelet aggregation in vitro, PDK4 plays a predominant role in regulating platelet secretion and arterial thrombosis in vivo. Secretion of prothrombotic mediators from platelet alpha and dense granules are highly critical for the activation of circulating platelets and their recruitment to the growing thrombus. A lack of effect on arterial thrombosis in PDK2−/− mice can therefore be partly attributed to normal GPVI receptor–mediated secretion in PDK2−/− platelets. Furthermore, we also speculate that, in contrast to PDK2, PDK4 in other cell types, including neutrophils (known to exhibit a high rate of aerobic glycolysis)10 contributes to arterial thrombosis in vivo.24,41 However, this speculation would require further investigation. These observations in platelets partly resonate with the previous reports that demonstrate a more prominent role of PDK4 in contributing to normal physiological functions in various other cell types and related pathologies.14 Although our investigation provides compelling evidence that suggests the role of PDK2/4 in regulating platelet activation, the role of PDK1 or PDK3 (or both) cannot be ruled out and requires further evaluation using mutant mice models.
Comorbidities such as type 2 diabetes, obesity, and cancer are well-established risk factors that can cause platelet hyperactivity and thrombosis.42, 43, 44 These comorbidities also demonstrate posttranscriptional upregulation of 1 or more PDKs, whereby affected cells favor ATP production by aerobic glycolysis relative to OXPHOS.45,46 Experimental targeting of PDKs to treat these disorders through pharmacological inhibition or genetic deletion has shown promising therapeutic outcomes.45,47,48 The previous findings combined with the data presented in this study suggest that targeting the PDK/PDH axis may confer a broad spectrum of protective effects in vivo. The development of the PDK/PDH axis into an antithrombotic target will require a comprehensive evaluation to ensure a balance between its ability to limit thrombosis and ensure a normal hemostatic response. Notably, the inhibition of thrombosis in PDK2/4−/− mice or treatment with DCA6 did not affect hemostasis.
While this study conclusively demonstrates the antithrombotic effects of targeting PDK2/4, it has limitations. We did not examine the role of PDK2/4 in platelets in the context of preexisting comorbidities, including diabetes, or in those from patients with coronary artery disease. As patients with coronary artery disease are at risk for arterial thrombosis, future investigations encompassing such comorbidities are warranted to provide key translational insights on the role of PDK2/4 in platelet function in the context of diseases. In summary, our findings suggest that PDKs regulate aerobic glycolysis in platelets to modulate multiple aspects of platelet activation, including aggregation, secretion, and molecular signaling (Figure 7F).
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Acknowledgments
The authors thank Adam Wende (University of Alabama) for sharing the PDK2+/−PDK4+/− mice.
The A.K.C. laboratory is supported by grants from the National Institutes of Health, National Heart, Lung, and Blood Institute (R35HL139926), and the National Institute of Neurological Disorders and Stroke (R01NS109910 and U01NS130587). The American Heart Association postdoctoral award supports G.D.F.
Authorship
Contribution: G.D.F., M.K.N., and A.K.C. were responsible for conceptualization; G.D.F., M.K.N., M.G. analyzed the data; G.D.F., M.K.N., M.G., M.K., and R.B.P. performed investigations; G.D.F. and A.K.C. wrote the original draft; and all authors reviewed and edited the manuscript.
Footnotes
∗G.D.F. and M.K.N. contributed equally to this work.
Data are available on request from the corresponding author, Gagan D. Flora (gagandeep-flora@uiowa.edu) or Anil K. Chauhan (anil-chauhan@uiowa.edu).
The full-text version of this article contains a data supplement.
Supplementary Material
References
- 1.Sanchez-Lopez E, Cheng A, Guma M. Can metabolic pathways be therapeutic targets in rheumatoid arthritis? J Clin Med. 2019;8(5):753. doi: 10.3390/jcm8050753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Heidker RM, Emerson MR, LeVine SM. Metabolic pathways as possible therapeutic targets for progressive multiple sclerosis. Neural Regen Res. 2017;12(8):1262–1267. doi: 10.4103/1673-5374.213542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Harris AL. Development of cancer metabolism as a therapeutic target: new pathways, patient studies, stratification and combination therapy. Br J Cancer. 2020;122(1):1–3. doi: 10.1038/s41416-019-0666-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mazumdar C, Driggers EM, Turka LA. The untapped opportunity and challenge of immunometabolism: a new paradigm for drug discovery. Cell Metab. 2020;31(1):26–34. doi: 10.1016/j.cmet.2019.11.014. [DOI] [PubMed] [Google Scholar]
- 5.Rizzieri D, Paul B, Kang Y. Metabolic alterations and the potential for targeting metabolic pathways in the treatment of multiple myeloma. J Cancer Metastasis Treat. 2019;5:26. doi: 10.20517/2394-4722.2019.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nayak MK, Dhanesha N, Doddapattar P, et al. Dichloroacetate, an inhibitor of pyruvate dehydrogenase kinases, inhibits platelet aggregation and arterial thrombosis. Blood Adv. 2018;2(15):2029–2038. doi: 10.1182/bloodadvances.2018022392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nayak MK, Ghatge M, Flora GD, et al. The metabolic enzyme pyruvate kinase M2 regulates platelet function and arterial thrombosis. Blood. 2021;137(12):1658–1668. doi: 10.1182/blood.2020007140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kulkarni PP, Tiwari A, Singh N, et al. Aerobic glycolysis fuels platelet activation: small-molecule modulators of platelet metabolism as anti-thrombotic agents. Haematologica. 2019;104(4):806–818. doi: 10.3324/haematol.2018.205724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.George MJ, Bynum J, Nair P, et al. Platelet biomechanics, platelet bioenergetics, and applications to clinical practice and translational research. Platelets. 2018;29(5):431–439. doi: 10.1080/09537104.2018.1453062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chacko BK, Kramer PA, Ravi S, et al. Methods for defining distinct bioenergetic profiles in platelets, lymphocytes, monocytes, and neutrophils, and the oxidative burst from human blood. Lab Invest. 2013;93(6):690–700. doi: 10.1038/labinvest.2013.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kramer PA, Ravi S, Chacko B, Johnson MS, Darley-Usmar VM. A review of the mitochondrial and glycolytic metabolism in human platelets and leukocytes: implications for their use as bioenergetic biomarkers. Redox Biol. 2014;2:206–210. doi: 10.1016/j.redox.2013.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ravi S, Chacko B, Sawada H, et al. Metabolic plasticity in resting and thrombin activated platelets. PLoS One. 2015;10(4):1–12. doi: 10.1371/journal.pone.0123597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Holness MJ, Sugden MC. Regulation of pyruvate dehydrogenase complex activity by reversible phosphorylation. Biochem Soc Trans. 2003;31(Pt 6):1143–1151. doi: 10.1042/bst0311143. [DOI] [PubMed] [Google Scholar]
- 14.Zhang S, Hulver MW, McMillan RP, Cline MA, Gilbert ER. The pivotal role of pyruvate dehydrogenase kinases in metabolic flexibility. Nutr Metab. 2014;11(1):1–9. doi: 10.1186/1743-7075-11-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Min BK, Park S, Kang HJ, et al. Pyruvate dehydrogenase kinase is a metabolic checkpoint for polarization of macrophages to the M1 phenotype. Front Immunol. 2019;10:1–14. doi: 10.3389/fimmu.2019.00944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Flora GD, Sahli KA, Sasikumar P, et al. Non-genomic effects of the Pregnane X Receptor negatively regulate platelet functions, thrombosis and haemostasis. Sci Rep. 2019;9(1):1–14. doi: 10.1038/s41598-019-53218-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sahli KA, Flora GD, Sasikumar P, et al. Structural, functional, and mechanistic insights uncover the fundamental role of orphan connexin-62 in platelets. Blood. 2021;137(6):830–843. doi: 10.1182/blood.2019004575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Unsworth AJ, Flora GD, Sasikumar P, et al. RXR ligands negatively regulate thrombosis and hemostasis. Arterioscler Thromb Vasc Biol. 2017;37(5):812–822. doi: 10.1161/ATVBAHA.117.309207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Upmacis RK, Shen H, Benguigui LES, et al. Inducible nitric oxide synthase provides protection against injury-induced thrombosis in female mice. Am J Physiol Heart Circ Physiol. 2011;301(2):H617–H624. doi: 10.1152/ajpheart.00667.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wong JH, Dukes J, Levy RE, et al. Sex differences in thrombosis in mice are mediated by sex-specific growth hormone secretion patterns. J Clin Invest. 2008;118(8):2969–2978. doi: 10.1172/JCI34957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Valéra M-C, Gratacap M-P, Gourdy P, et al. Chronic estradiol treatment reduces platelet responses and protects mice from thromboembolism through the hematopoietic estrogen receptor α. Blood. Blood. 2012;120(8):1703–1712. doi: 10.1182/blood-2012-01-405498. [DOI] [PubMed] [Google Scholar]
- 22.Unsworth AJ, Flora GD, Gibbins JM. Non-genomic effects of nuclear receptors: insights from the anucleate platelet. Cardiovasc Res. 2018;114(5):645–655. doi: 10.1093/cvr/cvy044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lam FW, Vijayan KV, Rumbaut RE. Platelets and their interactions with other immune cells. Compr Physiol. 2015;5(3):1265–1280. doi: 10.1002/cphy.c140074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Swystun LL, Liaw PC. The role of leukocytes in thrombosis. Blood. 2016;128(6):753–762. doi: 10.1182/blood-2016-05-718114. [DOI] [PubMed] [Google Scholar]
- 25.Bergmeier W, Boulaftali Y. Adoptive transfer method to study platelet function in mouse models of disease. Thromb Res. 2014;133 Suppl 1(0 1):S3–S5. doi: 10.1016/j.thromres.2014.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boulaftali Y, Hess PR, Getz TM, et al. Platelet ITAM signaling is critical for vascular integrity in inflammation. J Clin Invest. 2013;123(2):908–916. doi: 10.1172/JCI65154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Prakash P, Kulkarni PP, Lentz SR, Chauhan AK. Cellular fibronectin containing extra domain A promotes arterial thrombosis in mice through platelet Toll-like receptor 4. Blood. 2015;125(20):3164–3172. doi: 10.1182/blood-2014-10-608653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Montilla A, Ruiz A, Marquez M, Sierra A, Matute C, Domercq M. Role of mitochondrial dynamics in microglial activation and metabolic switch. Immunohorizons. 2021;5(8):615–626. doi: 10.4049/immunohorizons.2100068. [DOI] [PubMed] [Google Scholar]
- 29.Raskob GE, Angchaisuksiri P, Blanco AN, et al. Thrombosis: a major contributor to global disease burden. Arterioscler Thromb Vasc Biol. 2014;34(11):2363–2371. doi: 10.1161/ATVBAHA.114.304488. [DOI] [PubMed] [Google Scholar]
- 30.Metharom P, Berndt MC, Baker RI, Andrews RK. Current state and novel approaches of antiplatelet therapy. Arterioscler Thromb Vasc Biol. 2015;35(6):1327–1338. doi: 10.1161/ATVBAHA.114.303413. [DOI] [PubMed] [Google Scholar]
- 31.Gachet C. Antiplatelet drugs: which targets for which treatments? J Thromb Haemost. 2015;13(Suppl 1):S313–S322. doi: 10.1111/jth.12947. [DOI] [PubMed] [Google Scholar]
- 32.Aibibula M, Naseem KM, Sturmey RG. Glucose metabolism and metabolic flexibility in blood platelets. J Thromb Haemost. 2018;16(11):2300–2314. doi: 10.1111/jth.14274. [DOI] [PubMed] [Google Scholar]
- 33.Kulkarni PP, Ekhlak M, Singh V, Kailashiya V, Singh N, Dash D. Fatty acid oxidation fuels agonist-induced platelet activation and thrombus formation: Targeting β-oxidation of fatty acids as an effective anti-platelet strategy. FASEB J. 2023;37(2):e22768. doi: 10.1096/fj.202201321RR. [DOI] [PubMed] [Google Scholar]
- 34.Kholmukhamedov A, Jobe S. Platelet respiration. Blood Adv. 2019;3(4):599–602. doi: 10.1182/bloodadvances.2018025155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Patel MS, Korotchkina LG. Regulation of mammalian pyruvate dehydrogenase complex by phosphorylation: complexity of multiple phosphorylation sites and kinases. Exp Mol Med. 2001;33(4):191–197. doi: 10.1038/emm.2001.32. [DOI] [PubMed] [Google Scholar]
- 36.Zheng J. Energy metabolism of cancer: Glycolysis versus oxidative phosphorylation (Review) Oncol Lett. 2012;4(6):1151–1157. doi: 10.3892/ol.2012.928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Krawczyk CM, Holowka T, Sun J, et al. Toll-like receptor-induced changes in glycolytic metabolism regulate dendritic cell activation. Blood. 2010;115(23):4742–4749. doi: 10.1182/blood-2009-10-249540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pearce EL, Pearce EJ. Metabolic pathways in immune cell activation and quiescence. Immunity. 2013;38(4):633–643. doi: 10.1016/j.immuni.2013.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Michalek RD, Rathmell JC. The metabolic life and times of a T-cell. Immunol Rev. 2010;236 (1):190–202. doi: 10.1111/j.1600-065X.2010.00911.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pfeiffer T, Schuster S, Bonhoeffer S. Cooperation and competition in the evolution of ATP-producing pathways. Science. 2001;292(5516):504–507. doi: 10.1126/science.1058079. [DOI] [PubMed] [Google Scholar]
- 41.Dehghani T, Panitch A. Endothelial cells, neutrophils and platelets: getting to the bottom of an inflammatory triangle. Open Biol. 2020;10(10):200161. doi: 10.1098/rsob.200161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kaur R, Kaur M, Singh J. Endothelial dysfunction and platelet hyperactivity in type 2 diabetes mellitus: molecular insights and therapeutic strategies. Cardiovasc Diabetol. 2018;17(1):121. doi: 10.1186/s12933-018-0763-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ranucci M, Aloisio T, Dedda UD, La Rovere MT, De Arroyabe BML, Baryshnikova E. Platelet reactivity in overweight and obese patients undergoing cardiac surgery. Platelets. 2019;30(5):608–614. doi: 10.1080/09537104.2018.1492108. [DOI] [PubMed] [Google Scholar]
- 44.Wang S, Li Z, Xu R. Human cancer and platelet interaction, a potential therapeutic target. Int J Mol Sci. 2018;19(4):1246. doi: 10.3390/ijms19041246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stacpoole PW. Therapeutic targeting of the pyruvate dehydrogenase complex/pyruvate dehydrogenase kinase (PDC/PDK) axis in cancer. J Natl Cancer Inst. 2017;109(11) doi: 10.1093/jnci/djx071. [DOI] [PubMed] [Google Scholar]
- 46.Lee IK. The role of pyruvate dehydrogenase kinase in diabetes and obesity. Diabetes Metab J. 2014;38(3):181–186. doi: 10.4093/dmj.2014.38.3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Anwar S, Shamsi A, Mohammad T, Islam A, Hassan MI. Targeting pyruvate dehydrogenase kinase signaling in the development of effective cancer therapy. Biochim Biophys Acta Rev Cancer. 2021;1876(1):188568. doi: 10.1016/j.bbcan.2021.188568. [DOI] [PubMed] [Google Scholar]
- 48.Jeoung NH. Pyruvate dehydrogenase kinases: therapeutic targets for diabetes and cancers. Diabetes Metab J. 2015;39(3):188–197. doi: 10.4093/dmj.2015.39.3.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.