Abstract
Background
In recent years, the assessment of global longitudinal strain (GLS) derived by speckle-tracking analysis has become a clinically feasible alternative to left ventricular (LV) ejection fraction (LVEF) for the assessment of myocardial function. However, the determinant factors of impaired GLS in structurally and functionally normal patients are unclarified. The objective of this study was to elucidate the determinant factors of impaired GLS in structurally and functionally normal patients.
Methods
We evaluated structurally and functionally normal patients scheduled to undergo noncardiac surgery. The evaluated patient characteristics were age, sex, presence of hypertension, presence of diabetes mellitus, presence of hyperlipidemia, systolic blood pressure, and body mass index. The concentrations of B-type natriuretic peptide and high-sensitivity troponin I were measured. Echocardiography was performed to determine the LVEF, GLS, transmitral early diastolic velocity/transmitral atrial velocity ratio, LV mass index (LVMI), and relative wall thickness (RWT). Patients with preserved LVEF (≥50%) were divided into the normal GLS group (GLS ≤ -20%) and the impaired GLS group (GLS > -20%). On the basis of the RWT and LVMI values, the patients were categorized as having four types of LV geometry. Logistic regression analysis was performed to ascertain the determinant factors of impaired GLS.
Results
The study cohort comprised 75 structurally and functionally normal patients (age 67.7 ± 12.6 years, 45 men). The GLS was normal in 43 patients and impaired in 32 patients. There was a significant difference in RWT between the impaired and normal GLS groups. The evaluation based on the LV geometry showed that six of seven patients with concentric hypertrophy geometry had impaired GLS, and the GLS was significantly more impaired in patients with concentric hypertrophy geometry than in patients with normal geometry or eccentric hypertrophy geometry. Logistic regression analysis revealed that LV concentric hypertrophy geometry was a significant determinant factor of impaired GLS (odds ratio 22.4, P = 0.042).
Conclusions
Global longitudinal strain is more impaired in structurally and functionally normal patients with concentric hypertrophy geometry compared with those with eccentric hypertrophy geometry or normal geometry. In addition, concentric hypertrophy geometry is a significant determinant for impaired GLS in patients with normal cardiac structure and function.
Keywords: Global longitudinal strain, Left ventricular ejection fraction, Left ventricular geometry, Concentric hypertrophy
1. Introduction
In cardiovascular medicine, left ventricular (LV) ejection fraction (LVEF) is one of the best-studied measures for the diagnosis and risk stratification of systolic LV function [1]. However, the assessment of global longitudinal strain (GLS) derived by speckle-tracking analysis of two-dimensional echocardiography has recently become a clinically feasible alternative to LVEF for the assessment of myocardial function. Compared with LVEF, evidence gathered over the last decade shows that GLS is more sensitive to LV dysfunction and provides additional prognostic information [2].
It is most valuable to measure GLS in patients with conditions involving subclinical LV dysfunction, such as heart failure with preserved LVEF, stage B heart failure, aortic stenosis (AS), and mitral regurgitation [3]. GLS assessment is of particular interest in patients with subtle LV systolic dysfunction despite preserved LVEF [4]. GLS measurements are also useful in assessing the effect of cardiotoxic chemotherapies on individual patients over time, and may be useful in assessing identifying acute subclinical rejection in cardiac transplant patients [1].
Studies have reported that GLS is impaired in aged, female, and overweight patients. [5,6] Several diseases have been associated with impaired GLS, including hypertension (HT), diabetes mellitus (DM), renal insufficiency, cardiomyopathies, and valvular heart disease (VHD) [1,[7], [8], [9], [10], [11], [12], [13]]. In patients with HT, impaired GLS is reportedly associated with concentric and dilated LV hypertrophy (LVH) patterns, independent of demographic and clinical parameters [14]. In addition, LV geometry and subclinical LV function as assessed by GLS are more impaired in hypertensive patients with type 2 DM than those without type 2 DM [11]. However, the determinant factors of impaired GLS in structurally and functionally normal patients have not yet been clarified.
The objective of the present study was to elucidate the determinant factors of impaired GLS in structurally and functionally normal patients.
2. Methods
The study protocol was approved by the institutional ethics committee (approval numbers: 1–06 on 25 June 2019, and 1–23 on 28 February 2020). All participants provided written informed consent.
The study cohort comprised patients who underwent preoperative evaluation before noncardiac surgery from January 2020 to December 2020 and agreed to participate in this study. The characteristics of our facility and patients who underwent preoperative evaluation for noncardiac surgery have been described previously [15]. To ensure that only structurally and functionally normal patients were recruited, the exclusion criteria were reduced LVEF (<50%), arrhythmia (atrial fibrillation or atrioventricular block), LVH (>12 mm), LV dilatation (LV end-diastolic dimension (LVDd) > 58 mm in men; LVDd >52 mm in women), more than moderate VHD, or evidence of ischemic heart disease (wall motion abnormality and/or thinning of the left ventricle) [14,[16], [17], [18], [19], [20], [21]].
The evaluated patient characteristics were age, sex, body mass index (BMI), presence of HT, presence of DM, presence of hyperlipidemia, and systolic blood pressure (BP).
The ARCHITECT i2000SR (Abbott Laboratories, Abbott Park, IL, USA) was used to measure the B-type natriuretic peptide (BNP) concentration using the ARCHITECT BNP-JP assay (Abbott Laboratories) and to measure the high-sensitivity troponin I (hsTnI) concentration with an ARCHITECT STAT hsTnI assay (Abbott Laboratories) [22].
Echocardiography was performed to obtain the LVEF using the modified Simpson method, GLS, transmitral early diastolic velocity/transmitral atrial velocity (E/A) ratio, LV mass index (LVMI), relative wall thickness (RWT), presence or absence of more than moderate VHD, and LVH. Ultrasonographic analysis with the speckle-tracking technique was used to calculate the systolic longitudinal strain. The systolic function was assessed by measuring the global longitudinal peak systolic strain (GLS [%]) [10]. Echocardiography was done with an EPIQ 7G ultrasound machine (Koninklijke Philips N.V., Amsterdam, the Netherlands). The echocardiogram was evaluated in accordance with the recommendations of the American Society of Echocardiography and the European Association of Cardiovascular Imaging [16,17]. The presence of more than moderate VHD and a LV wall thickness of >12 mm were considered significant findings. LVEF ≥50% and GLS ≤ -20% were considered normal findings [[16], [17], [18], [19]].
The LV mass (LVM) and RWT were calculated using the following formulas used in previous studies [16,20,21].
| LVM (g) = 0.8 × 1.04 × [(IVSth + LVDd + PWth)3 - LVDd3] + 0.6 |
| RWT = 2 × PWth/LVDd |
where IVSth is the interventricular septum thickness, LVDd is the LV end-diastolic dimension, PWth is the posterior wall thickness, and LVDs is the LV end-systolic dimension.
RWT was measured at end-diastole. The LVM was indexed for the body surface area to give the LVMI.
The cut-off values for LVDd, LVMI, and RWT were taken from recently published guidelines [14,16]. The upper limit of normal for the LVMI was 95 g/m2 for women and 115 g/m2 for men. The upper limit of normal for the RWT was 0.42. The upper limit of normal for the LVDd was 52 mm for women and 58 mm for men.
The RWT and LVMI were used to categorize patients as having either normal geometry (normal RWT and LVMI), concentric remodeling geometry (increased RWT and normal LVMI), concentric hypertrophy geometry (increased RWT and LVMI), or eccentric hypertrophy geometry (normal RWT and increased LVMI) [1,16,23].
2.1. Statistical analysis
Continuous variables were presented as means and standard deviations. All continuous variables were checked for normality of distribution using the Kolmogorov-Smirnov test. Normally distributed data were compared using the Student's t-test. Fisher's exact test was used to analyze categorical variables. Pearson's correlation analysis was used to assess the correlation between GLS and LVEF. One-way analysis of variance (ANOVA) was used to identify statistically significant differences between the mean GLS among the four LV geometry groups. As ANOVA revealed a significant difference in mean GLS between the four groups, Bonferroni post-hoc testing was performed. Logistic regression analysis was used to evaluate the association of the assessed variables with impaired GLS. A two-sided p-value of <0.05 was considered statistically significant in all cases. All analyses were performed using STATA 17.0 statistical software (StataCorp LLC, College Station, TX, USA).
3. Results
Ninety-nine patients (age 68.7 ± 12.5 years, 62 men) provided written informed consent for study participation. In accordance with the exclusion criteria, 24 patients were excluded from the analysis, including nine patients with LVEF <50%, two with atrial fibrillation, one with 2nd degree atrioventricular block, two with LVH, three with LV dilatation, one with VHD, and six with ischemic heart disease. The final study cohort comprised 75 patients (age 67.7 ± 12.6 years, 45 men). These patients were to undergo the following surgeries: 49 (65.3%) genitourinary system, 10 (13.3%) respiratory system, 7 (9.3%) head and neck, 5 (6.7%) orthopedic, 3 (4.0%) gastrointestinal system, and 1 (1.3%) surface. Perioperative complications are as follows: 2 patients developed hypotension, and a patient developed syncope due to bradyarrhythmia. No patient developed heart failure or acute coronary syndrome.
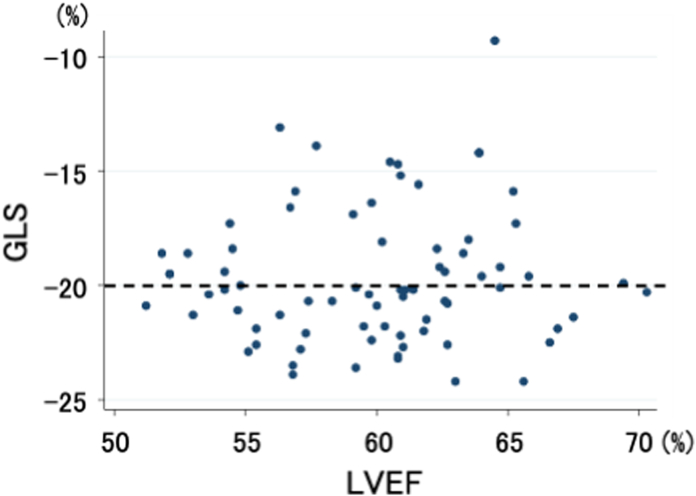
The LVEF and GLS of the 75 included patients are shown in Fig. 1. LVEF ranged from 51.2% to 70.3%, while GLS ranged from −24.2% to −9.3%. There was no significant correlation between GLS and LVEF (r = 0.058, P = 0.62).
Fig. 1.
Left ventricular ejection fraction (LVEF) and global longitudinal strain (GLS) in 75 structurally and functionally normal patients. The dotted line denotes −20% as the cut-off value for normal GLS.
The patients were divided into the impaired GLS group (GLS > -20%) and the normal GLS group (GLS ≤ -20%). The characteristics of the patients in each group are shown in Table 1. There were no significant differences between the impaired and normal GLS groups in age, sex, BMI, prevalence of HT, prevalence of DM, hyperlipidemia, systolic BP, BNP concentration, hsTnI concentration, LVEF, E/A ratio, and LVMI. By definition, the GLS was significantly lower in the normal GLS group than the impaired GLS group. The RWT was significantly smaller in the normal GLS group than the impaired GLS group.
Table 1.
Patient characteristics.
| normal GLS |
impaired GLS |
P value |
|
|---|---|---|---|
| (n = 43) | (n = 32) | ||
| Age, years | 67.8 ± 11.9 | 67.6 ± 13.6 | 0.54 |
| Sex, male (%) | 24 (56%) | 21 (66%) | 0.48 |
| BMI, kg/m2 | 23.3 ± 3.9 | 24.3 ± 3.5 | 0.15 |
| Hypertension, n (%) | 21 (49%) | 17 (55%) | 0.79 |
| Diabetes mellitus, n (%) | 11 (26%) | 7 (22%) | 0.79 |
| Hyperlipidemia, n (%) | 10 (23%) | 4 (13%) | 0.37 |
| systolic BP, mm Hg | 130.8 ± 20.5 | 134.7 ± 18.2 | 0.19 |
| BNP, pg/ml | 23.2 ± 28.1 | 19.1 ± 18.4 | 0.77 |
| hsTnI, pg/ml | 11.4 ± 10.4 | 13.8 ± 24.9 | 0.29 |
| LVEF, % | 59.8 ± 4.2 | 60.1 ± 4.7 | 0.37 |
| GLS, % | −21.7 ± 1.2 | −17.0 ± 2.5 | <0.001 |
| E/A ratio | 0.88 ± 0.30 | 0.80 ± 0.22 | 0.90 |
| LVMI, g/m2 | 81.9 ± 21.7 | 87.9 ± 23.0 | 0.13 |
| RWT | 0.38 ± 0.06 | 0.40 ± 0.06 | 0.04 |
BMI, body mass index; BNP, B-type natriuretic peptide; BP, blood pressure.
E/A, transmitral early diastolic velocity/transmitral atrial velocity.
GLS, global longitudinal strain; hsTnI, high-sensitivity troponin I.
LVEF, left ventricular ejection fraction; LVMI, left ventricular mass index.
RWT, relative wall thickness.
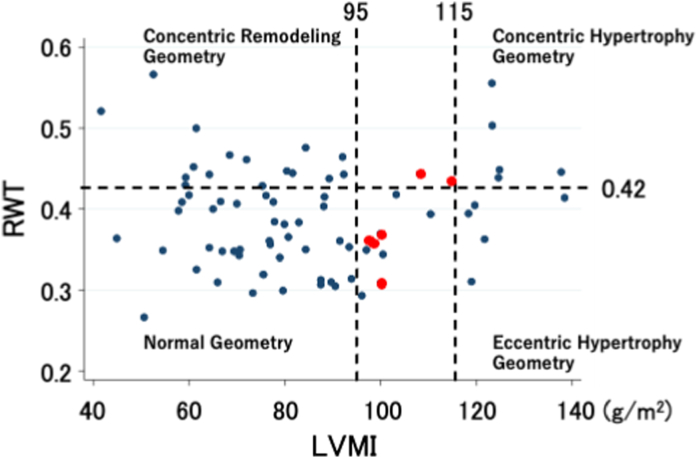
As the RWT significantly differed between the normal and impaired GLS groups, the patients were divided into four groups by LV size and mass categorized based on the ratio of the RWT to the total LVMI (Fig. 2). The distribution of patients with a normal or impaired GLS in the four LV geometry groups is shown in Table 2; Fisher's exact test showed that the distribution did not significantly differ between the four LV geometry groups (P = 0.053). In the concentric hypertrophy geometry group, six of seven patients had impaired GLS. In the eccentric hypertrophy geometry group, seven of nine patients had normal GLS.
Fig. 2.
Relative wall thickness (RWT) and left ventricular mass index (LVMI) in 75 structurally and functionally normal patients. The horizontal dotted line denotes 0.42 as the cut-off value for normal RWT. The vertical dotted lines denote the cut-off values for normal LVMI (95 g/m2 in women and 105 g/m2 in men). The red dots indicate women with LVMI 95–115 g/m2. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Table 2.
Distribution of normal and impaired GLS among patients with four left ventricular geometry patterns.
| normal geometry | concentric remodeling geometry | concentric hypertrophy geometry | eccentric hypertrophy geometry | total | |
|---|---|---|---|---|---|
| normal GLS | 27 | 8 | 1 | 7 | 43 |
| impaired GLS | 16 | 8 | 6 | 2 | 32 |
| total | 43 | 16 | 7 | 9 | 75 |
Fisherʼs exact test, P=0.053; GLS, global longitudinal strain.
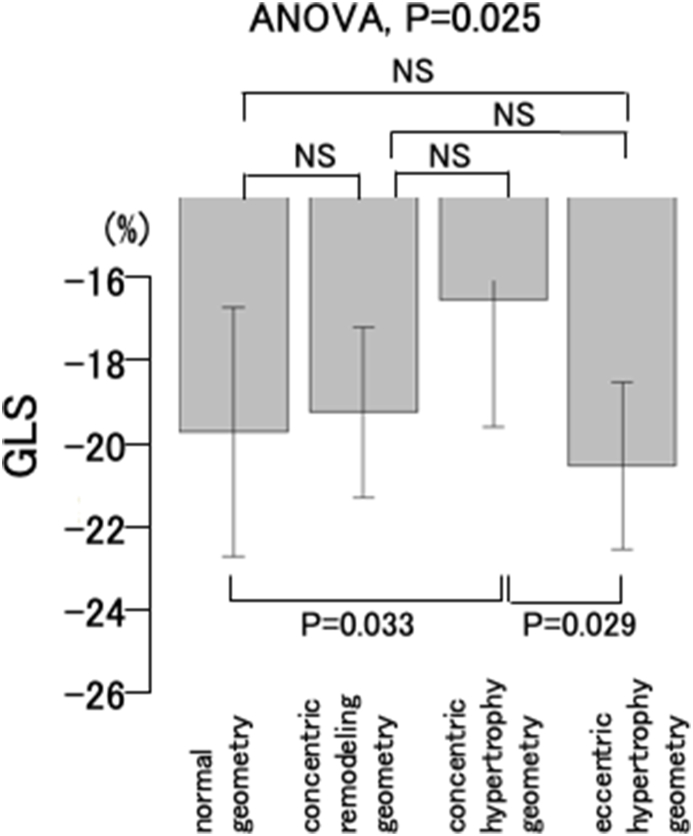
The GLS values in the four LV geometry groups are shown in Fig. 3. The mean GLS was −20.0 ± 3.1% in the normal geometry group, −19.5 ± 2.1% in the concentric remodeling geometry group, −16.7 ± 3.2% in the concentric hypertrophy geometry group, and −20.8 ± 2.1% in the eccentric hypertrophy geometry group. ANOVA revealed that the mean GLS significantly differed between the four LV geometry groups (P = 0.025). Bonferroni post-hoc testing revealed significant differences in mean GLS between the concentric hypertrophy geometry group and the normal geometry group (P = 0.033), and between the concentric hypertrophy geometry group and the eccentric remodeling geometry group (P = 0.029).
Fig. 3.
Global longitudinal strain (GLS) in the four left ventricular geometry groups. One-way analysis of variance (ANOVA) revealed that the mean GLS significantly differed between the four LV geometry groups (P = 0.025). Bonferroni post-hoc testing shows that the GLS is significantly different between the concentric hypertrophy geometry group and the normal geometry group (P = 0.033), and between the concentric hypertrophy geometry group and the eccentric remodeling geometry group (P = 0.029).
NS: not significant.
Logistic regression analysis revealed that concentric hypertrophy geometry was a significant determinant of impaired GLS (odds ratio 22.4, 95% confidence interval 1.12–448.5, P = 0.042) (Table 3).
Table 3.
Results of logistic regression analysis for impaired GLS.
| Risk factor | odds ratio | 95% confident interval | P value | |
|---|---|---|---|---|
| Age | 0.97 | 0.91 | 1.03 | 0.27 |
| Sex | 2.00 | 0.59 | 6.77 | 0.26 |
| BMI | 1.02 | 0.87 | 1.18 | 0.82 |
| systolic BP | 0.99 | 0.97 | 1.02 | 0.91 |
| BNP | 0.99 | 0.97 | 1.03 | 0.92 |
| hsTnI | 0.99 | 0.95 | 1.02 | 0.52 |
| E/A ratio | 0.13 | 0.01 | 3.09 | 0.25 |
| normal geometry* | 1.0 (reference) | – | – | – |
| concentric remodeling geometry | 2.21 | 0.61 | 8.02 | 0.23 |
| concentric hypertrophy geometry | 22.4 | 1.12 | 448.5 | 0.042 |
| eccentric hypertrophy geometry | 0.83 | 0.10 | 6.96 | 0.87 |
BMI, body mass index; BNP, B-type natriuretic peptide; BP, blood pressure.
E/A, transmitral early diastolic velocity/transmitral atrial velocity.
GLS, global longitudinal strain; hsTnI, high-sensitivity troponin I.
*Normal geometry was defined as reference among the four left ventricular geometry groups.
Characteristics in patients with concentric hypertrophy geometry and other LV geometry were compared (Table 4). The E/A ratio was significantly smaller in patients with concentric hypertrophy geometry than those with the other LV geometry. There were no significant differences in other parameters in the comparison.
Table 4.
Patient characteristics in patients with concentric hypertrophy geometry and other left ventricular geometry.
| concentric hypertrophy geometry |
other left ventricular geometry |
P value |
|
|---|---|---|---|
| (n = 7) | (n = 68) | ||
| Age, years | 80.3 ± 6.3 | 66.4 ± 12.4 | 1.00 |
| Sex, male (%) | 3 (43%) | 42 (62%) | 0.43 |
| BMI, kg/m2 | 25.2 ± 2.8 | 23.6 ± 3.8 | 0.86 |
| Hypertension, n (%) | 5 (71%) | 33 (49%) | 0.43 |
| Diabetes mellitus, n (%) | 2 (29%) | 16 (24%) | 0.67 |
| Hyperlipidemia, n (%) | 0 (0%) | 14 (21%) | 0.34 |
| systolic BP, mm Hg | 152.6 ± 15.2 | 130.4 ± 18.8 | 1.00 |
| BNP, pg/ml | 27.7 ± 14.8 | 20.8 ± 25.1 | 0.76 |
| hsTnI, pg/ml | 36.9 ± 53.4 | 9.9 ± 5.6 | 1.00 |
| LVEF, % | 63.1 ± 3.2 | 59.6 ± 4.4 | 0.98 |
| GLS, % | −16.7 ± 3.2 | −20.0 ± 2.8 | 1.00 |
| E/A ratio | 0.62 ± 0.19 | 0.87 ± 0.27 | 0.01 |
| LVMI, g/m2 | 122.3 ± 9.2 | 80.6 ± 19.4 | 1.00 |
| RWT | 0.47 ± 0.05 | 0.38 ± 0.06 | 1.00 |
BMI, body mass index; BNP, B-type natriuretic peptide; BP, blood pressure.
E/A, transmitral early diastolic velocity/transmitral atrial velocity.
GLS, global longitudinal strain; hsTnI, high-sensitivity troponin I.
LVEF, left ventricular ejection fraction; LVMI, left ventricular mass index.
RWT, relative wall thickness.
4. Discussion
Preoperative evaluation showed that the GLS was impaired in 32 of 75 structurally and functionally normal patients scheduled for noncardiac surgery. The RWT was significantly greater in patients with impaired GLS than in those with normal GLS. Six of seven patients with concentric hypertrophy geometry had impaired GLS, and the GLS was significantly higher in patients with concentric hypertrophy geometry than in patients with normal geometry or eccentric hypertrophy geometry. Logistic regression analysis showed that LV concentric hypertrophy geometry was the only significant determinant factor for impaired GLS.
4.1. Assessment of factors correlated with impaired GLS
4.1.1. Age, sex, and BMI
There were no significant differences in age, sex, and BMI between the normal and impaired GLS groups. Previous studies have reported that GLS is impaired in aged, female, or overweight patients [5,6]. Furthermore, GLS reportedly declines with age without a significant decrease in LVEF [24]. Sex has a significant impact on normal GLS values. In the general population without cardiovascular disease or traditional risk factors, the absolute GLS difference between men and women is >1%, and women have better (lower) GLS [5]. Obese candidates for bariatric surgery have a high prevalence of preclinical systolic dysfunction and LVH or concentric remodeling, which is detectable in the early stage with echocardiography [6]. The lack of correlation between these factors and impaired GLS in our cohort may be due to the small sample size.
4.1.2. Disease status
There were no significant differences in HT, DM, and systolic BP between the normal and impaired GLS groups.
-
1)
Cardiomyopathy and VHD
Impaired GLS is reportedly associated with cardiomyopathies and VHD [1,[7], [8], [9]].
In patients with AS, lower average GLS is related to greater LV mass, concentric geometry, and more severe AS [7,8]. LVEF markedly underestimates the extent of myocardial systolic impairment in the presence of LV concentric hypertrophy, which often occurs in patients with AS [8].
The GLS of patients with dilated cardiomyopathy with type 2 DM is reportedly significantly more impaired than the GLS of patients with dilated cardiomyopathy without type 2 DM, despite having similar conventional LV function [9]. Because we excluded patients with more than moderate VHD and LVEF <50%, our results cannot be directly compared with these previous results.
-
2)
HT, type 2 DM, and hyperlipidemia
Diseases reportedly associated with impaired GLS include HT, type 2 DM associated with obesity, and renal insufficiency [1,[10], [11], [12]].
Decreased GLS is reportedly detected in 15%–42% of patients with HT, depending on both the severity and control of HT. In patients with HT, early impairment of LV function (detected as impaired GLS) is associated with long-term, uncontrolled BP, increased BMI, and related metabolic changes, and is more pronounced in patients with LVH [10]. In addition, patients with type 2 DM have early impairment of GLS, which is worsened by coexistent HT [11]. GLS is significantly impaired in patients with type 2 DM with a BMI ≥25 kg/m2, but not among those with a BMI ≥25 kg/m2 without type 2DM [12].
There were no significant differences between the normal and impaired GLS groups regarding HT, DM, hyperlipidemia, and systolic BP. This may be because our cohort was mainly composed of stable patients scheduled to undergo noncardiac surgery, and may have been too small to detect these differences.
4.1.3. Biomarkers
There were no significant differences between the normal and impaired GLS groups in the BNP and hsTnI concentrations. BNP is a marker of cardiac failure or LV dysfunction [18,22,25], while hsTnI is well-established specific and sensitive marker of myocardial injury [18,22,25,26]. The reason that BNP and hsTnI were not significantly associated with impaired GLS was probably because our cohort comprised patients with stable general and cardiac statuses.
4.1.4. LVEF and GLS
LVEF is a simple measure of global systolic function that pervades the risk evaluation and management of many cardiovascular diseases. However, LVEF is limited not only by technical challenges but also by pathophysiological entities where the ratio of the stroke volume to the LV cavity size is preserved [2].
Two-dimensional speckle-tracking echocardiography enables assessment of myocardial strain, thereby providing detailed information on global and regional LV deformation [4]. Global strain, particularly GLS, has emerged as an important measure of cardiac performance that adds incremental predictive value to standard measures such as the LVEF [1,27].
GLS assessment accurately detects early subclinical alterations in LV longitudinal function that occur before LVEF impairment [17,28,29]. GLS is expressed as a percentage; as the overall deformation is toward compression (negative), values closer to zero represent worse LV function [1].
The advantages of GLS over LVEF in the assessment of LV systolic function have been demonstrated in several studies. GLS is more sensitive than LVEF in detecting subtle changes in LV systolic function. In addition, LV end-diastolic pressure varies directly with GLS in patients with heart failure with preserved LVEF, suggesting that GLS may be useful as a surrogate indicator of LV filling pressures. Furthermore, impaired GLS has superior prognostic value compared with LVEF [4].
Current guidelines for the diagnosis and treatment of acute and chronic heart failure include LVEF as one of the main determinants of the characterization and risk stratification of patients. However, GLS may be able to refine the risk stratification of these patients [4]. The GLS is increasingly being used in clinical practice because of its utility in the detection of early subclinical LV dysfunction, identification of regional variation in specific cardiomyopathies, monitoring improvement with therapy, and as a prognostic marker in a variety of cardiac conditions [13].
GLS also appears to predict survival or heart failure development following myocardial infarction. Global strain measurements are also useful in assessing the effect of cardiotoxic chemotherapies on individual patients over time and may be useful in identifying acute subclinical rejection in cardiac transplant patients [1].
GLS was impaired in 32 of 75 patients. Impaired myocardial contractility appears to first affect the endocardial layers; therefore, longitudinal motion markers may be more sensitive than LVEF in identifying early changes in myocardial contractility [[30], [31], [32]]. In the early hypertensive stages when clinically defined LVH has not yet developed and the LVEF is still normal, increased afterload could indicate early LV systolic dysfunction that is detectable by GLS and midwall fractional shortening assessment [33]. The relationship between circumferential midwall and longitudinal function in patients with HT is nonlinear and dependent on LV geometry. In these patients, systolic impairment occurs earlier in longitudinal than circumferential performance [34].
In general, longitudinal LV mechanics, which are predominantly governed by the subendocardial layer, are the most vulnerable and most sensitive to the presence of myocardial disease. If unaffected, midmyocardial and epicardial function may result in normal or nearly normal circumferential and twist mechanics with relatively preserved LV pump function and LVEF [28]. Therefore, GLS should be assessed to provide a quantitative analysis of LV longitudinal function [17,28,29].
Those with impaired GLS in our cohort may have had increased afterload indicating early LV systolic dysfunction.
4.1.5. LV geometry
There were no significant differences between the normal and impaired GLS groups in the LVEF, E/A ratio, and LVMI. However, there was a significant difference in RWT between these two groups. RWT is reported to be the strongest determinant of the relative efficacy of circumferential and longitudinal LV contraction [34]. Increased LV wall thickness or reduced LV diameter contribute to preserved LVEF in the presence of reductions in especially longitudinal, but also circumferential shortening. Thus, patients with myocardial dysfunction but a small LV cavity and LVH have a preserved LVEF in the presence of impaired GLS [35].
Alterations in LV size and mass are categorized based on the ratio of the RWT to the LVMI. The specific pattern of LV remodeling is related to the prognosis of a variety of both myocardial and valvular diseases [1,16,20]. RWT and LVMI are used to categorize patients as having normal geometry, concentric remodeling geometry, concentric hypertrophy geometry, or eccentric hypertrophy geometry [1,16,23]. In our study, six of seven patients with concentric hypertrophy geometry had impaired GLS, while only two of nine patients with eccentric hypertrophy geometry had impaired GLS. GLS was significantly smaller in patients with concentric hypertrophy geometry than in patients with normal geometry or eccentric hypertrophy geometry. Furthermore, logistic regression analysis revealed that LV concentric hypertrophy geometry was the only significant determinant factor for impaired GLS in structurally and functionally normal patients. In addition, the patients with concentric hypertrophy geometry had a significantly smaller E/A ratio, suggesting diastolic dysfunction existing with impaired GLS.
In the logistic regression analysis, LV concentric hypertrophy geometry was the only significant determinant of impaired GLS, and the odds ratio of concentric hypertrophy geometry has a broad 95% confidential interval (1.12–448.5) and borderline P value (P = 0.042). The reasons for these will be that LV geometry and only seven parameters have been analyzed due to the small number of patients, and the similarity of the characteristics of the patients with normal GLS and impaired GLS. Caution should be required for the interpretation of the results because of the broad confidential interval and borderline P value.
It might also be possible to be underestimated the result of the logistic regression analysis due to the small number of patients. As shown in Fig. 3, the GLS of the patients with concentric hypertrophy geometry is significantly impaired in the small number of patients. If we could enroll more patients, the E/A ratio or eccentric hypertrophy geometry might become significant determinants. But in this study, the concentric hypertrophy geometry was the only determinant due to the small number of patients included in the study, and the similarity of the characteristics of the patients with normal GLS and impaired GLS.
The VALIANT echocardiographic study reported concentric LVH carries the greatest risk of adverse cardiovascular events, including death in patients with high-risk myocardial infarction [36].
Several studies have evaluated patients with HT. A recent study of hypertensive patients with conserved LVEF reported that LV geometry is more impaired and more prone to concentric remodeling geometry and concentric hypertrophy geometry in hypertensive patients with type 2 DM than in those without type 2 DM [11]. Other studies of hypertensive patients have demonstrated that only concentric hypertrophy geometry but not eccentric hypertrophy geometry or concentric remodeling geometry is associated with impaired GLS, independent of age, BMI, systolic BP, and LVMI [14,37].
The findings of these previous studies of patients with HT are similar to our findings in structurally and functionally normal patients. Our study further confirmed and expanded the results of previous studies on patients with old myocardial infarction or HT to structurally and functionally normal patients. Even in structurally and functionally normal patients with no significant LVH, concentric hypertrophy geometry contributed to impaired GLS.
5. Clinical implications
Structurally and functionally normal patients with impaired GLS may have subclinical concentric hypertrophy. Therefore, clinicians should evaluate such patients for infiltrative diseases such as amyloidosis. Although the effects were not statistically significant in this study, DM, HT, or obesity should be managed carefully.
6. Limitations
Because we did not follow-up these patients, we could not analyze the changes in GLS, LVEF, or LV wall thickness over time. Furthermore, the lack of renal function data meant that we could not evaluate the influence of renal function on GLS. There were no patients on dialysis in our cohort.
7. Conclusions
Global longitudinal strain is more impaired in structurally and functionally normal patients with concentric hypertrophy geometry compared with those with eccentric hypertrophy geometry or normal geometry. In addition, concentric hypertrophy geometry is a significant determinant of impaired GLS in patients with normal cardiac structure and function.
Funding
None.
Credit authors statement
Ryuichiro Anan: Conceputualization, Methodology, Validation, Formal analysis, Investigation, Resources, Data curation, Writing-Original draft preparation, Writing-Reviewing and Editing, Visualization, Supervision, Project administration. Tatsuya Imoto: Validation, Investigation, Data curation, Writing-Original draft preparation, Visualization. Kumi Onizuka: Investigation. Hideaki Watanabe: Investigation. Wakako Mori: Data curation. Mayu Murakoso: Investigation.
Author contribution statement
Ryuichiro Anan: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Tatsuya Imoto: Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Kumi Onizuka: Hideaki Watanabe: Mayu Murakoso: Performed the experiments.
Wakako Mori: Contributed reagents, materials, analysis tools or data.
Data availability statement
Data will be made available on request.
Additional information
No additional information is available for this paper.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
We are greatly indebted to the study participants. We thank Ms. Emiko Matsumoto and Dr. Masanori Tsurugida for their help with the study. We also thank Dr. Kelly Zammit, BVSc, from Edanz (https://jp.edanz.com/ac) for editing a draft of this manuscript.
References
- 1.Wu J.C., Gillam L.D., Solomon S.D. In: Braunwald's Heart Disease, A Textbook of Cardiovascular Medicine. twelfth ed. Libby R.P., Bonow R.O., Mann D.L., Tomaselli G., Bhatt D.L., Solomon S.D., et al., editors. Elsevier; Philadelphia: 2022. Echocardiography; pp. p196–p267. [Google Scholar]
- 2.Potter E., Marwick T.H. Assessment of left ventricular function by echocardiography: the case for routinely adding global longitudinal strain to ejection fraction. J. Am. Coll. Cardiol. Img. 2018;11:260–274. doi: 10.1016/j.jcmg.2017.11.017. [DOI] [PubMed] [Google Scholar]
- 3.Haji K., Marwick T.H. Clinical Utility of echocardiographic strain and strain rate measurements. Curr. Cardiol. Rep. 2021;23:18. doi: 10.1007/s11886-021-01444-z. [DOI] [PubMed] [Google Scholar]
- 4.Tops L.F., Delgado V., Marsan N.A., Bax J.J. Myocardial strain to detect subtle left ventricular systolic dysfunction. Eur. J. Heart Fail. 2017;19:307–313. doi: 10.1002/ejhf.694. [DOI] [PubMed] [Google Scholar]
- 5.Cheng S., Larson M.G., McCabe E.L., Osypiuk E., Lehman B.T., Stanchev P., et al. Age and sex-based reference limits and clinical correlates of myocardial strain and synchrony: clinical perspective. Circ. Cardiovasc. Imaging. 2013;6:692–699. doi: 10.1161/CIRCIMAGING.112.000627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Frea S., Andreis A., Scarlatta V., Rovera C., Vairo A., Pistone E., et al. Subclinical left ventricular dysfunction in severe obesity and reverse cardiac remodeling after bariatric surgery. J. Cardiovasc. Echogr. 2020;30:22–28. doi: 10.4103/jcecho.jcecho_50_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cramariuc D., Gerdts E., Davidsen E.S., Segadal L., Matre K. Myocardial deformation in aortic valve stenosis: relation to left ventricular geometry. Heart. 2010;96:106–112. doi: 10.1136/hrt.2009.172569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pibarot P., Dumesnil J.G. Longitudinal myocardial shortening in aortic stenosis: ready for prime time after 30 years of research? Heart. 2010;96:95–96. doi: 10.1136/hrt.2009.177345. [DOI] [PubMed] [Google Scholar]
- 9.Tanaka H., Tatsumi K., Matsuzoe H., Matsumoto K., Hirata K.I. Impact of diabetes mellitus on left ventricular longitudinal function of patients with non-ischemic dilated cardiomyopathy. Cardiovasc. Diabetol. 2020;19:84. doi: 10.1186/s12933-020-01063-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Soufi Taleb Bendiab N., Meziane-Tani A., Ouabdesselam S., Methia N., Latreche S., Henaoui L., et al. Factors associated with global longitudinal strain decline in hypertensive patients with normal left ventricular ejection fraction. Eur. J. Prev. Cardiol.C. 2017;24:1463–1472. doi: 10.1177/2047487317721644. [DOI] [PubMed] [Google Scholar]
- 11.Soufi Taleb Bendiab N., Ouabdesselam S., Henaoui L., Lopez-Sublet M., Monsuez J.J., Benkhedda S. Impact of diabetes on cardiac function in patients with high blood pressure. Int. J. Environ. Res. Publ. Health. 2021;18:6553. doi: 10.3390/ijerph18126553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Suto M., Tanaka H., Mochizuki Y., Mukai J., Takada H., Soga F., et al. Impact of overweight on left ventricular function in type 2 diabetes mellitus. Cardiovasc. Diabetol. 2017;16:145. doi: 10.1186/s12933-017-0632-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Trivedi S.J., Altman M., Stanton T., Thomas L. Echocardiographic strain in clinical practice. Heart Lung Circ. 2019;28:1320–1330. doi: 10.1016/j.hlc.2019.03.012. [DOI] [PubMed] [Google Scholar]
- 14.Tadic M., Cuspidi C., Majstorovic A., Kocijancic V., Celic V. The relationship between left ventricular deformation and different geometric patterns according to the updated classification: findings from the hypertensive population. J. Hypertens. 2015;33:1954–1961. doi: 10.1097/HJH.0000000000000618. [DOI] [PubMed] [Google Scholar]
- 15.Anan R., Watanabe H., Takahama H., Mori W. Ventricular tachycardia in patients undergoing 24-h Holter monitoring as preoperative evaluation for noncardiac surgery. Heart Ves. 2021;36:1047–1055. doi: 10.1007/s00380-021-01779-1. [DOI] [PubMed] [Google Scholar]
- 16.Lang R.M., Badano L.P., Mor-Avi V., Afilalo J., Armstrong A., Ernande L., et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2015;28:1–39. doi: 10.1016/j.echo.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 17.Galderisi M., Cosyns B., Edvardsen T., Cardim N., Delgado V., Di Salvo G., et al. Standardization of adult transthoracic echocardiography reporting in agreement with recent chamber quantification, diastolic function, and heart valve disease recommendations: an expert consensus document of the European Association of Cardiovascular Imaging. Eur. Heart J. Cardiovasc. Imaging. 2017;18:1301–1310. doi: 10.1093/ehjci/jex244. [DOI] [PubMed] [Google Scholar]
- 18.Heidenreich P.A., Bozkurt B., Aguilar D., Allen L.A., Byun J.J., Colvin M.M., et al. AHA/ACC/HFSA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Joint Committee on clinical practice guidelines. J. Am. Coll. Cardiol. 2022;79(2022):e263–e421. doi: 10.1016/j.jacc.2021.12.012. [DOI] [PubMed] [Google Scholar]
- 19.D'Elia N., Caselli S., Kosmala W., Lancellotti P., Morris D., Muraru D., et al. Normal global longitudinal strain: an individual patient meta-analysis. J. Am. Coll. Cardiol. Img. 2020;13:167–169. doi: 10.1016/j.jcmg.2019.07.020. [DOI] [PubMed] [Google Scholar]
- 20.Stewart M.H., Lavie C.J., Shah S., Englert J., Gilliland Y., Qamruddin S., et al. Prognostic implications of left ventricular hypertrophy. Prog. Cardiovasc. Dis. 2018;61:446–455. doi: 10.1016/j.pcad.2018.11.002. [DOI] [PubMed] [Google Scholar]
- 21.Ganau A., Devereux R.B., Roman M.J., de Simone G., Pickering T.G., Saba P.S., et al. Patterns of left ventricular hypertrophy and geometric remodeling in essential hypertension. J. Am. Coll. Cardiol. 1992;19:1550–1558. doi: 10.1016/0735-1097(92)90617-v. [DOI] [PubMed] [Google Scholar]
- 22.Sugawa S., Masuda I., Kato K., Yoshimura M. Increased levels of cardiac troponin I in subjects with extremely low B-type natriuretic peptide levels. Sci. Rep. 2018;8:5120. doi: 10.1038/s41598-018-23441-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Konstam M.A., Kramer D.G., Patel A.R., Maron M.S., Udelson J.E. Left ventricular remodeling in heart failure: current concepts in clinical significance and assessment. J. Am. Coll. Cardiol. Img. 2011;4:98–108. doi: 10.1016/j.jcmg.2010.10.008. [DOI] [PubMed] [Google Scholar]
- 24.Zghal F., Bougteb H., Réant P., Lafitte S., Roudaut R. Assessing global and regional left ventricular myocardial function in elderly patients using the bidimensional strain method. Echocardiography. 2011;28:978–982. doi: 10.1111/j.1540-8175.2011.01476.x. [DOI] [PubMed] [Google Scholar]
- 25.Ibrahim N.E., Januzzi J.L., Jr. Established and emerging roles of biomarkers in heart failure. Circ. Res. 2018;123:614–629. doi: 10.1161/CIRCRESAHA.118.312706. [DOI] [PubMed] [Google Scholar]
- 26.Cardinale D., Sandri M.T., Colombo A., Colombo N., Boeri M., Lamantia G., et al. Prognostic value of troponin I in cardiac risk stratification of cancer patients undergoing high-dose chemotherapy. Circulation. 2004;109:2749–2754. doi: 10.1161/01.CIR.0000130926.51766.CC. [DOI] [PubMed] [Google Scholar]
- 27.Hernandez-Suarez D.F., Lopez-Candales A. Strain imaging echocardiography: what imaging cardiologists should know. Curr. Cardiol. Rev. 2017;13:118–129. doi: 10.2174/1573403X12666161028122649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mor-Avi V., Lang R.M., Badano L.P., Belohlavek M., Cardim N.M., Derumeaux G., et al. Current and evolving echocardiographic techniques for the quantitative evaluation of cardiac mechanics: ASE/EAE consensus statement on methodology and indications endorsed by the Japanese Society of Echocardiography. Eur. J. Echocardiogr. 2011;12:167–205. doi: 10.1093/ejechocard/jer021. [DOI] [PubMed] [Google Scholar]
- 29.Thomas J.D., Badano L.P. EACVI-ASE-industry initiative to standardize deformation imaging: a brief update from the co-chairs. Eur. Heart J. Cardiovasc. Imaging. 2013;14:1039–1040. doi: 10.1093/ehjci/jet184. [DOI] [PubMed] [Google Scholar]
- 30.Nahum J., Bensaid A., Dussault C., Macron L., Clémence D., Bouhemad B., et al. Impact of longitudinal myocardial deformation on the prognosis of chronic heart failure patients. Circ. Cardiovasc. Imaging. 2010;3:249–256. doi: 10.1161/CIRCIMAGING.109.910893. [DOI] [PubMed] [Google Scholar]
- 31.Koyama J., Ray-Sequin P.A., Falk R.H. Longitudinal myocardial function assessed by tissue velocity, strain, and strain rate tissue Doppler echocardiography in patients with AL (primary) cardiac amyloidosis. Circulation. 2003;107:2446–2452. doi: 10.1161/01.CIR.0000068313.67758.4F. [DOI] [PubMed] [Google Scholar]
- 32.Serri K., Reant P., Lafitte M., Berhouet M., Le Bouffos V., Roudaut R., et al. Global and regional myocardial function quantification by two-dimensional strain: application in hypertrophic cardiomyopathy. J. Am. Coll. Cardiol. 2006;47:1175–1181. doi: 10.1016/j.jacc.2005.10.061. [DOI] [PubMed] [Google Scholar]
- 33.Lembo M., Santoro C., Sorrentino R., Canonico M.E., Fazio V., Trimarco B., et al. Interrelation between midwall mechanics and longitudinal strain in newly diagnosed and never-treated hypertensive patients without clinically defined hypertrophy. J. Hypertens. 2020;38:295–302. doi: 10.1097/HJH.0000000000002257. [DOI] [PubMed] [Google Scholar]
- 34.Ballo P., Quatrini I., Giacomin E., Motto A., Mondillo S. Circumferential versus longitudinal systolic function in patients with hypertension: a nonlinear relation. J. Am. Soc. Echocardiogr. 2007;20:298–306. doi: 10.1016/j.echo.2006.08.024. [DOI] [PubMed] [Google Scholar]
- 35.Marwick T.H. Ejection fraction pros and cons: JACC state-of-the-art review. J. Am. Coll. Cardiol. 2018;72:2360–2379. doi: 10.1016/j.jacc.2018.08.2162. [DOI] [PubMed] [Google Scholar]
- 36.Verma A., Meris A., Skali H., Ghali J.K., Arnold J.M., Bourgoun M., et al. Prognostic implications of left ventricular mass and geometry following myocardial infarction: the VALIANT (VALsartan in Acute myocardial iNfarcTion) Echocardiographic Study. J. Am. Coll. Cardiol. Img. 2008;1:582–591. doi: 10.1016/j.jcmg.2008.05.012. [DOI] [PubMed] [Google Scholar]
- 37.Tadic M., Sala C., Carugo S., Mancia G., Grassi G., Cuspidi C. Myocardial strain and left ventricular geometry: a meta-analysis of echocardiographic studies in systemic hypertension. J. Hypertens. 2021;39:2297–2306. doi: 10.1097/HJH.0000000000002911. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.