Abstract
Lentil belonging to the fabaceae family is a proteinaceous cool-season legume consumed across the world. However, lentil is low yielding with a narrow genetic base compared to other grain legumes such as chickpea, faba bean, and cowpea. In the present study, we intended to investigate the effect of two different mutagens viz., caffeine and lead nitrate on the bio-physiological and agronomical traits of lentil. Unlike other mutagens like ethyl methanesulphonate, sodium azide, and hydrazine hydrates very little is known about the mutagenic potency of caffeine and lead nitrate. The results revealed contrasting effects as lower doses of caffeine-induced a substantial increase in mean values of physiological and agronomical traits whereas both lower and higher doses of lead nitrate negatively impacted the agronomical traits of lentil. Among the mutagen doses, 0.1% caffeine was most efficient in inducing a substantial increase in mean values of bio-physiological and quantitative traits. The present study also revealed the successful conduct of induced mutagenesis in lentil and present a protocol that could be followed in future breeding programs aimed at increasing the yielding potential of legumes.
Keywords: Lentil, Mutation breeding, Yield improvement, Caffeine, Lead nitrate
1. Introduction
Pulses have proven an important asset to the agriculture and its allied sectors and have meticulously played a worthy role in the protein-deficient cereal-based human diet. Pulse crops belonging [1] to the fabaceae family are mainly harvested for their edible seeds that are rich in protein and diverse micronutrients. Besides their food uses pulses can fix atmospheric nitrogen and help enhance soil fertility [2]. Among pulses, lentil (Lens culinaris Medik.), 2n = 14 is a self-pollinating annual herb growing to a length of up to 40 cm [3]. The leaves are small sessile, compound, and alternate, and flowers are usually white, pink, and purple arising in clusters of four on the long slender stems. Lentil is believed to be an ancient crop of Western Asian origin around 2000 BCE that later spread rapidly to other parts of the world (Zohary et al., 2012). It is cultivated in the most sub-tropical, Northern Hemisphere, and Pacific Northwest regions. It is rich in carbohydrates, dietary fiber, protein, thiamine, calcium, folate, iron, magnesium, potassium and zinc [4]. After soybean, lentil has the highest ratio of protein per calorie. The data provided by the USDA National Nutrient Database suggested that 100 gm of raw and cooked lentils provide 353 and 116 kilocalories, respectively. The folate in lentils helps lower the homocysteine levels in the blood and prevents the progress of coronary heart disease [5]. Consuming cooked lentils could prevent iron deficiency, an essential mineral required daily, particularly for pregnant women and adolescents (Soltan S., 2013). It is considered suitable for pregnant women in lowering breast cancer and the congenital disabilities in newborns.
According to FAOSTAT, 2020 lentil in India occupies an area of about 2.22 million hectares, with an annual production of 1.62 million tonnes in 2018. The average yield of 731 kg/ha is less than the global average of 1038 kg/ha, which is insufficient regarding the country's increasing demands. India is ranked first in the area (39.79%) of lentil cultivation and second in production (22.79%) after Canada (41.16%), which may be attributed to the low productivity of lentil in India (611 kg/ha) compared to Canada (1633 kg/ha). However, the productivity could be improved by enhancing the yielding potential of existing lentil cultivars. Various traditional and new plant breeding techniques have been implemented to increase grain yield. Among breeding approaches, mutagenesis has proven a coherent tool in improving the agronomical traits of lentil and offers an opportunity to improve a single trait without altering the entire genetic constitution [[6], [7], [8], [9], [10]]. However, the success of mutagenesis is determined by the efficiency and effectiveness of the mutagen dose (Chowdhury and Tah 2013; [[11], [12], [13]]. Therefore, we employed a chemical mutagenesis experiment in the present study in lentil variety L-4076 with the objectives to study the effects of mutagens on seed germination, plant survival, pollen fertility, chlorophyll and carotenoid contents, cytological aberrations and quantitative traits. The variety L-4076 was chosen due to its wide adaptability to North Indian climatic conditions, including present site of study (http://dpd.gov.in/).The present study may also provide an idea about optimum mutagen doses and high yielding putative mutants and set a working mutagenesis protocol for cool-season legumes.
2. Materials and methods
2.1. Plant material and mutagenic treatment
Lentil seeds var. L-4076 was procured from the Indian Agricultural Research Institute, New Delhi, and treated with different doses of caffeine and lead nitrate (Pb(NO3)2). The stock solution of caffeine and Pb(NO3)2 were prepared in pH 3.0 and 7.0, respectively. Prior to mutagenic treatment, seeds were sterilized using 5% sodium hypochlorite for 15 min, followed by a thorough washing under tap water for 30 min. Healthy and dry seeds (10–12% moisture content) were pre-soaked in double-distilled water and then treated with 0.1%, 0.25%, 0.5%, 0.75%, 1% of caffeine and 5, 10, 15, 20, 25 ppm of Pb(NO3)2 for 9 h. During mutagen treatment, seeds were continuously stirred to ensure a uniform mutagen and seed interaction. At the beginning of the present mutation breeding experiment, LD50, a dose at which germination percentage is 50%, was calculated. The results revealed that doses beyond 1% caffeine and 25 ppm Pb(NO3)2 treatments induced more than 50% reduction in seed germination. Therefore, such doses were not selected for future generations. After the treatment, seeds were thoroughly washed under running tap water for about 30 min to ensure the removal of excess mutagen adhered to the seed surface. Thoroughly washed 100 seeds/treatment were sown in earthen pots in five replications of twenty seeds and kept in the Net House.
2.2. Seed germination
The percentage of inhibition was calculated by using the following formula:
2.3. Plant survival
In the mutagen-treated population rate of plant survival was measured at the time of maturity. The percentage of survival and lethality was calculated using the following formula.
2.4. Pollen fertility
Fresh and young floral buds were collected from 30 randomly chosen plants for evaluating pollen fertility. Staining of the pollen grains was done using a 2% acetocarmine solution. The pollen grains that were stained and depicted circular or regular outlines were referred to as fertile pollen grains, while unstained pollen grains with a shrunken outline were considered sterile. Finally, the pollen fertility and sterility (%) was calculated using the following formula.
2.5. Chlorophyll and carotenoid contents
Following the protocol of [14] leaves were picked from fifteen days old young plants. Leaves weighing 1 g were ground to a fine pulp in 80% acetone (20 ml) and then centrifuged at 10000 rpm for 2 min. The supernatant was transferred in a 100 ml volumetric flask and the residue was washed three times using 80% acetone. The absorbance of each sample was recorded at 645 and 663 nm for chlorophyll and 480 and 510 nm for carotenoid. The pigment contents (mg.g−1 leaf fresh mass) were calculated according to formula of Arnon (1949).
where,
OD645, OD663, OD480, OD510 = Optical densities at respective wavelengths
V = Volume of an extract
W = Mass of leaf tissues
d = Length of light path (d = 1.4 cm)
2.6. Cytological studies
Young floral buds from mutagen-treated plants and control were collected in the early morning hours and fixed in freshly prepared Carnoy's fixative, absolute alcohol: chloroform: acetic acid in the ratio of 6:3:1 respectively, augmented with crystals of ferric chloride and stored in 70% alcohol for 24 h. The anthers from the collected floral buds were then squashed in 1% propionocarmine. Permanent slides were prepared by the NBA-GAA series and then used to study mutagen-induced meiotic abnormalities.
2.7. Quantitative traits
In M1 generation, mean values of various traits such as plant height (PH), pod length (PL), branches per plant (BPP), seeds per pod (SPP), pods per plant (PPP), seed weight (SW) and total seed yield (TSY) were recorded and subjected to recommended statistical analysis to evaluate the effect of mutagens on the degree of divergence among the putative mutants. In the present study, plant height was measured by taking the mean length from base to the apex in ten randomly chosen plants, mean pod length was measured by taking the length of pods in ten randomly chosen plants, and the mean number of branches and pods were calculated by counting the number of branches, and pods in ten randomly chosen plants. The number of seeds per pod was calculated by counting the seeds in ten pods each from ten randomly chosen plants. The seed weight was measured by weighing the 100 seeds randomly selected from ten plants. The mean total seed yield per plant was calculated by measuring the total weight of seeds in ten randomly chosen plants.
3. Results
3.1. Seed germination
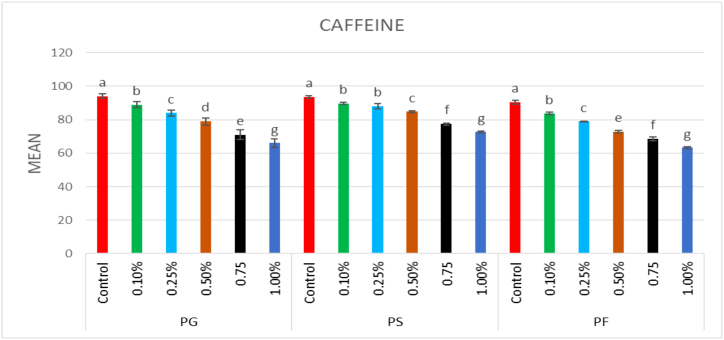
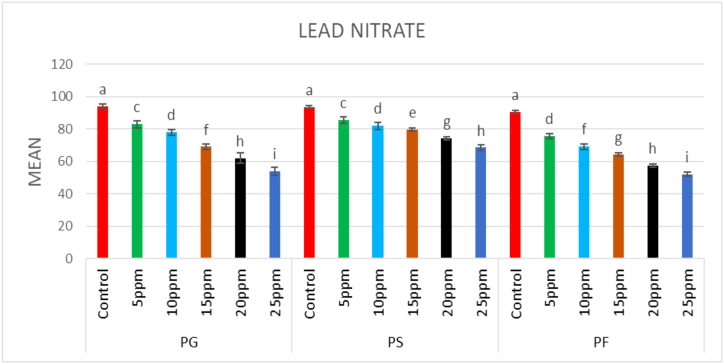
In control, seed germination was 94% and in the mutagenized population, it decreased dose-dependently. The highest seed germination across the treated population was 89% in 0.1% caffeine, while the lowest seed germination was 54% in 25 ppm Pb(NO3)2. Overall germination percentage reduced significantly in all the treatments of the mutagens; however, caffeine proved more effective than Pb(NO3)2 (Fig. 1, Fig. 2).
Fig. 1.
Effect of different concentrations of (0.1%–1%) caffeine on plant germination (PG), plant survival (PS) and pollen fertility (PF) (Mean ± SE) (n = 5).
Fig. 2.
Effect of different concentrations (5ppm–25 ppm) of lead nitrate (Pb(NO3)2 on plant germination (PG), plant survival (PS), and pollen fertility (PF) (Mean ± SE) (n = 5).
3.2. Plant survival
The plant survival at maturity was 93.61% in the control population, and it decreased in a dose-dependent manner in mutagen-treated populations. The minimum reduction in plant survival was recorded in 0.1% caffeine (89.8%) and the maximum reduction in plant survival was recorded in 25 ppm Pb(NO3)2 (68.51%) (Fig. 1, Fig. 2).
3.3. Pollen fertility
In the control population, pollen fertility was 90.52%, and it was reduced in a dose-dependent manner in mutagen-treated treated plants. Among the treated populations, the maximum pollen fertility was recorded in 0.1% caffeine (83.6%), while the minimum pollen fertility was recorded in 25 ppm Pb(NO3)2 (52.17%) (Fig. 1, Fig. 2).
3.4. Chlorophyll and carotenoid contents
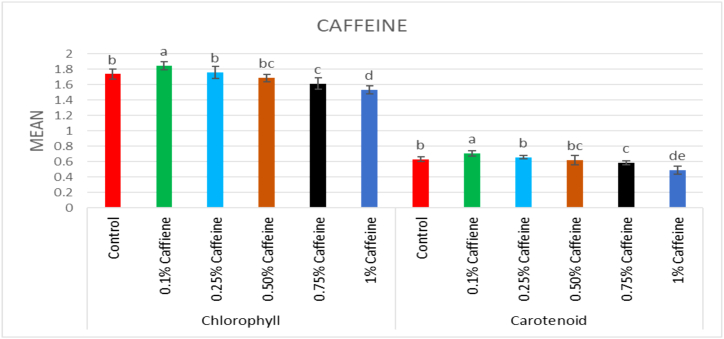
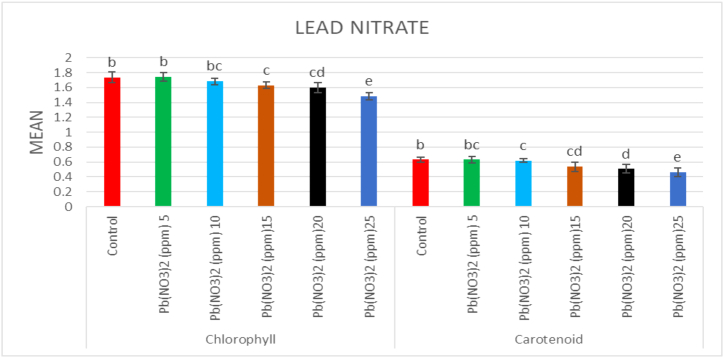
The chlorophyll content in the control population was 1.737 mg g−1 leaf fresh mass. In the mutagenized plants, a considerable increase in the chlorophyll content was recorded at lower doses of both mutagens. However, the highest increase among treated populations was 1.845 mg g−1 leaf fresh mass in 0.1% caffeine and the lowest chlorophyll content was recorded in 25 ppm Pb(NO3)2 (1.485 mg g−1 leaf fresh mass) (Fig. 3, Fig. 4). The carotenoid content in the control population was 0.632 mg g−1 leaf fresh mass. The highest carotenoid content was recorded in 0.1% caffeine (0.71 mg g−1 leaf fresh mass) and the lowest carotenoid content was recorded in 25 ppm Pb(NO3)2 (0.46 mg g−1 leaf fresh mass) (Fig. 3, Fig. 4).
Fig. 3.
Effect of different concentrations of (0.1%–1%) caffeine on chlorophyll and carotenoid content in Lens culinaris (M1 generation) (Mean ± SE) (n = 5).
Fig. 4.
Effect of different concentrations (5ppm–25 ppm) of lead nitrate (Pb(NO3)2) on chlorophyll and carotenoid content in Lens culinaris (M1 generation) (Mean ± SE) (n = 5).
3.5. Quantitative traits
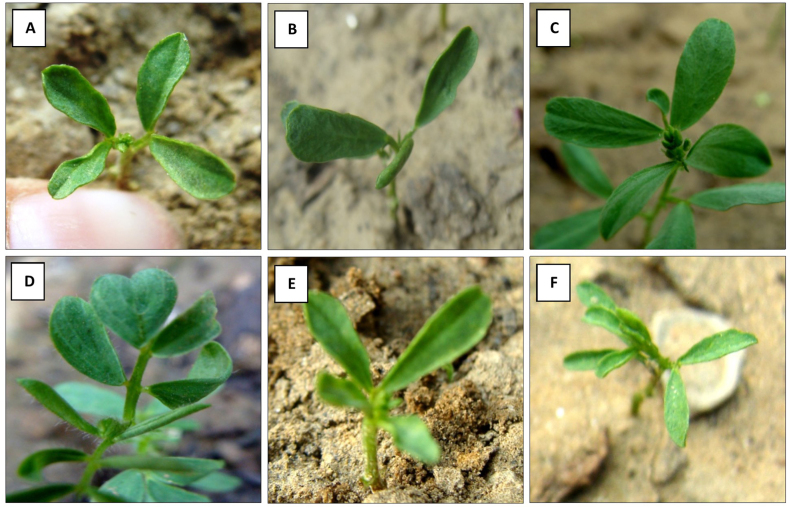
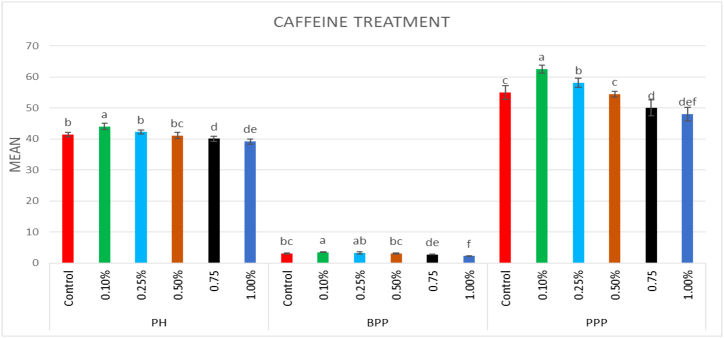
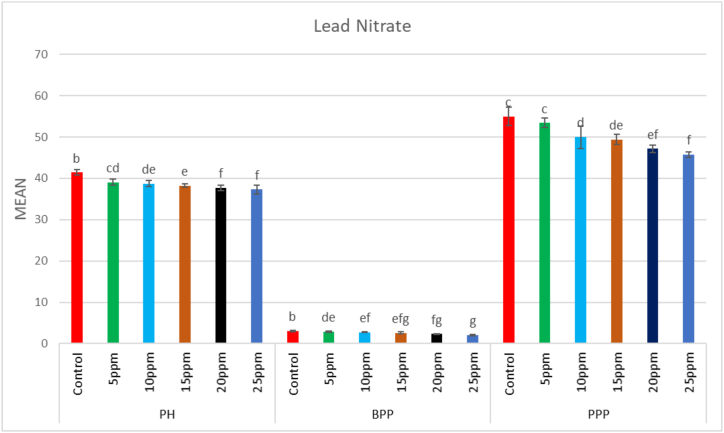
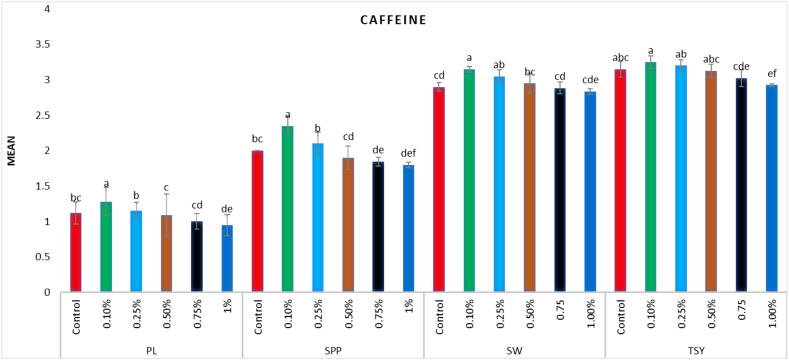
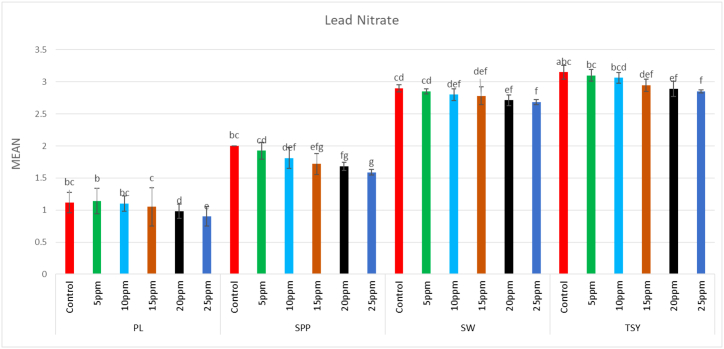
In the M1 generation, a wide range of putative mutants with varying numbers and shapes of leaves were isolated in treated population (Fig. 5A–F). Besides putative mutants with altered morphology including tall mutants (isolated in 0.75% caffeine), dwarf mutants (isolated in 15 ppm Pb(NO3)2), and bushy mutants (isolated in 0.25% caffeine) (Fig. 6A–D). The mean plant height in the control population was 41.4 cm, whereas in the treated population, the maximum plant height was 44.07 cm in 0.1% caffeine and the minimum plant height was 37.35 cm in 25 ppm Pb(NO3)2 (Fig. 7, Fig. 8). The results revealed that in caffeine-treated plants, lower doses induced a significant increase whereas in Pb(NO3)2 treated plants all mutagen doses induced a substantial decrease in mean values of quantitative traits (plant height, branches per plant, number of pods per plant, number of seeds per pod, seed weight, seed yield per plant) compared to control. The mean number of branches per plant in the control population was 3.1, while in the treated populations the highest number of branches per plant was 3.5 in 0.1% caffeine, and the lowest number of branches per plant was 2.04 in 25 ppm Pb(NO3)2 (Fig. 7, Fig. 8). The mean number of pods per plant in the control population was 55, while in the treated population the highest number of pods per plant was 62.5 in 0.1% caffeine, and the lowest number of pods per plant was 45.89 in 25 ppm Pb(NO3)2 (Fig. 7, Fig. 8). Pod length in the control population was 1.12 cm and the highest pod length was 1.28 cm in 0.1% caffeine, while the lowest pod length was 0.9 cm in 25 ppm Pb(NO3)2. In both caffeine and Pb(NO3)2 treated plants, lower and higher mutagen doses induced a proportionate increase and decrease in the mean pod length (Fig. 9, Fig. 10). In the control population, the mean number of seeds per pod was 2; in the treated population, the maximum number of seeds per pod was 2.35 in 0.10% caffeine and the lowest number of seeds per pod was 1.59 in 25 ppm Pb(NO3)2 (Fig. 9, Fig. 10). The mean 100 seeds weight (gm) in the control population was 2.90 gm, while the highest seed weight among the treated population was 3.15 gm in 1% caffeine and the lowest seed weight was 2.68 gm in 25 ppm Pb(NO3)2 (Fig. 9, Fig. 10). The total seed yield (gm) in the control population was 3.15 gm, and the highest seed yield among all the treated population was 3.25 gm in 0.1% caffeine, while the lowest seed yield was 2.85 gm in 25 ppm Pb(NO3)2 (Fig. 9, Fig. 10).
Fig. 5.
(A) Four vegetative leaflets (control), (B) Two long and broad leaflets (0.50% caffeine), (C) A very small curved leaflet (0.25% caffeine), (D) Broad obcordate (heart-shaped leaflet) [15 ppm Pb(NO3)2], (E) Unequal leaflets; two large, broad and another two small [20 ppm Pb(NO3)2], (F) Thick, small and light green leaflets (1.0% caffeine). (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Fig. 6.
(A) Control plant with normal height and yield, (B) Tall and a bushy variant with high yield (0.75% caffeine), (C) Tall variant with an increased number of branches and pods (0.25% caffeine), (D) Dwarf variant with long pods [15 ppm Pb(NO3)2.].
Fig. 7.
Effect of different concentrations (0.10%–1%) of caffeine on plant height (PH) and branches per plant (BPP), pods per plant (PPP) in Lens culinaris (M1 generation) (Mean ± SE) (n = 5).
Fig. 8.
Effect of different concentrations (5ppm–25 ppm) of lead nitrate (Pb(NO3)2) on plant height (PH) and branches per plant (BPP), pods per plant (PPP) in Lens culinaris (Mean ± SE) (n = 5).
Fig. 9.
Effect of different concentrations of (0.1%–1%) caffeine on pod length (PL), seeds per pod (SPP), seed weight (SW) and total seed yield (TSY) in Lens culinaris (Mean ± SE) (n = 5).
Fig. 10.
Effect of different concentrations (5ppm–25 ppm) of lead nitrate (Pb(NO3)2) on pod length (PL), seeds per pod (SPP), seed weight (SW) and total seed yield (TSY) in Lens culinaris (Mean ± SE) (n = 5).
3.6. Cytological aberrations
In the present study, meiotic analysis in control and treated populations were performed in the M1 generation to investigate the changes in chromosomal behavior. Normal meiotic phases were observed in the control plants. However, various chromosomal aberrations were recorded in the pollen mother cells of mutagen-treated plants. In caffeine and Pb(NO3)2 treated populations, meiotic aberrations such as stickiness, laggards, bridges, and disturbed polarity were observed. The most frequent aberration at the anaphase stage were laggards followed by bridges and unequal separation of chromosomes (Table 1). The results demonstrated increased meiotic abnormalities with increased concentrations of mutagens. Comparatively, Pb(NO3)2 induced a higher frequency of meiotic aberrations than caffeine. Moreover, the results indicated that abnormalities were more frequent at metaphase followed by anaphase and telophase (Table 1).
Table 1.
Frequency of chromosomal abnormalities induced by caffeine and Pb(NO3)2 in Lens culinaris Medik.
| Conc.of mutagens (%) and (ppm) | Total No. of PMCs Obser. |
Prophase-I |
Metaphase-I/II |
Anaphase-I/II |
Telophase-I/II |
Total No. of Abn. PMCs Obser. |
Total % of Abn. PMCs Obser. |
|||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Univalents | Multivalents | % of Abn. PMCs | Univalents | Multivalents | Precocious Mov | Stray chromosomes | Stickiness | % of Abn. PMCs | Laggards | Bridges | Unequal Sep. | % of Abn. PMCs | Laggards | Bridges | Unequal Sep. | Micro nucleate | Multi nucleate | Disturbed Polarity poleritl |
Cytomixis | % of Abn. PMCs | ||||
| Control | 275 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| Caffeine (%) | ||||||||||||||||||||||||
| 0.10 | 236 | – | 1 | 0.42 | – | 1 | – | 1 | 1 | 1.27 | 1 | – | – | 0.42 | 1 | – | – | 1 | 1 | – | – | 1.27 | 8 | 3.38 |
| 0.25 | 252 | 1 | 1 | 0.79 | – | 1 | – | 1 | 2 | 1.58 | 1 | – | 1 | 0.79 | 1 | – | – | 1 | 1 | 1 | – | 1.58 | 12 | 4.74 |
| 0.50 | 263 | 2 | 2 | 1.52 | 1 | – | 2 | 1 | 2 | 2.28 | 2 | 1 | – | 0.82 | 2 | 1 | 1 | 1 | 2 | 2 | 1 | 3.80 | 23 | 8.42 |
| 0.75 | 275 | 2 | 3 | 1.81 | 2 | 2 | 2 | 3 | 4 | 4.72 | 2 | 1 | 2 | 1.81 | 3 | 2 | 2 | 3 | 3 | 3 | 2 | 6.54 | 41 | 14.88 |
| 1.0 | 285 | 3 | 3 | 2.10 | 1 | 3 | 2 | 5 | 4 | 5.26 | 3 | 2 | 2 | 2.45 | 3 | 2 | 2 | 4 | 5 | 3 | 3 | 7.71 | 50 | 17.52 |
| Pb(NO3)2 (ppm) | ||||||||||||||||||||||||
| 5 | 247 | 1 | 1 | 0.76 | – | 1 | 1 | 1 | 1 | 1.61 | 1 | – | 1 | 0.80 | 1 | – | – | 1 | 1 | 1 | – | 1.61 | 12 | 4.78 |
| 10 | 258 | 1 | 2 | 1.20 | 1 | – | 1 | 2 | 2 | 2.32 | 2 | – | 1 | 1.16 | 2 | 1 | 1 | 2 | 2 | 1 | 1 | 3.87 | 22 | 8.55 |
| 15 | 262 | 1 | 3 | 1.92 | 1 | 2 | 2 | 3 | 3 | 4.19 | 2 | 1 | 1 | 1.52 | 3 | 1 | 2 | 3 | 3 | 2 | 2 | 6.10 | 35 | 13.73 |
| 20 | 270 | 2 | 3 | 2.29 | 2 | 3 | 2 | 4 | 4 | 5.55 | 2 | 1 | 2 | 1.85 | 3 | 2 | 3 | 3 | 4 | 4 | 2 | 7.77 |
46 | 17.46 |
| 25 | 278 | 3 | 3 | 2.57 | 3 | 4 | 5 | 5 | 6 | 8.27 | 4 | 2 | 4 | 3.59 | 4 | 2 | 3 | 4 | 4 | 4 | 3 | 8.63 1111 |
63 | 23.06 |
4. Discussion
4.1. Bio-physiological traits
Seed germination, a progression of the metabolically active processes needed for growth is an important factor in demonstrating the plant-generated response to different mutagenic treatments [15]. In the present study, a dose-dependent decrease in seed germination may be attributed to mutagen-induced regression in metabolic processes, chromosomal abnormalities, delayed replication and reduced activity of phytohormones [16]. The present study was supported by previous studies in Coriandrum sativum [17] and Capsicum annuum [18]. Chlorophyll plays a vital role in photosynthesis and influences all the key processes such as plant growth and development [19]. The enhanced chlorophyll content in the lower doses of mutagenic treatments may be attributed to mutagen-induced upregulation of chlorophyll biosynthesizing genes. Enhanced chlorophyll content could lead to improved photosynthetic rate and productivity. On the contrary, decreased chlorophyll content in the higher mutagen treated plants may be attributed to increased chlorophyllase activity. The results are in propinquity with the previous studies in Vigna unguiculata [20]; Triticum aestivum [21] and in Euryale ferox [22]. Carotenoids are an important class of photosynthetic pigments that play a diverse roles such as quenching excess light energy and scavenging free radicals and providing first line of plant defense against the formation of reactive oxygen species (ROS) in the chloroplast. In the present study, mutagens altered the carotenoid biosynthesizing genes resulted in putative mutants with increased carotenoid contents. The results aligned with the previous studies [23]. Such putative mutants may possess a better ability to survive oxidative stress with more efficient photosynthesis under various conditions [24].
The results revealed a dose-dependent reduction in plant survival, and previous studies also reported the same response in mutagenized plants such as Lycopersicon esculentum [25], and Lathyrus sativus [26]. Mutagens interact with the genetic material and alter the vital process of growth and development that eventually reduce the survival of plants [[27], [28], [29]]. At maturity, a varying degree of pollen sterility in mutagenized plants could be attributed to chromosomal abnormalities, physiological changes, and genetic variations in pollen mother cells that lead to the production of non-viable gametes [16,30]. The extent of pollen sterility increased with higher concentrations of the mutagen, and these observations were following the results of previous studies [26]; [16]. However, pollen sterility is insignificant in a lower mutagen dose-treated population, and such doses may be considered optimum for future breeding programs.
4.2. Cytological aberrations
Analysis of cytological aberrations following mutagenic treatments forms an essential component of the mutation breeding programs. This analysis provides an idea about the mutagenic potency, and genotypic sensitivity and to analyze the most effective mutagen dose for a particular crop to maximize the desirable results. In the present study, dose-dependent increase in the frequency of meiotic abnormalities could be attributed to the interaction of mutagens with the chromosomes. Previous studies have also reported cytological aberrations following mutagenic treatments in different plants viz., Plantago ovata [31], Vicia faba [32,33], and Linum usitatissimum [34]. In the present study, univalents were observed in the PMCs of treated plants. Univalents could be attributed to early chiasma terminalization [35] or mutagen-induced cryptic structural changes in chromosomes, that prevent the normal pairing of homologous chromosomes [36]. Previous studies have also recorded univalents in crops such as Nigella sativa [37,38], Vicia faba [39], and Cichorium intybus [40]. Chromosome stickiness (chromosomes clumped into one, two, or more than two groups) was most frequently observed anomaly in the treated population. Chromosome stickiness could be attributed to the improper functioning of some specific nonhistone proteins needed for chromatid separation and segregation [41]. The stickiness of chromosomes could also cause an unequal separation of chromosomes in daughter nuclei towards poles at anaphase due to the nondisjunction of homologous chromosomes at metaphase [42]. According to Ref. [30]; unequal chromosomal separation in meiosis could be attributed to non-oriented bivalent formation due to spindle malfunction or the creation of univalents during metaphase or diakinesis. Similar results were reported by [34] in Linum usitatissimum, [43] in Capsicum annum, and [44] in Trigonella foenum-graecum. In the present study, multivalent chromosomes may be ascribed to mutagen-induced irregular pairing and breakage followed by translocation and inversion [39,42]. Previous workers also reported multivalent chromosomes in mutagenized plants such as [45] in Trigonella foenum-graecum [46,47], in Cicer arietinum. In the present study, precocious separation of chromosomes may be attributed to a lack of chiasmata formation that is vital for maintaining the bivalents owing to normal segregation of the chromosomes. Besides, mutagen-induced breaks in the nucleoprotein backbone could also result in precocious separation [30]. Several workers reported precocious separation in different plants such as [48] in Hordeum vulgare [46], in Cicer arietinum, [45]; and in Trigonella foenum-graecum [44]. At anaphase, discrepancies in the spindle formation that eventually lead to unsynchronized bivalents or laggards [49]. The laggard formation at Anaphase has been earlier reported by Ref. [30] in Zea mays, [44] in Trigonella foenum-graecum [43], in Capsicum annum, and [33] in Vicia faba. Chromosomal bridges with or without fragments may be due to delayed terminalization and the inability of chromosome movement [50]. Several workers reported chromosomal bridges in different plants [34]. Micronuclei formation may be due to the adhesion of chromosomal fragments and irregular segregation [51,52]. The disturbed polarity at Anaphase could be due to mutagen-induced disturbances and divergences in the spindle formation. Disturbed polarity has also been reported by Ref. [33] in Vicia faba and [30] in Zea mays. Cytomixis, transmigration of chromatin between adjoining cells could be attributed to mutagen altered gene function [53], changes in the biochemical process that involves microsporogenesis [54].
4.2. Quantitative traits
In the present study, lower doses of mutagens caused a substantial increase in mean values of quantitative traits. Plant height decreased gradually at higher concentrations of the mutagen, and similar findings were reported by Ref. [55]. The reduction in plant height may be due to chromosomal abnormalities induced by mutagenic treatments [56, 57] also reported tall and dwarf mutants of lentil treated with different doses of caffeine, EMS, lead and cadmium nitrate [58]. suggested that reduced plant height could be due to inhibitory effects of the mutagen on cell division and elongation [59]. reported that mutagen induced alterations in the gibberllic acid synthesis could be attributed to the induction of plant height mutants. These plant height mutants especially dwarfs showed few desired attributes like lodging resistance, increased root nodules, and higher seed yield, hence these mutants could be tested in future breeding programs. In the present study, bushy mutants may be attributed to mutagen induced increase in lateral branches and increased photosynthesis that imparted a bushy appearance. Bushy growth habits might be attributed to altered physiological properties such as leaf senescence. Similarly, putative mutants with increased pods also depicted an augmented number of floral buds, and therefore, we concluded that mutations in genes associated with flowering might have been altered. Such putative mutants are higher-yielding and have the potential to yield more in subsequent generations. [60] reported that the physiological effects of lower and medium doses of gamma rays and sodium azide and their hydrolysis products might be attributed to the augmented number of pods in cowpea mutants. Previous studies have also reported mutants with increased pods [20,23,61,62]. In lentil seed weight is an important character for overall seed yield. In the present study, few bold-seeded putative mutants revealed substantial enhancement in yield as compared to control. Such mutants are also valuable genetic resources and could be released as new cultivars after multi-location trails. Mutagen induced bold-seeded mutants have also been reported in Vicia faba [63,64], Vigna mungo [65], Cicer arietinum [66], and Linum usitatissimum [67]. The putative bold-seeded mutants observed in the present study revealed a significant increase in yield and could prove promising donor parents for the bold character or released directly as new cultivars after multi-location trials. Another desired mutation in the putative mutants was an increased number of seeds per pod. The seed number is also an important yield attributing trait, and mutants with increased seeds per pod should be prioritized in selection programs. Earlier studies also reported increased seeds per pod in mutagenized crops [68]. In plant breeding programs, yield improvement is the ultimate aim of plant breeders and geneticists. We were able to isolate a few putative mutants that yielded higher compared to the control population; however, such mutants must be advanced to subsequent generations at multiple locations to check the trait stability. Since yield is a complex trait dependent on many genes therefore it is not possible to report which genes have been mutated.
5. Conclusion
The present study revealed that lower doses of caffeine were effective in enhancing the bio-physiological and agronomical traits in lentil. All the doses of lead nitrate were detrimental and must be avoided in mutation breeding experiments. Moreover, few morphological mutants such as tall, dwarf, and bushy isolated in the present study could be used as a source of elite genes and may be exploited in future breeding programs. Most importantly putative mutants with improved yield and bio-physiological traits have the potential to be released as a new variety after subjecting to multi-location trials. Both mutagens are rarely used in plant breeding, and the present study may be used for reference by plant breeders and geneticists.
Author contribution statement
Janib Yousuf: Conceived and designed the experiments; Performed the experiments.
Aamir Raina, Zubair Altaf and Shiekh Rasik: Analyzed and interpreted the data; Wrote the paper.
Durre Shahwar: Contributed reagents, materials, analysis tools or data; Wrote the paper.
Data availability statement
Data included in article/supplementary material/referenced in article.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors thank the Chairperson, Department of Botany, Aligarh Muslim University, Aligarh, India, for providing basic research facilities.
Contributor Information
Aamir Raina, Email: aamir854@gmail.com.
Durre Shahwar, Email: dshahwar123@gmail.com.
References
- 1.Zohary D., Hopf M. third ed. Oxford university press; 2000. Domestication of Plants in the Old World: the Origin and Spread of Cultivated Plants in West Asia, Europe and the Nile Valley(No. [Google Scholar]
- 2.Joshi P.K. This Volume. 1998. Performance of grain legumes in the indo-gangetic plain. [Google Scholar]
- 3.Alabboud I., Szilagyi L., Roman G.V. Molecular research on the genetic diversity of lentil genotypes (Lens culinaris Medik) using the RAPD method. Lucrări Științifce, Universitatea de Stiinte Agricole Și Medicină Veterinară" ion ionssescu de la Brad" iași. Seria Agronomie. 2009;52(1):753–757. [Google Scholar]
- 4.United States Department of Agriculture, USDA Nutrient Database. 2011. [Google Scholar]
- 5.Singh P. Lambert Academic Publishers; Saarbrucken, Germany: 2012. Genetic Analysis in Lentil: Genetic Analysis and Breeding Tools for Improvement of Lentil; p. 117. [Google Scholar]
- 6.Khursheed S., Raina A., Laskar R.A., Khan S. Effect of gamma radiation and EMS on mutation rate: their effectiveness and efficiency in faba bean (Vicia faba L.). Caryologia: inter. J. Cyto., Cytosyst. Cytog. 2018;71:397–404. [Google Scholar]
- 7.Raina A., Khursheed S., Khan S. Optimisation of mutagen doses for gamma rays and sodium azide in cowpea genotypes. Trends Biosci. 2018;11:2386–2389. [Google Scholar]
- 8.Laskar R.A., Khan S., Deb C.R., Tomlekova N., Wani M.R., Raina A., Amin R. Springer; Cham: 2019. Lentil (Lens Culinaris Medik.) Diversity, Cytogenetics and Breeding. Advances in Plant Breeding Strategies: Legumes; pp. 319–369. [Google Scholar]
- 9.Goyal S., Wani M.R., Raina A., Laskar R.A., Khan S. Quantitative assessments on induced high yielding mutant lines in urdbean [Vigna mungo (L.) hepper] Legume Sci. 2021;e125 [Google Scholar]
- 10.Goyal S., Wani M.R., Raina A., Laskar R.A., Khan S. Phenotypic diversity in mutagenized population of urdbean (Vigna mungo (L.) Hepper) Heliyon. 2021;7 doi: 10.1016/j.heliyon.2021.e06356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Devi A.S., Mullainathan L. Effect of gamma rays and ethyl methane sulphonate (EMS) in M3 generation of blackgram (Vigna mungo L. Hepper) Afr. J. Biotechnol. 2012;11(15):3548–3552. [Google Scholar]
- 12.Goyal S., Wani M.R., Laskar R.A., Raina A., Khan S. Assessment on cytotoxic and mutagenic potency of Gamma rays and EMS in Vigna mungo L. Hepper. Biotecnol. Veg. 2019;19:193–204. [Google Scholar]
- 13.Goyal S., Wani M.R., Laskar R.A., Raina A., Khan S. Mutagenic effectiveness and efficiency of individual and combination treatments of gamma rays and ethyl methanesulfonate in black gram (Vigna mungo (L.) hepper) Adv. in Zool. Bot. 2020;8:163–168. [Google Scholar]
- 14.Mackinney G. Absorption of light by chlorophyll solutions. J. Biol. Chem. 1941;140(2):315–322. [Google Scholar]
- 15.Shah T.M., Mirza J.I., Haq M.A., Atta B.M. Radio sensitivity of various chickpea genotypes in M1 generation I-Laboratory studies. Pakistan J. Bot. 2008;40(2):649–665. [Google Scholar]
- 16.Raina A., Khan S., Wani M.R., Laskar R.A., Mushtaq W. In: Advances in Plant Breeding Strategies: Legumes. Al-Khayri J.M., Jain M., Johnson D.V., editors. 2019. Chickpea (Cicer arietinum L.) cytogenetics, genetic diversity and breeding; pp. 53–112. [Google Scholar]
- 17.Kumar G., Kumar R. Chromotoxic and mito- inhibitory effects of pesticide in Trigonella foenum graecum L. J. Cytol.Genet. 2000;1:11–15. [Google Scholar]
- 18.Aslam R., Bhat T.M., Choudhary S., Ansari M.Y.K., Shahwar D. Estimation of genetic variability, mutagenic effectiveness and efficiency in M2 flower mutant lines of Capsicum annuum L. treated with caffeine and their analysis through RAPD markers. J. King Saud Univ. Sci. 2017;29(3):274–283. [Google Scholar]
- 19.Li Y., He N., Hou J., Xu L., Liu C., Zhang J., Wang Q., Zhang X., Wu X. Factors influencing leaf chlorophyll content in natural forests at the biome scale. Front. Ecol. Evol. 2018;6:64. [Google Scholar]
- 20.Raina A., Laskar R.A., Tantray Y.R., Khursheed S., Wani M.R., Khan S. Characterization of induced high yielding cowpea mutant lines using physiological, biochemical and molecular markers. Sci. Rep. 2020;10:3687. doi: 10.1038/s41598-020-60601-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Borzouei A., Kafi M., Khazaei H., Naseriyan B., Majdabadi A. Effects of gamma radiation on germination and physiological aspects of wheat (Triticum aestivum L.) seedlings. Pakistan J. Bot. 2010;42(4):2281–2290. [Google Scholar]
- 22.Verma A.K., Banerji B.K., Chakrabarty D., Datta S.K. Studies on makhana (Euryale ferox Salisbury) Curr. Sci. 2010:795–800. [Google Scholar]
- 23.Laskar R.A., Laskar A.A., Raina A., Khan S., Younus H. Induced mutation analysis with biochemical and molecular characterization of high yielding lentil mutant lines. Int. J. Biol. Macromol. 2018;109:167–179. doi: 10.1016/j.ijbiomac.2017.12.067. [DOI] [PubMed] [Google Scholar]
- 24.Uarrota V.G., Stefen D.L.V., Leolato L.S., Gindri D.M., Nerling D. Antioxidants and Antioxidant Enzymes in Higher Plants. Springer; Cham: 2018. Revisiting carotenoids and their role in plant stress responses: from biosynthesis to plant signaling mechanisms during stress; pp. 207–232. [Google Scholar]
- 25.Jayabalan N., Rao G.R. Effect of physical and chemical mutagens on seed germination and survival of seedlings in Lycopersicon essculentum Mill. J. Indian Bot. Soc. 1987;10:133–137. [Google Scholar]
- 26.Kumar S., Dubey D.K. Mutagenic efficiency and effectiveness of separate and combined treatment of Gamma rays, EMS and DES in Khesari (Lathyrus sativus L.) J. Indian Bot. Soc. 1998;77:1–4. [Google Scholar]
- 27.Rao G.M., Rao V.M. Mutagenic efficiency, effectiveness and factor of the effectiveness of physical and chemical mutagens in rice. Cytologia. 1983;48:427–436. [Google Scholar]
- 28.Kirtane S., Dhumal K.N. Studies on induced mutations in onion: biological and cytological effects of mutagens in M2 generation. Int. J. Mend. 2004;21:11–13. [Google Scholar]
- 29.Raina A., Laskar R.A., Khursheed S., Khan S., Parveen K., Amin R., Khan S. Induce physical and chemical mutagenesis for improvement of yield attributing traits and their correlation analysis in chickpea. Int. Lett. Nat. Sci. 2017;16:14–22. [Google Scholar]
- 30.Kumar G., Rai P.K. EMS induced karyomorphological variations in maize (Zea mays L.) inbreds. Turk. J. Biol. 2007;31(4):187–195. [Google Scholar]
- 31.Kumar G., Shrivatava U. Cytomictic variations in Isabgol (Plantago ovata forsk) Nucleus. 2001;44:180–182. [Google Scholar]
- 32.Bhat T.A., Khan A.H., Parveen S.A.H.B.A. Comparative analysis of meiotic abnormalities induced by gamma rays, EMS and MMS in Vicia faba L. J. Indian Bot. Soc. 2005;84:45–48. [Google Scholar]
- 33.Shahwar D., Ansari M.Y.K., Bhat T.M. Assessment of genetic variability, morphology and productivity response of Vicia faba under the stress of lead nitrate. Int. J. Adv. Life Sci. 2016;9:58–65. [Google Scholar]
- 34.Alka, Ansari M.Y.K., Bhat T.M., Choudhary S., Aslam R. Genotoxic effect of ethylmethane sulphonate and sodium azide in Linum usitatissimum L. Int. J. Plant, Ani. Environ. Sci. 2012;1:1–6. [Google Scholar]
- 35.Sidhu M.C. Meiotic studies in the hybrids of wheat-rye additional lines and wheat (Triticum aestivum L.) varieties. J. Indian Bot. Soc. 2008;87(1&2):86–91. [Google Scholar]
- 36.Zeerak N.A. Cytogenetical effects of gamma rays and ethyl ethanosulfonate in tomato (Lycopersicon esculentum var. Cerasiforme) Phytomorphology. 1992;42:81–86. [Google Scholar]
- 37.Saha A., Datta A.K. Gamma-rays induced reciprocal translocation in black cumin (Nigella sativa L.) Cytologia. 2002;67(4):389–396. [Google Scholar]
- 38.Amin R., Wani M.R., Raina A., Khursheed S., Khan S. Induced morphological and chromosomal diversity in the mutagenized population of black cumin (Nigella sativa L.) using single and combination treatments of gamma rays and ethyl methane sulfonate. Jordan J. Biol. Sci. 2019;12:23–30. [Google Scholar]
- 39.Bhat T.A., Khan A.H., Parveen S. Effect of gamma rays on certain cytomorphological parameters in two varieties of Vicia faba (L.) Adv. Plant Sci. 2006;19(1):227. [Google Scholar]
- 40.Aslam R., Choudhary S., Ansari M.Y.K., Alka H.I., Bhat T.M. Assessment of genetic variability induced by MMS and 6-AP in a medicinal herb Cichorium intybus L. Biosci. Int. 2012;1(2):30–39. [Google Scholar]
- 41.Gaulden M.E. Hypothesis: some mutagens directly alter specific chromosomal proteins (DNA topoisomerase II and peripheral proteins) to produce chromosomal stickiness, which causes chromosomal aberrations. Mutagenesis. 1987;2:357–365. doi: 10.1093/mutage/2.5.357. [DOI] [PubMed] [Google Scholar]
- 42.Anis M., Wani A.A. Caffeine lnduced Morpho-cytological variability in fenugreek, Trigonella foenum-graecum L. Cytologia. 1997;62(4):343–349. [Google Scholar]
- 43.Gulfishan M., Khan A.H., Haneef I., Bhat T.A. Genotoxic effect of diethyl sulphate in two varieties of Capsicum annum L. Nucleus. 2011;54(2):107–111. [Google Scholar]
- 44.Shrivastva A., Kapoor K. Seed yield is not impaired by chromosome stickiness in sodium azide treated Trigonella foenum graecum L. Cytologia. 2008;73:115–127. 12. [Google Scholar]
- 45.Abbasi N., Anis M. Clastogenic effect of chemical mutagens in Trigonella foenum-geaceumL. J.Cytol. Genet. 2002;3:109–114. [Google Scholar]
- 46.Ganai F.A., Khan A.H., Bhat Parveen S., Wani N.A. Cytogenic effects of methyl methanesulphonate (MMS) on two varities of chikepea (Cicer arietinium L.) J. Cytol. Genet. 2005;6:97–102. [Google Scholar]
- 47.Sharma V., Kumar G. Meiotic studies in two cultivars of Cicer arietinum L. after EMS treatment. Cytologia. 2004;69:243–248. [Google Scholar]
- 48.Umar G., Singh V. Comparative analysis of meiotic abnormalities induced by gamma rays and EMS in barley. J. Indian Bot. Soc. 2003;82:19–22. [Google Scholar]
- 49.Tarar J.L., Dnyansagar V.R. Comparison of ethyl methanesulfonate and radiation induced meiotic abnormalities in Turnera ulmifolia Linn. var. angustifolia Willd. Cytologia. 1980;45(1/2):221–231. [Google Scholar]
- 50.Iqbal M., Datta A.K. Cytogenetic studies in Withania somnifera (l.) dun. (Solanaceae) Cytologia. 2007;72(1):43–47. [Google Scholar]
- 51.Anis M., Aijaza W., Khursheed T. Cytotoxic effect of leaf extract of Ipomoea cornea on root tip cells of Trigonella foenum-graecum L. J. Cytol. Genet. 1999;34:111–114. [Google Scholar]
- 52.Khursheed S., Laskar R.A., Raina A., Amin R., Khan S. Comparative analysis of cytological abnormalities induced in Vicia faba L. genotypes using physical and chemical mutagenesis. Chromosome Sci. 2015;18(3–4):47–51. [Google Scholar]
- 53.Nirmala C. Male sterility in pea II Male sex specific dys-synapsis. Cytologia. 1993;58(1):67–76. [Google Scholar]
- 54.Kaul K.K. Cytomixis in Pollen mother cells of Alopecurus arundinaceus poir. Cytologia. 1990;55:169–173. [Google Scholar]
- 55.Banu M.R., Ashok S., Kalmani M. Effect of mutagenic treatments in M1 generation of cowpea (Vigna unguiculata (L.)Walp.) Int. J. Mendal. 2004;21:63–64. [Google Scholar]
- 56.Shahwar D., Ansari M.Y.K., Park Y. Physio-biochemical analysis and molecular characterization of induced lentil mutant lines. PLoS One. 2022;17(10) doi: 10.1371/journal.pone.0274937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kumar G., Tripathi R. Lead-induced cytotoxicity and mutagenicity in grass pea. Turk. J. Biol. 2008;32(2):73–78. [Google Scholar]
- 58.Sparrow A.H., Cuany R.L., Schairer L.A. Some factors affecting the responses of plants to acute and chronic radiation exposures. Radiat. Bot. 1961;1:10–34. [Google Scholar]
- 59.Cheng Q., Dong L., Su T., Li T., Gan Z., Nan H., Kong F. CRISPR/Cas9-mediated targeted mutagenesis of GmLHY genes alters plant height and internode length in soybean. BMC Plant Biol. 2019;19(1):1–11. doi: 10.1186/s12870-019-2145-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Raina A., Laskar R.A., Wani M.R., Jan B.L., Ali S., Khan S. Gamma rays and sodium azide induced genetic variability in high-yielding and biofortified mutant lines in cowpea [Vigna unguiculata (L.) Walp.] Front. Plant Sci. 2022;13 doi: 10.3389/fpls.2022.911049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Horn L.N., Ghebrehiwot H.M., Shimelis H.A. Selection of novel cowpea genotypes derived through gamma irradiation. Front. Plant Sci. 2016;7:262. doi: 10.3389/fpls.2016.00262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Laskar R.A., Wani M.R., Raina A., Amin R., Khan S. Morphological characterisation of gamma rays induced multipodding mutant (mp) in lentil cultivar Pant L 406. Int. J. Radiat. Biol. 2018;94:1049–1053. doi: 10.1080/09553002.2018.1511927. [DOI] [PubMed] [Google Scholar]
- 63.Joshi P., Verma R.C. Radiation induced pod and seed mutant in faba bean (Vicia faba L.) Indian J. Genet. 2004;64:155–156. [Google Scholar]
- 64.Alghamdi S.S., Migdadi H.M. Morphological diversity of faba bean (Vicia faba L.) M2 mutant populations induced by gamma radiation and diethyl sulfate. J. King Saud Univ. Sci. 2020;32(2):1647–1658. [Google Scholar]
- 65.Singh R.K. Gamma rays induced bold seeded mutant in Vigna mungo (L.) Hepper. Indian J. Genet. 1996;56:104–108. [Google Scholar]
- 66.Gumber R.K., Singh S., Singh K. Frequency and spectrum of mutations induced by gamma rays in Desi and Kabuli Chickpea. Int. Chickpea Newsletter. 1995;2:8. [Google Scholar]
- 67.Badre R.S., Choudary A.D. Induced mutations in linseed (Linum usitatissimum L.) Indian J. Genet. 2004;64:159–160. [Google Scholar]
- 68.Rasik S., Raina A., Laskar R.A., Wani M.R., Jan B.L., Ali S., Khan S. Comparative mutagenic effectiveness and efficiency of gamma rays and sodium azide in inducing chlorophyll and morphological mutants of cowpea. Plants. 2022;11:1322. doi: 10.3390/plants11101322. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data included in article/supplementary material/referenced in article.