Abstract
Background:
Management of <2 cm pancreatic neuroendocrine tumors (PanNETs) is controversial. Although often indolent, the oncologic heterogeneity of these tumors particularly related to lymph node (LN) metastases poses challenges when deciding between resection versus surveillance.
Methods:
WE analyzed all patients who underwent resection of primary non-functional <2 cm with curative-intent at 8 institutions of the US Neuroendocrine Tumor Study Group from 2000–2016. PanNETs with poor-differentiation and Ki-67>20% were excluded. Our primary aim was to create a Lymph Node Risk Score (LNRS) that predicted LN metastases accurately for <2 cm PanNETs utilizing readily available preoperative data.
Results:
Of 695 patients with resected PanNETs, 309 were <2 cm. Of these smal PanNETs , 25% were proximal (head/uncinate), 23% had a Ki-67≥3%, and only 8% were moderately-differentiated. Also, only 9% of all <2 cm PanNETs were LN(+); indeed lymph node positivity was associated with worse 5-year recurrence-free survival compared to LN(−) disease (80% vs 96%; p=0.007). Factors known preoperatively to be associated with LN metastases were proximal location (OR 4.0; p=0.002) and Ki-67≥3% (OR 2.7; p=0.05). Moderate-differentiation was not associated with LN(+) disease. Location and Ki-67 were assigned a value weighted by their odds ratio: (distal= 1, proximal= 4, and Ki-67<3%= 1and Ki-67≥3%=3), which formed a LNRS ranging from 1–7. Scores were categorized into low (1–2), intermediate (3–4), and high (5–7) risk groups. Incidence of LN metastases increased progressively based on risk group, with Low= 3.2%, Intermediate= 13.8%, and High= 20.5% (Table). Only 3.4% of PanNETs with a Ki-67<3% in the distal pancreas were LN(+) compared to 21.4% of PanNETs with a Ki-67≥3% in the head/uncinate.
Conclusion:
This simple and novel LN risk score utilizes readily available preoperative factors (tumor location and Ki-67) to stratify risk of LN metastase accurately s for <2 cm PanNETs and may help guide management strategy.
INTRODUCTION
Pancreatic neuroendocrine tumors (PanNETs) are a rare and heterogeneous tumor type ,and currently represent about 3% of all pancreatic malignancies.1 Although PanNETs are relatively indolent neoplasms, 5-year survival can be as great as 90–100% and as low as 25%.2 Nearly 20% of PanNETs are considered to be “functional” tumors, manifesting with clinical signs and symptoms secondary to hormonal activation.1,3 The large majority of PanNETs, however, are classified as “non-functional,” and thus either present late in their disease course due to tumor burden or are identified by chance.2,3 As cross-sectional imaging increases in frequency and quality, non-functional PanNETs are being diagnosed at an increasingly small size.4,5 The behavioral heterogeneity that distinguishes PanNETs has been noted even among PanNETs < 2 cm, with multiple studies reporting instances of nodal metastasis, distant metastasis, and disease recurrence among this group.5–9 As a result, standardizing management, follow-up surveillance, and prognosis for these tumors remains a challenge.10
The sevenfold increase in the incidence of small PanNETs in the United States over the last two decades, as well as the uncertainty of their malignant potential, has created controversy over the optimal management of these neoplasms.11,12 While operative resection remains the only cure for PanNETs,10 several studies have proposed observation for tumors <2 cm as the preferred management strategy.11–14 Yet, guidelines remain unclear, because both the European Neuroendocrine Tumor Society (ENETS) and the National Comprehensive Cancer Network (NCCN) offer several viable treatment strategies, including both resection and observation.15,16 Without a definitive consensus, health care teams must utilize known prognostic factors to predict tumor behavior and to help choose the appropriate approach for each individual patient.
While a number of poor prognostic factors have been implicated in the natural history of PanNETs, the impact of lymph node metastases on survival in non-functional PanNETs has been demonstrated clearly.17–23 According to the American Joint Committee on Cancer, nodal disease automatically renders a Stage III diagnosis of Stage III disease in neuroendocrine tumors.24 As such, even in the indolent group of <2 cm PanNETs, lymph node metastases may serve as a surrogate for aggressive tumor biology, which can aid in the decision to resect or observe the seneoplasms.
Given that only an average of 40% of all non-functional PanNETs have lymph node metastases, an accurate preoperative method for staging nodal status may be used to inform the surgical plan.17 The aim of this study was to create a lymph node risk score (LNRS) that accurately predicts lymph node metastas accurately es for <2 cm non-functional PanNETs utilizing readily available preoperative data.
METHODS
Study Population
The U.S. Neuroendocrine Tumor Study Group (US-NETSG) is a collaboration of 8, high-volume institutions from across the United States, including Emory University, Michigan University, the Ohio State University, Stanford University, Vanderbilt University, Virginia Mason Clinic, Washington University in St. Louis, and University of Wisconsin. This database is comprised of all patients from these institutions with neuroendocrine tumors of the abdomen, specifically those located in the stomach, duodenum, ampulla, pancreas, liver, gallbladder, small bowel, appendix, colon, rectum, spleen, and peritoneum, who underwent operative resection from January 1, 2000 to December 31, 2016. For the purpose of this study, we included only patients with <2 cm, non-functional primary PanNETs who underwent curative-intent resection. Poorly differentiated and metastatic PanNETs , as well as those with a Ki-67>20% were excluded. All 30-day mortalities and R2 resections were also excluded. The primary end-point was presence of lymph node metastases, with the aim to create a preoperative Lymph Node Risk Score (LNRS) to predict accurately the presence of nodal metastases for <2 cm PanNETs.
Study Variables
Pertinent baseline demographic, preoperative, intraoperative, pathologic, and postoperative data were collected retrospectively through review of the medical records. Comorbidities were defined using the American College of Surgeons National Surgical Quality Improvement Program (ACS NSQIP) risk-calculator. Cancer staging was assigned per the guidelines of the American Joint Committee on Cancer 7th edition.25 Neoadjuvant and adjuvant therapy, disease recurrence, and survival data were also collected. Approval by the Institutional review board was obtained at each institution prior to any data retrieval, and survival information was verified with the Social Security Death Index when appropriate.
Statistical Analyses
All statistical tests were executed using SPSS version 23.0 (Armonk New York Software, IBM Inc.) with statistical significance predefined as p<0.05. Descriptive and comparative analyses were performed for the entire cohort. Chi-squared analyses and Fisher’s exact tests were used to compare categorical variables, and Student’s t-test was used for continues variables, where indicated. Kaplan-Meier log-rank plots were calculated for recurrence-free survival (RFS), and univariable logistic regression was performed to evaluate both the clinicopathologic variables associated with lymph node positivity and the preoperative LNRS generated subsequently.
RESULTS
Patient Variables
Of 2,182 patients with neuroendocrine tumors in the US-NETSG database, 695 patients had low-to-intermediate, non-functional PanNETs who underwent curative-intent resection with a curative intent, 309 of which were <2 cm in size. Baseline demographics and clinicopathologic features of this study cohort are summarized in Table 1. Mean age was 58 years, 48% were male (n=147), and 76% (n=231) were Caucasian. Mean tumor size was 1.3 cm, and given that 75% (n=232) of tumors were located distally in the neck, body, or tail of the pancreas, only 25% (n=77) were located proximally in the head or uncinate. With regard to pathologic data, 93% (n=258) of tumors were well-differentiated, 77% (n=177) had a Ki-67 index <3%, and 9% (n=22) were lymph node-positive.
Table 1.
Baseline Demographics and Clinicopathologic Variables of Patients with <2 cm Low/Intermediate Grade,Non-functional, Pancreatic Neuroendocrine Tumors (PNETs) from the US-NETSG Database who underwent Curative-intent Resection from 2000–2016 (n=309).
| Baseline Variables | n (%) |
|---|---|
| Age (y), mean ± SD | 58 ± 12 |
|
| |
| Male, n (%) | 147 (48) |
|
| |
| BMI, mean ± SD | 29 ± 6 |
|
| |
| Comorbidities, n (%)a | |
| 0 | 106 (34) |
| 1 | 91 (29) |
| ≥2 | 108 (35) |
|
| |
| Race, n (%) | |
| White | 231 (76) |
| Black | 32 (11) |
| Other | 40 (13) |
|
| |
| ASA class, n (%) | |
| 1 | 6 (2) |
| 2 | 143 (47) |
| 3 | 151 (50) |
| 4 | 3 |
|
| |
| CgA (ng/L), mean ± SD | 176 ± 332 |
|
| |
| Operative/ Pathologic Data | n (%) |
|
| |
| Tumor Size (cm), mean ± SD | 1.3 ± 0.4 |
|
| |
| Location of Tumor in Pancreas, n (%) | |
| Head/uncinate (Proximal) | 77 (25) |
| Neck/body/tail (Distal) | 232 (75) |
|
| |
| OperativeTechnique, n (%) | |
| Open | 198 (64) |
| Laparoscopic | 70 (23) |
| Other | 31 (13) |
|
| |
| Type of Resection, n (%) | |
| Enucleation | 28 (9) |
| Classic pancreatoduodenectomy | 25 (8) |
| Pylorus preserving pancreatoduodenectomy | 36 (12) |
| Central pancreatectomy | 22 (7) |
| Distal pancreatectomy | 195 (63) |
| Total pancreatectomy | 3 (1) |
|
| |
| Tumor Differentiation, n (%) | |
| Well | 258 (93) |
| Moderate | 21 (8) |
|
| |
| Ki-67 Index, n (%) | |
| < 3% | 177 (77) |
| 3–20% | 52 (23) |
|
| |
| Lymph Node-Positive, n (%) | 22 (9) |
|
| |
| Lymph Node Yield, median (IQR) | 8 (3–13) |
| *ELSEVIER SEE BELOW Pancreatoduodenectomy | 11 (7–17) |
| Distal Pancreatectomy | 8 (4–12) |
| Enucleation/Central pancreatectomy | 1 (1–2) |
|
| |
| Post-operative Data | n (%) |
|
| |
| Clavien-Dindo Classification, n (%)b | |
| I | 48 (26) |
| II | 67 (37) |
| IIIa | 43 (23) |
| IIIb | 11 (6) |
| IVa | 11 (6) |
| IVb | 3 (2) |
|
| |
| Disease Recurrence | 13 (4) |
|
| |
| Region of Recurrence | |
| Locoregional | 6 (46) |
| Locoregional + Distant | 0 (0) |
| Distant | 7 (54) |
|
| |
| Deaths | 11 (4) |
|
| |
| Time to Death | |
| <30 days | 0 (0) |
| 31–60 days | 0 (0) |
| 61–90 days | 0 (0) |
| ≥90 days | 11 (100) |
Abbreviations: SD, standard deviation; BMI, body mass index; ASA, American Society of Anesthesiologists; CgA, chromogranin A; IQR, interquartile range;
Comorbidities are defined as any concurrent medical condition, including but not limited to, heart disease, chronic pulmonary disease, diabetes, renal disease, and liver disease as per the American College of Surgeons National Surgical Quality Improvement Program Risk Calculator.
Clavien-Dindo is a grading scale for ranking the severity of post-operative complications ranging from I-V, where I represents the lowest acuity complication (one not requiring pharmacological treatment or surgical, endoscopic, or radiologic intervention), and V represents death.
ELSEVIER THE THREE OPERATIVE PROCEDURES ARE IN A UNIQUE GROUP OF PATIENTS THAT NEEDS TOP BE DEFINED IN THE TABLE – I THINK THESE REPRESENT THE PATIENTS WITH LYMPH NODE-POSTITIVE DISEASE THIS NEEDS TO BE SPECIFIED BY THE AUTHORS
Recurrence-Free Survival Analysis:
Median follow-up was 35 months (IQR 13.9–59.1), and 13 patients (4%) experienced recurrence of disease after resection. Of those patients who developed a recurrence, , 6 recurred loco-regionally and 7 recurred distantly (1 in bone, 4 in liver, and 2 in lung). On Kaplan-Meier analysis, the 5-year RFS for patients with lymph node-positive disease was 80% compared to 96% for those with node-negative disease (p=0.007) (Figure 1). On univariable Cox regression, there was a 6-fold increase in risk of recurrence among patients with lymph node-positive versus negative disease (HR 5.9, 95% CI 1.4–25.3; p=0.016). Other pathologic factors, including final margin status, lymphovascular invasion, perineural invasion, tumor grade, and T-stage, were not associated with RFS in this cohort.
Figure 1.
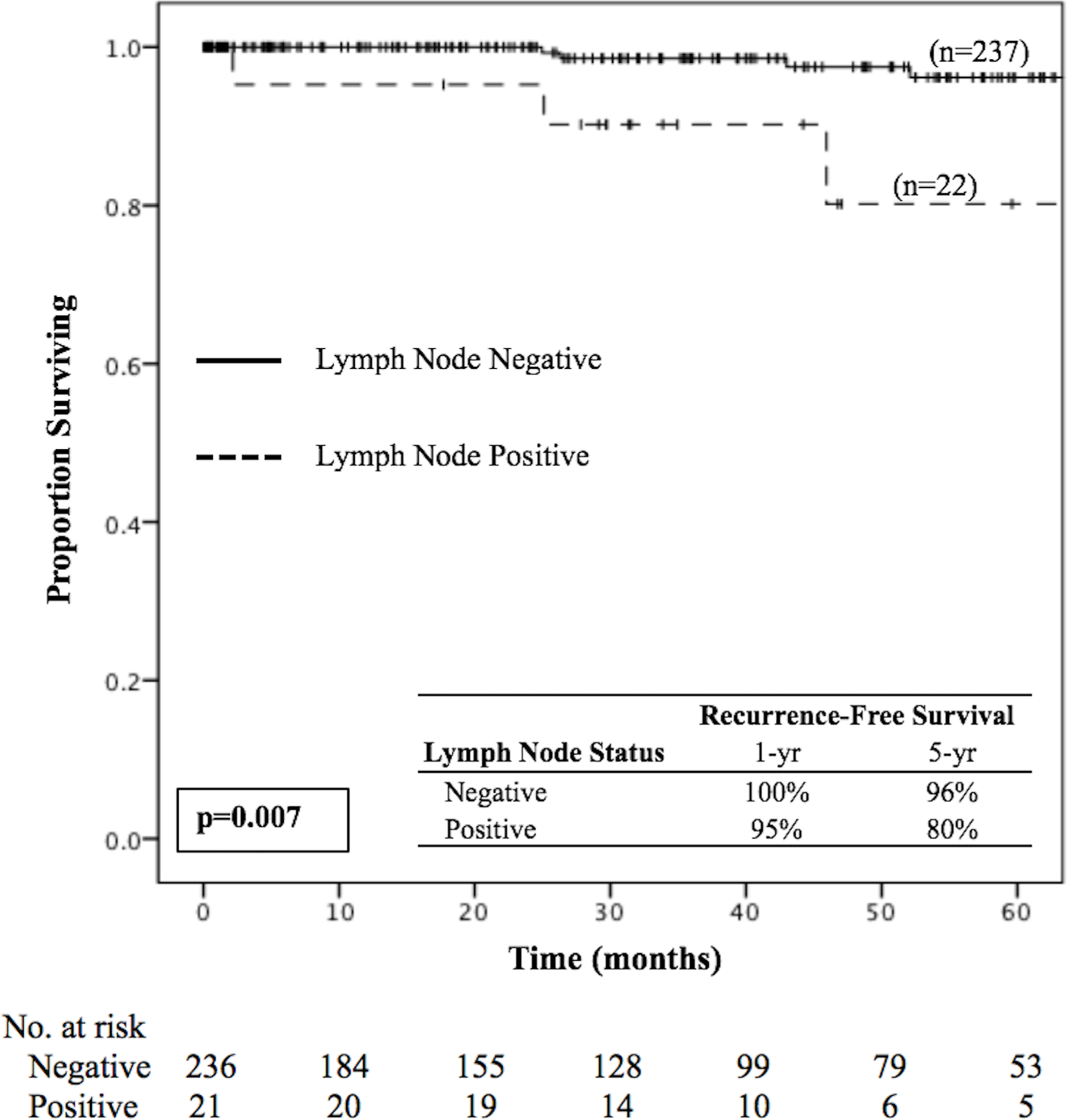
Kaplan-Meier survival curve for recurrence-free survival in lymph node–positive versus lymph node–negative patients with <2 cm low/intermediate grade nonfunctional pancreatic neuroendocrine tumors.
Lymph Node-Positivity and the Lymph Node Risk Score
Lymph node-positive patients when compared to those with lymph node-negative disease were more likely to have tumors located proximally in the pancreas (55% vs 23%; p=0.003), to have an advanced T-stage of T3/T4 (18% vs 3%; p=0.006), lymphovascular invasion (61% vs 8%; p<0.001), and perineural invasion (39% vs 12%; p=0.005) (Table 2). On binary logistic regression, proximal tumor location (OR 4.0, 95% CI 1.6–9.7; p=0.002), Ki-67 >3% (OR 2.7, 95% CI 1.0–7.2; p=0.054), positive margins (OR 3.3, 95% CI 1.0–11.0; p=0.052), lymphovascular invasion (OR 17.2, 95% CI 5.9–50.1; p<0.001), perineural invasion (OR 4.9, 95% CI 1.7–14.0; p=0.003), and advanced T-stage (OR 8.6, 95% CI 2.2–33.1; p=0.002) were all associated with an increased risk for lymph node-positivity (Table 3). When assessing lymph node-positivity simply by tumor location, 10 of the 193 (5.2%) distal tumors had lymph node4 positive disease compared to 12 of 67 (17.9%) proximal tumors (p<0.01).
Table 2.
Distribution of Pathologic Factors among Patients with <2 cm Low/Intermediate Grade, Non-functional, PNETs from the US-NETSG Database who underwent Curative-intent Resection from 2000–2016, Stratified by Lymph Node-Positivity.
| Pathologic Factors | Lymph Node Negative (n=287) |
Lymph Node Positive (n=22) |
p-valuea |
|---|---|---|---|
| Location of Tumor in Pancreas, n (%) | 0.003 | ||
| Proximal | 55 (23) | 12 (55) | |
| Distal | 183 (77) | 10 (45) | |
|
| |||
| Tumor size | 0.677 | ||
| <1 cm | 57 (24) | 4 (18) | |
| 1–1.5 cm | 114 (48) | 10 (46) | |
| ≥1.5 cm | 67 (28) | 8 (36) | |
|
| |||
| AJCC T-Stage, n (%) | 0.006 | ||
| T1/T2 | 231 (97) | 18 (82) | |
| T3/T4 | 6 (3) | 4 (18) | |
|
| |||
| Tumor Differentiation, n (%) | 0.646 | ||
| Well | 199 (92) | 16 (89) | |
| Moderate | 17 (8) | 2 (11) | |
|
| |||
| Ki-67 Index, n (%) | 0.090 | ||
| <3% | 136 (77) | 10 (56) | |
| 3–20% | 41 (23) | 8 (44) | |
|
| |||
| Mitotic Rate (per 10 HPF), n (%) | 0.081 | ||
| <2 | 141 (92) | 9 (75) | |
| 2–20 | 12 (8) | 3 (25) | |
|
| |||
| Final Resection Status, n (%) | 0.065 | ||
| R0b | 222 (94) | 18 (82) | |
| R1b | 15 (6) | 4 (18) | |
|
| |||
| Lymphovascular Invasion, n (%) | <0.001 | ||
| Negative | 186 (92) | 7 (39) | |
| Positive | 17 (8) | 11 (61) | |
|
| |||
| Perineural Invasion, n (%) | 0.005 | ||
| Negative | 161 (88) | 11 (61) | |
| Positive | 21 (12) | 7 (39) | |
Abbreviations: AJCC, American Joint Committee on Cancer; HPF, high power fields;
Statistical significance is indicated by a p<0.05.
R0 resection refers to negative margins on pathologic review of the specimen, while R1 resection refers to positive margins on pathologic review of the specimen.
Table 3.
Association of Pathologic Factors with Risk for Lymph Node Positivity in Patients with <2 cm Low/Intermediate Grade, Non-functional, PNETs from the US-NETSG Database who underwent Curative-intent Resection from 2000–2016.
| Logistic Regression | ||
|---|---|---|
| Lymph Node Positivity | ||
| Pathologic Factors | OR (95% CI) | p-valuea |
| Tumor Location in Pancreas | ||
| Distal | Ref | -- |
| Proximal | 4.0 (1.6–9.7) | 0.002 |
|
| ||
| Tumor Size | ||
| <1 cm | Ref | -- |
| 1–1.5 cm | 1.25 (0.4–4.2) | 0.716 |
| ≥1.5 cm | 1.7 (0.5–5.9) | 0.405 |
|
| ||
| Tumor Differentiation | ||
| Well | Ref | -- |
| Moderate | 1.5 (0.3–6.9) | 0.631 |
|
| ||
| Ki-67 Index | ||
| <3% | Ref | -- |
| 3–20% | 2.7 (1.0–7.2) | 0.054 |
|
| ||
| Final Resection Status | ||
| R0 | Ref | -- |
| R1 | 3.3 (1.0–11.0) | 0.052 |
|
| ||
| Mitotic Rate (per 10 HPF) | ||
| <2 | Ref | -- |
| 2–20 | 3.9 (0.9–16.4) | 0.062 |
|
| ||
| Lymphovascular Invasion | ||
| Negative | Ref | -- |
| Positive | 17.2 (5.9–50.1) | <0.001 |
|
| ||
| Perineural Invasion | ||
| Negative | Ref | -- |
| Positive | 4.9 (1.7–14.0) | 0.003 |
|
| ||
| Advanced T Stage | ||
| T1/T2 | Ref | -- |
| T3 | 8.6 (2.2–33.1) | 0.002 |
Abbreviations: OR, odds ratio; CI, confidence interval; HPF, high power fields;
Statistical significance is indicated by a p<0.05.
Bold values indicate preoperatively measurable variables through imaging or biopsy.
The LNRS was created by assigning a value for the risk score based on the odds ratio on logistic regression for the preoperatively available factors of tumor location and Ki-67 index. For proximal tumor location, the OR was 4; thus, a score of 1 was given for distal tumor location and a score of 4 for proximal tumor location. For the Ki-67 index ≥3%, the OR was 2.7, so a score of 1 was given for a Ki-67 <3% and a score of 3 for a Ki-67 ≥3%. The final risk score ranged from 1–7, depending on the presence of one or both preoperative factors. The scores were then re-stratified into three groups, where a score of 1–2 corresponded with “low risk,” a score of 3–4 with “intermediate risk,” and a score of 5–7 with “high risk” for lymph node-positivity; 63%(n=195) of the cohort was classified as having a low LNRS, 20% (n=61) as having an intermediate LNRS, and 17% (n=53) as having a high LNRS. The incidence of lymph node-positivity ranged from 3% to 14% to 21% for a low, intermediate, and high LNRS, respectively (Table 4). Using binary logistic regression, an intermediate LNRS corresponded with a nearly 5-fold increase in risk for positive lymph nodes compared to a low LNRS (OR 4.9, 95% CI 1.5–15.7; p=0.007), while a high LNRS was associated with an almost 8-fold increase in risk for lymph node-positivity (OR 7.9, 95% CI 2.5–24.9; p<0.001) (Table 4). For patients with both a distally located tumor and a Ki-67 <3%, lymph node metastases occurred at a rate of 3%. Conversely, 21% of patients with tumors having both a proximal location and a Ki-67 ≥3% had node-positive disease.
Table 4.
Rate and Risk of Lymph Node-Positivity According to the Preoperative Lymph Node Risk Score among Patients with <2 cm, Low/Intermediate Grade, Non-functional ,PNETs from the US-NETSG Database who underwent Curative-intent Resection from 2000–2016.
| Lymph Node Positivity* | ||||
|---|---|---|---|---|
| Incidence | p-value† | OR (95% CI) | p-value† | |
| Lymph Node Risk Score | <0.001 | |||
| Low (Score 1–2) (n=195) | 3% | Ref | -- | |
| Intermediate (Score 3–4) (n=61) | 14% | 4.9 (1.5–15.7) | 0.007 | |
| High (Score 5–7) (n=53) | 21% | 7.9 (2.5–24.9) | <0.001 | |
Abbreviations: OR, odds ratio; CI, confidence interval;
Chi-squared analysis was used to compare incidence of lymph node positivity among the risk groups, while binary logistic regression was used to evaluate risk for nodal disease based on the assigned lymph node risk score.
Statistical significance is indicated by a p<0.05.
DISCUSSION
Small pancreatic neuroendocrine tumors are a heterogeneous group of neoplasms with controversy regarding their best management. This study showed an association between lymph node metastases and decreased RFS among <2 cm, non-functional PanNETs. The preoperatively available factors of tumor location and Ki-67 index were utilized to create a simple and novel LNRSto stratify accurately these small tumors for risk of lymph node metastases. Indeed, using this LNRS ranging from1–7 based on the odds ratios on logistic regression for tumor location and Ki-67 index, <2 cm PanNETs were grouped into low (score 1–2), intermediate (score 3–4), and high (score 5–7) risk groups. The incidence of lymph node-positivity increased with increases in the LNRS, from 3% to 14% to 21%, respectively. Compared to low risk tumors, PanNETs classified as intermediate-risk were 5 times more likely to be lymph node-positive (OR 4.9; p=0.007), while high-risk PanNETs had a nearly 8-fold increase in lymph node metastases (OR 7.9; p<0.001).
Although the diagnosis of small, non-functional PanNETs has increased in the last few decades due in large part to the increase in cross-sectional imaging, current guidelines for the optimal management of these tumors remain ambiguous and not consistent.11 ENETS recommends either surveillance or resection for <2 cm PanNETs, with careful weighing of the risks and benefits on a case-by-case basis.16 Likewise, NCCN guidelines offer enucleation +/− regional lymphadenectomy, anatomic resection, and observation as viable management strategies.15 Even the North American Neuroendocrine Tumor Society (NANETS) has an unclear consensus, suggesting enucleation or operative resection versus observation depending on individual patient characteristics.26 Findings in the literature have been conflicting as well. Although several studies, such as those of Lee et al., and Sadot et al., have concluded that it is safe to manage <2 cm PanNETs by observation,11,13,27 others including Gratian et al., and Haynes et al., have suggested that these small tumors may still display an aggressive course.6,9,12,28 Of course, with advances in functional imaging modalities such as DOTATATE, for example, the ability to preoperatively assess aggressive behavior via evaluation for lymph node metastasis may improve. In the current study, 9% (n=22) of patients had lymph node metastases at resection, and 13 recurred within a median follow-up of 35 months. Importantly, those patients with a high LNRS had a rate of lymph node metastases as great as 21%. These findings support the evidence that <2 cm PanNETs may manifest aggressive behavior, thus highlighting the need for both optimizing and standardizing their management.
When considering the characteristics of PanNETs that may predict poor outcomes and inform the decision to resect versus observe, there is substantial evidence supporting lymph node metastases as a marker of worse disease.17,18,21,22 A study by Partelli et al., demonstrated a 5-year disease-free survival of 70% for N1 disease versus 97% for N0 disease among patients with non-functional PanNETs.17 Similarly, the study of Postlewait et al.’s showed that the 5-year RFS was 40% for patients with nodal metastasis compared with 85% in patients with lymph node-negative disease.29 These findings are in accordance with the current study, because lymph node-positive patients in our cohort had a 5-year RFS of 80% compared to 96% for those who were lymph node-negative. Not only has lymph node metastasis in PanNETs been shown to correlate with decreased survival, but PanNETs specifically <3 cm metastasize at a rate as great as 33%.30 Given this high incidence, the ability to predict nodal metastasis preoperatively in small PanNETs is of paramount importance and clinical relevance.
A number of clinicopathologic factors are correlated with lymph node-positivity in PanNETs, including tumor location in the head of the pancreas, increasing tumor size, lymphovascular invasion, and an increasedKi-67 index.29,31,32 In the current study, although multiple factors were associated with an increased risk for lymph node metastasis, only tumor location and Ki-67 index were reliably available preoperatively. Hashim et al., showed previously that PanNETs located proximally in the pancreas had a 2.8 times increase in risk for nodal disease compared to those in the body/tail.31 In accordance with Hashim et al, our study demonstrated that proximal tumors had a 4-fold increase in risk for nodal metastases and that a Ki-67 index ≥3% had a nearly 3-fold increase in risk for nodal positivity. Because Ki-67 on preoperative biopsy has been found to correlate well with Ki-67 of the resected specimen, and because it is more sensitive than other clinicopathologic factors at predicting malignant behavior of neuroendocrine neoplasms, Ki-67 appears to be able to serve as a useful preoperative prognostic indicator.33–35 Thus, tumor location and Ki-67 were combined to create a reliable LNRS from which to predict accurately the risk for lymph node metastasis among patients with <2cm PanNETs. Access to such information preoperatively may be used to guide and optimize patient management.
This study is limited by its retrospective design which may involve incomplete preoperative, pathologic, and survival data for the entire cohort. Specific limitations include incomplete data on preoperative, radiographically measured tumor size, preoperative radiographically assessed lymph node status, and Ki-67 data based on the preoperative biopsy specimen. It is also limited by the small overall number of patients who ultimately had positive lymph nodes (n=22;9%) from which we based the Lymph Node Risk Score, however this low rate of lymph node-positive disease is an inherent characteristic of this cohort of patient with small <2cm pancreatic NETs. Furthermore, because only operatively resected neuroendocrine tumors were included in this database, those with worse tumor biology or worrisome features may have been selected. Nonetheless, this study is one of the largest cohorts of <2 cm PanNETs reported, and the use of 8, geographically diverse, academic institutions from across the U.S. eliminates single-institution bias.
In conclusion, a simple LNRSutilizing the readily available preoperative factors of tumor location and Ki-67 can stratify accurately the risk of lymph node metastases for <2 cm PanNETs. This risk score may be used to guide future treatment strategies by informing the decision to resect versus observe patients with small, non-functional PanNETs. This study demonstrated a rate of lymph node metastasis as great as 21% in patients with a high-riskLNRS. Ultimately, this LNRS may provide useful information to inform a meaningful conversation in the preoperative setting to determine the optimal management strategy for patients with <2cm PanNETs.
Acknowledgements
Supported by the National Center for Advancing Translational Sciences of the National Institutes of Health under Award Number UL1TR000454 and TL1TR000456. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Contribution: Funding provided in part by the Katz Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICTS OF INTEREST
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Strosberg JR, Cheema A, Weber JM, et al. Relapse-free survival in patients with nonmetastatic, surgically resected pancreatic neuroendocrine tumors: an analysis of the AJCC and ENETS staging classifications. Ann Surg 2012;256(2):321–325. [DOI] [PubMed] [Google Scholar]
- 2.Metz DC, Jensen RT. Gastrointestinal neuroendocrine tumors: pancreatic endocrine tumors. Gastroenterology 2008;135(5):1469–1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shanahan MA, Salem A, Fisher A, et al. Chromogranin A predicts survival for resected pancreatic neuroendocrine tumors. J Surg Res 2016;201(1):38–43. [DOI] [PubMed] [Google Scholar]
- 4.Zerbi A, Falconi M, Rindi G, et al. Clinicopathological features of pancreatic endocrine tumors: a prospective multicenter study in Italy of 297 sporadic cases. Am J Gastroenterol 2010;105(6):1421–1429. [DOI] [PubMed] [Google Scholar]
- 5.Vagefi PA, Razo O, Deshpande V, et al. Evolving patterns in the detection and outcomes of pancreatic neuroendocrine neoplasms: the Massachusetts General Hospital experience from 1977 to 2005. Arch Surg 2007;142(4):347–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kuo EJ, Salem RR. Population-level analysis of pancreatic neuroendocrine tumors 2 cm or less in size. Ann Surg Oncol 2013;20(9):2815–2821. [DOI] [PubMed] [Google Scholar]
- 7.Falconi M, Zerbi A, Crippa S, et al. Parenchyma-preserving resections for small nonfunctioning pancreatic endocrine tumors. Ann Surg Oncol 2010;17(6):1621–1627. [DOI] [PubMed] [Google Scholar]
- 8.Ferrone CR, Tang LH, Tomlinson J, et al. Determining prognosis in patients with pancreatic endocrine neoplasms: can the WHO classification system be simplified? J Clin Oncol 2007;25(35):5609–5615. [DOI] [PubMed] [Google Scholar]
- 9.Haynes AB, Deshpande V, Ingkakul T, et al. Implications of incidentally discovered, nonfunctioning pancreatic endocrine tumors: short-term and long-term patient outcomes. Arch Surg 2011;146(5):534–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cloyd JM, Poultsides GA. Non-functional neuroendocrine tumors of the pancreas: Advances in diagnosis and management. World J Gastroenterol 2015;21(32):9512–9525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sadot E, Reidy-Lagunes DL, Tang LH, et al. Observation versus Resection for Small Asymptomatic Pancreatic Neuroendocrine Tumors: A Matched Case-Control Study. Ann Surg Oncol 2016;23(4):1361–1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gratian L, Pura J, Dinan M, Roman S, Reed S, Sosa JA. Impact of extent of surgery on survival in patients with small nonfunctional pancreatic neuroendocrine tumors in the United States. Ann Surg Oncol 2014;21(11):3515–3521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee LC, Grant CS, Salomao DR, et al. Small, nonfunctioning, asymptomatic pancreatic neuroendocrine tumors (PNETs): role for nonoperative management. Surgery 2012;152(6):965–974. [DOI] [PubMed] [Google Scholar]
- 14.Bettini R, Partelli S, Boninsegna L, et al. Tumor size correlates with malignancy in nonfunctioning pancreatic endocrine tumor. Surgery 2011;150(1):75–82. [DOI] [PubMed] [Google Scholar]
- 15.Clark OH, Benson AB 3rd, Berlin JD, et al. NCCN Clinical Practice Guidelines in Oncology: neuroendocrine tumors. J Natl Compr Canc Netw 2009;7(7):712–747. [DOI] [PubMed] [Google Scholar]
- 16.Falconi M, Eriksson B, Kaltsas G, et al. ENETS Consensus Guidelines Update for the Management of Patients with Functional Pancreatic Neuroendocrine Tumors and Non-Functional Pancreatic Neuroendocrine Tumors. Neuroendocrinology 2016;103(2):153–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Partelli S, Gaujoux S, Boninsegna L, et al. Pattern and clinical predictors of lymph node involvement in nonfunctioning pancreatic neuroendocrine tumors (NF-PanNETs). JAMA Surg 2013;148(10):932–939. [DOI] [PubMed] [Google Scholar]
- 18.Bettini R, Boninsegna L, Mantovani W, et al. Prognostic factors at diagnosis and value of WHO classification in a mono-institutional series of 180 non-functioning pancreatic endocrine tumours. Ann Oncol 2008;19(5):903–908. [DOI] [PubMed] [Google Scholar]
- 19.Schindl M, Kaczirek K, Kaserer K, Niederle B. Is the new classification of neuroendocrine pancreatic tumors of clinical help? World J Surg 2000;24(11):1312–1318. [DOI] [PubMed] [Google Scholar]
- 20.Sarmiento JM, Farnell MB, Que FG, Nagorney DM. Pancreaticoduodenectomy for islet cell tumors of the head of the pancreas: long-term survival analysis. World J Surg 2002;26(10):1267–1271. [DOI] [PubMed] [Google Scholar]
- 21.Hochwald SN, Zee S, Conlon KC, et al. Prognostic factors in pancreatic endocrine neoplasms: an analysis of 136 cases with a proposal for low-grade and intermediate-grade groups. J Clin Oncol 2002;20(11):2633–2642. [DOI] [PubMed] [Google Scholar]
- 22.Tomassetti P, Campana D, Piscitelli L, et al. Endocrine pancreatic tumors: factors correlated with survival. Ann Oncol 2005;16(11):1806–1810. [DOI] [PubMed] [Google Scholar]
- 23.Scarpa A, Mantovani W, Capelli P, et al. Pancreatic endocrine tumors: improved TNM staging and histopathological grading permit a clinically efficient prognostic stratification of patients. Mod Pathol 2010;23(6):824–833. [DOI] [PubMed] [Google Scholar]
- 24.Amin MB, Greene FL, Edge SB, et al. The Eighth Edition AJCC Cancer Staging Manual: Continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA Cancer J Clin 2017;67(2):93–99. [DOI] [PubMed] [Google Scholar]
- 25.Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol 2010;17(6):1471–1474. [DOI] [PubMed] [Google Scholar]
- 26.Kunz PL, Reidy-Lagunes D, Anthony LB, et al. Consensus guidelines for the management and treatment of neuroendocrine tumors. Pancreas 2013;42(4):557–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Regenet N, Carrere N, Boulanger G, et al. Is the 2-cm size cutoff relevant for small nonfunctioning pancreatic neuroendocrine tumors: A French multicenter study. Surgery 2016;159(3):901–907. [DOI] [PubMed] [Google Scholar]
- 28.Cherenfant J, Stocker SJ, Gage MK, et al. Predicting aggressive behavior in nonfunctioning pancreatic neuroendocrine tumors. Surgery 2013;154(4):785–791; discussion 791–783. [DOI] [PubMed] [Google Scholar]
- 29.Postlewait LM, Ethun CG, Baptiste GG, et al. Pancreatic neuroendocrine tumors: Preoperative factors that predict lymph node metastases to guide operative strategy. J Surg Oncol 2016;114(4):440–445. [DOI] [PubMed] [Google Scholar]
- 30.Parekh JR, Wang SC, Bergsland EK, et al. Lymph node sampling rates and predictors of nodal metastasis in pancreatic neuroendocrine tumor resections: the UCSF experience with 149 patients. Pancreas 2012;41(6):840–844. [DOI] [PubMed] [Google Scholar]
- 31.Hashim YM, Trinkaus KM, Linehan DC, et al. Regional lymphadenectomy is indicated in the surgical treatment of pancreatic neuroendocrine tumors (PNETs). Ann Surg 2014;259(2):197–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Curran T, Pockaj BA, Gray RJ, Halfdanarson TR, Wasif N. Importance of lymph node involvement in pancreatic neuroendocrine tumors: impact on survival and implications for surgical resection. J Gastrointest Surg 2015;19(1):152–160; discussion 160. [DOI] [PubMed] [Google Scholar]
- 33.Hasegawa T, Yamao K, Hijioka S, et al. Evaluation of Ki-67 index in EUS-FNA specimens for the assessment of malignancy risk in pancreatic neuroendocrine tumors. Endoscopy 2014;46(1):32–38. [DOI] [PubMed] [Google Scholar]
- 34.Weynand B, Borbath I, Bernard V, et al. Pancreatic neuroendocrine tumour grading on endoscopic ultrasound-guided fine needle aspiration: high reproducibility and inter-observer agreement of the Ki-67 labelling index. Cytopathology 2014;25(6):389–395. [DOI] [PubMed] [Google Scholar]
- 35.Lowe K, Khithani A, Liu E, et al. Ki-67 labeling: a more sensitive indicator of malignant phenotype than mitotic count or tumor size? J Surg Oncol 2012;106(6):724–727. [DOI] [PubMed] [Google Scholar]


